SUMMARY
Radical chemistry has emerged as a cornerstone in modern organic synthesis, providing chemists with numerous new tools to rapidly expand reactivity and chemical space in academic and industrial research. In this regard, titanium complexes have been recognized as an attractive class of catalysts owing to their rich redox activities in addition to the abundance and low toxicity of this early transition metal. Traditionally employed for the activation of epoxides and carbonyl compounds, Ti radical redox catalysis has broken into new grounds in recent years, giving rise to a diverse repertoire of useful transformations. In this Perspective, we highlight recent developments in the area of TiIII/IV catalysis with respect to the activation of different types of chemical bonds. Furthermore, we discuss future opportunities in integrating Ti radical chemistry with other catalytic systems as well as with emerging new technologies such as photochemistry and electrochemistry.
Keywords: titanium, radical, redox catalysis, bond activation
Graphical Abstract
eTOC Blurb
Radical chemistry has emerged as a cornerstone in modern organic synthesis, enabling chemists to rapidly expand reactivity and chemical space. Titanium complexes have been recognized as attractive catalysts owing to their rich redox activities, abundance, and low toxicity. In this Perspective, we highlight recent developments in TiIII/IV catalysis with respect to the activation of different types of chemical bonds. Furthermore, we discuss future opportunities in integrating Ti radical chemistry with other catalytic systems as well as with emerging new technologies.
INTRODUCTION
As the second most abundant transition metal, titanium has seen wide use in synthetic transformations1 and industrial processes.2 Titanium-based complexes often exhibit strong Lewis acidity and display high reactivity towards various common organic functional groups. In particular, chiral titanium catalysts have been employed in numerous stereoselective reactions including the Sharpless epoxidation3 and nucleophilic additions.4 In addition to their canonical Lewis acid activities, organotitanium compounds also present rich redox properties and can adopt three oxidation states at the metal from TiII to TiIV. This feature has led to the development of a myriad of important transformations such as the McMurry reaction,5 the pinacol coupling,6 the Kulinkovich reaction,7 the Barbier-type allylation,8 the Pauson-Khand reaction,9 and very recently, the [2+2+1]-pyrrole synthesis.10 Similarly, titanium complexes also show promising applications in the activation of small molecules including nitrogen11 and carbon monoxide.12
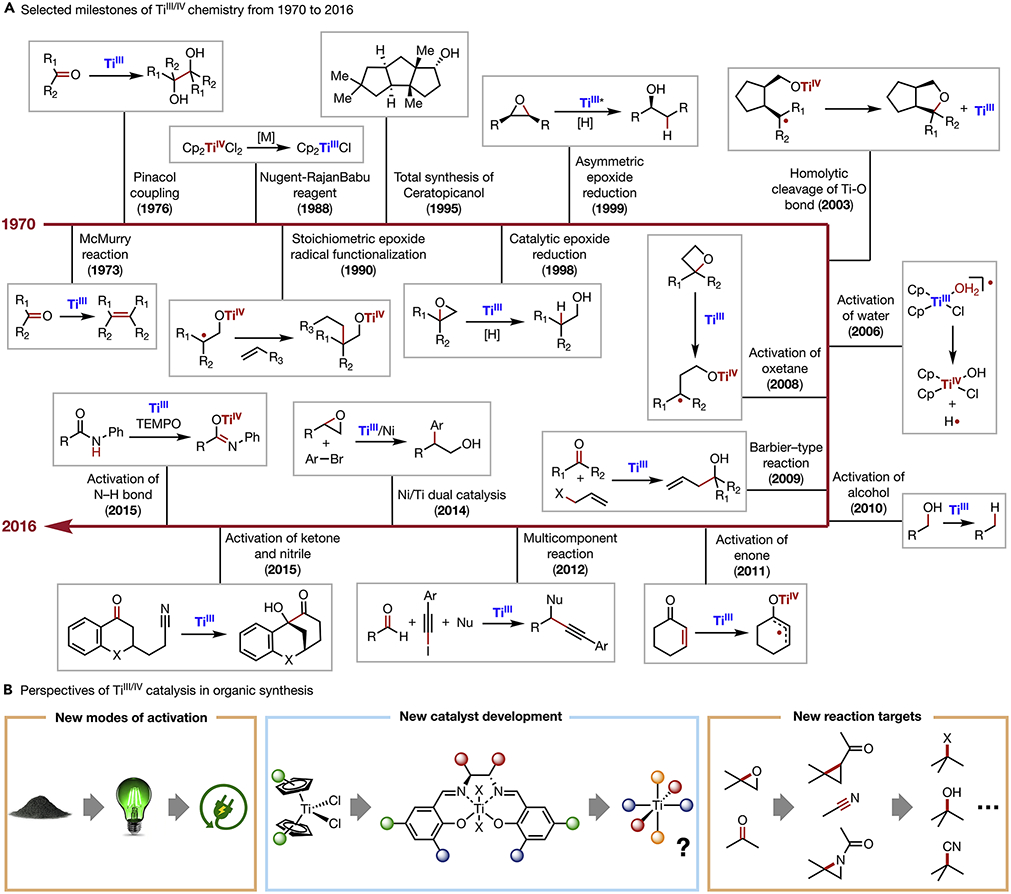
Titanium-mediated redox organic transformations are most often powered by the TiIII/IV or TiII/IV redox cycles.13-15 In this perspective, we are particularly interested in highlighting TiIII/IV catalysis, which has been shown to promote efficient organic radical generation. Selected milestones highlighted in Scheme 1A summarize the development of TiIII/IV chemistry since the 1970s. Cp2TiCl, first reported by Wilkinson in 195517 by treating titanocene dichloride with reducing metals, has since found numerous synthetic applications in free radical and organometallic chemistry. This reagent exists as a Cl-bridged dimer [Cp2TiCl]2, which is in equilibrium with the active Cp2TiCl form via solvent-assisted dissociation.15 Intrigued by these initial findings, Nugent and RajanBabu reported a series of epoxide reductive functionalization reactions using stoichiometric amounts of Cp2TiCl.17,18 In the context of total synthesis, the first use of Cp2TiCl was demonstrated by Clive in the synthetic campaign towards Ceratopicanol.19 Gansäuer developed the first catalytic variant of TiIII-promoted reductive epoxide functionalization in 199820 and subsequently reported the asymmetric synthesis of anti-Markovnikov alcohols using a chiral titanocene complex.21 Beyond transformations of epoxides22, TiIII/IV redox couples have been widely employed towards the functionalization of various oxygen-containing organic motifs, including water,23 hydrogen peroxide,24 oxetanes,25 alcohols,26 ozonides27 and enones.28 In many of these examples, TiIII acts as a single electron reductant and activates C─O bonds to furnish radical species, which often participate in subsequent functionalization to form C─C bonds or C─H bonds. In addition, a wide range of other substrate classes have also been used in TiIII-promoted radical reactions, including allylic halides,8 α-halo carbonyl compounds,29 and nitriles.30 The expansion of activation targets significantly increased the synthetic values of TiIII/IV redox chemistry. Meanwhile, innovations in radical chemistry have driven the development of a plethora of elegant new reaction strategies by merging TiIII chemistry with cross-coupling,31-34 proton-coupled electron transfer (PCET),35 and multicomponent catalysis.36
Scheme 1. TiIII/IV Radical Chemistry.
(A) Selected milestones of TiIII/IV chemistry from 1970 to 2016
(B) Perspectives of TiIII/IV catalysis in organic synthesis
In this Perspective, we highlight recent development (c. 2016) in TiIII/IV redox catalysis and discuss emerging opportunities in the field. The main section is largely divided according to the type of chemical bond that is activated by Ti: (1) C─O bond activation, (2) C─N bond activation, (3) C─C bond activation, and (4) C─halogen and C─S bond activation. In the Outlook section, we provide a review of new strategies to achieve the generation and turnover of TiIII employing electrochemistry and photoredox chemistry, and discuss opportunities in each respective area. Finally, we anticipate that more comprehensive mechanistic studies of known reaction methodologies will guide the development of new ligands and new reactivities amenable to Ti radical catalysis.
C─O Bond Activation
The functionalization of carbon─oxygen bonds in organic molecules such as alcohols, epoxides, and carbonyl compounds are most frequently achieved via electrophilic activation through the use of Lewis acidic catalysts or reagents. Recent advances in transition metal catalysis and radical chemistry have provided complementary strategies for the cleavage and functionalization of C─O bonds, giving rise to a diverse range of new transformations. In this context, titanium radical catalysis represents an attractive approach as it often offers mild reaction conditions and complementary reactivity in comparison with Lewis acid and late transition metal catalysts.
