Abstract
This evidence synthesis applying realist concepts and behavioural science aimed to identify behavioural mechanisms and contexts that facilitate prescribers tapering opioids. We identified relevant opioid‐tapering interventions and services from a 2018 international systematic review and a 2019 England‐wide survey, respectively. Interventions and services were eligible if they provided information about contexts and/or behavioural mechanisms influencing opioid‐tapering success. A stakeholder group (n = 23) generated draft programme theories based around the 14 domains of the Theoretical Domains Framework. We refined these using the trial and service data. From 71 articles and 21 survey responses, 56 and 16 respectively were included, representing primary care, hospital, specialist pain facilities and prison services. We identified 6 programme theories comprising 5 behavioural mechanisms: prescribers' knowledge about how to taper; build prescribers' beliefs about capabilities to initiate tapering discussions and manage psychological consequences of tapering; perceived professional role in tapering; the environmental context enabling referral to specialists; and facilitating positive social influence by aligning patient: prescriber expectations of tapering. No interventions are addressing all 6 mechanisms supportive of tapering. Work is required to operationalise programme theories according to organisational structures and resources. An example operationalisation is combining tapering guidelines with information about local excess opioid problems and endorsing these with organisational branding. Prescribers being given the skills and confidence to initiate tapering discussions by training them in cognitive‐based interventions and incorporating access to psychological and physical support in the patient pathway. Patients being provided with leaflets about the tapering process and informed about the patient pathway.
Keywords: addiction, behaviour change, deprescribing, implementation, overprescribing, polypharmacy, substance misuse, survey, synthesis, systematic review, tapering
What is already known about this subject
Opioid over prescribing is a global health problem causing significant early and preventable mortality.
Initiatives to address this have largely focussed on patient behaviour change, the use of guidelines, reporting and feedback on prescribing statistics.
Evidence suggests that these approaches in isolation have limited long‐term impact.
What this study adds
We identified 5 behavioural mechanisms that support prescribers to taper opioids, all of which must be addressed to achieve the necessary change in opioid prescribing.
No existing opioid‐tapering intervention addresses all 5 mechanisms.
Organisations should identify and implement components to address behavioural mechanisms that are missing from their opioid‐tapering strategy.
1. INTRODUCTION
Opioid use for chronic noncancer pain has rapidly increased over the past 20 years, spanning broad geographical areas across North America, Europe and Australia. 1 However, there are major concerns that any potential benefits of these long‐term opioids are markedly outweighed by the harms. The act of initiating opioids is a relatively quick and easy intervention for busy prescribers to alleviate the patient's discomfort in the short‐term. 2 After opioid initiation, there can be a tendency for the dosages and frequency of administration to gradually increase as the patient develops opioid tolerance or psychological dependence. 2 This leads to significant challenges in dose tapering and ultimately achieving cessation, despite limited evidence of long‐term effectiveness. 3
Strategies to address the growth in opioid prescribing for chronic noncancer pain have focussed around 2 key areas of work: patient behaviour change and practitioner behaviour change. Patient behaviour change interventions have commonly included nonpharmacological modalities for pain control and education about the harms of opioid use. The ongoing i‐WOTCH trial across England is an example of such an intervention; it comprises group sessions supporting patients to self‐manage chronic pain. The intervention circumvents the need to change the behaviour of the existing prescribing workforce by providing expert clinical facilitators (trained in motivational interviewing) to initiate and manage the tapering process. 4 This model of funding expert clinical facilitators in addition to the existing prescribing workforce may not be feasible beyond the trial setting for all healthcare organisations.
The mainstay of practitioner behaviour change strategies has been the development of guidelines. 5 , 6 , 7 , 8 The marginal progress achieved in stemming the opioid epidemic since the advent of these tapering guidelines suggests that alone they are insufficient. Guidelines primarily address knowledge gaps, yet it is widely recognised within the behavioural science field that providing knowledge alone rarely achieves substantive behaviour change. 9 Other reported strategies to support practitioners have largely focussed on monitoring and feedback. 10 , 11 The barriers and enablers (determinants) of practitioners changing their behaviour from prescribing to tapering or deprescribing opioids are wide ranging. It is unsurprising therefore, that simple interventions have had marginal impact on stemming the rapid growth in opioid prescribing. 1
Numerous trials have tested interventions to support patients to taper their prescribed opioid use. A recent systematic review of such trials reported effective interventions to be complex in nature, addressing a range of patient determinants to opioid tapering. 12 It does, however, recognise that little attention is given to exploring strategies to facilitate practitioners to deliver these interventions in the real‐world as intended. The systematic review explicitly recommends, “Future studies should examine interventions that are feasible in busy primary care settings and scalable across multiple health systems.”
Reviews adopting a realist approach do not require the effect of interest to be the primary outcome of the intervention. The substantive volume of interventions designed to taper opioids through targeting patient behaviour may therefore provide useful data to inform a strategy to support practitioners to taper opioids. We applied behavioural theory to conduct a synthesis using methodological concepts specific to realist reviews to combine data from opioid‐tapering interventions in the trial and real‐world environment with the knowledge and experience of experts. Using a realist approach enabled us to establish the causal behavioural mechanisms via which complex opioid‐tapering interventions are facilitating practitioners to taper opioids and how context influences these effects. Understanding the causal mechanisms and effects of context enables us to describe what needs to be done to change practitioner behaviour (intervention components) without dictating exactly how each intervention component should be operationalised. This approach facilitates organisations to operationalise the intervention components according to available resources and infrastructure whilst preserving efficacy. We aimed to determine which opioid‐tapering intervention components effect practitioner behaviour‐change leading to effective opioid tapering. We also aimed to understand the contexts and behavioural mechanisms that are conducive to the outcomes being achieved to better understand how to implement in the real‐world environment.
2. METHODS
Our data collection and analysis processes were based on a previously reported novel methodological approach combining realist synthesis methods with behavioural science. 13 , 14 , 15 We sought to generate programme theories that identify the behavioural mechanisms of action (MoAs) by which prescribers are facilitated to taper opioids and to explore the contexts that determine whether the different MoAs produce intended outcomes. An MoA in the field of behavioural science is the process by which the behaviour change technique (active ingredient in an intervention) brings about change in behaviour.
There are numerous behaviour change theories with overlapping constructs; thus, selecting the most appropriate is challenging. Often several theories are necessary in order to try and represent the full breadth of the behavioural complexity yet the risk of behavioural determinants being missed by the selected theories remains. We used the Theoretical Domains Framework (TDF) as our a priori framework for generating initial programme theories. 16 The TDF is a synthesis of 33 behaviour change theories with related constructs clustered into 14 domains with each domain representing an MoA.
Combining the TDF with methodological concepts specific to realist reviews, to synthesise data from multiple sources facilitates exploration of the breath of implementation, causal and contextual factors that may not be considered using empirical evidence alone. We finally sought to collate our programme theories into a mid‐range theory describing MoAs that support practitioners to taper opioids.
2.1. Data collection
We used 3 data sources for our evidence synthesis to develop and finalise the programme theories: (i) experiential evidence from stakeholder group discussions; (ii) evidence from published opioid‐tapering trials; and (iii) data from commissioners/managers and evaluators of opioid‐tapering services in England. We reported our findings according to the RAMESES standards for realist reviews 17 and STROBE guidelines for reporting cross‐sectional studies. 18
2.1.1. Stakeholder group discussions
The research team (n = 6)—comprising researchers, prescribers and pharmacists familiar with the opioid‐tapering literature or with relevant clinical experience—formulated initial programme theories from the 14 TDF domains (supplementary file 1). We presented these programme theories to a stakeholder group for refinement and prioritisation through discussions using realist principles. 13 , 14 The stakeholder group (n = 23) comprised prescribers, pharmacists and policy makers with expertise and experience in managing patients prescribed opioids. We tested the resulting programme theories with data from opioid‐tapering trials and the survey. 15
2.1.2. Published international opioid‐tapering trials
We identified trials of opioid‐tapering interventions from a 2018 systematic review of dose reduction/discontinuation interventions for long‐term opioid therapy. 12 We excluded studies if they investigated interventions evaluated by the research team as inappropriate and/or unlawful for 1 or more public healthcare setting. We supplemented the systematic review with snowballing 15 to identify additional relevant literature related to emerging programme theories. We excluded opioid‐tapering guidelines and toolkits from the data for testing programme theories because they lack detail regarding the underlying causal mechanisms of effectiveness. 14 We did review these information sources for any references relevant for inclusion as a data source.
2.1.3. Survey of opioid‐tapering service providers in England, UK
We developed an electronic survey for capturing details of all opioid‐tapering services implemented in any healthcare setting. We distributed the draft survey to the stakeholder group for feedback regarding face and content validity and refined as necessary based on the feedback. We then piloted the survey with the local primary care organisation.
We distributed a link to the fully refined survey in November 2018 to all primary care organisations in England via email to the clinical commissioning group prescribing lead and used the following national networks to distribute the survey link to prisons and hospitals:
Faculty of Pain Medicine
National Health Service England medicines safety group
Royal Pharmaceutical Society
Royal College of Physicians
Primary Care Pharmacist's Association
Regional Medicine information service
Specialist Pharmacy services lead
Royal college of General Practice
Substance Misuse Management in General Practice
We generated awareness of the survey via social media and announcements at relevant professional conferences/meetings. The survey link, accessible from the project website, was open to responses from November 2018 to February 2019. Responses were eligible if they described experiences and learning from an organisational perspective or as a synthesis of the experiences of practitioners and patients involved in a service. We excluded responses describing the approaches and experiences of an individual. The survey collected a description of the service; details about who developed and delivered the service; the patient groups targeted; reasons for the approach taken; details of any results; and details about what had/had not worked and why. A copy of the survey is available here: UEA opioid‐tapering toolkit.
2.2. Data analysis
H.W. and E.T. reviewed the published literature for relevance in terms of whether it provided sufficient detail to contribute to refining and/or adding to the initial programme theories. We graded articles as high, moderate, low and no relevance. We excluded studies of no relevance or if the intervention was inappropriate to a publicly funded healthcare setting. A third researcher (D.B.) assessed all excluded studies and presented any disagreements to the research team for arbitration. D.B. And E.T. reviewed and graded survey results for relevance using the same criteria as the published literature.
We extracted from the published literature and survey results, data relating to trial/service design, location and relevance to the initial and emerging programme theories. For the published literature, we piloted a data extraction form on 3 studies. H.W. and E.T. extracted the data, each reviewing half of the studies; a randomly selected 10% of studies were reviewed by both to ensure consistency. We extracted the electronic survey data into the same data extraction form used for the published literature and replicated the process described above. We iteratively tested the initial programme theories using the extracted data and refined the programme theories as necessary. We assessed the rigour of the published literature and survey data through continuous and iterative discussions within the review team. Discussions focused on assessing the quality of the data in terms of reliability, credibility and plausibility, and to ensure a consistent approach when making judgements about and interpreting the data from multiple sources.
