Abstract
Objective
To quantify rates of influenza illness and assess value of influenza vaccination among pregnant women in Panama and El Salvador.
Methods
Pregnant women were enrolled and followed each week in a prospective cohort study to identify acute respiratory illnesses (ARI). Nasopharyngeal swabs obtained from women with febrile ARI were tested by reverse‐transcription polymerase chain reaction for influenza and other respiratory viruses.
Results
We enrolled 2556 women between October 2014 and April 2017. Sixteen percent developed at least one ARI; 59 had two ARI, and five had three ARI for a total of 463 ARI. Women in El Salvador and Panama contributed 297 person‐years (py) and 293 py, respectively, during influenza circulation. Twenty‐one (11%) of 196 sampled women tested positive for influenza. Influenza incidence was 5.0/100 py (5.7/100 py in El Salvador and 4.3/100 py in Panama). Only 13% of women in El Salvador and 43% in Panama had been vaccinated against influenza before influenza epidemics (P < 0.0001).
Conclusions
One in six pregnant women developed ARI and more than one in ten ARI were attributable to vaccine‐preventable influenza. While women were at risk of influenza, few had been vaccinated before each epidemic. Such findings suggest the utility of evaluations to optimize vaccine timing and coverage.
Keywords: infection, influenza, pregnant, respiratory, syncytial, vaccine, virus, women
Short abstract
One in six pregnant women developed ARI and ≥10% were attributable to vaccine‐preventable influenza. Few pregnant women, however, were vaccinated against influenza before each epidemic.
1. INTRODUCTION
The sustainability of vaccination programs depends on understanding risk of vaccine‐preventable illnesses and benefits of vaccination. Pregnant women are at increased risk of influenza hospitalization, 1 adverse birth outcomes, 2 , 3 and death as a result of influenza infection. Their disproportionate risk of influenza complications is attributed to anatomical, physiological, and immune changes during pregnancy. 4 Vaccination protects women and their unborn infants from influenza illnesses. 5 , 6 , 7 , 8 In 2012, the World Health Organization (WHO) recommended members prioritize pregnant women for influenza vaccination. 9 In 2016, important gaps in knowledge about risk of influenza illnesses among pregnant women were identified. 1 Such gaps in knowledge threaten the sustainability of newly established vaccination programs. For example, pregnant women seem to underutilize vaccines 10 because they and their providers are often unaware of illness risks and uncertain about the benefits of vaccination. 11 , 12 This is reflected in the modest 2018 influenza vaccination coverage among pregnant women in 22 of the 45 PAHO countries reporting such data in the Joint Reporting Form (median 72.5%, interquartile range 38%–82%). 13
To address a gap in knowledge about the risk of influenza, and better understand the benefits of influenza vaccination, we established mother/baby cohorts in Panama and El Salvador. Our primary objective was to quantify rates of influenza illnesses among pregnant women and their offspring in each country. As secondary objectives, we quantified rates of rhinovirus, parainfluenza 1–3, respiratory syncytial viruses (RSV), human metapneumovirus, and adenovirus. In this manuscript we present pregnant women's risk of acute respiratory illnesses (ARI) by viral etiology. While we were not powered to estimate vaccine effectiveness, we quantify and time vaccine coverage in relation to the timing of influenza epidemics. Influenza illness and vaccination knowledge, attitudes, and practices among pregnant women are part of a separate investigation.
2. METHODS
2.1. Study population
In 2014, we established a multi‐center longitudinal prospective cohort study of pregnant women in two primary health care centers in Panama (i.e., Tocumén and 24 de Diciembre Health Centers) and two in El Salvador (i.e., Tomas Piñeda and San Rafael Health Units). The cohort was named INFLUMIKA for its Spanish language acronym (i.e., Influenza maternal e Infantil y Zika). We chose these four peri‐urban health centers because their staff managed prenatal care for 300–400 women per year and provided influenza vaccination free‐of‐charge as part of prenatal care. We sought to enroll all pregnant women aged 15 or older who resided in the catchment of these health centers and were willing and able to provide written informed consent to participate.
2.2. Patient involvement
Upon enrollment, study staff documented demographic, socioeconomic, and clinical histories using standardized survey instruments into an open‐source software (OpenClinica, LLC, Waltham, MA, USA). Information about ultrasound gestational age dates and vaccination was updated during subsequent pre‐natal visits. When feasible, self‐reports were compared with medical records. Participants were instructed to contact study staff if they developed any ARI symptoms including cough, sore throat, or rhinorrhea. Participants were also called and/or texted each week throughout their pregnancy to identify ARI. Women with new onset measured fever of ≥38°C or subjective fever and ARI within 7 days of contact were asked to come to the clinic for respiratory virus testing. If women were unreachable for two consecutive prenatal check‐ups or after three phone calls and/or texts, they were considered lost to follow‐up.
