Abstract
Background
The effectiveness and long‐term outcomes of spinal cord stimulation (SCS) are not fully established, especially considering that data from patients who withdrew from the trial are rarely analysed, which may lead to overestimation of SCS efficacy. We evaluated short‐ and long‐term effects of SCS on chronic pain and perceived health, beyond natural variability in these outcomes.
Methods
In a prospective design, 176 chronic pain patients referred to SCS were evaluated five times (baseline; retest ~6 weeks later; post‐SCS trial; 8 and 28 weeks post‐permanent implantation). Patients whose SCS trial failed (Temp group) were followed up and compared to those who underwent permanent SCS (Perm group).
Results
Analyses revealed a non‐linear (U‐shaped) trend significantly different between the two groups. In the Perm group, a significant improvement occurred post‐SCS implantation in pain severity, pain interference, health‐related quality of life and self‐rated health, which was followed by gradual worsening and return to baseline values at end of follow‐up. In the Temp group, only minor changes occurred in these outcomes over time. On average, baseline and end of follow‐up values in the Perm and Temp groups were similar: ~40% in each group exhibited an increase in pain severity over time and 38% and 33%, respectively, exhibited reductions in pain severity over time.
Conclusions
Since the greatest improvement in the outcome measures occurred from baseline to post‐SCS trial (T1–T3) followed by a gradual decline in the effect, it appears that SCS may not be effective for the majority of chronic pain patients.
Significance
This longitudinal study evaluated short and long term effects of spinal cord stimulation (SCS) on chronic pain outcome measures, beyond their natural variation in time. Despite significant short term improvements, by the end of the seven months' follow‐up, the outcomes in the treatment group (people who received the permanent implantation) were similar to those of the control group (people whose SCS trial failed and did not continue to permanent implantation) suggesting SCS may not be cost‐effective for chronic pain patients.
1. INTRODUCTION
In recent years, conventional spinal cord stimulation (SCS), a minimally invasive and non‐pharmacological treatment, has been recommended for pain management when other treatments failed to yield a satisfactory outcome (e.g., Geurts et al., 2017; Harmsen et al., 2021) particularly for patients with neuropathic pain, failed back syndrome, complex regional pain syndrome and angina pectoris (Brinzeu et al., 2019; de Vos et al., 2014; Kumar et al., 2008; Rigoard et al., 2019; Simpson et al., 2009; Taylor et al., 2014; Tsigaridas et al., 2015; Visnjevac et al., 2017). In most instances, the ‘conventional’ or ‘paresthesia‐based’ SCS was evaluated, wherein permanently implanted percutaneous cylindrical leads are placed in the epidural space.
Although the number of randomized controlled trials (RCTs) is continuously growing, current evidence for conventional SCS is limited and large‐scale, well‐designed studies are needed to evaluate effectiveness and long‐term outcomes (e.g., Duarte et al., 2020; Geurts et al., 2017). A recent systematic review of 34 eligible RCTs of SCS for chronic pain (McNicol et al., 2021) has noted three major limitations: (1) relatively small samples (median of 38 participants); (2) relatively short follow‐up (median of 12 weeks, although it ranged between 0 and 208 weeks) and, (3) the majority of the studies were sponsored by the devices' manufacturers, a potential bias towards higher likelihood of positive outcomes (North & Shipley, 2018). The authors also raised a concern that in some instances, the samples included patients who did not fulfil the criteria for chronic pain (pain lasting 3 months or more). Furthermore, many studies analysed data only from the participants who completed the entire trial; lack of data from participants who withdrew from the trial may artificially increase the likelihood of SCS efficacy. Thus, additional studies and RCTs that can address these limitations are called for.
Importantly, SCS is an expensive treatment relative to pharmacological and physical interventions, with potential complications such as: infections (2.5%–14% of cases), pain around the implantation region (5%–10%), lead migration (1.4%–22%) and spinal cord injury (about 2%) due to dural puncture, epidural haematoma and/or blunt trauma (Eldabe et al., 2020; Kumar et al., 2008; Mekhail et al., 2011; Taccola et al., 2020). The rather high cost of SCS thus necessitates careful evaluation of its long‐term effectiveness not only in large cohorts but also considering the natural variation in the chronic pain outcomes. Chronic pain severity often fluctuates over time and spontaneous increases or decreases can occur regardless of any treatment utilization (Gibson et al., 2005; Serrano et al., 2017). It is therefore immensely important to evaluate chronic pain and other SCS outcomes beyond their natural variance over time, which would indicate a real clinical difference, and which to the best of our knowledge has not been attempted previously.
Our aim was therefore to investigate the short‐ and long‐term effects of conventional SCS on chronic pain and health perceptions. Specifically, we assessed the trajectory of the outcome measures among patients who underwent permanent SCS implantation following the trial SCS in comparison to that among patients whose SCS trial failed but who remained in the study. Clinically significant changes in the outcome measures were assessed based on test–retest evaluation and the calculation of cutoff values prior to the trial. The study was not industry sponsored.
2. METHODS
2.1. Study population
The sample included 176 patients (69 women and 107 men) who suffered from chronic pain (defined as pain lasting >6 months) due to a multitude of origins as follows: peripheral neuropathic pain (63 out of the 176 patients, 35.8%), failed back surgery syndrome (64, 36.4%), lower back pain (24, 13.6%), complex regional pain syndrome (18, 10.2%) and headaches or other types of pain (7, 4%). All the patients were referred to SCS for the first time, and were at the age 21 and over, with no language barriers. Patients were excluded from the study if they were using other ongoing neuromodulatory treatments, started a new treatment in the 3 weeks prior to enrolment, suffered from an acute or a terminal disease, had chronic pain due to malignancy, were pregnant, had systemic infection, suffered from any damage to the spinal cord and/or abnormal condition as evident by CT or MRI scan that could interfere with implantation, had wounds or scars in the tested body regions, or had psychiatric diagnoses. Patients were also excluded if they were participating in other concurrent clinical trials or other studies.
The patients were recruited on a voluntary basis from the units for pain treatment of two major general hospitals in central Israel: Tel‐Aviv Sourasky Medical Center and Sheba Medical Center. The physicians in each centre made a preliminary screening of eligibility of patients referred to SCS in order to verify whether the patients had any contraindications for SCS, whether the patients fully understood the procedure and were interested in it, and in order to learn whether there is any need for psychological evaluation. Afterwards, the physicians approached these patients at their scheduled visit to the clinic, introduced the study to them, and provided information about the study. Patients who requested additional information were provided with a phone number they could contact. Eligible patients who agreed to participate in the study signed an informed consent form and were set a date for the first evaluation session at their convenient time. The study was approved by the Institutional Review Boards of the Tel Aviv Sourasky Medical Center and the Sheba Medical Center.
