Abstract
Background
Cannabidiol (CBD) and cannabidiolic acid (CBDA) are reported to have antinociceptive, immunomodulatory and anti‐inflammatory actions.
Objectives
To determine if CBD/CBDA is an effective therapy for canine atopic dermatitis (cAD).
Animals
Thirty‐two privately owned dogs with cAD.
Materials and methods
Prospective, randomised, double‐blinded, placebo‐controlled study. Concurrent therapies were allowed if remained unchanged. Dogs were randomly assigned to receive either 2 mg/kg of an equal mix of CBD/CBDA (n = 17) or placebo for 4 weeks. On Day (D)0, D14 and D28, Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) and pruritus Visual Analog Scale (pVAS) scores were determined by investigators and owners, respectively. Complete blood count, serum biochemistry profiles and cytokine bioassays were performed on serum collected on D0 and D28.
Results
There was no significant difference in CADESI‐04 from D0 to D14 (p = 0.42) or D28 (p = 0.51) in either group. pVAS scores were significantly lower for the treatment group at D14 (p = 0.04) and D28 (p = 0.01) and a significant change in pVAS from baseline was seen at D14 (p = 0.04) and not D28 (p = 0.054) between groups. There was no significant difference in serum levels of interleukin (IL)‐6, IL‐8, monocyte chemoattractant protein ‐ 1, IL‐31 or IL‐34 between groups at D0 or D28. Elevated alkaline phosphatase was observed in four of 17 treatment group dogs.
Conclusions and clinical relevance
CBD/CBDA as an adjunct therapy decreased pruritus, and not skin lesions associated with cAD in dogs.
Keywords: acid, atopic, canine, cannabidiol, cannabidiolic, dermatitis, hemp
Background– Cannabidiol (CBD) and cannabidiolic acid (CBDA) are reported to have antinociceptive, immunomodulatory and anti‐inflammatory actions. Objectives – To determine if CBD/CBDA is an effective therapy for canine atopic dermatitis (cAD). Conclusions and clinical relevance – Cannabidiol/CBDA as an adjunct therapy decreased pruritus, and not skin lesions associated with cAD in dogs.
Résumé
Contexte ‐ Le cannabidiol (CBD) et l'acide cannabidiolique (CBDA) auraient des actions antinociceptives, immunomodulatrices et anti‐inflammatoires.
Objectifs – Déterminer si le CBD/CBDA est une thérapie efficace pour la dermatite atopique canine (cAD).
Animaux – Trente‐deux chiens de propriétaires privés atteints de cAD
Matériels et méthodes – Étude prospective, randomisée, en double aveugle, contrôlée versus placebo. Les thérapies concomitantes étaient autorisées si elles restaient inchangées. Les chiens ont été répartis au hasard pour recevoir soit 2 mg/kg d'un mélange égal de CBD/CBDA (n = 17) soit un placebo pendant quatre semaines. Aux jours (J)0, J14 et J28, les scores Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) et prurit Visual Analog Scale (pVAS) ont été déterminés respectivement par les investigateurs et les propriétaires. Une formule sanguine complète, des profils biochimiques sériques et des dosages biologiques des cytokines ont été réalisés sur le sérum prélevé à J0 et J28.
Résultats – Il n'y avait pas de différence significative au CADESI‐04 de J0 à J14 (P = 0,42) ou J28 (P = 0,51) dans les deux groupes. Les scores pVAS étaient significativement inférieurs pour le groupe de traitement à J14 (P = 0,04) et J28 (P = 0,01) et un changement significatif de la pVAS par rapport à l'inclusion a été observé à J14 (P = 0,04) et non à J28 (P = 0,054) entre les groupes. Il n'y avait pas de différence significative dans les taux sériques d'interleukine (IL)‐6, IL‐8, protéine chimiotactique des monocytes‐1, IL‐31 ou IL‐34 entre les groupes à J0 ou J28. Une phosphatase alcaline élevée a été observée chez quatre des 17 chiens du groupe de traitement.
Conclusions et pertinence clinique ‐ Le CBD/CBDA en tant que traitement d'appoint a diminué le prurit, et non les lésions cutanées associées à la DAC chez les chiens.
Resumen
Introducción‐ se ha descrito que el cannabidiol (CBD) y el ácido cannabidiólico (CBDA) tienen acciones antinociceptivas, inmunomoduladoras y antiinflamatorias.
Objetivos‐ determinar si el CBD/CBDA es una terapia eficaz para la dermatitis atópica canina (CAD).
Animales ‐ Treinta y dos perros de propietarios privados con cAD
Materiales y métodos – Estudio prospectivo, aleatorio, doble ciego, controlado con placebo. Se permitieron terapias concurrentes si permanecían sin cambios. Los perros fueron asignados al azar para recibir 2 mg/kg de una mezcla igual de CBD/CBDA (n = 17) o placebo durante cuatro semanas. En el día (D)0, D14 y D28, los investigadores y los propietarios determinaron las puntuaciones del índice de extensión y gravedad de la dermatitis atópica canina, cuarta revisión (CADESI‐04) y la escala análoga visual de prurito (pVAS), respectivamente. Se realizaron hemogramas completos, perfiles bioquímicos séricos y bioensayos de citoquinas en suero obtenido en D0 y D28.
Resultados‐ no hubo diferencias significativas en CADESI‐04 de D0 a D14 (P = 0,42) o D28 (P = 0,51) en ninguno de los grupos. Las puntuaciones de pVAS fueron significativamente más bajas para el grupo de tratamiento en D14 (P = 0.04) y D28 (P = 0.01) y se observó un cambio significativo en pVAS desde el inicio en D14 (P = 0.04) y no en D28 (P = 0.054) entre grupos . No hubo diferencias significativas en los niveles séricos de interleuquina (IL)‐6, IL‐8, proteína quimioatrayente de monocitos‐1, IL‐31 o IL‐34 entre los grupos en D0 o D28. Se observó fosfatasa alcalina elevada en cuatro de los 17 perros del grupo de tratamiento.
Conclusiones y relevancia clínica‐ CBD/CBDA como terapia adjunta disminuyó el prurito y no las lesiones cutáneas asociadas con la CAD en perros.
Zusammenfassung
Hintergrund – Eine antinozizeptive, immunmodulatorische und entzündungshemmende Wirkung von Cannabidiol (CBD) und Cannabidiolsäure (CBDA) ist beschrieben.
Ziele – Es sollte festgestellt werden, ob CBA/CBDA eine wirksame Therapie für die atopische Dermatitis (cAD) des Hundes darstellt.
Materialien und Methoden – Es handelt sich um eine prospektive, randomisierte, doppelblinde, Plazebo‐kontrollierte Studie. Begleitende Behandlungen waren erlaubt, sofern sie nicht verändert wurden. Die Hunde wurden zufällig eingeteilt, um entweder 2 mg/kg einer gleichen Mischung von CBD/CBDA (n = 17) oder Plazebo vier Wochen lang zu erhalten. Am Tag (D) 0, D14 und D28 wurden mittels Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) bzw Pruritus Visual Analog Scale (pVAS) entsprechende Werte durch UntersucherInnen bzw BesitzerInnen bestimmt. Es wurden ein großes Blutbild, Serum Biochemie und Zytokin Assays am Serum, welches an D0 und D28 genommen wurde, durchgeführt.
