Abstract
The number and intensity of flood events will likely increase in the future, raising the risk of flooding stress in terrestrial plants. Understanding flood effects on plant physiology and plant‐associated microbes is key to alleviate flooding stress in sensitive species and ecosystems. Reduced oxygen supply is the main constrain to the plant and its associated microbiome. Hypoxic conditions hamper root aerobic respiration and, consequently, hydraulic conductance, nutrient uptake, and plant growth and development. Hypoxia favours the presence of anaerobic microbes in the rhizosphere and roots with potential negative effects to the plant due to their pathogenic behaviour or their soil denitrification ability. Moreover, plant physiological and metabolic changes induced by flooding stress may also cause dysbiotic changes in endosphere and rhizosphere microbial composition. The negative effects of flooding stress on the holobiont (i.e., the host plant and its associated microbiome) can be mitigated once the plant displays adaptive responses to increase oxygen uptake. Stress relief could also arise from the positive effect of certain beneficial microbes, such as mycorrhiza or dark septate endophytes. More research is needed to explore the spiralling, feedback flood responses of plant and microbes if we want to promote plant flood tolerance from a holobiont perspective.
Keywords: flood resilience, holobiont, inundation, pathogens, phyllosphere, plant endophytes, rhizosphere, waterlogging
1. INTRODUCTION
Seasonal flooding is a common phenomenon in certain ecosystems, in which it contributes positively to biodiversity and productivity. Flooding can support agriculture by replenishing soil nutrients in floodplains, create new habitats for biodiversity, and recharge wetlands (Talbot et al., 2018; Tockner & Stanford, 2002; Tonkin et al., 2018). In contrast, unanticipated and uncontrolled floods driven by weather extremes and infrastructural errors rank as one of the most destructive natural disasters, with potential to cause massive damage in agriculture and forestry, destroy infrastructure, and hazard the public health (Kron, 2005). Flood events affect approximately 23% of the world population (Rentschler et al., 2021) and are steadily increasing since the 1950s in all continents (except Oceania; Planchet et al., 2017). Furthermore, flood intensity is expected to increase with ongoing climate change (Kahraman et al., 2021). The rising temperature creates warmer air masses that hold more water, which potentiates increasing frequency and magnitude of local precipitation extremes (Westra et al., 2014). Torrential rains often lead to flash floods that have devastating ecological and socioeconomic consequences (Price & Vojinovic, 2008). The global sea level rise is accelerating (Dangendorf et al., 2019; Nerem et al., 2018), adding to the risk of flooding damage in coastal and riparian areas, which are usually highly populated. Flood is an increasingly important threat to consider in management of any terrestrial ecosystems, including planted or natural forests and agricultural environments. In the areas where flooding is a natural process, plants have evolved morphological, anatomical, physiological, metabolic and phenological traits that allow them to cope with the flooding stress (Jia et al., 2021). However, similar to most terrestrial plants, trees suffer more of flooding than desiccation (Scherer, 1995) and, in the absence of evolutionary adaptations and acclimation capacity, they are prone to damage and death (Fukao et al., 2019). Because of the predicted increases in flood risk due to global climate changes, hydrological disruptions and land use changes, protection of vulnerable forest and agricultural ecosystems against flood and reinforcement of plant tolerance and resilience to flooding stress should have a high priority in research.
Two basic strategies can be implemented to mitigate flooding stress: traditional water management infrastructures (e.g., drainage, dikes) and nature‐based solutions (Zölch et al., 2017). Nature‐based solutions utilise living organisms and/or landscape features to reduce climate change hazards (Hobbie & Grimm, 2020), and are expected to be more flexible and adaptable than traditional approaches upon the uncertain and dynamic future climate. An interactive network that is likely to be strongly affected by waterlogging and submergence is the plant‐associated microbiome, composed of fungi, bacteria and other microorganisms that occupy the external and internal spaces of the plants (Baldrian, 2016). The microbiome is increasingly recognised as a key component of plant health and resilience to environmental stress (Compant et al., 2019; Koskella et al., 2017; Lau & Lennon, 2012; Lau et al., 2017; Turner et al., 2013). The short generation times and high mutation and recombination rates of microorganisms favour plant adaptability to change (Grandaubert et al., 2019; Lamb et al., 2008). Thus, the microbiome could be an integral component of flooding stress tolerance and resilience in plants. Multidirectional relationships between flooding and the holobiont (i.e. the host plant and its associated microbiome) are plausible. Flooding stress may alter the composition and the functional characters of the microbiome directly or indirectly via alterations in plant metabolism, morphology, physiology, or chemistry (Trivedi et al., 2022) promoting detrimental plant‐microbe interactions. However, recruitment of stress‐relieving microbiomes, with the ability to adjust to or tolerate the new stressful condition and metabolise stress‐induced signals, can occur in some cases (Liu et al., 2020a). The consequences of flooding events for microbial diversity, the extent to which plant reaction and acclimation to flood is mediated by the microbiome, and the potential for innovative use of the plant microbiome against flooding are still not thoroughly explored.
The goal of this review is to summarise the current understanding of plant‐microbe interactions under flood conditions and to identify major knowledge gaps to guide future research efforts. We have briefly reviewed flood effects on the host plant, which has already been the topic of many comprehensive reviews, and have focused on the direct and indirect (i.e., plant‐mediated) effects of flood on the host‐associated microbial components: the rhizosphere, the mycorrhizal fungi, and the endobiome of below‐ and above‐ground plant organs. We have also emphasised how flood effects on the microbiome affect the plant, especially the susceptibility to plant disease. Because of the crucial role of the microbiome in plant health and stress resilience, we highlight the importance of considering the microbiome in studies of plant adaptation and resilience to flooding events.
2. FLOOD EFFECTS ON PLANT PHYSIOLOGY
There is a number of comprehensive reviews on the effects of flood on plant molecular, physiological and anatomical mechanisms, some of them focusing on particular plant organs or species (e.g., Bailey‐Serres & Voesenek, 2008; Gill, 1970; Jia et al., 2021; Kozlowski, 1997; Mittal et al., 2022; Sauter, 2013; Tewari & Mishra, 2018). Depending on the species, provenances or genotypes, and the timing and duration of the flooding event, variable alterations in plant metabolism can occur that, over time, scale up to organ physiology, anatomy and whole‐plant performance. Poor soil aeration first alters root energy metabolism. Reduced respiration stimulates glycolysis as part of a deep reorchestration of energy, carbon (C) and nitrogen (N) metabolism. As a strategy to acclimate to low O2 levels, plants downregulate ATP‐consuming pathways and favour nicotinamide adenine dinucleotide (NAD+) regeneration for use in glycolysis (Bailey‐Serres & Voesenek, 2008). Thus, toxic ethanol accumulates, faster in flood‐intolerant than in flood‐tolerant species (Figure 1) (McManmon & Crawford, 1971). This energy crisis, together with root mortality and reduced water uptake and transport in living roots under hypoxia limit the absorption and assimilation of mineral nutrients. Deficiencies in mineral macro‐ and micro‐nutrients occur in flooded plants, in both the root and aerial tissues, which feed forward on growth reductions caused by hormonal imbalances and low energy provision (Bailey‐Serres & Voesenek, 2008; Kozlowski, 1997; Steffens et al., 2005).
Figure 1.
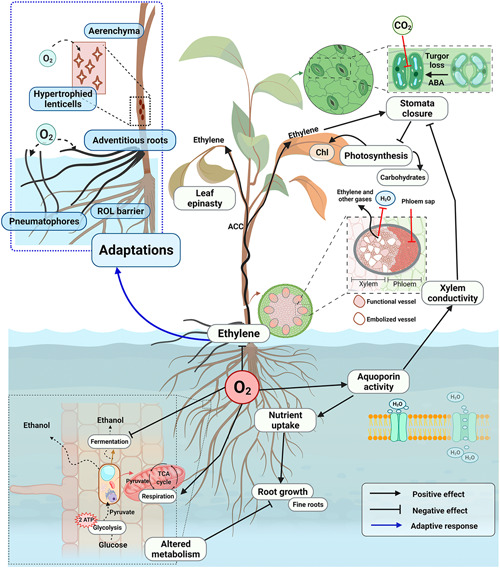
Main physiological changes experienced by plants during flooding stress. Oxygen (O2) depletion is the main direct effect produced by flooding. Poor soil aeration alters root energy metabolism due to the inhibition of the TCA cycle in the mitochondria and the stimulation of glycolysis and fermentation, which leads to the accumulation of toxic ethanol in roots. Hypoxia also leads to the inhibition of the aquaporin activity, which together with the altered root metabolism, contribute to stop the formation and elongation of lateral roots. Alterations in root functioning rapidly extend to the aerial parts, with hydraulic and chemical signals dominating the root to shoot communication. Low aquaporin activity and increased resistance to apoplastic water movement reduce xylem conductivity and, together with abscisic acid (ABA) accumulation in the leaves, enhance stomatal closure. Stomatal closure and impaired photosynthesis reduce CO2 uptake and the production of carbohydrates. Leaf chlorophyll concentration (chl) decreases due to photoinhibition and deficient root nitrogen uptake. The gaseous hormone ethylene is rapidly synthesised under hypoxic conditions in the roots, or in the leaves from its precursor ACC (1‐aminocyclopropane‐1‐carboxylate), causing leaf epinasty, reducing leaf growth, and inducing stomatal closure. Ethylene accumulation also leads to the formation of aerenchyma, which facilitates gas diffusion, suberin‐rich tissues, which prevent radial O2 loss (ROL), and hypertrophied lenticels, adventitious roots and pneumatophores, which favour O2 transport to submerged plant parts. Created with BioRender.com [Color figure can be viewed at wileyonlinelibrary.com]
One of the first alterations induced by flood in the root system architecture is that the formation and elongation of lateral roots stops as a consequence of the interference of ethylene with local auxin signalling, which is the hormone responsible for the formation of lateral roots (Shukla et al., 2019). Alterations in root function rapidly extend to the aerial parts via hydraulic and chemical signals. The capacity to transport water in the xylem can be compromised after flooding, both initially (Else et al., 1995) and in the longer term (Li et al., 2015; Rodríguez‐Gamir et al., 2011) due to low aquaporin activity under hypoxia and reduced apoplastic water movement (Figure 1) (Else et al., 1995; Rodríguez‐Gamir et al., 2011; Sauter, 2013). Low xylem hydraulic conductance reduces the transport of gibberellins and cytokinins diluted in water to the shoot but favours the movement of gases in the xylem conduits (Figure 1). Ethylene is a gaseous hormone that accumulates in flooded plants and rapidly causes epinasty in some species and reduces leaf growth (Jackson, 2002; Jaeger et al., 2009). Ethylene synthesis occurs in the shoot after oxidation of 1‐aminocyclopropane‐1‐carboxylate (ACC) coming from the root. However, this hormone also reaches the shoot from the root and soil (Figure 1), being produced by the root and plant‐associated microbes (Ravanbakhsh et al., 2018). In fact, ethylene signalling regulation is a paradigm of plant‐microbe interactions under stress (see below), and the synthesis and regulation of plant hormones (e.g., cytokinins, ABA, ethylene) are increasingly considered from a holobiont perspective (Eichmann et al., 2021). Hydraulic or chemical signals induce stomatal closure and eventually contribute to reduce leaf growth (Figure 1). Stomatal closure occurs in leaves of flooded plants exhibiting lower (Else et al., 1995, 2001) or more typically similar or higher (Else et al., 1995, 2009; Li et al., 2015; Rodríguez‐Gamir et al., 2011) leaf water potentials than non‐flooded plants. The hormone ABA can accumulate in the leaves (Jackson, 2002; Wilkinson, 1999). However, while stomatal closure is universal in flooded plants, leaf ABA concentration can remain unchanged or increase later than stomata close, suggesting multiple mechanisms of stomatal closure.
Another general response to flooding is a decrease in photosynthesis (Figure 1). Stomatal closure limits carbon dioxide (CO2) diffusion to the chloroplasts and net CO2 assimilation (Camisón et al., 2020; Jaeger et al., 2009; Li et al., 2015). The limited use of reducing power and energy in reducing CO2 triggers alternative mechanisms of energy dissipation such as heat exchange and photorespiration (Else et al., 2009), which may not prevent permanent photoinhibition, chlorophyll degradation, and leaf shedding (Jaeger et al., 2009). The restriction in photosynthesis is likely to be faster and greater when the plant is completely submerged, because irradiance is attenuated and diffusion limitations extend to all gases (Pedersen et al., 2013). Moreover, a deficiency in N uptake and assimilation can limit the photosynthetic capacity of flooded plants (Kozlowski, 1997). The extent to which reduction in C use parallels that in C gain varies across species and affects the concentration of non‐structural carbohydrates in plant organs. Thus, in spite of photosynthetic limitations, non‐structural carbohydrate concentrations are often higher in flooded than in non‐flooded plants in both submerged and non‐submerged organs (Camisón et al., 2020; Jaeger et al., 2009). The increase in non‐structural carbohydrates in leaves can also be related to arrested phloem transport (Peuke et al., 2015). Important from a holobiont perspective is that, besides changes in carbohydrate reserves, flooding stress results in dramatic changes in C and N metabolism (Jaeger et al., 2009; Tewari & Mishra, 2018) and can alter the partitioning of metabolites within and outside the plant (e.g., via root exudates; Henry et al., 2007).
Most terrestrial plants cannot tolerate flooding stress over long periods. Sustained or repeated flood exposure can kill the plant by accumulation of toxic substances, C starvation, cytoplasmic acidification, or disease, the earlier the more sensitive the species (or genotype) is (Kozlowski, 1997; Limami, 2014). Wetland species exhibit adaptive changes that minimise and/or postpone physiological stress upon flooding. For example, semi‐aquatic herbaceous plants such as rice can switch the energy metabolism from an energy‐saving, quiescent state under water to an active energy‐consuming metabolism to escape submersion (Hattori et al., 2011; Mittal et al., 2022). In fact, the ability to minimise C use and maximise C gain determines how much C can be allocated to sustain hypoxic organs and it is a key factor in enduring flood and recovering afterwards (Jaeger et al., 2009). In turn, the capacity to manage O2 concentrations is vital for maintaining photosynthesis and respiration. Wetland and riparian species are capable of forming suberin‐rich tissues preventing radial O2 loss (ROL, Ejiri et al., 2021), hypertrophied lenticels, and adventitious roots and pneumatophores (i.e., aerial roots emerging from the soil) favoring O2 transport to submerged plant parts (Figure 1) (Kozlowski, 1997). Hormonal changes, mainly driven by ethylene accumulation, are responsible for these modifications (Visser & Voesenek, 2005). When thriving in waterlogged soils, these species often form aerenchyma, a specialised tissue formed in the stems, roots, adventitious roots, pneumatophores and symbiotic nodules with large intercellular spaces that facilitate internal gas transport (Takahashi et al., 2014). The aerenchymatous phellem formed in the root nodules of some species can improve both O2 and atmospheric nitrogen (N2) delivery to N2‐fixing bacteria, playing a key role in surviving waterlogging and flood (Takahashi et al., 2014). On the other hand, aerenchyma can also facilitate the diffusion of CO2 derived from root cells and root‐ and soil‐associated microbes to the leaves, alleviating photosynthetic limitations (Greenway et al., 2006).
The changes in the plant host (e.g., in anatomy, C and N metabolism and allocation, gas concentrations, sap pH, etc.) can affect the microbiome (e.g., via food supply, physico‐chemical microenvironment, etc.) (Fry et al., 2020). Conversely, the ability of plants to adjust their metabolism, physiology and anatomy under flood conditions (i.e., flood tolerance) could be affected by the microbiota, i.e., by the relative abundance or stress sensitivity of certain taxa, and their capacity to recolonise the plant or the rhizosphere upon a flood‐induced change in microbial community composition. In the following sections we address flood effects on the plant‐microbe interactions occurring in the rhizosphere, the roots, and the above‐ground plant parts.