2.2. Study design
Figure 1 describes the timeline of the study. Data were collected in each unit, in five waves: (T1) At referral for SCS implantation (baseline evaluation); (T2) several weeks later, still before the trial SCS implantation (median 6.0 weeks, interquartile range, IQR = 2.6–8.9). This evaluation was performed in order to determine whether the outcome measures were stable across time prior to the intervention, and for the purpose of determining if a clinically significant change had occurred in the outcome measures due to the intervention. Patients whose trial implantation was scheduled a week or more after the baseline (T1) evaluation were asked to take part in T2 (n = 49, 43% of the baseline sample); (T3) a few days after the trial (temporary) SCS implantation. This evaluation was performed in order to assess the acute effect of SCS on the outcome measures. The questionnaire at this time point was shorter and did not include the McGill Pain Questionnaire; (T4) about 2 months after implantation of a permanent SCS electrode. This evaluation was performed in order to assess the short‐term effect of SCS on the outcome measures; (T5) about 7 months after the permanent implantation. This evaluation was performed in order to assess the longer term effect of SCS on the outcome measures. Participants who did not proceed to the permanent implantation completed the T4 and T5 evaluations at the estimated time they would have reached these waves, had they undergone the permanent procedure. These patients served as a comparison group to those who underwent the permanent implantation (the groups are hereafter labelled Temp and Perm respectively). Overall retention rates were 69%, 73% and 66%, for T3, T4 and T5 respectively. Participants who missed a wave for some reason were not excluded from the study, they were invited to take part in the next wave. Retention rates were similar in the Temp and Perm groups up to and including T4. At T5, they were higher in the Perm group (78% of the group compared with 46% in the Temp group). If patients were unable to complete the questionnaire during their evaluation visit to the pain unit, they were provided with the option to return the questionnaires by mail with a return envelope.
FIGURE 1.
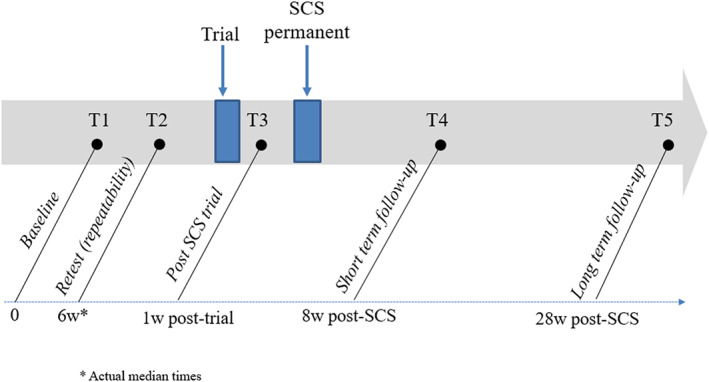
The experimental protocol; longitudinal evaluation times
2.3. The recruitment and participation flow chart
Figure 2 presents the flow of participants through the stages of the study (modified from the CONSORT [Consolidated Standards of Reporting Trials] diagram). During the recruitment period, from February 2010 until October 2014, 418 patients with chronic pain were referred to SCS. Of these patients, 75 were excluded from the study (30 due to language barriers, 28 due to health problems, 9 due to change of referral decision by the physician and 8 for technical reasons), 120 refused to participate and 223 took part in the study (65% of eligible patients). Of the 223 participants, 47 eventually did not undergo SCS (25 of their own choice, 11 because of a medical problem that arose after they were referred to SCS, 9 because their procedure was not approved by their healthcare service and two for unknown reasons), leaving a final sample of 176 participants who underwent a trial SCS implantation. Of these, 113 (64%) went on to the permanent implantation (‘Perm’ group). Sixty‐three patients (36%) underwent only the trial (temporary) SCS and as it was deemed unsuccessful, they did not proceed with the treatment but remained in the study as a comparison group (‘Temp’ group). Sample size calculation conducted with G*Power (Faul et al., 2007) showed that for detecting a medium‐sized effect (d = 0.5) when comparing the permanent SCS group and the trial only group, in a one‐tailed test under the assumption of a ratio of 2 to 1 in the size of the groups, a total sample of 156 patients (in groups of n = 104 and n = 52, for the Perm and Temp respectively), would be required to reach power >0.90 (for power of 0.80 a total N = 114 would suffice).
FIGURE 2.
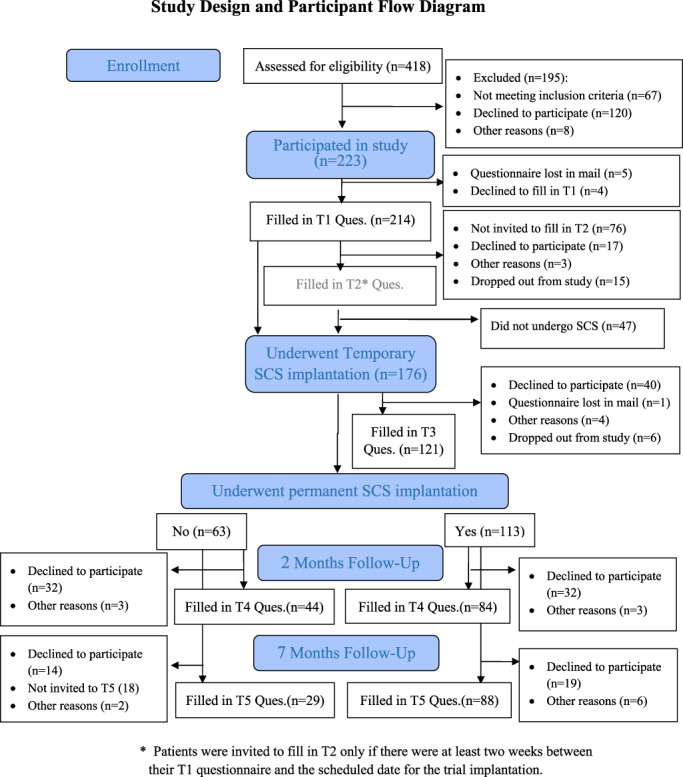
Study design and participant flow
2.4. The SCS intervention
Trial and permanent implantation included spinal cord stimulator devices of three companies: Medtronic, Abbott and Boston Scientific. All the patients underwent the ‘conventional’ or ‘paresthesia‐based’ SCS procedure. The participants underwent a percutaneous tubular lead implantation trial under sedation. According to each patient's painful region, the electrodes were introduced in the epidural space based on anatomical landmarks and their final position as well as the fine tuning was paresthesia based, so that the pain area was to be coved in order to achieve optimal pain relief. In patients in which the pain was felt in a single limb or in a restricted area, one lead was placed and in patients in which the pain was bilateral we placed two leads accordingly. The patients were instructed to activate the stimulator for a 7‐day period defined as the ‘SCS test trial’. The patients were trained in and allowed to change the SCS program (there were 3–5 options) which meant changing the amplitude and/or frequency (this was also encouraged during the post‐implantation period). Patients who responded positively to the test trial were then scheduled for implantation of a permanent, non‐rechargeable pulse generator. The median duration between the test trial and the permanent implantation was 10.0 weeks (IQR = 6.3–15.0). A positive response to the test trial, that is satisfactory pain relief, was determined if the patient reported at least 50% pain relief, reduced analgesic intake and no adverse effects. These patients with a positive response were then scheduled to undergo a permanent SCS implantation. The patients were encouraged to return to the outpatient clinic if pain relief was not satisfactory for adjustment/reprogramming which was done by the technician and physician as needed. The patients who underwent permanent implantation as well as those who did not have a positive response to the trial implantation were followed up at equivalent times, as described above.
2.5. Outcome measures
Sociodemographic information was collected at baseline with a structured questionnaire that included questions on age, family status, education and employment status.
