Abstract
A multicolor flow cytometry panel was designed and optimized to define the following nine mouse T cell subsets: Treg (CD3+ CD4+ CD8− FoxP3+), CD4+ T naïve (CD3+ CD4+ CD8−FoxP3− CD44int/low CD62L+), CD4+ T central memory (CD3+ CD4+ CD8− FoxP3− CD44high CD62L+), CD4+ T effector memory (CD3+ CD4+ CD8− FoxP3− CD44high CD62L−), CD4+ T EMRA (CD3+ CD4+ CD8− FoxP3− CD44int/low CD62L−), CD8+ T naïve (CD3+ CD8+ CD4− CD44int/low CD62L+), CD8+ T central memory (CD3+ CD8+ CD4− CD44high CD62L+), CD8+ T effector memory (CD3+ CD8+ CD4− CD44high CD62L−), and CD8+ T EMRA (CD3+ CD8+ CD4− CD44int/low CD62L−). In each T cell subset, a dual staining for Ki‐67 expression and DNA content was employed to distinguish the following cell cycle phases: G0 (Ki67−, with 2n DNA), G1 (Ki67+, with 2n DNA), and S‐G2/M (Ki67+, with 2n < DNA ≤ 4n). This panel was established for the analysis of mouse (C57BL/6J) spleen.
Keywords: cell cycle, DNA content, flow cytometry, Ki‐67, mouse T cells, spleen
1. BACKGROUND
The periodicity of cell proliferation is a fundamental aspect of biology that, for example, discriminates neural stem cells from terminally differentiated neurons. Likewise, it is a key feature of adaptive immunity that depends upon clonal expansion of primed T and B cells with appropriate antigen specificity, thereupon generating a vast progeny of short‐lived effector cells and a few long‐lived memory cells. There are two main subsets of T cells, CD4+ and CD8+ T cells, having a predominant helper and cytotoxic effector function, respectively. Maintenance of memory CD4+ and CD8+ T cells over time is a dynamic process, relying on a fine equilibrium among cell death, survival, and low level of homeostatic proliferation [1, 2]. Under steady‐state conditions, most T cells in the spleen of untreated mice are quiescent cells, although a tiny proportion divides, possibly reflecting immune responses to unknown environmental antigens and/or cytokine‐driven homeostatic proliferation. Such cell cycling in the absence of intentional immunization is more prominent among the so‐called memory‐phenotype T cells, that share a set of membrane markers with antigen‐primed T cells [3]. Furthermore, some Treg cells (a subset of CD4+ T cells with regulatory function defined by the expression of the transcription factor FoxP3 [4]) have an activated/proliferative phenotype, possibly reflecting continuous self‐renewal in adult mice [5].
In fact, memory‐phenotype T cells comprise a heterogeneous pool of cells of undefined antigen‐specificity, that are considered to include T cells primed by environmental antigens, as well as some antigen‐inexperienced T cells having self‐ligand‐ and/or cytokine‐dependent development [6, 7]. In C57BL/6 mice memory‐phenotype T cells have a high expression of CD44, an adhesion molecule that binds to hyaluronic acid, and can thus be distinguished from naïve‐phenotype T cells, that have an intermediate/low CD44 expression [6]. Similarly, in humans naïve and memory‐phenotype T cells can be identified by high and low expression of CD45RA, respectively [8].
Proliferative potential is one of the features that, together with lymph node (LN) homing capabilities, and effector function, differentiates additional subsets among memory T cells. Thus, central memory (CM) T cells have a LN homing receptor typically expressed by naïve T cells, specifically CD62L, also named L‐selectin, a glycan receptor [9], whereas effector memory (EM) T cells lack it [10]. According to this classification, originally proposed for human blood T cells using CCR7, the chemokine receptor for CCL19/CCL21, as a marker [11], T CM are LN‐homing cells with high potential to expand after stimulation, while T EM cells are tissue‐homing cells able to display rapid effector function [12, 13]. An additional subset, the T EMRA cells, comprises effector memory T cells that re‐acquire a naïve phenotype (CD45RA+ in humans, CD44int/low in mice). Based on this, the following four naïve/memory T cell subsets can be identified among mouse CD4+ and CD8+ T cells: CD44int/low CD62L+ naïve, CD44high CD62L+ CM, CD44high CD62L− EM, and CD44int/low CD62L− EMRA [10, 14].
