Abstract
Purpose of review
The primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD) relies on optimizing cardiovascular health and appropriate pharmacotherapy, a mainstay of which is low-density lipoprotein-cholesterol (LDL-C) lowering. Typically, statin therapy remains the first line approach. Advances in technology and understanding of lipid metabolism have facilitated the development of several novel therapeutic targets and medications within the last decade. This review focuses on medications recently approved by the U.S. Food and Drug Administration (FDA) for the reduction of LDL-C and ASCVD risk, as well as new therapies in the pipeline.
Recent findings
Novel lipid therapies aim to lower risk of ASCVD by targeting reduction of atherogenic compounds, such as LDL, lipoprotein(a) (Lp(a)), and triglyceride-rich lipoproteins. Evolocumab and alirocumab, monoclonal antibody proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors which lower LDL-C by approximately 60%, have emerged as important therapies for use in patients with ASCVD as well as familial hypercholesterolemia (FH). Bempedoic acid, an ATP citrate lyase inhibitor, is an oral medication recently approved that can lower LDL-C by approximately 18% alone and 38% when combined with ezetimibe. Inclisiran, a small-interfering RNA (siRNA) molecule which inhibits the translation of PCSK9, is the most recently FDA-approved LDL-C lowering medication, and can reduce LDL-C by approximately 50% with twice yearly subcutaneous dosing. The cardiovascular outcome trials for bempedoic acid and inclisiran are still on-going. Evinacumab, a monoclonal antibody which targets angiopoietin-like protein 3 (ANGPTL3), has been approved for use in patients with homozygous FH. SiRNAs and anti-sense oligonucleotides (ASO) facilitating selective inhibition of the production of targeted proteins including Lp(a) and ANGLPTL3 are active areas of clinical investigation.
Summary
Recently several novel LDL-C lowering medications have been approved. New therapeutic targets have been identified and present additional means of lowering LDL-C and other atherogenic compounds for patients who remain at high ASCVD risk.
Keywords: Low density lipoprotein cholesterol, lipoprotein(a), apolipoprotein B, triglycerides, lipids, atherosclerotic cardiovascular disease, prevention
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of death globally.(1) ASCVD is a highly prevalent condition which is a major cause of morbidity and mortality worldwide. The role of low density lipoprotein cholesterol (LDL-C) in the development of atherosclerosis is one of the most intensively studied issues in medicine and its causal role is well established.(2–5) The importance of cumulative exposure to elevated LDL-C levels (i.e. “cholesterol-years”) in the development of ASCVD is increasingly recognized.(6–8) Sustained exposure to elevated LDL-C levels in late adolescence and early adulthood is associated with elevated risk even if levels are lowered later in life.(2, 9, 10) These findings might help to explain the failure to eliminate later risk which has been accumulated during early exposures. It also suggests that early interventions to target LDL-C reduction may be more effective than later ones.(6)
Across all guidelines, statin therapy remains first line approach for LDL-C reduction for the prevention of ASCVD.(3, 4, 11) Typically ezetimibe is the most commonly used add-on oral agent to statins for patients who need additional LDL-C lowering, with bile acid sequestrants now less commonly used due to side effect profile.(3) Nevertheless, despite the effectiveness of these different agents to lower LDL-C and reduce ASCVD risk, recurrent vascular events still occur in statin-treated patients, which is termed “residual risk”.(12, 13) Some of this residual risk is due to other sub-optimally controlled risk factors such as elevated blood pressure, insulin resistance, smoking, and inflammatory risk. However, some of the residual risk is due to persistent elevation of atherogenic lipid particles like LDL and the triglyceride (TG)-rich lipoproteins (TRLs).(12, 13)
Many high-risk patients retain levels of LDL-C at unacceptably high levels despite treatment with maximally tolerated doses of statins and other established LDL-lowering agents, like ezetimibe.(14) It is also important to note that patients with relatively low LDL-C continue to have events and that statin therapy may not be enough to achieve lower LDL-C targets now being targeted.(15) The presence of residual risk and difficulties achieving sufficiently low LDL-C levels has prompted the research and development of novel agents to further reduce risk of primary and secondary ASCVD.
Trials of LDL-C lowering therapy have consistently demonstrated better outcomes in the group that has received more aggressive LDL-C lowering. Many trials have achieved LDL-C levels below 70 mg/dL (1.8 mmol/L), which conferred additional risk reduction and confirmed safety.(16) This has been true for high-dose statins in the Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER trial), ezetimibe therapy on-top of statins in the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT),(15) and for the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors in the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER)(17) and Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY) Outcomes(18) trials.