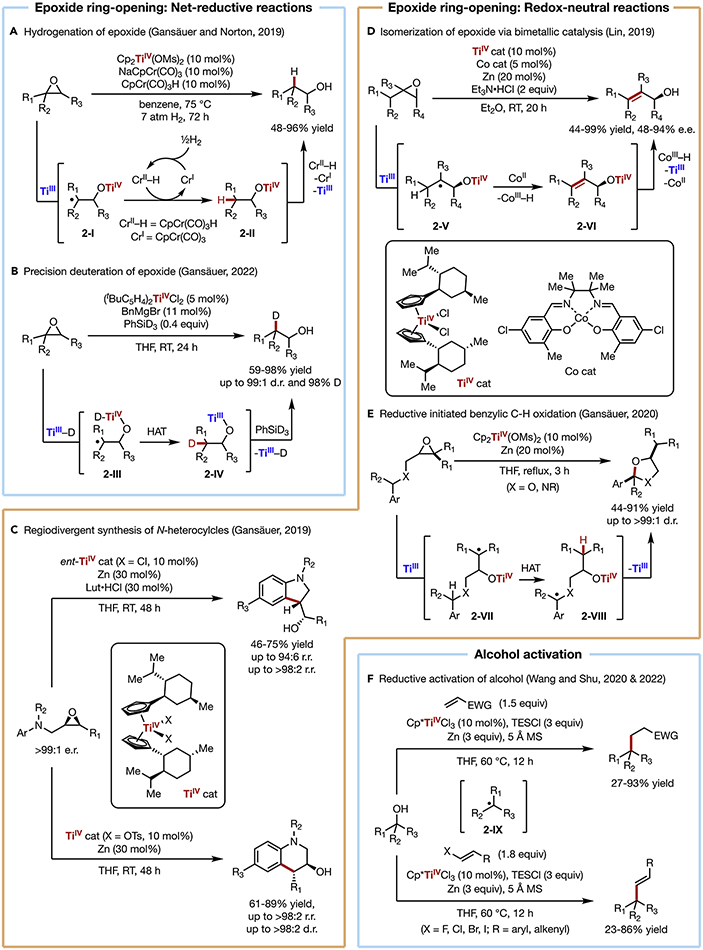
Epoxides are highly versatile electrophiles that can undergo stereospecific ring-opening with a wide array of nucleophiles via a traditional two-electron pathway. In contrast, TiIII typically mediates ring-opening of epoxides via C─O bond homolysis induced by an inner-sphere single electron transfer (SET) from TiIII to the substrate. This event triggers the regioselective cleavage of one of the two C─O bonds, resulting in the more stable carbon-centered radical that can subsequently participate in various radical transformations such as hydrogen atom transfer (HAT), addition to unsaturated bonds, and elimination. Notably, the use of chiral Ti complexes often enables stereoselective functionalization of epoxides to give chiral alcohol products. Conventionally, reductive ring-opening of epoxides often requires a stoichiometric amount of metal reductant (e.g., Zn, Mn) to achieve catalyst turnover, which could hamper their application in synthesis beyond laboratory scale. To circumvent this limitation, Norton and Gansäuer developed a dual Ti/Cr catalytic system for the anti-Markovnikov hydrogenation of epoxides (Scheme 2A).37 In this reaction, the carbon-centered radical (2-I) arising from epoxide ring rupture accepts a hydrogen atom from a catalytic chromium-hydride complex, CpCr(CO)3H. Upon release of the resultant alcohol product, the TiIV and Cr0 catalysts undergo SET with each other to return to their catalytically active oxidation states, TiIII and CrI. The latter then takes up a H atom from hydrogen gas, reforming the Cr─H species. With H2 as the only stoichiometric reagent, the Norton-Gansäuer strategy provides a more atom-economical approach for the reductive ring-opening of epoxides.
Scheme 2. C─O Bond Activation.
(A) Hydrogenation of epoxide
(B) Precision deuteration of epoxide
(C) Regiodivergent synthesis of N-heterocycles
(D) Isomerization of epoxide via bimetallic catalysis
(E) Reductive initiated benzylic C─H oxidation
(F) Reductive activation of alcohol
The preparation of isotopically labeled molecules represents an emerging research field in organic chemistry owing to its significance in modern synthetic and biomedical research. Recently, Gansäuer incorporated the TiIII-induced epoxide ring-opening in the preparation of β-deuterated anti-Markovnikov alcohols with high D-incorporation and excellent diastereoselectivity (Scheme 2B).38 In this example, (tBuC5H4)2TiCl2 is first activated by BnMgBr to generate (tBuC5H4)2TiBn, which can undergo σ-bond metathesis with PhSiD3 to form [(tBuC5H4)2Ti─D]. This species promotes the regioselective transformation of an epoxide to carbon-centered radical (2-III), upon which an intramolecular D-atom transfer (2-III to 2-IV) completes the deuteration.
Compared with net-reductive transformations, redox-neutral reactions catalyzed by titanium remain relatively underdeveloped. However, the desire to eliminate the need for stoichiometric reductants, while simultaneously expanding the reactivity of Ti to new territories has led to increasing interest in searching for alternative means to achieve catalytic turnover. For example, following their early work on Ti-catalyzed radical arylation,39 Gansäuer recently demonstrated the regiodivergent synthesis of tetrahydroquinolines and indolines (Scheme 2C).40 Upon TiIII-induced reductive epoxide ring-opening, subsequent intramolecular radical addition to the pendant aromatic ring generates a cyclohexadienyl radical. This intermediate undergoes SET to TiIV followed by rearomatization, giving rise to the product and turning over the catalyst. The electron transfer step is driven by the restoration of aromaticity. Interestingly, the regioselectivity of the ring-opening step is controlled by the absolute configuration of the enantiopure Kagan’s complex and its anionic ligands X, furnishing either indoline or tetrahydroquinoline products in excellent selectivity.
In another recent example, Lin reported the stereoselective isomerization of epoxides to allylic alcohols via dual Ti/Co catalysis, leveraging TiIII-induced epoxide opening (initiation) and CoII-mediated HAT (termination) in the same catalytic system (Scheme 2D).41 The combination of Kagan’s complex and cobalt salen complex enables excellent regio- and stereochemical control over bond-breaking and -forming events of the isomerization. Notably, this transformation is overall redox-neutral, with the catalytic system sustaining itself without the need for a stoichiometric reductant. Specifically, upon TiIII-mediated epoxide ring-opening, CoII extracts an H atom β-to the carbon-center radical (2-V) to furnish the alkene (2-VI). Subsequent proton transfer from CoIII─H to TiIV-alkoxide releases the allylic alcohol product, which is followed by an SET between the resultant pair of metal complexes (CoI to TiIV) re-establishing the active CoII and TiIII catalysts. Compared with the conventional base-mediated isomerization, this reaction operates under neutral conditions, thereby preserving labile functional groups and stereogenic centers sensitive to epimerization.
In another elegant example, Gansäuer recently reported the Ti-catalyzed synthesis of cyclic acetals and hemiaminals from benzylic ethers and benzylic amines, respectively, with a pendent epoxide (Scheme 2E).42 On the basis of their early developments in Ti-catalyzed tetrahydrofuran synthesis,22 the authors incorporated a new elementary step in the catalytic cycle to expand the reaction scope. Specifically, after TiIII-induced epoxide ring-opening, radical translocation via 1,5-hydrogen atom shift generates a more stable benzylic radical (2-VII to 2-VIII). Subsequent reductive elimination via SET between the benzylic radical and Ti-alkoxide affords the cyclized product. This overall redox-neutral reaction achieves benzylic C─H oxidation under redox-neutral conditions with the epoxide serving as the electron acceptor. We note that in all the three examples summarized above, only catalytic quantities of Zn reductant are needed to reduce the commercially available TiIV precatalysts because the overall reactions are redox neutral.
In recent years, the scope of Ti-catalyzed C─O bond activation has further diversified to include alcohol substitution. Alcohols are among the most common functional groups in organic compounds. However, the direct dehydroxylative C─C bond formation from alcohols remains a challenging synthetic problem. In their seminal contributions, Barrero described the activation of alcohols using stoichiometric amounts of Cp2TiCl.26 Following this initial establishment of reactivity, Wang and Xu recently reported the Ti-catalyzed dehydroxylative functionalization of tertiary alcohols (Scheme 2F).43 This reaction begins with inner-sphere reductive C─O bond cleavage by Cp*TiCl2 upon catalyst-substrate coordination affording a carbon-centered radical along with Cp*Ti(OH)Cl. The nucleophilic radical (2-IX) undergoes addition to an activated alkene, upon which further reduction and protonation delivers the Giese-type product. The TiIII catalyst is regenerated with the combination of a metal reducing agent (e.g., Zn) and a chlorosilane. In 2022, Shu further expanded the reactivity to cross-coupling of alcohols and vinyl halides (Scheme 2F).44 The radical (2-IX) generated from reductive activation of the alcohol adds to a vinyl halide. The resultant carbon-centered radical recombines with TiIII, and the ensuing Ti-alkyl intermediate undergoes β-halogen elimination to afford the E-selective product.
Despite recent progress, the search for reactions that can selectively activate inert oxygen-containing molecules remains a challenging objective. We anticipatethatthe desireto expand Ti-catalyzed activation of inert C─O bonds will continue to drive the innovation of ligand scaffolds and development of new catalytic strategies that can provide access to highly reactive low-valent titanium intermediates.