3. FLOOD EFFECTS ON RHIZOSPHERE MICROORGANISMS
The rhizosphere is the fraction of soil which is biochemically affected by plant roots; it encompasses a soil layer of circa 1–5 mm from the root surface (Hartman & Tringe, 2019). The rhizosphere microbiome is constituted by the microbiota inhabiting the rhizosphere (bacteria, archaea, fungi, viruses, and oomycetes) and their associated genomes. The release of mucilage, hormones and other substances from roots influences microbial growth, attracts certain microbes and can modify the rhizosphere properties (Bulgarelli et al., 2012). Some of the microbes attracted are specifically recruited by the plant to enhance stress tolerance through the so‐called “cry for help” mechanism (Liu et al., 2021; Rizaludin et al., 2021). Thus, the rhizosphere microbiome has the potential to influence plant performance (Vandenkoornhuyse et al., 2015). Studies on the impact of flooding stress on rhizosphere microbiome have been mainly carried out on herbaceous plants, particularly on the economically important paddy rice, while studies on woody species are scarce. Most of these studies have addressed the influence of flood on bacteria, while fungi, archaea, oomycetes and viruses have received considerably less attention. Microbial communities of bulk and rhizosphere soil change upon flooding (Francioli et al., 2021; Graff & Conrad, 2005; Hamonts et al., 2013; Lin et al., 2011). Because the bulk soil is the main source of microorganisms recruited by plant roots towards the rhizosphere (Bonito et al., 2014; Bulgarelli et al., 2012), the effects of flooding on the microbial composition of the bulk soil can also affect the composition of the rhizosphere.
A direct effect of flooding on soil rhizosphere microbiota originates from the progressive depletion of O2 in the soil pores as they fill with water. The transition from oxygenated to anoxic soil changes the microbial composition from a predominance of aerobic organisms to a higher presence of facultative anaerobes, and finally to dominance of strict anaerobes (Ponnamperuma, 1984). A recent research on spring wheat (Triticum aestivum) has shown that flooding stress induces dramatic shifts in the composition of the rhizosphere microbiota (Francioli et al., 2021). The proportion of bacteria with anaerobic respiratory capabilities within phyla Firmicutes and Desulfobacterota increased together with plant‐detrimental taxa such as Clostridium or Geobacter, whereas the proportion of Actinobacteria and Proteobacteria (particularly in families Rhizobiaceae and Xanthobacteraceae) and plant‐beneficial bacterial taxa such as genera Streptomyces and Sphingomonas decreased (Francioli et al., 2021). To tolerate flooding stress some microbes produce osmoprotectants or endospores and switch their metabolism to anaerobic respiration, fermentation or microaerophily (Bardgett & Caruso, 2020; Unger et al., 2009). However, more research is needed to know how flooding interacts with other factors modulating plant microbial composition. Soil factors such as flood duration, drainage conditions, pH, redox status and C dynamics have the potential to modify the rhizosphere microbiota structure and composition (Moche et al., 2015; Pett‐Ridge & Firestone, 2005). Moreover, the rhizosphere bacterial composition is influenced by host species‐specific traits and by host genotype‐specific traits (Bonito et al., 2014; Liu et al., 2014); therefore, the impact of flood on rhizosphere microbiota can strongly depend on specific host traits.
Indirectly, the rhizosphere microbiota can be altered by flood‐induced stress in the plant. For example, plant stress can induce changes in the quantity and quality of root exudates which, in turn, can modify the biochemical composition of the rhizosphere and the metabolism of heterotrophic bacteria (Figure 2) (Vives‐Peris et al., 2020). The compounds released by roots during the process of rhizodeposition (e.g., in the form of exudates, border cells or mucilage) may account for circa 25% of C allocated below ground (Jones et al., 2009) and represent around 17% of the net C fixed by photosynthesis (Nguyen, 2009). Alteration in C inputs to the rhizosphere can have consequences to the so‐called rhizosphere priming effect, which occurs when input of new soil C stimulates decomposition of old soil C (Liu et al., 2020b). The mechanisms underlying the rhizosphere priming effect are not fully understood (Liu et al., 2020b), possibly because C and N flow in the rhizosphere is extremely complex and highly dependent on plant and environmental factors (Jones et al., 2009). One explanation to the rhizosphere priming effect was that, under N limitation in the rhizosphere, microbes use C supplied by easily assimilable compounds to synthesise enzymes that hydrolyze, in turn, more complex organic materials to acquire additional N (Blagodatskaya & Kuzyakov, 2008). Other studies suggest that the C to N ratio is more important for the rhizosphere priming effect than C input per se (Liu et al., 2020b). Although it is widely accepted that abiotic stress alters the composition and amount of root exudates (Badri & Vivanco, 2009; Karst et al., 2017), the way in which flooding stress affects the total organic C exuded by roots is not well known. Henry et al. (2007) reported an increase of 45% in total organic C exuded after flooding in Agropyron cristatum, but this result should be interpreted with caution due to low replication in the experiment. Other authors have suggested that since soil anoxia leads to reduction in net photosynthesis the amount of root‐derived C in the rhizosphere can be limited after flooding (Hamonts et al., 2013). Possibly, factors affecting the magnitude of photosynthesis reduction (e.g., host specific traits or flood duration) will ultimately determine the amount of root‐derived C under flooding. Alteration of C inputs to the soil seems to have higher impact on microbial functions than on overall microbial diversity (Mounier et al., 2004). Moreover, specific phytotoxic substances such as ethanol, acetaldehyde, and cyanogenic compounds can be released by plant roots under anoxic conditions to avoid cell damage caused by flooding (Blom, 1999), and some of these compounds may have significant effects in the rhizosphere microbiome (Figure 2). For instance, ethanol release has been demonstrated to act as a chemical attractant for different root pathogens, such as Phytophthora sp. and Fusarium sp. (Cameron & Carlle, 1978; Smucker & Erickson, 1987).
Figure 2.
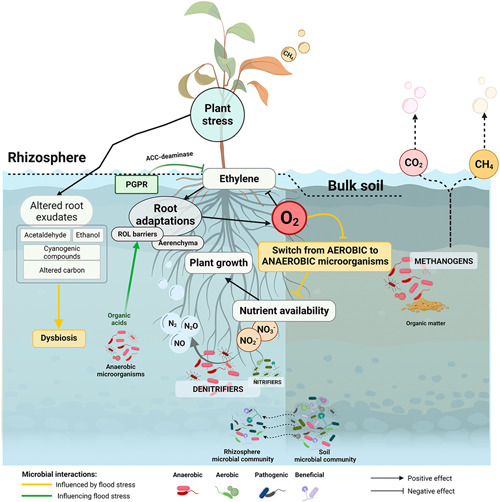
Main flood effects on bulk soil and rhizosphere microorganisms. Low oxygen (O2) conditions induced by flooding impact the rhizosphere and bulk soil microbial composition by producing a switch from aerobic to anaerobic microorganisms. The continuous flow of microbes from bulk soil to rhizosphere (indicated in the lower part of the diagram) can impact the taxonomic composition of the rhizosphere. One of the most important effects of soil microbiome alterations due to flooding is the variation in the nutritional status of the soil. Regarding nitrogen, the inhibition of nitrifying microorganisms and the consequent boost of denitrifiers induce the consumption of nitrate (NO3 −) and nitrite (NO2 −) to produce different gaseous nitrogen forms (NO, N2O, and N2). Nitrogen limitation may ultimately affect different aspects of plant growth. Hypoxic conditions on bulk soil also induce methanogenic processes due to the degradation of the soil organic matter by anaerobic methanogenic microbes. This process culminates with the production of methane (CH4) and CO2, which can be released to the atmosphere through soil or plant tissues. These changes on rhizosphere and soil microbes can be mitigated through specific plant adaptations favoring O2 transport (e.g., aerenchyma). Plant stress can alter the composition of the root exudates and, therefore, produce a dysbiosis on the rhizosphere. Plant stress (and indirectly microbial stress) could be mitigated by the presence of specific groups of microbes, such as the plant growth promoting rhizobacteria (PGPR), which cleaves the ethylene precursor ACC (1‐aminocyclopropane‐1‐carboxylate) through the release of ACC‐deaminases. Created with BioRender.com [Color figure can be viewed at wileyonlinelibrary.com]
Plants and soil microbes compete for O2 in flooded soils (Drew, 1997). As previously mentioned, wetland plants adapted to anoxic conditions form aerenchyma through which O2 from aerial tissues is translocated to roots (while CO2, ethylene, and methane are transported from the soil to the shoots and atmosphere). Oxygen transported in this way reaches the root cells, but is also partly released to the rhizosphere to a distance of around 1–3 mm from the root surface (Gilbert & Frenzel, 1998), a process known as radial O2 loss (ROL). The microbes living in the rhizosphere under flooding receive O2 from the plant (Figure 2) while bulk soil microorganisms continue under anaerobic conditions. The input of O2 supports the activity of aerobic bacteria in the rhizosphere, such as heterotrophic bacteria, methane‐oxidising bacteria, and autotrophic bacteria involved in nitrification of ammonium to nitrate (Cheng et al., 2020), while anaerobic processes such as denitrification, N2‐fixation and methanogenesis occur mainly in the bulk soil (Figure 2) (Faußer et al., 2012; Neori & Agami, 2017). Some species such as maize, wheat and barley do not form aerenchyma under well‐drained soil conditions, but its formation can be induced under flooding stress (Colmer, 2003; Yamauchi et al., 2013) and help to recover aerobic microbial processes in the rhizosphere during flood progression.
The effect of flooding on rhizosphere microorganisms can affect the nutritional status of the soil, especially regarding N availability. Low O2 levels in the rhizosphere can hinder the metabolism of aerobic autotrophic bacteria responsible of nitrification which, together with plant uptake of nitrate (NO3 −), may cause a progressive NO3 − loss from the soil (Figure 2) (Blom, 1999). Under anoxia, NO3 − and nitrite (NO2 −) are used by heterotrophic facultative anaerobic bacteria as terminal electron acceptors to finally release nitric oxide (NO), nitrous oxide (N2O) and dinitrogen (N2) gases by denitrification, a process that can lead to significant N loss from the soil (Hamonts et al., 2013). Limited N available to plants due to denitrification can ultimately affect plant growth, leaf area, photosynthesis and leaf longevity (Mu & Chen, 2021; Vos et al., 2005).
The beneficial effects of some members of the rhizosphere microbiota can improve plant tolerance to abiotic stress, including flooding or waterlogging. Probably the best‐known group of beneficial rhizosphere microorganisms are the so‐called plant growth promoting rhizobacteria (PGPR), which enhance plant growth and resilience against a variety of abiotic stressors (Figure 2) (Babalola et al., 2021; Chauhan et al., 2022; Sagar et al., 2021). Certain taxa of PGPRs reduce the level of ethylene accumulated in plants under flood conditions, counteracting the detrimental effects of high ethylene levels on plant growth and development (Barnawal et al., 2012; Gamalero & Glick, 2015). PGPR produce ACC deaminase in the rhizosphere or in the root endosphere (Figure 2), an enzyme that cleaves ACC, the immediate precursor of ethylene in plants, limiting the quantity of ethylene accumulated in plant tissues. Thus, selection and artificial inoculation of PGPRs to improve plant performance against different stressors such as flooding, salinity, heavy metals or drought is a promising field of research. Several PGPRs in the genera Bacillus, Microbacterium, Methylophaga, and Paenibacillus have been artificially applied to rice plants and found to provide beneficial effects against flooding stress by substantially reducing ethylene levels (Bal & Adhya, 2021). Some PGPRs in the genera Achromobacter, Serratia, Herbaspirillum and Ochrobactrum showed potential to protect Ocimum plants (poorly adapted to waterlogging) against stress under waterlogged conditions (Barnawal et al., 2012). Furthermore, Pseudomonas putida showed the capacity to counteract the inhibitory effects of hypoxic stresses on cucumber plant biomass (Li et al., 2013). Another example of flood tolerance induction is the development of ROL barriers by soil anaerobic microbes. This effect seems to be stimulated by the secretion of organic acids (acetic, propionic, butyric, and hexanoic acids) by anaerobic microbes while decomposing the organic matter in the soil (Armstrong & Armstrong, 2001; Colmer et al., 2019). The previous studies exemplify the high potential of engineering the rhizosphere microbiome to enhance plant tolerance to flood or waterlogging stress, an aspect of high interest to improve crop production but also the success of reforestations in flood‐prone areas.
4. FLOOD EFFECTS ON ROOT MICROORGANISMS
4.1. Mycorrhizal fungi
In recent years there has been an increasing interest in assessing the impact of flooding and waterlogging on the abundance and diversity of mycorrhizal fungi (Barnes et al., 2018; Johnson, 2018; Thomas, 2021). Inhibition of arbuscular mycorrhizae (AM) formation by flooding varies considerably among species of mycorrhizal fungi (Figure 3 and Table 1) and has been attributed to hypoxia, which inhibits AM spore germination and hyphal growth (Keeley, 1980; Kozlowski, 1997; Le Tacon et al., 1983). The degree of AM colonisation usually decreases with increased flooding along wetland gradients (Cooke & Lefor, 1998; Wang et al., 2010), in riparian plants living in floodplains with fluctuating water levels (Klymiuk & Sikes, 2019; Ray & Inouye, 2006), and in lowland and flooded rice roots (Chialva et al., 2020). Nonetheless, some AM associations benefit from short‐term flooding (Sah et al., 2006 in Table 1), and AM colonisation of wetland plants is now believed to be more common than previously thought (Cooke & Lefor, 1998). Several studies along wet to dry transects assessed seasonal trends in AM colonisation of roots (Bohrer et al., 2004; Escudero & Mendoza, 2005), and changes of AM in waterlogged soils across soil depth (Harner et al., 2011; Miller, 2000). The occurrence of some AM species within waterlogged environments is related to the presence of aerenchyma within the cortex of the root system (Voesenek & Bailey‐Serres, 2015; Wang et al., 2010). As stated above, this tissue benefits the plant, but also its mycorrhizal and other aerobic microorganisms through the facilitation of gas exchange (Cooke & Lefor, 1998).
Figure 3.
