Abstract
Background and Aim
Gastro‐esophageal reflux (GER) is the main predisposing factor for Barrett's esophagus (BE). A more precise estimate of the association of GER symptoms with the risk of BE would be important to prioritize endoscopic screening. We conducted a systematic review and meta‐analysis to examine this issue.
Methods
MEDLINE, EMBASE, and EMBASE Classic were searched to identify cross‐sectional studies that reported the prevalence of BE based on presence of GER symptoms. The prevalence of BE was compared according to presence or absence of GER symptoms using an odds ratio (OR), with a 95% confidence interval (CI). Specificity and sensitivity of GER symptoms for predicting BE was calculated.
Results
Of 10,463 citations evaluated, 19 studies reported the prevalence of BE in 43,017 subjects. The pooled OR among individuals with weekly GER symptoms compared with those without was 1.67 (95% CI 1.30–2.15) for endoscopically suspected BE, and 2.42 (95% CI 1.59–3.68) for histologically confirmed BE. No significant association was found between weekly GER symptoms and the presence of short segment BE (OR 1.30; 95% CI 0.86–1.97), whereas a strong association was present with long segment BE, with an OR of 6.30 (95% CI 2.26–17.61).
Conclusions
Gastro‐esophageal reflux symptoms are associated with an increased odds of BE, with a further increase when weekly symptoms are present. Overall, GER symptoms showed low sensitivity and specificity for predicting BE; however, a strong association was found between weekly GER symptoms and long segment BE, but not short segment BE, suggesting that it may be worth considering screening individuals with weekly GER symptoms to rule out long segment BE.
Keywords: Barrett's esophagus, epidemiology, esophageal cancer, gastro‐esophageal reflux disease
Introduction
Barrett's esophagus (BE) represents a metaplastic transformation of the normal squamous esophageal epithelium into columnar epithelium. 1 This condition is asymptomatic but can predispose to the development of esophageal adenocarcinoma (EAC), which is often diagnosed at an advanced stage and, therefore, carries a poor prognosis. 2 In contrast, early treatment of pre‐malignant BE mucosa leads to favorable outcomes, and its complete eradication can be achieved in most cases. 3 , 4
In this regard, identification of patients with BE who are at high risk of progression to EAC remains paramount. Several risk factors have been described to be associated with BE and might contribute to its development and progression. Gastro‐esophageal reflux (GER) symptoms are common in the community, and it is widely accepted that chronic exposure to GER is the main predisposing factor to both BE and EAC. 3 , 5 For many other risk factors, including alcohol consumption, tobacco smoking, or obesity, the associations with BE are weak or non‐significant among individuals with GER symptoms, suggesting that these might represent confounders associated with an increased risk of GER, rather than being independently associated with the development of BE. 6
In a previous meta‐analysis, we reported that up to 14% of individuals worldwide reporting gastro‐esophageal reflux symptoms were found to have histologically confirmed Barrett's esophagus. 7 However, almost 50% of patients with BE do not report GER symptoms and, in some studies, BE has been reported to occur in up to 25% of asymptomatic subjects, suggesting that GER symptoms are a poor predictor of BE. 8 , 9 A prior systematic review and meta‐analysis demonstrated that GER symptoms did not predict short segment BE but were associated with a five‐fold increased risk of long segment BE (LSBE). 10 Although subsequent studies have evaluated the prevalence of BE in patients with and without GER symptoms, the impact of symptoms and the association with the risk of BE remains unclear.
A more precise estimate of the association between symptomatic GER and BE would be important to better inform patients during consultation and prioritize endoscopic evaluation to confirm or refute the presence of BE. Therefore, we conducted a systematic review and meta‐analysis of cross‐sectional surveys to evaluate the association of GER symptoms with presence of BE, comparing the prevalence of BE in patients undergoing upper endoscopic assessment for GER symptoms with those undergoing the same procedure for other indications.
