Abstract
Aims
To analyse the prevalence of prescribed medications among people with type 2 diabetes, their relationship to HbA1c levels and transitions between medications.
Methods
The data included all 18‐ to 85‐year‐old adults with type 2 diabetes (identified from the electronic health records), who lived in North Karelia, Finland, between 2013 and 2019. Type 2 diabetes medication was defined based on prescriptions. Logistic and linear regressions with generalized estimating equations were used to assess the differences between years.
Results
Metformin as a monotherapy was the most used medication (33%–35%) with the largest percentage of those in good glycaemic control. After metformin, the most used medications were long‐acting and short‐acting insulin and gliptin (16%–24% per group). In insulin groups, there were the smallest percentage of people in good glycaemic control. The use of SGLT2‐i increased most during the follow‐up (from 1.6% to 11%), but at the same time the percentage of those meeting the target HbA1c level decreased the most (from 83% to 53%). The use of GLP‐1 RA and other medications were under 3.5%. SGLT2‐i and insulin were the most stable medication groups. The most common transitions were from SGLT2‐i to long‐acting insulin and between insulin groups.
Conclusions
The sequencing of prescribing additional type 2 diabetes medication or replacing current medication with new ones seems to occur according to guidelines. However, more attention should be paid to the intensification of treatment and the possibilities for new treatment choices in the management of T2D taking into account the persons’ characteristics.
Keywords: care, HbA1c, intensification, medicines, treatment, type 2 diabetes
Novelty statement
What is already known?
Evidence‐based guidelines provide recommendations about the selection of appropriate glucose‐lowering medication to achieve treatment targets.
What this study has found?
The sequencing of prescribing additional type 2 diabetes medication or replacing current medication with new ones seems to occur according to the guidelines in North Karelia, Finland. However, challenges exist in the good management of disease progression and the implementation of insulin treatment.
What are the implications of the study?
Attention should be paid to the intensification of type 2 diabetes treatment, better inclusion of new treatments and appropriate support for self‐care especially concerning insulin treatment.
1. BACKGROUND
Type 2 diabetes is a multisystem disease which is characterized by the development of macro‐ and microvascular complications. 1 To avoid or delay the complications after a type 2 diabetes diagnosis, it is important to aim at achieving good glycaemic, lipid and blood pressure control by proper medication, good self management and regular monitoring. 2 , 3 The Finnish current care guidelines for the management of type 2 diabetes recommend that the goal of glycaemic control should be HbA1c less than 53 mmol/mol or 7%, but the targets should be tailored individually based on the patient’s risk factor levels, such as their age, and the duration and severity of the disease. 3 In addition, several factors such as the degree of hyperglycaemia, comorbidities and patient preferences need to be considered separately when choosing a diabetic medication. 2 , 3 , 4 , 5 , 6
Therefore, healthcare professionals have a space for the interpretation of how to set customized HbA1c objectives and how to select the appropriate first‐, second‐ or even third‐line treatment for each individual with type 2 diabetes. In addition, the range of antidiabetic agents to treat type 2 diabetes has increased within the last decade and professionals have more treatment options than ever. 7 , 8 Although the evidence‐based guidelines have been developed to assist professionals with the clinical decision‐making, the difficulties with keeping up to date with changing recommendations and new treatment options have been shown to be barriers to implementing recommendations and achieving treatment targets. 9
In Finland, information is scarce on the prevalence of prescribed medications and their relationship to glucose control among people with type 2 diabetes. The study by Ramirez 10 identified differences in the medications used by age and gender as well as in glycaemic control according to age and treatment options. Based on the results, it seemed that the description practices followed the main principles of guidelines. However, the study used cross‐sectional data from Finnish health care and pointed out the need for longitudinal analyses. This study aimed to analyse the prevalence of the prescribed medications, their relationship to HbA1c levels and transitions between medications during a 6‐year follow‐up in North Karelia, in Finland.
2. DATA AND METHODS
The data include all adults with type 2 diabetes aged from 18 to 85 years who lived in North Karelia, Finland, between 2013 and 2019. They were identified from the regional electronic health records using ICD‐10 code E11. Local clinicians checked the electronic health records of patients having overlapping diagnoses E10/E11 and confirmed the correct one. Newly diagnosed or deceased were excluded from that year’s follow‐up so that all those included in the analyses had full follow‐up years annually. Age, sex and HbA1c measurements of the study population were also retrieved from the electronic health records. Only those who had HbA1c measurements (annual range, Table 1) were included in the analyses. Every year, the values of the last HbA1c measurement were used in the analysis.
TABLE 1.
