Abstract
The stable fly Stomoxys calcitrans (Diptera: Muscidae) is considered as the main mechanical vector of the lumpy skin disease virus (LSDV). In addition, the mosquito species Aedes aegypti (Diptera: Culicidae) was shown to transmit the virus from donor to receptor animals. Retention of the virus for several days was shown for two additional tropical mosquito species and the biting midge Culicoides nubeculosus (Diptera: Ceratopogonidae). In the present study, viral retention for 10‐ or 7‐days post feeding on virus‐spiked blood through a membrane was shown for field‐collected Aedes japonicus and laboratory‐reared Culex pipiens, two widely distributed mosquito species in temperate regions. Viral DNA could be detected from honey‐coated Flinders Technology Associates (FTA) cards and shedded faeces for 1 or 4 days after an infectious blood meal was given to Ae. aegypti. Virus increase over time and virus dissemination was observed in laboratory‐reared C. nubeculosus, but the virus could be isolated from field‐collected biting midges only from the day of exposure to the blood meal. Thus, mosquitoes might serve as mechanical vectors of LSDV in case of interrupted feeding. A putative biological virus transmission by Culicoides biting midges, as suspected from field observations, deserves further investigations.
Keywords: Aedes aegypti, Aedes japonicus, Culex pipiens, Culicoides nubeculosus, field‐collected Culicoides , transmission
Lumpy skin disease virus, mainly transmitted by Stomoxys calcitrans, was retained in different mosquito species for up to 10 days after feeding on virus‐spiked blood.
Virus was detected from honey‐coated Flinders Technology Associates (FTA) cards (virus presumably deposited with saliva) and shedded faeces for 1 or 4 days, respectively, in the yellow fever mosquito Aedes aegypti.
Virus propagation and dissemination was observed in laboratory‐reared Culicoides nubeculosus biting midges, but not in field‐collected Culicoides spp.
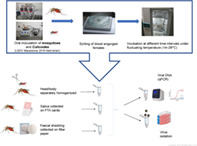
INTRODUCTION
Lumpy skin disease (LSD) is caused by a virus in the genus Capripoxvirus from the Poxviridae family, affecting mainly cattle. The disease is characterized by skin nodules, which may cover the whole body, and by similar lesions in the internal mucosa of the digestive and respiratory tracts. Systemic effects include pyrexia, anorexia, dysgalactia, and pneumonia. Though case fatality is low, morbidity is high, making LSD an economically important disease which is categorized as notifiable by The World Organization of Animal Health (OIE) (Davies, 1991; Sanz‐Bernardo et al., 2020; Tuppurainen & Oura, 2012). LSD used to be endemic to Sub‐Saharan Africa but first epidemics occurred in the Middle East in the 1980s (Tuppurainen & Oura, 2012). In 2015, the disease was diagnosed in south‐eastern Europe (Greece, the Balkans) where it rapidly spread but was controlled by the implementation of concerted measures (Tuppurainen et al., 2020). Flying blood‐feeding insects have been incriminated as vectors by circumstantial evidence (seasonality of transmission, lack of transmission in insect‐proof environments) and mathematical models (Gubbins et al., 2020; Kahana‐Sutin et al., 2017; Magori‐Cohen et al., 2012; Sprygin et al., 2019). The main suspected vector is the biting stable fly Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae), and experimental transmission of LSD virus (LSDV) from infected to susceptible animals via blood‐fed S. calcitrans was proven (Issimov et al., 2020; Sohier et al., 2019). A very recent study demonstrated the retention of LSDV in S. calcitrans for 4 days (7/47 flies positive for LSDV DNA in quantitative polymerase chain reaction [qPCR] on homogenized whole insects) and in rare cases (1/41) up to day 8 after feeding on an infected animal (Sanz‐Bernardo et al., 2021). In another recent study that addressed the mode of LSDV transmission by S. calcitrans, flies were fed with virus‐spiked blood through a membrane, and heads, bodies, regurgitated blood, and faeces were investigated by qPCR and virus isolation in cell culture. Thus, viral DNA was detected in all specimens up to the full duration of the experiment of 3 days, and viable virus up to 2 days (Paslaru et al., 2021). Viral loads did not increase over time, consolidating S. calcitrans as mechanical vector.
Additional blood‐feeding insects have experimentally been investigated for their potential role as LSDV vectors, namely laboratory colonies of mosquitoes (Diptera: Culicidae; Aedes aegypti [Linnaeus, 1762], yellow fever mosquito; Anopheles stephensi Liston, 1901, Asian malaria vector; Culex quinquefasciatus Say, 1823, southern house mosquito) and Culicoides biting midges (Diptera: Ceratopogonidae; Culicoides nubeculosus [Meigen, 1830], Western Palearctic species), collectively termed ‘model vector species’ by Sanz‐Bernardo et al. (2021). They were fed on lesions of LSDV‐infected donor animals or virus‐spiked blood through membranes, and virus transmission to recipient animals was investigated (Chihota et al., 2001, 2003). LSDV could be isolated from all six recipient animals exposed to Ae. aegypti between 2 and 6 days after feeding on donor animals, and 5/6 displayed clinical signs. In contrast, no animals challenged with An. stephensi, Cx. quinquefasciatus or C. nubeculosus became infected. In another recent study, viral retention in insects after feeding on infected cattle was analysed (Sanz‐Bernardo et al., 2021). The virus was detected in homogenates of whole insects by qPCR up to 2 (Cx. quinquefasciatus), 4 (C. nubeculosus) or 8 days (Ae. aegypti) after feeding.
Culicidae were incriminated as LSDV vectors in a single report due to high populations at areas of the first disease outbreaks in Kenya, (Burdin & Prydie, 1959, cited in Sprygin et al., 2019). Culicoides biting midges were recently considered as putative vectors, because the explosive spread of LSDV in the Balkan countries was reminiscent of the bluetongue epizootic in Western Europe whose causative virus is efficiently spread by Culicoides spp. Indeed, LSDV DNA was recorded in field‐collected, non‐engorged Culicoides punctatus (Meigen, 1804) during the 2014–2015 LSDV outbreak in Turkey (Sevik & Dogan, 2017).
