Abstract
Transformation of Bacillus subtilis by a plasmid requires a circular multimeric form. In contrast, linearized plasmids can be circularized only when homologous sequences are present in the host genome. A recombinational transfer system was constructed with this intrinsic B. subtilis recombinational repair pathway. The vector, pGETS103, a derivative of the θ-type replicating plasmid pTB19 of thermophilic Bacillus, had the full length of Escherichia coli plasmid pBR322. A multimeric form of pGETS103 yielded tetracycline-resistant transformants of B. subtilis. In contrast, linearized pGETS103 gave tetracycline-resistant transformants only when the recipient strain had the pBR322 sequence in the genome. The efficiency and fidelity of the recombinational transfer of DNAs of up to 90 kb are demonstrated.
Horizontal DNA transfer between bacteria is currently regarded as ubiquitous and as being involved in genome evolution (6). Indications from comparative genomic studies are that transfer of DNA occurs between the plasmid and integrated forms in the genome. The fate of a plasmid in horizontal transfer is important because it is suggested that one of the two chromosomes of Vibrio cholerae might be a plasmid (8), and the second chromosome of Deinococcus radiodurans might be derived from Thermus thermophilus (23). However, experimental approaches to studying the dynamics of horizontal DNA transfer between genomes, between plasmids, and between genomes and plasmids have been limited. Bacillus subtilis has historically been used as a recipient for horizontal transfer due to its ability to develop competency (4, 5, 16). B. subtilis develops a competent state at which exogenous DNAs are trapped and processed to yield single-stranded DNAs in cytoplasm (7). Through recombinational and replicational processes, these DNAs are established as plasmids or integrated into the genome where homologous sequences are present (7).
We constructed a system to analyze the dynamics of noncognate genomic DNA horizontal transfer by using a multicopy plasmid, pTB522, from a thermophilic Bacillus strain that shows θ-type replication in B. subtilis (10, 11). There are two advantages to the use of this multicopy plasmid. In contrast to the previously used origin of DNA replication (oriN), which rendered a single copy per cell (15, 17), approximately 9 copies are obtained with pTB522, which is closer to the polyploid nature of bacterial chromosome (e.g., the genomic number of copies per cell of Synechocystis sp. strain PCC6803 is about 12 [20]). The θ-type replication was demonstrated to carry large DNAs stably (18) compared with the rolling circle replicating form common to many plasmids in gram-positive bacteria.
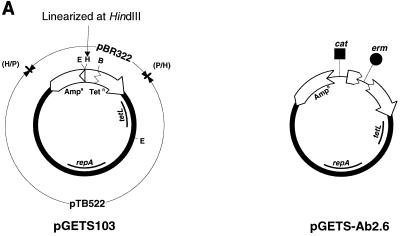
Plasmid pTB522 (11) was linearized at the unique HindIII site by HindIII digestion and blunted by T4 DNA polymerase. The blunted pTB522 was ligated with an Escherichia coli plasmid, pBR322, which had been linearized by PvuII and dephosphorylated with alkaline phosphatase. Recombinant plasmid pGETS103 (Fig. 1A) has the full length of pBR322 and can shuttle between E. coli and B. subtilis. The copy numbers of pGETS103 were estimated to be 9 for B. subtilis and 20 for E. coli (data not shown). Luria-Bertani (LB) broth was used for all bacteria at 37°C. B. subtilis plasmids were prepared by an alkaline method (3). Large-scale preparation of plasmids was performed by an alkaline-sodium dodecyl sulfate (SDS) method, followed by equilibrium centrifugation with a CsCl-ethidium bromide gradient (21). The preparation of competent B. subtilis cells was done by the two-step culture method developed by Anagnostopoulos and Spizizen (1), followed by freezing at −70°C for storage. E. coli JA221 (F− hsdR hsdM+ trp leu lacY recA1) (12) was routinely used as a host for molecular cloning. E. coli LE392 (supE44 supF58 hsdR514 galK2 galT22 metB1 trpR55 lacY1) (21) was used to obtain the multimeric form of pGETS103. Linearized pGETS103 (1 μg) was prepared by digestion with 10 U of HindIII at 37°C for 1 h, followed by phenol extraction and ethanol precipitation.
