Abstract
Background
Conditional survival estimates provide critical prognostic information for patients with advanced renal cell carcinoma (aRCC). Efficacy, safety, and conditional survival outcomes were assessed in CheckMate 214 (ClinicalTrials.gov identifier NCT02231749) with a minimum follow‐up of 5 years.
Methods
Patients with untreated aRCC were randomized to receive nivolumab (NIVO) (3 mg/kg) plus ipilimumab (IPI) (1 mg/kg) every 3 weeks for 4 cycles, then either NIVO monotherapy or sunitinib (SUN) (50 mg) daily (four 6‐week cycles). Efficacy was assessed in intent‐to‐treat, International Metastatic Renal Cell Carcinoma Database Consortium intermediate‐risk/poor‐risk, and favorable‐risk populations. Conditional survival outcomes (the probability of remaining alive, progression free, or in response 2 years beyond a specified landmark) were analyzed.
Results
The median follow‐up was 67.7 months; overall survival (median, 55.7 vs 38.4 months; hazard ratio, 0.72), progression‐free survival (median, 12.3 vs 12.3 months; hazard ratio, 0.86), and objective response (39.3% vs 32.4%) benefits were maintained with NIVO+IPI versus SUN, respectively, in intent‐to‐treat patients (N = 550 vs 546). Point estimates for 2‐year conditional overall survival beyond the 3‐year landmark were higher with NIVO+IPI versus SUN (intent‐to‐treat patients, 81% vs 72%; intermediate‐risk/poor‐risk patients, 79% vs 72%; favorable‐risk patients, 85% vs 72%). Conditional progression‐free survival and response point estimates were also higher beyond 3 years with NIVO+IPI. Point estimates for conditional overall survival were higher or remained steady at each subsequent year of survival with NIVO+IPI in patients stratified by tumor programmed death ligand 1 expression, grade ≥3 immune‐mediated adverse event experience, body mass index, and age.
Conclusions
Durable clinical benefits were observed with NIVO+IPI versus SUN at 5 years, the longest phase 3 follow‐up for a first‐line checkpoint inhibitor‐based combination in patients with aRCC. Conditional estimates indicate that most patients who remained alive or in response with NIVO+IPI at 3 years remained so at 5 years.
Keywords: advanced renal cell carcinoma, CheckMate 214, dual checkpoint inhibition, durable response, long‐term follow‐up, nivolumab plus ipilimumab, phase 3
Short abstract
In the longest phase 3 follow‐up of a checkpoint inhibitor combination therapy in advanced renal cell carcinoma together with the first long‐term conditional survival analyses in the CheckMate 214 trial, nivolumab plus ipilimumab demonstrated durable survival and response benefits versus sunitinib at 5 years. These results establish a new benchmark for both the magnitude and durability of benefit possible with first‐line immunotherapy‐based regimens in this setting.
Introduction
Survival outcomes for patients with advanced or metastatic renal cell carcinoma (aRCC) have improved significantly in recent years, with immunotherapy‐based combination regimens further prolonging survival over single‐agent, targeted therapies. 1 , 2 , 3 , 4 However, because limited follow‐up is available for most phase 3 trials of newer first‐line aRCC treatments, additional analyses that comprehensively assess long‐term clinical benefits in this setting remain of critical importance. Nivolumab (NIVO) plus ipilimumab (IPI) (NIVO+IPI) is approved for patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate‐risk/poor‐risk disease based on the primary phase 3 CheckMate 214 trial results. 2
Traditional prognostic risk models like the IMDC classification are routinely used to inform upfront prognosis and treatment decisions to help predict patient outcomes in aRCC. 5 However, such prognostic estimates based on baseline risk factors were established in the era of single‐agent tyrosine kinase inhibitors and cytokines, and do not adequately address outcomes for patients with aRCC who achieve a durable response and survival benefits with immunotherapy. 6 , 7 As the aRCC treatment landscape evolves and long‐term outcomes continue to improve, an updated prognostic framework is needed. Although clinical response and survival projections at diagnosis using standard prognostic models can help individualize therapy regimens, prognoses may change over time, particularly among patients with a poor outlook according to baseline assessments. 8 , 9 Conditional survival has since emerged as a clinically relevant measure of prognosis that estimates survival probability for patients as the length of survival increases in response to treatment. 9 , 10 Conditional survival assessments may account for the time alive since randomization or treatment initiation and can help provide critical long‐term prognostic information as prespecified survival milestones are reached. Previous conditional survival analyses in patients with aRCC who received VEGF‐targeted therapy demonstrated improved outcomes over time with length of survivorship. 8 , 11 However, limited conditional survival data exist in patients with aRCC who received NIVO monotherapy or first‐line immunotherapy combinations. 11
With a minimum follow‐up of 5 years, we report the longest phase 3 follow‐up for a checkpoint inhibitor combination therapy in aRCC together with the first long‐term conditional survival analyses of patients in CheckMate 214.
Materials and Methods
Patients and Treatment
CheckMate 214 is a global, open‐label, randomized, phase 3 trial. Study design and statistical analyses details have been described previously, and additional details are included in the online Supporting Information. 12 CheckMate 214 was approved by institutional review boards or ethics committees at each site and was conducted following Good Clinical Practice Guidelines according to the International Conference for Harmonisation. All patients provided written informed consent in accordance with Declaration of Helsinki principles. This study is registered with ClinicalTrials.gov (ClinicalTrials.gov identifier NCT02231749).
Assessments
The co‐primary trial end points were overall survival (OS), progression‐free survival (PFS) according to an independent radiology review committee, and the objective response rate (ORR) according to the independent radiology review committee (with duration of response [DOR]) in intermediate‐risk/poor‐risk patients (primary), intent‐to‐treat (ITT) patients (secondary), and favorable‐risk patients (exploratory). Conditional OS, conditional PFS, and conditional response estimates, together with an evaluation of patients who had durable clinical benefits and an assessment of treatment‐free interval in responders, were analyzed post hoc. Additional assessment details are included in the Supporting Methods.
Statistical Analysis
Conditional survival was analyzed using a landmark approach based on Kaplan‐Meier estimates and was calculated for patients who were either alive, progression free, or in response at 1‐year increments from time zero. Conditional OS, conditional PFS (time zero was the date of randomization for both), and conditional response (time zero was the date of first confirmed response) were assessed until death or censoring at the date of last follow‐up. Data from patients who died before the landmark timepoint or whose follow‐up interval was less than the landmark time were excluded. Statistical analysis details for OS, PFS, ORR, and health‐related quality of life (HRQoL) were previously reported, and additional information is included in the Supporting Information.