2.3. Laboratory testing for core outcome
Swabs were transported at 2–8°C to the national reference laboratories within 24–48 h of sample collection. Gorgas Memorial Institute for Health Studies in Panama or the Laboratorio Nacional de Referencia in El Salvador tested specimens through reverse‐transcription polymerase chain reaction to identify respiratory virus RNA using a protocol and primers and probes provided by US Centers for Disease Control and Prevention (CDC).
2.4. Data management and analysis
We estimated the incidence of respiratory viruses during pregnancy using two risk periods. First, we divided the number of ARI and the number of laboratory‐confirmations of each virus by the total person‐time mothers contributed to study. Second, we divided the number of laboratory‐confirmations of each virus by the person‐time mothers contributed to the study during weeks when that virus was circulating nationally as detected by that country's National Influenza Centres (NICs). We subtracted 2 weeks from this risk period for every laboratory‐confirmed illness because we presumed convalescing women would not be susceptible to a new infection with the same virus within that time. We also assumed ARI were separate events if they occurred after >14‐day symptom‐free period.
We estimated the proportion of women with ARI, but without respiratory swabs, that could have tested positive for each virus. To estimate such missed detections, we multiplied the number of women with untested ARI by the proportion of persons aged 15–49 years with respiratory swabs that tested positive for that virus in that country's national surveillance systems during that week. In doing so, we assumed that untested women with ARI in the Panama and El Salvador cohorts would have been as likely to test positive for those respiratory viruses as persons aged 15–49 years identified through each country's national surveillance system. We adjusted by the proportion of influenza detections among afebrile vs. febrile adults with ARI (i.e., 0.24) 14 because untested women were typically afebrile, while those tested were febrile. To partially account for uncertainty, we incorporated the variance in the proportion of persons aged 15–49 years testing positive for each virus per week among all tested and the variance in the proportion testing positive for influenza among afebrile vs. febrile persons. To assess whether women had been vaccinated against influenza before the start of same‐year influenza epidemics, we defined epidemics as the first week when the proportion of samples testing positive for influenza was above the annual median for ≥3 consecutive weeks. 15 We used STATA SE 15.1 (StataCorp., College Station, TX, USA) for all statistical analyses.
3. RESULTS
3.1. Enrollment, demographics and follow
During October 11, 2014 to April 10, 2017, we approached 2604 pregnant women and enrolled 2556 (98%) (Table 1); 1051 in El Salvador contributed 464 person‐years (py) and 1505 women in Panama contributed 533 py (Table 2). In El Salvador, women were at risk for influenza during 297 py (64%) of 464 py and in Panama during 293 py (55%) of 533 py. Women were more frequently primigravidae in El Salvador (487, 47%) and had <7th grade education (265, 28%) and <400 USD income per month (741, 89%) when compared to women in Panama (423, 28%, 212, 18%, and 335, 38%, respectively, P < 0.0001). Women in Panama had a maximum vaccination coverage of 77%. Only 43% of women were vaccinated against influenza before the start of the influenza epidemic period (Figure 1a). Similarly, women in El Salvador had a maximum vaccination coverage of 66% and only 13% were vaccinated against influenza before the start of the influenza epidemic period (Figure 1b). On average, pregnant women were vaccinated at 17 weeks gestation (IQR 11–25).
TABLE 1.
