Abstract
Background
Patients with short‐bowel syndrome and intestinal failure (SBS‐IF) require parenteral support (PS) and experience various symptoms and comorbidities. This survey assessed the impact of SBS‐IF and PS on patients and their health‐related quality of life (HRQoL).
Methods
An online survey of adult patients who had a self‐reported clinician diagnosis of SBS‐IF and were receiving PS was conducted in France, Germany, Italy, the UK, and the USA. Patients reported symptoms, comorbidities, and treatment satisfaction; the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP) and the Home Parenteral Nutrition‐Quality of Life (HPN‐QoL) questionnaire assessed impact on work and HRQoL, respectively.
Results
Patients (N = 181; aged 52.0 ± 15.1 years; 56.9% women) experienced fatigue (75.1%), anemia (49.7%), and difficulty spending time with family (36.5%) and friends (30.4%). A total work productivity loss of 37.5% was calculated in patients reporting employment (29.3%). Patients typically (64.0%) reported some degree of satisfaction with their PS treatment. Almost two‐thirds (59.7%) reported that their PS was either “not,” “a little,” or “moderately” convenient. The mean HPN‐QoL scores were higher for patients who were satisfied with treatment (n = 116; 17.1 ± 21.0 [median, 16.7; interquartile range, 0.0–31.7]) than for patients who were dissatisfied/neither (n = 65; 1.7 ± 19.7 [median, 0.0; interquartile range, –13.3–13.3]).
Conclusions
Patients with SBS‐IF who are receiving PS experience burdensome symptoms and comorbidities and report impacts on work productivity and time spent with friends and family. This study can increase awareness of the impacts of SBS‐IF and PS and how treatment satisfaction may influence patients’ health and HRQoL.
Keywords: impact, intestinal failure, short‐bowel syndrome, WPAI:SHP
CLINICAL RELEVANCY STATEMENT
Patients with short‐bowel syndrome and intestinal failure (SBS‐IF) are dependent on parenteral support (PS). They experience a range of symptoms and comorbid conditions that impact interactions with friends and family as well as reducing their productivity and their ability to maintain full employment. Findings from this study also demonstrate that patients with SBS‐IF who are receiving PS experience fatigue and report reduced emotional and sexual function. Furthermore, healthcare providers should be aware that treatment satisfaction may influence patient health and health‐related quality of life. Although patients express high levels of satisfaction with PS, their lives are impacted by this chronic condition and by on‐going, life‐sustaining treatment with PS.
INTRODUCTION
Short‐bowel syndrome and intestinal failure (SBS‐IF) is a rare, chronic, life‐threatening condition with a reported prevalence ranging from 0.4 to 25.0 per million people across Europe and the USA. 1 , 2 , 3 , 4 , 5 By definition, patients with short‐bowel syndrome (SBS) have a remaining functional small‐bowel length of <200 cm. This may result from various causes, including extensive surgical resection due to Crohn's disease, mesenteric infarction, surgical complications, and abdominal trauma. 6 , 7 Following intestinal resection, if the absorptive capacity of the remaining intestine is not sufficient to provide the macronutrients, fluids, electrolytes, and minerals to sustain life, then the patient experiences intestinal failure (IF). 8 Patients with SBS and IF, therefore, require parenteral support (PS), which provides nutrition and hydration requirements intravenously. 8
Because of their SBS‐IF, patients may experience fatigue, diarrhea, abdominal pain, and dehydration. 3 , 4 , 9 , 10 PS, although lifesaving, is invasive and time‐consuming. 7 , 8 Furthermore, it is associated with various adverse events and potentially life‐threatening complications, such as catheter‐related issues, bacterial infections, blood clots, kidney disease, and liver problems. 11 , 12
SBS‐IF and treatment with PS can also have an impact on the lives of patients socially and emotionally, which can impair health‐related quality of life (HRQoL). 13 Impacts can include social restrictions, difficulty maintaining employment, and financial limitations. 10 , 14 , 15 In addition, reductions in PS volume and the number of days of PS required per week have both been associated with improvements in HRQoL for adult patients with SBS‐IF. 16 , 17
The objective of this study was to further characterize the disease burden of SBS‐IF in adult patients and the impact of treatment with PS on employment and HRQoL.
MATERIALS AND METHODS
An online, noninterventional, cross‐sectional survey of adult patients with SBS‐IF was conducted in France, Germany, Italy, the UK, and the USA. Data collection took place from January 2019 through July 2019.
Recruitment
Patients were recruited via patient advocacy organizations, healthcare providers (HCPs), online patient panels, social media outreach, and physician referrals.
Inclusion criteria
Patients were aged ≥18 years, had a self‐reported clinician diagnosis of SBS‐IF, and were currently receiving PS (for a minimum of 3 months).
Survey platform and consent
The web‐based survey was hosted on a web server, which was secured using a “Secure Sockets Layer” protocol. Potential participants were provided with links to the survey via email. To proceed with the survey, participants had to be eligible for the study and had to provide consent.
Instruments
Instruments used to assess the impact of SBS and PS on patients with SBS‐IF included the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI:SHP) and the Home Parenteral Nutrition‐Quality of Life (HPN‐QoL) questionnaire.
