Abstract
Background
Pneumonia is commonly caused by Streptococcus pneumoniae (pneumococcus) and associated with subsequent cardiovascular complications and increased mortality. Potential short‐term survival benefits conferred by acetylsalicylic acid (ASA) use in pneumonia remain controversial, and long‐term outcomes have not been studied.
Objectives
To evaluate the association between ASA use and survival for up to 1 year following bacteremic pneumococcal pneumonia.
Methods
All bacteremic pneumococcal episodes in Iceland from 1975 to 2019 were reviewed. The study cohort consisted of individuals at least 18 years of age with symptoms and imaging results consistent with pneumonia. Differences in survival were assessed at 30 days, 90 days and 1 year using propensity score weighting (inverse probability weighting). Splitting and stratifying on survival at 7 days was done for the 30‐day survival, because of nonproportionality.
Results
In total, 815 bacteremic pneumococcal pneumonia episodes (median age 67 years, females 48%) were identified. Cox regression using propensity score weighting on the association of ASA with survival at 30 days showed an average hazard ratio (HR) of 0.60 (95% confidence interval [CI] 0.34–1.05). A significantly improved survival was observed within 7 days (HR = 0.42, 95% CI 0.19–0.92) but not during days 7–30 (HR = 1.08, 95% CI 0.46–2.55). ASA was associated with survival at 90 days (HR = 0.53, 95% CI 0.32–0.87) and 1 year (HR = 0.48, 95% CI 0.31–0.75).
Conclusion
Use of ASA upon admission for bacteremic pneumococcal pneumonia is associated with significantly reduced mortality for up to 1 year after diagnosis. ASA therapy in patients with pneumonia and other infectious syndromes warrants further study.
Keywords: aspirin, pneumonia, population‐based, Streptococcus pneumoniae, survival
Introduction
Pneumonia is an exceedingly common disease with high mortality rates. Even before the current COVID‐19 pandemic, an estimated 2 million lives were lost due to pneumonia annually [1].
Streptococcus pneumoniae (pneumococcus) is one of the most common pathogens identified worldwide among adults hospitalized with community‐acquired pneumonia (CAP) [2, 3, 4, 5, 6]. Importantly, only a minority of pneumococcal pneumonia cases are associated with bacteremia or sepsis, but a positive blood culture establishes the etiologic diagnosis.
Cardiovascular complications within 30 days of admission to hospital have been reported in 27%–32% of patients admitted with pneumonia [7, 8], with half of those complications developing within 24 h [8]. While these complications are most prominent during the first days, patients may remain at increased risk for months or years when compared to age‐matched controls [9]. The cardiovascular risk associated with bacteremic pneumonia is higher than that of pneumonia without bacteremia and other respiratory infections, and the more severe the respiratory infection, the longer the cardiovascular risk remains elevated [10].
Pneumonia caused by S. pneumoniae has been linked to subsequent cardiovascular complications [11, 12, 13]. A retrospective study found a 20‐fold increased risk of myocardial infarction and a 26‐fold increased risk of stroke during the first 3 days following invasive S. pneumoniae infection compared to a reference period. An increased, albeit smaller, risk was noted with respiratory viruses [14].
Acetylsalicylic acid (ASA), commonly known as aspirin, reduces clustering of platelets and inhibits cyclooxygenase 1 and production of prostaglandins [15]. Furthermore, ASA reduces the risk of myocardial and cerebral infarction when used in secondary prevention [16]. The potential benefits conferred by ASA among patients with CAP remain controversial, with some studies suggesting decreased short‐term mortality [17, 18] while other investigators have failed to find a significant short‐term benefit [19, 20]. ASA is a low‐cost generic drug that is widely used without prescription, impeding studies of its effects using information from prescription databases. Pneumonia represents a heterogeneous group of patients with multiple underlying microbial etiologies, some of which may have varying clinical courses. Therefore, studying a well‐defined and detailed cohort of patients with severe pneumonia caused by a single pathogen, such as S. pneumoniae, is a suitable approach to address this question in a rigorous manner.
The goal of this study was to estimate the association between ASA use and overall survival and causes of death in a nationwide cohort of adults with microbiologically confirmed bacteremic pneumococcal pneumonia.
Material and methods
All necessary permits were obtained in accordance with Icelandic law and the Declaration of Helsinki. Research permission was granted by the National Bioethics Committee.
Study design and data sources
A national database of confirmed invasive S. pneumoniae infections was generated by identifying all positive cultures with this pathogen from sterile sites in clinical microbiology records. All positive cultures with retrievable medical records over the 45‐year period from 1975 to 2019 were subsequently reviewed in detail with respect to demographics, clinical characteristics, underlying medical conditions and medication use upon admission (including ASA), infection site, imaging and laboratory test results, treatment, and vital status. Information regarding vital status and eventual date of death was derived from the Icelandic National Registry in January of 2021. Data on cause of death were collected from the Icelandic Causes of Death Register for cases diagnosed from 1975 to 2015.
Criteria for inclusion in the pneumonia group were (1) a blood culture positive for S. pneumoniae, (2) infiltrates consistent with pneumonia as judged by a radiologist, and (3) clinical symptoms consistent with pneumonia. The exposure of interest was the current use of ASA (any dosage) according to medication history at admission. Underlying medical conditions listed in medical records were included for adjustment.
Study outcomes
The study outcomes were the survival at 30 days, 90 days, and 1 year of patients receiving ASA at admission compared to patients not receiving the drug. Secondary outcomes were the causes of death of studied individuals.