Basic characteristics of the study population, all 18–85 years old in 2013–2019 with HbA1c measurement
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | p‐value a | |
|---|---|---|---|---|---|---|---|---|
| N | 8059 | 8365 | 8519 | 8818 | 9015 | 7391 | 7690 | |
| Sex, % of women | 47 | 46 | 46 | 45 | 45 | 45 | 44 | 0.019 |
| Age, mean ± SD | 68 ± 11 | 68 ± 10 | 68 ± 10 | 68 ± 10 | 68 ± 10 | 69 ± 10 | 69 ± 10 | <0.001 |
| Age <65 years, % | 36 | 35 | 34 | 33 | 32 | 29 | 29 | <0.001 |
p‐values for the differences between years from logistic (percentages) and linear (mean) regression with GEE.
Subgroup analyses were carried out for the 2013 cohort, that is, those who were diagnosed with type 2 diabetes before 2013 and who were still in the follow‐up in 2019 and were measured at least every second year to find out if having the same patient cohort during the time and regular measuring makes any difference to the results. The year 2013 cohort was also divided into two parts according to the first HbA1c measurement: those who were at target (HbA1c <53 mmol/mol or 7%) and those not at target in the beginning of the follow‐up. These two datasets were analysed separately to see how they differ from each other.
Type 2 diabetes medication was defined based on prescriptions using Anatomic Therapeutical Chemical (ATC) codes. The following treatment variables were used in the classification: biguanides (Metformin; ATC code A10BA02), gliptins or dipeptidyl peptidase 4 inhibitors (Gliptins; ATC code A10BH), sodium‐glucose co‐transporter 2 inhibitors (SGLT2‐i; ATC code A10BK), glucagon‐like peptide‐1 analogues (GLP‐1 RA; ATC code A10BJ), long‐acting insulin (ATC code A10AC or A10AE) and short‐acting insulin (ATC code A10AB or A10AD). The following classification was used in analyses based on the previous groups: (1) no medication, (2) metformin as a monotherapy, (3) gliptins without SGLT2‐i or GLP‐1 RA or insulin, (4) SGLT2‐i without GLP‐1 RA or insulin, (5) GLP‐1 RA without insulin, (6) long‐acting insulin without short‐acting insulin, (7) short‐acting insulin, and (8) other, such as sulfonylureas, thiazolidinediones (pioglitazones) and other blood‐glucose‐lowering medications (repaglinide). The next category can always include medicines from the previous category except for the category other, which includes only medications not mentioned previously.
2.1. Statistical methods
Descriptive statistics such as frequencies, percentages, mean and standard deviation were used to describe the data. Stability of medication use was reported as a percentage of those taking medications from the same medication group in subsequent years. Transition between medication groups was reported as a percentage of those changing their medication from one group to another in subsequent years. Percentage points (%p) were used for the differences between percentages. Logistic and linear regressions with generalized estimating equations (GEE) were used to assess the differences between the years in percentages and mean values of HbA1c. Subject id was used as a subject variable and year as an explanatory factor variable in these models. GEE can model data where there is more than one observation per person. The R language and environment for statistical computing (Version 4.0.3) (ref) and IBM SPSS Statistics for Windows (Version 27.0) were used in the statistical analyses. 11 , 12 p < 0.05 were regarded as statistically significant.
3. RESULTS
The data included individuals with at least one HbA1c measurement in the follow‐up period, with an annual number ranging from 7391 to 9015 (Table 1). The annual percentage of women varied between 44% and 47%. The mean±SD age increased from 68 ± 11 years to 69 ± 10 years and the percentage of those under 65 years old decreased from 36% to 29% during the follow‐up period.
3.1. Type 2 diabetes medication among the entire study population during the follow‐up
The annual percentage of those with no medication varied between 8.2% (2015) and 9.1% (2019) during 2013–2019 (Table 2). Metformin as a monotherapy was the most common medication; the percentage of those getting metformin as a monotherapy was quite stable over the years and was around 33%–35%. Medication including short‐acting insulin was the second most common treatment in 2013 (18%), but its percentage declined quite smoothly during the follow‐up time to the fourth position at 12% in 2019. The percentage of gliptin users started from 17% and increased in the first couple of years up to 19% but declined back to its lowest level in 2019 (14%). The percentage of those using long‐acting insulin without short‐acting insulin was quite stable (16%–17%) moving from the fourth position to the second position. The users of SGLT2‐i started with 2.0% in 2013 but their percentage increased during the follow‐up time up to 11%. The percentage of GLP‐1 RA users was quite stable at 2%–3% during the follow‐up period. The percentage of other medication users declined from 3.3% to 0.5% during the follow‐up time.
TABLE 2.
