Abstract
Aims
Cardiac tamponade is a high morbidity complication of transseptal puncture (TSP). We examined the associations of TSP‐related cardiac tamponade (TRCT) for all patients undergoing left atrial ablation at our center from 2016 to 2020.
Methods and Results
Patient and procedural variables were extracted retrospectively. Cases of cardiac tamponade were scrutinized to adjudicate TSP culpability. Adjusted multivariate analysis examined predictors of TRCT. A total of 3239 consecutive TSPs were performed; cardiac tamponade occurred in 51 patients (incidence: 1.6%) and was adjudicated as TSP‐related in 35 (incidence: 1.1%; 68.6% of all tamponades). Patients of above‐median age [odds ratio (OR): 2.4 (1.19–4.2), p = .006] and those undergoing re‐do procedures [OR: 1.95 (1.29–3.43, p = .042] were at higher risk of TRCT. Of the operator‐dependent variables, choice of transseptal needle (Endrys vs. Brockenbrough, p > .1) or puncture sheath (Swartz vs. Mullins vs. Agilis vs. Vizigo vs. Cryosheath, all p > .1) did not predict TRCT. Adjusting for operator, equipment and demographics, failure to cross the septum first pass increased TRCT risk [OR: 4.42 (2.45–8.2), p = .001], whilst top quartile operator experience [OR: 0.4 (0.17–0.85), p = .002], transoesophageal echocardiogram [TOE prevalence: 26%, OR: 0.51 (0.11–0.94), p = .023], and use of the SafeSept transseptal guidewire [OR: 0.22 (0.08–0.62), p = .001] reduced TRCT risk. An increase in transseptal guidewire use over time (2016: 15.6%, 2020: 60.2%) correlated with an annual reduction in TRCT (R 2 = 0.72, p < .001) and was associated with a relative risk reduction of 70%.
Conclusions
During left atrial ablation, the risk of TRCT was reduced by operator experience, TOE‐guidance, and use of a transseptal guidewire, and was increased by patient age, re‐do procedures, and failure to cross the septum first pass.
Keywords: cardiac tamponade, SafeSept guidewire, transoesophageal echocardiogram, transseptal puncture
In this retrospective analysis of 3239 consecutive transseptal punctures performed during left atrial ablation, we present the independent risk factors for the occurrence of cardiac tamponade.
We also propose a novel classification system for the causes of cardiac tamponade during left atrial ablation.

1. INTRODUCTION
Transseptal puncture (TSP) is the preferred route of access to the left atrium for catheter ablation and structural intervention. 1 Recent therapeutic developments, including the advent of cryoballoon pulmonary vein isolation, percutaneous left atrial appendage occlusion, and transcatheter mitral valve intervention, necessitate the delivery of wide‐bore sheaths and devices across the interatrial septum. 2 , 3 , 4 Whilst innovations in intracardiac imaging, needle technology, guidewire assistance, and 3D electro‐anatomical mapping have reduced procedural morbidity, TSP remains a high risk step during left‐sided catheter‐based interventions. 5 , 6 , 7
Cardiac tamponade remains the most frequent, potentially fatal complication of left atrial ablation; large, contemporary studies report an incidence of 0.45–1.3%, however, this attenuates with operator experience. 8 , 9 , 10 As mis‐directed TSP is the most common cause of cardiac tamponade, measures to improve TSP safety can ameliorate patient outcomes; for example, Žižek at al. 11 performed 524 consecutive TSPs guided by intracardiac echocardiography (ICE), and reported an overall cardiac tamponade rate of 0.2%.
As part of our quality assurance process, we sought to identify patient, procedural and operator‐dependent factors associated with cardiac tamponade during TSP for left atrial ablation, with a view to establishing best practice at our UK tertiary center.
2. METHODS
Data were extracted retrospectively from a secure internal registry comprising all patients undergoing catheter ablation at our institution from January 1st 2016 to December 31st 2020. “Left atrial ablation” included de novo and re‐do ablations for atrial fibrillation (AF) or atrial tachycardia requiring transseptal access to the left atrium. To reduce heterogeneity, left sided accessory pathway and ventricular tachycardia ablations were excluded. Procedures in which left atrial access was obtained via a patent foramen ovale were also excluded. Cardiac tamponade was defined as an accumulation of pericardial blood leading to sufficient clinical or haemodynamic deterioration as to warrant an attempt at percutaneous or surgical drainage. All cases of cardiac tamponade in this study were identified within 12 h of the ablation procedure. The decision as to whether the TSP was the most likely cause of the tamponade was made following examination of procedural reports and imaging and, in cases of discrepancy, discussion with the operators. Final adjudication was made according to consensus between study authors, with reference to a novel classification system (Table 2). Operator experience was quantified according to the number of TSPs for left atrial ablation performed per year.