Statistical analysis
Statistical tests of significance were two sided, and p‐values of less than 0.05 were deemed significant. Survival plotting was done with Kaplan–Meier graphs. Cox regression using propensity score weighting (PSW) was performed. Data were collected from medical records and registered using FileMaker Pro 8th edition (Claris International Inc., USA). Subsequently, data were exported to R (R core Team 2021) and Rstudio (Rstudio Team 2021) for statistical analysis (Survival and WeightIt R packages) [21, 22].
Survival analysis
The entry time was the gathering of blood culture, and the endpoint was all‐cause death. The unit of survival time was number of days (or the fraction of a day if less than 1 day). Individuals were censored at end of study or upon re‐infection. Cox regression with clustering of individuals was performed. We assessed the proportionality of the hazards and outlying observations. In cases where the effect of ASA violated the proportionality assumption, the survSplit function was used to split the survival time into chunks guided by changes in the effect slope [23]. The average hazard ratio (HR) when the proportionality assumption was violated was also presented [24].
Propensity score weighting (inverse probability weighting)
Based on the current literature and availability, we selected a priori for PSW [25, 26]: age in tertiles (18–57.9, 57.9–74.7,74.8–101.0), sex, ischemic heart disease (IHD), dementia, malignancy, statin use, cerebrovascular disease (CeVD), chronic obstructive pulmonary disease (COPD), heart failure (HF), chronic kidney failure (CKF), diabetes mellitus (DM), glucocorticosteroid use, liver disease, alcoholism, and macrolide antibiotic treatment. Variable selection is explained in the supplementary chapter.
Propensity score was created using logistic regression utilizing the WeightIt package in R [21, 22, 27]. Using the PSW, inverse probability weighting was performed to estimate the average treatment effect of the treated (ATT) [28]. The ATT estimates the difference in outcome if those treated with ASA had not received ASA [29]. To increase the balance using the propensity score, multiple interactions between variables were done [26]. The balance was deemed acceptable if the mean difference was less than 0.1 for each variable and overall.
Sensitivity analysis
Cox regression adjusting for underlying diseases with direct covariate adjustment was performed. It was planned following the general rule of minimum of 10 endpoints per covariate [30, 31, 32]. Variables were chosen based on the current literature.
Sensitivity analyses—Cox regressions using PSW—were carried out assessing the effect of beta blockers, statins, proton pump inhibitors (PPIs), and macrolides on mortality.
Missing data
Survival information was missing for individuals who moved abroad (14 cases) but was otherwise universally available. Information on active smoking was missing in 273/815 (33.5%) of episodes. The smoking variable was included in the propensity score in two sensitivity analyses—a complete case analysis and in the use of multiple imputations by chained equations (MICE). For the sensitivity analysis utilizing MICE, 20 imputations were performed, and the within approach was used in assembling PSW from the imputed datasets using the MatchThem package in R [33].
Results
Characteristics of the population‐based sample
A flow chart describing the generation of the final patient cohort is shown in Fig. 1. The yield of the chart review was 1457/1505 (96.8%) (Fig. 1). Individuals in the ASA group were older than their counterparts and more commonly had DM, hypertension, IHD, CeVD, and HF. They were also more likely to be receiving statins, beta blockers, or PPIs on admission (Table 1). On average, they had a higher Acute Physiology And Chronic Health Evaluation II (APACHE II) severity score. However, their current smoking and alcoholism rates were lower than in the group not taking ASA (Table 1). The balance before and after PSW is shown in Fig. S1.
Fig. 1.
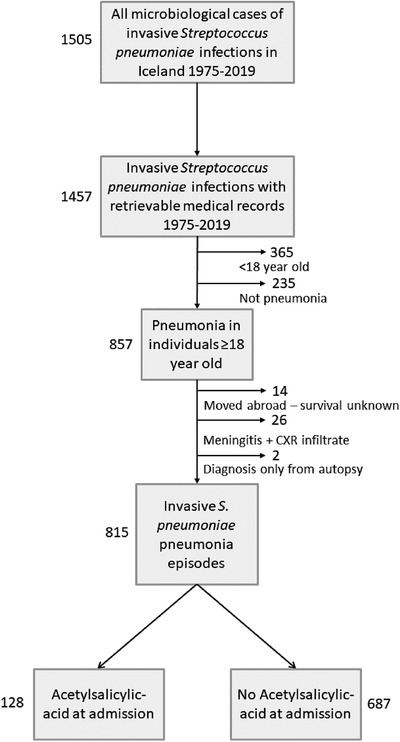
Patient flow chart illustrating the definition of the final cohort. In total, 1505 Streptococcus pneumoniae bloodstream infections were diagnosed in the country during 1975–2019. Medical records were available for 1457 of those, 365 episodes were diagnosed in children, and 235 were not pneumonia. Of the remaining 857 pneumonia cases, clinical information was not available in 42. The final cohort consisted of 815 episodes of bacteremic pneumococcal pneumonia in 795 adults. Twenty re‐infections occurred over the whole 45‐year period, thereof nine within 1 year of the first infection. One patient had two episodes with different serotypes 15 days apart; therefore, these were judged to be two separate events.
Table 1.
