Abstract
Immune‐mediated diseases (IMDs) are chronic conditions that have an immune‐mediated etiology. Clinically, these diseases appear to be unrelated, but pathogenic pathways have been shown to connect them. While inflammation is a common occurrence in the body, it may either stimulate a favorable immune response to protect against harmful signals or cause illness by damaging cells and tissues. Nanomedicine has tremendous promise for regulating inflammation and treating IMIDs. Various nanoparticles coated with nanotherapeutics have been recently fabricated for effective targeted delivery to inflammatory tissues. RNA interference (RNAi) offers a tremendous genetic approach, particularly if traditional treatments are ineffective against IMDs. In cells, several signaling pathways can be suppressed by using RNAi, which blocks the expression of particular messenger RNAs. Using this molecular approach, the undesirable effects of anti‐inflammatory medications can be reduced. Still, there are many problems with using short‐interfering RNAs (siRNAs) to treat IMDs, including poor localization of the siRNAs in target tissues, unstable gene expression, and quick removal from the blood. Nanotherapeutics have been widely used in designing siRNA‐based carriers because of the restricted therapy options for IMIDs. In this review, we have discussed recent trends in the fabrication of siRNA nanodelivery systems, including lipid‐based siRNA nanocarriers, liposomes, and cationic lipids, stable nucleic acid‐lipid particles, polymeric‐based siRNA nanocarriers, polyethylenimine (PEI)‐based nanosystems, chitosan‐based nanoformulations, inorganic material‐based siRNA nanocarriers, and hybrid‐based delivery systems. We have also introduced novel siRNA‐based nanocarriers to control IMIDs, such as pulmonary inflammation, psoriasis, inflammatory bowel disease, ulcerative colitis, rheumatoid arthritis, etc. This study will pave the way for new avenues of research into the diagnosis and treatment of IMDs.
Keywords: autoimmunity, drug delivery, inflammation, nanotechnology, nanotherapeutics, siRNA
Abbreviations
- ARDS
acute respiratory distress syndrome
- CDs
carbon dots
- CNTs
carbon nanotubes
- DCs
dendritic cells
- DOPE
dioleoylphosphatidylethanolamine
- DOTAP
1,2‐dioleoyl‐3‐trimethylammoniumpropane
- FDA
Food and Drug Administration
- IBD
inflammatory bowel disease
- ICH
ntracerebral hemorrhage
- IL‐6
interleukin 6
- IMDs
immune‐mediated diseases
- LPNs
lipid‐polymer nanoparticles
- NF
nuclear factor
- NK
natural killer
- NLC
nanostructured lipid carriers
- NMs
nanomaterials
- NPs
nanoparticles
- PDT
photodynamic therapy
- PEI
polyethylenimine
- PLGA
polyglycolic acid
- PLL
poly‐l‐Lysine
- RA
rheumatoid arthritis
- RNAi
RNA interference
- siRNAs
short‐interfering RNAs
- SLE
systemic lupus erythematosus
- SNALPs
stable nucleic acid‐lipid particles
- TLRs
toll‐like receptors
- TNF‐α
tumor necrosis factor‐alpha
- UC
ulcerative colitis
1. INTRODUCTION
Immune‐mediated diseases (IMDs), including rheumatoid arthritis (RA), systemic lupus erythematosus, inflammatory bowel disease (IBD), ulcerative colitis (UC), Crohn's disease, psoriasis, and other inflammatory conditions, are complex conditions affected by both environmental and genetic factors. The relationships between genetic markers and predisposition to these disorders have been widely investigated, and hundreds of risk factors have emerged over the last 20 years, with scientists identifying similar inherited patterns between them (Axelrad, 2021; Borren & Ananthakrishnan, 2019; González‐Serna et al., 2020; Lo et al., 2021). The inflammation process could be stimulated by physical, chemical, biological, or psychological events in acute and chronic states (Chen et al., 2017). On the other hand, chronic inflammation is a significant influencer in diseases like cancer, autoimmune disorders, asthma (Hunter, 2012), bone diseases [arthritis (Moudgil & Choubey, 2011; Rojas et al., 2018), osteoporosis (Hoffmann et al., 2016) and gout (Bohatá et al., 2021)], heart diseases, diabetes (Lopez‐Candales et al., 2017), neurodegenerative diseases (Barcelos et al., 2019), Crohn's disease and UC (Deepak et al., 2019). The human immune response to protect the steady functionality of the body is a complex network of immune cells and molecules. Autoimmune diseases refer to abnormal responses with systemic or localized disorder origins, which could include the mechanisms like cytokine dysregulation (Moudgil & Choubey, 2011), T‐cell mediated (Sasaki et al., 2019), B cell receptor‐mediated (Taher et al., 2017), dendritic cell (DC) apoptosis (Ganguly et al., 2013), molecular mimicry (Rojas, Restrepo‐Jiménez, 2018), etc. Abnormal inflammation response contributes to several autoimmune diseases and inflammation regulatory methods are promising therapeutic options (Duan et al., 2019). As a treatment view of IMDs, gene expression knockdown could stop the disease's main pathways. Regulation of gene expression could modify DNA, transcriptional, posttranscriptional, and translational regulations by changing the rates, structure, factors, and stabilities. Gene knockdown therapies with RNA interference (RNAi) pathways have recently received great attention (Mocellin & Provenzano, 2004).
Small interfering RNAs (siRNAs) are silencing RNAs (noncoding double‐stranded RNA molecules) with approximately 20–25 base pairs (bps) that with phosphorylated 5′ end, hydroxylated 3′ end, and two overhanging nucleotides, involved in RNAi pathways. Stable siRNA could deliver as stem‐loops in multiple promoter/shRNA, long hairpin RNA (hpRNA), and microRNA (miRNA)‐embedded structures. The siRNA could be introduced to cells by exogenous origin, uptaking, vectors (virus, transposons) (Cambon & Déglon, 2013), electroporation (Muller et al., 2015), or direct injection. According to clinical trials, there are currently 90 registered siRNA‐related projects, including Cosdosiran, Nedosiran, Tivanisiran, Teprasiran, Vutrisiran, and Fitosiran in phase 3, which 40 of them stated as completed (2022). Commercial siRNA therapeutics are patisiran, givosiran, Inclisiran, and lumasiran, which gained the US Food and Drug Administration (FDA) approvals. Patisiran is formulated with a lipid nanoparticle (NP) delivery system for polyneuropathy patients (FDA, 2018). Givosiran is formulated with GalNAc conjugation for hepatic porphyria disease (FDA, 2019). Lumasiran formulation with GalNAc conjugation is approved to treat primary hyperoxaluria type 1 (FDA, 2020).
Nanotechnology's emergence in medicine has excellent applications in pharmaceutical delivery (Mei et al., 2013), high‐quality diagnostics (Mukhtar et al., 2021; Sheervalilou et al., 2021), and regenerative medicine (Garbayo et al., 2020). Several commercial nanotechnology‐enabled drugs have been FDA‐approved, including Cabenuva, Rapamune, Doxil, Abraxane, VYXEOS, Copaxone, Onivyde, Abelcet Estrasorb, Avinza, Feraheme, etc. (2022, Albalawi et al., 2021; FDA, 2022). Furthermore, the application of nanotechnology has a great impact on medical devices such as thin‐film protection, sensors, actuators, and delivery mechanisms, which reduces the size while improving efficiency. In this connection, nanotechnology‐assisted drug delivery is a promising solution to the issues above and precise control of the delivery process. A broad range of nanomaterials (NMs), including organic and inorganic NPs, are fabricated in the forms of nanoemulsions, dendrimers, polymers, liposomes, lipids, mesoporous silicon, etc. (Patra et al., 2018). Nanodelivery has advantages of targeted stability, biocompatibility, and bioavailability. Despite the advantages, the toxicity and biodegradability of NMs pose a great challenge to in vivo applications, which could be viewed as an opportunity (Albalawi et al., 2021). Inflammation‐induction issues and immune stimulation of NMs are the main concerns as they could easily cross barriers and persist in organs which require accurate controlling of the physicochemical properties (Jeong et al., 2022). Proinflammatory cytokines are activated in case of lung inflammation by triggering tumor necrosis factor‐α (TNF‐α) production for initiation in the preliminary lung infections, mainly referred to as chronic obstructive pulmonary disease, asthma, acute respiratory distress syndrome, acute lung injury, and COVID‐19 (Subhan et al., 2022). Therefore, siRNA can be modulated for localized and targeted delivery through airways by inhalation via nasal route, as shown in Figure 1, to exert its action directly on the inflamed tissues and achieve rapid onset of action, reduced dosing frequency, and reduced side effects (Mahinfar et al., 2022).
Figure 1.
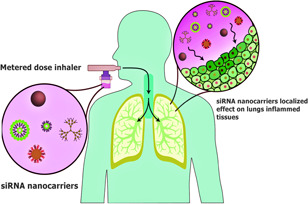
siRNA can be modulated for localized and targeted delivery through airways by inhalation via nasal route. siRNA, short‐interfering RNA
In MS complex autoimmune cases, nanocarriers like nanostructured lipid carriers could cross the blood‐brain barrier (BBB) (Chountoulesi & Demetzos, 2020), carrying lipophilic and hydrophilic drug loads with high capacity and release controls (Chauhan et al., 2020). In a RA case, Sun et al. (2019b) synthesized pH‐sensitive polymeric NPs for siRNA‐delivery with neutral degradation products, rapid‐controlled release, and a faster degradation profile. Pandi et al. (2018) had compared dendriplexes and lipoplex carriers for topical siRNA‐delivery safe formulation to treat psoriasis. Such studies show nanomedicine's important role in overcoming the specific challenges in inflammation/autoimmune diseases treatment.
The ideal in vivo siRNA delivery mechanism should be biocompatible and nonimmunogenic, protect siRNA against serum nucleases, and allow siRNA delivery by endocytosis to target cells after systemic administration (Oh & Park, 2009). Over the last decade, nanotechnology has proven to be a fast and effective way to specifically target cell types with potential therapies such as siRNA in autoimmune conditions (Tarner & Fathman, 2001; Verma et al., 2021). The small size of immune‐inert NPs leads to targeted delivery of cargo to the destination, as well as small biodegradable NPs can significantly evade trap by the reticuloendothelial system that serves as a system for particles cleaning and soluble substances in the blood circulatory system resulting in prolonging the presence of the therapeutics (Alexis, Tang et al., 2019). Positively charged peptide NPs could embed the negatively charged siRNA with charge‐charge interaction, assembled on a core NP to treat breast cancer (Lee et al., 2017).
Carbon nanotubes (CNTs) have toxicity that could accumulate in the body, and researchers have studied the functionalized CNTs to overcome the disadvantages (Li et al., 2016). Virus‐like particles assembly of siRNA in the lumen area to treat the osteoporosis in a rat model (Hoffmann, Böker, 2016). In photodynamic therapy (PDT) for cancer treatment, NMs have increased efficiency, specificity, stability, drug load capacity, upconversion, and therapy resistance (Chen et al., 2020). Laroui et al. have combined noninvasive PDT and gene silencing methods with a novel cationic guanidylated porphyrin (H2‐PG)‐siRNA NPs therapy to treat breast cancer, indicating the potential of nanomedicine in modern therapies (Laroui et al., 2019). Li et al. (2021) have synthesized polyethyleneimine (PEI) modified carbon dots (CDs) for siRNA‐based downregulation of HDGF with enhanced stability, emission‐independent excitation (traceable), and transfection efficiency (Li et al., 2021), which indicates nanomedicine potential in novel treatment methods.
Many therapeutic goals against IMDs are ineffective due to their lack of focus on the specific target cells for treatment. However, the increase in the number of patients with autoimmune disorders and the convergence of nanotechnology and biotechnology has led to the development of methods based on defective gene silencing specifically, which can be an appropriate solution to coverage of traditional methods disadvantages.
Following the granted patents, commercial application, and approval of the siRNA‐based gene expression regulatory drugs, great interests have focused on the siRNA‐based therapeutics market to treat various diseases. This review article will attempt to briefly discuss the mechanisms of siRNA‐based Gene knockdown therapies and by focusing on nanotherapeutic approaches against IMDs. In addition, the possible challenges and potential strategies from treatment view to commercial scale‐up are addressed. Finally, we will discuss the challenges of applying siRNA‐based nontherapeutic approaches and future prospects.
2. IMDS
2.1. Psoriasis
Psoriasis is an immune modulated chronic inflammatory skin infection affecting about 2–3% of the population worldwide (Roslan et al., 2020). As far as the pathophysiology of psoriasis is concerned, it is multifactorial, depending on either genetic causes or epigenetic changes (Novelli et al., 2021). However, it can be characterized as excessive epidermal proliferation via lymphocytes and neutrophils (Novelli et al., 2021). Furthermore, due to the involvement of immune responses, inflamed tissues can be infiltrated by leucocytes (Novelli et al., 2021; Sun et al., 2019a). Overexpression of the psoriasis phenotype is due to the underlying interaction between innate immunity cells, cytokines like interleukins (IL‐17, IL‐22, and IL‐23), as well as TNF‐α (Ricardo & Lipner, 2020). Symptomatically, psoriasis is diagnosed by the presence of plaques in the squamous and erythematous skin layers (Zhang, 2019). In addition, as far as treatment of psoriasis is concerned, various strategies have been utilized to overcome the severity and progression of the disease (Arshad et al., 2019). Most common topical therapies include corticosteroids, vitamin D, and antibodies (Hosseinikhah et al., 2021). Systemic‐based therapies include the use of retinoid, immuno‐suppressants, and methotrexate (uz Zaman et al., 2021). However, these treatments are limited owing to their compromised safety profile and reduced therapeutic index (Arshad et al., 2021c).
2.2. Inflammatory bowel disease
Intestinal epithelial cells are embellished with various microorganisms, dietary antigens, and specific antimicrobial proteins and toxoids to maintain homeostasis and activate the normal immune response of the immune system in the form of inflammation (Goto, 2019). Inflammation is the most important component of the immune system's defense against intestinal infections or injuries (Jarmakiewicz‐Czaja et al., 2020). To keep the digestive system in balance, the body's immune cells go through normal inflammatory processes. These processes account for intestinal mucosal and epithelial barriers, intestinal immune components, and proinflammatory pathways (Curciarello et al., 2019; Yue et al., 2019). All these three natural mechanistic intestine approaches help limit the over‐burden of notorious pathogenic bacteria and over‐activated immune responses (De La Fuente et al., 2019). Notwithstanding, any alteration in the intestinal flora, such as stress, genetics, and age, can alter the balance when preserving intestinal homeostasis and allow pathogenic bacteria to enter the intestinal epithelium, contributing to excessive inflammation and IBD pathogenesis (Kim et al., 2019b). Moreover, IBD can be further propagated towards chemical phenomena of CD and UC, and the situation can be worsened with the relapse of this disease followed by chronic inflammation at various sites resulting in abdominal pain, diarrhea, and most importantly, lead to colorectal cancer. We have previously reported several advancements in the treatment and detection of IBD using NP‐based systems (Barani et al., 2021).
2.3. Ulcerative colitis
IBD is associated with further inflammatory responses leading toward UC, affecting mucosal and sub‐mucosal linings of the intestine (Arshad et al., 2021b). UC is disastrous because it leads to an increased risk of colorectal cancer if the prognosis is left unaddressed (Arshad et al., 2021c). However, now a day, treatment protocols for UC involve 5‐ASA (5‐aminosalicylic acid) and steroids, but this therapy leads to the remission of infection, thus needing novel therapeutic evaluations (Kanwal et al., 2019). In recent years, novel antibody‐based medications have been involved in treating UC with high levels of effectiveness in severe cases. However, antibodies related therapy leads to therapeutic failure in case of systemic infections. Moreover, antibodies‐based treatments originated from animal sources and are very expensive compared to other treatments. Systemic infection of UC cannot be effectively treated with the siRNA due to rapid degradation via nucleases. It seems urgent to develop nanotechnology‐based drug delivery platforms for the targeted delivery of siRNAs (Arshad et al., 2021a).
2.4. Rheumatoid arthritis
RA is an autoimmune disease associated with other disorders affecting bone structure and framework leading to disability. However, with time as diagnosis techniques have been improved, pathogenesis has been simplified to some extent. Current treatment‐based strategies highlight the need for quick onset prognosis time and therapeutic evaluation and interventions based on the use of anti‐rheumatic and nonsteroidal anti‐inflammatory medications (Janakiraman et al., 2018). Therefore, novel treatment strategies based on nanotechnology and siRNA leading to RNA silencing proved to be a hallmark in drug development technology. RA has been characterized by raised cytokines and immune cells, leading to inflammatory endothelial cells and activating the accumulation of immune responses. In addition, in RA, inflammation is associated with the infiltration of many macrophages in the synovial tissues, leading to cartilage damage and infiltration leading to proinflammatory cytokines like TNF‐α, IL‐1, and IL‐6. One of the main issues regarding the delivery of siRNA is its degradable nature. Therefore, cationic ligands are in demand for penetration enhancement and targeted delivery and can be considered a gold standard for gene delivery owing to the presence of ample amino acid (Han et al., 2021).
2.5. Brain inflammation
Intracerebral hemorrhage (ICH) is the worst health condition with increased morbidity and mortality (Almarghalani & Shah, 2021; Mukhtar et al., 2020; Zheng et al., 2018). However, survival cases after this deadliest disease are determined based on its levels of severity (Mishra et al., 2022). ICH is often associated with inflammation reactions and modulation of inflammatory responses (Mishra, Ashique et al., 2022). Yet, current treatment opportunities for the hemorrhage involved no particular involvement in the therapeutic preferences (Kandil et al., 2020). Similarly, glioblastoma (GBM) is also related to aggression in chronic inflammation, where IL‐6 cytokines are highly associated with GBM (Akhilesh et al., 2022). One of the novel treatment strategies is gaining huge interest due to the successful target binding and crossing of biological brain barriers (Del prado‐Audelo et al., 2019; Zhang et al., 2021).
