Abstract
The timely management of skin wounds has been an unmet clinical need for centuries. While there have been several attempts to accelerate wound healing and reduce the cost of hospitalisation and the healthcare burden, there remains a lack of efficient and effective wound healing approaches. In this regard, stem cell‐based therapies have garnered an outstanding position for the treatment of both acute and chronic skin wounds. Stem cells of different origins (e.g., embryo‐derived stem cells) have been utilised for managing cutaneous lesions; specifically, mesenchymal stem cells (MSCs) isolated from foetal (umbilical cord) and adult (bone marrow) tissues paved the way to more satisfactory outcomes. Since angiogenesis plays a critical role in all four stages of normal wound healing, recent therapeutic approaches have focused on utilising stem cells for inducing neovascularisation. In fact, stem cells can promote angiogenesis via either differentiation into endothelial lineages or secreting pro‐angiogenic exosomes. Furthermore, particular conditions (e.g., hypoxic environments) can be applied in order to boost the pro‐angiogenic capability of stem cells before transplantation. For tissue engineering and regenerative medicine applications, stem cells can be combined with specific types of pro‐angiogenic biocompatible materials (e.g., bioactive glasses) to enhance the neovascularisation process and subsequently accelerate wound healing. As such, this review article summarises such efforts emphasising the bright future that is conceivable when using pro‐angiogenic stem cells for treating acute and chronic skin wounds.
Keywords: angiogenesis, differentiation, exosome, mesenchymal, stem cells, wound healing
List of Abbreviations
- AHA
acrylated hyaluronic acid
- Ang‐2
angiopoietin‐2
- ASCs
adipose‐derived stem cells
- BM
bone marrow
- BM‐MSCs
bone marrow‐derived mesenchymal stem cells
- BMP4
bone morphogenetic protein‐4
- BMSSCs
bone marrow stromal stem cells
- CM
conditioned medium
- COX‐2
cycloxygenase‐2
- CFU‐F
colony‐forming unit fibroblasts CFU‐F
- CTGF
connective tissue growth factor
- DFO
deferoxamine
- DDM
decellularized dermal matrix DDM
- EC
endothelial cell
- ECM
extracellular matrix
- EGFP
enhanced green fluorescent protein
- EPCs
endothelial progenitor cells
- ESCs
embryonic stem cells
- ET‐1
endothelin‐1
- EVs
extracellular vesicles
- FGFs
fibroblast growth factors
- Flk 1
fetal liver kinase 1
- hCB‐EPCs
human cord blood‐derived EPCs
- hESC
human embryonic stem cell
- hESC‐EPCs
human ESC‐derived EPCs
- HIF‐1α
hypoxia‐induced factor‐1α
- hUCB‐MSCs
human UCB‐derived MSCs
- HUCPVCs
human umbilical cord perivascular cells
- hUC‐MSC‐exos
human UC‐MSCs‐derived exosomes
- HUCPVCs
human umbilical cord perivascular cells
- Klf4
Kruppel‐like factor 4
- ICM
inner cell mass
- IFATS
International Fat Applied Technology Society
- M
Integra® matrix
- iPSCs
induced pluripotent stem cells
- iPSCs‐MVs
iPSCs‐derived microvesicles
- MMPs
matrix metallopeptidases
- MMP‐9
matrix metalloproteinase‐9
- MRSA
methicillin‐resistant Staphylococcus aureus
- MSCs
mesenchymal stem cells
- PDGF
platelet‐derived growth factor
- PF‐127
pluronic F‐127
- PTX3
pentraxin‐3
- PVA
poly(vinyl alcohol)
- SAP
sodium ascorbyl phosphate
- SMCs
smooth muscle cells
- SIS
small intestinal submucosa
- SMA
smooth muscle actin
- SVF
stromal vascular fraction
- TGF‐α
transforming growth factor‐α
- tPA
tissue‐type plasminogen activator
- UCB
umbilical cord blood
- UCB‐MSCs
umbilical cord blood‐derived MSCs
- uPA
urokinase‐type plasminogen activator
- UV
ultraviolet
- VE
vascular endothelial
- VEGF
vascular endothelial growth factor
- vWF
Von Willebrand factor
- WJ
Wharton's Jelly
- WJMSCs
Wharton's Jelly‐derived MSCs
1. INTRODUCTION
Skin is a multi‐layer tissue that serves as the first barrier against life‐threatening pathogens (e.g., bacteria) and radiation [e.g., ultraviolet (UV) rays]. Due to its extension, the skin is commonly exposed to a broad range of damages and injuries, which may cause acute and chronic wounds. In general, skin exhibits a self‐repairing ability, albeit only to some extent. Indeed, skin self‐healing is a complex and dynamic biological process that comprises four overlapping stages of: (I) haemostasis, (II) inflammation, (III) proliferation, and (IV) maturation (remodelling). 1 However, this self‐healing potential is limited in the case of severe and extensive skin injuries (e.g., deep burns, lacerations, and diabetic foot ulcers to name a few). In most cases, bacterial infections [e.g., methicillin‐resistant Staphylococcus aureus (MRSA)] are considered as one of the main reasons for the delay in the regeneration of damaged skin. Therefore, necessary medical interventions need to be applied to support the repair and regeneration processes of the skin. 2 , 3 For this matter, a plenty of conventional therapies have been investigated and prasticed, but stem cell‐based approaches have provided the greatest opportunities to date for accelerating the wound healing process. 4 All types of stem cells, including embryonic, foetal, and adult stem cells, have been confirmed to manage acute and chronic skin wounds. Recently, the particular use of induced pluripotent stem cells (iPSCs) has been suggested to find a promising alternative stem cell source for wound healing. 5 Several experimental studies have clarified the ability of stem cells to differentiate into skin cells (e.g., epidermal lineages), indicating their critical role in the reconstruction of damaged skin. For instance, chemical cues and mechanical stimuli have been successfully applied to facilitate stem cells differentiation into epidermal cell lineages. 6 , 7
Still, the risk of tumorigenicity and immune rejection is among the main restriction of specific stem cell‐based therapies. Moreover, there is a controversial issue on whether adult stem cells [e.g., mesenchymal stem cells (MSCs)] possess the ability to fully differentiate into endothelial cell (EC) lineages in vivo. 8 In addition, the risk of stem cell rejection has been mentioned as another restriction ahead of stem cell transplantation. Therefore, trends have gradually shifted towards using cell‐free approaches, such as stem cell‐derived extracellular vesicles (EVs), exosomes and microvesicles. 9 Based on definition, EVs represent heterogeneous plasma membrane vesicles with a size of 40–150 nm in diameter. EVs are commonly released from cells into biological fluids and classified into three primary groups including apoptotic bodies (∼>500 nm), microvesicles (∼100–1000 nm), and exosomes (∼40–120 nm). Regarding the literature, it can be stated that exosomes are being extensively used for wound healing as to their mediatory roles in cutaneous wound healing. 10 , 11 In fact, exosomes are lipid bilayer‐enclosed particles (∼30–140 nm) with an endosomal origin, which contain therapeutic molecules (e.g., proteins, lipids, and RNAs). 12 These extracellular vehicles are effective in promoting wound healing by enhancing cell proliferation and migration as well as neovascularisation. 13 , 14 Indeed, the use of exosomes offers some specific benefits over stem cell therapy; for example, they overcome the risk of immune rejection in vivo.
Since postnatal neovascularisation is one of the most important biological events in all stages of tissue wound healing, many researchers have been trying benefit from stem/progenitor cells for pro‐angiogenic strategies that accelerate healthy skin repair and regeneration. 15 Stem/progenitor cells can enhance neovessel formation at the wound site, including: (I) direct differentiation into ECs and (II) secreting pro‐angiogenic exosomes. Although preliminary studies have emphasised the importance of the differentiation of stem cells towards ECs, recent reports have revealed that pro‐angiogenic exosomes secreted by stem cells play a central role in advancing angiogenesis and wound healing. 16 As an illustration, McBride et al. reported that bone marrow (BM)‐MSC‐derived CD63+ exosomes can stimulate the proliferation and migration of dermal fibroblasts and improve endothelial angiogenesis in vitro. 17 In mammalian cells, specific signalling pathways, including the Wnt4/β‐catenin pathway, were activated under the influence of pro‐angiogenic exosomes, thereby advancing new blood vessel formation. 18 Recent studies have focused on stimulating stem/progenitor cells to secrete higher amounts of pro‐angiogenic exosomes. In this regard, pre‐conditioning cells in a hypoxic environment has been one of the most promising approaches. 19 However, the large‐scale production, as well as the high purity isolation of exosomes, remains an unsolved issue, which should be addressed in future studies. In addition, the repeated administration of exosomes is required to obtain a desired outcome in vivo.
The present review aims to highlight the pivotal role of stem/progenitor cells in promoting angiogenesis and subsequently accelerating skin wound healing. For this purpose, previously published reports were collected and critically reviewed to determine the major advantages and disadvantages of stem cell‐based approaches as a resource for researchers, scientists, and clinicians who work on this important topic of biomedicine. Finally, this review may be helpful for preparing guidelines and developing clinical trial protocols to form a bridge between basic science and the clinic.
