Summary
Arbuscular mycorrhizal fungi (AMF) can help mitigate plant responses to water stress, but it is unclear whether AMF do so by indirect mechanisms, direct water transport to roots, or a combination of the two. Here, we investigated if and how the AMF Rhizophagus intraradices transported water to the host plant Avena barbata, wild oat.
We used two‐compartment microcosms, isotopically labeled water, and a fluorescent dye to directly track and quantify water transport by AMF across an air gap to host plants.
Plants grown with AMF that had access to a physically separated compartment containing 18O‐labeled water transpired almost twice as much as plants with AMF excluded from that compartment. Using an isotopic mixing model, we estimated that water transported by AMF across the air gap accounted for 34.6% of the water transpired by host plants. In addition, a fluorescent dye indicated that hyphae were able to transport some water via an extracytoplasmic pathway.
Our study provides direct evidence that AMF can act as extensions of the root system along the soil–plant–air continuum of water movement, with plant transpiration driving water flow along hyphae outside of the hyphal cell membrane.
Keywords: arbuscular mycorrhizal fungi, fluorescence microscopy, hyphal transport, nutrient transport, plant–microbe interactions, plant–water relations, Rhizophagus intraradices, stable isotopes
Introduction
Arbuscular mycorrhizal fungi (AMF) form symbiotic associations with 80% of surveyed land plant species and are well‐recognized for accessing and transferring nutrients to plants (Smith & Read, 2008). Yet, AMF also perform other essential functions, notably improving plant–water relations (Augé, 2001). Plants with AMF symbionts can have different rates of water movement into and out of roots, which affect tissue hydration and leaf physiology, and often lead to higher drought tolerance (Augé, 2001). Indeed, mycorrhizal plants typically have higher water contents than non‐mycorrhizal plants in the same environment (Faber et al., 1991) and have been shown to access soil water below the permanent wilting point of non‐mycorrhizal plants (Dakessian et al., 1986; Bethlenfalvay et al., 1988; Franson et al., 1991). The role of AMF in plant–water relations is most commonly attributed to indirect mechanisms such as enhancement of plant nutrition and osmoregulation in the host plants (Ruiz‐Lozano, 2003; Porcel & Ruiz‐Lozano, 2004; Augé et al., 2015; Mo et al., 2016). However, some evidence suggests AMF may directly transport water to plants (Faber et al., 1991; Ruiz‐Lozano & Azcón, 1995; Khalvati et al., 2005; Püschel et al., 2020). Overall, the relative contribution of direct and indirect AMF mechanisms to the amelioration of plant–water relations remains unclear.
Arbuscular mycorrhizal fung can improve plant–water relations via several indirect mechanisms (as reviewed by Augé, 2001). By enhancing plant nutrition, AMF not only improve plant health, which boosts plants’ resilience to environmental stresses, but also increase plants’ ability to osmoregulate via the production of nontoxic compatible solutes (Ruiz‐Lozano, 2003; Wu & Xia, 2006; Zulfiqar et al., 2020). In addition, AMF can reduce drought‐induced oxidative stress in their host plants (Porcel & Ruiz‐Lozano, 2004; Talbi et al., 2015), and help roots absorb more water by improving soil water retention properties and modulating root hydraulic conductivity (Aroca et al., 2007, 2009; Maurel et al., 2008; Querejeta, 2017; Bitterlich et al., 2018; Chen et al., 2018; Quiroga et al., 2019a,b). The mycelia of AMF improve soil structure and soil moisture characteristics, so plants with AMF can more efficiently deplete soil water (Augé et al., 2001; Querejeta, 2017; Bitterlich et al., 2018; Chen et al., 2018). Root hydraulic conductivity and symplastic flow also tend to increase in plants colonized by AMF, possibly through increased expression of root aquaporins, which allow plants to uptake more water (Aroca et al., 2007, 2009; Maurel et al., 2008; Ruiz‐Lozano et al., 2009; Li et al., 2013; Quiroga et al., 2019a,b). Indeed, non‐mycorrhizal roots have decreased levels of water permeability and cell hydraulic conductivity when water‐stressed, whereas mycorrhizal roots maintain the same levels as non‐water‐stressed counterparts (Quiroga et al., 2019a,b). AMF may also help regulate stomatal conductance in host plants, leading to 50% higher conductance rates during moderate drought and > 100% higher rates during severe drought compared to non‐mycorrhizal plant hosts (Kaschuk et al., 2009; Augé et al., 2015). By consuming plant‐fixed carbon (C), which amplifies the translocation of C out of leaves and reduces its concentration in the mesophyll, AMF also stimulate stomatal opening (Jarvis & Davies, 1998; Kaschuk et al., 2009).
Relatively little is known about direct mechanisms of water transport via AMF to plants. While investigating nutrient transport, Faber et al. (1991) discovered that mycorrhizal plants with intact hyphae transpired about 20% more than mycorrhizal plants with severed hyphae. In two experiments where AMF were allowed to access a separate compartment where roots had been excluded, the water content of the soil in the no‐plant compartment declined, and it was estimated that AMF contributed 4–20% of the water transpired by their host plants (Khalvati et al., 2005; Ruth et al., 2011). However, a more recent study using deuterated water found that although AMF transported some water to plants, the volume carried was low compared to the transpiration demand of the plants (Püschel et al., 2020). Thus, the ability of AMF to transport a significant volume of water to host plants remains ill‐defined.
The physical pathways of direct water movement from AMF hyphae to roots are also unknown. Water could be transferred via hyphae by travelling along the outside of the fungal cell wall or through the cell wall matrix itself (Allen, 2007). The composition of cell walls can differ between fungal genera, developmental stages and conditions, and includes different proportions of polysaccharides and glycoproteins, primarily chitin and glucan (as reviewed by Bowman & Free, 2006; Feofilova, 2010). The cross‐linking of polysaccharides and glycoproteins forms a complex network (Bago et al., 1996, 1998) that creates a space and surface outside the plasma membrane where water can travel (Allen, 2007). We refer to this external pathway as ‘extracytoplasmic’, in contrast to an internal ‘cytoplasmic’ pathway where transport occurs inside the fungal cell membrane. Arbuscular mycorrhizal fungi hyphae are coenocytic so the cytoplasm can stream long distances in the space within the plasma membrane of the hyphae without being slowed or stopped by septa (Jany & Pawlowska, 2010; Purin & Morton, 2011).
In order to resolve these knowledge gaps, we investigated if and how the AMF Rhizophagus intraradices transported water to the host plant Avena barbata, wild oat. In a glasshouse experiment, we used isotopically labeled water and a fluorescent dye to directly track and quantify water transport by AMF to plants. We specifically assessed whether AMF could access water in soil unavailable to roots and transport it across an air gap to their host plant. Finally, we estimated the relative contribution of direct and indirect AMF mechanisms to the improvement of plant–water relations.
Materials and Methods
Experimental set‐up
Avena barbata seeds gathered from the Hopland Research and Extension Center in Hopland, CA, were de‐husked and sterilized in chlorine gas for 4 h to kill any potential fungal pathogens on the surface and inside the seeds. Seeds were then germinated in Petri dishes on autoclaved filter paper and watered with autoclaved distilled water. The Petri dishes were placed in the dark at room temperature for 2 wk.