WPAI:SHP
The WPAI:SHP 18 is a six‐item self‐assessment instrument that assesses work and activity impairment during the past 7 days due to a specific health condition. In this study, patients responded based on the impact of SBS‐IF and PS. The instrument generates four scores that relate to absenteeism, presenteeism, work productivity, and activity impairment. The scores are given as percentages, with higher values indicating greater impairment and reduced work productivity. 19
HPN‐QoL
The HPN‐QoL 20 is a self‐assessment scale used to determine the impact that receiving PS has on patient quality of life. The instrument comprises 48 items divided across 20 subscales. Each subscale belongs to either a functional, symptom, or home parenteral nutrition (HPN)–specific scale. A raw score is calculated within each subscale by averaging the scores of all items included in the subscale. The scores (S) are normalized to a 100‐point scale by applying a linear transformation (S = [(RS − 1)/range] × 100). The range is the difference between the maximum and minimum possible values on the respective subscale. On the 0–100 scale, 50 represents the average score for an idealized general population, with a standard deviation (SD) of 10. For the functional and HPN scales, a higher score indicates a higher HRQoL or better functioning. For the symptom scales, a higher score indicates a more severe symptom or lower HRQoL.
Survey items assessing other impacts of SBS and PS
Additional items included in the survey addressed symptoms and comorbidities associated with SBS‐IF, as well as the impacts of SBS‐IF and PS on daily patient activities, relationships, emotions, employment, and productivity. The satisfaction, difficulties, and complications associated with receiving PS were also addressed.
Translation of instruments and survey items for the multinational survey
The translations were carried out by ICON Language Services. The HPN‐QoL and WPAI:SHP were translated from US English into French, German, and Italian. Survey questions were forward‐ and back‐translated into the target languages by independent translators and then proofread for accuracy and clarity by a professional linguist.
Ethical compliance
Ethical approval was obtained from Salus Institutional Review Board (IRB) and an IRB accredited by the Association for the Accreditation of Human Research Protection Programs. All data were collected in a manner that complies with the principles that have their origin in the Declaration of Helsinki. All participants provided consent through a web link before completing the survey.
Statistical analysis
Data were summarized with descriptive statistics (mean ± SD, median [interquartile range], or percentage).
RESULTS
Patient characteristics and recruitment
Patients (N = 181; mean age, 52.0 ± 15.1 years; 56.9% female) were predominantly recruited in Germany (29.3%, 53/181), followed by the US (24.9%, 45/181) and the UK (24.3%, 4/181); 59.7% (108/181) of respondents had a household income of <$50,000 or equivalent (Table 1). The etiologies associated with SBS‐IF included Crohn's disease, ulcerative colitis, and blocked blood vessels (mesenteric ischemia), reported by 18.2% (33/181), 3.3% (6/181), and 5.5% (10/181) of patients, respectively; most (72.9%, 132/181) were classified as “other” (Table S1). Patients had received their diagnosis of SBS a mean of 9.44 years ago and had been receiving PS for a mean of nearly 8 years (Table 1). Just under half of the patients (47.0%, 85/181) reported having a stoma, which they had lived with for an average of 14 years (Table S1).
Table 1.
Characteristics of patients who have short‐bowel syndrome and intestinal failure and are receiving PS.
| Characteristic | Overall (N = 181) |
|---|---|
| Age, mean (SD), years | 52.01 (15.08) |
| Sex, n (%) | |
| Male | 78 (43.1) |
| Female | 103 (56.9) |
| Country, n (%) | |
| France | 8 (4.4) |
| Germany | 53 (29.3) |
| Italy | 31 (17.1) |
| UK | 44 (24.3) |
| USA | 45 (24.9) |
| Employment status, n (%) | |
| Employed | 53 (29.3) |
| Unemployed | 115 (63.5) |
| Other | 13 (7.2) |
| <$50,000 or equivalent | 108 (59.7) |
| $50,000–$99,000 or equivalent | 26 (14.4) |
| ≥$100,000 or equivalent | 12 (6.6) |
| Time since diagnosis, mean (SD), years | 9.44 (10.9) |
| Time receiving PS, mean (SD), years | 7.95 (9.7) |
| Average volume of PS, mean (SD), L/week | 13.15 (7.7) |
| Average duration of PS administration, mean (SD), h | 9.59 (4.8) |
| Received additional fluids, n (%) | 111 (61.3) |
Abbreviations: PS, parenteral support; SD, standard deviation.
SBS comorbid conditions and symptoms
Patients reported the most common comorbid condition of SBS to be anemia (49.7%, 90/181) (Figure 1A). The most commonly reported SBS symptom was fatigue (75.1%, 136/181), followed by diarrhea (71.8%, 130/181) (Figure 1B).
Figure 1.
