Abstract
Background and study aims
We conducted a systematic review and meta‐analysis of population‐based studies to explore pooled prevalence and magnitude of electrolyte changes after bowel preparation for colonoscopy based on the most recent guidelines.
Patients and methods
PubMed and Cochrane were queried for population‐based studies examining changes in electrolyte values after bowel preparation, published by July 1, 2021. We report prevalences of serum hypokalemia, hyponatremia, hyperphosphatemia, and hypocalcemia after bowel preparation and changes in mean electrolyte values after vs. before bowel preparation using sodium phosphate (NaP) and polyethylene glycol (PEG).
Results
Thirteen studies met the inclusion criteria; 2386 unique patients were included. Overall, hypokalemia was found in 17.2% (95% CI 6.7, 30.9) in the NaP group vs. 4.8% (95% CI 0.27, 13.02) in the PEG group. The magnitude of potassium decrease after NaP bowel preparation was significantly increased compared to PEG (mean difference −0.38; 95% CI −0.49 to −0.27, P < 0.001). No study reported on major complications.
Conclusions
Hypokalemia was found in 17.2% of patients after bowel preparation with NaP and in 4.8% of patients with PEG, a finding that is clinically relevant with respect to choosing the type of bowel preparation. The magnitude of the potassium decrease after NaP was significantly higher compared to PEG. These data provide the evidence that supports the recommendation of the European Society of Gastrointestinal Endoscopy against routine use of NaP for bowel preparation.
Keywords: bowel preparation, colonoscopy, electrolyte disorder, hypokalemia, low potassium
INTRODUCTION
Colonoscopy is considered as a safe procedure. An optimally cleansed bowel is a prerequisite for diagnosis and treatment of colorectal disorders. However, bowel preparation should not cause clinically important shifts in systemic electrolytes, in fluids, or in patient comfort.
Two main groups of bowel preparation solutions are available: the high‐volume and low‐volume solutions. High‐volume polyethylene glycol (PEG) is included in the high‐volume bowel preparation solutions, while sodium phosphate (NaP), low‐volume PEG, and sodium picosulfate with magnesium citrate (SPMC) are included in the low‐volume bowel preparation solutions.
High‐volume PEG solutions may cause discomfort, due to unpleasant smell or taste or occurrence of gastrointestinal symptoms, i.e., cramping and bloating. Due to lower prevalences of these side‐effects with low‐volume solutions, these solutions are increasingly used in endoscopy units. SPMC and low‐volume PEG solutions provide efficient bowel preparation with only minimal adverse effects. Among the low‐volume PEG solutions, a commonly used solution is 2 L polyethylene glycol with ascorbic acid (PEG‐asc) as additive 1 , 2 for its more pleasant taste and low risk for side‐effects.
Bowel preparations may cause electrolyte disturbances which remain asymptomatic and unrecognized in the majority of cases. A recent publication reported on two patients who died because of cardiac arrhythmias that occurred after low‐volume PEG resulting in severe postcolonoscopy hypokalemia. 3 This publication was the first to report on patients with fatal consequences of electrolyte disturbances after bowel preparation for colonoscopy.
Up to now, the extent, magnitude, and risk factors for electrolyte disturbances remain unclear. In a meta‐analysis, Tan and colleagues have studied the mean differences in serum potassium levels occurring in patients before vs. after bowel preparation with NaP or PEG. Based on 16 studies, the authors concluded that decreases in serum potassium levels were significantly more often associated with use of NaP than with use of PEG solutions. 4 However, the pooled prevalence of electrolyte disturbances was not evaluated in their study. Apart from this meta‐analysis, only a few small sample size population‐based studies have examined the frequency of electrolyte disturbances and mean differences in serum electrolyte levels after vs. before bowel preparation. 5 Current clinical guidelines do not include recommendations on electrolyte measurement before or after bowel preparation for colonoscopy. The European Society of Gastrointestinal Endoscopy (ESGE) guideline strongly recommends against the routine use of NaP, while the evidence level for this recommendation is low. 6 To provide an upgrade for the level of evidence on which clinical recommendations are given, we conducted a systematic review and meta‐analysis of population‐based studies, up to July 1, 2021, examining the pooled prevalence rate of electrolyte disturbances and mean differences after bowel preparation. In addition, pooled changes in mean electrolyte levels after vs. before bowel preparation were analyzed.
MATERIAL AND METHODS
The preferred reporting items for systematic reviews and meta‐analyses (PRISMA) methodology was employed for reporting systematic reviews. 7 A local protocol for conducting a meta‐analysis was applied, which is available on request. 8 , 9
Selection criteria
The studies included in this systematic review are population‐based studies of plasma electrolyte disorders or serum electrolyte measurement after bowel preparation. Randomized, retrospective, and prospective studies were included. We report on: (i) studies that evaluated serum electrolyte measurements after bowel preparation vs. before bowel preparation; and (ii) studies that examine prevalences of serum electrolyte disorders after bowel preparation. We defined serum electrolyte measurements as measurement of one or more of the following: potassium, sodium, magnesium, phosphorus, and/or calcium. Electrolyte concentrations had to be checked both before and after bowel preparation. Prevalences of serum electrolyte disorders were defined as the prevalence of hypokalemia (serum potassium <3.5 mmol/L or mEq/L), hyponatremia (serum sodium <135 mmol/L or mEq/L), or hypernatremia (serum sodium >150 mmol/L or mEq/L) after bowel preparation. Definitions of electrolyte disorders were equal among the included studies.
Search strategy
A systematic search was conducted in PubMed and the Cochrane Library using search terms ‘colonoscopy’ and ‘hypokalemia’ or ‘potassium’. We retrieved key original population‐based studies, whenever available up to July 1, 2021. The following key words were used: Colonoscopy AND (bowel cleansing OR bowel preparation) AND (potassium OR hypokalemia) AND (sodium OR hyponatremia) including corresponding Mesh terms. Studies in English language were included. We reviewed all reference lists of eligible studies identifying additional population‐based studies.
Case reports, review articles, studies reporting bowel preparation quality, studies investigating bowel preparation effects on electrolytes for other purposes than colonoscopy, and studies only reporting mean serum electrolyte values after bowel preparation were excluded. Studies investigating only prevalences of electrolyte disorders after bowel preparation were included.
Two reviewers (A.R. and Q.Z.) screened all studies independently. Study characteristics (first author, year of publication, country), study design (randomized controlled trial, prospective cohort study, retrospective cohort study), study outcomes (mean electrolyte values with standard deviations [SD], when available, prevalence of electrolyte disorders), and mean age with ranges were retrieved. In case of discrepancy between the two reviewers, a senior investigator (S.S.) reviewed the data to achieve consensus.
We used published criteria 7 , 10 , 11 , 12 to evaluate the quality of clinical prevalence studies. The most suitable subsets of questions of the quality assessment tool for diagnostic accuracy studies‐2 (QUADAS‐2) tool 13 and the Loney scale 10 were used, as summarized in Table S1.
Endpoints
The primary end‐point was to estimate pooled prevalences of hypokalemia after bowel preparation and to calculate pooled changes in mean potassium values after vs. before bowel preparation.