Three 2‐wk‐old A. barbata seedlings were planted in the ‘plant compartment’ of 18 two‐compartment microcosms (Fig. 1). The plant compartment was separated from the ‘no‐plant compartment’ by a 3.2‐mm air gap. The purpose of the air gap was to prevent liquid water from travelling via mass flow between compartments. Each side of the air gap had nylon mesh, either 18 μm, allowing hyphae but excluding roots, or 0.45 μm, excluding both hyphae and roots. A total of 18 microcosms were used, 12 with 18‐μm mesh and six with 0.45‐μm mesh. The microcosms were made of laser‐cut, 3.2‐mm‐thick acrylic panels glued together into single compartments; two compartments were screwed together tightly to make a microcosm (Supporting Information Fig. S1). The plant compartment was (W × D × H) 10 × 2.5 × 26.5 cm and packed up to 2 cm from the top with a sand‐clay mixture, 1 : 1 (v/v), to a 1.21 g cm−3 density (referred to herein as the ‘sand mix’). The no‐plant compartment was 10 × 0.75 × 26.5 cm and was packed up to 2 cm from the top with a soil‐sand mixture, 1 : 1 (v/v), to a 1.21 g cm−3 density (referred to as the ‘soil mix’). Sand mix was used instead of soil mix in the plant compartment to ensure that AMF would be the only fungal symbiont to colonize roots. Biologically intact soil can contain fungal pathogens; autoclaving soil would have killed pathogens but would have released nutrients, and we wanted to be able to control and limit nutrients in the plant compartment to encourage AMF colonization. We used soil mix in the no‐plant compartment so that hyphae would find nutrients there. The sand and clay were washed three times in distilled water, air‐dried, and then autoclaved three times 48 h apart. Soil (0–10 cm) was collected at the Hopland Research and Extension Center in Hopland, CA (lat. 38°59′35″N, long. 123°4′3″W) where A. barbata was the dominant vegetation type. The soil was then air‐dried and sieved to 2 mm to remove large rocks and roots. The microcosms were covered with acrylic black panels on the outside to prevent growth of moss and algae. The no‐plant compartment was covered at the top with AeraSeal® Films (Spectrum Chemicals, Gardena, CA, USA) to limit the dispersal of potential fungal pathogens from the soil mix in the no‐plant compartment to the plant compartment.
Fig. 1.
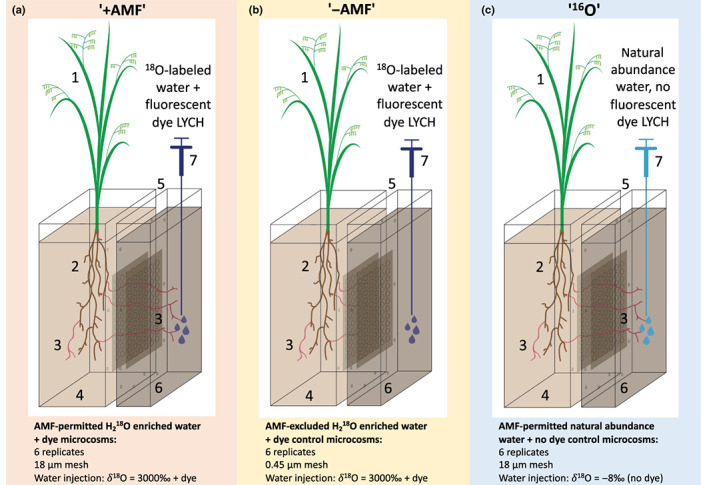
Experimental set‐up designed to test the movement of water to plants by arbuscular mycorrhizal fungi (AMF) hyphae. (a) AMF permitted 18O + dye microcosms (‘+AMF’) where AMF are able to access a no‐plant compartment, and 18O‐labeled water and fluorescent dye lucifer yellow carbohydrazide (LYCH) injected into the no‐plant compartment. (b) Arbuscular mycorrhizal fungi excluded 18O + dye microcosms (‘−AMF’) where AMF are not able to access the no‐plant compartment, and 18O‐labeled water and fluorescent dye LYCH injected into the no‐plant compartment. (c) Arbuscular mycorrhizal fungi permitted 16O + no dye microcosms (‘16O’) where AMF are able to access the no‐plant compartment, and unenriched water without a fluorescent dye is injected into the no‐plant compartment. In (a–c): 1, Avena barbata shoots; 2, A. barbata roots; 3, AMF Rhizophagus intraradices; 4, Plant compartment filled with ½ sand : ½ clay mixture; 5, 3.2 mm air gap; 6, No‐plant compartment filled with ½ soil : ½ sand mixture; 7, Syringe illustration of injection of solutions into the no‐plant compartment.
The AMF R. intraradices was chosen for this experiement because it naturally colonizes A. barbata roots at our annual grassland field site near Hopland, CA, where our A. barbata seeds were collected (Kakouridis, 2021). In the plant compartment, the sand mix was inoculated with 26 g of whole inoculum of R. intraradices (accession number AZ243, International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM), West Virginia University, Morgantown, WV, USA). Before packing, 20 g of the inoculum was mixed into the sand mix, and the remaining 6 g was poured in a layer 3 cm from the top of the sand mix. The inoculum was added in these two ways to ensure that roots would encounter AMF spores as they grew down through the inoculum layer and continue to have access to spores as they grew through the sand mix matrix. In addition, 78 mg of autoclaved bone meal (Jobe’s Organics, Bone Meal Organic Fertilizer, Waco, TX, USA) was mixed into the sand mix before packing to encourage AMF growth and establishment. In the no‐plant compartment, 78 mg of autoclaved bone meal was mixed into the soil mix before packing to act as a bait for AMF to cross the air gap. Data for total nitrogen (N), phosphorus (P) and iron (Fe) added to each compartment are given in Table S1.
The microcosms were incubated in growth chambers in the Environmental Plant Isotope Chamber (EPIC) facility, located in the Oxford Tract Greenhouse at UC Berkeley, where environmental conditions were monitored and controlled. Three chambers were used, with six microcosms in each, organized in a randomized fashion. Each microcosm was individually raised on two autoclaved metal bars to allow drainage and prevent water flow between microcosms. Chambers were thoroughly cleaned with a 10% bleach solution and a 70% ethanol solution before use. Each chamber had a fan with a cooling system to maintain temperature below 25°C. The fan also encouraged root growth as a consequence of the effect of simulated wind on shoots. Each compartment of the microcosms had three drain holes, 1 cm in diameter. Autoclaved glass wool was inserted into the drain holes to retain soil, and 18‐μm mesh was glued on the drain holes to prevent roots from growing out, while still allowing water to drain. Volumetric water content was monitored with electronic probes (EC‐5; Decagon Services, Pullman, WA, USA) that measure the dielectric constant of the media. In each chamber, two microcosms had soil moisture probes, and the other four microcosms were assumed to have the same volumetric water content. For all microcosms, plants were well‐watered for the first 3 wk, then watering volumes were adjusted as needed to maintain volumetric water content at c. 17% (mean water potential = −1.5 Mpa). The volume of water added to the plant compartment of microcosms were adjusted so that the growth medium had the same water potential in all treatments. It was only for the last 3 d of the experiment, when we stopped watering the plant compartments, that plants of different treatments experienced different water conditions. Both compartments of the microcosms were watered three times a week with autoclaved distilled water. After the distilled water, 10 ml of filter‐sterilized Rorison’s nutrient solution (Rorison & Rorison, 1987) was added to the plant compartment (low P) and no‐plant compartment (high P) once a week. The plant compartment of microcosms with 0.45‐μm mesh received twice as much nutrient solution as the plant compartment of microcosms with 18‐μm mesh, to make up for nutrients that plants could obtain in the no‐plant compartment via AMF in microcosms with 18‐μm mesh (this was accomplished by replacing 10 ml of distilled water by 10 ml of Rorison’s nutrient solution).
