ABSTRACT
Animal behaviour is remarkably sensitive to disruption by chemical pollution, with widespread implications for ecological and evolutionary processes in contaminated wildlife populations. However, conventional approaches applied to study the impacts of chemical pollutants on wildlife behaviour seldom address the complexity of natural environments in which contamination occurs. The aim of this review is to guide the rapidly developing field of behavioural ecotoxicology towards increased environmental realism, ecological complexity, and mechanistic understanding. We identify research areas in ecology that to date have been largely overlooked within behavioural ecotoxicology but which promise to yield valuable insights, including within‐ and among‐individual variation, social networks and collective behaviour, and multi‐stressor interactions. Further, we feature methodological and technological innovations that enable the collection of data on pollutant‐induced behavioural changes at an unprecedented resolution and scale in the laboratory and the field. In an era of rapid environmental change, there is an urgent need to advance our understanding of the real‐world impacts of chemical pollution on wildlife behaviour. This review therefore provides a roadmap of the major outstanding questions in behavioural ecotoxicology and highlights the need for increased cross‐talk with other disciplines in order to find the answers.
Keywords: animal, behaviour, contaminant, ecology, ecotoxicology, environmental change, fitness, pollutant, population, wildlife
I. INTRODUCTION
Chemical pollution represents a leading threat to human health, wildlife, and ecosystems around the world (Landrigan et al., 2018). In fact, increases in the diversity and volume of novel synthetic chemicals released into the environment now far outpace other key drivers of global change (e.g. rising atmospheric CO2, habitat loss; Bernhardt, Rosi & Gessner, 2017). This trend is the result of rapidly increasing chemical use, with the global chemicals industry, currently valued at >US$5 trillion, projected to double in size between 2017 and 2030 (UNEP, 2019). The spread of novel environmental contaminants is concerning given that many ecosystems are already inundated with myriad organic and inorganic chemicals released by human activities, including metals, pesticides, pharmaceuticals, and per‐ and polyfluoroalkyl substances (PFAS) (Bertram et al., 2022). Moreover, chemical pollution continues to accelerate large‐scale wildlife losses (Groh et al., 2022), including dramatic declines in the abundance and biodiversity of insects (Sánchez‐Bayo & Wyckhuys, 2019), avifauna (Rosenberg et al., 2019), and aquatic species (Tickner et al., 2020).
Adverse impacts of chemical pollution on wildlife are not limited to mortality at acutely toxic levels. Indeed, exposure to environmental pollutants at sublethal concentrations acts as an often‐cryptic driver of wildlife declines by disrupting a wide range of fundamental biological processes [e.g. development (Gore et al., 2015); reproduction (Aulsebrook et al., 2020)]. In this regard, a growing body of research has demonstrated that chemical pollutants can have both direct and indirect impacts at multiple levels of organisation by altering animal behaviour (reviewed in Saaristo et al., 2018). In fact, behaviour can be remarkably sensitive to perturbation by even low pollutant concentrations, and is often disturbed at much lower exposure levels than more conventional endpoints in ecotoxicology, such as development, reproduction, and mortality, which is routinely estimated using LC50 (i.e. the concentration at which 50% of the animals in an exposed population are expected to die) (Melvin & Wilson, 2013). This is alarming given that behaviour represents the link between an organism and its environment, meaning that an inability to appropriately produce and maintain behaviour can have dire consequences for individual‐ and population‐level fitness (Wong & Candolin, 2015).
Clearly, there is an urgent need to better establish the causes and consequences of pollutant‐induced behavioural changes in wildlife (discussed in Peterson et al., 2017). However, conventional approaches applied in behavioural ecotoxicology are often insufficient to address the complexity of real‐world exposure scenarios (Pyle & Ford, 2017), that is exposing individuals of a single species to a single contaminant and measuring movement in a confined arena. This limits our ability to achieve meaningful biological interpretations of pollutant impacts at the individual level. Moreover, such approaches hamper the accurate extrapolation of findings to predict effects on populations and communities, which is vital to environmental protection efforts (Ford et al., 2021).
At the same time, significant methodological and technological innovations promise to transform the study of wildlife behavioural responses to pollutants, originating from within behavioural ecotoxicology and related disciplines such as analytical, environmental, and computational chemistry, comparative toxicology, behavioural ecology, collective behaviour, movement ecology, automated sensing, and computer vision. These advances open the door to the collection of data of high environmental and ecological relevance at an unprecedented resolution and scale in both the laboratory and the field. Crucially, this is enabling behavioural ecotoxicologists to confront the formidable complexity that often characterises real‐world exposure scenarios (e.g. chemical interactions, chemical and non‐chemical stressor interactions, complex and/or large‐scale behaviours). Indeed, recent work embracing these innovations has revealed effects of pollutants on behavioural processes that were previously not possible to capture using conventional approaches. These range from neurotoxic insecticide‐induced migration delays in songbirds (Eng, Stutchbury & Morrissey, 2019) to the breakdown of behavioural diversity in fish populations exposed to pharmaceutical pollution (Polverino et al., 2021).
The aim of this review is to guide the field of behavioural ecotoxicology towards increased environmental realism, ecological complexity, and mechanistic understanding by highlighting major research opportunities and experimental innovations. First, we examine how recent advances in analytical and environmental chemistry can increase environmental realism and experimental quality in behavioural ecotoxicology, and discuss underused approaches to prioritising contaminants of concern for behavioural testing. We then argue for targeting behavioural endpoints of high ecological significance, and outline concepts in ecology that have been broadly overlooked in behavioural ecotoxicology to date but which have the potential to provide key insights. We go on to present a suite of cutting‐edge tools and technologies that can be incorporated into behavioural ecotoxicology studies during design, implementation, and analysis. Further, we consider developments in statistical sophistication necessary to evaluate behavioural impacts of pollutants occurring in complex systems. Finally, we provide open‐access and freely available tools and resources, wherever possible, in order to increase the accessibility of behavioural ecotoxicology research.
II. INCREASING ENVIRONMENTAL REALISM
Here, we outline how recent advances in analytical and environmental chemistry can be harnessed to enhance environmental realism and experimental quality in behavioural ecotoxicology, and discuss underused approaches for prioritising contaminants and mixtures of concern for behavioural testing.
(1). Confronting real‐world exposure scenarios
Given the sheer number of chemicals in commerce, one of the challenges we face is how to identify what potentially behaviour‐modifying chemicals and mixtures are present in the environment and at what levels. There are at least 350,000 chemicals and chemical combinations registered for use around the world (Wang et al., 2020), several thousand of which have been detected in the environment (Hollender et al., 2017). However, regulatory monitoring is still limited to a small selection of well‐known contaminants, representing only a fraction of overall chemical and mixture risk (discussed in Brack et al., 2019a). For example, the United States Clean Water Act regulates 126 priority pollutants (EPA, 2021a), while the European Water Framework Directive is based on the analysis of 45 priority substances, as well as pollutants (~300 total) defined nationally by the different European Union member states (European Union, 2013). This limitation is partly a consequence of traditional environmental surveillance methodologies relying on targeted analyses of contaminants (discussed in Brack et al., 2019b), which requires that compounds are already ‘known’ and that chemical analytical standards are available to support their measurement. Fortunately, rapid advances in analytical and environmental chemistry, such as non‐targeted screening using high‐resolution mass spectrometry coupled to chromatography, now allow for near‐simultaneous identification of thousands of contaminants that are not preselected for study across a range of environmental compartments (e.g. water, air, dust, soil) and biological matrices (e.g. tissue, blood, plasma) (Hollender et al., 2017; McCord, Groff II & Sobus, 2022). The sensitivity of analytical instrumentation for both targeted and non‐targeted analyses has also increased markedly, allowing for the detection of even trace concentrations (e.g. pg/l or kg, ng/l or kg) of chemicals that could cause sub‐lethal changes to animal behaviour (Jiang & Li, 2020). However, equipment and analysis costs are still a limiting factor in the widespread application of these approaches (see Fernandez, André & Cardeal, 2020). Thankfully, data on complex exposure scenarios in the wild are also increasingly accessible due to the availability of large‐scale, open‐access databases documenting the occurrence of environmental contaminants, for example the EPA NCOD Database (EPA, 2021b), the NORMAN EMPODAT Database (NORMAN, 2022), the UBA Pharmaceuticals in the Environment Database (UBA, 2021), and the Global Monitoring of Pharmaceuticals Project (Wilkinson et al. 2022). Taken together, these developments increasingly allow behavioural ecotoxicologists to place their work in a real‐world context when targeting contaminants and mixtures of concern (e.g. based on environmental prevalence, persistence, co‐occurrence, and/or relevance to particular species or research questions). This targeted approach is especially important given that testing all relevant chemicals and combinations of chemicals for potential behavioural effects would be prohibitively costly, inefficient, and ethically problematic. Moreover, these advances facilitate extreme precision, accuracy, and breadth in characterising and validating exposure scenarios (e.g. confirming contaminants and transformation products in exposure media and/or experimental animal tissues).
(2). Prioritising contaminants of concern
The next major challenge is identifying and prioritising contaminants and mixtures that are likely to have deleterious effects on wildlife behaviour. To this end, we must look beyond traditional laboratory exposure experiments alone, again adopting a targeted approach given that more chemicals exist than can be adequately examined in a timely manner. It is therefore crucial to predict how chemicals interact with biological systems, which requires developing predictive approaches to identify and prioritise contaminants and mixtures that are likely (or not) to alter behaviour. In addition to facilitating the analytical chemistry advances discussed previously, computational chemistry can help to identify inherent chemical attributes and properties that are likely to interact with specific biomolecules and elicit downstream biological and behavioural responses in organisms. For example, an explosion of molecular genetic information across phyla is facilitating in silico advances in predictive toxicology and pharmacology during drug development (Raies & Bajic, 2016). Such approaches are translatable from human to environmental protection efforts when the structural alerts for specific chemicals align with predictions of evolutionarily conserved molecular initiating events (MIEs, i.e. a molecular interaction between a chemical and a specific biomolecule; LaLone et al., 2016). These MIEs may then lead to a sequential series of higher‐order effects (e.g. gene activation, changed cell signalling, disturbed homeostasis, altered tissue development and/or function) across biological scales (e.g. molecular, cellular, tissue, and/or organ levels). This may produce adverse outcomes – for instance, on behaviour – of relevance to individuals and populations [summarised in the ‘Adverse Outcome Pathway’ (AOP) concept; Ankley et al., 2010]. Adding a layer of complexity, AOPs can also be used to predict mixture effects (reviewed in Escher et al., 2017), which may be produced by combinations of chemicals that act via the same or different MIEs, and could include additive, antagonistic, or synergistic interactive effects on animal behaviour. Supporting this approach, large‐scale computational toxicology initiatives are examining thousands of chemicals with hundreds of targets in in vitro models to identify MIEs that may cause adverse outcomes in the environment (e.g. Tox21, 2020; ToxCast, 2020; ToxPi, 2021). Although very little integration of computational chemistry, comparative toxicology, and behavioural ecotoxicology has occurred to date, this approach could be extremely valuable in facilitating effective prioritisation of contaminants of concern for behavioural testing, and promises to improve mechanistic understanding. Moreover, because groups of chemicals within contaminant classes often act on the same or similar physiological pathways and molecular mechanisms (e.g. serotonin re‐uptake inhibiting pharmaceuticals; Gunnarsson et al., 2019), this targeted approach promises to reduce substantially the number of candidate chemicals and mixtures for behavioural testing.
III. ADDRESSING ECOLOGICAL COMPLEXITY
A common critique of behavioural ecotoxicology research is the perception that such studies lack sufficient ecological relevance to be applied to real‐world scenarios (discussed in Ågerstrand et al., 2020). This is true even though, when assessed correctly, behavioural responses can be invaluable in predicting higher‐order population‐ and community‐level outcomes (Saaristo et al., 2018). Here, we outline practices to improve ecological realism in behavioural ecotoxicology research and highlight seldom‐studied but significant new research avenues in this field.
(1). Key experimental design considerations
When designing studies testing the potential behavioural impacts of contaminants, how do we decide which behaviours we should test and how to test them most appropriately? Most importantly, behaviours need to be ecologically relevant to the focal species and must be biologically targeted by the contaminant(s) in question via some direct or indirect mechanism(s) of action, and/or predicted to impact an organism's spatial pollution attraction/avoidance (discussed in Araújo et al., 2020). Contaminants that are expected to affect fundamental behaviours for a species' ecology and fitness should be prioritised, as these will likely have more immediate and adverse impacts. For instance, contaminants that target social‐cue mechanisms may disproportionately affect organisms that rely on social information and/or the ability to form cohesive groups (e.g. schools, flocks) that are essential to their antipredator behaviour, feeding and foraging, and mating biology (e.g. Ward et al., 2008; Martin et al., 2019; Mason et al., 2021). Similarly, certain pollutants might interfere with a species' ability to avoid predators by targeting neural pathways involved in predator recognition (e.g. Polo‐Cavia, Burraco & Gomez‐Mestre, 2016) or that support locomotion for escape (e.g. Sievers et al., 2018). Further, behavioural experiments should ideally be tailored to mimic the species' natural social environment and habitat (or be conducted in the wild; see Section IV.2). Social species, for instance, should be tested in the presence of conspecifics (see Martin & McCallum, 2021), both to increase ecological relevance and because of the known impacts of social isolation on animal physiology and behaviour (e.g. Shams, Chatterjee & Gerlai, 2015; Tunbak et al., 2020; Munson, Michelangeli & Sih, 2021). Likewise, species that rely on specific environmental resources as part of their behavioural repertoire should be tested with those resources (e.g. refuge structures, substrate, perches), and if movement‐related behaviours are being measured then adequately sized experimental environments should be provided for natural space use and avoidance behaviours to occur. These details will vary from species to species but their absence may lead to unnatural behaviours and erroneous biological interpretations.
(2). Novel research directions in behavioural ecotoxicology: what are the knowns and the unknowns?
Keeping the above experimental considerations in mind, we next feature a range of future research avenues that, despite being of high ecological significance, have received relatively little attention in behavioural ecotoxicology (Fig. 1; see also Table 1 for examples of studies that have been carried out in each of these research areas to date). We by no means see this as an exhaustive list or as mutually exclusive groupings. In fact, integrating across these groupings will only help to address the complexities of how pollutant exposure affects animals in the wild.
Fig. 1.
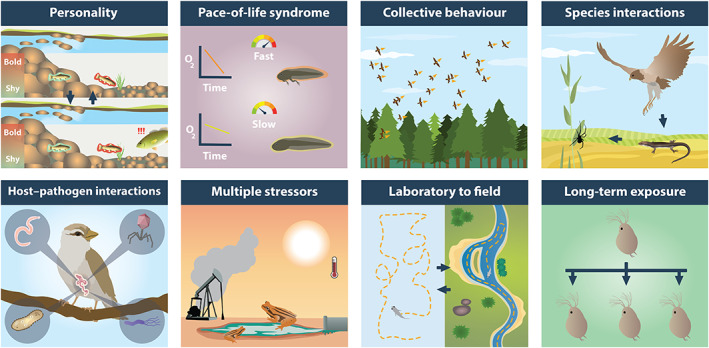
Important future avenues for research investigating behavioural impacts of chemical pollution on wildlife, which have received relatively little attention in behavioural ecotoxicology, especially when compared with more conventional ecotoxicological endpoints (e.g. development, reproduction, and LC50). Examples of studies that have been conducted in each of these research areas to date are provided in Table 1.
Table 1.