Number and percentage of individuals, mean HbA1c, percentage of those at the target HbA1c level less than 53 mmol/mol (7%) and with a poor level of HbA1c more or equal to 75 mmol/mol (9%) in each medication group a for the entire study population
| Medication group | Statistic | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | p‐value b |
|---|---|---|---|---|---|---|---|---|---|
| No medication | N (%) | 665 (8.3) | 700 (8.4) | 701 (8.2) | 765 (8.7) | 753 (8.4) | 653 (8.8) | 703 (9.1) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 41 (5.9) | 42 (6.0) | 41 (5.9) | 41 (5.9) | 42 (6.0) | 41 (5.9) | 42 (6.0) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 96 | 95 | 96 | 97 | 96 | 98 | 97 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 1.1 | 0.9 | 0.7 | 0.3 | 0.4 | 0.3 | 0.6 | 0.392 | |
| Metformin only | N (%) | 2713 (34) | 2879 (34) | 2923 (34) | 2994 (34) | 3156 (35) | 2418 (33) | 2574 (34) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 42 (6.0) | 44 (6.1) | 44 (6.1) | 43 (6.1) | 44 (6.2) | 44 (6.2) | 44 (6.2) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 95 | 93 | 93 | 94 | 93 | 92 | 91 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 0.4 | 0.9 | 0.8 | 0.6 | 0.5 | 0.6 | 0.6 | <0.001 | |
| Gliptin without SGLT2‐i or GLP−1 RA or insulin | N (%) | 1340 (17) | 1508 (18) | 1636 (19) | 1676 (19) | 1532 (17) | 1120 (15) | 1061 (14) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 47 (6.4) | 48 (6.6) | 48 (6.6) | 48 (6.5) | 49 (6.6) | 48 (6.5) | 49 (6.6) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 82 | 78 | 76 | 79 | 76 | 79 | 75 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 2.2 | 2.2 | 2.7 | 2.1 | 3.2 | 2.5 | 2.5 | <0.001 | |
| SGLT2‐i without GLP−1 RA or insulin | N (%) | 132 (1.6) | 176 (2.1) | 214 (2.5) | 404 (4.6) | 631 (7.0) | 678 (9.2) | 854 (11) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 47 (6.5) | 48 (6.6) | 50 (6.7) | 52 (6.9) | 53 (7.0) | 53 (7.0) | 53 (7.0) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 83 | 74 | 68 | 62 | 56 | 56 | 53 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 3.0 | 0.6 | 3.3 | 3.5 | 4.8 | 4.0 | 4.2 | 0.001 | |
| GLP−1 RA without insulin | N (%) | 164 (2.0) | 179 (2.1) | 194 (2.3) | 205 (2.3) | 225 (2.5) | 203 (2.7) | 238 (3.1) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 49 (6.6) | 50 (6.8) | 54 (7.0) | 54 (7.1) | 55 (7.2) | 57 (7.3) | 57 (7.4) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 73 | 68 | 55 | 54 | 49 | 43 | 41 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 1.8 | 3.4 | 5.7 | 6.3 | 6.7 | 7.9 | 8.4 | 0.042 | |
| Long‐acting insulin without short acting insulin | N (%) | 1327 (17) | 1333 (16) | 1374 (16) | 1373 (16) | 1449 (16) | 1277 (17) | 1293 (17) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 56 (7.2) | 58 (7.4) | 58 (7.5) | 58 (7.5) | 60 (7.6) | 60 (7.6) | 60 (7.7) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 49 | 42 | 39 | 39 | 34 | 35 | 32 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 11 | 12 | 12 | 13 | 15 | 14 | 15 | 0.004 | |
| Short‐acting insulin | N (%) | 1456 (18) | 1394 (17) | 1335 (16) | 1290 (15) | 1191 (13) | 991 (13) | 932 (12) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 61 (7.7) | 62 (7.8) | 62 (7.8) | 62 (7.8) | 64 (8.0) | 63 (7.9) | 65 (8.1) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 33 | 31 | 29 | 28 | 25 | 27 | 23 | <0.001 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 17 | 20 | 19 | 19 | 21 | 19 | 23 | 0.022 | |
| Other | N (%) | 262 (3.3) | 196 (2.3) | 142 (1.7) | 112 (1.3) | 78 (0.9) | 51 (0.7) | 35 (0.5) | <0.001 |
| Mean HbA1c, mmol/mol (%) | 46 (6.4) | 46 (6.4) | 46 (6.4) | 45 (6.2) | 46 (6.4) | 45 (6.3) | 47 (6.5) | <0.001 | |
| HbA1c < 53 mmol/mol (7%), % | 87 | 84 | 85 | 85 | 85 | 84 | 86 | 0.002 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 2.3 | 1.0 | 0.7 | 0.9 | 1.3 | 0.0 | 5.7 | 0.379 |
Each category can include medications from the previous categories except the category other, which includes only medications not mentioned previously.
p‐values for the differences between years from logistic (percentages) and linear (mean) regression with GEE.
