Abstract
Ciprofol is a propofol analogue with improved pharmacokinetic properties. A multi‐centre, non‐inferiority trial was conducted to compare the deep sedation properties of ciprofol and propofol with a non‐inferiority margin of 8% in patients undergoing gastroscopy and colonoscopy. In total, 289 patients were randomly allocated for surgery (259 colonoscopy and 30 gastroscopy) at a 1:1 ratio to be given intravenous injections of ciprofol (0.4 mg/kg) or propofol (1.5 mg/kg). The primary outcome was the success rate of colonoscopy defined as colonoscopy completion with no need for an alternative sedative or >5 ciprofol or propofol top up doses within any 15‐min time period. The success rate of colonoscopy was 100% in the ciprofol group vs. 99.2% in the propofol group (mean difference 0.8%, 95% CI: −2.2% to 4.2%). Except for the gastrointestinal lesions found during the gastroscopy and colonoscopy procedures, the occurrence rates of adverse drug reactions in the ciprofol and propofol groups were 31.3% and 62.8%, respectively (P < 0.001). Pain on injection was less common in the ciprofol group (4.9% vs. 52.4%, P < 0.001). The outcomes demonstrated that ciprofol was non‐inferior to propofol with regard to successful sedation for gastroscopy or colonoscopy procedures and no obvious important adverse events occurred.
Keywords: ciprofol, colonoscopy, deep sedation, gastroscopy, propofol
1. INTRODUCTION AND BACKGROUND
Patients undergoing gastrointestinal endoscopy must be properly sedated, typically with benzodiazepines alone or together with an opioid drug. 1 , 2 , 3 Propofol has a rapid onset of action and a short half‐life and has been increasingly used for sedation in gastrointestinal endoscopy. 4 , 5 Despite these desirable features, propofol also has some disadvantages, such as producing hypotension and bradycardia, 6 , 7 , 8 as well as pain on injection. 9 Therefore, several alternatives have been developed to potentially resolve the aforementioned issues. 10 , 11 , 12 Ciprofol (2‐[(1R)‐[1‐cyclopropylethyl]]‐6‐isopropylphenol) is a structural analogue of propofol (Figure 1). The incorporation of an R‐ chiral centre and a cyclopropyl group improves the pharmacological and physicochemical properties, most notably making it more potent than propofol as well as eliciting less pain on injection. 13 , 14 A phase 1 trial showed that ciprofol is safe at doses of 0.15–0.90 mg/kg and that most AEs were mild to moderate. 15 In another trial, ciprofol was shown to be safe at doses of 0.4–0.9 mg/kg and exhibited a similar onset time and four to five times more potency than propofol. 14 Due to the higher potency of ciprofol compared to propofol, the amount of drug necessary to achieve anaesthesia is reduced, which in turn needs less solvent and also might reduce side effects other than pain at the injection side.
FIGURE 1.
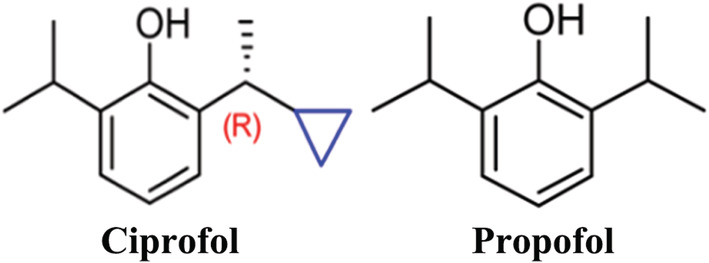
Structures of ciprofol and propofol
We conducted a multi‐centre, non‐inferiority trial to test the hypothesis that ciprofol at a dose of 0.4 mg/kg is non‐inferior to propofol 1.5 mg/kg for the induction of sedation in patients undergoing gastroscopy and colonoscopy and has a tolerable safety profile. A non‐inferiority design was chosen because we wanted to show that ciprofol is not inferior to propofol regarding sedation success rate but possibly with less adverse events (AEs).
2. MATERIALS AND METHODS
2.1. Study design and patient selection
This multi‐centre, non‐inferiority trial was conducted at 10 teaching hospitals in China. The Institutional Review Board of each participating hospital approved the trial protocols. Informed consent was obtained from each patient prior to enrollment in the trial, which was registered at ClinicalTrials.gov (NCT03674008). The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies. 16 Adult patients (18 ~ 65 years), with an American Society of Anesthesiologists physical classification status of I ~ II who were due to undergo elective gastroscopy or colonoscopy, were eligible. A detailed list of inclusion and exclusion criteria is presented in Table S1.
Eligible patients were randomized at a 1:1 ratio to be given either ciprofol (0.4 mg/kg) or propofol (1.5 mg/kg). Randomization was stratified based on the type of procedure (gastroscopy vs. colonoscopy). Concealment was achieved using an online central randomization interactive web response system (IWRS). Both patients and assessors were blinded to the group assignment. The physical appearance of ciprofol was identical to propofol (both supplied in 20 ml ampoules), and the intravenous line and syringe were covered with a drape.
