Abstract
Bartonella species are facultative intracellular bacteria and recognized worldwide as emerging zoonotic pathogens. Bartonella were isolated or identified by polymerase chain reaction (PCR) in bats and their ectoparasites worldwide, whereas the association between them was scarce, especially in Asia. In this study, a retrospective analysis with frozen samples was carried out to identify the genetic diversity of Bartonella in bats and their ectoparasites and to investigate the relationships of Bartonella carried by bats and their ectoparasites. Bats and their ectoparasites (bat flies and bat mites) were collected from caves in Hubei Province, Central China, from May 2018 to July 2020. Bartonella were screened by PCR amplification and sequencing of three genes (gltA, rpoB, and ftsZ). Bats, bat flies, and bat mites carried diverse novel Bartonella genotypes with a high prevalence. The sharing of some Bartonella genotypes between bats and bat flies or bat mites indicated a potential role of bat flies and bat mites as vectors of bartonellae, while the higher genetic diversity of Bartonella in bat flies than that in bats might be due to the vertical transmission of this bacterium in bat flies. Therefore, bat flies might also act as reservoirs of Bartonella. In addition, human‐pathogenic B. mayotimonesis was identified in both bats and their ectoparasites, which expanded our knowledge on the geographic distribution of this bacterium and suggested a potential bat origin with bat flies and bat mites playing important roles in the maintenance and transmission of Bartonella.
Keywords: Bartonella, bat, bat fly, bat mite, Nycteribiidae, Spinturnicidae
1. INTRODUCTION
As the second largest order of mammals, bats are unique long‐lived gregarious flying mammals with a worldwide distribution, and they have been considered as the most likely sources of several viruses causing severe emerging infectious diseases in humans, including SARS‐CoV, MERS‐CoV, Nipah virus, Hendra virus, Ebola virus, Marburg virus, and the currently rampant SARS‐CoV‐2 (Han et al., 2015; Zhou et al., 2020). In the last two decades, a large number of novel viruses have been found in bats (Chen et al., 2014), whereas the study of bacterial agents in bats has been far more neglected (Mühldorfer, 2013).
Bartonella species are Gram‐negative, fastidious, and facultative intracellular bacteria that parasitize erythrocytes and endothelial cells of a variety of mammals, including rodents, insectivores, carnivores, ungulates, and marine mammals such as dolphins (Birtles, 2005; Dehio, 2001; Eicher & Dehio, 2012; Kosoy et al., 2010). Several Bartonella species are capable of causing infections in humans, including Bartonella henselae (cat‐scratch disease), B. quintana (trench fever), and B. bacilliformis (Carrión's disease) (Angelakis & Raoult, 2014; Jacomo et al., 2002; Rolain et al., 2004). Besides, an increasing number of novel Bartonella species associated with human endocarditis were reported (Edouard et al., 2015; Okaro et al., 2017).
There was evidence that bat‐borne bartonellae were associated with infections in humans and other animals. Bartonella mayotimonensis was first isolated from the aortic valve of a patient with endocarditis in the United States (E. Y. Lin et al., 2010). Subsequently, Bartonella related to B. mayotimonensis were identified in bats from Finland (Veikkolainen et al., 2014), the United States (Lilley et al., 2017), the United Kingdom (Concannon et al., 2005), France, and Spain (Stuckey, Boulouis, et al., 2017). In addition, some Bartonella genotypes found in bats from Georgia were genetically related to those identified in dogs from Thailand and humans from Poland (Urushadze et al., 2017). Moreover, a novel Bartonella species, Bartonella rousetti, was isolated from fruit bats in Nigeria, and serological studies revealed that infection with this bacterium was prevalent in the local population (Bai et al., 2018). These reports highlighted the zoonotic potential of bat‐borne bartonellae and underscored the need for expanded surveillance and investigation of these pathogens.
Bats harbour numerous ectoparasites, including bat flies (Diptera: Nycteribiidae and Streblidae), bat bugs (Hemiptera: Cimicidae and Polyctenidae), bat fleas (Siphonaptera: Ischnopsyllidae), bat ticks (Ixodida: Ixodidae and Argasidae), and bat mites (Mesostigmata: Spinturnicidae and Macronyssidae) (Szentiványi et al., 2019). In the last decade, diverse novel Bartonella strains/genotypes were identified in bats and their ectoparasites worldwide (Figure 1, Table S1). Besides, a recent study inferred that bats had a great influence on both the origin and spread of Bartonella among other mammals and geographic regions (Mckee et al., 2021). However, there was a lack of understanding on the maintenance and transmission of Bartonella in bat populations. Bat flies and bat mites are obligate hematophagous ectoparasites of bats, and they may play important roles in the transmission and maintenance of bat pathogens. Bat flies were the most studied ectoparasites of bats in terms of bartonellae. The presence of identical genotypes of Bartonella in bat flies and their bat hosts was frequently reported (Brook et al., 2015; Dietrich et al., 2016; Judson et al., 2015; Kamani et al., 2014; Qiu et al., 2020); therefore, bat flies were generally considered as vectors for transmitting bartonellae among bats. Few studies reported the occurrence of Bartonella in bat mites (Hornok et al., 2012; Ikeda et al., 2020; Reeves et al., 2016), and a recent study in Poland found that identical genotypes of Bartonella were shared among bats and their Spinturnix myoti mites, indicating the possible role of bat mites in the acquisition and transmission of Bartonella (Szubert‐Kruszyńska et al., 2019).
FIGURE 1.
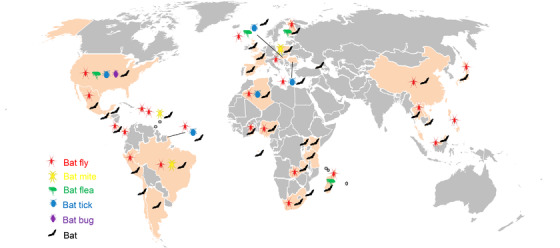
Map showing our current understanding of Bartonella in bats and their ectoparasites worldwide. Countries or regions with reports of Bartonella in bats or their ectoparasites are highlighted in gold. The map was created based on data from Table S1
Studies on the genetic diversity of Bartonella in bats and their ectoparasites in Asia were quite limited (Figure 1), and there was a lack of research on the relationship between them. In China, Bartonella were previously identified in bats from Shandong Province by our team (Han et al., 2017) and from Taiwan (J.‐W. Lin et al., 2012). However, there were hardly any reports of Bartonella in bat ectoparasites other than one bat fly in China (Morse et al., 2012). Considering the highly diverse novel Bartonella genotypes identified in bats from China in our previous work (Han et al., 2017), there was a need for further investigation on the relationship of Bartonella in bats and their ectoparasites.
In this study, a retrospective analysis with frozen samples was carried out to identify the genetic diversity of Bartonella in bats and their ectoparasites and to investigate the relationships of Bartonella carried by bats and their ectoparasites. Our study will provide insight into the evolution and ecology of Bartonella in bat populations.
2. MATERIALS AND METHODS
2.1. Ethics statement
Collection of bats for microbiological studies was approved by the Ethics Committee of the Medical School, Wuhan University (WHU2020‐YF0023), and every effort was made to minimize the discomfort of bats.
2.2. Sampling and species identification of bats and their ectoparasites
Frozen bats, bat flies, and bat mites stored in our laboratory, which were sampled for an ongoing programme aiming at identifying pathogens in bats, were used for the analysis of Bartonella. These bats were collected with mist nets, which were settled near the entrance of karst caves at sunset when bats left roosts for night feeding, and bats were collected in the next early morning. Captured bats were put in a bag, sacrificed by inhaling of ethyl ether in the field, and then transported back to the laboratory on ice as soon as possible. Once arrived at the laboratory, bats were checked for ectoparasites with forceps, with bat flies in the fur and bat mites on the membrane wings. After collection of ectoparasites, thoracic and abdominal organs of bats were collected. All the specimen were stored at −80℃ until use. Bat species were preliminary identified by morphology, and then confirmed by polymerase chain reaction (PCR) amplification and sequencing of the cytochrome B (cytB) gene as described previously (Li et al., 2021). Bat flies and bat mites were molecularly identified by the cytochrome oxidase subunit I (COI) and mitochondrial 16S rRNA genes (Bruyndonckx et al., 2009) (Table S2; Castro et al., 2002; Folmer et al., 1994).