Characteristics at the time of admission for the 815 pneumonia episodes
| ASA prior to admission (n = 128) | Not on ASA prior to admission (n = 687) | p‐value a | Standard mean difference | |
|---|---|---|---|---|
| Re‐infections within 1 year of prior episode | 0 | 9 (1.3%) | 0.37 | 0.16 |
| Age median (IQR) | 75 (68–82) | 65 (48–77) | <0.001 | 0.79 |
| Females | 56 (43.8%) | 331 (48.2%) | 0.39 | 0.09 |
| Malignancy | 27 (21.1%) | 139 (20.2%) | 0.81 | 0.02 |
| Diabetes mellitus | 19 (14.8%) | 43 (6.3%) | 0.002 | 0.28 |
| Kidney failure | 13 (10.2%) | 50 (7.3%) | 0.28 | 0.10 |
| Hypertension | 46 (35.9%) | 127 (18.5%) | <0.001 | 0.40 |
| Liver disease | 1 (0.8%) | 12 (1.7%) | 0.70 | 0.09 |
| HIV or AIDS | 0 | 8 (1.2%) | 0.62 | 0.15 |
| Chronic obstructive pulmonary disease | 25 (19.5%) | 97 (14.1%) | 0.14 | 0.15 |
| Dementia | 8 (6.2%) | 26 (3.8%) | 0.23 | 0.11 |
| Ischemic heart disease | 61 (47.7%) | 79 (11.5%) | <0.001 | 0.86 |
| Cerebrovascular disease | 17 (13.3%) | 27 (3.9%) | <0.001 | 0.34 |
| Heart failure | 19 (14.8%) | 42 (6.1%) | 0.002 | 0.29 |
| Current smoking (missing 273/815 = 33.5%) | 29 (34.5%) (missing 34.4%) | 245 (53.5%) (missing 33.3%) | 0.002 | 0.39 |
| Asthma | 9 (7.0%) | 35 (5.1%) | 0.39 | 0.08 |
| Alcoholism | 4 (3.1%) | 75 (10.9%) | 0.005 | 0.31 |
| Statin therapy | 34 (26.6%) | 26 (3.8%) | <0.001 | 0.67 |
| Beta blockers | 58 (45.3%) | 97 (14.1%) | <0.001 | 0.73 |
| Proton pump inhibitors | 31 (24.2%) | 88 (12.8%) | 0.002 | 0.30 |
| Long‐term glucocorticoid therapy | 13 (10.2%) | 65 (9.5%) | 0.75 | 0.02 |
| APACHE II score, median (IQR) | 13 (10–16) | 12 (8–16) | 0.02 | 0.15 |
| Intensive care in admission | 26 (20.3%) | 139 (20.2%) | 1.00 | 0.00 |
| Treatment included macrolide | 35 (27.3%) | 190 (27.7%) | 1.00 | 0.01 |
| Treatment in the first 2 days included beta‐lactam antibiotics | 118 (92.2%) | 630 (91.7%) | 1.00 | 0.00 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; APACHE II, Acute Physiology And Chronic Health Evaluation II; ASA, acetylsalicylic acid; HIV, human immunodeficiency virus; IQR, interquartile range.
p‐value was calculated using Fisher's exact test for categorical values and independent two‐group Mann–Whitney U test for continuous variables.
Unadjusted results
Overall, the all‐cause mortality rate for the entire group was 110/814 (13.5%) at 30 days, 147/811 (18.1%) at 90 days, and 203/806 (25.2%) at 1 year (Table S1). All but eight of 110 deaths within 30 days occurred while the patients were still in‐hospital. Unadjusted all‐cause mortality rates for patients taking ASA were 19/128 (14.8%), 23/128 (18.0%), and 29/128 (22.7%) for 30‐day, 90‐day, and 1‐year mortality, respectively. Corresponding figures were 91/686 (13.3%), 124/683 (18.2%), and 174/678 (25.7%) for patients not taking ASA (Table S1). The unadjusted 30‐day Kaplan–Meier survival curves are shown in Fig. 2a.
Fig. 2.
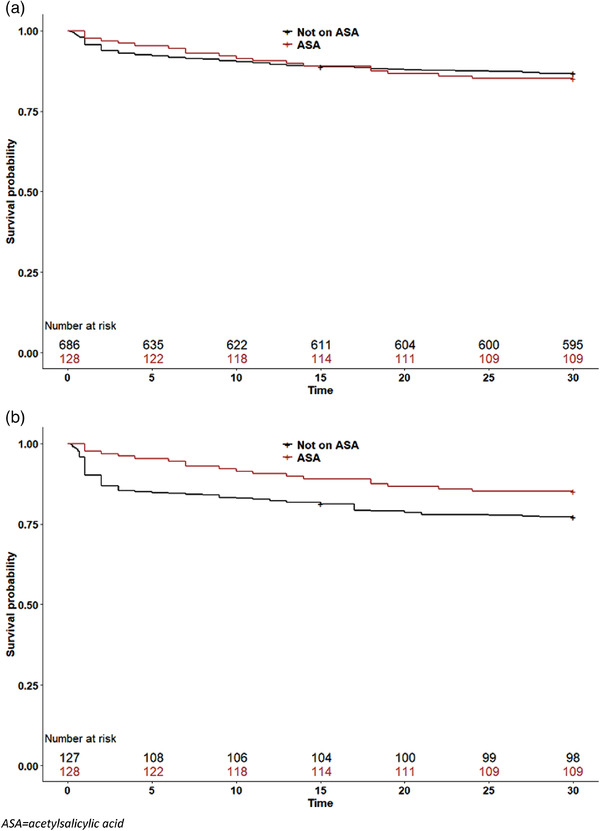
Survival of patients with bacteremic pneumococcal pneumonia by acetylsalicylic acid (ASA) use on admission. Patients taking ASA are indicated by red lines, whereas patients not taking ASA are shown with black lines. (a) Unadjusted 30‐day survival analysis; (b) 30‐day survival curves adjusted with propensity score weighting.
Results after covariate adjustment
Kaplan–Meier survival curves adjusted with PSW are depicted in Fig. 2b. As shown, a steep increase in mortality occurs during the first few days for patients with pneumococcal pneumonia, affecting ASA users less than nonusers. The maximal separation between the adjusted curves was reached at around 1 year after the infection, and then the separation between the lines decreases (Fig. 3).