Results
Patients and Treatment Outcomes
In total, 1096 patients were randomized to NIVO+IPI (ITT patients, n = 550; intermediate‐risk/poor‐risk patients, n = 425; favorable‐risk patients, n = 125) or sunitinib (SUN) (ITT patients, n = 546; intermediate‐risk/poor‐risk patients, n = 422; favorable‐risk patients, n = 124). Overall, 547 patients in the NIVO+IPI arm and 535 in the SUN arm received treatment and were included in the safety analyses. The database lock for this analysis was February 24, 2021. At a minimum 5‐year study follow‐up (median follow‐up, 67.7 months), 34 of 547 (6%) treated patients in the NIVO+IPI arm and 9 of 535 (2%) treated patients in the SUN arm continued therapy (see Supporting Fig. 1). Key baseline characteristics were generally similar between treatment arms in ITT patients, as previously reported (see Supporting Table 1). 2 , 12 , 13 , 14 The median duration of therapy was 7.9 months (quartile 1 [Q1]‐Q3, 2.1‐21.8 months) in the NIVO+IPI arm and 7.8 months (Q1‐Q3, 3.5‐19.6 months) in the SUN arm. Subsequent systemic therapy was received by 305 of 550 (55%) ITT patients in the NIVO+IPI arm and by 372 of 546 (68%) ITT patients in the SUN arm (see Supporting Table 2).
OS, PFS, and ORR in ITT, Intermediate‐Risk/Poor‐Risk, and Favorable‐Risk Patients
With a minimum follow‐up of 5 years, OS superiority was maintained with NIVO+IPI versus SUN in the ITT population (hazard ratio [HR], 0.72; 95% confidence interval [CI], 0.62‐0.85). The median OS was 55.7 versus 38.4 months with NIVO+IPI versus SUN, respectively, and the 5‐year OS probability was greater with NIVO+IPI (48% vs 37%) (Fig. 1A). In intermediate‐risk/poor‐risk patients, OS also remained superior with NIVO+IPI versus SUN, with a median OS of 47.0 versus 26.6 months, respectively (HR, 0.68; 95% CI, 0.58‐0.81), and 5‐year OS probabilities of 43% versus 31%, respectively (Fig. 2A). In favorable‐risk patients, the HR for OS was 0.94 (95% CI, 0.65‐1.37). The median OS was 74.1 months with NIVO+IPI versus 68.4 months with SUN, and the 5‐year OS probability was 63% with NIVO+IPI versus 55% with SUN (Fig. 3A). OS benefits were observed with NIVO+IPI versus SUN in both intermediate‐risk patients (HR, 0.74) and poor‐risk patients (HR, 0.58) and in ITT patients regardless of tumor programmed death ligand 1 (PD‐L1) expression status (<1%: HR, 0.77; ≥1%: HR, 0.57) (Fig. 4).
Figure 1.
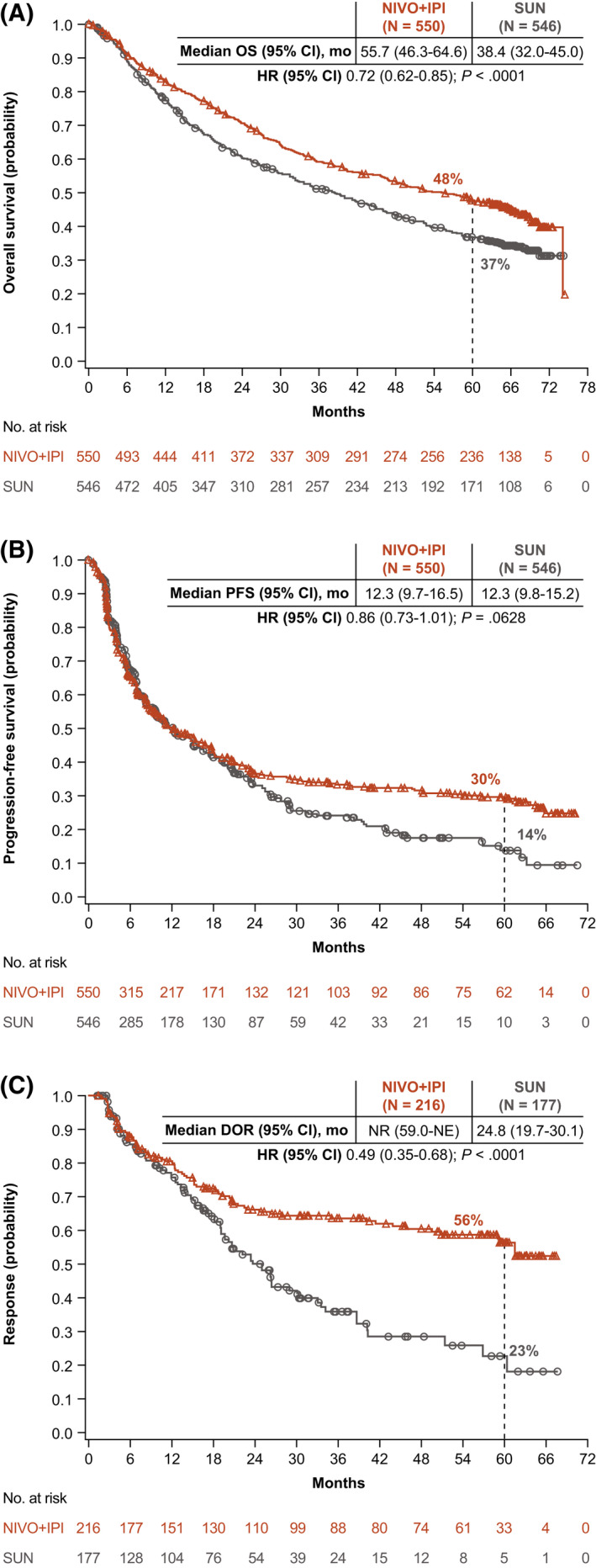
(A) Overall survival (OS), (B) progression‐free survival (PFS), and (C) duration of response (DOR) are illustrated according to an independent radiology review committee using Response Evaluation Criteria in Solid Tumors, version 1.1, among patients in the intent‐to‐treat group. CI indicates confidence interval; HR, hazard ratio; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; NR, not reached; SUN, sunitinib.
Figure 2.