Ergebnisse – Es bestand in keiner der Gruppen ein signifikanter Unterschied des CADESI‐04 zwischen D0 und D14 (P = 0,42) oder D28 (P = 0,51). pVAS Werte waren in der Behandlungsgruppe am D14 (P = 0,04) und am D28 (P = 0,01) signifikant niedriger und eine signifikante Veränderung der pVAS Basiswerte zwischen den Gruppen wurde am D14 (P = 0,04) und nicht am D28 (P = 0,054) gefunden. Es bestand kein signifikanter Unterschied zwischen den Serumwerten von Interleukin (IL)‐6, IL‐8, Monozyten Chemoattractant Protein‐1 , IL‐31 oder IL‐34 zwischen den Gruppen am D0 oder D28. Es wurden bei Hunden in vier der 17 Behandlungsgruppen erhöhte Werte der Alkalischen Phosphatase gefunden.
Schlussfolgerungen und klinische Bedeutung – CBD/CBDA als Zusatztherapie verminderte den Juckreiz, aber nicht die Hautveränderungen, die mit cAD bei Hunden einhergehen.
要約
背景 ‐ カンナビジオール (CBD) およびカンナビジオール酸 (CBDA) は、抗侵害受容作用、免疫調節作用、抗炎症作用を有すると報告されている。
目的 – 本研究の目的は、CBD/CBDAが犬アトピー性皮膚炎 (cAD) に対して有効な治療法であるかどうかを明らかにすることであった。
被検動物 ‐ cAD を有するオーナー所有犬 32 頭
材料と方法 ‐ 前向き無作為化二重盲検プラセボ対照試験。併用療法は、変化がない場合は許可された。犬はCBD/CBDAの等量混合物2mg/kg(n = 17) またはプラセボのいずれかを4週間投与するよう無作為に割り当てられた。D0、D14およびD28に、犬アトピー性皮膚炎の程度および重症度指数 (CADESI‐04)、痒みのビジュアルアナログスケール (pVAS) スコアをそれぞれ調査員、飼い主が決定した。D0とD28に採取した血清について、全血球数、血清生化学プロファイル、サイトカイン・バイオアッセイ法を実施した。
結果 ‐ CADESI‐04のD0からD14(P = 0.42) またはD28(P = 0.51) まで、いずれの群でも有意差はなかった。pVASスコアはD14(P = 0.04) およびD28(P = 0.01) で治療群に有意に低く、ベースラインからのpVASの有意変化はD14(P = 0.04) で見られ、D28(P = 0.054) では認められなかった。インターロイキン (IL)‐6、IL‐8、単球走化性タンパク質‐1、IL‐31、IL‐34の血清レベルには、D0とD28で群間に有意な差はなかった。アルカリフォスファターゼの上昇が17頭中4頭で観察された。
結論および臨床的意義 ‐ CBD/CBDAを補助療法として投与することで、cADに関連する皮膚病変ではなく、犬の痒みを減少させることができた。
摘要
背景‐据报告, 大麻二醇(CBD)和大麻二酚酸(CBDA)具有镇痛、免疫调节和抗炎作用。
目的‐确定CBD/CBDA是否是犬特应性皮炎(cAD)的有效疗法。
动物‐32只患有cAD的私家犬。
材料和方法‐前瞻性、随机、双盲、安慰剂对照研究。如果保持不变, 允许合并治疗。将犬随机分配至2 mg/kg CBD/CBDA等混合物组(n = 17)或安慰剂组, 持续4周。在第(D)0天、第14天和第28天, 由研究者和犬主人分别测定犬特应性皮炎程度和严重指数、第4版(CADESI‐04)和瘙痒视觉模拟量表(pVAS)评分。对D0和D28采集的血清进行全血细胞计数、血清生化特征和细胞因子生物测定。
结果‐两组中从D0至D14(P = 0.42)或D28(P = 0.51)的CADESI‐04无显著差异。治疗组在D14(P = 0.04)和D28(P = 0.01)的pVAS评分显著降低, 在D14(P = 0.04)而非D28(P = 0.01)观察到pVAS相对于基线的显著变化.054) 。D0或D28时, 组间血清白细胞介素(IL)‐6、IL‐8、单核细胞趋化蛋白‐1、IL‐31或IL‐34水平无显著差异。在17只治疗组犬的4只中观察到碱性磷酸酶升高。
结论和临床相关性–CBD/CBDA作为辅助治疗减少了犬中的瘙痒, 而对cAD相关的皮肤病变无效。
Resumo
Contexto – O canabidiol (CBD) e ácido canabidiólico (CBDA) são relatados como tendo ações antinociceptivas, imunomoduladoras e anti‐inflamatórias.
Objetivos – Determinar se CBD/CBDA é eficaz no tratamento da dermatite atópica canina (CAD)
Animais ‐ Trinta e dois cães de propriedade privada com DAC.
Materiais e métodos ‐ Estudo prospectivo, randomizado, duplo‐cego, placebo‐controle. As terapias concomitantes foram permitidas se permanecessem inalteradas. Os cães foram divididos aleatoriamente em dois grupos, o que receberia 2 mg/kg de uma mistura igual de CBD/CBDA (n = 17) ou placebo durante quatro semanas. No Dia (D) 0, D14 e D28, o Índice de Extensão e Gravidade da Dermatite Atópica Canina, 4ª iteração (CADESI‐04) e os escores da Escala Visual Analógica de Prurido (pVAS) foram determinados pelos investigadores e proprietários, respectivamente. Hemograma completo, perfis bioquímicos séricos e ensaios de citocinas foram realizados no soro coletado em D0 e D28.
Resultados ‐ Não houve diferença significativa no CADESI‐04 de D0 a D14 (P = 0,42) ou D28 (P = 0,51) em nenhum dos grupos. Os escores de pVAS foram significativamente menores para o grupo de tratamento no D14 (P = 0,04) e D28 (P = 0,01) e observou‐se uma alteração significativa no pVAS do D0 comparado ao D14 (P = 0,04) e não ao D28 (P = 0,054) entre os grupos. Não houve diferença significativa nos níveis séricos de interleucina (IL)‐6, IL‐8, proteína quimiotática de monócitos‐1, IL‐31 ou IL‐34 entre os grupos em D0 ou D28. Elevação na fosfatase alcalina foi observada em quatro dos 17 cães do grupo de tratamento.
Conclusões e relevância clínica – CBD e CBDA como uma terapia adjuvante é capaz de reduzir prurido, mas não lesões cutâneas associadas à DAC em cães.