FIG. 1.
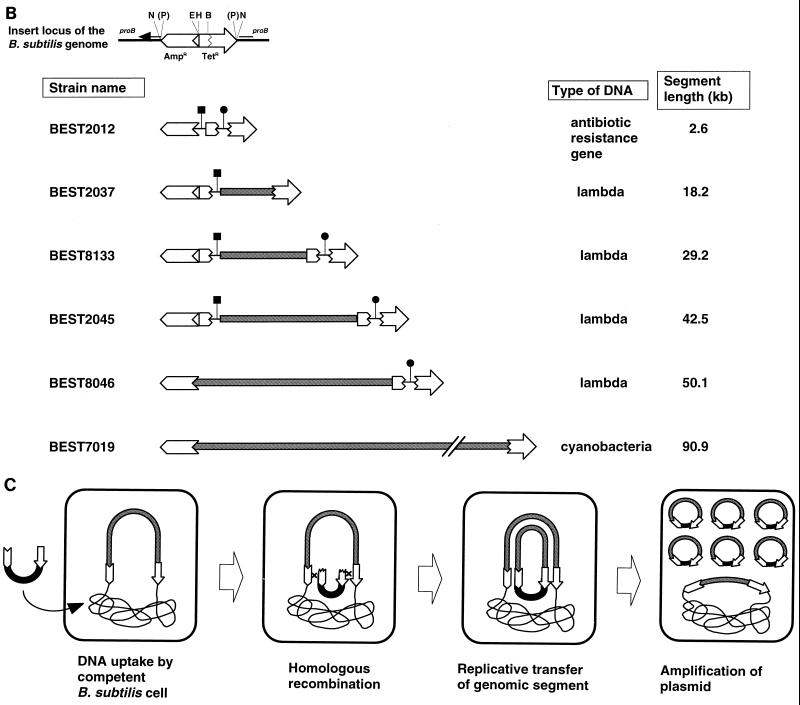
Transfer of genomic segments to plasmid by recombinational transfer. (A) Structures of pGETS103 and pGETS-Ab2.6. Details of the construction are described in the text. pGETS103 was constructed with pBR322 sequences (open arrows) and the pTB522 sequence (bold circle). pGETS103 is linearized at the unique HindIII site. (B) Structure of the insert in the B. subtilis genome. These clones, except for BEST7019, are described in references 13, 14, and 16. leuB::tet of BEST2042 and BEST2046 (14) was converted to leu+ of BEST8133 and BEST8046, which are tetracycline sensitive, because the B. subtilis transformant obtained with pGETS103 was selected by using tetracycline. This conversion was done by transfer of the lambda DNA insert of BEST2042 and BEST2046 to strain 1A1. The pBR322 sequences at the ends are the same as those in panel A. Fragment EcoRI-PvuII is illustrated with two segments: a 375-bp EcoRI-BamHI fragment and 1,689-bp BamHI-PvuII fragment (open torn arrow). A duplicate of the former segment in BEST2037 and BEST2045 produced two recombinants described in the text. Segment length indicates the insert between the genomic pBR322 fragments at the end. ▪ and ● indicate genes for chloramphenicol resistance (cat) and erythromycin resistance (erm), respectively. They were cloned in the BamHI site of pBR322, except for the cat gene of BEST2012, which was cloned in the EcoRI site of pBR322. (C) Recombinational transfer proceeds by homologous recombination between pBR322 sequences (open arrows).
