Abstract
Objective
To investigate the functional and quality of life (QoL) outcomes of treatments for localised prostate cancer and inform treatment decision‐making.
Patients and Methods
Men aged 50–69 years diagnosed with localised prostate cancer by prostate‐specific antigen testing and biopsies at nine UK centres in the Prostate Testing for Cancer and Treatment (ProtecT) trial were randomised to, or chose one of, three treatments. Of 2565 participants, 1135 men received active monitoring (AM), 750 a radical prostatectomy (RP), 603 external‐beam radiotherapy (EBRT) with concurrent androgen‐deprivation therapy (ADT) and 77 low‐dose‐rate brachytherapy (BT, not a randomised treatment). Patient‐reported outcome measures (PROMs) completed annually for 6 years were analysed by initial treatment and censored for subsequent treatments. Mixed effects models were adjusted for baseline characteristics using propensity scores.
Results
Treatment‐received analyses revealed different impacts of treatments over 6 years. Men remaining on AM experienced gradual declines in sexual and urinary function with age (e.g., increases in erectile dysfunction from 35% of men at baseline to 53% at 6 years and nocturia similarly from 20% to 38%). Radical treatment impacts were immediate and continued over 6 years. After RP, 95% of men reported erectile dysfunction persisting for 85% at 6 years, and after EBRT this was reported by 69% and 74%, respectively (P < 0.001 compared with AM). After RP, 36% of men reported urinary leakage requiring at least 1 pad/day, persisting for 20% at 6 years, compared with no change in men receiving EBRT or AM (P < 0.001). Worse bowel function and bother (e.g., bloody stools 6% at 6 years and faecal incontinence 10%) was experienced by men after EBRT than after RP or AM (P < 0.001) with lesser effects after BT. No treatment affected mental or physical QoL.
Conclusion
Treatment decision‐making for localised prostate cancer can be informed by these 6‐year functional and QoL outcomes.
Keywords: localised prostate cancer, treatments, patient‐reported outcomes, functional outcomes, quality of life, #PCSM, #ProstateCancer, #uroonc
Introduction
Radical treatments for localised prostate cancer have shown oncological benefits in several randomised trials compared to active surveillance or watchful waiting with comparable disease‐specific survival [1]. To assess the ‘trade‐off’ between oncological outcomes and future quality of life (QoL) after treatment, informed shared treatment decisions require knowledge of the sexual, urinary and bowel functional and QoL impacts of localised prostate cancer treatments, which are optimally assessed with patient‐reported outcome measures (PROMs). Several cohorts [2, 3] and the UK National Institute of Health Research (NIHR) Prostate Testing for Cancer and Treatment (ProtecT) randomised trial [4, 5], which diagnosed 1643 men with population‐based PSA testing and biopsies [5] have published PROMs, but differences between studies hinder their utility for clinicians and patients [6]. For example, PROMs from the Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) cohort [2] and the Prostate Cancer Comparative Effectiveness and Survivorship Study (ProCESS) [3] were analysed according to treatments received, whilst ProtecT analysed by intention‐to‐treat (ITT) i.e., random allocation. The ProtecT ITT analysis potentially underestimates the harms of radical treatments, as 54% of men randomised to active monitoring (AM) changed treatment over 10 years’ follow‐up [5]. Studies also differ in the active surveillance comparator for radical treatment impacts, PROMs, follow‐up periods and response rates, which complicates the interpretation of findings for patients and clinicians. The United States Preventive Services Task Force systematic review of prostate cancer [6] could not meta‐analyse radiotherapy (RT) PROMs due to their heterogeneity across studies.
The objective of the present study was to analyse the functional and QoL PROMs for treatments received by men randomised to or selecting their own treatments in the ProtecT trial over 6 years, to generate long‐term side‐effect profiles and assist patients and clinicians in treatment decision‐making. We also compared these PROMs with those experienced by men who received radical treatments after a period of AM and by age group.
Patients and Methods
Study Design and Participants
Men aged 50–69 years were invited for PSA testing at primary care practices at nine UK urology centres between 1999 and 2009 [7]. Of 2640 men diagnosed with clinically localised prostate cancer following biopsies, 1643 were randomised to AM or three‐dimensional‐conformal external‐beam RT (EBRT; a precursor to intensity‐modulated RT [IMRT], without image‐guided RT, 74 Gy in 37 fractions to the prostate) with 3–6 months neoadjuvant and androgen‐deprivation therapy (ADT) concurrent with RT, or open retropubic radical prostatectomy (RP) [8]. The AM protocol included PSA testing every 6–12 months (3‐monthly in the first year) with an increase of ≥50% over a 12‐month period triggering a clinical review (including potentially imaging and/or repeat biopsies as required) about possible change of management. There were 997 men who declined randomisation and chose a ProtecT treatment or low‐dose‐rate brachytherapy (BT) [9]. Research nurse follow‐up, with supervision by a senior urologist, was by annual record review and a participant visit. Ethics committee approval was obtained from the UK Trent Multicentre Research Ethics Committee (01/4/025). Participants provided written informed consent. At baseline, participants reported their age and ethnicity, and nurses collected other sociodemographic and clinical information.
Outcomes
Validated PROMs were collected until the median 10‐year analysis timepoint (23 November 2015) at diagnostic biopsy clinics, 6 months, then annually from the time of randomisation or treatment choice by mailed questionnaires with a structured reminder system [8]. Functional impacts were evaluated with the International Consultation on Incontinence Questionnaire (ICIQ) [10], the ICS urinary ICSmaleSF [11] and the 50‐item Expanded Prostate Index Composite (EPIC‐50, added in 2005 before EPIC‐26 was available, excluding the hormone domain to reduce participant burden as related only to EBRT at diagnosis) [12]. The Hospital Anxiety and Depression Scale (HADS) [13], Medical Outcomes Study 12‐Item Short Form Health Survey (SF‐12) [14] measured general health‐related QoL. Key items and QoL scores were presented graphically as previously [4]: urinary leakage (EPIC – at least 1 incontinence pad/day); erectile dysfunction (EPIC – erections not firm enough for intercourse); bloody stools (EPIC – ≥50% of the time); nocturia (ICSmaleSF – urinating at least once a night); anxiety and depression (HADS case score of ≥8); general mental and physical function (SF‐12 subscores). EPIC scores and SF‐12 subscales were reversed for display (higher score – worse impact) to align with other outcomes.
Statistical Analysis
This analysis of treatments in the randomised and ‘treatment choice’ cohorts (n = 2565) (as in the trial statistical analysis plan) [15] included men whose treatment commenced within 12 months of diagnosis. As previously [4, 16, 17], treatment was considered received for AM if there were at least two PSA tests recorded within 12 months of diagnosis and EBRT (including ADT) or BT was completed within 15 months. The date radical treatments commenced was time zero (randomisation/choice date for AM) and time on treatment calculated thereafter. Questionnaire data were censored on subsequent prostate cancer treatments to identify specific impacts of initial treatments.