Most analyses have suggested a continuous log-linear benefit with LDL-C lowering.(19, 20) In a landmark meta-analysis of 26 trials involving nearly 170,000 patients of statin therapy in a broad range of patients and baseline LDL-C levels, there was an approximate 22% reduction in the rate of major vascular events per 1 mmol/L (39 mg/dL) decrease in LDL-C without any evidence of therapeutic threshold within the LDL-C range studied.(21) Additionally initial safety concerns surrounding cognitive decline or risk of hemorrhagic stroke in targeting very low LDL-C have not been borne out in the large randomized controlled trials to date.(16, 22, 23)
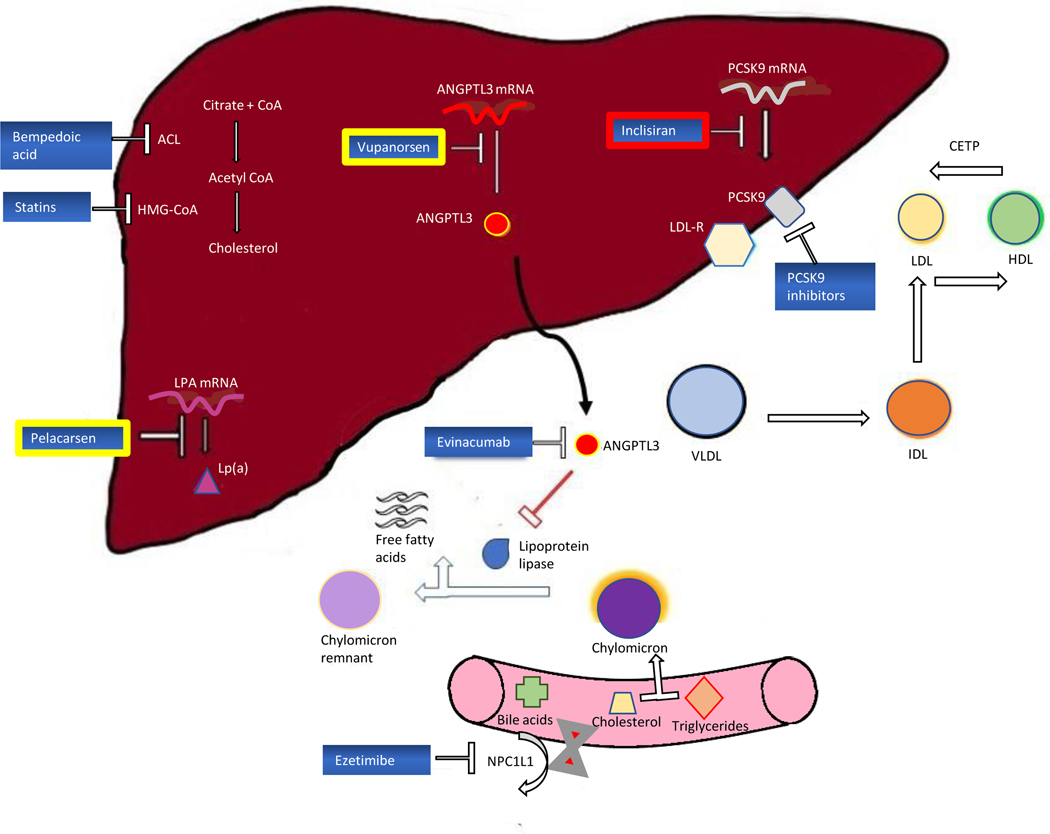
This review focuses medications recently approved by the U.S. Food and Drug Administration (FDA) for the further reduction of LDL-C and ASCVD risk, as well as new therapies in the pipeline. We will discuss their mechanisms of action (Figure 1), their clinical benefit, and FDA approval indications (Table 1).
Figure 1:
Targets of action for current and emerging LDL-C lowering therapeutics
Table 1:
Summary of recently FDA approved LDL-C lowering therapeutics
| Agent | Mechanism of Delivery | Percentage LDL reduction | Side effects | FDA Approved status |
|---|---|---|---|---|
| Alirocumab | 75–150mg Q2 weekly or 300mg subcutaneously Q4 weekly | 50–60% | Injection-site reactions, hypersensitivity reactions | HeFH, ASCVD, HoFH |
| Evolocumab | 140mg Q2 weeks or 420mg subcutaneously Q2-Q4 weekly | |||
| Inclisiran | 284mg subcutaneously single injection, again at 3 months, Q6 monthly thereafter | 50% | Antibody development, injection-site reactions | HeFH, ASCVD |
| Bempedoic acid | 180mg PO daily | 17–25% | Hyperuricemia/Gout, possibly tendon rupture | HeFH, ASCVD |
| Bempedoic acid/ezetimibe | 180mg PO daily/10mg PO daily | 36% | Hyperuricemia/Gout, possibly tendon rupture | HeFH, ASCVD |
| Evinacumab | 15mg/kg IV Q4 weekly | 50% | Injection site reactions, hypersensitivity reactions, nasopharyngitis | HoFH |
Abbreviations: FDA, Food and Drug Administration; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; ASCVD, atherosclerotic cardiovascular disease
PCSK9-inhibitor monoclonal antibodies (evolocumab and alirocumab)
The PCSK9 protein is an important regulator of circulating LDL‐C levels, through its inhibitory action on recycling of the LDL receptor. The LDL receptor which is expressed on the liver cell surface binds to LDL-C and the LDL receptor–LDL complex is internalized, and LDL-C degraded after which the LDL receptor is recycled back to the cell surface. Secreted PCSK9 binds to the LDL receptor on the surface of the hepatocyte, leading to the internalization and degradation of the LDL receptor in the lysosomes, and reducing the number of available LDL receptors on the cell surface.(24) When PCSK9 activity is suppressed, hepatocytes recycle and express a larger proportion of LDL receptors surface receptors which more efficiently clear circulating LDL-C from the plasma. Initial genetic studies showed that gain-of-function mutations in PCSK9 were associated with high LDL-C levels and onset of premature coronary heart disease (CHD), whereas loss-of-function nonsense mutations were associated with low LDL-C level and reduced CHD thus identifying PCSK9 as a potential therapeutic target.(25)
Evolocumab and alirocumab are monoclonal antibody inhibitors of PCSK9 that confer LDL-C reductions of approximately 60% even on background statin therapy.(17, 18, 26) The FOURIER trial enrolled patients with established stable ASCVD and LDL-C >70 mg/dL despite maximally tolerated statins. In this study, evolocumab was shown to significantly lower LDL-C levels to a median of 30 mg/dL and confer a relative risk reduction of major adverse cardiovascular events (MACE) compared with placebo by 15% at a median of 2.2 years.(17) Alirocumab was next examined in the ODYSSEY OUTCOME trial, which showed that patients with recent acute coronary syndrome (ACS) and LDL-C >70 mg/dL on maximally tolerated statin therapy experienced a significant 15% relative reduction in MACE at median of 2.8 years.(18) The recent Effects of the PCSK9 Antibody Alirocumab on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction (PACMAN-AMI) trial provided mechanistic insight for the benefit of alirocumab by showing significant coronary plaque regression at 1-year in non-infarct related arteries as an explanation for the significant reduction MACE in this population.(27)
Moreover, post-trial analyses of FOURIER showed that higher-risk patients such as those with recent myocardial infarction (MI), more than one MI, multivessel disease or peripheral arterial disease (PAD) derived even greater benefit with more intensive LDL-C lowering with evolocumab compared to their lower risk counterparts.(28) The relative risk reductions with evolocumab for the primary end point were 20%, 18% and 21% for those with more recent MI, multiple prior MIs, and residual multivessel coronary artery disease, compared with 5%, 8%, and 7% in those without, respectively. Similarly, evolocumab conferred a 21% reduction in MACE in patients with PAD compared to a 14% reduction in those without PAD.(29) Evolocumab also reduced major adverse limb events in this PAD population as well.