Two B. subtilis strains, wild-type strain RM125 and strain BEST2012, were used as recipients. BEST2012 had a pBR322 sequence in the genome with two antibiotic resistance genes (a 2.6-kb segment), as illustrated in Fig. 1B. One hundred microliters of competent cell culture was incubated with 1 μg of linearized or circular pGETS103 for 30 min at 37°C. The solution, supplemented with 300 μl of LB medium, was incubated at 37°C for 1.5 h to allow expression of the tetracycline resistance gene. Recombinants were selected on LB plates containing tetracycline at 10 μg/ml by incubation at 37°C for 24 h. A covalently closed circular multimeric form of pGETS103 prepared from LE392 (recA+) yielded an equal number of tetracycline-resistant colonies (approximately 104) from both strains. Plasmid DNAs from 20 randomly selected tetracycline-resistant colonies revealed that they carried pGETS103 itself for both strains. In contrast, when linearized pGETS103 was used, no tetracycline-resistant colony was obtained from RM125, as expected, but 43 such colonies were formed from BEST2012. Plasmid DNAs prepared from the 20 randomly selected tetracycline-resistant clones of BEST2012 were all identical to the structure of pGETS-Ab2.6 carrying a segment including two antibiotic markers originating from the genomic pBR322 structure of BEST2012 (Fig. 1A and B). The fragment digested with SalI shown in Fig. 2 revealed a 2.0-kb fragment and a 1.0- kb fragment carrying the erythromycin and chloramphenicol resistance genes, respectively. These results demonstrated that the linearized pGETS103 was not established as plasmid by a mechanism other than the model described in Fig. 1C. We will refer to this mechanism as recombinational transfer.
FIG. 2.
Segments transferred to pGETS103. (A) SalI digests of plasmid from the BEST2012 transformant. Two antibiotic resistance genes (cat and erm) originating from genomic pBR322 (Fig. 1B) were transferred to all plasmids. (B) BstEII digests of the lambda DNA insert in the plasmid are compared with intact lambda digested by BstEII. Plasmids from 20 transformants each of BEST2037 (18.2-kb segment), BEST8133 (29.2-kb segment), BEST2045 (42.5-kb segment), and BEST8046 (50.1-kb segment) showed faithful recombinational transfer, except for clones denoted by the downward arrow as described in the text.
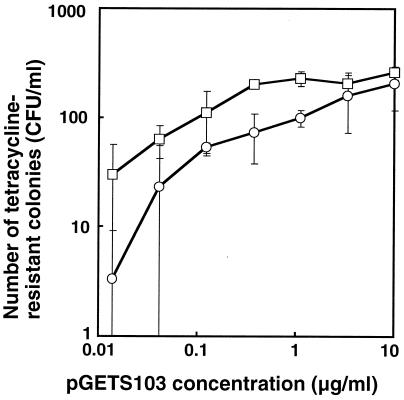
The requirement and efficiency of larger genomic inserts for recombinational transfer were investigated by using a series of B. subtilis strains that have various lengths of E. coli phage lambda DNA (up to 50.1 kb) (13, 14, 16), as indicated in Fig. 1B. The common pBR322 structure at both ends of the lambda DNA insert is basically the same as that of BEST2012; thus, we expected recombinational transfer of the lambda DNA insert to pGETS103 in a manner similar to that of the two antibiotic resistance genes. To obtain profiles of recombinational transfer efficiency, the numbers of recombinants from BEST8046, which had the complete lambda DNA insert (50.1 kb), and BEST2012, which had the smallest segment (2.6 kb), were directly compared over a broad range of pGETS103 concentrations from 0.01 to 10 μg/ml. The results of these experiments are shown in Fig. 3. In both strains, the number of tetracycline-resistant transformants increased as the concentration of linearized pGETS103 DNA increased up until 1 μg/ml for BEST2012 and 10 μg/ml for BEST8046. Below 1 μg/ml, BEST8046 always gave a smaller number (approximately 10-fold) than BEST2012. At 10 μg/ml, the numbers from both strains were indistinguishable. Similar numbers of transformants at 10 μg/ml were also obtained for BEST2037 (19.2-kb insert), BEST8133 (28.2-kb insert), and BEST2045 (39.5-kb insert) (data not shown). The structure of the plasmid in 20 randomly selected clones from the respective host (BEST2037, BEST8133, BEST2043, and BEST8046) was analyzed by digestion with restriction endonuclease BstEII. Although there are only two exceptional clones (indicated by downward arrows in Fig. 2), all of BstEII fragments of the 20 independent clones presented in Fig. 2 had identical patterns. Each pattern of the BstEII fragments derived from various strains was consistent with the structure of the relevant phage insert of the respective host. From these results, we concluded that tetracycline-resistant transformants obtained by using linearized pGETS103 were formed only by a recombinational transfer mechanism. Two exceptions, one from BEST2037 and the other from BEST2045, were pGETS103 itself, which also accounted for the recombinational transfer caused by a contiguous sequence around the EcoRI site of BEST2037 and BEST2045 (Fig. 1B). The recombinational transfer mechanism leaves the original insert in the genome unaltered. Genomic Southern analysis of NotI digests of recombinational transferred strains revealed that this was the case (data not shown).