2.3. Molecular detection of Bartonella in bats, bat flies, and bat mites
DNA was extracted from bat flies, bat mites, and bat liver tissue samples using QIAamp DNA Mini Kit (Qiagen, Valencia, CA, Spain). Bartonella were screened by PCR amplification and sequencing of gltA, rpoB, and ftsZ genes as described previously (Bai et al., 2015; Gonçalves‐Oliveira et al., 2020) (Table S2).
The PCR reaction was performed in a 50 μl mixture containing 0.25 μl 5 U/μl TaKaRa Ex Taq (TaKaRa, Shiga, Japan), 5 μl 10× Ex Taq buffer, 4 μl 25 mM MgCl2, 4 μl 2.5 mM dNTP mixture, 5 μl 10 μM each forward and reverse primer (Sangon Biotech, Shanghai, China), 27.75 μl nuclease‐free water, and 5 μl sample DNA. PCR was performed with one denaturation cycle at 95˚C for 5 min followed by 40 cycles at 95˚C for 30 s, 55˚C for 30 s, and 72˚C for 90 s, and an additional final cycle at 72˚C for 10 min. Each PCR assay included nuclease‐free water as a negative control. PCR products were analyzed by 1.2% agarose gel electrophoresis. Bands of expected size were excised from the gels, and purified using a Gel Extraction Kit (TSINGKE Biological Technology, Wuhan, China). The purified amplicons were cloned into the pMD19‐T vector (TaKaRa, Shiga, Japan), and at least three positive clones were selected for sequencing with the universal primers M13‐47/ M13‐48. Chromatograms were checked with Chromas 2.5.1 (Technelysium, Tewantin, QLD) to ensure sequencing accuracy. The sequences were compared with those in public databases using the Nucleotide Basic Local Alignment Search Tool (BLASTn) on the website of the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.4. Phylogenetic analysis
Sequences of interest were imported into MEGA 7.0, and primers were removed after alignment with ClustalW. Phylogenetic trees were constructed based on the neighbour‐joining method using the Kimura 2‐parameter model in MEGA 7.0, and bootstrap values were calculated using 1000 replicates.
2.5. Statistical analyses
Since information on the age, sex, reproduction status, or ectoparasite intensity of bats was not collected in this study, statistical analyses were not performed for these factors. Chi‐squared test and Fisher's exact test were used to evaluate the Bartonella prevalence by bat species, and differences were statistically significant if p‐values <.05.
3. RESULTS
3.1. Sampling and species identification of bats and their ectoparasites
Frozen bat tissue samples and bat ectoparasites used in this study were collected from bats sampled from three cities (Songzi, Jingmen, and Xianning) in Hubei Province, Central China, during May 2018 to July 2020. In each of the three cities, bats were sampled once from a single karst cave (Table 1).
TABLE 1.
Sampling information and prevalence of Bartonella in bats, bat flies, and bat mites from Hubei Province, China
| Sampling date | Sampling area | Sample type | Family | Species | Number of samples | Number of positive samples (%) | p‐Value |
|---|---|---|---|---|---|---|---|
| May 2018 | Songzi, Hubei, China (30°17´N, 111°77´E) | Bat fly | Nycteribiidae | Penicillidia monoceros | 15 | 10 (66.7) | – |
| Nycteribia sp. | 1* | 1* (100) | – | ||||
| Bat | Vespertilionidae | Myotis adversus | 4 | 2 (50.0) | – | ||
| Myotis davidii | 6 | 0 (0) | – | ||||
| September 2019 | Jingzhou, Hubei, China (30°35´N, 112°19´E) | Bat fly | Nycteribiidae | Penicillidia monoceros | 16* | 16* (100) | – |
| Nycteribia sp. | |||||||
| Phthiridium sp. | |||||||
| Bat | Vespertilionidae | Myotis davidii | 13 | 3 (23.1) | – | ||
| Miniopteridae | Miniopterus schreibersii | 3 | 0 (0) | – | |||
| July 2020 | Xianning, Hubei, China (29°85´N, 114°30´E) | Bat fly | Nycteribiidae | Penicillidia monoceros | 119 | 60 (50.4) | – |
| Nycteribia sp. | 6* | 6* (100) | – | ||||
| Phthiridium sp. | |||||||
| Bat mite | Spinturnicidae | Spinturnix sp. | 11* | 11* (100) | – | ||
| Eyndhovenia sp. | |||||||
| Bat | Vespertilionidae | Myotis adversus | 29 | 1 (3.4) | <.05 | ||
| Myotis davidii | 105 | 17 (16.2) | |||||
| Rhinolophidae | Rhinolophus pusillus | 69 | 3 (4.3) | ||||
| Total | 397 | 130 (32.7) |
Note: Asterisk (*) represents pooled samples, and en‐dash (‐) indicates that no statistical analysis was performed.
In September 2019, bat flies were collected from 16 bats sampled from a karst cave in Jingzhou, Hubei Province, China. Initially, these bat flies were divided into 16 pools (bat flies from several bats were mixed as a pool). Based on the COI and 16S rRNA genes, 16 bat fly pools consisted of three bat fly species belonging to three genera (Nycteribia, Penicillidia, and Phthiridium) in the family Nycteribiidae. Based on the COI gene, the three bat fly species shared 98.3%, 98.3%, and 96.4% nucleotide identity with Penicillidia monoceros (GenBank accession number: MW590972), Nycteribia sp. (Korea) (GenBank accession number: MT362944), and Phthiridium hindlei (GenBank accession number: AB632568), respectively. Based on the 16S rRNA gene, the three bat fly species shared 98.2%, 95.8%, and 96.3% nucleotide identity with P. monoceros (GenBank accession number: AB632585), N. pleuralis (GenBank accession number: AB632581), and P. hindlei (GenBank accession number: AB632587), respectively. Therefore, the three bat fly species found in this study were identified as P. monoceros, Nycteribia sp. (Korea), and a potentially novel Phthiridium sp. (Figures 2 and 3, Table 1). The 16 bat hosts were identified as Myotis davidii (13) and Miniopterus schreibersii (3) based on the cytB gene.
FIGURE 2.
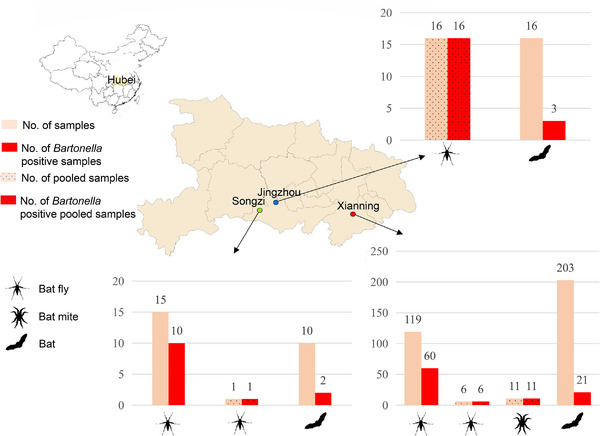
Map showing sampling of bats and their ectoparasites (bat flies and bat mites) and the positive rate of Bartonella
FIGURE 3.