Fig. 3.
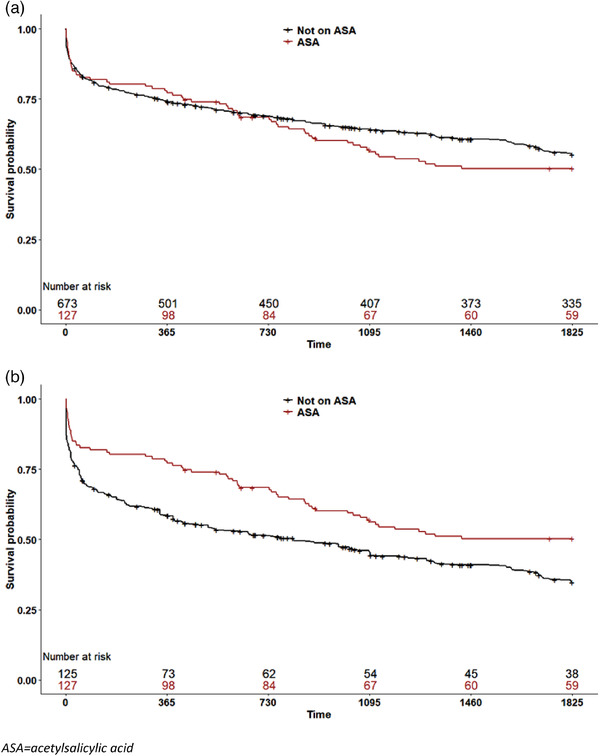
Survival of patients with bacteremic pneumococcal pneumonia by acetylsalicylic acid (ASA) use on admission, 5 years following infection. Patients taking ASA are indicated by red lines, whereas patients not taking ASA are shown with black lines. (a) Unadjusted; (b) adjusted using propensity score weighting.
The Cox regression using PSW did not meet the assumption of proportional hazards at the 30‐day survival (Fig. S2). However, the average HR was 0.60 (95% confidence interval [CI] 0.34–1.05) (Fig. 4). To address the nonproportionality, survival time was split into two periods (less than 7 days and 7–30 days). This yielded an HR of 0.42 (95% CI 0.19–0.92) for the association between ASA and survival in the first 7 days and 1.08 (95% CI 0.46–2.55) for the 7–30 day survival (Fig. 4). The 90‐day and 1‐year mortality resulted in an HR of 0.53 (95% CI 0.32–0.87) and an HR of 0.48 (95% CI 0.31–0.75), respectively.
Fig. 4.
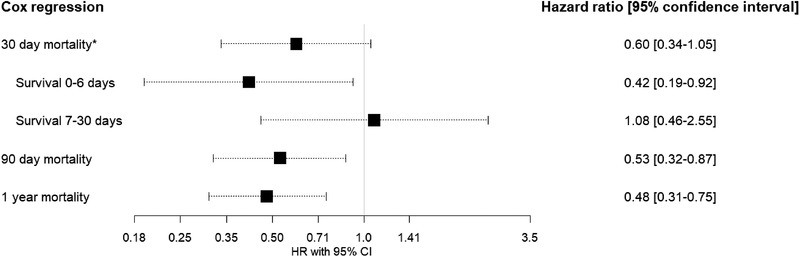
Cox regression with propensity score weighting on the association of acetylsalicylic acid use and mortality at 30 and 90 days and 1 year after diagnosis of bacteremic pneumococcal pneumonia. The proportional hazard assumption did not hold for the 30‐day survival; after observing the changes in the slope of the hazard, the survival analysis was split at 7 days into two intervals—less than 7 days and 7–30 days.
Causes of death
Unadjusted underlying causes of death of patients diagnosed with invasive pneumococcal pneumonia in the period 1975–2015 are summarized in Table 2. Cardiovascular diseases were the underlying cause of death in 2/108 (1.9%) patients receiving ASA and in 13/573 (2.3%) among the non‐ASA patients within 30 days, in 3/108 (2.8%) patients receiving ASA and in 16/569 (2.8%) among the non‐ASA patients at 90 days, and in 5/108 (4.6%) patients receiving ASA and in 22/567 (3.9%) among the non‐ASA patients at 1 year. Malignancies were the underlying cause of death in 2.8% of ASA patients and 3.3% of non‐ASA patients within 30 days, and in 4.6% of ASA patients and 10.2% of non‐ASA patients at 1 year. The differences were nonsignificant.
Table 2.
Underlying causes of death for patients diagnosed during 1975–2015
| 30‐day mortality | 90‐day mortality | 1‐year mortality | ||||
|---|---|---|---|---|---|---|
| ASA (n = 108) | Not on ASA (n = 573 a ) | ASA (n = 108) | Not on ASA (n = 569 a ) | ASA (n = 108) | Not on ASA (n = 567 a ) | |
| Cardiovascular, n (%) | 2 (1.9) | 13 (2.3) | 3 (2.8) | 16 (2.8) | 5 (4.6) | 22 (3.9) |
| Malignancy, n (%) | 3 (2.8) | 19 (3.3) | 3 (2.8) | 31 (5.4) | 5 (4.6) | 58 (10.2) |
| Pneumonia, n (%) | 6 (5.6) | 31 (5.4) | 6 (5.6) | 37 (6.5) | 6 (5.6) | 37 (6.5) |
| Respiratory disease, n (%) | 1 (0.9) | 1 (0.2) | 1 (0.9) | 2 (0.4) | 2 (1.9) | 8 (1.4) |
| Accidents, n (%) | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.2) | 1 (0.9) | 1 (0.2) |
| Other illnesses, n (%) | 1 (0.9) | 6 (1.0) | 1 (0.9) | 9 (1.6) | 2 (1.9) | 15 (2.6) |
| Missing information, n (%) | 0 | 3 | 0 | 4 | 0 | 4 |
Abbreviation: ASA, acetylsalicylic acid.