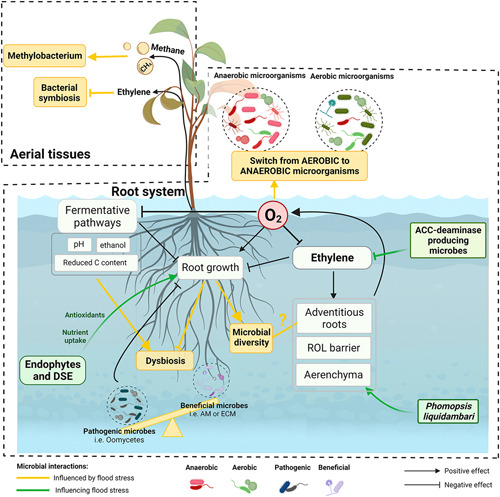
Main flood effects on plant endosphere microbiome, including the root system and the aerial part of the plant. Low oxygen (O2) conditions induced by flooding impact the root microbiome by producing a switch from aerobic to anaerobic microorganisms. Alterations of the chemical composition of the root system due to the activated fermentative pathways, and the reduced growth of the root system due to hypoxia or ethylene synthesis, produced dysbiotic changes on the microbial community composition of the root system. These dysbiotic changes are usually produced by the increase in the competitive ability of certain microbes, i.e. oomycete pathogens or ethanol‐catabolizing microorganisms, and the inhibition of the presence of beneficial aerobes such as endophyte or mycorrhizal fungi. Variations on root system architecture may also cause adjustments in the root microbial community, with a probable reduction in the richness and diversity of species due to low nutrient provision. The extent to which new developed adventitious roots contribute to alterations on the root microbiome remains unknown. Furthermore, the signals generated on the root system, especially the diffusible ones such as ethylene or methane (CH4), can affect the phyllosphere microbiome by inhibiting bacterial symbiosis or enhancing the presence of Methylobacterium, a bacterial genus with the ability to use CH4 as a carbon source. Root adaptations induced by the synthesis and signalling of ethylene, such as adventitious roots, radial oxygen loss (ROL) barriers and aerenchyma attenuates flooding (hypoxic) stress on root microbial composition. Futhermore, the presence of certain microbes can improve flooding stress tolerance by enhancing aerenchyma formation (as in the case of the fungi Phomopsis liquidambari) or the development of ROL barriers (as in the case of certain anaerobic microbes). Microbes producing ACC‐deaminase can mitigate ethylene stress. Other endophytes, such as dark septate endophytes (DSE), can increase root growth by enhancing nutrient uptake and ameliorating the plant antioxidant response. Created with BioRender.com [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Effects of flooding and waterlogging on arbuscular mycorrhizal and ectomycorrhizal symbiosis reported in 16 studies
| Type of mycorrhiza | Species | Host (age) | Type of stressa (duration) | Effect | Reference | |
|---|---|---|---|---|---|---|
| Arbuscular | Diversispora spurca | Citrus junos (0.5 year) | W (37 days) | Less colonisation. Mycorrhization ameliorated effects of waterlogging on growth, RSA and antioxidant enzyme activities | Wu et al. (2013) | |
| Gigaspora margarita | Poncirus trifoliata (0.5 year) | W (3 months) | Reduced hyphal density and colonisation | Matsumura et al. (2008) | ||
|
Gigaspora margarita + G. rosea and Glomus intraradices + Entrophospora columbiana mixes |
Phaseolus vulgaris (2–7 weeks) |
F (4 weekly 8 h duration) | No effect | Sah et al. (2006) | ||
| Glomus intraradices | Citrus aurantium and Poncirus trifoliata x C. sinensis (0.5 year) | F (21 days) | Not affected | Hartmond et al. (1987) | ||
| Glomus intraradices | Citrus sinensis (1 year) | F (21 days) | 40% less colonisation | Hartmond et al. (1987) | ||
| Glomus intraradices + Entrophospora columbiana mix |
Phaseolus vulgaris (2–7 weeks) |
F (3 weekly 8‐h duration) | Increased colonisation | Sah et al. (2006) | ||
| Glomus mosseae | Medicago trunculata (2 weeks) | W (8 days) | Decrease of infection rate at saturate conditions | Reid & Bowen (1979) | ||
| Several species | Panicum hemitomon and Leersia hexandra | F |
Inhibition of colonisation with increasing water depth |
Miller (2000) | ||
| Ectomycorrhiza | Cenococcum geophilum | Quercus ilex (>70 years) | F (2 months) | Higher relative abundance in stream banks than in upper slopes | Corcobado et al. (2015) | |
| Thelephora terrestris, Laccaria laccata, Hebeloma crustuliniforme, Suillus flavidus and S. bovinus | Pinus sylvestris (7 weeks) | F (2 min–6h per day, 4 days a week) | First three species not sensitive, the last two highly sensitive | Stenström (1991) | ||
| Tuber aestivum | Quercus robur (0.5 year) | F (7–65 days) | Reduction of ectomycorrhizae in the upper zone, then in the lowest zone | Thomas (2021) | ||
| Several species | Populus deltoids and Salix nigra (0.5 year) | F (3 months) | Inhibition of colonisation in favour to AM symbiosis | Lodge (1989) | ||
| Several species | Alnus glutinosa and A. incana | W |
Dry plots harboured three times more unique species than temporarily waterlogged plots |
Tedersoo et al., (2009) | ||
| Several species | Liquidambar styraciflua, Quercus nuttallii, Q. phellos, and Q. lyrata | F | Temporal decline but recovery by the end of the growing season | Filer (1975) | ||
| Several species | Pinus densiflora (2–4 years) | F (6 months) | Less diversity and different composition in flooded area | Cho et al. (2021) | ||
| Several species | Pinus taeda (40 years) | F | Reduction on flat sites during wet periods | Lorio et al. (1972) | ||
| Several species | Salix viminalis (10 years) | F (days) | Decline in the abundance and species richness, only in hydrophobic EM | Barnes et al. (2018) | ||
Flooding (F; water standing above soil level) and waterlogging (W; only the soil is flooded).
Compared to other mycorrhizal associations, AM tend to dominate in soils frequently subjected to water saturation (Thomas, 2021). Few plants are known to regularly form both AM and ectomycorrhizae (ECM), like several species of Populus and Salix. However, in periodically inundated and poorly drained soils, these species almost exclusively form AM (Lodge, 1989). Because the proximal roots are better supplied with O2 than distal roots (Keeley, 1980), AM fungi, infecting the proximal parts of root systems (Keeley, 1980), may have an advantage over ECM fungi in anoxic soils (Lodge, 1989). Although there are many examples of reduced ECM colonisation and richness because of flooding (Barnes et al., 2018; Unger et al., 2009 and other references in Table 1), some ECM fungi inhabit environments which are permanently waterlogged (Baar et al., 2002; Cho et al., 2021). The mycelium produced by ECM fungi can be categorised as either hydrophobic or hydrophilic (Barnes et al., 2018), with hydrophilic fungi being often more resistant to flooding (Barnes et al., 2018; Stenström, 1991; Unestam & Sun, 1995). As such, there is ample variation in flood resistance among ECM species. Laccaria spp. and Thelephora terrestris are tolerant to flooding (Cho et al., 2021; Hashimoto & Higuchi, 2003), Cenococcum geophilum benefits from flooding (Corcobado et al., 2015), while there is a variable resistance in Suillus spp. (Cho et al., 2021). The mycorrhizae formed by Tuber aestivum can survive at least 65 days of flood (Thomas, 2021). Although full submersion for 7 days is enough to cause a significant impact on ECM colonisation, extending the duration of submersion does not mean inoculum extinction, and ECM recovery can occur even in deep zones of the soil (Thomas, 2021). From a practical point of view, flood‐tolerant mycorrhizal fungi could be used for inoculating seedlings to be planted in wet forest areas. Mycorrhizal associations tolerant to wet conditions may help improve plant vitality by reducing pathogen infection (see below) and assisting plant recovery from short‐term flood events and root rot.
4.2. Root endophytes
Plants suffering soil water stress have been described to undergo a reduction in colonisation of microbial endophytes proportional to the level and duration of flooding (Li et al., 2010). This event can be explained by the fact that many of the endophytes colonising terrestrial roots are obligate aerobes, and their survival is limited under hypoxic conditions (Figure 3). Tian et al. (2015) described a reduced endophyte diversity under anaerobic conditions in Myricaria laxiflora, a riparian shrub usually suffering periodic summer flooding. Similarly, reduced bacterial diversity was observed in flooded rice roots (Vishwanathan et al., 2020). Contrary to AM, ECM and endophytic fungi, bacterial or fungal taxa with anaerobic respiratory capabilities tend to increase during flood. For example, flooding stress promoted the enrichment of strictly root anaerobic bacteria within Deltaproteobacteria or Firmicutes (Chialva et al., 2020; Ferrando et al., 2015; Francioli et al., 2021), or within Proteobacteria, such as the genus Aquapirillium in the rhizoplane (the external root layer) of submerged Populus roots (Graff & Conrad, 2005). Anaerobic yeasts, including the beneficial Cryptococcus, Exophiala, Sporobolomyces, or Rhodorotorula, may also increase their presence under hypoxic conditions (Freed et al., 2019). As a consequence of anaerobiosis, methanobacteria (Phylum Euryarcheota) frequently proliferate, as described in submerged rice roots (Chialva et al., 2020) and the root endosphere of Taxodium distichum trees, a deciduous conifer living in swamps (Lumibao et al., 2020).
The flood‐induced alteration of root chemical composition may also produce a microbial community re‐structuration due to changes in the chemistry of the ecological niche (Figure 3) (Hadacek & Kraus, 2002). Graff and Conrad (2005) described that high ethanol concentration favoured the presence of ethanol‐catabolizing microorganisms in Populus roots. Alterations in carbohydrate, Mg, S, and Mn concentrations under flooding conditions can modify the competitive interaction among microbial members, as observed in roots of Phragmites australis (Neubert et al., 2006). In line with this, a low number of fungal species was reported by Duan et al. (2019) in the roots of Distilyum chinese, a Chinese waterlogging tolerant endemic plant, with Phomopsis being the dominant member of the community probably due to its rapid growth or its ability to produce bioactive secondary metabolites.
In addition to the hypoxic conditions or the chemical alterations produced on root tissues during flood, variations in root system architecture and anatomy may also cause adjustments in the microbial community (Galindo‐Castañeda et al., 2022; Figure 3). Reduced lateral root growth and the development of new adventitious roots to maximise O2 uptake are central alterations observed in the root system (Karlova et al., 2021; Sauter, 2013; see above). The reduced development of fine roots during flooding may influence the diversity and the richness of the microbial community, as those roots are typically richer in nutrients and metabolites, and have greater surface area than large roots (Saleem et al., 2018). Therefore, reduced endophytic diversity, with a consequent lower abundance of beneficial mutualists (Saleem et al., 2018), can be expected in flooded roots; however, this issue remains understudied. The reduced microbial colonisation of fine roots may also limit the transference and the establishment of specialist microbes in secondary and primary roots (Saleem et al., 2018). Despite some general patterns commonly observed in response to flooding in different plant species, endophytic microbial community alterations are usually very different between the wide array of plant species studied, which ranges from annual plants to trees. The ability of flood‐tolerant plant species to enhance internal tissue aeration (e.g., via aerenchyma) will buffer microbial community alterations under flood by reducing abundance and activity of anaerobic microorganisms, and increasing microorganisms with aerobic metabolism (Galindo‐Castañeda et al., 2022). The increased root growth and nutrient uptake upon aerenchyma formation may also indirectly influence the root microbial community under flooding stress. Nevertheless, Galindo‐Castañeda et al. (2022) stated that the formation of aerenchyma will reduce the apoplastic space for microbial colonisation, reducing their abundance and diversity. Therefore, the flood‐adaptation level of the host plant species may shape the impact of flood on root microbial community.
The flood tolerance of some plant species may partly rely on the presence of root microbes with the ability to tolerate flooding stress and exert beneficial effects despite the stress. The recognition and understanding of those beneficial interactions may be of interest to alleviate flooding stress on the plant. One group of endophytic fungi with the ability to survive despite flooding damage on root tissues, and with a putative beneficial role against flood, is the dark septate endophyte (DSE) group (Figure 3) (de Marins & Carrenho, 2017). DSEs form a diverse group of ascomycetous, sterile or conidial root‐associated endophytes that are commonly found in plant roots in various environments without causing disease symptoms (Gaber et al., 2020; Mandyam & Jumpponen, 2005; Santos et al., 2021). The presence of melanin in DSE hyphae cell walls and their ability to produce microsclerotia enhance their survival probability under a wide array of plant‐stress conditions (Barrow, 2003; Berthelot et al., 2020; Zhan et al., 2011)—although salt tolerance has been found to be decoupled from melanin (Gaber et al., 2020). The beneficial effect of DSE on the host plant include improved growth, enhanced nutrient uptake, and an increased ability to resist biotic and abiotic stress (Santos et al., 2021). Root associations with DSE have been commonly described in plants living in a wide array of terrestrial environments (Mandyam & Jumpponen, 2005), but also in wetland and rainforest habitats (Kohout et al., 2012). Plants living in riparian habitats may also take advantage of this association to survive inundation periods. For instance, Zhao et al. (2016) reported a high DSE colonisation rate in diverse riparian plant species living in the Three Gorges Reservoir in China, suggesting that the success of these species to survive under temporary flooding patterns might rely on their ability to associate with DSE, which sometimes occurred in combination with AMF. Duan et al. (2019) also reported that members of the DSE group, including Diaporthe sp., Phomopsis sp. or Periconia sp., accounted for 13% of the fungi composing the root microbiome in the flood‐tolerant plant Distilyum chinense, further linking the presence of DSE with flood tolerance.
Other root endophytes can enhance flood tolerance by influencing the development of specific anatomical structures, such as the aerenchyma or the ROL barriers (Figure 3). For example, rice plants artificially inoculated with the root endophytic fungi Phomopsis liquidambari were able to develop significantly larger aerenchyma compared with non‐inoculated plants (Hu et al., 2018). Phomopsis liquidambari seems to stimulate both the auxin and the ethylene signalling pathways in host plants, which enables elevated concentrations of both hormones. This double activation appears to be responsible for the upregulation of some of the genes involved in aerenchyma formation, leading to rapid and effective aerenchyma development in rice roots and enhancing its potential to mitigate anaerobic processes during flood stress (Hu et al., 2018).
Plant physiological improvements induced by endophytic microbes can also contribute to ameliorating flooding stress tolerance. The production of antioxidant molecules or the synthesis of the ACC‐deaminase enzyme by symbiotic microorganisms seems to be involved in the modulation of the plant antioxidant system or plant ethylene levels (Figure 3). It is hypothesised that the antioxidant capacity of certain endophytes colonising the roots of submerged plants can promote the adaptation of those plants to excess soil water conditions. For instance, two fungal endophytes isolated from the riparian species Myricaria laxiflora were able to synthesise two different antioxidants: Z‐N‐4‐hydroxystyryl formamide and chaetoglobosin A (Xue et al., 2021). The application of these antioxidants in rice and Arabidopsis seedlings before flooding application demonstrated their role in mitigating flooding stress effects by stimulating the plant antioxidant system through NADPH oxidases (Xue et al., 2020, 2021). Some endophytic fungi and bacteria producing the enzyme ACC‐deaminase (that cleave the ethylene precursor ACC into α‐ketobutyrate and ammonia) inhibit the biosynthesis of ethylene (Jia et al., 1999), which at high concentrations can arrest plant growth, and cause chlorosis and even plant death (Ali & Kim, 2018). The bacterium Pseudomonas fluorescens and the fungus Trichoderma asperellum are two examples of endophytic microorganisms isolated from waterlogged roots exhibiting ACC‐deaminase activity (Etesami et al., 2014; Rauf et al., 2021). The artificial inoculation of these ACC‐deaminase‐producing endophytes in rice and wheat plants, respectively, improved plant growth and photosynthesis under flooding stress (Etesami et al., 2014; Rauf et al., 2021), and generally mitigated the detrimental consequences of stress (Ali & Kim, 2018; Grichko & Glick, 2001). The previous examples imply that flooding stress can be mitigated by means of root symbiotic microorganisms.
5. FLOOD EFFECTS ON PHYLLOSPHERE MICROORGANISMS
The phyllosphere refers to the total above‐ground parts of a plant (Vacher et al., 2016); it comprises the phylloplane, i.e., the surfaces of leaves, stems, branches, flowers and seeds, and the internal compartments (endosphere) (Lau et al., 2013; Müller et al., 2016). With an estimated global span of 6.4 × 108 km2 (Morris & Kinkel, 2002), the phyllosphere constitutes a huge, temporally and spatially dynamic matrix of habitats, where the microbial communities are shaped by their species interactions, abiotic factors (e.g., UV‐light or nutrient availability), and host biology (e.g., phenological, genotypic or organ‐specific traits) (Hartmann et al., 2009; Lindow & Brandl, 2003; Lindow & Leveau, 2002). Phyllosphere microbiomes have been found to contribute to important ecosystem processes, such as N2 fixation and nitrification (Guerrieri et al., 2015; Moyes et al., 2016), and absorption of air pollutants (Bringel & Couée, 2015; Wei & Jousset, 2017). These microbiomes may also support host plant health and adaptation to environmental stress caused by salinity, temperature extremes, drought, natural enemies or flood (Saleem et al., 2017; Stone et al., 2018).