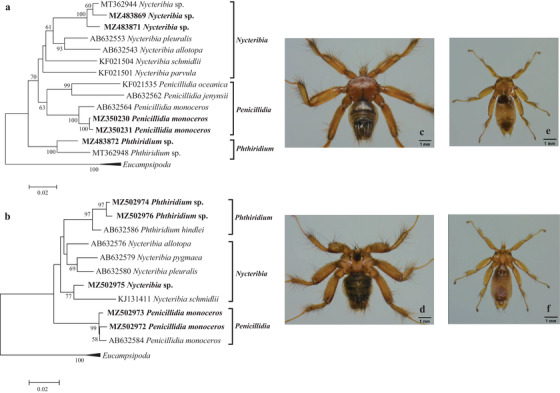
Molecular identification and photos of bat flies. Phylogenetic trees based on the (a) COI and (b) 16S rRNA genes of bat flies, and photos of the dorsal and ventral sides of (c and d) Penicillidia Monoceros and (e and f) Nycteribia sp. under an optical microscope
Due to inability to identify bat flies and bat mites using morphological keys, molecular identification was performed to confirm their species. To meet the requirement of DNA extraction, for ectoparasites collected from Songzi in 2018, and Xianning in 2020, big bat flies were individually processed, while small bat flies and bat mites were randomly pooled for Bartonella analysis.
In May 2018, 15 big bat flies and one small bat fly pool (about five bat flies) were collected from six bats from a karst cave in Songzi City, Hubei Province, China. The six bats were identified as M. davidii (2) and Myotis adversus (4) based on the cytB gene. Based on the COI and 16S rRNA genes, the 15 big bat flies were identified as P. monoceros, and the one pool of small bat flies was identified as Nycteribia sp. (Korea) (Figures 2 and 3, Table 1).
In July 2020, 203 bats were sampled from a karst cave in Xianning City, Hubei Province, China. Based on the cytB gene, the 203 bats were identified as M. davidii (105), M. adversus (29), and Rhinolophus pusillus (69). Bat flies collected from these bats included 119 big bat flies and six small bat fly pools (approximately 20 bat flies per pool). Based on the COI and 16S rRNA genes, the 119 big bat flies were all identified as P. monoceros, and the small bat fly pools consisted of Nycteribia sp. (Korea) and Phthiridium sp. (Figures 2 and 3, Table 1).
Bat mites collected from bats sampled in 2020 were randomly divided into 11 pools (approximately 20 bat mites per pool). Phylogenetic analysis based on the COI and 16S rRNA genes showed that bat mites in this study included two species belonging to the genus Spinturnix and the genus Eyndhovenia in the family Spinturnicidae, respectively. Based on the COI gene, Spinturnix sp. of this study shared 90.1% nucleotide identity with S. myotis (GenBank accession number: FJ225911), and Eyndhovenia sp. of this study shared 90.3% nucleotide identity with Eyndhovenia euryalis (GenBank accession number: EU784906). Based on the 16S rRNA gene, Spinturnix sp. of this study shared 96.7% nucleotide identity with S. myotis (GenBank accession number: FJ225969), and Eyndhovenia sp. of this study shared 94.3% nucleotide identity with E. euryalis (GenBank accession number: EU784846). Therefore, Spinturnix sp. and Eyndhovenia sp. identified in this study represented two novel species (Figures 2 and 4, Table 1).
FIGURE 4.
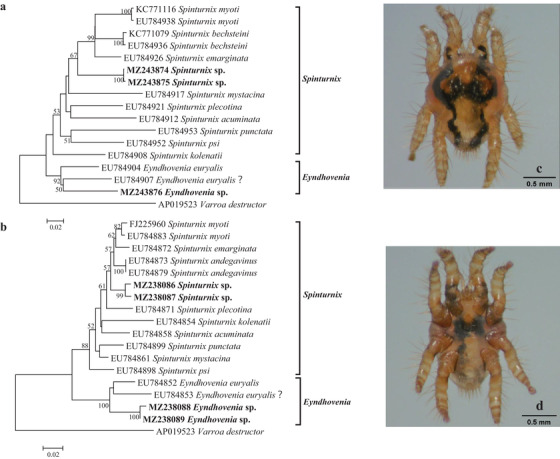
Molecular identification and photos of bat mites. Phylogenetic trees based on the (a) COI and (b) 16S rRNA genes of bat mites (Spinturnicidae), and photos of the dorsal and ventral sides of (c and d) Spinturnix sp. under an optical microscope
3.2. Prevalence of Bartonella in bats, bat flies, and bat mites
Bartonella positive rates for bats collected in 2018, 2019, and 2020 were 20.0% (2/10), 18.8% (3/16), and 10.3% (21/203), respectively. For individual big bat flies (P. monoceros) collected in 2018 and 2020, Bartonella infection rates were 66.7% (10/15) and 50.4% (60/119), respectively. One small bat fly pool of 2018 (Nycteribia sp.), 16 bat fly pools of 2019 (P. monoceros, Nycteribia sp., and Phthiridium sp.), six small bat fly pools (Nycteribia sp. and Phthiridium sp.), and 11 bat mite pools (Spinturnix sp. and Eyndhovenia sp.) of 2020 were all positive for Bartonella (Figure 2, Table 1, and Table S3).
3.3. Molecular characterization of Bartonella in bats, bat flies, and bat mites
For pooled samples (bat flies and bat mites) and bats, all the samples were screened for Bartonella with the three genes (gltA, rpoB, and ftsZ). For individual big bat fly samples, they were firstly screened with the gltA gene. Based on the gltA gene, Bartonella identified in individual big bat flies clustered into only two groups. Therefore, representative samples were further selected for Bartonella characterization by the rpoB and ftsZ genes to reduce workload (Table S3).
Phylogenetic analysis showed that bats, bat flies, and bat mites carried diverse novel genotypes of Bartonella, clustered with previously reported bat/bat fly‐borne bartonellae. Based on the gltA gene, some Bartonella genotypes identified in this study were closely related to those identified in bats from China, as well as in bat flies from South Korea. Based on the rpoB gene, some Bartonella genotypes identified in this study were clustered with those identified in bats from other places of China as well as from other countries such as Georgia and Japan. Based on the ftsZ gene, some Bartonella genotypes identified in this study were clustered with bat‐borne Bartonella from China, Georgia, Japan, and Kenya (Figures 5, 6, 7).
FIGURE 5.
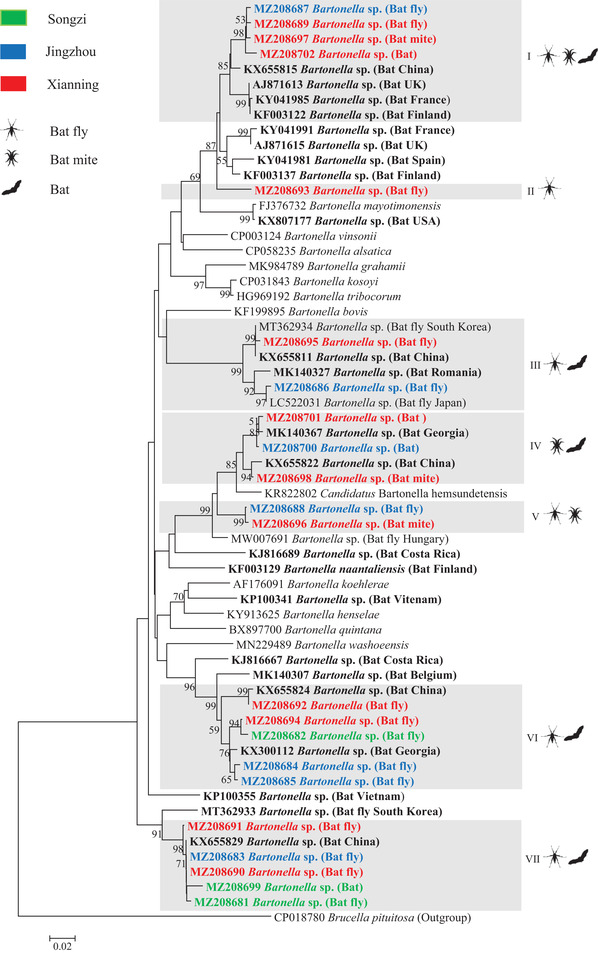
Phylogenetic analysis of bat‐borne Bartonella based on the gltA gene (327 bp). Phylogenetic tree was constructed using the neighbour‐joining method with the Kimura 2‐parameter model in MEGA 7.0. Bootstrap values more than 50% were indicated at nodes. Representative Bartonella sequences with hosts (bat, bat fly, and bat mite) from each sampling area (green: Songzi, Blue: Jingzhou and red: Xianning) were included in the analysis. Genotypes of Bartonella defined in this study are highlighted with grey box. Reference Bartonella sequences downloaded from GenBank were included in the analysis, and bat‐derived Bartonella sequences are shown in bold
FIGURE 6.