Chronic pain was diagnosed prior to the study by the pain experts. The type of chronic pain was diagnosed according to clinical examination and diagnostic tests as needed (e.g., Electromyography, CT scans and nerve blocks). In the study, participants were questioned about the duration, intensity, quality and location of the pain in the body. Participants also completed the McGill Pain Questionnaire (MPQ), which enables quantitative evaluation of the patients' pain (Melzack, 1975). The quantitative indices were as follows: (1) the pain rating index (PRI)—the total values assigned to the words chosen from a list of 64 pain descriptors; and (2) the number of words chosen (NWC) from that list. Since the two indices were highly correlated we used the PRI for subsequent analyses. The MPQ included a body chart on which the patient could mark the location\s of their pain. In order to calculate the number of painful body regions, the body was divided into the following regions: feet, shins, knees, thighs, buttocks, groins, lower back, abdomen, waist, upper back, chest, hand, forearm, arm, shoulder, neck, head/face. Then the number of areas with chronic pain was summed for each patient (in cases of bilateral pain, both sides were counted). In addition, the participants were asked to rate the average, highest and lowest severity of their chronic pain in the last 2 weeks, on visual analogue scales (VAS) that consisted of 10 cm lines with endpoints denoted as ‘no pain’ and ‘worst pain imaginable’ (0 and 100 mm respectively). The ratings were calculated by measuring the distance in mm from ‘0’ to the patient's mark on the line.
Health‐related quality of life was assessed with the 12‐item Short Form Health Survey (SF‐12; Ware et al., 1994). This questionnaire includes 1–2 items from each of the eight dimensions assessed by the original SF‐36: Physical functioning, role‐physical, general health, bodily pain, vitality, social functioning, role‐emotional and mental health. Internal reliability for the full scale was high at all five time points (range α = 0.80 to α = 0.85).
Two of the SF‐12 items, self‐rated health and pain interference were also used separately:
Self‐rated health was assessed with the commonly used question that has been consistently documented as a predictor of future health outcomes (Idler & Benyamini, 1997). It is also the first question in the SF‐12: ‘How would you rate your health status, in general’? (response options ranged from 1 = ‘bad’ to 5 = ‘very good’).
Pain interference was assessed with the question from the SF‐12: ‘During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?’ (response options ranged from 1 = ‘not at all’ to 5 = ‘extremely’).
Post‐implantation treatment information—at T4 and T5, participants who had not undergone permanent SCS or were no longer using the device were asked whether they are currently receiving other treatments (an open‐ended question). Participants in the permanent implantation group were asked whether they operated the electrode in the past week.
2.6. Statistical analyses
SAS statistical software, version 9.4 (SAS Institute Inc) was used for data analysis. A linear mixed model with SAS Proc Mixed with REML estimation was used to estimate the effect of time (repeated measures) on the study measures. In this model, the effects of time (linear and curvilinear effects), age, gender and SCS group (Temp vs. Perm) were tested, as well as the moderating effects of the SCS group upon the linear and curvilinear effects of time. This procedure allows for estimating the effects using all available data regardless of missing values.
Percent of change in pain levels was assessed prospectively: We subtracted pain at a later time point from baseline pain, divided the difference by baseline pain and multiplied by 100 (e.g. if pain at baseline was rated as 75 and after the implantation the rating was 50, then there was a 33% improvement). We followed the recommendations of Dworkin et al. (2008), which indicate that a 15% to 20% or approximately a 10 mm improvement on a 10 cm VAS represents minimal or little change, a change of ≥30% (or about 2.0–2.7 cm) represents a moderately important improvement and a change of ≥50% (or about 4 cm) represents a substantial improvement in pain intensity.
Test–retest repeatability of the outcome measures was tested among the patients who participated in evaluations T1 and T2, by calculating the difference and standard deviation (SD) of, and correlation between, outcomes reported at Tests 1 and 2 using paired t‐tests and intraclass correlations (ICC) respectively. Then, the standard error of measurement (SEM) was calculated with the following equation SEM = SD × √(1−ICC).
3. RESULTS
3.1. Sample and chronic pain characteristics
Participants' average age was 55 (±15), 39% were female, 67% were married and 85% had one or more children. Their average educational level was 13 years (±3), 57% had above high school education. A third of them were employed, a little over a third were not working because of their pain disability, and the remainder were mostly retired or unemployed. Participants reported on average 2.6 (±2.3) current diseases and medical conditions. The most common conditions reported were hypertension (41%), gastrointestinal problems (28%), nephrological or urological problems (27%), coronary heart disease (16%) and diabetes (16%).
The mean time since onset of chronic pain was 8.6 (±9.7) years. The median of chronic pain duration was 5 years with an interquartile range of 2.5–10.0. All but two patients reported chronic pain lasting 1 year or longer. The average pain severity over the past 2 weeks as rated by the participants at baseline was 78.2 (±16.0) out of 100 on the VAS.
At baseline the two study groups, that is patients who eventually had permanent implantation after the trial (Perm group) and patients who did not undergo a permanent implantation after the trial (Temp group), did not significantly differ in the chronic pain types (χ2[3] = 2.33, p = 0.67) or in any of the demographic and pain characteristics or any other study measure.
3.2. Test–retest repeatability of the outcome measures
Table 1 presents the repeatability analysis for the main outcome measures in the entire sample in the first two time points, both of which took place before any intervention. The outcome measures were relatively stable, as indicated by the lack of difference between test and retest, the moderate to high ICCs and the relatively small SEMs. As the SEM reflects a cutoff value for a clinically significant difference, changes observed in the outcome measures from T1 to T3‐T5 time points were examined relative to the SEM.
TABLE 1.
Test–retest repeatability of the main outcome measures
| Variable | Time 1 (mean ± SD) | Time 2 (mean ± SD) | p‐value | ICC | SEM |
|---|---|---|---|---|---|
| Pain severity‐average | 77.7 ± 16.5 | 78.8 ± 15.6 | 0.50 | 0.72 | 8.2 |
| Pain severity‐highest | 88.7 ± 14.0 | 89.2 ± 12.1 | 0.78 | 0.67 | 6.9 |
| Pain severity‐lowest | 48.7 ± 22.6 | 48.3 ± 21.3 | 0.86 | 0.66 | 12.9 |
| Pain rating index | 38.4 ± 18.1 | 39.1 ± 17.8 | 0.74 | 0.75 | 8.9 |
| Number of painful regions | 8.4 ± 6.3 | 8.3 ± 5.8 | 0.82 | 0.34 | 2.1 |
| Self‐rated health | 2.3 ± 1.0 | 2.4 ± 1.1 | 0.63 | 0.62 | 0.5 |
| Quality of life | 29.6 ± 19.4 | 28.1 ± 20.0 | 0.34 | 0.84 | 7.5 |
| Pain interference | 4.3 ± 0.9 | 4.3 ± 0.7 | 0.74 | 0.81 | 0.3 |
Note: p‐value in paired t‐test between test and retest.
Abbreviations: ICC, Intra class correlation coefficient; SD, Standard deviation; SEM, Standard error of measurement.
3.3. Changes over time in chronic pain, health and quality of life outcomes
In contrast with the relative stability of the outcome measures in the first two time points (T1 and T2), the Perm and Temp groups exhibited different patterns of changes over time in the outcome measures, starting from the trial SCS and onward (i.e. from T3 to T5). Means and standard deviations of the outcome measures for each group at the five time points (T1–T5) are presented in Table 2. The absolute difference in each outcome measures between baseline and T3–T5 time points within each group was compared to the particular SEM value in order to determine whether the observed difference over time was clinically significant. Then, Statistical significance was ascertained using linear mixed models that tested the linear and non‐linear trends of these changes over time in each outcome measure both within and between the two study groups (see Table 3). For each of these trends over time, we also tested its interaction with group, in order to learn whether these trends differ significantly over time between the groups. The analyses revealed a group X time squared interaction, namely a non‐linear trend (U‐shaped, as explained below) of change over time from T1 to T5, as well as a difference between the two groups in this trend (Importantly, whenever significant non‐linear interactions are found, main effects of time and group, and linear trends over time can be misleading and should be controlled).
TABLE 2.