Methods
Search strategy and study selection
We searched the literature using EMBASE CLASSIC and EMBASE (1947 to December 2021), and MEDLINE (1948 to December 2021) to identify only cross‐sectional surveys published in full that reported the prevalence of BE in adults (aged ≥ 16 years) referred for upper endoscopy. Studies were required to recruit consecutive participants undergoing upper endoscopy to investigate upper gastrointestinal symptoms or as a voluntary health check and report the number of subjects undergoing endoscopy with GER symptoms who had BE and the number of subjects without GER symptoms who had BE. Studies that recruited convenience samples, such as employees at an institution, university students, or veterans were not eligible for inclusion.
Other eligibility criteria, which we defined prospectively, included prospective recruitment of at least 100 participants, a definition of GER that included one or more of the following: heartburn and/or regurgitation of any severity, or symptoms felt to be compatible with GER as diagnosed by a clinician or according to a questionnaire, a definition of endoscopic BE compatible with presence of columnar‐lined esophagus (proximal displacement of the squamo‐columnar junction above the upper end of the gastric folds or gastro‐esophageal junction), or a definition of histologically‐confirmed BE in the presence of specialized intestinal metaplasia on biopsies obtained from the columnar‐lined esophagus.
We searched the medical literature using the following terms: esophageal neoplasm, esophageal adenocarcinoma, Barrett esophagus, dysplasia, intestinal metaplasia, NERD, non‐erosive reflux disease, ERD (both as medical subject heading (MeSH) and free text terms). We combined these using the set operator AND with studies identified with the terms: heartburn, GERD, gastroesophageal reflux disease, gastroesophageal reflux, esophageal reflux (both as MeSH and free text terms), acid regurgitation, GORD, or upper gastrointestinal symptoms (as free text terms). We limited the initial search to study title and abstract. The resulting studies were screened independently by two investigators for potential suitability, and we retrieved those that appeared relevant to examine them in more detail. We translated foreign language articles, if needed. We conducted a recursive search using the bibliographies of all included articles. If multiple studies from the same population where found, we included the most recent study. Eligibility assessment was performed independently by two investigators, and we resolved disagreements by consensus. We assessed the quality of included studies using the JBI Critical Appraisal Tool for analytical cross sectional studies. 11 We conducted the systematic review according to the MOOSE checklist, 12 and published the study protocol on the PROSPERO international prospective register of systematic reviews (registration number CRD 42020164811).
Data analysis
Two investigators (LHE and GGC) extracted data independently on to a Microsoft Excel spreadsheet (XP professional edition; Microsoft, Redmond, WA, USA). We resolved any discrepancies by mutual consensus between the reviewers. We collected the following data for each study: year(s) conducted, country and geographical region, setting where the study was conducted, method of symptom data collection (interview‐administered questionnaire, self‐completed questionnaire, or face‐to‐face interview), symptom frequency and duration used to define presence of GER symptoms, number of subjects providing complete data, age range and mean age of subjects, proportion of male subjects, the number of subjects with an endoscopically suspected and/or histologically confirmed