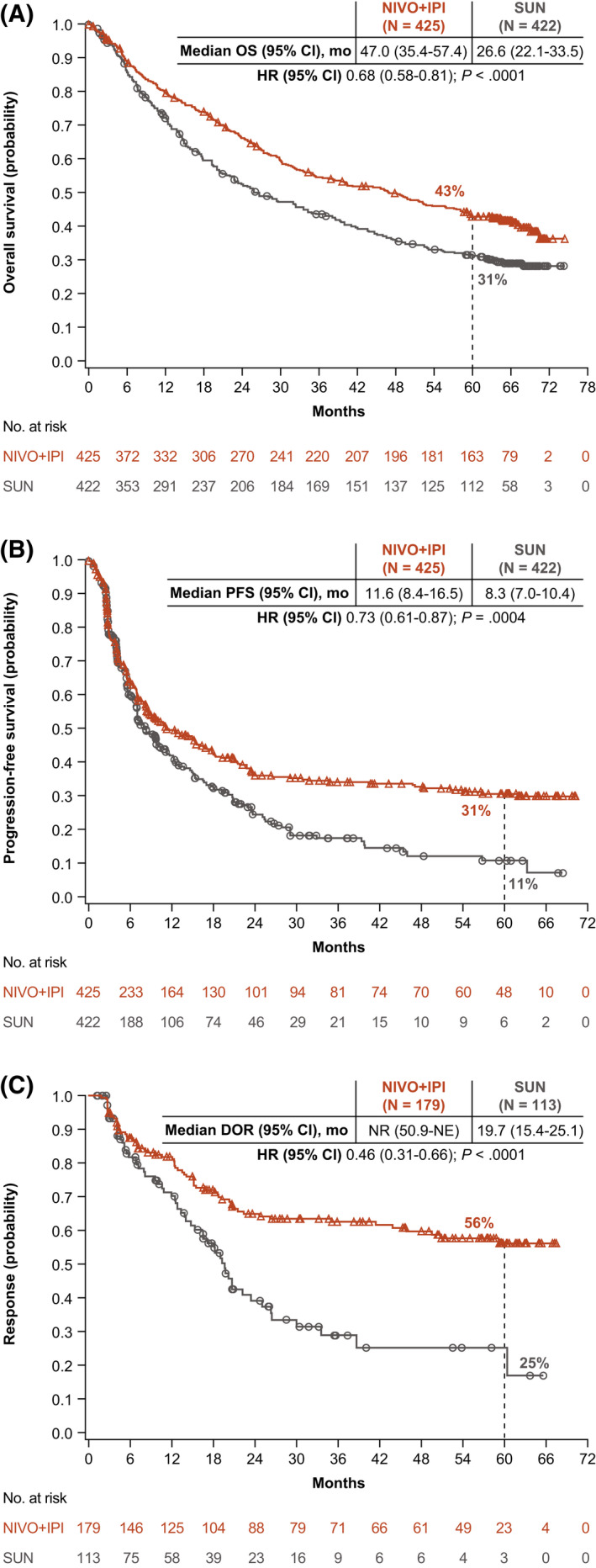
(A) Overall survival (OS), (B) progression‐free survival (PFS), and (C) duration of response (DOR) are illustrated according to an independent radiology review committee using Response Evaluation Criteria in Solid Tumors, version 1.1, in patients with intermediate‐risk/poor‐risk renal cell carcinoma according to the International Metastatic Renal Cell Carcinoma Database Consortium. CI indicates confidence interval; HR, hazard ratio; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; NR, not reached; SUN, sunitinib.
Figure 3.
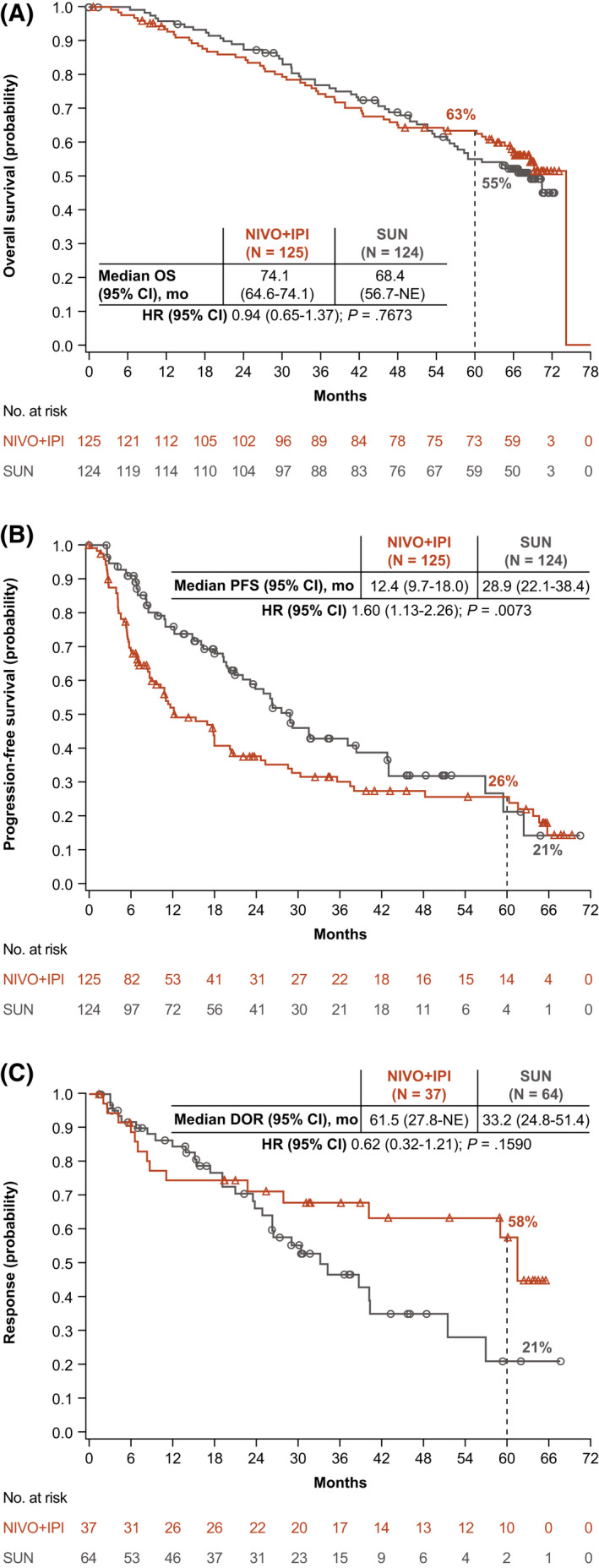
(A) Overall survival (OS), (B) progression‐free survival (PFS), and (C) duration of response (DOR) are illustrated according to an independent radiology review committee using Response Evaluation Criteria in Solid Tumors, version 1.1, in patients with favorable‐risk renal cell carcinoma according to the International Metastatic Renal Cell Carcinoma Database Consortium. CI indicates confidence interval; HR, hazard ratio; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; SUN, sunitinib.
Figure 4.
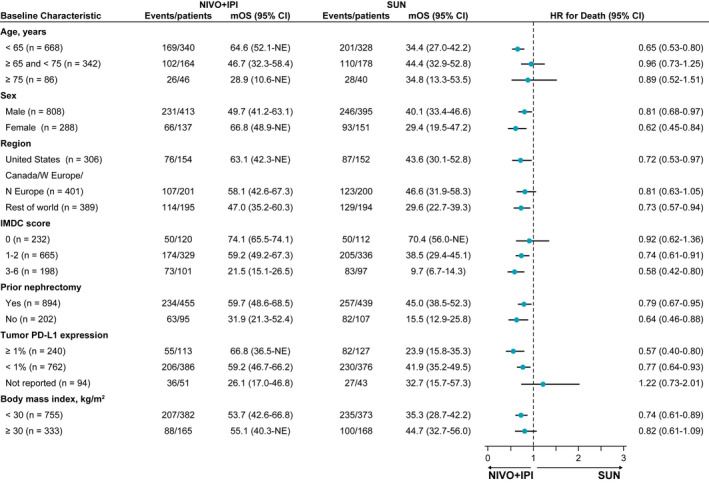
Subgroup analysis of overall survival is illustrated in intent‐to‐treat patients. Note that hazard ratios (HRs) were not computed for subset categories that included <21 patients per treatment group. CI indicates confidence interval; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; mOS, median overall survival; N Europe, northern Europe; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; PD‐L1, programmed death ligand 1; SUN, sunitinib; W Europe, western Europe.