2. ANGIOGENESIS IN WOUND HEALING: A MOLECULAR POINT OF VIEW
The process of neovessel formation from pre‐existing blood vessels, known as angiogenesis, plays a vital role in all wound healing stages. Efficient and successful neovascularisation depends on well‐orchestrated interactions of various cell types [e.g., ECs, endothelial progenitor cells (EPCs), pericytes, etc.], ECM components, and biomolecules (e.g., GFs, cytokines, hormones, etc.). 20 In brief, angiogenesis is initially induced by a mixture of GFs and cytokines released from the damaged tissue, such as vascular endothelial growth factor (VEGF), angiopoietin‐2 (Ang‐2), and fibroblast growth factors (FGFs). 21 Furthermore, platelets via secreting several GFs [e.g., platelet‐derived growth factor (PDGF), VEGF, transforming growth factor‐α (TGF‐α), TGF‐β, and bFGF], contribute to EC proliferation, migration, and tube formation. 22 It has been observed that monocytes and macrophages release various pro‐angiogenic macromolecules (e.g., PDGF, VEGF, Ang‐1, TGF‐α, bFGF, IL‐8, and TNF‐α) during the inflammatory phase of the normal wound healing process. 23 Afterward, hypoxia present in the wound bed leads to the expression of hypoxia‐induced factor‐1α (HIF‐1α) and subsequent overexpression of VEGF, which consequently results in vascular cell proliferation. 24 The newly formed vessels are then stabilised as a result of recruited smooth muscle cells (SMCs) and pericytes as well as the deposition of a vascular basement membrane. 25 Finally, the restoration of hypoxia and reduced pro‐inflammatory cytokines and GFs at the last stage of the normal wound healing process lead to decreased pro‐angiogenic factors, which in turn yield the suppression of neovascularisation. 26
3. ROLE OF STEM CELLS IN ANGIOGENESIS: A SHORT SURVEY
Angiogenesis is a complicated biological process that comprises several interactions between vascular cells and the extracellular environment. The critical role of stem/progenitor cells in enhancing neovessel formation has been previously identified as they can induce angiogenesis through either direct differentiation towards ECs or secretion of pro‐angiogenic EVs. 27 , 28 , 29 , 30 The latter is currently acknowledged as the main biological phenomenon behind promoted angiogenesis and accelerated wound healing. 31 , 32 , 33
Prior experiments have shown that stem cells may differentiate into ECs under specific conditions, including co‐culturing with an extracellular matrix (ECM), exposure to different GFs (e.g., VEGF), preconditioning in a hypoxic environment, and implementing mechanical stimuli (e.g., shear stress). 34 , 35 For instance, Shang et al. recently reported that treating adipose‐derived stem cells (ASCs) with VEGF and bone morphogenetic protein‐4 (BMP4) under hypoxia may induce stem cell differentiation into ECs through the demethylation of ephrinB2, 36 although there is limited convincing evidence for the in vivo differentiation of adult stem cells (e.g., ASCs) towards ECs. In recent years, an increased body of scientific reports has indicated that the pro‐angiogenic potential of stem/progenitor cells (e.g., MSCs) is associated with their capability of secreting pro‐angiogenic bioactive molecules, which can be enhanced by exposing the cells to some specific situations (e.g., hypoxic conditions) (Figure 1). For example, stem/progenitor cells could secret EVs containing angiogenic GFs (e.g., bFGF, PDGF, TGF‐β, and VEGF) (Table 1). 38 In addition to GFs, the neovascularisation process may be improved via particular kinds of enzymes, including tissue‐type plasminogen activator (tPA), urokinase‐type plasminogen activator (uPA), and matrix metallopeptidases (MMPs). 39 Over the last years, several published works have highlighted the critical role of miRNAs, that is, small non‐coding RNAs with a length of 19–23 nucleotides, in promoting angiogenesis. 40
FIGURE 1.

Role of hypoxia on angiogenesis. After exposure to hypoxia, mesenchymal stem cells (MSCs) release EVs containing a series of bioactive molecules (e.g., active pSTAT3 and miR‐31), which are transferred to recipient endothelial cell (ECs) and thereby can promote the transcription of pro‐angiogenic proteins. Source: Reproduced with permission from Ref. 37
TABLE 1.
A summary of bioactive molecules (e.g., growth factors, cytokines, etc.) that have the ability to induce angiogenesis
| Category | Bioactive molecule | Cognate receptor/mechanism of action |
|---|---|---|
| Growth factors | VEGF | Tyrosine kinase receptors (VEGFR1, VEGFR2, and VEGFR3) |
| PDFG | Tyrosine kinase receptors (PDGFRα and β) | |
| FGF | Tyrosine kinase receptors (FGFR1, FGFR2, FGFR3, and FGFR4) | |
| EGF | Tyrosine kinase receptors: EGFR (ErbB1, HER1), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4) | |
| TGF | Serine/threonine kinase receptors (type I and type II) | |
| TNF | Tyrosine kinase receptors (TNFRI and TNFRII) | |
| Angiopoietin | Tyrosine kinase receptors (Tie‐1 and Tie‐2) | |
| Cytokines | IL‐8 | CXCR1 and CXCR2 and thereby VEGFR2 |
| CSF‐1 | CSFR1, CSFR 2, and CXCR4 | |
| Bioactive lipids | PGE2 | EP1‐4 receptors |
| Matrix‐degrading enzymes | MMPs | Low‐density LRP |
| Heparanases | HBP | |
| Small mediators | NO | Tyrosine kinase receptors (VEGFR1, VEGFR2) |
| Serotonin | 5‐HT1 and 5‐HT2 | |
| Histamine | H1R and H2R | |
| Micro RNA | miR‐10a | MAP3K7/EC |
| miR‐21 | Pten, Bcl2, PDCD4, Sprouty‐2, PPAR/VSMCs | |
| miR‐31 | UD/HUVECs | |
| miR‐132 | RasGTPase activating protein, methyl‐CpG‐binding protein 2/Pericytes | |
| miR‐145 | Klf‐2, Elk‐1, Klf‐4/VSMCs | |
| miR‐150 | Zeb1/hESCs | |
| miR‐155 | ATR1/ECs | |
| miR‐181a | Prox1/hESCs | |
| miR‐181b | Prox1/hESCs | |
| miR‐210 | Ephrin A3/ECs | |
| miR‐217 | SirT1/ECs | |
| miR‐424 | CUL2/ECs |
Source: Reprinted with some modifications from Ref. 38.
Abbreviations: CXCR1, CXC chemokine receptor 1; CXCR2, CXC chemokine receptor 2; CSF1R, colony‐stimulating factor 1 receptor; EC, endothelial cell; EP1‐4, E‐prostanoid receptors 1–4; ErbB1, erythroblastic leukaemia viral oncogene homologue 1; 5‐HT1, 5‐hydroxytryptamine; elk‐1, E‐26‐like protein; HBP, heparin‐binding protein; hESC, human embryonic stem cells; HUVEC, human umbilical vein endothelial cells; HER1, 2,3,4, human epidermal growth factor receptor 1, 2, 3, 4; hESCs, human embryonic stem cells; HUVECs, human umbilical vein endothelial cell; KLF2/4, Kruppel‐like factor 2/4; LRP, lipoprotein receptor related protein; PGE2, prostaglandin E2; VSMCs, vascular smooth muscle cells.
In the following sections, the pro‐angiogenic potentials of different types of stem/progenitor cells are introduced and compared, discussing their usefulness in accelerating wound healing. A selection of studies is also reported in Table 2.
TABLE 2.
A short list of experimental studies in which different stem cells were used for promoting angiogenesis and wound healing.
| Type of stem cells | Wound model | Route of administration | Remarks | Ref (s) |
|---|---|---|---|---|
| ESC‐derived EPCs | Dermal excisional wound/mice model | Topical transplantation |
Greater proliferation rate and secretion of VEGF and Ang‐1 compare to hCB‐derived EPCs Accelerated re‐epithelialisation |
41 |
| iPSC‐derived early vascular cells | Full‐thickness diabetic wound/mice model | Transplantation by acrylated hyaluronic acid (AHA) hydrogels |
Stimulating recruitment and infiltration of macrophages into the hydrogel, facilitating host neovascularisation Promoted granulation tissue formation |
42 |
| hiPSC‐derived ECs and SMCs | Full‐thickness excisional wound/mice model | Intradermal injection | Enhanced neovascularisation at the wound site and accelerated wound closure | 43 |
| hUCB‐MSCs | Irradiated wound/mice model | Transplanting via SIS hydrogel |
Increased secretion of HGF, VEGF‐A, and Ang‐1 Increased recruitment of vascular ECs to the wound bed |
44 |
| hUCB‐derived CD34+ cells | Full‐thickness excision wound/NOD/SCID mice model | Intravenous injection |
Reduced expression of pro‐inflammatory cytokines (i.e., TNF‐α, IL‐1β, IL‐6 and NOS2A), while increasing IL‐10 Promoted re‐epithelialisation and vascularisation, as well as decreased MMP expression |
45 |
| WJMSCs | Full‐thickness excisional wound/mice model | Topical transplantation thorough PF‐127 hydrogel |
Propagated dermal thickness, noeformation of hair follicles, and collagen fibre deposition, and reduced scar width Greater infiltration of M2 macrophages and proliferating cells as well as promoted neovascularisation |
46 |
| HUCPVCs | Full‐thickness diabetic wound/rat model | Topical transplantation by decellularised dermal matrix (DDM) | Enhanced wound closure rate, re‐epithelisation, granulation tissue formation and reduced collagen deposition greater expression of VEGFR‐2 and vascular density | 47 |
| ASCs | Full‐thickness diabetic wound/rat model | Intradermal injection |
Enhanced re‐epithelialisation and granulation tissue formation Promoted secretion of VEGF, HGF, and FGF2 resulting in raised neovascularisation via paracrine effects |
48 |
| BM‐MSCs | Full‐thickness diabetic wound/rat model | Intradermal injection |
Increased re‐epithelialisation, cellular repopulation, and vascularisation Greater expression of VEGF and Ang‐1 |
49 |
| BM‐MSCs | Full‐thickness excisional wound/mice model | Intravenous injection | BM‐MSCs recruitment to the wound site and further differentiation to the keratinocytes, ECs, and pericytes | 50 |
4. EMBRYONIC STEM CELLS
Embryonic stem cells (ESCs) are self‐regenerating pluripotent cells that originate from the blastocyst's inner cell mass (ICM). ESCs can differentiate into three germ layers (ectoderm, mesoderm, and endoderm) and form a complete organism under appropriate conditions. This feature makes ESCs promising candidates for tissue engineering and regenerative medicine. 51 These cells can be maintained in standard cell culture conditions for years without losing their differentiation potential. However, the possible risk of immunogenicity and tumorigenicity still limits their application in the clinic.