While identification of memory T cells with high proliferative potential can impact the success of adoptive transfers [15], accurate measurement of in vivo proliferation is essential to track the dynamics of T cell responses [16]. Proliferation of mouse T cells has been measured by a few cytofluorimetric methods, that can be divided into “static”—that is, those which provide a snapshot of the cell cycle phases at the time of analysis—and “dynamic,” that is, those which give information on the proliferation that occurred over a few hours or days prior to the analysis. The most widely used “static” method relies on the Ki‐67 marker, an intranuclear protein that supports chromosome architecture organization, and nucleus and nucleolar assembly after cell division [17, 18]. However, Ki‐67 is expressed by cells in any phase of cell cycle (i.e., in G1, S, G2, M), while it is only low or absent in quiescent cells (i.e., in G0 state). The “dynamic” methods include carboxyfluorescein succinimidyl ester (CFSE) and Bromodeoxyuridine (BrdU) labelling, which identify proliferating cells that have undergone cell division and S‐phase, respectively [2]. We chose to combine Ki‐67 staining and DNA content analysis, thereby to distinguish between cells in G1 and those in S‐G2/M phases of cell cycle, a discrimination that is relevant to the proliferative fate of the cell. Indeed, cells in S are duplicating their DNA and are committed to proceed into G2/M and divide. In contrast, cells that are in G1 might proceed into S‐G2/M, return to G0, or stay in G1 for a prolonged period. Thus, Ki‐67+ cells might not be actively proliferating if they are in G1 phase or are returning to G0. For example, this might be the case for antigen‐specific T cell progeny at the end of clonal expansion [19].
In this OMIP, we offer a staining panel for ex vivo cell cycle analysis of CD4+ and CD8+ naïve/memory‐phenotype T cell subsets, and of Treg cells from mouse spleen, using Ki‐67/DNA dual staining to distinguish cells in G0, G1, and S‐G2/M (Table 1; Figure 1). Panel optimization and protocol details are reported in the online Supporting Information. We used standard markers for T cell subset identification. CD3 expression was used to identify T cells, and the mutually exclusive expression of CD4 and CD8 to distinguish CD4+ and CD8+ T cells, respectively. Treg cells were identified among CD4+ T cells based on their expression of the intranuclear protein FoxP3, and by this marker distinguished from conventional CD4+ T cells, that is, FoxP3− CD3+ CD4+ CD8− cells. CD4+ and CD8+ naïve/memory T cell subsets were subsequently identified among conventional CD4+ and CD8+ T cells, respectively. The four classical naïve/memory subsets were defined based on their CD62L and CD44 membrane phenotype (see above). For each T cell subset, cells in G0, G1, and S‐G2/M were discriminated based on Ki‐67 and DNA staining (Table 2; Figure 1).
TABLE 1.
Summary table for the application of OMIP‐079
| Purpose | Cell cycle analysis of CD4 and CD8 naïve/memory T cell subsets, and of Treg cells |
| Species | Mouse |
| Cell types | Splenocytes |
| Cross‐references | No similar OMIPs |
FIGURE 1.