The HR for PFS with NIVO+IPI versus SUN was 0.86 (95% CI, 0.73‐1.01) in ITT patients and 0.73 (95% CI, 0.61‐0.87) (Fig. 2B) in patients with intermediate‐risk/poor‐risk disease. PFS benefits were observed in these patients, as demonstrated by greater 5‐year PFS probabilities with NIVO+IPI versus SUN in both populations (ITT, 30% vs 14%; intermediate‐risk/poor‐risk, 31% vs 11%) (Figs. 1B and 2B). In patients with favorable‐risk disease, the HR for PFS favored SUN (HR, 1.60; 95% CI, 1.13‐2.26), yet the 5‐year PFS probability was 26% with NIVO+IPI versus 21% with SUN (Fig. 3B).
The ORR was greater with NIVO+IPI versus SUN both in ITT patients (39% vs 32%) and in intermediate‐risk/poor‐risk patients (42% vs 27%) (see Supporting Table 3). Among favorable‐risk patients, the ORR was lower with NIVO+IPI versus SUN (30% vs 52%); however, a higher proportion of patients achieved a complete response (CR) with NIVO+IPI regardless of risk group (ITT population, 12% vs 3%; intermediate‐risk/poor‐risk population, 11% vs 2%; favorable‐risk population, 13% vs 6%) (see Supporting Table 3). The median time to response was shorter with NIVO+IPI versus SUN (2.8 months [Q1‐Q3, 2.7‐4.0 months] vs 4.0 months [Q1‐Q3, 2.8‐5.6 months]), and the median DOR was longer (not reached vs 24.8 months) in the ITT population, with more ongoing responses at 5 years in those who received NIVO+IPI across risk groups (ITT population, 63% vs 50%; intermediate‐risk/poor‐risk population, 64% vs 50%; favorable‐risk population, 59% vs 52%) (Figs. 1C, 2C, and 3C; see Supporting Table 3). More ITT responders experienced a treatment‐free interval without requiring subsequent systemic therapy with NIVO+IPI (103 of 216 patients; 48%) versus SUN (43 of 177 patients; 24%).
Conditional Survival in ITT, Intermediate‐Risk/Poor‐Risk, and Favorable‐Risk Patients
In the NIVO+IPI arm, point estimates for the probability of remaining alive for an additional 2 years (conditional OS) were higher or stable at each subsequent year of survival after time zero (randomization) in ITT patients (randomization, 71%; year 1, 71%; year 2, 76%; year 3, 81%) and in intermediate‐risk/poor‐risk patients (randomization, 66%; year 1, 68%; year 2, 75%; year 3, 79%), and remained high in favorable‐risk patients (randomization, 85%; year 1, 80%; year 2, 77%; year 3, 85%) (Fig. 5; see Supporting Table 4). Point estimates for 2‐year conditional OS were also higher with NIVO+IPI versus SUN from the 3‐year landmark regardless of IMDC risk group (ITT patients, 81% vs 72%; intermediate‐risk/poor‐risk patients, 79% vs 72%; favorable‐risk patients, 85% vs 72%).
Figure 5.
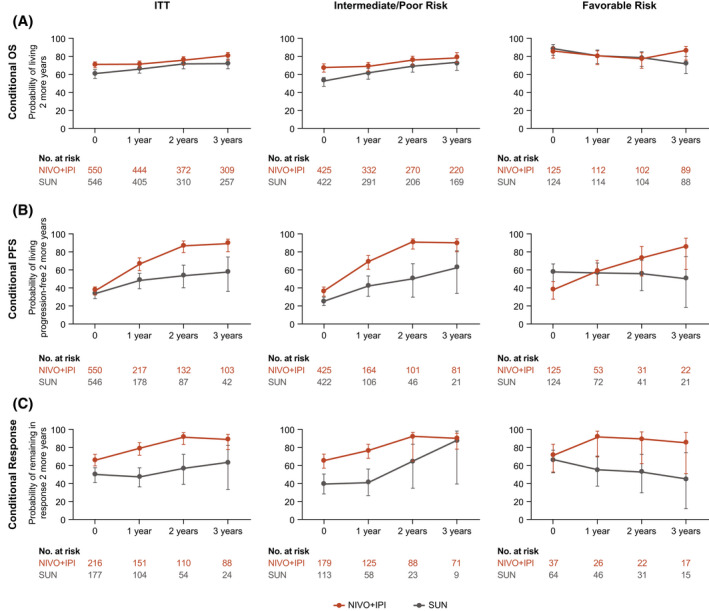
(A) Conditional overall survival (OS), (B) conditional progression‐free survival (PFS), and (C) and conditional responses are illustrated among patients in the intent‐to‐treat (ITT) group, patients who had International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate‐risk/poor‐risk disease, and patients who had IMDC favorable‐risk disease. X‐axes indicate the landmark time from randomization (conditional OS and PFS) or the landmark time from the first confirmed response (conditional response). Error bars indicate 95% confidence intervals. OS, PFS, and response probabilities were conditioned on the time alive, the time progression‐free, or the time in response after time zero. NIVO+IPI indicates nivolumab plus ipilimumab; SUN, sunitinib.
With NIVO+IPI, point estimates for 2‐year conditional PFS were higher or remained stable from time zero (randomization) at each subsequent year of survival in ITT (randomization, 37%; year 1, 66%; year 2, 87%; year 3, 89%), intermediate‐risk/poor‐risk (randomization, 36%; year 1, 69%; year 2, 91%; year 3, 90%), and favorable‐risk (randomization, 38%; year 1, 58%; year 2, 73%; year 3, 85%) patients (Fig. 5; see Supporting Table 4). At the 3‐year landmark, point estimates for conditional PFS were higher with NIVO+IPI versus SUN in ITT (89% vs 57%), intermediate‐risk/poor‐risk (90% vs 62%), and favorable‐risk (85% vs 50%) patients. The probability of remaining in response with NIVO+IPI for an additional 2 years (conditional response) was also higher or remained stable from time zero (first response) according to point estimates at each subsequent year of survival in ITT (first response, 66%; year 1, 79%; year 2, 91%; year 3, 89%), intermediate‐risk/poor‐risk (first response, 65%; year 1, 76%; year 2, 92%; year 3, 90%), and favorable‐risk (first response, 71%; year 1, 91%; year 2, 89%; year 3, 85%) patients (Fig. 5; see Supporting Table 4). Point estimates for 2‐year conditional response from the 3‐year landmark were higher with NIVO+IPI versus SUN regardless of IMDC risk group (ITT patients, 89% vs 63%; intermediate‐risk/poor‐risk patients, 90% vs 88%; favorable‐risk patients, 85% vs 45%).