Means and standard deviations (SD) of outcome measures in the study groups across time
| TEMP (only trial SCS) | PERM (trial and permanent SCS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wave a | Wave | |||||||||
| T1 | T2 | T3 | T4 | T5 | T1 | T2 | T3 | T4 | T5 | |
| N | 61 | 34 | 39 | 44 | 29 | 110 | 49 | 82 | 84 | 88 |
| Pain severity‐average b | ||||||||||
| Mean | 79.97 | 78.75 | 67.82 | 71.33 | 69.90 | 77.17 | 79.21 | 44.73 | 56.67 | 68.36 |
| SD | 13.74 | 12.58 | 23.97 | 17.43 | 20.89 | 17.14 | 17.40 | 25.87 | 26.47 | 22.93 |
| Pain severity‐highest b | ||||||||||
| Mean | 90.76 | 90.42 | 80.35 | 86.75 | 81.66 | 88.80 | 89.39 | 62.23 | 70.13 | 81.18 |
| SD | 9.90 | 9.11 | 20.20 | 12.68 | 23.81 | 13.04 | 13.33 | 28.92 | 25.73 | 20.54 |
| Pain severity‐lowest b | ||||||||||
| Mean | 50.87 | 44.89 | 46.56 | 48.78 | 40.90 | 50.87 | 50.52 | 26.20 | 35.99 | 47.46 |
| SD | 23.31 | 19.85 | 29.29 | 24.12 | 26.44 | 25.11 | 23.33 | 24.00 | 27.24 | 26.87 |
| Pain rating index c | ||||||||||
| Mean | 33.69 | 39.13 | 33.71 | 35.01 | 39.91 | 39.27 | 37.35 | 36.88 | ||
| SD | 14.83 | 15.22 | 15.93 | 14.63 | 17.90 | 19.37 | 23.17 | 19.85 | ||
| Number of painful regions c | ||||||||||
| Mean | 7.76 | 7.88 | 8.16 | 9.03 | 8.32 | 8.48 | 7.80 | 7.22 | ||
| SD | 5.86 | 5.45 | 4.99 | 5.21 | 5.18 | 6.07 | 5.25 | 4.66 | ||
| Self‐rated health | ||||||||||
| Mean | 2.52 | 2.57 | 2.72 | 2.38 | 2.66 | 2.35 | 2.27 | 3.05 | 2.94 | 2.57 |
| SD | 1.16 | 1.09 | 1.07 | 1.09 | 1.20 | 1.06 | 1.16 | 1.02 | 1.07 | 1.06 |
| Health‐related quality of life | ||||||||||
| Mean | 30.15 | 32.33 | 32.90 | 28.59 | 33.01 | 27.41 | 24.94 | 46.01 | 40.21 | 32.46 |
| SD | 17.84 | 20.82 | 20.53 | 19.72 | 20.03 | 17.91 | 18.66 | 22.96 | 24.20 | 21.45 |
| Pain interference | ||||||||||
| Mean | 4.16 | 4.03 | 4.05 | 4.06 | 3.79 | 4.39 | 4.44 | 3.14 | 3.66 | 3.99 |
| SD | 1.01 | 0.74 | 0.89 | 1.08 | 1.05 | 0.77 | 0.71 | 1.13 | 1.05 | 1.01 |
T1 = at referral to SCS; T2 = between referral and trial SCS; T3 = several days after trial SCS; T4/T5 = ~2/7 months after permanent SCS (or equivalent time for Temp group).
Rated for the past 2 weeks on a Visual Analogue Scale (VAS) measured in millimetres.
These measures were not included at the third time point.
TABLE 3.
Multivariate repeated measures analysis of variance for changes over time a in the study variables b
| Variable | Intercept | SCS group c | Time | Time squared | SCS X time | SCS X time squared | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SD | Estimate | SD | Estimate | SD | Estimate | SD | Estimate | SD | Estimate | SD | |
| Pain severity‐average | 57.89*** | 1.48 | −18.82*** | 3.14 | −2.20*** | 0.27 | 0.51*** | 0.06 | −1.40* | 0.56 | 0.50** | 0.13 |
| Pain severity‐highest | 72.85*** | 1.38 | −17.72*** | 2.94 | −1.80*** | 0.25 | 0.41*** | 0.06 | −1.42** | 0.52 | 0.49*** | 0.12 |
| Pain severity‐lowest | 37.23*** | 1.76 | −16.36*** | 3.77 | −1.34*** | 0.32 | 0.34*** | 0.07 | −1.41* | 0.67 | 0.59*** | 0.16 |
| Pain rating index | 49.64*** | 4.40 | 2.87 | 3.42 | −0.21 | 0.21 | 0.02 | 0.06 | −0.20 | 0.44 | 0.02 | 0.13 |
| Number of painful regions | 9.18*** | 1.24 | 0.03 | 0.96 | −0.03 | 0.06 | 0.00 | 0.02 | −0.16 | 0.12 | −0.01 | 0.04 |
| Self‐rated health | 2.82*** | 0.07 | 0.46** | 0.16 | 0.05*** | 0.01 | −0.01*** | 0.00 | 0.07** | 0.03 | −0.02** | 0.01 |
| Health‐related quality of life | 38.44*** | 1.40 | 12.13*** | 2.96 | 1.07*** | 0.25 | −0.26*** | 0.06 | 1.67** | 0.52 | −0.44*** | 0.12 |
| Pain interference | 3.66*** | 0.07 | −0.61*** | 0.14 | −0.07*** | 0.01 | 0.01*** | 0.00 | −0.08** | 0.02 | 0.03*** | 0.01 |
From referral to spinal cord stimulation to about 7 months after the permanent implantation or equivalent time for those who did not proceed after the temporary implantation.
All analyses were adjusted for age and gender, which had no significant contributions except for a significant effect of age for highest pain and depressive symptoms.
SCS group: Permanent vs. only temporary implantation.
p < 0.05
p < 0.01
p < 0.001.
These tests show that following the SCS trial, there was a significant linear and non‐linear trends, which significantly differed between the two groups in all the outcome measures, except for the PRI and the number of painful body regions. The non‐linear effect (SCS X Time squared in Table 3) revealed a complex picture: In the Perm group, a U‐shaped trend was found, which began with an improvement seen in all outcome measures from T1 (baseline) to T3 (a few days post‐trial implantation), substantially larger than the SEMs, suggesting a clinically significant change. Then, from T3 to T4 (about 2 months post permanent implantation), some worsening had occurred in the outcome measures despite a substantial overall improvement compared to T1 levels. With the exception of the PRI and the number of painful regions, the observed improvement from T1 to T4 in the outcome measures was still greater than their SEM, suggesting a clinically significant change. From T4 to T5 (about 7 months post permanent implantation) further worsening had occurred in the outcome measures. At this time point, only the average and the highest pain severity and pain interference exhibited a change beyond their SEM values, yet they did not exceed their SEMs by much. The overall U‐shaped trajectory thus suggests temporary improvement followed by a return to baseline values.
The linear effect (SCS X Time) was significant in the models shown in Table 3, which controlled for the non‐linear U‐shaped effect described above. This remaining independent linear effect reflects the slight improvement from baseline to T5, as can be seen in the means shown in Table 2. However, in only three of the measures—average pain level, highest pain level and pain interference—this improvement was greater than the SEMs (computed on the basis of T1–T2 assessments preceding the trial implantation). For example the average pain level improved from T1 to T5 by about 9 mm on the VAS, which is only slightly more than the SEM for this measure (8.2).