3. IMMUNOSTIMULATORY SIRNAS AND THEIR MECHANISM OF ACTION
siRNAs are characterized by low cellular uptake and are vulnerable to degradation by nucleases in the blood circulation or within cells (Alshaer et al., 2021). They can suppress the expression of target genes and provide an alternative treatment option to treat various disorders, including inflammatory diseases (Jiang et al., 2013; Judge et al., 2009; Yang et al., 2011). However, determining the underlying mechanism of their therapeutic benefits remains a major challenge. RNAi‐specific messenger RNA (mRNA) cleavage products were also detected in diseased cells, and their abundance was linked to the duration of target mRNA suppression. RNAi‐mediated gene suppression substantially decreases the target's biological activity, according to histological biomarkers (Judge, Robbins, 2009).
To modulate gene expression, briefly, the Dicer enzyme (an endo‐ribonuclease 3) cleaves the long dsRNA/hpRNA to RNAi/siRNA, which in cells (cytoplasm) forms RNA‐Induced Silencing Complex (RISC) with single‐stranded antisense part of siRNA (guide strand) as sense strand take apart by RNA Helicase enzyme after attachment (Bernstein et al., 2001). The low thermodynamically stability of siRNAs (nucleotides in 5′ end) allows them to stand as a part of the RISC complex to bind a complementary messenger RNA (mRNA) entirely from 5′ seed part (2nd to 8th nucleotides) and induce a slicing mechanism by Argonaute protein (AGO part of RISC). The cleaved mRNAs degrade as abnormal molecules before translation. miRNA, as a small single‐stranded noncoding RNA molecule (~22 nucleotides), has a similar mechanism to siRNA, in which they bind to the target mRNA partially in the seed part and inhibit the translation process. hpRNAs (siRNAs) can be processed via Dicer in cell cytoplasm before the RNAi pathway (Figure 2) (Mack, 2007).
Figure 2.
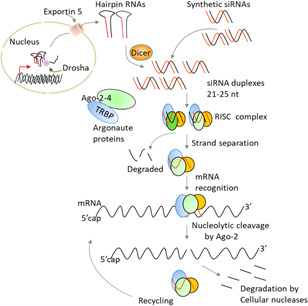
Schematic of siRNA/miRNA silencing pathway. RISC, RNA‐induced silencing complex (Sioud, 2019). miRNA, microRNA; mRNA, messenger RNA; siRNA, small interfering RNA; TRBP, TAR RNA‐binding protein
RNAi mediated gene therapies have recently gained much attention in treating IMDs (Bunse et al., 2014; Courties et al., 2009; Jakymiw et al., 2006). In common cases, multiple mechanisms such as decreased drug absorption (Longley & Johnston, 2005), drug efflux (Zhang et al., 2020), detoxifying systems activation, inhibition of apoptosis (Gillet & Gottesman, 2010; Mishra et al., 2017), and silencing of drug‐resistant genes (Edson & Kwon, 2014). However, a higher dose of drugs can increase the adverse effects (Cooper et al., 2019). RNAi has been widely used in molecular medicine research as an alternative therapeutic method in the last decade. In particular, significant achievements have been made in various diseases treatment, including autoimmune conditions (Li et al., 2020; Scheinman et al., 2011).
Mammalian innate immunity relies on Toll‐like receptors (TLRs), which recognize pathogen‐associated molecular patterns, and conserved components of microbes (PAMPs). siRNAs may activate TLRs, including TLR3, TLR7, and TLR8, which can detect double‐stranded RNAs in the viral genome (Sioud, 2006). When employed therapeutically, siRNA detection via TLR7 of innate immune cells might lead to undesired inflammatory effects (Sioud, 2007). A variety of transcription factors, including interferon regulatory factor 3 (IRF‐3) and 7 (IRF‐7), and nuclear factor (NF)‐B, are activated by siRNAs in inflammatory processes. Activation of TLRs via siRNAs can contribute to the activation of DCs and T lymphocytes in particular tissues (Hornung et al., 2005). Evidence supports the hypothesis that 5′‐triphosphate siRNA can activate natural killer cells and DCs via triggering the RIG‐I pathway (Poeck et al., 2008; Wang et al., 2013). To eliminate diseased cells, therapeutic DC vaccines depend on immune responses. Such vaccines can be turned into powerful immune stimulators by using anti‐immunosuppressive siRNAs in the development process of DCs. For adoptive immunotherapy, siRNA alteration of ex‐vivo‐expanded T cells increased their ability to destroy diseased cells (Figure 3). In preclinical and clinical trials, siRNAs that block most common immune inhibitory components have shown the greatest therapeutic outcomes. There is a compelling argument for the future development of DC vaccines using siRNA‐modified DCs in therapeutic modalities (Sioud, 2019). The potential activation of TLRs has been identified as a major disadvantage for the development of anti‐inflammatory siRNAs (Avenoso et al., 2018), as inflammatory response stimulation goes in the opposite direction as expected gene inhibition of cytokines.
Figure 3.
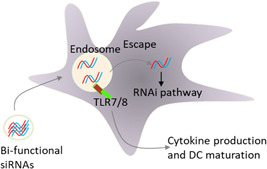
Schematic representation of boosting the immune responses by combining siRNAs with DC vaccinations. For this purpose, DCs may be electroporated with siRNAs. The assembly process is found to be easy and does not add any additional manufacturing expenses (Sioud, 2019). DC, dendritic cell; siRNA, small interfering RNA; TLR7/8, Toll‐like receptors 7 and 8
When considering RNAi as a therapeutic approach, stability and delivery of siRNA molecules in in vitro and in vivo models are vital factors. The siRNA molecules must overcome rapid degradation by plasma enzymatic system, membrane uptake, immune system response as extracellular barriers, and endosomal escape, off‐target effects as intracellular limitations. However, the bare siRNA molecule has little chance of targeting gene silencing due to its short half‐life (Pauley & Cha, 2013; Sajid et al., 2020). To prevent the stimulation of the immune system, as a side effect of siRNA‐based therapies, using more efficient methods of delivery or performing chemical modifications can be applied (Gorabi et al., 2020). Although various chemical modifications have been applied to enhance their function and stability, siRNAs are still vulnerable to degradation by plasma and nucleases (Watts et al., 2008).
The treatment of autoimmune diseases with siRNA by NPs‐mediated with prolonged blood circulation and associated with extravasation along leaky vasculature and subsequent inflammatory cell‐mediated sequestration (ELVIS) (Wei et al., 2017; Wei et al., 2018) has recently focused much consideration because of its capacity to specifically select defective genes and overcome the disadvantages of standard therapeutic processes (Khurana et al., 2010; Merlo et al., 2020; Yi Xue et al., 2015). Below, we will briefly discuss the potential of different nanovehicles to deliver siRNAs to target locations.
4. SIRNAS NANO‐DELIVERY SYSTEMS
siRNA plays a crucial role in gene silencing as one of the most critical drug candidates, so many siRNA delivery systems have been developed (Naik et al., 2021). It is approved that siRNAs can disrupt the translation process, silence genes, and even inhibit corresponding proteins' expression via targeting a specific mRNA. Nevertheless, naked siRNA is not long‐lasting in circulation, and it is difficult for siRNA to enter cells, requiring appropriate gene carriers to aid intracellular and systemic delivery (Sousa et al., 2019). Different delivery systems have been offered for siRNA delivery. Although viral vectors have been the most effective in siRNA delivery, there are many concerns over biosafety, especially regarding immunogenicity (Jiang et al., 2021). Therefore, there is an interest in investigating and studying nonviral carriers. These carriers are typically easier to create and safer to use within the body, albeit less efficient than viral carriers. Nonviral carriers that have been proposed for siRNA delivery include lipid‐based, polymeric‐based, and inorganic material‐based systems (Goswami, 2021). The ideal nonviral siRNA carriers must meet the following prerequisites: biocompatibility, biodegradability, stability, non‐toxicity, compress siRNA to NP size, induce endosomal/lysosomal escape, facilitate cellular uptake, and protect siRNA from enzymatic degradation and/or inactivation (Raftery et al., 2013). Although the most common nonviral carriers of siRNA include lipids, polymers, and inorganic NMs, modified naked siRNAs have also shown remarkable ability to obtain further benefits such as more stability and circulation in vivo (Hu et al., 2020).
4.1. Lipid‐based siRNA nanocarriers
Due to amphipathic properties, lipid nanocarriers can spontaneously form lipid bilayers consisting of hydrophobic tails and hydrophilic head groups so that they will be able to entrap hydrophilic drugs in the core and hydrophobic drugs in the bilayer membrane. Since cellular membranes are composed of lipids and phospholipids, lipid‐based siRNA nanocarriers can interact favorably with the cell membrane and increase siRNA uptake (Yi Xue, Guo, 2015). The use of lipid‐based nanocarriers is further practical for siRNA delivery because they are commercially available without chemical synthesis; however, the most prominent problem with lipid systems is their clinical toxicity and nonspecific activation of inflammatory cytokines (Dokka et al., 2000). In addition, it has been confirmed that encapsulating RNA with lipids reduces the RNA degradation rates and increases the nucleic acid material cellular uptake (Gomes‐da‐Silva et al., 2012; Mashaghi et al., 2013). Several lipid‐based nanocarriers have been examined for siRNA delivery, including liposomes, lipoplexes, and stable nucleic acid‐lipid particles (SNALPs) (Nguyen et al., 2021) (Figure 4).
Figure 4.
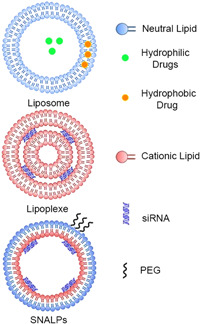
Schematic representation of lipid‐based siRNA nanocarriers. siRNA, small interfering RNA
4.1.1. Liposomes and cationic lipids
Lipid‐based nanocarrier systems are broadly utilized in pharmaceutical sciences due to their structural similarity to cell membranes and desirable properties such as great biodegradability (Pathak et al., 2011). The spherical nanostructure of liposomes contains an aqueous core entrapped by one or more phospholipids bilayers. As nanocarriers, these powerful platforms have the potential to encapsulate a wide range of therapeutic agents, antibodies, proteins, peptides, and nucleic acids; they can also serve as a vehicle for photosensitizers (PS), which are essential for enhancing PDT (Guimarães et al., 2021).
Liposomes, both cationic and neutral, have been shown to be effective carriers of siRNA. There are three major elements in cationic lipids, and they are as follows: (I) the lipid tail(s); (II) the cationic head group; and (III) the linkers that connect the two components together. These nanovehicles can enhance RNA encapsulation efficiency (EE) due to electrostatic interactions; this process sometimes results in siRNA/lipid complexes formation known as lipoplexes (Berger et al., 2021). Although the preparation of lipoplexes is straightforward and has good transfection efficiency, their drawback is poor stability and low reproducibility. Also, some RNAs may be exposed to the carrier surface, promoting immunogenic responses; therefore, minimizing undesired toxicity remains vital when forming lipoplexes (Yi et al., 2015). Cationic liposomes (e.g., DOTAP, AtuFECT01, and DC‐Cholesterol) combined with associate lipids (e.g., DPhyPE, Cholesterol, and DOPE) have shown excellent results in siRNA delivery at optimized N/P ratios. (Tagami et al., 2011). Neutral liposomes [e.g., 1, 2‐dioleoyl‐sn‐glycero‐3‐phosphatidyl‐choline (DOPC)] have reduced such toxicity, although their entrapment efficiency might be decreased. Various strategies have been offered to reduce lipoplex toxicity and improve the in vivo delivery of siRNA. To this end, researchers have coated the cationic lipoplexes with nontoxic and biodegradable anionic polymers, such as poly(acrylic acid) sodium salt, dextran sulfate sodium salt, hyaluronic acid sodium salt, alginic acid sodium salt, heparin sulfate sodium salt, carboxymethylcellulose sodium salt, and poly‐l‐glutamic acid sodium salt. Among these anionic polymers, polyglutamate did not have any apparent toxicity over many sizes. The coated cationic liposomes showed enhanced siRNA delivery in the liver and lung tissues compared to uncoated lipoplexes (Figure 5) (Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes).
Figure 5.
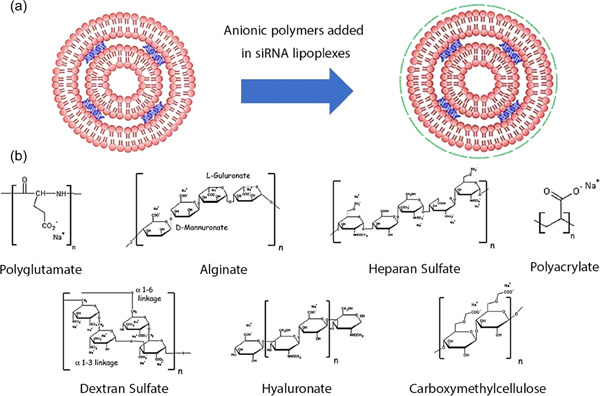
(a) Schematic representation of the cationic lipid (lipoplex) coating with anionic polymers for siRNA delivery. (b) Schematic representation of anionic polymers chemical structure added in siRNA lipoplexes. siRNA, small interfering RNA
To achieve efficient delivery of siRNA mediated by lipid‐based systems, various parameters must be considered: cargo encapsulation ratio, electrostatic charge, and particle size. Also, to increase the stability of the nanocarrier system and efficient nucleic acid delivery, polymeric compounds can be used alongside lipid‐based siRNA delivery systems (Ozpolat et al., 2014). Recently, ONPATTRO® (patisiran) was developed by Alnylam® Pharmaceuticals as the first FDA‐approved therapeutic For the treatment of polyneuropathy in cases with hereditary transthyretin‐mediated amyloidosis, in addition to the first targeted RNA‐based gene therapy, siRNA‐lipid NPs mediated (Garber, 2018; Hoy, 2018). Osteoporosis and cartilage degradation are common complications of RA (Goldring, 2003). During the developmental cycle of the RA, several genes and regulatory pathways lead to the production of inflammatory cytokines such as TNF‐α, nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB), and interleukin 1 beta (IL‐1β)are involved, silencing these genes and pathways by siRNA represents a promising treatment approach (Yonezawa et al., 2020). Herman et al. (2015) developed a liposome‐based siRNA delivery system to silence Heterogeneous nuclear ribonucleoproteins A2/B1 (hnRNP A2/B1) in mouse models due to collagen‐induced arthritis. hnRNP A2/B1 appears to be a fundamental coactivator for involved transcription factors in the proinflammatory processes (Cloonan & Choi, 2012). Cationic liposome RPR209120/DOPE was used as a lipid‐based delivery system (Khoury et al., 2006). Dynamic light scattering (DLS) and zeta potential determination were carried out to characterize formed lipoplex particles. Eventually, the results showed that inhibition of hnRP A2/B1 as an innate immune system regulator affects the release of proinflammatory mediators levels (TNF‐α, IL‐23) and alleviates the disease.
4.1.2. Stable nucleic acid‐lipid particles
Protecting cationic liposomes using hydrophilic molecules, such as poly (ethylene glycol) (PEG), have been used to extend their circulation and reduce inflammatory responses as they reduce phagocytic opsonization activity (Qelliny et al., 2021). SNALPs are among the PEGylated cationic liposomes that provide hydrophilicity and a neutral layer to stabilize these particles in circulation (Ali et al., 2021). An exciting feature of SNALPs is that they show a positive charge at acidic pH while neutral at physiological pH (Rudorf & Rädler, 2012). These systems comprise a systemic lipid bilayer based on fusogenic and cationic lipids that enable endosomal release and facilitate the absorption of siRNA into cells, respectively (Figure 6) (Abd Ellah et al., 2021). SNALPs have become a promising platform for silencing therapeutically relevant genes in different animal models (Subhan & Torchilin, 2019). Nonhuman primate Apo B gene expression was silenced using SNALPs for the first time by Zimmermann et al. (2006). Moreover, SNALP has been successfully used to silence mTTR gene expression, and 100 clinical investigations proved the practicality of this SNALP‐siRNA carrier in humans (Lin & Tam, 2019; Nikam & Gore, 2018).
Figure 6.
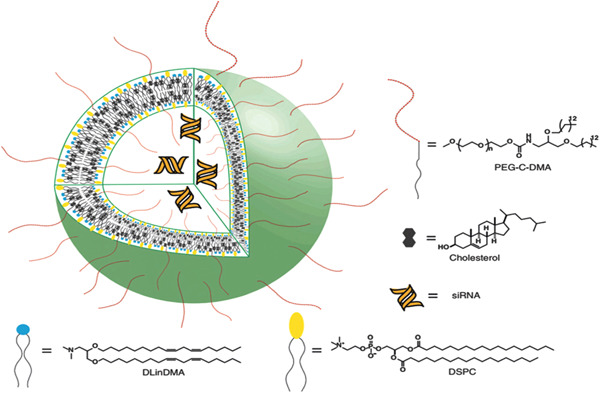
Schematic of the SNALP delivery system. Reprinted from ref (Alabi et al., 2012) (Copyright 2022 Elsevier). SNALP, stable nucleic acid‐lipid particle
4.2. Polymeric‐based siRNA nanocarriers
In the last decades, natural or synthetic nano‐polymers as colloidal solids have been extensively developed to degrade in vivo without having toxic ingredients (Rozema et al., 2007). The advantage of polymeric carriers is their diverse and flexible nature; moreover, they do not strongly stimulate the immune response as liposomes do (Srivastava et al., 2015). Polymeric carriers are generally divided into polycations and polymeric NPs (Cavallaro et al., 2017). These polymers' high positive charge content fuses with the negative charge of nucleic acids to form a polyplex. Electrostatic binding of siRNA is readily achieved by the presence of positively charged units on polymeric platforms such as thiol‐maleimide or disulfide linkages, making it feasible to covalent involving siRNA and polymers (Parmar et al., 2014). Polymers' versatility and simple manipulation have resulted in different polymeric carriers for reliable delivery to the target tissue/cell (Gary et al., 2007). Cationic polymers are divided into two branches of synthetic such as PEI, poly‐l‐lysine, and natural Polymers (i.e., chitosan, atelocollagen) that usually contain a cationic part involved in siRNA and polymer formulations (Farshbaf et al., 2018). Late, the advancement of natural cationic polymers has led to a highly efficient delivery strategy with non‐cytotoxicity, biodegradability, and biocompatibility. The siRNA rigid structure provokes weak interactions with polycations. As a result, polyplexes are less efficient in protecting siRNA against nucleases. Increasing the polycations amount can improve this imperfection but increases toxicity (Miele et al., 2012). Also, dendrimers have been considered synthetic globular lipid nanostructures with high flexibility in their physicochemical features, great potential in pharmaceutical applications, and proficient loading of siRNA (Figure 7) (Kim et al., 2019a).