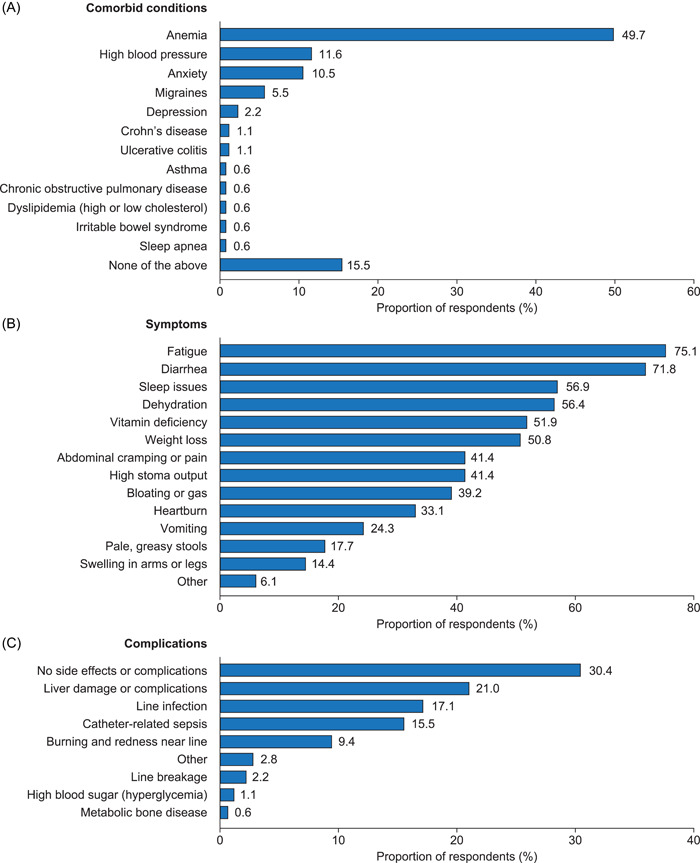
(A) Comorbid conditions, (B) symptoms, and (C) complications reported by patients who have short‐bowel syndrome and intestinal failure and are receiving parenteral support.
Impact of SBS on employment and work productivity
The most common life impacts of SBS‐IF reported by patients were difficulty advancing in their career (21.0%, 38/181) and an inability to work full‐time (19.9%, 36/181) (Table S2). Almost two‐thirds of patients (65.7%, 119/181) reported that they either stopped working completely or worked a reduced number of hours (44.8% [81/191] and 21.0% [38/181], respectively) (Figure S1A). Of those patients who reported being unemployed, over half (56.4%, 102/181) stated it was at least partially related to their SBS (35.9% [65/181] completely related and 20.4% [37/181] partially related) (Figure S1B).
53 of 181 patients (29.3%) reported being employed either full‐time or part‐time (Table 1). Of those, 90.6% (48 of 53) answered questions on absenteeism, and 84.9% (45 of 53) answered questions on presenteeism using the WPAI:SHP assessment. Patients were found to have missed a mean of 16.3% of work hours over the past week and were present but not productive for a mean of 32.0% of the time (Table 2). The mean total work productivity loss for the past week was calculated to be 37.5%. All patients (181 of 181) reported on activity impairment using the WPAI:SHP assessment (including patients who were unemployed and those who responded “other”); patients experienced activity impairment for a mean of 58.0% of the previous week.
Table 2.
Impact of short‐bowel syndrome and parenteral support on work and productivity.
| WPAI:SHP | Absenteeisma (n = 48) | Presenteeismb (n = 45) | Work productivity lossc (n = 45) | Activity impairmentd (N = 181) |
|---|---|---|---|---|
| Mean | 16.26 | 32.00 | 37.47 | 58.01 |
| Standard deviation | 27.48 | 25.01 | 28.26 | 26.80 |
| Median | 0.00 | 30.00 | 30.00 | 60.00 |
| Interquartile range | 0.00–18.83 | 10.00–50.00 | 10.00–61.03 | 40.00–80.00 |
Abbreviations: WPAI:SHP, Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
The percentage of hours of work reported as missed in the previous week.
The percentage of reported time present at work but not productive in the previous week.
The estimated percentage of productivity lost during the previous week.
The percentage of time that patients reported being impaired in their activities in the previous week.
Other impacts of SBS‐IF
Approximately one‐third of patients reported difficulty spending time with either family (36.5%, 66/181) or friends (30.4%, 55/181) because of their SBS‐IF. Reduced intimacy with their spouse/partner was reported by 7.2% (13/181) of patients (Table S2).
PS administration, complications/difficulties, and satisfaction
Patients typically received PS 7 days a week (59.7%, 108/181), with most receiving treatment at night (72.9%, 132/181) (Table S1). Patients received an average of 13.2 L/week, with each administration taking a mean time of 9.6 h (Table 1). The most common complication reported by patients was liver damage or complications (21.0%), followed by line infection (17.1%, 31/181), catheter‐related sepsis (15.5%, 28/181), and burning and redness near the line (9.4%, 17/181). Just under one‐third of patients (30.4%, 55/181) reported no side effects or complications (Figure 1C). Although nearly two‐thirds (64.1%, 116/181) of patients reported being satisfied to extremely satisfied with their PS treatment (Figures 2A), 59.7% (108/181) of patients reported their PS was either “not,” “a little,” or only “moderately” convenient (Figure 2B). Patients reported the greatest difficulty associated with PS treatment was schedule arrangement (60.2%, 109/181) (Figure S2).
Figure 2.