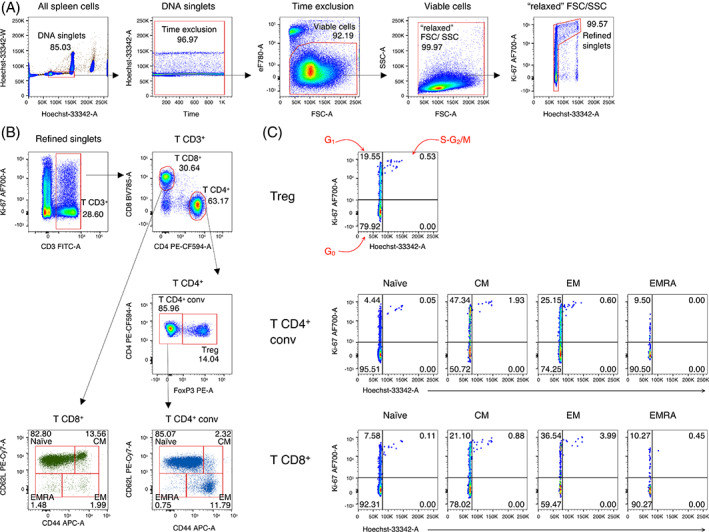
Cell cycle analysis of mouse T cell subsets. Example of analysis of spleen cells from a 3‐months old C57BL/6J mouse, using manual gating strategy. (A) Refined gating of viable single cells from the spleen in five steps: (1) DNA singlets. Single cells having 2n ≤ DNA content ≤4n were selected on the Hoechst‐33342 area (A) versus (vs) Hoechst‐33342 width (W) plot; (2) time exclusion. Stable acquisition over time (seconds) was monitored on the time vs Hoechst‐33342‐A plot and any events collected in case of pressure fluctuations were excluded; (3) viable cells. Live cells were selected using FSC‐A vs eFluor 780 (eF780) viability dye; (4) FSC/SSC “relaxed” gate. A “relaxed” gate was used on the FSC‐A vs SSC‐A plot, to include highly activated and cycling lymphocytes [19]; (5) refined singlets. A few remaining doublets composed by one cell sitting on top of another (so called “shadow” doublets) were excluded as Ki‐67int/− events having >2n DNA content [20]. This gating strategy was used as a base for the subsequent gates. (B) CD3+ T cells were gated on CD3‐A vs Ki‐67‐A plot, then CD4+ and CD8+ T cells on CD4‐A vs CD8‐A plot. CD4+ Treg cells were distinguished based on their FoxP3 expression from conventional FoxP3− CD4+ T cells. Subsequently, the following naïve/memory subsets of conventional CD4+ T cells were identified: CD44int/lowCD62L+ naïve, CD44highCD62L+ central memory (CM), CD44highCD62L− effector memory (EM), and CD44int/lowCD62L− EMRA. Similarly, naïve/memory subsets were identified among CD8+ T cells. (C) Cell cycle phases of Treg cells and of naïve/memory CD4+ and CD8+ T cell subsets were defined on Hoechst‐33342‐A vs Ki67‐A plot as follows: Cells in G0 were identified as DNA 2n/ Ki67− (bottom left quadrant); cells in G1 as DNA 2n/ Ki67+ (upper left quadrant); cells in S‐G2/M as DNA > 2n/ Ki67+ (top right quadrant) [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Reagents used for OMIP‐079
| Fluorochrome | Specificity | Clone | Function |
|---|---|---|---|
| FITC | CD3 | 145‐2C11 | Pan T cell marker |
| APC | CD44 | IM7 | NaÏve/Memory subset identification |
| Alexa Fluor 700 | Ki‐67 | SolA15 | Quiescence/cell cycle |
| eFluor 780 | Dead cells | N/A | Live/Dead cell discrimination |
| Hoechst 33342 | DNA | N/A | DNA content/cell cycle |
| PE | FoxP3 | FJK‐16s | Treg identification |
| PE‐CF594 | CD4 | RM4‐5 | Helper T cell identification |
| PE‐Cy7 | CD62L | MEL‐14 | NaÏve/Memory subset identification |
| BV785 | CD8 | 53‐6.7 | Cytotoxic T cell identification |
Abbreviation: N/A, not applicable.
This OMIP can be exploited for in depth‐analysis of T cell cycle in conditions characterized by altered proportions, numbers and proliferative state of spleen T cell subsets, for example in aged mice having higher percentages of memory CD4+ and CD8+ T cells, with or without oligoclonal expansion [21, 22, 23]; in lymphopenic mice having compensatory T cell proliferation [24]; or in genetically modified mice with abnormal Treg cells representation [25]. Furthermore, this panel may be instrumental in identifying hitherto overlooked changes in Treg and/or naïve/memory T cell subset cycling in a variety of settings such as vaccination, infection, autoimmunity, and cancer.
2. SIMILARITY TO PUBLISHED OMIPS
The new ground trodden by this OMIP is the examination of cell cycle of naïve/memory CD4+ and CD8+ T cell subsets and of Treg cells by Ki‐67/DNA dual staining, with no similarities to other OMIPs.
OMIP‐031 and ‐032 examined naïve/memory T cells, with different purposes. OMIP‐031 used a combination of CD44, CD62L, CD27, CD45RA for T cell subset definition, plus a panel of activation and exhaustion markers, with the aim to analyze inhibitor checkpoint expression. OMIP‐032 was designed for assessing innate and adaptive immune subsets from mouse organs, including naïve/memory T cell subsets, that were identified based on CD44 and CD62L expression. OMIP‐032 did not include any analysis of proliferation or cell cycle.