diagnosis of BE, and the length of BE detected (short‐segment BE (SSBE) (< 3 cm of columnar‐lined esophagus) versus LSBE (≥ 3 cm of columnar‐lined esophagus)). Where studies reported the prevalence of GER symptoms according to different symptom frequency thresholds, we extracted the number of subjects with GER symptoms according to each threshold. Subjects undergoing upper endoscopy for bothersome GER symptoms that were reported at a frequency of at least weekly were considered to have gastro‐esophageal reflux disease (GERD) in line with the Montreal definition. 13
We combined the proportion of individuals with BE from each study to give a pooled prevalence for all studies. The prevalence of BE was pooled separately in all study participants according to the presence or absence of GER symptoms at the time of the endoscopic evaluation. Moreover, the pooled prevalence of BE in subjects with GER symptoms at the time of the endoscopic evaluation was compared with those without GER symptoms using an unadjusted odds ratio (OR), with a 95% confidence interval (CI). We assessed heterogeneity between studies using the I 2 statistic with values of 25% to 49%, 50% to 74%, and ≥ 75% typically considered low, moderate, and high levels of heterogeneity, respectively, and the χ 2 test with a P value < 0.10, as the threshold used to define statistically significant heterogeneity. 14 Data were pooled using a random effects model, to give a more conservative estimate of the prevalence of BE and the odds of BE in these various groups. 15 StatsDirect version 3.2.10 (StatsDirect Ltd, Sale, Cheshire, England) was used to generate Forest plots of pooled ORs with 95% CIs. We planned to assess for evidence of publication bias by applying Egger's test to funnel plots of ORs, 16 where a sufficient number of studies (≥ 10) were available. 17
Results
The search strategy identified 10,463 citations. From these, we identified 73 that appeared relevant (Fig. 1). There were 19 articles that fulfilled the eligibility criteria, 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 of which two reported the prevalence of endoscopically suspected BE only, 27 , 33 13 reported the prevalence of histologically confirmed BE only, 18 , 19 , 20 , 21 , 22 , 24 , 25 , 29 , 30 , 31 , 32 , 34 , 36 and four reported the prevalence of BE according to both definitions. 23 , 26 , 28 , 35 Agreement between investigators for assessment of study eligibility was excellent (kappa statistic = 0.92). All articles were published in English. Seven studies were high quality according to the JBI Critical Appraisal Tool (Table S1). The 19 included studies recruited 43,017 subjects and were geographically diverse, with four from Europe, 18 , 23 , 26 , 28 three from North America, 19 , 25 , 35 three from the Middle east, 21 , 29 , 34 and nine from Asia. 20 , 22 , 24 , 27 , 30 , 31 , 32 , 33 , 36 Detailed characteristics of all included studies are provided in Table 1.
Figure 1.
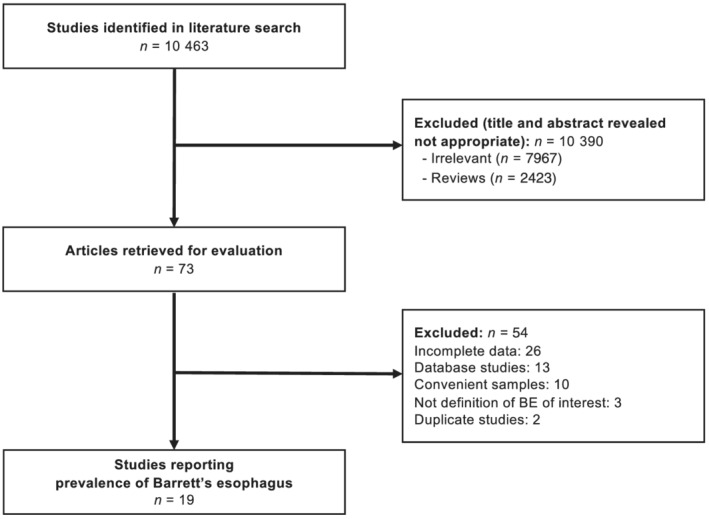
Flow diagram of studies identified in the systematic review.
Table 1.
Characteristics of included eligible studies
| Author and year of publication | Country | Diagnosis of GER symptoms | Weekly symptoms | Total no. included patients | Endoscopically suspected and/or histologically diagnosed BE | Number with GER symptoms (BE/cases) | Number without GER symptoms (BE/cases) |
|---|---|---|---|---|---|---|---|
| Mantynen 2002 18 | Finland | Interview‐administered questionnaire | No | 3378 | Histologically diagnosed | 11/760 | 13/2618 |
| Rex 2003 19 | US | Self‐completed questionnaire | No | 961 | Histologically diagnosed | 30/393 | 35/568 |
| Yes | 961 | Histologically diagnosed | 14/150 | 51/811 | |||