Repeated infections within the relevant endpoint were omitted along with cases with missing information on cause of death. One individual had a repeated episode within 30 days of the prior episode, four had repeated episodes within 90 days, and nine within 1 year.
Sensitivity analysis
The multivariable Cox models for the 30‐day, 90‐day, and 1‐year survival did not meet the proportionality assumption. The average HR was 0.66 (95% CI 0.39–1.09) for the 30‐day survival, 0.59 (95% CI 0.36–0.98) for the 90‐day survival, and 0.54 (95% CI 0.35–0.82) for the 1‐year survival (Table S2).
Complete case analysis with the active smoking variable added to the PSW (n = 541) yielded an average HR of 0.70 (95% CI 0.29–1.66) for 30‐day mortality, 0.52 (95% CI 0.25–1.05) for 90‐day mortality, and 0.47 (95% CI 0.27–0.85) for 1‐year mortality (Table S3). Imputation of missing information on smoking resulted in an average HR of 0.60 (95% CI 0.34–1.07) for 30‐day mortality, 0.52 (95% CI 0.31–0.86) for 90‐day mortality, and 0.49 (95% CI 0.31–0.78) for 1‐year mortality (Table S4).
No significant association with 30‐day, 90‐day, or 1‐year survival was found with use of beta blockers, PPIs, or statins at admission and macrolide therapy after admission in Cox regression models with PSW using the same covariates as in prior analysis, including ASA (Tables S5, S6, S7, and S8). Statin use showed a slight trend towards protection, especially in the first 30 days, but this was nonsignificant. Sensitivity analyses on the putative effects of ASA within decades all showed a protective trend (Table S9). The apparent protective effect of ASA was more pronounced within the influenza season (December to May; Table S10).
Discussion
In this large study of bacteremic pneumococcal pneumonia spanning 45 years in a nationwide cohort, ASA use at the time of diagnosis was associated with improved survival at 90 days and 1 year in the propensity score‐weighted models. Our observations indicate a protective effect of ASA on medium‐term survival (90 days to 1 year) resulting in HRs of around 0.5–0.6. However, the proportionality assumption did not hold for the 30‐day survival, and the effect varied noticeably within the period. A significantly improved survival was noted within 7 days but not for days 7–30.
The highest death rates occurred during the first few days following diagnosis; however, rates were higher in the first days for the non‐ASA patients than the ASA patients. Even without adjustment, the survival curve indicates a plausible protective effect among patients on ASA. This group of patients is otherwise at highest risk of death, being older with more severe disease and more underlying comorbidities at diagnosis. The results were robust in various different sensitivity analyses.
Interpretation of the time‐varying effects of ASA observed here within 30 days is not straightforward. The benefit seen during the first days could be explained by a protective effect against the dramatically increased rate of acute cardiovascular events observed during the first 3 days following an acute infection [14]. Highlighting this possibility is the recommended use of ASA along with other measures in the immediate treatment of myocardial infarction [34]. The fact that ASA was not significantly protective in the 7–30‐day period is harder to explain but could be due to reduced treatment effect following the acute inflammatory episode or simply represents a lack of statistical power within the period.
Prior studies on the association of ASA with survival following pneumonia have only examined short‐term mortality (30 days) [17, 18, 35], and they have lacked information regarding causes of death. In the present study, analysis of unadjusted underlying causes of death indicated that patients with pneumonia receiving ASA did not die more frequently from cardiovascular causes than other patients despite having a four times higher IHD prevalence.
Prior observational studies on short‐term mortality (30 days) in patients with pneumonia have given inconsistent results with respect to ASA use [17, 18, 19, 20]. A recent study utilizing diagnostic codes identified a protective effect of the drug against subsequent cardiovascular complications among pneumonia patients in primary care [36]. The only randomized study examining ASA treatment following diagnosis of pneumonia was open‐label and included patients with certain cardiovascular risk factors but excluded patients with prior coronary artery disease, malignancy, and heart failure [35]. Only 198 of 1223 screened individuals were enrolled and randomized [35]. While there were significantly fewer acute coronary syndrome events and cardiovascular‐related deaths in the ASA‐treated group, the difference in all‐cause mortality at 30 days was not statistically significant (p = 0.15) [35].
A prior observational study on bloodstream infections found ASA treatment to be associated with lower mortality among those infected with Staphylococcus aureus but not Escherichia coli [37]. Possible protective effects of ASA in the setting of sepsis could therefore depend on the clinical syndrome of infection as well as the specific microbial etiology. The site of infection may also play a role, as urinary tract infections seem to have a weaker association with these complications than pneumonia [38]. Indeed, there seems to be a specific relationship between pulmonary inflammation and subsequent cardiovascular disease, as reviewed by Van Eeden et al. [39].
Cardiovascular complications in the setting of pneumonia are thought to be caused by a combination of factors, including hypoxemia, damage to the myocardium, inflammatory response, plaque destabilization, platelet activation, and diminished perfusion of coronary arteries [10, 13, 20, 40, 41]. Finally, pneumococcal antigen persistence can result in a chronic inflammatory response, which could also play a role [42, 43].