Conditional OS in Subgroups
Point estimates for conditional OS with NIVO+IPI varied to some extent across age groups in ITT patients at time zero yet were stable or consistently higher with NIVO+IPI at each subsequent year of conditional survival regardless of IMDC risk group, except for favorable‐risk patients younger than 65 years (Fig. 6A; see Supporting Table 5). Point estimates for conditional OS with NIVO+IPI in ITT patients were similar regardless of body mass index (BMI), grade ≥3 immune‐mediated adverse event (AE) experience, or tumor PD‐L1 expression and were either higher or stable at each subsequent year of survival in each subgroup (Fig. 6B‐D; see Supporting Table 5). Point estimates for conditional OS with NIVO+IPI in intermediate‐risk/poor‐risk patients were also higher or stable from time zero at each subsequent year of survival regardless of BMI, grade ≥3 immune‐mediated AEs, or tumor PD‐L1 expression and generally remained high (approximately ≥80%) at 3 years in favorable‐risk patients (see Supporting Table 5). Point estimates for conditional OS with NIVO+IPI remained high (>96%) in ITT patients who had a CR from all landmark timepoints assessed (Fig. 6E; see Supporting Table 5); similar trends were observed in patients who had a CR regardless of IMDC risk group (see Supporting Table 5). Point estimates for conditional OS with SUN were higher or remained stable at each subsequent year of survival from time zero across all subgroups among ITT and intermediate‐risk/poor‐risk patients, except for patients aged 75 years and older (see Supporting Table 6). Interestingly, point estimates for conditional OS with SUN decreased with subsequent years of survival across almost all subgroups in favorable‐risk patients, except for those with tumor PD‐L1 expression ≥1% and those who achieved a CR.
Figure 6.
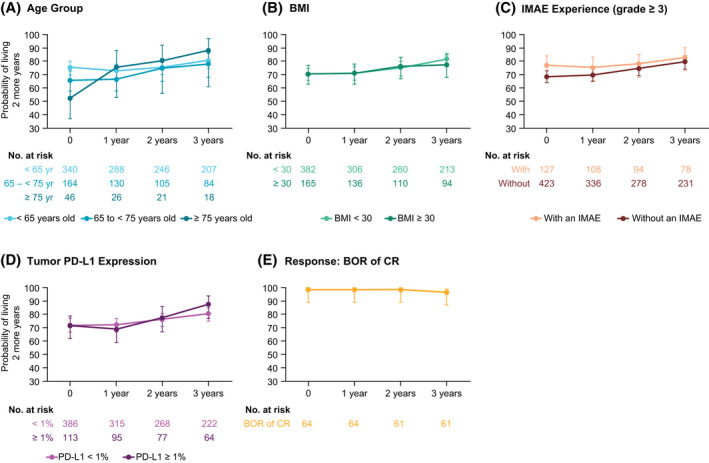
Conditional overall survival (OS) is illustrated according to baseline clinical subgroups and complete responses among patients in the intent‐to‐treat group from the nivolumab plus ipilimumab arm. X‐axes indicate the landmark time from randomization. Error bars are 95% confidence intervals. OS probabilities were conditioned on the time survived after time zero and were stratified by (A) age (<65, 65‐75, or ≥75 years), (B) body mass index (BMI) (<30 or ≥30 kg/m2), (C) grade ≥3 immune‐mediated adverse event (IMAE) experience (yes or no), (D) tumor programmed death ligand 1 (PD‐L1) expression (<1% or ≥1%), and (E) and among patients who had a complete response (CR) as their best overall response (BOR).
Durable Clinical Benefit
More patients achieved a CR and did not progress with NIVO+IPI (n = 53 of 550; 9.6%) versus SUN (n = 13 of 546; 2.4%). Among complete responders without progression, baseline characteristics were largely similar to those of ITT patients in the NIVO+IPI arm. However, a higher proportion of complete responders without progression in the SUN arm had just 1 site with target/nontarget lesions at baseline (5 of 13 patients [38%] vs 118 of 546 patients [22%]), and more had favorable‐risk disease (6 of 13 patients [46%] vs 124 of 546 patients [23%]) compared with ITT patients; none of the complete responders without progression had poor‐risk disease in the SUN arm (see Supporting Table 1).
Few patients discontinued treatment because of maximum clinical benefit in either treatment arm (NIVO+IPI, n = 18; SUN, n = 7) (see Supporting Fig. 1). Among this small subgroup, all 18 patients in the NIVO+IPI arm, versus 4 patients in the SUN arm, achieved an objective response: 5 versus 2 patients had CRs, respectively; 13 versus 2 patients had a partial response, respectively; and zero versus 3 patients had stable disease, respectively. The median duration of study therapy among these patients was 32.8 months (Q1‐Q3, 24.2‐45.1 months) in the NIVO+IPI arm and 7.8 months (Q1‐Q3, 6.5‐19.1 months) in the SUN arm.
Safety and HRQoL
Comparable overall rates of treatment‐related AEs of any grade occurred with NIVO+IPI (515 of 547 patients; 94%) versus SUN (522 of 535 patients; 98%) with extended follow‐up (see Supporting Table 7). However, fewer grade 3 and 4 treatment‐related AEs were reported in patients who received NIVO+IPI versus SUN (48% vs 64%). Treatment‐related AEs leading to discontinuation occurred in 127 patients (23%) in the NIVO+IPI arm and 70 patients (13%) in the SUN arm. The overall incidence of treatment‐related, select (potentially immune‐mediated) AEs with NIVO+IPI (see Supporting Table 7) was similar to previous reports. 12 In total, 162 of 547 patients (30%) treated with NIVO+IPI received corticosteroids (≥40 mg prednisone daily or equivalent [PDE]) to manage any‐grade, treatment‐related, select AEs, as reported within 30 days of the last dose of NIVO+IPI; 108 patients (20%) received ≥40 mg PDE continuously for ≥2 weeks, and 56 (10%) received ≥40 mg PDE continuously for ≥30 days.
With 5 years of follow‐up, the overall difference in the mean change from baseline between treatment arms was statistically significant in favor of NIVO+IPI according to the National Comprehensive Cancer Network Functional Assessment of Cancer Therapy‐Kidney Symptom Index total and disease‐related symptoms subscale scores (P < .05) in both ITT patients and intermediate‐risk/poor‐risk patients (see Supporting Table 8).
