Abstract
It has been suggested that pets play a critical role in the maintenance of methicillin‐resistant (MR) and multidrug‐resistant (MDR) Staphylococcus spp. in the household. We examined risk factors for carriage of antimicrobial‐resistant coagulase‐positive staphylococci, with particular attention to Staphylococcus aureus and Staphylococcus pseudintermedius isolated from pets living in households of people diagnosed with methicillin‐resistant S. aureus (MRSA) skin or soft‐tissue infection. We analyzed data collected cross‐sectionally from a study conducted in 2012 that evaluated the transmission of MRSA and other staphylococci from humans, their pets and the environment (Pets and Environmental Transmission of Staphylococci [PETS] study). We used unadjusted and adjusted stratified logistic regression analyses with household‐clustered standard errors to evaluate the association between demographic, healthcare‐related, contact‐related and environmental risk factors and MDR Staphylococcus spp. isolated from dogs and cats. Staphylococcal isolates obtained from dogs (n = 63) and cats (n = 47) were included in these analyses. The use of oral or injectable antimicrobials by the pets during the prior year was the main risk factor of interest. Based on our results, 50% (12/24) of S. aureus, 3.3% (1/30) of S. pseudintermedius and 25% (14/56) of other coagulase‐positive staphylococci (CPS) were determined to be MDR. S. aureus isolates were more likely to be MDR compared with S. pseudintermedius. We did not find a significant statistical association between the use of oral or injectable antimicrobials in the prior year and the presence of MDR bacteria. The results suggest that drivers of antimicrobial resistance in household staphylococci may vary by bacterial species, which could have implications for one health intervention strategies for staphylococci and inform the investigation of other reverse zoonoses, such as COVID‐19.
Keywords: antimicrobial resistance, domestic animals, multidrug resistance, Staphylococcus
Impacts.
We evaluated odds of MDR in coagulase‐positive staphylococci.
S. aureus was more likely to be MDR than S. pseudintermedius isolated from pets.
Risk factors driving antimicrobial nonsusceptibility may differ among Staphylococcus spp.
1. INTRODUCTION
Antimicrobial resistance is a critically important global health challenge. Recent attention has been given to the contribution of pets to the increased prevalence of antimicrobial‐resistant bacteria among humans due to their frequent and close contact with humans and their environment. As the burden of community‐associated methicillin‐resistant Staphylococcus (MRS), particularly Staphylococcus aureus (MRSA), has increased in recent years, pets could play an important role in this relatively understudied transmission setting (Davis et al., 2012; Pantosti, 2012). Antimicrobial‐resistant bacteria carried by pets can be transmitted to humans and can be a source of resistance genes that can be horizontally transferred from commensal to pathogenic organisms (and vice versa) present in a shared community of microbes (Davis et al., 2012; Pantosti, 2012). Antimicrobial usage in both pets and humans could contribute to the selective survival of multidrug‐resistant S. aureus (MDRSA) in the environment (Shahbazian et al., 2017). Therefore, household settings of people infected with MRSA is a particular scenario where pets could potentially remain carriers of resistant staphylococci even after humans have been treated, thus serving as a source for human re‐exposure or a source of resistant genes. Additionally, pets can be exposed to staphylococci from animals and humans outside the home, bringing new strains to the home environment that could re‐colonize and re‐infect humans. Both scenarios could lead to the persistence of staphylococci in the household setting and the apparent failure of decolonization therapies. However, the exact role animals play and whether they may have modifiable risk factors related to the carriage of methicillin‐ and multidrug‐resistant staphylococci (MDRS) have not been well‐studied.
Coagulase‐positive Staphylococcus (CPS) species of medical and veterinary importance (e.g. S. aureus and S. pseudintermedius) frequently develop antimicrobial resistance to beta‐lactam drugs. Although S. aureus is more common among humans and S. pseudintermedius causes infection primarily in dogs, both organisms can be transmitted between animals and humans and both can cause severe skin and soft‐tissue infections (SSTIs) (Davis et al., 2012; Saputra et al., 2017; Soares Magalhães et al., 2010).
Previous studies of MRSA and methicillin‐resistant S. pseudintermedius (MRSP) from domestic animals have identified several potential risk factors for carriage. However, most of these studies have evaluated factors associated with veterinary health care settings in inpatient or outpatient animals, such as previous use of antimicrobials and contact with veterinary clinics (Qekwana et al., 2017; Saputra et al., 2017; Soares Magalhães et al., 2010). Our team's previous work in households of people diagnosed with MRSA has demonstrated that household characteristics, practices in the household and environmental factors are significantly associated with the presence of MDRSA (defined as nonsusceptibility to four or more classes of antimicrobials) in the environment (Shahbazian et al., 2017). Despite this, little is known about whether household and environmental factors also contribute to the risk of MDR in colonizing staphylococci among household pets. To address this gap in the literature regarding risk factors for animal carriage of MDRS, which may contribute to both human and animal health risks, we sought to investigate demographic, healthcare‐related, contact‐related and environmental risk factors for the carriage of MDRS; and to describe the patterns of antimicrobial resistance. This study focussed on the human pathogen Staphylococcus aureus and the veterinary pathogen Staphylococcus pseudintermedius isolated from pets living in the households of people diagnosed with community‐associated MRSA infections. Understanding the risk factors for MRSA carriage among these pets could shed light on One Health interventions to prevent re‐colonization and re‐infection among humans and animals.
2. MATERIALS AND METHODS
Details about household recruitment, data collection, environmental sampling, and microbiological methods can be found in the Appendix S1 in this article's online repository.
2.1. Study design
This study is a secondary analysis of data collected in the ‘Pets and Environmental Transmission of Staphylococci’ (PETS) study, which was a cross‐sectional study nested in the ‘CURE’ randomized controlled trial (NCT00966446) (Cluzet et al., 2016). Secondary data analysis for the PETS study was approved by the Johns Hopkins Bloomberg School of Public Health Review Board (IRB 00006259).
2.2. Household recruitment
The PETS sub‐study included a subset of RCT study participants that also owned at least one pet. This sub‐study enrolled 67 index participants, representing 70 households in the mid‐Atlantic region. Samples from all pets of any species – according to availability and temperament of the animal – were obtained at two household visits, 3 months apart.