| Rajendra 2004 20 | Malaysia | Clinical assessment | Yes | 1985 | Histologically diagnosed | 76/661 | 47/1324 |
| Toruner 2004 21 | Turkey | Self‐completed questionnaire | No | 395 | Histologically diagnosed | 3/61 | 26/334 |
| Kim 2005 22 | Korea | Clinical assessment | No | 992 | Histologically diagnosed | 7/164 | 29/828 |
| Ronkainen 2005 23 | Sweden | Self‐completed questionnaire | No | 1000 | Endoscopically suspected | 50/400 | 53/600 |
| Histologically diagnosed | 9/400 | 7/600 | |||||
| Yes | 1000 | Endoscopically suspected | 28/200 | 75/800 | |||
| Histologically diagnosed | 5/200 | 11/800 | |||||
| Amano 2006 24 | Japan | Self‐completed questionnaire | No | 1668 | Histologically diagnosed | 32/497 | 74/1171 |
| Ward 2006 25 | US | Self‐completed questionnaire | Yes | 300 | Histologically diagnosed | 21/106 | 29/194 |
| Johansson 2007 26 | Sweden | Self‐completed questionnaire | Yes | 604 | Endoscopically suspected | 43/207 | 52/397 |
| Histologically diagnosed | 10/207 | 11/397 | |||||
| Tseng 2008 27 | Taiwan | Self‐completed questionnaire | Yes | 19,812 | Endoscopically suspected | 12/3171 | 44/16641 |
| Zagari 2008 28 | Italy | Self‐completed questionnaire | No | 1033 | Endoscopically suspected | 7/458 | 18/575 |
| Histologically diagnosed | 19/458 | 6/575 | |||||
| Yes | 1033 | Endoscopically suspected | 15/245 | 22/788 | |||
| Histologically diagnosed | 7/245 | 6/788 | |||||
| Odemis 2009 29 | Turkey | Interview‐administered questionnaire | No | 1000 | Histologically diagnosed | 6/351 | 6/649 |
| Peng 2009 30 | China | Interview‐administered questionnaire | No | 2580 | Histologically diagnosed | 16/310 | 11/2270 |
| Kuo 2010 31 | Taiwan | Self‐completed questionnaire | Yes | 736 | Histologically diagnosed | 13/344 | 0/392 |
| Xiong 2010 32 | China | Clinical assessment | No | 2022 | Histologically diagnosed | 3/139 | 18/1883 |
| Zou 2011 33 | China | Self‐completed questionnaire | No | 1029 | Endoscopically suspected | 2/187 | 17/842 |
| Yes | 1029 | Endoscopically suspected | 1/48 | 18/981 | |||
| Ege 2015 34 | Turkey | Interview‐administered questionnaire | No | 1370 | Histologically diagnosed | 1/180 | 10/1190 |
| Sami 2015 35 | US | Self‐completed questionnaire | No | 205 | Endoscopically suspected | 24/115 | 14/90 |
| Histologically diagnosed | 12/115 | 4/90 | |||||
| Yes | 205 | Endoscopically suspected | 7/29 | 31/176 | |||
| Histologically diagnosed | 6/29 | 10/176 | |||||
| Quach 2020 36 | Vietnam | Clinical assessment | No | 1947 | Histologically diagnosed | 0/788 | 8/1159 |
GER, gastro‐esophageal reflux; BE, Barrett's esophagus.
Association between gastro‐esophageal reflux symptoms or gastro‐esophageal reflux disease and endoscopically suspected Barrett's esophagus
Six studies reported the prevalence of endoscopically suspected BE in individuals undergoing endoscopy due to GER symptoms of any frequency and in individuals undergoing endoscopy for other upper gastrointestinal symptoms, containing a total of 23,683 subjects (4538 with GER symptoms of any frequency). 23 , 26 , 27 , 28 , 33 , 35 Three studies were conducted in Europe, 23 , 26 , 28 two in Asia, 27 , 33 and one in North America. 35 When data from all six study populations were pooled, the overall prevalence of endoscopically suspected BE was 6.6% (95% CI 1.7% to 14.4%). Among subjects with GER symptoms the prevalence of endoscopically suspected BE was 7.7% (95% CI 1.7% to 17.5%), compared with 5.6% (95% CI 1.5% to 12.2%) among those without. The pooled OR for the presence of endoscopically suspected BE among individuals with GER symptoms of any frequency compared with those without was 1.48 (95% CI 1.17 to 1.87), with no heterogeneity between studies (I 2 = 0%, P = 0.77) (Fig. 2).
Figure 2.