INTRODUCTION
Canine atopic dermatitis (cAD) is a common inflammatory and pruritic allergic skin disease that is characterised by excessive immunoglobulin (Ig)E production directed against allergens. Due to the multifactorial and progressive nature of the disease, multimodal and individualised therapeutic regimens often are indicated. 1
Cannabinoids are a unique group of chemical compounds found in Cannabis sativa plants. Cannabidiol (CBD) is the predominant nonpsychotropic cannabinoid found in the cannabis plant and as such, CBD‐rich plants are classified as hemp rather than marijuana. Cannabidiolic acid (CBDA) is the precursor carboxylic acid form of CBD, and a recent study showed similar and possibly better absorption than CBD in dogs. 2
Immunomodulatory and anti‐inflammatory actions of CBD/CBDA in mammals have been reported. 3 , 4 These cannabinoids appear to be well‐tolerated and effective at reducing osteoarthritic pain and seizures in dogs. 2 , 5 , 6 , 7 , 8
The G‐protein‐coupled cannabinoid receptors (CB1 and CB2) are both expressed in canine keratinocytes, with immunoreactivity to CB1 and CB2 being heightened in atopic dogs compared to healthy dogs. 9 Cannabinoid receptor agonists reduce skin lesions and pruritus in atopic dogs, and attenuated inflammation in the skin of mice in a model of contact hypersensitivity. 10 , 11
Cannabidiol does not appear to interact with CB1 or CB2 receptors directly, yet has been implicated in altering endogenous levels of naturally derived endocannabinoids such as anandamide. CBD also may interact with other receptor systems in the inflammatory cells or neurons such as the transient receptor activation channels (TRPV), adenosine reuptake inhibitor as well as peroxisome proliferation and activation receptors (PPAR) based on in vitro and in vivo assessments in humans and rodents. 12 , 13 , 14 , 15 , 16 , 17 , 18
To the best of the authors' knowledge, no studies investigating the efficacy of CBD/CBDA as a treatment for cAD have been conducted. The primary objective of this study was to determine if CBD/CBDA‐rich hemp extract decreased pruritus and cutaneous lesions in dogs with cAD. Secondary objectives included determining whether CBD‐rich hemp extract caused any adverse effects through client surveys, routine complete blood counts, serum biochemistry and evaluating serum cytokines before and after its administration for 28 days.
MATERIALS AND METHODS
Ethics
This study was approved by the Cornell University Institutional Animal Care and Use Committee (IACUC) 2019–0119.
Dog owners signed a written informed consent form before inclusion and could withdraw at any time.
Overview
The study was a 4 week, randomised, double‐blinded and placebo‐controlled trial. Client‐owned dogs were assigned randomly to a CBD/CBDA group or placebo group using a computer‐based random number generator.
Enrolment criteria
Client‐owned dogs (irrespective of breed, age or sex) with visible signs of pruritic skin disease and diagnosed with cAD based on published guidelines were enrolled. 19 Dogs included in the study met the following criteria: (i) current on flea prevention; (ii) confirmed to not have an adverse food reaction after an 8 weeks diet trial (hydrolysed or novel protein) with subsequent negative challenge or if previously confirmed to have an adverse food reaction were controlled on a diet; (iii) Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) score was >9 and <60; (iv) pruritus Visual Analog Scale (pVAS) of between 3 and 8 cm on a previously validated scale; 20 and (v) maintained flea prevention throughout study.
Dogs were excluded if: (i) there was clinical evidence of bacterial or fungal skin infections; (ii) systemic or dermatological causes of pruritus other than cAD were present; (iii) oral antibiotics were used 7 days before enrollment or throughout study; (iv) treatment with lokivetmab or long‐acting corticosteroid injection were used less than 8 weeks before enrollment; (v) allergen‐specific immunotherapy (ASIT) was introduced <52 weeks before enrollment; (vi) administration of dietary supplements or nutraceuticals was changed or introduced less than 2 weeks before enrollment; (vii) oral glucocorticoids, oclacitinib, azole antifungals, terbinafine and antihistamine dosing was changed less than 2 weeks before enrollment; (viii) ciclosporin dosing was changed less than 8 weeks before enrollment; (ix) antimicrobial topical protocol was changed less than 2 weeks before enrollment; (x) bathing frequency was changed less than 2 weeks before enrollment; and (xi) patient's pre‐existing medication regimen changed in any other way throughout the study.
Study protocol
Dogs were evaluated on the day of enrollment, which was classified as Day (D)0, D14 and D28. A validated owner‐assessed pVAS was used to determine severity of pruritus at each evaluation. 20 CADESI‐04 was performed at each evaluation. 21 Complete blood counts (CBC) and biochemistry profiles were performed on D0 and D28. Serum from D0 and D28 were collected and stored at −20°C for cytokine analysis, shipped on dry ice within 4 months and stored at −80°C until analysis.
All adverse events (AEs) were reported on D14 and D28. Owners were asked if they thought the treatment was effective at the conclusion of the study in a standard written survey (see Supporting information Appendix S1).
Assessment of efficacy
The outcome measures were the differences in pruritus and lesion severity at D14 and D28 from baseline. Treatment was considered successful if the pVAS and/or CADESI‐04 scores showed a statistically significant reduction compared with the initial value at D0 when compared to the placebo group. We also quantified the reduction in pruritus using an improvement of ≥2 cm as indicating treatment success, as has been used in other similar studies, and additionally we assessed the number of dogs achieving ≤1.9 cm on the scale. 10 , 22 , 23 Assessment of treatment effect using these outcome measures was assessed as responder and nonresponder based on client exit survey at the end of treatment through Fisher's exact testing.
Tolerability
Tolerability was assessed by recording AEs and withdrawals at any time during the study. All AEs that occurred during the study were recorded by a noninvestigator to maintain blinding on an AE reporting form, together with their onset, severity and perceived association with the study product.
Study product
The study product was an equal mix of CBD/CBDA in a sesame oil vehicle (Ellevet) with third‐party analysis of the product showing that it contained approximately 30 mg/ml CBD, 31 mg/ml CBDA, 1.2 mg Δ9‐THC and 1.3 mg/ml THCA provided in gelatin capsules in 5, 10, 20 and 30 mg/ml CBD/CBDA increments for dosing. The placebo was sesame oil‐filled capsules of similar increments. Dogs were administered CBD/CBDA (approximately 2 mg/kg) or placebo, twice daily with a meal for the entire study period of 28 days.
Cytokine assays
Serum from all patients at D0 and D28 were analysed for selected cytokines using commercially available canine‐specific enzyme‐linked immunosorbent assay (ELISA) techniques. Serum levels of interleukin (IL)‐6 and IL‐8 (Luminex Platform, Millipore), monocyte chemoattractant protein (MCP)‐1(Millipore), and IL‐31 and IL‐34 (MBS039863, MyBiosource) were evaluated from serum on D0 and D28. All kits were used according to the manufacturers' recommendations, running each sample in duplicate.
Serum CBD/CBDA concentrations
Analysis was performed using gas chromatography and mass spectrometry based on previously published methods for evaluation on stored serum from dogs in the treatment group that were sent to a contract research laboratory (University of Illinois at Chicago, Toxicology Laboratory) for validated CBD and CBDA analysis for D0 and D28 samples. 2
Statistical methods
Statistical analysis was performed with a commercially available software package (JMP 12.0, JMP). All continuous data were assessed utilising a Shapiro–Wilk test for normality. Considering the majority of our blood cell count, serum biochemistry and inflammatory cytokine data were normally distributed, a two‐way ANOVA with repeated measures (ANOVA‐RM) was used to analyse these outcomes, including the fixed effects of treatment, time, and treatment × time. Pairwise comparisons between all time points of both groups were corrected for multiple comparisons with Tukey's post hoc tests to examine the interaction of time and treatment variables, and to assess differences between change from baseline at any time point as they related to treatment. For ordinal veterinary surgeon and owner VAS scoring data (CADESI‐04 and pVAS) also were analysed using ANOVA‐RM as Shapiro–Wilk testing revealed normality of the data. In addition, a change from baseline pVAS for both the treatment and placebo groups was assessed using an unpaired unequal variance Student's t‐test at D14 and D28 to assess significant differences between groups. A p‐value of <0.05 was defined as significant for all analyses.