2.3. Inclusion criteria
Only dogs and cats recruited at the first visit were included in this analysis. Samples taken from reptiles, pocket pets and birds were excluded due to the small sample size.
2.4. Pet sampling
Sampling techniques used in this study have been described previously (Iverson et al., 2015). Briefly, swabs were collected from the nares, mouth, inguinal region and perineum of each pet using dry culture swabs with transport media (BBL™ Culture Swabs, Becton Dickinson).
2.5. Bacterial culture
Swabs and cloths (environmental sampling, Appendix S1) were cultured for methicillin‐susceptible and methicillin‐resistant CPS as previously described (Iverson et al., 2015). All presumptive CPS (based on Baird‐Parker (BP) agar phenotype and additional tube coagulase testing for MRS isolates that did not demonstrate lecithinase activity on BP) were stored in Microbank™ tubes (Pro‐Lab Diagnostics) at 80°C for further analyses.
2.6. Single‐locus spa‐typing
To further characterize the MRSA isolates, we conducted a DNA sequence analysis of the protein A gene (spa) as previously described (Cotter et al., 2022; Mellmann et al., 2007; Shopsin et al., 1999). The antimicrobial resistance/susceptibility percent concordance was calculated by dividing the total number of antimicrobials that were concordant (susceptible or resistant) between the pet's and environmental isolate by the total number of antimicrobials tested.
2.7. PCR analysis
One presumptive CPS isolate from each pet was selected for PCR analysis. Multiplex PCR that amplifies species‐specific segments of the CPS nuclease gene (nuc) was used to confirm isolates as S. aureus and S. pseudintermedius (Sasaki et al., 2012). Organisms negative for S. aureus or S. pseudintermedius nuc genes were not further identified to species, so they were considered as ‘other coagulase‐positive staphylococci (CPS)’ (S. schleiferi, S. intermedius, S. delphini, among others). To detect methicillin‐resistant strains, primers targeting a universal mecA/C sequence were used; control organisms were ATCC43300 and LGA251, respectively (García‐Alvarez et al., 2011).
2.8. Antimicrobial susceptibility testing
Antimicrobial susceptibility testing (AST) was performed on isolates from pets prior to cryopreservation. Kirby–Bauer disc diffusion was performed to evaluate eleven antimicrobials (belonging to 10 families): quinupristin/dalfopristin (Q/D), tetracycline, ciprofloxacin, erythromycin, chloramphenicol, trimethoprim‐sulfamethoxazole, amikacin, gentamicin, clindamycin, linezolid and cefoxitin. Clinical Laboratory Standards Institute 2018 guidelines were used to categorize strains isolated from pets as susceptible, intermediate, or resistant (Clinical and Laboratory Standards Institute M100, 2019). Breakpoints standardized for human isolates were used to harmonize findings and interpretation with data from the same study focussed on human and environmental isolates (Shahbazian et al., 2017). Strains that were intermediate or resistant to three or more classes of antimicrobials were considered multidrug‐resistant according to the criteria published by Magiorakos et al. (Magiorakos et al., 2012). The Infectious Diseases Society of America (IDSA) recommends that humans with SSTI caused by MRSA are treated with clindamycin, trimethoprim‐sulfamethoxazole, a tetracycline, or linezolid (Liu et al., 2011). Therefore, these antimicrobials were considered ‘clinically important’ and resistance to these individual antimicrobials is shown in Figure S1.
2.9. Variables of interest
Four types of potential risk factors for the carriage of MDR Staphylococcus isolates were evaluated: demographic, healthcare‐related, contact‐related and environmental risk factors. A priori, the primary risk factor of interest was defined as systemic antimicrobial use (oral or injectable) in the prior year by the pet. The variables of interest, their categories and the variable definition and construction are shown in Table S1.
2.10. Statistical analysis
The outcome of interest was carriage of multidrug‐resistant Staphylococcus (MDRS) defined as resistant to at least one agent in three or more antimicrobial classes of the ten that were tested (Magiorakos et al., 2012). The proposed relation of the risk factors with the outcome is shown in Figure 1. For the investigation of risk factors, unadjusted and adjusted logistic regression models were developed using cluster adjustment to account for the nonindependence of multiple animals within the same household. Since all pets included in this study were colonized with Staphylococcus, the comparison group for this analysis was formed by all the pets that were colonized by non‐MDR Staphylococcus spp. Due to the low prevalence of MDR among S. pseudintermedius and other CPS isolates, we also conducted a secondary analysis to test whether the risk factors remained the same for being resistant to an increasing number of antimicrobial classes (see Appendix S1). Post hoc stratified analyses by species of Staphylococcus were performed given our observation that the relationship between the hypothesized risk factors and the outcome appeared to vary across species of Staphylococcus, which was expected based on the design of the parent study to include households with a human index patient diagnosed with MRSA SSTI. For unadjusted models, MDR S. pseudintermedius (MDRSP) isolates were combined with other CPS isolates because only one S. pseudintermedius isolate was MDR. We adjusted for multiple comparisons using the False Discovery Rate.
FIGURE 1.
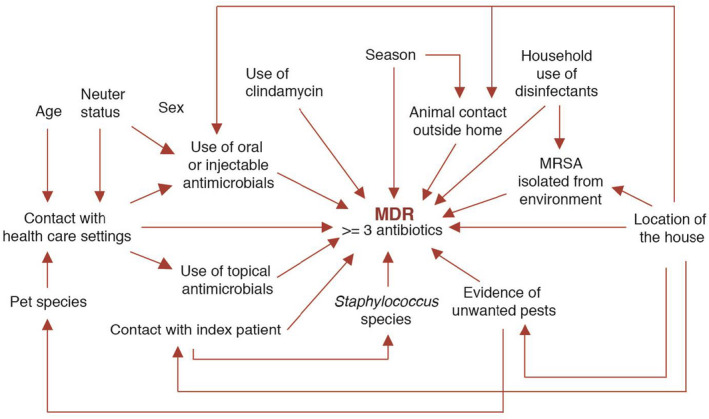
Conceptual framework of possible demographic, healthcare‐related, contact‐related and environmental risk factors for multidrug‐resistant Staphylococcus in dogs and cats. The proposed relationship of the variables of interest and the outcome of the primary model (MDR, defined as acquired nonsusceptibility to at least one agent in three or more antimicrobial classes of the ten that were tested) (Magiorakos et al., 2012) are shown in this figure. According to this conceptual framework, some variables have direct effects on MDR, while others are indirectly related to the outcome of interest
In adjusted models, our sample size was insufficient for stratified analysis and therefore, we included species of pets and species of Staphylococcus into the different models a priori. All variables with a p‐value of less than .15 in the unadjusted analysis were included in the adjusted models. Variables included in the adjusted models are listed in Table 4 and Table S4. For all statistical tests, a p‐value of less than .05 was considered statistically significant.