The changes in the Temp group were much smaller than in the Perm group. From T1 to T3 they reported very small improvements that did not reach the SEM values in most of the measures, except for average and highest pain levels that slightly exceeded their SEMs. These reports correspond with the decision not to continue to the permanent implantation. A slight worsening occurred from in the Perm group from T3 to T4, and a slight improvement occurred afterwards but neither represented a statistically or clinically significant change.
In summary, the significant SCS group X time‐squared interaction reflected a non‐linear U‐shaped trend of improvement and then some worsening among the Perm group's levels of pain severity, pain interference, health‐related quality of life and self‐rated health, compared to minor changes over time in these measures among the Temp group.
A visualization of these effects can be seen in Figure 3, which shows the average chronic pain severity in the past 2 weeks, as rated on the VAS by participants of the two groups at the five time points. The non‐linear effects can be clearly seen in the Perm group: Average pain severity significantly decreased from 77 ± 17 at baseline (T1) to 45 ± 26 mm immediately after the trial period (T3) (p < 0.001), namely by 42% and exactly four SEMs. The decrease in chronic pain severity from baseline to 2 months after the permanent implantation (T4) (57 ± 26 mm) almost halved (27%, p < 0.001) but was still above two SEMs. However, pain severity at 7 months post permanent implantation (T5) was only 11% below pre‐trial level (68 ± 23) and just 0.3 units above SEM. In contrast, in the Temp group, chronic pain severity showed a slight positive effect of the trial SCS but relatively stable pain severity levels onward, which were smaller than the SEM (Figure 3). The pattern of changes for all the other outcome measures was similar except for the PRI and the number of painful body regions, which were not affected by the intervention in either group.
FIGURE 3.
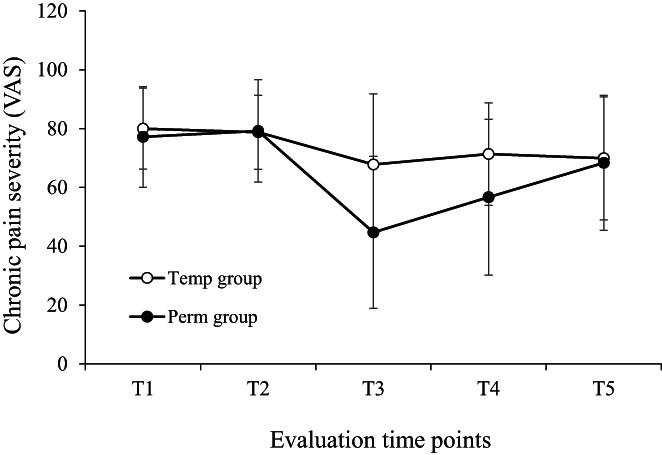
Mean (±SD) reported average pain severity over the past 2 weeks (measured on a 1–100 visual analogue scale) in the Temp (only temporary implantation) and Perm groups (permanent implantation) over the five evaluation time points: T1 – At referral; T2 – Several weeks later; T3 – After temporary implantation; T4 and T5 – About 2 and 7 months, respectively, after permanent implantation (or equivalent time for the TEMP group).
Finally, at T5, the Temp and Perm groups did not significantly differ on any of the outcome measures (p = 0.08 for the number of pain areas, all other ps >0.25). Thus, both groups did not differ at baseline, their pain severity levels decreased over time but no improvement was seen in other outcome measures, and at T5 they again did not differ. Namely, the only difference between the two groups was the improvement in pain severity levels (VAS) in the Perm group following the implantation, and the worsening they experienced later (i.e. from T4 to T5).
3.4. Variability within groups in the extent of changes in pain intensity
Tables 2 and 3 present average changes over time in each group. However, there was great variability within each group. To illustrate this variability, we also examined the data according to the cut‐off points proposed by Dworkin et al. (2008), for percent of change in pain severity and number of VAS units of change (see methods section). Figure 4 shows the distribution of percent of change in average pain severity in the two study groups from baseline to T5. As can be seen in the Figure, roughly 40% of the patients in both groups exhibited an increase in pain severity over time. As for reductions in pain severity, 38% of the Perm group and 33% of the Temp group exhibited a moderately important or a substantial improvement in pain levels (i.e., a reduction of 30% or more in pain levels at T5 compared to baseline levels). Using Dworkin et al.'s (2008) cut‐off points for improvement measured in absolute change in centimetres on the VAS revealed a similar picture. The extent of change in pain intensity over time did not differ by pain diagnoses when comparing the distribution of diagnoses among those whose VAS scores improved by 30% and above versus those whose VAS score improved by less than 30% (T1–T4: χ2(3) = 1.35, p = 0.85; T1‐T5: χ2(3) = 4.82, p = 19).
FIGURE 4.
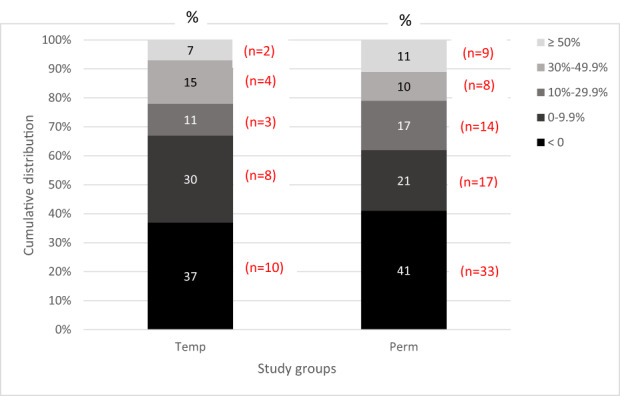
Cumulative distribution of participants according to categories of the extent of change in pain severity (visual analogue scale) from T1 (baseline) to T5 (about 7‐month post permanent implantation) in the Perm group or equivalent time for those in the Temp group who underwent only temporary implantation. Change is defined as percentage reduction at T5 compared to T1 values; change categories are shown in the legend. The numbers in the bars represent the percentage of participants from their group in each pain change category. The distributions in the two groups did not significantly differ (χ2(4) = 2.05, p = 0.73).
3.5. Reports on usage of the SCS device and adverse effects
Of the 113 participants in the Perm group, 84 participated in the T4 evaluation. Of them, 70 patients responded to the question about SCS usage, and almost all of them (n = 68, 97%) reported that they still operate the SCS device. Of the 88 patients in the Perm group who participated in T5 evaluation, 75 were still using the device (85%) and 13 were no longer using it. Of those who were not using the device, 12 reported receiving other treatments (six were taking pain medication, six did not report the type of other treatment). In total, out of the 113 patients who had a permanent implantation, around the time of T5, 20 patients (18%) either had the SCS device removed or did not operate it (this information was obtained by phone calls regardless of whether the patient participated in T5 or not).
Participants in the Temp group were also asked about other treatments at both T4 and T5. Out of the 44 patients of the Temp group who participated in the T4 evaluation, 27 (64%) reported that they are receiving various types of treatments for their pain as follows: 22 received pain medications (including in decreasing order opioids, non‐steroidal anti‐inflammatory drugs = NSAIDs, serotonine‐noradrenaline reuptake inhibitors = SNRIs and cannabis), five received invasive interventions (including intravenous lidocaine and radiofrequency), two received physical therapy and/or hydrotherapy; some received more than one type of treatment. Out of the 29 patients of the Temp group who participated in the T5 evaluation, 21 (72%) reported receiving pain medication (including opioids, NSAIDSs, SNRIs), three received physical therapy and two received hydrotherapy.
Adverse effects among patients in the Perm group included lead migration (12%), pain around the location of the implant (15%) and superficial tissue infection (3%).