We presented the research findings to the stakeholder group for discussion and considered whether the evidence was supportive, contradictive or insufficient to warrant inclusion in the mid‐range theory.
The initial programme theories were categorised as:
- Retain;
included in mid‐range theory. We found sufficient evidence to support this programme theory.
- Uncertain;
included in mid‐range theory. We found insufficient evidence to support this programme theory; however, stakeholder discussions indicated that it may be a contributory factor in the MoA of another programme theory.
- Exclude;
excluded from mid‐range theory. We found that there was insufficient evidence to support this programme theory.
3. RESULTS
The research team generated 36 initial programme theories spanning all domains of the TDF except beliefs about capabilities and goals. We worked with our stakeholder group to refine and prioritise these initial programme theories. Supplementary file 1 summarises the iterative refinement of initial programme theories from 36 to the 6 that we tested with the evidence.
Figure 1 illustrates the flow of data from 71 identified to 56 eligible studies and 21 survey respondents to 16 included responses (services 1–16). The primary reason for excluding studies was interventions comprising components incompatible with a publicly funded health service such as alternative or complementary therapies. Survey respondents were excluded because their responses represented their individual experiences of opioid tapering.
FIGURE 1.
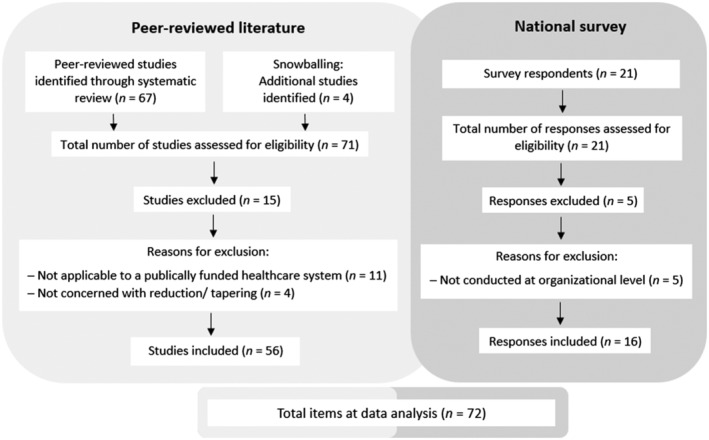
Flow of data identification, and screening of peer reviewed studies and national survey responses
Table 1 summarises the published literature and survey data that we used to test the initial 6 programme theories. The published literature was dominated by USA hospital‐based interventions delivered from specialist pain facilities. The England survey data, in contrast, were dominated by primary care‐based interventions led by doctors and pharmacists.
TABLE 1.
Service characteristics and outcomes extracted from the literature and survey responses
| Peer‐reviewed literature characteristics | ||||
|---|---|---|---|---|
| Author, y | Setting, country | Patient group | Intervention summary | Outcome |
| Baron et al., 2006 19 | Inpatient psychiatric facility with outpatient follow‐up, USA | Patients referred by pain physicians for opioid detoxification | Ibuprofen only or ibuprofen plus buprenorphine taper. | 100% discontinued opioid medications. No significant difference in pain severity between treatment groups. |
| Berland et al., 2013 20 | Two hospital inpatient settings with outpatient follow‐up, USA | Patients experiencing worsening pain and function despite escalating doses of opioids | Intramuscular or sublingual buprenorphine assisted taper. Inpatient conversion then outpatient follow‐up monthly. | 100% discontinued opioid medications at follow‐up, 54% on buprenorphine, 26% resumed opioid and 10% not on opioids. |
| Blondell et al., 2010* 21 | Outpatient multidisciplinary pain management programme, USA | Chronic pain patient with coexistent opioid addiction | Comparison of steady state and tapering doses of buprenorphine. Follow‐up was monthly for 6 mo. | 100% discontinued opioid medications. At 6 mo, 8/10 patients on buprenorphine and 2/10 resumed opioid medications. |
| Buckley et al., 1986 22 | Inpatient multidisciplinary pain centre, USA | Chronic noncancer pain patients admitted during an 18‐mo period | Blinded methadone/phenobarbital pain cocktail tapering. | 94% (116/124) discontinued opioid medications. |
| Cowan et al., 2003 23 | Hospital outpatient multidisciplinary pain clinic, UK | Chronic noncancer pain patients prescribed controlled‐release oral morphine and/or fentanyl patches | Multimodal pharmacological and nonpharmacological intervention. Opioid discontinuation was not part of the intervention. | 57% (59/104) discontinued opioid medications; 17% (13/78) reported opioid withdrawal symptoms |
| Cowan et al., 2005 24 | Outpatient pain clinic, UK | Chronic noncancer pain patients treated with 12‐hourly controlled‐release oral morphine for at least 30 d | Randomised, double‐blinded placebo, cross‐over study where morphine was substituted with placebo for 60 h at either first or second period. | 100% discontinued opioids during 60‐h abstinence period 30% (3/10) reported withdrawal symptoms. |
| Crisostomo et al., 2008* 25 | Outpatient multidisciplinary pain rehabilitation programme, USA | Chronic low back pain patients | 3‐wk intensive multidisciplinary pain rehabilitation programme using cognitive behavioural model and incorporating opioid withdrawal. | Proportion of patients using opioid medications decreased 79% at discharge vs. admission. |
| Cunningham et al., 2016* 26 | Outpatient multidisciplinary pain rehabilitation centre, USA | Fibromyalgia patients who completed programme | 3‐wk intensive multidisciplinary pain rehabilitation programme using cognitive behavioural model and incorporating opioid withdrawal. | 100% (55/55) discontinued opioid medications; opioid dose and duration were not determinants of withdrawal symptoms. |
| Daitch et al., 2012 27 | Interventional pain management practice, USA | Chronic noncancer pain patients converted onto sublingual buprenorphine for ≥60 d | Patients converted from opioids to sublingual buprenorphine. | Significant reduction in pain severity after conversion to buprenorphine vs. baseline. |
| Daitch et al., 2014 28 | Interventional pain management practice, USA | Chronic pain patients on high dose opioids converted onto sublingual buprenorphine for ≥60 d | Patients converted from opioids to sublingual buprenorphine. | Significant reduction in pain severity after conversion to buprenorphine vs. baseline. |
| Darchuk et al., 2010* 4 | Outpatient multidisciplinary pain rehabilitation centre, USA | Geriatric patients with chronic noncancer pain | 3‐wk outpatient interdisciplinary pain rehabilitation programme using cognitive behavioural model and incorporating opioid withdrawal. | 94% (239/253) discontinued opioid medications at discharge 15% (44/292) reported opioid use at 6‐mo follow‐up. |
| Dersh et al., 2008 5 | Multidisciplinary functional restoration programme, USA | Patients with chronic disabling occupational spinal disorders and prescription opioid dependence | Intensive physical reactivation and pain/disability management interventions, including opioid withdrawal. | Opioid discontinuation not specifically reported but required for programme completion. 91% programme completion rate. |
| Drossman et al., 2012 6 | Inpatient gastroenterology consult service and outpatient gastroenterology clinic, USA | Patients with severe chronic abdominal pain on opioids | Inpatient or outpatient opioid withdrawal using a local detoxification protocol. | 100% decreased opioid dose; 90% (35/39) discontinued opioids at programme completion. |
| Hanson et al., 2009 7 | Tertiary care inflammatory bowel disease referral centre, USA | Patients with a diagnosis of Crohn’s disease, ulcerative colitis and ileal pouchitis using opioids | Patient seen at inflammatory bowel disease clinic with ≥1 follow‐up visit/ | 56% (22/39) of patients who returned for follow‐up discontinued opioid medications. |
| Harden et al., 2015 8 | Veteran medical centre, USA | Patients with noncancer pain on opioids for ≥90 consecutive d | Opioid tapering implemented by primary care providers, the pain service, or the pharmacist‐run pain management clinic. | 94% (47/50) decreased opioid dose at 12‐mo follow up 13% (6/50) discontinued opioid medications. |
| Hassamal et al., 2016* 29 | Outpatient multidisciplinary opioid reduction programme, USA | Presurgical spine surgery candidates on chronic opioid analgesia | Opioid‐tapering programme incorporating physical and psychological therapies. Opioid dose reduction goal ≥10% per wk. | No patients (0/5) discontinued opioids. Mean morphine equivalent dose was decreased (238 to 139 mg). |
| Hooten et al., 2007* 30 | Outpatient multidisciplinary pain rehabilitation centre, USA | Fibromyalgia patients | Multidisciplinary pain rehabilitation programme based on a cognitive behavioural model, incorporating physical and occupational therapy and opioid withdrawal. | 93% (57/61) of patients on opioids discontinued by programme completion. |
| Hooten et al., 2007* 31 | Outpatient multidisciplinary pain rehabilitation centre, USA | Fibromyalgia patients | Multidisciplinary pain rehabilitation programme based on a cognitive–behavioural model, physical therapy and opioid withdrawal. Patients assessed at admission and interviewed 1 y post discharge. | 95% (20/21) discontinued opioid medications. |
| Hooten et al., 2009* 32 | Outpatient pain rehabilitation centre, USA | Chronic pain patients consecutively admitted to the clinic during defined period | Multidisciplinary pain rehabilitation programme using cognitive behavioural model and incorporating opioid discontinuation. Comparison of smokers and nonsmokers. | Success of opioid tapering not dependent on smoking status. Overall proportion of patients using opioid medications decreased. |
| Hooten et al., 2010 33 | Outpatient pain rehabilitation pain centre, USA | Consecutively admitted chronic pain patients on a daily morphine equivalent dose ≥30 mg morphine equivalent >1 mo duration | Multidisciplinary pain rehabilitation programme using cognitive behavioural model and incorporating opioid discontinuation. | 98% (99/101) of programme completers discontinued opioids. |
| Hooten et al., 2015* 34 | Outpatient multidisciplinary pain rehabilitation centre, USA | Chronic noncancer pain patients on a daily morphine equivalent dose ≥60 mg morphine equivalent >6 mo duration | A randomised, single‐blinded, placebo‐controlled pilot trial where patient received either varenicline or placebo as part of a programme using cognitive behavioural therapy (CBT). |
95% (20/21) of study completers discontinued opioids. Withdrawal symptoms decreased in 5/7 patients in the varenicline group and 4/11 patients in the placebo group. |
| Huffman et al., 2013 35 | Outpatient academic medical centre, USA | Chronic noncancer pain patients with therapeutic opioid addiction | 3–4‐wk intensive interdisciplinary outpatient programme including physical/occupational therapy, psychotherapy, substance‐use education and opioid withdrawal. | 82% (459/558) of programme completers discontinued opioid medications, 23% (27/120) resumed an opioid at 1 y. |
| Huffman et al., 2017* 36 | Multidisciplinary chronic pain rehabilitation programme, USA | Patients on high‐dose chronic opioid therapy | 3–4‐wk intensive interdisciplinary outpatient programme with optional aftercare including physical and psychological therapy, substance‐use education and opioid withdrawal. | 87% (654/754) discontinued opioids, 4% (30/754) discharged on buprenorphine, 10% (77/754) continued full‐agonist opioids. 31% (128/417) resumed opioid use by 12‐mo follow‐up. |
| Kidner et al., 2009 37 | Regional rehabilitation facility, USA | Patients with a chronic disabling occupational musculoskeletal disorder | Functional restoration programme consisting of exercise programme with a multimodal disability management component. Patients consented to be weaned from all opioid medications. | 74% (441/596) of patients on opioids at baseline discontinued opioid medications. |