Previously, ESCs were well documented as a source for generating ECs. 52 In culture, the differentiation of ESCs into ECs can be facilitated through endothelial‐specific markers, including foetal liver kinase 1 (Flk 1), platelet EC adhesion molecule, vascular endothelial (VE)‐cadherin, and von Willebrand factor. 52 It has been reported that the BMP family may induce sprouting angiogenesis of ECs derived from human ESCs. 53 In 2007, researchers succeeded in differentiating human ESCs into ECs and forming durable blood vessels in vivo (see Figure 2). 54 In 2013, Park et al. compared the potential of human ESC‐derived EPCs (hESC‐EPCs) with human cord blood‐derived EPCs (hCB‐EPCs) for the treatment of mouse dermal excisional wounds. 41 They reported that hESC‐EPCs have a higher proliferation rate in comparison with hCB‐EPCs as well as express increased levels of pro‐angiogenic factors (e.g., VEGF and Ang‐1). 41
FIGURE 2.
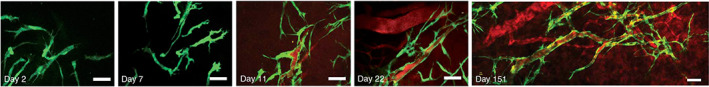
Human embryonic stem cell (hESC)‐derived endothelial cells can create functional vessels in vivo. hESCs derived endothelial cells (GFP+) and the mouse mesenchymal precursor cell line (10 T1/2) were mixed in a collagen gel and then implanted into cranial windows in SCID mice for 2, 7, 11, 22, and 151 days. Rhodamine‐dextran was injected into the tail vein at Day 11 post‐transplantation in order to highlight perfused vessels. Green, hES cells expressing enhanced green fluorescent protein (EGFP); red, functional blood vessels with contrast‐enhanced by rhodamine‐dextran. Scale bar, 50 μm. Source: Reproduced with permission from Ref. 54
Some studies indicated the use of ESCs‐derived EVs for promoting angiogenesis and consequently, improving cutaneous wound healing. 55 For instance, ESC‐EVs enhanced the therapeutic effects of MSCs in vivo via increasing epithelial and dermal cell proliferation, angiogenesis, and dermal collagen synthesis, which led to accelerated skin wound healing in Balb/c mice. 56 In another study, Lee et al. investigated the wound‐healing effect of the conditioned medium (CM) from hESC‐EPC in the healing of skin excisional wounds. The CM had various pro‐angiogenic cytokines and GFs, including EGF, bFGF, IL‐6, IL‐8, PDGF‐AA, and VEGF, which are all favourable for enhanced skin wound healing. 57
In summary, ESCs may be considered as a notable source for generating EC lineages in culture; however, the existence of regulatory hurdles, ethical concerns, and legal prohibitions are the main limitations ahead of the translation of ESC‐based therapies into the clinic. Additionally, the tendency to utilise ESCs for tissue engineering and wound healing dramatically declined with the emergence of iPSCs.
5. INDUCED PLURIPOTENT STEM CELLS
iPSCs are a new class of pluripotent stem cells which can differentiate into cells of all three germ layers. 58 These cells are generated by reprogramming somatic cells (e.g., dermal fibroblasts) via exposure to four specific genes encoding transcription factors, that is, octamer 3/4 (Oct3), sex‐determining region Y‐box (Sox2), Kruppel‐like factor 4 (Klf4), and v‐Myc myelocytomatosis viral oncogene homologue (c‐Myc). 59 In 2006, Takahashi et al. successfully developed iPSCs from postnatal somatic cells. 60 The iPSCs exhibited superior advantages for cell therapy in comparison with embryonic stem cells, including a lack of ethical issues and no risk of immune rejection. In addition, there are no actual limitations for the high‐scale production of iPSCs. 29 , 59
As mentioned above, iPSCs give rise to ectoderm, mesoderm, and endoderm lineages; for example, the successful differentiation of fibroblast‐generated iPSCs into ECs was previously reported. 61 The angiogenic potential of ECs derived from porcine iPSCs has been previously documented both in vitro and in vivo (Matrigel plug angiogenesis assay), opening new horizons for autologous transplantation of iPSC‐derived ECs for therapeutic angiogenesis in large animal models. 62
In the context of tissue engineering, Shen et al. utilised endothelial progenitors or early vascular cells derived from hiPSCs and administrated them via engineered vascularised hydrogel constructs in a full‐thickness diabetic wound mouse model. They reported that this construct increased neovascularisation and reperfusion and subsequently enhanced skin wound healing. 42 In another study, human iPSCs differentiated into ECs and SMCs, which were then co‐transplanted into a murine full‐thickness wound model. 43 The data indicated enhanced vascularisation and improved wound healing, even more than those observed in the animals receiving the differentiated ECs alone. These results were further proved in another study where iPSC‐derived ECs loaded into polycaprolactone/gelatin‐based electrospun scaffolds increased the angiogenesis process (over‐expression of pro‐angiogenic growth factors and cytokines). 63
iPSCs‐derived microvesicles (iPSCs‐MVs), as the main paracrine mediators, can play important roles in the repair and regeneration of skin wounds. In this regard, Yan et al. reported that iPSCs‐MVs increased the number of newly formed blood vessels in deep second‐degree burn wounds in mice models. 64 Moreover, it has been well demonstrated that conditioned medium from iPSC‐derived MSCs has multiple impacts in the healing of full‐thickness excisional wounds as compared with umbilical cord‐derived MSCs. Indeed, they stated that this conditioned medium could stimulate HUVEC proliferation, tube formation, and energy metabolism via the ERK pathway. 65 According to the literature, it is clear that there is an outstanding opportunity for utilising iPSCs for skin wound healing applications by promoting angiogenesis.
6. FOETAL STEM CELLS
6.1. Umbilical cord‐derived MSCs
Similar to the above‐mentioned stem/progenitor cells, angiogenesis can also be promoted in wounds through either the exogenous administration of MSCs derived from the umbilical cord and extra‐embryonic foetal tissues or their released exosomes. As a matter of fact, the umbilical cord comprises two main types of MSCs, including umbilical cord blood‐derived MSCs (UCB‐MSCs) and Wharton's Jelly‐derived MSCs (WJMSCs). 66 It should be mentioned that the bulk of Wharton's Jelly itself contains another type of mesenchymal stromal cells, termed human umbilical cord perivascular cells (HUCPVCs). 67 UC‐MSCs represent a suitable candidate for skin tissue engineering approaches thanks to their outstanding features, including ease of availability, high proliferation and differentiation capacity, low immunogenicity, 68 a painless collection procedure, faster self‐renewal properties, and recruitment to the wound area. 68 , 69 , 70 , 71 , 72 , 73 An increasing body of scientific evidence demonstrates that UC‐MSCs can promote neovascularisation, re‐epithelialisation, and formation of skin appendages with no serious adverse effects after treatment. 73 For example, EVs derived from human UC‐MSCs were able to facilitate diabetic wound healing via miR‐17‐5p‐mediated enhancement of angiogenesis. 74
Despite a great number of promising research efforts aimed at differentiating MSCs to osteogenic, chondrogenic, and adipogenic lineages, there have been no reports so far in the literature in which UC‐MSC differentiate towards vascular cell lineages in order to accelerate angiogenesis and subsequently improve skin wound healing. It is worth mentioning that the micro‐environment of chronic wounds (e.g., diabetic ulcers) cannot support the survival, proliferation, and differentiation of exogenous stem cells after a long time. In order to overcome this limitation, UC‐MSCs‐derived extracellular vesicles were utilised for promoting the neovascularisation process, collagen deposition, and wound closure. 18 , 75 However, some obstacles exist in terms of the topical application of exosomes at the wound site; for example, these vesicles are promptly cleared from the wound bed. 76 Thus, the combination of exosomes with biomaterial‐based scaffolds has been proposed for ensuring the prolonged existence of exosomes at the wound site. For example, human UC‐MSCs‐derived exosomes (hUC‐MSC‐exos) encapsulated in a thermosensitive Pluronic F‐127 (PF‐127) hydrogel was topically applied in a diabetic wound rat model. The combination of a PF‐127 hydrogel with hUCMSC‐exos led to increased expression of CD31, VEGF, and TGF‐β1 and consequently enhanced wound angiogenesis and healing. 77 In another study, human UC‐MSCs‐derived exosomes were encapsulated in bioactive scaffolds made of poly(vinyl alcohol) (PVA)/alginate (Alg) nanohydrogel (exo@H) for improving wound healing in diabetic rats. 78 The obtained in vitro and in vivo data confirmed that exo@H positively affected the expression of a series of bioactive molecules involved in wound healing, including smooth muscle actin (SMA), the scavenger receptor, class B type 1 (SR‐B1), and CD31. In addition, exo@H up‐regulated the VEGF levels via regulating the ERK1/2 pathway, leading to promoted angiogenesis and subsequently accelerated healing of diabetic wounds in vivo (Figure 3).