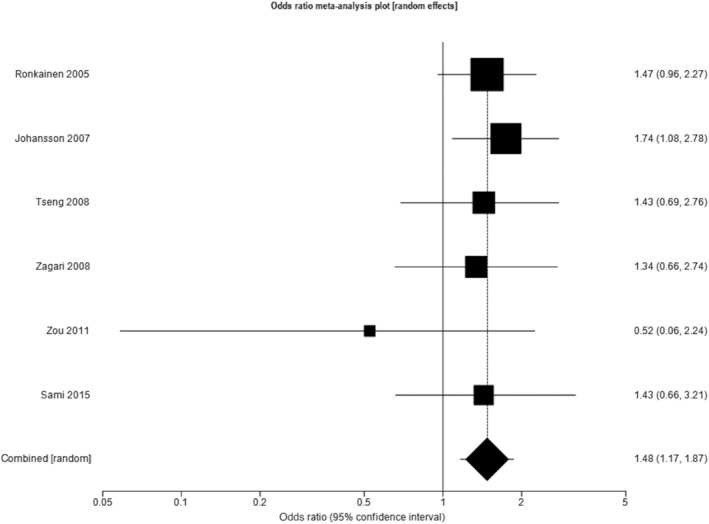
Odds ratio for endoscopically suspected Barrett's esophagus in individuals with gastro‐esophageal reflux symptoms of any frequency compared with those without.
The same six studies reported data regarding the prevalence of endoscopically suspected BE among subjects undergoing endoscopy due to GERD, as per the Montreal definition, as well as among subjects with other upper gastrointestinal symptoms. 23 , 26 , 27 , 28 , 33 , 35 In this analysis, the total number of subjects with GERD was 3900. The pooled prevalence of BE among subjects with GERD was 9.2% (95% CI 1.7% to 21.7%), compared with 5.8% (95% CI 1.6% to 12.6%) among those without. The pooled OR for the presence of endoscopically suspected BE among individuals with GERD compared with those without was 1.67 (95% CI 1.30 to 2.15), again with no heterogeneity between studies (I 2 = 0%, P = 0.93) (Fig. 3).
Figure 3.
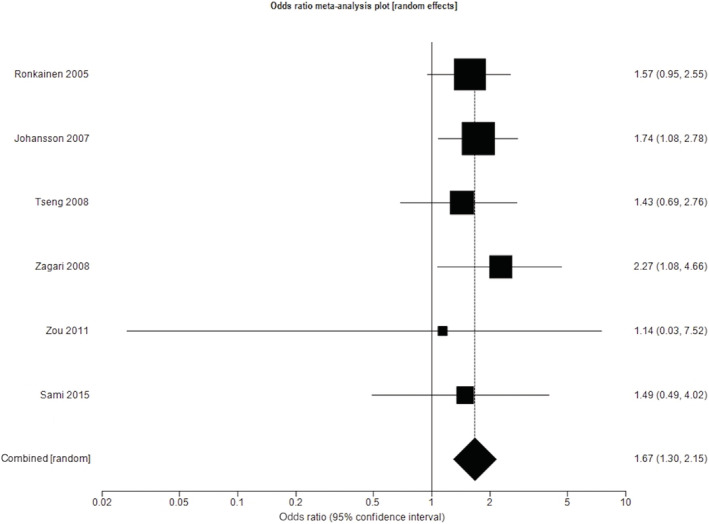
Odds ratio for endoscopically suspected Barrett's esophagus in individuals with gastro‐esophageal reflux disease compared with those without.