On day (D)7 of week 10, the plant compartments of all microcosms received their last watering. On D1 of week 11 at 22:00 h, 20 ml of water was injected using syringes with 15.2‐cm‐long spinal tap needles into the no‐plant compartment of all 18 microcosms (Fig. 1). Water labeled with 18O (δ18O 3000‰) and the fluorescent dye lucifer yellow carbohydrazide (LYCH, 0.01% w/v in water, MW 457) was used for six microcosms with 18‐μm mesh (these AMF‐permitted H2 18O enriched water + dye microcosms are hereafter referred to as ‘+AMF’), and six microcosms with 0.45‐μm mesh (these AMF‐excluded H2 18O enriched water + dye control microcosms are hereafter referred to as ‘−AMF’). The 18O‐labeled water and dye were added in order to trace the path of water from the no‐plant compartment, through fungal hyphae crossing the air gap, to the plant roots. Six microcosms with 18‐μm mesh received unenriched water that did not contain the fluorescent dye (these AMF‐permitted natural abundance water + no dye control microcosms are hereafter referred to as ‘16O’). The unenriched water used was laboratory distilled water at natural abundance δ18O that had been autoclaved three times for 30 min, 24 h apart, to ensure that it was free of fungal contaminants. The autoclaving process raised the δ18O value of the water from −12.23 ± 0.03‰ to −8.24 ± 0.03‰, but it remained in the natural abundance range. The 16O microcosms helped to establish a baseline for the 18O : 16O ratio in transpired water, identify natural isotope fractionation, and provide a control for autofluorescence in plant and fungal tissues. The LYCH dye has been used in previous studies to investigate the path of hydraulically lifted water from plants to the soil through their mycorrhizal networks (Querejeta et al., 2003; Egerton‐Warburton et al., 2007; Plamboeck et al., 2007), the reverse path of our experiment.
On D2, D3 and D4 of week 11 from 06:00 h to 22:00 h, a Ziploc® storage plastic bag (3.7 l capacity, 27 × 29.5 cm) was placed over the shoots of each microcosm to collect transpired water. At 22:00 h on each of the 3 d, the bags were carefully removed, sealed and placed on ice overnight to allow the water to condense. Then, the bags were weighed to measure the volume of water transpired and the water was pipetted into tubes for isotopic analysis. We collected water each day for 3 d because we did not know how long it would take for 18O‐labeled water to be transported by AMF across the air gap and to be detectable in transpired water.
Harvest and sample processing
On D5 of week 11, all microcosms were destructively sampled. Shoots were cut at the base and into 2.5‐cm pieces, dried at 60°C until a stable weight was reached, and weighed for aboveground biomass measurements. Roots were gently separated from surrounding soil and divided into several aliquots: (1) roots for staining with acid fuchsin were placed in distilled water, (2) roots for fluorescence microscopy were placed on wet Kimwipes in Petri dishes and kept in the dark, (3) roots for molecular analysis were placed in cell release buffer (Brodie et al., 2011), and (4) roots for belowground biomass measurements (all of the remaining roots) were placed in paper envelopes and dried at 60°C until a stable weight was reached.
The sand mix was collected and split into several aliquots: (1) sand mix for gravimetric water content was placed in 50‐ml Falcon tubes and stored at 4°C, then 10 g was weighed and oven‐dried at 105°C until a stable weight was reached; (2) sand mix for water extraction was placed in a whirlpack bag, flash frozen in liquid N2, and stored at −80°C; and (3) sand mix for nutrient measurements was placed in a whirlpack bag, flash frozen in liquid N2, and stored at −80°C.
The soil mix was also collected and split into several aliquots: (1) soil mix for gravimetric water content was placed in 50‐ml Falcon tubes and stored at 4°C, then 10 g was weighed and oven‐dried at 105°C until a stable weight was reached; (2) soil mix for molecular analysis was placed in a whirlpack bag, flash frozen in liquid N2, and stored at −80°C; (3) soil mix for water extraction was placed in a whirlpack bag, flash frozen in liquid N2, and stored at −80°C; (4) soil mix for nutrient measurements was placed in a whirlpack bag, flash frozen in liquid N2, and stored at −80°C; and (5) soil mix for spore and hyphae extraction was placed in 50‐ml Falcon tubes and stored at 4°C.
Hyphae evident in the air gap were collected on the mesh facing the inside of the air gap using tweezers and scalpels and placed into the first tube of the DNeasy PowerSoil kit (Qiagen LLC, Germantown, MD, USA) for DNA extraction on the same day.
Microscopy
Acid fuchsin
Roots were stained in acid fuchsin using a protocol modified from Habte & Osorio (2001). Roots were washed in distilled water, placed in a 10% KOH solution for 12 h, then rinsed in distilled water for 5 min. The roots then were placed in a 1% HCl solution for 12 h, followed by a 0.01% acid fuchsin solution (85% 1 M lactic acid, 5% glycerol in water) for 48 h. Finally, the roots were moved to a destaining solution (85% 1 M lactic acid, 5% glycerol in water) for 48 h. The stained roots were mounted on slides using the destaining solution and observed under both bright field and fluorescence with excitation/emission peaks of 596/615 nm (Habte & Osorio, 2001).
Fluorescent dye LYCH
For each microcosm, five 1‐cm root segments were mounted on slides using a 50% glycerol solution in water. Fluorescence microscopy was conducted on the day of the harvest at the Biological Imaging Facility at UC Berkeley. Lucifer yellow carbohydrazide has excitation/emission peaks of 428/536 nm (Oparka, 1991; Querejeta et al., 2003; Egerton‐Warburton et al., 2007; Plamboeck et al., 2007).
Molecular methods
For each microcosm, roots were washed twice in cell release buffer (Brodie et al., 2011) to remove microbial cells from their surface. Roots then were centrifuged to remove excess buffer, and ground in a tissue lyser with tungsten beads at 30 rpm for 20 min. DNA was extracted from 50 mg of ground roots for each microcosm using the DNeasy PowerPlant Pro Kit (Qiagen LLC).
In order to extract AMF spores and hyphae from the soil mix, 4 g of homogenized soil mix were mixed with 50 ml of distilled water and 6 ml of hexametaphosphate solution (35 gl−1 in water) and stirred for 30 min using a magnetic stirrer. The mixture was decanted through a 35‐μm sieve and the spores and hyphae caught in the sieve were collected. This process was repeated twice for each microcosm to obtain enough spores and hyphae. DNA was extracted separately from spores and hyphae from the sand mix, and from all the air gap hyphae collected for each microcosm using the DNeasy PowerSoil kit (Qiagen LLC).
DNA extracted from roots, soil mix spores and hyphae, and air gap hyphae was quantified by Quant‐iT™ PicoGreen™ dsDNA Assay Kit (Invitrogen) and the concentrations were normalized. PCR was conducted on the normalized DNA samples using forward universal eukaryotic primer WANDA (Dumbrell et al., 2011) and reverse AMF‐specific primer AML2 (Lee et al., 2008). This primer pair spans a variable 530‐bp region in the SSU rRNA gene (Egan et al., 2018). The PCR products were run on a gel to confirm the presence of DNA at 530 bp, then sequenced by Sanger sequencing at the UC Berkeley DNA Sequencing Facility. Sequencing results were compared to the MaarjAM database (Öpik et al., 2010) using the nucleotide Blast function to confirm the presence of R. intraradices in roots, air gap and soil mix. A sequence was considered a match for R. intraradices if query coverage and percentage identity were both > 97%.