Research in behavioural ecotoxicology has increased markedly in ecological realism, especially in recent years. This includes studies conducted within each of the key areas for future research identified in Fig. 1, although such studies are still relatively rare. Examples of these studies are provided here, grouped by research area
| Research area | Compound(s)/stressor(s) | Species | Major result(s) | Reference |
|---|---|---|---|---|
|
Personality Behavioural differences between individuals that are consistent over time and across contexts (see Montiglio & Royauté, 2014; Jacquin et al., 2020) |
Phosmet (organophosphate insecticide) | Jumping spider (Eris militaris) | Repeatability of personality traits declined in the exposed group, mostly mediated by an increase in within‐individual variance. | Royauté, Buddle & Vincent (2015) |
| Esfenvalerate (pyrethroid insecticide) | Damselfly (Coenagrion puella) | Exposure changed average activity and behavioural covariation (activity and boldness) but not behavioural repeatability. | Tüzün et al. (2017) | |
| Mixture of metals, mainly lead, copper, and zinc | Great tit (Parus major) | Exploration behaviour and aggressiveness during nest defence were repeatable across years. Birds with high levels of lead in their blood and high levels of multiple metals in their feathers exhibited slower exploration behaviour but no effect of exposure was seen on aggressiveness. | Grunst et al. (2019) | |
| Fluoxetine (antidepressant pharmaceutical) | Guppy (Poecilia reticulata) | Homogenised individuals' activity (i.e. reduced consistent variation between individuals). | Tan et al. (2020) | |
| Fluoxetine (antidepressant pharmaceutical) | Guppy (Poecilia reticulata) | Homogenised individuals' activity (i.e. reduced variation between but not within individuals). | Polverino et al. (2021) | |
|
Pace‐of‐life syndrome The correlation between an individual's average behaviour (e.g. activity) and averages of its other phenotypic traits (e.g. metabolic rate, growth rate), with all traits significantly repeatable after repeated measures. |
Chlorpyrifos (organophosphate insecticide) | Ischnura damselfly species (I. elegans, I. genei, I. graellsii and I. pumilio) | Exposure affected covariation of life‐history and boldness in the most fast‐lived species (I. pumilio). | Debecker et al. (2016) |
| Zinc (heavy metal) | Blue‐tailed damselfly (Ischnura elegans) | Fast pace‐of‐life was associated with higher zinc sensitivity. Zinc exposure made larvae less active, less exploratory, and less risk‐taking. Exposure to zinc did not change the covariation patterns between traits (behavioural and physiological). | Debecker & Stoks (2019) | |
| Fluoxetine (antidepressant pharmaceutical) | Fairy shrimp (Branchipodopsis wolf) | Fluoxetine disrupted sex‐specific relationships between body size (proxy for growth rate) and activity. | Thoré, Brendonck & Pinceel (2021b) | |
|
Collective behaviour A form of social behaviour involving the coordinated behaviour of groups of similar individuals, and the emergent properties of these groups. |
4‐nonylphenol (endocrine‐disrupting chemical) | Banded killifish (Fundulus diaphanus) | Unexposed fish oriented away from dosed conspecifics. Shoals of all exposed fish had larger nearest‐neighbour distances (less‐tight shoals). | Ward et al. (2008) |
| Imidacloprid (neonicotinoid insecticide) | Bumblebee (Bombus impatiens) | Impaired nursing behaviour and altered social and spatial dynamics of workers within nests. | Crall et al. (2018) | |
| Crude oil | Atlantic croaker (Micropogonias undulatus) | Reduced shoal cohesion in shoals with all exposed fish and in shoals with only one exposed fish. | Armstrong et al. (2019) | |
| Benzo[a]pyrene (polycyclic aromatic hydrocarbon; PAH) | Zebrafish (Danio rerio) | Increased inter‐individual distances in exposed shoals. Exposed shoals moved less overall. | Hamilton et al. (2021) | |
| Oxazepam (anxiolytic pharmaceutical) | Brown trout (Salmo trutta) | Fish were less aggressive at higher doses and subordinate fish became more competitively successful at low doses (dominant and subordinate fish affected differently). | McCallum et al. (2021) | |
|
Species interactions Behavioural interactions between individuals of different species, such as predator–prey and competitive interactions (see Saaristo et al., 2018; Fleeger, 2020; Fisher et al., 2021) |
Carbaryl (carbamate insecticide), malathion (organophosphate insecticide) | Amphibian prey (gray treefrog, Hyla versicolor; green frog, Rana clamitans; American bullfrog, R. catesbeiana) and a predator (red‐spotted newt, Notophthalmus viridescens) | Exposure to either insecticide reduced the activity of all three tadpole prey species, and reduced the predation rate of newts on one tadpole species. | Relyea & Edwards (2010) |
| Urban and industrial contamination, including polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), dioxins, and metals (e.g. copper, lead, zinc) | Killifish (Fundulus heteroclitus), bluefish (Pomatomus saltatrix), fiddler crab (Uca pugnax), blue crab (Callinectes sapidus), grass shrimp (Palaemonetes pugio) | At contaminated sites, all five species showed reduced activity and feeding. Complex behavioural changes were also seen within species due to contamination, including reduced predator avoidance in killifish but increased predator avoidance in fiddler crabs and blue crabs. | Weis et al. (2011) | |
| Trifloxystrobin (TFS, strobilurin fungicide) | Eel (Synbranchus marmoratus), four anuran prey species (Rhinella arenarum, Physalaemus santafecinus, Leptodactylus latrans, Elachistocleis bicolor) | Exposure altered the outcome of eel–tadpole interactions by decreasing prey movement and prey detection, increasing tadpole survival. Eels preyed selectively upon non‐exposed tadpoles. | Junges et al. (2012) | |
| 17β‐oestradiol (E2, oestrogen steroid hormone) | Fathead minnow (Pimephales promelas), bluegill sunfish (Lepomis macrochirus) | Exposure reduced anti‐predator escape behaviour of larval minnows and they were more likely to be predated by a sunfish predator. | Rearick et al. (2018) | |
| Dichlorodiphenyltrichloroethane (DDT, organochlorine insecticide) | African clawed frog (Xenopus laevis), mosquito (Culex sp.) | Significant exposure × prey cue interaction. Exposure reduced frog foraging behaviour towards live prey cues, although no effect was seen in response to olfactory prey cues. Mosquito larvae exhibited reduced antipredator behaviour. | South et al. (2019) | |
|
Host–pathogen interactions The interactions between microbes (e.g. parasites, bacteria, viruses) and their host organism(s), which may alter the behaviour of the host. |
Imidacloprid (neonicotinoid insecticide), entomopathogenic fungi (Metarhizium robertsii and Beauveria bassiana) | Citrus root weevil (Diaprepes abbreviatus) | Application of either fungus had no effect on the movement of larvae in soil, although insecticide exposure was found to impair larval movement. Moreover, exposure to both imidacloprid and a fungus acted synergistically to produce more severe impairment to larval movement. | Quintela & McCoy (1998) |
| Chlorpyrifos (organophosphate insecticide), trematode parasite (Euhaplorchis californiensis) | California killifish (Fundulus parvipinnis) | Insecticide exposure reduced activity and decreased average swimming speed following a simulated predator attack. No singular or interactive effects of parasite infection were observed. | Renick et al. (2016) | |
| Mixture of metals (cadmium, copper, and zinc), immune challenge (antigen mixture mimicking a parasite infection) | Gudgeon (Gobio occitaniae) | Single stressors increased immune defences and oxidative stress at the expense of body mass (metal contamination) or swimming activity (immune challenge). Multiple stressors produced fewer interactive effects than expected but primarily resulted in antagonistic effects on swimming activity. | Petitjean et al. (2021) | |
|
Multiple stressors The potentially interacting effects of combined exposure to multiple contaminants (within and/or across contaminant classes), or to contaminants and other external stressors (e.g. temperature, light, noise) (see Halfwerk & Slabbekoorn, 2015; Hale, Piggott & Swearer, 2017; Jacquin et al., 2020) |
Copper (heavy metal), imidacloprid (insecticide) | Spotted marsh frog (Limnodynastes tasmaniensis) | Copper increased erratic swimming at the lower imidacloprid concentration. Limited overall evidence for interactive effects (both stressors produced independent effects). | Sievers et al. (2018) |
| Chlorpyrifos (organophosphate insecticide), flow speed | California killifish (Fundulus parvipinnis), polychaete worm (Polydora cornuta) | Contamination and differing flow speeds resulted in complex effects on predator–prey interactions, including reducing prey‐patch selection in contaminated killifish and reducing feeding behaviour in worms. | Hayman et al. (2019) | |
| 17β‐trenbolone (androgenic steroid), temperature | Eastern mosquitofish (Gambusia holbrooki) | Contamination increased boldness, with effects of exposure on some behaviours (i.e. exploration and predator‐escape behaviour) being dependent on temperature. | Lagesson et al. (2019) | |
| Fluoxetine (antidepressant pharmaceutical), acute temperature stress | Guppy (Poecilia reticulata) | No evidence for interactive effects on reproductive behaviours and activity levels (both stressors produced independent effects). | Wiles et al. (2020) | |
| Chlorpyrifos (organophosphate insecticide), acute, developmental, and transgenerational warming | Mosquito (Culex pipiens) | Particularly developmental and transgenerational warming reduced larvae antipredator behaviours. Contamination decreased heat tolerance and antipredator behaviours. | Meng et al. (2021) | |
|
Laboratory to field Using paired laboratory‐ and field‐based approaches to investigate potentially behaviour‐modifying effects of contaminants. Note: also included here are semi‐field and field‐based studies without a paired laboratory component (see Hellström et al., 2016b; Saaristo et al., 2018) |
Urban and industrial contamination, including copper, cadmium, and polycyclic aromatic hydrocarbons (PAHs). | Round goby (Neogobius melanostomus) | In the laboratory, fish collected from contaminated sites exhibited reduced activity and exploration, although this was not reflected in distance moved in a mark–recapture field study. | Marentette et al. (2012) |
| Neonicotinoid insecticide mixture (thiamethoxam and imidacloprid) | European honey bee (Apis mellifera) | Individual honeybees near treated fields disappeared more quickly but this was buffered by the colonies' demographic regulation response. | Henry et al. (2015) | |
| Oxazepam (anxiolytic pharmaceutical) | Atlantic salmon (Salmo salar) | Promoted downward migratory behaviour in the laboratory and in a natural river tributary. | Hellström et al. (2016a) | |
| Oxazepam (anxiolytic pharmaceutical) | European perch (Perca fluviatilis) | Increased boldness and activity both in the laboratory and in a lake ecosystem. | Klaminder et al. (2016) | |
| Clothianidin (neonicotinoid insecticide) | Bumblebee (Bombus terrestris audax) | In a semi‐field experiment, exposure produced subtle changes in patterns of foraging activity and pollen foraging, with a colony census at the end of the experiment revealing that treated colonies had fewer adults (workers, drones, and gynes) compared to control colonies. | Arce et al. (2017) | |
|
Long‐term exposure Examining potential behavioural effects of long‐term exposure to contaminants, including chronic, transgenerational, and multigenerational exposures. |
Carbamazepine (anticonvulsant pharmaceutical), gemfibrozil (blood lipid‐regulating pharmaceutical) | Zebrafish (Danio rerio) | Parental exposure to either drug reduced male courtship behaviour in unexposed offspring. Effects on courtship displays were compound‐specific. | Galus et al. (2014) |
| Fluoxetine (antidepressant pharmaceutical) | Zebrafish (Danio rerio) | Developmental exposure produced hypocortisolism and reduced exploratory behaviours in two consecutive generations of unexposed descendants. | Vera‐Chang et al. (2018) | |
| Lambda‐cyhalothrin (LCT, pyrethroid insecticide) | Mustard leaf beetle (Phaedon cochleariae) | Parental exposure altered aspects of mating behaviour in both the parental generation and unexposed offspring. | Müller, Römer & Müller (2019) | |
| Urban contaminant mixture, including bisphenol‐A (BPA, xenoestrogen), N,N‐diethyl‐meta‐toluamide (DEET, insect repellent), and 4‐nonylphenol (xenoestrogen) | Fathead minnow (Pimephales promelas) | Exposure for three generations altered the behaviour (foraging, courtship, boldness) of larvae and adults, which were magnified in the F1 and F2 generations. | Swank et al. (2021) | |
| Fluoxetine (antidepressant pharmaceutical) and 3,4‐dichloroaniline (pesticide) | Turquoise killifish (Nothobranchius furzeri) | Behavioural effects differed in single‐chemical versus mixture exposure treatments, and across two successive generations. | Thoré et al. (2021) |
At the individual level, behavioural ecotoxicology research to date has largely focused on how exposure to chemicals alters the average level of ecologically relevant behaviours (e.g. activity, aggression, boldness). Such observations have been pragmatic from a laboratory experimental design perspective but often ignore behavioural variation. This is concerning because phenotypic plasticity, that is an individual's capacity for phenotypic variation under different environments, is a key determinant of performance under fluctuating conditions within their lifetime (often called ‘acclimation’; see Donelson et al., 2019). Moreover, phenotypic variation among individuals is fundamental for adaptation (and evolution) to occur, meaning that the extent of variation within a population is an important indicator of how buffered that population will be to environmental change, including chemical pollution (Sih, Ferrari & Harris, 2011). Accordingly, the field of behavioural ecology has seen a shift towards quantifying and understanding consistent within‐ and among‐individual behavioural variation (i.e. animal personality; Sih, Bell & Johnson, 2004). This includes both phenotypic plasticity and other sources of behavioural variation such as circadian/circannual rhythms (e.g. Melvin, 2017; Thoré, Brendonck & Pinceel, 2021a), and life‐history events (e.g. Thoré et al., 2019). This has produced new insights into how consistent individual behavioural differences are correlated with key traits related to behaviour, such as cognitive ability (Sih & Del Giudice, 2012; Griffin, Guillette & Healy, 2015), metabolic rate (Careau et al., 2008; Biro & Stamps, 2010), and space‐use patterns (Spiegel et al., 2017; Michelangeli et al., 2021). Furthermore, individual behavioural variation is heritable (Dochtermann, Schwab & Sih, 2015) but can also be shaped by experience via transgenerational and developmental phenotypic plasticity (Groothuis & Taborsky, 2015), including exposure to chemical pollutants (Tüzün et al., 2017). Importantly, differences in behaviour among individuals may also lead to variation in sensitivity and exposure to chemical pollutants. For example, recent research has demonstrated that social status modulates the extent of uptake of the pharmaceutical pollutant oxazepam by juvenile brown trout (Salmo trutta), with subordinate fish absorbing more of the drug than dominant ones, likely due to higher ventilation and respiration rates (McCallum et al., 2021). Given that only a relatively small number of recent studies have examined how chemical stressors alter aspects of behavioural variation (e.g. Tüzün et al., 2017; Polverino et al., 2021), or how consistent individual differences affect behavioural responses to chemical stressors (Nanninga, Scott & Manica, 2020), the integration of behavioural ecotoxicology and consistent individual variation is clearly an important direction for future study (see Montiglio & Royauté, 2014).
Scaling up to the group and population levels, we know that animals display a range of social phenotypes (Gartland et al., 2021) and that interactions among conspecifics can underlie many aspects of animal fitness (e.g. territory defence, reproduction, parental care). Yet very little attention has been given to understanding how contaminants might affect animal groups and intraspecific interactions (beyond simple dyads). As such, another significant future direction will be to measure the impact of chemical pollutants in natural social settings on endpoints like: (i) social network structure, including the formation and consequences of dominance hierarchies; (ii) collective behaviours such as group shoaling, flocking, foraging, or collective decision‐making; (iii) how individual traits (e.g. personality variation) affect group or collective behaviour; (iv) animal contests over valued resources (e.g. territory, mates); and (v) classic reproductive behaviours like courtship, mating and alternative strategies, and parental care. This work can also operate beyond a single generation because an individual's behaviour can be shaped by both past and current exposure, as well as past conspecific experiences via indirect maternal/paternal effects (Bell & Hellmann, 2019; Donelan et al., 2020).
