Summary
Background
Healthcare settings, where invasive procedures are frequently performed, may play an important role in the transmission dynamics of blood‐borne pathogens when compliance with infection control precautions is suboptimal.
Aims
To understand and quantify the role of hospital‐based invasive procedures on hepatitis C virus (HCV) transmission.
Methods
We conducted a systematic review and meta‐analysis to identify recent studies reporting association measures of HCV infection risk that are linked to iatrogenic procedures. Based on expert opinion, invasive procedures were categorised into 10 groups for which pooled measures were calculated. Finally, the relationship between pooled measures and the country‐level HCV prevalence or the Healthcare Access and Quality (HAQ) index was assessed by meta‐regression.
Results
We included 71 studies in the analysis. The most frequently evaluated procedures were blood transfusion (66 measures) and surgery (43 measures). The pooled odds ratio (OR) of HCV infection varied widely, ranging from 1.46 (95% confidence interval: 1.14–1.88) for dental procedures to 3.22 (1.7–6.11) for transplantation. The OR for blood transfusion was higher for transfusions performed before 1998 (3.77, 2.42–5.88) than for those without a specified/recent date (2.20, 1.77–2.75). In procedure‐specific analyses, the HCV infection risk was significantly negatively associated with the HAQ for endoscopy and positively associated with HCV prevalence for endoscopy and surgery.
Conclusions
Various invasive procedures were significantly associated with HCV infection. Our results provide a ranking of procedures in terms of HCV risk that may be used for prioritisation of infection control interventions, especially in high HCV prevalence settings.
Association between HCV infection and 10 groups of hospital‐based procedures was investigated. This metaanalysis shows that healthcare settings remain a gateway of HCV infection; the provided ranking of procedures could be used to improve infection control interventions.

INTRODUCTION
Hepatitis C virus (HCV) is mainly a blood‐borne virus associated with an estimated global sero‐prevalence of 2.5%. 1 However, wide between‐country discrepancies are observed, with Egypt and Pakistan having high anti‐HCV prevalence in the general population. 1 Chronic HCV infection, which is most of the time asymptomatic, may lead to serious complications like cirrhosis or hepatocellular carcinoma (HCC), with about 20% developing HCC. 2 Highly effective and well‐tolerated direct‐acting antiviral treatments (DAAs) are now available, but these treatments remain costly. An estimated 79% of people chronically infected with HCV worldwide still did not know their status in 2019, 3 limiting the population impact of DAAs. In addition, the residual risk of HCC is suspected to remain relatively high among patients who cured chronic HCV infection. 4 Moreover, HCV treatment should not be the only way of fighting new infections, as accumulating evidence suggests that even after the DAA therapy‐induced sustained virological response reinfection can occur, especially in people who inject drugs. 5 Therefore, prevention interventions to limit the risk of HCV contamination remain key in the global response against HCV. 6
Although injection drug use has been established to be the most important risk factor for acquiring a new HCV infection globally, 7 healthcare settings may play an important role in HCV transmission dynamics, due to the high frequency of invasive procedures and over‐representation of HCV‐infected individuals among hospitalised patients. Medical procedures have been linked to multiple HCV outbreaks worldwide, for instance in Egypt, 8 India 9 or the United States. 10 In healthcare settings where compliance with infection control interventions is suboptimal, patients may still become infected following blood transfusion or haemodialysis. 11 , 12 Previous hospitalisation has been identified as a major risk factor for HCV infection in many countries. 13 In the countries where HCV prevalence is high, infection control interventions may not be systematically implemented, and data are scarce regarding HCV infection risks.
In this context, a better control of hospital‐acquired infections would significantly help in the fight against HCV epidemics. This requires a clear understanding of the role played by different high‐risk procedures within healthcare settings. We conducted a systematic review and meta‐analysis to synthesise the strength of association between different hospital‐based invasive procedures and HCV infection risk, in order to rank these procedures according to the risk of iatrogenic HCV transmission.
MATERIAL AND METHODS
Search strategy and selection criteria
We searched PubMed, Web of Science and Scopus for studies published between January 2000 and December 2020 using the following concepts: hepatitis AND risk factor AND hospital AND procedure (Table S1). This systematic review was registered in PROSPERO (ID: CRD42021224886) and reported according to PRISMA guidelines.
Studies were eligible if: (i) exposure group was composed of adults, who were hospitalised or who had outpatient visit; and (ii) they reported measures of association (odds ratios, ORs; risk ratios, RRs; or prevalence ratios, PRs) by comparing the proportion of people positive for HCV RNA or anti‐HCV antibody between a group who underwent a specific healthcare procedure and another group not exposed to the procedure. There was no restriction in terms of the study design and non‐exposure group.
Studies were excluded if: (i) they exclusively included paediatric patients, drug users or blood donors; (ii) patient recruitment started before 2000; (iii) they were not written in French or English; or (iv) they did not present original results.
Article titles and abstracts were screened by two independent investigators (M.C. and P.H.) using the Covidence review tool. 14 Full‐text articles were then retrieved and assessed for their eligibility by the same two authors. Any conflict in articles screening or full‐text assessment were resolved by a third senior researcher (K.J. or L.T.). For each study, the following data were extracted by P.H.: total number of patients; patient type; study design; and measures of association and sample size of the exposed/non‐exposed groups. Any doubt concerning data extraction was resolved by a senior researcher (K.J. or L.T.).
Data analysis
The procedures were categorised into 10 groups and validated by medical experts (L.B.L.N. and Y.S.) (Table 1; Table S10, Text S2). Risk of blood‐borne infection was supposed to be the same within these groups. Measures associated with the same procedure but reported across different populations (e.g. by gender) were considered independently in the meta‐analysis.
TABLE 1.
Aggregation of procedures found within selected articles
| Group | Procedures within group (as found in selected studies) |
|---|---|
| Surgery | Surgery, operation, bloody operation, splenectomy, laparoscopy, organ biopsy, caesarean section |
| Transplantation | Transplantation, renal transplantation |
| Blood transfusion | dated blood transfusion/non‐dated blood transfusion |
| Intravenous (IV)/Catheter | Cholangiography, IV pyelography, IV injection, IV‐line, sclerotherapy, catheter, central venous catheter, cannula, phlebotomy |
| Haemodialysis | Haemodialysis |
| Wound care | Wound suture, wound care |
| Injection | IM injection, percutaneous injection, subcutaneous injection, injection/vaccination, injection |
| Other procedures | Electromyography, haemorrhoids treatment, tapping ascites, abscess drainage, abortion, biopsy, ligation of oesophageal varices, urinary catheter |
| Endoscopy | Endoscopy, colonoscopy, gastroscopy |
| Dental care | Tooth extraction, dental anaesthesia, dental procedure, dental care, tooth filling |
Note: Procedure names are reported as mentioned in each of the articles.
Abbreviation: IM, intramuscular.
We performed random‐effect meta‐analyses to pool ORs for an association between each procedure group and the risk of HCV infection using the R ‘meta’ package. 15 When two or more distinct procedures were classified in the same procedure group within the same study population, a three‐level model 16 was used to account for the dependence between the measures of association. I 2 statistics were used to assess the heterogeneity across the studies. Procedure‐specific forest plots were visually inspected for potential outliers. Measures that were not ORs (RRs and PRs) were considered to be equivalent to ORs and included in the same analyses. When both adjusted ORs (AORs) and crude ORs were available in the same study, the AORs were preferred. In a same study, if risk estimates were available for old‐dated and undated (without specified date) blood transfusion, estimates associated with undated blood transfusion were preferred.