TABLE 4.
Adjusted analysis of possible demographic, healthcare‐related, contact‐related and environmental risk factors for multidrug‐resistant Staphylococcus a
| Variable | Adjusted OR (95% CI) | p‐value |
|---|---|---|
| Cats | 1.57 (0.44, 5.63) | .49 |
| Staphylococcus species | ||
| Other CPS | Ref. | – |
| S. pseudintermedius | 0.13 (0.01, 1.16) | .07 |
| S. aureus | 3.58 (0.89, 14.34) | .07 |
| Neutered animals | 1.26 (0.31, 5.01) | .75 |
| Animal contact outside home | 0.52 (0.14, 1.91) | .32 |
| Household use of disinfectants | 0.76 (0.52, 1.08) | .12 |
Only variables with a p‐value of less than .15 in any of the strata in the unadjusted analysis of possible demographic, clinical, contact‐related and environmental risk factors for having a class of antimicrobial nonsusceptibility stratified by species of Staphylococcus were included in the adjusted model.
Additionally, a data‐driven backward stepwise selection of risk factors for adjusted logistic regression, based on the likelihood ratio statistic, was conducted. All analyses were performed using Stata version 15 (Stata Corp.).
3. RESULTS
3.1. Description of study population
A total of 179 animals were sampled during the first household visit, including 71 dogs, 63 cats, 10 small mammals, 23 reptiles and birds and 11 fish. Only Staphylococcus spp. isolated from dogs and cats that had results from both the antimicrobial resistance testing and PCR testing of the nuclease gene (classified as S. aureus, S. pseudintermedius or other CPS) were included in this study. The distribution of demographic, health care‐related, household‐related and environmental risk factors considered relevant for the carriage of MDRS are presented in Table 1, separately for dogs and cats.
TABLE 1.
Companion animal characteristics
| Dogs (N = 63) | Cats (N = 47) | p‐value* | |
|---|---|---|---|
| Demographic factors | |||
| Female sex | 34 (54%) | 32 (68%) | .17 |
| Age in months (Mean (SD)) | 49.03 (48.68) | 41.23 (52.66) | .23 |
| Neutered animals | 20 (32%) | 20 (43%) | .32 |
| Clinical factors | |||
| Contact with health care settings | .48 | ||
| Between 6 and 12 months ago | 7 (11%) | 4 (9%) | |
| Last 6 months | 21 (33%) | 11 (23%) | |
| Use of oral or injectable antimicrobials within last year | 5 (8%) | 5 (11%) | .74 |
| Use of topical antimicrobials within last year | 7 (11%) | 2 (4%) | .30 |
| Contact factors | |||
| Animal contact outside home | 21 (33%) | 15 (32%) | 1.00 |
| Contact with index patient | .58 | ||
| Intermediate contact | 23 (37%) | 17 (36%) | |
| High contact | 19 (30%) | 18 (38%) | |
| Environmental factors | |||
| Home area | .01 | ||
| Urban | 26 (41%) | 30 (64%) | |
| Suburban | 13 (21%) | 11 (23%) | |
| Rural | 24 (38%) | 6 (13%) | |
| MRSA isolated from the environment | 32 (51%) | 35 (74%) | .02 |
| Household use of disinfectants (mean (SD)) | 4.28 (0.99) | 4.86 (1.58) | <.01 |
| Presence of pests in the house** | 54 (86%) | 27 (57%) | <.01 |
| Season | .42 | ||
| Fall or winter | 20 (32%) | 19 (40%) | |
| Spring or summer | 43 (68%) | 28 (60%) | |
| Microbial factors | |||
| Pet carriage of | <.01 | ||
| Other CPS | 22 (35%) | 34 (72%) | |
| S. pseudintermedius | 28 (44%) | 2 (4%) | |
| S. aureus | 13 (21%) | 11 (23%) | |
p‐values were calculated using the Fisher exact test for categorical variables and t‐test or the Mann–Whitney U test for continuous variables.
Includes mice and cockroaches.
3.2. Distribution of staphylococcal species
Of the total samples collected during the first visit, 110 pets tested positive for CPS by PCR (dogs n = 63, 88.7%; cats = 47, 74.6%). The number of dogs and cats from which each type of Staphylococcus was isolated is summarized in Table 2. MRSA staphylococcal protein A types (spa types) found in the 11 MRSA‐positive pets (living in 8 houses) included: t008, t121, t216, t334 and t12500, with 5/11 (45.5%) being t008 (Figure 2).
TABLE 2.
Distribution of Staphylococcal species
| Species of Staphylococcus | Dogs (N = 63) | Cats (N = 47) |
|---|---|---|
| S. aureus | 13 (20.6%) | 11 (23.4%) |
| MRSA | 4 (6.3%) | 7 (14.9%) |
| S. pseudintermedius | 28 (44.4%) | 2 (4.3%) |
| MRSP | 1 (1.6%) | 0 (0.0%) |
| Other CPS | 22 (35%) | 34 (72.0%) |
| MDR | 12 (19.0%) | 15 (31.9%) |
FIGURE 2.