Further assessment of the treatment effect was assessed as responder and nonresponder based on client exit survey, dogs with ≥2 cm reduction in pVAS during treatment, and a pVAS of ≤1.9 cm were deemed to be normalised through Fisher's exact testing with a p‐value set at 0.05.
Regression analysis was performed on changes over time in pVAS compared to total CBD/CBDA concentration in the serum using Pearson's linear regression. A p‐value <0.05 was considered significant. The correlation R values were considered weak if ≤0.3, mild if >0.3 and ≤0.5, moderate if >0.5 and ≤0.7, and strong if >0.7.
Data graphing was performed using prism software (v6, GraphPad Software Inc.).
RESULTS
Thirty‐two dogs were enrolled (Table 1). Three dogs were excluded from the full data analysis: one dog dropped out after 2 weeks as a result of perceived behavioural changes; one dog was withdrawn from the study as a result of failure of its owner to comply with consistent antiparasitic prophylaxis; and one dog was withdrawn as a consequence of a dosing error. Twenty‐nine dogs (17 treatment, 12 placebo) were included in the complete statistical evaluation. The individual daily dose for analysed dogs in the treatment group was 4.48 mg/kg daily equally divided in a twice‐daily dosing regimen (range: 3.69–5.97, median: 4.40).
TABLE 1.
Case data for dogs included in the cannabidiol (CBD) treatment study
| Dog no. | Signalment | Group | CBD dose (mg/kg) twice daily |
|---|---|---|---|
| 1 | 13 y/o M/N schnoodle | CBD | 2.2 |
| 3 | 9 y/o M/N English bulldog | CBD | 2.4 |
| 9 | 9 y/o M/N shih tzu | CBD | 2.3 |
| 11 | 8 y/o M/N mixed breed | CBD | 1.9 |
| 12 | 8 y/o M/N goldendoodle | CBD | 2.2 |
| 16 | 9 y/o F/S shih tzu | CBD | 2.1 |
| 18 | 10 y/o F/S mixed breed | CBD | 2.1 |
| 19 | 4 y/o F/S German shepherd dog | CBD | 1.9 |
| 20 | 4 y/o M/N mixed breed | CBD | 2.1 |
| 21 | 3 y/o F/S Labrador retriever | CBD | 2.1 |
| 22 | 3 y/o M/N shih tzu | CBD | 2.5 |
| 25 | 7 y/o M/I German shepherd dog | CBD | 2.1 |
| 26 | 6 y/o M/N Tibetan terrier | CBD | 2.4 |
| 28 | 3 y/o F/S golden retriever | CBD | 2.1 |
| 30 | 8 y/o F/S Havanese | CBD | 3 |
| 31 | 6 y/o M/N Dachshund | CBD | 3 |
| 32 | 3 y/o F/S French bulldog | CBD | 2.2 |
| 2 | 1 y/o M/I Labrador retriever | PB | |
| 4 | 10 y/o F/S cockapoo | PB | |
| 5 | 10 y/o M/N mixed breed | PB | |
| 7 | 9 y/o M/N bichon frise | PB | |
| 8 | 2 y/o M/N shiba inu | PB | |
| 10 | 6 y/o M/I mixed breed | PB | |
| 13 | 6 y/o M/I Rottweiler | PB | |
| 14 | 5 y/o F/S French bulldog | PB | |
| 23 | 3 y/o M/N mixed breed | PB | |
| 24 | 2 y/o M/N mixed breed | PB | |
| 27 | 5 y/o M/I cane corso | PB | |
| 29 | 8 y/o F/S Havanese | PB |
Note: Dogs 6, 15 and 17 did not complete the study and therefore are not included in this table.
Abbreviations: A, appetite; B, behaviour; L, lethargy; M/I, male intact; M/N, male neutered; PB, placebo; R, regurgitation; S/F, female spayed; y/o, year‐old.
Pruritus
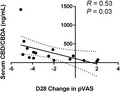
Mean pVAS (±SD) scores at D0, D14 and D28 in the treatment group, were 5.5 ± 1.2, 3.5 ± 2.0 and 3.3 ± 2.3, respectively. Mean pVAS at D0, D14 and D28 in the placebo group were 5.6 ± 1.4, 5.1 ± 1.7 and 5.0 ± 2.0, respectively. The pVAS scores were statistically significantly lower for the treatment group at both time points (D14: p = 0.04, D28: p = 0.01), and a significant decrease in pVAS from baseline was seen at D14 (p = 0.04) and not D28 (p = 0.054) when comparing treatment and placebo groups (Figure 1a–c). Using criteria of ≥2 cm improvement to evaluate clinical significance, nine of 17 dogs improved at D14 (p = 0.003) when compared to zero of 12 in the placebo group. In the treatment group, 11 of 17 had a decrease of ≥2 cm at D28 (p = 0.022) when compared to two of 17 dogs improving at D28 in the placebo group. Only three of 12 dogs at D14 achieved a normalised PVAS of 1.9 cm compared to zero of 12 in the placebo group (p = 0.25). Six dogs at D28 in the treatment group achieved a pVAS of ≤1.9 cm, while no dogs in the placebo group achieved a “normal” score, per derivation of a normal range of ≤1.9 for the pVAS (p = 0.028). 24
FIGURE 1.