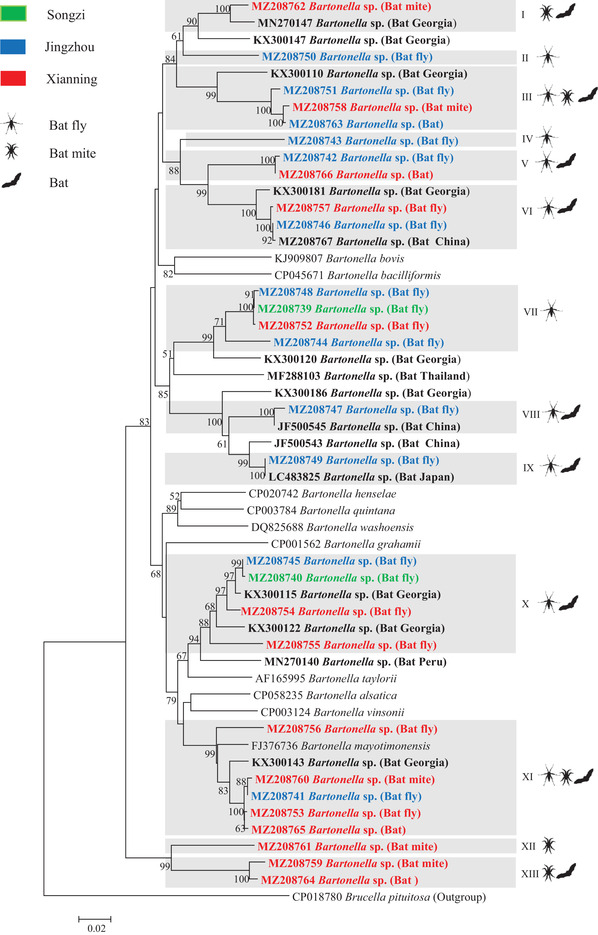
Phylogenetic analysis of bat‐borne Bartonella based on the rpoB gene (825 bp). Phylogenetic tree was constructed using the neighbour‐joining method with the Kimura 2‐parameter model in MEGA 7.0. Bootstrap values more than 50% were indicated at the nodes. Representative Bartonella sequences with hosts (bat, bat fly, and bat mite) from each sampling area (green: Songzi, blue: Jingzhou and red: Xianning) were included in the analysis. Genotypes of Bartonella defined in this study were highlighted with grey box. Reference Bartonella sequences downloaded from GenBank were included in the analysis, and bat‐derived Bartonella sequences are shown in bold
FIGURE 7.
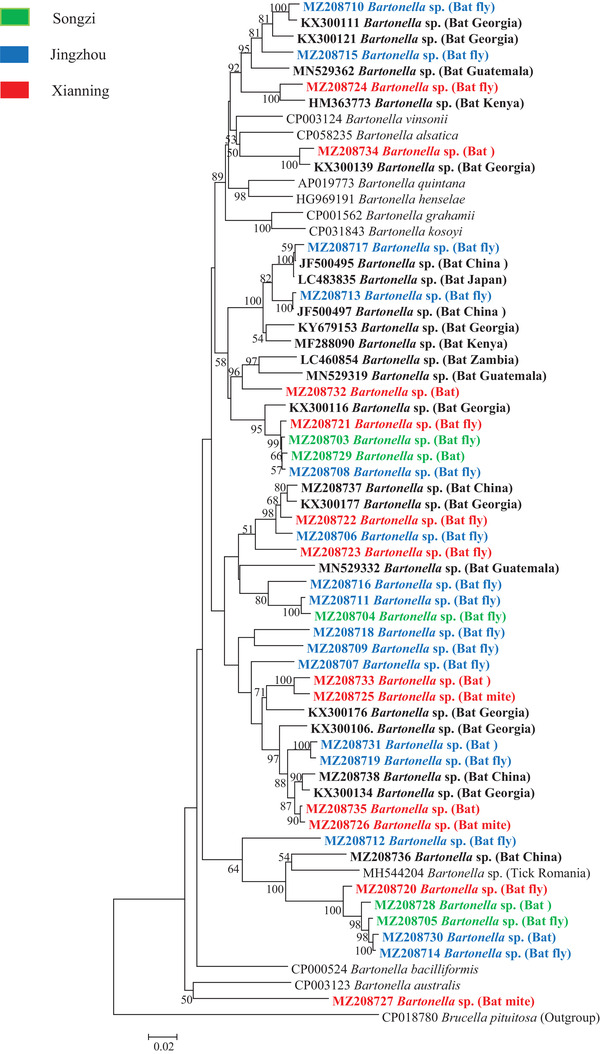
Phylogenetic analysis of bat‐borne Bartonella based on the ftsZ gene (885 bp). Phylogenetic tree was constructed using the neighbour‐joining method with the Kimura 2‐parameter model in MEGA 7.0. Bootstrap values more than 50% were indicated at the nodes. Representative Bartonella sequences with hosts (bat, bat fly, and bat mite) from each sampling area (green: Songzi, Blue: Jingzhou and red: Xianning) were included in the analysis. Reference Bartonella sequences downloaded from GenBank were included in the analysis, and bat‐derived Bartonella sequences are shown in bold
In 2003, La Scola et al. (2003) proposed a gene‐sequence‐based criterion for species identification of Bartonella: Bartonella isolates should be considered as a new species if the gltA fragment (327 bp) and the rpoB fragment (825 bp) share less than 96.0% and 95.4% sequence similarity, respectively, to those of validated species. According to this criterion, bartonellae identified in bats and their ectoparasites (bat flies and bat mites) in this study could be divided into seven and 13 Bartonella genotypes by gltA and rpoB, respectively, among which three Bartonella genotypes (II, V, and VII) defined by gltA, and 7 Bartonella genotypes (II, IV, V, VII, VIII, XII, and XIII) defined by rpoB represented novel genotypes of Bartonella. Based on the gltA gene, three, six, and three Bartonella genotypes were identified in bats, bat flies, and bat mites, respectively, and based on the rpoB gene, there were 4, 10, and 5 genotypes, respectively. Genotypes of Bartonella identified in bats (I, IV, and VIII by gltA; III, V, XI, and XIII by rpoB) were shared by their ectoparasites (bat flies, and bat mites). Some genotypes of Bartonella were identified in bat flies and bat mites, but not in bats in this study (II, III, V, and VI by gltA; I, II, IV, VI, VII, VIII, IX, X, and XII by rpoB) (Figures 5 and 6).
Based on the 825 bp rpoB fragment, Bartonella genotype (XI) identified in this study in both bats and their ectoparasites (bat flies and bat mites) shared 95.1%−95.8% nucleotide identity with B. mayotimonesis (GenBank accession number: FJ376736), the causative agent of human endocarditis. It was close to the cutoff value (95.4%) by the gene‐sequence‐based criteria for Bartonella species definition for rpoB; therefore, Bartonella genotype (XI) could be identified as B. mayotimonesis (Figure 6).
3.4. Statistical analyses
Statistical analyses of Bartonella prevalence by bat species were performed for bats collected from a cave in Xianning in 2020, and the Bartonella prevalence among the three bat species (M. adversus, M. davidii, and R. pusillus) was statistically significant (χ 2 = 8.031, p = .018 < .05). Exactly, significant difference of the Bartonella prevalence was found between M. davidii and R. pusillus (χ 2 = 5.740, p = .017 < .05).