3.2. Medication in relation to HbA1c levels among the entire study population during the follow‐up
Persons who had no medication most often achieved a target level of less than 53 mmol/mol (7%) of HbA1c (Table 2). The annual percentage of those meeting the target HbA1c level were quite stable over the years, ranging between 95% and 98%. For metformin users, the annual percentage of those at the target HbA1c level declined −3.5%p during the follow‐up time from 95% to 91%. SGLT2‐i users had biggest decline of −31%p in annual percentage of those meeting the target level during the follow‐up starting from 83%, together with biggest increase of 9.5%p in the percentage of users. Gliptin users started from a lower level of 82% in the percentage of those who were the target compared with SGLT2‐i users but had a smaller decline of −6.4%p during the follow‐up time. Insulin users had the worst situation in the percentage of those meeting the target HbA1c level: those using long‐acting insulin without short‐acting insulin had a larger decline during the follow‐up time (−17%p from 49% to 32%) compared with those using also short‐acting insulin (−10%p from 33% to 23%), but it stayed at a higher level during the whole follow‐up time. On the other hand, the percentage of users also declined among those using short‐acting insulin, but the percentage was quite stable among those using long‐acting insulin without short‐acting insulin.
3.3. Medication and its relation to HbA1c levels in the year 2013 cohort
The number of people in the year 2013 cohort was 4587 with percentage of women 46% and mean±SD age 64 ± 9 in 2013. The percentage of those with no medication was lower when compared with the entire study population (Table 3), and it was decreasing, whereas in the overall data it was stable (Figure 1a,b). The percentage of those using metformin as a monotherapy started from the same level as in the overall data but declined during the follow‐up by about −12%p. In contrast, the percentage of those using long‐acting insulin without short‐acting insulin increased by about 8%p even though it was quite stable in the overall data and the percentage of those using short‐acting insulin increased a bit rather than decreased.
TABLE 3.
Number and percentage of individuals, mean HbA1c, percentage of those at the target HbA1c level (<53 mmol/mol or 7%) and with a poor HbA1c level (>=75 mmol/mol or 9%) in each medication group a for the year’s 2013 cohort still followed in 2019 (N = 4587)
| Medication group | Statistic | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|
| No medication | N (%) | 241 (5.9) | 226 (5.3) | 199 (4.7) | 188 (4.4) | 174 (4.1) | 155 (3.9) | 155 (3.9) |
| Mean HbA1c, mmol/mol (%) | 40 (5.8) | 42 (6.0) | 41 (5.9) | 41 (5.9) | 41 (5.9) | 42 (6.0) | 42 (6.0) | |
| HbA1c < 53 mmol/mol (7%), % | 97 | 97 | 99 | 97 | 95 | 96 | 99 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 1.2 | 0.9 | 0.5 | 0.0 | 0.0 | 0.6 | 0.0 | |
| Metformin only | N (%) | 1458 (36) | 1454 (34) | 1342 (32) | 1209 (29) | 1174 (28) | 988 (25) | 933 (24) |
| Mean HbA1c, mmol/mol (%) | 42 (6.0) | 44 (6.2) | 44 (6.1) | 43 (6.1) | 44 (6.2) | 44 (6.2) | 45 (6.2) | |
| HbA1c < 53 mmol/mol (7%), % | 96 | 93 | 94 | 95 | 93 | 93 | 90 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 0.5 | 1.0 | 0.5 | 0.2 | 0.2 | 0.0 | 0.2 | |
| Gliptin without SGLT2‐i or GLP−1 RA or insulin | N (%) | 750 (18) | 837 (20) | 877 (21) | 886 (21) | 802 (19) | 684 (17) | 623 (16) |
| Mean HbA1c, mmol/mol (%) | 46 (6.4) | 48 (6.6) | 48 (6.5) | 48 (6.5) | 48 (6.6) | 48 (6.5) | 48 (6.6) | |