| Krumova et al., 2013* 38 | Inpatient pain management service with ongoing outpatient clinics, Germany | Consecutive patients with severe chronic noncancer pain despite opioid medication | Opioid‐tapering programme using nonmedical treatments including CBT and physiotherapy. | 76% (78/102) discontinued opioid medications; 24% (24/102) reduced dose by an average of 82%; 42% (31/73) resumed opioid medications at follow‐up. |
| Lake et al., 2009* 39 | Inpatient headache treatment centre, USA | Patients with intractable chronic daily headache (including migraine) | Multimodal programme including intravenous and oral medication protocols, drug withdrawal when indicated, and physical and/or psychological interventions. | 100% (n = 267) of programme completers discontinued opioid medications. |
| Maclaren et al., 2006 40 | Multidisciplinary functional restoration programme, USA | Patients with chronic pain related to work injuries | 4–6 wk interdisciplinary functional restoration programme including psychoeducation, physical and occupational therapy. | 14/70 (20%) patients decreased their opioid dose and 10/70 (14%) discontinued during treatment. |
| Malinoff et al., 2005 41 | Outpatient treatment programme, USA | Patients experiencing worsening pain despite escalating doses of short‐ and long‐acting opioids | Outpatient clinic conversion to sublingual buprenorphine with monthly follow‐up. | 94% discontinued long‐term opioid therapy and initiated buprenorphine. No patients resumed opioid medications. |
| Mehl‐Madrona et al., 2016 42 | Medical centre, USA | Patients on long‐term opioids completing at least 6 mo of a group medical visit programme, with opioid tapering | Pain‐management group medical visit providing patient education on nonpharmacological pain management methods and weekly physical activity. | 19% (8/42) of intervention group discontinued opioids and 43% (18/42) reduced opioid dose. In treatment‐as‐usual group, 1/42 decreased opioid dose. |
| Miller et al., 2006 43 | Inpatient addiction facility, USA | Patient with a diagnosis of opioid prescription medication dependence | Abruptly withdrawal from opioids on admission with self‐reported pain monitoring. Diazepam and/or clonidine were used to manage withdrawal symptoms. | Study only included patients who discontinued opioid medications pain severity improved significantly at programme completion vs. baseline. |
| Murphy et al., 2016 44 | Hospital inpatient chronic pain rehabilitation programme, USA | Exploration of differences between female and male veterans engaged in a chronic pain rehabilitation programme | 3‐wk residential rehabilitation programme. Programmes aims to teach self‐management skills and includes cessation of all opioids and centrally acting muscle relaxants. | 100% discontinued opioid medications at programme discharge. At 3‐mo follow‐up, 17% reported opioid use. There was no difference in follow‐up opioid use by sex. |
| Murphy et al., 2013* 45 | Hospital inpatient programme, USA | Veterans/active‐duty service members with chronic noncancer pain admitted to the chronic pain rehabilitation programme | 3‐wk inpatient, interdisciplinary pain programme with a cognitive–behavioural model. Gradual opioid taper using hydromorphone cocktail fruit drink. | 100% (221/221) discontinued opioid medications at programme discharge. |
| Naylor et al., 2010* 46 | University medical centre, USA | Completers of an 11‐wk group CBT programme | Random assignment to 1 of 2 study conditions. Experimental group received 4 mo of CBT maintenance programme via the therapeutic interactive voice response programme. Control group received standard care only. | 21% (3/14) therapeutic interactive voice response patients discontinued opioids at 8‐mo. At 8‐mo, opioid dose decreased in the experimental group and increased significantly in the control group. |
| Nilsen et al., 2010* 47 | Hospital multidisciplinary pain centre, Norway | Chronic pain patients prescribed codeine referred to 2 pain/rehab clinics | Tapering of codeine within 8 wk and CBT sessions. | 55% (6/11) patients discontinued opioids; 45% (5/11) remained off codeine at 3 mo; mean opioid dose decreased by 81% post‐treatment. |
| Nissen et al., 2001 48 | Hospital multidisciplinary pain centre, Australia | Consecutive in‐patient admissions | Assessment by a multidisciplinary pain team; a 2‐wk educational programme at a multidisciplinary inpatient pain centre on drugs, activities of daily living, posture, back care, relaxation, exercise, diet and with chronic pain. | Average opioid dose decreased at discharge vs. admission (36.9 mg vs. 88.7 mg morphine equivalent dose); proportion of patients taking an opioid decreased (58% at discharge vs. 83% at admission). |
| Ralphs et al., 1994* 49 | Hospital inpatient unit, UK | Patient with chronic pain | Multimodal programme including psychological (CBT) and physical interventions with medication reduction over 4 wk. Choice of patient‐controlled opioid reduction or cocktail reduction method. | At discharge, 89% of the cocktail group discontinued opioids vs. 68% of the patient‐controlled reduction group. At 6‐mo, abstinence rate equivalent with 55% of patients remaining off opioids. |
| Rome et al., 2004* 50 | Inpatient pain rehabilitation centre, USA | Patients with chronic pain | Rehabilitative treatment based on a CBT model with opioid withdrawal. | 98% (132/135) of patients discontinued opioids by programme discharge. |
| Rosenblum et al., 2012 51 | Outpatient pain practice, USA | Patients with moderate to severe chronic pain on long term opioid therapy exhibiting 1 or more aberrant drug‐related behaviours | Discontinued all opioids and substituted with buprenorphine/naloxone in‐office. Regular outpatient clinic reviews with additional telephone review if required. | 33% (4/12) patients completed transition to buprenorphine 83% (10/12) experienced an adverse effect, 7 discontinued treatment as a result; 1 patient hospitalised. |
| Roux et al., 2013 52 | Inpatient research unit, USA | Patients with mild to moderate chronic, nonmalignant pain meeting DSM‐IV criteria for opioid dependence | Conversion of patients to sublingual buprenorphine/naloxone and administration at double blind doses. Patient self‐administration of oxycodone as required. Monthly clinic visits and 12‐mo follow up. | 72% (31/43) completed the 7‐wk study. Higher doses of buprenorphine/naloxone associated lower doses of oxycodone opioid withdrawal symptoms reported in 83% of study sessions. |
| Schneider et al., 2010 53 | Private outpatient pain clinic, USA | Consecutive patients prescribed opioids at the clinic | Chart review of patients receiving ≥1‐y treatment by a single pain specialist. | 15% (29/197) decreased opioid dose during follow‐up; 2% (3/197) patients with aberrant behaviours discontinued opioids. |
| Schwarzer et al., 2015 54 | Hospital inpatient unit, Germany | Patients admitted to inpatient unit for opioid withdrawal (after an opioid intake >6 mo) | 3‐wk inpatient opioid tapering with pharmacological management of withdrawal symptoms and outpatient multidisciplinary follow‐up. Patients received individual physical, psychological and occupational therapies. | 100% (18/18) patients discontinued opioids; 1/18 resumed low‐dose opioids. |
| Streltzer et al., 2015 55 | Outpatient psychiatric pain clinic, USA | Patients referred to the pain clinic with a diagnosis of opioid dependence | Conversion from opioids to buprenorphine with counselling. Methadone additionally used in some patients to allow rapid reduction of high dose opioids prior to initiating buprenorphine. | 100% (43/43) discontinued opioids; 44% (19/43) maintained buprenorphine treatment; 7% (3/43) successfully detoxed. |
| Sullivan et al., 2017* 56 | Outpatient medicine centre, USA | Patients receiving long‐term opioid therapy for chronic pain and interested in tapering their opioid dose | 22‐wk prescription opioid‐taper support intervention involving psychiatric consultation, opioid dose tapering and 18 weekly meetings exploring motivation for tapering and pain self‐management education. | 39% (7/18) intervention and 12% (2/17) usual care reduced opioid dose by ≥50% at 22 wk; 1 patient in each group discontinued opioids. 22% (4/18) in intervention and 47% (8/17) in usual care did not reduce dose at 22 wk. |
| Taylor et al., 1980 57 | Inpatient pain clinic, USA | Patients experiencing continuous abdominal or headache pain and exceeding prescribed doses of controlled drugs for at least 6 mo and exceeding prescribed doses of controlled drugs | Detoxification from analgesic medications and relaxation techniques education with supportive therapy. | 100% (n = 7) discontinued opioids over an average of 3.7 d (range, 1–6 d); 50% (3/6) patients reported taking an opioid at 6‐mo. |
| Tennant et al., 1982 58 | Multidisciplinary outpatient pain programme, USA | Patients voluntarily seeking outpatient withdrawal from prescription opioid dependence. | 21‐d detoxification then psychotherapy vs. 21‐d detoxification then psychotherapy with optional maintenance, regular follow up and gradual withdrawal of methadone/propoxyphene, | 24% (5/21) in psychotherapy alone group discontinued opioid medications. At 90 d, 10% (2/21) patients in each group abstinent from opioids; at 180 d 4/21 additional patients in opioid maintenance group discontinued opioids. |
| Thieme et al., 2003 59 | Hospital inpatient unit, Germany | Female fibromyalgia patients | Operant pain treatment compared with a standard inpatient medical treatment programme with physical therapy components. | Intervention patients reported a significant reduction in opioid medication use. |
| Townsend et al., 2008* 60 | Outpatient multidisciplinary rehabilitation programme, USA | Chronic noncancer pain patients | Opioid‐tapering programme incorporating physical therapy, occupational therapy, biofeedback and relaxation training, stress management, wellness instruction, chemical health education and pain management training. | 93% (176/190) discontinued opioids by programme completion 14% (33/238) of patients were taking opioids at 6‐mo follow‐up. |
| Vines et al., 1996* 61 | Hospital rehabilitation unit, USA | Patient with chronic pain for which there was no further useful medical or surgical intervention | 4‐wk chronic pain programme employing pain management, pain coping strategies, relaxation/stress management techniques and exercise. Patients asked to self‐report their opioid use before and after intervention. | 70% (16/23) discontinued opioids by follow‐up 3–11 mo after programme completion. |
| Wang et al., 2011 62 | Outpatient orthopaedic surgery clinic, Germany | Patients with chronic low back pain on opioid theory for at least 3 mo | Prospective cohort study to investigate pain sensitivity after tapering opioids in patients with chronic low back pain. Dose of opioids was halved every 3 d until opioid clear. Doxepin was prescribed for withdrawal symptoms and continued for 2 wk after opioid clean. | 91% (32/35) discontinued opioids by d 21; 15% (3/20) of patients were taking an opioid medication at 6‐mo follow‐up. |
| Webster et al., 2016 63 | Inpatient clinical trial setting, USA | Chronic pain patients on 80–220‐mg morphine equivalent dose | Double‐blind, placebo‐controlled, crossover study comparing 24‐h periods on 50% of baseline morphine equivalent dose as full opioid agonist vs. buccal buprenorphine. | No significant differences in pain ratings between treatments. 2 patients experienced opioid withdrawal; 1 patient during both 24‐h periods and 1 patient with full agonist only. |