In a second step, pooled ORs for low HCV prevalence (<5%) and high HCV prevalence (>5%) countries were computed following the previously described method and compared through subgroup analyses for each procedure group. Prevalence data for each country were collected from the MapCrowd online global data on hepatitis C (Table S9). 17 Procedure‐specific pooled ORs were stratified by country for the two most represented procedure groups. In addition, subgroup analyses were performed to compare estimates between cohort and non‐cohort studies.
Third, the pooled OR for blood transfusion based on transfusions performed with either recent dated or an unspecified date of realisation was compared to the one based on transfusions performed before 1998, which is the latest cut‐off date used within selected articles. Indeed, we assumed cut‐off dates found in the studies selected to reflect local implementation of mandatory HCV screening in blood donors.
Finally, meta‐regressions were performed to investigate the potential effect modifier of: (i) the HCV prevalence level (as a continuous variable), and (ii) the Healthcare Access and Quality (HAQ) index, a 0–100 score quantifying the strength of healthcare quality and access based on amenable mortality data in each country (Table S9). 18 The HAQ index was retrieved for 2000, 2005, 2010 and 2015 and was associated with each study based on their publication year.
The risk of bias was assessed by P.H. on the articles included. Nine bias assessment criteria were adapted from Lam et al., 19 and publication bias was assessed by testing for asymmetry in the overall and procedure‐specific (for procedures with more than 10 measures of association) funnel plots. 20
RESULTS
Among the 1961 initially identified studies, 71 were included in the review and meta‐analysis, 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 as described in the flow diagram (Figure 1). The total number of participants across all selected studies was 120,568.
FIGURE 1.
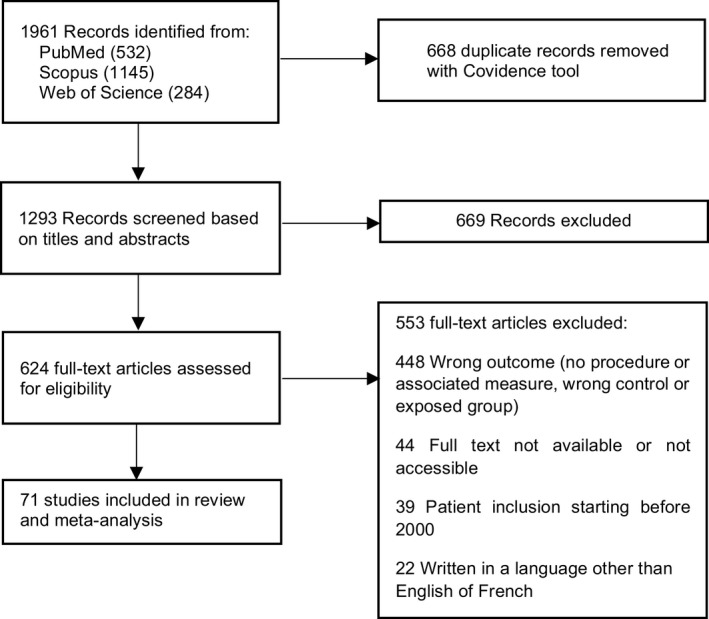
Study selection.
Figure 2A shows an increase in publications related to HCV risk assessment within hospitals between 2000 and 2015, while the number of publications remained stable between 2015 and 2020. Together, Pakistan (14 studies) and Egypt (9 studies) represented one‐third of all included studies (Figure 2C). Over a fifth of studies were focused on haemodialysis patients (22.5%) (Figure 2B). Most studies were cross‐sectional (55%) or case–control (22.5%) studies (Figure 2D).
FIGURE 2.
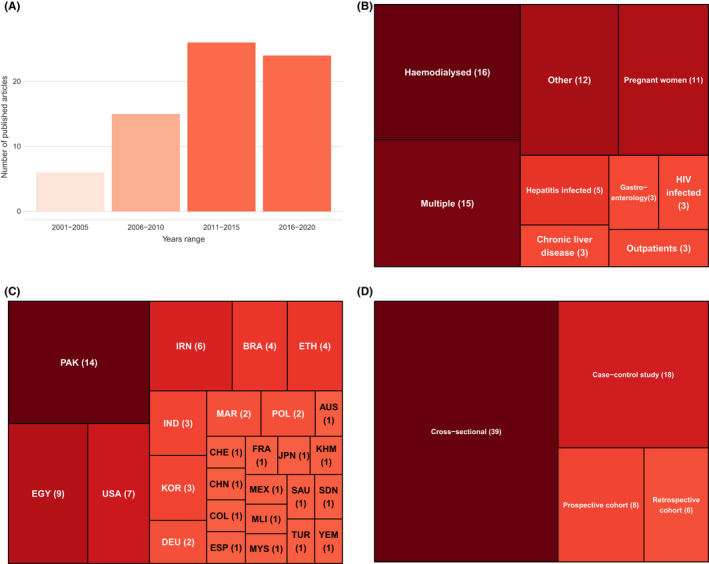
Study characteristics. Number of included studies by: (A) year of publication; (B) type of patients; (C) country; (D) Study designs. Total number of studies, 71.
Forty‐five different procedures were assessed (Table 1). The number of outcome measures by each of the 10 procedure groups ranged from 5 to 66, with surgery and transfusion as the largest groups (43 and 66 respectively) (Figure 3; Table S8). Visual inspection of procedure‐specific forest plots (Figures S6–S15) led to the exclusion of a single outlier for haemodialysis (Figure S6).
FIGURE 3.
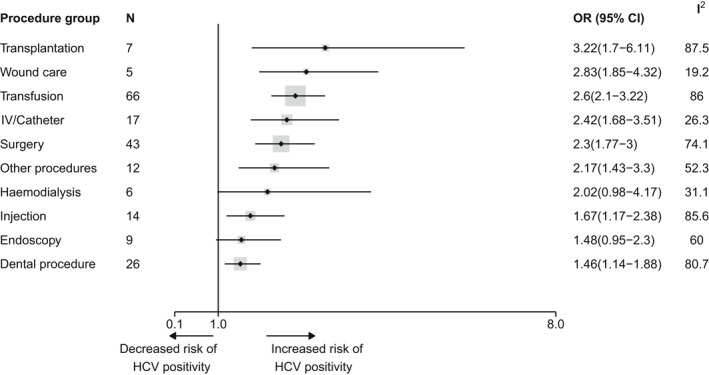
Forest plot reporting pooled OR estimates for HCV infection risk associated with different groups of iatrogenic procedures. Points correspond to average estimates and error bars correspond to 95% CI of these estimates. The solid line represents the limit for which OR = 1 and the dotted line the value of the overall estimate. The total number of observations used for each pooled OR calculation is specified in the column N and represented as the size of the grey area around each point estimate; the heterogeneity for each estimation is depicted in the column I 2. A given study could be used in the OR estimation of multiple procedures groups. Procedures are sorted based on the value of their associated mean estimate, from the highest to the lowest. CI, confidence interval; HCV, hepatitis C virus; OR, odds ratio.