The secondary end‐point was to estimate pooled prevalences after bowel preparation and pooled changes in mean electrolyte values after vs. before bowel preparation for sodium, magnesium, phosphorus, and calcium.
Statistical analysis
Random effects model was used to calculate pooled prevalences of electrolyte disorders and corresponding 95% confidence intervals (CI) from meta‐analysis. Double arcsine transformations for these prevalences were applied because of the low expected prevalences and possibly negative lower limits of the confidence intervals. Using double arcsine back‐conversion, prevalences were translated to the original scale. 14 , 15 Heterogeneity among the studies was measured using I 2 statistics. 16 Pooled overall prevalences of hypokalemia, hyponatremia, hyperphosphatemia, and hypocalcemia were calculated. From the studies that included both groups (NaP and PEG) pooled odds ratios (ORs), corresponding 95% CIs and P‐values were computed using a random effects model. Pooled changes in mean electrolyte values after vs. before bowel preparation were calculated for potassium, sodium, magnesium, phosphorus, and calcium. In case standard deviations were not reported, standard deviations were estimated using information such as standard errors, 95% CI, or test results, where possible. Pooled mean difference in change scores between NaP and PEG were obtained using a random effects model. We followed the Cochrane Handbook (section 16.1.3.2) in imputing standard deviations for change scores using a correlation coefficient from another study. 17 Statistical analyses were performed using the metafor package 18 in R statistics 3.1.2. 19 Pooled meta‐analysis data were presented in forest plots and tables.
RESULTS
Included studies
The study selection flowchart is shown in Figure 1. The PubMed search resulted in 103 studies and Cochrane search in seven studies. Six duplicates were traced and deleted. A total of 104 studies were identified, of those 44 studies could be excluded based on title. Six additional records were identified via hand searching of reference lists. Of the 66 screened abstracts, 53 were excluded (i.e., two reviews, five retrospective cohort studies, two case reports, 38 studies that contained only information on bowel preparation, seven studies reported only differences in electrolytes [no means] or only means after bowel preparation, and two studies did not report standard deviations or provided no information to estimate standard deviations 20 , 21 ).
Figure 1.
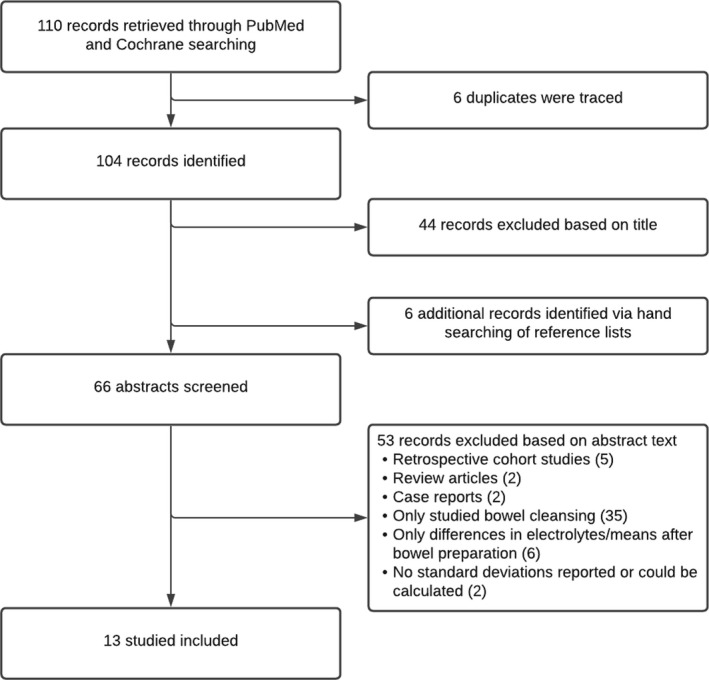
Study selection flowchart of studies who examined pooled prevalence and magnitude of electrolyte changes after bowel preparation for colonoscopy.
Summary data of the 13 included studies are described in Table 1. All studies were published between January 1, 1995 and July 1, 2021. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Nine studies were randomized controlled trials, 23 , 25 , 26 , 27 , 29 , 30 , 31 , 32 , 34 four were prospective cohort studies. 22 , 24 , 28 , 33 Six studies originated from Europe, 22 , 26 , 27 , 29 , 30 , 33 four from the United States, 25 , 28 , 31 , 34 one from Asia, 23 one from Canada, 32 and one from the Middle East. 24 Overall, 2386 (range 32–147,832) unique patients were included in the studies 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 (Table 1). In the included studies, mean age of subjects varied from 46.9 to 80.5 years 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 (Table 1). Ten studies reported means of serum electrolytes, mostly with changes after vs. before bowel preparation; 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 34 11 studies reported prevalences of electrolyte disorders after bowel preparation. 22 , 24 , 25 , 27 , 28 , 29 , 30 , 32 , 33
Table 1.
Characteristics of included studies
| Study | Country | Study design | Bowel preparation | Mean age ± SD (range), in years † | Participants undergoing colonoscopy |
Electrolyte prevalences or means (SD)/ range |
Blood samples | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Ainley et al. 22 | United Kingdom | Prospective cohort study | NaP 90 mL | 61.6 (19–89) | 100 outpatients | Prevalences | After bowel preparation | No exclusion criteria |
| Bae et al. 23 | Korea | Randomized |
PEG 4 L PEG 2 L plus NaP ‡ |
53.7 ± 14 53.4 ± 14 |
271 outpatients | Means plus SD | Before and after bowel preparation |
Renal insufficiency Congestive heart failure Bowel obstruction/surgery |
| Beloosesky et al. 24 | Israel | Prospective cohort study | NaP 90 mL or barium enema ‡ | 80.5 ± 6 (65–90) | 36 inpatients | Means plus SD, prevalence of hypokalemia | Before and after bowel preparation |
Renal or liver insufficiency Congestive heart failure Myocardial infarction ≤6 months CVA <3 months Active IBD or diverticulitis Bowel obstruction |
| Bitoun et al. 33 | France | Prospective cohort study |
PEG‐asc 2L NaP 90 mL |
53.0 | 340 outpatients | Prevalences | Before and after bowel preparation |
Age >75 years Renal insufficiency Congestive heart failure IBD Bowel obstruction/surgery |
| Clarkston et al. 25 | United States of America | Randomized |
PEG 4L NaP 90 mL |
57.0 ± 2 (27–85) | 98 outpatients | Means plus SD, prevalences | Before and after bowel preparation |
Age >65 years Renal insufficiency Congestive heart failure Bowel surgery |
| Huppertz‐Hauss et al. 26 | Norway | Randomized |
PEG 4L PEG 2 L + bisacodyl NaP |
58.0 | 231 outpatients | Means | Before and after bowel preparation |
Renal or liver insufficiency Congestive heart failure Serum electrolyte abnormalities |
| Johanson et al. 34 | United States of America | Randomized |
PEG 2L + bisacodyl NaP tablets 90 mL |
56.1 (20–83) | 411 outpatients | Means plus SD | Before and after bowel preparation |
Renal insufficiency Congestive heart failure Serum electrolyte abnormalities Use of digitalis preparations Myocardial infarction IBD Bowel obstruction/surgery |
| Klare et al. 27 | Germany | Randomized |
PEG 4L SPMC 300 mL |
56.4 ± 16 | 200 out/inpatients | Means plus SD, prevalences | Before and after bowel preparation |
Renal insufficiency Congestive heart failure ASA V or VI Urgent procedures |
| Lieberman et al. 28 | United States of America | Prospective cohort study | NaP 90 mL | 62.3 (29–77) | 32 outpatients | Means plus range | Before and after bowel preparation |
Non‐veterans Renal insufficiency |
| Marin Gabriel et al. 29 | Spain | Randomized |
PEG 4L NaP 90 mL |
57.9 ± 16 46.9 ± 18 |
42 outpatients | Means plus SD, prevalences | Before, immediately after and 1 h after the end of colonoscopy |
Renal or liver insufficiency Congestive heart failure Bowel obstruction/surgery Urgent procedures |
| Mathus‐Vliegen et al. 30 | The Netherlands | Randomized |
PEG 4L NaP 90 mL |
48.8 ± 17 55.0 ± 17 |
94 outpatients | Means plus SD, prevalences | Before and after bowel preparation |
Renal or liver insufficiency (NaP) Congestive heart failure Severe gastrointestinal ulcers (PEG) Electrolyte imbalances (NaP) Salt‐restricted diet (NaP) Use of calcium‐blockers, diuretics, digoxin, lithium (NaP) |
| Rex et al. 31 | United States of America | Randomized |
NaP 90 mL SPMC 10 mg |
58 (21–83) | 338 outpatients | Means plus SD | Before and after bowel preparation |
Renal or liver insufficiency Congestive heart failure Serum electrolyte abnormalities Bowel obstruction/surgery IBD |
| Rostom et al. 32 | Canada | Randomized |
PEG 4L NaP 90 mL |
55 52 |
193 outpatients | Prevalences | Before and after bowel preparation |
Renal or liver insufficiency Congestive heart failure Bowel obstruction/surgery |
†Not in all studies available.