Table 2.
Proposed classification system for the causes of cardiac tamponade during left atrial ablation, stratified by TSP culpability
| Classification of cardiac tamponade | Cause of tamponade | Supporting evidence | Incidence (n = 51) |
|---|---|---|---|
| TSP‐related (n = 35) | |||
| Type A | Tamponade diagnosed following an attempted TSP with the needle or guidewire; no sheaths passed beyond the interatrial septum | Repeated or challenging attempts at TSP; abnormal pressure trace obtained from TSP needle (e.g. suggestive of aortic or pericardial puncture) | 10 (19.6%) |
| Type B | Tamponade diagnosed following passage of the sheath(s) beyond the interatrial septum; no ablation performed | Repeated or challenging attempts at TSP; difficult manipulation of the sheaths or mapping catheter; sheath or mapping catheter seen to pass outside the cardiac silhouette immediately following TSP; abnormal pressure trace obtained from sheath | 19 (37.3%) |
| Type C | Tamponade diagnosed immediately following withdrawal of sheaths on conclusion of the procedure | Repeated or challenging attempts at TSP; haemodynamically stable on conclusion of ablation treatment, however sudden haemodynamic deterioration documented following sheath withdrawal | 6 (11.8%) |
| Non‐TSP related (n = 16) | |||
| Type D | Tamponade diagnosed during ablation treatment or during manipulation of the ablation catheter | High force noted on ablation catheter; clear temporal association between the onset of ablation treatment and haemodynamic deterioration; haemodynamic deterioration whilst ablation catheter within the left atrium; visualization of ablation catheter outside of the cardiac silhouette or left atrial geometry during treatment phase | 13 (25.5%) |
| Type E | Tamponade diagnosed during ablation in association with a steam pop | Impedance spike noted during ablation; audible ‘pop’ noted by operator | 2 (3.9%) |
| Type F | Tamponade diagnosed during recovery from procedure | Haemodynamically stable following withdrawal of sheaths, but subsequent subacute deterioration documented during recovery | 1 (1.9%) |
Abbreviation: TSP, transseptal puncture.
2.1. Procedures
Patients attended for left atrial ablation without interruption of their oral anticoagulation or antiarrhythmic drugs, and procedures took place under either local anaesthetic and conscious sedation, or general anaesthetic. In all cases, following a chlorhexidine scrub and subcutaneous injection of 1% lidocaine, the right femoral vein was accessed under ultrasound guidance. The equipment and technique used for TSP was at the operators' discretion: this included the decision to puncture through a dedicated transseptal sheath (either the Swartz™ SL0, SL1 or SR0 guiding introducers, or Cook Medical's Mullins guiding sheath) or through a long steerable sheath (Abbott Agilis™ steerable introducer, Biosense Webster Vizigo™ guiding sheath, Medtronic FlexCath Advance™, or Boston Scientific steerable POLARSHEATH™). Punctures were made using a matched‐length Medtronic Brockenbrough (BRK‐0 or BRK‐1 with or without the extra sharp modification) or a Cook Medical Endrys needle. The Pressure Products SafeSept® 150 cm, 0.0315″ nitinol guidewire was available for all procedures according to operator preference, and transoesophageal echo (TOE) imaging was performed in all cases performed under general anaesthetic. A pressure line was connected directly to the TSP needle for all procedures in which the transseptal guidewire was not used. For procedures using a transseptal guidewire, a pressure line was either connected to the TSP needle via an O‐ring, or no pressure line was used.