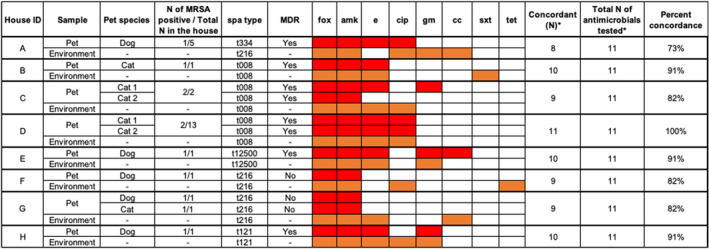
Patterns of antimicrobial resistance among pet's and environmental MRSA isolates. A red box shows the pet's isolate was resistant. An orange box shows the environmental isolate was resistant. fox – cefoxitin; amk – amikacin; e – erythromycin; cip – ciprofloxacine; gm – gentamicin; cc – clindamycin; sxt – trimethoprim/sulfamethoxazole; tet – tetracycline. *Quinupristin/dalfopristin, chloramphenicol and linezolid were excluded from this figure because none of the isolates were resistant to these antimicrobials. House IDs were harmonized with Table 3 (spa typing at baseline) in Cotter et al., 2022
3.3. Patterns of resistance
Of the 110 isolates, 27 (25%) were MDR. The prevalence of MDR (resistance to three or more classes of antimicrobials) was 50.0% (12/24) for S. aureus, 3.3% (1/30) for S. pseudintermedius and 25.0% (14/56) for other CPS (Figure S1). In unadjusted analysis, the prevalence of MDR was higher in S. aureus compared with other CPS (OR 3.00 [95% CI: 0.96, 9.39], p = .06), and significantly lower in S. pseudintermedius compared with other CPS (OR 0.10 [95% CI: 0.01, 0.88], p = .04). S. pseudintermedius also was significantly less likely to be MDR compared with S. aureus (OR 0.03 [95% CI: 0.003, 0.33], p < .01). Importantly, S. pseudintermedius comparisons were limited by the small number of MDR isolates detected (n = 1).
All the MRSA isolates (those isolated from the pets and the environment) were resistant to cefoxitin and amikacin; while all were susceptible to quinupristin/dalfopristin, chloramphenicol and linezolid. Most of the pet's MRSA isolates (64%) were MDR. The antimicrobial resistance/susceptibility percent concordance between the pet's and environmental isolates ranged between 73%–100% (Figure 2).
3.4. Unadjusted analysis
The results of unadjusted logistic regressions stratified by species of Staphylococcus and with MDR as the outcome are presented in Table 3. We did not find a significant statistical association between the use of oral or injectable antimicrobials in the prior year and the presence of MDR bacteria. The association between neuter status and MDR carriage was modified by the species of Staphylococcus present on the animal (p < .01). While being neutered was a protective factor for MDR among animals with S. aureus (OR 0.10 [95% CI: 0.02, 0.58], p = .05), it had a tendency towards being a risk factor for the isolation of MDRS from animals with other CPS (OR 3.37 [95% CI: 1.08, 10.46], p = .15). Stratified sensitivity analysis suggested this was driven by neutered males (data not shown). Finally, the odds of other CPS being MDR decreased as more rooms in the house were cleaned more frequently with a disinfectant on the EPA list of MRSA‐cidal products (OR 0.60 [95% CI: 0.40, 0.90], p = .06), although this was not statistically significant.
TABLE 3.
Unadjusted analysis of possible demographic, healthcare‐related, contact‐related and environmental risk factors for multidrug‐resistant Staphylococcus stratified by staphylococcal species c
| Variables | All other CPS (N = 86) | S. aureus (N = 24) | Interaction term* | ||
|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p‐value*** | Unadjusted OR (95% CI) | p‐value*** | p‐value | |
| Demographic factors | |||||
| Female sex | 0.57 (0.18, 1.81) | .73 | 0.47 (0.08, 2.72) | .80 | .94 |
| Age (in months) | 1.00 (0.98, 1.01) | .92 | 1.00 (0.98, 1.02) | .94 | – |
| Cats | 2.44 (0.67, 8.93) | .50 | 1.40 (0.31, 6.36) | .94 | .70 |
| Neutered animals | 3.37 (1.08, 10.46) | .15 | 0.10 (0.02, 0.58) | .05 | <.01 |
| Healthcare factors | |||||
| Contact with health care settings d | |||||
| Prior 6–12 months | 4.00 (0.57, 28.15) | .50 | 1.67 (0.24, 11.35) | .92 | .61 |
| Prior 6 months | 1.50 (0.38, 5.85) | .92 | 0.33 (0.05, 2.40) | .68 | .40 |
| Use of oral or injectable antimicrobials | 0.94 (0.10, 8.84) | .98 | 3.67 (0.26, 51.63) | .73 | .60 |
| Use of topical antimicrobials | 2.57 (0.42, 15.72) | .73 | Omitted a | – | – |
| Clindamycin use | 1.70 (0.44, 6.51) | .84 | 0.71 (0.13, 4.03) | .94 | .57 |
| Contact with veterinary health care settings and use of oral or injectable antimicrobials in the past year d | |||||
| Vet visit without antimicrobials | 2.01 (0.53, 7.65) | .73 | 0.41 (0.07, 2.64) | .73 | .32 |
| Vet visit with pet antimicrobial use | 1.20 (0.12, 11.93) | .94 | 2.5 (0.18, 35.49) | .86 | .83 |
| Contact factors | |||||
| Contact with index patient (score) d | |||||
| Intermediate contact | 0.93 (0.19, 4.57) | .97 | 1.25 (0.17, 9.00) | .94 | .91 |
| High contact | 1.25 (0.29, 5.31)** | .94 | 1.67 (0.24, 11.80)** | .92 | .91 |
| Animal contact outside home | 1.11 (0.27, 4.57) | .94 | 0.14 (0.03, 0.78) | .11 | .15 |
| Environmental factors | |||||
| Location of the house d | |||||
| Suburban | 1.26 (0.28, 5.75) | .94 | 0.38 (0.07, 2.05) | .66 | .39 |
| Rural | 0.30 (0.05, 1.89) | .55 | 0.31 (0.02, 5.32) | .83 | .98 |
| Household use of disinfectants | 0.60 (0.40, 0.90) | .06 | 2.05 (0.86, 4.90) | .34 | – |
| MRSA isolated from the environment | 1.07 (0.18, 6.51) | .97 | Omitted b | – | – |
| Evidence of unwanted pests | 2.20 (0.57, 8.51) | .66 | 0.70 (0.10, 4.81) | .94 | .46 |
| Season d | |||||
| Spring and summer | 0.39 (0.11, 1.42) | .49 | 1.00 (0.16, 6.14) | 1.00 (1.00) | .53 |
There were no MDR S. aureus positive animals given topical Abx in prior year.