Characteristics of pregnant women at enrolment in the INFLUMIKA a cohort
| El Salvador | Panama | P value | Total | |
|---|---|---|---|---|
| n | 1051 | 1505 | 2556 | |
| Age, median (interquartile) | 23 (19–27) | 24 (20–27) | 0.012 | |
| Race | ||||
| Indigenous | 1 (0.1%) | 400 (26.6%) | <0.001 | 401 (15.7%) |
| Mestizo | 1049 (99.8%) | 758 (50.4%) | 1807 (70.7%) | |
| White | 0 (0%) | 247 (16.4%) | 247 (9.7%) | |
| Black | 1 (0.1%) | 97 (6.4%) | 98 (3.8%) | |
| Other | 0 (0%) | 3 (0.2%) | 3 (0.1%) | |
| Primary school or less | 265 (28.3%) | 212 (17.7%) | <0.001 | 477 (22.3%) |
| Income <$400 USD | 741 (88.8%) | 335 (37.8%) | <0.001 | 1076 (62.6%) |
| Drinks alcohol | 5 (0.5%) | 46 (3.1%) | <0.001 | 51 (2.0%) |
| Smoker | 6 (0.6%) | 12 (0.8%) | 0.633 | 18 (0.7%) |
| Pre‐existing condition | 42 (4%) | 171 (11.6%) | <0.001 | 213 (8.4%) |
| Asthma | 19 (1.8%) | 59 (3.9%) | 0.002 | 78 (3.0%) |
| Hypertension | 6 (0.6%) | 27 (1.8%) | 0.007 | 33 (1.3%) |
| Kidney disease | 0 (0%) | 5 (0.3%) | 0.082 | 5 (0.2%) |
| Heart disease | 1 (0.1%) | 3 (2.0%) | 0.648 | 4 (0.2%) |
| Diabetes | 3 (0.3) | 2 (0.1%) | 0.407 | 5 (0.2%) |
| Cancer | 0 (0%) | 2 (0.1%) | 0.516 | 2 (0.1%) |
| HIV | 0 (0%) | 2 (0.1%) | 0.516 | 2 (0.1%) |
| Primigravid | 487 (46.5%) | 423 (28.2%) | <0.001 | 910 (35.7%) |
| Influenza vaccines | ||||
| Ever vaccinated | 693 (65.9%) | 1232 (81.9%) | <0.001 | 1925 (75.3%) |
| During pregnancy | 625 (59.5%) | 1074 (71.4%) | <0.001 | 1699 (66.5%) |
| Before influenza epidemics b | 133 (12.6%) | 652 (43.3%) | <0.001 | 785 (30.7%) |
INFLUMIKA is the Spanish language acronym of the cohort (i.e., Influenza maternal e Infantil y Zika).
Typical influenza epidemic period April‐September. 15
TABLE 2.
Acute respiratory illnesses (ARI), risk periods, and incidence rates by viral etiology among pregnant women enrolled in the INFLUMIKA a cohort
| El Salvador | Panama | P‐value | Total | ||
|---|---|---|---|---|---|
| Laboratory‐tested ARI | 149 (69.0%) | 47 (19.0%) | 196 (42.2%) | ||
| Rhinoviruses | 30 (20%) | 12 (26%) | <0.001 | 42 (21%) | |
| Influenza viruses b | 15 (10%) | 6 (13%) | 0.006 | 21 (11%) | |
| Parainfluenza 1 | 3 (2%) | 0 (0%) | 0.069 | 3 (2%) | |
| Parainfluenza 2 | 5 (3%) | 0 (0%) | 0.012 | 5 (3%) | |
| Parainfluenza 3 | 7 (5%) | 2 (4%) | 0.038 | 9 (5%) | |
| Respiratory syncytial virus | 11 (7%) | 3 (6%) | 0.006 | 14 (7%) | |
| Human metapneumovirus | 3 (2%) | 1 (2%) | 0.312 | 4 (2%) | |
| Estimated viral ARI in untested c | 67 | 201 | 268 | ||
| Rhinoviruses | 1.7 (1.3–2.1) | 28.0 (25.1–30.9) | 29.7 (26.4–33.0) | ||
| Influenza viruses b | 1.9 (0.0–3.7) | 6.5 (5.8–7.2) | 8.4 (5.8–10.9) | ||
| Parainfluenzas | 0.2 (0.0–0.4) | 6.0 (4.5–7.4) | 6.2 (4.5–7.8) | ||
| Respiratory syncytial virus | 0.8 (0.5–1.1) | 2.9 (2.1–3.8) | 3.7 (2.6–4.9) | ||
| Human metapneumovirus | 0.1 (0.0–3.0) | 2.2 (1.3–3.1) | 3.0 (2.1–4.1) | ||
| Total risk period in years | 464 | 533 | 997 | ||
| Influenza viruses b , d | 297 (64%) | 293 (55%) | 590 (59%) | ||
| Respiratory Syncytial virus | 331 (71%) | 413 (77%) | 744 (75%) | ||
| Human metapneumovirus | 349 (75%) | 402 (75%) | 751 (75%) | ||
| Parainfluenza viruses | 223 (48%) | 481 (90%) | 704 (71%) | ||
| Rhinoviruses | 455 (98%) | 528 (99%) | 983 (99%) | ||
| Adjusted rates per 100 py e | |||||
| Rhinoviruses | 7.0 (6.9–7.1) | 7.7 (7.1–8.2) | 7.3 (7.0–7.7) | ||
| Influenza viruses b | 5.7 (5.0–6.3) | 4.3 (4.0–4.5) | 5.0 (4.5–5.4) | ||
| Parainfluenzas | 6.8 (6.7–6.9) | 1.7 (1.4–2.0) | 3.3 (3.1–3.5) | ||
| Respiratory syncytial virus | 3.6 (3.5–3.7) | 1.4 (1.2–1.6) | 2.4 (2.2–2.5) | ||
| Human metapneumovirus | 0.9 (0.9–0.9) | 1.0 (0.8–1.2) | 0.9 (0.8–1.1) | ||
INFLUMIKA is the Spanish language acronym of the cohort (i.e., Influenza maternal e Infantil y Zika).