| Weimer et al., 2016 64 | Academic medical centre, USA | Opioid prescribed patients at the clinic | Implementation of a provider education intervention and a dose limitation policy which requires patients prescribed doses over 120 mg morphine equivalent to initiate a 3–6‐mo opioid taper. | 37% (41/112) patients reduced opioid dose below 120 mg morphine equivalent dose; 12% (13/112) discontinued opioids. Mean opioid dose decreased from 263 to 199 mg. |
| Whitten et al., 2013* 65 | Primary care veteran clinic, USA | Chronic pain patients at the clinic | 6‐wk group CBT programme. Telephone reviews with participants between sessions. | 18% (4/22) discontinued opioids. |
| Williams et al., 1996* 66 | Hospital pain management unit, UK | Chronic pain which significantly disrupted patients’ life | 4‐wk inpatient programme or 9‐wk outpatient programme involving exercise, goal setting, pacing of activities, education sessions, CBT, reduction of pain‐related drugs (patient choice of cocktail or self‐controlled reduction), relaxation, sleep management, relapse planning, family involvement. | 50% (21/42) discontinued opioids at 1 mo. At 1 year, 80% (24/30) and 55% (17/31) not using opioids in inpatient and outpatient groups. Inpatient group achieved a significant dose reduction at 1 year. |
| Younger et al., 2008 67 | Inpatient multidisciplinary pain programme, USA | Chronic pain patients on long‐term opioid analgesic treatment | Individualised biopsychosocial approach toward pain management incorporating voluntary opioid titration. | 58% (7/12) discontinued opioid therapy; 2 patients greatly reduced high‐dose therapy (i.e., ≥400 mg morphine equivalent dose). |
| Zgierska et al., 2016*, 68 2016* 69 | Outpatient unit, USA | Adults with chronic low back pain, prescribed 30 mg/d of morphine equivalent dose for at least 3 mo | In addition to usual care provided by their regular clinicians, 8‐weekly group CBT and meditation sessions supplemented with patient self‐directed practice at home vs. wait‐list control receiving usual care. | Opioid dose reduction not significant in either group at 26 wk. Proportion on >200 mg morphine equivalent dose decreased in the intervention group (29 to 20%) but not control (21 to 23%) |
| Characteristics of services reported from England‐wide survey | ||||
|---|---|---|---|---|
| Service | Setting | Patient group | Intervention summary | Outcome |
| Service 1 | Primary care, community pharmacies and prisons | Prescribed high‐dose opioids for chronic noncancer pain | A standardised form provided to guide audits of registered patients to identify those prescribed high dose opioids. Staff encouraged to reflect on their audit findings. Resources provided to support clinicians with prescribing decisions. | 1022 patients prescribed high dose opioids identified. 80% of practices submitted reflections and action plans for changes to patient care. Overarching theme from practice reflections was to treat high‐dose opioids like any other high‐risk drugs. |
| Service 2 | Primary care | Prescribed high‐dose opioids (morphine equivalent >100 mg/24 h) for 6 mo or more | General practitioner training delivered by specialist psychiatrist in opioid dependence, sharing of opioid awareness resources via monthly newsletter and a peer support session. A standardised form provided to guide audits of registered patients to identify those prescribed high‐dose opioids. | Ongoing project. |
| Service 3 | Primary care | Prescribed high‐dose opioids | Prescribing incentive scheme requiring practices to conduct an audit and develop an action plan to reduce the prescribing of high dose opioids (>120 mg morphine or equivalent per d). Target set to reduce prescribing per 1000 patients by 15%. | Achieved a lower national ranking for opioid prescribing. |
| Service 4 | Primary care | Prescribed high‐dose opioids | A pilot multidisciplinary team reviewed referrals into the pain service and managed them according to best practice as defined by the British pain society. Training of general practitioners in pharmacological and nonpharmacological pain management through group pain education classes. | Ongoing project. |
| Service 5 | Primary care | Prescribed high‐dose opioids | A standardised form generated for medical practices to audit patients prescribed high dose opioids. Individual results and benchmarking data discussed at annual meeting with all general practitioners and nonmedical prescribers. An incentive scheme to measure formulary choice as a percentage of all opioids prescribing. Pharmacological and nonpharmacological pain management training day for general practitioners. |
Patients prescribed opioids on repeats has not reduced compared to previous year—remains at 79%. Substantial increase in the number of patients receiving a medication review of their opioid prescription. Reduction in inappropriate use of opioid patches. Little change in numbers of patients taking high doses. |
| Service 6 | Primary care | Prescribed 120‐mg morphine equivalent dose or higher | Prescribing incentive scheme associated with review of each patient with noncancer pain in primary care prescribed >120‐mg morphine equivalent dose; review conducted by general practitioners or practice pharmacists with the aim to reduce or withdraw opioids. | 52% (119/227) undergoing a dose reduction; 31% (70/227) with dose decreased to <120‐mg morphine equivalent dose; 26% (59/227) declined a dose reduction. Remainder would be those not reviewed yet, referred to specialists or pharmacist. |
| Service 7 | Primary care | Patients on 120‐mg morphine equivalent dose or higher | Searches written, presentations held and resources produced for general practitioners to enable them to carry out reviews with their patients confidently. An audit developed for practices to use. | Ongoing project. The initial feeling is that the general practitioners were engaged but hesitant to have difficult conversations within this cohort of patients. They also seemed concerned with workload and additional time required to tackle this problem. |
| Service 8 | Primary care | Patients on 120‐mg morphine equivalent dose or higher | Monthly pharmacist opiate reduction clinics running simultaneously with general practice clinic. Referral when needed to general practitioners or pain clinic for specialist advice, their support and help provided reassurance for the patient that all health care professionals were working together to provide the best outcome for them. Consultation includes an explanation of harms of opiates in chronic noncancer pain, how to support them in reducing their opiate use and living with pain. | Ongoing project. 42% (61/94) undergone a dose reduction since start of project. |
| Service 9 | Primary care pain clinic, | Adults with noncancer chronic pain | Group education sessions comprising cover pacing, importance of exercise and sleeping. Pharmacist providing medication management, cognitive behavioural therapist providing mindfulness and stress management. | 100% of patients referred agreed to a dose reduction schedule; 60% successful in adhering to the regime. |
| Service 10 | Primary care | Patients on 120‐mg morphine equivalent dose or higher | Audit conducted by clinical commissioning group to identify medical practices prescribing high levels of opioids. Patients prescribed high levels of opioids highlighted to general practitioners and the list was accompanied with recommendations to review and commence tapering if appropriate. | Ongoing project. |
| Service 11 | Primary care | Patients on opioids for noncancer persistent pain | Development of guidance for opioid prescribing in persistent pain. Commissioning of a new community persistent pain management service. Medical practice level reduction targets for opioid prescribing and opioid audits included in medical practice budget funding model. Educational sessions for and development of additional resources to support prescribers. Patient information leaflets and signposts to national support websites were made available. Practices were supported to sign up for a multicentre trial of a patient behaviour change opioid‐tapering intervention. |
1360 patients were identified from audit, including 169 patients without a review within 12 mo, and 132 patients were over‐ordering. Practices have action plans to manage these patients. A reduction in the total volume (as total oral morphine equivalence/1000 patients), items dispensed and spend on opioids. |
| Service 12 | Outpatient pain clinic, | Patients on 120‐mg morphine equivalent dose or higher who had inadequate pain relief | Face to face, 1 on 1 education sessions, educational materials, blood tests to check for specific opioid‐induced hormonal derangement. Group educational sessions for patients on potential risks/benefits, nonpharmacological strategies for managing persistent pain; reduction of opioids voluntarily if they are ineffective and to manage possible misuse/diversion/dependence issues. Converting higher‐risk patients to safer forms of opioids, e.g. nondivertible patches, longer‐acting opioids. | Many patients voluntarily reducing or even coming off opioids completely. Follow ups showed that patients had appreciated the sessions and felt more confident about self‐managing flare‐ups. All patients who expressed a desire for opioid reduction were weaned off their short‐acting opioids. Doses of opioids in these patients have remained stable with no escalation. Mood and behaviour improved in the men who received testosterone supplementation. |
| Service 13 | Primary care, | Patients prescribed high‐dose opioids | Pain consultant operating an opioid‐tapering service provided a training session to general practitioners. Incentive scheme for practices that included a high dose opioid audit, requesting practices to review all patients on high dose opioids with the aim of reducing or stopping where appropriate. | Ongoing project. Prescribing data year to date has shown a reduction in the number of items and spend on opioids as well as some reduction in high‐dose prescribing |
| Service 14 | Primary care | Patients prescribed opioids for chronic pain | Medical practice‐based pharmacists and technicians audited prescribed opioids in all medical practices across 1 region. | Identified >600 patients prescribed high‐dose opioids. Recommendations made to practices regarding prescribing processes for opioids. |
| Service 15 | Primary care | Unclear | A patient leaflet regarding the problems of high‐dose opioid use prepared. A delivered for general practitioner trainees. A session on this topic held for local deep end group. Running of an audit for patients taking tramadol. | Ongoing project. |
| Service 16 | Primary care | High‐dose opioid users | Education for general practitioners regarding problems of excess opioid use. Searches conducted by the clinical commissioning group to establish number of patients prescribed large quantities and high doses of opioids. Performance results fed back to medical practices including performance of other medical practices. Some practices used the information as a credible source to help initiate discussion with the patient. | Larger reduction in opioid prescribing achieved in intervention practices relative to control. |
All studies comprised complex interventions; the most frequent components of commonality were patient and/or prescriber education, psychological intervention, primarily in the form of cognitive behavioural therapy (n = 25, indicated in Table 2 by*), and pharmacological support of opioid tapering. Interventions were mainly doctor‐led; however, multidisciplinary approaches were frequently adopted. These disciplines included psychologists, nurses, pharmacists and physiotherapists.