| HbA1c < 53 mmol/mol (7%), % | 83 | 77 | 78 | 80 | 77 | 80 | 76 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 1.9 | 1.8 | 2.1 | 1.7 | 2.1 | 1.5 | 1.4 | |
| SGLT2‐i without GLP−1 RA or insulin | N (%) | 94 (2.3) | 122 (2.9) | 146 (3.5) | 236 (5.6) | 335 (7.9) | 392 (9.8) | 429 (11) |
| Mean HbA1c, mmol/mol (%) | 46 (6.3) | 47 (6.5) | 49 (6.7) | 51 (6.8) | 53 (7.0) | 53 (7.0) | 54 (7.1) | |
| HbA1c < 53 mmol/mol (7%), % | 88 | 80 | 70 | 64 | 57 | 50 | 49 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 2.1 | 0.0 | 0.7 | 2.1 | 3.3 | 2.6 | 3.3 | |
| GLP−1 RA without insulin | N (%) | 126 (3.1) | 133 (3.1) | 140 (3.3) | 141 (3.3) | 148 (3.5) | 147 (3.7) | 158 (4.0) |
| Mean HbA1c, mmol/mol (%) | 49 (6.6) | 51 (6.8) | 53 (7.0) | 54 (7.1) | 56 (7.2) | 56 (7.3) | 58 (7.4) | |
| HbA1c < 53 mmol/mol (7%), % | 71 | 65 | 52 | 50 | 45 | 40 | 39 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 0.8 | 3.0 | 5.0 | 5.0 | 7.4 | 6.1 | 8.9 | |
| Long‐acting insulin without short‐acting insulin | N (%) | 654 (16) | 723 (17) | 771 (18) | 821 (19) | 885 (21) | 931 (23) | 943 (24) |
| Mean HbA1c, mmol/mol (%) | 55 (7.2) | 58 (7.4) | 58 (7.5) | 58 (7.5) | 60 (7.6) | 60 (7.7) | 61 (7.7) | |
| HbA1c < 53 mmol/mol (7%), % | 53 | 43 | 37 | 37 | 31 | 32 | 29 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 9.0 | 11 | 11 | 12 | 13 | 15 | 15 | |
| Short‐acting insulin | N (%) | 643 (16) | 662 (16) | 669 (16) | 681 (16) | 682 (16) | 663 (17) | 680 (17) |
| Mean HbA1c, mmol/mol (%) | 61 (7.7) | 61 (7.7) | 62 (7.8) | 62 (7.9) | 64 (8.0) | 63 (7.9) | 66 (8.2) | |
| HbA1c < 53 mmol/mol (7%), % | 31 | 30 | 26 | 24 | 23 | 25 | 20 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 16. | 17 | 17 | 19 | 20 | 20 | 24 | |
| Other | N (%) | 127 (3.1) | 101 (2.4) | 82 (1.9) | 68 (1.6) | 45 (1.1) | 39 (1.0) | 26 (0.7) |
| Mean HbA1c, mmol/mol (%) | 45 (6.2) | 45 (6.3) | 45 (6.3) | 43 (6.1) | 46 (6.3) | 45 (6.2) | 46 (6.3) | |
| HbA1c < 53 mmol/mol (7%), % | 92 | 87 | 92 | 90 | 87 | 82 | 96 | |
| HbA1c ≥ 75 mmol/mol (9%), % | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 |
Each category can include medications from the previous categories except the category other, which includes only medications not mentioned previously.
FIGURE 1.
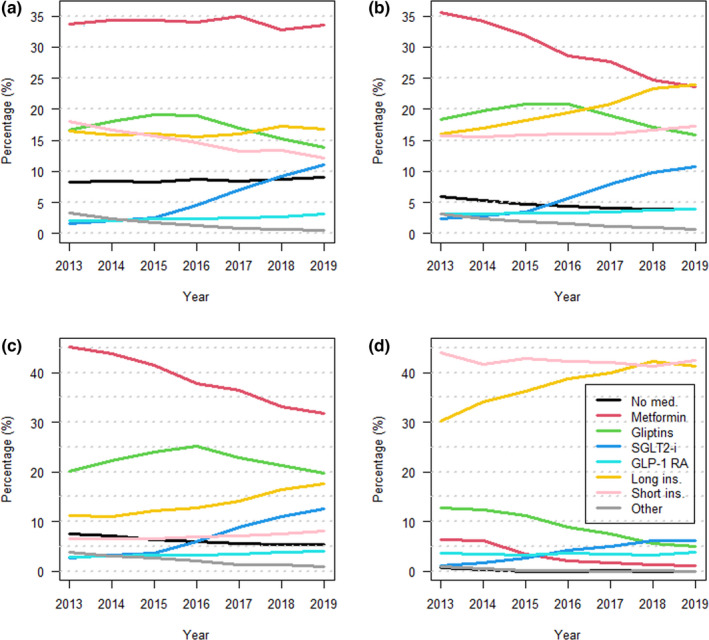
Annual percentages of individuals with type 2 diabetes in each glucose‐lowering medication groups: no medication (black), metformin as a monotherapy (red), gliptins without SGLT2 or GLP‐1 or insulin (green), SGLT2 without GLP‐1 or insulin (blue), GLP‐1 without insulin (light blue), long‐acting insulin without short‐acting insulin (yellow), short‐acting insulin (pink), and other (grey). Each category can include medications from the previous categories except the category other, which includes only medications not mentioned previously. Figure (a) presents the entire study population, (b) 2013 cohort, (c) 2013 cohort with HbA1c < 7% (53 mmol/mol) or (d) 2013 cohort with HbA1c >=7% (53 mmol/mol) at the beginning of follow‐up
Those who were not meeting the treatment target at the beginning of follow‐up time most often had insulin treatment: the percentage of users of short‐acting and/or long‐acting insulin together was around 80% during the whole follow‐up time (Figure 1d). Among those who were at the target level of HbA1c at the beginning, the most common medications were metformin and/or gliptin (altogether about 50%–65% during the follow‐up) (Figure 1c). The third most common medicine was long‐acting insulin without short‐acting insulin, covering 70% of the study population together with the previous ones.