FIG. 3.
Number of tetracycline transformants of BEST2012 (2.6-kb insert; □) and BEST8046 (50.1-kb insert; ○) with linearized pGETS103 at the indicated concentrations. The number of tetracycline-resistant transformants counted directly was normalized for comparison by measuring efficiency of competence. Efficiency of competence was obtained by scoring tetracycline-resistant colonies in a leuB+→leuB::tet frequency by using genomic DNA of strain BEST2204 (leuB::tet), which had a tetracycline resistance gene at the leuB locus (16). Values are the mean of three independent experiments with error bars showing standard deviation.
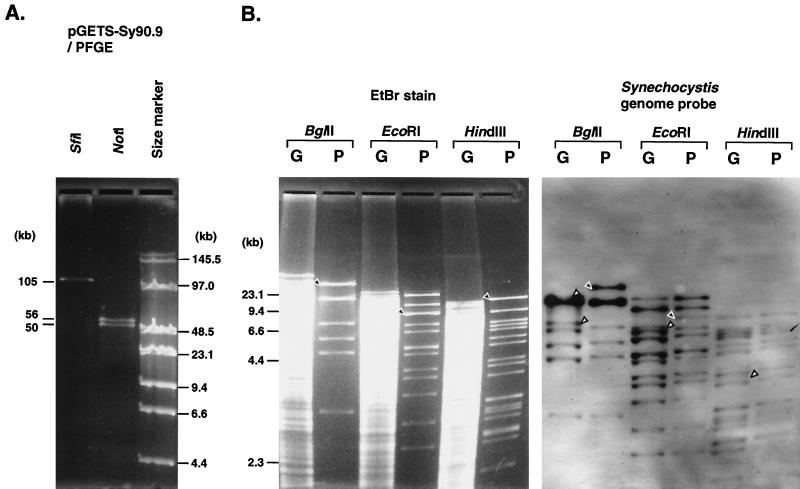
To exploit the recombinational transfer functions for DNAs different from and larger than lambda DNA, a B. subtilis strain, BEST7019, that had genomic DNA of Synechocystis sp. strain PCC6803 was used (M. Itaya et al., unpublished data). Cyanobacterial genomic DNA as long as 90.9 kb from bp 25603 to 115223 in the genome sequence (DDBJ/EMBL/GenBank accession no. AB001339) (Itaya et al., unpublished) was inserted in the genomic pBR322 in a structure similar to that of the lambda insert as shown in Fig. 1B. On transformation of BEST7019 by linearized pGETS103 (approximately 1 μg), 10 tetracycline-resistant colonies were isolated. All 10 of the plasmids had identical HindIII fragment patterns (data not shown). A representative plasmid, pGETS-Sy90.9, was further analyzed by using other enzymes to determine the size of the insert. An SfiI digestion of pGETS-Sy90.9 gave a fragment (Fig. 4A) consistent with the calculated value (105.4 kb) of the insert (90.9 kb) and pGETS vector (14.5 kb) component. The two NotI fragments of the plasmid shown in Fig. 4A were also consistent with the calculated value (49.6 and 55.9 kb). To confirm the structure of the insert, digests of genome DNA of strain BEST7019 and pGETS-Sy90.9 treated with BglII, EcoRI, and HindIII were directly compared by Southern analysis with Synechocystis sp. strain PCC6803 total genome DNA as a probe. Southern hybridization experiments with digoxigenin-labeled probe were performed as previously described (12). The result shown in Fig. 4B that Southern bands from both DNAs were identical indicated that the 90.9-kb insert of BEST7019 was faithfully transferred to pGETS103 by recombinational transfer.