CONFLICT OF INTEREST
A.C.H. is a board member and equity holder in ImmunoQure, AG., and Gamma Delta Therapeutics, and is an equity holder in Adaptate Biotherapeutics.
AUTHOR CONTRIBUTIONS
Ambra Natalini: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); writing – review and editing (supporting). Sonia Simonetti: Conceptualization (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); writing – review and editing (supporting). Gabriele Favaretto: Data curation (supporting); formal analysis (supporting); methodology (supporting); writing – review and editing (supporting). Giovanna Peruzzi: Data curation (supporting); formal analysis (supporting); writing – review and editing (supporting). Fabrizio Antonangeli: Data curation (supporting); formal analysis (supporting); writing – review and editing (supporting). Angela Santoni: Writing – review and editing (supporting). Miguel Munoz‐Ruiz: Methodology (supporting); writing – review and editing (supporting). Adrian Hayday: Supervision (supporting); writing – review and editing (supporting). Francesca Di Rosa: Conceptualization (lead); supervision (lead); writing – original draft (lead).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/cyto.a.24509.
Supporting information
Appendix S1. MIFlowCyt MIFlowCyt Item Checklist: MIFlowCyt‐Compliant Items.
Appendix S2. Supporting Information.
Figure S1 Spillover spreading error (SSE) matrix (SSM). SSM was obtained with compensation beads, except for eF780 viability dye and Hoechst‐33342, which were compensated using live and dead splenocytes (1:1 mix) and bone marrow cells, respectively. SSE values >10 are highlighted in orange.
Figure S2 Titration of the DNA dye Hoechst‐33342 (Hoechst) and of the fixable viability dye eFluor 780 (eF780). (A) Hoechst titration with four 2‐fold dilutions. Titration was performed in parallel using either spleen (top) or bone marrow (BM) cells (bottom). Histogram plots show Hoechst fluorescence intensity on the x‐axis, using a linear scale in arbitrary units. The left and right gates correspond to the G0/G1 and the G2/M DNA peak, respectively, and the numbers represent the coefficients of variation (CV) of each peak. (B) Spreading of signal of Hoechst at 2 and 4 μg/ml in the indicated channels. All the channels were set identically at the two concentrations, except for UV laser (bandpass 530/30), i.e., the Hoechst channel, that was adapted for visualization of the DNA peaks by changing PMT voltage (decreasing PMT voltage for increasing Hoechst concentration). Histograms show fluorescence intensity in arbitrary units of unstained spleen cells (dotted empty line) and spleen cells stained with Hoechst (gray filled line) at either 2 or 4 μg/ml, as indicated. Laser (color code) and bandpass are indicated on top of each channel. Laser color code as follows: blue for 488 nm (blue) laser; red for 639 nm (red) laser; green for 561 nm (yellow/green) laser; black for 355 nm (UV) laser; violet for 405 nm (violet) laser. Where appropriate, fluorochromes used in this OMIP are indicated, although only Hoechst was used here. Hoechst concentration at 2 μg/ml (in red) was chosen as it resulted in DNA peaks having CV ≤ 5 (A), and lower signal spreading than Hoechst at 4 μg/ml (B). (C) Viability dye eF780 titration with five 2‐fold dilutions. The titer is shown on the x‐axis as dilution factor of the reagent, and the fluorescence intensity on the y‐axis, in arbitrary units (corresponding laser is indicated, with bandpass in parentheses). Files were concatenated for analysis using FlowJo version 10.7.1 (BD software). (D) eF780 Stain Index (SI) and percentage of positive cells. The eF780 titer is shown on the x‐axis as dilution factor, and SI and percentage of positive cells are represented on the left and right y‐axis, respectively. The titer with maximal or near maximal percentage of positive cells and high SI was chosen. The chosen titer (1:500) is highlighted with a red frame (C) and a red font (D).