Finally, ixodid ticks were experimentally demonstrated to act as mechanical vectors of LSDV, but their lifecycle does not fit with the rapid spread of LSD as described above. Vertical transmission of LSDV was observed in ticks, and thus their epidemiological significance might be a role as reservoirs (summarized in Berg et al., 2015; Sprygin et al., 2019).
The present study expands on the findings of the insect ‘model vector species’. The LSDV vector suitability of further species was investigated using an artificial feeding method under laboratory conditions. The species included were Cx. pipiens Linnaeus, 1758 (northern house mosquito, laboratory‐reared), Aedes japonicus (Theobald, 1901) (Asian bush mosquito, invasive to Europe and Northern America and locally highly abundant [Koban et al., 2019], field‐collected) in addition to laboratory‐reared Ae. aegypti, C. nubeculosus, and field‐collected Culicoides spp.
MATERIALS AND METHODS
Insects
Laboratory strains of the mosquito species Ae. aegypti (AAE_IPNC strain; generation 22 in our laboratory) and Cx. pipiens (CPI_BRW strain; maintained in our laboratory since 2017, generations not recorded) were initially provided by the Institut Pasteur, New Caledonia, and The Pirbright Institute UK, respectively. Eggs of Ae. japonicus mosquitoes were collected with ovitraps in the field in the Zürich area (Switzerland) as described (Verhulst et al., 2020). Mosquitoes were reared according to standard protocols published earlier by our group (Glavinic et al., 2020; Verhulst et al., 2020; Wagner et al., 2018).
Culicoides nubeculosus were originally provided by The Pirbright Institute and reared as described (Kaufmann et al., 2015). Live Culicoides spp. were collected at a farm in the outskirts of Zürich with OVI UV light traps as earlier described in detail (Paslaru et al., 2018).
Oral exposure of insects to virus
The LSDV (Macedonia 2016, field strain) (Möller et al., 2019) was propagated five times on Madin‐Darby bovine kidney epithelial cells (MDBK NBL‐1; CCL‐22 line, ATCC), reaching a final titre of 6.25 log10TCID50/ml. The virus was mixed in a ratio of 1:9 with heparinised bovine blood (obtained from a local abattoir, Zürich), yielding a final titre of 5.75 log10TCID50/ml in the infectious blood meals.
Mosquitoes (7–9 days old) were exposed to infectious blood meals for 45 min as earlier described (Veronesi et al., 2018) but by using Parafilm M (Sigma–Aldrich, Buchs, Switzerland) instead of pig intestine as a membrane for the Hemotek feeders (Hemotek Ltd., Lancashire, UK). Field‐collected Culicoides and 2–3 days old C. nubeculosus were exposed to infectious blood meals for 30–45 min at 25 ± 4 °C precisely as described (Paslaru et al., 2018). Freshly engorged insects were collected after immobilization by exposure to −20 °C for 4–5 min. Ten were immediately stored at −80 °C (Day 0 specimens) for further tests whereas the remaining ones were incubated in batches of approximately 30 under a fluctuating temperature regime (14–28 °C, mean 22 °C; 80 %–85 % relative humidity) in a climate chamber. At different time intervals post‐incubation, the live insects of a batch were collected after immobilization as described above, individually transferred into 1.5 ml Eppendorf tubes, and stored at −80 °C until molecular analysis. Also, 1 ml aliquots of the infectious inoculums were collected immediately after the blood‐feeding (Day 0 samples).
All the feeding, manipulation, and incubation of LSDV‐exposed insects were done under biosafety containment level 3 (BSL3) conditions.
Processing of insects
Heads and bodies (abdomens and thoraxes) were chopped by using sterile needles 26 G × ½″ (Fine‐Ject, Tuttlingen, Germany) and separately transferred into 1.5 ml Eppendorf tubes containing either 180 μl TE (Tris ethylenediaminetetraacetic acid) buffer (heads) or 400 μl Glasgow Minimum Essential Media (GMEM) (Gibco, Thermo Fisher Scientific, Reinach, Switzerland) supplemented with 2 % antibiotics and fungizone (1000 IU/ml penicillin/streptomycin; 4 μg/ml amphotericin; Gibco) (bodies). In an additional experiment with C. nubeculosus, heads, wings, and abdomens were separately homogenized in 400 μl GMEM. Samples were manually homogenized for 30 s using sterilized polypropylene pestles (Sigma–Aldrich, Gillingham, UK) mounted to a motorized grinder (Micro Handrührer, Carl Roth, Karlsruhe, Germany), centrifuged for 5 min at 13,000g and stored at −80 °C until further use.
Collection of saliva and faeces of Ae. aegypti
Aedes aegypti females (n = 20) exposed to LSDV‐spiked blood as described above were individually incubated in cardboard boxes (Adelphi healthcare packaging 4 oz., APD Deep, Haywards Heath, UK), covered with nets at both sides. Every box was provided with a Flinders Technology Associates card (FTA®, Sigma–Aldrich, Darmstadt, Germany) coated with Manuka honey (nu3 Manuka‐Honig MGO 400, Alnatura, Zürich, Switzerland) and blue food colouring (Dr Oetker‐Lebensmittelfarben‐Set, Coop, Zürich, Switzerland) to collect expectorated saliva (Hall‐Mendelin et al., 2010). Also, a filter paper (Whatman 1541‐185, Grade 541 Ashless Fast Filter Paper, Tisch Scientific, Cleves, OH, U.S.A.) was provided at the bottom of each box to collect the mosquitoes' faeces. The FTA cards and filter papers were exchanged at Days 1, 4, 7, and 10 post exposure to the infectious blood meals.