FIGURE 3.

(A) Gross anatomical images displaying diabetic skin wounds treated with PVA/Alg nanohydrogels (NH), exosome, human UC‐MSCs‐derived exosomes (exo), and exo encapsulated in PVA/Alg nanohydrogels (exo@H) at Days 0, 6, 12 and 18 post‐surgery. (Scale bars: 5 mm). (B) The graph shows the wound closure rate in the untreated and treated diabetic rats. (C) H&E staining of the harvested tissues of NH, exo, and exo@H groups after 10 days of surgery. (D) Masson staining of the samples at the same time (Day 10) (Scale bar: 100 μm). *p ≤ 0.05, **p ≤ 0.01, ns represents lack of significance. Source: Reproduced with permission from Ref. 78
6.2. Umbilical cord blood‐MSCs
Human UCB‐derived MSCs (hUCB‐MSCs) have gained much attention in skin wound repair and regeneration due to their relative ease of isolation, availability, immunomodulatory responses, and the lack of ethical issues. 79 , 80 , 81 , 82 , 83 However, the poor retention and survival at the wound bed limit the extensive use of hUCB‐MSCs in the clinical setting. In this regard, the combination of UCB‐MSCs with natural or synthetic porous matrices has been suggested in order to enhance cell adhesion, proliferation, differentiation, and migration during the healing process. 84 , 85 For example, hUCB‐MSCs were incorporated into a natural‐based scaffold [i.e., porcine small intestinal submucosa (SIS)] and implanted into a radiation wound in a mouse model. 86 The results showed that the cell‐containing constructs could enhance the secretion of pro‐angiogenic GFs, including VEGF, HGF, and Ang‐1. Additionally, the levels of neovascularisation markers of CD31 and von Willebrand Factor (vWF) were significantly enhanced in the mice treated with SIS gel containing hUCB‐MSCs after 21 days of implantation. 44
To date, several studies have demonstrated the pro‐angiogenic and skin tissue regeneration potential of UCB‐MSCs‐derived exosomes both in vitro and in vivo. 87 This capacity may be due to a two‐step process, that is, (I) a first transient and immediate‐acting secreted cytokines and other soluble factors and (II) exosome‐delivered nucleic acids (e.g., miRNAs and mRNAs) for a prolonged response. 88 In this regard, Montemurro et al. assessed the pro‐angiogenic and anti‐inflammatory behaviour of UCB‐MSCs in terms of their soluble secretomes and EVs. 9 Based on molecular analyses, UCB‐MSCs were recognised to significantly secrete high levels of pro‐angiogenic factors [heparin‐binding (HB)‐EGF, HGF, FGF, and VEGF]. In addition, multiple angiogenesis‐inducing transcripts were detected in EVs, including connective tissue growth factor (CTGF), FGF, IL‐6, TGF‐β1, and VEGF mRNAs. Furthermore, the tube formation assay was performed using the conditioned medium (CM) of UCB‐MSCs containing both soluble factors and EVs. The UCB MSC‐CM could promote capillary‐like structure formation, which was confirmed by the expression of vWF and CD31 as well as enhanced neovessel densities. 88
6.3. Wharton's Jelly‐MSCs
It is well understood that MSCs derived from Wharton's Jelly (WJ) are suitable candidates for allogeneic cell therapy and tissue engineering applications since they possess immunosuppressive properties as well as express pro‐angiogenic factors. 89 , 90 , 91 , 92 In addition, WJ‐MSCs can give rise to specific cell lineages, such as ECs. As an illustration, the differentiation capacity of WJ‐MSCs into ECs was previously reported in the presence of celecoxib. 93 In fact, celecoxib, as a cycloxygenase‐2 (COX‐2) inhibitor, facilitates trans‐differentiation of WJ‐MSCs into ECs, supporting accelerated wound healing through promoted neovascularisation. Other experimental studies have also confirmed the use of WJ‐MSCs for skin wound healing; sodium ascorbyl phosphate (SAP)‐containing PF‐127 hydrogels loaded with WJ‐MSCs can increase engraftment in the dermis of an excisional wound in a mouse model. 46 The reported data suggested that an accelerated and more efficient wound healing process took place as a result of greater angiogenesis and M2 macrophage formation.
The pro‐angiogenic and wound healing applications of WJ‐MSCs were investigated both in vitro and in vivo by Edwards et al. 92 The presence of an enriched secretome of pro‐angiogenic GFs [e.g., IL‐8, Ang‐1, and matrix metalloproteinase‐9 (MMP‐9)] was observed in the UC‐MSCs group as compared to adipose tissue‐derived MSCs (ASCs) in vitro. In vivo experiments evaluated the relative rate of wound angiogenesis using an immunocompetent mouse model of angiogenesis. A proteome assay was performed in order to identify whether the released pro‐angiogenic and tissue‐repair molecules correlated to the secretomes from WJ‐MSC or endogenous expression of cells residing in the wound bed. The Integra® matrix (IM) revealed no expression of such molecules, while WJ‐MSCs‐enriched IM led to a significant expression and secretion of pro‐and anti‐angiogenic GFs (e.g., VEGF‐A, angiogenin, and Serpin E1, respectively), inflammatory molecules (such as HGF and TIMP‐4), tissue repair biomolecules (e.g., Activin and IL‐8), and migratory factors (e.g., MMP‐8 and TIMP‐1).
6.4. Human umbilical cord perivascular cells
HUCPVCs are an alternative rich source of MSCs with phenotypic properties similar to hBM‐MSCs but in a higher frequency of colony‐forming unit fibroblasts (CFU‐F) than hBM MSCs. 94 These stem cells exhibit appropriate immunosuppressive features, 95 which make them suitable candidates for cell therapy. The pro‐angiogenic capacity of HUCPVCs in skin wound healing was previously evaluated in vitro and in vivo. For example, HUCPVCs at a density of 106 cells were loaded into fibrin and then transplanted into a full‐thickness wound in the dorsum of female Balb/c nude mice in order to better repair infected skin wounds. 96 Enhanced vessel densities were detected in the animals treated with HUCPVCs after 7 days of transplantation. In another study, the intradermal injection of HUCPVCs led to a promoted expression of TGF‐β1, VEGF‐1, and Ang‐1 at the wound site, enhancing wound angiogenesis. Taking advantage of natural scaffolds, Milan et al. utilised HUCPVCs and a decellularised dermal matrix (DDM) in order to increase angiogenesis and the wound healing rate in a diabetic rat model. 47 After 7 and 14 days of implantation, a significant increase in the neovascularisation, mature blood vessel densities, and the expression of VEGF‐R2 occurred in the animals receiving HUCPVCs loaded DDM as compared to cell‐free counterparts. Despite valuable and efficient outcomes gained from the pro‐angiogenesis role of UCPVCs in skin wound tissue healing, there have been limited reports about the use of exosomes for promoting wound angiogenesis. Therefore, researchers are encouraged to design and conduct reasonable experimental studies to determine the effectiveness of HUCPVCs derived EVs in promoting angiogenesis, either in vitro or in vivo.