We also need to look beyond any single species to answer questions concerning multi‐species interactions and how behaviour is affected by other ecological processes across environmental gradients. These types of questions will allow us to better approximate the complexities of natural environments and might include, for example, uncovering whether and how contaminants could decouple or change host–parasite or host–pathogen relationships (Blanken, van Langevelde & van Dooremalen, 2015; Rumschlag et al., 2019), alter interspecific information transfer or eavesdropping (Gil et al., 2018), and affect predator–prey interactions (Weis & Candelmo, 2012; Hayden et al., 2015). Moreover, considering that various pollutants (e.g. psychoactive pharmaceuticals) have been shown to alter predator avoidance and escape behaviours in prey species (e.g. Polo‐Cavia, Burraco & Gomez‐Mestre, 2016; Martin et al., 2017), key questions that have received surprisingly little attention to date are whether exposure of predators and/or prey to (the same or different) contaminants might shift the ‘landscape of fear’ (Gaynor et al., 2019), or shape animal communities and trophic cascades through altered patterns of competition and/or consumption (e.g. Rohr, Kerby & Sih, 2006; Weis et al., 2011).
Finally, to reveal patterns of effects of chemicals on individuals, animal groups, and whole community/ecosystem outcomes, a crucial need is to understand the relative impact of chemical stressors and other abiotic and biotic stressors that can have unexpected and complex effects on animal behaviour, that is we need a multi‐stressor approach (Orr et al., 2020). Numerous studies show that even very low levels of chemical stressors can severely reduce fitness if combined with low food levels (e.g. Rohr et al., 2004), predation risk (Relyea, 2003), high parasite load (e.g. Rumschlag et al., 2019), or variable temperature (e.g. Brown et al., 2015; Delnat et al., 2019). Yet relatively few studies have examined how behaviour might mediate these multi‐stressor outcomes via animal dispersal or avoidance, seasonal or diurnal shifts in behaviour, or increasing/decreasing behaviours to compensate for changing metabolic demands (e.g. a foraging–detoxification trade‐off). Among species, some of the variation in negative impacts of chemical stressors will be due to differences in physiology that underlie inherent differences in vulnerability to exposure. However, differences in behavioural responses will likely play a major role in explaining dissimilar exposure to chemicals, and in the ability of animals to compensate behaviourally, for example by increasing energy intake or via social buffering. Thus, more accurate behavioural assessments of chemical pollutants will often require a multi‐stressor approach in order to assess the simultaneous or sequential effects of chemicals with other key environmental factors within ecologically relevant conditions.
IV. TOOLS AND TECHNOLOGIES FOR ADVANCING BEHAVIOURAL ECOTOXICOLOGY
Here, we provide an overview of recent methodological and technological innovations that allow for the possible effects of contaminant exposure on organismal behaviour to be investigated at a greater resolution and scale than ever before (Fig. 2). See the online Supporting Information for a comprehensive overview of suppliers and resources for laboratory software and hardware (Tables S1 and S2, respectively), and field software and hardware (Tables S3 and S4).
Fig. 2.
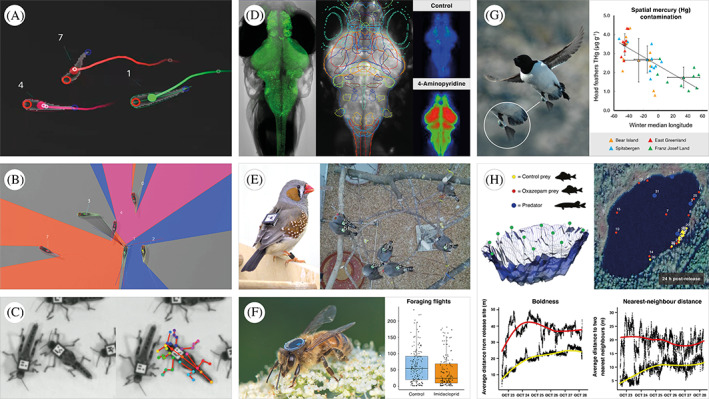
A wide variety of tools and technologies are now available to study potential impacts of contaminants on animal behaviour. Automated systems allow for (A) simultaneous tracking of large numbers of individuals (TRex; Walter & Couzin, 2021), and quantification of traits beyond movement, including (B) 2D visual fields (TRex; Walter & Couzin, 2021) and (C) posture analysis (DeepPoseKit; Graving et al., 2019). New technologies are also increasing mechanistic understanding in behavioural ecotoxicology, such as (D) functional neuroimaging with genetically encoded calcium sensors, used to quantify whole‐brain drug activity in larval fish (Winter et al., 2017). Further, innovative approaches enable tracking individuals in semi‐field and field settings: (E) automated barcode systems (Alarcón‐Nieto et al., 2018); (F) radio‐frequency identification (RFID) tagging, employed to reveal lifetime reductions in neonicotinoid‐exposed honey bee foraging (Colin et al., 2019); (G) Global Location Sensors (GLS), deployed to study spatial and seasonal mercury contamination of migratory little auks (Alle alle; Renedo et al., 2020); and (H) high‐resolution acoustic telemetry, used in a whole‐lake experiment to demonstrate reduced anxiety in prey fish exposed to pharmaceutical pollution (Klaminder et al., 2016). Image credits: A and B, T. Walter; C, J.M. Graving; D, M.J. Winter; E, G. Alarcón‐Nieto (left) and D. Farine (right); F, T. Colin; G, J. Fort; and H, J. Klaminder.
(1). Laboratory‐based experimental innovations
There is now a tremendous variety of laboratory software and hardware options available to ecotoxicologists for capturing potential behavioural responses to contaminant exposure. For instance, cameras with high frame rates offer the temporal resolution to track behaviours that were previously not possible to capture. This includes recording high‐speed locomotion on land (e.g. walking parameters of insects; Pfeffer et al., 2019), in water (e.g. propulsion in swimming fish; Johansen et al., 2017), and in the air (e.g. honeybee flight paths; Guiraud, Roper & Chittka, 2018), as well as feeding performance of predators (Rossoni & Niven, 2020), and escape performance of prey (e.g. Jonsson et al., 2019). Similarly, cameras with wide spectral‐ or thermal‐sensing capabilities enable researchers to record behaviours in the dark (e.g. Mitchell & Clarke, 2019), while multi‐camera systems track animal movement in three‐dimensional space (Macrì et al., 2017). Regarding high‐throughput approaches, lab‐on‐a‐chip devices, which make use of the changes in laminar flow within a chamber, have been developed for an array of aquatic organisms (Campana & Wlodkowic, 2018). These small chips are versatile to a variety of sensors and can measure behaviour following or during chemical exposure, in binary conditions as well as complex chemical gradients (Campana & Wlodkowic, 2018).
In addition to monitoring behaviours of pre‐exposed organisms or those exposed in real time, many new technologies have the ability to stimulate behaviours using light, vibration/noise, or electrical signals (discussed in Ågerstrand et al., 2020). This allows for the measurement of behavioural traits such as reaction time and speed, turning angles, space use, and predator avoidance. More sophisticated technologies are also being adapted to stimulate social, cognitive, and learning behaviours. For instance, state‐of‐the‐art robots can now be used to interact with fish in real time to study the underpinnings of social and anxiety‐related behaviours (e.g. Polverino et al., 2019), and even reveal the long‐lasting consequences of brief behavioural alterations on the life‐history, reproduction, and ecological success of targeted animals (Polverino et al., 2022). Indeed, despite being underexploited in behavioural ecotoxicology to date, bioinspired robots offer an autonomous, customisable, and repeatable approach that overcomes challenges associated with more traditional methods, in which live stimuli are an extra source of variation, although at the cost of some degree of biological and ecological realism. In addition to visual stimuli, new approaches also allow for increasingly sophisticated analysis of behavioural responses to chemical signals. For example, wind‐tunnel assays are allowing researchers to determine the preference of pollinating insects for non‐altered versus ozone‐contaminated floral blends (Cook et al., 2020), while two‐current choice flumes are increasingly used to examine chemosensory function in aquatic species (see Jutfelt et al., 2017), including attraction towards or avoidance of contaminants (Horký et al., 2021).
In parallel, automated visual tracking is fast becoming an indispensable tool for quantifying animal behaviour (reviewed in Henry & Wlodkowic, 2020; Panadeiro et al., 2021). There is now a wide variety of programs available for tracking animal movement, including a number of recently developed open‐source systems, such as TRex (Fig. 2A, Walter & Couzin, 2021) and ToxTrac (Rodriguez et al., 2018). Both of these systems are fast, powerful, user‐friendly, and versatile across a wide range of model species. Furthermore, these programs represent a major advance given their capacity to track large groups of animals simultaneously, for example ≤1000 individuals using TRex, including deep‐learning‐based visual identification of up to 100 unmarked individuals (Walter & Couzin, 2021). This provides an unparalleled opportunity to study the potential effects of contaminants on complex social behaviours in animal groups, such as dominance hierarchies and collective motion. Moreover, although distinctly underutilised in ecotoxicology to date, newly available software packages [e.g. DeepPoseKit (Graving et al., 2019); TRex (Walter & Couzin, 2021)] also allow for the quantification of an array of behavioural measures in addition to positional data, such as stereotyped movements (e.g. courtship displays), visual field (Fig. 2B), and posture analysis (e.g. body shape, head/tail position; Fig. 2C). As alluded to above, the integration of artificial intelligence and machine learning algorithms into these systems continues to power a revolution in motion‐tracking technology, including using deep learning to identify specific animals, determine posture, and even detect behaviours that were previously invisible to human observers (discussed in Graving et al., 2019).
From a mechanistic perspective, an increasing number of methods in molecular neurotoxicology are now available for uncovering drivers of pollutant‐induced behavioural changes (reviewed in Bownik & Wlodkowic, 2021). These methods range from transcriptomics (i.e. gene transcript analysis) to proteomics (i.e. analysis of proteins) and enzymatic assays, metabolomics (i.e. analysis of chemical processes involving metabolites in cells and tissues), and examination of sub‐cellular processes such as programmed cell death (Bownik & Wlodkowic, 2021). An important recent advance that promises to increase mechanistic understanding in behavioural ecotoxicology is large‐scale neurotransmitter profiling, enabled by the above‐mentioned rapid developments in analytical chemistry (see Section II.1). Here, an array of neurotransmitters can be monitored, as well as their precursors and degradation products, across different brain regions, providing a direct mechanistic link between pollutant‐induced behavioural changes and altered neuronal transmission (Mayol‐Cabré et al., 2020). For example, pioneering research harnessing neurotransmitter profiling recently demonstrated direct effects of the pervasive herbicide glyphosate on fish monoaminergic systems, with resulting effects on oxidative stress and anxiety‐related behaviours (Faria et al., 2021). Furthermore, significant recent breakthroughs mean that we can now dissect neural mechanisms for chemically induced brain activation and disruption using genetically encoded (bio)sensor systems coupled with high‐speed fluorescence light‐sheet microscopy (LSM; Winter et al., 2017; Fig. 2D). Illustrating this, transgenic zebrafish (Danio rerio) with pan‐neuronal expression of the calcium indicator GcaMP6, coupled with LSM, allow for functional imaging with sub‐cellular resolution across the whole brain, which has been applied to uncover mechanisms associated with behavioural responses to drug treatments (Winter et al., 2021).
(2). Scaling up: experimental advances in the field
When assessing possible impacts of contaminants on behaviour, a holistic approach is ideal, that is studies relating mechanistic explanations to experiments spanning laboratory, semi‐field, and field contexts. While laboratory assays provide valuable insights in a standardised setting and can be important for validating field observations (both of which are fundamental to ecotoxicity studies to support various tiers of chemical risk assessments), semi‐field‐ and field‐based ecotoxicology is crucial for understanding the potential impacts of contaminants in large‐scale, complex, and dynamic natural systems. Encouragingly, experimental and technological innovations continue to emerge that allow for effects of chemical pollution on animal behaviour to be measured in the field with ever‐greater efficiency and accuracy. This includes advances in remote‐sensing technology, which have revolutionised our capacity to gather high‐resolution behavioural and physiological data from animals in the wild (Smith & Pinter‐Wollman, 2021; Nathan et al., 2022). Cheaper, smaller, and increasingly more capable transmitters and sensors allow researchers to tag and track animals over large spatial and temporal scales (Smith & Pinter‐Wollman, 2021; Nathan et al., 2022). For example, automated barcode systems enable whole‐group or even whole‐population tracking over months or potentially years (Alarcón‐Nieto et al., 2018; Fig. 2E). Meanwhile, radio‐frequency identification (RFID) tagging has revealed changes in the lifetime foraging behaviour of free‐ranging honeybees exposed to a neonicotinoid pesticide (Colin et al., 2019; Fig. 2F), and Global Location Sensors (GLS) have been used to study spatial and seasonal trends of mercury contamination in seabirds from Arctic breeding colonies (Renedo et al., 2020; Fig. 2G).
Perhaps the most transformative use of remote‐sensing technology can be found in aquatic environments, where tools such as acoustic telemetry are leapfrogging our understanding of the behaviour and ecology of aquatic organisms by enabling detailed spatial monitoring of individuals over whole freshwater systems (e.g. rivers and lakes; Fig. 2H) and even oceans (reviewed in Hellström et al., 2016b). Data previously difficult or impossible to obtain from the field, such as survival, schooling behaviour, home ranges, diel activity patterns, and predator–prey interactions can now be investigated in the wild at a level of detail comparable to that of laboratory studies (Hellström et al., 2016b). Further, we anticipate that future research pairing acoustic telemetry with newly developed methods of remote contaminant exposure (e.g. slow‐release implants; McCallum et al., 2019) and/or spatial contaminant modelling (Fonseca et al., 2020) will provide valuable insights into the real‐world behavioural impacts of chemical pollution. Parallel to the progress made in animal tracking technology, the ‘golden age of bio‐logging’ is providing an ever‐expanding variety of small physiological and behavioural sensors that can record everything from an animal's heart rate, body temperature, and acceleration, to detailed data on foraging and spawning behaviour (reviewed in Whitford & Klimley, 2019). In combination, these advances in remote‐sensing technology provide a powerful, yet largely unexplored, experimental toolkit to study impacts of pollution on behavioural and ecological processes. Harnessing these novel approaches will also enable research attempting to establish a causal chain from pollutant‐induced changes in individual‐ and group‐level behavioural variation to population responses and ecosystem change. Still, given the complexity of natural environments, it is important to note that careful experimental design (including the use of suitable controls), and appropriate statistical and biological interpretation of collected data, will be critical to the validity and reliability of such studies.
V. IMPROVING STATISTICAL SOPHISTICATION
Historically, ecotoxicology experiments for use in chemical risk assessment follow strict regulatory guidelines (Chapman et al., 1996). These guidelines have generally employed simple experimental designs and straightforward statistical analyses to maximise external validity and ensure protocols are broadly accomplishable by researchers (Chapman et al., 1996). However, ever‐increasing environmental and ecological complexity is being incorporated into behavioural ecotoxicology studies (see Sections II and III, respectively), and new technologies have changed the nature of the data extracted from these experiments (see Section IV). As a result, various statistical techniques conventionally used in ecotoxicology may not always be appropriate for use in modern behavioural ecotoxicology.