Preparation for gastroscopy and colonoscopy procedures were conducted in accordance with the local protocol or national guidelines of China. 17 Intravenous access was established by placing an 18‐gauge cannula in the upper dorsal vein, and all patients received 300–500 ml of sterile 0.9% sodium chloride solution before sedation was initiated. Pulse oxygen saturation (SpO2), respiratory rate (RR), systolic blood pressure (SBP), mean arterial pressure (MAP), heart rate (HR), and a standard 12‐lead ECG were continuously monitored using a multi‐parameter monitor (T6, Mindray, Shenzhen, China). All patients received 50‐μg fentanyl 1 min before the intravenous infusion of either ciprofol (0.4 mg/kg) or propofol (1.5 mg/kg) over a time period of 1 min. A colonoscope or gastroscope was inserted when the MOAA/S score was ≤1 (Table S2). During the induction of sedation, the anaesthesiologist evaluated the MOAA/S score every 30 s. If the MOAA/S score remained >1 after 2 min of initial administration of the study drug, a top‐up dose of 1/2 the initial dose was injected over 10 s. In the maintenance phase, a top‐up dose was given upon signs of agitation or inadequate sedation and repeated at 2‐min intervals as required. Sedation was regarded as unsuccessful if more than five top‐up doses were required within 15 min, and then, propofol was the only permitted alternative sedative in this trial.
Oxygen at a flow rate of 10 L/min was administered continuously through a face mask until each patient was completely alert (3 sequential/S scores of MOAA = 5). After completion of the operation procedure, patients were transported to a post‐anaesthesia care unit. Post‐procedure recovery was assessed every 2 min using the Modified Aldrete Score, and discharge readiness was defined by a score of ≥9. Before discharge, each patient was asked to complete a satisfaction questionnaire (Table S3).
Safety was evaluated by the anaesthesiologist during the procedure and during the recovery time, including vital signs, ECG, SpO2, and AEs. AE data were also collated via telephone interviews 24 h after the initial procedure, and the patients were asked to go back to the hospital for laboratory inspections on post‐procedure days 2 or 3. The out‐of‐range results with clinical significance would be retested until they returned back to normal or became without clinical significance as determined by the primary investigator. In addition, the necessity for airway assistance was evaluated. When apnoea or hypoxemia occurred, a jaw‐thrust manoeuvre was used to open the airway. Positive mask ventilation and tracheal intubation were necessary if severe hypoxia could not be corrected by opening the airway which was decided by the anaesthesiologist. The following ECG parameters were measured: PR, RR, QRS, and QT intervals and the QTc interval corrected using the Bazett and Fridericia formula. 18 , 19
2.2. Primary outcome
The primary outcome was the success rate of colonoscopy, defined as requiring ≤5 top‐up doses of ciprofol or propofol within any 15‐min time period to completion of the surgical procedure.
2.3. Secondary outcomes
Secondary outcomes included (1) the success rate of gastroscopy; (2) the success rate of colonoscopy and gastroscopy; (3) induction time (MOAA/S ≤ 1 after administration of the first dose); (4) insertion time (for the gastroscope or colonoscope) and insertion success rates; (5) time for a patient to be fully aware (time to reach 3 consecutive MOAA/S scores of 5); (6) recovery time (time to reach the Modified Aldrete Score ≥ 9); (7) requirements for alternative or top‐up dose during the procedure and (8) patient satisfaction.
2.4. Safety and definitions of terms used for safety evaluation
An AE is any adverse medical event that occurred in the patients who participated in the clinical trial but not necessarily causally related to the study drug. Thus, an AE can be any adverse, unexpected indicator (including abnormal clinically significant laboratory values), symptom, or disease (new onset or exacerbation of a pre‐existing condition), which is associated with, but not necessarily caused by, the study drug. AEs not included were sleepiness, drowsiness or even loss of consciousness related to the effects of anaesthetics during ciprofol/propofol administration; AEs caused by chronic diseases prior to participating in the clinical trial or caused by selective medical examination or surgery prior to participation as well as overuse of the study drug or the study drug combined with other drugs without causing any symptoms or signs.
Severity of AEs was defined as Grade 1 (mild): asymptomatic or mild, clinically or diagnostic, without treatment; Grade 2 (moderate): required minor, local or non‐invasive treatment; age‐related limitations in instrumental activities of daily living; or Grade 3 (severe): serious or medically significant but not immediately life‐threatening; resulting in hospitalization or prolonged hospitalization; disabled; self‐rational activities of daily living were limited.
Serious adverse events (SAEs) were defined as a serious medical event resulting in (1) death; (2) life‐threatening (e.g., subject is at risk of immediate death if a necessary intervention was not undertaken at the time; does not include fatal events if more serious); (3) required hospitalization or extending existing hospitalization; (4) permanent or significant loss of functions or disability; (5) birth malformations or birth defects and (6) other important medical events.
Adverse drug reactions (ADRs) were defined as drug‐related AEs that occurred while patients were given the study drug.
Adverse drug reactions of special interest (ADRSI) included those due to pharmacological effects (hypoxemia, apnoea, bradycardia and hypotension); hypoxemia: SPO2 < 90% and duration >30 s, evaluated from first administration of the study drug to discharge; apnoea: thoracic motion disappeared for >30 s; evaluation was discontinued from the first dose of the study drug until the patient was fully alert (consecutive 3 measurements of MOAA/S = 5); bradycardia: defined as HR < 45 beats/min and duration >30 s, evaluated from the first dose of the study drug to discharge of the patient; hypotension: defined as SBP < 90 mmHg or 30% decrease from baseline and duration >2 min from the time of first treatment to discharge.
Adverse events of special interest (AESI) included AEs of special interest and serious adverse drug reactions (SADRs).
2.5. Statistical analysis
Sample size requirements were estimated using the following assumptions: (1) sedation success rate in the propofol control; (2) non‐inferiority margin of 8% 20 and (3) one‐sided type I error of 0.025 and a power of 80%. Assuming 6% dropout, a total of 250 patients (125 per group) were needed for the trial. A pilot trial involving 30 patients was also conducted. Thus, 280 patients were included in the primary outcome analysis of successful sedation.