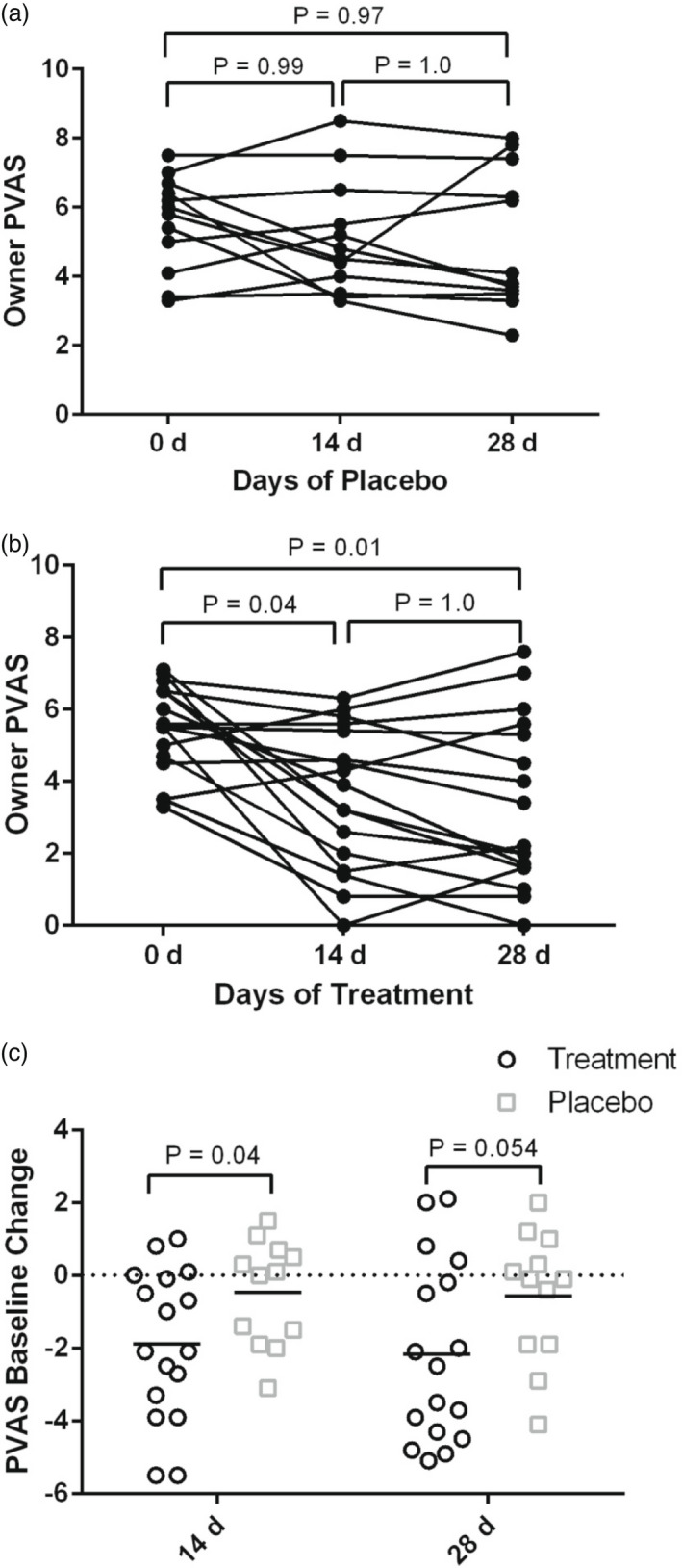
Dot plots for owner pruritus Visual Analog Scale (pVAS) scores following treatment with cannabidiol (CBD)/ cannabidiolic acid (CBDA) or placebo. (a) Placebo group at Day (D)0, D14 and D28. P‐values indicate no significance from baseline scores. (b) treatment group at D0, D14 and D28. Significant p ‐values were observed from D0 to D14 (p = 0.04) and D0–D28 (p = 0.01), and not D14–D28 (p = 1.0). (c) Mean change in score from baseline at D14 and D28 in treatment and placebo groups. Statistical significance was observed when examining change from baseline pVAS at D14 between treatment and placebo groups (p = 0.04), and not at D28 (p = 0.054)
Ten of 17 treatment group owners answered “yes” to the question “Did you notice an improvement in your dog's itch throughout the study?” (Appendix S1). Only two of 13 placebo group owners answered “yes” with one dog dropping out before the conclusion of the study, which was statistically significant (p = 0.026).
Lesions
Mean CADESI‐04 scores at D0, D14 and D28 in the treatment group were 27.8 ± 9.4, 25.3 ± 10.3 and 25.0 ± 11.0, respectively. Mean scores at D0, D14 and D28 in the placebo group were 29.0 ± 8.9, 27.6 ± 10.4 and 26.3 ± 13.7, respectively. There was no significant difference in CADESI‐04 from D0 to D14 (p = 0.42) or D28 (p = 0.51) in either group (Figure 2a and b).
FIGURE 2.
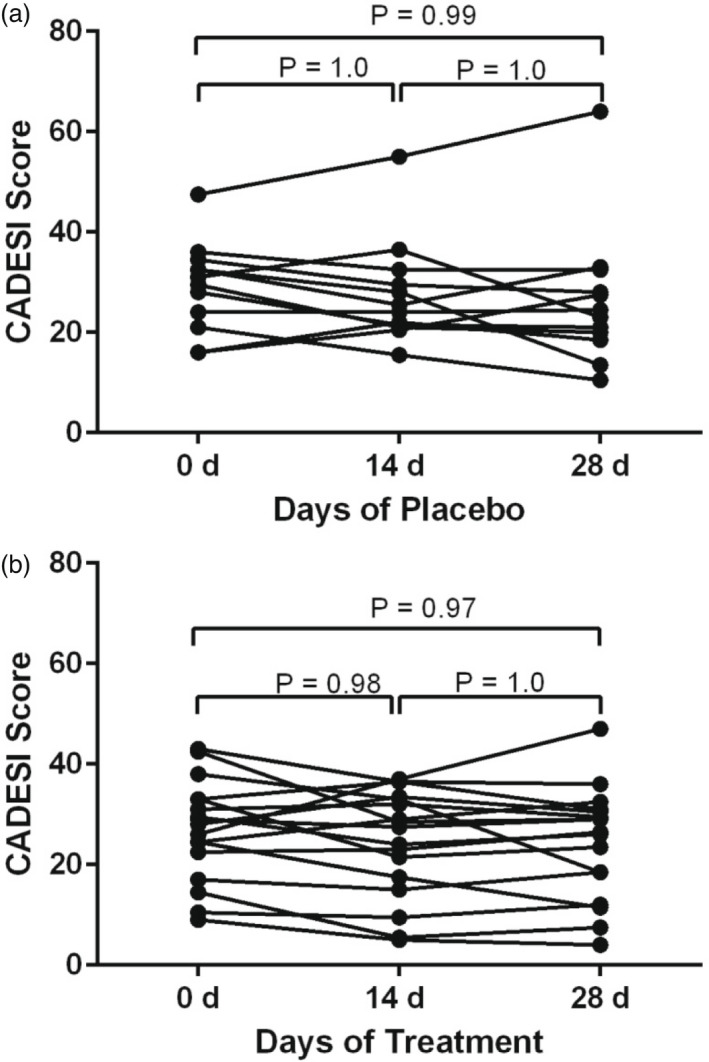
Canine Atopic Dermatitis Extent and Severity Index, 4th iteration (CADESI‐04) lesion scoring by veterinary surgeons.
(a) Dot plots at Day (D)0, D14 and D28 in the placebo group. No statistical significance from baseline or across days of treatment was observed. (b) Dot plots at D0, D14 and D28 in the treatment group. No statistical significance from baseline or across days of treatment was observed
Cytokines
Cytokine means (±SD) including MCP‐1, IL‐6, IL‐8, IL‐31 and IL‐34 can be found in Table 2. There were no significant differences noted between treatment, time, or treatment × time for any of the cytokines measured at D0 and D28 (Table 3).
TABLE 2.