C─N Bond Activation
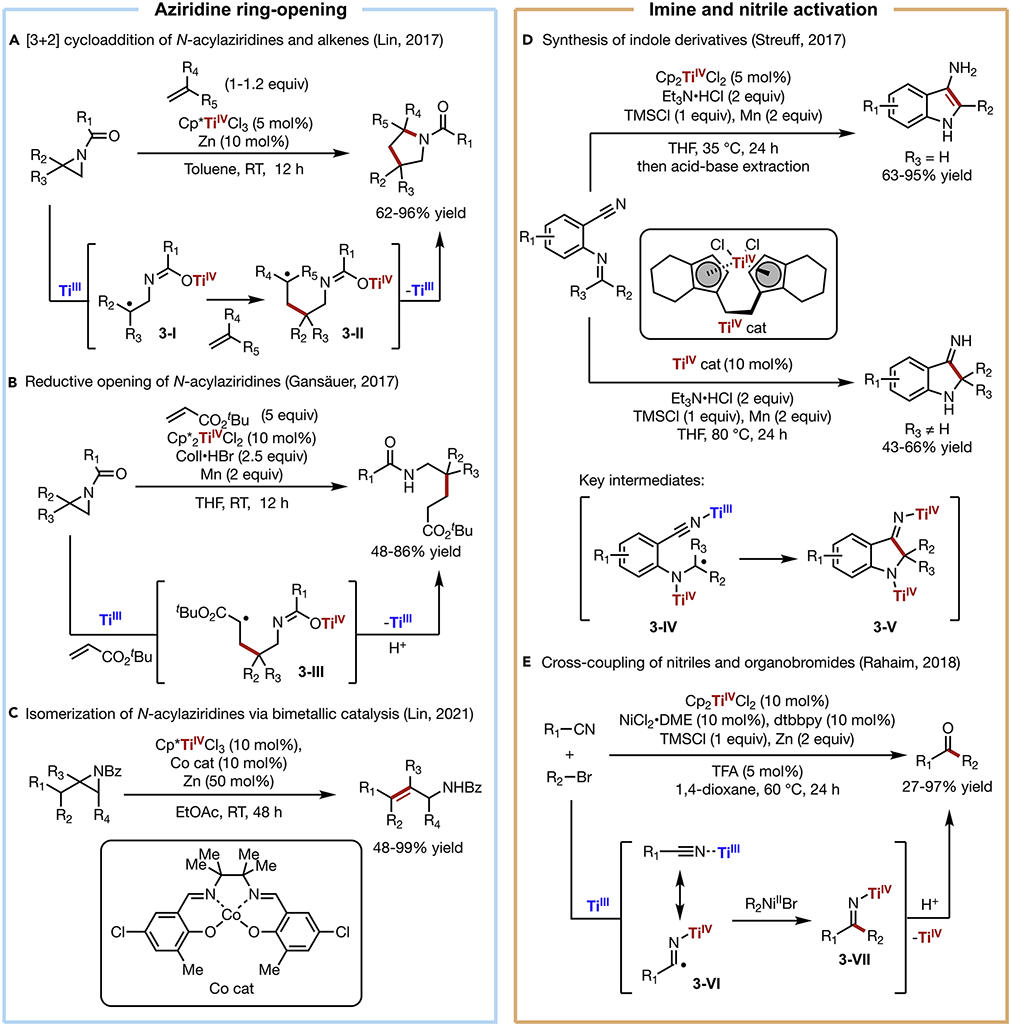
Owing to the prevalence of nitrogen-containing motifs in medicinally active molecules, the development of efficient and selective methods for the formation and functionalization of C─N bonds is of critical importance. In recent years, the scope of titanium radical chemistry has broadened to incorporate the reductive activation of C─N bonds towards the construction of nitrogen-containing targets. In 2017, Lin developed a Ti-catalyzed formal [3+2] cycloaddition of N-acylaziridines and alkenes (Scheme 3A).45 In this transformation, the coordination of TiIII to N-acylaziridines induces the cleavage of a C─N bond via intramolecular SET. The ensuing carbon-centered radical (3-I) then adds to an alkene to furnish a new radical intermediate (3-II), which cyclizes onto the pendant TiIV-azaenolate to give the desired N-acylpyrrolidine product with simultaneous catalyst turnover. Notably, this strategy requires only a catalytic amount of Zn to reduce the Cp*TiCl3 pre-catalyst, as the overall reaction is redox-neutral. Lin termed this strategy radical redox-relay catalysis,13,46 which generally describes overall redox-neutral reactions that are initiated, regulated, and terminated by a redox-active catalyst via a series of electron transfer and radical relocation steps. It is important to note that the inception of radical redox-relay catalysis in the context of the [3+2] cycloaddition was inspired by previous work from Knowles,35 who showed that low-valent TiIII complexes can weaken strong chemical bonds in associated ligands, and Zakarian33,34 and Moreira47, who showed that TiIV-enolates display biradical characters and can react with carbon-centered radicals.
Scheme 3. C─N Bond Activation.
(A) [3+2] cycloaddition of N-acylaziridines and alkenes
(B) Reductive opening of N-acylaziridines
(C) Isomerization of N-acylaziridines via bimetallic catalysis
(D) Synthesis of indole derivatives
(E) Cross-coupling of nitriles and organobromides
Concurrently, Gansäuer reported the net-reductive functionalization of N-acylaziridines via Ti catalysis (Scheme 3B).48 In contrast to Lin’s redox-neutral transformation, the carbon-centered radical (3-III) generated from TiIII-mediated aziridine ring-opening and alkene addition undergoes further reduction and protonation to afford an acyclic C─C bond coupling product. This different reactivity is presumably controlled by the pronounced steric effect of the pair of pentamethylcyclopentadienyl ligands bound to Ti, which hinders cyclization and thus favors the formation of a linear product.
Following their previous demonstration of Ti/Co co-catalyzed epoxide isomerization (Scheme 2D), Lin further expanded this reactivity to the rearrangement of N-acylaziridines to allylic amides (Scheme 3C).49 Here, Ti-catalyzed reductive aziridine ring-opening and Co-catalyzed HAT operate in synergy to achieve the overall redox-neutral transformation. A series of polysubstituted aziridines were selectively converted into allylic benzamide products under mild, base-free conditions.
TiIII is also capable of reductively activating unsaturated polar functionalities including aldehydes and ketones,5,6 nitriles,28 and imines.50 Recently, Streuff explored this reactivity in the synthesis of five-membered nitrogen-containing heterocycles (Scheme 3D).51 In this approach, an N-cyanoaryl or N-cyanoalkenyl imine was activated via TiIII-mediated SET to form stabilized α-aminoalkyl radical (3-IV). Subsequent catalyst-controlled radical addition to the tethered nitrile generates cyclized intermediate (3-V). Depending on the substitution of the imine (i.e., ketimine or aldimine), an aminoindole or iminoindoline product can then be obtained.
The merger of Ti radical chemistry with late transition metal catalysis has recently been shown to enable new cross-coupling reactions. In 2014, Weix reported an early example of ring-opening coupling of epoxides with aryl bromides via dual Ni/Ti catalysis.32 In 2018, Rahaim further expanded the scope of this dual catalytic strategy to the cross-electrophile coupling of nitriles and organobromides (Scheme 3E).52 This reaction was initiated by TiIII-mediated nitrile activation to form an TiIV-bound imidoyl radical (3-VI). Meanwhile, NiI undergoes oxidative addition to an organohalide to furnish an organonickel intermediate. Transmetallation between this pair of metal complexes followed by reductive elimination from the resultant NiIII complex delivers the desired coupling product (3-VII), which is converted to ketone upon acidic work-up. Notably, the Ni/Ti system can be used to prepare unsymmetrical ketones from readily available starting materials.
Recent advances in Ti-catalyzed C─N bond activation provide innovative strategies for the synthesis and functionalization of nitrogen-containing molecules. The examples discussed in this section enabled complementary reactivities that are typically not observed in existing methods using late transition metal catalysis. However, current methods are still limited to the cleavage of activated C─N bonds in π-systems or strained rings. Developing catalysts capable of breaking and functionalizing unactivated C─N bonds constitutes an attractive future objective in the field of Ti catalysis.