Continuous variables are expressed as the mean ± SD and were analysed using Student's t test or Wilcoxon rank‐sum test. Categorical variables were compared by employing a chi‐squared test or Fisher's exact test. P < 0.05 (one‐sided for the primary outcome, and two‐sided for all others) was considered a statistically significant difference. All statistical analyses were performed using SAS version 9.4. The trial was overseen by an independent data monitoring committee.
3. RESULTS
3.1. Baseline demographics and clinical characteristics
A total of 366 patients were screened, 81 of whom who did not meet the inclusion or meet the exclusion criteria and were therefore excluded from the trial. Five screened failed patients were re‐selected, and one patient withdrew informed consent without receiving any medication after randomization. Overall, 289 patients underwent randomization and completed the procedures (259 for colonoscopy and 30 for gastroscopy) between 11 September 2018 and 28 February 2019 (Figure 2). Demographic and baseline characteristics of the participating patients including their Modified Mallampati Scores are listed in Table 1.
FIGURE 2.
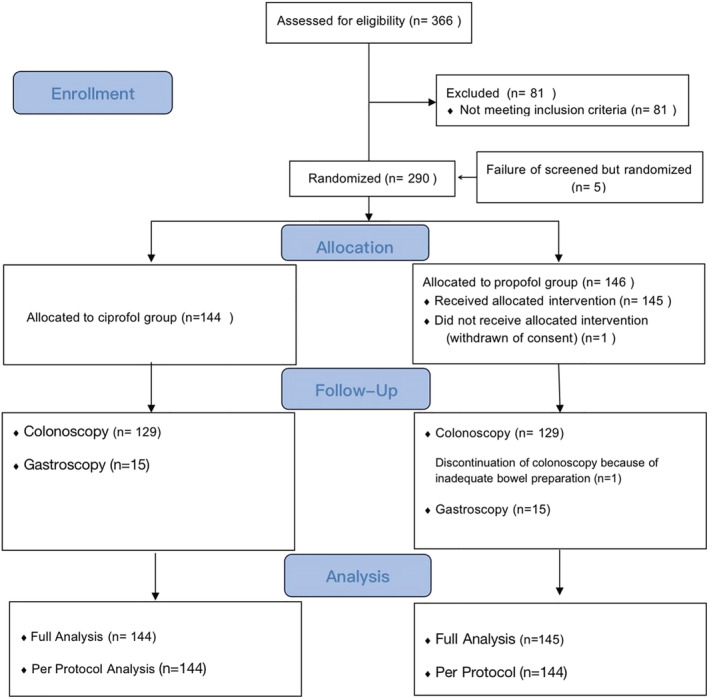
Trial enrolment and follow‐up flow diagram
TABLE 1.
Demographic variables of the two study groups
| Variables | Ciprofol (n = 144) | Propofol (n = 145) | P value |
|---|---|---|---|
| Age (mean ± SD, years) | 43.8 ± 11.8 | 44.1 ± 11.3 | 0.929 |
| Sex | 0.364 | ||
| Males, n (%) | 55 (38.2) | 63 (43.4) | |
| Females, n (%) | 89 (61.8) | 82 (56.6) | |
| Height (mean ± SD, cm) | 161.5 ± 8.2 | 163.1 ± 8.4 | 0.103 |
| Weight (mean ± SD, kg) | |||
| Screening | 60.8 ± 9.6 | 62.4 ± 10.0 | 0.193 |
| Baseline | 60.0 ± 9.6 | 61.5 ± 9.7 | 0.212 |
| BMI (mean ± SD, kg/m2) (Screening) | 23.2 ± 2.5 | 23.4 ± 2.6 | 0.671 |
| ASA status, n (%) | 0.744 | ||
| I | 115 (79.9) | 118 (81.4) | |
| II | 29 (20.1) | 27 (18.6) | |
| Modified Mallampati Score, n (%) | 0.904 | ||
| I | 99 (68.8) | 97 (66.9) | |
| II | 43 (29.9) | 45 (31.0) | |
| III | 2 (1.4) | 3 (2.1) |
3.2. Primary outcome
3.2.1. Success rates of colonoscopy
The success rate of colonoscopy was 100% in the ciprofol group and 99.2% in the propofol group, with a mean difference at 0.8% (95% CI: −2.2%, 4.2%).
3.3. Secondary outcomes
3.3.1. Overall success rates of gastroscopy and colonoscopy and of gastroscopy
The overall success rate of gastroscopy and colonoscopy was 100% in the ciprofol group and 99.3% in the propofol group, and the success rate of gastroscopy was 100% in both groups. However, one patient in the propofol group did not complete colonoscopy due to insufficient intestinal preparation, but all the other patients completed colonoscopy. The sedation success rate of insertion for gastroscopy, colonoscopy or gastroscopy and colonoscopy was 100% for both groups.
3.3.2. Induction time and insertion time
The induction time was 1.1 ± 0.5 min for the ciprofol group and 1.1 ± 0.4 min for the propofol group (P = 0.405). There was no significant difference between the 2 groups for either colonoscopy (1.1 ± 0.4 vs. 1.1 ± 0.4 min, P = 0.218) or for gastroscopy (1.2 ± 0.8 vs. 1.0 ± 0.2 min, P = 0.499).
The insertion time was 1.5 ± 0.5 min for the ciprofol group and 1.5 ± 0.5 min for the propofol group (P = 0.911). In the subgroup analysis, insertion time did not differ between the two groups (1.6 ± 0.8 vs. 1.2 ± 0.2 min for gastroscopy, P = 0.081; 1.5 ± 0.5 vs. 1.5 ± 0.5 min for colonoscopy, P = 0.504) (Table 2).