In all cases, TSP was performed under fluoroscopic guidance. In the left anterior oblique (LAO) projection, the selected sheath and needle were drawn down from the superior vena cava into the right atrium and subsequently the fossa ovalis. A damped pressure trace was considered a sign of opposition of the needle against the interatrial septum. The antero‐posterior orientation of the needle was assessed in the right anterior oblique projection, with manual readjustment performed using the anatomical landmarks of the spine and a diagnostic catheter placed within the coronary sinus as the posterior and anterior borders of the left atrium, respectively. If adequately positioned, operators returned to the LAO projection and advanced either the needle or, if selected, the transseptal guidewire. In the case of the former, the sheath and dilator were advanced only if a left atrial pressure trace was observed. In cases using a transseptal guidewire, the wire was ideally advanced beyond the border of the cardiac silhouette to the left superior or inferior pulmonary vein, and the needle and sheath advanced subsequently. In some cases, the guidewire would coil within the left atrium rather than enter a vein, and so observation of it moving freely within this chamber was also used to confirm its position. For procedures in which two sheaths were required within the left atrium simultaneously, operators either performed a second TSP (“double puncture” technique), or a second sheath was advanced across the same puncture site over an ablation catheter under fluoroscopic guidance. At the operators' discretion, intravenous contrast was administered via an injection manifold to confirm the position of the needle tip either against the fossa ovalis (the “septal staining” technique) or within the left atrium.
Following TSP, intravenous heparin was administered repeatedly to maintain an activated clotting time (ACT) of 300–400 s during left atrial dwell time. On conclusion of the ablation, sheaths were withdrawn from the left atrium and intravenous protamine (dosed between 50 and 100 mg according to the patients' bodyweight and final ACT) administered before femoral hemostasis. Transthoracic echocardiography was performed to exclude pericardial effusion before transfer to the recovery unit; this scan was not repeated before discharge unless clinically indicated.
In the event of intra‐ or postprocedural cardiac tamponade being identified, percutaneous drainage was attempted and, in accordance with our institution's major hemorrhage protocol, a stepwise combination of protamine, concentrated clotting factors, Vitamin K, fresh frozen plasma or cryoprecipitate was administered to reverse anticoagulation or acquired coagulopathy. If blood transfusion was required, either cross‐matched or type O rhesus negative units were administered, or auto‐transfusion performed via an existing femoral sheath. If percutaneous drainage was unsuccessful or did not improve the patient's haemodynamic status, surgical drainage was initiated either following transfer to an operating theater or emergently in the catheter laboratory.
2.2. Ethics
The project was registered with our Clinical Effectiveness Unit. This was a retrospective service evaluation performed as part of our quality assurance framework; as such, the need for formal ethical approval was waived by our institution.
2.3. Statistics
Statistical analysis was performed using R. The Shapiro–Wilk test discerned whether data were normally distributed. Categorical group variables were compared using a Z‐test for differences of proportion. Continuous variables were analysed using two‐tailed unpaired t tests for normally distributed data or the Mann–Whitney U test for non‐normally distributed data. Group outcomes were compared using Fisher's exact test. Univariate logistic regression analysis for the prediction of TSP‐related cardiac tamponade was performed for patients' baseline characteristics, procedural, and operator‐dependent variables. Stepdown multivariate analysis was performed subsequently for all univariate factors in which p < .25; a variance inflating factor (VIF) was calculated to assess for multicollinearity with a cut‐off of 2.5 set for categorical variables and 10 for continuous variables. Normally distributed data are presented as mean ± standard deviation and non‐normally distributed data as median (interquartile range). Odds ratios are provided with 95% confidence intervals; the level of significance for all tests was set at α < .05.
3. RESULTS
A total of 2696 patients underwent 3239 left atrial ablations. Cardiac tamponade occurred in 51 procedures (1.6%). A total of six patients required surgical drainage via median sternotomy; two of these tamponades were TSP‐related. The mean blood volume drained by pericardiocentesis was 780 (±538) mls. There were no peri‐procedural deaths. Following the diagnosis and management of cardiac tamponade, further ablation was abandoned in all but two cases. Patients' baseline characteristics, procedural, and operator‐dependent variables are shown in Table 1, stratified according to the incidence of TSP‐related cardiac tamponade. For the purposes of this analysis, a “high‐volume operator” was defined as any electrophysiologist performing a top quartile number of TSPs (>45) per year in our institution. During each procedure, if the operator was unable—or elected not—to advance the sheath into the left atrium following the first attempted septal puncture with the needle, this was considered an “unsuccessful first pass.”
Table 1.