An additional benefit of PCSK9 inhibitors also appears to derive from their effect on circulating lipoprotein(a) [Lp(a)] levels. PCSK9 inhibitors have demonstrated significant Lp(a) lowering ability by unclear mechanism possibly involving increased clearance and reduced production.(30) Both alirocumab and evolocumab have also been shown to lower Lp(a) by about 25% and the clinical benefit may be the result of combined effects on different atherogenic compounds rather than LDL-C lowering alone.(31) Importantly patients with higher baseline Lp(a) levels tended to experience greater reduction in MACE with PCSK9 inhibitors than individuals with lower Lp(a) levels.(31, 32)
The U.S. FDA has approved both evolocumab and alirocumab to reduce the risk of MACE in patients with clinical ASCVD and as an adjunct to diet and maximally tolerated statin therapy to lower LDL-C in patients with heterozygous familial hypercholesterolemia (HeFH). They have also been approved as adjunct to other LDL-C lowering therapies in patients with homozygous familial hypercholesterolemia (HoFH), although given that their mechanism of action is to upregulate the LDL receptor, they have limited efficacy in the HoFH population.
Novel siRNA PCSK9 inhibitor (Inclisiran)
Inclisiran is a novel long-acting, subcutaneously delivered, synthetic small-interfering RNA (siRNA) directed against PCSK9.(33, 34) The siRNA approach uses an RNA interference pathway to bind to the RNA-induced silencing complex, enabling it to specifically cleave mRNA molecules encoding PCSK9 and thereby prevent translation. This provides a novel mechanism for targeting LDL-C reduction.
The siRNA oligonucleotide is conjugated to a ligand containing three N-acetylgalactosamine (GalNAc) motifs that allows for binding to the liver-specific asialoglycoprotein receptor and subsequent targeted delivery to hepatocytes.(35) It further benefits from a long pharmacological duration and only needs to be administered subcutaneously initially as a single-injection, once again at 3 months and then every 6 months thereafter to achieve a 50% reduction in LDL-C, which is a considerably longer duration than currently available monoclonal antibody therapies (every 2 weeks or once per month).
Inclisiran was studied in the phase 3 ORION-10 and ORION-11 trials where it achieved reductions in LDL-C levels of approximately 50% when administered subcutaneously every 6 months in patients already on statin therapy and notably showed no evidence of muscle related side-effects which can limit statin use. It also showed significant benefit in the secondary exploratory cardiovascular endpoint 7.4% compared with 10.2% in the placebo arm.(34) The highly-anticipated dedicated cardiovascular outcomes trial (ORION-4, NCT03705234) is currently examining the effect of inclisiran on cardiovascular morbidity and mortality. Similar to the effect seen in PCSK9 monoclonal antibody inhibitors, inclisiran has been shown to lower Lp(a) by 18–26% which may provide additive benefit in terms of ASCVD risk reduction.