Isotopic analyses
Analyses of transpired water and water extracted from the soil mix and the sand mix were conducted at the Center for Stable Isotope Biogeochemistry (CSIB) at UC Berkeley. The stable oxygen isotope composition of transpired water samples was determined by Isotope Ratio Infrared Spectroscopy (IRIS), using a L2140‐I (Picarro Inc., Santa Clara, CA, USA) analyzer. Long‐term external precision is ± 0.3‰.
Soil mix and sand mix water was extracted using a vacuum evaporation system and liquid nitrogen condensation trap. Stable oxygen isotope composition of soil mix and sand mix water extracts was measured by continuous flow (CF) using a Thermo Gas Bench II interfaced to a Thermo Delta V Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) using a CO2‐H2O equilibration method. In brief, 200 μl of water for both laboratory water standards and samples were pipetted into 10‐ml glass vials (Exetainer®; Labco Ltd, Lampeter, UK) and quickly sealed. The vials then were purged with 0.2% CO2 in helium and allowed to equilibrate at RT for 48 h. The 18O:16O value of CO2 then was determined; long‐term external precision is ± 0.12‰.
18O calculations
Detailed calculations and associated assumptions are explained in Methods S1 and the data used to perform these calculations are presented in Table S1. Calculations of the volume of water transported by AMF across the air gap and taken up by roots were performed using the following standard isotope mixing model (after Hayes, 2004):
(V K, volume of water from the no‐plant compartment transpired by plants (ml); V T, total volume of water transpired by plants (m); F T, 18O value of transpired water (atom% 18O); F I, 18O value of water in the sand mix in plant compartment (atom% 18O); and F K, 18O value of water in the soil mix in no‐plant compartment (atom% 18O)).
We performed these calculations for −AMF microcosms to determine the volume of 18O‐labeled water that crossed the air gap as a result of liquid or vapor diffusion and not hyphal transport. We then subtracted this value from the 18O‐labeled water that crossed the air gap in +AMF microcosms to obtain the amount of AMF‐transported water.
We used four assumptions to perform these calculations: (1) the same volume of water crossed the air gap via liquid or vapor diffusion (i.e. not via AMF hyphae) from the no‐plant compartment to the plant compartment in all microcosms; (2) for +AMF and −AMF microcosms, the 18O content of the water in the soil mix (no‐plant compartment) was the same at t = 0 and harvest, 300.75‰ δ18O or 0.2601 atom% 18O on average; (3) water crossing the air gap from the no‐plant compartment to the plant compartment had the 18O content of water in the no‐plant compartment, 300.75‰ δ18O or 0.2601 atom% 18O; and (4) the 18O content of the water in the sand mix in the plant compartment between t = 0 and harvest (t = 3.5) was the same for all microcosms, −4.89‰ δ18O or 0.1991 atom% 18O on average.
Statistical analyses
Statisticalanalyses were conducted using R v.3.6.1 (R Core Team, 2019). A one‐way ANOVA coupled with Fisher’s least‐significant difference (LSD) test (agricolae package) was used to differentiate means of 18O content, volume of water transpired, K1 and K2, gravimetric water content, above‐ and belowground biomass mass, %C, %N, C : N and %P in shoots from different treatment groups. Means ± SE are averages of six microcosms per treatment, except for transpired water volumes and 18O contents that are averages of 18 samples per treatment (six microcosms × three individual days of transpired water collection). Means ± SE are reported in the main text and Methods S1, except where we report 95% confidence interval (CI) instead of SE, which we note after the means.
Results
Rhizophagus intraradices colonized roots and extended hyphae into the no‐plant compartment
We confirmed that roots of all microcosms were colonized by R. intraradices by sequencing. Sequences from the root samples of all microcosms were a match for R. intraradices with query coverage and percentage identity > 97%. Sequences from air gap hyphae and soil mix (no‐plant compartment) samples from +AMF and 16O microcosms also were a match for R. intraradices with query coverage and percent identity > 97%. Sequencing of soil mix (no‐plant compartment) samples from −AMF microcosms did not detect AMF.
Using light and fluorescence microscopy, we confirmed that R. intraradices was active in roots by staining them with acid fuchsin and observing hyphae, spores and arbuscules (Figs 2a–d, S2). This verifies that R. intraradices was growing in roots over the course of the experiment and the plant–AMF interaction was functional (Bago et al., 1998).
Fig. 2.
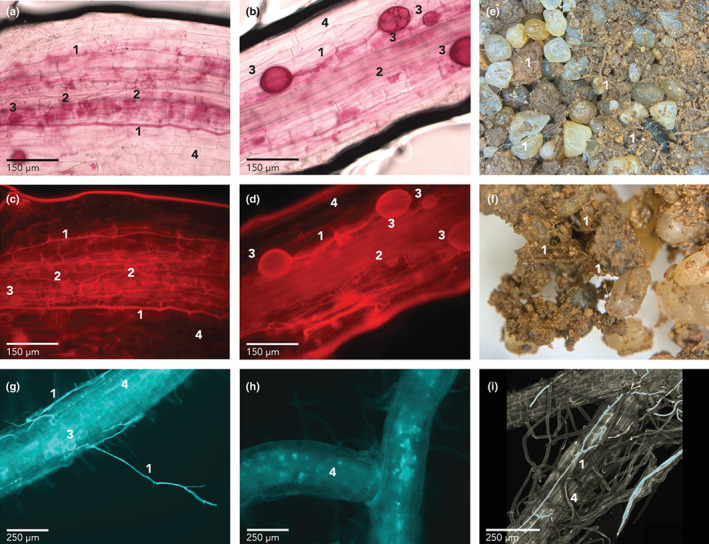
(a–d) Avena barbata roots dyed with acid fuchsin showing arbuscular mycorrhizal fungi (AMF) structures. (a, b) Bright field micrographs. (c, d) Fluorescence images at AMF wavelengths (λex 596 nm; λem 615 nm). (e, f) Soil–sand mixture from the no‐plant compartment of a +AMF microcosm with numerous AMF hyphae visible under a dissecting microscope. (g–i) Fluorescence micrographs of roots at lucifer yellow carbohydrazide (LYCH) wavelengths (λex 428 nm; λem 536 nm). (g) Root from a +AMF microcosm with hyphae and vesicles visible in blue. (h) Root autofluorescence from a 16O control microcosm in which hyphae and vesicles are not visible. (i) Reconstituted 3D model from confocal images of a root from a +AMF microcosm; fluorescing tissues are blue, nonfluorescing tissues are gray. In (a–i): 1, hypha; 2, arbuscule; 3, vesicle; 4, root.
We visually observed hyphae crossing the air gap, and also observed extensive hyphal networks in the soil mix of the no‐plant compartment of +AMF and 16O microcosms (Fig. 2e,f). In −AMF microcosms, we did not observe hyphae crossing the air gap nor hyphal networks in the soil mix.
+AMF plants transpired more than −AMF plants
We found that +AMF plants transpired almost twice as much water as −AMF plants, 7.67 ± 1.10 ml vs 4.03 ± 0.52 ml, respectively, over 3 d (P < 0.05) (Fig. 3a; Table S1). The gravimetric water content (and water potential) in the plant and no‐plant compartments, the mass of aboveground biomass (a proxy for leaf area index), and the root : shoot ratios were not significantly different among treatments (Table S1). We observed no difference in C : N and %N of the plant shoots between treatments. −AMF plants had a significantly lower P content than +AMF plants (P < 0.05; Table S1), but no significant differences in P and N contents were found between +AMF and 16O plants. The N : P ratio was c. 3 for +AMF and 16O plants and c. 6 for −AMF plants. In all treatments, general plant stunting and tissue nutrient content (and N : P) suggested that plant biomass was limited by N availability.
Fig. 3.