Patient baseline characteristics, procedural, and operator‐dependent variables stratified according to the incidence of TSP‐related cardiac tamponade
| All procedures (n = 3239) | |||
|---|---|---|---|
| Parameter | No TSP‐related cardiac tamponade (n = 3204) | TSP‐related cardiac tamponade (n = 35) | p value |
| Male | 63.6% | 60% | .67 |
| Age (years) | 64 (16) | 67 (10) | .08 |
| EHRA class | 2 (1) | 2 (1) | .39 |
| Ischemic heart disease | 8.1% | 14.3% | .19 |
| Dilated cardiomyopathy | 3.8% | 2.8% | .87 |
| Hypertrophic cardiomyopathy | 3.6% | 2.8% | .92 |
| Congenital heart disease | 3.1% | 2.8% | .94 |
| Previous sternotomy | 8% | 2.8% | .25 |
| Left atrial dilatation (>40 mm) | 30.7% | 25.7% | .52 |
| Anticoagulated with warfarin (remainder DOAC) | Warfarin: 11.6% | Warfarin: 2.8% | .09 |
| Re‐do procedure | 29.4% | 40% | .15 |
| Cryoablation | 46.8% | 51.4% | .11 |
| Transoesophageal echo | 26.3% | 17.1% | .22 |
| High‐volume operator | 57.8% | 42.9% | .07 |
| TSP puncture sheath | Cryosheath: 5.5% | Cryosheath: 5.7% | .96 |
| Agilis: 0.9% | Agilis: 0% | N/A | |
| Vizigo: 0.1% | Vizigo: 0% | N/A | |
| Swartz: 72% | Swartz: 74.3% | .69 | |
| Mullins: 21.5% | Mullins: 20% | .88 | |
| TSP puncture needle | Brockenbrough: 50.8% | Brockenbrough: 57.1% | .48 |
| Endrys: 49.2% | Endrys: 42.9% | .4 | |
| Transseptal guidewire | 37% | 14.2% | .004 |
| First pass unsuccessful | 2.7% | 11.4% | .002 |
| Procedures using 3D electro‐anatomical mapping (n = 1716) | |||
|---|---|---|---|
| Parameter | No TSP‐related cardiac tamponade (n = 1693) | TSP‐related cardiac tamponade (n = 17) | p value |
| Double TSP technique | 14.7% | 11.8% | .71 |
Note: Significant p values in bold.
Abbreviation: TSP, transseptal puncture.
3.1. Adjudication of the cause of cardiac tamponade
As part of this analysis, we propose a novel classification system for the adjudication of the cause of cardiac tamponade during left atrial ablation procedures, and this is shown in Table 2. These events can be divided broadly into six categories, three of which relate to the TSP, and three which are not related. The adjudication process was based on retrospective analysis of procedure documentation and when necessary, scrutiny of fluoroscopic imaging and/or 3D electro‐anatomical mapping system data. Based on this novel classification system, 35 of the 51 procedures in which cardiac tamponade occurred were adjudicated to be TSP‐related (incidence: 1.1%; 68.6% of all tamponades).
3.2. Risk factors for cardiac tamponade
Univariate and subsequently stepdown multivariate analyses were performed and are shown in Table 3, with Figure 1 demonstrating the independent predictors of transseptal‐related cardiac tamponade (final model concordance: 0.79; VIF < 1.5 for all variables). As multivariate analysis suggested the transseptal guidewire was a modifiable predictor of TSP‐related cardiac tamponade, further analysis was performed to investigate this relationship. The prevalence of transseptal guidewire use at our institution increased annually (2016: 15.6%, 2020: 60.2%) and inversely correlated with TSP‐related cardiac tamponade (Figure 2: R 2 = 0.72, p < .001). A total of 988 procedures made use of the transseptal guidewire; four TSP‐related tamponades occurred in this subgroup versus 31 in the remaining patients (incidence: 0.4% vs. 1.3%, p = .015; relative risk reduction: 70%). Notably, of the 849 procedures (26%) under TOE‐guidance, 190 also made use of the transseptal guidewire: no TSP‐related tamponades occurred in this subgroup versus six (0.88%) in the remaining TOE‐guided TSPs performed without the transseptal guidewire.
Table 3.