Figure 7.
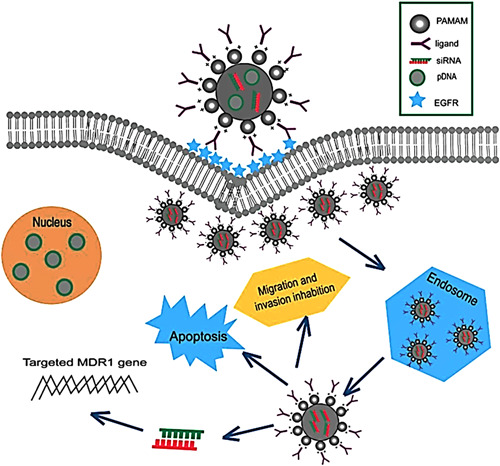
Schematic illustration of PAMAM‐mediated siRNA and pDNA delivery for EGFR‐targeted tumor therapy. Specific binding to the EGFR overexpressing receptors on the tumor cells causes receptor‐mediated endocytosis captured by the lysosomes, lysosomal escape, gene release, and induces apoptosis. Reprinted from ref (Li et al., 2018) (Copyright 2022 Elsevier). siRNA, small interfering RNA
4.2.1. PEI‐based nanosystems
The PEI synthetic polymer has a high utility due to its high efficiency in siRNA transfection and is considered the gold standard for gene transfer. However, its transfection efficiency also relies on its molecular weight and the number of branches. Although high molecular weight PEI has good transfection potential, it shows considerable toxicity. Many studies examined different bonding agents to reduce cytotoxicity while preserving the gene delivery capacity (Fischer et al., 1999). Less toxicity is expected using lower molecular weight PEIs or fusing them with liposomes in the structure of lipopolyplex (Ewe et al., 2017). The lipopolyplex gene delivery system combines polynucleotide molecules, liposomes, and cationic polymers with noncovalent bonds to escape the endosome (Rezaee et al., 2016) (Figure 8). Lipopolyplex, due to its extensive surface area, can connect many functional groups to a liposomal or polymeric part and has been proposed as a manipulative platform (Emilian Leucuta, 2013). Therefore, depending on the composition of liposomes and polymer types, various lipopolyplex have been designed. For example, dexamethasone‐loaded lipopolyplex bound to PEI has been transferred to mouse A2Neuro neuroblastoma cells (Malaekeh‐Nikouei et al., 2018). In a study that compared PEI and PEI‐PEG polyplexes for siRNA transfer to the lung, the PEI‐PEG system showed higher efficiency in silencing the EGFP gene by up to 42%. However, PEI‐PEG polyplexes also had moderate proinflammatory effects on elevated levels of IL‐6 and TNF‐α cytokines (Merkel et al., 2009). Various studies have been performed to modify the structure of PEI to increase the transmutability of a genetic agent (siRNA) into the cell and reduce the toxicity of this realistic vector. Xu et al. (2008) used Poly (ester amine)‐mediated PEG to modulate PEI's high toxicity and biodegradability in siRNA in vivo delivery. The transfer of this siRNA‐carrying complex to lung cancer cells to reduce Akt1 gene expression in mice showed a significant reduction in cancer cell progression without significant toxic effects (Xu et al., 2008). Polymers such as PEI can escape the endosome. This ability called the proton sponge is due to the PEI high buffering capacity, in which polymer protonates (releases protons) over a wide range of pH, causing CL‐ions to enter the endosome, then water followed enters the endosomes. Eventually, the endosome ruptures, releasing its contents into the cytoplasm due to the high osmotic pressure (Creusat et al., 2010). Although in synthetic polymers such as PEI, the transfection rate is high due to the proton sponge effect, cytotoxicity remains as a serious challenge (Brunot et al., 2007).
Figure 8.
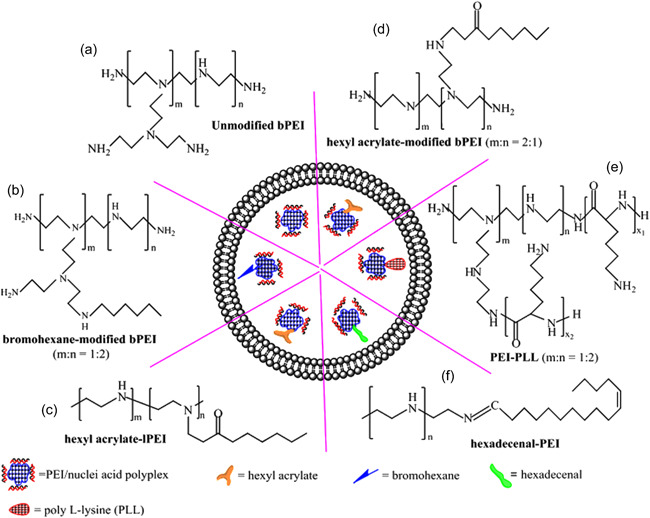
Schematic structures of various modified PEIs in LPPs formulations. (a) Unmodified branched polyethylenimine (bPEI); (b) bromohexane‐modified bPEI; (c) hexyl acrylate‐modified linear polyethylenimine (lPEI); (d) hexyl acrylate‐modified bPEI; (e) poly l‐lysine (PLL) conjugated bPEI; (f) reversible conjugation of hexadecenal to PEI. Reprinted from ref (Rezaee et al., 2016) (Copyright 2022 Elsevier). PEI, polyethylenimine
4.2.2. Chitosan‐based nanoformulations
Chitosan has received great attention as a potential vector in siRNA transfection due to its naturalness, biocompatibility, and biodegradability properties. We recently showed that chitosan nanomaterials could be extensively used for gene (i.e., microRNA) delivery (Sargazi et al., 2022). Unfortunately, the only drawback of using this polymer is moderate transfection efficiency in in vitro and inv vivo environments because it cannot escape the endosome. To improve siRNA transfection efficiency using chitosan NPs, an ionic gelation method has been proposed, which is produced using sodium triphosphate. Comparing the typical chitosan‐based siRNA nanocarriers and ionic gelation nanocarriers showed the improved ability of the ionic gelation nanocarriers to turn off the target gene in vitro (Katas & Alpar, 2006). Figure 9 illustrates cationic polymer‐based siRNA nanocarriers.
Figure 9.
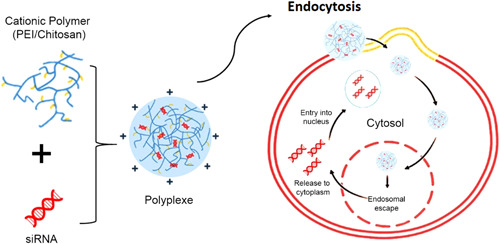
Scheme of cationic polymer‐based siRNA nanocarriers. siRNA, small interfering RNA
4.3. Inorganic material‐based siRNA nanocarriers
Inorganic nanoparticles (INPs) have been designed as an alternative to lipid‐based nanovehicles for in vitro and in vivo siRNA delivery (Shariatinia, 2022). Their extensive surface‐area‐to‐volume ratio allows efficient loading of genetic agent (siRNA) through direct conjugation or noncovalent encapsulation (Khan et al., 2021). Ability to modify the surface chemistry of these NPs provides promising platforms to overcome the challenges of in vitro and in vivo siRNA delivery (Baccaro et al., 2015). The siRNA immobilization strategies on the surface of INPs are shown in Figure 10. INPs have been widely used as genetic agent carriers due to their efficient transduction capacity, surface functionalization, and size diversity. Their extensive surface has provided siRNA's efficient attachment onto INPs via chemisorption and electrostatic interaction. However, there are critical immune responses and long‐term cytotoxicity for INPs‐based applications. Further investigations seem to be required to describe physical and chemical characteristics of INPs affect their biological responses.
Figure 10.
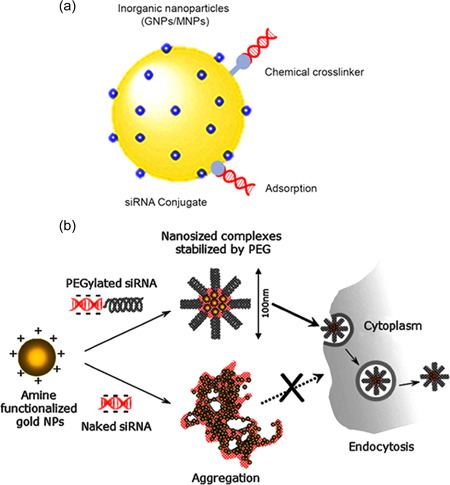
(a) The siRNA immobilization strategies onto the surface of INPs. (b) Schematic illustration for polyelectrolyte complexes formed from amine‐functionalized gold nanoparticles (AF‐AuNPs) with siRNA and siRNA–PEG conjugate. Reprinted from ref (Lee et al., 2008) (Copyright 2022 Elsevier). siRNA, small interfering RNA
Furthermore, the unique optical and physical characteristics of inorganic frameworks, such as magnetic NPs and AuNPs, have been used to monitor the delivery of siRNA inside the body for in vivo imaging (Shrestha et al., 2019). For example, Ngamcherdtrakul et al. (2018) developed 50 nm INPs modified with PEI, PEG, and an antibody. These siRNA delivery nanosystems were stable, showed luciferase silencing inhibition, and displayed antiproliferative impact in vitro. In addition, mesoporous silica NPs have been utilized as outstanding carriers to deliver siRNAs to cancer cells (Wang & Gu, 2015) (Figure 11).
Figure 11.
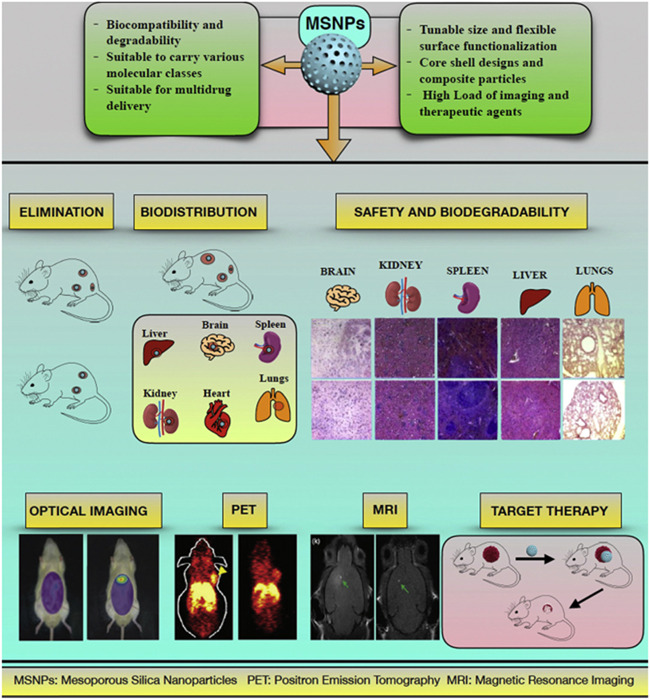
Overview of the classification, functional abilities, applications, and biological fate of the MSNPs in drug delivery research. Reprinted from ref (Barkat et al., 2021) (Copyright 2022 Elsevier)
4.4. Hybrid‐based delivery system
As discussed earlier, lipid and polymer‐based strategies played a crucial function as a vector in the siRNA delivery to the target cell or tissue. However, by electrostatic dealings between the siRNA negative charge and these cationic systems, immunogenicity, nonspecific targeting, and lack of particle size control are formed. Combining different siRNA delivery systems to achieve high efficiency targeted siRNA delivery and to overcome the disadvantages of previous methods has led to the generation of hybrid‐based systems. To achieve more efficient delivery, siRNA has been merged with diverse nano‐composites, including lipid‐polymer nanoparticles (LPNs), peptide‐polymer, and aptamer, to provide a safe and specific treatment method to manage autoimmune diseases. Recently, the hybrid system that has been considered is poly (lactic‐co‐glycolic acid) or PLGA and the cationic lipid 1,2‐dioleoyl‐3‐trimethylammonium propane (DOTAP) as LPNPs that are involved in the efficient delivery of siRNA (Figure 12) (Colombo et al., 2015; te Boekhorst et al., 2012).
Figure 12.
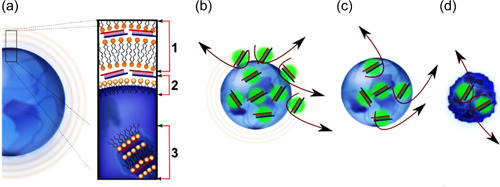
Model for the structural characteristics of siRNA‐loaded LPNs and their release dynamics. For LPNs the siRNA is loaded in (1) surface lamellar layers, (2) surface‐grafted DOTAP–siRNA complexes, and (3) matrix‐entrapped siRNA–DOTAP complexes (a). The release from these structures occurs as the release of siRNA–DOTAP complexes by disassembly of surface structures (b), sustained release of siRNA–DOTAP complexes by diffusion (c), and matrix erosion (d). Reprinted from ref (Colombo et al., 2015) (Copyright 2022 Elsevier). siRNA, small interfering RNA
Jansen et al. (2019) designed a hybrid nanocomplex by incorporating PLGA polymer with DOTAP cationic lipid and TNF‐α siRNA to achieve a promising treatment for severe inflammations caused by autoimmunity. SiRNA was closely packed in LPNs, but LPNs did not conjugate with others as compared with SNALPs. The hydrodynamic diameter (approximately 210 nm) and polydispersity index (PDI) (approximately 0.11) represent a relatively narrow size distribution of synthesized NPs. The LPNs had a better effect in vitro than the SNALPs in the RAW 264.7 macrophage cell line that was activated with lipopolysaccharide (LPS). Both nanodelivery platforms employed macropinocytosis to enhance cell uptake. When injected intraarticularily, TNF siRNA‐encapsulated LPNs markedly prevented inflammation in a murine arthritis model. However, this study indicated that LPN‐mediated TNF knockdown is a promising strategy for treating arthritis and other chronic inflammatory conditions mediated by TNF (Jansen et al., 2019). However, additional investigations are required to optimize the dose and progress towards safe treatment. These conclusions could supply a rationale for the future of therapeutics employing gene therapy NP‐based mediated delivery technology to decrease symptoms of autoimmune disorders. Here, we summarized the Nano‐based delivery system used for siRNA delivery to create innovative treatment modalities for autoimmune disorders (Table 1).
Table 1.
Recent nano‐based siRNA delivery systems in autoimmune disorder models
| Target | Disorder | carrier | Outcome | Model | Reference |
|---|---|---|---|---|---|
| TyRP‐1a | Vitiligo | Liquid crystalline NPs | Inhibition of target protein involved in melanocyte destruction | in vitro | (Tofani et al., 2018) |
| HMGB1/DHA | SLE | PEGylated TAT peptide‐cationic liposomes | Reduced inflammatory response by affecting HMGB1/TLR4/NF‐кB signaling pathways | in vitro | (Diao et al., 2019) |
| PTPN22 | TD1 | Lipoplexes | Targeted inhibition of autoimmunity related pathophysiological factor | in vivo (PBMC*) | (Pellegrino et al., 2019) |
| TNF‐α | Psoriasis | polymer‐lipid NPs | Decrease disease activity; suppressed inflammatory cytokines | Mice | (Suzuki et al., 2021) |
| TNF‐ α | Autoimmune hepatitis | cationic helical polypeptide PPABLG | Decrease disease activity; suppressed inflammatory cytokines | Mice | (He et al., 2016) |
| IL‐15 | Celiac | transglutaminase‐2 (TG2) | reduce proinflammatory cytokines in models | Mice | (Attarwala et al., 2021) |
| NF‐κB (p65) | RA | micelle | Inhibit inflammatory cytokines involved in disease | in vitro | (Chen et al., 2022) |
| TNF‐ α | IBD | Galactosylated trimethyl chitosan‐cysteine (GTC) | Decreased intestinal permeability and inflammation in the model | UC*‐Mice | (Cheng et al., 2015) |
| TNF‐α | RA | acid‐sensitive sheddable PEGylated solid‐lipid NPs | Decrease disease activity; suppressed inflammatory cytokines | CAIA*‐Mice | (Aldayel et al., 2018) |
| MOG | MS | Phosphatidylserine‐rich liposomes | Reduce incidence, delay in onset and reduce disease severity. | in vitro/mice (EAE*) | (Pujol‐Autonell et al., 2017) |
| STAT5 | Atherosclerosis | Lipofectamine | inhibited the secretion levels of inflammatory factors | Mice‐(HFD*) | (Wang et al., 2021) |
| Notch1 | RA | Thiolated glycol chitosan (tGC) | Prevents arthritis progression; decreased cartilage and bone damage | CIA*‐Mice | (Kim et al., 2015) |
| TNF‐ α | IBD | Chitosan NPs | In the treated model with lipopolysaccharide; the expression of TNF‐ α decreased | Mice | (Laroui et al., 2011) |
| NF‐kB (p65) | RA | Peptide modified polymeric micelle | Reduced disease progression due to decreased inflammatory cytokines | CIA‐Mice | (Kanazawa et al., 2016) |
| HIF‐2α | RA | Chondrocyte‐homing peptide/PEI | Decreased cartilage destruction; attenuated synovial inflammation | ACLT*‐Mice | (Pi et al., 2015) |
| TNF‐α | psoriasis | Nanostructured lipid carriers (NLCs) | overexpression of TNF‐α in The psoriasis model inhibited | Mice | (Viegas et al., 2020) |
| TNF‐α | RA | Biodegradable Cationic Polymer | Decrease inflammation and cartilage damage by inhibiting TNF‐α expression | CIA‐Mice | (Song et al., 2016) |
| TNF‐α | IBD | Gelatin (NiMOS*) | TNF‐α silencing and decrease proinflammatory cytokines production level | Mice | (Kriegel & Amiji, 2011) |
| Ihh | RA | LNPs | delayed cartilage destruction mediated by Chondroprotective | CIA‐Mice | (Zhou et al., 2014a) |
Abbreviations: ACLT, anterior cruciate ligament transection; CAIA, collagen antibody induced arthritis; CIA, collagen‐induced arthritis; EAE, experimental autoimmune encephalomyelitis; HFD, high‐fat diet; NF‐kB, nuclear factor kappa B; NiMOS, NPs‐in‐microsphere oral delivery systems; PBMCs, peripheral blood mononuclear cells; TNF, tumor necrosis factor; UC, ulcerative colitis.