TABLE 2.
Secondary endpoints (induction time, insert time, fully alert time, recovery time and top‐up doses)
| Variable | Ciprofol | Propofol | ||||
|---|---|---|---|---|---|---|
| Colonoscopy (n = 129) | Gastroscopy (n = 15) | Colonoscopy and gastroscopy (n = 144) | Colonoscopy (n = 130) | Gastroscopy (n = 15) | Colonoscopy and gastroscopy (n = 145) | |
| Induction time (min) | ||||||
| Mean ± SD | 1.1 ± 0.4 | 1.2 ± 0.8 | 1.1 ± 0.5 | 1.1 ± 0.4 | 1.0 ± 0.2 | 1.1 ± 0.4 |
| Median (min, max) | 1.0 (0.5, 3.3) | 1.0 (0.5, 3.0) | 1.1 (0.5, 3.3) | 1.0 (0.5, 3.5) | 1.0 (0.5, 1.2) | 1.0 (0.5, 3.5) |
| P value a | 0.218 | 0.499 | 0.405 | |||
| Insert time (min) | ||||||
| Mean ± SD | 1.5 ± 0.5 | 1.6 ± 0.8 | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.2 ± 0.2 | 1.5 ± 0.5 |
| Median (min, max) | 1.3 (0.8, 3.6) | 1.5 (0.8, 3.4) | 1.4 (0.8, 3.6) | 1.3 (0.6, 3.6) | 1.3 (0.7, 1.5) | 1.3 (0.6, 3.6) |
| P value a | 0.504 | 0.081 | 0.911 | |||
| Fully alert time (min) | ||||||
| Mean ± SD | 3.2 ± 3.2 | 4.4 ± 2.3 | 3.3 ± 3.1 | 1.8 ± 2.0 | 3.0 ± 2.7 | 2.0 ± 2.1 |
| Median (min, max) | 2.0 (0.0, 13.0) | 4.0 (0.2, 9.2) | 2.0 (0.0, 13.0) | 1.0 (0.1, 3.0) | 3.0 (0.1, 11.0) | 1.1 (0.0, 13.0) |
| P value a | < 0.001 | 0.070 | < 0.001 | |||
| Recovery time (min) | ||||||
| Mean ± SD | 7.2 ± 3.2 | 8.4 ± 2.3 | 7.4 ± 3.1 | 5.8 ± 2.0 | 7.1 ± 2.8 | 6.0 ± 2.1 |
| Median (min, max) | 6.0 (3.9, 17.0) | 8.0 (4.0, 13.1) | 6.0 (3.9, 17.0) | 5.1 (4.0, 17.0) | 7.0 (4.0, 15.0) | 5.1 (4.0, 17.0) |
| P value a | < 0.001 | 0.076 | < 0.001 | |||
| Top‐up dosing times (n) | ||||||
| Median (min, max) | 0.0 (0.0, 7.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 7.0) | 1.0 (0.0, 3.0) | 0.0 (0.0, 1.0) | 1.0 (0.0, 3.0) |
| P value a | 0.052 | 0.150 | 0.136 | |||
| Top‐up doses required | ||||||
| No | 74 (57.4%) | 11(73.3%) | 85 (59.0%) | 58 (44.6%) | 14 (93.3%) | 72 (49.7%) |
| Yes | 55 (42.6%) | 4 (26.7%) | 59 (41.0%) | 72 (56.4%) | 1 (6.7%) | 73 (50.3%) |
| P value a | 0.193 | 0.132 | 0.340 | |||
Note: Insert time: colonoscopy or gastroscopy insertion; fully alert time: from colonoscope withdrawal to full alertness; recovery time: from colonoscope withdrawal to patient discharge.
Ciprofol compared to propofol.
3.3.3. Time to patients being fully alert and their discharge
The mean time for a patient to become fully alert was 3.3 ± 3.1 min in the ciprofol group vs. 2.0 ± 2.1 min for the propofol group (P < 0.001) in the overall analysis. In the subgroup analysis, the time to being fully alert was longer in the ciprofol group who underwent colonoscopy (3.2 ± 3.2 vs. 1.8 ± 2.0 min, P < 0.001) but not in patients who underwent gastroscopy (4.4 ± 2.3 vs. 3.0 ± 2.7 min, P = 0.070).
The time to discharge was 7.4 ± 3.1 min for the ciprofol group vs. 6.0 ± 2.1 min for the propofol group (P < 0.001). In the subgroup analysis, the time to being fully alert was longer in the ciprofol group (7.2 ± 3.2 vs. 5.8 ± 2.0 min, P < 0.001) who underwent colonoscopy, but not for patients who underwent gastroscopy (8.4 ± 2.3 vs. 7.1 ± 2.8, P = 0.076) (Table 2).
3.3.4. Requirements for top‐up doses during a procedure
During the colonoscopy and gastroscopy procedures, 85 patients (59.0%) in the ciprofol group and 72 (49.7%) in the propofol group did not require top‐up doses (Table 2). There were 45 patients (34.9%), 9 patients (7.0%) and 1 patient (0.8%) who received 1 top‐up, 2 top‐up and 7 top‐up doses in the ciprofol group, whereas 60 patients (46.2%), 9 patients (6.9%) and 3 patients (2.3%) needed 1 top‐up, 2 top‐up and 3 top‐up doses in the propofol group during colonoscopy (P = 0.193). For gastroscopy, there were 11 patients (73.3%) in the ciprofol group and 14 patients (93.3%) in the propofol group who did not require any top‐up doses; 3 patients (20.0%) and 1 patient (6.7%) needed 1 top‐up and 2 top‐up doses in the ciprofol group, while 1 patient (6.7%) in the propofol group needed 1 top‐up dose (P = 0.132). Patient satisfaction scores were 9.9 ± 0.4 for the ciprofol group vs. 9.7 ± 0.7 for the propofol group (P = 0.001) during the colonoscopy procedure.