Univariate and stepdown multivariate analyses for predicting TSP‐related cardiac tamponade during left atrial ablation
| Covariate | Odds ratio (CI): Univariate analysis | p value | Adjusted odds ratio (CI): Final step of multivariate analysis | p value |
|---|---|---|---|---|
| Male | 1.29 (0.65–2.55) | .47 | ||
| Age >65 years | 1.84 (1.05–3.56) | .029 | 2.4 (1.19–4.2) | .006 |
| EHRA class | 1.16 (0.24–2.74) | .74 | ||
| Ischemic heart disease | 1.66 (0.89–4.83) | .11 | 1.63 (0.67–4.05) | .54 |
| Dilated cardiomyopathy | 0.91 (0.22–3.79) | .89 | ||
| Hypertrophic cardiomyopathy | 1.9 (0.87–3.55) | .09 | 1.44 (0.77–3.1) | .35 |
| Congenital heart disease | 1.28 (0.81–2.4) | .32 | ||
| Previous sternotomy | 0.77 (0.3–1.16) | .15 | 0.64 (0.18–2.3) | .47 |
| Left atrial dilatation | 0.87 (0.48–1.29) | .13 | 1.12 (0.77–1.88) | .33 |
| Anticoagulation | Warfarin: 0.62 (0.23–1.67) | .31 | ||
| Direct oral anticoagulant: 1.38 (0.73–7.4) | .22 | |||
| Re‐do procedure | 1.97 (1.19–2.43) | .035 | 1.95 (1.29–3.43) | .042 |
| Cryoablation | 1.53 (0.86–3.1) | .26 | ||
| Transoesophageal echo | 0.51 (0.27–0.89) | .021 | 0.51 (0.11–0.94) | .023 |
| High‐volume operator | 0.5 (0.31–0.77) | .008 | 0.4 (0.17–0.85) | .002 |
| TSP puncture sheath | Cryosheath: 0.93 (0.22–3.88) | .92 | ||
| Agilis: 0.77 (0.41–3.1) | .39 | |||
| Vizigo: 0.67 (0.31–4.2) | .76 | |||
| Mullins: 1.09 (0.52–2.31) | .82 | |||
| Swartz: 1.42 (0.72–2.81) | .31 | |||
| TSP puncture needle | Brockenbrough: 1.25 (0.66–2.36) | .48 | ||
| Endrys: 0.88 (0.45–1.72) | .71 | |||
| Transseptal guidewire | 0.38 (0.15–0.81) | .002 | 0.22 (0.08–0.62) | .001 |
| First pass unsuccessful | 3.93 (2.13–6.85) | .019 | 4.42 (2.45–8.2) | .001 |
Note: Significant p values in bold.
Abbreviations: CI, confidence interval; TSP, transseptal puncture.
Figure 1.
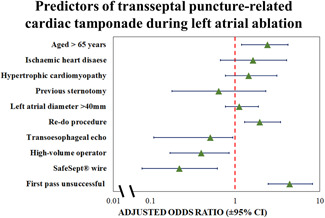
Forest plot of multivariate analysis for predicting TSP‐related cardiac tamponade; adjusted odds ratios are provided with 95% confidence intervals. TSP, transseptal puncture
Figure 2.
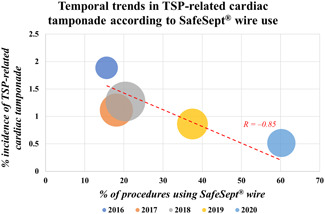
Bubble plot demonstrating the prevalence of transseptal guidewire use and its association with the incidence of TSP‐related cardiac tamponade for 5 consecutive years. Procedure numbers—2016: 371, 2017: 807, 2018: 786, 2019: 696, 2020: 579. TSP, transseptal puncture
4. DISCUSSION
This analysis sought to identify the key predictors of cardiac tamponade as a result of TSP during left atrial ablation. In addition to patient demographics and procedural characteristics, we examined operator‐dependent factors—in particular, choice of equipment—with a view to establishing best practice and improving patient safety. Even allowing for the heterogeneity in TSP technique across 23 Consultant operators, the present study identified consistent predictors of cardiac tamponade that have also been demonstrated in other analyses, including patient age [adjusted odds ratio (OR) if >65 years of age: 2.4 (1.19–4.2), p = .006] and re‐do procedures [adjusted OR: 1.95 (1.29–3.43), p = .042]. 12 , 13 Whilst these two factors are not modifiable, an appreciation of their impact on the risk of complications is nevertheless important for patient selection, particularly during the consent process.