Figure S3 Antibody titration on spleen cells stained with eF780 and Hoechst. Eight 2‐fold dilutions were tested for each antibody. Titration of CD4 PE‐CF594, CD8 BV785, CD62L PE‐Cy7, and Ki‐67 AF700 was performed on CD3 FITC co‐stained cells. Titration of CD44 APC, and FoxP3 PE was performed on CD3 FITC and CD4 PE‐CF594 co‐stained cells. The concentration is shown on the x‐axis in μg/ml (final concentration, in a total staining volume of 100 μl for FoxP3 and Ki‐67 mAb, and 50 μl for all the other mAbs). For each antibody titration, concatenated files were represented (top), and SI and percentage of positive cells plotted (bottom), as in Online Figure 2C and D. The concentration with maximal or near maximal percentage of positive cells and high SI was chosen. The chosen titer is highlighted with a red frame (top) and a red font (bottom).
Figure S4 Antibody titration on spleen cells stained with eF780. Titration was performed following the same scheme as in Online Figure 3, but without Hoechst.
Figure S5 Fluorescence Minus One (FMOs). FMOs were used as quality controls for assessment of the accuracy of gates used to identify cell populations in Figure 1B and C. The following FMO plots are represented: (A) Ki‐67; (B) CD3; (C) CD4; (D) CD8; (E) FoxP3; (F) CD44; (G) CD62L. Please, note that the CD44int/low and CD44high populations were defined based on the level of expression of CD44, rather than on the FMO control.
Figure S6 eF780 staining control. Freshly obtained spleen cells were stained with eF780, then either incubated with surface mAbs as in Figure 1 or not (gray filled circles). In parallel, spleen cells were either incubated with surface mAbs as in Figure 1 or not, then stained with eF780 (black filled triangles). All samples were then fixed and permeabilized according to our protocol. Surface mAb stained samples were stained with intracellular mAbs as in Figure 1 (Full stained), while the remaining samples were not (no mAbs). Finally, all samples were incubated with Hoechst. The percentage of viable cells was determined using the gating strategy shown in Figure 1. The panel summarizes the results of 4 independent experiments. Mean values and Standard Deviations are represented. Statistical analysis was performed using Friedman test with Dunn's multiple comparison (*P < 0.05).
Figure S7 Antibody titration on spleen cells stained with eF780 and Hoechst. Six or eight 2‐fold dilutions were tested for each antibody. Titration of CD4 PerCp‐Cy5.5, CD4 BUV737, CD8 BUV805, CD8 PE‐Cy5 and CD8 PE‐Cy7 was performed on CD3 FITC co‐stained cells. The concentration is shown on the x‐axis in μg/ml (final concentration, in a total staining volume of 50 μl). For each antibody titration, concatenated files were represented (top), and SI and percentage of positive cells plotted (bottom), as in Online Figure 3. The concentration with maximal or near maximal percentage of positive cells and high SI was chosen. The chosen titer is highlighted with a red frame (top) and a red font (bottom). The mAbs in this figure were not included in the final panel (reason for exclusion in Online Table 3).
Figure S8 Alternative options for Ki67 and FoxP3 mAbs. Freshly obtained spleen cells were stained using either mAbs and reagents of this OMIP panel (A) or a modified panel to test the following mAbs (indicated in red): (B) Ki‐67 PE and FoxP3 AF700; (C) Ki‐67 APC, FoxP3 AF700 and CD44 PE; (D) Ki‐67 PE‐Cy7, FoxP3 AF700, CD44 PE and CD62L APC. Each of these mAbs replaced the corresponding marker‐specific mAb in this OMIP panel. Data were analyzed using the gating strategy shown in Figure 1, and representative plots are shown, as indicated. The mAbs in this figure were not included in the final panel (reasons for exclusion in Online Table 3).
Figure S9 Alternative options for CD44 and CD62L mAbs. Freshly obtained spleen cells were stained using either mAbs and reagents of this OMIP panel (A‐C), or CD44 PE‐Cy7 and CD62L APC (in red) replacing the corresponding marker‐specific mAbs in this OMIP panel (D‐F). Data were analyzed using the gating strategy shown in Figure 1, and representative plots are shown, as indicated. Fully stained samples are shown in A and D; CD44 FMO controls in B and E; CD62L FMO controls in C and F. Please, note that the CD44int/low and CD44high populations were defined based on the level of expression of CD44, rather than on the FMO control. CD44 PE‐Cy7 and CD62L APC mAbs were not included in the final panel (reasons for exclusion in Online Table 3).
Figure S10 Scheme of gating strategy.