5. SIRNA‐BASED NANOCARRIERS TO CONTROL IMDS
Several studies have reported that specific genes and pathways play crucial roles in the induction of autoimmunity disorders, which includes TNF‐α or IL‐6 as a multifunctional cytokine; it has been currently recognized to have extra vital capabilities as a pathological aspect of autoimmune diseases (Ishihara & Hirano, 2002; Jang et al., 2021).
5.1. Pulmonary inflammation
In an exciting experiment, Bohr et al. (2020) successfully fabricated three polyamidoamine (PAMAM) dendrimers to deliver siRNA to inflamed pulmonary cells. Dendriplex were prepared in the 10 mM of 4‐(2‐hydroxyethyl)−1‐piperazineëthanesulfonic acid (HEPES) buffer, a zwitterionic sulfonic acid buffering agent, at nitrogen to phosphate (N/P) ratio of various concentrations (5–40). Dendriplexes were characterized for particle size distribution and PDI by DLS using the photon correlation spectroscopy technique, cell culture, cellular uptake, cell viability, and cell transfection. Results concluded that all dendriplexes demonstrated an average size between 127 nm and a PDI of 0.27. The dendriplexes showed an excellent aptitude to condensate siRNA, a high cellular internalization rate, and a specific and effective gene silencing of TNF‐α. In vivo studies in a murine acute lung inflammation model also presented silencing of TNF‐α. The outcomes recommend that TNF‐α targeting siRNA can be used as local management for the overall suppression of lung inflammation prophylactically (Bohr et al., 2020). In another investigation, Zhai and co‐workers (2022) developed a nanosystem endured with reactive oxygen species (ROS) to downregulate inflammatory responses. Therefore, in this project, dexamethasone acetate (DEX) was used to be encapsulated into the polymers‐based thiolated pockets. Thiolated pockets constituted small cleaving units in the form of urethane. Repeating urethane units can be cleaved only in the presence of oxidative stress and raised ROS levels, leading to targeted release. Hence, the synthesized formulations were characterized via in vitro, ex vivo, and in vivo parameters. Results concluded that the accumulation at pulmonary inflammation sites for releasing the encapsulated payloads rapidly followed by a decline in ROS levels (Zhai et al., 2022).
5.2. Psoriasis
Technological advancements in pharmaceutical industries claimed the effectiveness of siRNA in the overexpression of the genes in specific inflammatory responses during infection. However, an effective and stabilized carrier system is highly recommended for transferring siRNA at the site of action (Kiani et al., 2021). Nuphar Veiga and associates investigated hybrid NPs as a carrier system showing high flexibility and versatility (Veiga et al., 2020). Hybrid polymer‐lipid NPs (PLNs) are an emerging nano‐particulate system for loading drugs, constituting polymers and lipids as components, for bestowing the advantages of both materials. PLNs can bypass the restrictions of “naked” siRNA, thus paving the way to develop new gene silencing strategies. However, another critical issue is the internalization of the siRNA in the endosomal vesicles. Therefore, this research investigates a PS by light irradiation for siRNA photo‐activation within the vesicles. In this context, coadministration of TNF‐α siRNA and PS (TPPS2a) using a nano‐carrier based on hybrid polymer‐lipid nanoparticles (PLNs) for topical administration is utilized. Moreover, PLNs were synthesized through a hot homogenization method followed by sonication and incubation at room temperature for 30 min. The PS TPPS2a solution in the phosphate‐buffered saline/dimethyl sulfoxide mixture was added in the aqueous phase called PLN‐TPPS2a. Furthermore, PLN‐TPPS2a was complexed with siRNA resulting in PLN‐TPPS2a‐siRNA, as shown in Figure 13.
Figure 13.
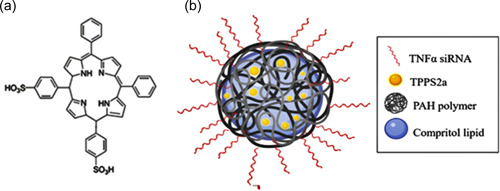
(a) Molecular structure of TPPS2a. (b) Graphic scheme of the PLN‐TPPS2a‐siRNA NPs. Reprinted from ref (Suzuki et al., 2021). siRNA, small interfering RNA
Moreover, PLNs were characterized based on size, NP tracking analysis, EE, Differential scanning calorimetry, complexation, integrity assay, in vitro skin permeation, and retention, cellular viability, and uptake. However, in vivo characterization includes the Imiquimod‐induced psoriasis model, TNF‐α silencing, and histopathological analysis. The characterization assays recommended worthy physicochemical characteristics for the PLN formulation, in addition to high EE %. The PLNs retention and penetration in the target cells were maximum. The in vitro and in vivo results confirmed the high prospective of this system in treating psoriatic lesions using the irradiation mechanism. Taken together, these outcomes clearly defined the prospective of using PLNs for co‐delivery of TNF‐α siRNA and TPPS2a as a promising topical therapy against psoriasis (Suzuki et al., 2021).
In another research, Rosa Viegas and coworkers developed tacrolimus (TAC), a macrolide immunosuppressive inhibitor, and siRNA‐based lipid nanocarriers formulated to deliver medications. NPs were characterized for in vitro, ex vivo, and in vivo parameters. However, results depicted the particle size of 230 nm with a zeta potential of +10 mV, respectively. The dissolution profile established the controlled release of TAC and siRNA with enhanced permeation and retention applicable for topical application. Findings showed a significant reduction in cytokines along with alleviation of psoriasis symptoms (Viegas, Praca, et al., 2020).
Moreover, another distinctive feature of the psoriasis is the immune responsive hyper‐proliferation and non‐differentiated keratinocytes as well as infiltrated inflammatory immune cells. Recent novel research in understanding the molecular mechanistic has proved that the signal transducer and activator of transcription 3 (STAT3) is the key factor in promoting human psoriatic skin lesions. There is evidence of the downregulation of the psoriasis factor aantolactone (ALT) and sesquiterpene lactone compound that could selectively suppress STAT3 activation. Hence in this research, ALT‐loaded polymeric chitosan/hyaluronic acid NPs (CHALT) were considered and explored the therapeutic application for psoriasis. It was identified that CHALT declined the hyper‐proliferation via inducing ROS‐mediated apoptosis by losing the mitochondrial matrix. Results designated that ALT‐based nanoformulation holds great potential for psoriasis therapy (Ferreira et al., 2017).
5.3. Inflammatory bowel disease
Nuphar Veiga and coworkers developed LNPs conjugated siRNA in an acetate buffer using a microfluidic mixing device. siRNA‐loaded LNPs were characterized for size, transmission electron microscopy, self‐assembly, EE, cell knockdown, dot blot, flow cytometry, ex vivo, and in vivo silencing assays. Results concluded that the size range of LNPs was 57.63 ± 3.2 nm with a ζ potential of 0.7 ± 0.35 mV. Generally, a distinguished reduction of IRF8 mRNA exhibited a targeted immunomodulatory effect ex vivo and in vivo in the DSS colitis model. Therefore, the authors claimed that a selective silencing of IRF8 in inflammatory leukocytes might function as a promising therapy against inflammatory disorders of the colon (Veiga et al., 2019).
In another research, Christina Kriegel and coworkers determined to down‐regulate TNF via oral RNAi therapy for treating IBD. However, in this therapeutic approach, (siRNA) was encapsulated in gelatin‐based NPs and further entrapped in PCL microspheres to develop NPs‐in‐microsphere oral system. Afterward, optimization and characterization tests lead to decreased levels of TNF‐α and other inflammatory mediators (Kriegel, Amiji & gastroenterology, 2011). Similarly, Mingzhen Zhang and researchers opted for green nanotechnology to target IBD. In this research, NPs can be synthesized from the edible ginger GDNPs and demonstrate efficient targeting capability. NPs exhibited a size of 230 nm with negative zeta potential. These NPs have the specialty of accumulating the high levels of lipids, few proteins, and ∼125 microRNAs (miRNAs) subunits. Ginger constitutes a large amount of bioactive constituents like 6‐gingerol and 6‐shogaol. Conclusively, it was depicted that it enhanced the cellular uptake in epithelial cells of the colon and macrophages. Conclusively, ginger‐based edible NPs proved to be a novel, and natural transporting agent in IBD preventive and therapeutic outcomes with synergistic with an added benefit of overcoming limitations such as potential toxicity and limited production scale that are common with synthetic NPs (Zhang et al., 2016).
5.4. Ulcerative colitis
Due to increased developments in drug discoveries, scientists tried to develop novel therapies based on the siRNA, through inexpensive chemical synthesis for targeting intracellular genes and macrophages.
HisakoIbaraki developed a drug delivery system, MPEG‐PCL‐CH2R4H2C, consisting of methoxy‐polyethylene glycol combined polycaprolactone and a cytoplasm‐responsive peptide, CH2R4H2C, for stabilized encapsulation of siRNA. MPEG‐PCL‐CH2R4H2C and siRNA were reacted using RNase‐free water, followed by mixing with nitrogen/phosphorous (N/P) in different molar ratios incubated for 30 min at 20 ± 5°C. The MPEG‐PCL‐CH2R4H2C/siRNA micelles were mixed in equal quantities and characterized by size, green exclusion assay, immuno‐histochemical analysis, therapeutic effects, hematoxylin & eosin staining. Results concluded that the intravenous administration of MPEG‐PCH‐CH2R4H2C/siRNA nanomicelles leads to accumulation in the inflamed large intestine and decreased inflammatory reactions in UC model mice, demonstrating a positive inhibitory effect of the nanoformulation. Hence, intravenous administration of these nanomicelles may be used as a potential treatment for UC (Ibaraki et al., 2022).
In another experiment, Muller et al. (2022) fabricated NPs loaded with NF‐κB p65‐specific siRNAs to treat UC in an in vivo murine model. For this purpose, dextran sulfate sodium was used to induce UC in animals. Silica‐coated calcium phosphate NPs (CaP/PEI‐Dy734/siRNA/SiO2) were fabricated and characterized, and then animals were administrated with 2.0 mg siRNA/kg body weight. The histopathological effects of the developed NPs were studied in different tissues, including the colon, and protein expression of NF‐κB and other associated proteins were assessed. Findings of their study revealed that the synthesized NPs downregulated NF‐κB‐related proteins (p65, p50, p52, and p100) in mice's colon and decreased the levels of inflammatory cytokines [interleukins, TNF‐α, interferon‐beta (IFN‐β), monocyte chemoattractant protein‐1 (MCP‐1)]. Together, CaP/PEI‐Dy734/siRNA/SiO2 were able to silence NF‐κB protein expression effectively and alleviate the clinical and histopathological markers of UA (Figure 14) (Müller et al., 2022).
Figure 14.
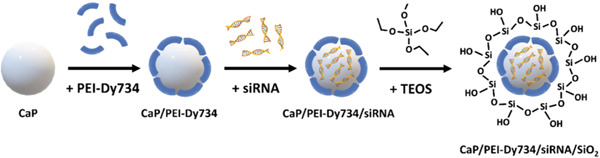
Schematic illustration of the process of fabricating Dy734‐labeled and siRNA‐loaded calcium phosphate NPs (CaP/PEI‐Dy734/siRNA/SiO2) for suppressing the NF‐κB p65 gene in mice. TEOS: tetraethoxysilane; CaP: calcium phosphate; Dy: Dyomics; PEI—polyethyleneimine; siRNA: short‐interfering RNA (Müller et al., 2022). siRNA, small interfering RNA
5.5. Rheumatoid arthritis
Xiao Hua Chen and coworkers (2022) developed a novel multifunctionalized LMW PEI–cholesterol–PEG (LPCE) delivery micelle against RA gene therapy. Furthermore, NF‐κB p65 siRNA was conjugated to the cationic polymeric micelles for regulating inflammation, immunity, cell proliferation, and cellular apoptosis for the targeted and safest delivery. Furthermore, the LPCE was characterized based on siRNA gel‐retarding assay, in vitro transfection, ribonuclease (RNase)‐degradation assay, quantitative real‐time polymerase chain reaction, validation of M1/M2‐Type Macrophages, cytotoxicity assay, cellular uptake, mechanism of the siRNA/LPCE complex, detection of IL‐10 expression level, histopathological examination, cytokine levels analysis in vivo. Results concluded that the NF‐κB p65 siRNA/LPCE multifunctionalized micelles successfully regulated the overexpressed inflammatory cells in the joint cavity. Moreover, NF‐κB p65 significantly contributed to the pathophysiological proinflammatory cascades during RA. So, it is noteworthy to confirm that silencing the expression of NF‐κB p65 in macrophages through siRNA‐based nano‐therapeutics can be a potential tactic for gene therapy against RA (Chen, Zhou et al., 2022).
In another related research, Scheinman and associates developed RGD functionalized poly(lactide‐co‐glycolytic) acid (PLGA)‐based NPs to deliver STAT1 siRNA, as RGD peptide is linked with the propagation of siRNA in cellular tissues. Therefore, RGD peptide was synthesized in preparing NPs for tracking via siRNA. Various in vitro, ex vivo, and in vivo characterization techniques were accessed. Conclusively, RGD functionalized PLGA NPs encapsulating STAT1‐targeted siRNAs were proved proficient in treating RA, perhaps through selective inhibition of macrophage and DC activation (Scheinman, Trivedi et al., 2011).
5.6. Brain inflammation
As mentioned before, ICH is frequently associated with inflammation and inflammatory response modulation (Mishra et al., 2022). Interestingly, siRNA are found to be involved in silencing inflammatory genes responsible for damaging the effects of hemorrhage (Jin et al., 2020; Poupot et al., 2018). However, siRNA therapy for GBM is hindered by multiple barriers, i.e., immunogenicity, poor cellular uptake, instability, and low BBB penetration (Jin, Chakraborty et al., 2020; Lu et al., 2020; Valentini et al., 2018).
In this connection, Kozielski and co‐workers developed bioreducible poly(beta‐amino ester) (PBAE) NPs for delivering siRNA to primary human GBM cells. NPs were synthesized via polymerization reaction. NPs can be characterized via size, cell killing in vitro, in vitro cell migration, Protein expression analysis, magnetic resonance imaging, tumor growth analysis, brain tissue, and tumor size evaluation. Bio‐reducible PBAE‐NPs showed significant results against human GBM cells through inhibition and migration of inflammatory cancer cells. It can be concluded without any doubt that this novel therapy is promising against GBM and other solid tumors (Charabati et al., 2020; Kozielski et al., 2019).
In another research, Hamideh Parhiz and coworkers (2022) worked on the induction of the acute inflammatory responses via activation of LPS and transferred them intratracheally, 1mg kg−1, or intravenously, via inflammatory models. Their findings revealed that the pretreatment with anti‐inflammatory drugs, such as corticosteroids, can moderately lessen IE response in mice. This highlights the significance of LNP‐mediated IE phenomena in gram‐negative bacterial inflammation. Still, the generalizability of using RNA‐LNPs in other forms against chronic or acute inflammation remains to be addressed (Parhiz et al., 2022).
The summary of siRNA‐based nanocarriers in the treatment of IMDs is given in Table 2.
Table 2.
Summary of key points of siRNA‐based nanocarriers against IMDs
| Nanocarrier | Outcome | References |
|---|---|---|
| siRNA‐PAMAM dendrimers | Local management for overall suppression of lung inflammation prophylactically | (Bohr et al., 2020) |
| TNF‐α–siRNA NPs | Co‐delivery of TNF‐α siRNA and TPPS2a, as an encouraging topical therapy against psoriasis | (Suzuki et al., 2021) |
| siRNA‐LNPs | Distinguished reduction of IRF8 mRNA exhibited a targeted immunomodulatory effect ex vivo and in vivo in the DSS colitis model | (Veiga, Goldsmith et al., 2019) |
| siRNA‐polymeric micelles | Accumulation in the inflamed large intestine, decreased inflammatory reactions in UC model mice, demonstrating a positive inhibitory effect | (Ibaraki et al., 2022) |
| NF‐κB p65‐siRNA multifunctionalized polymeric micelles | Potential tactic for gene therapy against RA | (Chen et al., 2022) |
| Bio‐reducible PbAE NPs | Significant results against human GBM cells through inhibition and migration of inflammatory cancer cells | (Kozielski, Ruiz‐Valls et al., 2019) |
| Au‐LNHy coated with AuNPs and comodified with PEG and α8 integrin, then loaded with dexamethasone and TGFβ1 siRNA | Treating glomerulonephritis via inhibiting local inflammation and fibrosis by delivering drugs directly to the glomerulus' Mesangial cells | (Fang et al., 2021) |
| PEGylated TAT peptide‐cationic liposomes to deliver anti‐HMGB1 siRNA and DHA | TLR4‐mediated inflammatory diseases, such as LN, may benefit from the therapy with TAT‐CLs‐DHA/siRNA. | (Diao et al., 2019) |
| Lipid nanoparticles (LNPs) with some cationic lipid component and polyethylene glycol (PEG) surfactants were used | Increased accumulation of siJAK1‐NPs within the subconfluent regions leads to uptake into immune cells near the epithelium. | (Hartwig et al., 2022) |
Abbreviations: Au‐LNHy, liposome‐nanoparticle hybrids; AuNPs, gold nanoparticles; DDS, drug delivery system; DHA, dihydroartemisinin; GBM, glioblastoma multiforme; HMGB1, high‐mobility group box 1; IRF8, interferon regulatory factor 8; LN, lupus nephritis; LNPs, lipid NPs; NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B; PAMAM, polyamidoamine; PbAE, poly(β‐amino ester)s; PEG, polyethylene glycol; RA, rheumatoid arthritis; siRNA, short‐interfering RNA; TGFβ1, transforming growth factor‐beta 1; TLR4, Toll‐like receptor 4; TNF‐α, tumor necrosis factor; TPPS2a, meso‐tetraphenyl porphyrin disulphonate; UC, ulcerative colitis.