Association between gastro‐esophageal reflux symptoms or gastro‐esophageal reflux disease and histologically confirmed Barrett's esophagus
Seventeen studies reported the prevalence of histologically confirmed BE among subjects undergoing endoscopy due to GER symptoms of any frequency and in individuals undergoing endoscopy for other upper gastrointestinal symptoms, containing a total of 22,176 subjects (5934 with GER symptoms of any frequency). 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 Seven studies were conducted in Asia, 20 , 22 , 24 , 30 , 31 , 32 , 36 four in Europe, 18 , 23 , 26 , 28 three in North America, 19 , 25 , 35 and three from the Middle East. 21 , 29 , 34 When data from all 17 studies were pooled, the prevalence of histologically confirmed BE in all individuals was 3.2% (95% CI 1.9% to 4.6%). Among subjects with GER symptoms the prevalence of histologically confirmed BE was 4.3% (95% CI 2.4% to 6.7%), compared with 2.4% (95% CI 1.4% to 3.7%) among those without. The pooled OR for the presence of histologically confirmed BE among individuals with GER symptoms of any frequency compared with those without was 1.88 (95% CI 1.27 to 2.79), with high heterogeneity between studies (I 2 = 72%, P < 0.001) (Fig. 4). Stratifying by geographical region demonstrated a stronger association in Asia than in Europe, but significant heterogeneity between studies remained. When studies from Asia were pooled the OR was 2.47 (95% CI 1.06 to 5.76; I 2 = 86.7%, P < 0.001), compared with 2.07 (95% CI 1.30 to 3.29; I 2 = 0%, P = 0.74) in European studies, 1.40 (95% CI 0.97 to 2.04; I 2 = 0%, P = 0.57) in North American studies, and 1.03 (95% CI 0.48 to 2.24; I 2 = 0%, P = 0.38) in Middle Eastern studies.
Figure 4.
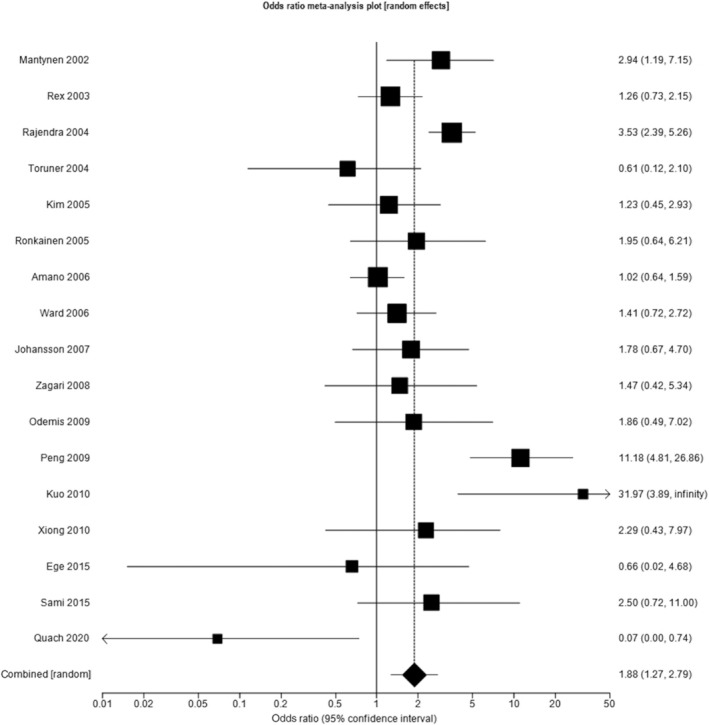
Odds ratio for histologically confirmed Barrett's esophagus in individuals with gastro‐esophageal reflux symptoms of any frequency compared with those without.
Eight studies reported data regarding the prevalence of histologically confirmed BE among subjects undergoing endoscopy due to GERD, as per the Montreal definition, as well as for other upper gastrointestinal symptoms. 19 , 20 , 23 , 25 , 26 , 28 , 31 , 35 These studies contained a total of 6824 subjects, 1942 of whom had GERD. Three studies were conducted in North America, 19 , 25 , 35 three in Europe, 23 , 26 , 28 and two in Asia. 20 , 31 The pooled prevalence of histologically confirmed BE among all subjects was 4.8% (95% CI 2.6% to 7.7%), with a higher pooled prevalence in subjects with GERD compared with those without ((7.9% (95% CI 4.4% to 12.2%) versus 3.4% (95% CI 1.5% to 6.0%)). The pooled OR for the presence of histologically confirmed BE among individuals with GERD compared with those without was 2.42 (95% CI 1.59 to 3.68), with moderate heterogeneity between studies (I 2 = 52.1%, P = 0.041) (Fig. 5).
Figure 5.