3.4. Safety assessments
In the trial, 109 patients (75.7%) experienced 211 AEs in the ciprofol group, and 100 patients (69.0%) experienced 200 AEs in the propofol group. Except for two occurrences (1.4%) of severe AEs in the ciprofol group and 1 (0.7%) in the propofol group, all other AEs were moderate (16.7% vs. 13.8%) or mild (70.1% vs. 64.8%) in severity, and patients recovered without or after transient treatment. No patients withdrew from the trial due to severe AEs in either group (Table 3).
TABLE 3.
Incidence of AEs and ADRs during colonoscopy and gastroscopy
| Ciprofol (n = 144) | Propofol (n = 145) | P value | |||
|---|---|---|---|---|---|
| Subjects (n, %) | Events (n) | Subjects (n, %) | Events (n) | ||
| AEs | 109 (75.7) | 211 | 100 (69.0) | 202 | 0.201 |
| ADRs | 41 (28.5) | 56 | 35 (24.1) | 54 | 0.403 |
| Severity of AEs | 0.603 | ||||
| Mild | 101 (70.1) | 179 | 94 (64.8) | 175 | |
| Moderate | 24 (16.7) | 30 | 20 (13.8) | 26 | |
| Severe | 2 (1.4) | 2 | 1 (0.7) | 1 | |
| Severity of ADRs | 0.692 | ||||
| Mild | 34 (23.6) | 46 | 33 (22.8) | 47 | |
| Moderate | 9 (6.3) | 10 | 7 (4.8) | 7 | |
| Severe | 0 (0.0) | 0 | 0 (0.0) | 0 | |
| Significant AEs | 13 (9.0) | 13 | 6 (4.1) | 7 | 0.094 |
| AESI | 21 (14.6) | 21 | 19 (13.1) | 22 | 0.716 |
| ADRSI | 21 (14.6) | 21 | 19 (13.1) | 22 | 0.716 |
| SAEs | 1 (0.7) | 1 | 1 (0.7) | 1 | 1.000 |
| SADRs | 0 (0.0) | 0 | 0 (0.0) | 0 | ‐ |
Note: ADRs, adverse drug reactions; AEs, adverse events; ADRSI, adverse drug reactions of special interest; AESI, adverse events of special interest; SADRs, serious adverse drug reactions; SAEs, serious adverse events.
ADRs are shown in Table 4. The occurrence rate of ADRs was 28.5% (41 of 144 patients) in the ciprofol group and 24.1% (35 of 145 patients) in the propofol group. Nine patients (6.3%) in the ciprofol group and 15 patients (10.3%) in the propofol group had respiratory‐related ADRs (including respiratory depression, apnoea and hypoxemia), all of which were mild or moderate in severity. No episodes of apnoea required intervention with positive pressure ventilation in either group and no patient received airway assistance treatments. Pain on injection was cited by 4.9% (7 of 144) of patients in the ciprofol group vs. 52.4% (76 of 145) in the propofol group (P < 0.001). In addition to the gastrointestinal lesions found during the gastroscopy and colonoscopy procedures, the overall incidence of ADRs in the two groups was 31.3% and 62.8%, respectively (P < 0.001).
TABLE 4.
Adverse drug reactions (ADRs, >2%) during colonoscopy and gastroscopy
| ADRs, n (%) | Ciprofol (n = 144) | Propofol (n = 145) | ||||||
|---|---|---|---|---|---|---|---|---|
| All | Mild | Moderate | Severe | All | Mild | Moderate | Severe | |
| Elevation of conjugated bilirubin | 4 (2.8) | 4 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prolongation of QT interval | 3 (2.1) | 3 (2.1) | 0 (0.0) | 0 (0.0) | 2 (1.4) | 2 (1.4) | 0 (0.0) | 0 (0.0) |
| Respiratory depression | 4 (2.8) | 2 (1.4) | 2 (1.4) | 0 (0.0) | 8 (5.5) | 8 (5.5) | 0 (0.0) | 0 (0.0) |
| Apnea | 2 (1.4) | 2 (1.4) | 0 (0.0) | 0 (0.0) | 6 (4.1) | 6 (4.1) | 0 (0.0) | 0 (0.0) |
| Hypoxemia | 4 (2.8) | 2 (1.4) | 2 (1.4) | 0 (0.0) | 7 (4.8) | 5 (3.4) | 3 (2.1) | 0 (0.0) |
| Pain on injection | 7 (4.9) | 7 (4.9) | 0 (0.0) | 0 (0.0) | 76 (52.4) | 76 (52.4) | 0 (0.0) | 0 (0.0) |
| Sinus bradycardia | 3 (2.1) | 0 (0.0) | 3 (2.1) | 0 (0.0) | 2 (1.4) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Hypotension | 18 (12.5) | 16 (11.1) | 2 (1.4) | 0 (0.0) | 11 (7.6) | 9 (6.2) | 2 (1.4) | 0 (0.0) |
| Dizziness | 6 (4.2) | 6 (4.2) | 0 (0.0) | 0 (0.0) | 9 (6.2) | 9 (6.2) | 0 (0.0) | 0 (0.0) |
Note: ADRs, adverse drug reactions; AEs, adverse events.