TABLE 2.
Refined programme theories
| Initial programme theory tested with the evidence (TDF domain) | Refined programme theory (TDF domain) | Supported N (%) | Contradicted N (%) | Not addressed N (%) | Example evidence supporting refined programme theory |
|---|---|---|---|---|---|
| 1. If patients are given comprehensive education regarding pain management and opioids which addresses their ideas, concerns and expectations then they are more likely to successfully reduce/taper their doses. (Knowledge) |
1.If patients are given comprehensive education to align patient: practitioner expectations of tapering then they are more likely to engage and persist with a tapering schedule. (Social influence) |
33 (47.1) | 0 | 37 (52.8) |
Patient education regarding the pharmacological and non‐pharmacological approaches to pain management, the role of opioids and what to expect from an opioid tapering programme (adverse effects and available support) facilitate patients to persevere with opioid tapering. High levels of attrition from the intervention were attributed to discrepant patient expectations that may be addressed through education. 64 Patients felt a sense of more control over their painful condition by having the knowledge of an array of non‐pharmacological approaches to use during the tapering process. 65 |
|
2a. If programmes incorporate psychological with/without physical interventions to improve confidence in function and address fears regarding recurrence of pain, then they are more likely to be successful in supporting patients to reduce their opioid use. (Skills) |
2a. If programmes incorporate access to psychological and physical support for patients, then practitioners find consultations regarding tapering easier as it allows them to offer an alternative to opioids and patients are better equipped to self‐manage pain during the tapering process. (Environmental context and resources) |
33 (47.1) | 0 | 37 (52.8) |
N = 24 studies reporting better outcomes when patients have access to CBT (Table 1). No interventions comprise physical support without psychological support hence the effects of physical support alone are unknown and therefore retained in this programme theory. Prescribers reported having greater success in opioid tapering discussions by offering an opioid alternative (service 16). 79 |
|
2b. If programmes adopt a multidisciplinary approach, then they are more likely to be successful in supporting patients to reduce their opioid use. (Skills) Combined with: 4. If there is effective communication between different care settings, then patients will be more successful in reducing their opioid use. (Environmental context and resources) |
2b and 4. If there is a consistent approach by all members of the healthcare team, then they will be more successful in supporting patients to taper and stop opioids. (Environmental context and resources) |
2b: 36 (51.4) 4: 8 (11.4) |
2b: 2 (2.8) 4: 0 |
2b: 32 (45.7) 4: 62 (88.6) |
2b. Survey data described situations where successful tapering by one practitioner had been overturned by others or where the planned care in supporting tapering had not been continued (services 11 and 16). 4. Only four interventions incorporated features of cross setting communication. 5 , 20 , 60 , 66 In all four interventions, this was communication from secondary/specialist care to primary care providing detailed information regarding the continuation of agreed tapering strategy. One successful programme specifically contacted all potential sources from which the patient may legitimately access opioids to ensure they are not re‐prescribed. 19 |
|
2c. If programmes adopt a pathway incorporating guidelines then they are more likely to be successful in supporting patients to reduce their opioid use. (Skills) |
2c If programmes have a defined pathway incorporating tapering guidelines, then practitioners know what is expected of them and what support is available when the complexity of a patient’s situation warrants referral. (Knowledge) |
30 (42.8) | 3 (4.3) | 37 (52.9) |
Absence of pathways and guidelines led to variation in practice and therefore outcomes (service 16). Effective interventions comprised a structured and defined pathway (service 12) incorporating guidelines regarding approaches to tapering. Knowledge regarding how to taper supports prescribers in their decision‐making with a credible source of information; Knowledge regarding when to refer supports prescribers to appropriately refer or signpost patients to other practitioners or resources respectively. The threshold for complexity at which referral is recommended will differ dependent upon the organisation’s capacity to offer timely access to specialist services such as psychological and physical support. 67 , 68 |
|
2d. If programmes ensure practitioners are equipped to deliver the intervention (through training or experience) then they will be successful in supporting patients to reduce their opioid use. (Skills) |
2d. If prescribers are equipped with cognitive behavioural intervention skills, then they have the confidence to initiate and manage tapering discussions. (Beliefs about capabilities) |
33 (47.1) | 0 | 37 (52.9) |
Appropriate training such as CBT gave physicians the confidence to initiate discussions and these ‘beliefs about capabilities’ led them to be more motivated to pursue tapering (service 16) Practitioners were more likely to initiate tapering discussions when they had basic training in cognitive based interventions (service 7), |
|
3. If patients perceive that they are being managed by an appropriately skilled clinician then they will be more receptive to the information provided. (Beliefs about consequences) |
Excluded | 1 (1.4) | 0 | 69 (98.6) | No evidence to support this programme theory |
|
5. If patients are allocated to an individual who is responsible for supporting them throughout their opioid tapering then patients are more likely to be successful in reducing/stopping opioids. (Social influences) |
Excluded | 12 (17.1) | 1 (1.4) | 57 (81.4) |
Strong presence of multi‐disciplinary teams in the published effective interventions. Furthermore, the mechanism via which allocation to an individual may be beneficial was proposed to be ensuring a consistent approach, which was therefore addressed by PT 4. |
|
6. If there is a clear expectation that opioid deprescribing is the responsibility of the clinicians, then they are more likely to initiate deprescribing discussions with patients. (Social and Professional role and identity) |
6. If there is a clear expectation that opioid deprescribing is the responsibility of the clinicians, then they are more likely to initiate deprescribing discussions with patients. (Social and Professional role and identity) |
34 (48.6) | 0 | 36 (51.4) | Widely supported by the survey as respondents described incentive schemes, campaigns and audits to highlight to practitioners that it is an expectation that they identify and effectively manage patients prescribed long‐term opioid therapy. This PT was less explicit in the published literature, however, it could be inferred that physicians involved in a trial felt responsible for opioid tapering. |
TDF, Theoretical Domains Framework.
Table 2 catalogues refinement of the initial programme theories based on the evidence and presents the resulting 6 refined programme theories with examples of the supporting evidence. Full details of the supporting and contradictory evidence are provided in supplementary file 2. Evidence was available for all of the programme theories prioritised for testing and was largely supportive. Programme theories (3) and (5) had the least evidence and were excluded.
Programme theory (1), relating to educating patients, was initially in the knowledge domain and transitioned to social influence. This was due to the mechanism of effect being alignment of patient expectations of the tapering process with that of the prescriber; the shared vision of prescribers and patients made the tapering process easier to navigate for the patient. From the prescriber perspective, patients being more receptive and committed to tapering, encouraged prescribers to follow tapering guidelines.
Programme theory (2a) related to availability of psychological and physical interventions transitioned from the skills to the environmental context and resources domain. The was due to the mechanism of effect being appropriate access to nonpharmacological approaches rather than all prescribers needing to have the skills to deliver psychological and physical interventions.
Programme theory (2b), regarding a multidisciplinary approach, did not exert its effect as initially hypothesised. Rather than giving prescribers access to a range of skills such as psychological and physical interventions (this was addressed by programme theory [2a]), the multidisciplinary team was providing the mechanism for a consistent approach by all. This programme theory therefore transitioned from the skills to the environmental context and resources domain. This was combined with programme theory (4) as effective communication is the mechanism by which a consistent approach can be delivered. This programme theory therefore remained in environmental context and resources.
Programme theory (2c) regarded guidelines transitioned from the skills domain to a focus on knowledge provision. The evidence supported the importance of tapering guidelines, but it was clear that guidelines alone are insufficient and must be combined with clear information about when and how to access specialist services. This was because a barrier to prescribers tapering opioids was not knowing the threshold at which referral to specialist services is appropriate. Some prescribers attempted to manage patients with highly complex circumstances that should be referred whilst other prescribers referred any patient prescribed opioids leading to overburdening of services.
Programme theory (2d) reflected the need for practitioners to be equipped with the necessary skills to taper; however, this transitioned to beliefs about capabilities as the mechanism by which the skills provision was exerting its effect was building the prescriber’s confidence to effectively manage tapering consultations.
Programme theory (6), regarding organisations demonstrating a clear expectation that opioid tapering is the role of the prescriber, remained unchanged after testing. This programme theory was therefore retained in the social and professional role and identity domain.
The final mid‐range theory is below with the numbers in brackets indicating the programme theory from which the statement is derived (Figure 2).
FIGURE 2.

Final programme theory
Table 3 provides an example of how the mid‐range theory may be operationalised. It demonstrates how 1 intervention component can be characterised to fulfil multiple programme theories. In the example, coproduced training materials with organisational branding address 4 of the 7 elements incorporated in the final mid‐range theory.
TABLE 3.
Example operationalisation of the 6 programme theories
| Example opioid‐tapering intervention component | Programme theory addressed by the intervention component | |||||
|---|---|---|---|---|---|---|
| Comprehensive education for patients | Access to appropriate levels of psychological and physical support | A consistent approach by all members of the health care team | A pathway including information about how to taper | Practitioners with the knowledge and skills to initiate tapering discussions and navigate the patient pathway | A clear expectation that opioid deprescribing is the responsibility of prescribers | |
| Patient friendly materials with organisation branding describing what to expect from opioid tapering including potential for opioid withdrawal symptoms and available support during the process. | ✓ | ✓ | ✓ | ✓ | ||
| Basic training in cognitive behavioural interventions giving practitioners the confidence to initiate tapering discussions and provide the ongoing nonpharmacological support to prevent patients feeling abandoned. | ✓ | ✓ | ✓ | |||
| Agreed thresholds (based on local resources and practitioner acceptability for when referrals to specialist services such as physiotherapists, psychiatric input and substance misuse programmes is required. Threshold descriptions incorporated in a treatment pathway with branding of the organisation. | ✓ | |||||
| Practitioner friendly opioid‐tapering training materials including guideline about how to taper, knowledge about local excess opioid use problems and the local patient pathway. Materials coproduced by primary and secondary care stakeholder organisations and incorporating their branding. An associated incentive scheme/recognition for adhering to the training/guideline recommendations. | ✓ | ✓ | ✓ | ✓ | ||
4. DISCUSSION
We have identified 6 programme theories that support practitioner behaviour change and are therefore essential to every effective organisational level strategy for opioid‐tapering services. Operationalisation of each programme theory should be determined by individual health systems according to existing structures and resources. The 6 interdependent MoAs address the barriers and enablers to opioid tapering from engaging prescribers in opioid‐tapering initiatives through to effective execution of opioid tapering in partnership with the patient. Existing guidelines may be adapted to address multiple programme theories but cannot fulfil all e.g. skills provision.