Due to the recent advances in metagenomic approaches, detailed data about the taxonomic diversity of the phyllosphere microbiomes and their importance for the plant holobiont is accumulating (Kaul et al., 2016; Vandenkoornhuyse et al., 2015). External factors such as humidity are known to shape microbial communities (Xin et al., 2016). Still, the knowledge about how hydrospheric disturbances, such as flash floods and river flooding, influence the recruitment and dynamics of the phyllosphere microbiomes has remained fragmented. Clearly, the hyperdiverse microbial communities and the difficulties in experimentally approaching the total microbiome and separating the effects of flooding from other factors complicate the studies. While the recruitment of phyllosphere microbiome is strongly dependent on the environment, results from several studies indicate that plant genotype, species, and physicochemical attributes of the organs appear to act as strong filters for the taxonomic assemblage of the microbiomes in the aerial parts (Agostinelli et al., 2018; Bálint et al., 2013; Knief et al., 2010; Romeralo et al., 2022). Bacteria and non‐pathogenic (asymptomatic) microbes that belong to a few predominant phylogenetic groups, such as classes Alphaproteobacteria and Gammaproteobacteria and the phyla Bacteroidetes and Actinobacteria, have often been found to dominate the phyllosphere communities (Cui et al., 2019; Herrmann et al., 2021; Vorholt, 2012). Rapidly sporulating species and yeasts colonise leaf surfaces actively, while filamentous fungi seem to occur on the phylloplane mainly as spores and be more frequent in the endosphere (Vacher et al., 2016). In response to flooding stress, rice culms showed a reduction of members within Gammaproteobacteria, while Firmicutes members, especially Bacillus species, seemed to adapt to the flooding stress condition (Cui et al., 2019). In the mangrove tree Rhizophora stylosa, which are frequently exposed to inundation, fungi dominated the endosphere microbiome (Purahong et al., 2019). In addition, in forest trees, the canopy position (top, mid, or bottom height) may significantly influence the bacterial community composition (Herrmann et al., 2021). Thus, there is increasing evidence showing that the species‐specific microenvironments support specific microbial communities (Camarena‐Pozos et al., 2019; Chaudhry et al., 2021). Consequently, also the direct and indirect impacts of flooding on the phyllosphere microbiome are likely to be variable among plant species.
The direct, physical impacts of flood on the phyllosphere microbiome can be both positive or negative in terms of frequency and diversity of microbial infections. In large trees, the direct effects of flooding on the microbiome may be less pronounced than in the rhizosphere (see above), because the site where the flooding occurs (soil and ground‐level) and the canopy, where the main part of the phyllosphere resides, are usually not directly connected. Thus, the most obvious location for the immediate, direct impact of flooding on the tree microbiome would be the lowest parts of the trunk and lower part of the canopy. However, flooding events are often preceded by heavy rainfalls, which influence the whole tree. Rain or flooding water can wash away epiphytes, but they may also seed microbial propagules to the phyllosphere. Flooding may also cause shifts in the epiphytic phyllosphere microbiome through direct, physical forces associated with water accumulation and movement, e.g., by flushing away the microbial propagules from the phyllosphere surfaces. This can reduce the likelihood of new recruitments by flushing away the nutrients deposited in the phylloplane from the atmosphere or exuded from the leaves (Inácio et al., 2002). To predict the outcome of direct effects of flooding on tree microbiomes is thus challenging.
The indirect effects of flooding on the phyllosphere microbiome are likely to involve complex physiological and metabolic processes that are induced in plants by flooding stress (see above). Plant responses to flooding stress may particularly target the microbes living in the endosphere, where they absorb nutrients from their hosts (Sarkar et al., 2021). Hormonal disbalances, ethanol synthesis or methane production, which are usually initiated in root tissues, may also influence the microbial communities in shoots. For example, the gaseous plant hormone ethylene may inhibit the endophytic colonisation processes of some bacterial endophytes (Nascimento et al., 2018). Furthermore, the synthesis of methane can enhance the presence of bacteria within Methylobacterium, which are known for using methane as a C source (Abanda‐Nkpwatt et al., 2006; Iguchi et al., 2015). In addition to indirect effects via the host tree physiology and metabolism, indirect ecological effects can also occur. For example, the process of microbial recruitment may be influenced by the impact of flood on the surrounding vegetation, including understorey herbs and shrubs that act as reservoirs for the environmental inoculum (Weißhaupt et al., 2016). Periodic flooding leads to enrichment of nutrients and thus supports the re‐growth of the surrounding vegetation. Thus, post‐flooding pulses in the production of environmental inoculum from vegetation can have positive effects on the diversity of microbiome in the phyllosphere.
Whether the different effects of flooding on recruitment and dynamics of phyllosphere microbiomes are transient or of permanent nature has rarely been systematically studied. The duration and magnitude of the changes will likely depend on the type of the disturbance, especially on the velocity and intensity of the flooding events, and the depth and duration of the inundation and waterlogging. If submergence and O2 deprivation are long‐lasting, the phyllosphere microbiome can be expected to switch towards anaerobic communities. The season and origin of the flood water also plays a significant role. For example, the O2 level, amount of nutrients, heavy metals, or salinity in the water may affect both the responses of the microbiome and plants (Schindler et al., 2016; Worms et al., 2006). In general, the damage to phyllosphere is likely to be less severe when flooding occurs during dormancy than if it occurs during the growing season and disturbs the development and growth of the trees (Glenz et al., 2006).
6. FLOOD EFFECTS ON MICROBIAL TROPHIC INTERACTIONS AND PLANT DISEASE SUSCEPTIBILITY
As described in the previous sections, flooding stress can profoundly modify the holobiont by affecting both the host physiology and microbiome structure and functions. Such flood‐induced changes are likely to have consequences for disease resistance by the host. In fact, flooding influences the occurrence and development of several plant diseases by affecting the susceptibility of the host and the survival and spreading of some pathogenic microorganisms. Causes of increased disease severity in plants subjected to hypoxia include energy deprivation due to reduction in ATP production in roots (Moslemi et al., 2018), suppression of oxidative burst and the immune response, and hypersensitive cell death (McDonald, 2002). Hypoxia may also stimulate the activity of pectate lyases secreted by pathogens, which contributes to tissue maceration during infection (Chung & Lee, 2020). For example, hypoxia induced by flooding increased the activity of pectate lyases of the bacterial pathogen Erwinia carotovora and reduced the expression levels of defence genes of the plant Solanum tuberosum, which resulted in the increased severity of bacterial soft rot on S. tuberosum tubers (Rumeau et al., 1990). In addition to bacterial diseases, flooding favours disease progress when plants become infected by fungal or oomycete pathogens of the genus Fusarium, Phytophthora, Pythium and Phytopythium (Table 2; Moslemi et al., 2018; Wilcox, 1985). Phytophthora root rot has frequently been associated with excessive soil moisture in poorly drained soils (Figure 4). This results from high Phytophthora tolerance to low soil O2 concentrations, the induction and dispersal of zoospores in water saturated soils (Burgess et al., 1999; Duniway, 1979; Hardham, 2001; Kenerley, 1984; Wilcox, 1985), and the increased predisposition of the plant to infection in flooded soils (over periods as short as 24 h; Blaker & McDonald, 1981; Kuan & Erwin, 1980). The duration of soil water saturation may be more important in disease expression than the number of saturation episodes (Browne & Mircetich, 1988; Stolzy et al., 1965; Wilcox, 1993). Two and a half months of waterlogging combined with subsequent water deprivation and P. cinnamomi infestation was the worst scenario for Quercus ilex survival, resulting in 100% mortality of plants (Corcobado et al., 2014a). Increased root exudation of ethanol, sugars and amino acids can also favour pathogen infection during flooding (Blaker & McDonald, 1981; Tyler, 2002). The exudation of these chemotactically active substances from roots under flood conditions has been attributed to an impaired functioning of root cell membranes under O2‐deficient conditions; thus, the predisposition of waterlogged plants to Phytophthora would partly result from an enhanced attraction of zoospore inoculum to flooded roots (Kuan & Erwin, 1980).
Table 2.
Combined effects of hypoxia and oomycetes on plants reported in 17 studies
| Host (age) | Type of stressa (duration) | Oomycete | Effect | Ref | |
|---|---|---|---|---|---|
| Abies fraseri (2 years) | F (24 and 48 h) | Phytophthora cinnamomi | Increased infection and seedling mortality | Kenerley (1984) | |
| Actinidia deliciosa (8 months) | F (3–5 days per week, 3 weeks) | Phytopythium chamaehyphon and P. vexans | Needed to induce kiwifruit decline | Savian et al., (2020) | |
| Capsicum annuum (1 month) | F (24 h) | Phytophthora capsici | Plant mortality increased as the number of flooding periods at 10‐day intervals increased | Bowers et al., (1990) | |
| Eucalyptus marginata (5 months) | 0.05 mg O2 L−1 (6 h) and 2 mg O2 L−1 (6 days) | Phytophthora cinnamomi | Larger lesions in roots exposed to anoxia but lower in roots exposed to hypoxia | Burgess et al., (1999) | |
| Glycine max (1–10 days) | F (2 and 5 days) | Pythium ultimum | Additive effect | Kirkpatrick et al., (2006) | |
| Malus ×domestica (8 weeks) | F (0, 24, 48, and 72 h each week for 4 months) |
Phytophthora cactorum, P. cambivora, P. cryptogea and P. megasperma |
Mean crown rot incidence of 2.5%, 6.3%, 19%, and 50% for 0, 24, 48, and 72 h flooding, respectively | Wilcox, (1993) | |
| Malus pumila (8 months) | F (4, 12, 24, 48 h every 2 weeks) |
Phytophthora cactorum, P. cambivora and P. cryptogea |
Increased root and crown rot especially by P. cryptogea | Browne & Mircetich (1988) | |
| Medicago sativa (3 weeks) | W (1 week) | Phytophthora megasperma | Water saturation before inoculation predisposed plants to root rot by increasing root damage and exudation of nutrients | Kuan & Erwin (1980) | |
| Nicotiana tabacum and Glycine max cells (7 days) | N atmosphere (4–9 h) | P. nicotianae and P. sojae | Failure of resistance expression; infection and colonisation of cells by incompatible isolates | McDonald (2002 | |
| ersea americana (1 year) | F (14 days) | Phytophthora cinnamomi | Additive and synergistic damage | Reeksting et al. (2014) | |
| Prunus mahaleb (4 months) | F (48 h every 2 wk) | Phytophthora cryptogea, P. cambivora, P. megasperma and P. drechsleri | Increased root damage and reduced growth | Wilcox (1985) | |
| Prunus mahaleb (8 weeks) | F (48 h every 2 weeks) | P. cryptogea and P. megasperma | 81%–99% of the root system diseased | Wilcox & Mircetich (1985) | |
| Prunus persica (10 weeks) | W (8 h every 5 days) | Pythium vexans and P. irregulare | Disease severity increased with P. vexans only | Biesbrock & Hendrix (1970) | |
| Quercus ilex (>70 years) | Wg (>2 months) | Phytophthora cinnamomi | Increased root damage | Corcobado et al. (2013) | |
| Quercus ilex (>70 years) | W (>2 months) | Phytophthora cinnamomi | Shifts in ectomycorrhizal abundance related to infection | Corcobado et al. (2014b) | |
| Quercus suber (2 months) | ≤1% oxygen (5 days) | Phytophthora cinnamomi | Higher disease incidence but similar disease severity | Jacobs et al., 1997) | |
| Rhododendron (1 year) | F (48 h before inoculation) | Phytophthora cinnamomi | severe symptoms of root and crown rot | Blaker & McDonald, (1981) | |
Flooding (F, water standing above soil level) and waterlogging (W, only the soil is flooded).
Figure 4.
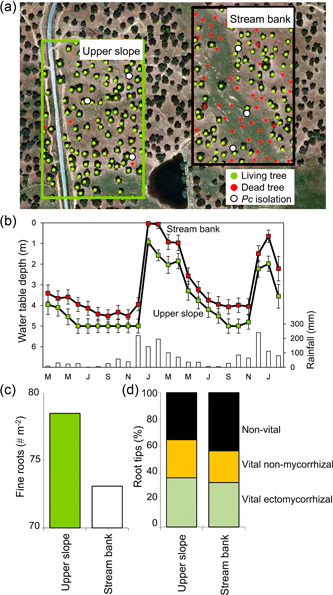
Increased tree mortality in a stream bank of a Quercus ilex forest in Extremadura, SW Spain, where flooding and the oomycete Phytophthora cinnamomi (Pc) co‐occur. (a) Image of the study site showing places of Pc isolation and distribution of living and dead trees. (b) Mean values of water table depth measured in stream banks and upper slopes from March 2009 to February 2011. Assuming sinker roots of Q. ilex growing deeper than 5 m (Moreno et al., 2005), about one‐third of the roots of trees located in stream banks would have been waterlogged for 4 months in 2010 and 2 months in 2011. Bars denote standard errors (n = 5 sites; adapted from Corcobado et al., 2013). (c) Fine roots of Q. ilex trees examined from soil pits (2.5 m wide and 1.5 m deep; n = 288) dug in upper slopes and stream banks. (d) Relative abundance of non‐vital, vital non‐mycorrhizal and vital ectomycorrhizal root tips of Q. ilex trees (n = 192) located in upper slopes and stream banks (n = 48; adapted from Corcobado et al., 2014b). [Color figure can be viewed at wileyonlinelibrary.com]
During flooding, the chemical or nutritional properties of the plant or the rhizosphere change. Bacterial and fungal species with different nutritional niches (Blumenstein et al., 2015) and belonging to different functional guilds (Talbot et al., 2015), respond differently to these changes. In flooded wheat roots, a reduction in the abundance of the beneficial member's Streptomyces, Sphingomonas, and different members within the genera Saccharimonadia, Massilia, and Flavobacterium was observed (Francioli et al., 2021). All these microbes were associated with induction of plant defences, improved nutrient uptake, or plant‐growth promotion (Kavamura et al., 2021; Soltani et al., 2010). The depletion of the beneficial symbionts may lower plant stress resilience, by reducing their antagonistic or antibiotic potential, or by weakening the induction of systemic resistance that restricts pathogen development. For example, Francioli et al. (2021) described an increase of Clostridium species in roots during flooding. These anaerobic species are usually associated with root rot diseases in waterlogged soils (da Silva et al., 2019). Similarly, Martínez‐Arias et al. (2020) observed changes in the root mycobiome composition in flooded Ulmus minor plants that involved a decrease of potentially beneficial members within Chaetothyriales, usually associated with the DSE group and healthy roots, and an increase of the root rot pathogen Plectosphaerella cucumerina. Moreover, the competitive interaction between ECM and Phytophthora contributes to prevent disease in non‐flooded soils. In Quercus ilex forests, higher tree mortality induced by Phytophthora cinnamomi was observed in stream banks than in upper slopes, even if the pathogen was successfully isolated in both areas (Figure 4a). Trees located in stream banks, usually subjected to waterlogging for approximately 1 month a year (Figure 4b), showed fewer fine roots and vital ectomycorrizal tips in comparison to trees located in upper slopes (Figure 4c,d). Non‐mycorrhizal root tips are vulnerable entry points for P. cinnamomi into the tree (Corcobado et al., 2014b; Malajczuk, 1988; Marx, 1972), and once they become infected, they rot and become non‐vital (Corcobado et al., 2014b).
Above ground tissues may also suffer detrimental pathogenic events due to flooding stress. High humidity is known as a disease‐promoting condition especially when it comes to bacterial infections (Xin et al., 2016). Raindrop impingement is a dispersal method for bacteria (Gilet & Bourouiba, 2015), and the level of bioaerosols (small water droplets suspended in the air and containing microbes) in the air is elevated after rain (Joung et al., 2017). Once landed on the phyllosphere, microbes can enter plant tissues through active penetration or through wounds, lenticels and stomata. Stomata were earlier considered as passive openings, but later studies showed that they are part of the plant innate immune system and can actively limit the entry of bacteria through the pathogen‐induced molecular pattern (PAMP)‐induced stomatal closure (McLachlan et al., 2014; Melotto et al., 2006, 2008). However, some pathogens, such as Pseudomonas syringae, can compromise the stomatal defence using the virulence factor coronatine, inducing stomatal reopening to enter the plant and subsequent closure to reduce water loss (Freeman & Beattie, 2009; Goel et al., 2008; Panchal & Melotto, 2017). Plant weakening due to flooding stress (Figure 1), makes above ground plant parts more vulnerable to insect attacks, and thus potentially increases the exposition of trees to microbes that are vectored by insects. For instance, Ranger et al. (2013) studied the influence of flooding stress on the attractiveness and susceptibility of Cornus florida (dogwood) to insects and microorganisms in field conditions. They found that as compared to non‐flooded dogwoods, flood‐stressed dogwoods were more attractive to Xylosandrus germanus, an ambrosia beetle that lives in nutritional symbiosis with ambrosia fungi. Ethanol, which is the most attractive semiochemical known for X. germanus, was detected in stem tissue of flooded dogwoods, but not in non‐flooded ones.