We also propose a novel classification system for the causes of cardiac tamponade during left atrial ablation (Table 2); this may provide a useful reference for clinical trialists examining the safety profile of new catheter ablation technology, as well as for physicians auditing ablation data within their department.
4.1. Operator experience
Another finding from the present study, having adjusted for patient parameters and equipment, is that procedures performed by high‐volume operators (>45 TSPs per year) were inversely associated with TSP‐related cardiac tamponade [adjusted OR: 0.5 (0.31–0.77)]; this is a recurrent finding which institutions should consider when selecting treatment strategies for patients at increased risk of complications. 14 As seen in Figure 2, the incidence of cardiac tamponade attenuated over time, and it is possible that this reflects increasing operator experience. However, as we were unable to quantify lifetime operating experience for each physician, we elected to use “TSPs for left atrial ablation per year” as a surrogate. Using this metric, those operators with top quartile experience remained constant throughout each year of our analysis. Furthermore, during the study period, three established operators left the center and four were newly appointed; it is plausible that these changes in the experience of faculty members may have influenced patient outcomes, however, the number of tamponade events per physician was insufficient to warrant meaningful comparisons.
4.2. Use of transoesophageal echocardiography
TOE‐guidance is well‐established as a tool for reducing TSP‐related complications, and conferred an independent safety benefit in the present study, with only six TSP‐related tamponades occurring under TOE‐guidance (incidence: 0.7%). For this reason, many centers around the world use TOE (or ICE) for all TSPs, but this option is not available at our institution or many other electrophysiological centers, both in the United Kingdom and elsewhere. Bayrak et al. 15 found that TOE led to a significant adjustment in catheter position immediately before TSP in 16.5% of cases, most notably in older individuals and those with a history of prior ablation. Our high‐volume center has recently demonstrated the feasibility and safety of same day discharge following AF ablation, particularly for those procedures performed under local anaesthetic and sedation, hence our use of general anaesthetic is attenuating over time. 16 In part, this stems from a desire to maximize procedure volume and efficiency in the EP lab by shortening case duration, but is also due to a lack of availability of anaesthesia for all cases; in the present analysis, only a minority (26%) of procedures were performed under general anaesthesia with associated TOE‐guidance. Alternative intracardiac imaging strategies are available, most significantly ICE, which has a favorable safety profile in large multicenter studies, however, ICE was not used routinely at our center during the study period. 7 Although access to ICE is increasing worldwide, it currently adds considerable financial cost to ablation procedures and, as such, given the relatively low incidence of TSP‐related tamponade, further analysis as to the cost‐utility of routine use may be necessary before more widespread uptake in the United Kingdom. 17
4.3. Use of a transseptal guidewire
Multicenter analysis has previously established the utility of the SafeSept® transseptal guidewire for TSP, and the present study found that the wire mitigated the risk of TSP‐related tamponade, independent of operator expertize [adjusted OR: 0.22 (0.08–0.62)]. 18 In our experience, the guidewire's narrow calibre and its propensity for crossing the thinnest part of the interatrial septum reduces the likelihood of inadvertent, traumatic pericardial or aortic puncture. Furthermore, by positioning the transseptal guidewire within the left upper pulmonary vein before advancing the puncture needle and sheath across the septum, use of the wire also diminishes the risk of left atrial appendage perforation during TSP. The number needed to treat in our analysis was 96 patients. When considering the unit cost of the SafeSept® guidewire (£60) versus the estimated cost of cardiac tamponade (approximately £7000), this implies that use of the wire at our center has been cost neutral. 19 Our retrospective analysis also demonstrated a reduction in the incidence of tamponade as transseptal guidewire use increased over a 5‐year time period (Figure 2). This finding raises the hypothesis that transseptal guidewire utilization is responsible for improved safety, but causality cannot be proven, and this effect would require testing as part of a randomized clinical trial.
4.4. Failure to cross the septum first pass
The finding in the present study that failure to cross the septum following the first pass of the TSP needle is both intuitive and expected, and was strongly associated with adverse outcomes. Whilst a causative relationship cannot be proven in this analysis, it is likely that failure to cross the septum first pass reflects a variation in the patient's anatomy—such as cardiac rotation or increased septal thickness—that may be challenging to appreciate and overcome using fluoroscopy alone. Although this phenomenon is impossible to predict before the procedure unless TOE or other imaging has been performed immediately beforehand, these data suggest that its occurrence constitutes an important safety marker; having failed to advance a sheath into the left atrium at the first attempt, the operator may wish to consider seeking assistance, introducing adjunctive TSP‐related technology into the procedure, or using additional imaging techniques.