FIG. 4.
Transfer of the 90.9-kb insert of the cyanobacterial genome cloned in BEST7019 to pGETS103. (A) pGETS-Sy90.9 digested by SfiI or NotI separated by contour-clamped homogeneous electric field electrophoresis. The calculated value is indicated on the left. Genomic DNA of BEST7019 is compared with the insert of pGETS-Sy90.9 by using restriction enzymes BglII, EcoRI, and HindIII. (Left) Ethidium bromide (EtBr)-stained DNA of genomic BEST7019 (G) and plasmid pGETS-Sy90.9 (P). (Right) Southern bands obtained with Synechocystis sp. strain PCC6803 total genomic DNA as a probe.
DNA fragments shuttling between the plasmid and the genome are one of the causes of genome structural alterations (15, 17). Similar DNA shuttling in Saccharomyces cerevisiae that proceeds between yeast artificial chromosomes and a DNA fragment of up to 130 kb has been reported (2). In these examples, cloned noncognate DNAs are used for further genetic manipulations. Recombinational transfer in B. subtilis was first reported between the plasmid and the genome (9, 22) in which transferred DNAs were intrinsic genomic segments with sizes of 4.0 and 4.7 kb (within the detection limit at the time). We first examined whether the recombinational transfer mechanism applies to noncognate DNA in B. subtilis. To allow systematic and quantitative analysis, noncognate DNAs ranging from 2.6 to 90.6 kb in the genomic pBR322 were used as target inserts.
In the transfer of the lambda DNA, to our surprise, the system worked with almost equal efficiency at high DNA concentrations. The process of recombinational transfer comprises four discrete steps. They are uptake of the linearized pGETS103, formation of homologous pairs, copying of the insert by replication, and amplification of the plasmid. The uptake process was unlikely to be the frequency-determining step, because BEST2012 and BEST8046 showed a similar level of competency, and the same linearized pGETS103 solution was used. The replication rate seems unlikely to be this step either, because it takes 2 min to copy the longest 48.5-kb insert if the rate by DNA polymerase (∼700 bases/s at 37°C) is adopted (19). The amplification of plasmid by DNA polymerases takes a similar amount of time; thus, the effect on frequency seems marginal. We think that the step for formation of simultaneous homologous pairs affects the frequency of the longer insert. Because completion of recombinational transfer absolutely requires two homologous recombinations, a low concentration of DNA results in a lower frequency of recombinogenic molecules. Thus, the chance of double crossover with the longer insert gives rise to abortive molecules in the cell. This lower frequency may be compensated for by providing a sufficient number of recombinogenic molecules, which is achieved at a high concentration of pGETS103. It remains to be demonstrated whether dual recombination requires any topological hindrance depending on the target insert length.
The technique of using a θ-type plasmid may be applied to recover not only the cloned insert, but also any inserts in the B. subtilis genome. An investigation of recombinational transfer with different cloned inserts is in progress.