Initially, four‐way comparisons of items by treatments were conducted, using a likelihood‐ratio test to compare a multilevel mixed effects linear regression model that included treatment as a covariate, to one where it was excluded. Two‐level models were used to incorporate the repeated PROMs for each individual over the 6 years following primary treatment. The mixed model appropriately distinguishes the within participant (level 1, the repeated measures) and between participant (level 2) variation, incorporating the latter as a normally distributed random effect. The randomised and treatment choice cohorts were previously shown to be comparable at baseline [9] and so were combined as previously for the clinical outcomes [17]. All analyses were adjusted for cohort (randomised or choice). Baseline PROMs were excluded from models as they can bias comparison of non‐randomised groups [18]. PROMs are presented as line graphs with each point including all questionnaires completed within 12 months (e.g., point 2 captures questionnaires completed between 1 and 2 years after treatment date and referred to as 2 years in the text and 1–2 years in tables). Men who had a radical treatment initially were compared with those who changed to the same radical treatment after AM on a treatment‐received basis with responses censored at a third treatment (time zero at second treatment date).
As symptoms were influenced by age at baseline [8], we also compared primary treatments for key items and scores by younger (<65 years) and older age (≥65 years) groups (BT excluded due to low numbers). The likelihood‐ratio tests compared models with and without an interaction term between treatment received and age (continuous) in years. All analyses used STATA 16.1 (StataCorp, College Station, TX, USA).
Results
Of 2565 men diagnosed with localised prostate cancer, 1135 received AM (628 randomised, 507 treatment choice) and over 6 years 174 of those men subsequently underwent surgery (15%) or 144 EBRT (13%) (Fig. S1). In total, 470 men received protocol EBRT, and 441 with ADT (93.8%), eight others did not receive ADT and 21 had missing ADT data. Non‐protocol EBRT was received by 34 men (mainly in the feasibility phase) with 13 with ADT (eight missing data) and there were 14 men who ceased EBRT due to complications (85 with missing dose data). Of 750 open RPs (488 randomised, 262 treatment choice), 427 men had a bilateral nerve‐sparing procedure and 60 a unilateral procedure, 119 men had non‐nerve‐sparing surgery and in 44 men nerve sparing was not recorded (Fig. S1). Questionnaire response rates exceeded 75% over 6 years (exemplars shown in Table S1).
Baseline Characteristics and Function
Participant characteristics and symptoms were similar at baseline across all treatments. The median (interquartile range) age was 62 (50–73) years, with BT men on average 2 years younger [16] and 99% of men reported White ethnicity. Baseline symptoms were generally infrequent but included nocturia reported by 20% of men, (Fig. 1g, Table S2), increased daytime urinary frequency by 30% (Table S2), erectile dysfunction by 35%, (Fig. 2a, Table S3), loose stools by 17% (Fig. 3c, Table S4), and anxiety by 20% (Fig. S2 and Table S6).
Fig. 1.
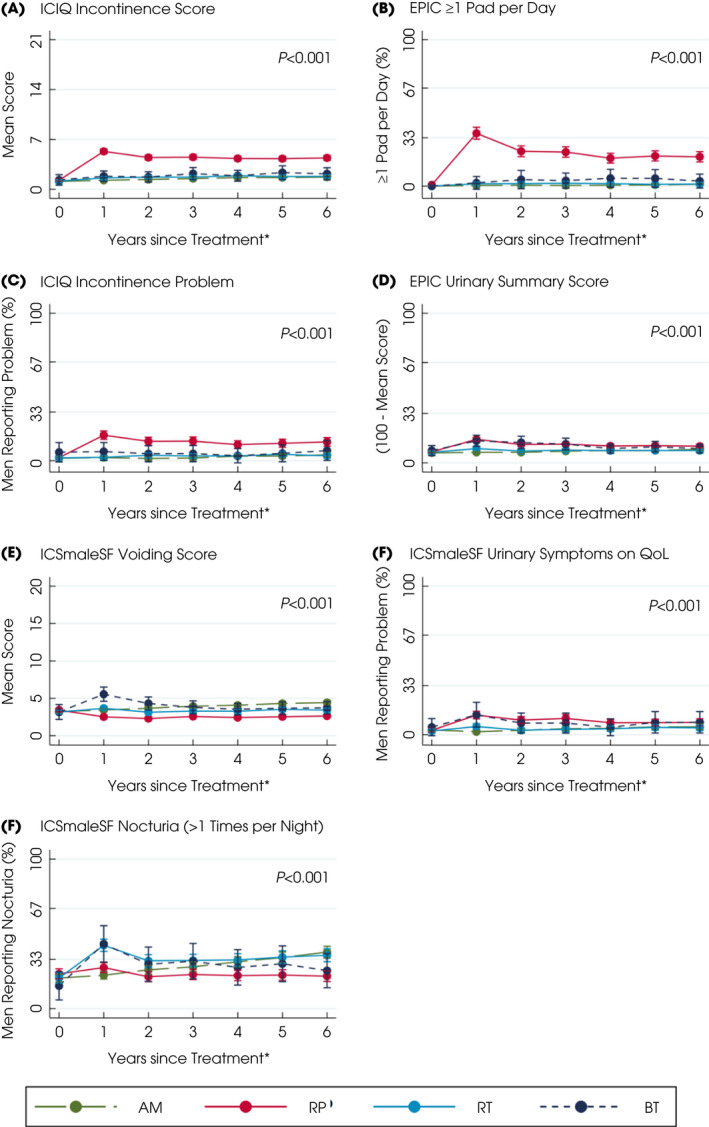
Patient‐reported urinary symptoms and QoL after primary treatments for localised prostate cancer over 6 years. *Questionnaires completed for e.g., year 2 as between 1 and 2 years after treatment. Points are estimated means from models with error bars representing 95% CIs. Higher scores or percentages indicate worse symptoms. P value based on likelihood‐ratio test for overall comparison of treatments. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig. 2.