7. ADULT STEM CELLS
7.1. Adipose‐derived mesenchymal stem cells
ASCs have shown great promise in cell therapy; they do not express MHC class II antigens on their surface and, therefore, are immunosuppressive cells. 97 ACSs exhibit some additional benefits for tissue engineering and regenerative medicine applications as compared to other cell types, including the ease of isolation, the lack of ethical issues, high in vitro proliferation rate, more genetic stability in long‐term cultures, and multi‐lineage capacity. According to the International Fat Applied Technology Society (IFATS), ASCs are identified as a plastic‐adherent multipotent cell population in adipose tissue. 59 Rodbell is known as the first scientific group able to isolate stem cells from adipose tissues in the early 1960s. 98 Later, Zuk et al. in 2001 reported the presence of these cells in human lipoaspirates as well. 99 The stromal vascular fraction (SVF) is the main compartment of adipose tissue, which contains ASCs, vascular SMCs (VSMCs), vascular ECs, and leukocytes. As an important point, it should be mentioned that both white and brown adipose tissues could be utilised for the isolation of ASCs; however, their biological characteristics are quite different per their source. 59 Experimental studies have indicated that ASCs via specific molecular pathways can effectively accelerate soft tissue repair and regeneration (e.g., skin wounds). 59 , 100 , 101 , 102
Previous studies have emphasised that ASCs may influence all four stages of the wound healing process, including haemostasis, inflammation, proliferation, and remodelling. 103 In this regard, ASCs could reduce inflammatory reactions 104 and scar formation, 105 while promoting neovascularisation. 106 All of these mentioned biological phenomena are in favour of wound healing. Since angiogenesis plays a critical role in all four stages of wound healing, many attempts have been made to leverage ASC capacity in promoting neovessel formation. 107 In this regard, two scenarios can be figured out for the role of ASCs in angiogenesis, including: (I) the direct differentiation towards ECs and (II) the secretion of pro‐angiogenic exosomes. According to the literature, most of the published data indicate that the paracrine effects of ASCs play the main role in promoting angiogenesis. For this matter, ASCs were proven to secret a wide range of pro‐angiogenic growth factors and cytokines, including PDGF, TGF‐β, VEGF, and hepatocyte growth factor (HGF). By secreting the mentioned bioactive molecules, ASCs can support the healing process of ischemic tissues such as chronic skin wounds. 100 , 108 , 109 It should be highlighted that ASCs can also secrete specific small molecules (miRNAs) as exosomal cargos, with potent pro‐angiogenic activity. As an illustration, overexpressing‐miR‐21 exosomes derived from ASCs enhance the vascularisation capacity of HUVECs. 110 As a rule of thumb, hypoxia‐induced stem cells were identified to significantly improve neovessel formation. In this regard, exosomes derived from hypoxic human ASCs could enhance the angiogenesis process through the PKA signalling pathway. 111 The activation of other molecular pathways involved in angiogenesis (e.g., VEGF/VEGF‐R) was also reported by hypoxia‐conditioned ASCs. 112
On the other side, some experimental studies claim that ASCs can give rise to ECs and incorporate them into blood vessels, where they release angiogenic growth factors to enhance recovery from ischemic perfusion. For instance, Zografou et al. have reported that the autologous transplantation of ASCs could improve the skin‐graft survival of full‐thickness wounds in rats. This improvement was associated with the differentiation of ASCs into ECs, thereby promoting the secretion of pro‐angiogenic growth factors like VEGF and TGFb3. 113 , 114 Aiming to evaluate the benefits of ASCs for wound healing, Nie et al. showed that ASCs enhance the epithelialisation and formation of granulation tissue, with subsequent acceleration of wound closure in diabetic rats. 48 The authors stated that this enhancement is associated with spontaneous site‐specific differentiation of ASCs into epithelial and endothelial cell lineages. However, other research groups have stated that accelerated wound healing is connected to both a direct (differentiation into ECs) and indirect (paracrine effects) impact of ASCs. 108
From a tissue engineering point of view, ASCs can be combined with biocompatible scaffolds and transplanted into the damaged site for improving wound healing. For this purpose, Ekea et al. prepared a series of hydrogels based on methacrylated gelatin (GelMA) and methacrylated hyaluronic acid, in which ASCs were incorporated to accelerate skin healing via possibly promoted neovascularisation. 115 The results showed that the hydrogels can actually provide a suitable microenvironment for ASC proliferation, and the cell‐loaded constructs can increase neovascularisation by up to 3‐folds compared to the cell‐free counterparts (Figure 4).
FIGURE 4.

(A) Histological and immunohistological evaluation for identifying the angiogenic activity of gelatin/hyaluronic acid hydrogels, in their pristine and loaded with adipose‐derived stem cells (ASCs) and VEGF forms, on Day 14 by using the chick embryo chorioallantoic membrane (CAM) assay. Arrows and arrow heads display large and small blood vessels, respectively. (B) The chart exhibits the quantified fluorescent α‐SMA positive blood vessel area (**p < 0.01, ***p < 0.005). (C) Images taken from (upper row) haematoxylin and eosin (H&E) stained samples indicating a lack of inflammation and slight perivascular inflammatory cell infiltration on the CAM tissue adjacent to the un‐loaded, ASC‐, and VEGF‐loaded hydrogels; (lower row), fluorescently stained samples indicating more α‐SMA stained blood vessels on the CAM underneath ASCs loaded and VEGF‐loaded hydrogels (Scale bars: 100 μm). Source : Reproduced with permission from Ref. 115
7.2. Bone marrow‐derived MSCs
Bone marrow (BM) is considered as the major source of MSCs in the human body. BM‐MSCs exhibit great potential towards differentiation for all the three germ lineages (i.e., ectoderm, mesoderm, and endoderm) in vitro. 59 In the BM, two main types of stem cells can be found including haemopoietic and stromal cells, which are responsible for generating all blood cells as well as fat, cartilage and bone, respectively. 59 Friedenstein et al. for the first time could recognise and characterise bone marrow stromal stem cells (BMSSCs or BM‐MSCs) from the BM. 116 , 117 Today, BM‐MSCs are being routinely harvested by aspiration from either the iliac crest or the tibia and femur. 59 This type of cell possesses outstanding features for cell therapy strategies such as promoting angiogenesis and subsequently promoting wound healing. 118 In this regard, previous studies have demonstrated that BM‐MSCs synthesise higher amounts of collagen, FGF, and VEGF as compared with dermal fibroblasts in vitro. 119 , 120 , 121 Apart from secreting such pro‐angiogenic cargo, BM‐MSCs exhibit the ability to differentiate into EC lineages and contribute to the neovascularisation process.
It has been previously shown that BM‐MSCs could restore the function of injured tissues through attenuating inflammation, recruiting native cells involved in the wound healing process, and promoting angiogenesis. 118 BM‐MSC secretomes contain a variety of pro‐angiogenic bioactive molecules, including angiogenin, angiopoietin‐1, CXCL16, endothelin‐1 (ET‐1), FGF‐7, heparin binding‐EGF, and pentraxin‐3 (PTX3), 119 , 122 , 123 which might be advantageous for improving skin wound repair and regeneration. In 2012, Schlosser and coworkers reported that paracrine effects of BM‐MSCs enhanced vascular regeneration in ischemic murine skin. 124 They found that the circulating BM‐MSCs home to perivascular sites in the ischemic tissue and augment microhemodynamics through a paracrine function, resulting in enhanced vascular regeneration. 124 In another study, exosomes derived from deferoxamine (DFO)‐stimulated BM‐MSCs (DFO‐Exos) were utilised for treating cutaneous wounds in rats. The downregulation of PTEN and activation of the PI3K/AKT signalling pathway were observed in the animals treated with DFO‐Exos, resulting in enhanced neovessel formation and consequently accelerated wound healing. 125
Wu et al. previously reported that the injection of allogeneic BM‐MSCs to the wound bed may significantly enhance wound healing in normal and diabetic mice through the differentiation and release of pro‐angiogenic factors. 49 Based on their data, accelerated wound closure with increased re‐epithelialisation and angiogenesis was observed in wounds treated with BM‐MSC (Figure 5). Furthermore, BM‐MSC‐conditioned medium promoted endothelial cell tube formation and a high‐level of VEGF and angiopoietin‐1 production in wounded sites. 49 On the other hand, Sasaki et al. claimed that BM‐MSCs can contribute to wound healing in mice by trans‐differentiation into various cell types, including keratinocytes, ECs, and pericytes. 50 However, there are a limited number of in vivo experimental studies that confirm the differentiation potential of BM‐MSCs towards ECs; therefore; more research should be performed to determine the actual capacity of BM‐MSC differentiation into EC lineages and improved skin wound healing.
FIGURE 5.

Representative images showing the effects of bone marrow (BM)‐derived mesenchymal stem cells (MSCs) on wound vascularity; (A) more blood vessels growing from surrounding tissue were observed in MSCs‐treated wounds as compared with vehicle medium (sham)‐ and fibroblast (FB)‐treated wounds. (B) Immunofluorescence for ECs on Days 7 (1 week) and 14 (1 week) in wound sections stained with an anti‐CD31 antibody and detected with Fluor 568 (red). Nuclei of cells were stained with Hoechst, and arrowheads indicate the epidermis layer (Scale bar = 20 μm). (C) CD31 staining of the samples for determining capillary density in the treated wounds on Day 14 (n = 6; *p < 0.0001). Source: Reproduced with permission from Ref. 49
8. CONCLUDING REMARKS AND OUTLOOK
Over the last decades, numerous scientific attempts have been made to provide complete and timely wound closure after skin injury. These efforts have led to the development of novel therapies for wound management in the clinic; the use of cells and cell‐derived products (e.g., exosomes) offer great opportunities in skin tissue repair and regeneration. For example, the spray‐based delivery of cells (e.g., keratinocytes, fibroblasts, and MSCs) to skin wounds could reduce the needed donor site area in comparison with conventional autologous skin grafting. 126 Regarding therapeutic properties, stem cells have been widely investigated in pre‐clinical studies for skin wound healing and show promise for use in human patients. Nowadays, updated perspectives have been given on utilising stem/progenitor cells for managing both acute and chronic wounds. In this regard, stem cells are being used for promoting angiogenesis as a potent therapeutic method in cutaneous lesions. It is now clear that angiogenesis plays a pivotal role in advancing the wound healing process and can be detected in all four stages of skin wound healing. 127 In fact, the formation of new blood vessels in the wound bed assists the quick transport of necessary substances (e.g., cells and bioactive molecules) to the area affected by damage while removing waste. There is sufficient scientific evidence on the potential of different types of stem cells, including ESCs and iPSCs, to differentiate towards EC lineages both in vitro and in vivo.