(1). Embracing mixed modelling to address biological complexity
Biological data, and particularly behavioural endpoints, are often inherently variable, unbalanced (i.e. unequal sample sizes for different classes), non‐normally distributed, and highly structured (i.e. containing groups of non‐independent units). Such data sets require statistical approaches that are capable of appropriately partitioning and quantifying behavioural variation, while also considering the hierarchical nature of the data (i.e. to avoid pseudoreplication and/or to deal with non‐independence). One increasingly popular approach is mixed‐effects modelling (i.e. multilevel or hierarchical modelling) of either normally distributed response variables (linear mixed model, LMM) or non‐normally distributed response variables, such as count, binary or proportion data (generalised linear mixed models, GLMMs). Mixed‐effects models are a sophisticated statistical tool that can be applied to analyse data that have both fixed effects (e.g. experimental treatment, sex) and one or more clustering (random) factors (e.g. individual ID, treatment replicate) (Bolker et al., 2009). Mixed models can therefore account for, and estimate, the variation contributed by hierarchical structures in the data. A common example of this is the use of mixed models to account for multiple observations made on the same individual, where individual IDs are included in the random structure of the model as random intercepts to account for, and estimate, the amount of variation within and among individuals. This capability has meant that mixed models are now widely used in behavioural ecology and evolutionary biology to partition the variance across response variables both within and across grouping factors, and reveal effects occurring at each level (Dingemanse & Dochtermann, 2013). As previously discussed in this review, inferring the magnitude of variation within and among groups can be highly informative in the context of understanding responses to chemical pollution, which is now made possible via mixed‐modelling approaches, including using movement data in the wild (Hertel et al. 2020). Mixed models can also accommodate multiple response variables simultaneously (i.e. multivariate mixed models), allowing for the direct estimation of both within‐ and between‐individual phenotypic (co)variances. Further, particularly relevant for complex experimental designs, data‐reduction techniques (e.g. principal component analysis) can be used in combination with mixed modelling to collapse multiple related variables into fewer uncorrelated predictors in order to avoid over‐parameterisation. Fortunately, many publications on mixed models include detailed step‐by‐step guides on applying such statistical approaches to answer specific biological questions (summarised in Table S5).
(2). Harnessing meta‐analytic approaches
We would also like to highlight the scarcity of meta‐analytic studies in the field of behavioural ecotoxicology. This is surprising given that sufficient data exist addressing behavioural effects of chemical pollutants to answer targeted meta‐analytic hypotheses/questions. Beyond this general call for meta‐analyses, we also suggest that behavioural ecotoxicologists draw from the recent advances in meta‐analytic methods developed in the fields of ecology and evolution (Nakagawa et al., 2017). For example, future meta‐analyses could focus on the impacts of chemical pollution on behavioural variance (i.e. total phenotypic variance of unexposed versus exposed populations), in parallel with a more ‘traditional’ mean‐focused meta‐analysis (Nakagawa et al., 2015). More specifically, in addition to comparing the means across different treatment groups of interest using standardised metrics such as Cohen's d, Hedges' g, or the response ratio, the natural logarithm of the ratio between the coefficients of variation (lnCVR) can also be extracted, allowing for meta‐analytical comparisons of differences between the variability in groups (for a detailed description, see Nakagawa et al., 2015). In addition, for broad research questions in behavioural ecotoxicology, systematic mapping will be beneficial to identify areas that require more research and those that are ready for meta‐analytic synthesis (see James, Randall & Haddaway, 2016), despite being currently underused in ecotoxicology (Wolffe et al., 2019). Further, to gain a more nuanced understanding of the structure of a research topic, a novel meta‐analytic technique called ‘research weaving’ could be used, which combines systematic mapping and bibliometrics (see Nakagawa et al., 2019).
VI. CONCLUSIONS
Behavioural studies represent a sensitive, powerful, and ecologically meaningful approach for assessing the effects of environmental contaminants.
As research in this area continues to grow and increase in complexity, we emphasise the importance of following basic principles of sound ecotoxicology wherever possible (Harris et al., 2014), for example using appropriate exposure route(s), testing a suitable number and span of exposure treatments, and quantifying exposure concentrations. Although not all principles apply to all studies, and certain principles can be challenging or impossible to implement in some cases (e.g. in large‐scale or long‐term studies), they are especially vital if researchers intend their work to be translationally applicable to chemicals risk assessment and/or regulation (Ågerstrand et al., 2020).
We encourage researchers working within behavioural ecotoxicology and related disciplines to explore the rich toolkit that is now available to propel our understanding of how chemical pollution impacts wildlife living in a rapidly changing world.
Supporting information
Table S1. Software available to be incorporated into behavioural ecotoxicology research in the laboratory.
Table S2. Hardware available to be incorporated into behavioural ecotoxicology research in the laboratory.
Table S3. Software available to be incorporated into behavioural ecotoxicology research in the field.
Table S4. Hardware available to be incorporated into behavioural ecotoxicology research in the field.
Table S5. (a) A brief overview of resources available for implementing mixed modelling approaches in behavioural ecotoxicology, and (b) examples of specific research questions and resources for testing potential effects of contaminant exposure on behavioural variation at the individual level.
VII. ACKNOWLEDGEMENTS
Support for this review was provided by a Swedish Research Council Formas Mobility Grant (2020‐02293 to M.G.B.), the Kempe Foundations (SMK‐1954 and SMK21‐0069 to M.G.B.), the Marie‐Claire Cronstedt Foundation (to M.G.B.), the ÅForsk Foundation (20‐51 to M.G.B.), the National Institute of Environmental Health Sciences (1P01ES028942 to B.W.B.), the EU Cross‐Channel Interreg Programme (RedPol; Interreg 5a #185 to A.T.F.), the Forrest Research Foundation (Forrest Fellowship to G.P.), an Australian Government Research Training Program (RTP) Scholarship (to J.A.B. and H.T.), the Australian Research Council (DP190100642 to B.B.M.W. and L.A.A., and DP220100245 and FT190100014 to B.B.M.W), and the Swedish Research Council Formas (2020‐00981 to E.S.M., and 2018‐00828 to T.B.).
REFERENCES
References marked with an asterisk (*) are cited in the online Supporting Information
- Ågerstrand, M. , Arnold, K. , Balshine, S. , Brodin, T. , Brooks, B. W. , Maack, G. , McCallum, E. S. , Pyle, G. , Saaristo, M. & Ford, A. T. (2020). Emerging investigator series: use of behavioural endpoints in the regulation of chemicals. Environmental Science: Processes & Impacts 22, 49–65. [DOI] [PubMed] [Google Scholar]
- Alarcón‐Nieto, G. , Graving, J. M. , Klarevas‐Irby, J. A. , Maldonado‐Chaparro, A. A. , Mueller, I. & Farine, D. R. (2018). An automated barcode tracking system for behavioural studies in birds. Methods in Ecology and Evolution 9, 1536–1547. [Google Scholar]
- * Allegue, H. , Araya‐Ajoy, Y. G. , Dingemanse, N. J. , Dochtermann, N. A. , Garamszegi, L. Z. , Nakagawa, S. , Réale, D. , Schielzeth, H. & Westneat, D. F. (2017). Statistical quantification of individual differences (SQuID): an educational and statistical tool for understanding multilevel phenotypic data in linear mixed models. Methods in Ecology and Evolution 8, 257–267. [Google Scholar]
- Ankley, G. T. , Bennett, R. S. , Erickson, R. J. , Hoff, D. J. , Hornung, M. W. , Johnson, R. D. , Mount, D. R. , Nichols, J. W. , Russom, C. L. , Schmieder, P. K. , Serrano, J. A. , Tietge, J. E. & Villeneuve, D. L. (2010). Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry 29, 730–741. [DOI] [PubMed] [Google Scholar]
- Araújo, C. V. , Laissaoui, A. , Silva, D. C. , Ramos‐Rodríguez, E. , González‐Ortegón, E. , Espíndola, E. L. , Baldó, F. , Mena, F. , Parra, G. , Blasco, J. , López‐Doval, J. , Sendra, M. , Banni, M. , Islam, M. A. & Moreno‐Garrido, I. (2020). Not only toxic but repellent: what can organisms' responses tell us about contamination and what are the ecological consequences when they flee from an environment? Toxics 8, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce, A. N. , David, T. I. , Randall, E. L. , Ramos Rodrigues, A. , Colgan, T. J. , Wurm, Y. & Gill, R. J. (2017). Impact of controlled neonicotinoid exposure on bumblebees in a realistic field setting. Journal of Applied Ecology 54, 1199–1208. [Google Scholar]
- Armstrong, T. S. , Khursigara, A. J. , Killen, S. S. , Fearnley, H. , Parsons, K. J. & Esbaugh, A. J. (2019). Oil exposure alters social group cohesion in fish. Scientific Reports 9, 13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Asadpour, M. , Tâche, F. , Caprari, G. , Karlen, W. & Siegwart, R. (2006). Robot‐animal interaction: perception and behavior of InsBot. International Journal of Advanced Robotic Systems 3, 93–98. [Google Scholar]
- Aulsebrook, L. C. , Bertram, M. G. , Martin, J. M. , Aulsebrook, A. E. , Brodin, T. , Evans, J. P. , Hall, M. D. , O'Bryan, M. K. , Pask, A. J. , Tyler, C. R. & Wong, B. B. M. (2020). Reproduction in a polluted world: implications for wildlife. Reproduction 160, R13–R23. [DOI] [PubMed] [Google Scholar]
- * Baktoft, H. , Gjelland, K. Ø. , Økland, F. , Rehage, J. S. , Rodemann, J. R. , Corujo, R. S. , Viadero, N. & Thygesen, U. H. (2017). Positioning of aquatic animals based on time‐of‐arrival and random walk models using YAPS (Yet Another Positioning Solver). Scientific Reports 7, 1–10. 10.1038/s41598-017-14278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Banner, K. M. , Irvine, K. M. & Rodhouse, T. (2020). The use of Bayesian priors in ecology: the good, the bad and the not great. Methods in Ecology and Evolution 11, 882–889. [Google Scholar]
- * Bartoń, K. (2020). MuMIn: multi‐model inference. R package version 1(43), 17. [Google Scholar]
- * Bates, D. , Machler, M. , Bolker, B. M. & Walker, S. C. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software 67, 1–48. [Google Scholar]
- *BehaviorCloud (in press). BehaviorCloud User Guide. Electronic file available at https://behaviorcloud.com/guide.html Accessed 10.07.2021.
- Bell, A. M. & Hellmann, J. K. (2019). An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. Annual Review of Ecology, Evolution, and Systematics 50, 97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt, E. S. , Rosi, E. J. & Gessner, M. O. (2017). Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment 15, 84–90. [Google Scholar]
- Bertram, M. G. , Martin, J. M. , Wong, B. B. M. & Brodin, T. (2022). Micropollutants. Current Biology 32, R17–R19. [DOI] [PubMed] [Google Scholar]
- * Beyan, C. & Browman, H. I. (2020). Setting the stage for the machine intelligence era in marine science. ICES Journal of Marine Science 77, 1267–1273. [Google Scholar]
- Biro, P. A. & Stamps, J. A. (2010). Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends in Ecology & Evolution 25, 653–659. [DOI] [PubMed] [Google Scholar]
- Blanken, L. J. , van Langevelde, F. & van Dooremalen, C. (2015). Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proceedings of the Royal Society B: Biological Sciences 282, 20151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Blumstein, D. T. & Daniel, J. C. (2007). Quantifying Behavior the JWatcher Way. Sinauer Associates Inc, Sunderland, MA. [Google Scholar]
- * Bolger, D. T. , Morrison, T. A. , Vance, B. , Lee, D. & Farid, H. (2012). A computer‐assisted system for photographic mark–recapture analysis. Methods in Ecology and Evolution 3, 813–822. [Google Scholar]
- Bolker, B. M. , Brooks, M. E. , Clark, C. J. , Geange, S. W. , Poulsen, J. R. , Stevens, M. H. H. & White, J. S. S. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology & Evolution 24, 127–135. [DOI] [PubMed] [Google Scholar]
- * Bonter, D. N. & Bridge, E. S. (2011). Applications of radio frequency identification (RFID) in ornithological research: a review. Journal of Field Ornithology 82, 1–10. [Google Scholar]
- Bownik, A. & Wlodkowic, D. (2021). Applications of advanced neuro‐behavioral analysis strategies in aquatic ecotoxicology. Science of the Total Environment 772, 145577. [DOI] [PubMed] [Google Scholar]
- Brack, W. , Aissa, S. A. , Backhaus, T. , Dulio, V. , Escher, B. I. , Faust, M. , Hilscherova, K. , Hollender, J. , Hollert, H. , Müller, C. , Munthe, J. , Posthuma, L. , Seiler, T.‐B. , Slobodnik, J. , Teodorovic, I. , et al. (2019a). Effect‐based methods are key. The European collaborative project SOLUTIONS recommends integrating effect‐based methods for diagnosis and monitoring of water quality. Environmental Sciences Europe 31, 10. [Google Scholar]
- Brack, W. , Hollender, J. , de Alda, M. L. , Müller, C. , Schulze, T. , Schymanski, E. , Slobodnik, J. & Krauss, M. (2019b). High‐resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources. Environmental Sciences Europe 31, 62. [Google Scholar]
- * Branson, K. , Robie, A. A. , Bender, J. , Perona, P. & Dickinson, M. H. (2009). High‐throughput ethomics in large groups of drosophila . Nature Methods 6, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Brooks, M. E. , Kristensen, K. , van Benthem, K. J. , Magnusson, A. , Berg, C. W. , Nielsen, A. , Skaug, H. J. , Mächler, M. & Bolker, B. M. (2017). glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R Journal 9, 378–400. [Google Scholar]
- Brown, A. R. , Owen, S. F. , Peters, J. , Zhang, Y. , Soffker, M. , Paull, G. C. , Hosken, D. J. , Wahab, M. A. & Tyler, C. R. (2015). Climate change and pollution speed declines in zebrafish populations. Proceedings of the National Academy of Sciences of the United States of America 112, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Browning, E. , Bolton, M. , Owen, E. , Shoji, A. , Guilford, T. & Freeman, R. (2018). Predicting animal behaviour using deep learning: GPS data alone accurately predict diving in seabirds. Methods in Ecology and Evolution 9, 681–692. [Google Scholar]
- * Bruneel, S. , Verhelst, P. , Reubens, J. , Baetens, J. M. , Coeck, J. , Moens, T. & Goethals, P. (2020). Quantifying and reducing epistemic uncertainty of passive acoustic telemetry data from longitudinal aquatic systems. Ecological Informatics 59, 101133. [Google Scholar]
- * Bürkner, P.‐C. (2017). Brms: an R package for Bayesian multilevel models using stan. Journal of Statistical Software 80, 1–28. [Google Scholar]
- * Bürkner, P.‐C. (2018). Advanced Bayesian multilevel modeling with the R package brms. R Journal 10, 395–411. [Google Scholar]
- * Burnham, K. P. , Anderson, D. R. & Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65, 23–35. [Google Scholar]
- Campana, O. & Wlodkowic, D. (2018). Ecotoxicology goes on a chip: embracing miniaturized bioanalysis in aquatic risk assessment. Environmental Science & Technology 52, 932–946. [DOI] [PubMed] [Google Scholar]
- * Campbell, H. A. , Watts, M. E. , Dwyer, R. G. & Franklin, C. E. (2012). V‐track: software for analysing and visualising animal movement from acoustic telemetry detections. Marine and Freshwater Research 63, 815–820. [Google Scholar]
- * Caravaggi, A. , Banks, P. B. , Burton, A. C. , Finlay, C. M. , Haswell, P. M. , Hayward, M. W. , Rowcliffe, M. J. & Wood, M. D. (2017). A review of camera trapping for conservation behaviour research. Remote Sensing in Ecology and Conservation 3, 109–122. [Google Scholar]
- Careau, V. , Thomas, D. , Humphries, M. & Réale, D. (2008). Energy metabolism and animal personality. Oikos 117, 641–653. [Google Scholar]
- * Cartlidge, R. , Nugegoda, D. & Wlodkowic, D. (2015). GammarusChip: innovative lab‐on‐a‐chip technology for ecotoxicological testing using the marine amphipod Allorchestes compressa . Proceedings of SPIE, Bio‐MEMS and Medical Microdevices II 9518, 951812. [Google Scholar]
- Chapman, P. F. , Crane, M. , Wiles, J. , Noppert, F. & McIndoe, E. (1996). Improving the quality of statistics in regulatory ecotoxicity tests. Ecotoxicology 5, 169–186. [DOI] [PubMed] [Google Scholar]
- * Chen, J. , Xu, H. , Wu, J. , Yue, R. , Yuan, C. & Wang, L. (2019). Deer crossing road detection with roadside LiDAR sensor. IEEE Access 7, 65944–65954. [Google Scholar]
- * Cheng, Y. , Fiedler, W. , Wikelski, M. & Flack, A. (2019). “Closer‐to‐home” strategy benefits juvenile survival in a long‐distance migratory bird. Ecology and Evolution 9, 8945–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Christin, S. , Hervet, É. & Lecomte, N. (2019). Applications for deep learning in ecology. Methods in Ecology and Evolution 10, 1632–1644. [Google Scholar]
- * Cleasby, I. R. , Nakagawa, S. , Schielzeth, H. & Hadfield, J. (2015). Quantifying the predictability of behaviour: statistical approaches for the study of between‐individual variation in the within‐individual variance. Methods in Ecology and Evolution 6, 27–37. [Google Scholar]
- Colin, T. , Meikle, W. G. , Wu, X. & Barron, A. B. (2019). Traces of a neonicotinoid induce precocious foraging and reduce foraging performance in honey bees. Environmental Science & Technology 53, 8252–8261. [DOI] [PubMed] [Google Scholar]
- * Colin, T. , Forster, C. C. , Westacott, J. , Wu, X. , Meikle, W. G. & Barron, A. B. (2021). Effects of late miticide treatments on foraging and colony productivity of European honey bees (Apis mellifera). Apidologie 52, 1–19. [Google Scholar]
- * Colot, A. , Caprari, G. & Siegwart, R. (2004). InsBot: design of an autonomous mini mobile robot able to interact with cockroaches. IEEE International Conference on Robotics and Automation 2418–2423.