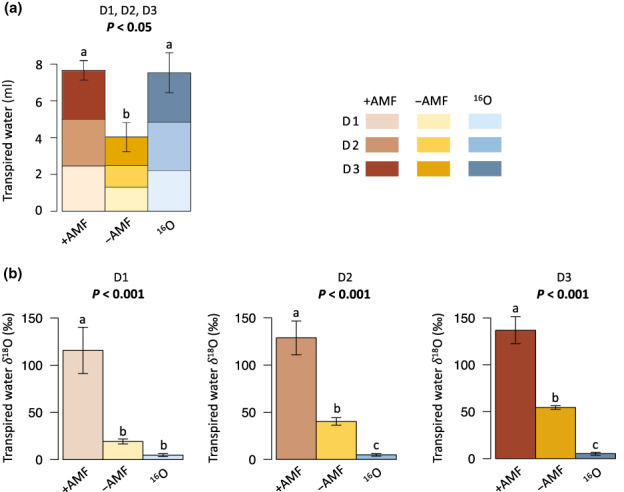
Volume (a) and isotope enrichment (b) of water transpired by Avena barbata shoots over 3 d (Day(D)1, 2, 3) in +AMF, −AMF and 16O microcosms. Each color and shade (light, medium, dark) represents 1 d of water transpired. In (a, b), different letters above bars represent statistically significant differences (one‐way ANOVA & Fisher LSD test); corresponding P‐values are indicated above each plot. The error bars represent SE (n = 18). AMF, arbuscular mycorrhizal fungi.
H2 18O injected in the no‐plant compartment was recovered in the transpired water of +AMF microcosms
In order to test whether +AMF plants obtained water from the no‐plant compartment via AMF, we quantified the 18O enrichment of the water transpired by the plants. On average, transpired water from +AMF plants was three times as 18O‐enriched as transpired water from −AMF plants (δ18O values of 127.09 ± 10.73‰ and 37.95 ± 3.86‰, respectively; P < 0.001) (Fig. 3b; Table S1). Although −AMF controls also had a higher δ18O in transpired water than 16O controls (δ18O values of 37.95 ± 3.86‰ and 4.95 ± 0.77‰, respectively; P < 0.001), there was significantly more 18O in transpired water in +AMF microcosms.
18O calculations
Plants in +AMF microcosms transpired an average of 2.56 ± 0.23 ml of water per day and plants in −AMF microcosms transpired an average of 1.34 ± 0.13 ml of water per day, during the three separate days when transpired water was collected. Although +AMF plants transpired an extra 1.22 ± 0.26 ml per day on average, some of the extra water transpired could be due to indirect benefits of AMF on plant–water relations. We used the isotope data to determine how much of this extra water was directly transported by AMF from the no‐plant compartment across the air gap and taken up by roots. In addition, to account for water from the no‐plant compartment that crossed the air gap as liquid or vapor diffusion (and not via AMF hyphae), we subtracted the volume of 18O water transpired by −AMF plants from the volume of 18O water transpired by +AMF plants. Using the measured volumes and 18O content of transpired water in +AMF and −AMF microcosms (Table S1), we estimated that the AMF‐transported water amounted to an average of 0.885 ± 0.268 ml (95% CI) per day per microcosm, each holding three plants. Thus, AMF‐transported water accounted for 34.6 ± 10.5% (95% CI) of the total water transpired each day by +AMF plants (detailed calculations are in Methods S1).
Water was transported on the outside of hyphal cell membrane
We used a fluorescent dye to test for extracytoplasmic water transport by fungal hyphae. The 18O‐labeled water that we injected into the no‐plant compartment of +AMF and −AMF microcosms also contained the membrane‐impermeant fluorescent dye LYCH. Lucifer yellow carbohydrazide can travel on the outer surface of the hyphal cell wall and inside the hyphal cell wall matrix, but it cannot cross cell membranes into the cytoplasm (Oparka, 1991). This property of LYCH allowed us to test for extracytoplasmic hyphal transport: if the dye injected in the no‐plant compartment is detected in the plant compartment, it must have travelled extracytoplasmically on hyphae to cross the air gap. In Fig. 2(g–i), this dye can be observed on hyphae outside and inside roots (labeled ‘1’), and in vesicle cell walls inside roots (labeled ‘3’), indicating that it had travelled via extracytoplasmic hyphal transport in the +AMF microcosms. LYCH has a high affinity for the cell wall matrix and does not diffuse out once taken up by hyphae (Pecková et al., 2016). In −AMF and 16O microcosms, the LYCH dye was not detected on hyphae or spores in the plant compartment, indicating that no LYCH dye was transported to the plants in these control microcosms; we observed only naturally occurring root autofluorescence (Roshchina, 2012; Fig. 2h).
Discussion
Water availability limits plant growth and is a pressing issue in the context of climate change as drought conditions are becoming more prevalent in many regions around the world (Kirkham, 2005). Plants have evolved multiple strategies to increase their tolerance of soil water deficit and alleviate its detrimental effects (Augé, 2001; Mo et al., 2016), including associations with AMF. Plants with AMF symbionts tend to cope with soil water limitations more effectively, due in part to indirect mechanisms including enhanced plant nutrition, osmoregulation and root hydraulic conductivity (Ruiz‐Lozano, 2003; Porcel & Ruiz‐Lozano, 2004; Augé et al., 2015; Quiroga et al., 2019a,b). However, it has remained unclear whether AMF also are able to directly transport water to their host plants. In our experiment, using 18O‐labeled water, we found that direct water transport by AMF accounted for 34.6 ± 10.5% (95% CI) of the water transpired by +AMF plants. Our results indicate that AMF have the ability to bridge air gaps in soil, penetrate small pores and access water that is inaccessible to roots, attributes that could be especially important in dry soils where water films are discontinuous.
We found that +AMF plants transpired an average of 1.22 ml more than −AMF plants each day, and 0.885 ml of this amount was derived from direct hyphal transport. Thus, in our experiment, direct water transport by AMF to roots accounted for over 2/3 of the extra 1.22 ml transpired by +AMF plants. We presume that indirect benefits of the AMF symbiosis accounted for the remaining 1/3 of the extra water transpired by +AMF plants each day. Notably, +AMF plants had a significantly higher P content than −AMF plants despite −AMF plants receiving more nutrient solution. Taken together, these results indicate that AMF improve plant–water relations both directly by helping plants access more water and indirectly by providing other benefits to plant health. We hypothesize that the relative contribution of direct and indirect AMF mechanisms to the improvement of plant–water relations may change depending on environmental conditions and the identity of the plant and fungal species involved in the symbiosis.
In order to investigate direct AMF water transport, Püschel et al. (2020) used an experimental design similar to ours, but employed deuterated water injected into the no‐plant compartment. Puschel and coworkers then measured deuterium incorporation in plant biomass, but did not assess isotope content in transpired water. They concluded that the amount of water AMF transported to plants was low compared to the volume of water that they estimated was transpired. Our direct measurement of isotope (18O) in transpired water indicated a much larger amount of water carried by AMF to their host plant. The differences between our findings could be due to the different plants and AMF taxa used, and/or to our direct measurement of isotope label in transpiration water. It seems likely that the specific amount of water AMF transport will depend on the plant–AMF species pairing as well as environmental conditions, in the same way that AMF can behave mutualistically or parasitically in their trade of nutrients for photosynthetic C (Johnson et al., 1997; Klironomos, 2000, 2003).