Elution of saliva from FTA cards and faeces from the filter papers was performed basically as described (Ritchie et al., 2013). Briefly, they were incubated at 4 °C for 20 min in a 2 ml Eppendorf tube with 400 μl GMEM supplemented with 2 % antibiotics (as above). After vortexing for 15 s, the supernatants were transferred into 1.5 ml Eppendorf tubes and stored at −80 °C for further analyses (DNA extraction and virus isolation, see below).
LSDV DNA detection
Extraction of DNA from 180 μl aliquots of the insect homogenates, the elutes or the virus‐spiked blood meals was carried out using the QIAamp viral DNA mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions (elution volume 100 μl). LSDV DNA was detected by qPCR with the primers and the probe described earlier (Stubbs et al., 2012) by using the EXPRESS qPCR SuperMix Universal (Invitrogen, Zug, Switzerland). Amplification using 5 μl of DNA solution was done in QuantiStudioFlex 7 (Applied Biosystems, Thermo Fischer, Zug, Switzerland) using the following program: 50 °C for 2 min (UDG incubation), 95 °C for 2 min (UDG inactivation, Taq polymerase activation), followed by 50 cycles of 15 s at 95 °C, 30 s at 58 °C, and 30 s at 72 °C.
A standard curve was generated using 10‐fold serial dilutions of the viral inoculum used in the experiments, revealing a cut‐off of Cq ≤ 34.6 (Figure S1). The virus‐spiked blood meals had Cq values ranging between 17.3 and 29.
Virus isolation
Aliquots (200 μl) of the supernatants of GMEM sample homogenates or elutes were added to 25 cm2 flasks seeded with 7 × 105 cells (MDBK NBL‐1; CCL‐22 line) 24 h before inoculation. The flasks were incubated for 1 h at 37 °C with 5 % CO2 after which growth medium (GMEM with 2.5 % horse serum [Biowest S0910, Lubio Science GmbH, Zürich, Switzerland] supplemented with 2 % antibiotics and fungizone) was added. The incubation was continued at the same conditions for 7 days, and the cells were examined daily for cytopathic effect (CPE).
Statistical analysis
A binomial (logistic) regression model was applied to assess whether the presence or absence of LSDV varied according to species and incubation period. The analyses were performed using R (https://www.r-project.org/). For quantitative analysis, the reciprocal of the Cq values were analysed. For Cq values above the cut‐off value of 34.6, the reciprocal was allocated a value of zero which was consistent with no virus particles in the sample. These transformed data were then analysed with a tweedie generalized linear model which can model data that have a positive mass at zero but is otherwise continuous.
RESULTS
Three mosquito species (Ae. aegypti, Ae. japonicus, Cx. pipiens) as well as biting midges (C. nubeculosus, field collected Culicoides spp.) were exposed to LSDV‐spiked blood and examined for the presence of the virus in bodies and head or wings (as proxy for virus dissemination) at different time points after feeding. In addition, saliva expectorated onto FTA cards and faeces shed by exposed Ae. aegypti were accordingly investigated.
Mosquitoes
Mosquitoes of three species (Ae. aegypti, Ae. japonicus, Cx. pipiens) were exposed to LSDV‐spiked blood, and the heads of 10 specimens per time point (0, 1, 2, 4, 7, 10 days post feeding) examined by qPCR for the presence of viral DNA (Table 1, Table S1). In the freshly blood‐fed mosquitoes (d 0) viral DNA was detectable in few (2, 3) heads of two species. All heads examined from Ae. japonicus tested negative, whereas single additional heads of the two other species were positive (d 1 for Ae. aegypti; d 2, 7 for Cx. pipiens). Five bodies (thorax and abdomen) per time point were examined, including the bodies from all specimens with positive qPCR in the head samples. Viral DNA was detected in all Day 0 body samples of all three mosquito species, and up to Day 4 in Ae. aegypti, Day 10 in Ae. japonicus and Day 7 in Cx. pipiens. Overall, from the total 25 body samples tested from the time points d 1–10 of each species, 9, 7, and 11, respectively, were positive. qPCR‐positive body samples were investigated for viable virus by cell culture. CPE caused by the presence of infectious virus particles was observed in all specimens from Day 0 (only Ae. japonicus samples investigated) and in total 19 of the 27 samples (d 1–10), originating from all three species, including one sample from d 10 (Table 1).
TABLE 1.
Analyses of different body parts of three mosquito species for the presence of lumpy skin disease virus DNA by quantitative polymerase chain reaction (qPCR) a or viable virus by cell culture inoculation at different time points after exposure to infectious blood meals.
| Species | Day post infectious blood meal | qPCR (head) | ||
|---|---|---|---|---|
| No. positive/no. tested | qPCR (body b ) | CPE in cell culture (body) | ||
| Aedes aegypti | 0 | 3/10 | 5/5 | nd |
| 1 | 1/10 | 4/5 | 2/4 | |
| 2 | 0/10 | 4/5 | 3/4 | |
| 4 | 0/10 | 1/5 | 1/1 | |
| 7 | 0/10 | 0/5 | nd | |
| 10 | 0/10 | 0/5 | nd | |
| Aedes japonicus | 0 | 0/10 | 5/5 | 5/5 |
| 1 | 0/10 | 0/5 | nd | |
| 2 | 0/10 | 2/5 | 0/2 | |
| 4 | 0/10 | 4/5 | 4/4 | |
| 7 | 0/10 | 0/5 | nd | |
| 10 | 0/10 | 1/5 | 1/1 | |
| Culex pipiens | 0 | 2/10 | 5/5 | nd |
| 1 | 0/10 | 3/5 | 3/3 | |
| 2 | 1/10 | 5/5 | 3/5 | |
| 4 | 0/10 | 2/5 | 1/2 | |
| 7 | 1/10 | 1/5 | 1/1 | |
| 10 | 0/10 | 0/5 | nd |
Abbreviations: CPE, cytopathic effect (all qPCR‐positive bodies examined); nd, not done.
Positive: Cq ≤ 34.6.