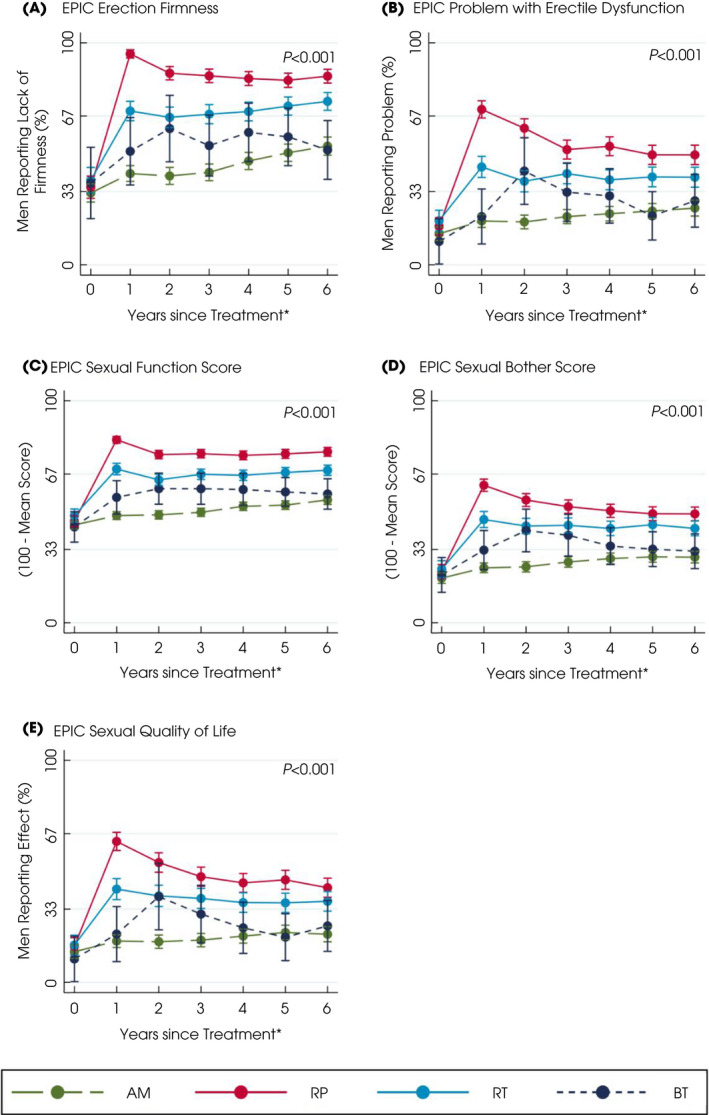
Patient‐reported sexual symptoms and QoL after primary treatments for localised prostate cancer over 6 years. *Questionnaires completed for e.g., year 2 as between 1 and 2 years after treatment. Higher scores or percentages indicate worse symptoms. Points are estimated means from models with error bars representing 95% CIs. P value based on likelihood‐ratio test for overall comparison of treatments. [Colour figure can be viewed at wileyonlinelibrary.com]
Fig. 3.
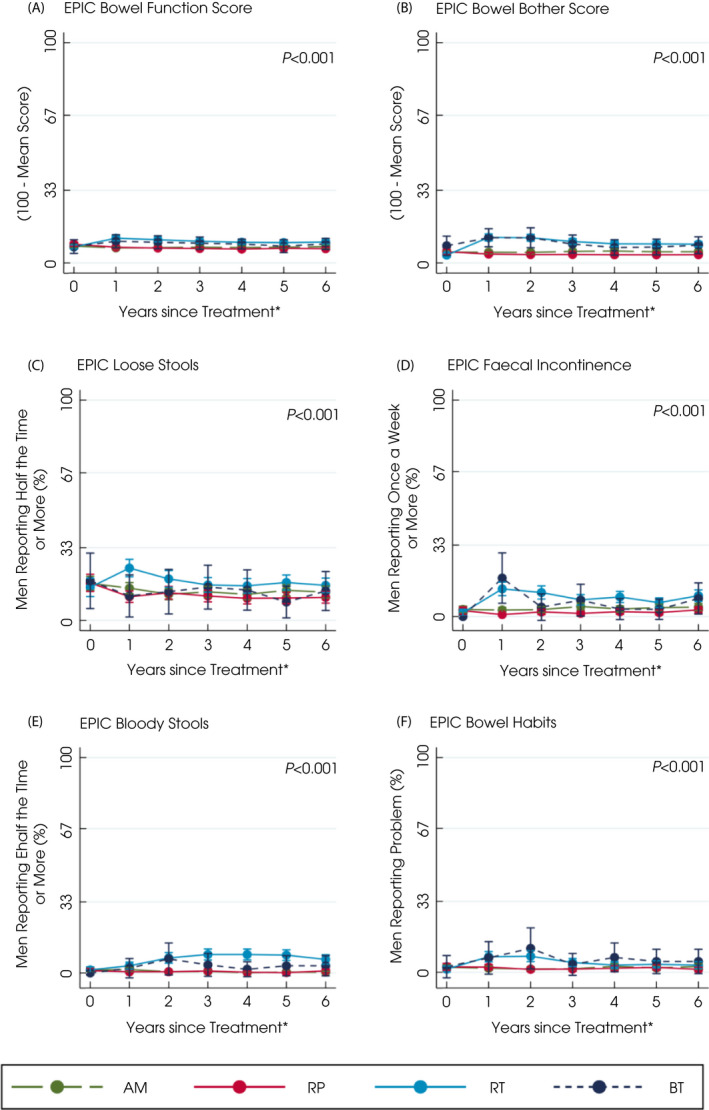
Patient‐reported bowel symptoms and QoL after primary treatments for localised prostate cancer over 6 years. *Defined as questionnaires completed for e.g., year 2 as between 1 and 2 years after treatment. Points are estimated means from models with error bars representing 95% CIs. Higher scores or percentages indicate worse symptoms. P value based on likelihood‐ratio test for overall comparison of treatments. [Colour figure can be viewed at wileyonlinelibrary.com]
Active Monitoring
Men receiving and staying on AM experienced functional deteriorations likely due to ageing. Voiding symptoms and nocturia gradually increased to 38% of men by 6 years from 20% at baseline with no changes in the use of urinary pads, urination frequency, urinary summary, functional, bother scores or worsening impact of urinary QoL (Fig. 1a–g, Table S2). Erectile dysfunction increased gradually to affect 53% of men at 6 years and its QoL impact increased from 14% of men at baseline to 22% at 6 years. Overall sexual function, bother or sexual‐related QoL did not alter (Fig. 2a–e, Table S3) nor did bowel symptoms (overall summary, function, bother or QoL), or physical and mental health (Fig. S2a–f, Table S4).
External‐beam RT and BT
Men receiving EBRT or BT experienced some changes in sexual, urinary and bowel function. Nocturia was reported by 42% of men immediately after EBRT and BT (43%) (P < 0.001 compared with RP or AM), which was increased from 20% at baseline but became comparable or less than AM by the sixth year (36% EBRT, 25% BT; Fig. 1g, Table S2). Men who received BT also reported more urinary voiding symptoms and higher irritative scores in the first year following treatment (EPIC irritative score and ICSmaleSF score; Fig. 1e, Table S2). Neither treatment affected urinary incontinence (1–3% of men reported pad use at 6 years; Fig. 1a–c, Table S2), urinary summary, functional, bother scores or QoL (Fig. 1d,f, Table S2). At 1 year after EBRT, 69% of men reported erectile dysfunction (compared with 38% of men at baseline), which was a moderate/big problem for 44% of men (20% of men at baseline). At 6 years, 74% of men still reported erectile dysfunction (problem for 39%) after EBRT compared with 53% for men on AM (problem for 26%, P < 0.001). In the first year following BT, 51% of men experienced erectile dysfunction (problem for 22%), which was comparable to AM by 6 years (52% of men and 29% a problem; Fig. 2a, Table S3). Sexual summary, function, bother and QoL scores were also better after BT than EBRT (Fig. 2c–e, Table S3). Bowel summary, function and bother scores and problems with symptoms worsened for 2 years after EBRT and BT (P < 0.001 with AM and RP; Fig. 3a,b,f, Table S4). Loose stools were most frequently experienced after EBRT (24% of men after 1 year and 19% by 2 years, P < 0.001 compared with other treatments; Fig. 3c, Table S4) and were still slightly higher than AM by 6 years (16% of men EBRT, 13% AM). Faecal incontinence increased after BT (reported by 18% of men) but declined to 8% by 6 years, whilst EBRT (13% of men at 1 year) remained higher than AM at 6 years (EBRT: 10% of men, AM: 4%; Fig. 3d, Table S4). Bloody stools were reported by 7% of men 2 years after EBRT, which continued over 6 years (6%; Fig. 3e, Table S4). Physical and mental health were unaffected by either treatment (Fig. S2a–d, Table S6).