C─C Bond Activation
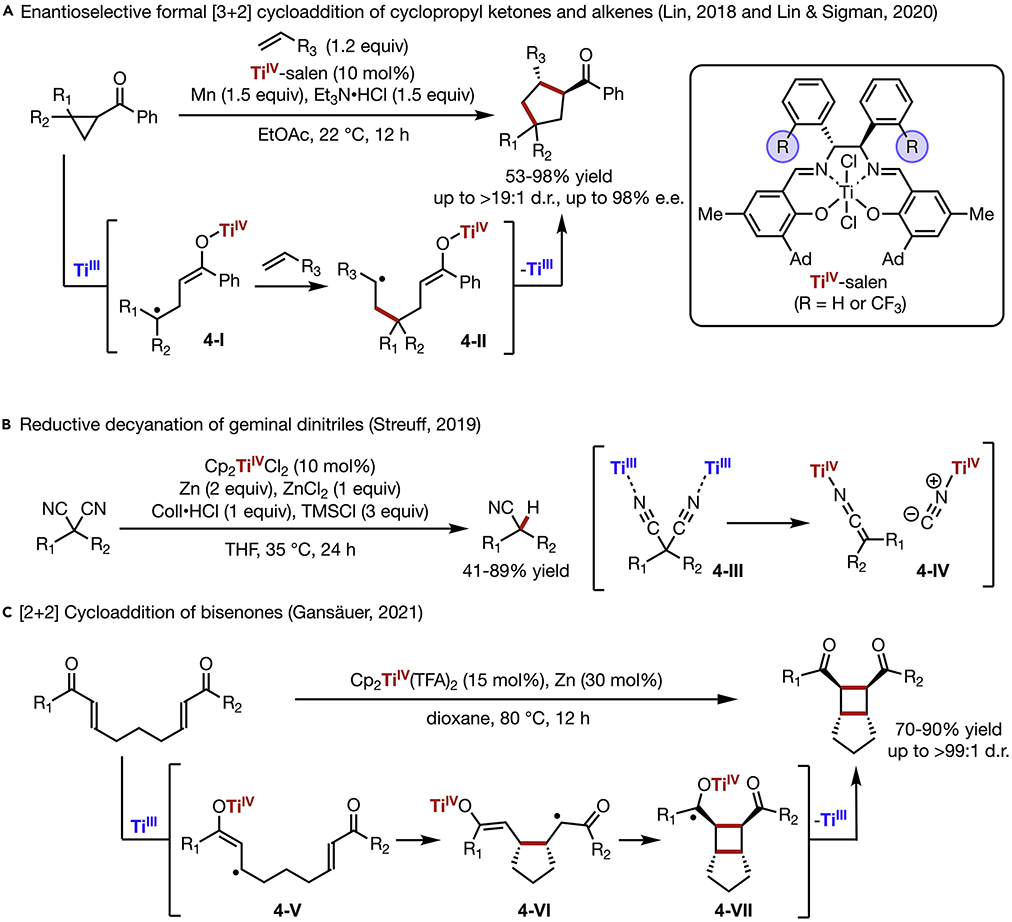
The catalyst-controlled cleavage of C─C bonds has recently developed into an efficient strategy towards molecular skeletal modification. In this context, TiIII complexes have been shown to be capable of inducing C─C bond homolysis, providing an attractive catalytic approach. Recent studies have demonstrated the ability of TiIII to not only activate polarized C─C bonds, but also promote non-polar C─C bond cleavage through strain release.53,56 In a follow-up study to their previous N-acylaziridine chemistry (Scheme 3A),45 Lin developed a diastereo- and enantioselective [3+2] cycloaddition of cyclopropyl ketones and alkenes by Ti-catalyzed radical redox relay (Scheme 4A).53 Through TiIII-coordination to the carbonyl group of a cyclopropylketone, reductive ring-opening of the cyclopropane generates a carbon-centered radical (4-I) tethered to a TiIV-enolate. Subsequent radical addition to an electrophilic alkene forges a new C─C bond, providing intermediate (4-II) that undergoes further cyclization to afford the cyclopentane product. Although stoichiometric amounts of Mn reductant are required to achieve high conversion, the overall reaction remains redox neutral, as TiIII is regenerated during the final ring-closing event. Importantly, with a chiral Ti-salen complex as the catalyst, highly substituted cyclopentanes are obtained with up to >19:1 d.r. and 98% e.e. In an effort to gain insight into the role of the catalyst’s structure on the stereochemical outcomes, Lin and Sigman carried out computational and mechanistic studies of the reaction system.54 Based on computational stereochemical models, optimization of the catalyst structure led to the incorporation of ortho-CF3 groups on the 1,2-diphenylethylene diamine backbone of the salen ligand. This new catalyst provides a higher degree of enantioinduction presumably through steric destabilization of the minor reaction transition states. The improved catalyst design enabled a more general transformation providing products with high stereoselectivity across a substantially broader substrate scope.
Scheme 4. C─C Bond Activation.
(A) Enantioselective formal [3+2] cycloaddition of cyclopropyl ketones and alkenes
(B) Reductive decyanation of geminal dinitriles
(C) [2+2] Cycloaddition of bisenones
In their previous work, Streuff showed that TiIII complexes can react with cyano groups upon coordination (Scheme 3D).51 Recently, they further developed a Ti-Catalyzed reductive decyanation of geminal dinitriles (Scheme 4B).55 Compared to previously reported free-radical decyanation reactions that required stoichiometric reagents (e.g., tin hydride, NHC-borane),57,58 this reaction proceeds under mild catalytic conditions and does not undergo a free-radical mechanism as suggested by a radical clock experiment. Instead, two equivalents of in situ generated Cp2TiCl coordinate to the geminal dinitrile (4-III), which triggers a dual single electron transfer process from Ti to the bound substrate to cleave the C─C bond, affording a pair of titanium-ketenimine and titanium-cyanide complexes (4-IV). From kinetic experiments, the reaction was found to be second order with respect to the catalyst, which is in line with the proposed mechanism. Protonation of the titanium-ketenimine delivers the nitrile product, while titanium-cyanide reacts with TMSCl to form TMSCN, releasing the Ti catalyst.
TiIII complexes have also been shown to effect homolysis of C─C π-bonds through coordination to conjugated polar functional groups. In 2021, Gansäuer reported the [2+2] cycloaddition of bis-enones catalyzed by Cp2Ti(TFA)2 (Scheme 4B).56 An inner-sphere electron transfer from TiIII to an enone substrate delivers a radical intermediate (4-V), which undergoes 5-exo cyclization onto the second enone group in the substrate to form α-carbonyl radical (4-VI). A 4-exo cyclization subsequently takes place to afford bicyclic intermediate (4-VII), and the resultant TiIV-bound ketyl radical undergoes electron transfer to furnish the bicyclic product while regenerating the active catalyst. Computational studies suggest that the 5-exo cyclization is the rate-determining step, followed by a thermodynamically favorable and kinetically facile 4-exo cyclization.
While a majority of examples of TiIII/IV catalysis involve the activation of polar C─O or C─N bonds, the recent advances highlighted in this section show the versatility of TiIII catalyst in engaging comparatively non-polar C─C bonds in radical functionalization reactions. Future work will likely further expand the scope of Ti radical catalysis towards a broader variety of substrates, including those with unactivated C─C bonds.
C─Halogen and C─S Bond Activation
Carbon─halogen (C─X) bonds are common motifs in organic compounds and widely used in transition metal-catalyzed cross-coupling reactions. Traditionally, C─X bonds are activated by palladium or other late transition metals through oxidative addition. In contrast, early transition metal catalysis has been underexplored for the conversion of organohalides. Furthermore, in comparison to transformations of C(sp)─X or C(sp2)─X bonds, the functionalization of unactivated alkyl halides, especially tertiary ones, remains a challenging problem in catalysis. A complementary approach to solving this challenge is to employ single-electron mediated reductive cleavage of C─X bonds, which can give rise to carbon-centered free radicals prior to further transformations.59 Recent studies demonstrated that TiIII species are capable of X-atom abstraction from unactivated alkyl halides owing to titanium’s high affinity to electronegative and “hard” anions.60-63
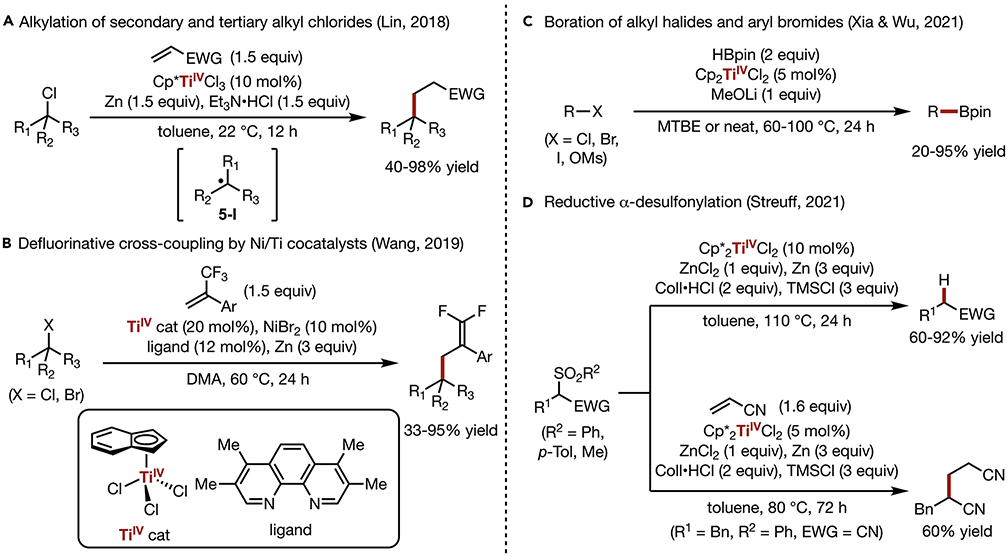
In 2018, Lin developed a TiIII-catalyzed radical alkylation of secondary and tertiary alkyl chlorides using Michael acceptors (Scheme 5A).60 This reaction is initiated by the reductive generation of the active TiIII species from catalyst precursor Cp*TiIVCl3, which is accelerated by the in situ generated Zn2+ Lewis acid. The TiIII complex abstracts a Cl atom from the alkyl chloride, and the resulting carbon-centered radical (5-I) then reacts with a Michael acceptor to form a new C─C bond. The driving force for this reaction is the formation a strong TiIV─Cl bond (BDE, bond dissociation energy, of 96 kcal/mol) at the expense of a weaker C─Cl bond (BDE of ca. 85 kcal/mol) in the substrate. While similar products may be obtained alternatively through the generation of Grignard reagents64 or Sn-mediated radical processes,65 the Ti-catalyzed protocol features milder reaction conditions and allows for chemoselective access to a broader scope of alkylation product. Recently, Koh and coworkers employed this reactivity in the Ti-catalyzed C-glycoside synthesis.61 Upon reductive dechlorination of a glycosyl chloride, the in-situ generated glycosyl radical undergoes C─C bond formation with an alkene or alkyne with high stereoselectivity.