Body: thorax and abdomen (bodies investigated from all specimens qPCR‐positive in the head samples, complemented with randomly chosen samples).
Saliva and faeces from 20 Ae. aegypti orally exposed to LSDV were collected with FTA cards and filter papers provided in the cages. qPCR with extracted DNA yielded Cq values below 50 up to 10 days post‐infection (Figure 1). However, when applying the cut‐off value generated from the standard curve (Cq ≤ 34.6; Figure S1) only 1 FTA card from Day 1, and 7 (d 1: 5; d 4: 2) filter papers up to Day 4 can be considered positive. No CPE was observed with these specimens.
FIGURE 1.
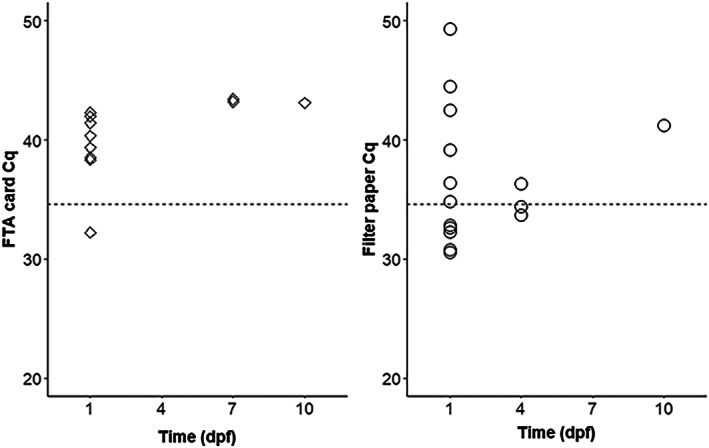
Detection of lumpy skin disease virus DNA by quantitative polymerase chain reaction from honey‐coated Flinders Technology Associates (FTA) cards and filter papers provided to 20 individually kept Aedes aegypti mosquitoes at different days post feeding (dpf) on virus‐spiked blood. The Cq values below 50 are shown (dotted line: threshold 34.6).
Culicoides
Similar to the experiments with the three mosquito species, the presence of LSDV was investigated in body parts of C. nubeculosus that fed on virus‐spiked blood, with an additional time point investigated (d 5 after blood meal; Table 2a, Figure 2; Table S1). The number of heads positive for virus DNA tended to be higher with increasing time span after the blood meal, with 3/10 being positive at both d 7 and d 10 post exposure (for statistical significance see below). qPCR with DNA from bodies of these head‐positive specimens, complemented to 5 samples by randomly chosen other insects, revealed no virus DNA in the biting midges at time points d 4 and d 5, but high positivity rates in the early phase of the experiment (d 0, d 1, d 2) and moderate ones towards the end (d 7, d 10). Viable virus was isolated by cell culture also from the body samples from d 10 (Table 2a). A second experiment focused on analysing more insects only at a later stage (d 10) and refining the analyses by investigating the abdomens and wings separately in addition to the heads (Table 2b). One of the 38 biting midges analysed at d 10 showed a disseminated infection, that is, qPCR and cell cultures were positive for all samples analysed.
TABLE 2.
Analyses of different body parts of Culicoides nubeculosus for the presence of lumpy skin disease virus DNA by quantitative polymerase chain reaction (qPCR) a or viable virus by cell culture inoculation at different time points after exposure to infectious blood meals.
| (a) First experiment | |||
|---|---|---|---|
| Day post‐infectious blood‐meal | qPCR (head) | qPCR (body b ) | CPE+ in cell culture (body) |
| No. positive/no. tested | |||
| 0 | 1/10 | 5/5 | 4/5 |
| 1 | 0/10 | 5/5 | 5/5 |
| 2 | 1/10 | 4/5 | 1/4 |
| 4 | 2/10 | 0/5 | nd |
| 5 | 1/10 | 0/5 | nd |
| 7 | 3/10 | 3/5 | 0/3 |
| 10 | 3/10 | 2/5 | 2/2 |
| (b) Second experiment | ||||||
|---|---|---|---|---|---|---|
| Day post‐infectious blood‐feeding | qPCR (head) | qPCR (abdomen) | qPCR (wings) | CPE in cell culture (head) | CPE in cell culture (abdomen) | CPE in cell culture (wings) |
| No. positive/no. tested | ||||||
| 1 | 3/10 | 2/5 | nd | 0/3 | 0/2 | nd |
| 10 | 1/38 | 1/20 | 1/16 | 1/1 | 1/1 | 1/1 |
Abbreviations: CPE, cytopathic effect (all qPCR‐positive specimens examined); nd, not done.
Positive: Cq ≤ 34.6.
Body: thorax and abdomen. Bodies (a) or abdomens only (b) investigated from all specimens qPCR‐positive in the head samples, complemented with randomly chosen samples.
FIGURE 2.
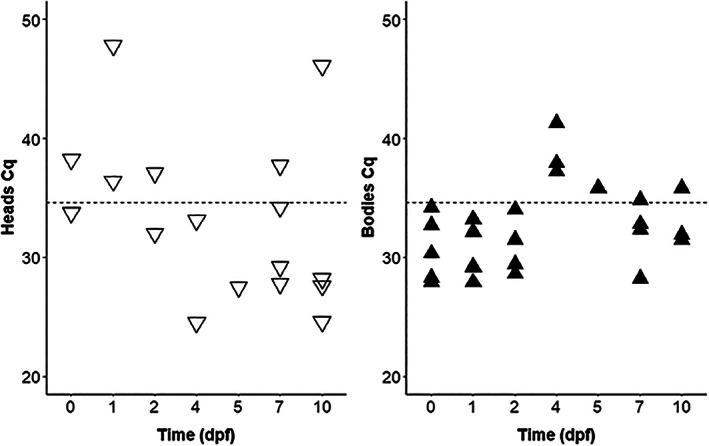
Detection of lumpy skin disease virus DNA by quantitative polymerase chain reaction (qPCR) in Culicoides nubeculosus heads and bodies at different days post feeding (dpf) on virus‐spiked blood. Ten heads were investigated per time point, and the bodies from all specimens qPCR‐positive in the head samples, complemented with randomly chosen samples to total 5. The Cq values below 50 are shown (dotted line: threshold 34.6).
