Abstract
Measuring vascularization in uterine fibroids is important for their diagnosis, treatment and prognosis. Vascularization can be measured by power Doppler ultrasound. The power Doppler signal depends on fibroid characteristics and on a variety of ultrasound‐machine settings. Literature describing which machine settings influence the power Doppler signal is limited. Each manufacturer names settings and presets at their own discretion, with little information available publicly. Consistency of machine settings is important for correct interpretation of images in daily practice and is essential in yielding reproducible data for research. The aims of this paper, drawing from both a literature search and semistructured interviews with ultrasound‐machine engineers and clinical experts in gynecological ultrasound, were: (1) to provide comprehensive background information on ultrasound physics and fibroid characteristics; (2) to present an overview of machine settings relevant to both two‐ and three‐dimensional power Doppler, including power Doppler frequency, pulse repetition frequency, gain, wall‐motion filter, acoustic power, persistence and signal rise; and (3) to provide a step‐by‐step tutorial on the optimal settings for vascular evaluation of uterine fibroids using power Doppler. The step‐by‐step tutorial comprises six steps to optimize the power Doppler signal, create a preset and acquire a reliable three‐dimensional volume. This step‐by‐step tutorial should help research groups and clinicians to use power Doppler correctly and reproducibly in the evaluation of uterine fibroids. © 2022 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: fibroid, power Doppler, ultrasound, vascularity, vascularization index
How to .…Practical advice on imaging‐based techniques and investigations with accompanying supporting information online
Background
Measurement of vascularization in uterine fibroids is important for their diagnosis and management. Evaluation of the vascularization pattern enables differentiation between fibroids and adenomyosis and between different histological types of fibroid 1 , 2 . The extent of a fibroid's vascularization is associated with clinical symptoms and growth potential 3 , 4 , 5 . Fibroid vascularization also has an influence on treatment response 6 , 7 , 8 . The importance of reporting fibroid vascularization has been emphasized by the ‘Morphological Uterus Sonographic Assessment’ (MUSA) working group 9 .
Doppler ultrasound is a sonographic imaging technique used for blood flow measurements. Details on Doppler physics can be found in Appendix S1. Fibroids have a distinctive vascularization pattern, including a highly vascularized peripheral rim and a core containing many slow‐flow capillaries. Other characteristics of fibroids important for power Doppler imaging are summarized in Appendix S2. The degree of vascularization may be reported using a subjective color score on two‐dimensional (2D) power Doppler ultrasound imaging 9 . This color score is easy to apply in daily practice, but depends on the observer's subjective appreciation and experience. Compared with color Doppler, power Doppler is three times more sensitive in detecting low flow velocities 10 , 11 . In addition, three‐dimensional (3D) power Doppler represents in a single image the amount and distribution of blood vessels in a fibroid. Furthermore, in contrast to a subjective color score, 3D power Doppler can provide an objective measurement of vascularity using Virtual Organ Computer‐aided AnaLysis (VOCAL™) software 9 . This ‘vascularization index’ is a ratio that is calculated automatically, and represents the amount of vasculature per unit surface area. The index is reproducible, is able to discriminate vascularity and has good correlation with histology 12 , 13 , 14 .
However, both subjective and objective quantification of vascularization depend on the settings and type of machine used. Transvaginal ultrasound is the primary technique for imaging uterine fibroids, and reveals features that differ from those on transabdominal ultrasound, the latter being required for large fibroids. Each manufacturer names settings and presets at their own discretion, with little public information available, adding to the complexity of the various different settings. Consistency of machine settings is important for correct interpretation of images in daily practice and is essential in yielding reproducible research data.
Herein, we aim to present an overview of machine settings relevant to both 2D and 3D power Doppler in the transvaginal ultrasound assessment of uterine fibroids, and provide a step‐by‐step tutorial. This tutorial is based on a literature search combined with semistructured interviews with ultrasound‐machine engineers and clinical experts in gynecological ultrasound (Appendix S3).
Practical points
The ultrasound machine settings of importance for 2D and 3D power Doppler are discussed herein taking into account ultrasound features (Appendix S1), fibroid characteristics (Appendix S2) and the literature (Table 1). First, we discuss the essential, adjustable machine settings, as well as relevant, but mostly standardized, settings, for performing 2D power Doppler, including color score. It should be borne in mind that there may be other ultrasound machine settings that might influence the power Doppler signal. Second, we provide tips for acquiring a 3D power Doppler volume to calculate the vascularization index. Definitions of the machine settings and details of how they affect the Doppler signal are presented in Table 2. Step‐by‐step instructions for optimal settings are summarized in Figure 1. Illustrative examples of ultrasound views are presented in Figure 2.