Influenza viruses are the only viruses listed in this table for which there is a licensed vaccine.
Estimates assume that untested women with ARI in the Panama and El Salvador cohorts would have been as likely to test positive for specific respiratory viruses as persons aged 15–49 years identified through each country's national surveillance system. For influenza, specifically, we adjusted the proportion of influenza detections among afebrile versus febrile adults with ARI (i.e., 0.24) 14 because untested women were typically afebrile, while those tested were febrile. To partially account for uncertainty in these estimates, we also incorporated the variance in the proportion of persons testing positive each week into the respiratory virus‐specific rates and the variance in the proportion of afebrile persons testing positive versus those who were febrile. The 95% confidence interval is provided in the parentheses.
Subset of risk period when virus was identified through each country's national surveillance system.
Adjusted rates use the sum of laboratory‐confirmed and estimated viral ARI as the numerator and the weeks when those viruses were identified through each country's national surveillance system as the risk period (i.e., denominator). Incidence rates are presented per 100 person‐years (py). The 95% confidence interval is provided in the parentheses.
FIGURE 1.
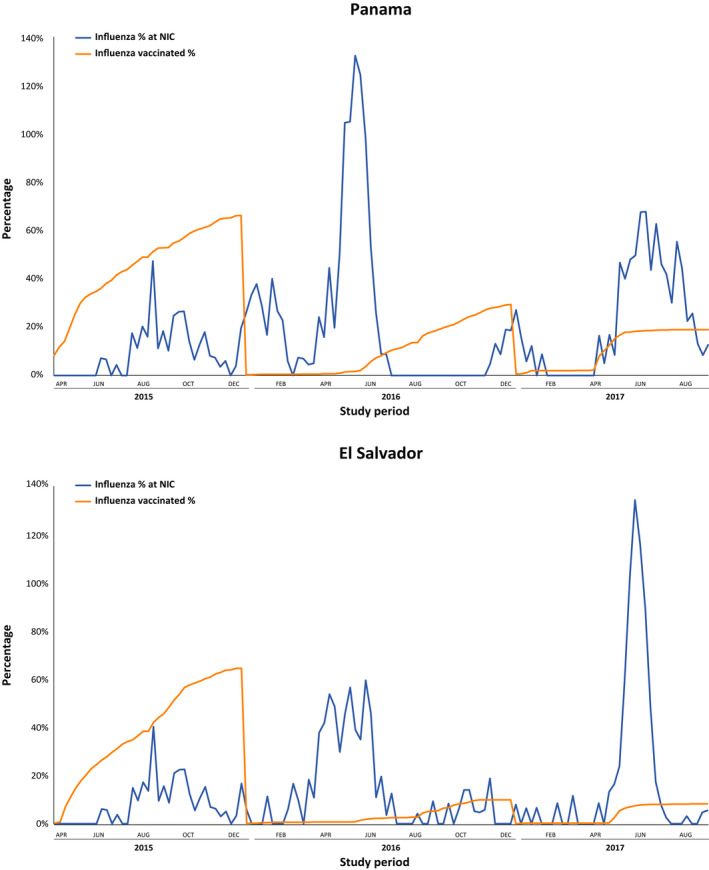
Influenza vaccinations among pregnant women in the INFLUMIKA cohort and proportion of influenza detections Panama and El Salvador National Influenza Centres, April 2015–August, 2017. NIC, National Influenza Centre; ARI, Acute respiratory illnesses
3.2. Syndromic and clinical characteristics
Approximately one in six women (399 [16%] of 2556 women) developed at least one ARI during follow‐up; 59 had two ARI, and five had three ARI and one had four ARI for a total of 464 ARI (Table 3). Only 212 (46%) of these 464 ARI were febrile and eligible for swabbing; 196 (92%) of these 212 were successfully swabbed. The median duration of ARI was 8 days (IQR, 6–10). Women sought care for 56 (11%) ARI. Care seeking occurred more frequently in El Salvador (n = 45, 8%) than in Panama (n = 11, 2%; P < 0.0001). Two women with ARI required referral to the hospital in El Salvador for the management of asthma exacerbations. One woman referred to the hospital had laboratory‐confirmed RSV and the second was untested but had illness when RSV was predominant.