Some clinical trials, recently reviewed by Huang et al., 128 actually showed enhanced wound healing after transplantation of MSCs in chronic wounds; major benefits were found in the treatment of lower extremity ulcers, pressure sores and radiation burns. However, these studies suffer from some limitations, especially associated with the small number of patients involved and few methodological flaws. Furthermore, comparison between results from different studies is often difficult due to different experimental parameters such as cell source, and route of administration, dosage and time.
Therefore, the use of pro‐angiogenic secretomes derived from stem/progenitor cells is currently regarded as a preferable approach for removing barriers ahead of stem cell therapies (e.g., immunological rejection). 129 , 130
In this sense, adult stem cells such as MSCs are ideal candidates for the isolation of pro‐angiogenic cargoes, either in normal or hypoxic conditions. Recent studies have emphasised that the pre‐treatment of stem cells with inflammatory cytokines may also result in promoted angiogenesis and thereby skin wound healing. 15 In addition, stimulation of stem/progenitor cells using specific types of biocompatible materials (e.g., bioactive glasses that release therapeutic ionic species with specific biological functions) can be taken into consideration as a simple and cost‐beneficial approach for enhancing the production of pro‐angiogenic exosomes and wound healing. 66 , 131 Although promising reports can be found in the literature in support of the effectiveness of pro‐angiogenic exosomes in accelerating wound healing, several unsolved questions still remain ahead of the clinical use of pro‐angiogenic EVs which should be addressed by upcoming research. For example, the high‐scale production of well‐defined media containing pro‐angiogenic EVs and the required concentrations are substantial issues ahead of clinical studies. Therefore, biologists, biomedical engineers, and medical specialists need to pay more attention to this critical topic to be able to imagine their bright future. Last but not least, the combination of biocompatible materials (e.g., biopolymers acting as 2D/3D matrices or porous scaffolds) with pro‐angiogenic stem cells may provide an outstanding opportunity for improving the skin wound healing process without any adverse effects and must be the focus of future studies. 66 , 132 , 133
CONFLICT OF INTERESTS
The authors declare no conflict of interest regarding the publication of this work.
ACKNOWLEDGMENT
Open Access Funding provided by Politecnico di Torino within the CRUI‐CARE Agreement.
Azari Z, Nazarnezhad S, Webster TJ, et al. Stem cell‐mediated angiogenesis in skin tissue engineering and wound healing. Wound Rep Reg. 2022;30(4):421‐435. doi: 10.1111/wrr.13033
Contributor Information
Francesco Baino, Email: francesco.baino@polito.it.
Saeid Kargozar, Email: kargozarsaeid@gmail.com.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated in the current study, which is a perspective paper.
REFERENCES
- 1. Basu A, Munir S, Mulaw MA, et al. A novel S100A8/A9 induced fingerprint of mesenchymal stem cells associated with enhanced wound healing. Sci Rep. 2018;8(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Milan PB, Pazouki A, Joghataei MT, et al. Decellularization and preservation of human skin: a platform for tissue engineering and reconstructive surgery. Methods. 2020;171:62‐67. [DOI] [PubMed] [Google Scholar]
- 3. Mohammadi MR, Kargozar S, Bahrami S, Rabbani S. An excellent nanofibrous matrix based on gum tragacanth‐poly (Ɛ‐caprolactone)‐poly (vinyl alcohol) for application in diabetic wound healing. Polym Degrad Stab. 2020;174:109105. [Google Scholar]
- 4. Kosaric N, Kiwanuka H, Gurtner GC. Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19(6):575‐585. [DOI] [PubMed] [Google Scholar]
- 5. Gorecka J, Kostiuk V, Fereydooni A, et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther. 2019;10(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunter‐Featherstone E, Young N, Chamberlain K, et al. Culturing keratinocytes on biomimetic substrates facilitates improved epidermal assembly in vitro. Cell. 2021;10(5):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwon Y‐W, Lee S‐H, Kim A‐R, et al. Plant callus‐derived shikimic acid regenerates human skin through converting human dermal fibroblasts into multipotent skin‐derived precursor cells. Stem Cell Res Ther. 2021;12(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendez JJ, Ghaedi M, Sivarapatna A, et al. Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials. 2015;40:61‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang B, Wang M, Gong A, et al. HucMSC‐exosome mediated‐Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158‐2168. [DOI] [PubMed] [Google Scholar]
- 11. Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin G, Prabhakaran MP, Ramakrishna S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomater. 2011;7(8):3113‐3122. [DOI] [PubMed] [Google Scholar]
- 14. Dos Santos JF, Borçari NR, da Silva AM, Nunes VA. Mesenchymal stem cells differentiate into keratinocytes and express epidermal kallikreins: towards an in vitro model of human epidermis. J Cell Biochem. 2019;120(8):13141‐13155. [DOI] [PubMed] [Google Scholar]
- 15. Zhu M, Chu Y, Shang Q, et al. Mesenchymal stromal cells pretreated with pro‐inflammatory cytokines promote skin wound healing through VEGFC‐mediated angiogenesis. Stem Cells Transl Med. 2020;9(10):1218‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang M, Wang C, Chen M, et al. Efficient angiogenesis‐based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13(9):10279‐10293. [DOI] [PubMed] [Google Scholar]
- 17. McBride JD, Rodriguez‐Menocal L, Guzman W, Candanedo A, Garcia‐Contreras M, Badiavas EV. Bone marrow mesenchymal stem cell‐derived CD63+ exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis in vitro. Stem Cells Dev. 2017;26(19):1384‐1398. [DOI] [PubMed] [Google Scholar]
- 18. Zhang B, Wu X, Zhang X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β‐catenin pathway. Stem Cells Transl Med. 2015;4(5):513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiu X, Liu J, Zheng C, et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020;53(8):e12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8(12):957‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet‐associated fibroblast growth factor‐2 (FGF‐2) and vascular endothelial growth factor (VEGF). Thromb Haemost. 2002;88(11):834‐842. [PubMed] [Google Scholar]
- 23. Yoshida S, Yoshida A, Matsui H, Takada Y‐i, Ishibashi T. Involvement of macrophage chemotactic protein‐1 and interleukin‐1β during inflammatory but not basic fibroblast growth factor–dependent neovascularization in the mouse cornea. Lab Invest. 2003;83(7):927‐938. [DOI] [PubMed] [Google Scholar]
- 24. Acker T, Plate KH. Role of hypoxia in tumor angiogenesis—molecular and cellular angiogenic crosstalk. Cell Tissue Res. 2003;314(1):145‐155. [DOI] [PubMed] [Google Scholar]
- 25. Inoki I, Shiomi T, Hashimoto G, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF‐induced angiogenesis. FASEB J. 2002;16(2):1‐27. [DOI] [PubMed] [Google Scholar]
- 26. Korff T, Kimmina S, Martiny‐Baron G, Augustin HG. Blood vessel maturation in a 3‐dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15(2):447‐457. [DOI] [PubMed] [Google Scholar]
- 27. Wang C, Li Y, Yang M, et al. Efficient differentiation of bone marrow mesenchymal stem cells into endothelial cells in vitro. Eur J Vasc Endovasc Surg. 2018;55(2):257‐265. [DOI] [PubMed] [Google Scholar]
- 28. Harding A, Cortez‐Toledo E, Magner NL, et al. Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. Stem Cells. 2017;35(4):909‐919. [DOI] [PubMed] [Google Scholar]
- 29. Clayton ZE, Tan RP, Miravet MM, et al. Induced pluripotent stem cell‐derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci Rep. 2018;38(4):BSR20180563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghodrat S, Hoseini SJ, Asadpour S, Nazarnezhad S, Alizadeh Eghtedar F, Kargozar S. Stem cell‐based therapies for cardiac diseases: the critical role of angiogenic exosomes. Biofactors. 2021;47(3):270‐291. [DOI] [PubMed] [Google Scholar]
- 31. Hu Y, Tao R, Chen L, et al. Exosomes derived from pioglitazone‐pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnol. 2021;19(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Dong R, Tang J, et al. Exosomes laden self‐healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem Eng J. 2022;430:132664. [Google Scholar]
- 33. Han Z‐F, Cao J‐H, Liu Z‐Y, Yang Z, Qi R‐X, Xu H‐L. Exosomal lncRNA KLF3‐AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract. 2022;183:109126. [DOI] [PubMed] [Google Scholar]
- 34. Bao X, Lian X, Dunn KK, et al. Chemically‐defined albumin‐free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res. 2015;15(1):122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dan P, Velot É, Decot V, Menu P. The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. J Cell Sci. 2015;128(14):2415‐2422. [DOI] [PubMed] [Google Scholar]