Acknowledgments
We thank Tadayuki Imanaka for the gift of plasmid pTB522.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradshaw M S, Bollekens J A, Ruddle F H. A new vector for recombination-based cloning of large DNA fragments from yeast artificial chromosomes. Nucleic Acids Res. 1995;23:4850–4856. doi: 10.1093/nar/23.23.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bron S. Plasmids. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus—1990. Chichester, England: John Wiley and Sons; 1990. pp. 75–174. [Google Scholar]
- 4.Cohan F M. Genetic exchange and evolutionary divergence in prokaryotes. Trends Ecol Evol. 1994;9:175–180. doi: 10.1016/0169-5347(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 5.Cutting S M, Horn P B V. Gene cloning techniques. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus—1990. Chichester, England: John Wiley and Sons; 1990. pp. 27–74. [Google Scholar]
- 6.Doolittle R F. Searching for the common ancestor. Res Microbiol. 2000;151:85–89. doi: 10.1016/s0923-2508(00)00124-8. [DOI] [PubMed] [Google Scholar]
- 7.Dubnau D. Genetic competence in Bacillus subtilis. Microbiol Rev. 1991;55:395–424. doi: 10.1128/mr.55.3.395-424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidelberg J F, Eisen J A, Nelson W C, Clayton R A, Gwinn M L, Dodson R J, Haft D H, Hickey E K, Peterson J D, Umayam L, Gill S R, Nelson K E, Read T D, Tettelin H, Richardson D, Ermolaeva M D, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann R D, Nierman W C, White O, Salzberg S L, Smith H O, Colwell R R, Mekalanos J J, Venter J C, Fraser C M. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglesias A, Bensi G, Canosi U, Trautner T A. Plasmid transformation in Bacillus subtilis: alterations introduced into the recipient-homologous DNA of hybrid plasmids can be corrected in transformation. Mol Gen Genet. 1981;184:405–409. doi: 10.1007/BF00352513. [DOI] [PubMed] [Google Scholar]
- 10.Imanaka T, Fujii M, Aiba S. Isolation and characterization of antibiotic resistance plasmid from thermophilic bacilli and construction of deletion plasmids. J Bacteriol. 1981;146:1091–1097. doi: 10.1128/jb.146.3.1091-1097.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imanaka T, Himeno T, Aiba S. Effect of in vitro DNA rearrangement in the NH2-terminal region of the penicillinase gene from Bacillus licheniformis on the mode of expression in Bacillus subtilis. J Gen Microbiol. 1985;131:1753–1763. doi: 10.1099/00221287-131-7-1753. [DOI] [PubMed] [Google Scholar]
- 12.Itaya M, Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991;220:631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- 13.Itaya M. Integration of repeated sequences (pBR322) in the Bacillus subtilis 168 chromosome without affecting the genome structure. Mol Gen Genet. 1993;241:287–297. doi: 10.1007/BF00284680. [DOI] [PubMed] [Google Scholar]
- 14.Itaya M. Toward a bacterial genome technology: integration of the Escherichia coli prophage lambda genome into Bacillus subtilis 168 chromosome. Mol Gen Genet. 1995;248:9–16. doi: 10.1007/BF02456608. [DOI] [PubMed] [Google Scholar]
- 15.Itaya M, Tanaka T. Experimental surgery to create subgenomes of Bacillus subtilis 168. Proc Natl Acad Sci USA. 1997;94:5378–5382. doi: 10.1073/pnas.94.10.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itaya M. Genetic transfer of large DNA inserts to designated loci of the Bacillus subtilis 168 genome. J Bacteriol. 1999;181:1045–1048. doi: 10.1128/jb.181.3.1045-1048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itaya M, Tanaka T. Fate of unstable Bacillus subtilis subgenome: reintegration and amplification in the main genome. FEBS Lett. 1999;448:235–238. doi: 10.1016/s0014-5793(99)00351-8. [DOI] [PubMed] [Google Scholar]
- 18.Jannière L, Bruand C, Ehrlich S D. Structurally stable Bacillus subtilis cloning vectors. Gene. 1990;87:53–61. doi: 10.1016/0378-1119(90)90495-d. [DOI] [PubMed] [Google Scholar]
- 19.Kornberg A, Baker T A. DNA replication. 2nd ed. New York, N.Y: W. H. Freeman and Company; 1992. [Google Scholar]
- 20.Labarre J, Chauvat F, Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989;171:3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Tanaka T. Transfer of a Bacillus subtilis chromosomal mutation to a homologous DNA region on a plasmid which is carried in the mutant. Agric Biol Chem. 1982;46:1101–1102. [Google Scholar]
- 23.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan J J, Lam P, McDonald L, Utterback T, Zalewski C, Makarova K S, Aravind L, Daly M J, Minton K W, Fleischmann R D, Ketchum K A, Nelson K E, Salzberg S, Smith H O, Venter J C, Fraser C M. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]