3.5. Nucleotide sequence accession numbers
The representative sequences of this study were deposited in the GenBank database with the accession numbers: MH888178 and MW085077‐MW085079 (bat cytB), MZ350230‐MZ350231, MZ483869, and MZ483871‐MZ483872 (bat fly COI), MZ502972‐MZ502976 (bat fly 16S rRNA), MZ483874‐MZ483876 (bat mite COI), MZ238086‐MZ238089 (bat mite 16S rRNA), MZ208681‐MZ208702 (Bartonella gltA) MZ208739‐MZ208766 (Bartonella rpoB), and MZ208703‐MZ208735 (Bartonella ftsZ).
4. DISCUSSION
Bats, bat flies, and bat mites were collected from Hubei Province in Central China for the detection of bartonellae. The positive rate of Bartonella in bats in this study was 3.9% (8/203; gltA) and 12.8% (26/203; gltA, rpoB, and ftsZ). Our previous study showed that the infection rate of Bartonella in bat blood from Shandong Province in East China was 25.2% (27/107) by gltA amplification (Han et al., 2017), and previous reports of Bartonella prevalence in bats were 83.2% (94/113) in Peru and 45% (36/80) in Belize by gltA amplification (Becker et al., 2018); 33.3% (21/63) in Costa Rica (Judson et al., 2015), 33.1% (39/118) in Guatemala (Bai et al., 2011), 24.1% (27/112) in Peru (Bai et al., 2012), and 30.2% (106/331) in Kenya (Kosoy et al., 2010) by blood culture. The prevalence of Bartonella in bats in this study was much lower than that reported in previous studies, which may be due to the use of liver tissue rather than blood samples, as Bartonella generally parasitize erythrocytes and endothelial cells (Dehio, 2001), blood samples should be the first choice for PCR screening (Kosoy et al., 2018).
4.1. The public health significance of bat‐associated Bartonella
Based on the 825 bp rpoB fragment, the Bartonella genotype (XI) identified in this study in bats, bat flies, and bat mites shared 95.2%−95.7% nucleotide identify with B. mayotimonesis (GenBank accession number: FJ376736), which was close to the cutoff value (95.4%) of the gene sequence‐based criteria for Bartonella species definition proposed by La Scola et al. (2003). While based on the 327 bp gltA fragment, Bartonella genotypes identified in this study (I and II) shared 91.2%−92.6% nucleotide identify with B. mayotimonesis (GenBank accession number: FJ376732), which was well below the cutoff value (96.0% by gltA). The inconsistency of the gltA and rpoB gene might be explained by recombination events as observed in rodent‐borne bartonellae (Paziewska et al., 2011). Several studies reported the discovery of Bartonella species related to B. mayotimonesis in bats collected in the Northern hemisphere (Finland, the United States, the United Kingdom, France, and Spain) (Concannon et al., 2005; Lilley et al., 2017; Stuckey, Boulouis, et al., 2017; Veikkolainen et al., 2014). These bat‐borne bartonellae related to B. mayotimonesis were characterized using a short gltA sequence (∼327 bp). With the exception of Bartonella identified in bats from the United States that shared 99.7% nucleotide identity with B. mayotimonesis (GenBank accession number: FJ376732), Bartonella found in bats from European countries (Finland, the United Kingdom, Spain, and France) shared 91.2%−92.6% nucleotide identity with B. mayotimonesis (Table S4). In terms of phylogenetic relationship, Bartonella identified in bats and their ectoparasites in China were comparable to those Bartonella identified in bats in Europe. With the discovery of Bartonella that were genetically related to B. mayotimonesis in bats and their ectoparasites in China, our knowledge on the geographic distribution of this bacterium was expanded, and it was very likely that this bacterium was circulating in bat populations with bat flies and bat mites as vectors, even reservoirs. In addition, this study identified a number of novel Bartonella genotypes whose pathogenicity and public health significance were still unknown. It will be necessary to isolate these bat‐borne novel bartonellae, evaluate their pathogenicity through animal experiments, and monitor the potential spillover events through the development of detection kits.
4.2. Bat flies and bat mites as potential vectors and reservoirs of Bartonella
As described in previous reports (Judson et al., 2015; Sándor et al., 2018; Szubert‐Kruszyńska et al., 2019), some Bartonella genotypes were shared by bats and their ectoparasites in this study. Bartonella genotypes identified in bats included I, IV, and VII by gltA, and III, V, XI, and XIII by rpoB, and these genotypes were also detected in bat flies and bat mites. Although the sharing of Bartonella among bats and their ectoparasites could be a result of bloodsucking, more and more evidence suggested that bat flies and bat mites were probably not simple carriers of Bartonella. A recent study showed that the ectoparasites intensity of bats was positively correlated with Bartonella infection, indicating the possible role of these ectoparasites as vectors of Bartonella (Stuckey, Chomel, et al., 2017). In addition, previous studies reported the isolation of Bartonella from bat flies (Billeter et al., 2012; Nabeshima et al., 2020), confirming the viability of Bartonella in bat flies, and highlighting the possibility of bat flies as vectors of Bartonella.
The genetic diversity of bartonellae identified in bat ectoparasites was higher than that in bats in this study, which was also reported in a previous study (Judson et al., 2015). Bat fly/bat mite‐unique Bartonella genotypes of this study clustered with bartonellae found in bats in previous studies, it was unlikely that they were symbiotic bacteria of bat flies and bat mites. The absence of some Bartonella genotypes in bats might be partially explained by the low detection rate of Bartonella in bats in this study due to the use of liver tissue rather than blood samples for Bartonella detection, or due to the immune response of bats to clear certain Bartonella species. Moreover, bat flies have evolved a unique reproductive strategy, viviparous pupation: The larval stage develops within a female bat fly, and when internal development is complete, a larva is laid, and it immediately forms a puparium, from which an unfed adult fly emerges after a pupal stage (Figure S1) (Dick & Dittmar, 2014). During the internal developmental stages, the larva feeds on the ‘milk’ glands, which may facilitate vertical transmission of some bacteria, including Bartonella (Szentiványi et al., 2019). A previous study reported the identification of Bartonella in female bat flies and their pupae, indicating vertical transmission of Bartonella across developmental stages (Morse et al., 2012), and suggesting the potential roles of bat flies as reservoirs of bartonellae.
Studies on the role of bat mites in the maintenance and transmission of bartonellae were scarce, and a study in Poland reported the presence of identical Bartonella genotypes in bats and their mites (Szubert‐Kruszyńska et al., 2019). The role of bat flies and bat mites for Bartonella will be better elucidated with epidemiological investigation of Bartonella across all life stages of these ectoparasites and laboratory infection experiments.
Although almost all Bartonella genotypes identified in bats in this study were found in bat flies and bat mites, there were still some Bartonella genotypes unique to bats (Figure 7). The role of other ectoparasites such as hard ticks, soft ticks, fleas, and bugs in the transmission of Bartonella in the bat population should be investigated. Interestingly, although bartonellae are generally considered as vector‐borne bacteria, they were also identified in the saliva and faeces of bats, indicating that biting and faecal exposure might also contribute to the transmission and maintenance of bartonellae among bat population (Becker et al., 2018; Veikkolainen et al., 2014). Future studies on the infection dynamics of bartonellae will shed light on how these bacteria circulated among bats.
4.3. Novel bat fly and bat mite species
Bat flies are obligate bloodsucking ectoparasites that commonly parasitize in the fur and wing membranes of bats. Bat flies are divided into two families: the wingless, spider‐like Nycteribiidae, and the more traditionally fly‐like Streblidae (Dick & Patterson, 2006). Currently, the family Nycteribiidae consists of 275 species in 21 genera, and the family Streblidae consists of 227 species in 31 genera. The family Nycteribiidae has a worldwide distribution, while the family Streblidae is mainly found in Western Hemisphere (Szentiványi et al., 2019). Based on the COI and 16S rRNA genes, three bat fly species belonging to three genera (Nycteribia, Penicillidia, and Phthiridium) in the family Nycteribiidae were identified in this study, with Phthiridium sp. as a potentially novel bat fly species.