4. DISCUSSION
Overall, a U‐shaped time trend was observed in which the greatest improvement in the outcome measures occurred from baseline to post‐trial implantation (T1‐T3), which then was followed by a gradual decline in the effect. All study participants were recruited in the same manner, had similar baseline values and were similarly eligible to receive SCS implantation. Eventually, about two‐thirds underwent the entire procedure of a trial and then permanent SCS (Perm group) whereas about a third underwent only the trial and served as a comparison group (Temp group). From baseline to post‐trial implantation, the Perm group exhibited a significant reduction in chronic pain intensity (VAS scores) and pain interference and an increase in self‐rated health and quality of life, in line with previous studies (for a review see O'Connell et al., 2021). The patients in the Temp group did not experience significant changes in the outcomes post‐trial, and did not continue to a permanent SCS implantation.
Previous studies have suggested that the trial SCS can predict successful pain relief with the permanent SCS. For example in one study, experiencing ≥50% pain relief during the trial was the variable most strongly associated with permanent SCS outcome (Williams et al., 2011). The results of the current study suggest otherwise: the longer term effects of permanent SCS varied and for some patients they disappeared altogether along the follow‐up, even though they experienced substantial pain relief during the trial. Another study also reported a lack of correlation between the trial outcomes and the permanent outcome, in the opposite direction: whereas average pain relief during the trial was 21%, that of the permanent system ranged between 44 and 83% (Oakley et al., 2008). In a recent study, there was no advantage to a SCS screening trial strategy over a no‐trial screening approach on pain severity or other outcomes measured at 6‐month follow‐up (Eldabe et al., 2020). Interestingly, patients who failed the trial did not exhibit worsening of their condition compared to baseline despite possible disappointment and/or investment in the procedure.
With regards to the long‐term effects in the Perm group, the outcome measures exhibited a non‐linear U‐shaped trend of a static phase (T1–T2) prior to the trial, followed by a statistically and clinically significant improvement immediately following the trial (T1‐T3), and then a reduced effect (T4, 2‐month post‐implantation), still improved compared to baseline, which then returned to baseline values (T5, 7‐month post‐implantation). Some T5 outcome measures exhibited slight improvement compared to baseline values, yet it was roughly equivalent to one SEM, indicating a non‐clinically significant change. The long‐term decline in SCS effects among the Perm group was unexpected, considering reports on ongoing effect of SCS for long durations, ranging from 3 to 48 months (e.g., Brinzeu et al., 2019; Campos et al., 2019; Eriksen et al., 2021; Hunter et al., 2018; Kumar et al., 2008; Rojo et al., 2021; Sears et al., 2011).
Several reasons may explain the relative low efficacy herein. First, we employed the most minimal exclusion criteria; dropout from the study was mainly due to patients' preferences and throughout the study, we did not exclude patients because of reasons such as reduced satisfaction, device malfunctioning, or adverse effects. All participants in both groups were contacted at follow‐up, regardless of their pain treatment at the time or the status and operation of their SCS device, even if they had not participated in the previous wave of data collection. Thus, although this was not a randomized controlled study, patients were followed up once the decision to undergo SCS implantation was made, regardless of its execution. Second, we included all patients who underwent the SCS trial and not only those whose trial was deemed successful. In this way, we reduced the selection bias of including only responsive patients. Studies in which only such patients were included would probably achieve greater pain relief. Third, many, though not all, studies measured pain relief retrospectively by asking patients to rate the percent of pain relief they had experienced. In the current study, we assessed pain relief prospectively, by comparing pain levels reported by participants at each time point. This method is less susceptible to self‐serving or to recall biases and may thus reflect the true condition of the participant. Fourth, we evaluated improvement in outcomes beyond their SEM, a conservative yet necessary criterion in clinical trials, seldom practiced, that controls for natural/spontaneous variations in chronic pain and in related outcomes over time.
The improvement in the outcome measures from T1 to T3, which continued in the Perm group in the 2‐month post‐implantation (T4), may be due to the actual stimulation of the dorsal horn or dorsal column, resulting in inhibition of upcoming nociceptive input and perhaps also by orthodromic activation of Aβ‐fibres (Heijmans & Joosten, 2020; Jensen & Brownstone, 2019). In such a case, the decline in the effect during the following months (up to T5) may be due to neuronal adaptation (Cliffer et al., 1992; O'Mara et al., 1988). The temporary improvement from T1 to T3/T4 could also reflect a placebo effect. Having the trial procedure and afterwards the permanent operation in a hospital setup can produce a strong placebo effect (Meissner & Linde, 2018) due to high expectations. Disappointment or gradually diminishing expectations may underlie the declined effect thereafter (T5). Notwithstanding, the decline in the pain‐relieving effect may have been due to decreased use of the SCS. However, this possibility seems unlikely as the majority of the patients did activate the device throughout the follow‐up period.
Indeed, recent review articles concluded that while it is almost certain that SCS provides short‐term benefit, there is no solid evidence that it can provide long‐term benefit (O'Connell et al., 2021; Palmer et al., 2019). Thus, although the dynamics of the outcome measures from baseline (T1) to end of follow‐up (T5; ~7 months) differed between the Perm and Temp groups, their values at T1 and T5 were overall similar. Additionally, 40% of the patients in both groups experienced worsening of chronic pain intensity and 38% and 33%, respectively, experienced clinically significant pain relief (≥30%). Considering all of the above, the results suggest that on average, SCS cannot provide a substantial long‐term relief for all chronic pain patients with the aetiologies included herein.
Although there exist a plethora of studies on SCS for chronic pain, a recent systematic review has concluded that the beneficial effect of this intervention is still not clear given previous studies' small sample size (average of 38), relatively short follow‐up periods (12 weeks), unclear definition of chronic pain and commercial sponsorship (North & Shipley, 2018). The present study addressed these four limitations: It included 176 patients who suffered from chronic pain as defined by IASP, followed up for 28 weeks, and no commercial sponsorship. Furthermore, in contrast with several previous reports, we continued to collect data from participants who withdrew from the study after the trial SCS or those who independently discontinued the use of the device. At the end of the follow‐up, the Perm and Temp groups resulted in a similar effect as those who failed the trial SCS. Noteworthy, those who failed the trial SCS went on trying other treatments (mostly pharmacological). However, apparently those treatments also failed to provide pain relief, judging by their VAS and PRI scores. Alternatively, the apparent lack of perceived pain relief in the Temp group despite turning to other treatments may reflect disappointment of the patients from the SCS procedure and not necessarily the actual level of their pain.
We could not identify any baseline characteristics that predicted successful outcomes among the patients. Similar to our study, others have also reported that pain interference and chronic pain severity could not predict SCS outcomes (Celestin et al., 2009; Taylor et al., 2014). The predictability of additional factors has been found inconsistent. For example various psychological factors such as somatization, depression and anxiety, as well as older age and chronic pain duration, were found to correspond with poorer treatment outcomes (Campos et al., 2019; Celestin et al., 2009; Stephens & Ward, 2014). However, others reported a lack of predictive ability of psychological factors such as pain catastrophizing (Poulsen et al., 2021) or a more complex relationship between predictors and outcomes depending on their nature (Fama et al., 2016). Thus, patients' selection for SCS is a major, open issue that requires further examination.