Discussion
With a minimum 5 years of follow‐up in CheckMate 214, NIVO+IPI demonstrated long‐term efficacy benefits versus SUN in ITT patients, establishing new benchmarks for the magnitude and durability of benefits possible using first‐line, immunotherapy‐based combinations for patients with aRCC. Notably, OS benefits with NIVO+IPI versus SUN were observed in the ITT population and in both IMDC intermediate‐risk and poor‐risk subgroups. Median OS was reached with NIVO+IPI in ITT patients for the first time and was numerically longer for the NIVO+IPI arm versus the SUN arm regardless of IMDC risk group. In addition, the ORR was higher in ITT patients, and the DOR was notably longer with NIVO+IPI versus SUN regardless of IMDC risk group. PFS probabilities appeared to stabilize above approximately 30% for both ITT patients and intermediate‐risk/poor‐risk patients after 3 years. Although the ORR was higher with SUN among favorable‐risk patients, 5‐year OS, PFS, and CR rates and DOR probabilities were all numerically higher with NIVO+IPI versus SUN in this subgroup. The proportion of patients who maintained a CR with NIVO+IPI was also relatively high versus SUN, yet responses were durable in those who had a CR in both treatment arms. In addition, approximately one‐half of all responders experienced a treatment‐free interval without initiating subsequent therapy in the NIVO+IPI arm. The overall incidence of treatment‐related AEs remained consistent with previous reports, and no new safety signals emerged. 2 , 12 Together with improved efficacy and safety benefits, NIVO+IPI treatment led to fewer symptoms and better HRQoL outcomes compared with SUN, further substantiating the long‐term benefits of NIVO+IPI for patients with aRCC. Of note, 75% of treated patients in the NIVO+IPI arm discontinued therapy by approximately 22 months, in alignment with the protocol amendment allowing for optional discontinuation of study treatment after 2 years; this approach has become common in clinical practice. However, the optimal duration of immunotherapy remains an important clinical question and continues to be investigated.
Conditional survival analyses estimate the probability of remaining event free (ie, remaining alive, progression free, or in response) for a defined period of time beyond reaching a landmark study milestone. These analyses are a novel, clinically relevant method to predict continued survival and response benefits as patients reach or exceed annual landmarks, thus providing meaningful insights for clinicians and patients. 10 In our analysis, point estimates for conditional OS with NIVO+IPI were higher or remained stable at each subsequent year of survival between randomization and the 3‐year landmark in ITT and intermediate‐risk/poor‐risk patients, and point estimates for conditional OS were higher for NIVO+IPI versus SUN at 3 years regardless of IMDC risk group. In favorable‐risk patients, conditional OS in those who received NIVO+IPI remained high over time, whereas conditional point estimates mostly declined with subsequent years of survival in those who received SUN. These data highlight that survival benefits with NIVO+IPI are largely durable with extended follow‐up, regardless of IMDC risk group. Furthermore, point estimates for conditional OS were higher or remained steady at each subsequent year of survival with NIVO+IPI in ITT patients stratified by age, BMI, grade ≥3 immune‐mediated AEs, and tumor PD‐L1 expression and remained consistently high in patients who achieved a CR, indicating that none of these baseline characteristics precluded patients from achieving durable survival benefits with NIVO+IPI.
The conditional survival analyses presented here only considered patients who were alive, progression free, or in response at a certain landmark timepoint, thus excluding those who died or were censored before the landmark time. In addition, these analyses were post hoc and descriptive and were intended to provide relevant information for possible scenarios that physicians and patients face, but not for inferential purposes. Limitations of the conditional survival analyses reported in this study include the increasingly small ITT patient numbers as the 3‐year landmarks were reached in the SUN arm and at later timepoints within some subgroups in both arms. Furthermore, because the conditional survival analyses were exploratory in nature, additional findings from other phase 3 prospective studies are needed to confirm the treatment effects reported in this analysis. Further investigation of long‐term efficacy in patients with aRCC treated with a first‐line tyrosine kinase inhibitor who subsequently receive either immunotherapy or antiangiogenic therapy in the refractory setting would help to shed light on which patient populations are likely to experience durable outcomes. Finally, it is important to point out that although baseline characteristics for this patient subgroup were largely balanced at the start of the trial, there may be imbalances in some clinical characteristics for those patients who remained alive at the landmark timepoints assessed, potentially affecting outcomes in the conditional survival analyses. Along these lines, the natural history of disease for patients with aRCC may vary considerably, depending on the biology of individual disease and the behavior of the tumor (aggressive vs indolent), both underlying the patient's response to therapy and also potentially affecting conditional survival outcomes. 15
In summary, the current results establish the durability of clinical benefit observed with NIVO+IPI over SUN in patients who have aRCC after a minimum follow‐up of 5 years. In addition, results from the first long‐term conditional survival analyses in CheckMate 214 show that most patients who remain alive or in response at the 3‐year landmark will remain alive or in response at 5 years with NIVO+IPI. These data provide a new prognostic framework critical to the improved clinical management of patients with aRCC in the current era.
Funding Support
This study was sponsored by Bristol Myers Squibb and Ono Pharmaceutical Company Limited. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672). Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by the Memorial Sloan Kettering Cancer Center Support Grant (core grant P30 CA008748).
Conflict of Interest Disclosures
Robert J. Motzer reports institutional grants or contracts from Aveo Pharmaceuticals, Bristol Myers Squibb (BMS), Eisai, Exelixis Inc, Genentech/Roche, Merck, Pfizer; and personal fees for advisory board participation from AstraZeneca, Aveo Pharmaceuticals, Eisai, EMD Serono, Exelixis Inc, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer, all outside the submitted work. David F. McDermott reports institutional research grants from Alkermes Inc, BMS, Genentech/Roche, Merck, Novartis, Peloton Therapeutics, and Prometheus Laboratories; consulting or advisory fees from Alkermes Inc, Array BioPharma, Aveo Pharmaceuticals, BMS, Calithera Biosciences, Checkmate Pharmaceuticals, CRISPR Therapeutics, Eisai, Eli Lilly and Company, EMD Serono, Exelixis Inc, Genentech/Roche, Iovance, Johnson & Johnson, Jounce Therapeutics, Merck, Novartis, Peloton Therapeutics, Pfizer, Synthekine Inc, Werewolf Therapeutics, and X4 Pharma; and other fees from the Beth Israel Deaconess Medical Center, all outside the submitted work. Bernard Escudier reports personal fees from Aveo Pharmaceuticals, BMS, Eisai, Ipsen, Oncorena, and Pfizer; honoraria from