- 36. Shang T, Li S, Zhang Y, Lu L, Cui L, Guo FF. Hypoxia promotes differentiation of adipose‐derived stem cells into endothelial cells through demethylation of ephrinB2. Stem Cell Res Ther. 2019;10(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat‐George F. Extracellular vesicles in angiogenesis. Circ Res. 2017;120(10):1658‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nazari‐Shafti TZ, Neuber S, Garcia Duran A, et al. Human mesenchymal stromal cells and derived extracellular vesicles: translational strategies to increase their proangiogenic potential for the treatment of cardiovascular disease. Stem Cells Transl Med. 2020;9(12):1558‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kraniak JM, Mattingly RR, Sloane BF, Janetka J. Roles of Pericellular proteases in tumor angiogenesis: therapeutic implications. Extracellular Targeting of Cell Signaling in Cancer; John Wiley & Sons, 2018:411‐446. [Google Scholar]
- 40. Ding M‐H, Lozoya EG, Rico RN, Chew SA. The role of angiogenesis‐inducing microRNAs in vascular tissue engineering. Tissue Eng Part A. 2020;26(23–24):1283‐1302. [DOI] [PubMed] [Google Scholar]
- 41. Park S‐J, Moon S‐H, Lee H‐J, et al. A comparison of human cord blood‐and embryonic stem cell‐derived endothelial progenitor cells in the treatment of chronic wounds. Biomaterials. 2013;34(4):995‐1003. [DOI] [PubMed] [Google Scholar]
- 42. Shen Y‐I, Cho H, Papa AE, et al. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials. 2016;102:107‐119. [DOI] [PubMed] [Google Scholar]
- 43. Kim KL, Song S‐H, Choi K‐S, Suh W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng Part A. 2013;19(21–22):2478‐2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee C, Shim S, Jang H, et al. Human umbilical cord blood–derived mesenchymal stromal cells and small intestinal submucosa hydrogel composite promotes combined radiation‐wound healing of mice. Cytotherapy. 2017;19(9):1048‐1059. [DOI] [PubMed] [Google Scholar]
- 45. Kanji S, Das M, Aggarwal R, et al. Nanofiber‐expanded human umbilical cord blood‐derived CD34+ cell therapy accelerates murine cutaneous wound closure by attenuating pro‐inflammatory factors and secreting IL‐10. Stem Cell Res. 2014;12(1):275‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deng Q, Huang S, Wen J, et al. PF‐127 hydrogel plus sodium ascorbyl phosphate improves Wharton's jelly mesenchymal stem cell‐mediated skin wound healing in mice. Stem Cell Res Ther. 2020;11(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Milan PB, Lotfibakhshaiesh N, Joghataie M, et al. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45:234‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose‐derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20(2):205‐216. [DOI] [PubMed] [Google Scholar]
- 49. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648‐2659. [DOI] [PubMed] [Google Scholar]
- 50. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581‐2587. [DOI] [PubMed] [Google Scholar]
- 51. Uluer E, Vatansever H, Aydede H, Ozbilgin M. Keratinocytes derived from embryonic stem cells induce wound healing in mice. Biotech Histochem. 2019;94(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 52. Li J, Stuhlmann H. In vitro imaging of angiogenesis using embryonic stem cell‐derived endothelial cells. Stem Cells Dev. 2012;21(2):331‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Richter A, Alexdottir MS, Magnus SH, et al. EGFL7 mediates BMP9‐induced sprouting angiogenesis of endothelial cells derived from human embryonic stem cells. Stem Cell Rep. 2019;12(6):1250‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25(3):317‐318. [DOI] [PubMed] [Google Scholar]
- 55. Loretelli C, Nasr MB, Giatsidis G, et al. Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020;57(7):883‐890. [DOI] [PubMed] [Google Scholar]
- 56. Zhang Y, Xu J, Liu S, et al. Embryonic stem cell‐derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics. 2019;9(23):6976‐6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee MJ, Kim J, Lee KI, Shin JM, Chae JI, Chung HM. Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy. 2011;13(2):165‐178. [DOI] [PubMed] [Google Scholar]
- 58. Lin W, Chen M, Hu C, et al. Endowing ipsc‐derived mscs with angiogenic and keratinogenic differentiation potential: a promising cell source for skin tissue engineering. Biomed Res Int. 2018;2018:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kargozar S, Mozafari M, Hamzehlou S, Brouki Milan P, Kim H‐W, Baino F. Bone tissue engineering using human cells: a comprehensive review on recent trends, current prospects, and recommendations. Appl Sci. 2019;9(1):174. [Google Scholar]
- 60. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861‐872. [DOI] [PubMed] [Google Scholar]
- 61. Niwa A, Umeda K, Chang H, et al. Orderly hematopoietic development of induced pluripotent stem cells via Flk‐1+ hemoangiogenic progenitors. J Cell Physiol. 2009;221(2):367‐377. [DOI] [PubMed] [Google Scholar]
- 62. Li X, Yu Y, Wei R, et al. In vitro and in vivo study on angiogenesis of porcine induced pluripotent stem cell‐derived endothelial cells. Differentiation. 2021;120:10‐18. [DOI] [PubMed] [Google Scholar]
- 63. Tan RP, Chan AH, Lennartsson K, et al. Integration of induced pluripotent stem cell‐derived endothelial cells with polycaprolactone/gelatin‐based electrospun scaffolds for enhanced therapeutic angiogenesis. Stem Cell Res Ther. 2018;9(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yan Y, Wu R, Bo Y, et al. Induced pluripotent stem cells‐derived microvesicles accelerate deep second‐degree burn wound healing in mice through miR‐16‐5p‐mediated promotion of keratinocytes migration. Theranostics. 2020;10(22):9970‐9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liang X, Lin F, Ding Y, et al. Conditioned medium from induced pluripotent stem cell‐derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res Ther. 2021;12(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kargozar S, Mozafari M, Hamzehlou S, Baino F. Using bioactive glasses in the Management of Burns. Front Bioeng Biotechnol. 2019;7(62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sarugaser R, Ennis J, Stanford WL, Davies JE. Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs). Stem Cells in Regenerative medicine. Springer; 2009:269‐279. [DOI] [PubMed] [Google Scholar]
- 68. Duscher D, Barrera J, Wong VW, et al. Stem cells in wound healing: the future of regenerative medicine? A mini‐review. Gerontology. 2016;62(2):216‐225. [DOI] [PubMed] [Google Scholar]
- 69. Maranda EL, Rodriguez‐Menocal L, Badiavas EV. Role of mesenchymal stem cells in dermal repair in burns and diabetic wounds. Curr Stem Cell Res Ther. 2017;12(1):61‐70. [DOI] [PubMed] [Google Scholar]
- 70. Motegi S‐i, Ishikawa O. Mesenchymal stem cells: the roles and functions in cutaneous wound healing and tumor growth. J Dermatol Sci. 2017;86(2):83‐89. [DOI] [PubMed] [Google Scholar]
- 71. Liu L, Yu Y, Hou Y, et al. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PloS One. 2014;9(2):e88348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang Y, Hao Z, Wang P, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF‐1α‐mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2):e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu H, Huang S, Wang J, et al. Enhanced cutaneous wound healing by functional injectable thermo‐sensitive chitosan‐based hydrogel encapsulated human umbilical cord‐mesenchymal stem cells. Int J Biol Macromol. 2019;137:433‐441. [DOI] [PubMed] [Google Scholar]
- 74. Wei Q, Wang Y, Ma K, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells facilitate diabetic wound healing through MiR‐17‐5p‐mediated enhancement of angiogenesis. Stem Cell Rev Rep. 2021;18:1025‐1040. [DOI] [PubMed] [Google Scholar]
- 75. Yang K, Li D, Wang M, et al. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019;10(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu X, Yang Y, Li Y, et al. Integration of stem cell‐derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430‐4438. [DOI] [PubMed] [Google Scholar]
- 77. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord‐derived mesenchymal stem cell‐derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine. 2020;15:5911‐5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C. 2021;120:111671. [DOI] [PubMed] [Google Scholar]
- 79. Oh W, Kim D‐S, Yang YS, Lee JK. Immunological properties of umbilical cord blood‐derived mesenchymal stromal cells. Cell Immunol. 2008;251(2):116‐123. [DOI] [PubMed] [Google Scholar]
- 80. Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3(4):248. [PMC free article] [PubMed] [Google Scholar]
- 81. Kim J‐Y, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood‐derived mesenchymal stem cells in disease models. World J Stem Cells. 2010;2(2):34‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tark K‐C, Hong J‐W, Kim Y‐S, Hahn S‐B, Lee W‐J, Lew D‐H. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg. 2010;65(6):565‐572. [DOI] [PubMed] [Google Scholar]
- 83. You H‐J, Namgoong S, Han S‐K, Jeong S‐H, Dhong E‐S, Kim W‐K. Wound‐healing potential of human umbilical cord blood–derived mesenchymal stromal cells in vitro—a pilot study. Cytotherapy. 2015;17(11):1506‐1513. [DOI] [PubMed] [Google Scholar]