Mean (±SD) of selected serum chemistry parameters measured at Day (D)0 and D28 for dogs in the placebo and treatment groups. Significance for treatment, time and treatment × time was set at p ≤ 0.05
| Parameter (ref. range) |
Treatment D0 |
Treatment D28 |
Placebo D0 |
Placebo D28 |
Time |
P Treatment |
Treatment × time |
|---|---|---|---|---|---|---|---|
| Glucose (74–143 mg/dl) | 103 ± 10 | 101 ± 17 | 104 ± 12 | 105 ± 10 | 0.92 | 0.6 | 0.63 |
| Creatinine (0.5–1.8 mg/dl) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.46 | 0.42 | 0.58 |
| Urea nitrogen (7–27 mg/dl) | 16 ± 6 | 16 ± 7 | 14.2 ± 3.0 | 14.3 ± 3.4 | 0.85 | 0.08 | 0.96 |
| Phosphorus (2.5–6.8 mg/dl) | 3.6 ± 0.8 | 3.6 ± 0.8 | 4.1 ± 0.6 | 4.3 ± 0.7 | 0.68 | <0.01 | 0.48 |
| Calcium (7.9–12.0 mg/dl) | 10.0 ± 0.4 | 9.7 ± 0.5 | 9.7 ± 0.5 | 9.7 ± 0.6 | 0.17 | 0.24 | 0.45 |
| Total protein (5.2–8.2 g/dl) | 6.9 ± 0.6 | 7 ± 0.5 | 6.8 ± 0.3 | 7.0 ± 0.6 | 0.65 | 0.84 | 0.48 |
| Albumin (2.3–4.0 g/dl) | 3.4 ± 0.2 | 3.3 ± 0.3 | 3.3 ± 0.3 | 3.4 ± 0.6 | 0.85 | 0.68 | 0.52 |
| Globulin (2.5–4.5 g/dl) | 3.6 ± 0.6 | 3.6 ± 0.4 | 3.5 ± 0.3 | 3.7 ± 0.3 | 0.59 | 0.93 | 0.78 |
| ALT (10–125 U/L) | 59 ± 35 | 55 ± 25 | 77 ± 52 | 59 ± 42 | 0.26 | 0.28 | 0.46 |
| AST (0–50 U/L) | 46 ± 21 | 40 ± 14 | 36 ± 10 | 47 ± 22 | 0.52 | 0.93 | 0.07 |
| ALP (23–212 U/L) | 117 ± 186 | 215 ± 303 | 102 ± 92 | 96 ± 79 | 0.32 | 0.11 | 0.27 |
| GGT (0–11 U/L) | 5 ± 3 | 5 ± 3.7 | 4.2 ± 2.8 | 4.5 ± 3.5 | 0.56 | 0.22 | 0.89 |
| Bilirubin (0.0–0.9 mg/dl) | 0.4 ± 0.2 | 0.4 ± 0.6 | 0.3 ± 0.2 | 0.5 ± 0.8 | 0.33 | 0.87 | 0.75 |
| Cholesterol (110–320 mg/dl) | 242 ± 57 | 237 ± 61 | 211 ± 49 | 207 ± 48 | 0.76 | <0.01 | 0.98 |
Abbreviations: ALP, alkaline phosphatase activity; ALT, alanine aminotransferase activity; AST, aspartate aminotransferase activity; GGT, gamma glutamyl transferase activity.
TABLE 3.
Mean (±SD) serum cytokine measurements at Day (D)0 and D28 including monocyte chemotactic protein‐1 (MCP‐1), interleukin (IL)‐6, IL‐8, IL‐31 and IL‐34. No significances in treatment, time or treatment × time were noted
| Cytokine | Treatment D0 | Treatment D28 | Placebo D0 | Placebo D28 | Time |
P Treatment |
Treatment × time |
|---|---|---|---|---|---|---|---|
| MCP‐1 (pg/ml) | 376.5 ± 404.5 | 325.8 ± 215.3 | 228.7 ± 123.9 | 258.4 ± 147.8 | 0.86 | 0.08 | 0.51 |
| IL‐6 (pg/ml) | 17.5 ± 8.7 | 17.6 ± 9.2 | 17.9 ± 17.4 | 18.1 ± 17.4 | 0.81 | 0.42 | 0.94 |
| IL‐8 (pg/ml) | 2778 ± 2938 | 2497 ± 2246 | 1802 ± 1697 | 1857 ± 1715 | 0.82 | 0.37 | 0.61 |
| IL‐31 (pg/ml) | 321.4 ± 238.4 | 386.9 ± 448.4 | 310.7 ± 248.5 | 364.3 ± 258.7 | 0.42 | 0.75 | 0.93 |
| IL‐34 (pg/ml) | 29.3 ± 28.4 | 29.2 ± 28.7 | 24.6 ± 10.3 | 23.6 ± 11.3 | 0.71 | 0.11 | 0.72 |
Overall owner satisfaction
In an end of study survey (Appendix S1) with most questions addressing adverse events, owners were also asked “Would you use this product in the future for your dog?” Ten of 17 treatment group owners answered that they would use the product again and seven of 17 would not. In the placebo group, two of 13 owners answered that they would use the product again, and 10 of 13 would not, with one dropping out before the end of the study as a consequence of adverse events and lack of efficacy.
Complete blood count and serum chemistry
Complete blood count assessment at D0 and D28 (data not shown) and serum chemistry evaluations (Table 2) revealed no significant changes over time or between groups for any parameter assessed. Of interest is that no significant changes in the treatment group or placebo group were observed for any of the hepatic enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST) and ALP] before D0 and after 28 days of twice‐daily treatment. While not significant, ALP was elevated outside the reference range in six of 17 treatment group dogs at D28. Two of these six dogs had elevated ALP at D0 and four dogs had ALP within the reference range at baseline. ALP that was elevated at baseline in one dog from the treatment group decreased from 771 to 744 IU/L, and elevated ALP in another dog increased from 336 to 1164 IU/L.
Adverse events
Adverse events are reported in Table 1. In the treatment group, AEs included lethargy, defined as loss of energy (dogs 1,18), behavioural changes (dogs 1, 3, 9, 18, 21, 32), regurgitation (Dog 11), increased flatulence (Dog 3), and inconsistent appetite (Dog 1). Behavioural changes reported included: somnolence, sleepiness (dogs 1, 18), decreased aggression (Dog 9) and increased calmness (dogs 9, 21, 32). Two dogs had increased energy/mobility (dogs 3, 11).
In the placebo group, one dog experienced diarrhoea and regurgitation (Dog 13). Lethargy and behavioural changes in one dog led to its exclusion after 14 days (Dog 6).
Cannabidiol/CBDA concentrations
Sixteen of the 17 dogs that had sufficient serum remaining after cytokine analysis had serum CBD/CBDA analysis. Median cannabidiol serum concentrations after 28 days of treatment was 112 ng/ml (range: 30–1350 ng/ml). The median CBDA serum level after 28 days of treatment was 48 ng/ml (range 10 168 mg/ml) (Table 1). Further regression statistics examining combined CBD/CBDA concentration in the serum compared to decrease in pVAS score at D28 showed a significant correlation with R = 0.53 suggesting a modest correlation with decrease in pVAS score and serum total CBD/CBDA concentration (p = 0.03; Figure 3).
FIGURE 3.
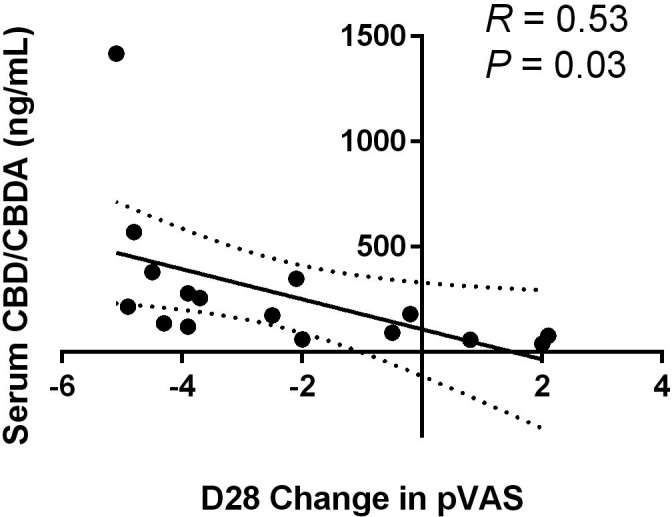
Regression of total cannabidiol (CBD)/ cannabidiolic acid (CBDA) concentration in serum relative to change in pruritus Visual Analog Scale (pVAS) score at Day 28 in 16 dogs on CBD‐rich hemp treatment
DISCUSSION
Results of this study indicated that CBD/CBDA does not affect lesion severity yet does have a positive effect on pruritus as an adjunct therapy in some dogs with cAD.