TABLE 3.
Signs, symptoms, and bronchopneumonia diagnosis associated with acute respiratory illnesses (ARI) in the INFLUMIKA a cohort
| El Salvador | Panama | P‐value | Total | |
|---|---|---|---|---|
| n | 1051 | 1505 | 2556 | |
| Acute respiratory illnesses (ARI) | 216 | 248 | 464 | |
| Cough | 167 (15.9%) | 139 (9.2%) | <0.001 | 306 (12.0%) |
| Rhinorrhea | 166 (15.8%) | 145 (9.6%) | <0.001 | 311 (12.2%) |
| Subjective fever | 133 (12.7%) | 60 (4.0%) | <0.001 | 193 (7.6%) |
| Sore throat | 91 (8.7%) | 82 (5.4%) | 0.002 | 173 (6.8%) |
| Headache | 41 (3.9%) | 69 (4.6%) | 0.429 | 110 (4.3%) |
| Fatigue | 21 (2.0%) | 25 (1.7%) | 0.548 | 46 (1.8%) |
| Shortness of breath | 11 (1.1%) | 32 (2.1%) | 0.042 | 43 (1.7%) |
| Myalgias | 3 (0.3%) | 19 (1.3%) | 0.008 | 22 (0.9%) |
| Prostration | 0 (0%) | 3 (0.2%) | 0.273 | 3 (0.1%) |
| Bronchopneumonia | 4 (0.4%) | 0 (0%) | 0.028 | 4 (0.2%) |
INFLUMIKA is the Spanish language acronym of the cohort (i.e., Influenza maternal e Infantil y Zika).
3.3. Viruses detected among tested ARI cases
Viral RNA was detected in 98 (50%) of 196 samples (Table 2). The most common detections were rhinovirus (n = 42, 21%), influenza (21, 11%), RSV (14, 7%), parainfluenza 3 (9, 5%), human metapneumovirus (4, 2%), parainfluenza 1 (3, 2%), and parainfluenza 2 (5, 2%). None tested positive for adenovirus (Table 3). Only two samples had co‐detections, one with respiratory syncytial virus and parainfluenza‐2 and another one with respiratory syncytial virus and rhinovirus. The majority of influenza positive samples were A(H3N2) (n = 16, 76%), followed by influenza B (n = 3, 14%), and influenza A(H1N1)pdm09 (n = 2, 10%). More than ¾ of influenza detections (16, 76%) occurred during the typical April through September southern hemisphere influenza season. 15
3.4. Estimated etiology of ARI among untested cases
Women in El Salvador had 67 (31%) of 216 ARI unsampled and women in Panama had 201 (81%) of 248 ARI unsampled (Table 2). Using El Salvador's national surveillance findings (Figure 1), we attributed 1.9 (95% confidence interval [CI] 0.0–3.7) additional ARI to influenza, 1.7 (95% CI 1.3–2.1) to rhinoviruses, 0.2 (95% CI 0.0–0.4) to parainfluenza viruses, 0.8 (95% CI 0.5–1.1) to RSV, and 0.1 (95% CI 0.0–0.3) to human metapneumovirus. Similarly, in Panama, we attributed 6.5 (95% CI 5.8–7.2) additional ARI to influenza, 28.0 (95% CI 25.1–30.9) to rhinoviruses, 6.0 (95% CI 4.5–7.4) to parainfluenza viruses, 2.9 (95% CI 2.1–3.8) to RSV, and 2.2 (95% CI 1.3–3.1) to human metapneumovirus.
3.5. Incidence of ARI and ARI by etiology
The febrile ARI incidence rate (19.7/100 py) was 42% of the incidence for all ARI (46.5/100 py) (Table 2). The incidence of laboratory‐confirmed influenza during the entire calendar follow‐up period was 2.1/100 py (1.5/100 py in El Salvador and 0.6/100 py in Panama). The incidence of influenza adjusted for untested ARI (i.e., laboratory‐confirmed influenza plus ARI attributable to influenza) during epidemic weeks when influenza was nationally detectable, was 5.0/100 py (95% CI 4.5–5.4/100 py) (Figure 2); 5.7/100 py (95% CI 5.0–6.3/100 py) in El Salvador and 4.3/100 py (95% CI 4.0–4.5/100 py) in Panama. Similarly, the adjusted incidence of rhinovirus during weeks when this virus was detectable nationally was 7.3/100 py (95% CI 7.0–7.7/100 py), parainfluenza viruses 3.3/100 py (95% CI 3.1–3.5/100 py), RSV 2.4/100 py (95% CI 2.2–2.5/100 py), and human metapneumovirus 0.9/100 py (95% CI 0.8–1.1/100 py). Based on the only laboratory‐confirmed RSV hospitalization, the RSV hospitalization rate in El Salvador was 3.0/1000 py.