Radical Prostatectomy
Men receiving a RP experienced urinary incontinence and sexual dysfunction. In the first year after surgery 36% of men reported urinary leakage (at least 1 incontinence pad/day) and 17% reported that incontinence caused interference with daily life (Fig. 1b,c and Table S2) and still affected 20% of men at 6 years (problem for 13%, P < 0.001 compared with other treatments). The EPIC, ICIQ and ICSmaleSF incontinence scores and ICSmaleSF QoL scores showed similar differences between treatments (P < 0.001; Fig. 1a,f, Table S2). In the first year after a RP, increased daytime frequency of urination was reported by 41% of men (from 36% at baseline) and nocturia also increased to 27% of men (23% at baseline) but both had reduced at 6 years (daytime urination 33% of men, nocturia 22%, 38%, P < 0.001; Fig. 1g and Table S2). The EPIC urinary summary and function scores remained worse than other treatments throughout and bother scores were increased following surgery but were similar to AM by 6 years (Fig. 1d and Table S2). The EPIC urinary irritative score and ICSmaleSF voiding scores remained low over 6 years (Fig. 1e and Table S2). Erectile dysfunction affected 95% of men after surgery (35% at baseline) and was a moderate/big problem for 70% (17% at baseline) and continued for 85% of men at 6 years (problem for 50%, P < 0.001; Fig. 2a,b and Table S3). Similar decrements occurred in the sexual summary, function, bother and QoL scores (Fig. 2c–e and Table S3). Bowel function (Fig. 3a–f and Table S4), physical and mental health, anxiety or depression were unaltered following surgery (Fig. S2a–d, Table S6).
Radical Treatments Following AM
Urinary leakage (at least daily pad use) was more frequent in men having surgery after AM (48%) compared with 36% initially, which persisted over 4 years (28% subsequently, 19% initially, P < 0.001; Fig. S3a, Table S5). Erectile dysfunction was frequently reported after immediate and later surgery with slightly more resolution over time (95% of men and 96% respectively immediately; 84% and 89% after 4 years, P = 0.033; Fig. S3c, Table S5). Nocturia and bloody stools showed no differences between immediate RP or surgery after AM (nocturia P = 0.277, bloody stools P = 0.478; Fig. S3b,d, Table S5). Depression was slightly worse following surgery after AM compared to immediate RP (P = 0.084; Fig. S4, Table S7) whereas anxiety, mental and physical health were comparable.
Nocturia was more frequently reported following EBRT after AM compared to immediate RT (52% of men subsequently, 42% immediately; at 4 years 41% and 33%, respectively, P = 0.087; Fig. S4b, Table S5,) as was erectile dysfunction (87% of men subsequently, 69% immediately; at 4 years 81% and 69%, respectively, P = 0.002; Fig. S4c, Table S5,). Urinary leakage (P = 0.911) and bloody stools (P = 0.907) were similar with immediate EBRT and after AM (Fig. S3a,b, Table S5). Mental health, anxiety and depression were comparable with immediate or delayed EBRT, whereas physical health was slightly worse after delayed EBRT than after immediate RT (Fig. S4, P = 0.02, Table S7).
Treatment Impacts by Age
Some treatment impacts were greater in men aged 65–69 years at diagnosis compared with younger men (50–64 years, utilising interaction tests of age and symptoms in Table S8). Nocturia was worse in older men receiving AM (baseline: younger 15% compared with 29% older; 4 years 29% compared to 42%, Fig. S5b, Table S8) and following radical treatments (interaction P = 0.016; Fig. S5b, Table S8). Erectile dysfunction after EBRT or RP was similar for older and younger men over 6 years (interaction P = 0.198; Fig. S5c, Table S8). Daily incontinence pads were used by 42% of older men after surgery compared with 33% of younger men (interaction P = 0.474; Fig. S5a, Table S8). Bloody stools after EBRT were comparable across age groups (interaction P = 0.340; Fig. S5d, Table S8).
Discussion
Men who make decisions about treatment for localised prostate cancer need to be able to assess the trade‐off between oncological benefits with treatment impacts on their QoL. Our report highlights radical treatment side‐effect profiles utilising PROMs over 6 years and deterioration in sexual and urinary function in men receiving AM. This analysis of PROMs related to the treatment actually received by ProtecT participants clearly shows the different impacts experienced over 6 years, without potential dilution by the ITT approach. Furthermore, larger numbers were analysed through combining men randomised with those who selected their treatment and were followed‐up over 6 years. Men remaining on AM experienced gradual declines in sexual and urinary function over time, and no change in bowel function or urinary incontinence. Impacts of radical treatments were more immediate and persistent. After RP, there was an immediate impact on sexual function with limited recovery and persistence of symptoms, worse than after other radical treatments. After RP, there was an immediate increase in urinary leakage requiring incontinence pads, with some improvement over the following few years, but persistent symptoms remaining in 20% of participants at 6 years. After EBRT with neoadjuvant ADT or BT, there was an immediate impact on sexual (particularly erectile) function that was less severe than for RP, and while this improved after 2 years with BT, the impact was sustained over time for EBRT, and was worse than in men receiving AM. Overall bowel function and bother were worse after EBRT and BT compared to AM or RP particularly for 2–3 years, with slightly lower scores for BT. Urinary voiding and nocturia were also adversely affected by BT and EBRT in the first year, but there was no impact on urinary continence. Mental and physical QoL were unaffected by any treatment. Overall, PROMs tended to be worse for older compared with younger men (<65 years).