BMS, Ipsen, Oncorena, and Pfizer; and support for meetings and/or travel accommodations/expenses from BMS, Ipsen, and MSD, all outside the submitted work. Mauricio Burotto reports honoraria from AstraZeneca, BMS, Merck, Novartis, and Roche; and participation on a data safety monitoring board at AstraZeneca, BMS, Merck, and Roche, all outside the submitted work. Toni K. Choueiri reports support in part by grants from the National Cancer Institute to the Dana‐Farber/Harvard Specialized Program of Research Excellence (2P50CA101942‐16) and Program 5P30CA006516‐56, the Kohlberg Chair at Harvard Medical School, and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana Farber Cancer Institute; institutional research funding for clinical trials from the Alliance Cooperative Group, AstraZeneca, Aveo Pharmaceuticals, Bayer, BMS, Eisai, EMD Serono, Exelixis Inc, Eli Lilly and Company, GlaxoSmithKline (GSK), Merck, Nikang Therapeutics, Novartis, Orien, Peloton, Pfizer, Roche, Sanofi‐Aventis, and Takeda; royalties/licensing fees from Up‐To‐Date (online textbook); personal fees from Analysis Group, Aptitude Health, AstraZeneca, Aravive, Aveo Pharmaceuticals, Bayer, BMS, Calithera Biosciences, Circle Pharma, Eisai, Eli Lilly and Company, EMD Serono, Exelixis Inc, GSK, IQVIA, Infinity Pharmaceuticals, Ipsen, Kanaph, Merck, Nikang Therapeutics, Novartis, Nuscan, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up‐To‐Date, and continuing medical education events (PeerView, PER, MJH Life Sciences, Research to Practice, France Foundation, Springer, WebMed, ASiM Ce, and Caribou Publishing); honoraria from Advent Health, the American Society of Clinical Oncology (ASCO)‐Society for the Immunotherapy of Cancer, ASiM Ce, CancerNet, Ipsen, The University of Texas MD Anderson Cancer Center, MJH Life Sciences, NAMC, PeerView, PER, Research to Practice, Springer, and WebMD; participation on a data safety monitoring board at Aravive; a leadership or fiduciary role in KidneyCan (unpaid), ASCO, the European Society for Medical Oncology, the National Comprehensive Cancer Network, and the Genitourinary Steering Committee of the National Cancer Institute; stock ownership in Nuscan, Osel, Pionyr, and Tempest; and principal investigator fees (institutional) from GSK, Roche, and Surface Oncology, all outside the submitted work. Hans J. Hammers reports clinical research funding from Aravive, BMS, Merck, and Surface Oncology; personal fees from ARMO Biosciences, Aveo Pharmaceuticals, BMS, Corvus, Merck, Pfizer, and Surface Oncology; and receipt of drugs for clinical trials from BMS, all outside the submitted work. Philippe Barthélémy reports personal fees from Amgen, Astellas Pharma, Bayer, BMS, Eisai, Ipsen, Janssen‐Cilag, Merck, Merck Sharp & Dohme (MSD), Novartis, Pfizer, and Roche; honoraria from Amgen, Astellas Pharma, AstraZeneca, Bayer, BMS, Ipsen, Janssen‐Cilag, Merck, Novartis, Pfizer, and Seagen; and support for travel accommodations/expenses from BMS, Ipsen, Janssen‐Cilag, Merck, MSD, Novartis, and Pfizer, all outside the submitted work. Elizabeth R. Plimack reports grants for clinical research from Astellas Pharma, BMS, Genentech, and Merck; personal fees from Astellas Pharma, AstraZeneca, Aveo Pharmaceuticals, BMS, Calithera Biosciences, Flatiron, Genentech, Infinity Pharmaceuticals, Janssen, MEI Pharma, Merck, Pfizer, Regeneron, and Seattle Genetics; honoraria from ACHL, Clinical Care Options, Creative Educational Solutions, CUA, Fox Chase Cancer Center, Georgetown University, American Society of Clinical Oncology Genitourinary Cancers Symposium (GU ASCO), JADPRO Bladder, Medscape, Mount Sinai, the National Comprehensive Cancer Network, Ohio State University, OncLive, Peak Medicals GmbH, PER, Research to Practice, Spire Learning, Total CME, the University of Hawaii, and the University of Pennsylvania; has applied for a patent for Screening Muscle Invasive Bladder Cancer Patients for Neoadjuvant Chemotherapy Responsiveness (US Patent Application No. 14/588,503); participates on a data safety monitoring board or advisory board for AstraZeneca, Infinity Pharmaceuticals, and Pfizer; and serves on the ASCO board of directors and the Bladder Cancer Advocacy Network Scientific Advisory Board, all outside the submitted work. Camillo Porta reports personal fees from Angelini, AstraZeneca, BMS, Eisai, EUSA Pharma, General Electric, Ipsen, Janssen, Merck, MSD, and Novartis; honoraria from Angelini, AstraZeneca, BMS, Eisai Medical Research, EUSA Pharma, General Electric, Ipsen, Janssen, Merck, MSD, Novartis, and Pfizer; fees for expert testimony from EUSA Pharma and Pfizer; steering committee member fees from BMS, Eisai, and EUSA Pharma; and support for travel accommodations/expenses from Roche, all outside the submitted work. Saby George reports institutional research funding from Aravive, Agensys, Aveo Pharmaceuticals, Bayer, BMS, Calithera Biosciences, Corvus Pharmaceuticals, Eisai, Exelixis Inc, Gilead, Merck, Novartis, Pfizer, Seattle Genetics, and Surface Oncology; personal fees from Aveo Pharmaceuticals, Bayer, BMS, Corvus Pharmaceuticals, Eisai, EMD Serono, Exelixis Inc, Merck, Immunomedics, Pfizer, QED Therapeutics, Sanofi, and Seattle Genetics; and honoraria from Aptitude Health, Curio Science, and DAVA Oncology, all outside the submitted work. Thomas Powles reports institutional research grants from Astellas Pharma, AstraZeneca, BMS, Eisai, Exelixis Inc, Ipsen, Johnson & Johnson, Merck, Merck Serono, MSD, Novartis, Pfizer, Roche, and Seattle Genetics; personal fees from Astellas Pharma, AstraZeneca, BMS, Eisai, Exelixis Inc, Incyte, Ipsen, Johnson & Johnson, Merck, Merck Serono, MSD, Novartis, Pfizer, Roche, and Seattle Genetics; and support for travel accommodations/expenses from Ipsen, MSD, Pfizer, and Roche, all outside the submitted work. Frede Donskov reports institutional research grants from Ipsen, MSD, and Pfizer outside the submitted work. Howard Gurney reports honoraria from BMS, Ipsen, Merck, and Pfizer; and participation on advisory boards for AstraZeneca, BMS, Ipsen, Janssen, Merck, MSD, and Pfizer, all outside the submitted work. Christian K. Kollmannsberger reports personal fees from Astellas Pharma, Bayer, BMS, Eisai, Ipsen, Janssen, Merck, and Pfizer outside the submitted work. Marc‐Oliver Grimm reports institutional research funding as a local/coordinating principal investigator from BMS, Intuitive Surgical, and Novartis; support for travel/accommodations from AstraZeneca, Ipsen, Merck, and Pfizer; participation on a data safety monitoring board or advisory board for Astellas, AstraZeneca, Bayer, BMS, Eisai, EUSA Pharma, Ipsen, Janssen‐Cilag, Merck Serono, MSD, Roche, and Takeda; honoraria from Astellas, AstraZeneca, BMS, EUSA Pharma, Ipsen, Janssen, Merck Serono, MSD, Oncinfo, and Pfizer; and is a member of the board of directors of Deutsche Gesellschaft fur Urologie, all outside the submitted work. Carlos Barrios reports institutional research funding from AB Science, AbbVie, Abraxis Biosciences, Amgen, Asana Biosciences, Astellas Pharma, AstraZeneca, BioMarin, BMS, Boehringer Ingelheim, Celgene, Clinica Atlantas, Covance, Daiichi Sankyo, GSK, Eli Lilly and Company, Exelixis Inc, Halozyme, ImClone Systems, INC Research, inVentiv Health, Janssen, LEO Pharma, Medivation, Merck KGaA, Merrimack, Millennium, Mylan, Novartis, Pfizer, PharmaMar, Polyphor, Roche/Genentech, Sanofi, Shanghai Henlius Biotech, and Taiho Pharmaceutical; consulting or advisory fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Eisai, GSK, Libbs, MSD