Table 1.
Ultrasound (US) machine settings described in the literature and their reported effect on power Doppler (PD) outcome
| Reference | Type of target | US machine | Blood flow velocity (cm/s) | PRF (kHz) | Gain | WMF (Hz) | PD frequency (MHz) | Signal power | Persistence/ signal rise | Speed of acquisition |
|---|---|---|---|---|---|---|---|---|---|---|
| Martins (2018) 22 | Phantom | Sonix | 6.0–40.0 | 0.6–10.0 | NR | 50–250 | 5.0 | NR | NR | NR |
| Nieuwenhuis (2018) 28 | Fibroids | Accuvix | NR | 0.6 | 50 dB | Low | 5.0–8.0 | NR | NR | NR |
| Soares (2016) 19 | Phantom | Voluson | 30.0 | 0.3–7.5 | 0 | Low | 5.0–9.0 | 100% | 2/2 | NR |
| Miyague (2015) 25 | Carotid artery | Voluson | NR | 0.6–9.0 | 0 | Mid | NR | 100% | 2/2 | NR |
| Sonix | NR | 0.6–10.0 | NR | 0–1500 | 5.0 | NR | NR | NR | ||
| Miyague (2013) 26 | Phantom | Voluson | 8.0–30.0 | 1.8 | –8 to + 8 dB | Mid | 5.0–9.0 | 100% | 2/2 | NR |
| Martins (2010) 27 | Phantom | Voluson | 0 | 0.6; 0.9 | –15 to + 15 dB | Low | 5.0–9.0 | NR | NR | NR |
| Raine‐Fenning (2008) 21 | Phantom | Voluson | 2.2 | 0.5–5.1 | 25–44 dB | 176–341 | 7.5 | –8 to + 4 dB | 0.1–1.2 | Slow–fast |
| Schulten‐Wijman (2008) 20 | Phantom | Voluson | 0.84–9.0 | 0.1–5.0 | –15 to + 15* | Min–max | 4.3–7.5 | NR | NR | NR |
| Mizushige (1999) 33 | Phantom | Aloka | 14 | Fixed | Fixed | NR | 7.5 | NR | NR | NR |
| Yoon (1999) 23 | Phantom | ATL HDI | 13.3–49.8 | 0.5–6 | 60–100% | Low–max | 5.0–10.0 | NR | NR | NR |
| Hoskins (1998) 30 | Phantom | Acuson | 60.0–80.0 | NR | 25–75* | NR | 0.0–2.0 | NR | 0.0–5.0 | NR |
Only first author of each study is given.
Reported as an index.
NR, not reported; PRF, pulse repetition frequency; WMF, wall‐motion filter.
Table 2.
Definitions of machine settings that influence power Doppler (PD) signal
| Step | Setting (unit) | Definition | Relation with PD signal | Optimal setting | If setting is lower than optimal | If setting is higher than optimal |
|---|---|---|---|---|---|---|
| 1a | Focus | Ultrasound waves converge to this point | Negative | Just behind target | Less differentiation of adjacent features/tissue | Delay in displaying Doppler signal |
| 1b | Depth/field‐of‐view | Distance and area where signal is displayed | Negative | Minimum | Incomplete image of fibroid | Delay in displaying Doppler signal |
| 1c | PD frequency (MHz) | Frequency transmitted by probe | Negative | Minimum | Lower resolution | Lower penetration |
| 2 | PRF (kHz) | Rate of pulses transmitted | Negative | 0.3–0.4 | Aliasing | No detection of low velocity |
| 3 | Gain (%, dB) | Amplifies incoming Doppler signal | Positive | Increase until noise artifacts appear, then lower until they just disappear | True PD signal not visible | Electronic and mirror‐image artifacts |
| 4 | WMF (Hz) | Clutter filter* for tissue signal | Negative | Minimum | False PD signal from tissue | True PD signal filtered out |
| 5a | Acoustic power (%) | Amplitude of pulse pressure | Positive | Maximum | Lower sensitivity | Safety issues |
| 5b | Persistence (index) | Averaging frames | Positive | Middle | Flow movements not visible | Delay in displaying subjective flow |
| Signal rise (index) | Temporal averaging technique | Negative | Middle | Noise artifacts | Afterimages | |
| 6 | Speed of acquisition (index) | Speed of constructing 3D volume | Negative | Quality medium–high, maximum scanning time 15 s | Movement artifacts | Flow movements not visible |
3D, three‐dimensional; PRF, pulse repetition frequency; WMF, wall‐motion filter.