Scheme 5. C─Halogen and C─S Bond Activation.
(A) Alkylation of secondary and tertiary alkyl chlorides
(B) Defluorinative cross-coupling by Ni/Ti cocatalysts
(C) Boration of alkyl halides and aryl bromides
(D) Reductive α-desulfonylation
A recent report by Wang shows that the alkyl radical intermediates generated by Ti-catalyzed activation of alkyl halides could also engage in cross-coupling reactions (Scheme 5B).62 Upon Ti-mediated C─X bond activation, the in-situ generated carbon centered radical undergoes Ni-mediated addition to the alkene, followed by β-F elimination to afford alkylated gem-difluoroalkene. In their previous study, Wang reported a similar transformation using Ni catalysis alone.66 However, the compatible substrates are limited to primary/secondary alkyl iodides and tertiary alkyl bromides due to the lack of reactivity of Ni towards cleaving stronger C─Cl or C─Br bonds. With the use of a Ti co-catalyst, the limitation is lifted and the reaction scope is further expanded to tertiary alkyl chlorides and primary/secondary alkyl bromides.
Recently, Xia and Wu reported Ti-catalyzed direct borylation of alkyl (pseudo)halides with pinacolborane (Scheme 5C).63 Previously reported alkyl halide borylation by transition metal catalysis (e.g., Cu, Ni, Pd) typically required the use of diboron compounds, with the use of boranes instead often leading to undesired hydrodehalogenation.67-69 In this work, the combination of a Ti catalyst and LiOMe base suppresses hydrodehalogenation and selectively provides the target borylation product. Radical clock experiments and EPR data suggest the formation of a carbon-centered radical intermediate and Cp2TiCl species in the reaction mixture. An alternative mechanism entailing alkene formation via based-promoted elimination followed by C═C bond hydroboration was ruled out.
Sulfones are versatile functional groups in organic synthesis that can be transformed into a variety of reactive intermediates.70 For example, the reductive desulfonylation has been observed in the presence of strong single-electron donors such as Na(Hg), Al(Hg), and SmI2.71 With the desire to achieve this transformation under milder and more practical conditions, Streuff developed a Ti-catalyzed reductive desulfonylation of α-sulfonyl nitriles and, in one instance, an α-sulfonyl ketone (Scheme 5D).72 In this reaction, the substrate first coordinates to TiIII via the cyano group, which induces homolytic cleavage of the C─S bond. This process generates a ketenimidotitanium complex and a sulfonyl radical, the former of which is protonated to provide the nitrile product and the latter is further reduced to a thiol. This protocol was further expanded to desulfonylative alkylation through Giese-type addition to acrylonitrile, providing a dinitrile product in 60% yield (Scheme 5D).
The examples highlighted in this section show that carbon─halogen bonds as well as carbon─sulfur bonds can be activated by TiIII to generate reactive radical intermediates. In addition to Giese-type addition, borylation, and hydrodefunctionalization, cross-coupling reactions could also be achieved through the incorporation of a Ni co-catalyst. These developments have opened a new avenue for the functionalization of a widely available class of electrophiles.
Future Outlook
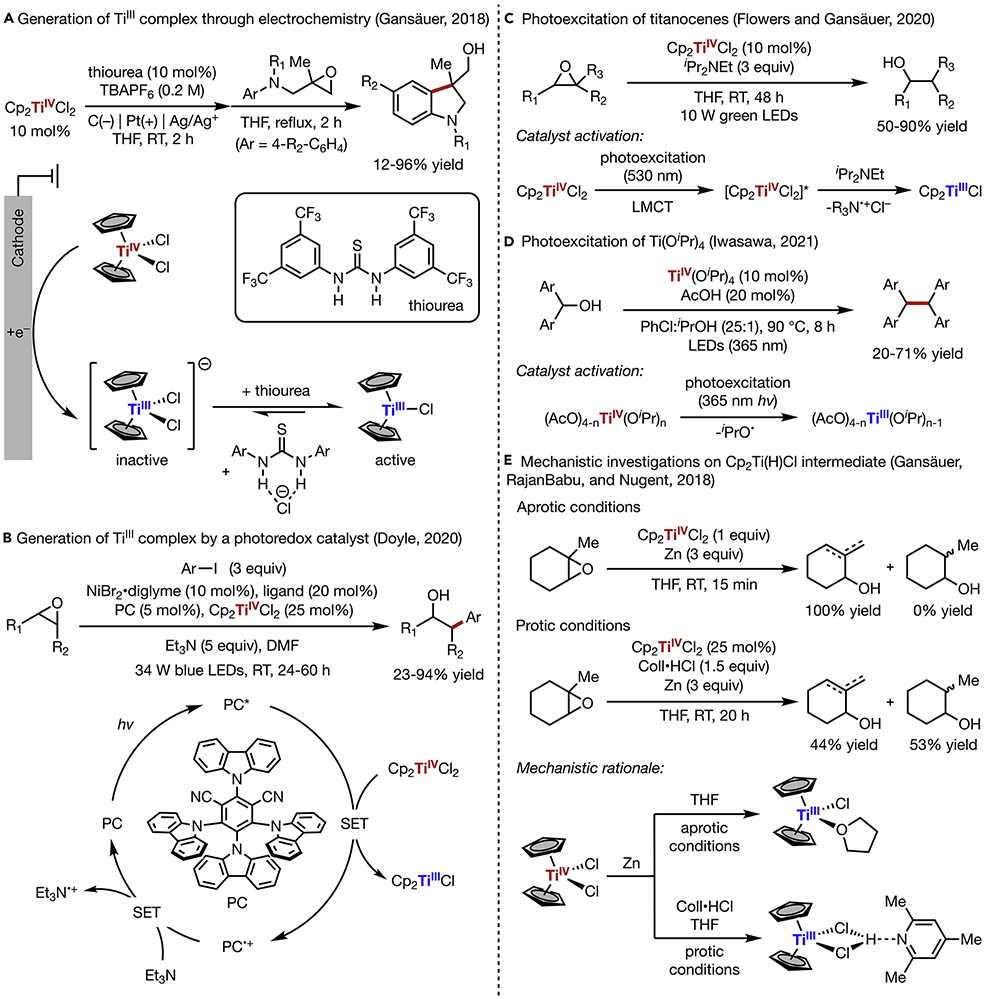
As progress has been made towards expanding the scope of Ti radical chemistry, the integration of TiIII/IV catalysis with other emerging branches of redox chemistry has further driven innovations in this area. For example, the generation of active TiIII species is being explored through alternative means, obfuscating stoichiometric heterogeneous reductants (e.g., Zn, Mg, Al, or Mn) that are conventionally used in Ti radical chemistry.17,73,74 In recent years, homogeneous reductants based on electron-rich organosilicon compounds have been employed for the generation of TiIII from titanocenes.75 Another attractive approach in this regard is the electrochemical reduction of TiIV species to TiIII. Previously, the electrochemical reduction of Cp2TiCl2 for in situ generation of Cp2TiCl has been studied using cyclic voltammetry,76,77 but the application of electrochemistry in Ti-mediated synthetic transformations remain underexplored. Recently, Gansäuer made use of electrochemically generated Cp2TiCl catalyst for the radical arylation of epoxides (Scheme 6A).78 Cathodic reduction of Cp2TiCl2 provides the catalyst resting state [Cp2TiCl2]− in equilibrium with the active catalyst Cp2TiCl. While the unreactive [Cp2TiCl2]− form is favored in the reaction medium, the equilibrium can be shifted in the presence of a thiourea additive as indicated by cyclic voltammetry data, thus turning on the desired epoxide ring-opening by TiIII.
Scheme 6. Future Outlook.
(A) Generation of TiIII complex through electrochemistry
(B) Generation of TiIII complex by a photoredox catalyst
(C) Photoexcitation of titanocenes
(D) Photoexcitation of Ti(OiPr)4
(E) Mechanistic investigations on Cp2Ti(H)Cl intermediate
An alternative strategy for the generation of TiIII species through photoredox chemistry has been demonstrated in Doyle’s Ni/Ti/photoredox-catalyzed cross-electrophile coupling of epoxides and aryl iodides (Scheme 6B).79 The irradiation of 4CzIPN (PC) gives rise to photoexcited PC*, which is sufficiently reducing (E1/2 = −1.18 V vs. SCE) to convert Cp2TiCl2 to Cp2TiCl (E1/2 = −0.57 V vs. SCE). This TiIII complex is then employed for the radical ring-opening of epoxides, forming carbon-centered radicals that engage in Ni-catalyzed cross-coupling reaction with aryl iodides. The photocatalytic cycle is closed by reducing PC radical cation to PC with Et3N.
The direct photoexcitation of titanocene has also been demonstrated by Gansäuer, exploiting the strong light absorption ability of the TiIV catalyst in the visible regime (Scheme 6C),81 while an alternative strategy for the generation of TiIII species was achieved through photoredox chemistry.80 The ligand-to-metal charge-transfer of photoexcited Cp2TiCl2* precedes its reductive quenching with iPr2NEt, thereby generating the active TiIII catalyst that promotes subsequent reductive epoxide ring-opening. Similarly, the use of Ti(OiPr)4 as a photocatalyst for dehydroxylative dimerization of benzylic alcohols was investigated by Iwasawa.82 Ligand exchange of Ti(OiPr)4 with acetic acid provides (AcO)4-nTiIV(OiPr)n, which undergoes photoinduced homolysis of a Ti─O bond to provide the catalytically active TiIII species towards alcohol activation.