In the corresponding experiment with field‐collected Culicoides spp., all surviving individuals per time point were analysed (n = 10–24; Table 3). qPCR was positive only with specimens of d 1 after blood feeding; homogenates from bodies were positive by DNA analyses and cell culture only from insects obtained at the day of feeding (d 0).
TABLE 3.
Analyses of different body parts of field‐collected Culicoides spp. for the presence of lumpy skin disease virus DNA by quantitative polymerase chain reaction (qPCR) a or viable virus by cell culture inoculation at different time points after exposure to infectious blood meals.
| Day post infectious blood meal | qPCR (head) | ||
|---|---|---|---|
| No positive/no tested | qPCR (body b ) | CPE in cell culture (body) | |
| 0 | 0/10 | 3/5 | 3/3 |
| 1 | 3/24 | 0/5 | nd |
| 3 | 0/15 | 0/5 | nd |
| 5 | 0/15 | 0/5 | nd |
| 7 | 0/20 | 0/5 | nd |
| 10 | 0/15 | 0/5 | nd |
Abbreviations: CPE, cytopathic effect (all qPCR‐positive bodies examined); nd, not done.
Positive: Cq ≤ 34.6.
Body: thorax and abdomen (bodies investigated from all specimens qPCR‐positive in the head samples, complemented with randomly chosen samples).
Statistical analysis
There was neither a significant difference detected between species of arthropods tested and the presence of LSDV nor a change over time in the proportion of insects from which virus could be detected by PCR. The only exception to the latter is the second experiment with C. nubeculosus (Table 2b; i.e., there were significantly less insect heads infected at d 10 as compared to d 1, p = 0.0245, Fisher test). The reciprocal of the Cq values significantly increased with time only in C. nubeculosus (d 68: t = 2.20, p = 0.0312). Therefore, the Cq values decreased, and viral load increased over time.
DISCUSSION
With the present study, two additional mosquito species (Ae. japonicus, Cx. pipiens) were shown to harbour viable LSDV in their bodies upon oral exposure for an extended period of time (10 and 7 days, respectively). Thus, retention of LSDV in mosquito vectors might be a general feature but the mechanism remains unknown (see below). The retention times of LSDV in different insects varied among the studies (Chihota et al., 2001, 2003; Sanz‐Bernardo et al., 2021), ranging from 4 to 9 days for Ae. aegypti and 0 to 10 days for C. nubeculosus. This might be due to the use of different virus strains and different feeding methods, that is, through membrane on infectious blood versus on lesion of infected animals. It was shown that LSDV was much more efficiently acquired by different vector species when feeding on clinical animals as compared to subclinical ones (Sanz‐Bernardo et al., 2021). By contrast with earlier studies, virus detection in the present study was done on homogenates of heads and body parts (thorax and abdomen; wings) (Figure S2) rather than on whole insects, allowing for estimating the dissemination of LSDV.
In all three mosquito species investigated, many more body samples were qPCR‐positive than head samples, indicating that the virus was not efficiently retained in the mouthparts and that there was no virus dissemination (Table 1). This is particularly exemplified with Ae. japonicus, of which all d 0 heads examined were negative and all bodies from this timepoint positive by PCR and, for confirmatory reasons, also by virus‐cell isolation. In Ae. aegpyti, PCR on heads and on FTA cards, containing expectorated saliva (Hall‐Mendelin et al., 2010) was only positive from Day 1 after feeding. Thus, mechanical transmission of LSDV by this species seems feasible in case of interrupted feeding; also, by considering that the LSDV minimum infective dose when inoculated intradermally is as low as 101 TCID50/ml to establish an infection (Carn & Kitching, 1995). Interrupted feeding is well known for the main vector S. calcitrans, whose bites are painful, triggering fierce defensive behaviour of hosts (Baldacchino et al., 2013). Such a behaviour against mosquitoes (Matherne et al., 2018) and therefore interrupted feeding is also evident for mosquitoes as, for example, substantiated by revealing mixed blood meals in engorged mosquitoes (Hernandez‐Colina et al., 2021; Schönenberger et al., 2016). Faecal shedding of LSDV from Ae. aegypti was observed until Day 4 after feeding. The significance of this finding is unclear. It might merely contribute to transfer the virus to naïve hosts upon interrupted feeding as mosquitoes, by contrast with S. calcitrans (Paslaru et al., 2021), do not regurgitate (i.e., reflux of gut content) (Day, 1955). However, such a transmission, corresponding to a transmission by contact of animals, is considered ineffective (Sprygin et al., 2019). Thus, mechanical transmission in the course of interrupted feeding seems possible for Ae. aegypti. Interestingly, this species was shown to transmit LSDV to recipient animals up to Day 6 after feeding in infected animals (Chihota et al., 2001). However, all animals involved in this study were exposed to infected mosquitoes, that is, there was no control animal involved (challenged with non‐infected mosquitoes). Though several lines of evidence suggest that direct or indirect transmission (e.g., through fomites, feed, and water contaminated with secretions from infected animals) do not play a significant role in LSDV epidemiology they may also be transmission routes (Berg et al., 2015). Animal‐to‐animal transmission experiments with S. calcitrans, involving LSDV (Issimov et al., 2020; Sohier et al., 2019) were done by allowing the flies to partially (10 min) feed on infected animals and then transferring them immediately to naïve animals. Such experiments with a re‐feeding after 24 h were unsuccessful (Chihota et al., 2003). In a study with Aedes vexans (Meigen, 1830), transmission of the porcine reproductive and respiratory syndrome virus was achieved after interrupted feeding for 1 min on viremic donor pigs and immediate transfer to healthy pigs (Otake et al., 2002).