Clutter filter removes low‐frequency Doppler signal.
Figure 1.
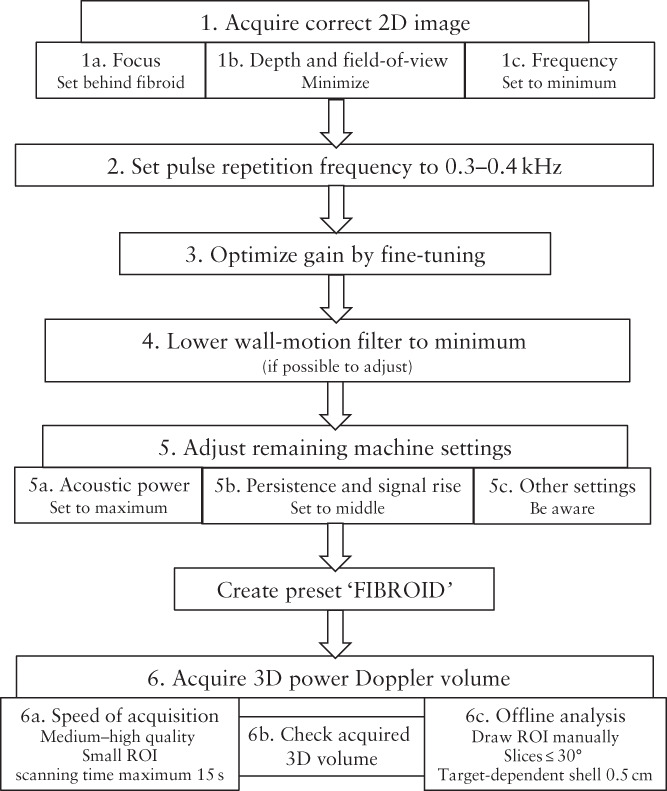
Flowchart summarizing step‐by‐step recommendations for adjusting settings to optimize power Doppler signal, creating a customized ‘FIBROID’ preset and acquiring a reliable three‐dimensional (3D) volume in transvaginal assessment of uterine fibroids. The two‐dimensional (2D) field‐of‐view is defined by the scanning area or box and the scanning angle of the probe. While the 3D region of interest (ROI) is also determined by the scanning area or box and the scanning angle, in the 3D ROI, these are perpendicular to each other, with the scanning area or box orientated left to right and the scanning angle from back to front.
Figure 2.
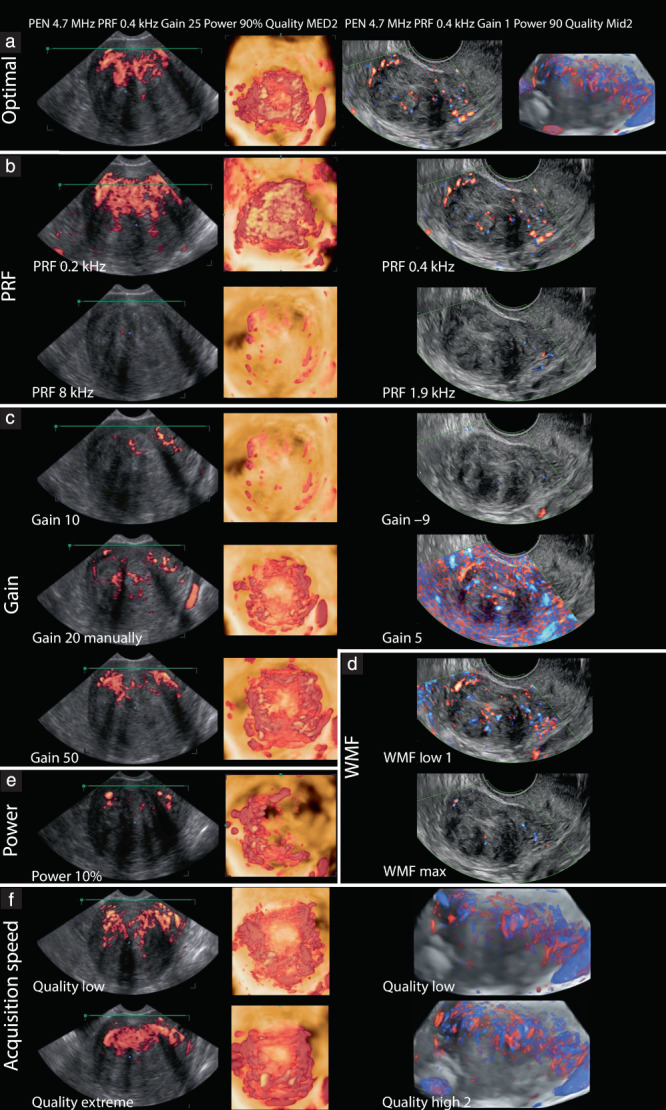
Examples of ultrasound views illustrating effect of adjusting different machine settings on power Doppler transvaginal imaging of uterine fibroids. In left column are sagittal power Doppler images, obtained using WS80 Samsung ultrasound machine; in middle column are three‐dimensional (3D) reconstructions performed onsite with same ultrasound machine; in right column are directional Doppler sagittal images or 3D reconstructions obtained using GE Voluson ultrasound machine. (a) Optimal imaging. (b–f) Effect of adjusting: (b) pulse repetition frequency (PRF); (c) gain; (d) wall‐motion filter (WMF); (e) power; (f) speed of acquisition or quality. MED, medium; Mid, mid range; PEN, penetration.
Step 1. Acquire correct 2D image: focus, field‐of‐view, depth and frequency
Set the focus just behind the fibroid for optimal lateral resolution; features adjacent to it will be better distinguished if they lie within the area of convergence of the ultrasound waves 15 , 16 . Minimize the depth and the field‐of‐view, defined by the scanning area and angle, to maintain a frame rate that will achieve optimal temporal resolution. A wide field‐of‐view delays the display of the Doppler signal 17 , 18 . The field‐of‐view should contain the entire vascular pseudocapsule and the area close to the probe to prevent reverberation artifacts 18 . B‐mode and Doppler frequency should be set as low as possible, resulting in high penetration.
Step 2. Optimize pulse repetition frequency to 0.3–0.4 kHz