FIGURE 2.

Rates of acute respiratory illnesses by viral etiology and their 95% confidence interval among pregnant women in the INFLUMIKA cohort, April 2015–August, 2017
4. DISCUSSION
One in six women in our study developed ARI during pregnancy and more than one in ten ARI were attributable to vaccine‐preventable influenza. The incidence of influenza was second only to rhinovirus among cohort women. CDC encourages persons to avoid exposure to respiratory viruses through the use of non‐pharmaceutical interventions. These include frequent handwashing, avoiding close contact with sick persons, and during epidemic periods, physical distancing, and mask‐wearing in public. Influenza specifically can be safely and effectively prevented 16 through vaccination. 7 , 17 Influenza illness increases pregnant women's risk of hospitalization, pregnancy loss, 18 and premature delivery. 19 While there were insufficient women in our study to assess such outcomes, influenza during pregnancy was associated with late pregnancy loss and reduction in mean birthweight in a concurrent three‐country pregnant women cohort. 18
Conversely, maternal influenza vaccination decreases the risk of influenza illness complications and passively protects infants from influenza during the first months of life. 5 , 7 , 16 Maternal immunization is important for infants because those younger than 6 months are still developing their immune system and ineligible for influenza vaccination. Our results reaffirm Panama and El Salvador's decisions to prioritize pregnant women for influenza vaccination and to annually invest in government‐purchased influenza vaccines for administration free‐of‐charge during prenatal care. While we were underpowered to quantify vaccine effectiveness, it is noteworthy that pregnant women in Panama were more likely to be vaccinated against influenza and ecologically had lower rates of influenza than women in El Salvador.
Our influenza rates were similar to those reported among pregnant women in China (3.8/100 py), 20 Nepal (5.0/100 py), 7 and South Africa (3.6/100 py). 21 Our rates were also similar to symptomatic persons aged 18–49 years in the U.S (5.3 patient‐years). 22 Our El Salvador rates (5.7 [5.0–6.3]/100 py) were also like Peru's (6.5 [4.5–9.5]/100 py) 18 possibly because both countries are middle‐income and have modest vaccination coverage when compared to higher‐income Panama. While our Panama and El Salvador cohorts were neither designed nor powered to examine associations between influenza and birth outcomes, the Peru study was part of three‐country cohort which identified adverse birth outcomes associated with influenza ARI. 18 It is therefore likely that pregnant women with influenza in El Salvador and Panama may have also been at higher risk of pregnancy loss and lower weight neonates when compared to uninfected women.
The self‐reported vaccination coverage among study women in 2015 was like that estimated through PAHO’s administrative methods in a 2014 publication (i.e., 50%–70%). 23 However, <43% of influenza vaccinations occurred before the start of the influenza epidemics in Salvador and Panama. Furthermore, influenza vaccination steadily decreased in both countries during 2015–2017 (Figure 1). Such findings raise concerns about these programs’ sustainability. The sustainability of vaccination programs is likely influenced by multiple factors, including population awareness of risk of influenza illness, 12 confidence in the value of mitigation measures, and competing health priorities. Until now, obstetricians in Central and South America would not have had sub‐regional influenza burden estimates from pregnant women to inform risk perception and reaffirm commitment to vaccination guidelines. Our study findings can therefore be used to develop risk communication messages for providers 24 and pregnant women to urge timely vaccination before the start of each influenza season.
Our study had noteworthy strengths; we conducted a prospective 3‐year, two‐country, active surveillance cohort. We estimated the incidence of influenza using sensitive molecular diagnostics to confirm influenza illness and previously used methods to correct for under‐ascertainment. 25 We then reported findings following STROBE guidelines.