‡Not implemented in the analysis.
ASA, American Association of Anesthesiologists; Asc, ascorbic acid; CVA, cerebrovascular accident; IBD, inflammatory bowel disease; NaP, sodium phosphate; OSS, oral sulfate solution; PEG, polyethylene glycol; SD, standard deviation; SPMC, sodium picosulfate plus magnesium citrate.
Since there were only two studies on means in SPMC, 27 , 31 two prevalence studies on SPMC, 27 and one on PEG‐asc, 33 a meta‐analysis based on these studies was not performed. In the study focusing on PEG‐asc, no electrolyte disorders were reported.
Pooled prevalences
Overall, the prevalence of hypokalemia was 17.2% (95% CI 6.7, 30.9) in patients after NaP bowel preparation for colonoscopy. After PEG bowel preparation hypokalemia was present in 4.8% (95% CI 0.3, 13.0; Table 2 and Fig. 2). The risk of hypokalemia after NaP was not significantly different from PEG (OR 2.10; 95% CI 0.2, 17.9, P = 0.49). Hyponatremia was found in 0.9% (95% CI 0.0, 4.1) in the NaP group vs. 3.3% (95% CI 0.0, 12.4) in the PEG group, hyperphosphatemia in 37.3% (95% CI 12.2, 66.5) vs. 0.65% (95% CI 0.0, 4.1), and hypocalcemia in 15.6% (95% CI 3.7, 32.9) vs. 8.1% (95% CI 1.4, 18.6; Table 2).
Table 2.
Pooled prevalences of electrolyte disorders after bowel preparation with NaP or PEG
| Bowel preparation | Electrolyte disorder | Number of studies included in the analysis | Number of patients included in the analysis | Pooled prevalence | 95% CI | I 2 |
|---|---|---|---|---|---|---|
| NaP | Hypokalemia 22 , 24 , 25 , 29 , 30 , 32 , 33 | 7 | 564 | 17.19% | 6.69, 30.95 | 92.37% |
| Hyponatremia 22 , 29 , 30 , 32 | 4 | 308 | 0.86% | 0.00, 4.07 | 54.8% | |
| Hyperphosphatemia 22 , 25 , 28 , 29 , 30 , 32 , 33 | 7 | 560 | 37.26% | 12.24, 66.45 | 97.8% | |
| Hypocalcemia 22 , 24 , 25 , 28 , 29 , 30 , 32 , 33 | 8 | 596 | 15.59% | 3.67, 32.94 | 95.6% | |
| High‐volume PEG | Hypokalemia 25 , 27 , 29 , 30 , 32 | 5 | 271 | 4.83% | 0.27, 13.02 | 80.19% |
| Hyponatremia 27 , 29 , 30 , 32 | 5 | 222 | 3.30% | 0.00, 12.41 | 82.3% | |
| Hyperphosphatemia 30 , 32 | 2 | 96 | 0.65% | 0.00, 4.06 | 5.12% | |
| Hypocalcemia 25 , 27 , 29 , 30 , 32 | 5 | 271 | 8.07% | 1.39, 18.63 | 83.43% |
CI, confidence interval; NaP, sodium phosphate; PEG, polyethylene glycol.
Figure 2.
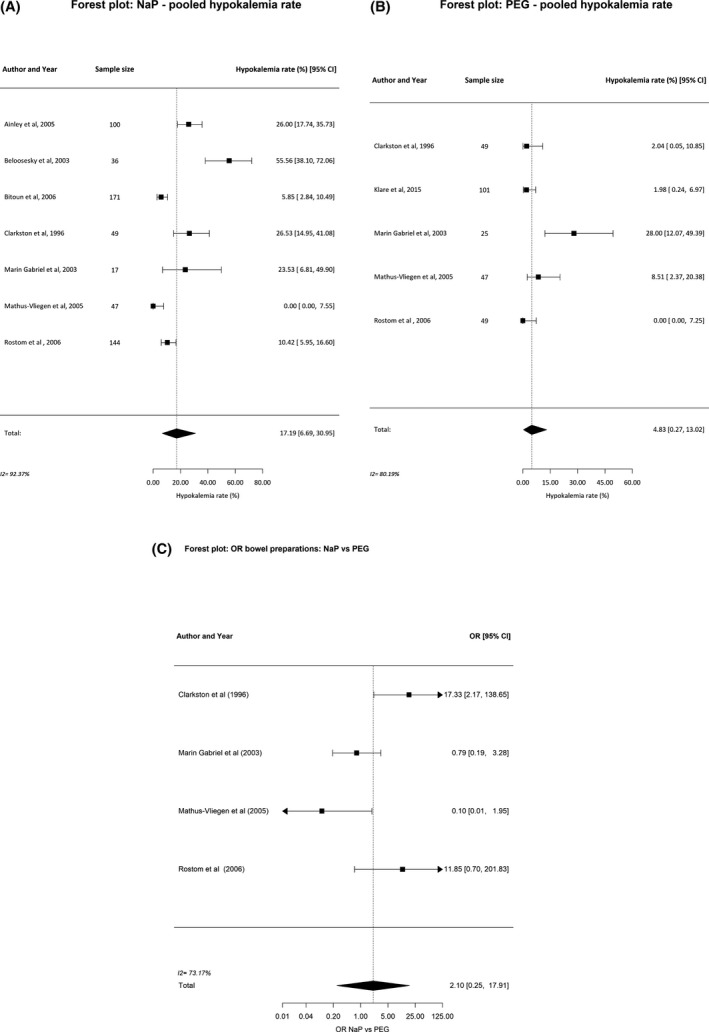
Pooled prevalences (%) of hypokalemia after bowel preparation with (A) sodium phosphate (NaP), (B) polyethylene glycol (PEG), and (C) odds ratio (OR) for NaP vs. PEG.