Parallel to the reaction discovery efforts outlined above, mechanistic investigations have also been carried out to gain a better understanding of the role of TiIII catalysts in these diverse sets of reaction systems.83 For example, Gansäuer, RajanBabu, and Nugent investigated the TiIII-mediated ring opening of trisubstituted epoxides (Scheme 6E).84 In an aprotic reaction medium with THF as the solvent, TiIII exists as Cp2TiCl(THF), which can readily displace THF ligand with carbon-centered radicals generated from reductive epoxide ring-opening. The resultant Ti-alkyl species will undergo β-H elimination to afford allylic alcohols along with Cp2Ti(H)Cl, which is an unstable compound and rapidly decomposes through a bimolecular pathway to generate TiIII complexes. However, in the presence of a proton source such as Coll•HCl, Cp2TiCl forms a stable complex with Coll•HCl, which suppresses its reaction with carbon-centered radicals. Therefore, fully reduced alcohols are typically observed as the major products upon HAT from the solvent, Coll•HCl, or Cp2Ti(H)Cl to the carbon-centered radicals. Alternatively, it was proposed that HAT between two molecules of the β-titanoxyl radical intermediate could afford both saturated and allylic alcohol products simultaneously. These mechanistic insights helped reveal the complementary reactivity of TiIII complexes towards epoxide activation under aprotic or protic conditions leading to divergent product selectivity.
Insights gleaned from mechanistic studies can often provide guiding principles for the design of new catalysts towards uncovering unknown reactivities and achieving stereoselective catalysis. As an example, the development Ti-salen complexes as an alternative to canonical titanocenes in radical catalysis has provided a new and more modular catalyst platform for the discovery of enantioselective transformations.53-54,85 Synergy between reaction development and mechanistic understanding will continue to drive innovations in titanium radical chemistry towards providing new catalytic tools to facilitate the exploration of new synthetic spaces.
The bigger picture.
Challenges and Opportunities:
Expanding the scope of TiIII catalysis to the functionalization of new functional groups beyond epoxide and carbonyls.
Designing new catalysts and catalytic strategies towards the activation of inert chemical bonds.
Integrating TiIII catalysis with other redox modalities such as late transition metal catalysis, photocatalysis, and electrocatalysis.
Elucidating catalytic intermediates and understanding reaction mechanisms, employing mechanistic insights to guide catalyst design and reaction discovery.
ACKNOWLEDGMENTS
This work is dedicated to the occasion of Prof. John McMurry's 80th birthday. Financial support is provided by the National Institute of Health (GM134088) and the American Chemical Society (Petroleum Research Fund). We thank Justin S. K. Ho for the help in the preparation of the manuscript and Yihuan Lai for designing the table of content graphic.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES*
- 1.Marek I (2002). Titanium and zirconium in organic synthesis (Wiley-VCH; ). [Google Scholar]
- 2.Collins RA, Russell AF, and Mountford P (2015). Group 4 metal complexes for homogeneous olefin polymerisation: a short tutorial review. Appl. Petrochem. Res, 5, 153–171. 10.1007/s13203-015-0105-2. [DOI] [Google Scholar]
- 3.Katsuki T and Sharpless KB (1980). The first practical method for asymmetric epoxidation. J. Am. Chem. Soc 102. 5974–5976. 10.1021/ja00538a077. [DOI] [Google Scholar]
- 4.Ramon DJ, and Yus M (2006). In the arena of enantioselective synthesis, titanium complexes wear the laurel wreath. Chem. Rev 106, 2126–2208. 10.1021/cr040698p. [DOI] [PubMed] [Google Scholar]
- 5.McMurry JE, and Fleming MP (1974). New method for the reductive coupling of carbonyls to olefins. Synthesis of β-carotene. J. Am. Chem. Soc 96, 4708–4709. 10.1021/ja00821a076. [DOI] [PubMed] [Google Scholar]
- 6.Corey EJ, Danheiser RL, and Chandrasekaran S (1976). New reagents for the intermolecular and intramolecular pinacolic coupling of ketones and aldehydes. J. Org. Chem 41, 260–265. 10.1021/jo00864a016. [DOI] [Google Scholar]
- 7.Kulinkovich OG, and de Meijere A (2000). 1,n-Dicarbanionic titanium intermediates from monocarbanionic organometallics and their application in organic synthesis. Chem. Rev 100, 2789–2834. 10.1021/cr980046z. [DOI] [PubMed] [Google Scholar]
- 8.Estévez RE, Justicia J, Bazdi B, Fuentes N, Paradas M, Choquesillo-Lazarte D, García-Ruiz JM, Robles R, Gansäuer A, Cuerva JM, et al. (2009).Ti-Catalyzed Barbier-type allylations and related reactions. Chem. Eur. J 15, 2774–2791. 10.1002/chem.200802180. [DOI] [PubMed] [Google Scholar]
- 9.Blanco-Urgoiti J, Añorbe L, Pérez-Serrano L, Domínguez G, and Pérez-Castells J (2004). The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules. Chem. Soc. Rev 33, 32–42. 10.1039/B300976A. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert ZW, Hue RJ, and Tonks IA (2016). Catalytic formal [2+2+1] synthesis of pyrroles from alkynes and diazenes via TiII/TiIV redox catalysis. Nat. Chem 8, 63–68. 10.1038/nchem.2386. [DOI] [PubMed] [Google Scholar]
- 11.Hanna TE, Lobkovsky E and Chirik PJ (2004) Dinitrogen activation by titanium sandwich complexes. J. Am. Chem. Soc 126, 14688–14689. 10.1021/ja045884r. [DOI] [PubMed] [Google Scholar]
- 12.hu S, Shima T, and Hou Z (2020). Hydrodeoxygenative cyclotetramerization of carbon monoxide by a trinuclear titanium polyhydride complex. J. Am. Chem. Soc 142, 19889–19894. 10.1021/jacs.0c10403. [DOI] [PubMed] [Google Scholar]
- 13.McCallum T, Wu X, and Lin S (2019). Recent advances in titanium radical redox catalysis. J. Org. Chem 84, 14369–14380. 10.1021/acs.joc.9b02465. [DOI] [PubMed] [Google Scholar]
- 14.Beaumier EP, Pearce AJ, See XY, and Tonks IA (2019). Modern applications of low-valent early transition metals in synthesis and catalysis. Nat. Rev. Chem 3, 15–34. 10.1038/s41570-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manßen M, and Schafer LL (2020). Titanium catalysis for the synthesis of fine chemicals – development and trends. Chem. Soc. Rev 49, 6947–6994. 10.1039/d0cs00229a. [DOI] [PubMed] [Google Scholar]
- 16.Birmingham JM, Fischer AK, and Wilkinson G (1955). The reduction of bis-cyclopentadienyl compounds. Naturwissenschaften. 42, 96. 10.1007/BF00617242. [DOI] [Google Scholar]
- 17.RajanBabu TV and Nugent WA (1989). Intermolecular addition of epoxides to activated olefins: a new reaction. J. Am. Chem. Soc 111, 4525–4527. 10.1021/ja00194a073. [DOI] [Google Scholar]
- 18.RajanBabu TV, Nugent WA, and Beattie MS (1990). Free radical-mediated reduction and deoxygenation of epoxides. J. Am. Chem. Soc 112, 6408–6409. 10.1021/ja00173a045. [DOI] [Google Scholar]
- 19.Clive DL, and Magnuson SR (1995). Synthesis of the sesquiterpene (±)-Ceratopicanol: Use of radicals derived from epoxides. Tetrahedron Lett. 36, 15–18. 10.1016/0040-4039(94)02158-8. [DOI] [Google Scholar]
- 20.Gansäuer A, Pierobon M, and Bluhm H (1998). Catalytic, highly regio-and chemoselective generation of radicals from epoxides: Titanocene dichloride as an electron transfer catalyst in transition metal catalyzed radical reactions. Angew. Chem. Int. Ed 37, 101–103. . [DOI] [Google Scholar]
- 21.Gansäuer A, Lauterbach T, Bluhm H, and Noltemeyer M (1999). A catalytic enantioselective electron transfer reaction: Titanocene-catalyzed enantioselective formation of radicals from meso-epoxides. Angew. Chem. Int. Ed 38, 2909–2910. . [DOI] [PubMed] [Google Scholar]
- 22.Gansäuer A, Rinker B, Pierobon M, Grimme S, Gerenkamp M and Mück-Lichtenfeld C (2003). A radical tandem reaction with homolytic cleavage of a Ti─O bond. Angew. Chem. Int. Ed 42, 3687–3690. 10.1002/anie.200351240. [DOI] [PubMed] [Google Scholar]