In accordance with previous evidence syntheses, a complex intervention is required to support prescribers in successfully tapering long‐term opioids. 12 The evidence gap that we address is identifying which components of these complex interventions work for whom, and when and why. Understanding why intervention components are effective enables adaptation for diverse health systems thus providing a service model suitable for global translation whilst maintaining efficacy. Furthermore, through combining research with practice evidence, our service model recognises the challenges of implementation beyond the trial setting. We have therefore generated a model that achieves feasibility and scalability whilst maintaining efficacy.
We identify that providing education to align patient expectations of opioid tapering with the actual experience contributes to successful tapering. This is achieved by addressing the negative social influence that patients may otherwise have on practitioners trying to taper opioids. The education should include preparation for the potential for opioid withdrawal symptoms and available support during the process. This is to a certain extent recognised in the US Centres for Disease Control and Prevention guidelines on prescribing opioids for chronic pain which refers to prescribers working with patients to taper. 70 The UK Opioids Aware resource goes further by advising prescribers to manage patient expectations that being pain free may be unrealistic. 71 The guidelines, however, do not extend to specifying that a key element of working together is fully preparing the patient regarding what to expect of tapering. The importance of adequate preparation of the patient prior to tapering aligns with 1 of the mechanisms via which cognitive‐based interventions were successful as their core function is facilitating patients to identify their barriers and enablers to tapering and working with them to problem‐solve.
Prescribers have reported reticence to discuss tapering as they feel ill‐equipped to deal with the “huge psychological toll on providers”. 72 , 73 They also have negative beliefs about their capability to provide the ongoing psychological support that they recognise is important for the patient. 74 Basic training for prescribers to deliver cognitive‐based interventions may therefore equip prescribers with the skills to initiate tapering discussions and provide the ongoing nonpharmacological support to prevent patients feeling abandoned. 74 , 75 This configuration may be most appropriate for service delivery in primary care where a multidisciplinary team is less accessible. 72 , 73
Whilst upskilling prescribers to deliver basic cognitive‐based interventions affords many benefits, our findings confirm that it is not feasible for 1 prescriber to fulfil the needs of all patients during the tapering process. We therefore recognise that restructuring the environment to provide a pathway for access to specialist services such as physiotherapists, psychiatric input and substance misuse programmes is required. We also acknowledge the importance of guidelines providing the knowledge to ensure consistency of practice both within and between care settings. Guidelines offering detailed knowledge about the tapering process whilst still allowing individualisation of care are particularly welcomed. 73 , 76 Our findings are therefore complemented by other programmes of research focussing on supporting patient behaviour change and refinements to tapering guidelines. 77 , 78
Misaligned goals of primary care providers perceiving that productivity is more important than investing the required time for complex tapering consultations hampers progress in achieving opioid‐tapering. 72 Incentive schemes to demonstrate that opioid tapering is an organisational expectation 76 challenge this perception. A rationale for the absence of such incentives in the published literature is the reactivity bias of data collection for a trial generating sufficient expectation of opioid tapering without the need for incentives.
Applying methodological concepts from realist reviews to behavioural science methods has enabled evidence from the trial and practice environment to be combined with the knowledge and experience of experts in a structured, transparent manner. Furthermore, a limitation of previous systematic reviews is the observational nature of most studies prohibiting inferences of causality 12 ; in contrast, our qualitative survey enabled inferences regarding causality.
Our literature focussed on intervention studies to provide high quality outcome data regarding opioid tapering; this may have limited our ability to derive data regarding the rationale for effective components. However, through combining these data with our survey findings, we have generated the richness of understanding necessary to identify the MoAs by which intervention components support practitioners to taper opioids. Through multiple recruitment strategies we have identified a diverse range of services in England, but we cannot be certain that we have identified all services. Bias in recruitment and reporting may have led to survey responses originating from services that are more successful. We attempted to mitigate these effects through emphasising the importance of sharing learning within healthcare. The inferences are limited by the survey data being derived only from 1 country; however, through combining these data with the published literature that represented numerous countries, we have greater confidence in the likely transferability of the findings. We also found that trial data were focussed on secondary care whereas much of the opioid tapering in real‐world practice took place in primary care. These findings are endorsed by a 2018 review of strategies adopted by primary care organisations across 1 US state. 76
We recommend that healthcare organisations commissioning opioid‐tapering programmes ensure that all 6 mechanisms represented in the mid‐range theory are incorporated. How the mid‐range theory is operationalised should be tailored to the individual organisation's infrastructure and resources through engaging with prescribers and patients, and using criteria such as affordability, practicability and acceptability to guide decision‐making. 79 For example, we state that programmes should incorporate “a pathway for patient management including access to appropriate levels of psychological and physical support”. The level that is deemed appropriate will differ according to factors such as availability of physiotherapists, practitioners trained in cognitive behavioural therapy and the level of patient complexity that primary care prescribers are willing to manage within their case load.
An example of how the programme theories may be operationalised is to build on the guidelines that most healthcare organisations already advocate. These guidelines will usually include information about how to taper but could be supplemented with knowledge about local excess opioid use problems and the local patient pathway. These guidelines may then be endorsed by the employer e.g. using an incentive scheme. The patient pathway may also function to facilitate all members of the healthcare system to adopt a consistent approach. Combining the guidelines with basic training in cognitive‐based interventions may provide prescribers with the required support, to initiate tapering discussions, particularly when the patient pathway incorporates access to psychological and physical support for the patient. Numerous credible educational resources are available for patients to ensure that their expectations of opioid tapering align with the guidelines and patient pathway.
5. CONCLUSIONS
This evidence synthesis has identified 6 programme theories by which the determinants of practitioners' opioid‐tapering behaviour should be addressed. Most opioid‐tapering interventions reported in the literature and real practice include components addressing some of these programme theories, but none address all 6.
The need remains to characterise behaviour change techniques that act on the MoAs identified to formulate practitioner behaviour change interventions bespoke for individual organisations. Whilst the facility for adaptation is important for supporting intervention implementation and makes our findings transferable and scalable across all healthcare systems, it recognises that further work is required by service delivery teams to design their local implementation strategy.
COMPETING INTERESTS
The authors declare that they have no competing interests.
PATIENT AND PUBLIC INVOLVEMENT
The group that worked with the research team to develop the research question and help with interpreting the findings included representation of 2 members of the public.
ROLE OF SPONSOR
The University of East Anglia had no role in the design, conduct or reporting of the study.
CONTRIBUTORS
All authors contributed to the study design led by Debi Bhattacharya. Hattie Whiteside and Emma Tang reviewed the published literature for relevance in terms of whether it provided sufficient detail to contribute to refining and/or adding to the initial programme theories. Debi Bhattacharya and Emma Tang reviewed and graded survey results for relevance using the same criteria as the published literature. Caroline Hill, Bethany Atkins, Kumud Kantilal, Hattie Whiteside and Debi Bhattacharya evaluated and analysed the data. Hattie Whiteside, Yoon Loke, Debi Bhattacharya, Bethany Atkins and Caroline Hill were major contributors in writing the manuscript. All authors read and approved the final manuscript.
OPEN RESEARCH BADGES
This article has been awarded Open Data and Open Materials Badges. All materials and data for this article are publicly accessible via the Open Science Framework.
Supporting information
TABLE S1 Development of initial program theories
ACKNOWLEDGEMENT
This research was funded by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) East of England. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Bhattacharya D, Whiteside H, Tang E, et al. A review of trial and real‐world data applying elements of a realist approach to identify behavioural mechanisms supporting practitioners to taper opioids. Br J Clin Pharmacol. 2022;88(9):4019‐4042. doi: 10.1111/bcp.15379
Funding information NIHR Applied Research Collaboration East of England; NIHR Applied Research Collaboration, Grant/Award Number: IE21
DATA AVAILABILITY STATEMENT
The datasets that support the findings of this study are openly available here: https://www.uea.ac.uk/pharmacy/research/chronic-opioid-use-in-non-cancer-pain.