The pulse repetition frequency (PRF) is the most important machine setting to adjust. The PRF is the rate of pulses transmitted consecutively, not to be confused with the ultrasound frequency of those pulses. The measureable blood flow velocity depends on the PRF according to the formula: velocity = distance × PRF, where distance is the difference between the depths of the target at two consecutive pulses. When the PRF is set too high, the power Doppler signal of low blood flow velocity, such as in fibroids, will not be detected. The PRF should not be set lower than half of the maximum velocity, to avoid aliasing (Appendix S1) 15 . In general, the PRF should be set as low as possible 19 , 20 , 21 , 22 , 23 , 24 . The PRF is associated negatively with the power Doppler signal (Figure 2b)19–22,25.
Step 3. Optimize power Doppler gain
The gain works as an amplifier of incoming Doppler signals 26 . Gain set too low might result in failure to visualize the blood flow, while gain set too high can result in electronic noise artifacts being displayed, even in the absence of flow. Also, mirror‐image artifacts can occur when the gain is set too high and the ultrasound machine can no longer distinguish the correct direction of flow, displaying the Doppler signal in the opposite or an incorrect direction 15 . Increasing the gain increases the 3D power Doppler signal (Figure 2c) 21 , 23 , 26 , 27 , 28 . The signal can be fine‐tuned by increasing the gain until noise artifacts become visible and then lowering the gain until the artifacts have just disappeared. Standardization of gain values can be considered if, within a hospital, only one type of machine with one specific setting is used 28 .
Step 4. Set wall‐motion filter to minimum
The wall‐motion filter removes low‐frequency Doppler signals 21 . It is used, for example, to filter out the reverberation of an arterial wall, which may interfere with the Doppler signal of arterial blood flow. However, it may remove true low‐frequency slow flow in small fibroid vessels 15 . The wall‐motion filter is related inversely to the power Doppler signal (Figure 2d) 20 , 21 , 22 , 23 . When measuring low‐velocity blood flow, it is recommended to use the lowest wall‐motion filter possible 20 .