All pooled ORs were significantly superior to one, except for endoscopy and haemodialysis (Figure 3).
The risk of HCV infection differed according to procedure groups with pooled OR estimates ranging from 1.46 (95% confidence interval [CI, 1.14–1.88]) for dental procedure up to 3.22 (95% CI [1.7–6.11]) for transplantation. The wound care and transplantation groups had OR estimates of 2.83 (95% CI [1.85–4.32]) and 3.22 (95% CI [1.7–6.11]), representing the higher risk groups. The haemodialysis, surgery, other procedure, intravenous (IV)/Catheter and transfusion groups had high/moderate pooled ORs, with estimates of 2.02 (95% CI [0.98–4.17]), 2.28 (95% CI [1.43–3.64]), 2.30 (95% CI [1.77–3.00]), 2.42 (95% CI [1.68–3.51]) and 2.6 (95% CI [2.1–3.22]) respectively. Injection was associated with a low/moderate risk of HCV infection with an estimate of 1.67 (95% CI [1.17–2.38]). Finally, the dental procedure and endoscopy groups presented the lowest risks of getting HCV infected (1.46 95% CI [1.14–1.88] and 1.48 95% CI [0.95–2.30] respectively).
Country‐stratified analyses were performed for transfusion and surgery (Figure 4). For these two procedure groups, estimated ORs varied widely between countries. The highest strength of association between blood transfusion and risk of HCV infection was reported in Germany (OR = 5.39 95% CI [2.67–10.89]) but this estimate corresponds to only one study and is associated with transfusions performed before 1991. Pooled ORs for the association between blood transfusion and risk of HCV infection were also high in Egypt (OR = 5.16 95% CI [1.86–14.28]), Iran (5.11 [1.04–24.99]), Turkey (4.50 [1.71–11.86]) and Malaysia (4.00 [2.18–7.35]), although the confidence intervals were quite wide. There were less high‐risk countries concerning surgery, for which Egypt and India had high pooled ORs (5.20 [2.50–11.00] and 4.62 [0.82–26.02] respectively).
FIGURE 4.
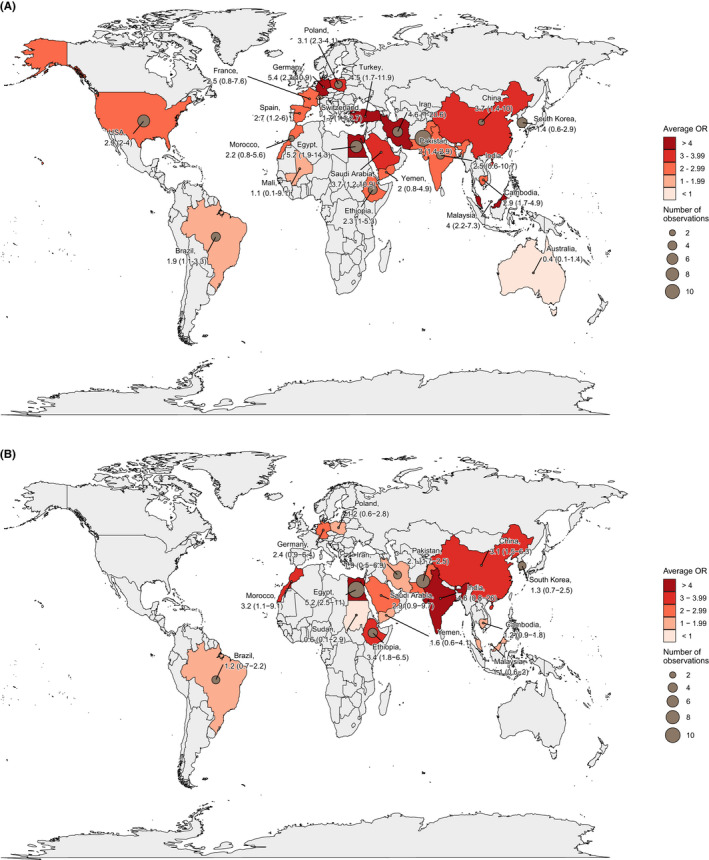
Maps of risks of HCV infection for: (A) Blood transfusion (B) Surgery in various countries. Brown points correspond to the number of observations (outcome measures) used to compute a given pooled OR. Each country is coloured based on the value of the estimated OR following a light red to dark red colour gradient. Values of average estimates are reported as well as their 95% CI. CI, confidence interval; HCV, hepatitis C virus; OR, odds ratio.
Overall, procedure‐ and prevalence‐specific pooled ORs tended to be higher in high prevalence countries (Figure 5; Tables S3 and S6) although this difference was not significant (p = 0.33, Figure 5). Nevertheless, a significant difference was observed for endoscopy (p = 0.0044, Table S3) and surgery (p = 0.013, Table S3). These results were supported by procedure‐specific meta‐regressions (Table S4) showing prevalence (as a continuous variable) to have a significant positive effect on the OR level of the endoscopy (p = 0.004) and surgery (p = 0.01) groups. However, prevalence (as a continuous variable) was not found to have a significant positive effect on the risk of iatrogenic HCV infection as a whole (p = 0.20, Table S6). In addition, no overall association was found between HAQ level and risk of iatrogenic infection (p = 0.93, Table S6), while in procedure‐specific analyses a negative significant impact of the HAQ level was observed for endoscopy only (p = 0.017, Table S5).
FIGURE 5.
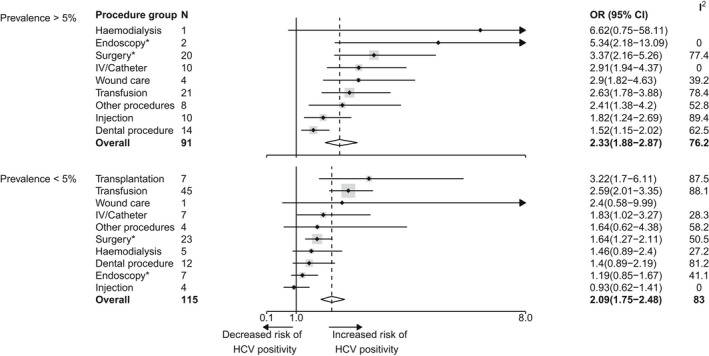
Forest plot reporting pooled OR estimates for HCV infection risk associated with different groups of iatrogenic procedures for high HCV prevalence countries (>5%) versus low HCV prevalence countries (<5%). The high prevalence group includes Egypt, Pakistan and Mali. Points correspond to average estimates and error bars correspond to 95% CI of these estimates. The solid line represents the limit for which OR = 1 and the dotted line the average value of the overall estimate. The total number of observations used for each pooled OR calculation is specified in the column N and the heterogeneity for each estimation is depicted in the column I 2. A given study could be used in the OR estimation of multiple procedures groups. Procedures are sorted based on the value of their associated mean estimate, from the highest to the lowest. There was no measure reported for transplantation in high HCV prevalence countries. *: procedures for which a significant different was found between high and low prevalence countries; Blanks within the I 2 column correspond to the absence of heterogeneity because of procedure group counting only one observation. Overall difference between high and low prevalence countries: p‐value = 0.33. CI, confidence interval; HCV, hepatitis C virus; OR, odds ratio.