Nevertheless, our study also had noteworthy limitations. We had insufficient resources to follow women more frequently than once a week. We asked women to present for swabbing only if they developed fever, when specimens would have been most likely to test positive for influenza. This strategy missed opportunities to laboratory‐confirm viral etiologies. Under‐ascertainment occurred more frequently in Panama, where women seemed less likely to report subjective fever than in El Salvador. Second, while Panama's and El Salvador's reference laboratories collected influenza and RSV data, which we used to determine risk periods, neither country collected parainfluenza data stratified by type and El Salvador did not collect human metapneumovirus and rhinovirus data during the entire study period. Therefore, we present aggregate parainfluenza rates and used the proportion of Panama's human metapneumovirus virus and rhinovirus risk weeks (79% of risk period) to estimate El Salvador risk weeks during 2016 and 2017. Last, while we are reassured that our findings are like those of other countries, 18 it is improbable that rates are representative of pregnant women in all tropical middle‐income countries.
Pregnant women in Panama and El Salvador frequently had ARI and more than one in ten febrile ARI were attributable to influenza. Our findings suggest the value of Panama's and El Salvador's investment in vaccination to protect pregnant women and their infants from influenza illnesses. During 2014–2018, however, less than half of the cohort of women in Panama had been vaccinated against influenza before the start of annual influenza epidemics. Indeed, less than one in five had been vaccinated in El Salvador, where influenza rates were higher than those of Panama. Pregnant women should receive inactivated influenza vaccines as soon as these become available at the start of each epidemic. 26 The influenza season starts approximately in April in countries with a southern hemisphere epidemic pattern and in October in countries with a northern hemisphere epidemic pattern. 27
As of 2021, Panama and El Salvador offer southern hemisphere formulation influenza vaccines to pregnant free‐of‐charge starting approximately April each year, but the lack of electronic vaccination registries makes it difficult to determine whether vaccine coverage has improved since 2014–2018. Such findings suggest Panama and El Salvador might optimize the benefit of their investments through World Health Organization‐recommended post‐introduction evaluations of their influenza vaccination programs. 28 The frequency of ARI with other respiratory viruses also suggests the value of promoting self‐protection through non‐pharmaceutical interventions as has been successfully done during the COVID‐19 pandemic. 29
CONFLICTS OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
EAB designed the study, assisted with protocol development, data cleaning, analyses, and manuscript writing. RG assisted with study design, drafted protocol, managed field teams during data collection, and assisted with interpretation of study findings. AC and VC assisted with data collection, cleaning, and interpretation. DF and JMP lad laboratory testing and interpretation. RD, RF, JA led field teams in El Salvador and assisted with data interpretation and manuscript development. AH assisted with interpretation of study findings and manuscript review.
ACKNOWLEDGEMENTS
The authors would like to thank the local health center personnel, staff at the National Influenza Centers and the participating families who made this work possible. We would also like to thank Mary Ann Hall, Susan Kaydos‐Daniels, Rachael Porter, Natalie Olson, and William Davis at CDC for their review of interim findings and advice about analytic approaches, and Nestor Sosa during his tenure as Director of the Gorgas Institute in Panama for his leadership in supporting this Gorgas‐CDC partnership. And a big thank you to IJG/Wiley staff for making this happen. Its a good end to an endeavor that is now eight years in the making.
Azziz‐Baumgartner E, Veguilla V, Calvo A, et al. Incidence of influenza and other respiratory viruses among pregnant women: A multi‐country, multiyear cohort. Int J Gynecol Obstet. 2022;158:359–367. doi: 10.1002/ijgo.14018
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention.