The PROMs capture localised prostate treatment impacts effectively, but published results vary due to underlying differences in study designs, interventions, and measures [19, 20, 21]. In the randomised Scandinavian Prostate Cancer Group‐4 (SPCG‐4) trial [22], urinary pad utilisation was greater after surgery (41%) than on watchful waiting at 12 years but in both groups erectile dysfunction was >80%. Conversely, radical treatment impacts improved over 10 years in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) cohort (based on 15% of the cohort) [23]. The LAParoscopic Prostatectomy Robot Open study (LAPPRO) trial showed better erectile function at 24 months after robot‐assisted RP (RARP) compared with an open RP (based on the International Index of Erectile Function) and a small benefit for open RP regarding urinary leakage (based on the urinary PROM used in SPCG‐4) [24]. A systematic review of prostate cancer RT trials [25] concluded that BT had less impact on sexual function than EBRT with insufficient evidence on urinary function. A trial of hypofractionated and conventional RT showed that no differences in bowel bother, overall urinary or sexual bother over 5 years for men with intermediate‐risk prostate cancer [21]. A more recent trial of ultra‐ and hypofractionated RT for intermediate‐risk prostate cancer showed that the increased urinary and bowel bother did not persist over 4 years, but sexual bother increased over time in both groups [26].
The ProtecT results are based on a large and well‐characterised population‐based cohort with 6 years annual follow‐up, low attrition, and outcomes assessed at diagnosis, thus minimising recall bias. AM men were comparable to those undergoing radical treatments, unlike many other cohorts [20], and received standard clinical follow‐up without watchful waiting/observation patients who may have greater co‐morbidities. The censoring of subsequent radical treatments highlighted the likely age‐related declines for men on AM acting as a comparator for radical treatments and complementing the ITT analysis [4], where men receiving radical treatments were analysed in the AM group. Results from the 270 men undergoing radical treatments after a period of AM is also potentially novel. These validated PROMs included core outcomes [27] whereas the diversity of PROMs in RT trials for localised prostate cancer prevented the completion of meta‐analyses [6].
There are limitations to this study. The ProtecT participants were mostly of White ethnicity (broadly reflecting the recruiting centres populations) [8], so these results may not be informative for other ethnicities. Radical treatment techniques have evolved since the trial commenced, so questions were raised whether ProtecT reflected contemporary approaches such as RARP and newer methods of irradiation. To investigate the potential similarities between ProtecT and other cohorts, we compared contemporary treatments (IMRT low‐risk group patients and RARP) from the CEASAR [2] cohort, which used the EPIC‐26 (domain scores correlate with EPIC‐50 in ProtecT) [28] with EBRT and open RP in ProtecT (Table S9). Urinary leakage was slightly greater following IMRT (6%) than after EBRT (1%) with comparable irritative scores. Erectile dysfunction was similar between EBRT and IMRT, whilst bloody stools only occurred after EBRT (8%), likely due to higher rectal exposure. BT results were comparable in both studies. Urinary leakage was slightly higher after RARP (10%) than open RP (6%) and a greater problem (6% vs 12%). Conversely, erectile dysfunction was less frequent after RARP (61% vs 71% after open RP), likely due to improved neurovascular bundle preservation, although 24% of the RARP group were aged ≥70 years [29] (ProtecT maximum 69 years at recruitment). Active surveillance men reported greater urinary and sexual dysfunction in the CEASAR study, as 25% received radical treatments but censored in these ProtecT treatment‐received analyses. However, the CEASAR active surveillance protocol had different selection criteria and monitoring compared with ProtecT that might have affected PROMs.
In order to inform patient treatment decision making, these functional and QoL outcomes need to be considered alongside the small reduction in disease‐specific mortality from radical treatments compared with AM (ProtecT treatment‐received analysis) [17], and the Prostate cancer Intervention vs Observation Trial (PIVOT) [30] 12‐year follow‐up, which suggested an overall mortality benefit for surgery over watchful waiting in the intermediate‐risk disease group.
In summary, these full 6‐year functional and QoL profiles should inform treatment decision‐making for men with newly diagnosed localised prostate cancer and their treating clinicians. The long‐term side‐effects of radical treatments, as well as naturally deteriorating sexual and urinary function when on AM, need to be weighed against the potential oncological benefits of radical treatments.
Disclosures of Interest
Ms Young: NIHR pre‐doctoral Fellowship. Professor Mason: Paid membership of Data Monitoring Committees for Clovis and Endocyte. Professor Martin: Receipt of Cancer Research UK grant at Univeristy of Bristol. The remaining authors have no disclosures.
Author Contributions
Professors Donovan, Hamdy, Lane and Metcalfe and Neal had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Donovan, Hamdy, Neal. Acquisition, analysis, or interpretation of the data: Lane, Avery, Blazeby, Davis, Turner, Wade, Walsh. Drafting of the manuscript: Lane, Donovan, Hamdy, Metacalfe, Neal. Critical revision of the manuscript for important intellectual content: All authors. Statistical design and analysis: Metcalfe, Peters, Young. Obtained funding: Donovan, Hamdy, Neal.
Funding
The study was supported by the NIHR Health Technology Assessment Programme (NIHR HTA: projects 96/20/06, 96/20/99, with University of Oxford as sponsor). Professor Lane is supported by the Bristol Randomised Trials Collaboration, a UKCRC registered Clinical Trials Unit (CTU) which, as part of the Bristol Trials Centre, is in receipt of NIHR CTU Support Funding. Professor Hamdy is supported by the Oxford NIHR Biomedical Research Centre Surgical Innovation and Evaluation Theme and Cancer Research UK Oxford Centre. Professors Blazeby, Lane and Martin and Dr Avery are supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol (BRC‐1215‐200011).
Role of the Funder/Sponsor
The funder had no role in the study design, data collection, analysis, interpretation, writing of the paper or decision to submit for publication.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect those of the UK Department of Health.
Abbreviations
- ADT
androgen‐deprivation therapy
- AM
active monitoring
- BT
low‐dose‐rate brachytherapy
- CEASAR
Comparative Effectiveness Analysis of Surgery and Radiation
- (EB)(IM)RT
(external‐beam) (intensity‐modulated) radiotherapy
- EPIC
Expanded Prostate Index Composite
- HADS
Hospital Anxiety and Depression Scale
- ICIQ
International Consultation on Incontinence Questionnaire
- ITT
intention‐to‐treat
- NIHR
UK National Institute of Health Research
- PROMs
Patient‐reported outcome measures
- ProtecT
Prostate Testing for Cancer and Treatment
- QoL
quality of life
- (RA)RP
(robot‐assisted) radical prostatectomy
- SF‐12
Medical Outcomes Study 12‐Item Short Form Health Survey
Supporting information
Fig. S1 Primary and secondary treatments for localised prostate cancer received over 6 years of follow‐up.
Fig. S2 Adjusted physical and mental health and psychological outcomes after primary treatments for prostate cancer over 6 years.
Fig. S3 Patient‐reported urinary, bowel and sexual symptoms after radical treatment for localised prostate cancer, either initially, or after a period of AM over 4 years.
Fig. S4 Adjusted QoL outcomes after receiving radical treatment after primary AM over 4 years.
Fig. S5 Patient‐reported urinary, bowel and sexual symptoms by age groups by primary localised prostate cancer treatments over 6 years.