6. CHALLENGES AND OPPORTUNITIES
Site‐specific distribution has remained the fundamental obstacle in siRNAs' targeted therapy, despite some previously resolved issues. Accessibility to the affected tissues is a major factor in determining the best delivery method. Local administration of siRNAs using intraocular, intranasal, or intra‐tumoral routes has been previously reported (Gomes‐da‐Silva et al., 2014). In terms of using siRNA‐based therapies, several concerns have been expressed about the potential for off‐target effects and the stimulation of the immune system. This can lead to adverse effects, which eventually lower their medicinal potential, depending on the sequencing of siRNAs. Since siRNAs are unable to concentrate in diseased locations, particularly those outside of the liver, they cannot be used in clinical practice. Because of siRNA's physicochemical properties, they are immediately eliminated from the circulation when administered systemically. As a result, the design of safe and effective drug delivery platforms is critical.
It has been established that a variety of factors, including cell line and concentration of siRNA, have been shown to influence cell survival, even siRNAs with scrambled sequences that presumably do not target any mRNA might exert cytotoxic effects through unknown mechanisms (Mendonça et al., 2010). Difficulties with large‐scale manufacturing, off‐target effects, stimulation of the innate immune system, high risk of mutagenesis, and the absence of cell‐specificity are among the main limitations of siRNA‐based therapies that should be overcome to reach clinical settings (Gomes‐da‐Silva et al., 2014). To overcome these obstacles, it has been suggested that poly‐U‐ and GU‐rich regions should not be avoided to prevent the siRNA immunostimulatory activity (Hornung, Guenthner‐Biller et al., 2005). Moreover, chemical modifications (at the base, sugar, or the backbone levels) that do not compromise siRNA's silencing ability can circumvent the stimulation of the immune system (Moreira et al., 2008).
Stimulation of the immune system might be the consequence of active endocytosis of siRNAs. In one study, siRNAs containing high levels of a GU motif were successfully delivered to desired tissues using LNPs, and it increased inflammatory mediators, such as interleukin‐6 (IL‐6) and interferon α (IFN‐α), in peripheral blood mononuclear cells (Judge et al., 2005). Interestingly, it has been shown that siRNA delivery via electroporation does not stimulate the immune system, while using cationic nanovehicles can trigger TLR7/8 receptors, therefore, leading to immune activation (Gomes‐da‐Silva et al., 2014; Heil et al., 2004). Unluckily, cationic RNAi NPs can be eliminated from the body via kidneys. They may have neutralized anionic proteins on the wall of glomerular capillaries, which may have led to siRNA's discharge on the capillary bed of the glomerulus and its eventual excretion. This obstacle can be circumvented by using PEGylated cationic RNAi NPs, which inhibit the renal clearance and lengthen the duration that siRNA is circulated (Zhou et al., 2014b). Furthermore, increasing the density of hydrophilic graft chains of cationic nanocarriers via linking them to copolymers can improve siRNAs' stability against plasma components and nucleases (Kano et al., 2008). Many osmolytic agents, such as polymers, small molecules, and peptides, have been integrated into siRNA nanocarrier formulations to escape the endosomal trap (Varkouhi et al., 2011). Despite their potential, however, some of these agents (e.g., PEI) might exert cytotoxicity depending on their physical size (Sonawane et al., 2003). Finally, due to their low toxicity and immunogenicity, aptamers can also be used to functionalize nanocarriers that are loaded with anti‐inflammatory drugs (Foy et al., 2007).
7. CONCLUSION
The discovery of RNAi has sparked an increased interest in using it to treat various conditions. When it comes to IMDs, RNAi may be a useful tool for modifying posttranscriptional pathways by targeting dysregulated genes. For knocking down specific genes, siRNAs have shown promise, but their application as therapeutic agents has limits due to their hydrophilicity and ease of degradation. As a result, most cell types can be damaged by artificial means of siRNA delivery; however, the transfection efficiency rate has remained low. It is possible to effectively transport and distribute siRNAs to a targeted recipient cell using NP‐based platforms, as circulating siRNAs are promptly degraded. Lately, siRNA‐based monotherapies have demonstrated great advantages to stir future activities in biomedicine. Still, there are some limitations in applying siRNA‐based therapeutics that need to be tackled. For example, glomerular filtration in the kidneys rapidly eliminates siRNAs from the body. Because glomerular filtration barriers have pore sizes of about 8 nm, NPs with particle sizes of about 20 nm cannot be easily filtered through them. Hence, encapsulating siRNAs in nanocarriers elongates their blood circulation time and increases their uptake by target cells.
NP‐based systems have been demonstrated to be efficient for siRNA delivery. To optimize the unique characteristics of NPs, such as their biocompatibility and cytotoxicity, studies have been focused on designing nontoxic nanosystems. Currently, lipid NPs, polymers, and hybrid NPs are the most widely employed types of NPs for siRNA loading. Despite some advancements, siRNA‐nanodelivery strategies are largely unexplored outside of cancer treatment. For example, exosomes with modified characteristics are now the subject of numerous studies in cancer research but are not well studied in terms of IMDs.
Recent advancements have been made in developing siRNA nanodelivery platforms to treat IMDs. Lipid‐based siRNA nanocarriers, cationic lipids and liposomes, SNALPs, polymeric‐based siRNA nanocarriers, PEG‐ and PEI‐based nanoplatforms, and chitosan‐based nanodelivery systems, hybrid‐based‐ and inorganic material‐based siRNA nanocarriers have been studied to alleviate UC, RA, IBD, pulmonary inflammations, and lupus nephritis. Nano‐delivery systems have also been embedded in hydrogels that preferentially degrade in the area of the inflamed colon to avoid the degradative effects of components in the GI tract. In addition, poor localization of the siRNAs in desired locations, high risks of immunogenicity, off‐target problems, and unstable gene expression have also limited their therapeutic application. The nanotoxicology of such nanodelivery systems in the human GI tract in IBD has received little attention, and it is likely to differ depending on the size and type of NPs. Structural stability during GI transit is another issue that requires further attention to avoid premature siRNA release in undesired targets. Moreover, enhanced circulation time of siRNAs in diseased tissue regions would help to optimize such therapies even further. Finally, using other delivery techniques, such as electroporation or nanocarriers with less toxicity, might help deliver siRNAs to target cells without causing toxic effects or immune activation.
To conduct clinical trials on the application of siRNA‐based nanosystems, it is necessary first to understand the benefits and drawbacks associated with such therapeutic modalities. Hopefully, fabricating novel multifunctional nanocarriers can be generalized to deliver many inflammation‐related siRNAs and drugs for a maximum therapeutic combination with minimal off‐targeting effects.
AUTHOR CONTRIBUTIONS
Conceptualization: Saman Sargazi; Writing‐original draft preparation: Rabia Arshad, Saman Sargazi, and Reza Ghamari; Writing‐review and editing: Saman Sargazi, Ali Bakhshi, Sonia Fathi Karkan, and Narges Ajalli; Supervision: Abbas Rahdar and Ana M. Díez‐Pascual. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Financial support from the Community of Madrid within the framework of the multiyear agreement with the University of Alcalá in the line of action “Stimulus to Excellence for Permanent University Professors”, Ref. EPU‐INV/2020/012, is gratefully acknowledged.
Sargazi, S. , Arshad, R. , Ghamari, R. , Rahdar, A. , Bakhshi, A. , Karkan, S. F. , Ajalli, N. , Bilal, M. , & Díez‐Pascual, A. M. (2022). siRNA‐based nanotherapeutics as emerging modalities for immune‐mediated diseases: A preliminary review. Cell Biology International, 46, 1320–1344. 10.1002/cbin.11841
Contributor Information
Abbas Rahdar, Email: a.rahdar@uoz.ac.ir.
Ana M. Díez‐Pascual, Email: am.diez@uah.es.
DATA AVAILABILITY STATEMENT
This article's data sharing is not applicable as no new data were created or analyzed in this study.
REFERENCES
- Abd Ellah, N. H. , Khalil, I. A. , & Harashima, H. (2021). Non‐viral gene delivery. The ADME Encyclopedia: A Comprehensive Guide on Biopharmacy and Pharmacokinetics, 1, 10. [Google Scholar]
- Akhilesh, A. , Uniyal, A. , Gadepalli, A. , Tiwari, V. , Allani, M. , Chouhan, D. , Ummadisetty, O. , Verma, N. , & Tiwari, V. (2022). Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy‐induced neuropathic pain. Life Sciences, 288, 120187. [DOI] [PubMed] [Google Scholar]
- Alabi, C. , Vegas, A. , & Anderson, D. (2012). Attacking the genome: Emerging siRNA nanocarriers from concept to clinic. Current Opinion in Pharmacology, 12, 427–433. [DOI] [PubMed] [Google Scholar]
- Albalawi, F. , Hussein, M. Z. , Fakurazi, S. , & Masarudin, M. J. (2021). Engineered nanomaterials: The challenges and opportunities for nanomedicines. International Journal of Nanomedicine, 16, 161–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldayel, A. M. , O'Mary, H. L. , Valdes, S. A. , Li, X. , Thakkar, S. G. , Mustafa, B. E. , & Cui, Z. (2018). Lipid nanoparticles with minimum burst release of TNF‐α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. Journal of Controlled Release, 283, 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, M. S. , Hooshmand, N. , El‐Sayed, M. , & Labouta, H. I. (2021). Microfluidics for development of lipid nanoparticles: paving the way for nucleic acids to the clinic. ACS Applied Bio Materials . 10.1021/acsabm.1c00732 [DOI] [PubMed]
- Almarghalani, D. A. , & Shah, Z. A. (2021). Progress on siRNA‐based gene therapy targeting secondary injury after intracerebral hemorrhage. Gene Therapy, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaer, W. , Zureigat, H. , Al Karaki, A. , Al‐Kadash, A. , Gharaibeh, L. , Hatmal, M. M. , Aljabali, A. , & Awidi, A. (2021). siRNA: Mechanism of action, challenges, and therapeutic approaches. European Journal of Pharmacology, 905, 174178. [DOI] [PubMed] [Google Scholar]
- Arshad, R. , Fatima, I. , Sargazi, S. , Rahdar, A. , Karamzadeh‐Jahromi, M. , Pandey, S. , Díez‐Pascual, A. M. , & Bilal, M. (2021a). Novel perspectives towards RNA‐based nano‐theranostic approaches for cancer management. Nanomaterials, 11, 3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad, R. , Sohail, M. F. , Sarwar, H. S. , Saeed, H. , Ali, I. , Akhtar, S. , Hussain, S. Z. , Afzal, I. , Jahan, S. , Anees‐Ur‐Rehman, & Shahnaz, G. (2019). ZnO‐NPs embedded biodegradable thiolated bandage for postoperative surgical site infection: In vitro and in vivo evaluation. PLoS One, 14, e0217079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad, R. , Tabish, T. A. , Kiani, M. H. , Ibrahim, I. M. , Shahnaz, G. , Rahdar, A. , Kang, M. , & Pandey, S. (2021b). A hyaluronic acid functionalized self‐nano‐emulsifying drug delivery system (SNEDDS) for enhancement in ciprofloxacin targeted delivery against intracellular infection. Nanomaterials, 11, 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad, R. , Tabish, T. A. , Naseem, A. A. , Hassan, M. R. , Hussain, I. , Hussain, S. S. , & Shahnaz, G. (2021c). Development of poly‐L‐lysine multi‐functionalized muco‐penetrating self‐emulsifying drug delivery system (SEDDS) for improved solubilization and targeted delivery of ciprofloxacin against intracellular Salmonella typhi. Journal of Molecular Liquids, 333, 115972. [Google Scholar]
- Attarwala, H. Z. , Suri, K. , & Amiji, M. M. (2021). Co‐Silencing of tissue transglutaminase‐2 and Interleukin‐15 genes in a celiac disease mimetic mouse model using a nanoparticle‐in‐microsphere oral system. Molecular Pharmaceutics, 18, 3099–3107. [DOI] [PubMed] [Google Scholar]
- Avenoso, A. , D'Ascola, A. , Scuruchi, M. , Mandraffino, G. , Calatroni, A. , Saitta, A. , Campo, S. , & Campo, G. M. (2018). The proteoglycan biglycan mediates inflammatory response by activating TLR‐4 in human chondrocytes: Inhibition by specific siRNA and high polymerized Hyaluronan. Archives of Biochemistry and Biophysics, 640, 75–82. [DOI] [PubMed] [Google Scholar]
- Axelrad, J. E. (2021). Immune‐mediated diseases—Are we closer to disease‐defining molecular signatures? Alimentary Pharmacology & Therapeutics, 53, 563–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaro, L. F. , Conde, D. M. , Costa‐Paiva, L. , & Pinto‐Neto, A. M. (2015). 15 years on siRNA delivery: Beyond the state‐of‐the‐art on inorganic nanoparticles for RNAi therapeutics. Nano Today, 10, 421–450. [Google Scholar]
- Barani, M. , Rahdar, A. , Sargazi, S. , Amiri, M. S. , Sharma, P. K. , & Bhalla, N. (2021). Nanotechnology for inflammatory bowel disease management: Detection, imaging and treatment. Sensing and bio‐sensing. Research; A Journal of Science and its Applications, 32, 100417. [Google Scholar]
- Barcelos, I. P. , Troxell, R. M. , & Graves, J. S. (2019). Mitochondrial dysfunction and multiple sclerosis. Biology (Basel), 8, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkat, A. , Beg, S. , Panda, S. K. , Alharbi, K. S. , Rahman, M. , & Ahmed, F. J. (2021). Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Seminars in Cancer Biology: Elsevier, 365, 75–375. [DOI] [PubMed] [Google Scholar]
- Berger, M. , Lechanteur, A. , Evrard, B. , & Piel, G. (2021). Innovative lipoplexes formulations with enhanced siRNA efficacy for cancer treatment: Where are we now? International Journal of Pharmaceutics, 605, 120851. [DOI] [PubMed] [Google Scholar]
- Bernstein, E. , Caudy, A. A. , Hammond, S. M. , & Hannon, G. J. (2001). Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature, 409, 363–366. [DOI] [PubMed] [Google Scholar]
- te Boekhorst, B. C. , Jensen, L. B. , Colombo, S. , Varkouhi, A. K. , Schiffelers, R. M. , Lammers, T. , Storm, G. , Nielsen, H. M. , Strijkers, G. J. , Foged, C. , & Nicolay, K. (2012). MRI‐assessed therapeutic effects of locally administered PLGA nanoparticles loaded with anti‐inflammatory siRNA in a murine arthritis model. Journal of Controlled Release, 161, 772–780. [DOI] [PubMed] [Google Scholar]
- Bohatá, J. , Horváthová, V. , Pavlíková, M. , & Stibůrková, B. (2021). Circulating microRNA alternations in primary hyperuricemia and gout. Arthritis Research & Therapy, 23, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr, A. , Tsapis, N. , Foged, C. , Andreana, I. , Yang, M. , & Fattal, E. (2020). Treatment of acute lung inflammation by pulmonary delivery of anti‐TNF‐α siRNA with PAMAM dendrimers in a murine model. European Journal of Pharmaceutics and Biopharmaceutics, 156, 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borren, N. Z. , & Ananthakrishnan, A. N. (2019). Safety of biologic therapy in older patients with immune‐mediated diseases: A systematic review and meta‐analysis. Clinical Gastroenterology and Hepatology, 17, 1736–1743 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunot, C. , Ponsonnet, L. , Lagneau, C. , Farge, P. , Picart, C. , & Grosgogeat, B. (2007). Cytotoxicity of polyethyleneimine (PEI), precursor base layer of polyelectrolyte multilayer films. Biomaterials, 28, 632–640. [DOI] [PubMed] [Google Scholar]
- Bunse, M. , Bendle, G. M. , Linnemann, C. , Bies, L. , Schulz, S. , Schumacher, T. N. , & Uckert, W. (2014). RNAi‐mediated TCR knockdown prevents autoimmunity in mice caused by mixed TCR dimers following TCR gene transfer. Molecular Therapy, 22, 1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon, K. , & Déglon, N. (2013). Lentiviral‐Mediated Gene Transfer of siRNAs for the Treatment of Huntington's Disease. In editors. Kohwi, Y. & McMurray, C. T. , Trinucleotide Repeat Protocols (pp. 95–109). Humana Press. [DOI] [PubMed] [Google Scholar]
- Cavallaro, G. , Sardo, C. , Craparo, E. F. , Porsio, B. , & Giammona, G. (2017). Polymeric nanoparticles for siRNA delivery: Production and applications. International Journal of Pharmaceutics, 525, 313–333. [DOI] [PubMed] [Google Scholar]
- Charabati, M. , Rabanel, J.‐M. , Ramassamy, C. , & Prat, A. (2020). Overcoming the brain barriers: From immune cells to nanoparticles. Trends In Pharmacological Sciences, 41, 42–54. [DOI] [PubMed] [Google Scholar]
- Chauhan, I. , Yasir, M. , Verma, M. , & Singh, A. P. (2020). Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Advanced Pharmaceutical Bulletin, 10, 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Fan, T. , Xie, Z. , Zeng, Q. , Xue, P. , Zheng, T. , Chen, Y. , Luo, X. , & Zhang, H. (2020). Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials, 237, 119827. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Deng, H. , Cui, H. , Fang, J. , Zuo, Z. , Deng, J. , Li, Y. , Wang, X. , & Zhao, L. (2017). Inflammatory responses and inflammation‐associated diseases in organs. Oncotarget, 9, 7204–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Zhou, B. , Gao, Y. , Wang, K. , Wu, J. , Shuai, M. , Men, K. , & Duan, X. (2022). Efficient treatment of rheumatoid arthritis by degradable LPCE nano‐conjugate‐delivered p65 siRNA. Pharmaceutics, 14, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W. , Tang, C. , & Yin, C. (2015). Effects of particle size and binding affinity for small interfering RNA on the cellular processing, intestinal permeation and anti‐inflammatory efficacy of polymeric nanoparticles. The Journal of Gene Medicine, 17, 244–256. [DOI] [PubMed] [Google Scholar]
- Chountoulesi, M. , & Demetzos, C. (2020). Promising nanotechnology approaches in treatment of autoimmune diseases of central nervous system. Brain Sciences, 10, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloonan, S. M. , & Choi, A. M. (2012). Mitochondria: Commanders of innate immunity and disease? Current Opinion in Immunology, 24, 32–40. [DOI] [PubMed] [Google Scholar]
- Colombo, S. , Cun, D. , Remaut, K. , Bunker, M. , Zhang, J. , Martin‐Bertelsen, B. , Yaghmur, A. , Braeckmans, K. , Nielsen, H. M. , & Foged, C. (2015). Mechanistic profiling of the siRNA delivery dynamics of lipid–polymer hybrid nanoparticles. Journal of Controlled Release, 201, 22–31. [DOI] [PubMed] [Google Scholar]
- Cooper, C. , Chapurlat, R. , Al‐Daghri, N. , Herrero‐Beaumont, G. , Bruyère, O. , Rannou, F. , Roth, R. , Uebelhart, D. , & Reginster, J. Y. (2019). Safety of oral non‐selective non‐steroidal anti‐inflammatory drugs in osteoarthritis: What does the literature say? Drugs & Aging, 36, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courties, G. , Presumey, J. , Duroux‐Richard, I. , Jorgensen, C. , & Apparailly, F. (2009). RNA interference‐based gene therapy for successful treatment of rheumatoid arthritis. Expert Opinion on Biological Therapy, 9, 535–538. [DOI] [PubMed] [Google Scholar]
- Creusat, G. , Rinaldi, A.‐S. , Weiss, E. , Elbaghdadi, R. , Remy, J.‐S. , Mulherkar, R. , & Zuber, G. (2010). Proton sponge trick for pH‐sensitive disassembly of polyethylenimine‐based siRNA delivery systems. Bioconjugate Chemistry, 21, 994–1002. [DOI] [PubMed] [Google Scholar]
- Curciarello, R. , Canziani, K. E. , Docena, G. H. , & Muglia, C. I. (2019). Contribution of non‐immune cells to activation and modulation of the intestinal inflammation. Frontiers in Immunology, 10, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak, P. , Axelrad, J. E. , & Ananthakrishnan, A. N. (2019). The role of the radiologist in determining disease severity in inflammatory bowel diseases. Gastrointestinal Endoscopy Clinics of North America, 29, 447–470. [DOI] [PubMed] [Google Scholar]
- Diao, L. , Tao, J. , Wang, Y. , Hu, Y. , & He, W. (2019). Co‐delivery of dihydroartemisinin and HMGB1 siRNA by TAT‐modified cationic liposomes through the TLR4 signaling pathway for treatment of lupus nephritis. International Journal of Nanomedicine, 14, 8627–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokka, S. , Toledo, D. , Shi, X. , Castranova, V. , & Rojanasakul, Y. (2000). Oxygen radical‐mediated pulmonary toxicity induced by some cationic liposomes. Pharmaceutical Research, 17, 521–525. [DOI] [PubMed] [Google Scholar]
- Duan, L. , Rao, X. , & Sigdel, K. R. (2019). Regulation of inflammation in autoimmune disease. Journal of Immunology Research, 2019, 7403796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson, J. A. , & Kwon, Y. J. (2014). RNAi for silencing drug resistance in microbes toward development of nanoantibiotics. Journal of Controlled Release, 189, 150–157. [DOI] [PubMed] [Google Scholar]
- Emilian Leucuta, S. (2013). Systemic and biophase bioavailability and pharmacokinetics of nanoparticulate drug delivery systems. Current Drug Delivery, 10, 208–240. [DOI] [PubMed] [Google Scholar]
- Ewe, A. , Panchal, O. , Pinnapireddy, S. R. , Bakowsky, U. , Przybylski, S. , Temme, A. , & Aigner, A. (2017). Liposome‐polyethylenimine complexes (DPPC‐PEI lipopolyplexes) for therapeutic siRNA delivery in vivo. Nanomedicine: Nanotechnology, Biology and Medicine, 13, 209–218. [DOI] [PubMed] [Google Scholar]
- Fang, P. , Han, L. , Liu, C. , Deng, S. , Zhang, E. , Gong, P. , et al. (2022). Dual‐regulated functionalized liposome–nanoparticle hybrids loaded with dexamethasone/TGFβ1‐siRNA for targeted therapy of glomerulonephritis. ACS Applied Materials & Interfaces. 14, 307– 323 [DOI] [PubMed] [Google Scholar]
- Farshbaf, M. , Davaran, S. , Zarebkohan, A. , Annabi, N. , Akbarzadeh, A. , & Salehi, R. (2018). Significant role of cationic polymers in drug delivery systems. Artificial Cells, Nanomedicine, and Biotechnology, 46, 1872–1891. [DOI] [PubMed] [Google Scholar]
- FDA . Drug Approval Package: Onpattro (patisiran). https://www.accessdata.fda.gov: US Food and Drug Administration (FDA). 2018.