- Cook, B. , Haverkamp, A. , Hansson, B. S. , Roulston, T. , Lerdau, M. & Knaden, M. (2020). Pollination in the Anthropocene: a moth can learn ozone‐altered floral blends. Journal of Chemical Ecology 46, 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Cooke, S. J. , Midwood, J. D. , Thiem, J. D. , Klimley, P. , Lucas, M. C. , Thorstad, E. B. , Eiler, J. , Holbrook, C. & Ebner, B. C. (2013). Tracking animals in freshwater with electronic tags: past, present and future. Animal Biotelemetry 1, 5. [Google Scholar]
- * Crall, J. D. , Gravish, N. , Mountcastle, A. M. & Combes, S. A. (2015). BEEtag: a low‐cost, image‐based tracking system for the study of animal behavior and locomotion. PLoS One 10, e0136487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crall, J. D. , Switzer, C. M. , Oppenheimer, R. L. , Versypt, A. N. , Dey, B. , Brown, A. , Eyster, M. , Guérin, C. , Pierce, N. E. , Combes, S. A. & de Bivort, B. L. (2018). Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362, 683–686. [DOI] [PubMed] [Google Scholar]
- * Crispim Junior, C. F. , Pederiva, C. N. , Bose, R. C. , Garcia, V. A. , Lino‐de‐Oliveira, C. & Marino‐Neto, J. (2012). ETHOWATCHER: validation of a tool for behavioral and video‐tracking analysis in laboratory animals. Computers in Biology and Medicine 42, 257–264. [DOI] [PubMed] [Google Scholar]
- * Dalgleish, F. , Ouyang, B. , Vuorenkoski, A. , Ramos, B. , Alsenas, G. , Metzger, B. , Cao, Z. & Principe, J. (2017). Undersea lidar imager for unobtrusive and eye safe marine wildlife detection and classification. OCEANS 2017‐Aberdeen 1–5.
- * Dammhahn, M. , Dingemanse, N. J. , Niemelä, P. T. & Réale, D. (2018). Pace‐of‐life syndromes: a framework for the adaptive integration of behaviour, physiology and life history. Behavioral Ecology and Sociobiology 72, 62–70. [Google Scholar]
- Debecker, S. , Sanmartin‐Villar, I. , De Guinea‐Luengo, M. , Cordero‐Rivera, A. & Stoks, R. (2016). Integrating the pace‐of‐life syndrome across species, sexes and individuals: covariation of life history and personality under pesticide exposure. Journal of Animal Ecology 85, 726–738. [DOI] [PubMed] [Google Scholar]
- Debecker, S. & Stoks, R. (2019). Pace of life syndrome under warming and pollution: integrating life history, behavior, and physiology across latitudes. Ecological Monographs 89, e01332. [Google Scholar]
- Delnat, V. , Tran, T. T. , Janssens, L. & Stoks, R. (2019). Daily temperature variation magnifies the toxicity of a mixture consisting of a chemical pesticide and a biopesticide in a vector mosquito. Science of the Total Environment 659, 33–40. [DOI] [PubMed] [Google Scholar]
- * Dingemanse, N. J. , Kazem, A. J. N. , Réale, D. & Wright, J. (2010). Behavioural reaction norms: animal personality meets individual plasticity. Trends in Ecology & Evolution 25, 81–89. [DOI] [PubMed] [Google Scholar]
- Dingemanse, N. J. & Dochtermann, N. A. (2013). Quantifying individual variation in behaviour: mixed‐effect modelling approaches. Journal of Animal Ecology 82, 39–54. [DOI] [PubMed] [Google Scholar]
- * Ditria, E. M. , Lopez‐Marcano, S. , Sievers, M. , Jinks, E. L. , Brown, C. J. & Connolly, R. M. (2020). Automating the analysis of fish abundance using object detection: optimizing animal ecology with deep learning. Frontiers in Marine Science 7, 429. [Google Scholar]
- Dochtermann, N. A. , Schwab, T. & Sih, A. (2015). The contribution of additive genetic variation to personality variation: heritability of personality. Proceedings of the Royal Society B: Biological Sciences 282, 20142201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Donaldson, M. R. , Hinch, S. G. , Suski, C. D. , Fisk, A. T. , Heupel, M. R. & Cooke, S. J. (2014). Making connections in aquatic ecosystems with acoustic telemetry monitoring. Frontiers in Ecology and the Environment 12, 565–573. [Google Scholar]
- Donelan, S. C. , Hellmann, J. K. , Bell, A. M. , Luttbeg, B. , Orrock, J. L. , Sheriff, M. J. & Sih, A. (2020). Transgenerational plasticity in human‐altered environments. Trends in Ecology & Evolution 35, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson, J. M. , Sunday, J. M. , Figueira, W. F. , Gaitán‐Espitia, J. D. , Hobday, A. J. , Johnson, C. R. , Leis, J. M. , Ling, S. D. , Marshall, D. , Pandolfi, J. M. , Pecl, G. , Rodgers, G. G. , Booth, D. J. & Munday, P. L. (2019). Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences 374, 20180186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Dujon, A. M. , Lindstrom, R. T. & Hays, G. C. (2014). The accuracy of Fastloc‐GPS locations and implications for animal tracking. Methods in Ecology and Evolution 5, 1162–1169. [Google Scholar]
- * Dunn, T. W. , Marshall, J. D. , Severson, K. S. , Aldarondo, D. E. , Hildebrand, D. G. C. , Chettih, S. N. , Wang, W. L. , Gellis, A. J. , Carlson, D. E. , Aronov, D. , Freiwald, W. A. , Wang, F. & Ölveczky, B. P. (2021). Geometric deep learning enables 3D kinematic profiling across species and environments. Nature Methods 18, 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng, M. L. , Stutchbury, B. J. M. & Morrissey, C. A. (2019). A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science 365, 1177–1180. [DOI] [PubMed] [Google Scholar]
- EPA (United States Environmental Protection Agency) (2021a). Clean Water Act (CWA). Electronic file available at https://www.epa.gov/laws-regulations/summary-clean-water-act. Accessed 01.03.2021.
- EPA (United States Environmental Protection Agency) (2021b). National contaminant occurrence database (NCOD). Electronic file available at www.epa.gov/sdwa/national‐contaminant‐occurrence‐database‐ncod.
- Escher, B. I. , Hackermüller, J. , Polte, T. , Scholz, S. , Aigner, A. , Altenburger, R. , Böhme, A. , Bopp, S. K. , Brack, W. , Busch, W. , Chadeau‐Hyam, M. , Covaci, A. , Eisenträger, A. , Galligan, J. J. , Garcia‐Reyero, N. , et al. (2017). From the exposome to mechanistic understanding of chemical‐induced adverse effects. Environment International 99, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Union (2013). Directive 2013/39/EU of the European Parliament and the council of 12. August 2013 amending directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official Journal of the European Union 226(1), 1–17. [Google Scholar]
- Faria, M. , Bedrossiantz, J. , Ramírez, J. R. , Mayol, M. , García, G. H. , Bellot, M. , Prats, E. , Garcia‐Reyero, N. , Gómez‐Canela, C. , Gómez‐Oliván, L. M. & Raldúa, D. (2021). Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environment International 146, 106253. [DOI] [PubMed] [Google Scholar]
- Fernandez, M. A. M. , André, L. C. & Cardeal, Z. L. (2020). Perspectives of biological analysis in Latin America using multi and comprehensive two‐dimensional gas chromatography: a mini‐review. Chromatographia 83, 1045–1053. [Google Scholar]
- Fisher, D. N. , Kilgour, R. J. , Siracusa, E. R. , Foote, J. R. , Hobson, E. A. , Montiglio, P. O. , Saltz, J. B. , Wey, T. W. & Wice, E. W. (2021). Anticipated effects of abiotic environmental change on intraspecific social interactions. Biological Reviews 96, 2661–2693. [DOI] [PubMed] [Google Scholar]
- Fleeger, J. W. (2020). How do indirect effects of contaminants inform ecotoxicology? A review. Processes 8, 1659. [Google Scholar]
- Fonseca, V. F. , Reis‐Santos, P. , Duarte, B. , Cabral, H. N. , Caçador, M. I. , Vaz, N. , Dias, J. M. & Pais, M. P. (2020). Roving pharmacies: modelling the dispersion of pharmaceutical contamination in estuaries. Ecological Indicators 115, 106437. [Google Scholar]
- Ford, A. T. , Ågerstrand, M. , Brooks, B. W. , Allen, J. , Bertram, M. G. , Brodin, T. , Dang, Z. , Duquesne, S. , Sahm, R. , Hoffmann, F. , Hollert, H. , Jacob, S. , Klüver, N. , Lazorchak, J. , Ledesma, M. , et al. (2021). The role of behavioral ecotoxicology in environmental protection. Environmental Science & Technology 55, 5620–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Fort, J. , Robertson, G. J. , Grémillet, D. , Traisnel, G. & Bustamante, P. (2014). Spatial ecotoxicology: migratory Arctic seabirds are exposed to mercury contamination while overwintering in the Northwest Atlantic. Environmental Science & Technology 48, 11560–11567. [DOI] [PubMed] [Google Scholar]
- * Francisco, F. A. , Nührenberg, P. & Jordan, A. L. (2020). High‐resolution, non‐invasive animal tracking and reconstruction of local environment in aquatic ecosystems. Movement Ecology 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Friard, O. & Gamba, M. (2016). BORIS: a free, versatile open‐source event‐logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330. [Google Scholar]
- * Gallois, B. & Candelier, R. (2021). FastTrack: an open‐source software for tracking varying numbers of deformable objects. PLoS Computational Biology 17, e1008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galus, M. , Rangarajan, S. , Lai, A. , Shaya, L. , Balshine, S. & Wilson, J. Y. (2014). Effects of chronic, parental pharmaceutical exposure on zebrafish (Danio rerio) offspring. Aquatic Toxicology 151, 124–134. [DOI] [PubMed] [Google Scholar]
- Gartland, L. A. , Firth, J. A. , Laskowski, K. L. , Jeanson, R. & Ioannou, C. C. (2021). Sociability as a personality trait in animals: methods, causes and consequences. Biological Reviews, 10.1111/brv.12823 [DOI] [PubMed] [Google Scholar]
- Gaynor, K. M. , Brown, J. S. , Middleton, A. D. , Power, M. E. & Brashares, J. S. (2019). Landscapes of fear: spatial patterns of risk perception and response. Trends in Ecology & Evolution 34, 355–368. [DOI] [PubMed] [Google Scholar]
- * Gibb, R. , Browning, E. , Glover‐Kapfer, P. & Jones, K. E. (2019). Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods in Ecology and Evolution 10, 169–185. [Google Scholar]
- Gil, M. A. , Hein, A. M. , Spiegel, O. , Baskett, M. L. & Sih, A. (2018). Social information links individual behavior to population and community dynamics. Trends in Ecology & Evolution 33, 535–548. [DOI] [PubMed] [Google Scholar]
- * Glover‐Kapfer, P. , Soto‐Navarro, C. A. & Wearn, O. R. (2019). Camera‐trapping version 3.0: current constraints and future priorities for development. Remote Sensing in Ecology and Conservation 5, 209–223. [Google Scholar]
- Gore, A. C. , Chappell, V. A. , Fenton, S. E. , Flaws, J. A. , Nadal, A. , Prins, G. S. , Toppari, J. & Zoeller, R. T. (2015). EDC‐2: the Endocrine Society's second scientific statement on endocrine‐disrupting chemicals. Endocrine Reviews 36, 1–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graving, J. M. , Chae, D. , Naik, H. , Li, L. , Koger, B. , Costelloe, B. R. & Couzin, I. D. (2019). DeepPoseKit, a software toolkit for fast and robust animal pose estimation using deep learning. eLife 8, e47994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, A. S. , Guillette, L. M. & Healy, S. D. (2015). Cognition and personality: an analysis of an emerging field. Trends in Ecology & Evolution 30, 207–214. [DOI] [PubMed] [Google Scholar]
- * Griffin, L. P. , Smith, B. J. , Cherkiss, M. S. , Crowder, A. G. , Pollock, C. G. , Hillis‐Starr, Z. , Danylchuk, A. J. & Hart, K. M. (2020). Space use and relative habitat selection for immature green turtles within a Caribbean marine protected area. Animal Biotelemetry 8, 1–13. [Google Scholar]
- Groh, K. , Vom Berg, C. , Schirmer, K. & Tlili, A. (2022). Anthropogenic chemicals as underestimated drivers of biodiversity loss: scientific and societal implications. Environmental Science & Technology 56, 707–710. [DOI] [PubMed] [Google Scholar]
- Groothuis, T. G. G. & Taborsky, B. (2015). Introducing biological realism into the study of developmental plasticity in behaviour. Frontiers in Zoology 12, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Grueber, C. E. , Nakagawa, S. , Laws, R. J. & Jamieson, I. G. (2011). Multimodel inference in ecology and evolution: challenges and solutions. Journal of Evolutionary Biology 24, 699–711. [DOI] [PubMed] [Google Scholar]
- Grunst, A. S. , Grunst, M. L. , Daem, N. , Pinxten, R. , Bervoets, L. & Eens, M. (2019). An important personality trait varies with blood and plumage metal concentrations in a free‐living songbird. Environmental Science & Technology 53, 10487–10496. [DOI] [PubMed] [Google Scholar]
- Guiraud, M. , Roper, M. & Chittka, L. (2018). High‐speed videography reveals how honeybees can turn a spatial concept learning task into a simple discrimination task by stereotyped flight movements and sequential inspection of pattern elements. Frontiers in Psychology 9, 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson, L. , Snape, J. R. , Verbruggen, B. , Owen, S. F. , Kristiansson, E. , Margiotta‐Casaluci, L. , Österlund, T. , Hutchinson, K. , Leverett, D. , Marks, B. & Tyler, C. R. (2019). Pharmacology beyond the patient – the environmental risks of human drugs. Environment International 129, 320–332. [DOI] [PubMed] [Google Scholar]
- * Hadfield, J. D. (2010). MCMC methods for multi‐response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software 33, 1–22. [PMC free article] [PubMed] [Google Scholar]
- * Hadfield, J. D. (2021). Package ‘MCMCglmm’. R package version 2.32.