Conversely, hypoxia induced by flooding can also reduce disease development in plants by increasing host defence responses as a consequence of the activation of a general stress response (Chung & Lee, 2020). In Arabidopsis, for example, flooding triggers innate immunity via WRKY transcription factors, a genetic response that may protect plants against pathogen infection either during or after flooding (Hsu et al., 2013). Root hypoxia in Eucalyptus marginata lead to the activation of several plant defence‐associated enzymes, and accumulation of soluble phenolics (Burgess et al., 1999). Hypoxia has also been found to negatively affect the survival of Collybia fusipes, Heterobasidion annosum and Fusarium poae (Camy et al., 2003; Martinez et al., 2019), and the biosynthesis of mycotoxins in several fungal plant pathogens (Paster et al., 1986; Tang et al., 2018). Indeed, soil flooding has been suggested as a practice against soil borne plant pathogens, as it is generally regarded as more environmentally friendly than soil fumigation (Niem et al., 2013).
7. CONCLUDING STATEMENTS, KNOWLEDGE GAPS AND FUTURE PROSPECTS
Plant resistance and resilience to flood in agricultural and forest environments should be further explored in view of the more frequent flooding episodes. The study of the holobiont offers a new, holistic perspective on flood tolerance mechanisms and flooding stress mitigation in sensitive plant species (Figure 5). Flooded plants modify their metabolism and physiology to acclimate to hypoxia, which triggers variations in microbial trophic interactions in different plant parts. On the other hand, some endophytic or rhizospheric fungi and bacteria may contribute to plant flooding stress signalling and tolerance (Figure 5). However, it is not known to what extent these plant functional changes are mediated by and leading to microbial changes. Important knowledge gaps remain to be filled before we can better understand how flooding and waterlogging shape the microbiome below and above ground, and what consequences this may have for the plants in different environments. The gap in knowledge is huge compared with that in plant‐microbe interactions under drought stress, nutrient stress or plant disease (e.g., Jones et al., 2019; Martínez‐Arias et al., 2021). The role of microbiome diversity and composition in flooding stress tolerance, and the direct and indirect effects of flood on the microbiome are still understudied in many important plant species. The difficulty in separating plant responses from symbiont‐mediated stress responses demands manipulative experiments where plant flood responses are examined with and without symbionts. These experiments, where microbes should be first isolated and their putative functional role characterised, could ultimately lead to plant inoculation with beneficial microbes to cope with flooding stress. Observational studies in different plant species will help to understand the intricate plant microbial network and how it can be challenged under flooding stress. More information about the temporal changes in species‐ and plant part‐specific microbial communities is needed to reveal the patterns in microbial diversity in different compartments before and after flood. Finally, we know very little about how long‐term flood acclimation and plant flood resilience are dependent on and condition the recolonisation of the plant by symbionts. All these research gaps deserve more attention if we are to study flooding stress from a holobiont perspective.
Figure 5.
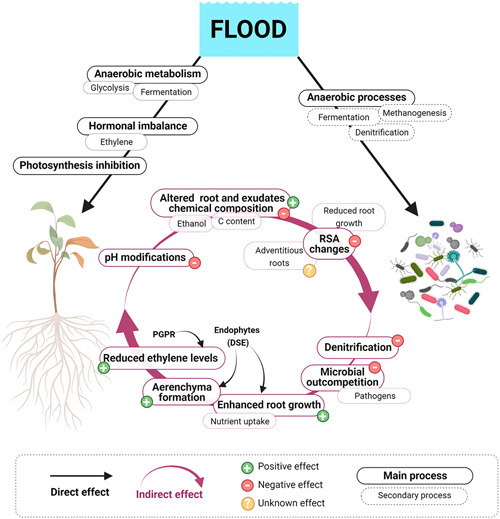
Main direct and indirect flood effects experienced by the plant and its associated microbiome. Direct effects of flooding on the plant and the plant microbiome are mainly driven by hypoxic soil conditions. Flooding changes the normal plant aerobic metabolism into a fermentative metabolism involving enhanced carbohydrate consumption through glycolysis. A hormonal imbalance due to increase ethylene synthesis also occurs, leading to different detrimental processes in the plants such as the inhibition of photosynthesis. Plant physiological and metabolic alterations drive the plant‐associated microbiome (including the rhizosphere and the endosphere microbiome). These changes include modifications in xylem sap pH, alterations on the chemical composition of root tissues or root exudates due to the production of ethanol during fermentation or changes on carbohydrate content, which usually lead to negative microbial recruitments. Nevertheless, the root exudation of specific chemical components may enhance the recruitment of certain microbes that counteract some negative stress effects (“cry‐for‐help” mechanism). Alterations on the root system architecture (RSA), e.g., reflected in reduced root growth, can alter the plant microbiome diversity; the development of new adventitious roots has an unknown effect on microbial composition. On the other hand, hypoxic soil conditions induced by flooding promote the enrichment of anaerobic microbes, which are usually linked with processes such as fermentation, methanogenesis or denitrification. The presence of these microorganisms produces indirect negative effects on the plant, such as soil denitrification or the outcompetition of certain microbial members by others with pathogenic behaviour. Nevertheless, the persistence of some microbes under stress conditions may have beneficial effects for the plant during the stress. For example, the establishment of endophytes such as the dark septate endophytes (DSE) or the endophytic fungi Phomopsis liquidambari may be involved in enhancing root growth, by improving nutrient uptake, or in stimulating the formation of aerenchyma. Other symbiotic microbes such as the plant‐growth promoting rhizobacteria (PGPR) are able to synthesise the enzyme ACC‐deaminase that cleaves ACC and reduces ethylene synthesis. Created with BioRender.com
ACKNOWLEDGEMENTS
This work was supported by the Spanish Ministry of Science and Innovation (PID2019‐107256RB‐I00) and the Swedish Research Council Formas (Grant Number 2016‐00907).
Martínez‐Arias, C. , Witzell, J. , Solla, A. , Martin, J. A. & Rodríguez‐Calcerrada, J. (2022) Beneficial and pathogenic plant‐microbe interactions during flooding stress. Plant, Cell & Environment, 45, 2875–2897. 10.1111/pce.14403
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abanda‐Nkpwatt, D. , Musch, M. , Tschiersch, J. , Boettner, M. & Schwab, W. (2006) Molecular interaction between Methylobacterium extorquens and seedlings: Growth promotion, methanol consumption, and localization of the methanol emission site. Journal of Experimental Botany, 57, 4025–4032. [DOI] [PubMed] [Google Scholar]
- Agostinelli, M. , Cleary, M. , Martín, J.A. , Albrectsen, B.R. & Witzell, J. (2018) Pedunculate Oaks (Quercus robur L.) Differing in vitality as reservoirs for fungal biodiversity. Frontiers in Microbiology, 9, 01758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, S. & Kim, W.C. (2018) Plant growth promotion under water: decrease of waterlogging‐induced ACC and ethylene levels by ACC deaminase‐producing bacteria. Frontiers in Plant Science, 9, 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. & Armstrong, W. (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany, 88, 1359–1370. [PubMed] [Google Scholar]
- Baar, L. , Bastiaans, T. , Coevering, M.V & R., J. (2002) Ectomycorrhizal root development in wet Alder carr forests in response to desiccation and eutrophication. Mycorrhiza, 12, 147–151. [DOI] [PubMed] [Google Scholar]
- Babalola, O.O. , Emmanuel, O.C. , Adeleke, B.S. , Odelade, K.A. , Nwachukwu, B.C. & Ayiti, O.E et al. (2021) Rhizosphere microbiome cooperations: strategies for sustainable crop production. Current Microbiology, 78, 1069–1085. [DOI] [PubMed] [Google Scholar]
- Badri, D.V. & Vivanco, J.M. (2009) Regulation and function of root exudates. Plant, Cell & Environment, 32, 666–681. [DOI] [PubMed] [Google Scholar]
- Bailey‐Serres, J. & Voesenek, L.A.C.J. (2008) Flooding stress: scclimations and genetic diversity. Annual Review Of Plant Biology, 59, 313–319. [DOI] [PubMed] [Google Scholar]
- Bal, H.B. & Adhya, T.K. (2021) Alleviation of submergence stress in rice seedlings by plant growth‐promoting rhizobacteria with ACC deaminase activity. Frontiers in Sustainable Food Systems, 5, 606158. [Google Scholar]
- Baldrian, P. (2016) Forest microbiome: diversity, complexity and dynamics. FEMS Microbiology Reviews, 41, 109–130. [DOI] [PubMed] [Google Scholar]
- Bálint, M. , Tiffin, P. , Hallström, B. , O'Hara, R.B. , Olson, M.S. & Fankhauser, J.D et al. (2013) Host genotype shapes the foliar fungal microbiome of balsam poplar (Populus balsamifera). PLoS One, 8, e53987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett, R.D. & Caruso, T. (2020) Soil microbial community responses to climate extremes: resistance, resilience and transitions to alternative states. Philosophical Transactions of the Royal Society, B: Biological Sciences, 375, 20190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnawal, D. , Bharti, N. , Maji, D. , Chanotiya, C.S. & Kalra, A. (2012) 1‐Aminocyclopropane‐1‐carboxylic acid (ACC) deaminase‐containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiology and Biochemistry, 58, 227–235. [DOI] [PubMed] [Google Scholar]
- Barnes, C.J. , van der Gast, C.J. , McNamara, N.P. , Rowe, R. & Bending, G.D. (2018) Extreme rainfall affects assembly of the root‐associated fungal community. New Phytologist, 220, 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow, J.R. (2003) Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza, 13, 239–247. [DOI] [PubMed] [Google Scholar]
- Berthelot, C. , Zegeye, A. , Gaber, D.A. , Chalot, M. , Franken, P. & Kovács, G.M. et al. (2020) Unravelling the role of melanin in Cd and Zn tolerance and accumulation of three dark septate endophytic species. Microorganisms, 8, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesbrock, J.A. & Hendrix, F.F. (1970) Influence of soil water and temperature on root necrosis of peach caused by Pythium spp. Phytopathology, 60, 880–882. [Google Scholar]
- Blagodatskaya, Е. & Kuzyakov, Y. (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biology and Fertility of Soils, 45, 115–131. [Google Scholar]
- Blaker, N.S. & McDonald, J.D. (1981) Predisposing effects of soil moisture extremes on the susceptibility of Rhododendron to Phytophthora Root and crown rot. Phytopathology, 71, 831. [Google Scholar]
- Blom, C.W.P.M. (1999) Adaptations to flooding stress: from plant community to molecule. Plant Biology, 1, 261–273. [Google Scholar]
- Blumenstein, K. , Albrectsen, B.R. , Martín, J.A. , Hultberg, M. , Sieber, T.N. & Helander, M et al. (2015) Nutritional niche overlap potentiates the use of endophytes in biocontrol of a tree disease. BioControl, 60, 655–667. [Google Scholar]
- Bohrer, K.E. , Friese, C.F. & Amon, J.P. (2004) Seasonal dynamics of arbuscular mycorrhizal fungi in differing wetland habitats. Mycorrhiza, 14, 329–337. [DOI] [PubMed] [Google Scholar]
- Bonito, G. , Reynolds, H. , Robeson, M.S. , Nelson, J. , Hodkinson, B.P. & Tuskan, G et al. (2014) Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Molecular Ecology, 23, 3356–3370. [DOI] [PubMed] [Google Scholar]
- Bowers, J.H. , Sonoda, R.M. & Mitchell, D.J. (1990) Path coefficient analysis of the effect of rainfall variables on the epidemiology of Phytophthora blight of pepper caused by Phytophthora capsici . Phytopathology, 80, 1439–1446. [Google Scholar]
- Bringel, F. & Couée, I. (2015) Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Frontiers in Microbiology, 06, 00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, G.T. & Mircetich, S.M. (1988) Effects of flood duration on the development of Phytophthora root and crown rots of apple. Phytopathology, 78, 846:851. [Google Scholar]
- Bulgarelli, D. , Rott, M. , Schlaeppi, K. , Ver Loren van Themaat, E. , Ahmadinejad, N. & Assenza, F. et al. (2012) Revealing structure and assembly cues for Arabidopsis root‐inhabiting bacterial microbiota. Nature, 488, 91–95. [DOI] [PubMed] [Google Scholar]
- Burgess, T. , McComb, J.A. , Colquhoun, I. & Hardy, G.E.S.S. (1999) Increased susceptibility of Eucalyptus marginata to stem infection by Phytophthora cinnamomi resulting from root hypoxia. Plant Pathology, 48, 797–806. [Google Scholar]
- Camarena‐Pozos, D.A. , Flores‐Núñez, V.M. , López, M.G. , López‐Bucio, J. & Partida‐Martínez, L.P. (2019) Smells from the desert: microbial volatiles that affect plant growth and development of native and non‐native plant species. Plant, Cell & Environment, 42, 1368–1380. [DOI] [PubMed] [Google Scholar]
- Cameron, J.N. & Carlle, M.J. (1978) Fatty acids, aldehydes and alcohols as attractants for zoospores of Phytophthora palmivora . Nature, 271, 448–449. [Google Scholar]
- Camisón, Á. , Ángela Martín, M. , Dorado, F.J. , Moreno, G. & Solla, A. (2020) Changes in carbohydrates induced by drought and waterlogging in Castanea sativa . Trees, 34, 579–591. [Google Scholar]
- Camy, C. , Delatour, C. , Caël, O. & Marçais, B. (2003) Inoculation of mature pedunculate oaks (Quercus robur) with the root rot fungus Collybia fusipes: relationships with tree vigour and soil factors. European Journal of Plant Pathology, 109, 545–553. [Google Scholar]
- Chaudhry, V. , Runge, P. , Sengupta, P. , Doehlemann, G. , Parker, J.E. & Kemen, E. (2021) Shaping the leaf microbiota: plant–microbe–microbe interactions. Journal of Experimental Botany, 72, 36–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan, A. , Saini, R. & Sharma, J.C. (2022) Plant growth promoting rhizobacteria and their biological properties for soil enrichment and growth promotion. Journal of Plant Nutrition, 45, 273–299. [Google Scholar]
- Cheng, H. , Liu, Y. , Jiang, Z.‐Y. & Wang, Y.‐S. (2020) Radial oxygen loss is correlated with nitrogen nutrition in mangroves. Tree Physiology, 40, 1548–1560. [DOI] [PubMed] [Google Scholar]
- Chialva, M. , Ghignone, S. , Cozzi, P. , Lazzari, B. , Bonfante, P. , Abbruscato, P , Lumini E. (2020) Water management and phenology influence the root‐associated rice field microbiota. FEMS Microbiology Ecology, 96, fiaa146. [DOI] [PubMed] [Google Scholar]
- Cho, Y. , Yoo, S. , Park, M.S. , Kim, J.S. , Kim, C.S. & Lim, Y.W. (2021) Ectomycorrhizal fungi associated with Pinus densiflora seedlings under flooding stress. Sustainability, 13, 4367. [Google Scholar]
- Chung, H. & Lee, Y.H. (2020) Hypoxia: a double‐edged sword during fungal pathogenesis? Frontiers in Microbiology, 11, 01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer, T.D. (2003) Long‐distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment, 26, 17–36. [Google Scholar]