- FDA . Drug Approval Package: GIVLAARI (givosiran) Injection. 2019.
- FDA . Drugs@FDA: FDA‐Approved Drugs. FDA. 2020.
- FDA . 2022.
- Ferreira, M. , Barreiros, L. , Segundo, M. A. , Torres, T. , Selores, M. , Costa Lima, S. A. , & Reis, S. (2017). Topical co‐delivery of methotrexate and etanercept using lipid nanoparticles. A Targeted Approach For Psoriasis Management, 159, 23–29. [DOI] [PubMed] [Google Scholar]
- Fischer, D. , Bieber, T. , Li, Y. , Elsässer, H.‐P. , & Kissel, T. (1999). A novel non‐viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharmaceutical Research, 16, 1273–1279. [DOI] [PubMed] [Google Scholar]
- Foy, J. W.‐D. , Rittenhouse, K. , Modi, M. , & Patel, M. (2007). Local tolerance and systemic safety of pegaptanib sodium in the dog and rabbit. Journal of Ocular Pharmacology and Therapeutics, 23, 452–466. [DOI] [PubMed] [Google Scholar]
- De La Fuente, M. K. , MacDonald, T. T. , & Hermoso, M. A. (2019). Intestinal homeostasis and disease: A complex partnership between immune cells, non‐immune cells and the microbiome. Frontiers in Immunology, 10, 2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly, D. , Haak, S. , Sisirak, V. , & Reizis, B. (2013). The role of dendritic cells in autoimmunity. Nature Reviews Immunology, 13, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbayo, E. , Pascual‐Gil, S. , Rodríguez‐Nogales, C. , Saludas, L. , Estella‐Hermoso de Mendoza, A. , & Blanco‐Prieto, M. J. (2020). Nanomedicine and drug delivery systems in cancer and regenerative medicine. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 12, e1637. [DOI] [PubMed] [Google Scholar]
- Garber, K. (2018). Alnylam launches era of RNAi drugs. Nature Biotechnology, 36, 777–779. [DOI] [PubMed] [Google Scholar]
- Gary, D. J. , Puri, N. , & Won, Y.‐Y. (2007). Polymer‐based siRNA delivery: Perspectives on the fundamental and phenomenological distinctions from polymer‐based DNA delivery. Journal of Controlled Release, 121, 64–73. [DOI] [PubMed] [Google Scholar]
- Gillet, J.‐P. , & Gottesman, M. M. (2010). Mechanisms of multidrug resistance in cancer. Multi‐Drug Resistance In Cancer: Springer , 47, 76. [DOI] [PubMed] [Google Scholar]
- Goldring, S. (2003). Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology, 42, ii11–ii16. [DOI] [PubMed] [Google Scholar]
- Gomes‐da‐Silva, L. C. , Simões, S. , & Moreira, J. N. (2014). Challenging the future of siRNA therapeutics against cancer: The crucial role of nanotechnology. Cellular and Molecular Life Sciences, 71, 1417–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes‐da‐Silva, L. C. , Fonseca, N. A. , Moura, V. , Pedroso de Lima, M. C. , Simões, S. , & Moreira, J. N. (2012). Lipid‐based nanoparticles for siRNA delivery in cancer therapy: Paradigms and challenges. Accounts of Chemical Research, 45, 1163–1171. [DOI] [PubMed] [Google Scholar]
- González‐Serna, D. , Villanueva‐Martin, G. , Acosta‐Herrera, M. , Márquez, A. , & Martín, J. (2020). Approaching shared pathophysiology in immune‐mediated diseases through functional genomics. Genes, 11, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorabi, A. M. , Kiaie, N. , Aslani, S. , Jamialahmadi, T. , Johnston, T. P. , & Sahebkar, A. (2020). Prospects for the potential of RNA interference in the treatment of autoimmune diseases: Small interfering RNAs in the spotlight. Journal of Autoimmunity, 114, 102529. [DOI] [PubMed] [Google Scholar]
- Goswami, P. (2021). RNA‐induced gene silencing. Bioinformatics in Rice Research: Springer, 541, 53–553. [Google Scholar]
- Goto, Y. (2019). Epithelial cells as a transmitter of signals from commensal bacteria and host immune cells. Frontiers in Immunology, 10, 2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães, D. , Cavaco‐Paulo, A. , & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601, 120571. [DOI] [PubMed] [Google Scholar]
- Han, Y. , Pang, X. , & Pi, G. (2021). Biomimetic and bioinspired intervention strategies for the treatment of rheumatoid arthritis. Advanced Functional Materials, 31, 2104640. [Google Scholar]
- Hartwig, O. , Loretz, B. , Nougarede, A. , Jary, D. , Sulpice, E. , Gidrol, X. , Navarro, F. , & Lehr, C. M. (2022). Leaky gut model of the human intestinal mucosa for testing siRNA‐based nanomedicine targeting JAK1. Journal of Controlled Release, 345, 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, H. , Zheng, N. , Song, Z. , Kim, K. H. , Yao, C. , Zhang, R. , Zhang, C. , Huang, Y. , Uckun, F. M. , Cheng, J. , Zhang, Y. , & Yin, L. (2016). Suppression of hepatic inflammation via systemic siRNA delivery by membrane‐disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano, 10, 1859–1870. [DOI] [PubMed] [Google Scholar]
- Heil, F. , Hemmi, H. , Hochrein, H. , Ampenberger, F. , Kirschning, C. , Akira, S. , Lipford, G. , Wagner, H. , & Bauer, S. (2004). Species‐specific recognition of single‐stranded RNA via toll‐like receptor 7 and 8. Science, 303, 1526–1529. [DOI] [PubMed] [Google Scholar]
- Herman, S. , Fischer, A. , Presumey, J. , Hoffmann, M. , Koenders, M. I. , Escriou, V. , et al. (2015). Inhibition of inflammation and bone erosion by RNA interference–mediated silencing of heterogeneous nuclear RNP A2/B1 in two experimental models of rheumatoid arthritis. Arthritis Rheumatology, 67, 2536–2546. [DOI] [PubMed] [Google Scholar]
- Hoffmann, D. B. , Böker, K. O. , Schneider, S. , Eckermann‐Felkl, E. , Schuder, A. , Komrakova, M. , Sehmisch, S. , & Gruber, J. (2016). In vivo siRNA delivery using JC virus‐like particles decreases the expression of RANKL in rats. Molecular Therapy. Nucleic Acids , 5, e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung, V. , Guenthner‐Biller, M. , Bourquin, C. , Ablasser, A. , Schlee, M. , Uematsu, S. , Noronha, A. , Manoharan, M. , Akira, S. , de Fougerolles, A. , Endres, S. , & Hartmann, G. (2005). Sequence‐specific potent induction of IFN‐α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nature Medicine, 11, 263–270. [DOI] [PubMed] [Google Scholar]
- Hosseinikhah, S. M. , Barani, M. , Rahdar, A. , Madry, H. , Arshad, R. , Mohammadzadeh, V. , & Cucchiarini, M. (2021). Nanomaterials for the diagnosis and treatment of inflammatory arthritis. International Journal of Molecular Sciences, 22, 3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy, S. M. (2018). Patisiran: First global approval. Drugs, 78, 1625–1631. [DOI] [PubMed] [Google Scholar]
- Hu, B. , Zhong, L. , Weng, Y. , Peng, L. , Huang, Y. , Zhao, Y. , & Liang, X. J. (2020). Therapeutic siRNA: State of the art. Signal Transduction and Targeted Therapy, 5, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, P. (2012). The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Reports, 13, 968–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibaraki, H. , Hatakeyama, N. , Arima, N. , Takeda, A. , Seta, Y. , & Kanazawa, T. (2022). Systemic delivery of siRNA to the colon using peptide modified PEG‐PCL polymer micelles for the treatment of ulcerative colitis. European Journal of Pharmaceutics and Biopharmaceutics, 170, 170–178. [DOI] [PubMed] [Google Scholar]
- Ishihara, K. , & Hirano, T. (2002). IL‐6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine & Growth Factor Reviews, 13, 357–368. [DOI] [PubMed] [Google Scholar]
- Jakymiw, A. , Ikeda, K. , Fritzler, M. J. , Reeves, W. H. , Satoh, M. , & Chan, E. K. (2006). Autoimmune targeting of key components of RNA interference. Arthritis Research & Therapy, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman, K. , Krishnaswami, V. , Rajendran, V. , Natesan, S. , & Kandasamy, R. (2018). Novel nano therapeutic materials for the effective treatment of rheumatoid arthritis‐recent insights. Materials Today Communications, 17, 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, D.‐I , Lee, A. H. , Shin, H.‐Y. , Song, H.‐R. , Park, J.‐H. , Kang, T.‐B. , Lee, S. R. , & Yang, S. H. (2021). The role of tumor necrosis factor alpha (TNF‐α) in autoimmune disease and current TNF‐α inhibitors in therapeutics. International Journal of Molecular Sciences, 22, 2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, M. A. A. , Klausen, L. H. , Thanki, K. , Lyngsø, J. , Skov Pedersen, J. , Franzyk, H. , Nielsen, H. M. , van Eden, W. , Dong, M. , Broere, F. , Foged, C. , & Zeng, X. (2019). Lipidoid‐polymer hybrid nanoparticles loaded with TNF siRNA suppress inflammation after intra‐articular administration in a murine experimental arthritis model. European Journal of Pharmaceutics and Biopharmaceutics, 142, 38–48. [DOI] [PubMed] [Google Scholar]
- Jarmakiewicz‐Czaja, S. , Piątek, D. , & Filip, R. (2020). The influence of nutrients on inflammatory bowel diseases. Journal of Nutrition and Metabolism, 2020, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, M.‐J. , Jeon, S. , Yu, H.‐S. , Cho, W.‐S. , Lee, S. , Kang, D. , Kim, Y. , Kim, Y. J. , & Kim, S. Y. (2022). Exposure to nickel oxide nanoparticles induces acute and chronic inflammatory responses in rat lungs and perturbs the lung microbiome. International Journal of Environmental Research and Public Health, 19, 522. 10.3390/ijerph19010522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Chen, W. , Zhu, G. , Zhang, L. , Tucker, B. , Hao, L. , Feng, S. , Ci, H. , Ma, J. , Wang, L. , Stashenko, P. , & Li, Y. P. (2013). RNAi‐mediated silencing of Atp6i and Atp6i haploinsufficiency prevents both bone loss and inflammation in a mouse model of periodontal disease. PLoS One, 8, e58599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Zhang, X. , Tang, Y. , Li, S. , & Chen, J. (2021). Progress on ocular siRNA gene‐silencing therapy and drug delivery systems. Fundamental & Clinical Pharmacology, 35, 4–24. [DOI] [PubMed] [Google Scholar]
- Jin, G.‐Z. , Chakraborty, A. , Lee, J.‐H. , Knowles, J. C. , & Kim, H.‐W. (2020). Targeting with nanoparticles for the therapeutic treatment of brain diseases. Journal of Tissue Engineering, 11, 2041731419897460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge, A. D. , Robbins, M. , Tavakoli, I. , Levi, J. , Hu, L. , Fronda, A. , Ambegia, E. , McClintock, K. , & MacLachlan, I. (2009). Confirming the RNAi‐mediated mechanism of action of siRNA‐based cancer therapeutics in mice. The Journal of Clinical Investigation, 119, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge, A. D. , Sood, V. , Shaw, J. R. , Fang, D. , McClintock, K. , & MacLachlan, I. (2005). Sequence‐dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotechnology, 23, 457–462. [DOI] [PubMed] [Google Scholar]
- Kanazawa, T. , Endo, T. , Arima, N. , Ibaraki, H. , Takashima, Y. , & Seta, Y. (2016). Systemic delivery of small interfering RNA targeting nuclear factor κB in mice with collagen‐induced arthritis using arginine‐histidine‐cysteine based oligopeptide‐modified polymer nanomicelles. International Journal of Pharmaceutics, 515, 315–323. [DOI] [PubMed] [Google Scholar]
- Kandil, R. , Xie, Y. , Mehta, A. , & Merkel, O. (2020). A method for targeted nonviral siRNA delivery in cancer and inflammatory diseases. Drug Delivery Systems: Springer, 2059, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano, A. , Yamano, T. , Choi, S. W. , & Maruyama, A. (2008). Polymer brush‐stabilized polyplex for a siRNA carrier with long blood circulatory half‐life. Advanced Materials Research: Trans Tech Publ, 762, 4–764. [Google Scholar]
- Kanwal, A. , Iqbal, A. , Arshad, R. , Akhtar, S. , Razzaq, S. , Ahmad, N. M. , Naz, H. , & Shahnaz, G. (2019). Formulation and evaluation of novel thiolated intra pocket periodontal composite membrane of doxycycline. AAPS PharmSciTech, 20, 1–14. [DOI] [PubMed] [Google Scholar]
- Katas, H. , & Alpar, H. O. (2006). Development and characterisation of chitosan nanoparticles for siRNA delivery. Journal of Controlled Release, 115, 216–225. [DOI] [PubMed] [Google Scholar]
- Khan, M. A. , Singh, D. , Ahmad, A. , & Siddique, H. R. (2021). Revisiting inorganic nanoparticles as promising therapeutic agents: A paradigm shift in oncological theranostics. European Journal of Pharmaceutical Sciences, 164, 105892. [DOI] [PubMed] [Google Scholar]
- Khoury, M. , Louis‐Plence, P. , Escriou, V. , Noel, D. , Largeau, C. , Cantos, C. , Scherman, D. , Jorgensen, C. , & Apparailly, F. (2006). Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor α in experimental arthritis. Arthritis & Rheumatism, 54, 1867–1877. [DOI] [PubMed] [Google Scholar]
- Khurana, B. , Goyal, K., A. , Budhiraja, A. , Arora, D. , & P Vyas, S. (2010). siRNA delivery using nanocarriers‐an efficient tool for gene silencing. Current Gene Therapy, 10, 139–155. [DOI] [PubMed] [Google Scholar]
- Kiani, M. H. , Ali, S. , Qadry, A. , Arshad, R. , Aslam, A. , & Shahnaz, G. (2021). Polyethylene imine conjugated supramolecular stereocomplexed nanomicelles for intracellular delivery of rifampicin against mycobacterium bovis. Colloids and Surfaces B: Biointerfaces, 206, 111976. [DOI] [PubMed] [Google Scholar]
- Kim, B. , Park, J. H. , & Sailor, M. J. (2019a). Rekindling RNAi therapy: Materials design requirements for in vivo siRNA delivery. Advanced Materials, 31, 1903637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. J. , Park, J.‐S. , Lee, S. J. , Jang, J. , Park, J. S. , Back, S. H. , Bahn, G. , Park, J. H. , Kang, Y. M. , Kim, S. H. , Kwon, I. C. , Jo, D. G. , & Kim, K. (2015). Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. Journal of Controlled Release, 216, 140–148. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Eun, H. S. , & Jo, E.‐K. (2019b). Roles of autophagy‐related genes in the pathogenesis of inflammatory bowel disease. Cells, 8, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielski, K. L. , Ruiz‐Valls, A. , Tzeng, S. Y. , Guerrero‐Cázares, H. , Rui, Y. , Li, Y. , Vaughan, H. J. , Gionet‐Gonzales, M. , Vantucci, C. , Kim, J. , Schiapparelli, P. , Al‐Kharboosh, R. , Quiñones‐Hinojosa, A. , & Green, J. J. (2019). Cancer‐selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials, 209, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel, C. , & Amiji, M. (2011). Oral TNF‐α gene silencing using a polymeric microsphere‐based delivery system for the treatment of inflammatory bowel disease. Journal of Controlled Release, 150, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel, C. , & Amiji, M. M. (2011). Dual TNF‐α/Cyclin D1 gene silencing with an oral polymeric microparticle system as a novel strategy for the treatment of inflammatory bowel disease. Clinicalal Translational Gastroenterology, 2, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroui, H. , Theiss, A. L. , Yan, Y. , Dalmasso, G. , Nguyen, H. T. , Sitaraman, S. V. , & Merlin, D. (2011). Functional TNFα gene silencing mediated by polyethyleneimine/TNFα siRNA nanocomplexes in inflamed colon. Biomaterials, 32, 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroui, N. , Coste, M. , Lichon, L. , Bessin, Y. , Gary‐Bobo, M. , Pratviel, G. , Bonduelle, C. , Bettache, N. , & Ulrich, S. (2019). Combination of photodynamic therapy and gene silencing achieved through the hierarchical self‐assembly of porphyrin‐siRNA complexes. International Journal of Pharmaceutics, 569, 118585. [DOI] [PubMed] [Google Scholar]