Step 5. Adjust remaining machine settings
Acoustic power
Additional settings may be adjusted, although this depends on the type of machine that is used. In some machines, these settings are fixed and cannot be adjusted. The acoustic or signal power has an influence on the power Doppler signal comparable to that of the gain 21 . The acoustic power is equal to the amplitude of the pulse pressure of the ultrasound beam multiplied by the displacement velocity 29 . Increasing the acoustic power may boost the Doppler signal, but most machines have an upper limit for reasons of safety. Some machines do not allow any adjustment. The acoustic power and 3D power Doppler signal are positively related (Figure 2e) 21 .
Persistence and signal rise
Persistence and signal rise are averaging techniques that influence detection of the power Doppler signal 21 . Persistence or ‘frame averaging’ is the averaging of frames acquired successively in order to reduce noise 15 . A higher index involves more averaging and therefore a greater number of previous frames is included in the averaged image 30 . When the persistence is increased, there is a delay in visualizing the Doppler flow. If the persistence is too low, blood flow cannot be detected and movements are missed. Details on which index corresponds to a particular number of frames on the weighted factor applied are not always shared by the manufacturer and this depends on the type of machine. Increasing the persistence moderately will result in a somewhat higher power Doppler signal 21 . Optimal persistence is mid‐range 30 .
Signal rise is another type of averaging technique. Increasing the signal rise delays the time at which the Doppler signal first appears. Afterimages can occur when a high signal rise is used, while too low a signal rise results in an image with unnatural‐looking flow and noise artifacts. A very high signal rise results in a decrease in the power Doppler signal 21 . Yet, low‐velocity blood flow is better visualized with a higher signal rise, which corresponds to greater frame averaging.
Step 6. Acquire 3D power Doppler volume
Speed of acquisition
The speed of acquisition defines the resolution or sensitivity of the 3D scan to detect flow. Also known as ‘quality’, the acquisition speed, along with the 3D region of interest, determines the scanning time. A low speed of acquisition (high quality) of a large 3D region of interest results in a long scanning time. The longer the scanning time, the higher the probability of movement artifacts, for example resulting from the patient breathing. The 3D region of interest is limited and its size varies between selected ultrasound probes 17 . The scanning area visible on the displayed plane, in combination with the scanning angle perpendicular to this plane, defines the 3D region of interest. The speed of acquisition is negatively related to the 3D power Doppler signal (Figure 2f) 21 . On most ultrasound machines, the speed of acquisition (quality) is expressed as an index. Details regarding which settings correspond to which resolutions and speeds are not made public by the manufacturers.
Check acquired 3D volume
It is important to check the acquired 3D power Doppler volume before saving it. There are multiple buttons on the ultrasound machine dashboard which allow adjustment of the volume in the sagittal, transverse and coronal planes. The 3D volume should include the entire fibroid; when this is not the case, or the 3D volume shows artifacts, the region of interest should be adjusted. This may affect the speed of acquisition. If artifacts remain or insufficient Doppler signal is visible, further adjusting one of the ultrasound settings discussed herein should be considered.
Offline analysis
After all settings have been optimized and the 3D image acquired, the data can be analyzed by VOCAL software. This software is applied mostly in the research setting to semiquantify vascularity and it is applicable to images acquired by machines from multiple manufacturers. The vascularization index represents the amount of vasculature per unit surface area, by calculating the proportion of color voxels (as a proxy for the blood vessels) relative to the total number of voxels, including both gray and colored ones (as a proxy for tissue and blood vessels) 21 . The software offers several options during the steps of the calculation and the choice of options will influence the value of the vascularization index. First, a region of interest can be defined automatically or manually, the latter being the preferred method 12 , using a certain number of virtual slices around a central axis. The most reliable is to use rotation steps of 9°, which is the smallest step size possible. For large uterine fibroids we advise using steps of 30° in order to be time‐effective while remaining sufficiently accurate. While delineating the region of interest, care should be taken to include the vascular pseudocapsule correctly. The vascular arcade of the uterus or uterine artery is easily mistaken for the fibroid's pseudocapsule on a Doppler image. Vascular indices are calculated automatically by a ‘histogram facility’. The histogram can include voxels of the total volume, or of a certain predefined ‘shell’, both based on the region of interest 31 . Whether to use the outer shell (Figure 3a), symmetric shell (Figure 3b), inner shell (Figure 3d) or no shell (Figure 3c) depends on the target 12 . For example, to calculate the fibroid pseudocapsule's flow, an outer shell of 0.5 cm is optimal 28 .