Mean differences in serum electrolyte concentrations
Table 3 (based on Tables 4, 5, 6) and Figure 3 show a pooled mean difference in serum potassium values of −0.58 mmol/L (95% CI −0.70, −0.45) in NaP patients and −0.25 mmol/L (95% CI −0.32, −0.17) in the PEG group. A pooled change in mean sodium of +2.4 mmol/L (95% CI 1.3, 3.5) was found in the NaP vs. +0.4 mmol/L (95% CI −0.1, 0.9) in the PEG group. For magnesium, phosphorus, and calcium minor alterations were shown (Table 3). The magnitude of potassium decrease after NaP bowel preparation was significantly increased compared to PEG (mean difference −0.38; 95% CI −0.49, −0.27, P < 0.001).
Table 3.
Pooled changes in mean serum electrolyte values after vs. before bowel preparation for NaP and PEG
| Bowel preparation | Serum electrolytes | Number of studies included in the analysis | Number of patients included in the analysis | Pooled change in means (after minus before) [mmol/L] | 95% CI | I 2 |
|---|---|---|---|---|---|---|
| NaP | Potassium 24 , 25 , 26 , 28 , 29 , 30 , 34 | 7 | 470 | −0.58 | −0.70, −0.45 | 87.9% |
| Sodium 24 , 25 , 26 , 28 , 29 , 30 , 34 | 7 | 470 | +2.39 | 1.25, 3.53 | 94.5% | |
| Magnesium 24 , 30 , 34 | 3 | 288 | −0.03 | −0.04, −0.02 | 0.0% | |
| Phosphorus 24 , 25 , 26 , 28 , 30 , 34 | 6 | 453 | +0.91 | 0.57, 1.25 | 99.1% | |
| Calcium 24 , 25 , 26 , 28 , 30 , 34 | 6 | 453 | −0.13 | −0.17, −0.09 | 92.0% | |
| High‐volume PEG | Potassium 23 , 25 , 26 , 27 , 29 , 30 , 34 | 7 | 645 | −0.25 | −0.32, −0.17 | 74.6% |
| Sodium 23 , 25 , 26 , 27 , 29 , 30 , 34 | 7 | 645 | +0.41 | −0.05, 0.87 | 95.7% | |
| Magnesium 27 , 30 , 34 | 3 | 354 | −0.03 | −0.05, −0.01 | 76.8% | |
| Phosphorus 23 , 25 , 26 , 27 , 30 , 34 | 6 | 620 | −0.02 | −0.08, 0.05 | 89.9% | |
| Calcium 23 , 25 , 26 , 27 , 30 , 34 | 6 | 620 | −0.06 | −0.12, −0.01 | 93.1% |
CI, confidence interval; NaP, sodium phosphate; PEG, polyethylene glycol.
Table 4.
Mean (±SD) electrolytes before and after bowel preparation with NaP and change in mean values (after minus before) in mmol/L
| Study | Number of patients | Electrolytes before and after bowel preparation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium | Sodium | Magnesium | Phosphorus | Calcium | ||||||||||||
| Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | ||
| Beloosesky et al. 24 | 36 | 4.5 ± 0.3 | 3.5 ± 0.5 | −1.0 | 137.8 ± 4.5 | 142.2 ± 4.2 | +4.4 | 1.07 ± 0.17 | 1.02 ± 0.17 | −0.05 | 1.15 ± 0.16 | 2.25 ± 0.48 | +1.10 | 2.29 ± 0.12 | 2.06 ± 0.15 | −0.23 |
| Clarkston et al. 25 | 49 | 4.1 ± 0.4 | 3.7 ± 0.4 | −0.4 | 139.7 ± 3.2 | 143.8 ± 2.5 | +4.1 | – | – | – | 1.13 ± 0.16 | 2.23 ± 0.58 | +1.10 | 2.38 ± 0.10 | 2.25 ± 0.13 | −0.13 |
| Huppertz‐Hauss et al. 26 | 84 | 4.4 ± 0.1 | 3.8 ± 0.1 | −0.6 | 142.1 ± 0.3 | 143.2 ± 0.5 | +1.1 | – | – | – | 1.06 ± 0.02 | 1.62 ± 0.05 | +0.56 | 2.37 ± 0.02 | 2.29 ± 0.02 | −0.08 |
| Johanson et al. 34 | 205 | 4.3 ± 0.4 | 3.7 ± 0.5 | −0.6 | 140.8 ± 2.5 | 142.7 ± 2.9 | −1.9 | 0.85 ± 0.07 | 0.82 ± 0.06 | −0.03 | 1.17 ± 0.17 | 2.38 ± 0.46 | +0.11 | 2.47 ± 0.09 | 2.33 ± 0.11 | −0.14 |
| Lieberman et al. 28 | 32 | 4.3 ± 0.3 | 3.8 ± 0.2 | −0.5 | 139.9 ± 1.8 | 144.6 ± 2.0 | +4.7 | – | – | – | 1.00 ± 0.12 | 2.20 ± 0.32 | +1.20 | 2.30 ± 0.13 | 2.15 ± 0.08 | −0.15 |
| Marin Gabriel et al. 29 | 17 | 3.7 ± 0.4 | 3.7 ± 04 | −0.1 | 139.4 ± 2.1 | 138.2 ± 2.1 | −1.2 | – | – | – | – | – | – | – | – | – |
| Mathus‐Vliegen et al. 30 | 47 | 4.1 ± 0.5 | 3.3 ± 0.4 | −0.8 | 138.0 ± 3.2 | 140.0 ± 2.6 | +2.0 | 0.84 ± 0.06 | 0.81 ± 0.06 | −0.05 | 1.10 ± 0.20 | 1.40 ± 0.48 | +0.30 | 2.40 ± 0.11 | 2.30 ± 0.09 | −0.10 |
NaP, sodium phosphate; SD, standard deviation; Δ, delta.
Table 5.