From the mosquito species so far investigated with regard to LSDV retention, Ae. aegypti and An. stephensi preferably bite humans (Scott & Takken, 2012) and are of little significance for virus transmission under field conditions. The other investigated mosquito species (Ae. japonicus, Cx. pipiens, Cx. quinquefasciatus) are opportunistic feeders and also bite large mammalian hosts (Becker et al., 2010; Schönenberger et al., 2016).
From experiments on biologically transmitted pathogens, it is known that insect populations from laboratory colonies and from field‐collections differ with regard to vector competence (Silaghi et al., 2017). To the best of our knowledge, nothing is known with this regard on mechanical transmission of animal pathogens. By contrast with the mechanical transmission of plant pathogens (designated non‐circulative), which involves specific interactions of the pathogens with receptors on mouthparts or in the alimentary tract, no such interaction is known for animal viruses (Blanc & Gutierrez, 2015). Consequently, no difference in mechanical transmission between field‐collected and laboratory‐reared populations are to be expected. Nevertheless, with the present study, the suitability of field‐collected mosquitoes (Ae. japonicus) to retain viable LSDV for an extended period of time (10 d, Table 1) and potentially serve as a vector was shown.
Other mosquito species that feed on cattle and which can be highly abundant in Europe (Schönenberger et al., 2016), for example, the flood‐plain species Ae. vexans and Aedes sticticus (Meigen, 1838), and also Aedes cantans (Meigen, 1818)/Aedes annulipes (Meigen, 1830), Anopheles maculipennis s.l. Meigen, 1818, Coquillettidia richiardi (Ficalbi, 1898), should be considered as putative LSDV vectors.
By contrast with the earlier study including C. nubeculosus, in which only insects at the day of feeding on an LSDV‐infected steer were PCR positive (Chihota et al., 2003), viable virus was isolated from homogenized bodies up to the end of the experiments (Day 10 post‐infectious blood meal) in the present study (Table 2a). Interestingly, Cq values decreased over time, and a disseminated infection at d 10 post feeding was identified in one insect (Table 2b). Considering the postulated absence of salivary gland barriers in Culicoides spp. (Fu et al., 1999), these findings indicate that the laboratory‐reared C. nubeculosus might behave as biological vectors of LSDV under laboratory conditions.
By contrast with these C. nubeculosus, no persistence of LSDV was observed in field‐collected biting midges (Table 3). However, only relatively few field‐collected biting midges could be investigated due to their inherent low feeding rates under laboratory conditions (Paslaru et al., 2018). Culicoides punctatus, the incriminated LSDV vector in Turkey (Sevik & Dogan, 2017) is widely distributed in the Northern hemisphere and can reach high abundances (e.g., The Netherlands; around 30% of specimens; Meiswinkel et al., 2008). This species, however, is virtually absent at the collection location of the biting midges used in the present study where the Culicoides fauna is nearly exclusively composed of Culicoides obsoletus (Meigen, 1818) and Culicoides scoticus Downes & Kettle, 1952 (Paslaru et al., 2018). Given the very high population densities that biting midges can reach, even low vector capabilities could be contributing to the transmission of pathogens. Experimentally investigating LSDV vector competence of Culicoides from locations with other species compositions is highly desired.
AUTHOR CONTRIBUTIONS
Anca I. Paslaru: Methodology; formal analyses; investigation; writing—original draft. Lena M. Maurer: Investigation; writing—review and editing. Andrea Vögtlin: Resources; writing—review and editing; funding acquisition. Bernd Hoffmann: Resources. Paul R. Torgerson: Formal analyses; writing—review and editing. Alexander Mathis: Conceptualization; writing—original draft; supervision; funding acquisition. Eva Veronesi: Conceptualization; methodology; formal analyses; writing—original draft; supervision; project administration. All authors edited, read, and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Table S1. Lumpy skin disease virus (LSDV) qPCR values of heads, bodies (abdomen1 and thorax), and wings2 of all the investigated arthropods. At least 10 individual's heads and 5 bodies were tested for each time point. All the surviving field‐collected Culicoides were tested.
Figure S1. Standard curve for LSDV Macedonia 2016 field strain (Möller et al., 2019). Conversion of viral DNA (Cq values) into log10TCID50/ml was determined.
Figure S2. Cytopathic effect in MDBK cell cultures of homogenates of Culicoides nubeculosus head (a), thorax/abdomen (b), and wings (c) 10 days post feeding.
ACKNOWLEDGEMENTS
We highly acknowledge the Swiss Federal Food Safety and Veterinary Office (FSVO) for financial support of the National Centre for Vector Entomology and for financing the project ‘Lumpy skin disease: improvement of diagnostic methods and assessment of the vector potential of insects of Switzerland’ (project number Aramis 1.18.08). We kindly thank Dr Simon Carpenter (The Pirbright Institute, UK) for providing Culicoides nubeculosus via the Infravec2 project (Research Infrastructures for the control of vector‐borne diseases, Infravec2, funded from the European Union's Horizon 2020 research and innovation programme under grant agreement No 731060); Dr Kurt Tobler (Institute of Virology, University of Zürich, Switzerland) for molecular analysis consulting; Jeannine Hauri and Jasmin Varga (Institute of Parasitology, University of Zürich, Switzerland) for technical assistance in the lab. Open access funding provided by Universitat Zurich.