For blood transfusions performed before 1998, 17 studies were selected for the pooled OR calculation, whereas there were 49 studies available for undated/most recent blood transfusions (Figures S1 and S2). The estimated pooled OR of HCV infection associated with blood transfusions performed before 1998 (OR = 3.77 95% CI [2.42–5.88]) was significantly higher than the pooled OR associated with undated/most recent blood transfusions (2.20 [1.77–2.75], p = 0.012).
Across all studies, bias was low for most criteria, but several studies presented a potentially high risk of bias for criteria related to exposure assessment (almost 90% of all studies) and to potential confounding (almost 60%, Figure S3; Table S2). No study had at least potentially high bias risk for more than two criteria (out of nine). Nevertheless, the overall funnel plot showed asymmetry and the associated Egger test was highly significant, suggesting potential publication bias (Figure S4).
Average ORs in non‐cohort studies were found to be higher than in cohort studies (2.26 95% CI [1.94–2.63] vs. 1.96 [1.41–2.72]) but this difference was not significant (p = 0.44). Per‐procedure comparisons between cohort and non‐cohort studies showed no significant differences, but the difference for the transplantation group was at the edge of statistical significance (p = 0.054).
DISCUSSION
In this systematic review and meta‐analysis, we identified 71 studies assessing the association between hospital‐based invasive procedures and the risk of HCV infection, representing a total of 120,568 participants. The overall strength of evidence remained of limited confidence as the bias analysis found a lack of high‐quality studies. Nevertheless, we estimated pooled ORs of HCV infection that were significantly associated with most invasive procedures performed in hospitals. Our results suggest a prioritisation of iatrogenic procedures: transplantation and wound care were associated with the highest risk of HCV infection whereas dental procedures and endoscopy were associated with the lowest risk. We also underlined a large between‐procedure and between‐country variability, and showed that the per‐procedure risk tended to be higher in countries with high HCV prevalence, while the level of healthcare quality and access in the country (as measured by the HAQ) only appeared to play a minor role.
The geographical coverage of selected studies was in line with global observed prevalence levels, with a third of these studies coming from the two countries with the highest HCV prevalence worldwide (Egypt and Pakistan). In addition, the most represented population was composed of haemodialysed patients, for which the risk of HCV infection is historically substantial. 92 Regrettably, only few studies used cohort data.
The estimated per‐procedure risks seemed to be mostly in line with the available literature concerning HCV. Generally, the risk was higher for procedures with frequent blood contact.
We found the highest risk of infection to be associated with transplantation. This risk is quite high considering that most of countries now require HCV testing in donors. 93 This might result from reverse causality; those infected with HCV have higher chance of undergoing these invasive procedures because of liver‐related complications. Surprisingly, we found a high risk of infection to be associated with wound care. This might be related with the fact that four of five studies assessing this procedure were conducted in Egypt, the country with high HCV prevalence. The estimate of the HCV infection risk through sutures found in the only previous meta‐analysis by El‐Ghitany et al. was lower than our estimate, but increased in the most recent studies. 13
The intermediate risk we found for blood transfusion could be explained by the fact that there is still high discrepancy in systematic screening of blood products between countries (only 80% of donations are screened in low‐income countries). 93 Haemodialysis was also associated with an intermediate risk of HCV infection. Our estimate was within the same order of magnitude than the one reported in the previous meta‐analysis. 13
We found injection to be one of the lowest at‐risk procedures. Injection was described in the past to be at high risk of HCV infection, in particular because of the reuse of contaminated syringes, 8 but this risk might have dropped over the last decade, in particular after the publication of multiple World Health Organization (WHO) and Centers for disease control and prevention (CDC) guidelines for safe injections. 94 The injection is one of the most frequently performed procedures and most adults have universally received it. There may be more chance of recall errors compared to other procedures as this is often given during childhood (e.g. vaccination).
Finally, dental procedures and endoscopy were found to be the lowest risk groups, in line with current literature findings. Indeed, only a few cases of HCV contamination after endoscopy have been described and dental practices are often at low risk of contamination. 92 , 95 This result is also consistent with previous estimations showing low risk associated with these two procedures. 13
Country‐level prevalence was overall found to be related to a higher risk of HCV contamination, although it was only significantly associated with endoscopy and surgery risks when looking at specific procedures. The lack of significance for other procedures may be explained in several ways. First, many countries were represented by less than three studies, leading to a lack of power for some procedures and countries and less accurate OR estimates. Second, these non‐significant associations may also indicate the presence of other causes of heterogeneity. In particular, there may be high differences in terms of prevalence between hospital settings within the same country. Procedure ranking differed between low and high prevalence countries. In particular, haemodialysis and endoscopy were found to be to most at‐risk procedures in high prevalence countries. However, considering that this was based, for these countries, on only one study (for haemodialysis) or two studies (for endoscopy), the associated confidence intervals were very wide and this ranking should not be over‐interpreted. Observed between‐country variations may also result from different infection control practices; we explored this assumption using the HAQ index, which is internationally validated and available for all countries in our analysis. However, we found no significant relationship between the risk of getting HCV contaminated and this index, except for the risk linked to endoscopy. The HAQ index may not be the right indicator to accurately reflect compliance with infection control intervention within hospitals. Second, there could be a high heterogeneity in this compliance between settings within the same country, that this index is unable to capture.
Our study carries several methodological as well as more study‐related limitations.
First, no grey literature or papers in languages other than French or English were included in this review, resulting in a potential information loss. Some other methodological simplifications could have influenced our final results. In fact, pooling adjusted and non‐adjusted measures could have led to overestimation of risks associated with some procedures. Our results also highly depend on the initial procedures classification we proposed, which may contribute to the high between‐study heterogeneity we observed for many procedure groups. However, this classification was necessary due to the overall high number of unique procedures identified in the review. In particular, we could not take into account the different type of surgeries because we did not have this information in approximately 75% of the studies concerned by this procedure. Moreover, the high heterogeneity could also result from other sources (e.g. prevalence or infection control).
This study highlighted several caveats in the existing literature, notably through the risk of bias tool that we adapted from another review 19 ; in particular, modifying the wording of criteria such as the outcome assessment method or confounders incorporation to better fit the specificities of our study. Overall, this bias analysis showed a lack of high‐quality studies. On the one hand, 90% of studies presented at least a probably high bias concerning the assessment of exposure to hospital‐based procedures, mostly using questionnaires to collect risk factors for HCV infection. This is consistent with the distribution of studies design, since almost 80% of studies were either cross‐sectional or case–control studies. This over‐representation of non‐longitudinal designs may in particular have led to overestimated ORs of HCV infection associated with invasive iatrogenic procedures, due to differential recall bias between HCV‐infected cases and controls. We investigated this through a separate assessment of pooled ORs in non‐cohort and cohort studies, lower ORs were found in cohorts although differences were not significant. However, ORs for all other procedures, as well as overall ORs, did not differ significantly between cohorts and non‐cohorts, supporting our choice to consider all study designs together. On the other hand, more than 50% of studies included a potential risk of bias due to incomplete or missing use of adjustment variables when estimating measures of association between HCV infection and iatrogenic procedures. Overall, heterogeneity in measures associated with iatrogenic procedures was shown and may reveal potential publication bias. In particular, non‐significant risk measures might have been voluntarily neglected resulting, again, in an overestimation of the risk associated with these two groups of procedures.