Mean (±SD) electrolytes before and after bowel preparation with PEG and change in mean values (after minus before) in mmol/L
| Study | Number of patients | Electrolytes before and after bowel preparation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium | Sodium | Magnesium | Phosphorus | Calcium | ||||||||||||
| Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | ||
| High‐volume PEG | ||||||||||||||||
| Bae et al. 23 | 141 | 4.1 ± 0.4 | 3.9 ± 0.4 | −0.2 | 141.1 ± 0.2 | 142.6 ± 0.2 | +1.5 | – | – | – | 1.23 ± 0.16 | 1.35 ± 0.19 | +0.12 | 2.25 ± 0.10 | 2.20 ± 0.09 | −0.05 |
| Bae et al. 23 | 49 | 4.2 ± 0.4 | 4.1 ± 0.4 | −0.1 | 140.4 ± 3.2 | 141.4 ± 2.6 | +1.0 | – | – | – | 1.20 ± 0.13 | 1.16 ± 0.16 | −0.04 | 2.40 ± 0.13 | 2.35 ± 0.18 | −0.05 |
| Bae et al. 23 | 76 | 4.4 | 4.1 | −0.3 | 141.7 | 141.5 | −0.2 | – | – | – | 1.10 | 1.07 | −0.03 | 2.40 | 2.37 | −0.08 |
| Bae et al. 23 | 101 | 4.5 ± 0.5 | 4.2 ± 0.4 | −0.4 | 140.7 ± 2.9 | 139.9 ± 2.5 | −0.8 | 0.84 ± 0.07 | 0.80 ± 0.06 | −0.04 | 1.16 ± 0.19 | 1.09 ± 0.19 | −0.07 | 2.37 ± 0.12 | 2.32 ± 0.13 | −0.05 |
| Marin Gabriel et al. 29 | 25 | 4.0 ± 0.5 | 3.9 ± 0.4 | −0.1 | 138.2 ± 2.3 | 136.9 ± 2.2 | −1.5 | – | – | – | – | – | – | – | – | – |
| Mathus‐Vliegen et al. 30 | 47 | 4.2 ± 0.4 | 3.8 ± 0.4 | −0.4 | 139.4 ± 2.8 | 140.0 ± 2.1 | +0.6 | 0.84 ± 0.06 | 0.80 ± 0.07 | −0.04 | 1.10 ± 0.23 | 0.97 ± 0.23 | −0.13 | 2.50 ± 0.13 | 2.40 ± 0.15 | −0.10 |
| Low‐volume PEG | ||||||||||||||||
| Johanson et al. 34 | 206 | 4.3 ± 0.4 | 4.2 ± 0.5 | −0.1 | 140.7 ± 2.7 | 140.2 ± 3.3 | −0.5 | 0.85 ± 0.07 | 0.84 ± 0.06 | −0.01 | 1.14 ± 0.18 | 1.13 ± 0.16 | −0.01 | 2.46 ± 0.08 | 2.46 ± 0.10 | 0.00 |
| Huppertz‐Hauss et al. 26 | 71 | 4.4 | 4.1 | −0.3 | 141.8 | 141.2 | −0.6 | – | – | – | 1.05 | 1.05 | 0.00 | 2.38 | 2.37 | −0.01 |
PEG, polyethylene glycol; SD, standard deviation; Δ, delta.
Table 6.
Mean (±SD) electrolytes before and after bowel preparation with SPMC and change in mean values (after minus before) in mmol/L
| Study | Number of patients | Electrolytes before and after bowel preparation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium | Sodium | Magnesium | Phosphorus | Calcium | ||||||||||||
| Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | Before | After | Δ | ||
| Klare et al. 27 | 99 | 4.5 ± 0.5 | 4.3 ± 0.5 | −0.20 | 140.7 ± 3.3 | 137.2 ± 4.2 | −2.8 | 0.84 ± 0.07 | 0.88 ± 0.08 | +0.04 | 1.09 ± 0.17 | 1.14 ± 0.18 | +0.05 | 2.39 ± 0.11 | 2.36 ± 0.11 | −0.03 |
| Rex et al. 31 | 169 | 4.3 ± 0.4 | 4.3 ± 0.4 | 0.0 | 140.0 ± 2.0 | 139.0 ± 3.0 | +1.0 | 1.29 ± 0.04 | 0.82 ± 0.08 | −0.47 | 1.16 ± 0.16 | 1.09 ± 0.16 | −0.07 | 2.40 ± 0.1 | 2.38 ± 0.1 | −0.02 |
SD, standard deviation; SPMC, sodium picosulfate plus magnesium citrate; Δ, delta.
Figure 3.
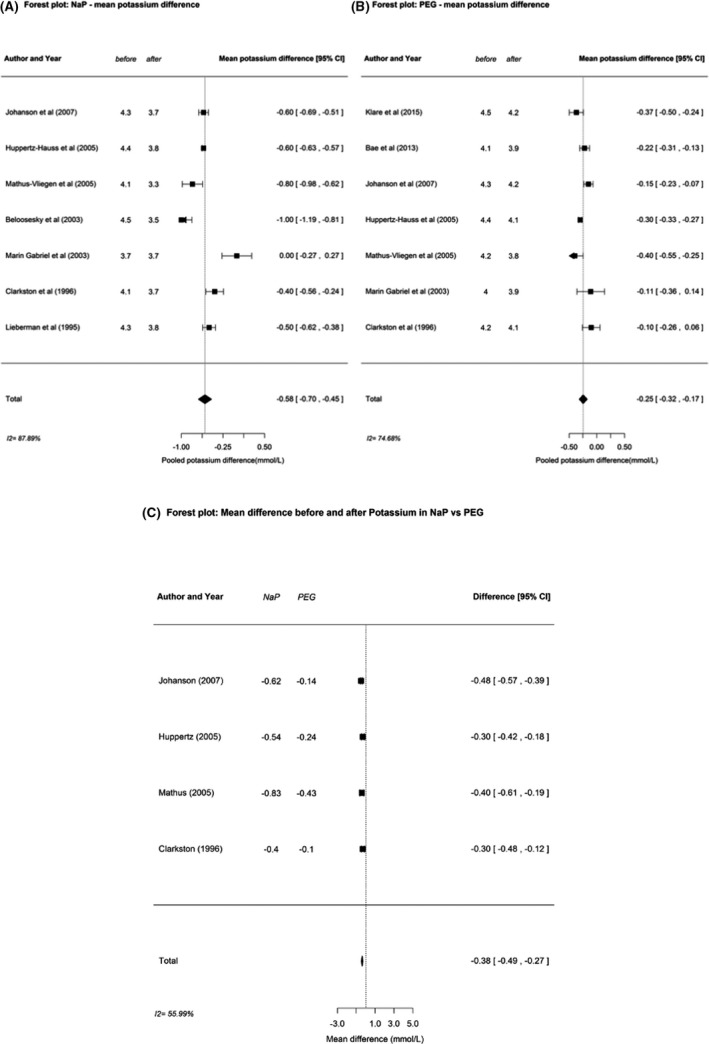
Pooled changes in mean potassium values (in mmol/L) after vs. before bowel preparation for (A) sodium phosphate (NaP), (B) polyethylene glycol (PEG), and (C) difference in mean change for NaP vs. PEG.
In all of the included studies, heterogeneity was high for the mean changes in electrolytes (I 2 = 74.6–99.1%, Table 3), except for magnesium (0.0%) in the NaP group. Heterogeneity was also high for all electrolyte prevalences (I 2 = 73.6–97.5%, Table 2). In none of the included studies major complications (e.g., cardiac arrhythmias, epileptic seizures, paralysis, coma, or death) related to electrolyte disturbances after bowel preparation were reported or specified.