In December 2021, the FDA recently approved inclisiran as a treatment to be used alongside of diet and maximally tolerated statin therapy for patients with HeFH or clinical ASCVD who require additional LDL-C lowering
Bempedoic acid
Bempedoic acid is an oral, first-in-class, small-molecule cholesterol synthesis inhibitor for the treatment of hypercholesterolemia. It is a pro-drug which requires conversion by hepatic enzyme ‘very long-chain acyl-CoA synthetase-1’ to become activated. This selective activation in hepatocytes is thought to minimise the risk of muscle-related adverse events which occur in statins. As an adenosine triphosphate (ATP)-citrate lyase (ACL) inhibitor, bempedoic acid blocks cholesterol synthesis at a step upstream of HMG-CoA reductase (the target of statins). ACL inhibition results in inhibition of cholesterol biosynthesis and increases LDL receptor expression on hepatocytes thereby reducing circulating LDL-C levels. Additionally, bempedoic acid enhances the activity of 5ʹ adenosine monophosphate-activated protein kinase (AMPK). AMPK activity prevents the phosphorylation of acetyl-CoA carboxylase and HMG-CoA reductase, reducing both glucose and lipid biosynthesis, thus contributing to LDL-C reduction by an alternate pathway.(36)
Bempedoic acid was studied in the Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen (CLEAR) Harmony trial(37) in patients with ASCVD with or without the presence of HeFH and in the CLEAR Wisdom trial(38) of patients with ASCVD with or without HeFH and LDL-C >70mg/dL on maximally tolerated lipid-lowering therapy. In both trials bempedoic acid was found to significantly lower LDL-C levels by 17–18% without an increase in adverse events compared with placebo in patients on statin-therapy.(37, 38) It has also been studied in patients unable to tolerate statins in the CLEAR Serenity trial, conferring a slightly greater LDL-C reduction of 21%.(39)
Fixed-dose combination therapy has also been an additional major focus with this medication. In a trial later nicknamed “CLEAR Combo”, the fixed dose combination therapy of bempedoic acid with ezetimibe in patients on background statin therapy significantly reduced LDL-C levels by 36.2%, compared with bempedoic acid alone of 17.2%, ezetimibe alone of 23.2%, and placebo of 1.8% at 12 weeks.(40) That is a placebo-corrected difference of 38% LDL-C lowering with combination therapy. This trial demonstrated that the benefit of the combination of bempedoic acid with ezetimibe together was better than either drug alone.(41)
Although LDL-C lowering is the primary target of this medication, an added potential benefit may be reduction in inflammation. Independent of lipids, high-sensitivity C-reactive protein (hsCRP) levels are also correlated with increased ASCVD risk and are frequently measured as a potential marker for efficacy in ASCVD reduction in trials.(42) Bempedoic acid has demonstrated significant hsCRP lowering effects with around 18–24% reduction in hsCRP levels as compared with placebo.(36) Moreover, with the fixed-dose combination regimen reductions in hsCRP as large as 35.1% have been demonstrated.(40)
An additional benefit of this agent is its favorable side effect profile. Notably, bempedoic does not seem to worsen glycemia or increase the risk of new onset diabetes(43), like has been demonstrated with statin therapy(44). Moreover, owing to its hepatocyte-selective activity it has not been associated with the same muscle-related side effects which limit statin-use in some patients.(36)
The CLEAR OUTCOME trial(45) is an ongoing highly anticipated cardiovascular outcome trial examining whether bempedoic acid can confer MACE reduction in patients who are intolerant of statin therapy. This trial enrolled nearly half of participants as women, which is a strength to examine sex differences, since female patients have historically been underrepresented in prior lipid-lowering trials.(46)
In Feb 2020, the FDA approved bempedoic acid for the treatment of patients with ASCVD or HeFH who require additional LDL-C lowering despite diet and maximally tolerated statin therapy.
Evinacumab
Angiopoietin-like protein 3 (ANGPTL3) is a hepatic protein which plays a key role in regulating circulating TGs and cholesterol levels through its reversible inhibition of lipoprotein lipase (LPL) and endothelial lipase (EL) which are enzymes responsible, in part, for metabolizing TGs and high-density lipoprotein cholesterol (HDL-C), respectively. Loss of function mutation of the ANGPTL3 gene has been identified in many individuals with familial combined hypolipidemia.(47) Furthermore, lower levels of TG and LDL-C have been observed with loss-of-function mutations in the ANGPTL3 gene.(48)
Evinacumab is monoclonal antibody that binds to and inhibits ANGPTL3, with the resulting disinhibition of LPL and EL reducing the levels of circulating LDL-C, TG and HDL-C. While the mechanism through which evinacumab reduces LDL-C is not entirely clear, this effect is independent of the LDL receptor and is therefore most likely due to the promotion of very low density lipoprotein (VLDL) processing and upstream clearance of LDL formation.(49)
The ELIPSE homozygous familial hypercholesterolemia trial evaluated evinacumab in patients with HoFH. These patients do not tend to benefit to the same degree from PCSK9 inhibition or statin therapy and fail to reach guideline-recommended LDL-C levels due to a complete lack of LDL receptor expression which often necessitates multiple agents and therapies such as apheresis to reduce ASCVD risk.(50) ANGPTL3 provides a potential new target in these patients as demonstrated in this study. In patients with HoFH receiving maximum doses of lipid-lowering therapy, evinacumab significantly reduced baseline LDL-C level 49% compared with placebo.
In February 2021, the FDA approved evinacumab for LDL-C reduction in patients with HoFH.
Vupanorsen
Vupanorsen is an N-acetyl galactosamine (GalNAc3)-modified anti-sense oligonucleotide (ASO) targeting hepatic ANGPTL3 mRNA. ASOs represent a novel mechanism to target specific disease-related mRNA and alter downstream protein synthesis by binding to the desired mRNA, in this instance ANGPTL3 mRNA, and promote its subsequent enzymatic degradation.(51) GalNAc3-modified ASOs represent a newer approach in ASO technology by targeting the ASO to the asialoglycoprotein receptor on hepatocytes, the same hepatocyte-specific binding receptor targeted by inclisiran. This results in a therapeutic efficacy similar to that of earlier unconjugated ASOs, but with 20- to 30-fold lower dosing and reduced systemic exposure.(52)
The TRANSLATE (Targeting ANGPTL3 with an Antisense Oligonucleotide in Adults with Dyslipidemia)–TIMI (Thrombolysis in Myocardial Infarction)-70 trial evaluated vupanorsen therapy in patients with elevated non-HDL-C >100 mg/dL and TGs 150–500mg/dL on statin therapy.(53) The study showed that in a dose-dependent fashion vupanorsen significantly reduced TG levels by 41–57% with additional modest reduction in apolipoprotein B (apoB) and LDL-C levels by 6–15% and 8–16.0% respectively. Despite these favourable improvements in the lipid profile, vuparnorsen also demonstrated increases in liver function tests compared with placebo and resulted in dose-dependent increases in hepatic fat fraction.(53) This led to the announcement by its industry sponsors that they are discontinuing the clinical development program for this agent.(54) Nevertheless, the investigational therapy provides another potential target and mechanism of action for reducing atherogenic compounds and ASCVD risk warranting further investigation with different related compounds.