Paslaru, A.I. , Maurer, L.M. , Vögtlin, A. , Hoffmann, B. , Torgerson, P.R. , Mathis, A. et al. (2022) Putative roles of mosquitoes (Culicidae) and biting midges (Culicoides spp.) as mechanical or biological vectors of lumpy skin disease virus. Medical and Veterinary Entomology, 36(3), 381–389. Available from: 10.1111/mve.12576
Funding information Bundesamt für Lebensmittelsicherheit und Veterinärwesen, Grant/Award Number: 1.18.08
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Baldacchino, F. , Muenworn, V. , Desquesnes, M. , Desoli, F. , Charoenviriyaphap, T. & Duvallet, G. (2013) Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): a review. Parasite, 20, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, N. , Petric, D. , Zgomba, M. , Boase, C. , Madon, M. , Dahl, C. et al. (2010) Mosquitoes and their control. Heidelberg: Springer. [Google Scholar]
- Berg, C. , Bøtner, A. , Browman, H. , De Koeijer, A. , Depner, K. , Domingo, M. et al. (2015) Scientific opinion on lumpy skin disease. EFSA Journal, 13, 3896. [Google Scholar]
- Blanc, S. & Gutierrez, S. (2015) The specifics of vector transmission of arboviruses of vertebrates and plants. Current Opinion in Virology, 15, 27–33. [DOI] [PubMed] [Google Scholar]
- Burdin, M.L. & Prydie, J. (1959) Observations on the first outbreak of lumpy skin disease in Kenya. Bulletin of Epizootic Diseases of Africa, 7, 21–26. [Google Scholar]
- Carn, V.M. & Kitching, R.P. (1995) The clinical response of cattle experimentally infected with lumpy skin disease (Neethling) virus. Archives of Virology, 140, 503–513. [DOI] [PubMed] [Google Scholar]
- Chihota, C.M. , Rennie, L.F. , Kitching, R.P. & Mellor, p.S. (2001) Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiology and Infection, 126, 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota, C.M. , Rennie, L.F. , Kitching, R.P. & Mellor, p.S. (2003) Attempted mechanical transmission of lumpy skin disease virus by biting insects. Medical and Veterinary Entomology, 17, 294–300. [DOI] [PubMed] [Google Scholar]
- Davies, F.G. (1991) Lumpy skin disease, an African capripox virus disease of cattle. The British Veterinary Journal, 147, 489–503. [DOI] [PubMed] [Google Scholar]
- Day, M.F. (1955) Mechanisms of transmission of viruses by arthropods. Experimental Parasitology, 4, 387–418. [DOI] [PubMed] [Google Scholar]
- Fu, H. , Leake, C.J. , Mertens, p.P. & Mellor, p.S. (1999) The barriers to bluetongue virus infection, dissemination and transmission in the vector, Culicoides variipennis (Diptera: Ceratopogonidae). Archives of Virology, 144, 747–761. [DOI] [PubMed] [Google Scholar]
- Glavinic, U. , Varga, J. , Paslaru, A.I. , Hauri, J. , Torgerson, P. , Schaffner, F. et al. (2020) Assessing the role of two populations of Aedes japonicus japonicus for Zika virus transmission under a constant and a fluctuating temperature regime. Parasites & Vectors, 13, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbins, S. , Stegeman, A. , Klement, E. , Pite, L. , Broglia, A. & Cortinas Abrahantes, J. (2020) Inferences about the transmission of lumpy skin disease virus between herds from outbreaks in Albania in 2016. Preventive Veterinary Medicine, 181, 104602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall‐Mendelin, S. , Ritchie, S.A. , Johansen, C.A. , Zborowski, P. , Cortis, G. , Dandridge, S. et al. (2010) Exploiting mosquito sugar feeding to detect mosquito‐borne pathogens. Proceedings of the National Academy of Sciences of the United States of America, 107, 11255–11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Colina, A. , Gonzalez‐Olvera, M. , Lomax, E. , Townsend, F. , Maddox, A. , Hesson, J.C. et al. (2021) Blood‐feeding ecology of mosquitoes in two zoological gardens in the United Kingdom. Parasites & Vectors, 14, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issimov, A. , Kutumbetov, L. , Orynbayev, M.B. , Khairullin, B. , Myrzakhmetova, B. , Sultankulova, K. et al. (2020) Mechanical transmission of lumpy skin disease virus by Stomoxys spp. (Stomoxys calcitrans, Stomoxys sitiens, Stomoxys indica), Diptera: Muscidae. Animals, 10, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana‐Sutin, E. , Klement, E. , Lensky, I. & Gottlieb, Y. (2017) High relative abundance of the stable fly Stomoxys calcitrans is associated with lumpy skin disease outbreaks in Israeli dairy farms. Medical and Veterinary Entomology, 31, 150–160. [DOI] [PubMed] [Google Scholar]
- Kaufmann, C. , Mathis, A. & Vorburger, C. (2015) Sugar‐feeding behaviour and longevity of European Culicoides biting midges. Medical and Veterinary Entomology, 29, 17–25. [DOI] [PubMed] [Google Scholar]
- Koban, M.B. , Kampen, H. , Scheuch, D.E. , Frueh, L. , Kuhlisch, C. , Janssen, N. et al. (2019) The Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in Europe, 17 years after its first detection, with a focus on monitoring methods. Parasites & Vectors, 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magori‐Cohen, R. , Louzoun, Y. , Herziger, Y. , Oron, E. , Arazi, A. , Tuppurainen, E. et al. (2012) Mathematical modelling and evaluation of the different routes of transmission of lumpy skin disease virus. Veterinary Research, 43, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherne, M.E. , Cockerill, K. , Zhou, Y. , Bellamkonda, M. & Hu, D.L. (2018) Mammals repel mosquitoes with their tails. Journal of Experimental Biology, 221, 15. [DOI] [PubMed] [Google Scholar]
- Meiswinkel, R. , Goffredo, M. , Leijs, P. & Conte, A. (2008) The Culicoides ‘snapshot’: a novel approach used to assess vector densities widely and rapidly during the 2006 outbreak of bluetongue (BT) in The Netherlands. Preventive Veterinary Medicine, 87, 98–118. [DOI] [PubMed] [Google Scholar]