3.4. Changes in medication during the follow‐up (transitions between medication groups) among the entire study population
When looking at the annual changes in medication among all subsequent measurements, SGLT2‐i was found to be the most stable medication group in the two first follow‐up years (stability 96% and 95% correspondingly, annual minimum 84%), followed by short‐acting insulin in the third year (stability 94%, annual minimum 90%) and long‐acting insulin without short‐acting insulin in the three last years (stability 93%, annual minimum 90%). The most common transition from SGLT2‐i was to long‐acting insulin (Table 4). In the insulin groups, the most common transitions were between each other. The annual stability in the metformin group ranged from 88% to 91% and the most common transitions were to gliptin or SGLT2‐i groups. GLP‐1 RA was nearly as stable as metformin with the most common transitions to long‐acting insulin or gliptins. The annual stability of gliptins ranged from 81% to 88% with the most common transitions to long‐acting insulin or SGLT2‐i. The annual stability in the no medication group ranged from 79% to 86% with the most common transitions to metformin and gliptins. The lowest stability was in the other medication group with transitions to gliptins, long‐acting insulin, metformin, SGLT2‐i and no medication groups.
TABLE 4.
Transitions between medication groups
| Medication group | Stability, annual range (%) | Most common transitions, annual range (%) | Other transitions per group, max (%) |
|---|---|---|---|
| No medication | 79–86 |
Metformin (3–8), Gliptins (1–6) |
2.1 |
| Metformin only | 88–91 |
Gliptins (3–8), SGLT2‐i (0–5) |
1.6 |
| Gliptins without SGLT2‐i or GLP−1 RA or insulin | 81–88 |
Long‐acting insulin (4–8), SGLT2‐i (1–11) |
2.0 |
| SGLT2‐i without GLP−1 RA or insulin | 84–96 | Long‐acting insulin (4–8) | 3.5 |
| GLP−1 RA without insulin | 83–91 | Long‐acting insulin (4–12), Gliptins (0–5) | 2.7 |
| Long‐acting insulin | 90–93 | Short‐acting insulin (5–7) | 1.3 |
| Short‐acting insulin | 90–94 | Long‐acting insulin (5–7) | 1.5 |
| Other | 67–75 |
Gliptins (8–19), Long‐acting insulin (2–6), Metformin only (3–5), SGLT2‐i (0–9), No medication (0–8) |
3.4 |
4. DISCUSSION
Metformin is recommended as the first‐line pharmacological treatment for type 2 diabetes. As the disease progresses, insulin may be needed to control blood glucose levels. Prior to initiating insulin therapy, treatment should be intensified with DPP4 inhibitors or with the newer medicines, such as SGLT2 inhibitors or GLP‐1 receptor agonists. The new international guidelines even emphasize the use of SGLT2 inhibitors or GLP1 receptor agonists as a second treatment choice. 2 , 3 , 4 , 5 , 6 Especially for people with arterial disease, heart failure, or diabetic kidney disease, treatment with an SGLT2 inhibitor or GLP‐1 receptor agonist is the most preferred option. 13 , 14 In this electronic health records‐based study, we investigated the real‐world utilization of type 2 diabetes medications, their relation to HbA1c levels and transitions between medications among prevalent adult population with type 2 diabetes in North Karelia, Finland.
Based on the results, lifestyle interventions and metformin were in many cases good options in the management of type 2 diabetes. However, the percentage of those with no medication or only metformin declined during the follow‐up indicating that the treatment was intensified with some other medication as the disease progressed. There were only a few cases with poor control in the metformin group over time. It is known that type 2 diabetes is a progressive disease and over time it becomes harder to achieve and maintain a good HbA1c level, and the need to intensify treatment with insulin becomes more likely. 15 In this study, the use of long‐ and short‐acting insulins was common, but a high percentage of those using insulin had poor glycaemic control, although insulin is highly effective at reducing hyperglycaemia. During the follow‐up, the percentage of those using short‐acting insulin declined evenly among the whole study population. One explanation for the declining percentage of those using short‐acting insulin in this aging population may be the updated guidelines that emphasize less strict treatment goals for the elderly. In addition, the increasing utilization of newer medications may have replaced the use of short‐acting insulins during the study period.