Pelacarsan
Lipoprotein(a) is an LDL-like lipoprotein containing an apoB moiety linked to apolipoprotein(a). The LPA gene is expressed mainly in the liver, followed by the kidneys.(55) Lp(a) potentially contributes to ASCVD through its proatherogenic, proinflammatory and prothrombotic effects.(56) Evidence accumulated over the past 20 years supports elevated plasma Lp(a) levels as an independent risk factor for CVDs such as MI, stroke and calcific aortic-valve stenosis.(57) Providing further support of a causal relationship, genetically determined elevated Lp(a) levels are associated with increased risk for aortic stenosis, MI, stroke, heart failure, and atrial fibrillation. (58–61)
Pelacarsen is a novel hepatocyte-directed ASO targeting the LPA gene mRNA currently being examined as potential therapy for ASCVD risk reduction. For example, the AKCEA-APO(a)-LRx study evaluated patients with established ASCVD and elevated Lp(a) levels >60 mg/dL and demonstrated effective plasma Lp(a) level lowering with pelacarsen.(62) In phase 2 trials, a weekly or monthly subcutaneous administration of pelacarsen dose-dependently decreased Lp(a) by 35–80%. Pelacarsen has also been shown to have a neutral-to-mild LDL-C lowering effect.(63) The ongoing phase 3 LP(a)HORIZON cardiovascular outcome trial (NCT04023552) is designed to support an indication for pelacarsen (TQJ23) for the reduction of cardiovascular events in patients with established ASCVD and baseline Lp(a)>70 mg/dL. This randomized, double-blind, placebo controlled, multicenter trial will assess the time to first MACE (cardiovascular death, non-fatal MI, and non-fatal stroke) comparing a monthly 80 mg subcutaneous injection of pelacarsen to placebo.
Emerging Lp(a) technology
Additionally, other LP(a)-directed therapeutics are also under investigation. For example, a novel siRNA targeting Lp(a) is showing promise as potential therapy.(64) Early results from the double-blind, phase I APOLLO trial showed that ascending doses of a single subcutaneous injection of siRNA, SLN360, were well tolerated and resulted in significant reductions in plasma Lp(a).(64) In this study, 32 asymptomatic participants free of known ASCVD with plasma Lp(a) levels of ≥150 nmol/L at baseline were randomly assigned to receive either placebo (n=8) or single subcutaneous doses of SLN360 at 30 mg (n=6), 100 mg (n=6), 300 mg (n=6), or 600 mg (n=6). Short term results at 150 days follow-up revealed minimal treatment-adverse events (most common reactions were headache and injection site reaction) and persistent, dose dependent changes in Lp(a) (maximal median change in the 300 mg and 600 mg groups: –96% (IQR, –98% to –89%) and –98% (IQR, –98% to –97%), respectively). There was also a 26% and 28% reduction in LDL-C and apoB, respectively, with max dose.
Olpasiran, AMG890, is a siRNA is currently being evaluated in a randomized, double-blind, placebo-controlled, single subcutaneous ascending dose study in asymptomatic adults with plasma concentrations of Lp(a) ≥ 70 to ≤ 199 nmol/L and ≥ 200 nmol/L (NCT0362662). Phase 1 results included 64 individuals (cohorts 1–5: AMG 890, n=30, doses: 3 mg, 9 mg, 30 mg, 75 mg, 225 mg; placebo, n=10; cohorts 6–7: AMG 890, n=18, doses: 9 mg and 75 mg; placebo, n=6). In adults with elevated Lp(a), a single dose of AMG 890 significantly reduced Lp(a) with observed approximate median percent reductions of >90% at doses of ≥9 mg in a dose-dependent manner, with Lp(a) reductions persisted for 3 to 6 months at doses of ≥9 mg.(65) The ongoing phase 2 trial (DOSE Finding Study - NCT04270760) will evaluate the safety/tolerability of subcutaneous AMG890 and change in Lp(a) among those with established ASCVD and Lp(a) > 150 nmol/L compared to placebo.
Although outcome data is required, siRNA technology hold promise considering durable reductions in Lp(a).
Gene therapy
Gene-editing technologies such as CRISPR-Cas nucleases and CRISPR base editors are being investigated to treat a variety of diseases ranging from cancers, inherited conditions such as sickle cell disease, cystic fibrosis and human immunodeficiency virus (HIV).(66) The same technology has also been recently employed in experimental studies in primates to target PCSK9 and LDL-C levels. Precise single-nucleotide loss-of-function mutations introduced using CRISPR adenine base editors delivered via lipid nanoparticles have been shown to result in durable reductions of plasma circulating levels of PCSK9 and LDL by 90% and 60% for at least 8 months.(67) This holds promise for a curative approach for individuals with the most severe forms of primary hypercholesterolemia such as HoFH.