Mites of the family Spinturnicidae are highly specialized bat ectoparasites. The taxonomy of Spinturnicidae was mainly based on morphology, and molecular identification of this family was explored by Bruyndonckx et al. (2009), making species identification of this family possible even for non‐experts in morphology. The family Spinturnicidae includes 12 genera. The genus Spinturnix is the most diverse of this family and includes more than 50 named species, most of which are associated with the Old World bats (Orlova et al., 2020). Currently, only two species are recognized in the genus Eyndhovenia, E. euryalis, and E. brachypus, with the latter only morphologically described from Rhinolophus rouxi bats in China (Luong et al., 2021, Yu‐Mei Sun, 1986). Eight bat mite species of three genera (Spinturnix, Eyndhovenia, and Paraperiglischrus) in the family Spinturnicidae were morphologically described in China, with four species in the genus Spinturnix (Spinturnix psi, S. myoti, S. sinicus, and S. kolenatoides) (Rui‐Yu Ye, 1996; Tian, 2009; Yu‐Mei Sun, 1986). Bat mites of this study were molecularly identified as two species in the family Spinturnicidae, with one species belonging to the genus Spinturnix, and the other to the genus Eyndhovenia. Whether the Spinturnix sp. identified in this study is S. sinicus or S. kolenatoides, or just another novel species, and whether the Eyndhovenia sp. identified in this study is the E. brachypus or another novel species need further study. The combination of morphological characteristics and molecular data will get these bat mites better defined in the future.
Due to the lack of knowledge on the morphology of bat flies and bat mites at the beginning of the study, they were not pooled by species for Bartonella analysis, which left a gap in understanding the host‐vector specificity of bartonellae. However, differences in the Bartonella genotypes carried by different bat fly species were observed in this study. Bartonella genotypes (I and VII by gltA; VII and XI by rpoB) were identified in the big bat fly (P. monoceros), while for the small bat fly pools (Nycteribia sp., and Phthiridium sp.), Bartonella genotypes (II, III, VI, and VII by gltA; X and VI by rpoB) were identified (Table S3). Further studies will be needed to reveal the roles of each bat fly or mite species in the transmission and maintenance of Bartonella.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure S1 Bat fly (Penicillidia monoceros) emerging from the pupa
Table S1 Summary of current knowledge on Bartonella in bats and their ectoparasites worldwide
Table S2 Primers used in this study
Table S3 Detailed information on Bartonella‐positive samples of this study
Table S4 Estimates of evolutionary divergence between Bartonella mayotimonensis and closely related bat‐borne Bartonella species based on the gltA gene (327 bp)
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Funds of China (grant number: 81971939), the China Postdoctoral Science Foundation Funded Project (grant number: 2019M662720), and the Innovation Research of Hubei Postdoctoral Science and Technology.
Han, H.‐J. , Li, Z.‐M. , Li, X.. , Liu, J.‐X. , Peng, Q.‐M. , Wang, R. , Gu, X.‐L. , Jiang, Y. , Zhou, C.‐M. , Li, D. , Xiao, X. , & Yu, X.‐J. (2022). Bats and their ectoparasites (Nycteribiidae and Spinturnicidae) carry diverse novel Bartonella genotypes, China. Transboundary and Emerging Diseases, 69, e845–e858. 10.1111/tbed.14357
Contributor Information
Xiao Xiao, Email: xiaoalltheway@gmail.com.
Xue‐Jie Yu, Email: yuxuejie@whu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/
REFERENCES
- Angelakis, E. , & Raoult, D. (2014). Pathogenicity and treatment of Bartonella infections. International Journal of Antimicrobial Agents, 44, 16–25. 10.1016/j.ijantimicag.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Bai, Y. , Hayman, D. T. S. , Mckee, C. D. , & Kosoy, M. Y. (2015). Classification of Bartonella strains associated with straw‐colored fruit bats (Eidolon helvum) across Africa using a multi‐locus sequence typing platform. PLoS Neglected Tropical Diseases, 9, e0003478. 10.1371/journal.pntd.0003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Kosoy, M. , Recuenco, S. , Alvarez, D. , Moran, D. , Turmelle, A. , Ellison, J. , Garcia, D. L. , Estevez, A. , Lindblade, K. , & Rupprecht, C. (2011). Bartonella spp. in bats, guatemala. Emerging Infectious Diseases, 17, 1269–1272. 10.3201/eid1707.101867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Osinubi, M. O. V. , Osikowicz, L. , Mckee, C. , Vora, N. M. , Rizzo, M. R. , Recuenco, S. , Davis, L. , Niezgoda, M. , Ehimiyein, A. M. , Kia, G. S. N. , Oyemakinde, A. , Adeniyi, O. S. , Gbadegesin, Y. H. , Saliman, O. A. , Ogunniyi, A. , Ogunkoya, A. B. , & Kosoy, M. Y. (2018). Human exposure to novel Bartonella Species from contact with fruit bats. Emerging Infectious Diseases, 24, 2317–2323. 10.3201/eid2412.181204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Recuenco, S. , Gilbert, A. T. , Osikowicz, L. M. , Gómez, J. , Rupprecht, C. , & Kosoy, M. Y. (2012). Prevalence and diversity of Bartonella spp. in bats in Peru. American Journal of Tropical Medicine and Hygiene, 87, 518–523. 10.4269/ajtmh.2012.12-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, D. J. , Bergner, L. M. , Bentz, A. B. , Orton, R. J. , Altizer, S. , & Streicker, D. G. (2018). Genetic diversity, infection prevalence, and possible transmission routes of Bartonella spp. in vampire bats. PLoS Neglected Tropical Diseases, 12, e0006786. 10.1371/journal.pntd.0006786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter, S. A. , Hayman, D. T. S. , Peel, A. J. , Baker, K. , Wood, J. L. N. , Cunningham, A. , Suu‐Ire, R. , Dittmar, K. , & Kosoy, M. Y. (2012). Bartonella species in bat flies (Diptera: Nycteribiidae) from western Africa. Parasitology, 139, 324–329. 10.1017/S0031182011002113 [DOI] [PubMed] [Google Scholar]
- Birtles, R. J. (2005). Bartonellae as elegant hemotropic parasites. Annals of the New York Academy of Sciences, 1063, 270–279. 10.1196/annals.1355.044 [DOI] [PubMed] [Google Scholar]
- Brook, C. E. , Bai, Y. , Dobson, A. P. , Osikowicz, L. M. , Ranaivoson, H. C. , Zhu, Q. , Kosoy, M. Y. , & Dittmar, K. (2015). Bartonella spp. in fruit bats and blood‐feeding Ectoparasites in Madagascar. Plos Neglected Tropical Diseases, 9, e0003532. 10.1371/journal.pntd.0003532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyndonckx, N. , Dubey, S. , Ruedi, M. , & Christe, P. (2009). Molecular cophylogenetic relationships between European bats and their ectoparasitic mites (Acari, Spinturnicidae). Molecular Phylogenetics and Evolution, 51, 227–237. 10.1016/j.ympev.2009.02.005 [DOI] [PubMed] [Google Scholar]