In the 2013 cohort, the percentage of those using long‐acting insulin without short‐acting insulin increased, and the percentage of those using short‐acting insulin increased a bit over time. Short‐acting insulin is only recommended when the glucose targets are not met with long‐acting insulin. 2 , 3 In this study, those using short‐term insulin had a mean HbA1c value higher than the recommendations and the percentage of those with poor glycaemic control was highest in this treatment group compared with the other treatment groups. In the long run, when the disease has highly progressed, achieving the treatment target becomes very challenging even when all possible treatment options are in use. 15 Additionally, the implementation of insulin treatment needs experience and professional knowledge and good self‐care skills from patients. 16
In this study, the percentage of those who used other medications such as sulfonylureas and thiazolidinediones, declined evenly during the follow‐up time. The declining trend in the use of these conventional medications is in accordance with the meta‐analysis by Ramzan et. al which showed a decrease in the prescription and use of sulfonylureas and thiazolidinediones. 17 During the follow‐up, the percentage of gliptin users decreased as the percentage of SGLT2‐inhibitors users increased, indicating an increase in the use of new medicines. The percentage of GLP‐1 RA users was quite stable over time. However, those who used gliptins, SGLT2 inhibitors and GLP‐1 receptor agonists less often had good glycaemic control compared with those on metformin monotherapy, even though these treatments are considered new and effective diabetic treatments. This may be partly explained by the progressive nature of the disease, but also the expenses of the treatment. People with diabetes might not use new treatments as recommended because they are more expensive than the old ones. In previous studies, the cost of the treatment has been shown to be associated with patients’ medication compliance and glycaemic control. 18 , 19
Due to cost containment pressures, the Finnish government decided to limit drug reimbursement in 2017, and therefore non‐insulin medicines for diabetes were transferred from the upper to the lower special reimbursement category, that is, only 65% of them are reimbursed. In addition, the patient must have a BMI of less than 30 kg/m2 to receive a special reimbursement for GLP‐1 analogues. Insulin remained in the upper 100% special reimbursement category. The recent study by Lavikainen et al. 20 showed an increase in average HbA1c levels after the Finnish government decided to limit drug reimbursement. The greatest decline in the treatment balance was observed, especially among those utilizing the new, more expensive medicines, such as GLP‐1 receptor agonists, SGLT2 inhibitors and DPP‐4 inhibitors. In addition, patients were also found to switch to cheaper medicines, that is, metformin and insulin, and patients reported financial difficulties with the purchase of diabetes medication. 20 , 21 In this study, the most common transitions from SGLT2‐inhibitors were to long‐acting insulin and insulin groups between each other. The recent changes in medicine reimbursements could be one explanation for the slightly increasing levels of HbA1c using newer medications in this study.
At the beginning of 2017, a major change in the organization of health services occurred when the Joint Municipal Authority for North Karelia Social and Health Services was established, joining the health service organizations of 14 municipalities. These organizational changes might also have had some effect on the continuity of the care of patients and further the outcomes of care. 22
The major strength of this study is that the electronic health records cover all adults with type 2 diabetes living in the North Karelia and includes data from both primary and specialized care, and therefore selection bias, non‐responsiveness and missing laboratory data were avoided. The validity of diabetes diagnosis in the electronic health records is high as diagnosis is based on explicit, predefined criteria (ICD‐10 code E11). In addition, in North Karelia, special attention has been paid to correctness of recording diagnoses to electronic health records. Discrepancies are also followed in the local quality of diabetes care register and diagnoses corrected when appropriate. We were not able to take into account the disease history of the individuals as the electronic health records in the area were established in 2010–2011 and information on clients prior to that in the electronic health records is not reliable. In addition, only information on prescriptions was available, which does not necessarily mean that patients had purchased all the medications prescribed.
Based on this study, the sequencing of prescribing additional type 2 diabetes medication or replacing current medication with new ones seems to occur according to the main principles of the guidelines. This partly reflects the implementation of national care guidelines generally in Finland. Thus, it could be assumed that the results of this study are generalizable to Finnish public health care. However, the results reveal the challenges in good management of disease progression and especially the implementation of insulin treatment, when it is finally needed after all other treatment lines have been tried. Much more attention should be paid to slowly deteriorating HbA1c levels and proactive modification of medications. Good register data and appropriate tools to easily follow‐up the treatment outcomes of patient population could provide remarkable support for clinical work.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS CONTRIBUTIONS
KW and ML contributed equally to this work. TL, KW and ML planned the study design. ML carried out the statistical analyses. All the authors participated in the interpretation of the results. KW and ML drafted the manuscript. PR and HT provided the clinical expertise in the analyses and contributed to a critical revision of the work. All the authors read and approved the final version of the manuscript. KW takes the authorial responsibility for the contents of the article.