Achieving targets
While these new therapies provide additional targets and mechanisms for ASCVD risk reduction, recent studies show that most high-risk patients with ASCVD are not at goal, and currently available combination therapy is sorely underutilized with available existing agents. (68, 69) In order to achieve guideline recommended LDL-C targets of <70 mg/dL (US)(4) or <55 mg/dL (ESC)(11), initial use of combination therapy might be reasonable in high-risk patients who have an LDL-C level more than 50% higher than target goal.(70) Potential available combination therapies include statins + ezetimibe, statins plus bempedoic acid, bempedoic acid plus ezetimibe, PCSK9 inhibitors or inclisiran plus statins.
Conclusions
In sum, LDL-C plays a central role in ASCVD pathogenesis and progression and as such remains the key target for ASCVD prevention. Statin therapy remains first line for the primary and prevention of ASCVD, with ezetimibe being the most frequently used add-on therapy when additional LDL-C lowering is needed. However, within the last decade there have been significant developments within the field of lipid management with several new medications which have been recently approved and multiple more still on-going clinical investigation. The development of monoclonal antibodies, siRNAs, small molecule inhibitors and ASOs have facilitated selective inhibition of the production of targeted proteins in lipid metabolism such as PCSK9, Lp(a), ANGPT3 and others. The FDA-approved monoclonal antibody (evolocumab, alirocumab) and siRNA (inclisiran) inhibitors of PCSK9 confer potent LDL-C lowering. The new oral agent of bempedoic acid also offers LDL-C lowering with or without background statin therapy, and can be used with ezetimibe in a fixed dose combination pill. New therapeutic targets have been identified and present additional means of lowering LDL and other atherogenic compounds. Myriad potential roles for these medications exist including the reduction of residual ASCVD risk in patients tolerating maximal doses of existing LDL-C lowering therapies, alternative agents in patients not tolerating existing therapies and additional means of targeting LDL-C or attaining reliable reduction where compliance is an issue.
Funding:
Dr. Michos is supported by the Amato Fund in Women’s Cardiovascular Health Research at Johns Hopkins University. Dr. Quispe is supported by a National Institutes of Health (NIH) T32 training grant (5T32HL007227).
Footnotes
Declarations:
Conflicts of Interest: Dr. Michos has served on advisory boards for AstraZeneca, Amarin, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, Pfizer.
Human and Animal Rights and Informed Consent:
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
● of importance
● of major importance
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022:CIR0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European heart journal. 2017;38(32):2459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michos ED, McEvoy JW, Blumenthal RS. Lipid Management for the Prevention of Atherosclerotic Cardiovascular Disease. The New England journal of medicine. 2019;381(16):1557–67. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019;73(24):3168–209. ●●(This is the last major U.S. cholesterol guideline released, which recommended the addition of non-statin therapy of ezetemibe and PCSK9 inhibitors if patients at very high ASCVD risk remained above an LDL-C threshold of 70 mg/dL despite maximally tolerated statin. This 2019 publication however was before the FDA approval of bempedoic acid and inclisiran, which were not discussed).
- 5.Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41(24):2313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro MD, Bhatt DL. “Cholesterol-Years” for ASCVD Risk Prediction and Treatment. Journal of the American College of Cardiology. 2020;76(13):1517–20. [DOI] [PubMed] [Google Scholar]
- 7. Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. Journal of the American College of Cardiology. 2018;72(10):1141–56. ●(This excellent state of the art review outlines the rationale that LDL-C is causually linked to atherosclerotic cariodvascular disease (ASCVD) and why lower LDL-C for longer periods of time is associated with reduction in (ASCVD).)
- 8.Martin SS, Michos ED. Mapping hyperlipidemia in young adulthood to coronary risk: importance of cumulative exposure and how to stay young. Circulation. 2015;131(5):445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pencina KM, Thanassoulis G, Wilkins JT, Vasan RS, Navar AM, Peterson ED, et al. Trajectories of Non-HDL Cholesterol Across Midlife: Implications for Cardiovascular Prevention. Journal of the American College of Cardiology. 2019;74(1):70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navar-Boggan AM, Peterson ED, D’Agostino RB Sr., Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131(5):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European heart journal. 2020;41(1):111–88. ●●(This is the last released European Society of Cardiology guideline for cholesterol management which set a recommenation for achieving both an LDL-C reduction of 50% and an LDL-C target of <55 mg/dL for patients at very high risk).
- 12.Joshi PH, Martin SS, Blumenthal RS. The remnants of residual risk. Journal of the American College of Cardiology. 2015;65(21):2276–8. [DOI] [PubMed] [Google Scholar]
- 13.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. Journal of the American College of Cardiology. 2008;51(7):724–30. [DOI] [PubMed] [Google Scholar]
- 14.Davidson MH. Reducing residual risk for patients on statin therapy: the potential role of combination therapy. Am J Cardiol. 2005;96(9A):3K–13K; discussion 34K-5K. [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. The New England journal of medicine. 2015;372(25):2387–97. [DOI] [PubMed] [Google Scholar]
- 16. Khan SU, Khan MU, Virani SS, Khan MS, Khan MZ, Rashid M, et al. Efficacy and safety for the achievement of guideline-recommended lower low-density lipoprotein cholesterol levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;28(18):2001–9. ●(This recent meta-analysis of 11 randomized clincial trials (over 130,000 participants) of LDL-C lowering therapies of statins, ezetimibe, and PCSK9 inhibitors showed that intensive lowering LDL-C to <70 mg/dL was associated with greater cardiovascualr benefit with safety data showing no signal for harm.)