- Castro, L. R. , Austin, A. D. , & Dowton, M. (2002). Contrasting rates of mitochondrial molecular evolution in parasitic diptera and hymenoptera. Molecular Biology and Evolution, 19, 1100–1113. 10.1093/oxfordjournals.molbev.a004168 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Liu, B. , Yang, J. , & Jin, Q.i (2014). DBatVir: The database of bat‐associated viruses. Database: The Journal of Biological Databases and Curation, 2014, bau021. 10.1093/database/bau021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon, R. , Wynn‐Owen, K. , Simpson, V. R. , & Birtles, R. J. (2005). Molecular characterization of haemoparasites infecting bats (Microchiroptera) in Cornwall, UK. Parasitology, 131, 489–496. 10.1017/S0031182005008097 [DOI] [PubMed] [Google Scholar]
- Dehio, C. (2001). Bartonella interactions with endothelial cells and erythrocytes. Trends in Microbiology, 9, 279–285. 10.1016/S0966-842X(01)02047-9 [DOI] [PubMed] [Google Scholar]
- Dick, C. W. , & Dittmar, K. (2014). Parasitic bat flies (Diptera: Streblidae and Nycteribiidae): Host specificity and potential as vectors. In Klimpel S. & Mehlhorn H. (Eds.), Bats (Chiroptera) as vectors of diseases and parasites: Facts and myths (pp. 131–155). Springer Berlin Heidelberg. [Google Scholar]
- Dick, C. W. , & Patterson, B. D. (2006). Bat flies: Obligate ectoparasites of bats. In Morand S., Krasnov B. R., & Poulin R. (Eds.), Micromammals and macroparasites: From evolutionary ecology to management (pp. 179–194). Springer Japan. [Google Scholar]
- Dietrich, M. , Tjale, M. A. , Weyer, J. , Kearney, T. , Seamark, E. C. J. , Nel, L. H. , Monadjem, A. , & Markotter, W. (2016). Diversity of Bartonella and Rickettsia spp. in bats and their blood‐feeding ectoparasites from South Africa and Swaziland. Plos One, 11, e0152077. 10.1371/journal.pone.0152077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard, S. , Nabet, C. , Lepidi, H. , Fournier, P.‐E. , & Raoult, D. (2015). Bartonella, a common cause of endocarditis: A report on 106 cases and review. Journal of Clinical Microbiology, 53, 824–829. 10.1128/JCM.02827-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher, S. C. , & Dehio, C. (2012). Bartonella entry mechanisms into mammalian host cells. Cellular Microbiology, 14, 1166–1173. 10.1111/j.1462-5822.2012.01806.x [DOI] [PubMed] [Google Scholar]
- Folmer, O. , Black, M. , Hoeh, W. , Lutz, R. , & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299. [PubMed] [Google Scholar]
- Gonçalves‐Oliveira, J. , Rozental, T. , Guterres, A. , Teixeira, B. R. , Andrade‐Silva, B. E. , da Costa‐Neto, S. F. , Furtado, M. C. , Moratelli, R. , D'andrea, P. S. , & Lemos, E. R. S. (2020). Investigation of Bartonella spp. in Brazilian mammals with emphasis on rodents and bats from the Atlantic Forest. International Journal for Parasitology: Parasites and Wildlife, 13, 80–89. 10.1016/j.ijppaw.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H.‐J. , Wen, H.‐L. , Zhao, L. , Liu, J.‐W. , Luo, L.‐M. , Zhou, C.‐M. , Qin, X.‐R. , Zhu, Y.‐L. , Zheng, X.‐X. , & Yu, X.‐J. (2017). Novel Bartonella species in insectivorous bats, northern China. Plos One, 12, e0167915. 10.1371/journal.pone.0167915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H.‐J. , Wen, H.‐L. , Zhou, C.‐M. , Chen, F.‐F. , Luo, L.i‐M. , Liu, J.‐W. , & Yu, X.‐J. (2015). Bats as reservoirs of severe emerging infectious diseases. Virus Research, 205, 1–6. 10.1016/j.virusres.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok, S. , Kovács, R. , Meli, M. L. , Gönczi, E. , Hofmann‐Lehmann, R. , Kontschán, J. , Gyuranecz, M. , Dán, Á. , & Molnár, V. (2012). First detection of Bartonellae in a broad range of bat ectoparasites. Veterinary Microbiology, 159, 541–543. 10.1016/j.vetmic.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Ikeda, P. , Marinho Torres, J. , Perles, L. , Lourenço, E. C. , Herrera, H. M. , De Oliveira, C. E. , Zacarias Machado, R. , & André, M. R. (2020). Intra‐ and inter‐host assessment of Bartonella diversity with focus on non‐hematophagous bats and associated ectoparasites from Brazil. Microorganisms, 8, 1822. 10.3390/microorganisms8111822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacomo, V. , Kelly, P. J. , & Raoult, D. (2002). Natural history of Bartonella infections (an exception to Koch's postulate). Clinical and Diagnostic Laboratory Immunology, 9, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson, S. D. , Frank, H. K. , & Hadly, E. A. (2015). Bartonellae are prevalent and diverse in costa rican bats and bat Flies. Zoonoses and Public Health, 62, 609–617. 10.1111/zph.12188 [DOI] [PubMed] [Google Scholar]
- Kamani, J. , Baneth, G. , Mitchell, M. , Mumcuoglu, K. Y. , Gutiérrez, R. , & Harrus, S. (2014). Bartonella species in bats (Chiroptera) and bat flies (Nycteribiidae) from Nigeria, West Africa. Vector Borne and Zoonotic Diseases, 14, 625–632. 10.1089/vbz.2013.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy, M. , Bai, Y. , Lynch, T. , Kuzmin, I. V. , Niezgoda, M. , Franka, R. , Agwanda, B. , Breiman, R. F. , & Rupprecht, C. E. (2010). Bartonella spp. in bats, Kenya. Emerging Infectious Diseases, 16, 1875–1881. 10.3201/eid1612.100601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy, M. , Mckee, C. , Albayrak, L. , & Fofanov, Y. (2018). Genotyping of Bartonella bacteria and their animal hosts: Current status and perspectives. Parasitology, 145, 543–562. 10.1017/S0031182017001263 [DOI] [PubMed] [Google Scholar]
- La Scola, B. , Zeaiter, Z. , Khamis, A. , & Raoult, D. (2003). Gene‐sequence‐based criteria for species definition in bacteriology: The Bartonella paradigm. Trends in Microbiology, 11, 318–321. 10.1016/S0966-842X(03)00143-4 [DOI] [PubMed] [Google Scholar]
- Li, Z.‐M. , Xiao, X. , Zhou, C.‐M. , Liu, J.‐X. , Gu, X.‐L. , Fang, L.‐Z. , Liu, B.‐Y. , Wang, L.‐R. , Yu, X.‐J. , & Han, H.‐J. (2021). Human‐pathogenic relapsing fever Borrelia found in bats from Central China phylogenetically clustered together with relapsing fever borreliae reported in the New World. PLoS Neglected Tropical Diseases, 15, e0009113. 10.1371/journal.pntd.0009113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley, T. M. , Wilson, C. A. , Bernard, R. F. , Willcox, E. V. , Vesterinen, E. J. , Webber, Q. M. R. , Kurpiers, L. , Prokkola, J. M. , Ejotre, I. , Kurta, A. , Field, K. A. , Reeder, D. M. , & Pulliainen, A. T. (2017). Molecular detection of Candidatus Bartonella mayotimonensis in North American bats. Vector Borne and Zoonotic Diseases, 17, 243–246. 10.1089/vbz.2016.2080 [DOI] [PubMed] [Google Scholar]
- Lin, J.‐W. , Hsu, Y.‐M. , Chomel, B. B. , Lin, L.‐K. , Pei, J.‐C. , Wu, S.‐H. , & Chang, C.‐C. (2012). Identification of novel Bartonella spp. in bats and evidence of Asian gray shrew as a new potential reservoir of Bartonella . Veterinary Microbiology, 156, 119–126. 10.1016/j.vetmic.2011.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, E. Y. , Tsigrelis, C. , Baddour, L. M. , Lepidi, H. , Rolain, J.‐M. , Patel, R. , & Raoult, D. (2010). Candidatus Bartonella mayotimonensis and endocarditis. Emerging Infectious Diseases, 16, 500–503. 10.3201/eid1603.081673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong, N. T. , Orlova, M. V. , Manh, V.u Q. , Loan, H.o T. , & Thong, V. D. (2021). First record of eyndhovenia (Mesostigmata: Gamasina: Spinturnicidae) from Vietnam. Parasitology International, 82, 102301. 10.1016/j.parint.2021.102301 [DOI] [PubMed] [Google Scholar]