We recognize that our glasshouse experiment took place in an artificial environment where conditions were crafted to encourage AMF root colonization and increase the likelihood of water transport by AMF to host plant. We designed the experiment so that if water transport by hyphae is a phenomenon that occurs, it would reveal itself in a context in which we could detect and measure it. Yet, the low nutrient and water content profiles plants and AMF experienced in our study can occur in the field. Plants and soil microbes experience intermittent or permanent conditions of low nutrient and/or water availability in numerous climatic zones and ecosystems, including in Mediterranean‐type climates and arid and semi‐arid regions around the world (Kirkham, 2005). In soil, fine roots and root hair can access water in macropores (> 80‐μm diameter) and mesopores (> 30‐μm diameter), but hyphae also can enter micropores down to 2‐μm diameter (Kirkham, 2005; Smith & Read, 2008; Brady & Well, 2009). In unsaturated soils and as previously saturated soils dry out, water is taken from larger pores first and the water films become discontinuous (Kirkham, 2005). Hyphae extend beyond zones that roots and root hairs inhabit, and enter smaller pores that remain water‐filled; they bridge the gap between soil particles by serving as a surface on which water can travel (Allen, 2007). These are the conditions we attempted to simulate with our microcosms with an air gap and a soil compartment that only hyphae could enter. Quantification of 18O in transpired water indicated that 34.6% of water transpired by +AMF plants was transported by AMF; this proportion may be on the higher end of what occurs in the field. Roots are more efficient than hyphae at water uptake when soil water is readily available, so water transport by hyphae to host plants will probably be most critical under drought conditions when hyphae can access water unavailable to roots. Nevertheless, we observed AMF transporting a significant volume of water to their host plants, with some of that water traveling via an extracytoplasmic pathway along hyphae.
Numerous studies have observed improved productivity in plants with AMF associations and have reported benefits of the AMF symbiosis in terms of increased above‐ and belowground biomass (as reviewed in Smith & Smith, 1996; Smith & Read, 2008; Diagne et al., 2020). Although AMF symbionts often have a positive effect on plant growth, in our study we found no significant difference between above‐ or belowground biomass in our +AMF and −AMF treatments. +AMF plants had significantly higher P content and transpired significantly more than −AMF plants, but this did not translate to increased above‐ or belowground growth. One possibility is that conditions other than P and water availability were limiting growth in all treatments. Another possibility is that +AMF plants with higher transpiration fluxes had higher fitness in metrics other than biomass. Indeed, the cost–benefit analysis of the plant–AMF symbiosis can be complicated, and biomass alone might not be a good indicator of reproductive fitness (Johnson et al., 1997). It also is worth noting that plants in both +AMF and −AMF treatments had AMF symbionts, but in the −AMF treatment, hyphae were not allowed to access the no‐plant compartment and the additional water and nutrients it contained. As described in the Materials and Methods section, −AMF plants received additional water and nutrients (to make up for what +AMF plants could obtain from the no‐plant compartment) because our goal was to grow plants of all treatments in the same conditions, except for the last few days of the experiment when the labeled water was injected into the no‐plant compartment. This also may explain why differences in above or below ground biomass between treatments were not observed.
A strength of our study is the complementary combination of isotopically labeled water and a fluorescent dye. Although the isotopically labeled water allows quantification, it can move across the airgap from the no‐plant to the plant compartment by non‐AMF means (e.g. in the gas phase), resulting in a background level of plant enrichment. By contrast, the fluorescent dye provides a complementary determination because it cannot cross the airgap in the gas phase, and as shown in our experiments, was not apparent at even a background level in the −AMF treatment. These orthogonal data provides solid evidence that the AMF in our study acted as a direct means of water transport from the no‐plant compartment to the plant.
In plants, roots can transport water via both apoplastic and symplastic pathways, and plants can regulate the relative contribution of each route based on environmental conditions (Bárzana et al., 2012). The symplastic pathway, which tends to be favored when water availability is limited (Steudle & Peterson, 1998; Steudle, 2000), is slower because water has to flow from cell to cell via the cytoplasm, crossing plasma membranes or plasmodesmata, following an osmotic gradient (Steudle & Peterson, 1998; Bárzana et al., 2012). The apoplastic pathway, which is favored when plants are not water‐stressed, is faster because water travels extracellularly through the cell wall and matrix and moves directly and continuously via the transpiration stream, facing little resistance (Steudle & Peterson, 1998; Steudle, 2000). Interestingly, Bárzana et al. (2012) found that plants with AMF associations have an increased apoplastic water flow in both drought and nondrought conditions, and have a greater ability to switch between water transport pathways, compared to plants with no AMF associations. They further suggest that AMF hyphae could contribute water to the apoplastic flow in roots, which is consistent with our observations. We found that the LYCH dye travelled from the no‐plant compartment to the plant compartment via hyphae. As this dye cannot cross cell membranes, it must have travelled extracellularly within the hyphal cell wall matrix and/or outside the cell wall. Indeed, we observed the dye on hyphae and even within the wall of fungal spores (Fig. 2g–i). This finding suggests that AMF can act as extensions of the root system along the soil–plant–air continuum of water movement, with plant transpiration driving water flow along hyphae outside of the hyphal cell membrane.
The soil solution generally contains nutrient ions that move to roots by diffusion and mass flow. When soil water content is very low, these nutrient supply paths are disrupted by the discontinuity of soil water films and plant nutrient deficiencies are common. Extracytoplasmic hyphal transport of water by AMF would not only supply water to roots during dry conditions, but also could enable the movement of nutrient ions to roots in dry soils. In addition, extracytoplasmic water flow along hyphae has been shown to be a way for AMF to carry phosphate‐solubilizing bacteria to areas with organic P and thereby enhance P mobilization (Jiang et al., 2021).
Our study provides strong evidence supporting the existence of extracytoplasmic water transport in hyphae. It is possible, however, that cytoplasmic transport also occurs in hyphae at the same time. Plants and AMF both have aquaporins at the soil interface and at the arbuscule–plant cell interface (Aroca et al., 2007, 2009; Maurel et al., 2008; Li et al., 2013) and, under drought conditions, the gene expression of AMF and plants aquaporins and the hydraulic conductivity and symplastic flow in roots with AMF have been shown to increase (Aroca et al., 2007, 2009; Maurel et al., 2008; Ruiz‐Lozano et al., 2009; Li et al., 2013; Sánchez‐Romera et al., 2017; Quiroga et al., 2019a,b). However, it has been argued that based on physical principles, notably the Hagen–Poiseuille equation, AMF hyphal diameters (2–20 μm) are too small and so their flow rates are too slow to transport significant volumes of water inside the cytoplasm (Allen, 2007). Evidence from the membrane‐impermeable tracer LYCH and physical principles of fluid dynamics strongly suggest that hyphae are able to transport water extracellularly to roots. That said, we cannot exclude cytoplasmic flow in hyphae, and it is possible that cytoplasmic water transport also plays an important complementary role in water transport to host plants; therefore, both pathways are illustrated in our conceptual diagram, Fig. 4.
Fig. 4.
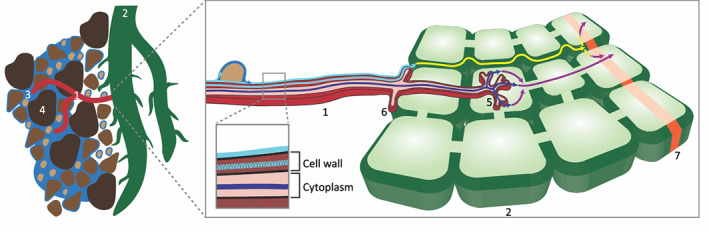
Simplified representation of water transport from soil through an arbuscular mycorrhizal fungus (AMF) hypha to a plant root. Extracytoplasmic water transport in a hypha, represented by a light blue arrow, joins apoplastic transport in a plant root, represented by a yellow arrow. Cytoplasmic transport in a hypha, represented by a dark blue arrow, joins symplastic transport in a plant root, represented by a purple arrow. 1, AMF hypha; 2, root; 3, soil water; 4, soil particles; 5, arbuscule; 6, appressorium; 7, Casparian strip.