- Hale, R. , Piggott, J. J. & Swearer, S. E. (2017). Describing and understanding behavioral responses to multiple stressors and multiple stimuli. Ecology and Evolution 7, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfwerk, W. & Slabbekoorn, H. (2015). Pollution going multimodal: the complex impact of the human‐altered sensory environment on animal perception and performance. Biology Letters 11, 20141051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Halloy, J. , Sempo, G. , Caprari, G. , Rivault, C. , Asadpour, M. , Tâche, F. , Said, I. , Durier, V. , Canonge, S. , Amé, J. M. , Detrain, C. , Correll, N. , Martinoli, A. , Mondada, F. , Siegwart, R. & Deneubourg, J. L. (2007). Social integration of robots into groups of cockroaches to control self‐organized choices. Science 318, 1155–1158. [DOI] [PubMed] [Google Scholar]
- Hamilton, T. J. , Krook, J. , Szaskiewicz, J. & Burggren, W. (2021). Shoaling, boldness, anxiety‐like behavior and locomotion in zebrafish (Danio rerio) are altered by acute benzo[a]pyrene exposure. Science of the Total Environment 774, 145702. [DOI] [PubMed] [Google Scholar]
- Harris, C. A. , Scott, A. P. , Johnson, A. C. , Panter, G. H. , Sheahan, D. , Roberts, M. & Sumpter, J. P. (2014). Principles of sound ecotoxicology. Environmental Science & Technology 48, 3100–3111. [DOI] [PubMed] [Google Scholar]
- * Harrison, X. A. , Donaldson, L. , Correa‐Cano, M. E. , Evans, J. , Fisher, D. N. , Goodwin, C. E. , Robinson, B. S. , Hodgson, D. J. & Inger, R. (2018). A brief introduction to mixed effects modelling and multi‐model inference in ecology. PeerJ 6, e4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Haulsee, D. E. , Fox, D. A. , Breece, M. W. , Clauss, T. M. & Oliver, M. J. (2016). Implantation and recovery of long‐term archival transceivers in a migratory shark with high site fidelity. PLoS One 11, e0148617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden, M. T. , Reeves, M. K. , Holyoak, M. , Perdue, M. , King, A. L. & Tobin, S. C. (2015). Thrice as easy to catch! Copper and temperature modulate predator‐prey interactions in larval dragonflies and anurans. Ecosphere 6, 1–7. [Google Scholar]
- Hayman, N. T. , Hentschel, B. T. , Renick, V. C. & Anderson, T. W. (2019). Combined effects of flow speed and sub‐lethal insecticide exposure on predator–prey interactions between the California killifish and an infaunal polychaete. Ecotoxicology 28, 117–131. [DOI] [PubMed] [Google Scholar]
- * Hays, G. C. , Ferreira, L. C. , Sequeira, A. M. , Meekan, M. G. , Duarte, C. M. , Bailey, H. , Bailleul, F. , Bowen, W. D. , Caley, M. J. , Costa, D. P. & Eguíluz, V. M. (2016). Key questions in marine megafauna movement ecology. Trends in Ecology & Evolution 31, 463–475. [DOI] [PubMed] [Google Scholar]
- * Hebert, L. , Ahamed, T. , Costa, A. C. , O'Shaughnessy, L. & Stephens, G. J. (2021). WormPose: image synthesis and convolutional networks for pose estimation in C. elegans . PLoS Computational Biology 17, e1008914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström, G. , Klaminder, J. , Finn, F. , Persson, L. , Alanärä, A. , Jonsson, M. , Fick, J. & Brodin, T. (2016a). GABAergic anxiolytic drug in water increases migration behaviour in salmon. Nature Communications 7, 13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström, G. , Klaminder, J. , Jonsson, M. , Fick, J. & Brodin, T. (2016b). Upscaling behavioural studies to the field using acoustic telemetry. Aquatic Toxicology 170, 384–389. [DOI] [PubMed] [Google Scholar]
- Henry, M. , Cerrutti, N. , Aupinel, P. , Decourtye, A. , Gayrard, M. , Odoux, J.‐F. , Pissard, A. , Rüger, C. & Bretagnolle, V. (2015). Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proceedings of the Royal Society B: Biological Sciences 282, 20152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. & Wlodkowic, D. (2020). High‐throughput animal tracking in chemobehavioral phenotyping: current limitations and future perspectives. Behavioural Processes 180, 104226. [DOI] [PubMed] [Google Scholar]
- Hertel, A. G. , Niemelä, P. T. , Dingemanse, N. J. , Mueller, T. (2020) A guide for studying among‐individual behavioral variation from movement data in the wild. Movement Ecology, 8(1), 10.1186/s40462-020-00216-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hill, A. P. , Prince, P. , Piña Covarrubias, E. , Doncaster, C. P. , Snaddon, J. L. & Rogers, A. (2018). AudioMoth: evaluation of a smart open acoustic device for monitoring biodiversity and the environment. Methods in Ecology and Evolution 9, 1199–1211. [Google Scholar]
- * Hodgson, J. C. , Mott, R. , Baylis, S. M. , Pham, T. T. , Wotherspoon, S. , Kilpatrick, A. D. , Raja Segaran, R. , Reid, I. , Terauds, A. & Koh, L. P. (2018). Drones count wildlife more accurately and precisely than humans. Methods in Ecology and Evolution 9, 1160–1167. [Google Scholar]
- Hollender, J. , Schymanski, E. L. , Singer, H. P. & Ferguson, P. L. (2017). Nontarget screening with high resolution mass spectrometry in the environment: ready to go? Environmental Science & Technology 51, 11505–11512. [DOI] [PubMed] [Google Scholar]
- Horký, P. , Grabic, R. , Grabicová, K. , Brooks, B. W. , Douda, K. , Slavík, O. , Hubená, P. , Sancho Santos, E. M. & Randák, T. (2021). Methamphetamine pollution elicits addiction in wild fish. Journal of Experimental Biology 224, jeb242145. [DOI] [PubMed] [Google Scholar]
- * Houslay, T. & Wilson, A. (2017). Avoiding the misuse of BLUP in behavioral ecology. Behavioral Ecology 28, 948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hussey, N. E. , Kessel, S. T. , Aarestrup, K. , Cooke, S. J. , Cowley, P. D. , Fisk, A. T. , Harcourt, R. G. , Holland, K. N. , Iverson, S. J. , Kocik, J. F. & Flemming, J. E. M. (2015). Aquatic animal telemetry: a panoramic window into the underwater world. Science 348, 1255642. [DOI] [PubMed] [Google Scholar]
- * Jackson, B. E. , Evangelista, D. J. , Ray, D. D. & Hedrick, T. L. (2016). 3D for the people: multi‐camera motion capture in the field with consumer‐grade cameras and open source software. Biology Open 5, 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin, L. , Petitjean, Q. , Côte, J. , Laffaille, P. & Jean, S. (2020). Effects of pollution on fish behavior, personality, and cognition: some research perspectives. Frontiers in Ecology and Evolution 8, 86. [Google Scholar]
- James, K. L. , Randall, N. P. & Haddaway, N. R. (2016). A methodology for systematic mapping in environmental sciences. Environmental Evidence 5, 7. [Google Scholar]
- * Javer, A. , Currie, M. , Lee, C. W. , Hokanson, J. , Li, K. , Martineau, C. N. , Yemini, E. , Grundy, L. J. , Li, C. , Ch'ng, Q. , Schafer, W. R. , Nollen, E. A. A. , Kerr, R. & Brown, A. E. X. (2018). An open‐source platform for analyzing and sharing worm‐behavior data. Nature Methods 15, 645–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, G. & Li, X. (2020). A New Paradigm for Environmental Chemistry and Toxicology: From Concepts to Insights. Springer, Singapore. [Google Scholar]
- Johansen, J. L. , Allan, B. J. M. , Rummer, J. L. & Esbaugh, A. J. (2017). Oil exposure disrupts early life‐history stages of coral reef fishes via behavioural impairments. Nature Ecology & Evolution 1, 1146–1152. [DOI] [PubMed] [Google Scholar]
- * Jolles, J. W. (2020). Pirecorder: controlled and automated image and video recording with the raspberry pi. Journal of Open Source Software 5, 2584. [Google Scholar]
- * Jolles, J. W. (2021). Broad‐scale applications of the raspberry pi: a review and guide for biologists. Methods in Ecology and Evolution 12, 1562–1579. [Google Scholar]
- Jonsson, M. , Andersson, M. , Fick, J. , Brodin, T. , Klaminder, J. & Piovano, S. (2019). High‐speed imaging reveals how antihistamine exposure affects escape behaviours in aquatic insect prey. Science of the Total Environment 648, 1257–1262. [DOI] [PubMed] [Google Scholar]
- * Joo, R. , Boone, M. E. , Clay, T. A. , Patrick, S. C. , Clusella‐Trullas, S. & Basille, M. (2020). Navigating through the R packages for movement. Journal of Animal Ecology 89, 248–267. [DOI] [PubMed] [Google Scholar]
- Junges, C. M. , Peltzer, P. M. , Lajmanovich, R. C. , Attademo, A. M. , Zenklusen, M. C. & Basso, A. (2012). Toxicity of the fungicide trifloxystrobin on tadpoles and its effect on fish–tadpole interaction. Chemosphere 87, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Jutfelt, F. , Sundin, J. , Raby, G. D. , Krang, A. S. & Clark, T. D. (2017). Two‐current choice flumes for testing avoidance and preference in aquatic animals. Methods in Ecology and Evolution 8, 379–390. [Google Scholar]
- * Kain, M. P. , Bolker, B. M. & McCoy, M. (2015). A practical guide and power analysis for GLMMs: detecting among treatment variation in random effects. PeerJ 3, e1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kass, R. E. , Caffo, B. S. , Davidian, M. , Meng, X. L. , Yu, B. & Reid, N. (2016). Ten simple rules for effective statistical practice. PLoS Computational Biology 12, e1004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kays, R. , Crofoot, M. C. , Jetz, W. & Wikelski, M. (2015). Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478. [DOI] [PubMed] [Google Scholar]
- * Kissling, W. D. , Pattemore, D. E. & Hagen, M. (2014). Challenges and prospects in the telemetry of insects. Biological Reviews 89, 511–530. [DOI] [PubMed] [Google Scholar]
- Klaminder, J. , Hellström, G. , Fahlman, J. , Jonsson, M. , Fick, J. , Lagesson, A. , Bergman, E. & Brodin, T. (2016). Drug‐induced behavioral changes: using laboratory observations to predict field observations. Frontiers in Environmental Science 4, 81. [Google Scholar]
- * Kopman, V. & Porfiri, M. (2013). Design, modeling, and characterization of a miniature robotic fish for research and education in biomimetics and bioinspiration. IEEE/ASME Transactions on Mechatronics 18, 471–483. [Google Scholar]
- * Kopman, V. , Laut, J. , Polverino, G. & Porfiri, M. (2013). Closed‐loop control of zebrafish response using a bioinspired robotic‐fish in a preference test. Journal of the Royal Society Interface 10, 20120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kuznetsova, A. , Brockhoff, P. B. & Christensen, R. H. B. (2013). lmerTest: tests for random and fixed effects for linear mixed effect models. R package version 2–6.
- Lagesson, A. , Saaristo, M. , Brodin, T. , Fick, J. , Klaminder, J. , Martin, J. M. & Wong, B. B. M. (2019). Fish on steroids: temperature‐dependent effects of 17β‐trenbolone on predator escape, boldness, and exploratory behaviors. Environmental Pollution 245, 243–252. [DOI] [PubMed] [Google Scholar]
- LaLone, C. A. , Villeneuve, D. L. , Lyons, D. , Helgen, H. W. , Robinson, S. L. , Swintek, J. A. , Saari, T. W. & Ankley, G. T. (2016). Sequence alignment to predict across species susceptibility (SeqAPASS): a web‐based tool for addressing the challenges of species extrapolation of chemical toxicity. Toxicological Sciences 153, 228–245. [DOI] [PubMed] [Google Scholar]
- Landrigan, P. J. , Fuller, R. , Acosta, N. J. , Adeyi, O. , Arnold, R. , Baldé, A. B. , Bertollini, R. , Bose‐O'Reilly, S. , Boufford, J. I. , Breysse, P. N. , Chiles, T. , Mahidol, C. , Coll‐Seck, A. M. , Cropper, M. L. , Fobil, J. , et al. (2018). The lancet commission on pollution and health. Lancet 391, 462–512. [DOI] [PubMed] [Google Scholar]
- * Lennox, R. J. , Aarestrup, K. , Cooke, S. J. , Cowley, P. D. , Deng, Z. D. , Fisk, A. T. , Harcourt, R. G. , Heupel, M. , Hinch, S. G. , Holland, K. N. & Hussey, N. E. (2017). Envisioning the future of aquatic animal tracking: technology, science, and application. Bioscience 67, 884–896. [Google Scholar]
- * Lin, Y. , Hsiung, J. , Piersall, R. , White, C. , Lowe, C. G. & Clark, C. M. (2017). A multi‐autonomous underwater vehicle system for autonomous tracking of marine life. Journal of Field Robotics 34, 757–774. [Google Scholar]
- *Loligo‐Systems (in press). Quick guide for LoliTrack. 5, 1–4. [Google Scholar]
- * Lüdecke, D. , Ben‐Shachar, M. S. , Patil, I. , Waggoner, P. & Makowski, D. (2021). Performance: an R package for assessment, comparison and testing of statistical models. Journal of Open Source Software 6, 3139. [Google Scholar]
- * Lyons, M. B. , Brandis, K. J. , Murray, N. J. , Wilshire, J. H. , McCann, J. A. , Kingsford, R. T. & Callaghan, C. T. (2019). Monitoring large and complex wildlife aggregations with drones. Methods in Ecology and Evolution 10, 1024–1035. [Google Scholar]
- * Magnusson, A. , Skaug, H. , Nielsen, A. , Berg, C. , Kristensen, K. , Maechler, M. , Van Bentham, K. , Bolker, B. , Brooks, M. & Brooks, M. M. (2017). Package ‘glmmTMB’. R package version 0.2.0.