- 17.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. The New England journal of medicine. 2017;376(18):1713–22. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. The New England journal of medicine. 2018;379(22):2097–107. [DOI] [PubMed] [Google Scholar]
- 19.Cholesterol Treatment Trialists C, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–405. [DOI] [PubMed] [Google Scholar]
- 20.Cholesterol Treatment Trialists C. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393(10170):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. The Lancet. 2010;376(9753):1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michos ED, Martin SS. Achievement of Very Low Low-Density Lipoprotein Cholesterol Levels: Is It Time to Unlearn Concern for Hemorrhagic Stroke? Circulation. 2019;140(25):2063–6. [DOI] [PubMed] [Google Scholar]
- 23.Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, et al. Cognitive Function in a Randomized Trial of Evolocumab. The New England journal of medicine. 2017;377(7):633–43. [DOI] [PubMed] [Google Scholar]
- 24.Seidah NG, Awan Z, Chrétien M, Mbikay M. PCSK9. Circulation Research. 2014;114(6):1022–36. [DOI] [PubMed] [Google Scholar]
- 25.Kent ST, Rosenson RS, Avery CL, Chen Y-DI, Correa A, Cummings SR, et al. PCSK9 Loss-of-Function Variants, Low-Density Lipoprotein Cholesterol, and Risk of Coronary Heart Disease and Stroke. Circulation: Cardiovascular Genetics. 2017;10(4):e001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. New England Journal of Medicine. 2015;372(16):1489–99. [DOI] [PubMed] [Google Scholar]
- 27. Raber L, Ueki Y, Otsuka T, Losdat S, Haner JD, Lonborg J, et al. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. Jama. 2022; epub ahead of print. ●(This recent proof of concept trial evaluating the initiation of PCSK9 inhibitors within 24 hours of an acute MI showed that intensive LDL-C lowering conferred reduction in atheroma plaque volume as seen on intravascular ultrasound, providing mechanism for the reduction in cardiovascular events seen in the PCSK9 inhibitor outcome trials).
- 28.Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, et al. Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease. Circulation. 2018;138(8):756–66. [DOI] [PubMed] [Google Scholar]
- 29.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, et al. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137(4):338–50. [DOI] [PubMed] [Google Scholar]
- 30.Lipoprotein Libby P. (a). JACC: Basic to Translational Science. 2016;1(6):428–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation. 2019;139(12):1483–92. ●(This secondary analysis of the FOURIER trial showed that evolocumab, a PCSK9 inhibitor, lowered LP(a) levels and that individuals with higher LP(a) derived greater benefit from PCSK9 inhibitor in terms of reduction in vascular events).
- 32.Reyes-Soffer G, Pavlyha M, Ngai C, Thomas T, Holleran S, Ramakrishnan R, et al. Effects of PCSK9 Inhibition With Alirocumab on Lipoprotein Metabolism in Healthy Humans. Circulation. 2017;135(4):352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. The New England journal of medicine. 2017;376(15):1430–40. [DOI] [PubMed] [Google Scholar]
- 34. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. The New England journal of medicine. 2020;382(16):1507–19. ●●(These two pivotal phase 3 trials demonstrated that inclisiran dosed subcutaenously twice yearly conferred 50% reduction in LDL-C with good safety data; this work supported the recent FDA approval of inclisiran for LDL-C lowering in patients with ASCVD and heterozygous familial hypercholesterlemia).
- 35.Cupido AJ, Kastelein JJP. Inclisiran for the treatment of hypercholesterolaemia: implications and unanswered questions from the ORION trials. Cardiovascular Research. 2020;116(11):e136–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwala A, Quispe R, Goldberg AC, Michos ED. Bempedoic Acid for Heterozygous Familial Hypercholesterolemia: From Bench to Bedside. Drug Des Devel Ther. 2021;15:1955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. The New England journal of medicine. 2019;380(11):1022–32. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, et al. Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. Jama. 2019;322(18):1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, et al. Efficacy and Safety of Bempedoic Acid in Patients With Hypercholesterolemia and Statin Intolerance. Journal of the American Heart Association. 2019;8(7):e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603. ●(This trial showed that the combination of bemepodic acid and ezetimibe lowered LDL-C better than either agent alone, with placebo corrected difference of 38%, similar to what can be achieved by moderate to high intensity statins).
- 41.Khan SU, Michos ED. Bempedoic acid and ezetimibe - better together. Eur J Prev Cardiol. 2019:2047487319864672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Speiser JL, Ye F, Tsai MY, Cainzos-Achirica M, Nasir K, et al. High-Sensitivity C-Reactive Protein Modifies the Cardiovascular Risk of Lipoprotein(a). Journal of the American College of Cardiology. 2021;78(11):1083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leiter LA, Banach M, Catapano AL, Duell PB, Gotto AM Jr., Laufs U, et al. Bempedoic acid in patients with type 2 diabetes mellitus, prediabetes, and normoglycaemia: A post hoc analysis of efficacy and glycaemic control using pooled data from phase 3 clinical trials. Diabetes Obes Metab. 2022, epub ahead of print. ●(This pooled post-hoc analysis of trial data showed that, unlike statins, bempedoic acid does not worsen glycemia or increase the risk for new onset diabetes).