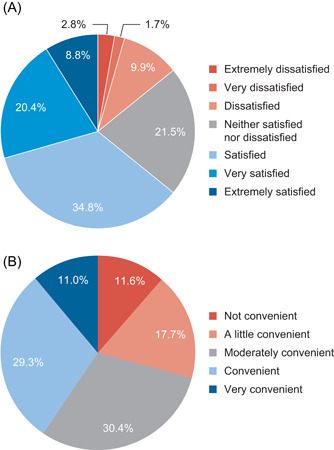
Level of (A) satisfaction with and (B) convenience of parenteral support reported by patients with short‐bowel syndrome and intestinal failure.
HPN‐QoL
HPN‐QoL scores were compared with an idealized general population mean score of 50. The HPN‐QoL functional subscale with the poorest mean patient‐reported score was emotional function, scoring >3 SDs below 50, followed by sexual function (>2 SDs below 50) (Table 3). The HPN‐QoL symptom subscale with the poorest mean patient‐reported score was fatigue (>1 SD above 50). General health of SBS‐IF patients was scored as higher than the mean for the representative general population (>1 SD above 50). Patients with SBS‐IF reported better gastrointestinal (GI) symptoms (2 SDs below 50) and a lower impact of issues related to pain (unrelated to GI symptoms), financial issues, and stoma care/bowel movements (each 1 SD below 50) compared with the reference population. Patients were asked questions about an ambulatory pump and its impact on their mobility, and 66.3% of patients answered these. Overall, these patients reported a mean score of 81.39, suggesting that they were more mobile owing to the presence of an ambulatory pump. Patients who were “extremely satisfied–satisfied” with treatment tended to have higher functional and HPN‐specific scores and lower symptom scores, indicating better HRQoL compared with patients who were “neither–extremely dissatisfied” (Table 3). The largest differences between scores based on patient levels of satisfaction with treatment were impact on weight, sleep pattern, coping, body image, and emotional function (Table 3). Patients with ambulatory pumps reported improved mobility; patients expressing some level of treatment satisfaction reported higher HRQoL than those who did not express treatment satisfaction (Table 3).
Table 3.
Patient‐reported HPN‐QoL scores.
| Scale | Extremely satisfied–satisfied (n = 116), mean (SD) | Neither–extremely dissatisfied (n = 65), mean (SD) | Overall (N = 181), mean (SD) |
|---|---|---|---|
| Functional scales (higher score = better functioning) | |||
| General health | 70.26 (22.58) | 60.00 (24.92) | 66.57 (23.90) |
| Ability to eat/drink | 56.90 (24.48) | 55.64 (22.30) | 56.45 (23.67) |
| Coping | 45.21 (23.11) | 32.31 (21.49) | 40.58 (23.32) |
| Physical function | 38.22 (21.79) | 33.33 (21.19) | 36.46 (21.65) |
| Employment | 36.35 (31.74) | 31.03 (30.03) | 34.44 (31.16) |
| Ability to holiday/travel | 30.93 (25.30) | 25.19 (23.85) | 28.87 (24.87) |
| Sexual function | 23.99 (26.77) | 23.85 (23.75) | 23.94 (25.66) |
| Emotional function | 23.92 (26.00) | 12.18 (19.82) | 19.71 (24.56) |
| Symptom scales (higher score = worse symptoms) | |||
| Fatigue | 58.48 (30.10) | 69.49 (25.78) | 62.43 (29.04) |
| Sleep pattern | 45.69 (32.46) | 60.00 (32.91) | 50.83 (33.25) |
| Weight | 42.53 (36.67) | 60.00 (32.38) | 48.80 (36.09) |
| Body image | 40.37 (26.57) | 52.82 (26.12) | 44.84 (27.01) |
| Immobility | 37.76 (26.17) | 44.51 (24.84) | 40.18 (25.83) |
| Presence of stoma or no stomab | 38.94 (22.02) | 41.20 (24.17) | 39.75 (22.78) |
| Financial issues | 36.49 (38.06) | 43.08 (37.60) | 38.86 (37.93) |
| Other painc | 35.34 (25.55) | 40.51 (29.61) | 37.20 (27.11) |
| Gastrointestinal symptoms | 28.54 (22.85) | 29.40 (23.03) | 28.85 (22.85) |
| HPN scales (higher score = better functioning) | |||
| Ambulatory pumpd | 87.08 (24.59) | 70.00 (32.73) | 81.39 (28.60) |
| Nutrition team | 68.10 (32.42) | 64.10 (25.21) | 66.67 (30.02) |
| HPN‐QoL numerical rating scalee | 17.10 (20.99) | 1.69 (19.67) | 11.57 (21.77) |
Abbreviations: HPN, home parenteral nutrition; HPN‐QoL, Home Parenteral Nutrition‐Quality of Life; SD, standard deviation.
aScores are standardized on a 0–100 scale; 50 represents the average score for the general population (SD = 10).
Questions related to stoma care or bowel movements.
Pain experienced by patients, other than pain related to gastrointestinal symptoms.
Of 181 patients, 120 answered questions about an ambulatory pump.
HPN‐QoL numerical rating scale raw scores were scaled from –33.3 to 66.6; higher score indicates higher quality of life.
DISCUSSION
This study shows that patients with SBS‐IF who are receiving PS experience a range of physical, social, and emotional impacts owing to their condition. As well as reporting fatigue and anemia, patients reported difficulty spending time with friends and family in addition to impaired work productivity and unemployment because of their condition. Patients also reported that PS had a negative impact on emotional and sexual function compared with that of the idealized reference population, despite reporting improved general health and GI symptoms (based on responses to the HPN‐QoL). Patients were also typically satisfied with their PS, although less than half of patients rated PS as being “convenient” or “very convenient.”