- Macrì, S. , Neri, D. , Ruberto, T. , Mwaffo, V. , Butail, S. & Porfiri, M. (2017). Three‐dimensional scoring of zebrafish behaviour unveils biological phenomena hidden by two‐dimensional analyses. Scientific Reports 7, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marentette, J. R. , Tong, S. , Wang, G. , Sopinka, N. M. , Taves, M. D. , Koops, M. A. & Balshine, S. (2012). Behavior as biomarker? Laboratory versus field movement in round goby (Neogobius melanostomus) from highly contaminated habitats. Ecotoxicology 21, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Martin, J. M. , Saaristo, M. , Bertram, M. G. , Lewis, P. J. , Coggan, T. L. , Clarke, B. O. & Wong, B. B. M. (2017). The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish. Environmental Pollution 222, 592–599. [DOI] [PubMed] [Google Scholar]
- Martin, J. M. , Saaristo, M. , Tan, H. , Bertram, M. G. , Nagarajan‐Radha, V. , Dowling, D. K. & Wong, B. B. M. (2019). Field‐realistic antidepressant exposure disrupts group foraging dynamics in mosquitofish. Biology Letters 15, 20190615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J. M. & McCallum, E. S. (2021). Incorporating animal social context in ecotoxicology: can a single individual tell the collective story? Environmental Science & Technology 55, 10908–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, R. T. , Martin, J. M. , Tan, H. , Brand, J. A. , Bertram, M. G. , Tingley, R. , Todd‐Weckmann, A. & Wong, B. B. M. (2021). Context is key: social environment mediates the impacts of a psychoactive pollutant on shoaling behavior in fish. Environmental Science & Technology 55, 13024–13032. [DOI] [PubMed] [Google Scholar]
- Mayol‐Cabré, M. , Prats, E. , Raldúa, D. & Gómez‐Canela, C. (2020). Characterization of monoaminergic neurochemicals in the different brain regions of adult zebrafish. Science of the Total Environment 745, 141205. [DOI] [PubMed] [Google Scholar]
- McCallum, E. S. , Cerveny, D. , Fick, J. & Brodin, T. (2019). Slow‐release implants for manipulating contaminant exposures in aquatic wildlife: a new tool for field ecotoxicology. Environmental Science & Technology 53, 8282–8290. [DOI] [PubMed] [Google Scholar]
- McCallum, E. S. , Dey, C. J. , Cerveny, D. , Bose, A. P. H. & Brodin, T. (2021). Social status modulates the behavioral and physiological consequences of a chemical pollutant in animal groups. Ecological Applications 31, e02454. [DOI] [PubMed] [Google Scholar]
- McCord, J. P. , Groff, L. C. II & Sobus, J. R. (2022). Quantitative non‐targeted analysis: bridging the gap between contaminant discovery and risk characterization. Environment International 158, 107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Meijering, E. , Dzyubachyk, O. & Smal, I. (2012). Methods for cell and particle tracking. Methods in Enzymology 504, 183–200. [DOI] [PubMed] [Google Scholar]
- Melvin, S. D. (2017). Effect of antidepressants on circadian rhythms in fish: insights and implications regarding the design of behavioural toxicity tests. Aquatic Toxicology 182, 20–30. [DOI] [PubMed] [Google Scholar]
- Melvin, S. D. & Wilson, S. P. (2013). The utility of behavioral studies for aquatic toxicology testing: a meta‐analysis. Chemosphere 93, 2217–2223. [DOI] [PubMed] [Google Scholar]
- Meng, S. , Tran, T. T. , Delnat, V. & Stoks, R. (2021). Transgenerational exposure to warming reduces the sensitivity to a pesticide under warming. Environmental Pollution 284, 117217. [DOI] [PubMed] [Google Scholar]
- Michelangeli, M. , Payne, E. , Spiegel, O. , Sinn, D. , Leu, S. T. , Gardner, M. & Sih, A. (2021). Personality, spatiotemporal ecological variation, and resident/explorer movement syndromes in the sleepy lizard. Journal of Animal Ecology 91, 210–223. [DOI] [PubMed] [Google Scholar]
- Mitchell, W. F. & Clarke, R. H. (2019). Using infrared thermography to detect night‐roosting birds. Journal of Field Ornithology 90, 39–51. [Google Scholar]
- Montiglio, P. & Royauté, R. (2014). Contaminants as a neglected source of behavioural variation. Animal Behaviour 88, 29–35. [Google Scholar]
- Müller, T. , Römer, C. I. & Müller, C. (2019). Parental sublethal insecticide exposure prolongs mating response and decreases reproductive output in offspring. Journal of Applied Ecology 56, 1528–1537. [Google Scholar]
- Munson, A. , Michelangeli, M. & Sih, A. (2021). Stable social groups foster conformity and among‐group differences. Animal Behaviour 174, 197–206. [Google Scholar]
- * Nakagawa, S. & Schielzeth, H. (2010). Repeatability for Gaussian and non‐Gaussian data: a practical guide for biologists. Biological Reviews 85, 935–956. [DOI] [PubMed] [Google Scholar]
- * Nakagawa, S. & Schielzeth, H. (2013). A general and simple method for obtaining R 2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution 4, 133–142. [Google Scholar]
- Nakagawa, S. , Poulin, R. , Mengersen, K. , Reinhold, K. , Engqvist, L. , Lagisz, M. & Senior, A. M. (2015). Meta‐analysis of variation: ecological and evolutionary applications and beyond. Methods in Ecology and Evolution 6, 143–152. [Google Scholar]
- * Nakagawa, S. , Johnson, P. C. D. & Schielzeth, H. (2017). The coefficient of determination R 2 and intraclass correlation coefficient from generalized linear mixed‐effects models revisited and expanded. Journal of the Royal Society Interface 14, 20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. , Noble, D. W. A. , Senior, A. M. & Lagisz, M. (2017). Meta‐evaluation of meta‐analysis: ten appraisal questions for biologists. BMC Biology 15, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. , Samarasinghe, G. , Haddaway, N. R. , Westgate, M. J. , O'Dea, R. E. , Noble, D. W. A. & Lagisz, M. (2019). Research weaving: visualizing the future of research synthesis. Trends in Ecology & Evolution 34, 224–238. [DOI] [PubMed] [Google Scholar]
- Nanninga, G. B. , Scott, A. & Manica, A. (2020). Microplastic ingestion rates are phenotype‐dependent in juvenile anemonefish. Environmental Pollution 259, 113855. [DOI] [PubMed] [Google Scholar]
- * Nath, T. , Mathis, A. , Chen, A. C. , Patel, A. , Bethge, M. & Mathis, M. W. (2019). Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nature Protocols 14, 2152–2176. [DOI] [PubMed] [Google Scholar]
- Nathan, R. , Monk, C. T. , Arlinghaus, R. , Adam, T. , Alós, J. , Assaf, M. , Baktoft, H. , Beardsworth, C. E. , Bertram, M. G. , Bijleveld, A. I. , Brodin, T. , Brooks, J. L. , Campos‐Candela, A. , Cooke, S. J. , Gjelland, K. Ø. , (2022) Big‐data approaches lead to an increased understanding of the ecology of animal movement. Science 375. 10.1126/science.abg1780 [DOI] [PubMed] [Google Scholar]
- NORMAN Network. (2022). Norman Empodat Database‐Chemical Occurrence Data. Electronic file available at https://www.norman-network.com/nds/empodat/. Accessed 26.02.2022.
- * Norouzzadeh, M. S. , Nguyen, A. , Kosmala, M. , Swanson, A. , Palmer, M. S. , Packer, C. & Clune, J. (2018). Automatically identifying, counting, and describing wild animals in camera‐trap images with deep learning. Proceedings of the National Academy of Sciences of the United States of America 115, E5716–E5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Norouzzadeh, M. S. , Morris, D. , Beery, S. , Joshi, N. , Jojic, N. & Clune, J. (2021). A deep active learning system for species identification and counting in camera trap images. Methods in Ecology and Evolution 12, 150–161. [Google Scholar]
- * Nussbaum‐Krammer, C. I. , Neto, M. F. , Brielmann, R. M. , Pedersen, J. S. & Morimoto, R. I. (2015). Investigating the spreading and toxicity of prion‐like proteins using the metazoan model organism C. elegans . Journal of Visualized Experiments 95, e52321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * O'Dea, R. E. , Noble, D. W. A. & Nakagawa, S. (2022). Unifying individual differences in personality, predictability and plasticity: a practical guide. Methods in Ecology and Evolution 13, 278–293. [Google Scholar]
- Orr, J. A. , Vinebrooke, R. D. , Jackson, M. C. , Kroeker, K. J. , Kordas, R. L. , Mantyka‐Pringle, C. , Van den Brink, P. J. , De Laender, F. , Stoks, R. , Holmstrup, M. , Matthaei, C. D. , Monk, W. A. , Penk, M. R. , Leuzinger, S. , Schäfer, R. B. , et al. (2020). Towards a unified study of multiple stressors: divisions and common goals across research disciplines. Proceedings of the Royal Society B: Biological Sciences 287, 20200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panadeiro, V. , Rodriguez, A. , Henry, J. , Wlodkowic, D. & Andersson, M. (2021). A review of 28 free animal‐tracking software applications: current features and limitations. Lab Animal 50, 246–254. [DOI] [PubMed] [Google Scholar]
- * Pérez‐Escudero, A. , Vicente‐Page, J. , Hinz, R. C. , Arganda, S. & de Polavieja, G. G. (2014). idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nature Methods 11, 743–748. [DOI] [PubMed] [Google Scholar]
- * Pereira, T. D. , Aldarondo, D. E. , Willmore, L. , Kislin, M. , Wang, S. S. H. , Murthy, M. & Shaevitz, J. W. (2019). Fast animal pose estimation using deep neural networks. Nature Methods 16, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pereira, T. D. , Tabris, N. , Li, J. , Ravindranath, S. , Papadoyannis, E. S. , Wang, Z. Y. , Turner, D. M. , McKenzie‐Smith, G. , Kocher, S. D. , Falkner, A. L. , Shaevitz, J. W. & Murthy, M. (2020). SLEAP: multi‐animal pose tracking. bioRxiv.
- Peterson, E. K. , Buchwalter, D. B. , Kerby, J. L. , LeFauve, M. K. , Varian‐Ramos, C. W. & Swaddle, J. P. (2017). Integrative behavioral ecotoxicology: bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Current Zoology 63, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean, Q. , Jacquin, L. , Riem, L. , Pitout, M. , Perrault, A. , Cousseau, M. , Laffaille, P. & Jean, S. (2021). Intraspecific variability of responses to combined metal contamination and immune challenge among wild fish populations. Environmental Pollution 272, 116042. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. E. , Wahl, V. L. , Wittlinger, M. & Wolf, H. (2019). High‐speed locomotion in the Saharan silver ant. Journal of Experimental Biology 222, jeb198705. [DOI] [PubMed] [Google Scholar]
- * Pinheiro, J. C. & Bates, D. M. (2000). Linear mixed‐effects models: basic concepts and examples. In Mixed‐Effects Models in S and S‐PLUS (eds Pinheiro J. C. and Bates D. M.). Springer, New York, NY. [Google Scholar]
- * Pinheiro, J. , Bates, D. , DebRoy, S. , Sarkar, D. & R Core Team . (2017). nlme: Linear and nonlinear mixed effects models. R Package version 3.1–131.
- Polo‐Cavia, N. , Burraco, P. & Gomez‐Mestre, I. (2016). Low levels of chemical anthropogenic pollution may threaten amphibians by impairing predator recognition. Aquatic Toxicology 172, 30–35. [DOI] [PubMed] [Google Scholar]
- Polverino, G. , Karakaya, M. , Spinello, C. , Soman, V. R. & Porfiri, M. (2019). Behavioural and life‐history responses of mosquitofish to biologically inspired and interactive robotic predators. Journal of the Royal Society Interface 16, 20190359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverino, G. , Martin, J. M. , Bertram, M. G. , Soman, V. R. , Tan, T. , Brand, J. A. , Mason, R. T. & Wong, B. B. M. (2021). Psychoactive pollution suppresses individual differences in fish behaviour. Proceedings of the Royal Society B: Biological Sciences 288, 20202294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverino, G. , Soman, V. R. , Karakaya, M. , Gasparini, C. , Evans, J. P. & Porfiri, M. (2022). Ecology of fear in highly invasive fish revealed by robots. iScience 25, 103529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle, G. & Ford, A. T. (2017). Behaviour revised: contaminant effects on aquatic animal behaviour. Aquatic Toxicology 182, 226–228. [DOI] [PubMed] [Google Scholar]
- Quintela, E. D. & McCoy, C. W. (1998). Synergistic effect of imidacloprid and two entomopathogenic fungi on the behavior and survival of larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in soil. Journal of Economic Entomology 91, 110–122. [Google Scholar]
- Raies, A. B. & Bajic, V. B. (2016). In silico toxicology: computational methods for the prediction of chemical toxicity. Wiley Interdisciplinary Reviews: Computational Molecular Science 6, 147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Réale, D. , Reader, S. M. , Sol, D. , McDougall, P. T. & Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biological Reviews 82, 291–318. [DOI] [PubMed] [Google Scholar]
- * Réale, D. , Garant, D. , Humphries, M. M. , Bergeron, P. , Careau, V. & Montiglio, P. O. (2010). Personality and the emergence of the pace‐of‐life syndrome concept at the population level. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 4051–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rearick, D. C. , Ward, J. , Venturelli, P. & Schoenfuss, H. (2018). Environmental oestrogens cause predation‐induced population decline in a freshwater fish. Royal Society Open Science 5, 181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relyea, R. A. (2003). Predator cues and pesticides: a double dose of danger for amphibians. Ecological Applications 13, 1515–1521. [Google Scholar]
- Relyea, R. A. & Edwards, K. (2010). What doesn't kill you makes you sluggish: how sublethal pesticides alter predator–prey interactions. Copeia 2010, 558–567. [Google Scholar]
- Renedo, M. , Amouroux, D. , Albert, C. , Bérail, S. , Bråthen, V. S. , Gavrilo, M. , Grémillet, D. , Helgason, H. H. , Jakubas, D. , Mosbech, A. , Strøm, H. , Tessier, E. , Wojczulanis‐Jakubas, K. , Bustamante, P. , et al. (2020). Contrasting spatial and seasonal trends of methylmercury exposure pathways of Arctic seabirds: combination of large‐scale tracking and stable isotopic approaches. Environmental Science & Technology 54, 13619–13629. [DOI] [PubMed] [Google Scholar]
- Renick, V. C. , Weinersmith, K. , Vidal‐Dorsch, D. E. & Anderson, T. W. (2016). Effects of a pesticide and a parasite on neurological, endocrine, and behavioral responses of an estuarine fish. Aquatic Toxicology 170, 335–343. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. , Zhang, H. , Klaminder, J. , Brodin, T. , Andersson, P. L. & Andersson, M. (2018). ToxTrac: a fast and robust software for tracking organisms. Methods in Ecology and Evolution 9, 460–464. [Google Scholar]
- Rohr, J. R. , Elskus, A. A. , Shepherd, B. S. , Crowley, P. H. , McCarthy, T. M. , Niedzwiecki, J. H. , Sager, T. , Sih, A. & Palmer, B. D. (2004). Multiple stressors and salamanders: effects of an herbicide, food limitation, and hydroperiod. Ecological Applications 14, 1028–1040. [Google Scholar]
- Rohr, J. R. , Kerby, J. L. & Sih, A. (2006). Community ecology as a framework for predicting contaminant effects. Trends in Ecology & Evolution 21, 606–613. [DOI] [PubMed] [Google Scholar]
- * Romero‐Ferrero, F. , Bergomi, M. G. , Hinz, R. C. , Heras, F. J. H. & de Polavieja, G. G. (2019). Idtracker.Ai: tracking all individuals in small or large collectives of unmarked animals. Nature Methods 16, 179–182. [DOI] [PubMed] [Google Scholar]
- Rosenberg, K. V. , Dokter, A. M. , Blancher, P. J. , Sauer, J. R. , Smith, A. C. , Smith, P. A. & Marra, P. P. (2019). Decline of the north American avifauna. Science 366, 120–124. [DOI] [PubMed] [Google Scholar]
- Rossoni, S. & Niven, J. E. (2020). Prey speed influences the speed and structure of the raptorial strike of a ‘sit‐and‐wait’ predator. Biology Letters 16, 20200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royauté, R. , Buddle, C. M. & Vincent, C. (2015). Under the influence: sublethal exposure to an insecticide affects personality expression in a jumping spider. Functional Ecology 29, 962–970. [Google Scholar]
- Rumschlag, S. L. , Halstead, N. T. , Hoverman, J. T. , Raffel, T. R. , Carrick, H. J. , Hudson, P. J. & Rohr, J. R. (2019). Effects of pesticides on exposure and susceptibility to parasites can be generalised to pesticide class and type in aquatic communities. Ecology Letters 22, 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaristo, M. , Brodin, T. , Balshine, S. , Bertram, M. G. , Brooks, B. W. , Ehlman, S. M. , McCallum, E. S. , Sih, A. , Sundin, J. , Wong, B. B. M. & Arnold, K. E. (2018). Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proceedings of the Royal Society B: Biological Sciences 285, 20181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Bayo, F. & Wyckhuys, K. A. (2019). Worldwide decline of the entomofauna: a review of its drivers. Biological Conservation 232, 8–27. [Google Scholar]
- * Schneider, C. A. , Rasband, W. S. & Eliceiri, K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sergio, F. , Tanferna, A. , Blas, J. , Blanco, G. & Hiraldo, F. (2019). Reliable methods for identifying animal deaths in GPS‐and satellite‐tracking data: review, testing, and calibration. Journal of Applied Ecology 56, 562–572. [Google Scholar]
- Shams, S. , Chatterjee, D. & Gerlai, R. (2015). Chronic social isolation affects thigmotaxis and whole‐brain serotonin levels in adult zebrafish. Behavioural Brain Research 292, 283–287. [DOI] [PubMed] [Google Scholar]
- Sievers, M. , Hale, R. , Swearer, S. E. & Parris, K. M. (2018). Contaminant mixtures interact to impair predator‐avoidance behaviours and survival in a larval amphibian. Ecotoxicology and Environmental Safety 161, 482–488. [DOI] [PubMed] [Google Scholar]
- Sih, A. , Bell, A. M. & Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution 19, 372–378. [DOI] [PubMed] [Google Scholar]
- Sih, A. , Ferrari, M. C. O. & Harris, D. J. (2011). Evolution and behavioural responses to human‐induced rapid environmental change. Evolutionary Applications 4, 367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih, A. & Del Giudice, M. (2012). Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philosophical Transactions of the Royal Society B: Biological Sciences 367, 2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Silk, M. J. , Harrison, X. A. & Hodgson, D. J. (2020). Perils and pitfalls of mixed‐effects regression models in biology. PeerJ 8, e9522. [Google Scholar]
- Smith, J. E. & Pinter‐Wollman, N. (2021). Observing the unwatchable: integrating automated sensing, naturalistic observations and animal social network analysis in the age of big data. Journal of Animal Ecology 90, 62–75. [DOI] [PubMed] [Google Scholar]
- * Smith, M. L. , Davidson, J. D. , Wild, B. , Dormagen, D. M. , Landgraf, T. & Couzin, I. D. (2021). The dominant axes of lifetime behavioral variation in honey bees. bioRxiv.