Only one S. aureus positive animal lived in a house from which S. aureus was not isolated from the home environment.
S. pseudintermedius was combined with other CPS for this analysis because only one S. pseudintermedius isolate was MDR, so it was not possible to make any comparison inside this category.
Reference categories for these variables were: (1) no contact with health care settings in the past year; (2) no contact with health care settings and no use of oral or injectable antimicrobials in the past year; (3) low contact, for contact with index patient; (4) urban, for location of the house; and (5) winter and fall, for season.
A significant interaction term's p‐value should be interpreted as the effect of each factor on MDR is different for S. aureus isolates compared with all other CPS isolates.
p for trend is 0.75 for all other CPS isolates and 0.61 for S. aureus isolates.
p‐values were adjusted using the false discovery rate ranking method to account for multiple comparisons.
The Significance of Bold values indicates p‐values less than 0.05 was considered statistically significant.
3.5. Adjusted analysis
None of the variables included in the adjusted logistic regression analysis were significantly associated with MDR (Table 4). However, as observed in other models, S. pseudintermedius isolates showed lower odds of being MDR compared with other CPS (OR 0.13 [95% CI: 0.01, 1.16], p = .07), while S. aureus isolates had more than 3 times the odds of being MDR compared with other CPS (OR 3.58 [95% CI: 0.89, 14.34], p = .07).
In the data‐driven backward stepwise logistic regression, no variables other than pet species and species of Staphylococcus (which were chosen a priori and artificially locked in), were retained. Adjusting for the variables that had a p‐value of less than .15 in the unadjusted analysis (neuter status, animal contact outside the home and household use of disinfectants), S. aureus isolates had significantly higher odds of being MDR compared with other CPS (OR 3.70 [95% CI: 1.09, 12.63], p = .04).
4. DISCUSSION
Understanding potentially modifiable risk factors for MDRSA among pets residing with a person diagnosed with MRSA SSTI is key to design effective household‐wide decolonization interventions. As it has been previously demonstrated (Gandolfi‐Decristophoris et al., 2013 ), we found that healthy dogs and cats can carry MDRS. These findings suggest that humans can potentially be re‐exposed to MDRS from their pets, increasing their risk of recurrent colonization and/or infection.
We found that, among pets exposed to a person with a laboratory‐confirmed MRSA infection, S. aureus isolates were more likely than other coagulase‐positive staphylococcal species recovered from the pets to be MDR, which may suggest an important role for pets as recipients of or reservoirs for MDRSA in households (Davis et al., 2012; Pantosti, 2012). Higher frequency of resistance among S. aureus isolates has been reported before (Couto, 2016; Morris et al., 2006; Qekwana et al., 2017). In the present study, the higher frequency of resistance among S. aureus isolates could be due to the fact that pet owners had been diagnosed with MRSA SSTIs and had already received antimicrobial treatment. Prior or current selective pressure could maintain MDRSA isolates among humans, pets and the environmental reservoir. Moreover, it is known that the environment can serve as a reservoir of resistant staphylococci (Davis et al., 2012; Shahbazian et al., 2017), and this study identified that a high prevalence of MRSA isolated from the household environment of people with confirmed MRSA infection could be associated with a higher risk of antimicrobial resistance in S. aureus isolates detected on pets (Table S3). We found that the antimicrobial‐resistant pattern of the pet's isolate is frequently similar to the environmental isolate, but they do not perfectly match, despite spa‐type concordance in almost 90% of the households (Figure 2). This shows that pets may be the reservoir of bacteria with some antimicrobial‐resistant genes that are not present in the isolates from the household environment.
Neuter status was significantly associated with MDR and with having a class of antimicrobial resistance in our unadjusted analyses. However, neutering was a protective factor among animals carrying S. aureus isolates, and a risk factor among animals carrying S. pseudintermedius and other CPS isolates. Neutered animals were more likely to have contact with veterinary healthcare settings and have received systemic antimicrobials during the last year. Therefore, these other associations may confound the association between neuter status and the presence of antimicrobial‐resistant staphylococci, meaning that the effect of neuter status might be mixed with the effect of contact with health care settings and the use of systemic antimicrobials, as conceptualized in Figure 1. Furthermore, it is possible that neuter status is a proxy for other, unmeasured factors as well. This is reinforced by the fact that the association between neuter status and the outcome was not maintained in adjusted analyses. A limited number of previous studies have considered neuter status as a risk factor for the carriage of MRS (Gandolfi‐Decristophoris et al., 2013; Hoet et al., 2013; Rynhoud et al., 2020). This variable should be evaluated to achieve an adequate epidemiological characterization of the animals included in research studies and to explore sex‐based differences and the effect of hormones in antimicrobial‐resistant bacteria carriage (Klein & Flanagan, 2016). Larger studies are needed to examine the potential for sex‐based differences and any potential effect modification by neuter status.
The main variable of interest in this study was the use of antimicrobials by the pet within the year prior to sampling because it has been well described as a risk factor for the acquisition of MDR bacteria among humans and animals (Eckholm et al., 2013; Salgado et al., 2003; Saputra et al., 2017; Weese et al., 2012). However, we did not find a significant association between prior pet antimicrobials use and carriage of resistant staphylococci. This could be due to the small number of pets that received antibiotics in the previous year, which is further diluted by the stratification across Staphylococcus species in the univariate analysis. It could also be due to the potential influence of human household members, since pet enrolment was contingent upon the enrolment of a person in the home diagnosed and treated for an MRSA SSTI. Finally, it could be due to the fact that, unlike prior studies of pets predicated upon veterinary patient status, this study enrolled community pets regardless of any prior veterinary contact. Indeed, for S. aureus isolates, the prevalence of MDR among pets with veterinary healthcare contact but without antimicrobial treatment was 33% (3/9), versus 75% (3/4) among those with both healthcare contact and antimicrobial treatment, but the small number of animals with these factors (n = 13 for pets with S. aureus and prior healthcare contact) precluded further evaluation. This distinction is important as the generalizability of data from this study is to pets living in a home with a person diagnosed with MRSA.