The protective effects of ASA reported here were independent of statin use, a potential confounder which has been associated with a survival benefit in pneumonia in some studies [44, 45]. Statin use was uncommon in our cohort, especially in the first half of the period. Because of the small number of patients on statins, the sensitivity analysis we performed on its effect on survival is underpowered to detect nondramatic effects. However, only 1/34 (2.9%) of patients on both statins and ASA died within 30 days, compared to the 13.5% overall mortality in the period. This raises questions regarding the possible combined protective effect of ASA and statins.
ASA is an inexpensive generic drug that is available over the counter and therefore difficult to study by analysis of prescriptions only. Many of its wide‐ranging effects are related to its inhibition of thromboxane A2 at low dose and cyclooxygenase 1, which is important in prostaglandin production [15]. ASA is known to reduce the risk of myocardial and cerebral infarction when used as secondary prevention or in individuals at high risk [16]. Furthermore, ASA has been shown to reduce the risk of acute cardiovascular events among previously healthy individuals with mildly elevated inflammatory markers [46].
Inflammation plays a part in the pathogenesis of many chronic illnesses [47, 48], and the ability of elderly patients to resolve inflammation following a triggering event such as pneumonia is often impaired, resulting in a prolonged hyperinflammatory state [49]. It is plausible that increased stress and inflammation associated with invasive pneumococcal pneumonia may both expose and accelerate undiagnosed or subclinical cardiovascular disease, with effects extending well beyond the infection episode, making ASA useful during the subsequent months. Therefore, observation extending beyond 30 days may be required to elucidate longer‐term benefits of ASA following the diagnosis of pneumonia. The potential benefits of ASA in pneumonia caused by other pathogens and in other infectious syndromes warrant further study.
Limitations
There are several limitations to our retrospective and observational study. Unobserved and unadjusted confounders or bias may have been present, although multiple sensitivity analyses to assess the robustness of the results were performed. Prior to the implementation of the ICD‐10 coding system, only the primary underlying cause of death was registered, making it more difficult to analyze other contributing causes. Further, we did not have information on medication changes during follow‐up, but such an occurrence would be expected to dilute the observed effects of ASA. The analysis was limited to the ATT. The observed effects of ASA usage could also theoretically be explained if ASA therapy leads to less severe pneumococcal pneumonia. The literature does not support this possibility [50, 51], and patients in this study who were taking ASA had, in fact, more severe illness, as determined by APACHE II. We were not able to directly adjust for a possible healthy user effect or a socioeconomic variable. However, given the public universal health care system, this effect is thought to be less notable in this population than in many other settings. Additionally, in sensitivity analyses excluding patients not taking any drugs, similar HRs were noted. The cohort studied is bacteremic pneumococcal CAP, and the results should be interpreted with that patient group in mind. Many patients on ASA have presumably been taking the drug for a long time, and those that did not tolerate it well or had adverse events can be expected to have stopped using it. It is possible that those that tolerate the drug well have some underlying favorable survival factors not otherwise adjusted for. Having information on changes to ASA therapy during follow‐up would have increased the precision of the longer‐term results.
Conclusions
In this large, nationwide, long‐term study of patients with documented bacteremic pneumococcal pneumonia, a significant survival benefit was demonstrated among patients who were receiving ASA upon admission. The accuracy of the diagnosis (with confirmed microbial etiology of the infection), medication use, and the clinical details of the underlying conditions and infection severity combined with long‐term follow‐up and cause of death grant this study novelty and robustness beyond previous work. The potential beneficial effects of ASA therapy upon admission in pneumonia patients should be studied further in other cohorts and infectious syndromes, with respect to cardiovascular complications and all‐cause mortality.
Conflict of interests
All authors declare no conflict of interests, including relevant financial interests, activities, relationships, and affiliations.
Author contributions
Kristján G. Rögnvaldsson: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; resources; software; validation; visualization; writing – original draft; writing – review and editing. Agnar Bjarnason: Conceptualization; funding acquisition; investigation; methodology; project administration; software; supervision; writing – original draft; writing – review and editing. Karl Kristinsson: Data curation; investigation; software; writing – review and editing. Hörður T. Bragason: Data curation; investigation; software; writing – review and editing. Helga Erlendsdóttir: Cenceptualization; data curation; project administration; resources; software; supervision; writing‐review and editing. Guðmundur Þorgeirsson: Conceptualization; supervision; visualization; writing – review and editing. Magnús Gottfreðsson: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing – original draft; writing – review and editing.
Supporting information
Figure S1: Balance in groups before (red) and after (black) propensity score weighting (PSW) for the main 30‐day, 90‐day and 1‐year survival analysis using Cox regression with PSW adjustment.
Figure S2: Time‐varying effect of ASA.
Table S1: Unadjusted crude mortality rates for the group receiving acetylsalicylic acid compared to the group not receiving acetylsalicylic acid at the time of admission.
Table S2: Cox regression on the association of acetylsalicylic acid with survival with direct covariate adjustment.
Table S3: Complete case analysis including all patients with current smoking information available (n = 542, thereof 84 receiving acetylsalicylic acid); Cox regression on the association of acetylsalicylic acid with survival performed with propensity score weighting*.
Table S4: Imputation of missing information (MICE) on active smoking followed by propensity score weighting on the imputed datasets using the “within” approach; Cox regression was then performed, and finally results were pooled.
Table S5: The association of beta blockers with survival analyzed with Cox regression using propensity score weighting with previously specified covariates, including acetylsalicylic acid.
Table S6: The association of proton pump inhibitors with survival analyzed with Cox regression using propensity score weighting with previously specified covariates including acetylsalicylic acid.
Table S7: The association of statins with survival analyzed with Cox regression using propensity score weighting with previously specified covariates, including acetylsalicylic acid.