DECLARATIONS
Ethics approval and consent to participate
The use of the data in this research was approved by the Ethics Committee of the North Savo Hospital District. The approval for data use was achieved from joint municipal authority for North Karelia social and health services administering the patient registers.
Informed consent
The Finnish legislation do not require informed consent for register‐based research, as the study is solely based on registers (involving no contact with the study subjects) and the study is considered to be of public health importance.
ACKNOWLEDGEMENTS
Not applicable
Wikström K, Lamidi M‐L, Rautiainen P, Tirkkonen H, Laatikainen T. Type 2 diabetes medication and HbA1c levels in North Karelia Finland, 2013–2019. Diabet Med. 2022;39:e14866. doi: 10.1111/dme.14866
Funding information
This study was partly funded by the Strategic Research Council of the Academy of Finland [project IMPRO 312703, 336325] and the Finnish Diabetes Association.
REFERENCES
- 1. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77. [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes‐2019. Diabetes Care. 2019;42(Suppl 1):S11‐S187. [Google Scholar]
- 3. Type 2 Diabetes Current Care Guidelines . Working group set up by the Finnish Medical Society Duodecim, the Finnish Society of Internal Medicine and the Finnish Diabetes Association, 2018. Accessed September 6, 2021. https://www.kaypahoito.fi/hoi50056
- 4. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461‐2498. [DOI] [PubMed] [Google Scholar]
- 6. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255‐323. [DOI] [PubMed] [Google Scholar]
- 7. Marín‐Peñalver JJ, Martín‐Timón I, Sevillano‐Collantes C, et al. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libianto R, Ekinci EI. New agents for the treatment of type 2 diabetes. Crit Care Clin. 2019;35(2):315‐328. [DOI] [PubMed] [Google Scholar]
- 9. Zafar A, Stone MA, Davies MJ, et al. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med. 2015;32(3):407‐413. [DOI] [PubMed] [Google Scholar]
- 10. Ramirez N. Treatment lines and glycaemic control among patients with type 2 diabetes in North Karelia. University of Eastern Finland; 2020. https://erepo.uef.fi/handle/123456789/22839 [Google Scholar]
- 11. R Core Team . A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. https://www.R‐project.org/
- 12. Corp IBM. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY. 2020.
- 13. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 14. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 15. Ceriello A, deValk HW, Guerci B, et al. The burden of type 2 diabetes in Europe: current and future aspects of insulin treatment from patient and healthcare spending perspectives. Diabetes Res Clin Pract. 2020;161:108053. [DOI] [PubMed] [Google Scholar]
- 16. Ellis K, Mulnier H, Forbes A. Perceptions of insulin use in type 2 diabetes in primary care: a thematic synthesis. BMC Family Pract. 2018;19(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramzan S, Timmins P, Hasan SS, et al. Trends in global prescribing of antidiabetic medicines in primary care: a systematic review of literature between 2000–2018. Prim Care Diabetes. 2019;13(5):409‐421. [DOI] [PubMed] [Google Scholar]
- 18. Pemminati S, Millis RM, Kamath A, et al. Are the Newer Antidiabetic Agents Worth the Cost? J Clin Diagn Res. 2016;10(3):FL01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunt J, Rozenfeld Y, Shenolikar R. Effect of patient medication cost share on adherence and glycaemic control. Manag Care. 2009;18(7):47‐53. [PubMed] [Google Scholar]
- 20. Lavikainen P, Aarnio E, Jalkanen K, et al. Impact of co‐payment level increase of antidiabetic medications on glycaemic control: an interrupted time‐series study among Finnish patients with type 2 diabetes. BMC Health Serv Res. 2020;20(1):1095‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavikainen P, Aarnio E, Niskanen L, et al. Short‐term impact of co‐payment level increase on the use of medication and patient‐reported outcomes in Finnish patients with type 2 diabetes. Health Policy. 2020;124(12):1310‐1316. [DOI] [PubMed] [Google Scholar]
- 22. Lamidi M‐L, Wikström K, Inglin L, et al. Trends in the process and outcome indicators of type 2 diabetes care: a cohort study from Eastern Finland, 2012–2017. BMC Fam Pract. 2020;21(1):253‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]