Table S1 Response rates for exemplar PROMs in the treatment received analysis.
Table S2 Adjusted urinary symptoms corresponding to Fig. 1 and subscales.
Table S3 Adjusted sexual symptoms corresponding to Fig. 2 and subscales.
Table S4 Adjusted bowel symptoms corresponding to Fig. 3 and subscales.
Table S5 Adjusted symptoms from immediate radical treatments or after AM corresponding to Fig. S3.
Table S6 Adjusted QoL items by treatment received corresponding to Fig. S2.
Table S7 Adjusted QoL of radical treatments after AM and immediate: radical treatment corresponding to Fig. S4.
Table S8 Adjusted interaction between treatment and age group on symptoms corresponding to Fig. S5.
Table S9 Adjusted symptoms and quality 5 years after enrolment in the CEASAR cohort or diagnosis in the ProtecT trial.
Acknowledgements
We thank all ProtecT participants and researchers, and members of the independent Trial Steering Committee, Data Monitoring Committee and Cause‐of‐death evaluation committee.
Principal investigators: Freddie C. Hamdy (Chief Investigator), Jenny L. Donovan, David E. Neal.
Trial co‐ordinator: Janet Athene Lane.
Trial statisticians: Chris Metcalfe, Tim J. Peters, Grace Young.
Urologist leads: Prasad Bollina, Andrew Doble, Alan Doherty, Vincent Gnanapragasam, Owen Hughes, David Gillatt, Roger Kockelbergh, Howard Kynaston, Alan Paul, Edgar Paez, Philip Powell, Stephen Prescott, Derek Rosario, Edward Rowe.
Others: John B. Anderson*, Jonathan Aning, James Catto, Garett Durkan, Anthony Kouparis, Hing Leung, Param Mariappan, Alan McNeill, Raj Persad, Hartwig Schwaibold, David Tulloch, Michael Wallace.
Nurses leads: Susan Bonnington*, Lynne Bradshaw, Deborah Cooper, Emma Elliott, Phillipa Herbert, Peter Holding, Joanne Howson, Amanda Jones, Teresa Lennon, Norma Lyons, Hilary Moody, Claire Plumb, Tricia O'Sullivan, Elizabeth Salter, Pauline Thompson, Sarah Tidball, Jan Blaikie, Catherine Grey. Others: Tonia Adam, Sarah Askew, Sharon Atkinson, Tim Baynes, Carole Brain, Viv Breen, Sarah Brunt, Sean Bryne, Jo Bythem, Jenny Clarke, Jenny Cloete, Susan Dark, Gill Davis, Rachael De La Rue, Jane Denizot, Elspeth Dewhurst, Anna Dimes, Nicola Dixon, Penny Ebbs, Ingrid Emmerson, Jill Ferguson, Ali Gadd, Lisa Geoghegan, Alison Grant, Collette Grant, Rosemary Godfrey, Louise Goodwin, Susie Hall, Liz Hart, Andrew Harvey, Chloe Hoult, Sarah Hawkins, Sharon Holling, Alastair Innes, Sue Kilner, Fiona Marshall, Louise Mellen, Andrea Moore, Sally Napier, Julie Needham, Kevin Pearse, Anna Pisa, Mark Rees, Ellie Richards, Lindsay Robson, Janet Roxburgh, Nikki Samuel, Irene Sharkey, Michael Slater, Donna Smith, Pippa Taggart, Helen Taylor, Vicky Taylor, Ayesha Thomas, Briony Tomkies, Nicola Trewick, Claire Ward, Christy Walker, Ayesha Williams, Colin Woodhouse, Elizabeth Wyber.
Oncologists lead: Malcolm Mason. Others: Amit Bahl, Richard Benson, Mark Beresford, Catherine Ferguson, John Graham, Chris Herbert, Grahame Howard, Nick James, Peter Kirkbride, Alastair Law, Carmel Loughrey, Duncan McClaren, Helen Patterson*, Ian Pedley, Trevor Roberts*, Angus Robinson, Simon Russell, John Staffurth, Paul Symonds, Narottam Thanvi, Subramaniam Vasanthan, Paula Wilson.
Histopathologists leads: Jon Oxley, Mary Robinson: Others: Selina Bhattarai, Neeta Deshmukh, John Dormer, Malee Fernando, John Goepel, David Griffiths, Ken Grigor, Nick Mayer, Murali Varma, Anne Warren.
Radiologists and medical physics: Helen Appleby, Dominic Ash, Dean Aston, Steven Bolton, Graham Chalmers, John Conway, Nick Early, Tony Geater, Lynda Goddall, Claire Heymann, Deborah Hicks, Liza Jones, Susan Lamb, Geoff Lambert, Gill Lawrence, Geraint Lewis, John Lilley, Aileen MacLeod, Pauline Massey, Alison McQueen, Rollo Moore, Lynda Penketh, Janet Potterton, Neil Roberts, Helen Showler, Pam Shuttleworth, Stephen Slade, Alasdair Steele, James Swinscoe, Marie Tiffany, John Townley, Jo Treeby, Michael Weston, Joyce Wilkinson, Lorraine Williams, Lucy Wills, Owain Woodley, Sue Yarrow.
Other researchers and data managers: Lucy Brindle, Linda Davies, Michael Davis, Dan Dedman, Elizabeth Down, Hanan Khazragui, Richard M. Martin, Sian Noble, Hilary Taylor, Marta Tazewell, Emma L. Turner, Julia Wade, Eleanor Walsh, Grace Young.
Administrative support: Susan Baker, Elizabeth Bellis‐Sheldon, Chantal Bougard, Joanne Bowtell, Catherine Brewer, Chris Burton, Jennie Charlton, Nicholas Christoforou, Rebecca Clark, Susan Coull, Christine Croker, Rosemary Currer, Claire Daisey, Gill Delaney, Rose Donohue, Jane Drew, Rebecca Farmer, Susan Fry, Jean Haddow, Alex Hale, Susan Halpin, Belle Harris, Barbara Hattrick, Sharon Holmes, Helen Hunt, Vicky Jackson, Donna Johnson, Mandy Le Butt, Jo Leworthy, Tanya Liddiatt, Alex Martin, Jainee Mauree, Susan Moore, Gill Moulam, Jackie Mutch, Kathleen Parker, Christopher Pawsey, Michelle Purdie, Teresa Robson, Lynne Smith, Carole Stenton, Tom Steuart‐Feilding, Beth Stott, Chris Sully, Caroline Sutton, Carol Torrington, Zoe Wilkins, Sharon Williams, Andrea Wilson, Ashleigh Weaver.
Joint ProtecT and CAP cause of death committee: Richard M. Martin (Research Lead), Peter Albertsen (Chair), Jan Adolfsson, Amit Bahl, Michael Baum, Anthony Koupparis, Jon McFarlane, Jon Oxley, Colette Reid, Mary Robinson, Emma Turner, Anthony Zietman.