4.5. Single versus double TSP technique
In a subset of 1716 ablations using 3D electro‐anatomical mapping, 1460 procedures (85.1%) were performed via a single TSP, with the second sheath (if required) passed through the same puncture site under fluoroscopic guidance. In the remaining procedures, operators performed an additional TSP to deliver the second sheath. Whilst the prevalence of the double puncture technique did not differ between patients with and without TSP‐related tamponade (Table 1), these data are subject to significant selection bias. In particular, operators who would ordinarily use the double puncture technique may instead opt to use a single puncture if the first TSP was difficult, which may skew the incidence of adverse events towards this patient group. Conversely, operators who prefer a single puncture may switch to the double puncture technique if the first sheath proves challenging to manipulate once passed across the interatrial septum. Furthermore, in 10 cases cardiac tamponade was diagnosed before delivery of the first sheath across the septum (Type A tamponade), precluding any attempt at a second TSP.
Similarly, operators also differ in their preference for using one or two long sheaths during radiofrequency ablations. In light of the above biases, we were unable to perform meaningful comparisons regarding the relative safety of these techniques in our cohort.
4.6. Limitations
This study has several limitations in addition to the above. As a single center analysis, the applicability of our findings to other populations is not assured. Unlike many other institutions, we perform a high percentage of cases without TOE‐guidance and so these data are particularly applicable to other institutions who adopt a similar practice. Likewise, routine use of ICE is currently prohibitively expensive in the United Kingdom and was not included in our study; our results may therefore not be generalizable to centers who have access to this technology.
The primary outcome of the study was TSP‐related cardiac tamponade; whilst our examination of the data and adjudication process was robust, in some cases TSP‐culpability cannot be assigned with absolute certainty without surgical or autopsy data, which was rarely available in our population. Similarly, it is feasible that tamponade can sometimes occur with two separate injuries in the same case (particularly Types C, D and F). Failure to cross the septum first pass was an important predictor of adverse outcomes, however it is possible that some operators may have failed to document, or fluoroscopically record this incident for every procedure in which it occurred. In addition to ICE, several other contemporary TSP‐related technologies—such as the radiofrequency needle or laser‐assisted puncture—were not used during the study period and hence are absent from this analysis. 20 Operator experience was calculated based on volumes of cases at our institution, however, many of the operators also perform left atrial ablation at other centers and these data are unavailable. Finally, whilst our multivariate analysis identified powerful independent predictors of adverse outcomes, our retrospective study may have failed to include additional important confounders.
5. CONCLUSIONS
At our high‐volume UK center, the incidence of TSP‐related cardiac tamponade was 1.1% (68.6% of all tamponades), and associated with a combination of fixed parameters and operator‐dependent factors. Whilst the chosen combination of TSP puncture sheath and needle did not appear to affect outcomes, use of a transseptal guidewire and, where available, TOE‐guidance may confer significant safety benefits. Use of the SafeSept® transseptal guidewire is cost neutral. A transseptal guidewire will be used routinely during left atrial ablation in an effort to further improve patient safety.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Maclean E, Mahtani K, Roelas M, et al. Transseptal puncture for left atrial ablation: risk factors for cardiac tamponade and a proposed causative classification system. J Cardiovasc Electrophysiol. 2022;33:1747‐1755. 10.1111/jce.15590
REFERENCES
- 1. Earley MJ. How to perform a transseptal puncture. Heart. 2009;95:85‐92. [DOI] [PubMed] [Google Scholar]