- Colmer, T.D. , Kotula, L. , Malik, A.I. , Takahashi, H. , Konnerup, D. , Nakazono, M , Pedersen O. (2019) Rice acclimation to soil flooding: low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant, Cell & Environment, 42, 2183–2197. [DOI] [PubMed] [Google Scholar]
- Compant, S. , Samad, A. , Faist, H. & Sessitsch, A. (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. Journal of Advanced Research, 19, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, J.C. & Lefor, M.W. (1998) The mycorrhizal status of selected plant species from Connecticut wetlands and transition zones. Restoration Ecology, 6, 214–222. [Google Scholar]
- Corcobado, T. , Cubera, E. , Juárez, E. , Moreno, G. & Solla, A. (2014a) Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi . Agricultural and Forest Meteorology, 192–193, 1–8. [Google Scholar]
- Corcobado, T. , Cubera, E. , Moreno, G. & Solla, A. (2013) Quercus ilex forests are influenced by annual variations in water table, soil water deficit and fine root loss caused by Phytophthora cinnamomi . Agricultural and Forest Meteorology, 169, 92–99. [Google Scholar]
- Corcobado, T. , Moreno, G. , Azul, A.M. & Solla, A. (2015) Seasonal variations of ectomycorrhizal communities in declining Quercus ilex forests: Interactions with topography, tree health status and Phytophthora cinnamomi infections. Forestry, 88, 257–266. [Google Scholar]
- Corcobado, T. , Vivas, M. , Moreno, G. & Solla, A. (2014b) Ectomycorrhizal symbiosis in declining and non‐declining Quercus ilex trees infected with or free of Phytophthora cinnamomi . Forest Ecology and Management, 324, 72–80. [Google Scholar]
- Cui, H.L. , Duan, G.L. , Zhang, H. , Cheng, W. & Zhu, Y.G. (2019) Microbiota in non‐flooded and flooded rice culms. FEMS Microbiology Ecology, 95, fiz036. [DOI] [PubMed] [Google Scholar]
- Dangendorf, S. , Hay, C. , Calafat, F.M. , Marcos, M. , Piecuch, C.G. , Berk, K , Jensen J. (2019) Persistent acceleration in global sea‐level rise since the 1960s. Nature Climate Change, 9, 705–710. [Google Scholar]
- Drew, M.C. (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 223–250. [DOI] [PubMed] [Google Scholar]
- Duan, X. , Xu, F. , Qin, D. , Gao, T. , Shen, W. , Zuo, S , Yu B., Xu J., Peng Y., Dong J. (2019) Diversity and bioactivities of fungal endophytes from Distylium chinense, a rare waterlogging tolerant plant endemic to the Three Gorges Reservoir. BMC Microbiology, 19, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duniway, J.M. (1979) Water relations of water molds. Annual Review of Phytopathology, 17, 431–460. [Google Scholar]
- Eichmann, R. , Richards, L. & Schäfer, P. (2021) Hormones as go‐betweens in plant microbiome assembly. The Plant Journal, 105, 518–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiri, M. , Fukao, T. , Miyashita, T. & Shiono, K. (2021) A barrier to radial oxygen loss helps the root system cope with waterlogging‐induced hypoxia. Breeding Science, 71, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else, M.A. , Coupland, D. , Dutton, L. & Jackson, M.B. (2001) Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiologia Plantarum, 111, 46–54. [Google Scholar]
- Else, M.A. , Davies, W.J. , Malone, M. & Jackson, M.B. (1995) A negative hydraulic message from oxygen‐deficient roots of tomato plants? (Influence of soil flooding on leaf water potential, leaf expansion, and synchrony between stomatal conductance and root hydraulic conductivity). Plant Physiology, 109, 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else, M.A. , Janowiak, F. , Atkinson, C.J. & Jackson, M.B. (2009) Root signals and stomatal closure in relation to photosynthesis, chlorophyll a fluorescence and adventitious rooting of flooded tomato plants. Annals of Botany, 103, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero, V. & Mendoza, R. (2005) Seasonal variation of arbuscular mycorrhizal fungi in temperate grasslands along a wide hydrologic gradient. Mycorrhiza, 15, 291–299. [DOI] [PubMed] [Google Scholar]
- Etesami, H. , Mirseyed Hosseini, H. & Alikhani, H.A. (2014) Bacterial biosynthesis of 1‐aminocyclopropane‐1‐caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiology and Molecular Biology of Plants, 20, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faußer, A.C. , Hoppert, M. , Walther, P. & Kazda, M. (2012) Roots of the wetland plants Typha latifolia and Phragmites australis are inhabited by methanotrophic bacteria in biofilms. Flora—Morphology, Distribution, Functional Ecology of Plants, 207, 775–782. [Google Scholar]
- Ferrando, L. & Fernández Scavino, A. (2015) Strong shift in the diazotrophic endophytic bacterial community inhabiting rice (Oryza sativa) plants after flooding. FEMS Microbiology Ecology, 91, fiv104. [DOI] [PubMed] [Google Scholar]
- Filer T.H. Jr. (1975) Mycorrhizae and soil microflora in a green‐tree reservoir. Forest Science, 21, 36–39. [Google Scholar]
- Francioli, D. , Cid, G. , Kanukollu, S. , Ulrich, A. , Hajirezaei, M.‐R. & Kolb, S. (2021) Flooding causes dramatic compositional shifts and depletion of putative beneficial bacteria on the Spring wheat microbiota. Frontiers in Microbiology, 12, 773116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed, G. , Schlatter, D. , Paulitz, T. & Dugan, F. (2019) Mycological insights into wetland fungal communities: the mycobiome of Camassia in the Pacific Northwest. Phytobiomes Journal, 3, 286–299. [Google Scholar]
- Freeman, B.C. & Beattie, G.A. (2009) Bacterial growth restriction during host resistance to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis thaliana . Molecular Plant‐Microbe Interactions, 22, 857–867. [DOI] [PubMed] [Google Scholar]
- Fry, E.L. , Zhu, F. & Greenwood, B. (2020) Adapting to environmental change, Microbiomes of soils, plants and animals. Cambridge University Press. pp. 154–181 [Google Scholar]
- Fukao, T. , Barrera‐Figueroa, B.E. , Juntawong, P. & Peña‐Castro, J.M. (2019) Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Frontiers in Plant Science, 10, 00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber, D.A. , Berthelot, C. , Camehl, I. , Kovács, G.M. , Blaudez, D. & Franken, P. (2020) Salt stress tolerance of dark septate endophytes is independent of melanin accumulation. Frontiers in Microbiology, 11, 562931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo‐Castañeda, T. , Lynch, J.P. , Six, J. & Hartmann, M. (2022) Improving soil resource uptake by plants through capitalizing on synergies between root architecture and anatomy and root‐associated microorganisms. Frontiers in Plant Science, 13, 827369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalero, E. & Glick, B.R. (2015) Bacterial modulation of plant ethylene levels. Plant Physiology, 169, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, B. & Frenzel, P. (1998) Rice roots and CH4 oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biology and Biochemistry, 30, 1903–1916. [Google Scholar]
- Gilet, T. & Bourouiba, L. (2015) Fluid fragmentation shapes rain‐induced foliar disease transmission. Journal of the Royal Society Interface, 12, 20141092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, C.J. (1970) The flooding tolerance of woody species—a review. Forestry Abstracts, 31, 671–688. [Google Scholar]
- Glenz, C. , Schlaepfer, R. , Iorgulescu, I. & Kienast, F. (2006) Flooding tolerance of Central European tree and shrub species. Forest Ecology and Management, 235, 1–13. [Google Scholar]
- Goel, A.K. , Lundberg, D. , Torres, M.A. , Matthews, R. , Akimoto‐Tomiyama, C. , Farmer, L , Dangl J.L., Grant S.R. (2008) The Pseudomonas syringae type III effector HopAM1 enhances virulence on water‐stressed plants. Molecular Plant‐Microbe Interactions, 21, 361–370. [DOI] [PubMed] [Google Scholar]
- Graff, A. & Conrad, R. (2005) Impact of flooding on soil bacterial communities associated with poplar (Populus sp.) trees. FEMS Microbiology Ecology, 53, 401–415. [DOI] [PubMed] [Google Scholar]
- Grandaubert, J. , Dutheil, J.Y. & Stukenbrock, E.H. (2019) The genomic determinants of adaptive evolution in a fungal pathogen. Evolution Letters, 3, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway, H. , Armstrong, W. & Colmer, T.D. (2006) Conditions leading to high CO2 (>5 kPa) in waterlogged–flooded soils and possible effects on root growth and metabolism. Annals of Botany, 98, 9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichko, V.P. & Glick, B.R. (2001) Amelioration of flooding stress by ACC deaminase‐containing plant growth‐promoting bacteria. Plant Physiology and Biochemistry, 39, 11–17. [Google Scholar]
- Guerrieri, R. , Vanguelova, E.I. , Michalski, G. , Heaton, T.H.E. & Mencuccini, M. (2015) Isotopic evidence for the occurrence of biological nitrification and nitrogen deposition processing in forest canopies. Global Change Biology, 21, 4613–4626. [DOI] [PubMed] [Google Scholar]
- Hadacek, F. & Kraus, G.F. (2002) Plant root carbohydrates affect growth behaviour of endophytic microfungi. FEMS Microbiology Ecology, 41, 161–170. [DOI] [PubMed] [Google Scholar]
- Hamonts, K. , Clough, T.J. , Stewart, A. , Clinton, P.W. , Richardson, A.E. , Wakelin, S.A , O'Callaghan M., Condron L.M. (2013) Effect of nitrogen and waterlogging on denitrifier gene abundance, community structure and activity in the rhizosphere of wheat. FEMS Microbiology Ecology, 83, 568–584. [DOI] [PubMed] [Google Scholar]
- Hardham, A.R. (2001) The cell biology behind Phytophthora pathogenicity. Australasian Plant Pathology, 30, 91–98. [Google Scholar]
- Harner, M.J. , Opitz, N. , Geluso, K. , Tockner, K. & Rillig, M.C. (2011) Arbuscular mycorrhizal fungi on developing islands within a dynamic river floodplain: an investigation across successional gradients and soil depth. Aquatic Sciences, 73, 35–42. [Google Scholar]
- Hartman, K. & Tringe, S.G. (2019) Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochemical Journal, 476, 2705–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, A. , Schmid, M. , Tuinen, D. & , van Berg, G. (2009) Plant‐driven selection of microbes. Plant and Soil, 321, 235–257. [Google Scholar]
- Hartmond, U. , Schaesberg, N.V. , Graham, J.H. & Syvertsen, J.P. (1987) Salinity and flooding stress effects on mycorrhizal and non‐mycorrhizal citrus rootstock seedlings. Plant and Soil, 104, 37–43. [Google Scholar]
- Hashimoto, Y. & Higuchi, R. (2003) Ectomycorrhizal and arbuscular mycorrhizal colonization of two species of floodplain willows. Mycoscience, 44, 339–343. [Google Scholar]
- Hattori, Y. , Nagai, K. & Ashikari, M. (2011) Rice growth adapting to deepwater. Current Opinion in Plant Biology, 14, 100–105. [DOI] [PubMed] [Google Scholar]
- Henry, A. , Doucette, W. , Norton, J. & Bugbee, B. (2007) Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. Journal of Environmental Quality, 36, 904–912. [DOI] [PubMed] [Google Scholar]
- Herrmann, M. , Geesink, P. , Richter, R. & Küsel, K. (2021) Canopy position has a stronger effect than tree species identity on phyllosphere bacterial diversity in a floodplain hardwood forest. Microbial Ecology, 81, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie, S.E. & Grimm, N.B. (2020) Nature‐based approaches to managing climate change impacts in cities. Philosophical Transactions of the Royal Society, B: Biological Sciences, 375, 20190124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, F.C. , Chou, M.Y. , Chou, S.J. , Li, Y.R. , Peng, H.P. & Shih, M.C. (2013) Submergence confers immunity mediated by the WRKY22 transcription factor in Arabidopsis . The Plant Cell, 25, 2699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L.Y. , Li, D. , Sun, K. , Cao, W. , Fu, W.Q. , Zhang, W , Dai C.C. (2018) Mutualistic fungus Phomopsis liquidambari increases root aerenchyma formation through auxin‐mediated ethylene accumulation in rice (Oryza sativa L.). Plant Physiology and Biochemistry, 130, 367–376. [DOI] [PubMed] [Google Scholar]
- Iguchi, H. , Yurimoto, H. & Sakai, Y. (2015) Interactions of methylotrophs with plants and other heterotrophic bacteria. Microorganisms, 3, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio, J. , Pereira, P. , Carvalho, M. , Fonseca, Á. , Amaral‐Collaço, M.T. & Spencer‐Martins, I. (2002) Estimation and diversity of phylloplane mycobiota on selected plants in a Mediterranean‐type ecosystem in Portugal. Microbial Ecology, 44, 344–353. [DOI] [PubMed] [Google Scholar]
- Jackson, M.B. (2002) Long‐distance signalling from roots to shoots assessed: the flooding story. Journal of Experimental Botany, 53, 175–181. [DOI] [PubMed] [Google Scholar]
- Jacobs, K.A. , MacDonald, J.D. , Berry, A.M. & Costello, L.R. (1997) Rooting responses of three oak species to low oxygen stress, proceedings of a symposium on oak woodlands: Ecology, management and urban interface issues. Albany, CA: Gen. Tech. Rep. USDA Forest Service. pp. 91–100 [Google Scholar]
- Jaeger, C. , Gessler, A. , Biller, S. , Rennenberg, H. & Kreuzwieser, J. (2009) Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. Journal of Experimental Botany, 60, 4335–4345. [DOI] [PubMed] [Google Scholar]
- Jia, W. , Ma, M. , Chen, J. & Wu, S. (2021) Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. International Journal of Molecular Sciences, 22, 1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Y.J. , Kakuta, Y. , Sugawara, M. , Igarashi, T. , Oki, N. , Kisaki, M. , Shoji, T. , Kanetuna, Y. , Horita, T. , Matsui, H. & Honma, M. (1999) Synthesis and degradation of 1‐aminocyclopropane‐1‐carboxylic acid by Penicillium citrinum. Bioscience, Biotechnology, and Biochemistry, 63, 542–9. [DOI] [PubMed] [Google Scholar]
- Johnson, D. (2018) Water, water everywhere … but how does it affect the functional diversity of ectomycorrhizal fungi? New Phytologist, 220, 950–951. [DOI] [PubMed] [Google Scholar]
- Jones, D.L. , Nguyen, C. & Finlay, R.D. (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant and Soil, 321, 5–33. [Google Scholar]
- Jones, P. , Garcia, B.J. , Furches, A. , Tuskan, G.A. & Jacobson, D. (2019) Plant host‐associated mechanisms for microbial selection. Frontiers in Plant Science, 10, 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung, Y.S. , Ge, Z. & Buie, C.R. (2017) Bioaerosol generation by raindrops on soil. Nature Communications, 8, 14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman, A. , Kendon, E.J. , Chan, S.C. & Fowler, H.J. (2021) Quasi‐stationary intense rainstorms spread across Europe under Climate Change. Geophysical Research Letters, 48, e2020GL092361. [Google Scholar]
- Karlova, R. , Boer, D. , Hayes, S. & Testerink, C. (2021) Root plasticity under abiotic stress. Plant Physiology, 187, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst, J. , Gaster, J. , Wiley, E. & Landhäusser, S.M. (2017) Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiology, 37, 154–164. [DOI] [PubMed] [Google Scholar]