For some procedure groups, only few data were available, which led to important uncertainty in the associated OR estimates. Although there might not be a clear minimum number of studies to include within a meta‐analysis, 96 it is recommended to have at least 10. 97 Almost half of procedure groups had less than 10 risk measures (and six of them were based on less than 10 studies), wound care being the procedure for which this number was the lowest with only five measures (and five studies). This lack of data could have interfered with the results of meta‐regressions and having more studies could have given us more accurate results. Also, some procedures like surgery might have described operations during which multiple systematic procedures were performed and not taken into account (pre‐surgical anaesthesia). More generally, we rarely had access to measures of risks associated with a unique realisation of each procedure. Patients might have undergone the same procedure multiple times but this information was not taken into account. In particular, the risk of getting HCV infected during haemodialysis is highly related to the duration of haemodialysis and the risk related to blood transfusion is somehow positively proportional to the number of received transfusions. 98 These limitations may have caused overestimation of the risk associated with hospital‐based procedures.
In addition, the date of realisation of a procedure was not always available, and some measures might have been based on procedures performed a long time ago, thus leading again to an overestimation of the risk for some of them, as standard operating procedures have evolved. Indeed, we found the pooled OR of blood transfusions performed before 1998 to be higher than the pooled OR of undated/most recent blood transfusions, strongly suggesting that the risk was higher for older procedures—all the more so that the undated procedures could also have included old procedures.
In conclusion, despite the uncertainty in our estimates and the probable decrease in the general risk of HCV infection through hospital‐based procedures over the last 20 years, this work shows that healthcare settings remain an important gateway of HCV infection and underline the importance of implementing efficient infection control.
Our results suggest a risk‐based ranking for iatrogenic procedures, transplantation and wound care being the most at‐risk procedures. In addition, they confirm the important role of blood screening in decreasing the risk of HCV infection during blood transfusion. We also show that the strength of association between HCV infection and iatrogenic procedures tend to be higher in high prevalence countries. This could partly be explained by the high global heterogeneity in infection and prevention control knowledge in healthcare workers, especially concerning blood‐borne pathogens. 99
Two main tracks appear to be key to control HCV iatrogenic infections. First, knowledge of existing infection control interventions should be improved in order to make them fully effective. Second, prevention efforts should be specifically targeted at high‐risk procedures, such as transplantation, wound care or procedures involving IV injection, and in high HCV prevalence settings. In this regard, our work provides support to identify the procedures on which these efforts should be focused and is therefore of high value to assist decision makers in setting priorities. Our results may also prove useful for future modelling studies assessing the effectiveness and cost‐effectiveness of control interventions to limit HCV transmission in healthcare settings.
In 2016, the WHO set targets for global HCV elimination as a public health problem by 2030—an 80% reduction in incidence and a 65% reduction in mortality from 2015 levels. 100 However, taking into account delays in implementation of the WHO recommendations and publication delays, studies published between 2016 and 2020 would probably not reflect the impact of these targets.
In order to reach WHO targets, high prevalence countries such as Egypt and Pakistan have engaged in elimination programmes based on large‐scale test‐and‐treat campaigns. 101 By helping efficiently scale up prevention interventions, our work has the potential to improve the cost‐effectiveness of these campaigns, reaching the WHO targets faster and more easily.
AUTHORSHIP
All authors approved the final version of the manuscript.
Guarantor of article: Paul Henriot
AUTHOR CONTRIBUTIONS
Paul Henriot: Conceptualization (equal); formal analysis (lead); funding acquisition (lead); methodology (equal); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Mathieu Castry: Writing – review and editing (equal). Liem Binh Luong Nguyen: Writing – review and editing (equal). Yusuke Shimakawa: Writing – review and editing (equal). Kevin Jean: Conceptualization (equal); formal analysis (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Laura Temime: Conceptualization (equal); formal analysis (equal); methodology (equal); supervision (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
We declare no competing interests.
Supporting information
Data S1
ACKNOWLEDGEMENTS
This study was funded by INSERM‐ANRS (France Recherche Nord and Sud Sida‐HIV Hépatites), grant number 12320 B115. Open access funding enabled and organized by ProjektDEAL.
Henriot P, Castry M, Luong Nguyen LB, Shimakawa Y, Jean K, Temime L. Meta‐analysis: risk of hepatitis C virus infection associated with hospital‐based invasive procedures. Aliment Pharmacol Ther. 2022;56:558–569. 10.1111/apt.17106
As part of AP&T’s peer preview process, a technical check of this meta‐analysis was performed by Dr. Y. Yuan. The Handling Editor for this article was Professor Grace Wong, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up‐date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maucort‐Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide: liver cancer attributable to hepatitis viruses worldwide. Int J Cancer. 2018;142:2471–7. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/341412. Accessed 15 Apr 2022. [Google Scholar]
- 4. Sapena V, Enea M, Torres F, Celsa C, Rios J, Maria Rizzo GE, et al. Hepatocellular carcinoma recurrence after direct‐acting antiviral therapy: an individual patient data meta‐analysis. Gut. 2022;71:593–604. [DOI] [PubMed] [Google Scholar]
- 5. Latham NH, Doyle JS, Palmer AY, Vanhommerig JW, Agius P, Goutzamanis S, et al. Staying hepatitis C negative: a systematic review and meta‐analysis of cure and reinfection in people who inject drugs. Liver Int. 2019;39:2244–60. [DOI] [PubMed] [Google Scholar]
- 6. Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155:1436–1450.e6. [DOI] [PubMed] [Google Scholar]
- 7. Trickey A, Fraser H, Lim AG, Peacock A, Colledge S, Walker JG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–91. [DOI] [PubMed] [Google Scholar]
- 9. Sarman Singh SN, Dwivedi RS. Hepatitis B, C and human immunodeficiency virus infections in multiply‐injected kala‐azar patients in Delhi. Scand J Infect Dis. 2000;32:3–6. [DOI] [PubMed] [Google Scholar]
- 10. Thompson ND. Nonhospital health care‐associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33–9. [DOI] [PubMed] [Google Scholar]
- 11. Alduraywish A, Ragheb M, Taher I, Louis N, Aldossari K, Kishk R. Prevalence, risk factors and impact of occult HCV infection on liver morbidity among haemodialysis patients: hospital‐based cross‐sectional study. Scand J Gastroenterol. 2020;55:963–9. [DOI] [PubMed] [Google Scholar]
- 12. Arnold S, Melville SK, Morehead B, Vaughan G, Moorman A, Crist MB. Notes from the field: hepatitis C transmission from inappropriate reuse of saline flush syringes for multiple patients in an acute care general hospital—Texas, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El‐Ghitany EM, Abdel Wahab MM, Abd El‐Wahab EW, et al. A comprehensive hepatitis C virus risk factors meta‐analysis (1989–2013); Do they differ in Egypt? Liver Int. 2015;35:489–501. [DOI] [PubMed] [Google Scholar]
- 14. Covidence systematic review software. Melbourne: Veritas Health Innovation; 2021. Available from: https://support.covidence.org/help/how‐can‐i‐cite‐covidence. Accessed 1 Dec 2021. [Google Scholar]
- 15. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Mental Health. 2019;22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van den Noortgate W, López‐López JA, Marín‐Martínez F, Sanchez‐Meca J. Three‐level meta‐analysis of dependent effect sizes. Behav Res. 2013;45:576–94. [DOI] [PubMed] [Google Scholar]
- 17. Médecins du Monde and Treatment Action Group . MapCrowd: online global data on hepatitis C; 2016. https://mapcrowd.org/. Accessed 19 Jul 2021.