Figure 3.
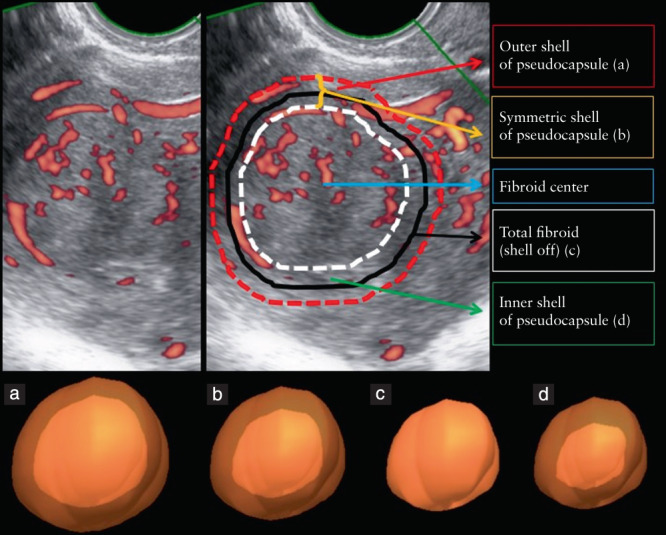
Power Doppler image of uterine fibroid, illustrating calculation of vascularization index. Sagittal view (top) and three‐dimensional reconstructions of regions of interest (bottom). Black line (c) corresponds to region of interest; this contour is delineated manually by the operator. Remaining lines (a,b,d) indicate shells applied automatically by VOCAL™ software. (a) Outer shell of pseudocapsule; (b) symmetric shell of pseudocapsule; (c) total fibroid (shell off); (d) inner shell of pseudocapsule.
Conclusion
Using power Doppler ultrasound in the transvaginal assessment of uterine fibroids is challenging. We have composed step‐by‐step guidance to help research groups and clinicians to use power Doppler signals in the evaluation of uterine fibroids. We have identified and discussed relevant machine settings which influence the power Doppler signal.
Some challenges will remain even after following this step‐by‐step guide. For example, some settings are interdependent: when keeping one setting constant and adjusting a second to increase the Doppler signal, a third setting may need to be adjusted to prevent the Doppler signal from becoming too high 23 . Moreover, the low blood flow velocity in vessels of small diameter 3 , 20 , 32 might be at the lower limit of what is technically possible to measure by power Doppler. Measuring multiple or large fibroids might be complex due to attenuation in fibroids more than 8–10 cm in diameter 4 and to the increased distance from the transducer to the proximal side of the fibroid 27 .
Despite these challenges, this step‐by‐step tutorial should contribute to the correct use and improved reproducibility of power Doppler ultrasound in the transvaginal assessment of uterine fibroids.
Supporting information
Appendix S1 Background information on ultrasound and Doppler physics
Appendix S2 Transvaginal power Doppler assessment of uterine fibroids
Appendix S3 Methods for selection of machine settings that influence power Doppler signal
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sharma K, Bora MK, Venkatesh BP, Barman P, Roy SK, Jayagurunathan U, Sellamuthu E, Moidu F. Role of 3D Ultrasound and Doppler in Differentiating Clinically Suspected Cases of Leiomyoma and Adenomyosis of Uterus. J Clin Diagn 2015; 9: QC08–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanos V, Berry KE. Benign and malignant pathology of the uterus. Best Pract Res Clin Obstet Gynecol 2018; 46: 12–30. [DOI] [PubMed] [Google Scholar]
- 3. Idowu BM, Ibitoye BO. Doppler sonography of perifibroid and intrafibroid arteries of uterine leiomyomas. Obstet Gynecol Sci 2018; 61: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieuwenhuis LL, Keizer AL, Stoelinga B, Twisk J, Hehenkamp W, Brölmann H, Huirne J. Fibroid vascularisation assessed with three‐dimensional power Doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG 2018; 125: 577–584. [DOI] [PubMed] [Google Scholar]
- 5. Keizer AL, Nieuwenhuis LL, Hehenkamp WJK, Twisk WJR, Brölmann HAM, Huirne JAF. Fibroid vascularisation assessed with 3D Power Doppler as predictor for fibroid related symptoms and quality of life; a pilot study. Facts Views Vis Obgyn 2021; 13: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleischer AC, Donnelly EF, Campbell MG, Mazer MJ, Grippo D, Lipsitz NL. Three‐dimensional color Doppler sonography before and after fibroid embolization. J Ultrasound Med 2000; 19: 701–705. [DOI] [PubMed] [Google Scholar]