REFERENCES
- 1. Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Reports. 2017;2(3):e599‐e599. doi: 10.1097/PR9.0000000000000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sandhu H, Underwood M, Furlan A, Noyes J, Eldabe S. What interventions are effective to taper opioids in patients with chronic pain? BMJ. 2018;362:k2990. doi: 10.1136/bmj.k2990 [DOI] [PubMed] [Google Scholar]
- 3. Chaparro LE, Furlan AD, Deshpande A, Mailis‐Gagnon A, Atlas S, Turk DC. Opioids Compared With Placebo or Other Treatments for Chronic Low Back Pain An Update of the Cochrane Review. Spine. 2014;39(7):556‐563. doi: 10.1097/BRS.0000000000000249 [DOI] [PubMed] [Google Scholar]
- 4. Darchuk KMT CO, Rome JD, Bruce BK, Hooten WM. Longitudinal Treatment Outcomes for Geriatric Patients with Chronic Non‐Cancer Pain at an Interdisciplinary Pain Rehabilitation Program. Pain Med. 2010;11:1352‐1364. doi: 10.1111/j.1526-4637.2010.00937.x [DOI] [PubMed] [Google Scholar]
- 5. Dersh J, Mayer TG, Gatchel RJ, Polatin PB, Theodore BR, Mayer EA. Prescription opioid dependence is associated with poorer outcomes in disabling spinal disorders. Spine. 2008;33(20):2219‐2227. doi: 10.1097/BRS.0b013e31818096d1 [DOI] [PubMed] [Google Scholar]
- 6. Drossman DA, Morris CB, Edwards H, et al. Diagnosis, characterization, and 3‐month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol. 2012;107(9):1426‐1440. doi: 10.1038/ajg.2012.142 [DOI] [PubMed] [Google Scholar]
- 7. Hanson KA, Loftus EV Jr, Harmsen WS, Diehl NN, Zinsmeister AR, Sandborn WJ. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: a case‐control study. Inflamm Bowel Dis. 2009;15:772‐777. doi: 10.1002/ibd.20847 [DOI] [PubMed] [Google Scholar]
- 8. Harden P, Ahmed S, Ang K, Wiedemer N. Clinical Implications of Tapering Chronic Opioids in a Veteran Population. Pain Med. 2015;16(10):1975‐1981. doi: 10.1111/pme.12812 [DOI] [PubMed] [Google Scholar]
- 9. Scott S, Wright DJ, Bhattacharya D. The role of behavioural science in changing deprescribing practice. Br J Clin Pharmacol. 2021;87(1):39‐41. doi: 10.1111/bcp.14595 [DOI] [PubMed] [Google Scholar]
- 10. Liu S, Gnjidic D, Nguyen J, Penm J. Effectiveness of interventions on the appropriate use of opioids for noncancer pain among hospital inpatients: A systematic review. Br J Clin Pharmacol. 2020;86(2):210‐243. doi: 10.1111/bcp.14203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alderson SL, Bald A, Carder P, Farrin A, Foy R. Establishing a primary care audit and feedback implementation laboratory: a consensus study. Implement Sci Commun. 2021;2(1):3. doi: 10.1186/s43058-020-00103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frank JW, Lovejoy TI, Becker WC, et al. Patient Outcomes in Dose Reduction or Discontinuation of Long‐Term Opioid Therapy: A Systematic Review. Ann Intern Med. 2017;167(3):181‐191. doi: 10.7326/M17-0598 [DOI] [PubMed] [Google Scholar]
- 13. Kantilal K, Hardeman W, Whiteside H, Karapanagiotou E, Small M, Bhattacharya D. Realist review protocol for understanding the realworld barriers and enablers to practitioners implementing self‐management support to people living with and beyond cancer. BMJ Open. 2020;(9):e037636. doi: 10.1136/bmjopen-2020-037636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manzano A. The craft of interviewing in realist evaluation. Evaluation. 2016;(3):22‐360. doi: 10.1177/1356389016638615 [DOI] [Google Scholar]
- 15. Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist Synthesis ‐ an Introduction. ESRC Working Paper Series. London: Economic and Social Research Council; 2004. [Google Scholar]
- 16. Cane J, Richardson M, Johnston M, Ladha R, Michie S. From lists of behaviour change techniques (BCTs) to structured hierarchies: comparison of two methods of developing a hierarchy of BCTs. Br J Health Psychol. 2015;20(1):130‐150. doi: 10.1111/bjhp.12102 [DOI] [PubMed] [Google Scholar]
- 17. Wong G, Westhorp G, Manzano A, Greenhalgh J, Jagosh J, Greenhalgh T. RAMESES II reporting standards for realist evaluations. BMC Med. 2016;14(1):96. doi: 10.1186/s12916-016-0643-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Little J, Higgins JP, Ioannidis JP, et al. STrengthening the REporting of Genetic Association Studies (STREGA)‐‐an extension of the STROBE statement. Genet Epidemiol. 2009;33(7):581‐598. doi: 10.1002/gepi.20410 [DOI] [PubMed] [Google Scholar]
- 19. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high‐dose opioids. J Opioid Manag. 2006;2(5):277‐282. doi: 10.5055/jom.2006.0041 [DOI] [PubMed] [Google Scholar]
- 20. Berland DW, Malinoff HL, Weiner MA, Przybylski R. When opioids fail in chronic pain management: the role for buprenorphine and hospitalization. Am J Ther. 2013;20(4):316‐321. doi: 10.1097/MJT.0b013e31827ab599 [DOI] [PubMed] [Google Scholar]
- 21. Blondell RD, Ashrafioun L, Dambra CM, Foschio EM, Zielinski AL, Salcedo DM. A Clinical Trial Comparing Tapering Doses of Buprenorphine with Steady Doses for Chronic Pain and Co‐existent Opioid Addiction. J Addict Med. 2010;4(3):140‐146. doi: 10.1097/ADM.0b013e3181ba895d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buckley PF, Sizemore WA, Charlton EJ. Medication management in patients with chronic non‐malignant pain. A review of the use of a drug withdrawal protocol. Pain. 1986;26:153‐165. doi: 10.1016/0304-3959(86)90071-0 [DOI] [PubMed] [Google Scholar]
- 23. Cowan DT, Wilson‐Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4:340‐351. doi: 10.1111/j.1526-4637.2003.03038.x [DOI] [PubMed] [Google Scholar]
- 24. Cowan DT, Wilson‐Barnett J, Griffiths P, Vaughan DJ, Gondhia A, Allan LG. A Randomized, Double‐Blind, Placebo‐Controlled, Cross‐Over Pilot Study to Assess the Effects of Long‐Term Opioid Drug Consumption and Subsequent Abstinence in Chronic Noncancer Pain Patients Receiving Controlled‐Release Morphine. Pain Med. 2005;6:113‐121. doi: 10.1111/j.1526-4637.2005.05020.x [DOI] [PubMed] [Google Scholar]
- 25. Crisostomo RA, Schmidt JE, Hooten WM, Kerkvliet JL, Townsend CO, Bruce BK. Withdrawal of analgesic medication for chronic low‐back pain patients: improvement in outcomes of multidisciplinary rehabilitation regardless of surgical history. Am J Phys Med Rehabil. 2008;87(7):527‐536. doi: 10.1097/PHM.0b013e31817c124f [DOI] [PubMed] [Google Scholar]
- 26. Cunningham JL, Evans MM, King SM, Gehin JM, Loukianova LL. Opioid Tapering in Fibromyalgia Patients: Experience from an Interdisciplinary Pain Rehabilitation Program. Pain Med. 2016;17(9):1676‐1685. doi: 10.1093/pm/pnv079 [DOI] [PubMed] [Google Scholar]
- 27. Daitch J, Frey ME, Silver D, Mitnick C, Daitch D, Pergolizzi J Jr. Conversion of chronic pain patients from full‐opioid agonists to sublingual buprenorphine. Pain Physician. 2012;3S;15(3S;7):15‐ES66. doi: 10.36076/ppj.2012/15/ES59 [DOI] [PubMed] [Google Scholar]
- 28. Daitch D, Daitch J, Novinson D, Frey M, Mitnick C, Pergolizzi J Jr. Conversion from high‐dose full‐opioid agonists to sublingual buprenorphine reduces pain scores and improves quality of life for chronic pain patients. Pain Med. 2014;15(12):2087‐2094. doi: 10.1111/pme.12520 [DOI] [PubMed] [Google Scholar]
- 29. Hassamal S, Haglund M, Wittnebel K, Danovitch I. A preoperative interdisciplinary biopsychosocial opioid reduction program in patients on chronic opioid analgesia prior to spine surgery: A preliminary report and case series. Scand J Pain. 2016;13:27‐31. doi: 10.1016/j.sjpain.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 30. Hooten WM, Townsend CO, Sletten CD, Bruce BK, Rome JD. Treatment Outcomes after Multidisciplinary Pain Rehabilitation with Analgesic Medication Withdrawal for Patients with Fibromyalgia. Pain Med. 2007;8(1):8‐16. doi: 10.1111/j.1526-4637.2007.00253.x [DOI] [PubMed] [Google Scholar]
- 31. Hooten WM, Townsend CO, Decker PA. Gender differences among patients with fibromyalgia undergoing multidisciplinary pain rehabilitation. Pain Med. 2007;8(8):624‐632. doi: 10.1111/j.1526-4637.2006.00202.x [DOI] [PubMed] [Google Scholar]
- 32. Hooten WM, Townsend CO, Bruce BK, Warner DO. The effects of smoking status on opioid tapering among patients with chronic pain. Anesth Analg. 2009;108(1):308‐315. doi: 10.1213/ane.0b013e31818c7b99 [DOI] [PubMed] [Google Scholar]
- 33. Hooten WM, Mantilla CB, Sandroni P, Townsend CO. Associations between Heat Pain Perception and Opioid Dose among Patients with Chronic Pain Undergoing Opioid Tapering. Pain Med. 2010;(11):1587‐1598. doi: 10.1111/j.1526-4637.2010.00962.x [DOI] [PubMed] [Google Scholar]
- 34. Hooten WM, Warner DO. Varenicline for opioid withdrawal in patients with chronic pain: a randomized, single‐blinded, placebo controlled pilot trial. Addict Behav. 2015;42:69‐72. doi: 10.1016/j.addbeh.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 35. Huffman KL, Sweis GW, Gase A, Scheman J, Covington EC. Opioid Use 12 Months Following Interdisciplinary Pain Rehabilitation with Weaning. Pain Med. 2013;14:1908‐1917. doi: 10.1111/pme.12201 [DOI] [PubMed] [Google Scholar]
- 36. Huffman KL, Rush TE, Fan Y, et al. Sustained improvements in pain, mood, function and opioid use post interdisciplinary pain rehabilitation in patients weaned from high and low dose chronic opioid therapy. Pain. 2017;158(7):1380‐1394. doi: 10.1097/j.pain.0000000000000907 [DOI] [PubMed] [Google Scholar]
- 37. Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91(4):919‐927. doi: 10.2106/JBJS.H.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krumova EK, Bennemann P, Kindler D, Schwarzer A, Zenz M, Maier C. Low Pain Intensity After Opioid Withdrawal as a First Step of a Comprehensive Pain Rehabilitation Program Predicts Long‐term Nonuse of Opioids in Chronic Noncancer Pain. Clin J Pain. 2013;29:760‐769. doi: 10.1097/AJP.0b013e31827c7cf6 [DOI] [PubMed] [Google Scholar]
- 39. Lake AE 3rd, Saper JR, Hamel RL. Comprehensive inpatient treatment of refractory chronic daily headache. Headache. 2009;49(4):555‐562. doi: 10.1111/j.1526-4610.2009.01364.x [DOI] [PubMed] [Google Scholar]
- 40. Maclaren JE, Gross RT, Sperry JA, Boggess JT. Impact of Opioid Use on Outcomes of Functional Restoration. Clin J Pain. 2006;22:392‐398. doi: 10.1097/01.ajp.0000208250.15572.01 [DOI] [PubMed] [Google Scholar]