- 18. Fullman N, Yearwood J, Abay SM, Abbafati C, Abd‐Allah F, Abdela J, et al. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, et al. A Systematic review and meta‐analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11:e0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. AbdulQawi K, Youssef A, Metwally MA, Ragih I, AbdulHamid M, Shaheen AA. Prospective study of prevalence and risk factors for hepatitis C in pregnant Egyptian women and its transmission to their infants. Croat Med J. 2010;51:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akhtar AM, Khan MA, Ijaz T, Maqbool A, Iqbal Z, Rehman A, et al. Hepatitis C virus infection in pregnant women in Lahore, Pakistan: an analytical cross‐sectional study. Int J Agric Biol. 2014;16:160–4. [Google Scholar]
- 23. Alavian SM, Einollahi B, Hajarizadeh B, Bakhtiari S, Mohsen N, Ahrabi S. Prevalence of hepatitis C virus infection and related risk factors among Iranian haemodialysis patients. Nephrology (Carlton). 2003;8:256–60. [DOI] [PubMed] [Google Scholar]
- 24. Amin Elzorkany KM, Zahran A. Hepatitis C virus status in hemodialysis patients in Menoufia Government, Egypt, five years apart: do we have any improvement? Saudi J Kidney Dis Transpl. 2017;28:1126–32. [DOI] [PubMed] [Google Scholar]
- 25. Amjad U, Ahmad SQ, Mir S, Ayub M. Association of anti‐HCV sero‐prevalence with blood transfusion and practice of haemodialysis from multiple centres in patients on maintenance haemodialysis. Pak J Med Sci. 2020;36:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asad M, Ahmed F, Zafar H, Farman S. Frequency and determinants of hepatitis B and C virus in general population of Farash Town, Islamabad. Pak J Med Sci. 2015;31:1394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asemahagn MA. Epidemiology of hepatitis B and C virus infections among patients who booked for surgical procedures at Felegehiwot referral hospital, Northwest Ethiopia. PLoS One. 2020;15:e0234822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aslam A, Munawar I, Zahid N. Blood transfusion as a risk factor for hepatitis C in pregnancy. Pak J Med Health Sci. 2016;10:233–4. [Google Scholar]
- 29. Ba‐Essa EM, Mobarak EI, Al‐Daghri NM. Hepatitis C virus infection among patients with diabetes mellitus in Dammam, Saudi Arabia. BMC Health Serv Res. 2016;16:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balew M, Moges F, Yismaw G, Unakal C. Assessment of hepatitis B virus and hepatitis C virus infections and associated risk factors in HIV infected patients at Debretabor hospital, South Gondar, Northwest Ethiopia. Asian Pac J Trop Dis. 2014;4:1–7. [Google Scholar]
- 31. Barut S, Barut S, Eǧri M. Healthcare related risk factors account for the majority of HCV transmissions in middle black sea region of Turkey: a case‐control study. Turk Klinikleri J Med Sci. 2011;31:142–7. [Google Scholar]
- 32. Beltrân M, Navas M‐C, De La Hoz F, Munoz MM, Jaramillo S, Etsrada C, et al. Hepatitis C virus seroprevalence in multi‐transfused patients in Colombia. J Clin Virol. 2005;34:S33–8. [DOI] [PubMed] [Google Scholar]
- 33. Bibi S, Dars S, Ashfaq S, Qazi RA, Akhund S. Seroprevalence and risk factors for hepatitis C virus (HCV) infection in pregnant women attending public sector tertiary care hospital in Hyderabad Sindh. Pak J Med Sci. 2013;29:505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouare B, Gothot A, Delwaide J, Bontems S, Vaira D, Seidel L, et al. Epidemiological profiles of human immunodeficiency virus and hepatitis C virus infections in malian women: risk factors and relevance of disparities. World J Hepatol. 2013;5:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calderon GM, Gonzalez‐Velaquez F, Gonzalez‐Bonilla CR, Novelo‐Garza B, Terrazas JJ, Martinez‐Rodriguez ML, et al. Prevalence and risk factors of hepatitis C virus, hepatitis B virus, and human immunodeficiency virus in multiply transfused recipients in Mexico. Transfusion. 2009;49:2200–7. [DOI] [PubMed] [Google Scholar]
- 36. Calles DL, Collier MG, Khudyakov Y, Mixson‐Hayden T, VanderBusch L, Weninger S, et al. Hepatitis C virus transmission in a skilled nursing facility, North Dakota, 2013. Am J Infect Control. 2017;45:126–32. [DOI] [PubMed] [Google Scholar]
- 37. da Silva NMO, Germano FN, Mendoza‐Sassi RA, Seuanez HN, Soares MA, Barral de Martinez AM. Evidence of association between hepatitis C virus genotype 2b and nosocomial transmissions in hemodialysis centers from southern Brazil. Virol J. 2013;10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Oliveira AM, White KL, Leschinsky DP, Beecham BD, Vogt TM, Moolenaar RL, et al. An outbreak of hepatitis C virus infections among outpatients at a hematology/oncology clinic. Ann Intern Med. 2005;142:898–902. [DOI] [PubMed] [Google Scholar]
- 39. De Weggheleire A, An S, De Baetselier I, Soeung P, Keath H, So V, et al. A cross‐sectional study of hepatitis C among people living with HIV in Cambodia: prevalence, risk factors, and potential for targeted screening. PLos One. 2017;12:e0183530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doblali T, Bahadi A, El Amrani M, Benyahia M. Prevalence and risk factors of hepatitis c virus infection in patients on hemodialysis: results of a Moroccan study. Med Sante Tropica. 2014;24:375–8. [DOI] [PubMed] [Google Scholar]
- 41. Ejeta E, Dabsu R. Prevalence of hepatitis C virus and HIV infection among pregnant women attending antenatal care clinic in Western Ethiopia. Front Med. 2019;5:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flisiak R, Halota W, Horban A, Juszczyk J, Pawlowska M, Simon K. Prevalence and risk factors of HCV infection in Poland. Eur J Gastroenterol Hepatol. 2011;23:1213–7. [DOI] [PubMed] [Google Scholar]
- 43. Galperim B, Mattos AA, Stein AT, Schneider NC, Buriol A, Fonseca A, et al. Hepatitis C in hemodialysis: the contribution of injection drug use, Brazilian. J Infect Dis. 2010;14:422–6. [PubMed] [Google Scholar]
- 44. Gasim GI, Hamdan HZ, Hamdan SZ, Adam I. Epidemiology of hepatitis B and hepatitis C virus infections among hemodialysis patients in Khartoum, Sudan. J Med Virol. 2012;84:52–5. [DOI] [PubMed] [Google Scholar]
- 45. Ghias M, Pervaiz MK, Marshall R, Thornley S. Identification of risk factors for hepatitis C infection in the Gujranwala district of Punjab, Pakistan. World Appl Sci J. 2012;20:94–101. [Google Scholar]