Using the criterion of ≥2 cm improvement in pVAS, nine of 17 dogs at D14 and 11 of 17 at D28 clinically and statistically improved in the treatment group, whereas zero of 12 dogs at D14 and two of 12 at D28 improved in the placebo group. Although there was a statistically significant decrease in mean pVAS scores from baseline between groups at D14, there was no significant difference at D28 even though there was a positive trend. There was a significant difference in affirmative answers to the question, “Did you notice an improvement in your dog's itch throughout the study?” between groups, supporting a treatment effect.
The pruritus reduction in the treatment group compared to placebo from baseline was observed at both time points, yet the significant difference shown between the groups at D14 only could be the result of several factors. Dogs may develop a tolerance for CBD over time, as has been seen in mice. 25 Due to CBD potential actions at the TRPV receptor systems, there may be desensitisation of these receptor systems to long‐term treatment. 12 , 17 In addition, tolerance may be caused by the hepatic metabolism of CBD induction of cytochrome p450 enzymes. This may require alteration of the dose to better tailor treatment to effect. Furthermore, it is recognised that some of the concurrent therapies that our population was receiving also may alter cytochrome p450 enzyme metabolism, further complicating the potential synergies between cannabinoids. Unfortunately, as a consequence of the low sample size, this could not be evaluated or elucidated so further studies are warranted. Based on our regression statistics, higher serum concentrations appear to be associated with better clinical effect for the reduction of pruritus. Serum concentration measurements may be indicated in the future to determine optimal dosing regimes.
There was a decrease in pruritus and not CADESI‐04. In a multicentre, open‐label, observational study using the same scoring methodology, PEA, an endocannabinoid‐like molecule, reduced skin lesions and pruritus in atopic dogs. 10 However, that study's duration was 8 weeks and, furthermore, PEA may have direct action at the CB1 and CB2 receptors which CBD does not, leaving this question open regarding CB1/CB2 agonists and cAD resolution. It is possible, however unlikely, that a longer study duration might be needed for the study of CBD/CBDA to see true anti‐inflammatory effects. Additionally, CADESI‐04 is less sensitive to changes in short‐term trials because secondary skin lesions resolve slowly. This also may have contributed to lack of improvement in skin lesions. 26
The lack of improvement in lesions also may be explained by CBD predominantly affecting the neurological (endocannabinoid) pathway rather than inflammatory cells. 12 , 17 , 18 Thus, pruritus may be improved without a change in the cytokine profile that has been associated with skin lesions in cAD. We found no significant change in serum cytokine levels over time, suggesting that the effects of CBD are through other mechanistic means of neuronal control rather than cytokine‐driven alterations. Five of the 17 dogs in the treatment group had reported lethargy or calmness, and it is possible that neurological status changed due to the CBD treatment, resulting in reduced perception or manifestations of pruritus. It is important to note that serum cytokine concentrations do not necessarily reflect those in skin lesions, and future studies evaluating the effects of CBD on skin cytokine concentrations would be useful.
A secondary objective of this study was to determine the AEs associated with CBD. Three clinical therapeutic studies in dogs using CBD noted elevations in serum ALP after therapy. 5 , 7 , 27 In our study, four dogs in the treatment group had elevations above the reference range after receiving CBD for 28 days. The ALP elevations presumably are due to upregulation of CYP450 enzymes. These elevations have been reported with prolonged exposure to hemp‐derived CBD or cannabis in humans and were considered adaptive and demonstrated reversibility. 28 Studies in dogs and cats did not demonstrate significant rises outside normal reference ranges in any enzymes associated with the hepatobiliary system. 2 , 29 A previous study employing the same product and dose as we used herein showed that healthy dogs treated twice daily for 3 months showed no evidence of hepatotoxicity, indicating that CBD at this dose is probably safe. 29
The role of CYP in the metabolism of CBD raises the question of drug interactions with concomitant medications. The four dogs on CBD with elevations in ALP outside the reference range were on concomitant medications, and three were on two or more. Ketoconazole, fluconazole and ciclosporin are all CYP450 substrates and oclacitinib and terbinafine are metabolised by the liver. 30 Interaction of CBD with one of these drugs, or possibly the combination, may be relevant and future studies are warranted.
Adverse events were considered mild, especially given that no dogs were withdrawn from the treatment group. Additionally, the majority of owners whose dogs were in the treatment group (10 of 17) answered that they would use the product again, and only two of 13 in the placebo group were willing to continue use. Only one owner (of Dog 1) in the treatment group cited adverse events as the reason for why he would not use this product again in their dog, although an improvement in itch was noted. One owner in the placebo group withdrew from the study as a consequence of perceived behavioural changes and no resolution of cAD in their dog.
The serum CBD concentrations described in this study were consistent with previously reported values at approximately 2 mg/kg of CBD. 5 , 31 All dogs in the treatment group had measurable CBD (30–1350 ng/ml) and CBDA (10–169 ng/ml) concentrations after treatment. The therapeutic level for serum CBD concentration is not currently known. The wide range in individual serum CBD concentrations is similar to three pharmacokinetic studies examining this product in the dog. 2 , 5 , 29 CBD administered to dogs orally in powder form was reported to have low bioavailability in an early pharmacokinetics study. 32 The absorption in our study may be greater because of the lipophilic oil‐based vehicle and the results cannot be extrapolated to all products marketed as “hemp‐rich CBD.” 32 , 33 However, the interindividual differences observed in our study and others suggest that serum concentration monitoring could be useful. 2 , 5 , 8 , 29
Limitations of this study include a small sample size and administration of concomitant therapies. This study also evaluated only short‐term effects, and a longer study duration is indicated in future studies to determine long‐term effects. Although this was a randomised study, more patients were on concomitant therapies in the treatment group than in the placebo group which were beyond our control during randomisation processes during enrollment. These differences were corrected for statistically, yet this is a limitation of this study.
CONCLUSIONS
Our results suggest that CBD as an adjunct therapy is useful in decreasing pruritus in some dogs with cAD. CBD at 2 mg/kg twice daily was well‐tolerated with minimal AEs.
CONFLICT OF INTEREST
Andrew Rosenberg serves on the advisory board of Ellevet Sciences and Joseph J. Wakshlag is currently the medical director of Ellevet Sciences. The funding was provided by Ellevet Sciences. Ellevet Sciences was not involved in the study design, collection, analysis, interpretation of data, writing or the decision to submit this article for publication.
AUTHOR CONTRIBUTIONS
Melissa Loewinger:Conceptualization; funding acquisition; investigation; methodology; project administration; writing – original draft. Joseph J. Wakshlag: Data curation; formal analysis; investigation; methodology; resources; software; validation; writing – review and editing. Daniel G. Bowden: Investigation; writing – review and editing. Jeanine Peters‐Kennedy: Investigation; writing – review and editing. Andrew S. Rosenberg: Conceptualization; investigation; methodology; resources; writing – review and editing.