- South, J. , Botha, T. L. , Wolmarans, N. J. , Wepener, V. & Weyl, O. L. (2019). Assessing predator‐prey interactions in a chemically altered aquatic environment: the effects of DDT on Xenopus laevis and Culex sp. larvae interactions and behaviour. Ecotoxicology 28, 771–780. [DOI] [PubMed] [Google Scholar]
- Spiegel, O. , Leu, S. T. , Bull, C. M. & Sih, A. (2017). What's your move? Movement as a link between personality and spatial dynamics in animal populations. Ecology Letters 20, 3–18. [DOI] [PubMed] [Google Scholar]
- * Spinello, C. , Macrì, S. & Porfiri, M. (2013). Acute ethanol administration affects zebrafish preference for a biologically inspired robot. Alcohol 47, 391–398. [DOI] [PubMed] [Google Scholar]
- * Spink, A. J. , Tegelenbosch, R. A. J. , Buma, M. O. S. & Noldus, L. P. J. J. (2001). The EthoVision video tracking system—a tool for behavioral phenotyping of transgenic mice. Physiology & Behavior 73, 731–744. [DOI] [PubMed] [Google Scholar]
- * Sridhar, V. H. , Roche, D. G. & Gingins, S. (2019). Tracktor: image‐based automated tracking of animal movement and behaviour. Methods in Ecology and Evolution 10, 815–820. [Google Scholar]
- * Stoffel, M. A. , Nakagawa, S. & Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed‐effects models. Methods in Ecology and Evolution 8, 1639–1644. [Google Scholar]
- * Stowers, J. R. , Hofbauer, M. , Bastien, R. , Griessner, J. , Higgins, P. , Farooqui, S. , Fischer, R. M. , Nowikovsky, K. , Haubensak, W. , Couzin, I. D. , Tessmar‐Raible, K. & Straw, A. D. (2017). Virtual reality for freely moving animals. Nature Methods 14, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sugai, L. S. M. , Silva, T. S. F. , Ribeiro, J. W. Jr. & Llusia, D. (2019). Terrestrial passive acoustic monitoring: review and perspectives. Bioscience 69, 15–25. [Google Scholar]
- Swank, A. , Wang, L. , Ward, J. & Schoenfuss, H. (2021). Multigenerational effects of a complex urban contaminant mixture on the behavior of larval and adult fish in multiple fitness contexts. Science of the Total Environment 791, 148095. [DOI] [PubMed] [Google Scholar]
- * Symonds, M. R. E. & Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behavioral Ecology and Sociobiology 65, 13–21. [Google Scholar]
- Tan, H. , Polverino, G. , Martin, J. M. , Bertram, M. G. , Wiles, S. C. , Palacios, M. M. , Bywater, C. L. , White, C. R. & Wong, B. B. M. (2020). Chronic exposure to a pervasive pharmaceutical pollutant erodes among‐individual phenotypic variation in a fish. Environmental Pollution 263, 114450. [DOI] [PubMed] [Google Scholar]
- * Taylor, P. , Crewe, T. , Mackenzie, S. , Lepage, D. , Aubry, Y. , Crysler, Z. , Finney, G. , Francis, C. , Guglielmo, C. , Hamilton, D. & Holberton, R. (2017). The Motus wildlife tracking system: a collaborative research network to enhance the understanding of wildlife movement. Avian Conservation and Ecology 12, 8. [Google Scholar]
- * Thomson, J. A. , Börger, L. , Christianen, M. J. A. , Esteban, N. , Laloë, J. O. & Hays, G. C. (2017). Implications of location accuracy and data volume for home range estimation and fine‐scale movement analysis: comparing Argos and Fastloc‐GPS tracking data. Marine Biology 164, 204. [Google Scholar]
- Thoré, E. S. J. , Grégoir, A. F. , Adriaenssens, B. , Philippe, C. , Stoks, R. , Brendonck, L. & Pinceel, T. (2019). Population‐, sex‐ and individual level divergence in life‐history and activity patterns in an annual killifish. PeerJ 7, e7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoré, E. S. J. , Brendonck, L. & Pinceel, T. (2021a). Natural daily patterns in fish behaviour may confound results of ecotoxicological testing. Environmental Pollution 276, 116738. [DOI] [PubMed] [Google Scholar]
- Thoré, E. S. J. , Brendonck, L. & Pinceel, T. (2021b). Neurochemical exposure disrupts sex‐specific trade‐offs between body length and behaviour in a freshwater crustacean. Aquatic Toxicology 237, 105877. [DOI] [PubMed] [Google Scholar]
- Thoré, E. S. J. , Van Hooreweghe, F. , Philippe, C. , Brendonck, L. & Pinceel, T. (2021). Generation‐specific and interactive effects of pesticide and antidepressant exposure in a fish model call for multi‐stressor and multigenerational testing. Aquatic Toxicology 232, 105743. [DOI] [PubMed] [Google Scholar]
- Tickner, D. , Opperman, J. J. , Abell, R. , Acreman, M. , Arthington, A. H. , Bunn, S. E. , Cooke, S. J. , Dalton, J. , Darwall, W. , Edwards, G. , Harrison, I. , Hughes, K. , Jones, T. , Leclère, D. , Lynch, A. J. , et al. (2020). Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. Bioscience 70, 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Toledo, S. , Kishon, O. , Orchan, Y. , Shohat, A. & Nathan, R. (2016). Lessons and experiences from the design, implementation, and deployment of a wildlife tracking system. 2016 IEEE International Conference on Software Science, Technology and Engineering (SWSTE) 51–60.
- * Toledo, S. , Shohami, D. , Schiffner, I. , Lourie, E. , Orchan, Y. , Bartan, Y. & Nathan, R. (2020). Cognitive map–based navigation in wild bats revealed by a new high‐throughput tracking system. Science 369, 188–193. [DOI] [PubMed] [Google Scholar]
- Tox21 (2020). Toxicology in the 21st Century (Tox21). Electronic file available at https://www.epa.gov/chemical-research/toxicology-testing-21st-century-tox21. Accessed 25.02.2021
- ToxCast (2020). Toxicity Forecaster (ToxCast). Electronic file available at https://www.epa.gov/chemical-research/toxicity-forecasting. Accessed 25.02.2021.
- ToxPi (2021). ToxPi: Toxicological Prioritization Index. https://toxpi.org/. Accessed 17.02.2021.
- Tunbak, H. , Vazquez‐Prada, M. C. , Ryan, T. M. , Kampff, A. R. & Dreosti, E. (2020). Whole‐brain mapping of socially isolated zebrafish reveals that lonely fish are not loners. eLife 9, e55863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüzün, N. , Müller, S. , Koch, K. & Stoks, R. (2017). Pesticide‐induced changes in personality depend on the urbanization level. Animal Behaviour 134, 45–55. [Google Scholar]
- UBA (UmweltBundesamt) . (2021). Pharmaceuticals in the Environment. Database. Electronic file available at https://www.umweltbundesamt.de/en/database-pharmaceuticals-in-the-environment-0. Accessed 17.02.2021.
- UNEP (United Nations Environment Programme) . (2019). Global chemicals outlook II: from legacies to innovative solutions. Electronic file available at https://wedocs.unep.org/bitstream/handle/20.500.11822/28113/GCOII.pdf?sequence=1&isAllowed=y. Accessed 22.04.2021.
- Vera‐Chang, M. N. , St‐Jacques, A. D. , Gagné, R. , Martyniuk, C. J. , Yauk, C. L. , Moon, T. W. & Trudeau, V. L. (2018). Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio . Proceedings of the National Academy of Sciences of the United States of America 115, E12435–E12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, T. & Couzin, I. D. (2021). TRex, a fast multi‐animal tracking system with markerless identification, and 2D estimation of posture and visual fields. eLife 10, e64000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Walker, G. W. , Muir, D. C. G. & Nagatani‐Yoshida, K. (2020). Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environmental Science & Technology 54, 2575–2584. [DOI] [PubMed] [Google Scholar]
- Ward, A. J. , Duff, A. J. , Horsfall, J. S. & Currie, S. (2008). Scents and scents‐ability: pollution disrupts chemical social recognition and shoaling in fish. Proceedings of the Royal Society B: Biological Sciences 275, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wario, F. , Wild, B. , Couvillon, M. J. , Rojas, R. & Landgraf, T. (2015). Automatic methods for long‐term tracking and the detection and decoding of communication dances in honeybees. Frontiers in Ecology and Evolution 3, 103. [Google Scholar]
- * Wark, J. D. , Cronin, K. A. , Niemann, T. , Shender, M. A. , Horrigan, A. , Kao, A. & Ross, M. R. (2019). Monitoring the behavior and habitat use of animals to enhance welfare using the ZooMonitor app. Animal Behavior and Cognition 6, 158–167. [Google Scholar]
- Weis, J. S. , Bergey, L. , Reichmuth, J. & Candelmo, A. (2011). Living in a contaminated estuary: behavioral changes and ecological consequences for five species. Bioscience 61, 375–385. [Google Scholar]
- Weis, J. S. & Candelmo, A. (2012). Pollutants and fish predator/prey behavior: a review of laboratory and field approaches. Current Zoology 58, 9–20. [Google Scholar]
- * Weiser, A. W. , Orchan, Y. , Nathan, R. , Charter, M. , Weiss, A. J. & Toledo, S. (2016). Characterizing the accuracy of a self‐synchronized reverse‐GPS wildlife localization system. 2016 15th ACM/IEEE International Conference on Information Processing in Sensor Networks (IPSN) 1–12.
- * Westneat, D. F. , Wright, J. & Dingemanse, N. J. (2015). The biology hidden inside residual within‐individual phenotypic variation. Biological Reviews 90, 729–743. [DOI] [PubMed] [Google Scholar]
- * Westneat, D. F. , Araya‐Ajoy, Y. G. , Allegue, H. , Class, B. , Dingemanse, N. , Dochtermann, N. A. , Garamszegi, L. Z. , Martin, J. G. A. , Nakagawa, S. , Réale, D. & Schielzeth, H. (2020). Collision between biological process and statistical analysis revealed by mean centring. Journal of Animal Ecology 89, 2813–2824. [DOI] [PubMed] [Google Scholar]
- Whitford, M. & Klimley, A. P. (2019). An overview of behavioral, physiological, and environmental sensors used in animal biotelemetry and biologging studies. Animal Biotelemetry 7, 26. [Google Scholar]
- * Wickham, H. & Chang, W. (2015). Package ‘ggplot2’. R package version 1.0.0.
- * Wild, B. , Dormagen, D. M. , Zachariae, A. , Smith, M. L. , Traynor, K. S. , Brockmann, D. , Couzin, I. D. & Landgraf, T. (2021). Social networks predict the life and death of honey bees. Nature Communications 12, 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles, S. C. , Bertram, M. G. , Martin, J. M. , Tan, H. , Lehtonen, T. K. & Wong, B. B. M. (2020). Long‐term pharmaceutical contamination and temperature stress disrupt fish behaviour. Environmental Science & Technology 54, 8072–8082. [DOI] [PubMed] [Google Scholar]
- Wilkinson, J. L. , Boxall, A. B. A. , Kolpin, D. W. , Leung, K. M. Y. , Lai, R. W. S. , Galbán‐Malagón, C. , Adell, A. D. , Mondon, J. , Metian, M. , Marchant, R. A. , Bouzas‐Monroy, A. , Cuni‐Sanchez, A. , Coors, A. , Carriquiriborde, P. , Rojo, M. , (2022) Pharmaceutical pollution of the world's rivers. Proceedings of the National Academy of Sciences, 119, e2113947119. 10.1073/pnas.2113947119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wilson, A. J. , Réale, D. , Clements, M. N. , Morrissey, M. M. , Postma, E. , Walling, C. A. , Kruuk, L. E. B. & Nussey, D. H. (2010). An ecologist's guide to the animal model. Journal of Animal Ecology 79, 13–26. [DOI] [PubMed] [Google Scholar]
- Winter, M. J. , Windell, D. , Metz, J. , Matthews, P. , Pinion, J. , Brown, J. T. , Hetheridge, M. J. , Ball, J. S. , Owen, S. F. , Redfern, W. S. , Moger, J. , Randall, A. D. & Tyler, C. R. (2017). 4‐dimensional functional profiling in the convulsant‐treated larval zebrafish brain. Scientific Reports 7, 6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, M. J. , Pinion, J. , Tochwin, A. , Takesono, A. , Ball, J. S. , Grabowski, P. , Metz, J. , Trznadel, M. , Tse, K. , Redfern, W. S. , Hetheridge, M. J. , Goodfellow, M. , Randall, A. D. & Tyler, C. R. (2021). Functional brain imaging in larval zebrafish for characterising the effects of seizurogenic compounds acting via a range of pharmacological mechanisms. British Journal of Pharmacology 178, 2671–2689. [DOI] [PubMed] [Google Scholar]
- Wong, B. B. M. & Candolin, U. (2015). Behavioral responses to changing environments. Behavioral Ecology 26, 665–673. [Google Scholar]
- Wolffe, T. A. , Whaley, P. , Halsall, C. , Rooney, A. A. & Walker, V. R. (2019). Systematic evidence maps as a novel tool to support evidence‐based decision‐making in chemicals policy and risk management. Environment International 130, 104871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Yamanaka, O. & Takeuchi, R. (2018). UMATracker: an intuitive image‐based tracking platform. Journal of Experimental Biology 221, jeb182469. [DOI] [PubMed] [Google Scholar]
- * Zimmerman, P. H. , Bolhuis, J. E. , Willemsen, A. , Meyer, E. S. & Noldus, L. P. J. J. (2009). The observer XT: a tool for the integration and synchronization of multimodal signals. Behavior Research Methods 41, 731–735. [DOI] [PubMed] [Google Scholar]
- * Zuur, A. , Ieno, E. N. , Walker, N. , Saveliev, A. A. & Smith, G. M. (2009). Mixed Effects Models and Extensions in Ecology with R. Springer, New York, NY. [Google Scholar]
- * Zuur, A. F. , Ieno, E. N. & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Software available to be incorporated into behavioural ecotoxicology research in the laboratory.
Table S2. Hardware available to be incorporated into behavioural ecotoxicology research in the laboratory.
Table S3. Software available to be incorporated into behavioural ecotoxicology research in the field.
Table S4. Hardware available to be incorporated into behavioural ecotoxicology research in the field.
Table S5. (a) A brief overview of resources available for implementing mixed modelling approaches in behavioural ecotoxicology, and (b) examples of specific research questions and resources for testing potential effects of contaminant exposure on behavioural variation at the individual level.