- Kaul, S. , Sharma, T., K. & Dhar, M. (2016) “Omics” tools for better understanding the plant–endophyte interactions. Frontiers in Plant Science, 7, 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavamura, V.N. , Mendes, R. , Bargaz, A. & Mauchline, T.H. (2021) Defining the wheat microbiome: towards microbiome‐facilitated crop production. Computational and Structural Biotechnology Journal, 19, 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley, J.E. (1980) Endomycorrhizae influence growth of Blackgum seedlings in flooded soils. American Journal of Botany, 67, 6–9. [Google Scholar]
- Kenerley, C.M. (1984) Effect of flooding on development of Phytophthora root rot in fraser fir seedlings. Phytopathology, 74, 401. [Google Scholar]
- Kirkpatrick, M.T. , Rupe, J.C. & Rothrock, C.S. (2006) Soybean response to flooded soil conditions and the association with soilborne plant pathogenic genera. Plant Disease, 90, 592–596. [DOI] [PubMed] [Google Scholar]
- Klymiuk, A.A. & Sikes, B.A. (2019) Suppression of root‐endogenous fungi in persistently inundated Typha roots. Mycologia, 111, 748–757. [DOI] [PubMed] [Google Scholar]
- Knief, C. , Ramette, A. , Frances, L. , Alonso‐Blanco, C. & Vorholt, J.A. (2010) Site and plant species are important determinants of the Methylobacterium community composition in the plant phyllosphere. The ISME journal, 4, 719–728. [DOI] [PubMed] [Google Scholar]
- Kohout, P. , Sýkorová, Z. , Ctvrtlíková, M. , Rydlová, J. , Suda, J. , Vohník, M , Sudová R. (2012) Surprising spectra of root‐associated fungi in submerged aquatic plants. FEMS Microbiology Ecology, 80, 216–235. [DOI] [PubMed] [Google Scholar]
- Koskella, B. , Meaden, S. , Crowther, W.J. , Leimu, R. & Metcalf, C.J.E. (2017) A signature of tree health? Shifts in the microbiome and the ecological drivers of horse chestnut bleeding canker disease. New Phytologist, 215, 737–746. [DOI] [PubMed] [Google Scholar]
- Kozlowski, T.T. (1997) Responses of woody plants to flooding and salinity. Tree Physiology, 17, 490. [Google Scholar]
- Kron, W. (2005) Flood risk = hazard • values • vulnerability. Water International, 30, 58–68. [Google Scholar]
- Kuan, T.L. & Erwin, D.C. (1980) Predisposition effect of water saturation of soil on Phytophthora root rot of Alfalfa. Phytopathology, 70, 981–986. [Google Scholar]
- Lamb, B.C. , Mandaokar, S. , Bahsoun, B. , Grishkan, I. & Nevo, E. (2008) Differences in spontaneous mutation frequencies as a function of environmental stress in soil fungi at ‘Evolution Canyon,’ Israel. Proceedings of the National Academy of Sciences, 105, 5792–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, J.A. & Lennon, J.T. (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proceedings of the National Academy of Sciences of the United States of America, 109, 14058–14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, J.A. , Lennon, J.T. & Heath, K.D. (2017) Trees harness the power of microbes to survive climate change. Proceedings of the National Academy of Sciences of the United States of America, 114, 11009–11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, M.K. , Arnold, A.E. & Johnson, N.C. (2013) Factors influencing communities of foliar fungal endophytes in riparian woody plants. Fungal Ecology, 6, 365–378. [Google Scholar]
- Li, H.Y. , Zhao, C.A. , Liu, C.J. & Xu, X.‐F. (2010) Endophytic fungi diversity of aquatic/riparian plants and their antifungal activity in vitro. The Journal of Microbiology, 48, 1–6. [DOI] [PubMed] [Google Scholar]
- Li, J. , McConkey, B.J. , Cheng, Z. , Guo, S. & Glick, B.R. (2013) Identification of plant growth‐promoting bacteria‐responsive proteins in cucumber roots under hypoxic stress using a proteomic approach. Journal of Proteomics, 84, 119–131. [DOI] [PubMed] [Google Scholar]
- Li, M. , López, R. , Venturas, M. , Pita, P. , Gordaliza, G.G. , Gil, L , Rodríguez‐Calcerrada J. (2015) Greater resistance to flooding of seedlings of Ulmus laevis than Ulmus minor is related to the maintenance of a more positive carbon balance. Trees, 29, 835–848. [Google Scholar]
- Limami, A.M. (2014) Adaptations of nitrogen metabolism to oxygen deprivation in plants, Low‐oxygen stress in plants. Vienna: Springer. pp. 209–221 [Google Scholar]
- Lin, X. , Zhu, D. & Lin, X. (2011) Effects of water management and organic fertilization with SRI crop practices on hybrid rice performance and rhizosphere dynamics. Paddy and Water Environment, 9, 33–39. [Google Scholar]
- Lindow, S.E. & Brandl, M.T. (2003) Microbiology of the phyllosphere. Applied and Environmental Microbiology, 69, 1875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow, S.E. & Leveau, J.H.J. (2002) Phyllosphere microbiology. Current Opinion in Biotechnology, 13, 238–243. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Brettell, L.E. , Qiu, Z. & Singh, B.K. (2020a) Microbiome‐mediated stress resistance in plants. Trends in Plant Science, 25, 733–743. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Li, J. , Carvalhais, L.C. , Percy, C.D. , Prakash Verma, J. & Schenk, P.M et al. (2021) Evidence for the plant recruitment of beneficial microbes to suppress soil‐borne pathogens. New Phytologist, 229, 2873–2885. [DOI] [PubMed] [Google Scholar]
- Liu, X. J. A. , Finley, B. K. , Mau, R. L. , Schwartz, E. , Dijkstra, P. & Bowker, M. A et al. (2020b) The soil priming effect: Consistent across ecosystems, elusive mechanisms. Soil Biology and Biochemistry, 140, 107617. [Google Scholar]
- Liu, Z. , Cheng, R. , Xiao, W. , Guo, Q. & Wang, N. (2014) Effect of off‐season flooding on growth, photosynthesis, carbohydrate partitioning, and nutrient uptake in Distylium chinense . PLoS One, 9, e107636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge, D. J. (1989) The influence of soil moisture and flooding on formation of VA‐endo‐ and ectomycorrhizae in Populus and Salix . Plant and Soil, 117, 243–253. [Google Scholar]
- Lorio, P.L. , Howe, V.K. & Martin, C.N. (1972) Loblolly pine rooting varies with microrelief on wet sites. Ecology, 53, 1134–1140. [Google Scholar]
- Lumibao, C.Y. , Kimbrough, E. , Formel, S. , Day, R.H. , From, A.S. , Conner, W.H , Krauss K.W., Van Bael S.A. (2020) Salinity, water level, and forest structure contribute to Baldcypress (Taxodium distichum) rhizosphere and endosphere community structure. Wetlands, 40, 2179–2188. [Google Scholar]
- Malajczuk, N. (1988) Interaction between Phytophthora cinnamomi zoospores and micro‐organisms on non‐mycorrhizal and ectomycorrhizal roots of Eucalyptus marginata . Transactions of the British Mycological Society, 90, 375–382. [Google Scholar]
- Mandyam, K. & Jumpponen, A. (2005) Seeking the elusive function of the root‐colonising dark septate endophytic fungi. Studies in Mycology, 79, 221–288. [Google Scholar]
- de Marins, J.F. & Carrenho, R. (2017) Arbuscular mycorrhizal fungi and dark septate fungi in plants associated with aquatic environments. Acta Botanica Brasilica, 31, 295–308. [Google Scholar]
- Martinez, M. , Arata, A.F. , Lázaro, L. , Stenglein, S.A. & Dinolfo, M.I. (2019) Effects of waterlogging stress on plant‐pathogen interaction between Fusarium poae and wheat/barley. Acta Scientiarum. Agronomy, 41, 42629. [Google Scholar]
- Martínez‐Arias, C. , Sobrino‐Plata, J. , MacAya‐Sanz, D. , Aguirre, N.M. , Collada, C. & Gil, L et al. (2020) Changes in plant function and root mycobiome caused by flood and drought in a riparian tree. Tree Physiology, 40, 886–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Arias, C. , Sobrino‐Plata, J. , Ormeño‐Moncalvillo, S. , Gil, L. , Rodríguez‐Calcerrada, J. & Martín, J.A. (2021) Endophyte inoculation enhances Ulmus minor resistance to Dutch elm disease. Fungal Ecology, 50, 101024. [Google Scholar]
- Marx, D.H. (1972) Ectomycorrhizae as biological deterrents to pathogenic root infections. Annual Review of Phytopathology, 10, 429–454. [DOI] [PubMed] [Google Scholar]
- Matsumura, A. , Horii, S. & Ishii, T. (2008) Observation of arbuscular mycorrhizal network system between trifoliate orange and some grasses under waterlogged conditions. ACTA Horticulturae, 773, 69–75. [Google Scholar]
- McDonald, K. (2002) Temporary hypoxia suppresses the oxidative burst and subsequent hypersensitive cell death in cells of tobacco and soybean challenged with zoospores of incompatible isolates of Phytophthora species. Physiological and Molecular Plant Pathology, 61, 133–140. [Google Scholar]
- McLachlan, D.H. , Kopischke, M. & Robatzek, S. (2014) Gate control: Guard cell regulation by microbial stress. New Phytologist, 203, 1049–1063. [DOI] [PubMed] [Google Scholar]
- McManmon, M. & Crawford, R.M.M. (1971) A metabolic theory of flooding tolerance: The significance of enzyme distribution and behaviour. New Phytologist, 70, 299–306. [Google Scholar]
- Melotto, M. , Underwood, W. & He, S.Y. (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annual Review of Phytopathology, 46, 101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto, M. , Underwood, W. , Koczan, J. , Nomura, K. & He, S.Y. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell, 126, 969–980. [DOI] [PubMed] [Google Scholar]
- Miller, S.P. (2000) Arbuscular mycorrhizal colonization of semi‐aquatic grasses along a wide hydrologic gradient. New Phytologist, 145, 145–155. [Google Scholar]
- Mittal, L. , Tayyeba, S. & Sinha, A.K. (2022) Finding a breather for Oryza sativa: Understanding hormone signalling pathways involved in rice plants to submergence stress. Plant, Cell & Environment, 45, 279–295. [DOI] [PubMed] [Google Scholar]
- Moche, M. , Gutknecht, J. , Schulz, E. , Langer, U. & Rinklebe, J. (2015) Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biology and Biochemistry, 90, 169–178. [Google Scholar]
- Moreno, G. , Obrador, J.J. , Cubera, E. & Dupraz, C. (2005) Fine root distribution in dehesas of central‐western Spain. Plant and Soil, 277, 153–162. [Google Scholar]
- Morris, C.E. & Kinkel, L. L. (2002) Fifty years of phyllosphere microbiology: Significant contributions to research in related fields, Phyllosphere Microbiology. St. Paul: APS. pp. 365–375 [Google Scholar]
- Moslemi, A. , Ades, P.K. , Groom, T. , Nicolas, M.E. & Taylor, P.W.J. (2018) Influence of waterlogging on growth of pyrethrum plants infected by the crown and root rot pathogens, Fusarium oxysporum, Fusarium avenaceum and Paraphoma vinacea . Australasian Plant Pathology, 47, 205–213. [Google Scholar]
- Mounier, E. , Hallet, S. , Cheneby, D. , Benizri, E. , Gruet, Y. , Nguyen, C. , Piutti, S. , Robin, C. , Slezack‐Deschaumes, S. , Martin‐Laurent, F. , Germon, J. C. & Philippot, L. (2004) Influence of maize mucilage on the diversity and activity of the denitrifying community. Environmental Microbiology, 6, 301–312. [DOI] [PubMed] [Google Scholar]
- Moyes, A.B. , Kueppers, L.M. , Pett‐Ridge, J. , Carper, D.L. , Vandehey, N. , O'Neil, J , Frank A.C. (2016) Evidence for foliar endophytic nitrogen fixation in a widely distributed subalpine conifer. New Phytologist, 210, 657–668. [DOI] [PubMed] [Google Scholar]
- Mu, X. & Chen, Y. (2021) The physiological response of photosynthesis to nitrogen deficiency. Plant Physiology and Biochemistry, 158, 76–82. [DOI] [PubMed] [Google Scholar]
- Müller, D.B. , Vogel, C. , Bai, Y. & Vorholt, J.A. (2016) The plant microbiota: systems‐level insights and perspectives. Annual Review of Genetics, 50, 211–234. [DOI] [PubMed] [Google Scholar]
- Nascimento, F.X. , Rossi, M.J. & Glick, B.R. (2018) Ethylene and 1‐Aminocyclopropane‐1‐carboxylate (ACC) in plant–bacterial interactions. Frontiers in Plant Science, 9, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neori, A. & Agami, M. (2017) The functioning of rhizosphere biota in wetlands—A review. Wetlands, 37, 615–633. [Google Scholar]
- Nerem, R.S. , Beckley, B.D. , Fasullo, J.T. , Hamlington, B.D. , Masters, D. & Mitchum, G.T. (2018) Climate‐change–driven accelerated sea‐level rise detected in the altimeter era. Proceedings of the National Academy of Sciences of the United States of America, 115, 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert, K. , Mendgen, K. , Brinkmann, H. & Wirsel, S.G.R. (2006) Only a few fungal species dominate highly diverse mycofloras associated with the common reed. Applied and Environmental Microbiology, 72, 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, C. (2009) Rhizodeposition of organic C by plant: Mechanisms and controls, sustainable agriculture. Dordrecht: Springer Netherlands. pp. 97–123 [Google Scholar]
- Niem, J. , Gundersen, B. & Inglis, D.A. (2013) Effects of soil flooding on the survival of two potato pathogens, Sclerotinia sclerotiorum and Verticillium dahliae . American Journal of Potato Research, 90, 578–590. [Google Scholar]
- Panchal, S. & Melotto, M. (2017) Stomate‐based defense and environmental cues. Plant Signaling & Behavior, 12, e1362517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster, N. , Barkai‐Golan, R. & Calderon, M. (1986) Control of T‐2 toxin production using atmospheric gases. Journal of Food Protection, 49, 615–617. [DOI] [PubMed] [Google Scholar]
- Pedersen, O. , Colmer, T.D. & Sand‐Jensen, K. (2013) Underwater photosynthesis of submerged plants—Recent advances and methods. Frontiers in Plant Science, 4, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett‐Ridge, J. & Firestone, M.K. (2005) Redox fluctuation structures microbial communities in a wet tropical soil. Applied and Environmental Microbiology, 71, 6998–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuke, A.D. , Gessler, A. , Trumbore, S. , Windt, C.W. , Homan, N. , Gerkema, E , VAN As H. (2015) Phloem flow and sugar transport in Ricinus communis L. is inhibited under anoxic conditions of shoot or roots. Plant, Cell & Environment, 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Planchet, E. , Lothier, J. & Limami, A. M. (2017) Hypoxic respiratory metabolism in plants: Reorchestration of nitrogen and carbon metabolisms, plant respiration: metabolix fluxes and carbon balance. Cham: Springer, pp. 209–226. [Google Scholar]
- Ponnamperuma, F.N. (1984) Effects of flooding on soils, flooding and plant Growth. Elsevier. pp. 9–45 [Google Scholar]
- Price, R.K. & Vojinovic, Z. (2008) Urban flood disaster management. Urban Water Journal, 5, 259–276. [Google Scholar]
- Purahong, W. , Sadubsarn, D. , Tanunchai, B. , Wahdan, S.F.M. , Sansupa, C. , Noll, M , Wu Y.T., Buscot F. (2019) First insights into the microbiome of a Mangrove tree reveal significant differences in taxonomic and functional composition among plant and soil compartments. Microorganisms, 7, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger, C.M. , Reding, M.E. , Schultz, P.B. & Oliver, J.B. (2013) Influence of flood‐stress on ambrosia beetle host‐selection and implications for their management in a changing climate. Agricultural and Forest Entomology, 15, 56–64. [Google Scholar]