Several studies have explored the burden of SBS‐IF and receiving PS and their impact on patient quality of life. 10 , 13 , 21 , 22 , 23 A strength of the current study is the robust sample size (for a rare disease) and the geographically diverse sample, both facilitated by using an online survey.
The broad inclusion criteria ensure that the findings are generalizable across patients with SBS‐IF receiving PS in countries with comparable healthcare systems. Reported patient demographics and clinical characteristics, including the proportion of the population with a stoma and the average volume of PS administered per week, are consistent with what is seen in clinical practice. 24 , 25
Anemia and fatigue are well‐documented comorbidities/symptoms associated with SBS‐IF and PS. 10 , 26 , 27 Patients may have been more inclined to report symptoms such as fatigue and diarrhea because they are frequently asked by HCPs whether they are experiencing these symptoms. The impacts of SBS‐IF and PS reported by patients are similar to those experienced by patients with other chronic health conditions, including end‐stage renal disease (ESRD). 28 Patients with ESRD also experience fatigue, which may in part be a consequence of anemia or emotional difficulties, such as depression. 29 , 30 In addition, it has been recognized that patients with ESRD undergoing dialysis experience greater sexual dysfunction when compared with healthy individuals. 31
The low number of responses to the WPAI:SHP absenteeism and presenteeism questions reflects the impact SBS‐IF may have on patient employment; low levels of patient employment may have also contributed to the typical household income of patients with SBS‐IF being “<$50,000 or equivalent” (Table 1).
As well as having an impact on their careers, SBS‐IF makes it difficult for patients to spend time with friends and family. These negative impacts on patients' professional and personal lives can impair HRQoL. Factors that affect patient lives are often intertwined, so alleviating the strain of one impact of SBS‐IF and PS could go on to affect several other factors that affect patients. For example, supporting patients who are feeling emotionally distressed by providing counseling or medication (such as antidepressants) could alleviate fatigue (to some degree) and help patients to interact with family and friends, which could improve HRQoL. 32
The impact on caregivers supporting patients with SBS‐IF was recently reported. 19 Much like the patients in this study, caregivers reported difficulty with working full‐time and spending time with family and friends.
Most patients experienced some form of complication resulting from PS; the most commonly communicated were associated with the liver (liver damage or other complications), likely a consequence of biochemical disruption of liver function consequent to PS. 2 , 33 However, it should be noted that IF‐associated liver disease is multifactorial and that PS may not necessarily be the sole contributing factor. 34 In addition, the terminology used to classify complications could vary between different medical centers and by country. This could have led to some complications not being reported because the terms that patients may have been familiar with were not present in the survey options. Furthermore, patients may have had difficulty differentiating between closely related complications (such as line infection, catheter‐related sepsis, and burning and redness near the line), making interpreting the relative proportions of complications reported more challenging.
Patients were predominantly satisfied with their PS and felt that their SBS‐IF was typically well to very well managed. These high levels of satisfaction could be a consequence of the treatment being lifesaving; although the majority of patients reported high levels of satisfaction, most patients reported that PS was not convenient and that arranging a schedule was the greatest difficulty associated with PS. These reported difficulties associated with PS have also been recognized in previous studies. 35 , 36 , 37 It has also previously been acknowledged that reducing the number of nights or hours necessary for patients to receive PS can have a positive impact on their HRQoL. 10 , 16 , 38
The physical and social burdens experienced by patients with SBS‐IF receiving PS could have contributed to the lower emotional function reported using the HPN‐QoL. Mean reported scores of 3 SDs below the reference general population for emotional function, and approaching 3 SDs for sexual function, suggest considerable impairment is experienced by many patients with SBS‐IF in these domains. For sexual function, this result from the HPN‐QoL suggests a greater impact than that observed in the individual survey items on relationship impact, in which only 7.2% of patients reported a reduction in intimacy with their partner due to their condition. The difference in response between the survey and HPN‐QoL questionnaire could be a consequence of the HPN‐QoL asking patients to rate both interest in sex and extent of sexual activity in the past 4 weeks rather than simply asking whether they have experienced reduced intimacy because of their condition. Patient levels of intimacy with their partners may have already been low, possibly because of a health condition that preceded their SBS‐IF diagnosis. The negative impact of SBS‐IF on patient sexual intimacy has previously been recognized. 10
Consistent with the responses to symptom‐related questions, fatigue had the poorest (highest) mean HPN‐QoL symptom subscale score. This is also consistent with low emotional and sexual function scores on the HPN‐QoL. The remaining symptom scores were within 1 SD of, or lower than, the reference general population score of 50, suggesting that their experiences are similar to or better than those of the general population. Such scores suggest that the patients can manage their expectations of the impacts of SBS‐IF and PS on their lives. The scores generated on the HPN‐QoL also had large SDs, which likely reflects the heterogeneity in patient responses.