- Lee, S. H. , Bae, K. H. , Kim, S. H. , Lee, K. R. , & Park, T. G. (2008). Amine‐functionalized gold nanoparticles as non‐cytotoxic and efficient intracellular siRNA delivery carriers. International Journal of Pharmaceutics, 364, 94–101. [DOI] [PubMed] [Google Scholar]
- Lee, S. K. , Law, B. , & Tung, C.‐H. (2017). Versatile nanodelivery platform to maximize siRNA combination therapy. Macromolecular Bioscience, 17, 1600294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Hao, Y. , Wang, N. , Wang, L. , Jia, S. , Wang, Y. , Yang, L. , Zhang, Y. , & Zhang, Z. (2016). DOTAP functionalizing single‐walled carbon nanotubes as non‐viral vectors for efficient intracellular siRNA delivery. Drug Delivery, 23, 830–838. [DOI] [PubMed] [Google Scholar]
- Li, J. , Liang, H. , Liu, J. , & Wang, Z. (2018). Poly (amidoamine)(PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. International Journal of Pharmaceutics, 546, 215–225. [DOI] [PubMed] [Google Scholar]
- Li, R. , Wei, F. , Wu, X. , Zhou, P. , Chen, Q. , Cen, Y. , Xu, G. , Cheng, X. , Zhang, A. , & Hu, Q. (2021). PEI modified orange emissive carbon dots with excitation‐independent fluorescence emission for cellular imaging and siRNA delivery. Carbon, 177, 403–411. [Google Scholar]
- Li, X. , Yu, C. , Meng, X. , Hou, Y. , Cui, Y. , Zhu, T. , Li, Y. , Teng, L. , Sun, F. , & Li, Y. (2020). Study of double‐targeting nanoparticles loaded with MCL‐1 siRNA and dexamethasone for adjuvant‐induced arthritis therapy. European Journal of Pharmaceutics And Biopharmaceutics: Official Journal of Arbeitsgemeinschaft für Pharmazeutische Verfahrenstechnik e.V, 154, 136–143. [DOI] [PubMed] [Google Scholar]
- Lin, P. J. , & Tam, Y. K. (2019). Controlling protein expression by delivery of RNA therapeutics using lipid nanoparticles. Nucleic Acid Nanotheranostics: Elsevier , 277, 310. [Google Scholar]
- Lo, C. H. , Khalili, H. , Lochhead, P. , Song, M. , Lopes, E. W. , Burke, K. E. , Richter, J. M. , Chan, A. T. , & Ananthakrishnan, A. N. (2021). Immune‐mediated diseases and risk of Crohn's disease or ulcerative colitis: A prospective cohort study. Alimentary Pharmacology & Therapeutics, 53, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley, D. , & Johnston, P. (2005). Molecular mechanisms of drug resistance. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 205, 275–292. [DOI] [PubMed] [Google Scholar]
- Lopez‐Candales, A. , Hernández Burgos, P. M. , Hernandez‐Suarez, D. F. , & Harris, D. (2017). Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. Journal of Natural Sciences, 3, e341. [PMC free article] [PubMed] [Google Scholar]
- Lu, L. , Qi, S. , Chen, Y. , Luo, H. , Huang, S. , Yu, X. , Luo, Q. , & Zhang, Z. (2020). Targeted immunomodulation of inflammatory monocytes across the blood‐brain barrier by curcumin‐loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials, 245, 119987. [DOI] [PubMed] [Google Scholar]
- Mack, G. S. (2007). MicroRNA gets down to business. Nature Biotechnology, 25, 631–638. [DOI] [PubMed] [Google Scholar]
- Mahinfar, P. , Mokhtarzadeh, A. , Baradaran, B. , & Torbati, E. S. (2022). Antiproliferative activity of CD44 siRNA‐PEI‐PEG nanoparticles in glioblastoma: Involvement of AKT signaling. Research in Pharmaceutical Sciences, 17, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaekeh‐Nikouei, B. , Gholami, L. , Asghari, F. , Askarian, S. , Barzegar, S. , Rezaee, M. , & Kazemi Oskuee, R. (2018). Viral vector mimicking and nucleus targeted nanoparticles based on dexamethasone polyethylenimine nanoliposomes: Preparation and evaluation of transfection efficiency. Colloids and Surfaces B: Biointerfaces, 165, 252–261. [DOI] [PubMed] [Google Scholar]
- Mashaghi, S. , Jadidi, T. , Koenderink, G. , & Mashaghi, A. (2013). Lipid nanotechnology. International Journal of Molecular Sciences, 14, 4242–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, L. , Zhang, Z. , Zhao, L. , Huang, L. , Yang, X.‐L. , Tang, J. , & Feng, S. S. (2013). Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Advanced Drug Delivery Reviews, 65, 880–890. [DOI] [PubMed] [Google Scholar]
- Mendonça, L. S. , Firmino, F. , Moreira, J. N. , Pedroso de Lima, M. C. , & Simões, S. (2010). Transferrin receptor‐targeted liposomes encapsulating anti‐BCR‐ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjugate Chemistry, 21, 157–168. [DOI] [PubMed] [Google Scholar]
- Merkel, O. M. , Beyerle, A. , Librizzi, D. , Pfestroff, A. , Behr, T. M. , Sproat, B. , Barth, P. J. , & Kissel, T. (2009). Nonviral siRNA delivery to the lung: Investigation of PEG−PEI polyplexes and their in vivo performance. Molecular Pharmaceutics, 6, 1246–1260. [DOI] [PubMed] [Google Scholar]
- Merlo, L. M. , Bowers, J. , Stefanoni, T. , Getts, R. , & Mandik‐Nayak, L. (2020). B‐Cell‐targeted 3DNA nanotherapy against indoleamine 2, 3‐dioxygenase 2 (IDO2) ameliorates autoimmune arthritis in a preclinical model. Clinical Pathology, 13, 2632010X20951812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele, E. , Spinelli, G. P. , Miele, E. , Di Fabrizio, E. , Ferretti, E. , Tomao, S. , & Gulino, A. (2012). Nanoparticle‐based delivery of small interfering RNA: Challenges for cancer therapy. International Journal of Nanomedicine, 7, 3637–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, D. K. , Balekar, N. , & Mishra, P. K. (2017). Nanoengineered strategies for siRNA delivery: From target assessment to cancer therapeutic efficacy. Drug Delivery and Translational Research, 7, 346–358. [DOI] [PubMed] [Google Scholar]
- Mishra, N. , Ashique, S. , Garg, A. , Rai, V. K. , Dua, K. , Goyal, A. , & Bhatt, S. (2022). Role of siRNA‐based nanocarriers for the treatment of neurodegenerative diseases. Drug Discovery Today, 27, 1431–1440. [DOI] [PubMed] [Google Scholar]
- Mocellin, S. , & Provenzano, M. (2004). RNA interference: Learning gene knock‐down from cell physiology. Journal of Translational Medicine, 2, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, J. , Santos, A. , Moura, V. , Pedroso, dL. , & Simoes, S. (2008). Non‐viral lipid‐based nanoparticles for targeted cancer systemic gene silencing. Journal of Nanoscience and Nanotechnology, 8, 2187–2204. [DOI] [PubMed] [Google Scholar]
- Moudgil, K. D. , & Choubey, D. (2011). Cytokines in autoimmunity: Role in induction, regulation, and treatment. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research, 31, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar, M. , Bilal, M. , Rahdar, A. , Barani, M. , Arshad, R. , Behl, T. , Brisc, C. , Banica, F. , & Bungau, S. (2020). Nanomaterials for diagnosis and treatment of brain cancer: Recent updates. Chemosensors, 8, 117. [Google Scholar]
- Mukhtar, M. , Sargazi, S. , Barani, M. , Madry, H. , Rahdar, A. , & Cucchiarini, M. (2021). Application of nanotechnology for sensitive detection of low‐abundance single‐nucleotide variations in genomic DNA: A review. Nanomaterials, 11, 1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, E. K. , Białas, N. , Epple, M. , & Hilger, I. (2022). Nanoparticles carrying NF‐κB p65‐Specific siRNA alleviate colitis in mice by attenuating NF‐κB‐related protein expression and pro‐inflammatory cellular mediator secretion. Pharmaceutics, 14, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, R. Y. , Hammond, M. C. , Rio, D. C. , & Lee, Y. J. (2015). An efficient method for electroporation of small interfering RNAs into ENCODE project tier 1 GM12878 and K562 cell lines. Journal of biomolecular techniques: JBT, 26, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik, S. , Shreya, A. B. , Raychaudhuri, R. , Pandey, A. , Lewis, S. A. , Hazarika, M. , Bhandary, S. V. , Rao, B. , & Mutalik, S. (2021). Small interfering RNAs (siRNAs) based gene silencing strategies for the treatment of glaucoma: Recent advancements and future perspectives. Life Sciences, 264, 118712. [DOI] [PubMed] [Google Scholar]
- Ngamcherdtrakul, W. , Sangvanich, T. , Reda, M. , Gu, S. , Bejan, D. , & Yantasee, W. (2018). Lyophilization and stability of antibody‐conjugated mesoporous silica nanoparticle with cationic polymer and PEG for siRNA delivery. International Journal of Nanomedicine, 13, 4015–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T. , Nguyen, T. T. D. , Tran, N.‐M.‐A. , & Van Vo, G. (2021). Lipid‐based nanocarriers via nose‐to‐brain pathway for central nervous system disorders. Neurochemical Research, 1, 22. [DOI] [PubMed] [Google Scholar]
- Nikam, R. R. , & Gore, K. R. (2018). Journey of siRNA: Clinical developments and targeted delivery. Nucleic Acid Therapeutics, 28, 209–224. [DOI] [PubMed] [Google Scholar]
- Novelli, L. , Motta, F. , Ceribelli, A. , Guidelli, G. M. , Luciano, N. , Isailovic, N. , Vecellio, M. , Caprioli, M. , Clementi, N. , Clementi, M. , Mancini, N. , Selmi, C. , & De Santis, M. (2021). A case of psoriatic arthritis triggered by SARS‐CoV‐2 infection. Rheumatology, 60, 21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, Y.‐K. , & Park, T. G. (2009). siRNA delivery systems for cancer treatment. Advanced Drug Delivery Reviews, 61, 850–862. [DOI] [PubMed] [Google Scholar]
- Ozpolat, B. , Sood, A. K. , & Lopez‐Berestein, G. (2014). Liposomal siRNA nanocarriers for cancer therapy. Advanced Drug Delivery Reviews, 66, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi, P. , Jain, A. , Kommineni, N. , Ionov, M. , Bryszewska, M. , & Khan, W. (2018). Dendrimer as a new potential carrier for topical delivery of siRNA: A comparative study of dendriplex vs. lipoplex for delivery of TNF‐α siRNA. International Journal of Pharmaceutics, 550, 240–250. [DOI] [PubMed] [Google Scholar]
- Parhiz, H. , Brenner, J. S. , Patel, P. N. , Papp, T. E. , Shahnawaz, H. , Li, Q. , Shi, R. , Zamora, M. E. , Yadegari, A. , Marcos‐Contreras, O. A. , Natesan, A. , Pardi, N. , Shuvaev, V. V. , Kiseleva, R. , Myerson, J. W. , Uhler, T. , Riley, R. S. , Han, X. , Mitchell, M. J. , … Muzykantov, V. R. (2022). Added to pre‐existing inflammation, mRNA‐lipid nanoparticles induce inflammation exacerbation (IE). Journal of Controlled Release, 344, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar, R. G. , Poslusney, M. , Busuek, M. , Williams, J. M. , Garbaccio, R. , Leander, K. , Walsh, E. , Howell, B. , Sepp‐Lorenzino, L. , Riley, S. , Patel, M. , Kemp, E. , Latham, A. , Leone, A. , Soli, E. , Burke, R. S. , Carr, B. , Colletti, S. L. , & Wang, W. (2014). Novel endosomolytic poly (amido amine) polymer conjugates for systemic delivery of siRNA to hepatocytes in rodents and nonhuman primates. Bioconjugate Chemistry, 25, 896–906. [DOI] [PubMed] [Google Scholar]
- Pathak, K. , Keshri, L. , & Shah, M. (2011). Lipid nanocarriers: Influence of lipids on product development and pharmacokinetics. Critical Reviews™ in Therapeutic Drug Carrier Systems, 28, 357–393. [DOI] [PubMed] [Google Scholar]
- Patra, J. K. , Das, G. , Fraceto, L. F. , Campos, E. V. R. , Rodriguez‐Torres, M. P. , Acosta‐Torres, L. S. , Diaz‐Torres, L. A. , Grillo, R. , Swamy, M. K. , Sharma, S. , Habtemariam, S. , & Shin, H. S. (2018). Nano based drug delivery systems: Recent developments and future prospects. Journal of Nanobiotechnology, 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley, K. M. , & Cha, S. (2013). RNAi therapeutics in autoimmune disease. Pharmaceuticals, 6, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino, M. , Ceccacci, F. , Petrini, S. , Scipioni, A. , De Santis, S. , Cappa, M. , Mancini, G. , & Fierabracci, A. (2019). Exploiting novel tailored immunotherapies of type 1 diabetes: Short interfering RNA delivered by cationic liposomes enables efficient down‐regulation of variant PTPN22 gene in T lymphocytes. Nanomedicine: Nanotechnology, Biology and Medicine, 18, 371–379. [DOI] [PubMed] [Google Scholar]
- Pi, Y. , Zhang, X. , Shao, Z. , Zhao, F. , Hu, X. , & Ao, Y. (2015). Intra‐articular delivery of anti‐Hif‐2α siRNA by chondrocyte‐homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Therapy, 22, 439–448. [DOI] [PubMed] [Google Scholar]
- Poeck, H. , Besch, R. , Maihoefer, C. , Renn, M. , Tormo, D. , Morskaya, S. S. , Kirschnek, S. , Gaffal, E. , Landsberg, J. , Hellmuth, J. , Schmidt, A. , Anz, D. , Bscheider, M. , Schwerd, T. , Berking, C. , Bourquin, C. , Kalinke, U. , Kremmer, E. , Kato, H. , … Hartmann, G. (2008). 5′‐Triphosphate‐siRNA: Turning gene silencing and Rig‐I activation against melanoma. Nature Medicine, 14, 1256–1263. [DOI] [PubMed] [Google Scholar]
- Poupot, R. , Bergozza, D. , & Fruchon, S. (2018). Nanoparticle‐based strategies to treat neuro‐inflammation. Materials, 11, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del prado‐Audelo, M. , Caballero‐Florán, I. , Meza‐Toledo, J. , Mendoza‐Muñoz, N. , González‐Torres, M. , Florán, B. , Cortés, H. , & Leyva‐Gómez, G. (2019). Formulations of curcumin nanoparticles for brain diseases. Biomolecules, 9, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol‐Autonell, I. , Mansilla, M.‐J. , Rodriguez‐Fernandez, S. , Cano‐Sarabia, M. , Navarro‐Barriuso, J. , Ampudia, R.‐M. , Rius, A. , Garcia‐Jimeno, S. , Perna‐Barrull, D. , Martinez‐Caceres, E. , Maspoch, D. , & Vives‐Pi, M. (2017). Liposome‐based immunotherapy against autoimmune diseases: Therapeutic effect on multiple sclerosis. Nanomedicine, 12, 1231–1242. [DOI] [PubMed] [Google Scholar]
- Qelliny, M. R. , Shimizu, T. , Elsadek, N. E. , Emam, S. E. , Takata, H. , Fathalla, Z. , Hussein, A. K. , Khaled, K. A. , Ando, H. , Ishima, Y. , & Ishida, T. (2021). Incorporating gangliosides into PEGylated cationic liposomes that complexed DNA attenuates Anti‐PEG antibody production but not Anti‐DNA antibody production in mice. Molecular Pharmaceutics, 18, 2406–2415. [DOI] [PubMed] [Google Scholar]
- Raftery, R. , O'brien, F. J. , & Cryan, S.‐A. (2013). Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules, 18, 5611–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee, M. , Oskuee, R. K. , Nassirli, H. , & Malaekeh‐Nikouei, B. (2016). Progress in the development of lipopolyplexes as efficient non‐viral gene delivery systems. Journal of Controlled Release, 236, 1–14. [DOI] [PubMed] [Google Scholar]