In contrast to previous studies, our analyses included environmental factors that could influence the carriage of antimicrobial‐resistant Staphylococcus spp. The odds of S. aureus strains being resistant to a class of antimicrobial increased as more rooms were cleaned more frequently with a disinfectant on the EPA list of MRSA‐cidal products (Table S3). Selective pressure may play an important role because previous studies have shown that biofilm‐producing S. aureus strains are less susceptible to certain disinfectants and also have a greater probability of being resistant to different antimicrobials (Almatroudi et al., 2016). As previously reported (Shahbazian et al., 2017) for the parent PETS study, household cleaning with MRSA‐cidal products was associated with higher recovery of MDRSA from home environments at the 3‐month visit, which is consistent with these findings from the pets at baseline. Finally, evidence of unwanted pests was a risk factor for having a class of antimicrobial resistance only among animals that carried other CPS (not S. aureus or S. pseudintermedius) (Table S3). Previous studies have isolated antimicrobial‐resistant staphylococci from cockroaches and rodents (Abdolmaleki et al., 2019; Ge et al., 2019), and this finding could suggest a role for unwanted pests in the dissemination of MDRS among pets. More studies are needed to further explore this potential association.
These findings should be interpreted under certain constrains. It is likely that the study lacked sufficient power to detect risk factors with more modest associations with the outcome. Also, only CPS isolates were included in this analysis, therefore we were not able to determine the risk factors for coagulase‐negative staphylococci (CNS), bacteria that also have the potential to be zoonotic and become MDR (Davis et al., 2013). Furthermore, only a cross‐sectional analysis was done, so we did not assess whether the risk factors for MDRS in pets changed after households were randomized to a household‐wide human decolonization intervention. Longitudinal studies should be conducted to further examine the relationship between the proposed risk factors and the development of MDRS. Additionally, pets included in this study could have acquired S. aureus, which are already resistant to humans (Harrison et al., 2014), which is likely because all the spa types were the same among pets and their owners, except for two households (A and G) where the human isolates were not available to be tested (Cotter et al., 2022). This means that selection for antimicrobial‐resistant bacteria may not have occurred on the animal. This makes the comparison of S. aureus isolates to S. pseudintermedius difficult. In fact, adjusted analyses showed that, regardless of the model used, species of Staphylococcus was the only variable associated with the outcome. S. pseudintermedius was always associated with lower odds of being resistant, while S. aureus had higher odds of being resistant. It is possible that pet‐specific factors in this population such as the low rate of veterinary healthcare contact and the low prevalence of antimicrobial use are partially responsible for the lower rates of antimicrobial resistance in the pet‐associated S. pseudintermedius. We also acknowledge that samples for this study were collected between 2011 and 2013, when the prevalence of MRSP in the USA was lower than current rates (Krapf et al., 2019). Future community‐based studies of populations of pets in the absence of human‐ or pet‐associated diagnosis of staphylococcal disease would be needed to better compare drug resistance phenotypes among staphylococcal populations circulating in the community.
In conclusion, none of the S. aureus isolates included in this study were resistant to trimethoprim‐sulfamethoxazole or linezolid, which is reassuring since these are critically important antimicrobials used in human medicine. Although the sample size was small and further reduced by stratification, we were able to observe that factors driving resistance among Staphylococcus isolates may be different for S. aureus and S. pseudintermedius. Therefore, future exploration of the role of Staphylococcus species as an effect modifier of risk factors for antimicrobial resistance and evaluation of the potential for horizontal gene transfer over time among species of staphylococci identified from household One Health reservoirs (humans, animals and the environment) should be conducted.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
Supporting information
Fig S1
Tab S1‐S4
App S1
ACKNOWLEDGEMENTS
We thank the study participants and study personnel, and the support of Francesca Schiaffino, Andree Valle and Carlos Culquichicon. This work was presented at the 2019 American Society of Tropical Medicine and Hygiene Conference (Ferradas et al., 2019).
Ferradas, C. , Cotter, C. , Shahbazian, J. H. , Iverson, S. A. , Baron, P. , Misic, A. M. , Brazil, A. M. , Rankin, S. C. , Nachamkin, I. , Ferguson, J. M. , Peng, R. D. , Bilker, W. B. , Lautenbach, E. , Morris, D. O. , Lescano, A. G. , & Davis, M. F. (2022). Risk factors for antimicrobial resistance among Staphylococcus isolated from pets living with a patient diagnosed with methicillin‐resistant Staphylococcus aureus infection. Zoonoses and Public Health, 69, 550–559. 10.1111/zph.12946
Funding information
Cusi Ferradas was supported by the Fogarty International Center of the National Institutes of Health (NIH) under Award Number D43TW009343 and the University of California Global Health Institute (UCGHI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or UCGHI. This project or its parent study was supported by the Commonwealth Universal Research Enhancement (CURE) Program of the Pennsylvania Department of Health (to E.L.), the Johns Hopkins Center for a Livable Future (to M.F.D.), a Johns Hopkins Faculty Innovation grant (to M.F.D.), the Morris Animal Foundation (to M.F.D.) and the American College of Veterinary Dermatology/American Academy of Veterinary Dermatology (to D.O.M.). Investigators were supported by an NIAID K24 grant (AI080942 to E.L.), a postdoctoral fellowship on a NIEHS T32 grant (ES7141‐29 for M.F.D.) and an ORIP K01 grant (K01OD019918 to M.F.D.). This parent study was supported by a CDC cooperative agreement (FOA#CK11‐001, Epicenters for the Prevention of Healthcare Associated Infections). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or UCGHI.
Contributor Information
Cusi Ferradas, Email: cusi.ferradas@upch.pe.
Meghan F. Davis, Email: mdavis65@jhu.edu.