Overall, patients with SBS‐IF receiving PS reported better scores than the reference general population for general health and GI symptom subscales. This could be due to their symptoms and other impacts being less severe and more manageable than they were before treatment with PS. The severity of negative impacts associated with SBS‐IF and PS on HRQoL may also be lower in patients who have had time to adapt to their condition or who can identify the positive impacts of their treatment. 39 In Figure 3, we present a graphic that summarizes the breadth of responses that patients with SBS‐IF may contend with. This heterogeneity of experience, and time‐dependent adaptation of attitudes, appears to have been captured in HPN‐QoL responses (evidenced by the size of the SDs on the subscale scores). Overall, patients reporting that they were more satisfied with their treatment typically scored better on the HPN‐QoL than those who were less satisfied. This suggests that having a more negative perception of treatment could influence patients' ability to cope with their condition and could lead to poorer outcomes compared with those of patients who have a more positive outlook.
Figure 3.
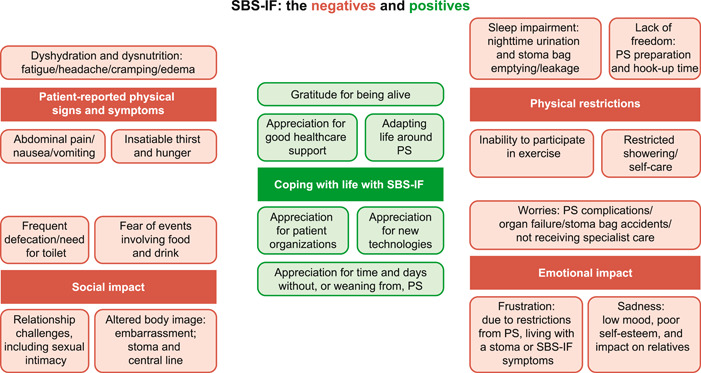
The potential impacts of short‐bowel syndrome and intestinal failure (SBS‐IF) on patient lives. 40 , 41 , 42 , 43 PS, parenteral support.
Limitations of the study
Patients directly reporting their symptoms and HRQoL is a strength of the study; however, the lack of cross‐validation with healthcare records is a limitation. There were few options for patients to provide free‐text responses to survey questions, so it is possible that granularity was lost from these responses. Selection bias may exist owing to the route of recruitment (in this case, referrals from patient associations, patient charities, patient panels, HCPs, or patient support groups). Using an online survey could have led to selection bias toward individuals with computer literacy and/or access to computers. Also, limited data were collected in France. Finally, willingness to participate in a survey may vary depending on disease severity and health status, leading to a potential for selection bias.
CONCLUSIONS
Patients with SBS‐IF who are receiving PS experience a range of symptoms and comorbidities, with the most common being fatigue and anemia, respectively; the most frequently reported complications were related to the liver, followed by line‐related infections. Patients also reported that their productivity and ability to maintain full‐time employment were impaired and that they experienced difficulty spending time with friends and family. In addition, many patients reported that PS was inconvenient and had a negative impact on emotional and sexual function. Patient satisfaction with PS is associated with better HPN‐QoL scores, which is indicative of higher HRQoL. Capturing information directly from patients with SBS‐IF receiving PS increases insights for HCPs and other stakeholders into the impacts of both SBS‐IF and PS on patients’ lives, including an awareness of how treatment satisfaction may influence patients’ health and quality of life.
AUTHOR CONTRIBUTIONS
Palle B. Jeppesen, Saeid Shahraz, Thomas Hopkins, Andrew Worsfold, and Elisabeth Genestin contributed to the conception and design of the research and the analysis and interpretation of the data. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
CONFLICTS OF INTEREST
Palle B. Jeppesen has received consultancy fees from Albumedix A/S; Protara Therapeutics; Baxter; Coloplast; Ferring Pharmaceuticals; Fresenius Kabi; GLyPharma Therapeutic; Naia Pharmaceuticals; Novo Nordisk Foundation; Shire, a Takeda company; Therachon; VectivBio AG; and Zealand Pharma. Saeid Shahraz is an employee of ICON plc, contracted by Takeda to conduct the survey. Thomas Hopkins and Elisabeth Genestin are employees of Takeda and stockholders of Takeda Pharmaceutical Company Limited. Andrew Worsfold is an employee of M‐Spective Limited, contracted by Takeda to provide evidence generation services.
FUNDING INFORMATION
This study was funded by Shire Human Genetic Therapies, Inc, a Takeda company, Cambridge, MA, USA, and was conducted by ICON plc. Editorial support was provided by Elizabeth Coe, PhD, and Richard Pye, PhD, of Oxford PharmaGenesis (Oxford, UK) and was funded by Takeda Development Center Americas, Inc.