- 2. Ströker E, De Greef Y, Schwagten B, et al. Over‐the‐needle trans‐septal access using the cryoballoon delivery sheath and dilator in atrial fibrillation ablation. PACE—Pacing Clin Electrophysiol. 2019;42:868‐873. [DOI] [PubMed] [Google Scholar]
- 3. Bajwa RJ, Kovell L, Resar JR, et al. Left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Clin Cardiol. 2017;40:825‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh GD, Smith TW, Rogers JH. Targeted transseptal access for MitraClip percutaneous mitral valve repair. Interv Cardiol Clin. 2016;5:55‐69. [DOI] [PubMed] [Google Scholar]
- 5. Imnadze G, Ajaj T, Bante H, Sohns C, Sommer P. Transseptal puncture without fluoroscopy using a radiofrequency needle: a case series. Cardiol J. 2021;28:655‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radinovic A, Mazzone P, Landoni G, Agricola E, Regazzoli D, Bella P. Different transseptal puncture for different procedures: optimization of left atrial catheterization guided by transesophageal echocardiography. Ann Card Anaesth. 2016;19:589‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman DJ, Pokorney SD, Ghanem A, et al. Predictors of cardiac perforation with catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. 2020;6:636‐645. [DOI] [PubMed] [Google Scholar]
- 8. Adamczyk M, Niedziela JT, Wasilewski J, Zembala MO, Kalarus Z, Gąsior M. Prevalence, management and outcomes of cardiac tamponade complicating 66,812 invasive cardiac procedures: single‐center clinical registry. Postep w Kardiol Interwencyjnej. 2021;17:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanagisawa S, Inden Y, Ohguchi S, et al. Periprocedural management of cardiac tamponade during catheter ablation for AF under uninterrupted DOAC and warfarin. JACC Clin Electrophysiol. 2020;6:786‐795. [DOI] [PubMed] [Google Scholar]
- 10. Bollmann A, Ueberham L, Schuler E, et al. Cardiac tamponade in catheter ablation of atrial fibrillation: German‐wide analysis of 21 141 procedures in the helios atrial fibrillation ablation registry (SAFER). Europace. 2018;20:1944‐1951. [DOI] [PubMed] [Google Scholar]
- 11. Žižek D, Antolič B, Prolič Kalinšek T, et al. Intracardiac echocardiography‐guided transseptal puncture for fluoroless catheter ablation of left‐sided tachycardias. J Interv Card Electrophysiol. 2021;61:595‐602. [DOI] [PubMed] [Google Scholar]
- 12. O'Brien B, Zafar H, Freitas SDe, Sharif F. Transseptal puncture—review of anatomy, techniques, complications and challenges. Int J Cardiol. 2017;233:12‐22. [DOI] [PubMed] [Google Scholar]
- 13. Marcus GM, Ren X, Tseng ZH, et al. Repeat transseptal catheterization after ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:55‐59. [DOI] [PubMed] [Google Scholar]
- 14. Abdur Rehman K, Wazni OM, Barakat AF, et al. Life‐threatening complications of atrial fibrillation ablation: 16‐Year experience in a large prospective tertiary care cohort. JACC Clin Electrophysiol. 2019;5:284‐291. [DOI] [PubMed] [Google Scholar]
- 15. Bayrak F, Chierchia GB, Namdar M, et al. Added value of transoesophageal echocardiography during transseptal puncture performed by inexperienced operators. Europace. 2012;14:661‐665. [DOI] [PubMed] [Google Scholar]
- 16. Creta A, Ventrella N, Providência R, et al. Same‐day discharge following catheter ablation of atrial fibrillation: a safe and cost‐effective approach. J Cardiovasc Electrophysiol. 2020;31:3097‐3103. [DOI] [PubMed] [Google Scholar]
- 17. Isath A, Padmanabhan D, Haider SW, et al. Does the use of intracardiac echocardiography during atrial fibrillation catheter ablation improve outcomes and cost? A nationwide 14‐year analysis from 2001 to 2014. J Interv Card Electrophysiol. 2021;61:461‐468. [DOI] [PubMed] [Google Scholar]
- 18. Chow AWC, Cobb V, Sepahpour A, McCready JW. Transseptal puncture performed with the new needle‐free ‘SafeSept’ guidewire: a multicentre experience. J Interv Card Electrophysiol. 2020;59:29‐34. [DOI] [PubMed] [Google Scholar]
- 19. Mansour M, Karst E, Heist K, et al. Reduction in costs after AF ablation and impact of clinical events. Hear Rhythm. 2016;42:13‐200. [Google Scholar]
- 20. Sharma SP, Nalamasu R, Gopinathannair R, Vasamreddy C, Lakkireddy D. Transseptal puncture: devices, techniques, and considerations for specific interventions. Curr Cardiol Rep. 2019;21:52. [DOI] [PubMed] [Google Scholar]