- Rauf, M. , Awais, M. , Ud‐Din, A. , Ali, K. , Gul, H. , Rahman, M.M , Hamayun M., Arif M. (2021) Molecular mechanisms of the 1‐Aminocyclopropane‐1‐Carboxylic Acid (ACC) Deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Frontiers in Plant Science, 11, 2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanbakhsh, M. , Sasidharan, R. , Voesenek, L.A.C.J. , Kowalchuk, G.A. & Jousset, A. (2018) Microbial modulation of plant ethylene signaling: ecological and evolutionary consequences. Microbiome, 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, A.M. & Inouye, R.S. (2006) Effects of water‐level fluctuations on the arbuscular mycorrhizal colonization of Typha latifolia L. Aquatic Botany, 84, 210–2116. [Google Scholar]
- Reeksting, B.J. , Taylor, N.J. & van den Berg, N. (2014) Flooding and Phytophthora cinnamomi: effects on photosynthesis and chlorophyll fluorescence in shoots of non‐grafted Persea americana (Mill.) rootstocks differing in tolerance to Phytophthora root rot. South African Journal of Botany, 95, 40–53. [Google Scholar]
- Reid, C.P.P. & Bowen, G.D. (1979) Effects of soil moisture on V/A mycorriza formation and root development in Medicago , The soil‐root interface. London: Academic Press, pp. 211–219. [Google Scholar]
- Rentschler, J. , Salhab, M. & Jafino, B. 2021. Flood exposure and poverty in 188 countries. 10.21203/rs.3.rs-965657/v1 [DOI] [PMC free article] [PubMed]
- Rizaludin, M.S. , Stopnisek, N. , Raaijmakers, J.M. & Garbeva, P. (2021) The chemistry of stress: understanding the ‘Cry for Help’ of plant roots. Metabolites, 11, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Gamir, J. , Ancillo, G. , González‐Mas, M.C. , Primo‐Millo, E. , Iglesias, D.J. & Forner‐Giner, M.A. (2011) Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiology and Biochemistry, 49, 636–645. [DOI] [PubMed] [Google Scholar]
- Romeralo, C. , Martín‐García, J. , Martínez‐Álvarez, P. , Muñoz‐Adalia, E.J. , Gonçalves, D. R. , Torres, E , Witzell J., Diez J.J. (2022) Pine species determine fungal microbiome composition in a common garden experiment. Fungal Ecology, 56, 101137. [Google Scholar]
- Rumeau, D. , Maher, E.A. , Kelman, A. & Showalter, A.M. (1990) Extensin and Phenylalanine Ammonia‐Lyase gene expression altered in potato tubers in response to wounding, hypoxia, and Erwinia carotovora infection. Plant Physiology, 93, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar, A. , Rathore, P. , Ramteke, P.W. , Ramakrishna, W. , Reddy, M.S. & Pecoraro, L. (2021) Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms, 9, 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah, S. , Reed, S. , Jayachandran, K. , Dunn, C. & Fisher, J.B. (2006) The effect of repeated short‐term flooding on mycorrhizal survival in snap bean roots. HortScience, 41, 598–602. [Google Scholar]
- Saleem, M. , Law, A.D. , Sahib, M.R. , Pervaiz, Z.H. & Zhang, Q. (2018) Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere, 6, 47–51. [Google Scholar]
- Saleem, M. , Meckes, N. , Pervaiz, Z.H. & Traw, M.B. (2017) Microbial interactions in the phyllosphere increase plant performance under herbivore biotic stress. Frontiers in Microbiology, 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, M. , Cesanelli, I. , Diánez, F. , Sánchez‐Montesinos, B. & Moreno‐Gavíra, A. (2021) Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. Journal of Fungi, 7, 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, S. , Dey, A. , Kumar, V. , Batiha, G.E. , El‐Esawi, M.A. , Tomczyk, M , Ray P. (2021) Fungal endophyte: an interactive endosymbiont with the capability of modulating host physiology in myriad ways. Frontiers in Plant Science, 12, 701800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter, M. (2013) Root responses to flooding. Current Opinion in Plant Biology, 16, 282–6. [DOI] [PubMed] [Google Scholar]
- Savian, F. , Ginaldi, F. , Musetti, R. , Sandrin, N. , Tarquini, G. , Pagliari, L , Firrao G., Martini M., Ermacora P. (2020) Studies on the aetiology of kiwifruit decline: interaction between soil‐borne pathogens and waterlogging. Plant and Soil, 456, 113–128. [Google Scholar]
- Scherer, H.W. (1995) Larcher, W. Ökophysiologie der Pflanzen, Zeitschrift für Pflanzenernährung und Bodenkunde. Stuttgart: Ulmer Verlag, p. 207. [Google Scholar]
- Schindler, S. , O'Neill, F.H. , Biró, M. , Damm, C. , Gasso, V. & Kanka, R. et al. (2016) Multifunctional floodplain management and biodiversity effects: a knowledge synthesis for six European countries. Biodiversity and Conservation, 25, 1349–1382. [Google Scholar]
- Shukla, V. , Lombardi, L. , Iacopino, S. , Pencik, A. , Novak, O. , Perata, P , Giuntoli B., Licausi F. (2019) Endogenous hypoxia in lateral root primordia controls root architecture by antagonizing auxin signaling in Arabidopsis . Molecular Plant, 12, 538–551. [DOI] [PubMed] [Google Scholar]
- da Silva, W.L. , Yang, K.‐T. , Pettis, G.S. , Soares, N.R. , Giorno, R. & Clark, C.A. (2019) Flooding‐associated soft rot of sweetpotato storage roots caused by distinct Clostridium isolates. Plant Disease, 103, 3050–3056. [DOI] [PubMed] [Google Scholar]
- Smucker, A.J.M. & Erickson, A.E. (1987) Anaerobic stimulation of root exudates and disease of peas. Plant and Soil, 99, 423–433. [Google Scholar]
- Soltani, A.‐A. , Khavazi, K. , Asadi‐Rahmani, H. , Omidvari, M. , Abaszadeh Dahaji, P. & Mirhoseyni, H. (2010) Plant growth promoting characteristics in some Flavobacterium spp. isolated from soils of Iran. Journal of Agricultural Science, 2, 106. [Google Scholar]
- Steffens, D. , Hütsch, B.W. , Eschholz, T. , Lošák, T. & Schubert, S. (2005) Waterlogging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant, Soil and Environment, 51, 545–552. [Google Scholar]
- Stenström, E. (1991) The effects of flooding on the formation of ectomycorrhizae in Pinus sylvestris seedlings. Plant and Soil, 131, 247–250. [Google Scholar]
- Stolzy, L.H. , Letey, J. , Klotz, L.J. & Dewolfe, T.A. (1965) Soil aeration and root‐rotting fungi as factors in decay of Citrus feeder roots. Soil Science, 99, 403–406. [Google Scholar]
- Stone, B.W.G. , Weingarten, E.A. & Jackson, C.R. (2018) The role of the phyllosphere microbiome in pant health and function. Annual Plant Reviews online, 1, 1–24. [Google Scholar]
- Le Tacon, F. , Skinner, F.A. & Mosse, B. (1983) Spore germination and hyphal growth of a vesicular–arbuscular mycorrhizal fungus, Glomus mosseae (Gerdemann and Trappe), under decreased oxygen and increased carbon dioxide concentrations. Canadian Journal of Microbiology, 29, 1280–1285. [Google Scholar]
- Takahashi, H. , Yamauchi, T. , Colmer, T.D. & Nakazono, M. (2014) Aerenchyma formation in plants, low‐oxygen stress in plants. Vienna: Springer. pp. 247–265 [Google Scholar]
- Talbot, C.J. , Bennett, E.M. , Cassell, K. , Hanes, D.M. , Minor, E.C. , Paerl, H. et al. (2018) The impact of flooding on aquatic ecosystem services. Biogeochemistry, 141, 439–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, J.M. , Martin, F. , Kohler, A. , Henrissat, B. & Peay, K.G. (2015) Functional guild classification predicts the enzymatic role of fungi in litter and soil biogeochemistry. Soil Biology and Biochemistry, 88, 441–456. [Google Scholar]
- Tang, G. , Zhang, C. , Ju, Z. , Zheng, S. , Wen, Z. , Xu, S , Chen Y., Ma Z. (2018) The mitochondrial membrane protein FgLetm1 regulates mitochondrial integrity, production of endogenous reactive oxygen species and mycotoxin biosynthesis in Fusarium graminearum . Molecular Plant Pathology, 19, 1595–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo, L. , Suvi, T. , Jairus, T. , Ostonen, I. & Põlme, S. (2009) Revisiting ectomycorrhizal fungi of the genus Alnus: differential host specificity, diversity and determinants of the fungal community. New Phytologist, 182, 727–735. [DOI] [PubMed] [Google Scholar]
- Tewari, S. & Mishra, A. (2018) Flooding Stress in Plants and Approaches to Overcome, Plant Metabolites and Regulation Under Environmental Stress. Academic Press. pp. 355–366 [Google Scholar]
- Thomas, P.W. (2021) Ectomycorrhiza resilience and recovery to extreme flood events in Tuber aestivum and Quercus robur . Mycorrhiza, 31, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, B.Y. , Cao, Y. & Zhang, K.Q. (2015) Metagenomic insights into communities, functions of endophytes and their associates with infection by root‐knot nematode, Meloidogyne incognita, in tomato roots. Scientific Reports, 5, 17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tockner, K. & Stanford, J.A. (2002) Riverine flood plains: present state and future trends. Environmental Conservation, 29, 208–330. [Google Scholar]
- Tonkin, J.D. , Merritt, D.M. , Olden, J.D. , Reynolds, L.V. & Lytle, D.A. (2018) Flow regime alteration degrades ecological networks in riparian ecosystems. Nature Ecology and Evolution, 2, 86–93. [DOI] [PubMed] [Google Scholar]
- Trivedi, P. , Batista, B.D. , Bazany, K.E. & Singh, B.K. (2022) Plant‐microbiome interactions under a changing world: responses, consequences, and perspective. New Phytologist n/a, 234, 1951–1959. Available at 10.1111/nph.18016 [DOI] [PubMed] [Google Scholar]
- Turner, T.R. , James, E.K. & Poole, P.S. (2013) The plant microbiome. Genome Biology, 14, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, B.M. (2002) Molecular basis of recognition between Phytophthora pathogens and their hosts. Annual Review of Phytopathology, 40, 137–167. [DOI] [PubMed] [Google Scholar]
- Unestam, T. & Sun, Y.‐P. (1995) Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza, 5, 301–311. [Google Scholar]
- Unger, I.M. , Kennedy, A.C. & Muzika, R.‐M. (2009) Flooding effects on soil microbial communities. Applied Soil Ecology, 42, 1–8. [Google Scholar]
- Vacher, C. , Hampe, A. , Porté, A.J. , Sauer, U. , Compant, S. & Morris, C.E. (2016) The phyllosphere: microbial jungle at the plant‐climate interface. Annual Review of Ecology, Evolution, and Systematics, 47, 1–24. [Google Scholar]
- Vandenkoornhuyse, P. , Quaiser, A. , Duhamel, M. , Le Van, A. & Dufresne, A. (2015) The importance of the microbiome of the plant holobiont. New Phytologist, 206, 1196–1206. [DOI] [PubMed] [Google Scholar]
- Vishwanathan, K. , Zienkiewicz, K. , Liu, Y. , Janz, D. , Feussner, I. , Polle, A , Haney C.H. (2020) Ectomycorrhizal fungi induce systemic resistance against insects on a nonmycorrhizal plant in a CERK1‐dependent manner. New Phytologist, 228, 728–740. [DOI] [PubMed] [Google Scholar]
- Visser, E. J. W. , Voesenek, L. A. C. J. (2005) Acclimation to Soil Flooding–sensing and signal‐transduction. Plant and Soil, 274, 197–214. [Google Scholar]
- Vives‐Peris, V. , de Ollas, C. , Gómez‐Cadenas, A. & Pérez‐Clemente, R.M. (2020) Root exudates: from plant to rhizosphere and beyond. Plant Cell Reports, 39, 3–17. [DOI] [PubMed] [Google Scholar]
- Voesenek, L.A.C.J. & Bailey‐Serres, J. (2015) Flood adaptive traits and processes: an overview. New Phytologist, 206, 57–73. [DOI] [PubMed] [Google Scholar]
- Vorholt, J.A. (2012) Microbial life in the phyllosphere. Nature Reviews Microbiology, 10, 828–840. [DOI] [PubMed] [Google Scholar]
- Vos, J. , Putten, P.E.L. & , van der Birch, C.J. (2005) Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Research, 93, 64–73. [Google Scholar]
- Wang, Y. , Qiu, Q. , Yang, Z. , Hu, Z. , Tam, N.F.Y. & Xin, G. (2010) Arbuscular mycorrhizal fungi in two mangroves in South China. Plant and Soil, 331, 181–191. [Google Scholar]
- Wei, Z. & Jousset, A. (2017) Plant breeding goes microbial. Trends in Plant Science, 22, 555–558. [DOI] [PubMed] [Google Scholar]
- Weißhaupt, S. , Köhl, L. , Kunz, S. , Hinze, M. , Ernst, M. , Schmid, A , Voegele R.T. (2016) Alternative inoculum sources for fire blight: the potential role of fruit mummies and non‐host plants. Plant Pathology, 65, 470–483. [Google Scholar]
- Westra, S. , Fowler, H.J. , Evans, J.P. , Alexander, L.V. , Berg, P. & Johnson, F et al. (2014) Future changes to the intensity and frequency of short‐duration extreme rainfall. Reviews of Geophysics, 52, 522–555. [Google Scholar]
- Wilcox, W.F. (1985) Effects of flooding duration on the development of Phytophthora root and crown rots of cherry. Phytopathology, 75, 1451. [Google Scholar]
- Wilcox, W.F. (1993) Incidence and severity of crown and root rots on four apple rootstocks following exposure to Phytophthora species and waterlogging. Journal of the American Society for Horticultural Science, 118, 63–67. [Google Scholar]
- Wilcox, W.F. & Mircetich, S.M. (1985) Pathogenicity and relative virulence of seven Phytophthora spp. on Mahaleb and Mazzard cherry. Phytopathology, 75, 221–226. [Google Scholar]
- Wilkinson, S. (1999) pH as a stress signal. Plant Growth Regulation, 29, 87–89. [Google Scholar]
- Worms, I. , Simon, D.F. , Hassler, C.S. & Wilkinson, K.J. (2006) Bioavailability of trace metals to aquatic microorganisms: importance of chemical, biological and physical processes on biouptake. Biochimie, 88, 1721–1731. [DOI] [PubMed] [Google Scholar]
- Wu, Q.‐S. , Zou, Y.‐N. & Huang, Y.‐M. (2013) The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of waterlogging on growth, root system architecture and antioxidant enzyme activities of citrus seedlings. Fungal Ecology, 6, 37–43. [Google Scholar]
- Xin, X.F. , Nomura, K. , Aung, K. , Velásquez, A.C. , Yao, J. , Boutrot, F , Chang J.H., Zipfel C., He S.Y. (2016) Bacteria establish an aqueous living space in plants crucial for virulence. Nature, 539, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Y. , Gao, Y. , Liu, C. & Liu, S. (2020) A styrene antioxidant NFA from riparian endophytic fungi enhances flooding tolerance in Arabidopsis. Journal of Plant Interactions, 15, 111–116. [Google Scholar]
- Xue, Y. , Liu, C. , Bai, X. , Cheng, F. , Chen, J. & Liu, S. (2021) Antioxidant metabolites from riparian fungal endophytes improve the tolerance of rice seedlings to flooding. Chemoecology, 31, 277–287. [Google Scholar]
- Yamauchi, T. , Shimamura, S. , Nakazono, M. & Mochizuki, T. (2013) Aerenchyma formation in crop species: a review. Field Crops Research, 152, 8–16. [Google Scholar]
- Zhan, F. , He, Y. , Zu, Y. , Li, T. & Zhao, Z. (2011) Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila . World Journal of Microbiology and Biotechnology, 27, 2483–2489. [Google Scholar]
- Zhao, X. , Yuan, S. , Song, H. , Su, X. , Mao, H. & Shen, W et al. (2016) Arbuscular mycorrhizal and dark septate fungal associations in riparian plants of the Three Gorges Reservoir Region, Southwest China. Aquatic Botany, 133, 28–37. [Google Scholar]
- Zölch, T. , Henze, L. , Keilholz, P. & Pauleit, S. (2017) Regulating urban surface runoff through nature‐based solutions—an assessment at the micro‐scale. Environmental Research, 157, 135–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