Table S8: The association of macrolide therapy with survival; Cox regression using propensity score weighting.
Table S9: The association of ASA with mortality, when dataset is split by decades from 1990 onwards.
Table S10: The dataset was divided into two by influenza season.
Supplementary Text: Variable Selection for the Propensity Score.
Acknowledgments
We thank Ubaldo Benitez Hernandez, Natural Scientist, at The Science Department/Biostatics Section at Landspitali for help with statistical analysis, and Sandra Halldórsdóttir, MD, at Landspitali for her contribution in terms of data collection. Neither of them received compensation for their role in the study.
The Icelandic Centre for Research (Rannís, grant number 217716‐051), The Doctoral Grants of The University of Iceland Research Fund, The Scientific Fund of Landspitali—The National University Hospital of Iceland, The Scandinavian Society for Antimicrobial Chemotherapy Foundation and the Foundation of St. Josef´s Hospital funded Kristján Godsk Rögnvaldsson´s work on this project. The funding sources had no role in the study's design, conduct, or reporting.
Rögnvaldsson KG, Bjarnason A, Kristinsson K, Bragason HT, Erlendsdóttir H, Þorgeirsson G, et al. Acetylsalicylic acid use is associated with improved survival in bacteremic pneumococcal pneumonia: A long‐term nationwide study. J Intern Med. 2022;292:321–332.
References
- 1. Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bjarnason A, Westin J, Lindh M, Andersson L‐M, Kristinsson KG, Löve A, et al. Incidence, etiology, and outcomes of community‐acquired pneumonia: a population‐based study. Open Forum Infect Dis. 2018;5(2):ofy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cilloniz C, Martin‐Loeches I, Garcia‐Vidal C, San Jose A, Torres A. Microbial etiology of pneumonia: epidemiology, diagnosis and resistance patterns. Int J Mol Sci. 2016;17(12):2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community‐acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aston SJ, Ho A, Jary H, Huwa J, Mitchell T, Ibitoye S, et al. Etiology and risk factors for mortality in an adult community‐acquired pneumonia cohort in Malawi. Am J Respir Crit Care Med. 2019;200(3):359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shoar S, Musher DM. Etiology of community‐acquired pneumonia in adults: a systematic review. Pneumonia. 2020;12(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Violi F, Cangemi R, Falcone M, Taliani G, Pieralli F, Vannucchi V, et al. Cardiovascular complications and short‐term mortality risk in community‐acquired pneumonia. Clin Infect Dis. 2017;64(11):1486–93. [DOI] [PubMed] [Google Scholar]
- 8. Corrales‐Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community‐acquired pneumonia incidence, timing, risk factors, and association with short‐term mortality. Circulation. 2012;125(6):773–81. [DOI] [PubMed] [Google Scholar]
- 9. Corrales‐Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C‐CH, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Musher DM, Abers MS, Corrales‐Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380(2):171–6. [DOI] [PubMed] [Google Scholar]
- 11. Shenoy AT, Beno SM, Brissac T, Bell JW, Novak L, Orihuela CJ. Severity and properties of cardiac damage caused by Streptococcus pneumoniae are strain dependent. PLoS One. 2018;13(9):e0204032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis. 2017;65(10):1736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Restrepo MI, Reyes LF. Pneumonia as a cardiovascular disease. Respirology. 2018;23(3):250–9. [DOI] [PubMed] [Google Scholar]
- 14. Ohland J, Warren‐Gash C, Blackburn R, Mølbak K, Valentiner‐Branth P, Nielsen J, et al. Acute myocardial infarctions and stroke triggered by laboratory‐confirmed respiratory infections in Denmark, 2010 to 2016. Eurosurveillance. 2020;25(17):1900199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desborough MJR, Keeling DM. The aspirin story—from willow to wonder drug. Br J Haematol. 2017;177(5):674–83. [DOI] [PubMed] [Google Scholar]
- 16. Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falcone M, Russo A, Shindo Y, Farcomeni A, Pieralli F, Cangemi R, et al. A hypothesis‐generating study of the combination of aspirin plus macrolides in patients with severe community–acquired pneumonia. Antimicrob Agents Chemother. 2019;63(2):e01556–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falcone M, Russo A, Cangemi R, Farcomeni A, Calvieri C, Barillà F, et al. Lower mortality rate in elderly patients with community‐onset pneumonia on treatment with aspirin. J Am Heart Assoc. 2015;4(1):e001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in community‐acquired pneumonia. Am J Med. 2008;121(11):1002–7.e1. [DOI] [PubMed] [Google Scholar]
- 20. Cangemi R, Casciaro M, Rossi E, Calvieri C, Bucci T, Calabrese CM, et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J Am Coll Cardiol. 2014;64(18):1917–25. [DOI] [PubMed] [Google Scholar]
- 21. RStudio Team . RStudio: Integrated development for R. http://www.rstudio.com/ (2021). Boston, MA: RStudio, Inc. Accessed 15 Oct 2021. [Google Scholar]
- 22. R Core Team . R: A language and environment for statistical computing. https://www.R‐project.org/ (2021). Vienna, Austria: R Foundation for Statistical Computing. Accessed 15 Oct 2021. [Google Scholar]
- 23. Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis‐Oudshoorn CGM. Time‐varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergstra SA, Sepriano A, Ramiro S, Landewé R. Three handy tips and a practical guide to improve your propensity score models. RMD Open. 2019;5(1):e000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greifer N. WeightIt: Weighting for covariate balance in observational studies. R package version 0.10.2. 2020.