Figure S11 Cell cycle analysis of spleen T cell subsets from a 6‐months old C57BL/6J mouse. Example of staining and gating of spleen cells from a 6‐months old C57BL/6J mouse, using the same gating strategy as in Figure 1.
Table S1 Instrument configuration of the cytometer BD LSR Fortessa.
Table S2 Commercially available reagents used in OMIP‐079.
Table S3 Antibodies tested but not used for the final panel (all tested with Hoechst).
ACKNOWLEDGMENTS
The authors wish to thank Hefin Rhys (Flow Cytometry Science Technology Platform, The Francis Crick Institute, London UK) for discussion, and Antonio Di Virgilio (Istituto Superiore di Sanità, Rome, Italy) for technical help in the mouse facility. Open Access Funding provided by Consiglio Nazionale delle Ricerche within the CRUI‐CARE Agreement.
Natalini A, Simonetti S, Favaretto G, Peruzzi G, Antonangeli F, Santoni A, et al. OMIP‐079: Cell cycle of CD4 + and CD8 + naïve/memory T cell subsets, and of Treg cells from mouse spleen. Cytometry. 2021;99:1171–1175. 10.1002/cyto.a.24509
[Correction added on 22 October 2021, after first online publication: The last name of the author has been updated from “Munoz‐Ruiz” to “Muñoz‐Ruiz”.]
Funding information Consiglio Nazionale delle Ricerche, Grant/Award Number: STM‐2019; Italian Minister of Research and University (MIUR), Grant/Award Number: 2017K55HLC
Contributor Information
Ambra Natalini, Email: ambra.natalini@ibpm.cnr.it.
Francesca Di Rosa, Email: francesca.dirosa@cnr.it.
REFERENCES
- 1. Farber DL, Netea MG, Radbruch A, Rajewsky K, Zinkernagel RM. Immunological memory: lessons from the past and a look to the future. Nat Rev Immunol. 2016;16:124–8. [DOI] [PubMed] [Google Scholar]
- 2. Di Rosa F. Two niches in the bone marrow: a hypothesis on life‐long T cell memory. Trends Immunol. 2016;37:503–12. [DOI] [PubMed] [Google Scholar]
- 3. Tough DF, Sprent J. Turnover of naive‐ and memory‐phenotype T cells. J Exp Med. 1994;179:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy‐like disease. J Exp Med. 2007;204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory‐phenotype cells. Nat Immunol. 2011;131:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White JT, Cross EW, Kedl RM. Antigen‐inexperienced memory CD8+ T cells: where they come from and why we need them. Nat Rev Immunol. 2017;17:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beverley PC. Functional analysis of human T cell subsets defined by CD45 isoform expression. Semin Immunol. 1992;4:35–41. [PubMed] [Google Scholar]
- 9. Ivetic A, Hoskins Green HL, Hart SJ. L‐selectin: a major regulator of leukocyte adhesion, migration and signaling. Front Immunol. 2019;10:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Del Zotto G, Principi E, Antonini F, Baratto S, Panicucci C, Bruno C, et al. Comprehensive phenotyping of peripheral blood T lymphocytes in healthy mice. Cytometry A. 2020;99(3):243–50. [DOI] [PubMed] [Google Scholar]
- 11. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. [DOI] [PubMed] [Google Scholar]
- 12. Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. [DOI] [PubMed] [Google Scholar]
- 14. Unsworth A, Anderson R, Haynes N, Britt K. OMIP‐032: two multi‐color immunophenotyping panels for assessing the innate and adaptive immune cells in the mouse mammary gland. Cytometry A. 2016;89:527–30. [DOI] [PubMed] [Google Scholar]
- 15. Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med. 2002;195:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borghans JA, de Boer RJ. Quantification of T‐cell dynamics: from telomeres to DNA labeling. Immunol Rev. 2007;216:35–47. [DOI] [PubMed] [Google Scholar]
- 17. Takagi M, Natsume T, Kanemaki MT, Imamoto N. Perichromosomal protein Ki67 supports mitotic chromosome architecture. Genes Cells. 2016;21:1113–24. [DOI] [PubMed] [Google Scholar]
- 18. Cuylen‐Haering S, Petrovic M, Hernandez‐Armendariz A, Schneider MWG, Samwer M, Blaukopf C, et al. Chromosome clustering by Ki‐67 excludes cytoplasm during nuclear assembly. Nature. 2020;587:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonetti S, Natalini A, Folgori A, Capone S, Nicosia A, Santoni A, et al. Antigen‐specific CD8 T cells in cell cycle circulate in the blood after vaccination. Scand J Immunol. 2019;89:e12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muñoz‐Ruiz M, Pujol‐Autonell I, Rhys H, Long HM, Greco M, Peakman M, et al. Tracking immunodynamics by identification of S‐G2/M‐phase T cells in human peripheral blood. J Autoimmun. 2020;112:102466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, et al. Differences in the expression profiles of CD45RB, Pgp‐1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–302. [PubMed] [Google Scholar]
- 22. Pinchuk LM, Filipov NM. Differential effects of age on circulating and splenic leukocyte populations in C57BL/6 and BALB/c male mice. Immun Ageing. 2008;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age‐associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. [DOI] [PubMed] [Google Scholar]
- 25. Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. MIFlowCyt MIFlowCyt Item Checklist: MIFlowCyt‐Compliant Items.