- 46. Haider J, Lufullah G, Nazli R, Akhtar T, Shah A. Screening of adult dental patients visiting Khyber College of Dentistry, Peshawar for HBV and HCV infections and identifying the associated risk factors. Pak J Med Sci. 2017;33:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hutton J, Doyle J, Zordan R, Weiland T, Cocco A, Howell J, et al. Point‐of‐care hepatitis C virus testing and linkage to treatment in an Australian inner‐city emergency department. Int J Drug Policy. 2019;72:84–90. [DOI] [PubMed] [Google Scholar]
- 48. Ishikawa T, Fukushima Y, Shiobara Y, Kishimoto T, Tanno S, Shoji I, et al. Outbreak of hepatitis C virus infection in an outpatient clinic. J Gastroenterol Hepatol. 2005;20:1087–93. [DOI] [PubMed] [Google Scholar]
- 49. Jaffery T, Tariq N, Ayub R, Yawar A. Frequency of hepatitis C in pregnancy and pregnancy outcome. J Coll Physicians Surg Pak. 2005;15:716–9. [PubMed] [Google Scholar]
- 50. Jamil Z, Waheed Y, Ahsan O, Najmi MH, Yousuf H. Familial clustering of hepatitis C virus in a Pakistani population. J Med Virol. 2020;92:3499–506. [DOI] [PubMed] [Google Scholar]
- 51. Paez Jimenez A, Mohamed MK, Eldin NS, Seif HA, El Aidi S, Sultan Y, et al. Injection drug use is a risk factor for HCV infection in urban Egypt. PLoS One. 2009;4:e7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kandeel AM, Talaat M, Afifi SA, el‐Sayed NM, Fadeel MAA, Hajjeh RA, et al. Case control study to identify risk factors for acute hepatitis C virus infection in Egypt. BMC Infect Dis. 2012;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khalid GG, Kyaw KWY, Bousquet C, Auat R, Donchuk D, Trickey A, et al. From risk to care: the hepatitis C screening and diagnostic cascade in a primary health care clinic in Karachi, Pakistan‐a cohort study. Int Health. 2019;12:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khan UR, Janjua NZ, Akhtar S, Hatcher J. Case‐control study of risk factors associated with hepatitis C virus infection among pregnant women in hospitals of Karachi‐Pakistan. Trop Med Int Health. 2008;13:754–61. [DOI] [PubMed] [Google Scholar]
- 55. Kim JY, Cho J, Hwang SH, Kil H, Bae SH, Kim YS, et al. Behavioral and healthcare‐associated risk factors for chronic hepatitis C virus infection in Korea. J Korean Med Sci. 2012;27:1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kundu A, Mehta S, Agrawal BK, Unakal C. Assessment of associated risk factors in hepatitis B virus and hepatitis C virus infections. JK Sci. 2017;18:229–32. [Google Scholar]
- 57. Lioussfi Z, Errami Z, Radoui A, Rhou H, Ezzaitouni F, Ouzeddoun N, et al. Viral hepatitis C and B among dialysis patients at the Rabat University Hospital: prevalence and risk factors. Saudi J Kidney Dis Transpl. 2014;25:672–9. [DOI] [PubMed] [Google Scholar]
- 58. Loras C, Saro C, Gonzalez‐Huix F, Minguez M, Merino O, Gisbert JP, et al. Prevalence and factors related to hepatitis B and C in inflammatory bowel disease patients in Spain: a nationwide, multicenter study. Am J Gastroenterol. 2009;104:57–63. [DOI] [PubMed] [Google Scholar]
- 59. Metwally A, Mohsen A, Saleh R, Foaud W, Ibrahim N, Rabaah T, et al. Prioritizing high‐risk practices and exploring new emerging ones associated with hepatitis C virus infection in Egypt. Iran J Public Health. 2014;43:1385–94. [PMC free article] [PubMed] [Google Scholar]
- 60. Mittal G, Gupta P, Thakuria B, Mukhiya GK, Mittal M. Profile of hepatitis B virus, hepatitis C virus, hepatitis D virus and human immunodeficiency virus infections in hemodialysis patients of a tertiary care hospital in Uttarakhand. J Clin Exp Hepatol. 2013;3:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mohd Suan MA, Said SM, Lim PY, Azman AZF, Abu Hassan MR. Risk factors for hepatitis C infection among adult patients in Kedah state, Malaysia: a case‐control study. PLoS One. 2019;14:e0224459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mohsen A, Bernier A, Lefouler L, Delarocque‐Astagneau E, El‐Daly M, El‐Kafrawy S, et al. Hepatitis C virus acquisition among Egyptians: analysis of a 10‐year surveillance of acute hepatitis C. Trop Med Int Health. 2015;20:89–97. [DOI] [PubMed] [Google Scholar]
- 63. Mostafa A, Ebeid FSE, Khaled B, Ahmed RHM, el‐Sayed MH. Micro‐elimination of hepatitis C through testing of Egyptian pregnant women presenting at delivery: implications for screening policies. Trop Med Int Health. 2020;25:850–60. [DOI] [PubMed] [Google Scholar]
- 64. Murad EA, Babiker SM, Gasim GI, Rayis DA, Adam I. Epidemiology of hepatitis B and hepatitis C virus infections in pregnant women in Sana'a, Yemen. BMC Pregnancy Childbirth. 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Neumeister AS, Pilcher LE, Erickson JM, Langley LL, Murphy MM, Haukaas NM, et al. Hepatitis‐C prevalence in an urban native‐American clinic: a prospective screening study. J Natl Med Assoc. 2007;99:389–92. [PMC free article] [PubMed] [Google Scholar]
- 66. Paez Jimenez A, Sharaf Eldin N, Rimlinger F, el‐Daly M, el‐Hariri H, el‐Hoseiny M, et al. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59:1554–60. [DOI] [PubMed] [Google Scholar]
- 67. Patil SR, Datkhile KD, Ghorpade MV, Patil SS, Kakade SV. Seroprevalence, risk factors and genotype distribution for hepatitis C infection: a study from rural hospital in Maharashtra. Indian J Med Microbiol. 2017;35:563–7. [DOI] [PubMed] [Google Scholar]
- 68. Prasad M, Saade GR, Sandoval G, Hughes BL, Reddy UM, Mele L, et al. Hepatitis C virus antibody screening in a cohort of pregnant women: identifying seroprevalence and risk factors. Obstet Gynecol. 2020;135:778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qureshi H, Arif A, Riaz K, Alam S, Ahmed W, Mujeeb S. Determination of risk factors for hepatitis B and C in male patients suffering from chronic hepatitis. BMC Res Notes. 2009;2:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ramsey SD, Unger JM, Baker LH, Little RF, Loomba R, Hwang JP, et al. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol. 2019;5:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosińska M, Parda N, Kołakowska A, Godzik P, Zakrzewska K, Madaliński K, et al. Factors associated with hepatitis C prevalence differ by the stage of liver fibrosis: a cross‐sectional study in the general population in Poland, 2012–2016. PLoS One. 2017;12:e0185055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ross RS, Viazov S, Clauberg R, Wolters B, Fengler I, Eveld K, et al. Lack of de novo hepatitis C virus infections and absence of nosocomial transmissions of GB virus C in a large cohort of German haemodialysis patients. J Viral Hepatitis. 2009;16:230–8. [DOI] [PubMed] [Google Scholar]