In the colonoscopy group, 34 patients (26.4%) experienced 45 ADRs in the ciprofol group, and 31 patients (23.8%) exhibited 46 ADRs in the propofol group. The most commonly reported events included hypotension (in 17 patients [13.2%] in the ciprofol group and in 10 patients [7.7%] in the propofol group), sinus bradycardia (in 3 patients [2.3%] in the ciprofol group and 2 patients [1.5%] in the propofol group), dizziness (in 4 patients [3.1%] in the ciprofol group and 8 patients [6.2%] in the propofol group), elevated conjugated bilirubin (in 3 patients [2.3%] in the ciprofol group), prolonged QT intervals (3 patients [2.3%] in the ciprofol group and 2 patients [1.5%] in the propofol group), respiratory depression (2 patients [1.6%] in the ciprofol group and 6 patients [4.6%] in the propofol group), apnoea (2 patients [1.6%] in the ciprofol group and 6 patients [4.6%] in the propofol group) and hypoxemia (2 patients [1.6%] in the ciprofol group and 4 patients [3.1%] in the propofol group). There were four patients in the ciprofol and two in the propofol group who received atropine for bradycardia, while two patients in the ciprofol group and two in the propofol group received ephedrine to treat hypotension.
In the gastroscopy group, ADRs occurred in 7 patients (7 of 15) in the ciprofol group and 4 (4 of 15) in the propofol group. The most commonly reported ADRs were hypoxemia, respiratory depression, dizziness, hypotension and elevated conjugated bilirubin. There were four patients in the ciprofol group and three in the propofol group who experienced hypoxemia or respiratory depression and who needed the chin lift manoeuvre.
There were no differences between the groups with regard to the incidence of clinically relevant abnormalities in laboratory test results such as aspartate amino transferase, glutamic‐pyruvic transaminase and conjugated bilirubin levels, or electrocardiographic findings.
4. DISCUSSION
This trial demonstrated that ciprofol 0.4 mg/kg was non‐inferior to 1.5 mg/kg propofol in the success rate of gastroscopy or colonoscopy. Except for the gastrointestinal lesions found during the gastroscopy and colonoscopy procedures, the overall incidence of ADRs in the ciprofol and propofol groups were 31.3% and 62.8%, respectively (P < 0.001). No patients withdrew from the trial because of severe AEs.
Propofol is one of the most commonly used agents for endoscopic sedation, either used alone or in combination with opioids. 21 Several studies reported the combination of fentanyl and other adjunctive analgesics to reduce the total dose of sedative required and also reduce serious AEs. 1 , 21 , 22 Accordingly, 50‐μg fentanyl was given prior to ciprofol or propofol in the present trial. During the procedure, all patients were maintained with a sedation level MOAA/S scores of ≤1. There were no requirements for an alternative sedative and no more than five top‐ups in any 15‐min period in either group during the procedure. The number of top‐up doses was also identical between the two groups. Notably, one patient in the ciprofol group received seven top‐up doses as a result of colonic polypectomy, and the procedure lasted for 67 min.
We found that the colonoscopy insert time under ciprofol was similar in the present study compared to a previous phase 2 study (1.5 vs. 1.9 min), but the fully alert time (3.2 ± 3.2 vs. 6.1 ± 4.5 min) and recovery time (7.2 ± 3.2 vs. 11.8 ± 4.4 min) in this phase 3 trial were shorter than in the previous phase 2 study. These data indicated that the higher number of patients assessed in the present study led to a results shift, particularly with regard to the fully alert time but also the recovery time, which should be taken into consideration when further clinical studies of ciprofol are conducted. In the present trial, ciprofol exhibited a rapid onset of action which was similar to that of propofol, but the patients in the ciprofol group had a longer recovery time during colonoscopy. No substantial difference was found in the recovery or discharge times after gastroscopy in the two groups. Such a discrepancy could be attributed to a longer colonoscopy procedure time (vs. gastroscopy) and thus the requirement for more top‐up doses. Such a finding is consistent with the longer duration of action and recovery time of ciprofol in a preclinical study. 14 Despite the longer times to full alertness and discharge, the ready for discharge time from the end of the surgical procedure was <15 min, which was not clinically significant. The overall incidence of AEs was similar in the two groups, and no severe AEs were observed. The most common AEs included hypotension, bradycardia, dizziness, apnoea, hypoxemia, hyperbilirubinemia and a prolonged QT interval. The AEs were generally mild to moderate in nature and did not interfere with the surgical procedure. The most concerning sedation‐related AEs are those associated with cardiovascular and respiratory systems. Ciprofol had a similar effect on the cardiovascular system to propofol which could produce transient hypotension. Several mechanisms may be involved including peripheral vasodilation, 23 or a reduction in ventricular preload, 24 sympathetic activity 25 or myocardial contractility. 26 Whether ciprofol has similar mechanisms of action to propofol still remains uncertain and requires further research. Hypoxia may have resulted from respiratory depression, apnoea or airway obstruction, with an incidence of 1.5–70%, which made it the most common cardiorespiratory complication during endoscopy. 27 In the present trial, respiratory complications (including respiratory depression, apnoea and hypoxemia) were rarer in the ciprofol group compared to the propofol group. We speculate that ciprofol may produce less respiratory depression in the CNS or airway collapse. However, this speculation requires further research in the near future. Laboratory tests such as aspartate amino transferase, glutamic‐pyruvic transaminase, conjugated bilirubin and electrocardiographic findings revealed no clinically significant differences between the two groups in the incidence of out‐of‐range values, all apparently related to pre‐procedure fasting and bowel preparation and therefore required no treatment.