Appendix S2. Supporting Information.
Figure S1 Spillover spreading error (SSE) matrix (SSM). SSM was obtained with compensation beads, except for eF780 viability dye and Hoechst‐33342, which were compensated using live and dead splenocytes (1:1 mix) and bone marrow cells, respectively. SSE values >10 are highlighted in orange.
Figure S2 Titration of the DNA dye Hoechst‐33342 (Hoechst) and of the fixable viability dye eFluor 780 (eF780). (A) Hoechst titration with four 2‐fold dilutions. Titration was performed in parallel using either spleen (top) or bone marrow (BM) cells (bottom). Histogram plots show Hoechst fluorescence intensity on the x‐axis, using a linear scale in arbitrary units. The left and right gates correspond to the G0/G1 and the G2/M DNA peak, respectively, and the numbers represent the coefficients of variation (CV) of each peak. (B) Spreading of signal of Hoechst at 2 and 4 μg/ml in the indicated channels. All the channels were set identically at the two concentrations, except for UV laser (bandpass 530/30), i.e., the Hoechst channel, that was adapted for visualization of the DNA peaks by changing PMT voltage (decreasing PMT voltage for increasing Hoechst concentration). Histograms show fluorescence intensity in arbitrary units of unstained spleen cells (dotted empty line) and spleen cells stained with Hoechst (gray filled line) at either 2 or 4 μg/ml, as indicated. Laser (color code) and bandpass are indicated on top of each channel. Laser color code as follows: blue for 488 nm (blue) laser; red for 639 nm (red) laser; green for 561 nm (yellow/green) laser; black for 355 nm (UV) laser; violet for 405 nm (violet) laser. Where appropriate, fluorochromes used in this OMIP are indicated, although only Hoechst was used here. Hoechst concentration at 2 μg/ml (in red) was chosen as it resulted in DNA peaks having CV ≤ 5 (A), and lower signal spreading than Hoechst at 4 μg/ml (B). (C) Viability dye eF780 titration with five 2‐fold dilutions. The titer is shown on the x‐axis as dilution factor of the reagent, and the fluorescence intensity on the y‐axis, in arbitrary units (corresponding laser is indicated, with bandpass in parentheses). Files were concatenated for analysis using FlowJo version 10.7.1 (BD software). (D) eF780 Stain Index (SI) and percentage of positive cells. The eF780 titer is shown on the x‐axis as dilution factor, and SI and percentage of positive cells are represented on the left and right y‐axis, respectively. The titer with maximal or near maximal percentage of positive cells and high SI was chosen. The chosen titer (1:500) is highlighted with a red frame (C) and a red font (D).
Figure S3 Antibody titration on spleen cells stained with eF780 and Hoechst. Eight 2‐fold dilutions were tested for each antibody. Titration of CD4 PE‐CF594, CD8 BV785, CD62L PE‐Cy7, and Ki‐67 AF700 was performed on CD3 FITC co‐stained cells. Titration of CD44 APC, and FoxP3 PE was performed on CD3 FITC and CD4 PE‐CF594 co‐stained cells. The concentration is shown on the x‐axis in μg/ml (final concentration, in a total staining volume of 100 μl for FoxP3 and Ki‐67 mAb, and 50 μl for all the other mAbs). For each antibody titration, concatenated files were represented (top), and SI and percentage of positive cells plotted (bottom), as in Online Figure 2C and D. The concentration with maximal or near maximal percentage of positive cells and high SI was chosen. The chosen titer is highlighted with a red frame (top) and a red font (bottom).