- 84. Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48(5):907‐913. [DOI] [PubMed] [Google Scholar]
- 85. Moon K‐C, Lee J‐S, Han S‐K, Lee H‐W, Dhong E‐S. Effects of human umbilical cord blood–derived mesenchymal stromal cells and dermal fibroblasts on diabetic wound healing. Cytotherapy. 2017;19(7):821‐828. [DOI] [PubMed] [Google Scholar]
- 86. Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27(9):1114‐1122. [DOI] [PubMed] [Google Scholar]
- 87. Cardoso RM, Rodrigues SC, Gomes CF, et al. Development of an optimized and scalable method for isolation of umbilical cord blood‐derived small extracellular vesicles for future clinical use. Stem Cells Transl Med. 2021;10(6):910‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Montemurro T, Viganò M, Ragni E, et al. Angiogenic and anti‐inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol. 2016;95(6–7):228‐238. [DOI] [PubMed] [Google Scholar]
- 89. Weiss ML, Anderson C, Medicetty S, et al. Immune properties of human umbilical cord Wharton's jelly‐derived cells. Stem Cells. 2008;26(11):2865‐2874. [DOI] [PubMed] [Google Scholar]
- 90. Shetty P, Thakur AM, Ravindran G, Viswanathan C. Directed therapeutic angiogenesis by mesenchymal stem cells from umbilical cord matrix in preclinical model of ischemic limb disease. Stem Cell Stud. 2011;1(1):e16‐e. [Google Scholar]
- 91. Hsieh J‐Y, Wang H‐W, Chang S‐J, et al. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PloS One. 2013;8(8):e72604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Edwards SS, Zavala G, Prieto CP, et al. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton's jelly in dermal regeneration. Angiogenesis. 2014;17(4):851‐866. [DOI] [PubMed] [Google Scholar]
- 93. Kaushik K, Das A. Cycloxygenase‐2 inhibition potentiates trans‐differentiation of Wharton's jelly–mesenchymal stromal cells into endothelial cells: transplantation enhances neovascularization‐mediated wound repair. Cytotherapy. 2019;21(2):260‐273. [DOI] [PubMed] [Google Scholar]
- 94. Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384‐1392. [DOI] [PubMed] [Google Scholar]
- 95. Ennis J, Götherström C, Le Blanc K, Davies J. In vitro immunologic properties of human umbilical cord perivascular cells. Cytotherapy. 2008;10(2):174‐181. [DOI] [PubMed] [Google Scholar]
- 96. Zebardast N, Lickorish D, Davies JE. Human umbilical cord perivascular cells (HUCPVC) a mesenchymal cell source for dermal wound healing. Organogenesis. 2010;6(4):197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lauritano D, Palmieri A, Vinci R, Azzi L, Taglabue A, Carinci F. Adipose derived stem cells: basic science fundaments and clinical application. An update. Minerva Stomatol. 2014;63(7–8):273‐281. [PubMed] [Google Scholar]
- 98. Rodhell M. Metabolism of isolated fat cells. J Biol Chem. 1964;299:375‐380. [PubMed] [Google Scholar]
- 99. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 2001;7(2):211‐228. [DOI] [PubMed] [Google Scholar]
- 100. Cheng N‐C, Lin W‐J, Ling T‐Y, Young T‐H. Sustained release of adipose‐derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017;51:258‐267. [DOI] [PubMed] [Google Scholar]
- 101. Maharlooei MK, Bagheri M, Solhjou Z, et al. Adipose tissue derived mesenchymal stem cell (AD‐MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract. 2011;93(2):228‐234. [DOI] [PubMed] [Google Scholar]
- 102. Guo J, Hu H, Gorecka J, et al. Adipose‐derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow‐derived cells. Am J Physiol. 2018;315(6):C885‐C896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose‐derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020;21(4):1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kim W‐S, Park B‐S, Sung J‐H. The wound‐healing and antioxidant effects of adipose‐derived stem cells. Expert Opin Biol Ther. 2009;9(7):879‐887. [DOI] [PubMed] [Google Scholar]
- 105. Yu J, Wang M‐Y, Tai H‐C, Cheng N‐C. Cell sheet composed of adipose‐derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018;77:191‐200. [DOI] [PubMed] [Google Scholar]
- 106. Zhou X, Ning K, Ling B, et al. Multiple injections of autologous adipose‐derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cells Dev. 2019;28(21):1463‐1472. [DOI] [PubMed] [Google Scholar]
- 107. Deleon NMD, Adem S, Lavin CV, et al. Angiogenic CD34+CD146+ adipose‐derived stromal cells augment recovery of soft tissue after radiotherapy. J Tissue Eng Regen Med. 2021;15(12):1105‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pu C‐M, Liu C‐W, Liang C‐J, et al. Adipose‐derived stem cells protect skin flaps against ischemia/reperfusion injury via IL‐6 expression. J Invest Dermatol. 2017;137(6):1353‐1362. [DOI] [PubMed] [Google Scholar]
- 109. Moriyama M, Sahara S, Zaiki K, et al. Adipose‐derived stromal/stem cells improve epidermal homeostasis. Sci Rep. 2019;9(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. An Y, Zhao J, Nie F, et al. Exosomes from adipose‐derived stem cells (ADSCs) overexpressing miR‐21 promote vascularization of endothelial cells. Sci Rep. 2019;9(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xue C, Shen Y, Li X, et al. Exosomes derived from hypoxia‐treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27(7):456‐465. [DOI] [PubMed] [Google Scholar]
- 112. Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia‐treated human adipose‐derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF‐R. Int J Biochem Cell Biol. 2019;109:59‐68. [DOI] [PubMed] [Google Scholar]
- 113. Zografou A, Tsigris C, Papadopoulos O, et al. Improvement of skin‐graft survival after autologous transplantation of adipose‐derived stem cells in rats. J Plast Reconstr Aesthet Surg. 2011;64(12):1647‐1656. [DOI] [PubMed] [Google Scholar]
- 114. Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet‐derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials. 2017;129:188‐198. [DOI] [PubMed] [Google Scholar]
- 116. Friedenstein A, Piatetzky‐Shapiro I, Petrakova K. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966; 16(3):381‐390. [PubMed] [Google Scholar]
- 117. Friedenstein A, Chailakhjan R, Lalykina K. The development of fibroblast colonies in monolayer cultures of Guinea‐pig bone marrow and spleen cells. Cell Prolif. 1970;3(4):393‐403. [DOI] [PubMed] [Google Scholar]
- 118. Costa MH, McDevitt TC, Cabral JM, da Silva CL, Ferreira FC. Tridimensional configurations of human mesenchymal stem/stromal cells to enhance cell paracrine potential towards wound healing processes. J Biotechnol. 2017;262:28‐39. [DOI] [PubMed] [Google Scholar]
- 119. Han S‐K, Yoon T‐H, Lee D‐G, Lee M‐A, Kim W‐K. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005;55(4):414‐419. [DOI] [PubMed] [Google Scholar]
- 120. Lee C‐H, Han S‐K, Choi W‐I, Kim W‐K. Effect of human bone marrow stromal cells and dermal fibroblasts on collagen synthesis and epithelization. Ann Plast Surg. 2007;59(6):713‐719. [DOI] [PubMed] [Google Scholar]
- 121. Han S‐K, Chun K‐W, Gye M‐S, Kim W‐K. The effect of human bone marrow stromal cells and dermal fibroblasts on angiogenesis. Plast Reconstr Surg. 2006;117(3):829‐835. [DOI] [PubMed] [Google Scholar]
- 122. Cui B, Zhang C, Gan B, et al. Collagen‐tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater Sci Eng C. 2020;109:110611. [DOI] [PubMed] [Google Scholar]
- 123. Watt SM, Gullo F, van der Garde M, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108(1):25‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schlosser S, Dennler C, Schweizer R, et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvasc Res. 2012;83(3):267‐275. [DOI] [PubMed] [Google Scholar]
- 125. Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126. Motamedi S, Esfandpour A, Babajani A, Jamshidi E, Bahrami S, Niknejad H. The current challenges on spray‐based cell delivery to the skin wounds. Tissue Eng Part C Methods. 2021;27(10):543‐558. [DOI] [PubMed] [Google Scholar]
- 127. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Huang YZ, Gou M, Da LC, Zhang WQ, Xie HQ. Mesenchymal stem cells for chronic wound healing: current status of preclinical and clinical studies. Tissue Eng Part B Rev. 2020;26:555‐570. [DOI] [PubMed] [Google Scholar]
- 129. Corradetti B, Gonzalez D, Pinto IM, Conlan RS. Exosomes as therapeutic systems. Front Cell Dev Biol. 2021;9:714743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zeng Q‐L, Liu D‐W. Mesenchymal stem cell‐derived exosomes: an emerging therapeutic strategy for normal and chronic wound healing. World J Clin Cases. 2021;9(22):6218‐6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kargozar S, Baino F, Hamzehlou S, Hill RG, Mozafari M. Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018;36(4):430‐444. [DOI] [PubMed] [Google Scholar]
- 132. Nazarnezhad S, Baino F, Kim H‐W, Webster TJ, Kargozar S. Electrospun nanofibers for improved angiogenesis: promises for tissue engineering applications. Nanomaterials. 2020;10(8):1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kargozar S, Singh RK, Kim H‐W, Baino F. “Hard” ceramics for “soft” tissue engineering: paradox or opportunity? Acta Biomater. 2020;115:1‐28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated in the current study, which is a perspective paper.