DISCUSSION
This systematic review and meta‐analysis examined the safety of bowel preparation for colonoscopy with respect to electrolyte disturbances. A significant proportion of patients developed serum electrolyte disturbances after bowel preparation: 35–360/1000 patients after NaP vs. 9–92/1000 patients after PEG. We found a pooled prevalence of hypokalemia of 17.2% (95% CI 6.7, 30.9) in patients after NaP vs. 4.8% (95% CI 0.27, 13.02) after PEG bowel preparation.
The difference in prevalence of hypokalemia after NaP vs. high‐volume PEG is in line with larger retrospective studies (1.2% vs. 0.1%), 35 although the amounts are smaller, probably due to higher study numbers. Practical clinical guidelines recommend to continuously monitor the quality and safety standards in colonoscopy. 36 , 37 Since bowel preparation is an essential part of the colonoscopy procedure, quality and safety requirements should be clearly defined and monitored. There should be a balance between the benefits (optimal luminal clearance, patient compliance) and harms (complications, i.e., electrolyte disturbances) in bowel cleansing. In general PEG is preferred over other bowel cleansing preparations in individuals of older age, in patients with renal impairment, heart failure, and inflammatory bowel disease. 38 The bowel cleansing agents NaP and SPMC have higher patient tolerance and compliance compared to PEG. 39 Routine use of NaP should be avoided in patients with impaired renal function. 38 In previous studies no significant differences in mean total cleansing scores were found between various preparation solutions, 39 , 40 while in one study 39 the mean total cleansing score was significantly worse in the NaP group vs. the PEG group.
Serum electrolyte values outside the normal range may increase patient and procedure risks. Electrolyte disturbances may vary from asymptomatic via mild and moderate symptoms (i.e., muscle weakness, constipation, nausea, and vomiting), to severe symptoms (i.e., paralysis, seizures, cardiac arrhythmias, coma, and death). 4 It should be noted that the degree of electrolyte disturbances is not directly related to the severity of the adverse event.
Bowel preparation solutions are supposed to effectively clean the colon with minimal or no side‐effects. NaP and PEG are among the most commonly used and studied preparations worldwide. 36 , 37 PEG is a nondigestible and nonabsorbable lavage solution. PEG is iso‐osmotic with plasma, causing no net absorption or excretion of water or ions. Therefore, PEG does not result in significant changes in systemic fluid and electrolyte balance. 41 NaP is a saline laxative, containing monobasic and dibasic sodium phosphate. NaP is highly osmotic and therefore results in fluid shifts from the systemic compartment to the gastrointestinal tract. 41 From a pharmacokinetic point of view, this may explain why NaP results more often in disturbances in the electrolytes balance compared to PEG.
As shown, the use of NaP results in higher prevalences of electrolyte disturbances after bowel preparation compared to use of PEG 22 , 24 , 25 , 27 , 28 , 29 , 30 , 32 , 33 (Table 2). Nowadays low‐volume preparations, such as SPMC and PEG‐asc, are more frequently used and these preparations may also result in electrolyte disturbances. 42 , 43 , 44 Up to now, SPMC or PEG‐asc induced electrolyte disturbances have not been extensively studied and reported in the literature. 5 , 27 , 33 To date, Di Nardo et al. 5 found no significant changes in serum potassium values after PEG‐asc bowel preparation in children. Bitoun et al. 33 reported that the hypokalemia risk was not increased after PEG‐asc (Table 7). In a retrospective study, Lee et al. 45 showed that hypokalemia was significantly more frequently seen in 2 L PEG‐asc compared to 4 L PEG. Considering the small number of (prospective) studies and the small sample sizes, the observed low risk of potassium disturbances after PEG‐asc should be interpreted with caution and cannot be considered to reliably represent the real‐life population‐based risk. Notably, severe hypokalemia and cardiac death in two patients following bowel preparation with low‐volume PEG‐asc has recently been reported. 3 Based on these cases, a study was undertaken to explore the magnitude of hypokalemia associated with bowel preparation in high risk patients. It was shown that 4.2% of patients had hypokalemia before bowel preparation and 23.6% developed hypokalemia after bowel preparation with low‐volume PEG‐asc. 46
Table 7.
Prevalence of electrolyte disorder after bowel preparation
| Bowel preparation | Study | Number of patients | Hypokalemia | Hyponatremia | Hypernatremia | Hyperphosphatemia | Hypocalcemia |
|---|---|---|---|---|---|---|---|
| High‐volume | |||||||
| PEG 4 L | Clarkston et al. 25 | 49 | 2.0% | – | – | – | 4.1% |
| Klare et al. 27 | 101 | 2.0% | 4.0% | – | – | 11.9% | |
| Marin Gabriel et al. 29 | 25 | 28.0% | 24.0% | – | – | 8.0% | |
| Mathus‐Vliegen et al. 30 | 47 | 8.3% | 0.0% | 0.0% | 2.9% | 0.0% | |
| Rostom et al. 32 | 49 | 0.0% | 0.0% | – | 0.0% | 26.0% | |
| Low‐volume | |||||||
| NaP | Ainley et al. 22 | 100 | 26.0% | 2.0% | 0.0% | 45.0% | 16.0% |
| Beloosesky et al. 24 | 36 | 56.0% | 14.0% | – | – | 58.0% | |
| Bitoun et al. 33 | 171 | 5.8% | – | – | 5.8% | 0.6% | |
| Clarkston et al. 25 | 49 | 26.5% | – | – | 98.0% | 12.2% | |
| Lieberman et al. 28 | 32 | – | – | – | 28.1% | 6.3% | |
| Marin Gabriel et al. 29 | 17 | 23.5% | 5.9% | – | 47.1% | 12.9% | |
| Mathus‐Vliegen et al. 30 | 47 | 0.0% | 2.4% | 2.4% | 39.0% | 5.0% | |
| Rostom et al. 32 | 144 | 10.4% | 0.0% | 0.7% | 6.3% | 41.7% | |
| PEG‐asc 2L | Bitoun et al. 33 | 169 | 0.0% | − | − | 0.0% | 0.0% |
| SPMC | Klare et al. 27 | 99 | 1.0% | 21.2% | − | − | 6.1% |
NaP, sodium phosphate; PEG, polyethylene glycol; PEG‐asc, polyethylene glycol ascorbic acid; SPMC, sodium picosulfate plus magnesium citrate.
In general, postcolonoscopy mortality is very low. 9 All doctors and medical workers ordering colonoscopies, especially (nurse) endoscopists, should be aware that colonoscopy related morbidity and mortality risks also include effects related to the use of bowel preparation regimens. Current gastroenterology‐ or endoscopy‐based professional guidelines do not recommend to routinely measure serum electrolyte levels prior to colonoscopy. 36 , 37 Unfortunately, risk profiles of patients developing hypokalemia after bowel preparation, especially with the low‐volume preparations, are lacking. To our knowledge, our manuscript is the first systematic review and meta‐analysis reporting on prevalences of electrolyte disturbances after use of NaP and PEG bowel preparations. We provided actual prevalences of electrolyte disturbances and changes in mean electrolytes levels after vs. before bowel preparation.