REFERENCES
- Abdolmaleki, Z. , Mashak, Z. , & Dehkordi, F. S. (2019). Phenotypic and genotypic characterization of antibiotic resistance in the methicillin‐resistant Staphylococcus aureus strains isolated from hospital cockroaches. Antimicrobial Resistance & Infection Control, 8(54). 10.1186/s13756-019-0505-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroudi, A. , Gosbell, I. B. , Hu, H. , Jensen, S. O. , Espedido, B. A. , Tahir, S. , Glasbey, T. O. , Legge, P. , Whiteley, A. , Dega, A. , & Vickery, K. (2016). Staphylococcus aureus dry‐surface biofilms are not killed by sodium hypochlorite: Implications for infection control. Journal of Hospital Infection, 93(3), 263–270. 10.1016/j.jhin.2016.03.020 [DOI] [PubMed] [Google Scholar]
- CLSI (2019). Performance Standards for Antimicrobial Susceptibility Testing (29th ed.). CLSI supplement M100. Clinical and Laboratory Standards Institute. [Google Scholar]
- Cluzet, V. C. , Gerber, J. S. , Metlay, J. P. , Nachamkin, I. , Zaoutis, T. E. , Davis, M. F. , Julian, K. G. , Linkin, D. R. , Coffin, S. E. , Margolis, D. J. , Hollander, J. E. , Bilker, W. B. , Han, X. , Mistry, R. D. , Gavin, L. J. , Tolomeo, P. , Wise, J. A. , Wheeler, M. K. , Hu, B. , … CDC Preventions Epicenters Program (2016). The effect of total household decolonization on clearance of colonization with methicillin‐resistant staphylococcus aureus. Infection Control and Hospital Epidemiology, 37(10), 1226–1233. 10.1017/ice.2016.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter, C. , Ferradas, C. , Ludwig, S. , Dalton, K. , Larsen, J. , Laucks, D. , Iverson, S. A. , Baron, P. , Tolomeo, P. C. , Brazil, A. M. , Ferguson, J. M. , Lautenbach, E. , Rankin, S. C. , Morris, D. O. , & Davis, M. F. (2022). Risk factors for methicillin‐resistant Staphylococcus aureus (MRSA) carriage in MRSA‐exposed household pets . In submission to Veterinary Dermatology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto, N. , Monchique, C. , Belas, A. , Marques, C. , Gama, L. T. , & Pomba, C. (2016). Trends and molecular mechanisms of antimicrobial resistance in clinical staphylococci isolated from companion animals over a 16 year period. Journal of Antimicrobial Chemotherapy, 71(6), 1479–1487. 10.1093/jac/dkw029 [DOI] [PubMed] [Google Scholar]
- Davis, M. F. , Cain, C. L. , Brazil, A. M. , & Rankin, S. C. (2013). Two coagulase‐negative staphylococci emerging as potential zoonotic pathogens: Wolves in sheep's clothing? Frontiers in Microbiology, 4, 1–4. 10.3389/fmicb.2013.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. F. , Iverson, S. A. , Baron, P. , Vasse, A. , Silbergeld, E. K. , Lautenbach, E. , & Morris, D. O. (2012). Household transmission of meticillin‐resistant Staphylococcus aureus and other staphylococci. The Lancet Infectious Diseases, 12(9), 703–716. 10.1016/S1473-3099(12)70156-1 [DOI] [PubMed] [Google Scholar]
- Eckholm, N. G. , Outerbridge, C. A. , White, S. D. , & Sykes, J. E. (2013). Prevalence of and risk factors for isolation of meticillin‐resistant Staphylococcus spp. from dogs with pyoderma in northern California, USA. Veterinary Dermatology, 24(1), 154–162. 10.1111/j.1365-3164.2012.01051.x [DOI] [PubMed] [Google Scholar]
- Ferradas, C. , Cotter, C. , Shahbazian, J. , Iverson, S. A. , Baron, P. , Misic, A. , Brazil, A. M. , Nachamkin, I. , Ferguson, J. M. , Lautenbach, E. , Morris, D. O. , Lescano, A. G. , & Davis, M. F. (2019). Patterns and risk factors for antibiotic resistance among coagulase‐positive Staphylococcus (CPS) isolated from dogs and cats that reside with a patient recently diagnosed with methicillin‐resistant Staphylococcus aureus skin or soft‐tissue infection. Poster presentation. American Society of Tropical Medicine and Hygiene. [Google Scholar]
- Gandolfi‐Decristophoris, P. , Regula, G. , Petrini, O. , Zinsstag, J. , & Schelling, E. (2013). Prevalence and risk factors for carriage of multi‐drug resistant Staphylococci in healthy cats and dogs. Journal of Veterinary Science, 14(4), 449–456. 10.4142/jvs.2013.14.4.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Alvarez, L. , Holden, M. T. G. , Lindsay, H. , Webb, C. R. , Brown, D. F. J. , Curran, M. D. , Walpol, E. , Brooks, K. , Pickard, D. , Teale, C. , Teale, C. , Parkhill, J. , Bentley, S. D. , Edwards, G. F. , Girvan, E. K. , Kearns, A. M. , Pichon, B. , Hill, R. L. R. , Larsen, A. R. , … Holmes, M. A. (2011). Meticillin‐resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. The Lancet Infectious Diseases, 11, 595–603. 10.1016/S1473-3099(11)70126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, J. , Zhong, X. , Xiong, Y. , Qiu, M. , Huo, S. , Chen, X. , Mo, Y. , Cheng, M. , & Chen, Q. (2019). Methicillin‐resistant Staphylococcus aureus among urban rodents, house shrews, and patients in Guangzhou, Southern China. BMC Veterinary Research, 15, 260. 10.1186/s12917-019-2012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, E. , Weinert, L. , Holden, M. T. G. , Welch, J. J. , Wilson, K. , Morgan, F. J. E. , Harris, S. R. , Loeffler, A. , Boag, A. K. , Peacock, S. J. , Paterson, G. K. , Waller, A. S. , Parkhill, J. , & Holmes, M. A. (2014). A shared population of epidemic methicillin‐resistant Staphylococcus aureus 15 circulates in humans and companion animals. Mbio 5(3), 1–10. 10.1128/mBio.00985-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet, A. , Balen, J. , Nava‐Hoet, R. C. , Bateman, S. , Hillier, A. , Dyce, J. , & Wittum, T. E. (2013). Epidemiological profiling of methicillin‐resistant Staphylococcus aureus ‐positive dogs arriving at a veterinary teaching hospital. Vector‐Borne and Zoonotic Diseases, 13(6), 385–393. 10.1089/vbz.2012.1089 [DOI] [PubMed] [Google Scholar]