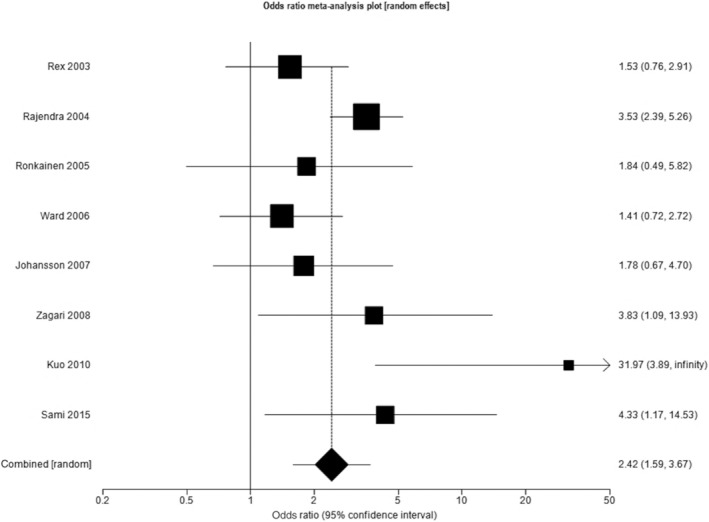
Odds ratio for histologically confirmed Barrett's esophagus in individuals with gastro‐esophageal reflux disease compared with those without.
Association between gastro‐esophageal reflux symptoms or gastro‐esophageal reflux disease and length of Barrett's esophagus
Five studies reported the prevalence of histologically confirmed BE according to the length of the segment of BE in individuals with GER symptoms of any frequency and in individuals undergoing endoscopy for other upper gastrointestinal symptoms. 19 , 23 , 25 , 26 , 28 The OR for SSBE in subjects with GER symptoms of any frequency compared with those without was not significant in these five studies (1.12; 95% CI 0.78 to 1.59), with no heterogeneity between studies (I 2 = 0%, P = 0.84) (Fig. S1). In contrast, the OR for LSBE in subjects with GER symptoms of any frequency was 5.27 (95% CI 1.97 to 14.11), with no heterogeneity between studies (I 2 = 0%, P = 0.62) (Fig. S2).
Four studies reported the prevalence of histologically confirmed BE according to segment length in individuals with and without GERD. 19 , 25 , 26 , 28 There was no significant association between presence of SSBE and GERD (OR 1.30; 95% CI 0.86 to 1.97, I 2 = 0%, P = 0.42), whereas for LSBE the summary OR was 6.30 (95% CI 2.26 to 17.61) with low heterogeneity between studies (I 2 = 7.3%, P = 0.36).
Discussion
We conducted a systematic review and meta‐analysis to estimate the association between GER symptoms and BE, including data from 19 cross‐sectional surveys that reported the prevalence of endoscopically suspected and/or histologically confirmed BE at upper gastrointestinal endoscopy, according to the presence or absence of GER symptoms. The odds of histologically confirmed BE were almost twice as high in subjects with GER symptoms, compared with those without, and more than twice as high in those with GERD, as per the Montreal definition. As expected, a smaller, but still statistically significant, association was found between endoscopically suspected BE and both GER symptoms and GERD. The degree of association varied according to geographical location, and was stronger in studies conducted in Asia and in Europe. There was no significant association between GER symptoms or GERD and histologically confirmed SSBE, whereas the association between both GER symptoms and GERD and histologically confirmed LSBE was strong, with ORs of > 5 and > 6, respectively.
Although both LSBE and SSBE seem to be a consequence of reflux of gastric contents, only LSBE seemed to be associated with GER. Reasons for this are speculative. Given that SSBE is located in the distal 3 cm of the esophagus, small amounts of acid reflux in this region may be less likely to be sensed as GER symptoms by patients, compared with more proximal reflux. Moreover, the diagnosis of SSBE is often more challenging, compared with LSBE, mainly due to the use of incorrect landmarks to define the presence and extent of the intestinal metaplasia, which has been observed in one‐in‐three endoscopists in observational studies. 37 This reinforces the need for standardized endoscopic classifications and validated biopsy protocols for BE to be implemented, such as the Prague classification 38 and the Seattle protocol, 39 in association with use of advanced endoscopic imaging techniques to increase accuracy of BE diagnosis.