The most recently published guideline of the ESGE recommends the use of both PEG‐based (high‐volume PEG or low‐volume PEG with ascorbate, citrate, or bisacodyl) and non‐PEG‐based (SPMC, oral sulfate sodium) agents taking into account the precisely defined contraindications. 6 The ESGE recommends against the routine use of NaP, but this recommendation is based on low quality evidence. Given the high prevalences of electrolyte disturbances reported in this systematic review and meta‐analysis, we now provide additional and high quality evidence to support the ESGE recommendation against the routine use of NaP based bowel preparation solutions.
At present, SPMC and PEG‐asc low‐volume bowel preparation regimens are increasingly used instead of NaP and high‐volume PEG. 36 , 37 More data on prevalences of electrolyte disturbances after low‐volume bowel preparations using SPMC and PEG‐asc should become available in order to examine pooled risks. Furthermore, 1 L PEG appears to be also promising in safety and efficacy, but electrolyte alterations were not studied. 47 Because of rapidly increasing numbers of colonoscopies for population‐based screening, surveillance, and regular care, the number of elderly patients and patients with comorbidity at risk for electrolyte disturbances will also increase in the near future. Therefore, population‐based data on patient‐specific risk factors for electrolyte disturbances and disorders after bowel preparation should become available. Only thereafter, evidence based recommendations on monitoring serum electrolyte levels, especially in high risk patients with specific regimens, can be made. Such recommendations are critical to ensure high quality and safety standards in colonoscopy.
Several limitations should be addressed with respect to our study. First, prevalences of electrolyte disturbances may have been underreported in the separate studies because not all cases could be identified (treatment in other hospital, gastroenterologist did not notify the electrolyte disturbance). Second, as shown in Table 1, patients who suffer from renal insufficiency, heart failure, and/or bowel problems have been excluded in almost all studies. These patients run a higher risk for electrolyte disturbances associated with bowel preparation. For example, diuretics are frequently prescribed in heart failure, renal disease, and hypertension. Hypokalemia is a common consequence of specific types of diuretics. 48 Unfortunately, diuretic use was not specifically reported in the included studies. In real life, patients with renal insufficiency, heart failure, or bowel problems regularly undergo colonoscopy for diagnostic, screening, or surveillance indications. To be informed about the real‐life prevalences of electrolyte disorders, these patient groups should be identified and examined in more detail in future studies. Third, not all studies provided bowel cleansing scores or reported every adverse event (Table S2). Furthermore, because of the limited amount of data available on SPMC and PEG‐asc, we could not present pooled prevalences or mean changes for these bowel preparation regimens. It should be taken into account that heterogeneity was high in most studies. No significant funnel plot asymmetry was seen (Fig. S1) for hypokalemia prevalences. In conclusion, electrolyte disturbances in response to bowel preparation have regularly been observed. Hypokalemia was found in 17.2% of patients after bowel preparation with NaP and in 4.8% of patients with PEG, a finding that is clinically relevant with respect to choosing the type of bowel preparation. The magnitude of the potassium decrease after NaP was significantly higher compared to PEG. These data provide the evidence that supports the recommendation of the ESGE against routine use of NaP for bowel preparation.
Conflict of interest
Authors declare no conflict of interest for this article.
Funding information
None.
Supporting information
Figure S1 Funnel plots pooled hypokalemia rate NaP (A) and PEG (B).
Table S1 The quality assessment of prevalence studies using a modified version of the QUADAS‐2 and the Loney scale.
Table S2 The bowel cleansing efficacy and serious adverse events per study.
Quirine van der Zander and Bjorn Winkens contributed equally to this work.
References
- 1. Aoun E, Abdul‐Baki H, Azar C et al. A randomized single‐blind trial of split‐dose PEG‐electrolyte solution without dietary restriction compared with whole dose PEG‐electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc 2005; 62: 213–8. [DOI] [PubMed] [Google Scholar]
- 2. Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: A randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol 2011; 23: 897–902. [DOI] [PubMed] [Google Scholar]
- 3. Reumkens A, Masclee AAM, Bakker CM. Postcolonoscopy mortality: Bowel preparation to blame? Gastrointest Endosc 2017; 86: 744–5. [DOI] [PubMed] [Google Scholar]
- 4. Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy ‐ A meta‐analysis. Colorectal Dis 2006; 8: 247–58. [DOI] [PubMed] [Google Scholar]
- 5. Di Nardo G, Aloi M, Cucchiara S et al. Bowel preparations for colonoscopy: An RCT. Pediatrics 2014; 134: 249–56. [DOI] [PubMed] [Google Scholar]
- 6. Hassan C, East J, Radaelli F et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline ‐ Update 2019. Endoscopy 2019; 51: 775–94. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Int J Surg 2010; 8: 336–41. [DOI] [PubMed] [Google Scholar]
- 8. Voorham QJ, Rondagh EJ, Knol DL et al. Tracking the molecular features of nonpolypoid colorectal neoplasms: A systematic review and meta‐analysis. Am J Gastroenterol 2013; 108: 1042–56. [DOI] [PubMed] [Google Scholar]
- 9. Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post‐colonoscopy complications: A systematic review, time trends, and meta‐analysis of population‐based studies. Am J Gastroenterol 2016; 111: 1092–101. [DOI] [PubMed] [Google Scholar]
- 10. Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: Prevalence or incidence of a health problem. Chronic Dis Can 1998; 19: 170–6. [PubMed] [Google Scholar]
- 11. Lovell RM, Ford AC. Prevalence of gastro‐esophageal reflux‐type symptoms in individuals with irritable bowel syndrome in the community: A meta‐analysis. Am J Gastroenterol 2012; 107: 1793–801. [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: A systematic review and meta‐analysis. Am J Gastroenterol 2014; 109: 1375–89. [DOI] [PubMed] [Google Scholar]
- 13. Whiting PF, Rutjes AW, Westwood ME et al. QUADAS‐2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. [DOI] [PubMed] [Google Scholar]
- 14. Miller JJ. The inverse of the Freeman‐Tukey double arcsine transformation. Am Stat 1978; 32: 138. [Google Scholar]
- 15. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health 2013; 67: 974‐8. [DOI] [PubMed] [Google Scholar]
- 16. Neyeloff JL, Fuchs SC, Moreira LB. Meta‐analyses and forest plots using a Microsoft Excel spreadsheet: Step‐by‐step guide focusing on descriptive data analysis. BMC Res Notes 2012; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions, 2nd edn. Chichester: John Wiley & Sons, 2019. [Google Scholar]