The most common AE elicited by propofol was pain at the injection site, with an incidence as high as 70%. 28 , 29 In the present trial, the rate of pain at injection site was 4.9% in the ciprofol group vs. 52.4% in the propofol group, which may be due to emulsion modification; that is, under the same conditions and at the same concentrations, ciprofol has a lower free drug concentration in the aqueous phase. In the trial, most patients reported being satisfied with their sedation experience. Interestingly, patients in the ciprofol group had much higher satisfaction scores (P < 0.05), which may be related to less pain on injection.
The present trial had several limitations. First, we defined the success of sedation as the need to administer no more than five top‐up doses of ciprofol or propofol within any 15‐min period to permit completion of the surgical procedure. The findings are reasonable, because most diagnostic gastroscopy or colonoscopy procedures are completed within 15 min and may overestimate the success rates of sedation. As a matter of fact, few patients needed more than three top‐up doses during a procedure in the present trial. Second, the level of sedation (MOAA/S score ≤ 1) may have been too deep in the trial. In western countries, moderate sedation is the target level of sedation in patients undergoing diagnostic endoscopy, while most painless gastrointestinal endoscopy procedures are performed under deep sedation in China. 30 , 31 However, there is no consensus regarding the most appropriate sedation used; it should be based on the local personnel and equipment available. Third, we assessed the sedation level using MOAA/S scores instead of BIS monitoring. Although research has shown that BIS monitor values correlate significantly with the MOAA/S score, 32 patients were prone to develop unexpected general anaesthesia with bolus administration of sedatives. They may require cardiopulmonary system support, but the cost‐effect benefit of BIS monitoring during gastrointestinal sedation should be considered. Finally, this trial was conducted in patients with ASA I or II, and whether the findings can be extrapolated to patients with higher ASA classifications requires further investigation.
5. CONCLUSION
With a dose of 0.4 mg/kg for deep sedation, ciprofol was non‐inferior to 1.5 mg/kg propofol in the success rate of gastroscopy and colonoscopy and exhibited a good safety profile.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Table S1. Inclusion and exclusion criteria.
Table S2. Modified Observer's Assessment of Alertness/Sedation Scale score (MOAA/S).
Table S3. Patient satisfaction questionnaire.
ACKNOWLEDGEMENTS
The research was funded and sponsored by the Haisco Pharmaceutical Group Co., Ltd. Thanks to all investigators from the following units for their great efforts in helping to complete the study: Affiliated Hospital of Zunyi Medical University; The Second Xiangya Hospital of Central South University; Sichuan Provincial People's Hospital, Sichuan Academy of Medical Sciences; The Third Xiangya Hospital of Central South University; Xiangya Hospital, Central South University; The 2nd Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University; Union Hospital, Tongji Medical College, Huazhong University of Science and Technology; General Hospital of Ningxia Medical University; Tianjian Medical University General Hospital. We are also grateful to Xiaoqing Li and Yang Zhang (Medkey Pharmaceutical Co., Ltd. Sichuan, China), Jiayu Yang (Fountain Medical Development Co., Ltd) for their assistance with data collection.
Li J, Wang X, Liu J, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: A multi‐centre, non‐inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022;131(2):138‐148. doi: 10.1111/bcpt.13761
Junxiang Li and Xiao Wang contributed equally to this manuscript.
Funding information Haisco Pharmaceutical Group Co., Ltd
Contributor Information
Jin Liu, Email: scujinliu@gmail.com.
Yunxia Zuo, Email: zuoyunxia@scu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Childers RE, Williams JL, Sonnenberg A. Practice patterns of sedation for colonoscopy. Gastrointest Endosc. 2015;82(3):503‐511. doi: 10.1016/j.gie.2015.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Froehlich F, Harris JK, Wietlisbach V, Burnand B, Vader JP, Gonvers JJ. Current sedation and monitoring practice for colonoscopy: an International Observational Study (EPAGE). Endoscopy. 2006;38(5):461‐469. doi: 10.1055/s-2006-925368 [DOI] [PubMed] [Google Scholar]
- 3. Porostocky P, Chiba N, Colacino P, Sadowski D, Singh H. A survey of sedation practices for colonoscopy in Canada. Can J Gastroenterol. 2011;25(5):255‐260. doi: 10.1155/2011/783706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vargo JJ, Zuccaro G Jr, Dumot JA, et al. Gastroenterologist‐administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology. 2002;123(1):8‐16. doi: 10.1053/gast.2002.34232 [DOI] [PubMed] [Google Scholar]
- 5. Vargo JJ. Propofol: a gastroenterologist's perspective. Gastrointest Endosc Clin N am. 2004;14(2):313‐323. doi: 10.1016/j.giec.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 6. Coté GA, Hovis RM, Ansstas MA, et al. Incidence of sedation‐related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8(2):137‐142. doi: 10.1016/j.cgh.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 7. Amornyotin S. Sedation and monitoring for gastrointestinal endoscopy. World J Gastrointest Endosc. 2013;5(2):47‐55. doi: 10.4253/wjge.v5.i2.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamperti M. Adult procedural sedation: an update. Curr Opin Anaesthesiol. 2015;28(6):662‐667. doi: 10.1097/ACO.0000000000000244 [DOI] [PubMed] [Google Scholar]