- 28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2013;25(5):1938–54. [DOI] [PubMed] [Google Scholar]
- 29. DuGoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9. [DOI] [PubMed] [Google Scholar]
- 31. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–8. [DOI] [PubMed] [Google Scholar]
- 32. van Smeden M, Moons KGM, de Groot JAH, Collins GS, Altman DG, Eijkemans MJC, et al. Sample size for binary logistic prediction models: beyond events per variable criteria. Stat Methods Med Res. 2018;28(8):2455–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pishgar F, Greifer N, Leyrat C, Stuart E. MatchThem:: matching and weighting after multiple imputation. The R Journal. 2021;13/2(2):292–305. [Google Scholar]
- 34. Collins R, Peto R, Baigent C, Sleight P. Aspirin, heparin, and fibrinolytic therapy in suspected acute myocardial infarction. N Engl J Med. 1997;336(12):847–60. [DOI] [PubMed] [Google Scholar]
- 35. Oz F, Gul S, Kaya MG, Yazici M, Bulut I, Elitok A, et al. Does aspirin use prevent acute coronary syndrome in patients with pneumonia: multicenter prospective randomized trial. Coron Artery Dis. 2013;24(3):231–37. [DOI] [PubMed] [Google Scholar]
- 36. Hamilton F, Arnold D, Henley W, Payne RA. Aspirin reduces cardiovascular events in patients with pneumonia: a prior event rate ratio analysis in a large primary care database. Eur Respir J. 2021;57(2):2002795. [DOI] [PubMed] [Google Scholar]
- 37. Osthoff M, Sidler JA, Lakatos B, Frei R, Dangel M, Weisser M, et al. Low‐dose acetylsalicylic acid treatment and impact on short‐term mortality in Staphylococcus aureus bloodstream infection: a propensity score–matched cohort study. Crit Care Med. 2016;44(4):773–81. [DOI] [PubMed] [Google Scholar]
- 38. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–8. [DOI] [PubMed] [Google Scholar]
- 39. Van Eeden S, Leipsic J, Paul Man SF, Sin DD. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med. 2012;186(1):11–6. [DOI] [PubMed] [Google Scholar]
- 40. Rae N, Finch S, Chalmers JD. Cardiovascular disease as a complication of community‐acquired pneumonia. Curr Opin Pulm Med. 2016;22(3):212–8. [DOI] [PubMed] [Google Scholar]
- 41. Mankowski RT, Yende S, Angus DC. Long‐term impact of sepsis on cardiovascular health. Intensive Care Med. 2019;45(1):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feldman C, Normark S, Henriques‐Normark B, Anderson R. Pathogenesis and prevention of risk of cardiovascular events in patients with pneumococcal community‐acquired pneumonia. J Intern Med. 2019;285(6):635–52. [DOI] [PubMed] [Google Scholar]
- 43. Feldman C, Anderson R. Platelets and their role in the pathogenesis of cardiovascular events in patients with community‐acquired pneumonia. Front Immunol. 2020;11:2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nielsen AG, Nielsen RB, Riis AH, Johnsen SP, Sørensen HT, Thomsen RW. The impact of statin use on pneumonia risk and outcome: a combined population‐based case‐control and cohort study. Critical Care. 2012;16(4):R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;55(11):1466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9. [DOI] [PubMed] [Google Scholar]
- 47. Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes. 2003;27(3):S29–34. [DOI] [PubMed] [Google Scholar]
- 48. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 49. Doyle R, Sadlier DM, Godson C. Pro‐resolving lipid mediators: agents of anti‐ageing? Semin Immunol. 2018;40:36–48. [DOI] [PubMed] [Google Scholar]
- 50. Chen YC, Chen YY, Yeh HW, Yeh T‐Y, Huang J‐Y, Liao P‐L, et al. Association between aspirin use and decreased risk of pneumonia in patients with cardio‐cerebra‐vascular ischemic disease: a population‐based cohort study. Front Public Health. 2021;9:625834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eisen DP, Leder K, Woods RL, Lockery JE, McGuinness SL, Wolfe PR, et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, double‐blind, placebo‐controlled primary prevention trial. Lancet Respir Med. 2021;9:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Balance in groups before (red) and after (black) propensity score weighting (PSW) for the main 30‐day, 90‐day and 1‐year survival analysis using Cox regression with PSW adjustment.
Figure S2: Time‐varying effect of ASA.
Table S1: Unadjusted crude mortality rates for the group receiving acetylsalicylic acid compared to the group not receiving acetylsalicylic acid at the time of admission.
Table S2: Cox regression on the association of acetylsalicylic acid with survival with direct covariate adjustment.
Table S3: Complete case analysis including all patients with current smoking information available (n = 542, thereof 84 receiving acetylsalicylic acid); Cox regression on the association of acetylsalicylic acid with survival performed with propensity score weighting*.
Table S4: Imputation of missing information (MICE) on active smoking followed by propensity score weighting on the imputed datasets using the “within” approach; Cox regression was then performed, and finally results were pooled.
Table S5: The association of beta blockers with survival analyzed with Cox regression using propensity score weighting with previously specified covariates, including acetylsalicylic acid.
Table S6: The association of proton pump inhibitors with survival analyzed with Cox regression using propensity score weighting with previously specified covariates including acetylsalicylic acid.
Table S7: The association of statins with survival analyzed with Cox regression using propensity score weighting with previously specified covariates, including acetylsalicylic acid.
Table S8: The association of macrolide therapy with survival; Cox regression using propensity score weighting.
Table S9: The association of ASA with mortality, when dataset is split by decades from 1990 onwards.
Table S10: The dataset was divided into two by influenza season.
Supplementary Text: Variable Selection for the Propensity Score.