- 23.Cuerva JM, Campaña AG, Justicia J, Rosales A, Oller-López JL, Robles R, Cárdenas DJ, Buñuel E and Oltra JE (2006). Water: The ideal hydrogen-atom source in free-radical chemistry mediated by TiIII and other single-electron-transfer metals? Angew. Chem. Int. Ed 45, 5522–5526. 10.1002/anie.200600831. [DOI] [PubMed] [Google Scholar]
- 24.Cannella R, Clerici A, Panzeri W, Pastori N, Punta C, and Porta O (2006). Free-radical version of the Strecker synthesis of α-aminoamides promoted by aqueous H2O2/TiCl3/HCONH2 system. J. Am. Chem. Soc 128, 5358–5359. 10.1021/ja061092g. [DOI] [PubMed] [Google Scholar]
- 25.Gansäuer A, Ndene N, Lauterbach T, Justicia J, Winkler I, Mück-Lichtenfeld C, and Grimme S (2008). Titanocene catalyzed opening of oxetanes. Tetrahedron, 64, 11839–11845. 10.1016/j.tet.2008.08.107. [DOI] [Google Scholar]
- 26.Dieguez HR, Lopez A, Domingo V, Arteaga JF, Dobado JA, Herrador MM, Quilez del Moral JF and Barrero AF (2010). Weakening C─O bonds: Ti(III), a new reagent for alcohol deoxygenation and carbonyl coupling olefination. J. Am. Chem. Soc 132, 254–259. 10.1021/ja906083c. [DOI] [PubMed] [Google Scholar]
- 27.Rosales A, Muñoz-Bascón J, López-Sánchez C, Álvarez-Corral M, Muñoz-Dorado M, Rodríguez-García I, and Oltra JE (2012). Ti-catalyzed homolytic opening of ozonides: a sustainable C─C bond-forming reaction. J. Org. Chem 77, 4171–4176. 10.1021/jo300344a. [DOI] [PubMed] [Google Scholar]
- 28.Streuff J (2011). A titanium(III)-catalyzed redox umpolung reaction for the reductive cross-coupling of enones with acrylonitriles. Chem. Eur. J 17, 5507–5510. 10.1002/chem.201100501. [DOI] [PubMed] [Google Scholar]
- 29.Estévez RE, Paradas M, Millan A, Jiménez T, Robles R, Cuerva JM, and Oltra JE (2008). Ti-catalyzed Reformatsky-type coupling between α-halo ketones and aldehydes. J. Org. Chem 73, 1616–1619. 10.1021/jo702189k. [DOI] [PubMed] [Google Scholar]
- 30.Frey G, Hausmann JN, and Streuff J (2015). Titanium-catalyzed reductive umpolung reactions with a metal-free terminal reducing agent. Chem. Eur. J 21, 5693–5696. 10.1002/chem.201500102. [DOI] [PubMed] [Google Scholar]
- 31.Campaña AG, Bazdi B, Fuentes N, Robles R, Cuerva JM, Oltra JE, Porcel S and Echavarren AM (2008). Divergent titanium-mediated allylations with modulation by nickel or palladium. Angew. Chem. Int. Ed 47, 7625–7629. 10.1002/anie.200802520. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, and Weix DJ (2014). Nickel-catalyzed regiodivergent opening of epoxides with aryl halides: Co-catalysis controls regioselectivity. J. Am. Chem. Soc 136, 48–51. 10.1021/ja410704d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaumont S, Ilardi EA, Monroe LR, and Zakarian A (2010). Valence tautomerism in titanium enolates: catalytic radical haloalkylation and application in the total synthesis of neodysidenin. J. Am. Chem. Soc 132, 1482–1483. 10.1021/ja910154f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Z, Herrmann AT, and Zakarian A (2011). Dual Ti─Ru catalysis in the direct radical haloalkylation of N-acyloxazolidinones. Angew. Chem. Int. Ed 50, 7136–7139. 10.1002/anie.201101364w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarantino KT, Miller DC, Callon TA, and Knowles RR (2015). Bond-weakening catalysis: Conjugate aminations enabled by the soft homolysis of strong N─H bonds. J. Am. Chem. Soc 137, 6440–6443. 10.1021/jacs.5b03428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gianino JB, and Ashfeld BL (2012). Titanocene-catalyzed multicomponent coupling approach to diarylethynyl methanes. J. Am. Chem. Soc 134, 18217–18220. 10.1021/ja308891e. [DOI] [PubMed] [Google Scholar]
- 37.Yao C, Dahmen T, Gansäuer A, and Norton J (2019). Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 364, 764–767. 10.1126/science.aaw391. [DOI] [PubMed] [Google Scholar]
- 38.Henriques DSG, Rojo-Wiechel E, Klare S, Mika R, Höthker S, Schacht JH, Schmickler N and Gansäuer A, (2021). Titanocene (III)-catalyzed precision deuteration of epoxides. Angew. Chem. Int. Ed 61, e202114198. 10.1002/anie.202114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gansäuer A, Behlendorf M, von Laufenberg D, Fleckhaus A, Kube C, Sadasivam DV, and Flowers RA (2012). Catalytic, atom-economical radical arylation of epoxides. Angew. Chem. Int. Ed 51, 4739–4742. 10.1002/anie.201200431 [DOI] [PubMed] [Google Scholar]
- 40.Mühlhaus F, Weißbarth H, Dahmen T, Schnakenburg G, and Gansäuer A (2019). Merging regiodivergent catalysis with atom-economical radical arylation. Angew. Chem. Int. Ed 58, 14208–14212. 10.1002/anie.201908860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye KY, McCallum T, and Lin S (2019). Bimetallic radical redox-relay catalysis for the isomerization of epoxides to allylic alcohols. J. Am. Chem. Soc 141, 9548–9554. 10.1021/jacs.9b04993. [DOI] [PubMed] [Google Scholar]
- 42.Gansäuer A, Grimme S, Funk P, Richrath R, and Bohle F (2021). Oxidizing under reductive conditions: From benzylic ethers to acetals with perfect atom-economy by titanocene (III)-catalysis. Angew. Chem. Int. Ed 60, 5482–5488. 10.1002/anie.202013561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie H, Guo J, Wang YQ, Wang K, Guo P, Su PF, Wang X and Shu XZ (2020). Radical dehydroxylative alkylation of tertiary alcohols by Ti catalysis. J. Am. Chem. Soc 142, 16787–16794. 10.1021/jacs.0c07492. [DOI] [PubMed] [Google Scholar]
- 44.Xie H, Wang S, Wang Y, Guo P, and Shu XZ (2022). Ti-Catalyzed reductive dehydroxylative vinylation of tertiary alcohols. ACS Catal. 12, 1018–1023. 10.1021/acscatal.1c05530. [DOI] [Google Scholar]
- 45.Hao W, Wu X, Sun JZ, Siu JC, MacMillan SN, and Lin S (2017). Radical redox-relay catalysis: formal [3+ 2] cycloaddition of N-acylaziridines and alkenes. J. Am. Chem. Soc 139, 12141–12144. 10.1021/jacs.7b06723. [DOI] [PubMed] [Google Scholar]
- 46.Huang HM, Garduño-Castro MH, Morrill C, and Procter DJ (2019). Catalytic cascade reactions by radical relay. Chem. Soc. Rev 48, 4626–4638. 10.1039/C8CS00947C. [DOI] [PubMed] [Google Scholar]
- 47.Moreira IDP, Bofill JM, Anglada JM, Solsona JG, Nebot J, Romea P, and Urpí F (2008). Unconventional biradical character of titanium enolates. J. Am. Chem. Soc 130, 3242–3243. 10.1021/ja076625f. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YQ, Vogelsang E, Qu ZW, Grimme S, and Gansäuer A (2017). Titanocene-catalyzed radical opening of N-acylated aziridines. Angew. Chem. Int. Ed 56, 12654–12657. 10.1002/anie.201707673. [DOI] [PubMed] [Google Scholar]
- 49.Wood DP, Guan W, and Lin S (2021). Titanium and cobalt bimetallic radical redox relay for the isomerization of N-Bz aziridines to allylic amides. Synthesis, 53, 4213–4220. 10.1055/s-0037-1610779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frey G, Luu HT, Bichovski P, Feurer M, and Streuff J (2013). Convenient titanium (III)-catalyzed synthesis of cyclic aminoketones and pyrrolidinones - Development of a formal [4+1] cycloaddition. Angew. Chem. Int. Ed 52, 7131–7134. 10.1002/anie.201302460. [DOI] [PubMed] [Google Scholar]
- 51.Leijendekker LH, Weweler J, Leuther TM, and Streuff J (2017). Catalytic reductive synthesis and direct derivatization of unprotected aminoindoles, aminopyrroles, and iminoindolines. Angew. Chem. Int. Ed 56, 6103–6106. 10.1002/anie.201702310. [DOI] [PubMed] [Google Scholar]
- 52.Chenniappan VK, Silwal S, and Rahaim RJ (2018). Ni/Ti Dual catalytic cross-coupling of nitriles and organobromides to access ketones. ACS Catalysis 8, 4539–4544. 10.1021/acscatal.8b00244. [DOI] [Google Scholar]
- 53.Hao W, Harenberg JH, Wu X, MacMillan SN, and Lin S (2018). Diastereo- and enantioselective formal [3+2] cycloaddition of cyclopropyl ketones and alkenes via Ti-catalyzed radical redox relay. J. Am. Chem. Soc 140, 3514–3517. 10.1021/jacs.7b13710. [DOI] [PubMed] [Google Scholar]