Figure S4 Antibody titration on spleen cells stained with eF780. Titration was performed following the same scheme as in Online Figure 3, but without Hoechst.
Figure S5 Fluorescence Minus One (FMOs). FMOs were used as quality controls for assessment of the accuracy of gates used to identify cell populations in Figure 1B and C. The following FMO plots are represented: (A) Ki‐67; (B) CD3; (C) CD4; (D) CD8; (E) FoxP3; (F) CD44; (G) CD62L. Please, note that the CD44int/low and CD44high populations were defined based on the level of expression of CD44, rather than on the FMO control.
Figure S6 eF780 staining control. Freshly obtained spleen cells were stained with eF780, then either incubated with surface mAbs as in Figure 1 or not (gray filled circles). In parallel, spleen cells were either incubated with surface mAbs as in Figure 1 or not, then stained with eF780 (black filled triangles). All samples were then fixed and permeabilized according to our protocol. Surface mAb stained samples were stained with intracellular mAbs as in Figure 1 (Full stained), while the remaining samples were not (no mAbs). Finally, all samples were incubated with Hoechst. The percentage of viable cells was determined using the gating strategy shown in Figure 1. The panel summarizes the results of 4 independent experiments. Mean values and Standard Deviations are represented. Statistical analysis was performed using Friedman test with Dunn's multiple comparison (*P < 0.05).
Figure S7 Antibody titration on spleen cells stained with eF780 and Hoechst. Six or eight 2‐fold dilutions were tested for each antibody. Titration of CD4 PerCp‐Cy5.5, CD4 BUV737, CD8 BUV805, CD8 PE‐Cy5 and CD8 PE‐Cy7 was performed on CD3 FITC co‐stained cells. The concentration is shown on the x‐axis in μg/ml (final concentration, in a total staining volume of 50 μl). For each antibody titration, concatenated files were represented (top), and SI and percentage of positive cells plotted (bottom), as in Online Figure 3. The concentration with maximal or near maximal percentage of positive cells and high SI was chosen. The chosen titer is highlighted with a red frame (top) and a red font (bottom). The mAbs in this figure were not included in the final panel (reason for exclusion in Online Table 3).
Figure S8 Alternative options for Ki67 and FoxP3 mAbs. Freshly obtained spleen cells were stained using either mAbs and reagents of this OMIP panel (A) or a modified panel to test the following mAbs (indicated in red): (B) Ki‐67 PE and FoxP3 AF700; (C) Ki‐67 APC, FoxP3 AF700 and CD44 PE; (D) Ki‐67 PE‐Cy7, FoxP3 AF700, CD44 PE and CD62L APC. Each of these mAbs replaced the corresponding marker‐specific mAb in this OMIP panel. Data were analyzed using the gating strategy shown in Figure 1, and representative plots are shown, as indicated. The mAbs in this figure were not included in the final panel (reasons for exclusion in Online Table 3).
Figure S9 Alternative options for CD44 and CD62L mAbs. Freshly obtained spleen cells were stained using either mAbs and reagents of this OMIP panel (A‐C), or CD44 PE‐Cy7 and CD62L APC (in red) replacing the corresponding marker‐specific mAbs in this OMIP panel (D‐F). Data were analyzed using the gating strategy shown in Figure 1, and representative plots are shown, as indicated. Fully stained samples are shown in A and D; CD44 FMO controls in B and E; CD62L FMO controls in C and F. Please, note that the CD44int/low and CD44high populations were defined based on the level of expression of CD44, rather than on the FMO control. CD44 PE‐Cy7 and CD62L APC mAbs were not included in the final panel (reasons for exclusion in Online Table 3).
Figure S10 Scheme of gating strategy.
Figure S11 Cell cycle analysis of spleen T cell subsets from a 6‐months old C57BL/6J mouse. Example of staining and gating of spleen cells from a 6‐months old C57BL/6J mouse, using the same gating strategy as in Figure 1.
Table S1 Instrument configuration of the cytometer BD LSR Fortessa.
Table S2 Commercially available reagents used in OMIP‐079.
Table S3 Antibodies tested but not used for the final panel (all tested with Hoechst).