Our study has several limitations. First, we did not include a group who received sham SCS and therefore the magnitude of a placebo effect could not be assessed. Second, though measuring pain relief prospectively is one of the strengths herein, we did not ask patients for their retrospective perception of pain relief and thus have no way to compare our findings with studies that used such measures. Third, the results may apply to the chronic pain types included in the study. In summary, after controlling for natural variations over time in the outcome measures, the results suggest that although SCS offers a short‐term pain relief to the majority of chronic pain patients, only about 40% of them experience adequate pain relief in the long run (7‐month post‐implantation) and these patients could not have been identified based on their baseline variables. However, considering the somewhat similar percentage of patients who experienced pain relief in the failed SCS group, it appears that the procedure may not be cost‐effective for the majority of chronic pain patients. As none of the perceived health and pain‐related variables herein predicted the outcomes, further research is needed to identify biomarkers for successful treatments.
FUNDING INFORMATION
The study was funded by a grant from the Israel Science Foundation.
CONFLICT OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGEMENT
The study was supported by the Israel Science Foundation (grant #745/10).
Brill, S. , Defrin, R. , Aryeh, I. G. , Zusman, A. M. , & Benyamini, Y. (2022). Short‐ and long‐term effects of conventional spinal cord stimulation on chronic pain and health perceptions: A longitudinal controlled trial. European Journal of Pain, 26, 1849–1862. 10.1002/ejp.2002
This accompanies the following article: Maarrawi J. Is spinal cord stimulation useful in the long term? A commentary on Brill et al. Eur J Pain. 2022; 26: 1825–1826. https://doi.org/10.1002/ejp.2023
REFERENCES
- Brinzeu, A. , Cuny, E. , Fontaine, D. , Mertens, P. , Luyet, P. P. , Van den Abeele, C. , & Djian, M. C. (2019). French SCS study group. Spinal cord stimulation for chronic refractory pain: Long‐term effectiveness and safety data from a multicentre registry. European Journal of Pain, 23(5), 1031–1044. 10.1002/ejp.1355 [DOI] [PubMed] [Google Scholar]
- Campos, W. , Linhares, M. , Sarda, J. , Santos, A. , Licinio, J. , Quevedo, J. , Lin, K. , & Walz, R. (2019). Determinants for meaningful clinical improvement of pain and health‐related quality of life after spinal cord stimulation for chronic intractable pain. Neuromodulation: Technology at the neural. Interface, 22, 280–289. 10.1111/ner.12891 [DOI] [PubMed] [Google Scholar]
- Celestin, J. , Edwards, R. R. , & Jamison, R. N. (2009). Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: A systematic review and literature synthesis. Pain Medicine, 10(4), 639–653. 10.1111/j.1526-4637.2009.00632.x [DOI] [PubMed] [Google Scholar]
- Cliffer, K. D. , Hasegawa, T. A. K. E. S. H. I. , & Willis, W. D. (1992). Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli: Basic characterization and antidromic activation from the thalamus. Journal of Neurophysiology, 68(3), 818–832. 10.1152/jn.1992.68.3.818 [DOI] [PubMed] [Google Scholar]
- de Vos, C. C. , Meier, K. , Zaalberg, P. B. , Nijhuis, H. J. , Duyvendak, W. , Vesper, J. , Enggaard, T. P. , & Lenders, M. W. (2014). Spinal cord stimulation in patients with painful diabetic neuropathy: A multicentre randomized clinical trial. PAIN®, 155(11), 2426–2431. 10.1016/j.pain.2014.08.031 [DOI] [PubMed] [Google Scholar]
- Duarte, R. V. , Nevitt, S. , McNicol, E. , Taylor, R. S. , Buchser, E. , North, R. B. , & Eldabe, S. (2020). Systematic review and meta‐analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain, 161(1), 24–35. 10.1097/j.pain.0000000000001689 [DOI] [PubMed] [Google Scholar]
- Dworkin, R. H. , Turk, D. C. , Wyrwich, K. W. , Beaton, D. , Cleeland, C. S. , Farrar, J. T. , Haythornthwaite, J. A. , Jensen, M. P. , Kerns, R. D. , Ader, D. N. , Brandenburg, N. , Burke, L. B. , Cella, D. , Chandler, J. , Cowan, P. , Dimitrova, R. , Dionne, R. , Hertz, S. , Jadad, A. R. , … Zavisic, S. (2008). Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain, 9(2), 105–121. 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Eldabe, S. , Duarte, R. V. , Gulve, A. , Thomson, S. , Baranidharan, G. , Houten, R. , Jowett, S. , Sandhu, H. , Chadwick, R. , Brookes, M. , Kansal, A. , Earle, J. , Bell, J. , Robinson, J. , Walker, S. , Rhodes, S. , & Taylor, R. S. (2020). Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost‐effectiveness (TRIAL‐STIM)? A randomised controlled trial. Pain, 161(12), 2820–2829. 10.1097/j.pain.0000000000001977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen, L. E. , Terkelsen, A. J. , Blichfeldt‐Eckhardt, M. R. , Sørensen, J. C. H. , & Meier, K. (2021). Spinal cord stimulation in severe cases of complex regional pain syndrome: A retrospective cohort study with long‐term follow‐up. European Journal of Pain, 25(10), 2212–2225. 10.1002/ejp.1834 [DOI] [PubMed] [Google Scholar]
- Fama, C. A. , Chen, N. , Prusik, J. , Kumar, V. , Wilock, M. , Roth, S. , & Pilitsis, J. G. (2016). The use of preoperative psychological evaluations to predict spinal cord stimulation success: Our experience and a review of the literature. Neuromodulation: Technology at the Neural Interface, 19(4), 429–436. 10.1111/ner.12434 [DOI] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A. G. , & Buchner, A. (2007). G* power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- Geurts, J. W. , Joosten, E. A. , & van Kleef, M. (2017). Current status and future perspectives of spinal cord stimulation in treatment of chronic pain. Pain, 158(5), 771–774. 10.1097/j.pain.0000000000000847 [DOI] [PubMed] [Google Scholar]
- Gibson, M. C. , Woodbury, M. G. , Hay, K. , & Bol, N. (2005). Pain reports by older adults in long‐term care: A pilot study of changes over time. Pain Research and Management, 10(3), 159–164. [DOI] [PubMed] [Google Scholar]
- Harmsen, I. E. , Hasanova, D. , & Elias, G. J. B. (2021). Boutet a, Neudorfer C, Loh a, Germann J, Lozano AM. Trends in clinical trials for spinal cord stimulation. Stereotactic and Functional Neurosurgery, 99(2), 123–134. 10.1155/2005/654192 [DOI] [PubMed] [Google Scholar]
- Heijmans, L. , & Joosten, E. A. (2020). Mechanisms and mode of action of spinal cord stimulation in chronic neuropathic pain. Postgraduate Medicine, 132, 17–21. 10.1080/00325481.2020.1769393 [DOI] [PubMed] [Google Scholar]
- Hunter, C. W. , Carlson, J. , Yang, A. , & Deer, T. (2018). Spinal cord stimulation for the treatment of failed neck surgery syndrome: Outcome of a prospective case series. Neuromodulation: Technology at the Neural Interface, 21(5), 495–503. 10.1111/ner.12769 [DOI] [PubMed] [Google Scholar]
- Idler, E. L. , & Benyamini, Y. (1997). Self‐rated health and mortality: A review of twenty‐seven community studies. Journal of Health and Social Behavior, 38, 21–37. 10.2307/2955359 [DOI] [PubMed] [Google Scholar]