- 44.Mansi IA, Chansard M, Lingvay I, Zhang S, Halm EA, Alvarez CA. Association of Statin Therapy Initiation With Diabetes Progression: A Retrospective Matched-Cohort Study. JAMA internal medicine. 2021;181(12):1562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholls S, Lincoff AM, Bays HE, Cho L, Grobbee DE, Kastelein JJ, et al. Rationale and design of the CLEAR-outcomes trial: Evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. American heart journal. 2021;235:104–12. [DOI] [PubMed] [Google Scholar]
- 46.Khan SU, Khan MZ, Raghu Subramanian C, Riaz H, Khan MU, Lone AN, et al. Participation of Women and Older Participants in Randomized Clinical Trials of Lipid-Lowering Therapies: A Systematic Review. JAMA Netw Open. 2020;3(5):e205202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noto D, Cefalù AB, Valenti V, Fayer F, Pinotti E, Ditta M, et al. Prevalence of ANGPTL3 and APOB Gene Mutations in Subjects With Combined Hypolipidemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(3):805–9. [DOI] [PubMed] [Google Scholar]
- 48.Lim GB. ANGPTL3: a therapeutic target for atherosclerosis. Nature Reviews Cardiology. 2017;14(7):381-. [DOI] [PubMed] [Google Scholar]
- 49.Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. Journal of Lipid Research. 2015;56(7):1308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: Guidelines and new therapies. Atherosclerosis. 2018;277:483–92. [DOI] [PubMed] [Google Scholar]
- 51.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nature Reviews Drug Discovery. 2020;19(10):673–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crooke ST, Liang X-H, Baker BF, Crooke RM. Antisense technology: A review. Journal of Biological Chemistry. 2021;296:100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, et al. Effect of Vupanorsen on Non-High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients With Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation. 2022;145(18):1377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfizer Announces Topline Results from Phase 2b Trial of Vupanorsen in Statin-treated Participants with Dyslipidemia. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-topline-results-phase-2b-trial-vupanorsen [Accessed March 12, 2022]. 2021. [Google Scholar]
- 55.Pećin I, Reiner Ž. Novel Experimental Agents for the Treatment of Hypercholesterolemia. Journal of Experimental Pharmacology. 2021;Volume 13:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsimikas S, Moriarty PM, Stroes ES. Emerging RNA Therapeutics to Lower Blood Levels of Lp(a): JACC Focus Seminar 2/4. Journal of the American College of Cardiology. 2021;77(12):1576–89. [DOI] [PubMed] [Google Scholar]
- 57.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. European Heart Journal. 2010;31(23):2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamstrup PR. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA. 2009;301(22):2331. [DOI] [PubMed] [Google Scholar]
- 59.Mohammadi-Shemirani P, Chong M, Narula S, Perrot N, Conen D, Roberts JD, et al. Elevated Lipoprotein(a) and Risk of Atrial Fibrillation: An Observational and Mendelian Randomization Study. Journal of the American College of Cardiology. 2022;79(16):1579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Y, Li H, Wang Y, Meng X, Wang Y. Causal Effect of Lp(a) [Lipoprotein(a)] Level on Ischemic Stroke and Alzheimer Disease: A Mendelian Randomization Study. Stroke. 2019;50(12):3532–9. [DOI] [PubMed] [Google Scholar]
- 61.Kamstrup PR, Nordestgaard BG. Elevated Lipoprotein(a) Levels, LPA Risk Genotypes, and Increased Risk of Heart Failure in the General Population. JACC Heart failure. 2016;4(1):78–87. [DOI] [PubMed] [Google Scholar]
- 62. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med. 2020;382(3):244–55. ●(Important phase 2 trial showing that pelacarsen can lower Lp(a) in dose-dependent manner up to 80%. This work set the stage for the on-going HORIZON cardiovascular outcome trial with pelacarsen.)
- 63.Yeang C, Karwatowska-Prokopczuk E, Su F, Dinh B, Xia S, Witztum JL, et al. Effect of Pelacarsen on Lipoprotein(a) Cholesterol and Corrected Low-Density Lipoprotein Cholesterol. Journal of the American College of Cardiology. 2022;79(11):1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nissen SE, Wolski K, Balog C, Swerdlow DI, Scrimgeour AC, Rambaran C, et al. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals With Elevated Plasma Lipoprotein(a) Levels. Jama. 2022;327(17):1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koren MJ, Moriarty PM, Neutel J, Baum SJ, Hernandez-Illas M, Weintraub HS, et al. Abstract 13951: Safety, Tolerability and Efficacy of Single-dose Amg 890, a Novel Sirna Targeting Lp(a), in Healthy Subjects and Subjects With Elevated Lp(a). Circulation. 2020;142(Suppl_3):A13951-A. [Google Scholar]
- 66.Adli M. The CRISPR tool kit for genome editing and beyond. Nature communications. 2018;9(1):1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–34. [DOI] [PubMed] [Google Scholar]
- 68.Ray KK, Haq I, Bilitou A, Aguiar C, Arca M, Connolly DL, et al. Evaluation of contemporary treatment of high- and very high-risk patients for the prevention of cardiovascular events in Europe – Methodology and rationale for the multinational observational SANTORINI study. Atherosclerosis Plus. 2021;43:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cannon CP, De Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Gao Q, et al. Use of Lipid-Lowering Therapies Over 2 Years in GOULD, a Registry of Patients With Atherosclerotic Cardiovascular Disease in the US. JAMA Cardiology. 2021;6(9):1060. ●(Registry data of U.S. patients with ASCVD showing two-thirds of patients remained suboptimally treated with LDL-C above goal of 70 mg/dL and only 17% had their lipid lowering therapy intensified over a 2-year period).
- 70.Michos ED, Ferdinand KC. Lipid-lowering for the prevention of cardiovascular disease in the new era: A practical approach to combination therapy. European Atherosclerosis Journal. 2022;1(1):eaj_22005. [Google Scholar]