- Mckee, C. D. , Bai, Y. , Webb, C. T. , & Kosoy, M. Y. (2021). Bats are key hosts in the radiation of mammal‐associated Bartonella bacteria. Infection, Genetics and Evolution, 89, 104719. 10.1016/j.meegid.2021.104719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, S. F. , Olival, K. J. , Kosoy, M. , Billeter, S. , Patterson, B. D. , Dick, C. W. , & Dittmar, K. (2012). Global distribution and genetic diversity of Bartonella in bat flies (Hippoboscoidea, Streblidae, Nycteribiidae). Infection, Genetics and Evolution, 12, 1717–1723. 10.1016/j.meegid.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Mühldorfer, K. (2013). Bats and bacterial pathogens: A review. Zoonoses and Public Health, 60, 93–103. 10.1111/j.1863-2378.2012.01536.x [DOI] [PubMed] [Google Scholar]
- Nabeshima, K. , Sato, S. , Kabeya, H. , Komine, N. , Nanashima, R. , Takano, A. , Shimoda, H. , Maeda, K. , Suzuki, K. , & Maruyama, S. (2020). Detection and phylogenetic analysis of Bartonella species from bat flies on eastern bent‐wing bats (Miniopterus fuliginosus) in Japan. Comparative Immunology, Microbiology and Infectious Diseases, 73, 101570. 10.1016/j.cimid.2020.101570 [DOI] [PubMed] [Google Scholar]
- Okaro, U. , Addisu, A. , Casanas, B. , & Anderson, B. (2017). Bartonella Species, an emerging cause of blood‐culture‐negative endocarditis. Clinical Microbiology Reviews, 30, 709–746. 10.1128/CMR.00013-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova, M. V. , Laverty, T. M. , Reeves, W. K. , Gratton, E. M. , & Davies, M. L. (2020). The first record of the spinturnicid mite Spinturnix kolenatii Oudemans, 1910 (Mesostigmata: Gamasina: Spinturnicidae) from the long‐tailed serotine bat Eptesicus hottentotus A. Smith, 1833 (Chiroptera: Vespertilionidae) in Africa. International Journal of Acarology, 46, 160–164. 10.1080/01647954.2020.1731596 [DOI] [Google Scholar]
- Paziewska, A. , Harris, P. D. , Zwolińska, L. , Bajer, A. , & Siński, E. (2011). Recombination within and between species of the alpha proteobacterium Bartonella infecting rodents. Microbial Ecology, 61, 134–145. 10.1007/s00248-010-9735-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y. , Kajihara, M. , Nakao, R. , Mulenga, E. , Harima, H. , Hang'ombe, B. M. , Eto, Y. , Changula, K. , Mwizabi, D. , Sawa, H. , Higashi, H. , Mweene, A. , Takada, A. , Simuunza, M. , & Sugimoto, C. (2020). Isolation of Candidatus Bartonella rousetti and Other Bat‐associated Bartonellae from Bats and Their Flies in Zambia. Pathogens, 9, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves, W. K. , Beck, J. , Orlova, M. V. , Daly, J. L. , Pippin, K. , Revan, F. , & Loftis, A. D. (2016). Ecology of bats, their ectoparasites, and associated pathogens on Saint Kitts Island. Journal of Medical Entomology, 53, 1218–1225. 10.1093/jme/tjw078 [DOI] [PubMed] [Google Scholar]
- Rolain, J. M. , Brouqui, P. , Koehler, J. E. , Maguina, C. , Dolan, M. J. , & Raoult, D. (2004). Recommendations for treatment of human infections caused by Bartonella species. Antimicrobial Agents and Chemotherapy, 48, 1921–1933. 10.1128/AAC.48.6.1921-1933.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui‐Yu Ye, L.‐M. M. (1996). A new species of Spinturnix and a new record of Steatonyssus from China (Acari: Spinturnicidae, Macronyssidae). Acta Zootaxonomica Sinica, 21, 421–424. [Google Scholar]
- Sándor, A. D. , Földvári, M. , Krawczyk, A. I. , Sprong, H. , Corduneanu, A. , Barti, L. , Görföl, T. , Estók, P. , Kováts, D. , Szekeres, S. , László, Z. , Hornok, S. , & Földvári, G. (2018). Eco‐epidemiology of novel Bartonella genotypes from parasitic flies of insectivorous bats. Microbial Ecology, 76, 1076–1088. 10.1007/s00248-018-1195-z [DOI] [PubMed] [Google Scholar]
- Stuckey, M. J. , Boulouis, H.‐J. , Cliquet, F. , Picard‐Meyer, E. , Servat, A. , Aréchiga‐Ceballos, N. , Echevarría, J. E. , & Chomel, B. B. (2017). Potentially zoonotic Bartonella in bats from France and Spain. Emerging Infectious Diseases, 23, 539–541. 10.3201/eid2303.160934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey, M. J. , Chomel, B. B. , Galvez‐Romero, G. , Olave‐Leyva, J. I. , Obregón‐Morales, C. , Moreno‐Sandoval, H. , Aréchiga‐Ceballos, N. , Salas‐Rojas, M. , & Aguilar‐Setién, A. (2017). Bartonella infection in hematophagous, insectivorous, and phytophagous bat populations of Central Mexico and the Yucatan Peninsula. American Journal of Tropical Medicine and Hygiene, 97, 413–422. 10.4269/ajtmh.16-0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentiványi, T. , Christe, P. , & Glaizot, O. (2019). Bat Flies and their microparasites: Current knowledge and distribution. Frontiers in Veterinary Science, 6, 115. 10.3389/fvets.2019.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szubert‐Kruszyńska, A. , Stańczak, J. , Cieniuch, S. , Podsiadły, E. , Postawa, T. , & Michalik, J. (2019). Bartonella and Rickettsia infections in haematophagous Spinturnix myoti mites (Acari: Mesostigmata) and their bat host, Myotis myotis (Yangochiroptera: Vespertilionidae), from Poland. Microbial Ecology, 77, 759–768. 10.1007/s00248-018-1246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Z. (2009). Taxonomic Studies on Ectoparasites Gamasid of Bats in China (Acari: Mesostigmata, Gamasina: ) Guizhou University. [Google Scholar]
- Urushadze, L. , Bai, Y. , Osikowicz, L. , Mckee, C. , Sidamonidze, K. , Putkaradze, D. , Imnadze, P. , Kandaurov, A. , Kuzmin, I. , & Kosoy, M. (2017). Prevalence, diversity, and host associations of Bartonella strains in bats from Georgia (Caucasus). PLoS Neglected Tropical Diseases, 11, e0005428. 10.1371/journal.pntd.0005428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veikkolainen, V. , Vesterinen, E. J. , Lilley, T. M. , & Pulliainen, A. T. (2014). Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis . Emerging Infectious Diseases, 20, 960–967. 10.3201/eid2006.130956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu‐Mei Sun, L.‐L. W. , & Wang, D.‐Q. (1986). A new species of Eyndhovenia from Fujian (Mesostigmata: Spinturnicidae). Acta Zootaxonomica Sinica, 11, 194–197. [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H.‐R. , Zhu, Y. , Li, B. , Huang, C.‐L. , Chen, H.‐D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R.‐D.i , Liu, M.‐Q. , Chen, Y. , Shen, X.u‐R. , Wang, X. … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579, 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Bat fly (Penicillidia monoceros) emerging from the pupa
Table S1 Summary of current knowledge on Bartonella in bats and their ectoparasites worldwide
Table S2 Primers used in this study
Table S3 Detailed information on Bartonella‐positive samples of this study
Table S4 Estimates of evolutionary divergence between Bartonella mayotimonensis and closely related bat‐borne Bartonella species based on the gltA gene (327 bp)
Data Availability Statement
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/