Supporting information
AppendixS1
ACKNOWLEDGEMENTS
The authors would like to thank Ellevet Sciences for providing funding, CBD/CBDA product and matching placebos.
Loewinger M, Wakshlag JJ, Bowden D, Peters‐Kennedy J, Rosenberg A. The effect of a mixed cannabidiol and cannabidiolic acid based oil on client‐owned dogs with atopic dermatitis. Vet Dermatol. 2022;33:329–337. 10.1111/vde.13077
Funding information
Ellevet Sciences.
This abstract was presented at the NAVDF on April 22, 2021. This study has not been published previously.
REFERENCES
- 1. Nuttall T, Uri M, Halliwell R. Canine atopic dermatitis – what have we learned? Vet Rec. 2013;172:201–7. [DOI] [PubMed] [Google Scholar]
- 2. Wakshlag JJ, Schwark WS, Deabold KA, Talsma BN, Cital S, Lyubimov A, et al. Pharmacokinetics of cannabidiol, cannabidiolic acid, Δ9‐tetrahydrocannabinol, tetrahydrocannabinolic acid and related metabolites in canine serum after dosing with three oral forms of hemp extract. Front Vet Sci. 2020;7:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silver RJ. The endocannabinoid system of animals. Animals (Basel). 2019;9:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Formato M, Crescente G, Scognamiglio M, Fiorentino A, Pecoraro MT, Piccolella S, et al. (−)‐cannabidiolic acid, a still overlooked bioactive compound: an introductory review and preliminary research. Molecules. 2020;25(2):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gamble L‐J, Boesch JM, Frye CW, Schwark WS, Mann S, Wolfe L, et al. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front Vet Sci. 2018;5:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGrath S, Bartner LR, Rao S, Packer RA, Gustafson DL. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J Am Vet Med Assoc. 2019;254:1301–8. [DOI] [PubMed] [Google Scholar]
- 7. Kogan LR, Hellyer PW, Downing R. The use of cannabidiol‐rich hemp oil extract to treat canine osteoarthritis‐related pain: a pilot study. Amer Vet Holist Med Assoc. 2020;58:35–44. [Google Scholar]
- 8. Bartner LR, McGrath S, Rao S, Hyatt LK, Wittenburg LA. Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs. Can J Vet Res. 2018;82:178–83. [PMC free article] [PubMed] [Google Scholar]
- 9. Campora L, Miragliotta V, Ricci E, et al. Cannabinoid receptor type 1 and 2 expression in the skin of healthy dogs and dogs with atopic dermatitis. Am J Vet Res. 2012;73:988–95. [DOI] [PubMed] [Google Scholar]
- 10. Noli C, Della Valle MF, Miolo A, Cristino L, Di Marzo V, Albanese F, et al. Efficacy of ultra‐micronized palmitoylethanolamide in canine atopic dermatitis: an open‐label multi‐centre study. Vet Dermatol. 2015;26:432, e101–40. [DOI] [PubMed] [Google Scholar]
- 11. Karsak M, Gaffal E, Date R, Wang‐Eckhardt L, Rehnelt J, Petrosino S, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–7. [DOI] [PubMed] [Google Scholar]
- 12. Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocannabinoids. Prog Chem Org Nat Prod. 2017;103:61–101. [DOI] [PubMed] [Google Scholar]
- 13. Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large‐spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367:3364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laprairie RB, Bagher AM, Kelly ME, Denovan‐Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid cb1 receptor. Br J Pharmacol. 2015;172:4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non‐psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–27. [DOI] [PubMed] [Google Scholar]
- 16. Mechoulam R, Peters M, Murillo‐Rodriguez E, Hanus LO. Cannabidiol‐‐recent advances. Chem Biodivers. 2007;4:1678–92. [DOI] [PubMed] [Google Scholar]
- 17. Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (cbdv) and cannabidiol (cbd), activate and desensitize transient receptor potential vanilloid 1 (trpv1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–41. [DOI] [PubMed] [Google Scholar]
- 18. Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. Trpv2 is activated by cannabidiol and mediates cgrp release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res. 2015;11:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill PB, Lau P, Rybnicek J. Development of an owner‐assessed scale to measure the severity of pruritus in dogs. Vet Dermatol. 2007;18:301–8. [DOI] [PubMed] [Google Scholar]
- 21. Olivry T, Saridomichelakis M, Nuttall T, Bensignor E, Griffin CE, Hill PB, et al. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)‐4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet Dermatol. 2014;25:77–85, e25. [DOI] [PubMed] [Google Scholar]
- 22. Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Souza CP, Rosychuk RAW, Contreras ET, Martin DD, Walsh KF, Harfst JA, et al. A retrospective analysis of the use of lokivetmab in the management of allergic pruritus in a referral population of 135 dogs in the western USA. Vet Dermatol. 2018;29:489–e164. [DOI] [PubMed] [Google Scholar]
- 24. Rybnicek J, Lau‐Gillard PJ, Harvey R. Further validation of a pruritus severity scale for use in dogs. Vet Dermatol. 2009;20:115–22. [DOI] [PubMed] [Google Scholar]
- 25. Borys HK, Ingall GB, Karler R. Development of tolerance to the prolongation of hexobarbitone sleeping time caused by cannabidiol. Br J Pharmacol. 1979;67:93–101. [PMC free article] [PubMed] [Google Scholar]
- 26. Olivry T. Could we use the erythema grading of the cadesi4 as a simple instrument for future short‐duration clinical trials of dogs with atopic dermatitis? Vet Dermatol. 2019;30:80–1. [DOI] [PubMed] [Google Scholar]
- 27. McGrath S, Bartner LR, Rao S, Kogan L, Hellyer P. A report of adverse effects associated with the administration of cannabidiol in healthy dogs. J Am Vet Holist Med Assoc. 2018;52:34–8. [Google Scholar]
- 28. U.S. Food and Drug Administration . Federal Drug Administration Application (210365orig1s000). Division of Neurology Products. GW Pharmaceuticals. Neurology Non‐clinical Reviews, 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365orig1s000toc.cfm
- 29. Deabold KA, Schwark WS, Wolf L, Wakshlag JJ. Single‐dose pharmacokinetics and preliminary safety assessment with use of cbd‐rich hemp nutraceutical in healthy dogs and cats. Animals (Basel). 2019;9:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ketoconazole tablets [updated April 1, 2020]. Accessed October 10, 2020. Available from: https://www.drugs.com/pro/ketoconazole‐tablets.html
- 31. Mejia S, Duerr FM, Griffenhagen G, McGrath S. Evaluation of the effect of cannabidiol on naturally occurring osteoarthritis‐associated pain: a pilot study in dogs. J Am Anim Hosp Assoc. 2021;57:81–90. [DOI] [PubMed] [Google Scholar]
- 32. Samara E, Bialer M. Pharmacokinetics of the dimethylheptyl homolog of cannabidiol in dogs. Drug Metab Dispos. 1988;16:875–9. [PubMed] [Google Scholar]
- 33. Garrett ER, Hunt CA. Physiochemical properties, solubility, and protein binding of delta9‐tetrahydrocannabinol. J Pharm Sci. 1974;63:1056–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AppendixS1