Supporting information
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Jeppesen PB, Shahraz S, Hopkins T, Worsfold A, Genestin E. Impact of intestinal failure and parenteral support on adult patients with short‐bowel syndrome: A multinational, noninterventional, cross‐sectional survey. J Parenter Enteral Nutr. 2022;46:1650‐1659. 10.1002/jpen.2372
REFERENCES
- 1. Van Gossum A, Bakker H, De Francesco A, et al. Home parenteral nutrition in adults: a multicentre survey in Europe in 1993. Clin Nutr. 1996;15(2):53‐59. 10.1016/s0261-5614(96)80019-7 [DOI] [PubMed] [Google Scholar]
- 2. Buchman AL, Iyer K, Fryer J. Parenteral nutrition‐associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006;43(1):9‐19. 10.1002/hep.20997 [DOI] [PubMed] [Google Scholar]
- 3. Jeppesen PB. Short bowel syndrome–characterisation of an orphan condition with many phenotypes. Expert Opin on Orphan Drugs. 2013;1(7):515‐525. [Google Scholar]
- 4. Kelly DG, Tappenden KA, Winkler MF. Short bowel syndrome: highlights of patient management, quality of life, and survival. J Parenter Enteral Nutr. 2014;38(4):427‐437. 10.1177/0148607113512678 [DOI] [PubMed] [Google Scholar]
- 5. Brandt CF, Hvistendahl M, Naimi RM, et al. Home parenteral nutrition in adult patients with chronic intestinal failure: the evolution over 4 decades in a tertiary referral center. JPEN J Parenter Enteral Nutr. 2017;41(7):1178‐1187. 10.1177/0148607116655449 [DOI] [PubMed] [Google Scholar]
- 6. Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124(4):1111‐1134. 10.1016/s0016-5085(03)70064-x [DOI] [PubMed] [Google Scholar]
- 7. Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34(2):171‐180. 10.1016/j.clnu.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 8. Pironi L, Arends J, Bozzetti F, et al; Home Artificial Nutrition & Chronic Intestinal Failure Special Interest Group of ESPEN. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247‐307. 10.1016/j.clnu.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 9. Huisman‐de Waal G, Bazelmans E, van Achterberg T. Predicting fatigue in patients using home parenteral nutrition: a longitudinal study. Int J Behav Med. 2011;18(3):268‐276. 10.1007/s12529-010-9116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sowerbutts AM, Panter C, Dickie G, et al. Short bowel syndrome and the impact on patients and their families: a qualitative study. J Hum Nutr Diet. 2020;33(6):767‐774. 10.1111/jhn.12803 [DOI] [PubMed] [Google Scholar]
- 11. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo‐controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902‐914. 10.1136/gut.2010.218271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuglsang KA, Brandt CF, Eliasson J, Jeppesen PB. Mortality and outcomes in patients with non‐malignant short bowel syndrome receiving home parenteral support. Gastroenterology. 2019;156(Suppl 6):S‐689. 10.1016/S0016-5085(19)38643-3 [DOI] [Google Scholar]
- 13. Nordsten CB, Molsted S, Bangsgaard L, et al. High parenteral support volume is associated with reduced quality of life determined by the short‐bowel syndrome quality of life scale in nonmalignant intestinal failure patients. J Parenter Enteral Nutr. 2021;45(5):926‐932. 10.1002/jpen.1958 [DOI] [PubMed] [Google Scholar]
- 14. Ablett J, Vasant DH, Taylor M, Cawley C, Lal S. Poor social support and unemployment are associated with negative affect in home parenteral nutrition‐dependent patients with chronic intestinal failure. J Parenter Enteral Nutr. 2019;43(4):534‐539. 10.1002/jpen.1457 [DOI] [PubMed] [Google Scholar]
- 15. Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. J Parenter Enteral Nutr. 2014;38(1 Suppl):32S‐37S. 10.1177/0148607113517717 [DOI] [PubMed] [Google Scholar]
- 16. Jeppesen PB, Pertkiewicz M, Forbes A, et al. Quality of life in patients with short bowel syndrome treated with the new glucagon‐like peptide‐2 analogue teduglutide–analyses from a randomised, placebo‐controlled study. Clin Nutr. 2013;32(5):713‐721. 10.1016/j.clnu.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 17. Chen K, Mu F, Xie J, et al. Impact of teduglutide on quality of life among patients with short bowel syndrome and intestinal failure. J Parenter Enteral Nutr. 2020;44(1):119‐128. 10.1002/jpen.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353‐365. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 19. Jeppesen PB, Chen K, Murphy R, Shahraz S, Goodwin B. Impact on caregivers of adult patients receiving parenteral support for short bowel syndrome with intestinal failure: a multinational, cross‐sectional survey. J Parenter Enteral Nutr . Accepted manuscript. Published online August 8, 2021. 10.1002/jpen.2248 [DOI] [PMC free article] [PubMed]
- 20. Baxter JP, Fayers PM, McKinlay AW. The development and translation of a treatment‐specific quality of life questionnaire for adult patients on home parenteral nutrition. Eur J Clin Nutr Metab. 2008;3(1):e22‐e28. 10.1016/j.eclnm.2007.10.001 [DOI] [Google Scholar]
- 21. Blüthner E, Bednarsch J, Stockmann M, et al. Determinants of quality of life in patients with intestinal failure receiving long‐term parenteral nutrition using the SF‐36 questionnaire: a German single‐center prospective observational study. J Parenter Enteral Nutr. 2020;44(2):291‐300. 10.1002/jpen.1531 [DOI] [PubMed] [Google Scholar]
- 22. Winkler MF, Hagan E, Wetle T, Smith C, Maillet JO, Touger‐Decker R. An exploration of quality of life and the experience of living with home parenteral nutrition. J Parenter Enteral Nutr. 2010;34(4):395‐407. 10.1177/0148607110362582 [DOI] [PubMed] [Google Scholar]
- 23. Berghofer P, Fragkos KC, Baxter JP, et al. Development and validation of the disease‐specific Short Bowel Syndrome‐Quality of Life (SBS‐QoL) scale. Clin Nutr. 2013;32(5):789‐796. 10.1016/j.clnu.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 24. Allard JP, Genestin E, Gondolesi G, et al. A prospective, multicenter registry for patients with short bowel syndrome and intestinal failure (SBS‐IF Registry): interim effectiveness analysis of teduglutide treatment. Presented at: ASPEN Nutrition Science & Practice Conference 2021. Virtual. March 20–23; 2021.