- Ricardo, J. W. , & Lipner, S. R. (2020). Considerations for safety in the use of systemic medications for psoriasis and atopic dermatitis during the COVID‐19 pandemic. Dermatologic Therapy, 33, e13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, M. , Restrepo‐Jiménez, P. , Monsalve, D. M. , Pacheco, Y. , Acosta‐Ampudia, Y. , Ramírez‐Santana, C. , Leung, P. , Ansari, A. A. , Gershwin, M. E. , & Anaya, J. M. (2018). Molecular mimicry and autoimmunity. Journal of Autoimmunity, 95, 100–123. [DOI] [PubMed] [Google Scholar]
- Roslan, R. B. , Razly, I. N. M. , Sabri, N. , & Ibrahim, Z. (2020). Evaluation of psoriasis skin disease classification using convolutional neural network. IAES International Journal of Artificial Intelligence, 9, 349. [Google Scholar]
- Rozema, D. B. , Lewis, D. L. , Wakefield, D. H. , Wong, S. C. , Klein, J. J. , Roesch, P. L. , Bertin, S. L. , Reppen, T. W. , Chu, Q. , Blokhin, A. V. , Hagstrom, J. E. , & Wolff, J. A. (2007). Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proceedings of the National Academy of Sciences, 104, 12982–12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudorf, S. , & Rädler, J. O. (2012). Self‐assembly of stable monomolecular nucleic acid lipid particles with a size of 30 nm. Journal of the American Chemical Society, 134, 11652–11658. [DOI] [PubMed] [Google Scholar]
- Sajid, M. I. , Moazzam, M. , Kato, S. , Yeseom Cho, K. , & Tiwari, R. K. (2020). Overcoming barriers for siRNA therapeutics: From bench to bedside. Pharmaceuticals, 13, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargazi, S. , Siddiqui, B. , Qindeel, M. , Rahdar, A. , Bilal, M. , Behzadmehr, R. , Mirinejad, S. , & Pandey, S. (2022). Chitosan nanocarriers for microRNA delivery and detection: A preliminary review with emphasis on cancer. Carbohydrate Polymers, 290, 119489. [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Himeno, A. , Nakagawa, T. , Sasaki, Y. , Kiyonari, H. , & Iwai, K. (2019). Modulation of autoimmune pathogenesis by T cell‐triggered inflammatory cell death. Nature Communications, 10, 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman, R. I. , Trivedi, R. , Vermillion, S. , & Kompella, U. B. (2011). Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine, 6, 1669–1682. [DOI] [PubMed] [Google Scholar]
- Shariatinia, Z. (2022). Inorganic material‐based nanocarriers for delivery of biomolecules. Nanoengineering of Biomaterials: Biomedical Applications, 2, 245–293. [Google Scholar]
- Sheervalilou, R. , Shirvaliloo, M. , Sargazi, S. , Shirvalilou, S. , Shahraki, O. , Pilehvar‐Soltanahmadi, Y. , Sarhadi, A. , Nazarlou, Z. , Ghaznavi, H. , & Khoei, S. (2021). Application of nanobiotechnology for early diagnosis of SARS‐CoV‐2 infection in the COVID‐19 pandemic. Applied Microbiology and Biotechnology, 105, 2615–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha, B. , Tang, L. , & Romero, G. (2019). Nanoparticles‐mediated combination therapies for cancer treatment. Advanced Therapeutics, 2, 1900076. [Google Scholar]
- Sioud, M. (2006). Innate sensing of self and non‐self RNAs by Toll‐like receptors. Trends in Molecular Medicine, 12, 167–176. [DOI] [PubMed] [Google Scholar]
- Sioud, M. (2007). RNA interference and innate immunity. Advanced Drug Delivery Reviews, 59, 153–163. [DOI] [PubMed] [Google Scholar]
- Sioud, M. (2019). Releasing the immune system brakes using siRNAs enhances cancer immunotherapy. Cancers, 11, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane, N. , Szoka, F. C. , & Verkman, A. (2003). Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine‐DNA polyplexes. Journal of Biological Chemistry, 278, 44826–44831. [DOI] [PubMed] [Google Scholar]
- Song, J. , Chen, Y. , Jiang, S. , Yang, K. , Li, X. , Zhao, X. , Ouyang, Y. , Fan, C. , & Yuan, W. (2016). Efficient and non‐toxic biological response carrier delivering TNF‐α shRNA for gene silencing in a murine model of rheumatoid arthritis. Frontiers in Immunology, 7, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, A. R. , Oliveira, A. V. , Oliveira, M. J. , & Sarmento, B. (2019). Nanotechnology‐based siRNA delivery strategies for metastatic colorectal cancer therapy. International Journal of Pharmaceutics, 568, 118530. [DOI] [PubMed] [Google Scholar]
- Srivastava, A. , Gowda, D. V. , Madhunapantula, S. V. , Shinde, C. G. , & Iyer, M. (2015). Mucosal vaccines: A paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS, 123, 275–288. [DOI] [PubMed] [Google Scholar]
- Subhan, A. , Attia, S. A. , Torchilin, P. V. (2022). Targeted siRNA nanotherapeutics against breast and ovarian metastatic cancer: A comprehensive review of the literature. Nanomedicine, 17, 41–64. [DOI] [PubMed] [Google Scholar]
- Subhan, M. A. , & Torchilin, V. (2019). Efficient nanocarriers of siRNA therapeutics for cancer treatment. Translational Research, 214, 62–91. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Liu, W. , & Zhang, L.‐J. (2019a). The role of toll‐like receptors in skin host defense, psoriasis, and atopic dermatitis. Journal of Immunology Research, 2019, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Dong, S. , Li, X. , Yu, K. , Sun, F. , Lee, R. J. , Li, Y. , & Teng, L. (2019b). Delivery of siRNA using folate receptor‐targeted pH‐sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomedicine: Nanotechnology, Biology and Medicine, 20, 102017. [DOI] [PubMed] [Google Scholar]
- Suzuki, I. L. , de Araujo, M. M. , Bagnato, V. S. , & Bentley, M. V. L. B. (2021). TNFα siRNA delivery by nanoparticles and photochemical internalization for psoriasis topical therapy. Journal of Controlled Release, 338, 316–329. [DOI] [PubMed] [Google Scholar]
- Tagami, T. , Uehara, Y. , Moriyoshi, N. , Ishida, T. , & Kiwada, H. (2011). Anti‐PEG IgM production by siRNA encapsulated in a PEGylated lipid nanocarrier is dependent on the sequence of the siRNA. Journal of Controlled Release, 151, 149–154. [DOI] [PubMed] [Google Scholar]
- Taher, T. E. , Bystrom, J. , Ong, V. H. , Isenberg, D. A. , Renaudineau, Y. , Abraham, D. J. , & Mageed, R. A. (2017). Intracellular B lymphocyte signalling and the regulation of humoral immunity and autoimmunity. Clinical Reviews in Allergy & Immunology, 53, 237–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y. , Wang, X. , Li, J. , Nie, Y. , Liao, G. , Yu, Y. , & Li, C. (2019). Overcoming the reticuloendothelial system barrier to drug delivery with a “don't‐eat‐us” strategy. ACS Nano, 13, 13015–13026. [DOI] [PubMed] [Google Scholar]
- Tarner, I. H. , & Fathman, C. G. (2001). Gene therapy in autoimmune disease. Current Opinion in Immunology, 13, 676–682. [DOI] [PubMed] [Google Scholar]
- Tofani, L. B. , Depieri, L. V. , Campos, P. M. , Riul, T. B. , Antonietto, K. S. , de Abreu Fantini, M. C. , & Bentley, M. (2018). In vitro TyRP‐1 knockdown based on siRNA carried by liquid crystalline nanodispersions: an alternative approach for topical treatment of vitiligo. Pharmaceutical Research, 35, 1–13. [DOI] [PubMed] [Google Scholar]
- Valentini, X. , Deneufbourg, P. , Paci, P. , Rugira, P. , Laurent, S. , Frau, A. , Stanicki, D. , Ris, L. , & Nonclercq, D. (2018). Morphological alterations induced by the exposure to TiO2 nanoparticles in primary cortical neuron cultures and in the brain of rats. Toxicology Reports, 5, 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkouhi, A. K. , Scholte, M. , Storm, G. , & Haisma, H. J. (2011). Endosomal escape pathways for delivery of biologicals. Journal of Controlled Release, 151, 220–228. [DOI] [PubMed] [Google Scholar]
- Veiga, N. , Diesendruck, Y. , & Peer, D. (2020). Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Advanced Drug Delivery Reviews, 159, 364–376. [DOI] [PubMed] [Google Scholar]
- Veiga, N. , Goldsmith, M. , Diesendruck, Y. , Ramishetti, S. , Rosenblum, D. , Elinav, E. , Behlke, M. A. , Benhar, I. , & Peer, D. (2019). Leukocyte‐specific siRNA delivery revealing IRF8 as a potential anti‐inflammatory target. Journal of Controlled Release, 313, 33–41. [DOI] [PubMed] [Google Scholar]
- Verma, P. , Srivastava, A. , Srikanth, C. V. , & Bajaj, A. (2021). Nanoparticle‐mediated gene therapy strategies for mitigating inflammatory bowel disease. Biomaterials Science, 9, 1481–1502. [DOI] [PubMed] [Google Scholar]
- Viegas, J. S. R. , Praça, F. G. , Caron, A. L. , Suzuki, I. , Silvestrini, A. V. P. , Medina, W. S. G. , Del Ciampo, J. O. , Kravicz, M. , & Bentley, M. (2020). Nanostructured lipid carrier co‐delivering tacrolimus and TNF‐α siRNA as an innovate approach to psoriasis. Drug Delivery and Translational Research, 10, 646–660. [DOI] [PubMed] [Google Scholar]
- Wang, K. , Chen, X. , Yan, F. , Xing, Y. , Yang, X. , Tu, J. , & Chen, Z. (2013). 5′‐triphosphate‐siRNA against survivin gene induces interferon production and inhibits proliferation of lung cancer cells in vitro. Journal of Immunotherapy, 36, 294–304. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Ding, X. , Yan, J. , Lu, Z. , Cao, H. , Ni, X. , & Ying, Y. (2021). STAT5 inhibitor attenuates atherosclerosis via inhibition of inflammation: the role of STAT5 in atherosclerosis. American Journal of Translational Research, 13, 1422–1431. [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , & Gu, H. (2015). Core–shell‐type magnetic mesoporous silica nanocomposites for bioimaging and therapeutic agent delivery. Advanced Materials, 27, 576–585. [DOI] [PubMed] [Google Scholar]
- Watts, J. K. , Deleavey, G. F. , & Damha, M. J. (2008). Chemically modified siRNA: Tools and applications. Drug Discovery Today, 13, 842–855. [DOI] [PubMed] [Google Scholar]
- Wei, X. , Li, F. , Zhao, G. , Chhonker, Y. S. , Averill, C. , Galdamez, J. , Purdue, P. E. , Wang, X. , Fehringer, E. V. , Garvin, K. L. , Goldring, S. R. , Alnouti, Y. , & Wang, D. (2017). Pharmacokinetic and biodistribution studies of HPMA copolymer conjugates in an aseptic implant loosening mouse model. Molecular Pharmaceutics, 14, 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X. , Wu, J. , Zhao, G. , Galdamez, J. , Lele, S. M. , Wang, X. , Liu, Y. , Soni, D. M. , Purdue, P. E. , Mikuls, T. R. , Goldring, S. R. , & Wang, D. (2018). Development of a Janus kinase inhibitor prodrug for the treatment of rheumatoid arthritis. Molecular Pharmaceutics, 15, 3456–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C.‐X. , Jere, D. , Jin, H. , Chang, S.‐H. , Chung, Y.‐S. , Shin, J.‐Y. , Kim, J. E. , Park, S. J. , Lee, Y. H. , Chae, C. H. , Lee, K. H. , Beck GR, J. r , Cho, C. S. , & Cho, M. H. (2008). Poly (ester amine)‐mediated, aerosol‐delivered Akt1 small interfering RNA suppresses lung tumorigenesis. American Journal of Respiratory and Critical Care Medicine, 178, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R. , Castriota, G. , Chen, Y. , Cleary, M. A. , Ellsworth, K. , Shin, M. K. , Tran, J. L. , Vogt, T. F. , Wu, M. , Xu, S. , Yang, X. , Zhang, B. B. , Berger, J. P. , & Qureshi, S. A. (2011). RNAi‐mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet‐induced obese mice. International Journal of Obesity, 35, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Xue, H. , Guo, P. , Wen, W.‐C. , & Lun Wong, H. (2015). Lipid‐based nanocarriers for RNA delivery. Current Pharmaceutical Design, 21, 3140–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa, S. , Koide, H. , & Asai, T. (2020). Recent advances in siRNA delivery mediated by lipid‐based nanoparticles. Advanced Drug Delivery Reviews, 154‐155, 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, B. , Luo, X. , Yu, Z. , Mani, S. , Wang, Z. , & Dou, W. (2019). Inflammatory bowel disease: A potential result from the collusion between gut microbiota and mucosal immune system. Microorganisms, 7, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- uz Zaman, S. , Arshad, R. , Tabish, T. A. , Naseem, A. A. , & Shahnaz, G. (2021). Mapping the potential of thiolated pluronic based nanomicelles for the safe and targeted delivery of vancomycin against staphylococcal blepharitis. Journal of Drug Delivery Science and Technology, 61, 102220. [Google Scholar]
- Zhai, Z. , Ouyang, W. , Yao, Y. , Zhang, Y. , Zhang, H. , Xu, F. , & Gao, C. (2022). Dexamethasone‐loaded ROS‐responsive poly(thioketal) nanoparticles suppress inflammation and oxidative stress of acute lung injury. Bioactive Materials, 14, 430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. X. , Ip, C. K. , Chung, S. K. , Lei, K. K. , Zhang, Y. Q. , Liu, L. , & Wong, V. (2020). Drug‐resistance in rheumatoid arthritis: The role of p53 gene mutations, ABC family transporters and personal factors. Current Opinion in Pharmacology, 54, 59–71. [DOI] [PubMed] [Google Scholar]
- Zhang, L.‐J (2019). Type1 interferons potential initiating factors linking skin wounds with psoriasis pathogenesis. Frontiers in Immunology, 10, 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Viennois, E. , Prasad, M. , Zhang, Y. , Wang, L. , Zhang, Z. , Han, M. K. , Xiao, B. , Xu, C. , Srinivasan, S. , & Merlin, D. (2016). Edible ginger‐derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis‐associated cancer. Biomaterials, 101, 321–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. M. , Bahal, R. , Rasmussen, T. P. , Manautou, J. E. , & Zhong, X.‐B (2021). The growth of siRNA‐based therapeutics: Updated clinical studies. Biochemical Pharmacology, 189, 114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, M. , Tao, W. , Zou, Y. , Farokhzad, O. C. , & Shi, B. (2018). Nanotechnology‐based strategies for siRNA brain delivery for disease therapy. Trends in Biotechnology, 36, 562–575. [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Chen, Q. , Lanske, B. , Fleming, B. C. , Terek, R. , Wei, X. , Zhang, G. , Wang, S. , Li, K. , & Wei, L. (2014a). Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1‐CreER T2; Ihh fl/fl mice. Arthritis Research & Therapy, 16, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Zhang, C. , & Liang, W. (2014b). Development of RNAi technology for targeted therapy—A track of siRNA based agents to RNAi therapeutics. Journal of Controlled Release, 193, 270–281. [DOI] [PubMed] [Google Scholar]
- Zimmermann, T. , Lee, A. C. , Akinc, A. , Bramlage, B. , Bumcrot, D. , Fedoruk, M. N. , Harborth, J. , Heyes, J. A. , Jeffs, L. B. , John, M. , Judge, A. D. , Lam, K. , McClintock, K. , Nechev, L. V. , Palmer, L. R. , Racie, T. , Rohl, I. , Seiffert, S. , Shanmugam, S. , … MacLachlan, I. (2006). RNAi‐mediated gene silencing in non‐human primates. Nature, 441, 111–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article's data sharing is not applicable as no new data were created or analyzed in this study.