- 9. Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998;53(5):468‐476. doi: 10.1046/j.1365-2044.1998.00405.x [DOI] [PubMed] [Google Scholar]
- 10. Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427‐437.e426. doi: 10.1016/j.gie.2018.04.2351 [DOI] [PubMed] [Google Scholar]
- 11. Cohen LB, Cattau E, Goetsch A, et al. A randomized, double‐blind, phase 3 study of fospropofol disodium for sedation during colonoscopy. J Clin Gastroenterol. 2010;44(5):345‐353. doi: 10.1097/MCG.0b013e3181c2987e [DOI] [PubMed] [Google Scholar]
- 12. Valk BI, Absalom AR, Meyer P, et al. Safety and clinical effect of i.v. infusion of cyclopropyl‐methoxycarbonyl etomidate (ABP‐700), a soft analogue of etomidate, in healthy subjects. Br J Anaesth. 2018;120(6):1401‐1411. doi: 10.1016/j.bja.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 13. Teng Y, Ou M, Wang X, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi‐center clinical trials. Eur J Pharm Sci. 2021;164:105904 doi: 10.1016/j.ejps.2021.105904 [DOI] [PubMed] [Google Scholar]
- 14. Qin L, Ren L, Wan S, et al. Design, synthesis, and evaluation of novel 2,6‐disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60(9):3606‐3617. doi: 10.1021/acs.jmedchem.7b00254 [DOI] [PubMed] [Google Scholar]
- 15. Teng Y, Ou MC, Wang X, et al. Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: a single center, open‐label, single‐arm dose‐escalation phase 1 study. Am J Transl Res. 2021;13(12): PMID: 13791–13802. [PMC free article] [PubMed] [Google Scholar]
- 16. Tveden‐Nyborg P, Bergmann TK, Jessen N, Simonsen U, Lykkesfeldt J. BCPT policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2021;128(1):4‐8. doi: 10.1111/bcpt.13492 [DOI] [PubMed] [Google Scholar]
- 17. Digestive Endoscopy Special Committee of Endoscopic Physicians Branch of Chinese Medical Association , Cancer Endoscopy Committee of China Anti‐Cancer Association . Chinese guideline for bowel preparation for colonoscopy (2019, Shanghai). Zhonghua Nei Ke Za Zhi. 2019;58(7):485‐495. [DOI] [PubMed] [Google Scholar]
- 18. Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353‐370. [Google Scholar]
- 19. Fridericia L. Duration of systole in electrocardiogram. Acta Med Scand. 1920;53:469‐486. doi: 10.1111/j.0954-6820.1920.tb18266.x [DOI] [Google Scholar]
- 20. Chen S, Wang J, Xu X, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive‐controlled, phase III clinical trial. Am J Transl Res. 2020;12(8):4594‐4603. [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou S, Zhu Z, Dai W, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. 2021;127(1):56‐64. doi: 10.1016/j.bja.2021.01.028 [DOI] [PubMed] [Google Scholar]
- 22. Dossa F, Medeiros B, Keng C, Acuna SA, Baxter NN. Propofol versus midazolam with or without short‐acting opioids for sedation in colonoscopy: a systematic review and meta‐analysis of safety, satisfaction, and efficiency outcomes. Gastrointest Endosc. 2020;91(5):1015‐1026.e1017. doi: 10.1016/j.gie.2019.12.047 [DOI] [PubMed] [Google Scholar]
- 23. Boer F, Ros P, Bovill JG, van Brummelen P, van der Krogt J. Effect of propofol on peripheral vascular resistance during cardiopulmonary bypass. Br J Anaesth. 1990;65(2):184‐189. doi: 10.1093/bja/65.2.184 [DOI] [PubMed] [Google Scholar]
- 24. Lepage JY, Pinaud ML, Helias JH, Cozian AY, le Normand Y, Souron RJ. Left ventricular performance during propofol or methohexital anesthesia: isotopic and invasive cardiac monitoring. Anesth Analg. 1991;73(1):3‐9. doi: 10.1213/00000539-199107000-00002 [DOI] [PubMed] [Google Scholar]
- 25. Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76(5):725‐733. doi: 10.1097/00000542-199205000-00010 [DOI] [PubMed] [Google Scholar]
- 26. Coetzee A, Fourie P, Coetzee J, et al. Effect of various propofol plasma concentrations on regional myocardial contractility and left ventricular afterload. Anesth Analg. 1989;69(4):473‐483. [PubMed] [Google Scholar]
- 27. Qadeer MA, Lopez AR, Dumot JA, Vargo JJ. Hypoxemia during moderate sedation for gastrointestinal endoscopy: causes and associations. Digestion. 2011;84(1):37‐45. doi: 10.1159/000321621 [DOI] [PubMed] [Google Scholar]
- 28. Jalota L, Kalira V, George E, et al. On behalf of the Perioperative Clinical Research CorePrevention of pain on injection of propofol: systematic review and meta‐analysis. BMJ. 2011;342(mar15 1):d1110 doi: 10.1136/bmj.d1110 [DOI] [PubMed] [Google Scholar]
- 29. Picard P, Tramèr MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90(4):963‐969. [DOI] [PubMed] [Google Scholar]
- 30. Dumonceau JM, Riphaus A, Schreiber F, et al. Non‐anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline—Updated June 2015. Endoscopy. 2015;47(12):1175‐1189. doi: 10.1055/s-0034-1393414 [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Deng X, Liu J. Consensus of Chinese experts on sedation and anesthesia for digestive endoscopic diagnosis. Chin J Pract Intern Med. 2014;34(34):756‐764. [Google Scholar]
- 32. Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109(6):1811‐1815. doi: 10.1213/ANE.0b013e3181c04e58 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria.
Table S2. Modified Observer's Assessment of Alertness/Sedation Scale score (MOAA/S).
Table S3. Patient satisfaction questionnaire.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