Funding information
The study was funded through CDC cooperative agreement 5U01IP000791‐05. CDC authors provided technical assistance in the development of the study protocol, de‐identified data analyses, and writing of the manuscript
REFERENCES
- 1. Mertz D, Geraci J, Winkup J, et al. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta‐analysis of observational studies. Vaccine. 2017;35(4):521‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fell DB, Savitz DA, Kramer MS, et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG. 2017;124(1):48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Regan AK, Feldman BS, Azziz‐Baumgartner E, et al. An international cohort study of birth outcomes associated with hospitalized acute respiratory infection during pregnancy. J Infect. 2020;81(1):48‐56. [DOI] [PubMed] [Google Scholar]
- 4. Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol. 2010;53(2):329‐336. [DOI] [PubMed] [Google Scholar]
- 5. Blanchard‐Rohner G, Siegrist CA. Vaccination during pregnancy to protect infants against influenza: why and why not? Vaccine. 2011;29(43):7542‐7550. [DOI] [PubMed] [Google Scholar]
- 6. Nunes MC, Madhi SA. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: a systematic review and meta‐analysis. Hum Vaccin Immunother. 2018;14(3):758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinhoff MC, Katz J, Englund JA, et al. Year‐round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo‐controlled trial. Lancet Infect Dis. 2017;17(9):981‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson MG, Kwong JC, Regan AK, et al. influenza vaccine effectiveness in preventing influenza‐associated hospitalizations during pregnancy: a multi‐country retrospective test negative design study, 2010–2016. Clin Infect Dis. 2019;68(9):1444‐1453. [DOI] [PubMed] [Google Scholar]
- 9. Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 — conclusions and recommendations. Wkly Epidemiol Rec. 2014;89(21):221‐236. [PubMed] [Google Scholar]
- 10. Ortiz JR, Perut M, Dumolard L, et al. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF joint reporting form on immunization. Vaccine. 2016;34(45):5400‐5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arriola CS, Vasconez N, Bresee J, et al. Knowledge, attitudes and practices about influenza vaccination among pregnant women and healthcare providers serving pregnant women in Managua, Nicaragua. Vaccine. 2018;36(25):3686‐3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reinders S, Romero C, Carcamo C, et al. A community‐based survey on influenza and vaccination knowledge, perceptions and practices in Peru. Vaccine. 2020;38(5):1194‐1201. [DOI] [PubMed] [Google Scholar]
- 13. Pan American Health Organization . Immunization in the Americas 2019 Summary. 2020. https://www3.paho.org/hq/index.php?option=com_docman&view=download&category_slug=brochures‐immunization‐1581&alias=50553‐immunization‐in‐the‐americas‐2019‐summary&Itemid=270&lang=en [Google Scholar]
- 14. Wesley MG, Tinoco Y, Patel A, et al. Performance of symptom‐based case definitions to identify influenza virus infection among pregnant women in middle‐income countries: findings from the pregnancy and influenza multinational epidemiologic (PRIME) study. Clin Infect Dis. 2020;ciaa1697. doi: 10.1093/cid/ciaa1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durand LO, Cheng PY, Palekar R, et al. Timing of influenza epidemics and vaccines in the American tropics, 2002–2008, 2011–2014. Influenza Other Respir Viruses. 2016;10(3):170‐175. doi: 10.1111/irv.12371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Azziz‐Baumgartner E, Grohskopf L, Patel M. Realizing the potential of maternal influenza vaccination. JAMA. 2021;325(22):2257‐2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quach THT, Mallis NA, Cordero JF. Influenza vaccine efficacy and effectiveness in pregnant women: systematic review and meta‐analysis. Matern Child Health J. 2020;24(2):229‐240. [DOI] [PubMed] [Google Scholar]
- 18. Dawood FS, Kittikraisak W, Patel A, et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle‐income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis. 2021;21(1):97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunnes N, Gjessing HK, Bakken IJ, et al. Seasonal and pandemic influenza during pregnancy and risk of fetal death: a Norwegian registry‐based cohort study. Eur J Epidemiol. 2020;35(4):371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Zhou S, Zhang Z, et al. Cohort profile: China respiratory illness surveillance among pregnant women (CRISP), 2015–2018. BMJ Open. 2018;8(4):e019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz MA, Gessner BD, Johnson J, et al. Incidence of influenza virus infection among pregnant women: a systematic review. BMC Pregnancy Childbirth. 2017;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis. 2018;66(10):1511‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ropero‐Álvarez AM, El Omeiri N, Kurtis HJ, et al. Influenza vaccination in the Americas: progress and challenges after the 2009 A(H1N1) influenza pandemic. Hum Vaccin Immunother. 2016;12(8):2206‐2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morales KF, Menning L, Lambach P. The faces of influenza vaccine recommendation: a literature review of the determinants and barriers to health providers’ recommendation of influenza vaccine in pregnancy. Vaccine. 2020;38(31):4805‐4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Azziz‐Baumgartner E, Bruno A, Daugherty M, et al. Incidence and seasonality of respiratory viruses among medically attended children with acute respiratory infections in an Ecuador birth cohort, 2011–2014. Influenza Other Respir Viruses. 2021;2011‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Committee on Obstetric Practices . Influenza vaccination durign pregnancy. Obstet Gynecol. 2018;131(4):e109‐114. [DOI] [PubMed] [Google Scholar]
- 27. Hirve S, Newman LP, Paget J, et al. Influenza seasonality in the tropics and subtropics ‐ when to vaccinate? PLoS One. 2016;11(4):e0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. WHO . Influenza Vaccine Post‐Introduction Evaluation (IPIE). 2021. https://www.who.int/immunization/research/development/ipie_influenza_post_introduction_evaluation/en/ [Google Scholar]
- 29. Olsen SJ, Azziz‐Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID‐19 pandemic—United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1305‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]