- Iverson, S. A. , Brazil, A. M. , Ferguson, J. M. , Nelson, K. , Lautenbach, E. , Rankin, S. C. , Morris, D. O. , & Davis, M. F. (2015). Anatomical patterns of colonization of pets with staphylococcal species in homes of people with methicillin‐resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI). Veterinary Microbiology, 176(1–2), 202–208. 10.1016/j.vetmic.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Klein, S. L. , & Flanagan, K. L. (2016). Sex differences in immune responses. Nature Reviews Immunology, 16, 626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Krapf, M. , Müller, E. , Reissig, A. , Slickers, P. , Braun, S. D. , Müller, E. , Ehricht, R. , & Monecke, S. (2019). Molecular characterisation of methicillin‐resistant Staphylococcus pseudintermedius from dogs and the description of their SCC mec elements. Veterinary Microbiology, 233, 196–203. 10.1016/j.vetmic.2019.04.002 [DOI] [PubMed] [Google Scholar]
- Liu, C. , Bayer, A. , Cosgrove, S. E. , Daum, R. S. , Fridkin, S. K. , Gorwitz, R. J. , Kaplan, S. L. , Karchmer, A. W. , Levine, D. P. , Murray, B. E. , Chambers, H. F. , & Rybak, M. J. , Infectious Disease Society of America (2011). Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐ resistant Staphylococcus aureus infections in adults and children, Clinical Infectious Diseases, 52, e18–e55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- Magiorakos, A. P. , Srinivasan, A. , Carey, R. B. , Carmeli, Y. , Falagas, M. E. , Giske, C. G. , Harbarth, S. , Hindler, J. F. , Kahlmeter, G. , Olsson‐Liljequist, B. , Paterson, D. L. , Rice, L. B. , Stelling, J. , Struelens, M. J. , Vatopoulos, A. , Weber, J. T. , & Monnet, D. L. (2012). Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection, 18(3), 268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- Mellmann, A. , Weniger, T. , Berssenbrügge, C. , Rothgänger, J. , Sammeth, M. , Stoye, J. , & Harmsen, D. (2007). Based Upon Repeat Pattern (BURP): An algorithm to characterize the long‐term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiology, 7(98), 1–6. 10.1186/1471-2180-7-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, D. O. , Rook, K. A. , Shofer, F. S. , & Rankin, S. C. (2006). Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: A retrospective review of 749 isolates. Veterinary Dermatology, 17(5), 332–337. 10.1111/j.1365-3164.2006.00536.x [DOI] [PubMed] [Google Scholar]
- Pantosti, A. (2012). Methicillin‐resistant Staphylococcus aureus associated with animals and its relevance to human health. Frontiers in Microbiology, 3, 1–12. 10.3389/fmicb.2012.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qekwana, D. N. , Oguttu, J. W. , Sithole, F. , & Odoi, A. (2017). Patterns and predictors of antimicrobial resistance among Staphylococcus spp. from canine clinical cases presented at a veterinary academic hospital in South Africa. BMC Veterinary Research, 13(1), 1–9. 10.1186/s12917-017-1034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynhoud, H. , Meler, E. , Gibson, J. S. , Price, R. , Maguire, T. , Farry, T. , Bennett, E. , Hartono, J. , & Soares Magalhães, R. J. (2020). Epidemiology of methicillin resistant Staphylococcus species carriage in companion animals in the Greater Brisbane Area, Australia. [Preprint]. BMC Veterinary Research, 136, 138–142. [DOI] [PubMed] [Google Scholar]
- Salgado, C. D. , Farr, B. M. , & Calfee, D. P. (2003). Community‐acquired methicillin‐resistant Staphylococcus aureus: A meta‐analysis of prevalence and risk factors. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 36(2), 131–139. 10.1086/345436 [DOI] [PubMed] [Google Scholar]
- Saputra, S. , Jordan, D. , Worthing, K. A. , Norris, J. M. , Wong, H. S. , Abraham, R. , Trott, D. J. , & Abraham, S. (2017). Antimicrobial resistance in coagulase‐positive staphylococci isolated from companion animals in Australia: A one year study. PLoS One, 12(4), 1–17. 10.1371/journal.pone.0176379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T. , Tsubakishita, S. , Tanaka, Y. , Sakusabe, A. , Ohtsuka, M. , Hirotaki, S. , Kawakami, T. , Fukata, T. , & Hiramatsu, K. (2012). Population genetic structures of Staphylococcus aureus isolates from cats and dogs in Japan. Journal of Clinical Microbiology, 50, 2152–2155. 10.1128/JCM.06739-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian, J. H. , Hahn, P. D. , Ludwig, S. , Ferguson, J. , Baron, P. , Christ, A. , Spicer, K. , Tolomeo, P. , Torrie, A. M. , Bilker, W. B. , Cluzet, V. C. , Hu, B. , Julian, K. , Nachamkin, I. , Rankin, S. C. , Morris, D. O. , Lautenbach, E. , & Davis, M. F. (2017). Multidrug and mupirocin resistance in environmental methicillin‐resistant Staphylococcus aureus (MRSA) isolates from homes of people diagnosed with community‐onset MRSA infection. Applied and Environmental Microbiology, 83(22), e01369‐17. 10.1128/AEM.01369-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopsin, B. , Gomez, M. , Montgomery, S. O. , Smith, D. H. , Waddington, M. , Dodge, D. E. , Bost, D. A. , Riehman, M. , Naidich, S. , & Kreiswirth, B. N. (1999). Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. Journal of Clinical Microbiology, 37(11), 3556–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Magalhães, R. J. , Loeffler, A. , Lindsay, J. , Rich, M. , Roberts, L. , Smith, H. , Lloyd, D. H. , & Pfeiffer, D. U. (2010). Risk factors for methicillin‐resistant Staphylococcus aureus (MRSA) infection in dogs and cats: A case‐control study. Veterinary Research, 41(5), 55. 10.1051/vetres/2010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese, J. C. , Faires, M. C. , Frank, L. A. , Reynolds, L. M. , & Battisti, A. (2012). Factors associated with methicillin‐resistant versus methicillin‐susceptible Staphylococcus pseudintermedius infection in dogs. Journal of the American Veterinary Medical Association, 240(12), 1450–1455. 10.2460/javma.240.12.1450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Tab S1‐S4
App S1