- 54.Robinson SG, Wu X, Jiang B, Sigman MS, and Lin S (2020). Mechanistic studies inform design of improved Ti(salen) catalysts for enantioselective [3 + 2] cycloaddition. J. Am. Chem. Soc 142, 18471–18482. 10.1021/jacs.0c07128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weweler J, Younas SL, and Streuff J (2019). Titanium(III)-catalyzed reductive decyanation of geminal dinitriles by a non-free-radical mechanism. Angew. Chem. Int. Ed 58, 17700–17703. 10.1002/anie.201908372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, Stückrath JB, Grimme S, and Gansäuer A (2021). Titanocene-catalyzed [2+2] cycloaddition of bisenones and comparison with photoredox catalysis and established methods. Angew. Chem. Int. Ed 60, 14339–14344. 10.1002/anie.202102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curran DP, and Seong CM (1990). Atom transfer addition, annulation, and macrocyclization reactions of iodomalononitriles. J. Am. Chem. Soc 112, 9401–9403. 10.1021/ja00181a057. [DOI] [Google Scholar]
- 58.Kawamoto T, Geib SJ, and Curran DP (2015). Radical reactions of N-heterocyclic carbene boranes with organic nitriles: Cyanation of NHC-boranes and reductive decyanation of malononitriles. J. Am. Chem. Soc 137, 8617–8622. 10.1021/jacs.5b04677. [DOI] [PubMed] [Google Scholar]
- 59.Lekkala R, Lekkala R, Moku B, Rakesh KP, and Qin H-L (2019). Recent developments in radical-mediated transformations of organohalides. Eur. J. Org. Chem 2019, 2769–2806. 10.1002/ejoc.201900098. [DOI] [Google Scholar]
- 60.Wu X, Hao W, Ye K-Y, Jiang B, Pombar G, Song Z, and Lin S (2018). Ti-Catalyzed radical alkylation of secondary and tertiary alkyl chlorides using Michael acceptors. J. Am. Chem. Soc 140, 14836–14843. 10.1021/jacs.8b08605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Y, Wang Q, Zhang X, and Koh MJ (2021). Synthesis of C-glycosides by Ti-catalyzed stereoselective glycosyl radical functionalization. Chem 7, 3377–3392. 10.1016/j.chempr.2021.09.008. [DOI] [Google Scholar]
- 62.Lin Z, Lan Y, and Wang C (2019). Reductive allylic defluorinative cross-coupling enabled by Ni/Ti cooperative catalysis. Org. Lett 21, 8316–8322. 10.1021/acs.orglett.9b03102. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Cui P, Xia C, and Wu L (2021). Catalytic boration of alkyl halides with borane without hydrodehalogenation enabled by titanium catalyst. Angew. Chem. Int. Ed 60, 12298–12303. 10.1002/anie.202100569. [DOI] [PubMed] [Google Scholar]
- 64.Chai G, Lu Z, Fu C, and Ma S (2009). Ferric chloride hexahydrate-catalyzed highly regio- and stereoselective conjugate addition reaction of 2,3-allenoates with Grignard reagents: An efficient synthesis of β,γ-alkenoates. Adv. Synth. Cata 351,1946–1954. 10.1002/adsc.200900091. [DOI] [Google Scholar]
- 65.Hanessian S, Di Fabio R, Marcoux JF, and Prud’homme MJ (1990). The synthesis of substituted lactones by intramolecular chirality transfer with stereodifferentiating chiral α-ester radical intermediates J. Org. Chem 55, 3436–3438. 10.1021/jo00298a005. [DOI] [Google Scholar]
- 66.Lan Y, Yang F, and Wang C (2018). Synthesis of gem-difluoroalkenes via nickel-catalyzed allylic defluorinative reductive cross-coupling. ACS Catal. 8, 9245–9251. 10.1021/acscatal.8b02784. [DOI] [PubMed] [Google Scholar]
- 67.Iwamoto H, Endo K, Ozawa Y, Watanabe Y, Kubota K, Imamoto T, and Ito H (2019). Copper(l)- catalyzed enantioconvergent borylation of racemic benzyl chlorides enabled by quadrant-by-quadrant structure modification of chiral bisphosphine ligands. Angew. Chem. Int. Ed 58, 11112–11117. 10.1002/anie.201906011. [DOI] [PubMed] [Google Scholar]
- 68.Dudnik AS and Fu GC (2012). Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon─boron bonds. J. Am. Chem. Soc 134, 10693–10697. 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi J, Liu J-H, Liang J-L, Dai J-J, Yang C-T, Fu Y, and Liu L (2012). Alkylboronic esters from palladium- and nickel-catalyzed borylation of primary and secondary alkyl bromides. Adv. Synth. Catal 354, 1685–1691. 10.1002/adsc.201200136. [DOI] [Google Scholar]
- 70.Trost BM, and Kalnmals CA (2019). Sulfones as chemical chameleons: Versatile synthetic equivalents of small-molecule synthons. Chem. Eur. J 25, 11193–11213. 10.1002/chem.201902019. [DOI] [PubMed] [Google Scholar]
- 71.Nájera C, and Yus M (1999). Desulfonylation reactions: Recent developments. Tetrahedron 55, 10547–10658. 10.1016/S0040-4020(99)00600-6. [DOI] [Google Scholar]
- 72.Kern C, Selau J, and Streuff J (2021). A titanium-catalyzed reductive α-desulfonylation. Chem. Eur. J 27, 6178–6182. 10.1002/chem.202005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coutts RSP, Wailes PC, and Martin RL (1973). Dimeric dicyclopentadienyltitanium(III) halides. J. Organomet. Chem 47, 375–382. 10.1016/S0022-328X(00)81748-9. [DOI] [Google Scholar]
- 74.Sekutowski DG, and Stucky GD (1975). Synthesis and structure of some bis(cyclopentadienyl)titanium(III) metal halides. Inorg. Chem 14, 2192–2199. 10.1021/ic50151a033. [DOI] [Google Scholar]
- 75.Saito T, Nishiyama H, Tanahashi H, Kawakita K, Tsurugi H, and Mashima K (2014). 1,4-Bis(trimethylsilyl)-1,4-diaza-2,5-cyclohexadienes as strong salt-free reductants for generating low-valent early transition metals with electron-donating ligands. J. Am. Chem. Soc 136, 5161–5170. 10.1021/ja501313s. [DOI] [PubMed] [Google Scholar]
- 76.Enemærke RJ, Larsen J, Skrydstrup T, and Daasbjerg K (2004). Revelation of the nature of the reducing species in titanocene halide-promoted reductions. J. Am. Chem. Soc 126, 7853–7864. 10.1021/ja0491230. [DOI] [PubMed] [Google Scholar]
- 77.Enemaerke RJ, Larsen J, Skrydstrup T, and Daasbjerg K (2004). Mechanistic investigation of the electrochemical reduction of Cp2TiX2. Organometallics 23, 1866–1874. 10.1021/om034360. [DOI] [Google Scholar]
- 78.Liedtke T, Spannring P, Riccardi L, and Gansäuer A (2018). Mechanism-based condition screening for sustainable catalysis in single-electron steps by cyclic voltammetry. Angew. Chem. Int. Ed 57, 5006–5010. 10.1002/anie.201800731. [DOI] [PubMed] [Google Scholar]
- 79.Parasram M, Shields BJ, Ahmad O, Knauber T, and Doyle AG (2020). Regioselective cross-electrophile coupling of epoxides and (hetero)aryl iodides via Ni/Ti/photoredox catalysis. ACS Catal. 10, 5821–5827. 10.1021/acscatal.0c01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z, Richrath RB, and Gansäuer A (2019). Merging catalysis in single electron steps with photoredox catalysis – Efficient and sustainable radical chemistry. ACS Catal. 9, 3208–3212. 10.1021/acscatal.9b00787. [DOI] [Google Scholar]
- 81.Zhang Z, Hilche T, Slak D, Rietdijk NR, Oloyede UN, Flowers RA II, and Gansäuer A (2020). Titanocenes as photoredox catalysts using green-light irradiation. Angew. Chem. Int. Ed 59, 9355–9359. 10.1002/anie.202001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sumiyama K, Toriumi N, and Iwasawa N (2021). Use of isopropyl alcohol as a reductant for catalytic dehydoxylative dimerization of benzylic alcohols utilizing Ti─O bond photohomolysis. Eur. J. Org. Chem 2021, 2474–2478. 10.1002/ejoc.202100286. [DOI] [Google Scholar]
- 83.Nugent WA, and RajanBabu TV (2021). Four mechanistic mysteries: The benefits of writing a critical review. Angew. Chem. Int. Ed 60, 2194–2201. 10.1002/anie.202011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gordon J, Hildebrandt S, Dewese KR, Klare S, Gansaüer A, RajanBabu TV, and Nugent WA (2018). Demystifying Cp2Ti(H)Cl and its enigmatic role in the reactions of epoxides with Cp2TiCl. Organometallics 37, 4801–4809. 10.1021/acs.organomet.8b00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilli G, Cruickshank DWJ, Beddoes RL, and Mills OS (1972). The crystal and molecular structure of dichloro-N,N’-ethylenebis(salicylideneiminato)titanium(IV)-tetrahydrofuran. Acta Cryst. B28, 1889–1893. [Google Scholar]