Other researchers and data managers: Elizabeth Hill, Siaw Yein Ng, Naomi Williams, Jessica Toole, Charlotte Davies, Laura Hughes, Mari‐Anne Rowlands, Lindsey Bell, Sean Harrison, and Jainee Mauree.
Independent data monitoring committee
Chairs: Adrian Grant* and Ian Roberts.
Members: Deborah Ashby, Richard Cowan, Peter Fayers, Killian Mellon, James N'Dow, Tim O'Brien, Michael Sokhal.
Trial steering committee: Michael Baum (Chair), Jan Adolfsson, Peter Albertsen, David Dearnaley, Fritz Schröder, Tracy Roberts, Anthony Zietman.
*Deceased.
J.L.D., F.C.H., J.A.L., C.M. and D.E.N. contributed equally.
Trial registration: ClinicalTrials.gov: NCT02044172, www.isrctn.com: ISCTRN20141.
Contributor Information
Janet Athene Lane, Email: athene.lane@bristol.ac.uk.
for the Prostate Testing for Cancer and Treatment (ProtecT) Study Group:
Andrew Doble, Philip Powell, Stephen Prescott, John B. Anderson, Jonathan Aning, Garett Durkan, Anthony Kouparis, Hing Leung, Param Mariappan, Alan McNeill, Raj Persad, Hartwig Schwaibold, David Tulloch, Michael Wallace, Susan Bonnington, Lynne Bradshaw, Deborah Cooper, Emma Elliott, Phillipa Herbert, Peter Holding, Joanne Howson, Amanda Jones, Teresa Lennon, Norma Lyons, Hilary Moody, Claire Plumb, Tricia O'Sullivan, Elizabeth Salter, Pauline Thompson, Sarah Tidball, Jan Blaikie, Catherine Grey, Tonia Adam, Sarah Askew, Sharon Atkinson, Tim Baynes, Carole Brain, Viv Breen, Sarah Brunt, Sean Bryne, Jo Bythem, Jenny Clarke, Jenny Cloete, Susan Dark, Gill Davis, Rachael De La Rue, Jane Denizot, Elspeth Dewhurst, Anna Dimes, Nicola Dixon, Penny Ebbs, Ingrid Emmerson, Jill Ferguson, Ali Gadd, Lisa Geoghegan, Alison Grant, Collette Grant, Rosemary Godfrey, Louise Goodwin, Susie Hall, Liz Hart, Andrew Harvey, Chloe Hoult, Sarah Hawkins, Sharon Holling, Alastair Innes, Sue Kilner, Fiona Marshall, Louise Mellen, Andrea Moore, Sally Napier, Julie Needham, Kevin Pearse, Anna Pisa, Mark Rees, Ellie Richards, Lindsay Robson, Janet Roxburgh, Nikki Samuel, Irene Sharkey, Michael Slater, Donna Smith, Pippa Taggart, Helen Taylor, Vicky Taylor, Ayesha Thomas, Briony Tomkies, Nicola Trewick, Claire Ward, Christy Walker, Ayesha Williams, Colin Woodhouse, Elizabeth Wyber, Amit Bahl, Richard Benson, Mark Beresford, Catherine Ferguson, John Graham, Chris Herbert, Grahame Howard, Nick James, Peter Kirkbride, Alastair Law, Carmel Loughrey, Duncan McClaren, Helen Patterson, Ian Pedley, Trevor Roberts, Angus Robinson, Simon Russell, Paul Symonds, Narottam Thanvi, Subramaniam Vasanthan, Paula Wilson, Mary Robinson, Selina Bhattarai, Neeta Deshmukh, John Dormer, Malee Fernando, John Goepel, David Griffiths, Ken Grigor, Nick Mayer, Murali Varma, Anne Warren, Helen Appleby, Dominic Ash, Dean Aston, Steven Bolton, Graham Chalmers, John Conway, Nick Early, Tony Geater, Lynda Goddall, Claire Heymann, Deborah Hicks, Liza Jones, Susan Lamb, Geoff Lambert, Gill Lawrence, Geraint Lewis, John Lilley, Aileen MacLeod, Pauline Massey, Alison McQueen, Rollo Moore, Lynda Penketh, Janet Potterton, Neil Roberts, Helen Showler, Pam Shuttleworth, Stephen Slade, Alasdair Steele, James Swinscoe, Marie Tiffany, John Townley, Jo Treeby, Michael Weston, Joyce Wilkinson, Lorraine Williams, Lucy Wills, Owain Woodley, Sue Yarrow, Lucy Brindle, Linda Davies, Dan Dedman, Elizabeth Down, Hanan Khazragui, Sian Noble, Hilary Taylor, Marta Tazewell, Susan Baker, Elizabeth Bellis‐Sheldon, Chantal Bougard, Joanne Bowtell, Catherine Brewer, Chris Burton, Jennie Charlton, Nicholas Christoforou, Rebecca Clark, Susan Coull, Christine Croker, Rosemary Currer, Claire Daisey, Gill Delaney, Rose Donohue, Jane Drew, Rebecca Farmer, Susan Fry, Jean Haddow, Alex Hale, Susan Halpin, Belle Harris, Barbara Hattrick, Sharon Holmes, Helen Hunt, Vicky Jackson, Donna Johnson, Mandy Le Butt, Jo Leworthy, Tanya Liddiatt, Alex Martin, Jainee Mauree, Susan Moore, Gill Moulam, Jackie Mutch, Kathleen Parker, Christopher Pawsey, Michelle Purdie, Teresa Robson, Lynne Smith, Carole Stenton, Tom Steuart‐Feilding, Beth Stott, Chris Sully, Caroline Sutton, Carol Torrington, Zoe Wilkins, Sharon Williams, Andrea Wilson, Ashleigh Weaver, Peter Albertsen, Jan Adolfsson, Michael Baum, Anthony Koupparis, Jon McFarlane, Colette Reid, Mary Robinson, Anthony Zietman, Elizabeth Hill, Siaw Yein Ng, Naomi Williams, Jessica Toole, Charlotte Davies, Laura Hughes, Mari‐Anne Rowlands, Lindsey Bell, Sean Harrison, Adrian Grant, Ian Roberts, Deborah Ashby, Richard Cowan, Peter Fayers, Killian Mellon, James N'Dow, Tim O'Brien, Michael Sokhal, David Dearnaley, Fritz Schröder, and Tracy Roberts
Data Availability Statement
Individual participant data that underlie the results reported in this article, after de‐identification (text, tables, figures, and appendices) will be made available (the study protocol and statistical analysis plan is published). The data will be available to approved researchers following submission of a proposal (directed to f.c.hamdy@nds.ox.uk); to gain access, data requestors will need to sign a data access agreement.