- Jensen, M. P. , & Brownstone, R. M. (2019). Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. European Journal of Pain, 23(4), 652–659. 10.1002/ejp.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, K. , Taylor, R. S. , Jacques, L. , Eldabe, S. , Meglio, M. , Molet, J. , Thomson, S. , O'Callaghan, J. , Eisenberg, E. , Milbouw, G. , Buchser, E. , Fortini, G. , Richardson, J. , & North, R. B. (2008). The effects of spinal cord stimulation in neuropathic pain are sustained: A 24‐month follow‐up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery, 63(4), 762–770. 10.1227/01.NEU.0000325731.46702.D9 [DOI] [PubMed] [Google Scholar]
- McNicol, E. , Ferguson, M. , Bungay, K. , Rowe, E. L. , Eldabe, S. , Gewandter, J. S. , Hayek, S. M. , Katz, N. , Kopell, B. H. , Markman, J. , Rezai, A. , Taylor, R. S. , Turk, D. C. , Dworkin, R. H. , & Thomson, S. (2021). Systematic review of research methods and reporting quality of randomized clinical trials of spinal cord stimulation for pain. The Journal of Pain, 22(2), 127–142. 10.1016/j.jpain.2020.05.001 [DOI] [PubMed] [Google Scholar]
- Meissner, K. , & Linde, K. (2018). Are blue pills better than green? How treatment features modulate placebo effects. International Review of Neurobiology, 139, 357–378. 10.1016/bs.irn.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Mekhail, N. A. , Mathews, M. , Nageeb, F. , Guirguis, M. , Mekhail, M. N. , & Cheng, J. (2011). Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Practice, 11(2), 148–153. 10.1111/j.1533-2500.2010.00407.x [DOI] [PubMed] [Google Scholar]
- Melzack, R. (1975). The McGill pain questionnaire: Major properties and scoring methods. Pain, 1(3), 277–299. 10.1016/0304-3959(75)90044-5 [DOI] [PubMed] [Google Scholar]
- North, R. B. , & Shipley, J. (2018). Neuromodulation. In Krames Elliot S., Hunter P. P., & Rezai Ali R. (Eds.), Chapter 4 ‐ Clinical Study Designs for Neuromodulation (2nd ed., pp. 41–51). Academic Press. [Google Scholar]
- Oakley, J. C. , Krames, E. S. , Stamatos, J. , & Foster, A. M. (2008). Successful long‐term outcomes of spinal cord stimulation despite limited pain relief during temporary trialing. Neuromodulation: Technology at the Neural Interface, 11(1), 66–73. 10.1111/j.1525-1403.2007.00145.x [DOI] [PubMed] [Google Scholar]
- O'Connell, N. E. , Ferraro, M. C. , Gibson, W. , Rice, A. S. , Vase, L. , Coyle, D. , & Eccleston, C. (2021). Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database of Systematic Reviews, 12, CD013756. 10.1002/14651858.CD013756.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mara, S. , Rowe, M. J. , & Tarvin, R. P. (1988). Neural mechanisms in vibrotactile adaptation. Journal of Neurophysiology, 59(2), 607–622. 10.1152/jn.1988.59.2.607 [DOI] [PubMed] [Google Scholar]
- Palmer, N. , Guan, Z. , & Chai, N. C. (2019). Spinal cord stimulation for failed Back surgery syndrome–patient selection considerations. Translational Perioperative and Pain Medicine, 6(3), 81. 10.31480/2330-4871/093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen, D. M. , Sørensen, J. C. H. , Blichfeldt‐Eckhardt, M. R. , Gulisano, H. A. , Knudsen, A. L. H. , Nikolajsen, L. , & Meier, K. (2021). Pain catastrophizing does not predict spinal cord stimulation outcomes: A cohort study of 259 patients with long‐term follow‐up. Neuromodulation: Technology at the Neural Interface, 24(1), 76–85. 10.1111/ner.13213 [DOI] [PubMed] [Google Scholar]
- Rigoard, P. , Basu, S. , Desai, M. , Taylor, R. , Annemans, L. , Tan, Y. , Johnson, M. J. , Van den Abeele, C. , North, R. , & PROMISE Study Group . (2019). Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: A multicenter randomized controlled trial. Pain, 160(6), 1410–1420. 10.1097/j.pain.0000000000001510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo, E. , Hernández, C. P. , Martínez, N. S. , Margarit, A. C. , Arias, T. B. , Martínez, M. M. , Crespo, C. , & Mazarro, D. O. (2021). Real‐world cost‐effectiveness analysis of spinal cord stimulation vs conventional therapy in the management of failed back surgery syndrome. Journal of Pain Research, 14, 3025–3032. 10.2147/JPR.S326092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears, N. C. , Machado, A. G. , Nagel, S. J. , Deogaonkar, M. , Stanton‐Hicks, M. , Rezai, A. R. , & Henderson, J. M. (2011). Long‐term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation: Technology at the Neural. Interface, 14(4), 312–318. 10.1111/j.1525-1403.2011.00372.x [DOI] [PubMed] [Google Scholar]
- Serrano, D. , Lipton, R. B. , Scher, A. I. , Reed, M. L. , Adams, A. M. , & Buse, D. C. (2017). Fluctuations in episodic and chronic migraine status over the course of 1 year: Implications for diagnosis, treatment and clinical trial design. The Journal of Headache and Pain, 18(1), 1–12. 10.1186/s10194-017-0787-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, E. L. , Duenas, A. , Holmes, M. W. , Papaioannou, D. , & Chilcott, J. (2009). Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: Systematic review and economic evaluation. Health technology assessment (Winchester, England), 13(17), iii–ix. 10.3310/hta13170 [DOI] [PubMed] [Google Scholar]
- Stephens, K. A. , & Ward, A. (2014). Patient selection for spinal cord stimulators: Mental health perspective. Current Pain and Headache Reports, 18(3), 398. 10.1007/s11916-013-0398-8 [DOI] [PubMed] [Google Scholar]
- Taccola, G. , Barber, S. , Horner, P. J. , Bazo, H. A. C. , & Sayenko, D. (2020). Complications of epidural spinal stimulation: Lessons from the past and alternatives for the future. Spinal Cord, 58(10), 1049–1059. [DOI] [PubMed] [Google Scholar]
- Taylor, R. S. , Desai, M. J. , Rigoard, P. , & Taylor, R. J. (2014). Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: A systematic review and meta‐regression analysis. Pain Practice, 14(6), 489–505. 10.1111/papr.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigaridas, N. , Naka, K. , Tsapogas, P. , Herios Pelechas, E. , & Damigos, D. (2015). Spinal cord stimulation in refractory angina. A systematic review of randomized controlled trials. Acta Cardiologica, 70(2), 233–243. 10.1080/AC.70.2.3073516 [DOI] [PubMed] [Google Scholar]
- Visnjevac, O. , Costandi, S. , Patel, B. A. , Azer, G. , Agarwal, P. , Bolash, R. , & Mekhail, N. A. (2017). A comprehensive outcome‐specific review of the use of spinal cord stimulation for complex regional pain syndrome. Pain Practice, 17(4), 533–545. 10.1111/papr.12513 [DOI] [PubMed] [Google Scholar]
- Ware, J. E. , Kosinski, M. , & Keller, S. (1994). SF‐36 physical and mental health summary scales. A user's manual. Health Assessment Lab, New England Medical Center, Boston, MA.
- Williams, K. A. , Gonzalez‐Fernandez, M. , Hamzehzadeh, S. , Wilkinson, I. , Erdek, M. A. , Plunkett, A. , Griffith, S. , Crooks, M. , Larkin, T. , & Cohen, S. P. (2011). A multi‐center analysis evaluating factors associated with spinal cord stimulation outcome in chronic pain patients. Pain Medicine, 12(8), 1142–1153. 10.1111/j.1526-4637.2011.01184.x [DOI] [PubMed] [Google Scholar]