- Möller, J. , Moritz, T. , Schlottau, K. , Krstevski, K. , Hoffmann, D. , Beer, M. et al. (2019) Experimental lumpy skin disease virus infection of cattle: comparison of a field strain and a vaccine strain. Archives of Virology, 164, 2931–2941. [DOI] [PubMed] [Google Scholar]
- Otake, S. , Dee, S.A. , Rossow, K.D. , Moon, R.D. & Pijoan, C. (2002) Mechanical transmission of porcine reproductive and respiratory syndrome virus by mosquitoes, Aedes vexans (Meigen). Canadian Journal of Veterinary Research, 66, 191–195. [PMC free article] [PubMed] [Google Scholar]
- Paslaru, A.I. , Mathis, A. , Torgerson, P. & Veronesi, E. (2018) Vector competence of pre‐alpine Culicoides (Diptera: Ceratopogonidae) for bluetongue virus serotypes 1, 4 and 8. Parasites & Vectors, 11, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslaru, A.I. , Verhulst, N.O. , Maurer, L.M. , Brendle, A. , Pauli, N. , Vögtlin, A. et al. (2021) Potential mechanical transmission of lumpy skin disease virus (LSDV) by the stable fly (Stomoxys calcitrans) through regurgitation and defecation. Current Research in Insect Science, 1, 100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S.A. , Cortis, G. , Paton, C. , Townsend, M. , Shroyer, D. , Zborowski, P. et al. (2013) A simple non‐powered passive trap for the collection of mosquitoes for arbovirus surveillance. Journal of Medical Entomology, 50, 185–194. [DOI] [PubMed] [Google Scholar]
- Sanz‐Bernardo, B. , Haga, I.R. , Wijesiriwardana, N. , Hawes, p.C. , Simpson, J. , Morrison, L.R. et al. (2020) Lumpy skin disease is characterized by severe multifocal dermatitis with necrotizing fibrinoid vasculitis following experimental infection. Veterinary Pathology, 57, 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Bernardo, B. , Haga, I.R. , Wijesiriwardana, N. , Basu, S. , Larner, W. , Diaz, A.V. et al. (2021) Quantifying and modeling the acquisition and retention of lumpy skin disease virus by hematophagus insects reveals clinically but not subclinically affected cattle are promoters of viral transmission and key targets for control of cisease outbreaks. Journal of Virology, 95, e02239–e02220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönenberger, A.C. , Wagner, S. , Tuten, H.C. , Schaffner, F. , Torgerson, P. , Furrer, S. et al. (2016) Host preferences of host‐seeking and blood‐fed mosquitoes in Switzerland. Medical and Veterinary Entomology, 30, 39–52. [DOI] [PubMed] [Google Scholar]
- Scott, T.W. & Takken, W. (2012) Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends in Parasitology, 28, 112–121. [DOI] [PubMed] [Google Scholar]
- Sevik, M. & Dogan, M. (2017) Epidemiological and molecular studies on lumpy skin disease outbreaks in Turkey during 2014‐2015. Transboundary and Emerging Diseases, 64, 1268–1279. [DOI] [PubMed] [Google Scholar]
- Silaghi, C. , Beck, R. , Capelli, G. , Montarsi, F. & Mathis, A. (2017) Development of Dirofilaria immitis and Dirofilaria repens in Aedes japonicus and Aedes geniculatus . Parasites & Vectors, 10, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohier, C. , Haegeman, A. , Mostin, L. , De Leeuw, I. , Van Campe, W. , De Vleeschauwer, A. et al. (2019) Experimental evidence of mechanical lumpy skin disease virus transmission by Stomoxys calcitrans biting flies and Haematopota spp. horseflies. Scientific Reports, 9, 20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprygin, A. , Pestova, Y. , Wallace, D.B. , Tuppurainen, E. & Kononov, A.V. (2019) Transmission of lumpy skin disease virus: a short review. Virus Research, 269, 197637. [DOI] [PubMed] [Google Scholar]
- Stubbs, S. , Oura, C.A.L. , Henstock, M. , Bowden, T.R. , King, D.P. & Tuppurainen, E.S.M. (2012) Validation of a high‐throughput real‐time polymerase chain reaction assay for the detection of capripoxviral DNA. Journal of Virological Methods, 179, 419–422. [DOI] [PubMed] [Google Scholar]
- Tuppurainen, E.S.M. & Oura, C.A.L. (2012) Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transboundary and Emerging Diseases, 59, 40–48. [DOI] [PubMed] [Google Scholar]
- Tuppurainen, E.S.M. , Antoniou, S.E. , Tsiamadis, E. , Topkaridou, M. , Labus, T. , Debeljak, Z. et al. (2020) Field observations and experiences gained from the implementation of control measures against lumpy skin disease in South‐East Europe between 2015 and 2017. Preventive Veterinary Medicine, 181, 104600. [DOI] [PubMed] [Google Scholar]
- Verhulst, N.O. , Brendle, A. , Blanckenhorn, W.U. & Mathis, A. (2020) Thermal preferences of subtropical Aedes aegypti and temperate Ae. japonicus mosquitoes. Journal of Thermal Biology, 91, 102637. [DOI] [PubMed] [Google Scholar]
- Veronesi, E. , Paslaru, A. , Silaghi, C. , Tobler, K. , Glavinic, U. , Torgerson, P. et al. (2018) Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitology Research, 117, 1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, S. , Mathis, A. , Schonenberger, A.C. , Becker, S. , Schmidt‐Chanasit, J. , Silaghi, C. et al. (2018) Vector competence of field populations of the mosquito species Aedes japonicus japonicus and Culex pipiens from Switzerland for two West Nile virus strains. Medical and Veterinary Entomology, 32, 121–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Lumpy skin disease virus (LSDV) qPCR values of heads, bodies (abdomen1 and thorax), and wings2 of all the investigated arthropods. At least 10 individual's heads and 5 bodies were tested for each time point. All the surviving field‐collected Culicoides were tested.
Figure S1. Standard curve for LSDV Macedonia 2016 field strain (Möller et al., 2019). Conversion of viral DNA (Cq values) into log10TCID50/ml was determined.
Figure S2. Cytopathic effect in MDBK cell cultures of homogenates of Culicoides nubeculosus head (a), thorax/abdomen (b), and wings (c) 10 days post feeding.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