References
- 1. Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA 2017; 317: 2532–42 [DOI] [PubMed] [Google Scholar]
- 2. Hoffman KE, Penson DF, Zhao Z et al. Patient‐reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA 2020; 323: 149–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen RC, Basak R, Meyer AM et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient‐reported quality of life among men with localized prostate cancer. JAMA 2017; 317: 1141–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donovan JL, Hamdy FC, Lane JA et al. Patient‐reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016; 375: 1425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamdy FC, Donovan JL, Lane JA et al. 10‐year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016; 375: 1415–24 [DOI] [PubMed] [Google Scholar]
- 6. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate‐specific antigen–based screening for prostate cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018; 319: 1914–31 [DOI] [PubMed] [Google Scholar]
- 7. Lane JA, Donovan JL, Davis M et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014; 15: 1109–18 [DOI] [PubMed] [Google Scholar]
- 8. Lane A, Metcalfe C, Young GJ et al. Patient‐reported outcomes in the ProtecT randomized trial of clinically localized prostate cancer treatments: study design, and baseline urinary, bowel and sexual function and quality of life. BJU Int 2016; 118: 869–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donovan JL, Young GJ, Walsh EI et al. A prospective cohort and extended comprehensive‐cohort design provided insights about the generalizability of a pragmatic trial: the ProtecT prostate cancer trial. J Clin Epidemiol 2018; 96: 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004; 23: 322–30 [DOI] [PubMed] [Google Scholar]
- 11. Donovan JL, Peters TJ, Abrams P, Brookes ST, De La Rosette JJ, Schafer W. Scoring the short form ICSmaleSF questionnaire. International continence society. J Urol 2000; 164: 1948–55 [PubMed] [Google Scholar]
- 12. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health‐related quality of life in men with prostate cancer. Urology 2000; 56: 899–905 [DOI] [PubMed] [Google Scholar]
- 13. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–70 [DOI] [PubMed] [Google Scholar]
- 14. Gandek B, Ware JE, Aaronson NK et al. Cross‐validation of item selection and scoring for the SF‐12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol 1998; 51: 1171–8 [DOI] [PubMed] [Google Scholar]
- 15. Metcalfe C, Peters T, Hamdy F. Prostate Testing for Cancer and Treatment (ProtecT) Study. University of Bristol, 2015. Available from: https://research‐information.bris.ac.uk/?_ga=2.54053480.1749328329.1650471175‐1539700392.1612986357 [Google Scholar]
- 16. Donovan J, Opmeer B, Young G et al. Factors associated with trial recruitment, preferences, and treatments received were elucidated in a comprehensive cohort study. J Clin Epidemiol 2019; 113: 200–13 [DOI] [PubMed] [Google Scholar]
- 17. Neal DE, Metcalfe C, Donovan JL et al. Ten‐year mortality, disease progression, and treatment‐related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur Urol 2020; 77: 320–30 [DOI] [PubMed] [Google Scholar]
- 18. Van Breukelen GJ. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol 2006; 59: 920–5 [DOI] [PubMed] [Google Scholar]
- 19. Lardas M, Liew M, van den Bergh RC et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur Urol 2017; 72: 869–85 [DOI] [PubMed] [Google Scholar]
- 20. Bellardita L, Valdagni R, van den Bergh R et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Eur Urol 2015; 67: 637–45 [DOI] [PubMed] [Google Scholar]
- 21. Staffurth JN, Haviland JS, Wilkins A et al. Impact of Hypofractionated radiotherapy on patient‐reported outcomes in prostate cancer: results up to 5 yr in the CHHiP trial (CRUK/06/016). Eur Urol Oncol 2021; 4: 980–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johansson E, Steineck G, Holmberg L et al. Long‐term quality‐of‐life outcomes after radical prostatectomy or watchful waiting: the Scandinavian prostate cancer Group‐4 randomised trial. Lancet Oncol 2011; 12: 891–9 [DOI] [PubMed] [Google Scholar]
- 23. Punnen S, Cowan JE, Chan JM, Carroll PR, Cooperberg MR. Long‐term health‐related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. Eur Urol 2015; 68: 600–8 [DOI] [PubMed] [Google Scholar]
- 24. Nyberg M, Hugosson J, Wiklund P et al. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24‐month follow‐up in the Swedish LAPPRO trial. Eur Urol Oncol 2018; 1: 353–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolff RF, Ryder S, Bossi A et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur J Cancer 2015; 51: 2345–67 [DOI] [PubMed] [Google Scholar]
- 26. Widmark A, Gunnlaugsson A, Beckman L et al. Ultra‐hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5‐year outcomes of the HYPO‐RT‐PC randomised, non‐inferiority, phase 3 trial. Lancet 2019; 394: 385–95 [DOI] [PubMed] [Google Scholar]
- 27. Chen RC, Chang P, Vetter RJ et al. Recommended patient‐reported core set of symptoms to measure in prostate cancer treatment trials. J Natl Cancer Inst 2014; 106(7): 7. 10.1093/jnci/dju132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szymanski KM, Wei JT, Dunn RL, Sanda MG. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health‐related quality of life among prostate cancer survivors. Urology 2010; 76: 1245–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barocas DA, Chen V, Cooperberg M et al. Using a population‐based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J Comp Eff Res 2013; 2: 445–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilt TJ, Vo TN, Langsetmo L et al. Radical prostatectomy or observation for clinically localized prostate cancer: extended follow‐up of the prostate cancer intervention versus observation trial (PIVOT). Eur Urol 2020; 77: 713–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Primary and secondary treatments for localised prostate cancer received over 6 years of follow‐up.
Fig. S2 Adjusted physical and mental health and psychological outcomes after primary treatments for prostate cancer over 6 years.
Fig. S3 Patient‐reported urinary, bowel and sexual symptoms after radical treatment for localised prostate cancer, either initially, or after a period of AM over 4 years.
Fig. S4 Adjusted QoL outcomes after receiving radical treatment after primary AM over 4 years.
Fig. S5 Patient‐reported urinary, bowel and sexual symptoms by age groups by primary localised prostate cancer treatments over 6 years.
Table S1 Response rates for exemplar PROMs in the treatment received analysis.
Table S2 Adjusted urinary symptoms corresponding to Fig. 1 and subscales.
Table S3 Adjusted sexual symptoms corresponding to Fig. 2 and subscales.
Table S4 Adjusted bowel symptoms corresponding to Fig. 3 and subscales.
Table S5 Adjusted symptoms from immediate radical treatments or after AM corresponding to Fig. S3.
Table S6 Adjusted QoL items by treatment received corresponding to Fig. S2.
Table S7 Adjusted QoL of radical treatments after AM and immediate: radical treatment corresponding to Fig. S4.
Table S8 Adjusted interaction between treatment and age group on symptoms corresponding to Fig. S5.
Table S9 Adjusted symptoms and quality 5 years after enrolment in the CEASAR cohort or diagnosis in the ProtecT trial.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after de‐identification (text, tables, figures, and appendices) will be made available (the study protocol and statistical analysis plan is published). The data will be available to approved researchers following submission of a proposal (directed to f.c.hamdy@nds.ox.uk); to gain access, data requestors will need to sign a data access agreement.