- 7. McLucas B, Perrella R, Goodwin S, Adler L, Dalrymple J. Role of uterine artery Doppler flow in fibroid embolization. J Ultrasound Med 2002; 21: 113–120; quiz 122–123. [DOI] [PubMed] [Google Scholar]
- 8. Isonishi S, Coleman RL, Hirama M, Lida Y, Kitai S, Nagase M, Ochiai K. Analysis of prognostic factors for patients with leiomyoma treated with uterine arterial embolization. Am J Obstet Gynecol 2008; 198: 270.e1–6. [DOI] [PubMed] [Google Scholar]
- 9. Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, Van Schoubroeck D, Landolfo C, Installe AJ, Guerriero S, Exacoustos C, Gordts S, Benacerraf B, D'Hooghe T, De Moor B, Brolmann H, Goldstein S, Epstein E, Bourne T, Timmerman D. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015; 46: 284–298. [DOI] [PubMed] [Google Scholar]
- 10. Guo Z, Fenster A. Three‐dimensional power Doppler imaging: a phantom study to quantify vessel stenosis. Ultrasound Med Biol 1996; 22: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 11. Yoo J, Je BK, Choo JY. Ultrasonographic Demonstration of the Tissue Microvasculature in Children: Microvascular Ultrasonography Versus Conventional Color Doppler Ultrasonography. Korean J Radiol 2020; 21: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieuwenhuis LL, Betjes HE, Hehenkamp WJ, Heymans MW, Brolmann HA, Huirne JA. The use of 3D power Doppler ultrasound in the quantification of blood vessels in uterine fibroids: feasibility and reproducibility. J Clin Ultrasound 2015; 43: 171–178. [DOI] [PubMed] [Google Scholar]
- 13. Minsart AF, Ntoutoume Sima F, Vandenhoute K, Jani J, Van Pachterbeke C. Does three‐dimensional power Doppler ultrasound predict histopathological findings of uterine fibroids? A preliminary study. Ultrasound Obstet Gynecol 2012; 40: 714–720. [DOI] [PubMed] [Google Scholar]
- 14. Raine‐Fenning NJ, Nordin NM, Ramnarine KV, Campbell BK, Clewes JS, Perkins A, Johnson IR. Determining the relationship between three‐dimensional power Doppler data and true blood flow characteristics: an in‐vitro flow phantom experiment. Ultrasound Obstet Gynecol 2008; 32: 540–550. [DOI] [PubMed] [Google Scholar]
- 15. Hoskins PR, Martin K, Thrush A. Diagnostic Ultrasound: Physics and Equipment. Cambridge University Press: Cambridge, 2010; 4–21, 28, 35, 36, 60, 91–94, 107. [Google Scholar]
- 16. Ng A, Swanevelder J. Resolution in ultrasound imaging. Cont Educat Anaesth Crit Care Pain 2011; 11: 186–192. [Google Scholar]
- 17. Terslev L, Diamantopoulos AP, Døhn UM, Schmidt WA, Torp‐Pedersen S. Settings and artefacts relevant for Doppler ultrasound in large vessel vasculitis. Arthritis Res Ther 2017; 19: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torp‐Pedersen ST, Terslev L. Settings and artefacts relevant in colour/power Doppler ultrasound in rheumatology. Ann Rheumatic Dis 2008; 67: 143–149. [DOI] [PubMed] [Google Scholar]
- 19. Soares CA, Pavan TZ, Miyague AH, Kudla M, Martins WP. Influence of Pulse Repetition Frequency on 3‐D Power Doppler Quantification. Ultrasound Med Biol 2016; 42: 2887–2892. [DOI] [PubMed] [Google Scholar]
- 20. Schulten‐Wijman MJ, Struijk PC, Brezinka C, De Jong N, Steegers EA. Evaluation of volume vascularization index and flow index: a phantom study. Ultrasound Obstet Gynecol 2008; 32: 560–564. [DOI] [PubMed] [Google Scholar]
- 21. Raine‐Fenning NJ, Nordin NM, Ramnarine KV, Campbell BK, Clewes JS, Perkins A, Johnson IR. Evaluation of the effect of machine settings on quantitative three‐dimensional power Doppler angiography: an in‐vitro flow phantom experiment. Ultrasound Obstet Gynecol 2008; 32: 551–559. [DOI] [PubMed] [Google Scholar]