- 41. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379‐384. doi: 10.1097/01.mjt.0000160935.62883.ff [DOI] [PubMed] [Google Scholar]
- 42. Mehl‐Madrona L, Mainguy B, Plummer J. Integration of Complementary and Alternative Medicine Therapies into Primary‐Care Pain Management for Opiate Reduction in a Rural Setting. J Altern Complement Med. 2016;22(8):621‐626. doi: 10.1089/acm.2015.0212 [DOI] [PubMed] [Google Scholar]
- 43. Miller NS, Swiney T, Barkin RL. Effects of Opioid Prescription Medication Dependence and Detoxification on Pain Perceptions and Self‐Reports. Am J Ther. 2006;13(5):436‐444. doi: 10.1097/01.mjt.0000212894.35705.90 [DOI] [PubMed] [Google Scholar]
- 44. Murphy JL, Phillips KM, Rafie S. Sex differences between Veterans participating in interdisciplinary chronic pain rehabilitation. J Rehabil Res Dev. 2016;53(1):83‐94. doi: 10.1682/JRRD.2014.10.0250 [DOI] [PubMed] [Google Scholar]
- 45. Murphy JL, Clark ME, Banou E. Opioid Cessation and Multidimensional Outcomes After Interdisciplinary Chronic Pain Treatment. Clin J Pain. 2013;29(2):109‐117. doi: 10.1097/AJP.0b013e3182579935 [DOI] [PubMed] [Google Scholar]
- 46. Naylor MR, Naud S, Keefe FJ, Helzer JE. Therapeutic Interactive Voice Response (TIVR) to reduce analgesic medication use for chronic pain management. J Pain. 2010;11(12):1410‐1419. doi: 10.1016/j.jpain.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nilsen HK, Stiles TC, Landro NI, Fors EA, Kaasa S, Borchgrevink PC. Patients with problematic opioid use can be weaned from codeine without pain escalation. Acta Anaesthesiol Scand. 2010;54(5):571‐579. doi: 10.1111/j.1399-6576.2009.02164.x [DOI] [PubMed] [Google Scholar]
- 48. Nissen LM, Tett SE, Cramond T, Williams B, Smith MT. Opioid analgesic prescribing and use ‐ an audit of analgesic prescribing by general practitioners and The Multidisciplinary Pain Centre at Royal Brisbane Hospital. Br J Clin Pharmacol. 2001;52:693‐698. doi: 10.1046/j.0306-5251.2001.01502.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ralphs JA, de C Williams AC, Richardson PH, Pither CE, Nicholas MK. Opiate reduction in chronic pain patients: a comparison of patient‐controlled reduction and staff controlled cocktail methods. Pain. 1994;56(3):279‐288. doi: 10.1016/0304-3959(94)90166-X [DOI] [PubMed] [Google Scholar]
- 50. Rome JD, Townsend CO, Bruce BK, Sletten CD, Luedtke CA, Hodgson JE. Chronic noncancer pain rehabilitation with opioid withdrawal: comparison of treatment outcomes based on opiod use status at admission. Mayo Clin Proc. 2004;79:759‐768. doi: 10.1016/S0025-6196(11)62628-1 [DOI] [PubMed] [Google Scholar]
- 51. Rosenblum A, Cruciani RA, Strain EC, et al. Sublingual buprenorphine/naloxone for chronic pain in at‐risk patients: development and pilot test of a clinical protocol. J Opioid Manag. 2012;8(6):369‐382. doi: 10.5055/jom.2012.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roux P, Sullivan MA, Cohen J, et al. Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain. 2013;154(8):1442‐1448. doi: 10.1016/j.pain.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schneider JP, Kirsh PKL. Defining clinical issues around tolerance, hyperalgesia, and addiction: A quantitative and qualitative outcome study of long‐term opioid dosing in a chronic pain practice. J Opioid Manag. 2010;6(6):385‐395. doi: 10.5055/jom.2010.0036 [DOI] [PubMed] [Google Scholar]
- 54. Schwarzer A, Aichinger‐Hinterhofer M, Maier C, Vollert J, Walther JW. Sleep‐disordered breathing decreases after opioid withdrawal: results of a prospective controlled trial. Pain. 2015;156(11):2167‐2174. doi: 10.1097/j.pain.0000000000000279 [DOI] [PubMed] [Google Scholar]
- 55. Streltzer J, Davidson R, Goebert D. An observational study of buprenorphine treatment of the prescription opioid dependent pain patient. Am J Addict. 2015;24(4):357‐361. doi: 10.1111/ajad.12198 [DOI] [PubMed] [Google Scholar]
- 56. Sullivan MD, Turner JA, DiLodovico C, D'Appollonio A, Stephens K, Chan YF. Prescription Opioid Taper Support for Outpatients With Chronic Pain: A Randomized Controlled Trial. J Pain. 2017;18(3):308‐318. doi: 10.1016/j.jpain.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor BC, Zlutnick SI, Corley MJ, Flora J. The effects of detoxification, relaxation, and brief supportive therapy on chronic pain. Pain. 1980;8:319‐329. doi: 10.1016/0304-3959(80)90077-9 [DOI] [PubMed] [Google Scholar]
- 58. Tennant FS Jr, Rawson RA, Miranda L, Obert J. Outpatient Treatment of Prescription Opioid Dependence: Comparison of Two Methods. Arch Intern Med. 1982;142(10):1845‐1847. doi: 10.1001/archinte.1982.00340230087016 [DOI] [PubMed] [Google Scholar]
- 59. Thieme K, Gromnica‐Ihle E, Flor H. Operant behavioral treatment of fibromyalgia: a controlled study. Arthritis Rheum. 2003;49(3):314‐320. doi: 10.1002/art.11124 [DOI] [PubMed] [Google Scholar]
- 60. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140(1):177‐189. doi: 10.1016/j.pain.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 61. Vines SW, Cox A, Nicoll L, Garrett S. Effects of a Multimodal Pain Rehabilitation Program: A Pilot Study. Rehabil Nurs. 1996;21(1):25‐30. doi: 10.1002/j.2048-7940.1996.tb01669.x [DOI] [PubMed] [Google Scholar]
- 62. Wang H, Akbar M, Weinsheimer N, Gantz S, Schiltenwolf M. Longitudinal observation of changes in pain sensitivity during opioid tapering in patients with chronic low‐back pain. Pain Med. 2011;12:1720‐1726. doi: 10.1111/j.1526-4637.2011.01276.x [DOI] [PubMed] [Google Scholar]
- 63. Webster L, Gruener D, Kirby T, Xiang Q, Tzanis E, Finn A. Evaluation of the Tolerability of Switching Patients on Chronic Full mu‐Opioid Agonist Therapy to Buccal Buprenorphine. Pain Med. 2016;pnv110. doi: 10.1093/pm/pnv110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weimer MB, Hartung DM, Ahmed S, Nicolaidis C. A chronic opioid therapy dose reduction policy in primary care. Subst Abus. 2016;37(1):141‐147. doi: 10.1080/08897077.2015.1129526 [DOI] [PubMed] [Google Scholar]
- 65. Whitten SK, Stanik‐Hutt J. Group cognitive behavioral therapy to improve the quality of care to opioid‐treated patients with chronic noncancer pain: a practice improvement project. J Am Assoc Nurse Pract. 2013;25(7):368‐376. doi: 10.1111/j.1745-7599.2012.00800.x [DOI] [PubMed] [Google Scholar]
- 66. de Williams CAC, Richardson PH, Nicholas MK, Pither CE, et al. Inpatient vs. outpatient pain management: results of a randomised controlled trial. Pain. 1996;66(1):13‐22. doi: 10.1016/0304-3959(96)02996-X [DOI] [PubMed] [Google Scholar]
- 67. Younger J, Barelka P, Carroll I, et al. Reduced cold pain tolerance in chronic pain patients following opioid detoxification. Pain Med. 2008;9(8):1158‐1163. doi: 10.1111/j.1526-4637.2008.00475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zgierska AE, Burzinski CA, Cox J, et al. Mindfulness Meditation and Cognitive Behavioral Therapy Intervention Reduces Pain Severity and Sensitivity in Opioid‐Treated Chronic Low Back Pain: Pilot Findings from a Randomized Controlled Trial. Pain Med. 2016;17(10):1865‐1881. doi: 10.1093/pm/pnw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zgierska AE, Burzinski CA, Cox J, et al. Mindfulness Meditation‐Based Intervention Is Feasible, Acceptable, and Safe for Chronic Low Back Pain Requiring Long‐Term Daily Opioid Therapy. J Altern Complement Med. 2016;22(8):610‐620. doi: 10.1089/acm.2015.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scott S, Twigg MJ, Clark A, et al. Development of a hospital deprescribing implementation framework: A focus group study with geriatricians and pharmacists. Age Ageing. 2019;49(1):102‐110. doi: 10.1093/ageing/afz133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;1:1‐49. [DOI] [PubMed] [Google Scholar]
- 72. Aware O Checklist for prescribers ‐ what to discuss with the patient when considering opioid treatment. Available from: https://fpm.ac.uk/opioids-aware-structured-approach-opioid-prescribing/checklist-prescribers.
- 73. Giannitrapani KF, Ahluwalia SC, McCaa M, Pisciotta M, Dobscha S, Lorenz KA. Barriers to Using Nonpharmacologic Approaches and Reducing Opioid Use in Primary Care. Pain Medicine (Malden, Mass). 2018;19(7):1357‐1364. doi: 10.1093/pm/pnx220 [DOI] [PubMed] [Google Scholar]
- 74. Kennedy LC, Binswanger IA, Mueller SR, et al. Those Conversations in My Experience Don't Go Well: A Qualitative Study of Primary Care Provider Experiences Tapering Long‐term Opioid Medications. Pain Medicine (Malden, Mass). 2018;19(11):2201‐2211. doi: 10.1093/pm/pnx276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Matthias MS, Johnson NL, Shields CG, et al. "I'm Not Gonna Pull the Rug out From Under You": Patient‐Provider Communication About Opioid Tapering. J Pain off J Am Pain Soc. 2017;18(11):1365‐1373. doi: 10.1016/j.jpain.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Henry SG, Paterniti DA, Feng B, et al. Patients' Experience With Opioid Tapering: A Conceptual Model With Recommendations for Clinicians. J Pain off J Am Pain Soc. 2019;20(2):181‐191. doi: 10.1016/j.jpain.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hartung DM, Alley L, Leichtling G, Korthuis PT, Hildebran C. A statewide effort to reduce high‐dose opioid prescribing through coordinated care organizations. Addict Behav. 2018;86:32‐39. doi: 10.1016/j.addbeh.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nichols VP, Abraham C, Eldabe S, Sandhu HK, Underwood M, Seers K. Process evaluation protocol for the I‐WOTCH study: an opioid tapering support programme for people with chronic non‐malignant pain. BMJ Open. 2019;9(10):e028998. doi: 10.1136/bmjopen-2019-028998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic pain. Edinburgh: SIGN; 2013. (SIGN; publication no. 136). Revised August 2019. [Available from: http://www.sign.ac.uk.] [Google Scholar]
- 80. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4:CD011279. doi: 10.1002/14651858.CD011279.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Development of initial program theories
Data Availability Statement
The datasets that support the findings of this study are openly available here: https://www.uea.ac.uk/pharmacy/research/chronic-opioid-use-in-non-cancer-pain.