Our experimental method pairing of H2 18O and a fluorescent tracer provides strong evidence that AMF are able to bridge air gaps in soil and bring water to plants that is inaccessible to roots. In addition, our results indicate that water can be transported to plants via an extracytoplasmic pathway in hyphae. Our findings have implications for the management of water and plant drought tolerance in the context of climate change. Plant‐AMF symbioses are key players in the maintenance of soil and plant productivity when water is limited, making them essential not only in arid and semi‐arid regions around the world, but also in areas experiencing short‐term droughts, especially as changing climatic conditions increase the occurrence of water‐limiting conditions.
Author contributions
AK and MKF designed the experiment with the assistance of JAH, DJH and JP‐R; AK, JAH and MPK performed the experiment with assistance from DJH; AK, JAH, MPK, SM, PKW and MKF analyzed the data with the assistance of LJF, DJH and JP‐R; and AK and MKF drafted the manuscript. AK, JAH, SM, LJF, PKW, JP‐R and MKF all contributed to the final manuscript.
Competing interests
None declared.
Supporting information
Fig. S1 Assembly of a microcosm.
Fig. S2 Fluorescence images of Avena barbata roots dyed with acid fuchsin showing arbuscular mycorrhizal fungi structures.
Methods S1 Detailed 18O calculations and assumptions.
Table S1 Data used in statistical analyses and 18O calculations.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
The authors thank: Thomas Bruns, John Taylor, Todd Dawson, Louise Glass, Angela Hodge, Greg Jedd and Mengting Yuan for their thoughtful advice on the project; Denise Schichnes and Steven Ruzin for their guidance with microscopy; Christina Wistrom and Katerina Estera‐Molina for their assistance with the glasshouse set‐up; and Hunter Jamison for his help with the isotope analyses. This research was supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research Genomic Science program under awards DE‐SC0020163, DE‐SC0010570 and DE‐SC0016247 to MKF at UC Berkeley and awards SCW1589 and SCW1678 to JP‐R at Lawrence Livermore National Laboratory (LLNL). Work conducted at LLNL was contributed under the auspices of the US Department of Energy under Contract DE‐AC52‐07NA27344. AK was supported by the Bennett Agricultural Fellowship, the Storie Memorial Fellowship, and the Jenny Fellowship in Soil Science awarded by the Department of Environmental Science, Policy & Management at UC Berkeley.
Contributor Information
Anne Kakouridis, Email: annekakouridis@berkeley.edu.
Mary K. Firestone, Email: mkfstone@berkeley.edu.
Data availability
The data that supports the findings of this study are available in the Supporting Information file of this article or from the corresponding author upon reasonable request.
References
- Allen MF. 2007. Mycorrhizal fungi: highways for water and nutrients in arid soils. Vadose Zone Journal 6: 291–297. [Google Scholar]
- Aroca R, Bago A, Sutka M, Paz A, Cano C, Amodeo G, Ruiz‐Lozano JM. 2009. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt‐stressed and nonstressed mycelium. Molecular Plant–Microbe Interactions 22: 1169–1178. [DOI] [PubMed] [Google Scholar]
- Aroca R, Porcel R, Ruiz‐Lozano JM. 2007. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytologist 173: 808–816. [DOI] [PubMed] [Google Scholar]
- Augé RM. 2001. Water relations, drought and vesicular‐arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42. [Google Scholar]
- Augé RM, Stodola AJ, Tims JE, Saxton AM. 2001. Moisture retention properties of a mycorrhizal soil. Plant and Soil 230: 87–97. [Google Scholar]
- Augé RM, Toler HD, Saxton AM. 2015. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta‐analysis. Mycorrhiza 25: 13–24. [DOI] [PubMed] [Google Scholar]
- Bago B, Azcón‐Aguilar C, Goulet A, Piché Y. 1998. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. The New Phytologist 139: 375–388. [Google Scholar]
- Bago B, Chamberland H, Goulet A, Vierheilig H, Lafontaine JG, Piché Y. 1996. Effect of nikkomycin Z, a chitin‐synthase inhibitor, on hyphal growth and cell wall structure of two arbuscular‐mycorrhizal fungi. Protoplasma 192: 80–92. [Google Scholar]
- Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez‐Ballesta MC, Carvajal M, Ruiz‐Lozano JM. 2012. Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well‐watered and drought stress conditions. Annals of Botany 109: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay GJ, Thomas RS, Dakessian S, Brown MS, Ames RN, Whitehead EE. 1988. Mycorrhizae in stressed environments: effects on plant growth, endophyte development, soil stability and soil water. In: Hutchinson CF, Timmermann BN, eds. Arid lands: today and tomorrow. Boulder, CO, USA: Westview, 1015–1029. [Google Scholar]
- Bitterlich M, Franken P, Graefe J. 2018. Arbuscular mycorrhiza improves substrate hydraulic conductivity in the plant available moisture range under root growth exclusion. Frontiers in Plant Science 9: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SM, Free SJ. 2006. The structure and synthesis of the fungal cell wall. BioEssays 28: 799–808. [DOI] [PubMed] [Google Scholar]
- Brady NC, Well RR. 2009. The nature and properties of soil , 13 th edn. Upper Saddle River, NJ, USA: Pearson Prentice Hall, Pearson Education Published in India by Dorling. Kindersley (India) Pvt. Ltd. [Google Scholar]
- Brodie EL, Joyner DC, Faybishenko B, Conrad ME, Rios‐Velazquez C, Malave J, Martinez R, Mork B, Willett A, Koenigsberg S et al. 2011. Microbial community response to addition of polylactate compounds to stimulate hexavalent chromium reduction in groundwater. Chemosphere 85: 660–665. [DOI] [PubMed] [Google Scholar]
- Chen M, Arato M, Borghi L, Nouri E, Reinhardt D. 2018. Beneficial services of arbuscular mycorrhizal fungi – from ecology to application. Frontiers in Plant Science 9: 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakessian S, Brown MS, Bethlenfalvay GJ. 1986. Relationship of mycorrhizal growth enhancement and plant growth with soil water and texture. Plant and Soil 94: 439–444. [Google Scholar]
- Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S. 2020. Roles of arbuscular mycorrhizal fungi on plant growth and performance: importance in biotic and abiotic stressed regulation. Diversity 12: 370. [Google Scholar]
- Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Fitter AH, Helgason T. 2011. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytologist 190: 794–804. [DOI] [PubMed] [Google Scholar]
- Egan C, Rummel A, Kokkoris V, Klironomos J, Lekberg Y, Hart M. 2018. Using mock communities of arbuscular mycorrhizal fungi to evaluate fidelity associated with Illumina sequencing. Fungal Ecology 33: 52–64. [Google Scholar]
- Egerton‐Warburton LM, Querejeta JI, Allen MF. 2007. Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. Journal of Experimental Botany 58: 1473–1483. [DOI] [PubMed] [Google Scholar]
- Faber BA, Zasoski RJ, Munns DN, Shackel K. 1991. A method for measuring hyphal nutrients and water uptake in mycorrhizal plants. Canadian Journal of Botany 69: 87–94. [Google Scholar]
- Feofilova EP. 2010. The fungal cell wall: modern concepts of its composition and biological function. Microbiology 79: 711–720. [PubMed] [Google Scholar]
- Franson RL, Milford SB, Bethlenfalvay GJ. 1991. The glycine‐glomus‐Bradyrhizobium symbiosis. XI. Nodule gas exchange and efficiency as a function of soil and root water status in mycorrhizal soybean. Physiologica Plantarum 83: 476–482. [Google Scholar]
- Habte M, Osorio NW. 2001. Arbuscular mycorrhizas: producing and applying arbuscular mycorrhizal inoculum. Manoa, HI, USA: College of Tropical Agriculture and Human Resources, University of Hawaii. [Google Scholar]
- Hayes JM. 2004. An introduction to isotopic calculations. Woods Hole, MA, USA: Woods Hole Oceanographic Institution. [Google Scholar]
- Jany JL, Pawlowska TE. 2010. Multinucleate spores contribute to evolutionary longevity of asexual glomeromycota. The American Naturalist 175: 424–435. [DOI] [PubMed] [Google Scholar]
- Jarvis AJ, Davies WJ. 1998. The coupled response of stomatal conductance to photosynthesis and transpiration. Journal of Experimental Botany 1: 399–406. [Google Scholar]
- Jiang F, Zhang L, Zhou J, George TS, Feng G. 2021. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytologist 230: 304–315. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Graham JH, Smith FA. 1997. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. The New Phytologist 135: 575–585. [Google Scholar]
- Kakouridis A. 2021. The role of arbuscular mycorrhizal fungi in ecosystems: water transport to plants, carbon transport to soil, and the assessment of drivers that shape their biodiversity . PhD thesis, University of California, Berkeley, CA, USA.
- Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE. 2009. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biology and Biochemistry 41: 1233–1244. [Google Scholar]
- Khalvati MA, Hu Y, Mozafar A, Schmidhalter U. 2005. Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biology 7: 706–712. [DOI] [PubMed] [Google Scholar]
- Kirkham MB. 2005. Principles of soil and plant water relations. Burlington, MA, USA: Elsevier/Academic Press. [Google Scholar]
- Klironomos JN. 2000. Host‐specificity and functional diversity among arbuscular mycorrhizal fungi. Microbial Biosystems: New Frontiers 1: 845–851. [Google Scholar]
- Klironomos JN. 2003. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84: 2292–2301. [Google Scholar]
- Lee J, Lee S, Young PW. 2008. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiology Ecology 65: 339–349. [DOI] [PubMed] [Google Scholar]
- Li T, Hu Y‐J, Hao Z‐P, Li H, Wang Y‐S, Chen B‐D. 2013. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices . New Phytologist 197: 617–630. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. 2008. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology 59: 595–624. [DOI] [PubMed] [Google Scholar]
- Mo Y, Wang Y, Yang R, Zheng J, Liu C, Li H, Ma J, Zhang Y, Wei C, Zhang X. 2016. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well‐watered and drought conditions. Frontiers in Plant Science 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ. 1991. Uptake and compartmentation of fluorescent probes by plant cells. Journal of Experimental Botany 42: 565–580. [Google Scholar]
- Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier U, Zobel M. 2010. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytologist 188: 223–241. [DOI] [PubMed] [Google Scholar]
- Pecková E, Tulová E, Soukup A. 2016. Tracing root permeability: comparison of tracer methods. Biologia Plantarium 60: 695–705. [Google Scholar]
- Plamboeck AH, Dawson TE, Egerton‐Warburton LM, North M, Bruns TD, Querejeta JI. 2007. Water transfer via ectomycorrhizal fungal hyphae to conifer seedlings. Mycorrhiza 17: 439–447. [DOI] [PubMed] [Google Scholar]
- Porcel R, Ruiz‐Lozano JM. 2004. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany 55: 1743–1750. [DOI] [PubMed] [Google Scholar]
- Purin S, Morton JB. 2011. In situ analysis of anastomosis in representative genera of arbuscular mycorrhizal fungi. Mycorrhiza 21: 505–514. [DOI] [PubMed] [Google Scholar]
- Püschel D, Bitterlich M, Rydlová J, Jansa J. 2020. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: a gordian knot of roots and hyphae. Mycorrhiza 30: 299–313. [DOI] [PubMed] [Google Scholar]
- Querejeta JI. 2017. Chapter 17 – soil water retention and availability as influenced by mycorrhizal symbiosis: consequences for individual plants, communities, and ecosystems. In: Johnson NC, Gehring C, Jansa J, eds. Mycorrhizal mediation of soil. Amsterdam, the Netherlands: Elsevier, 299–317. [Google Scholar]
- Querejeta JI, Egerton‐Warburton LM, Allen MF. 2003. Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134: 55–64. [DOI] [PubMed] [Google Scholar]
- Quiroga G, Erice G, Aroca R, Chaumont F, Ruiz‐Lozano JM. 2019a. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environmental and Experimental Botany 167: 103821. [Google Scholar]
- Quiroga G, Erice G, Ding L, Chaumont F, Aroca R, Ruiz‐Lozano JM. 2019b. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant, Cell & Environment 42: 2274–2290. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL http://www.R‐project.org/. [Google Scholar]
- Rorison B, Rorison IH. 1987. Root hairs and plant growth at low nitrogen availabilities. New Phytologist 107: 681–693. [Google Scholar]
- Roshchina VV. 2012. Vital autofluorescence: application to the study of plant living cells. International Journal of Spectroscopy 2012: 1–14. [Google Scholar]
- Ruiz‐Lozano JM. 2003. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13: 309–317. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Lozano JM, Azcón R. 1995. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiologia Plantarum 95: 472–478. [Google Scholar]
- Ruiz‐Lozano JM, del Mar Alguacil M, Bárzana G, Vernieri P, Aroca R. 2009. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and non mycorrhizal maize plants through regulation of PIP aquaporins. Plant Molecular Biology 70: 565–579. [DOI] [PubMed] [Google Scholar]
- Ruth B, Khalvati M, Schmidhalter U. 2011. Quantification of mycorrhizal water uptake via high‐resolution on‐line water content sensors. Plant and Soil 342: 459–468. [Google Scholar]
- Sánchez‐Romera B, Ruiz‐Lozano JM, Zamarreño ÁM, García‐Mina JM, Aroca R. 2017. Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza 26: 111–122. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. Cambridge, UK: Academic Press/Elsevier. [Google Scholar]
- Smith FA, Smith SE. 1996. Mutualism and parasitism: diversity in function and structure in the ‘arbuscular’ (VA) mycorrhizal symbiosis. Advances in Botanical Research 22: 1–43. [Google Scholar]
- Steudle E. 2000. Water uptake by roots: effects of water deficit. Journal of Experimental Botany 51: 1532–1542. [DOI] [PubMed] [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49: 775–788. [Google Scholar]
- Talbi S, Romero‐Puertas M, Hernández A, Terrón Camero L, Ferchichi A, Sandalio L. 2015. Drought tolerance in a Saharian plant Oudneya africana: role of antioxidant defenses. Environmental and Experimental Botany 111: 114–126. [Google Scholar]
- Wu QS, Xia RX. 2006. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well‐watered and water stress conditions. Journal of Plant Physiology 163: 417–425. [DOI] [PubMed] [Google Scholar]
- Zulfiqar F, Akram NA, Ashraf M. 2020. Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta 251: 3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Assembly of a microcosm.
Fig. S2 Fluorescence images of Avena barbata roots dyed with acid fuchsin showing arbuscular mycorrhizal fungi structures.
Methods S1 Detailed 18O calculations and assumptions.
Table S1 Data used in statistical analyses and 18O calculations.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information file of this article or from the corresponding author upon reasonable request.