- 25. Allard JP, Genestin E, Gondolesi G, et al. A prospective, multicenter registry for patients with short bowel syndrome and intestinal failure (SBS‐IF Registry): interim safety analysis of teduglutide treatment. ASPEN Nutrition Science & Practice Conference 2021. Virtual. March 20–23; 2021.
- 26. Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol. 2016;30(2):173‐185. 10.1016/j.bpg.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 27. Massironi S, Cavalcoli F, Rausa E, Invernizzi P, Braga M, Vecchi M. Understanding short bowel syndrome: current status and future perspectives. Dig Liver Dis. 2020;52(3):253‐261. 10.1016/j.dld.2019.11.013 [DOI] [PubMed] [Google Scholar]
- 28. NKF . National Kidney Foundation. Kidney Disease Quality Outcomes Initiative (K/DOQI). 2020. Accessed June 2, 2021. http://www.kidney.org/professionals/kdoqi/guidelines.cfm
- 29. Jhamb M, Liang K, Yabes J, et al. Prevalence and correlates of fatigue in chronic kidney disease and end‐stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol. 2013;38(6):489‐495. 10.1159/000356939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. KDOQI . KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl 3):S11‐S145. 10.1053/j.ajkd.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 31. Coelho‐Marques FZ, Wagner MB, Poli de Figueiredo CE, d'Avila DO. Quality of life and sexuality in chronic dialysis female patients. Int J Impot Res. 2006;18(6):539‐543. 10.1038/sj.ijir.3901470 [DOI] [PubMed] [Google Scholar]
- 32. Simon GE. Treating depression in patients with chronic disease: recognition and treatment are crucial; depression worsens the course of a chronic illness. West J Med. 2001;175(5):292‐293. 10.1136/ewjm.175.5.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pironi L, Boeykens K, Bozzetti F, et al. ESPEN guideline on home parenteral nutrition. Clin Nutr. 2020;39(6):1645‐1666. 10.1016/j.clnu.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 34. Lal S, Pironi L, Wanten G, et al. Home Artificial Nutrition & Chronic Intestinal Failure Special Interest Group of the European Society for Clinical Nutrition and Metabolism (ESPEN) . Clinical approach to the management of intestinal failure associated liver disease (IFALD) in adults: a position paper from the Home Artificial Nutrition and Chronic Intestinal Failure Special Interest Group of ESPEN. Clin Nutr. 2018;37(6 Pt A):1794‐1797. 10.1016/j.clnu.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 35. Dreesen M, Foulon V, Spriet I, et al. Epidemiology of catheter‐related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2013;32(1):16‐26. 10.1016/j.clnu.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 36. Dreesen M, Pironi L, Wanten G, et al. Outcome indicators for home parenteral nutrition care: point of view from adult patients with benign disease. J Parenter Enteral Nutr. 2015;39(7):828‐836. 10.1177/0148607114536926 [DOI] [PubMed] [Google Scholar]
- 37. Huisman‐de Waal G, Schoonhoven L, Jansen J, Wanten G, van Achterberg T. The impact of home parenteral nutrition on daily life‐a review. Clin Nutr. 2007;26(3):275‐288. 10.1016/j.clnu.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 38. Hu M. Evaluating the quality of life of adult patients on home parenteral nutrition in North East England and Cumbria. Clin Nutr ESPEN. 2017;22:116. 10.1016/j.clnesp.2017.07.006 [DOI] [Google Scholar]
- 39. Carr AJ, Gibson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322(7296):1240‐1243. 10.1136/bmj.322.7296.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Misiakos EP, Macheras A, Kapetanakis T, Liakakos T. Short bowel syndrome: current medical and surgical trends. J Clin Gastroenterol. 2007;41(1):5‐18. 10.1097/01.mcg.0000212617.74337.e9 [DOI] [PubMed] [Google Scholar]
- 41. Jeppesen PB, Langholz E, Mortensen PB. Quality of life in patients receiving home parenteral nutrition. Gut. 1999;44(6):844‐852. 10.1136/gut.44.6.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlsson E, Bosaeus I, Nordgren S. Quality of life and concerns in patients with short bowel syndrome. Clin Nutr. 2003;22(5):445‐452. 10.1016/s0261-5614(03)00042-6 [DOI] [PubMed] [Google Scholar]
- 43. Carlsson E, Berglund B, Nordgren S. Living with an ostomy and short bowel syndrome: practical aspects and impact on daily life. J Wound Ostomy Continence Nurs. 2001;28(2):96‐105. 10.1067/mjw.2001.113261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supplemental Data.
Supporting information.
Supporting information.
Supporting information.
Supporting information.