- 18. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Soft 2010; 36: 1–48. [Google Scholar]
- 19. R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- 20. Kastenberg D, Chasen R, Choudhary C et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: Two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54: 705–13. [DOI] [PubMed] [Google Scholar]
- 21. Holte K, Nielsen KG, Madsen JL, Kehlet H. Physiologic effects of bowel preparation. Dis Colon Rectum 2004; 47: 1397–402. [DOI] [PubMed] [Google Scholar]
- 22. Ainley EJ, Winwood PJ, Begley JP. Measurement of serum electrolytes and phosphate after sodium phosphate colonoscopy bowel preparation: An evaluation. Dig Dis Sci 2005; 50: 1319–23. [DOI] [PubMed] [Google Scholar]
- 23. Bae SE, Kim KJ, Eum JB et al. A comparison of 2 L of polyethylene glycol and 45 mL of sodium phosphate versus 4 L of polyethylene glycol for bowel cleansing: A prospective randomized trial. Gut Liver 2013; 7: 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beloosesky Y, Grinblat J, Weiss A, Grosman B, Gafter U, Chagnac A. Electrolyte disorders following oral sodium phosphate administration for bowel cleansing in elderly patients. Arch Intern Med 2003; 163: 803–8. [DOI] [PubMed] [Google Scholar]
- 25. Clarkston WK, Tsen TN, Dies DF, Schratz CL, Vaswani SK, Bjerregaard P. Oral sodium phosphate versus sulfate‐free polyethylene glycol electrolyte lavage solution in outpatient preparation for colonoscopy: A prospective comparison. Gastrointest Endosc 1996; 43: 42–8. [DOI] [PubMed] [Google Scholar]
- 26. Huppertz‐Hauss G, Bretthauer M, Sauar J et al. Polyethylene glycol versus sodium phosphate in bowel cleansing for colonoscopy: A randomized trial. Endoscopy 2005; 37: 537–41. [DOI] [PubMed] [Google Scholar]
- 27. Klare P, Poloschek A, Walter B et al. Single‐day sodium picosulfate and magnesium citrate versus split‐dose polyethylene glycol for bowel cleansing prior to colonoscopy: A prospective randomized endoscopist‐blinded trial. J Gastroenterol Hepatol 2015; 30: 1627–34. [DOI] [PubMed] [Google Scholar]
- 28. Lieberman DA, Ghormley J, Flora K. Effect of oral sodium phosphate colon preparation on serum electrolytes in patients with normal serum creatinine. Gastrointest Endosc 1996; 43: 467–9. [DOI] [PubMed] [Google Scholar]
- 29. Marín Gabriel JC, Rodríguez Muñoz S, de la Cruz Bértolo J et al. Electrolytic disturbances and colonoscopy: Bowel lavage solutions, age and procedure. Rev Esp Enferm Dig 2003; 95: 863–75. [PubMed] [Google Scholar]
- 30. Mathus‐Vliegen EM, Kemble UM. A prospective randomized blinded comparison of sodium phosphate and polyethylene glycol‐electrolyte solution for safe bowel cleansing. Aliment Pharmacol Ther 2006; 23: 543–52. [DOI] [PubMed] [Google Scholar]
- 31. Rex DK, DiPalma JA, McGowan J, Cleveland M. A comparison of oral sulfate solution with sodium picosulfate: Magnesium citrate in split doses as bowel preparation for colonoscopy. Gastrointest Endosc 2014; 80: 1113–23. [DOI] [PubMed] [Google Scholar]
- 32. Rostom A, Jolicoeur E, Dubé C et al. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol‐based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc 2006; 64: 544–52. [DOI] [PubMed] [Google Scholar]
- 33. Bitoun A, Ponchon T, Barthet M, Coffin B, Dugue C, Halphen M. Results of a prospective randomised multicentre controlled trial comparing a new 2‐L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther 2006; 24: 1631–42. [DOI] [PubMed] [Google Scholar]
- 34. Johanson JF, Popp JW Jr, Cohen LB et al. A randomized, multicenter study comparing the safety and efficacy of sodium phosphate tablets with 2L polyethylene glycol solution plus bisacodyl tablets for colon cleansing. Am J Gastroenterol 2007; 102: 2238–46. [DOI] [PubMed] [Google Scholar]
- 35. Kan WC, Wang HY, Chien CC, Tan CK, Lin CY, Su SB. Intermediate bioelectrolyte changes after phospho‐soda or polyethylene glycol precolonoscopic laxatives in a population undergoing health examinations. Nephrol Dial Transplant 2012; 27: 752–7. [DOI] [PubMed] [Google Scholar]
- 36. Hassan C, Bretthauer M, Kaminski MF et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013; 45: 142–50. [DOI] [PubMed] [Google Scholar]
- 37. Wexner SD, Beck DE, Baron TH et al. A consensus document on bowel preparation before colonoscopy: Prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc 2006; 63: 894–909. [DOI] [PubMed] [Google Scholar]
- 38. Lim YJ, Hong SJ. What is the best strategy for successful bowel preparation under special conditions? World J Gastroenterol 2014; 20: 2741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jung YS, Lee CK, Kim HJ, Eun CS, Han DS, Park DI. Randomized controlled trial of sodium phosphate tablets vs polyethylene glycol solution for colonoscopy bowel cleansing. World J Gastroenterol 2014; 20: 15845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seo EH, Kim TO, Kim TG et al. Efficacy and tolerability of split‐dose PEG compared with split‐dose aqueous sodium phosphate for outpatient colonoscopy: A randomized, controlled trial. Dig Dis Sci 2011; 56: 2963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsu CW, Imperiale TF. Meta‐analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc 1998; 48: 276–82. [DOI] [PubMed] [Google Scholar]
- 42. Cohen SM, Wexner SD, Binderow SR et al. Prospective, randomized, endoscopic‐blinded trial comparing precolonoscopy bowel cleansing methods. Dis Colon Rectum 1994; 37: 689–96. [DOI] [PubMed] [Google Scholar]
- 43. Tjandra JJ, Chan M, Tagkalidis PP. Oral sodium phosphate (Fleet) is a superior colonoscopy preparation to Picopre (sodium picosulfate‐based preparation). Dis Colon Rectum 2006; 49: 616–20. [DOI] [PubMed] [Google Scholar]
- 44. Ell C, Fischbach W, Keller R et al. A randomized, blinded, prospective trial to compare the safety and efficacy of three bowel‐cleansing solutions for colonoscopy (HSG‐01*). Endoscopy 2003; 35: 300–4. [DOI] [PubMed] [Google Scholar]
- 45. Lee SP, Park E, Kim HV et al. Does 2 L polyethylene glycol plus ascorbic acid increase the risk of renal impairment compared to 4 L polyethylene glycol? Dig Dis Sci 2016; 61: 3207–14. [DOI] [PubMed] [Google Scholar]
- 46. Reumkens A, Masclee AA, Winkens B, van Deursen CT, Sanduleanu S, Bakker CM. Prevalence of hypokalemia before and after bowel preparation for colonoscopy in high‐risk patients. Gastrointest Endosc 2017; 86: 673–9. [DOI] [PubMed] [Google Scholar]
- 47. Yoshida N, Naito Y, Murakami T et al. Safety and efficacy of a same‐day low‐volume 1 L PEG bowel preparation in colonoscopy for the elderly people and people with renal dysfunction. Dig Dis Sci 2016; 61: 3229–35. [DOI] [PubMed] [Google Scholar]
- 48. Zorginstituut Nederland . Pharmacotherapeutic Compass. Diemen: Care Institute of the Netherlands; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Funnel plots pooled hypokalemia rate NaP (A) and PEG (B).
Table S1 The quality assessment of prevalence studies using a modified version of the QUADAS‐2 and the Loney scale.
Table S2 The bowel cleansing efficacy and serious adverse events per study.