Oncology, Novartis, Pfizer, Roche/Genentech, and United Medical; honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Eli Lilly and Company, GSK, MSD, Novartis, Pfizer, Roche/Genentech, Sanofi, and Zodiac Pharma; participation on an independent data monitoring committee for Roche/Genentech; travel accommodations/expenses from AstraZeneca, BMS Brazil, Eli Lilly and Company, MSD Oncology, Novartis, Pfizer, and Roche/Genentech; and stock ownership in Biomark, MEDSIR, and Tummi, all outside the submitted work. Yoshihiko Tomita reports institutional research grants from Astellas Pharma, Ono Pharmaceutical, and Takeda; and honoraria from Astellas Pharma, BMS, Ono Pharmaceutical, and Pfizer, all outside the submitted work. Daniel Castellano reports institutional research grants from Janssen Oncology; and personal fees from Astellas Pharma, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eli Lilly and Company, Ipsen, Janssen Oncology, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, and Sanofi, alloutside the submitted work. Viktor Grünwald reports institutional research grants from BMS, Ipsen, MSD, and Pfizer; personal fees from BMS, Eisai, EUSA Pharma, Ipsen, Janssen‐Cilag, Merck Serono, MSD, Nanobiotix, Pfizer, and Roche; honoraria from Apogepha, AstraZeneca, BMS, Eisai, EUSA Pharma, Ipsen, Janssen‐Cilag, Merck Serono, MSD, Pfizer, and Roche; support for travel/accommodations from AstraZeneca, BMS, and Pfizer; is on the faculty of the European Society for Medical Oncology; owns stock in AstraZeneca, BMS, GenMab, MSD, and Seattle Genetics; and is a member of the BMS, Ipsen, and Novartis steering committees, all outside the submitted work. Brian I. Rini reports institutional research funding from Aravive, Arrowhead, AstraZeneca, BMS, Merck, and Pfizer; grants from Aravive, Arrowhead Pharmaceuticals, AstraZeneca, BMS, Dragonfly Therapeutics, Exelixis Inc, Hoffmann‐La Roche, Immunomedics, Incyte, Janssen, Merck, Mirati Therapeutics, Pfizer, Seattle Genetics, and Surface Oncology; personal fees from 3DMedicines, Alkermes, Aravive, Arrowhead, Aveo Pharmaceuticals, BMS, Compugen, Corvus, Eisai, Genentech/Roche, GSK, Merck, Nikang Therapeutics, Pfizer, Shionogi, Surface Oncology, and Synthorx; support for travel/accommodations from Merck and Pfizer; participation on an AstraZeneca data safety monitoring board or advisory board; and owns stock in PTC Therapeutics, all outside the submitted work. M. Brent McHenry is an employee of BMS and owns stock in the company. Chung‐Wei Lee is an employee of BMS and owns stock in the company. Jennifer McCarthy is an employee of BMS. Flavia Ejzykowicz is an employee of BMS and owns stock in the company. Nizar M. Tannir reports institutional funding for clinical trials from Arrowhead, BMS, Calithera Biosciences, Eisai, Nektar Therapeutics, and Novartis; personal fees or honoraria from BMS, Calithera Bioscience, Eisai, Eli Lilly and Company, Exelixis Inc, Ipsen, MSD, Nektar Therapeutics, Novartis, Oncorena, Pfizer, and Surface Oncology; participation as a member of the US Renal Cell Carcinoma Advisory Board Committee; and owns stock in Amgen, Arcturus, Arcus, Bellus Health, BioCryst Pharmaceuticals, Corvus Pharmaceuticals, First Trust Amex Biotech, Johnson & Johnson, Merck, Nuvation Bio Inc Cl A (NUVB), Revolution Medicines, Spdr S&P Pharmaceuticals ETF, Surface Oncology, Vanguard Health Care ETF, and Xencor, all outside the submitted work.
Author Contributions
Robert J. Motzer had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Robert J. Motzer and Nizar M. Tannir contributed to the conception and design of the study. All authors provided study materials or patients. M. Brent McHenry completed the statistical analyses. Chung‐Wei Lee reviewed the clinical data. All authors contributed to the data analysis and interpretation, drafting, and revising of the article and provided final approval to submit the article for publication.
Supporting information
Supplementary Material
Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, Barthélémy P, Plimack ER, Porta C, George S, Powles T, Donskov F, Gurney H, Kollmannsberger CK, Grimm M‐O, Barrios C, Tomita Y, Castellano D, Grünwald V, Rini BI, McHenry MB, Lee C‐W, McCarthy J, Ejzykowicz F, Tannir NM. Conditional survival and long‐term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022. 10.1002/cncr.34180
We thank the patients who participated in this study, the clinical study teams, and the representatives of the sponsor who were involved in data collection and analyses. Medical writing support was provided by Rachel Maddente, PhD, of Parexel, and was funded by Bristol Myers Squibb.
The authors received no financial support or compensation for the publication of this article.
See referenced editorial on pages 2058–2060, this issue.
Correction added on 26 April 2022, after first online publication: Figures 1‐3 have been updated in this version.
Data Availability
Bristol Myers Squibb's policy on data sharing may be found online (see https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharingrequest‐process.html).
References
- 1. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal‐cell carcinoma. N Engl J Med. 2017;376:354‐366. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med. 2018;378:1277‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2019;380:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 4. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med. 2021;384:829‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hall JP, Zanotti G, Kim R, et al. Treatment patterns, outcomes and clinical characteristics in advanced renal cell carcinoma: a real‐world US study. Future Oncol. 2020;16:3045‐3060. [DOI] [PubMed] [Google Scholar]
- 6. Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal‐Cell Carcinoma Database Consortium prognostic model: a population‐based study. Lancet Oncol. 2013;14:141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530‐2540. [DOI] [PubMed] [Google Scholar]
- 8. Harshman LC, Xie W, Bjarnason GA, et al. Conditional survival of patients with metastatic renal‐cell carcinoma treated with VEGF‐targeted therapy: a population‐based study. Lancet Oncol. 2012;13:927‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi M, Fuller CD, Thomas CR Jr, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988‐2001. Gynecol Oncol. 2008;109:203‐209. [DOI] [PubMed] [Google Scholar]
- 10. Xing Y, Chang GJ, Hu CY, et al. Conditional survival estimates improve over time for patients with advanced melanoma: results from a population‐based analysis. Cancer. 2010;116:2234‐2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao N, Wan F, Zhu Y, Ye D. Conditional survival in patients with advanced renal cell carcinoma treated with nivolumab. Med Sci Monit. 2019;25:6518‐6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first‐line treatment for advanced renal cell carcinoma: extended follow‐up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42‐month follow‐up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8:e000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first‐line treatment of advanced renal cell carcinoma: extended 4‐year follow‐up of the phase III CheckMate 214 trial. ESMO Open. 2020;5:e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cairns P. Renal cell carcinoma. Cancer Biomark. 2010;9:461‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Bristol Myers Squibb's policy on data sharing may be found online (see https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharingrequest‐process.html).