- 22. Martins MR, Martins WP, Soares CAM, Miyague AH, Kudla MJ, Pavan TZ. Understanding the Influence of Flow Velocity, Wall Motion Filter, Pulse Repetition Frequency, and Aliasing on Power Doppler Image Quantification. J Ultrasound Med 2018; 37: 255–261. [DOI] [PubMed] [Google Scholar]
- 23. Yoon DY, Choi BI, Kim TK, Han JK, Yeon KM. Influence of instrument settings on flow signal and background noise in power Doppler US. An experimental study using a flow phantom with hyperechoic background. Invest Radiol 1999; 34: 781–784. [DOI] [PubMed] [Google Scholar]
- 24. Frijlingh M, De Milliano I, Hehenkamp WJK, Huirne JAF. Differences in fibroid vascularity after three months of pre‐treatment with leuprolide acetate or ulipristal acetate: A pilot study. Eur J Obstet Gynaecol Reprod Biol 2020; 245: 186–192. [DOI] [PubMed] [Google Scholar]
- 25. Miyague AH, Pavan TZ, Soares CA, De Catte L, Nastri CO, Welsh AW, Martins WP. Importance of Pulse Repetition Frequency Adjustment for 3‐ and 4‐Dimensional Power Doppler Quantification. J Ultrasound Med 2015; 34: 2245–2251. [DOI] [PubMed] [Google Scholar]
- 26. Miyague AH, Raine‐Fenning NJ, Pavan TZ, Polanski LT, Baumgarten MN, Nastri CO, Martins WP. Influence of gain adjustment on 3‐dimensional power Doppler indices and on spatiotemporal image correlation volumetric pulsatility indices using a flow phantom. J Ultrasound Med 2013; 32: 1831–1386. [DOI] [PubMed] [Google Scholar]
- 27. Martins WP, Raine‐Fenning NJ, Ferriani RA, Nastri CO. Quantitative three‐dimensional power Doppler angiography: a flow‐free phantom experiment to evaluate the relationship between color gain, depth and signal artifact. Ultrasound Obstet Gynecol 2010; 35: 361–368. [DOI] [PubMed] [Google Scholar]
- 28. Nieuwenhuis LL, Hehenkamp WJK, Brolmann HAM, Huirne JAF. 3D power Doppler in uterine fibroids; influence of gain, cardiac cycle and off‐line measurement techniques. J Obstet Gynaecol 2018; 38: 103–109. [DOI] [PubMed] [Google Scholar]
- 29. Rabe H, Grohs B, Schmidt RM, Schloo R, Bömelburg T, Jorch G. Acoustic power measurements of Doppler ultrasound devices used for perinatal and infant examinations. Pediatr Radiol 1990; 20: 277–281. [DOI] [PubMed] [Google Scholar]
- 30. Hoskins PR, Prattis J, Wardlaw J. A flow model of cerebral aneurysms for use with power Doppler studies. Br J Radiol 1998; 71: 76–80. [DOI] [PubMed] [Google Scholar]
- 31. Raine‐Fenning NJ, Campbell BK, Clewes JS, Kendall NR, Johnson IR. The reliability of virtual organ computer‐aided analysis (VOCAL) for the semiquantification of ovarian, endometrial and subendometrial perfusion. Ultrasound Obstet Gynecol 2003; 22: 633–639. [DOI] [PubMed] [Google Scholar]
- 32. Aitken E, Khaund A, Hamid SA, Millan D, Campbell S. The normal human myometrium has a vascular spatial gradient absent in small fibroids. Hum Reprod 2006; 21: 2669–2678. [DOI] [PubMed] [Google Scholar]
- 33. Mizushige K, Ueda T, Yuba M, Seki M, Ohmori K, Nozaki S, Matsuo H. Dependence of power Doppler image on a high pass filter instrumented in ultrasound machine. Ultrasound Med Biol 1999; 25: 1389–1393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Background information on ultrasound and Doppler physics
Appendix S2 Transvaginal power Doppler assessment of uterine fibroids
Appendix S3 Methods for selection of machine settings that influence power Doppler signal
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