- 73. Russmann S, Dowlatshahi EA, Printzen G, Habicht S, Reichen J, Zimmermann H. Prevalence and associated factors of viral hepatitis and transferrin elevations in 5036 patients admitted to the emergency room of a Swiss university hospital: cross‐sectional study. BMC Gastroenterol. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sahajian F, Vanhems P, Bailly F, Fabry J, Trepo C, Sepetjan M, et al. Screening campaign of hepatitis C among underprivileged people consulting in health centres of Lyon area, France. Eur J Public Health. 2007;17:263–71. [DOI] [PubMed] [Google Scholar]
- 75. Samimi‐Rad K, Hosseini M, Mobeini G, Asgari F, Alavian SM, Tahaei ME, et al. Hepatitis C virus infection among multi‐transfused patients and personnel in haemodialysis units in central Islamic Republic of Iran. East Mediterr Health J. 2012;18:227–35. [DOI] [PubMed] [Google Scholar]
- 76. Samimi‐Rad K, Hosseini M, Shahbaz B. Hepatitis C virus infection and HCV genotypes of hemodialysis patients. Iran J Public Health. 2008;37:146–52. [Google Scholar]
- 77. Samimi‐Rad K, Shahbaz B. Hepatitis C virus genotypes among patients with thalassemia and inherited bleeding disorders in Markazi province, Iran. Haemophilia. 2007;13:156–63. [DOI] [PubMed] [Google Scholar]
- 78. Schmidt AJ, Rockstroh JK, Vogel M, An der Heiden M, Baillot A, Krznaric I, et al. Trouble with bleeding: risk factors for acute hepatitis C among HIV‐positive gay men from Germany‐a case‐control study. PLoS One. 2011;6:e17781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Senosy SA, El Shabrawy EM. Hepatitis C virus in patients on regular hemodialysis in Beni‐Suef Governorate, Egypt. J Egypt Public Health Assoc. 2016;91:86–9. [DOI] [PubMed] [Google Scholar]
- 80. Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol. 2013;85:1724–33. [DOI] [PubMed] [Google Scholar]
- 81. Shahriari‐Fard F, Alavian SM, Farajzadegan Z, Rabiei A, Ataei B, Ataie M. Assessment of hepatitis C risk factors in center of Iran: a case‐control study. J Res Med Sci. 2018;23:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shimokura G, Chai F, Weber DJ, Samsa GP, Xia GL, Nainan OV, et al. Patient‐care practices associated with an increased prevalence of hepatitis c virus infection among chronic hemodialysis patients. Infect Control Hosp Epidemiol. 2011;32:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Silva JL, de Souza VS, Vilella TA, et al. HBV and HCV serological markers in patients with the hepatosplenic form of mansonic schistosomiasis. Arq Gastroenterol. 2011;48:124–30. [DOI] [PubMed] [Google Scholar]
- 84. Sohn HS, Kim JR, Ryu SY, Lee YJ, Lee MJ, Min HJ, et al. Risk factors for hepatitis C virus (HCV) infection in areas with a. high prevalence of HCV in the Republic of Korea in 2013. Gut Liver. 2016;10:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Somi MH, Etemadi J, Ghojazadeh M, Farhang S, Faramarzi M, Foroutan S, et al. Risk factors of HCV seroconversion in hemodialysis patients in Tabriz, Iran. Hepatitis Mon. 2014;14:e17417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Su Y, Yan R, Duan Z, Norris JL, Wang L, Jiang Y, et al. Prevalence and risk factors of hepatitis C and B virus infections in hemodialysis patients and their spouses: a multicenter study in Beijing, China. J Med Virol. 2013;85:425–32. [DOI] [PubMed] [Google Scholar]
- 87. Taye M, Daka D, Amsalu A, Hussen S. Magnitude of hepatitis B and C virus infections and associated factors among patients scheduled for surgery at Hawassa University comprehensive specialized Hospital, Hawassa City, Southern Ethiopia. BMC Res Notes. 2019;12:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ullah M, Hasan F, Najmudin , Masroor Alam M, Zahoor Zaidi SS, Suleman Rana M, et al. Seroprevalence of hepatitis C virus infection in Kohat division, Khyber Pakhtoonkhwa, Pakistan. Pak J Zool. 2016;48:1721–5. [Google Scholar]
- 89. Ver Hoeve E, Codlin AJ, Jawed F, Khan AJ, Samad L, Vatcheva KM, et al. Persisting role of healthcare settings in hepatitis C transmission in Pakistan: cause for concern. Epidemiol Infect. 2013;141:1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vidales‐Braz BM, Da Silva NMO, Lobato R, Germano FN, da Mota LD, Barros EJG, et al. Detection of hepatitis C virus in patients with terminal renal disease undergoing dialysis in southern Brazil: prevalence, risk factors, genotypes, and viral load dynamics in hemodialysis patients. Virol J. 2015;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weisbord JS, Trepka MJ, Zhang G, Smith IP, Brewer T. Prevalence of and risk factors for hepatitis C virus infection among STD clinic clientele in Miami, Florida. Sex Transm Infect. 2003;79:E1–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pozzetto B, Memmi M, Garraud O, Roblin X, Berthelot P. Health care‐associated hepatitis C virus infection. World J Gastroenterol. 2014;20:17265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. World Health Organization . Blood safety and availability (fact sheet, 2021). Available from: https://www.who.int/news‐room/fact‐sheets/detail/blood‐safety‐and‐availability. Accessed 1 Dec 2021.
- 94. Defendorf CM, Paul S, Scott GJ. Iatrogenic hepatitis C virus transmission and safe injection practices. J Am Osteopath Assoc. 2018;118:311–20. [DOI] [PubMed] [Google Scholar]
- 95. Morris J, Duckworth GJ, Ridgway GL. Gastrointestinal endoscopy decontamination failure and the risk of transmission of blood‐borne viruses: a review. J Hosp Infect. 2006;63:1–13. [DOI] [PubMed] [Google Scholar]
- 96. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta‐analysis. J Educ Behav Stat. 2010;35:215–47. [Google Scholar]
- 97. JPT Higgins, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.3. Cochrane; 2022. [updated 2022 Feb]. Available from: www.training.cochrane.org/handbook
- 98. Prati D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. J Hepatol. 2006;45:607–16. [DOI] [PubMed] [Google Scholar]
- 99. Alhumaid S, Al Mutair A, Al Alawi Z, Alsuliman M, Ahmed GY, Rabaan AA, et al. Knowledge of infection prevention and control among healthcare workers and factors influencing compliance: a systematic review. Antimicrob Resist Infect Control. 2021;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. World Health Organization . Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/246177. Accessed 1 Dec 2021. [Google Scholar]
- 101. Lim AG, Qureshi H, Mahmood H, Hamid S, Davies CF, Trickey A, et al. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol. 2018;47:550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