This study has several strengths. In order to maximize the likelihood of identifying all relevant studies, an exhaustive and contemporaneous literature search strategy was used. The eligibility of all the studies was performed by two investigators independently who also carried out the data extraction. Any discrepancies were resolved by consensus. Where necessary, we contacted the corresponding authors of studies to minimize the probability of including duplicate publications from the same cohort of patients, and to obtain additional data. A random effects model was used to pool data, to provide a more conservative estimate of the odds of BE according to presence of GER symptoms or GERD. Finally, we excluded studies conducted among convenience samples, to minimize the likelihood of overestimating the association between BE and GER symptoms or GERD.
Limitations of our analyses arise from the reporting of data within the available studies, specifically the variability in the criteria used to define the presence of GER symptoms, as well as the frequency and duration of symptoms. Therefore, we reported the results of studies pooled separately based on symptom frequency, estimating the association of BE only in studies that reported at least weekly symptoms, in line with the Montreal definition of GERD. However, none of the studies provided extractable data for a symptom frequency above this, meaning that we were unable to assess if daily symptoms of GER, for example, were more strongly associated with presence of BE. Moreover, no data were available regarding duration of GER symptoms, considered an important factor for the development of BE. Earlier age at onset of GER symptoms has been associated with increased risk of BE, suggesting that age at symptom onset could help practitioners decide which patients with GER symptoms to refer for endoscopic screening for BE. 40 In contrast, the use of standard landmarks, such as the displacement of the squamo‐columnar junction above the proximal end of the gastric folds to define endoscopically suspected BE, was homogeneous across the studies. However, the sampling of endoscopically suspected BE mucosa across the studies was not standardized, and was conducted according to local experience, which may have led to an underestimation of the true prevalence of BE. In order to reduce variability in the diagnoses across studies, and since it is not clear whether cardia‐type mucosa in the esophagus has the same malignant predisposition, 41 we only included studies that considered intestinal metaplasia with goblet cells (specialized intestinal metaplasia) as histologically confirmed BE in our analyses. Finally, there was moderate to high heterogeneity between studies in a few of our analyses, the reasons for which are unclear, but may relate to geographical region or other demographic differences between study populations, including ethnicity, which it was not possible to examine using the available data. Similarly, this could explain the discrepancies we found in the degree of association of GER symptoms with BE according to geographical location.
There have been previous systematic reviews examining the association of GER symptoms with BE. The most recent of these was published in 2010 by Taylor and Rubenstein. 10 The authors included cross‐sectional and case–control studies and reported a summary OR for the association between GER symptoms and BE of almost three, but with significant heterogeneity between studies. Among studies that conducted upper endoscopies irrespective of any symptoms, they also found a strong association between GERD and LSBE, with an OR of almost five, but no association with SSBE. 10 However, the different study designs they considered, as well as the fact that a considerable amount of data has been published since this meta‐analysis was conducted, reinforces the need for a more contemporaneous systematic assessment of the relationship between BE and GER symptoms. Moreover, in order to obtain a homogeneous study population, we included only cross‐sectional studies recruiting consecutive participants undergoing endoscopy comparing those with and without GER symptoms and excluding studies that recruited convenience samples.
The findings of this meta‐analysis have implications for both research and clinical practice. GER symptoms are recognized as a major risk factor for the development of BE, and our meta‐analysis supports this association and also demonstrates a stronger association in subjects meeting the Montreal definition of GERD. Moreover, the odds of LSBE were also higher when GERD was present. This reinforces the utility of assigning a diagnosis of GERD based on a minimum symptom severity and frequency, and consisting of moderate or severe symptoms occurring ≥ 1 day/week or mild symptoms occurring ≥ 2 days/week. 13 However, studies that have used such criteria remain scarce.
Population level screening to detect cases of BE is difficult to endorse, because overall progression rates to EAC are extremely low, ranging from between 0.1% to 0.5% per year. 42 , 43 In particular, the absolute risk of progression is substantially lower in patients with SSBE compared with LSBE, suggesting that the extent of intestinal metaplasia is an independent predictor for the risk of progression to EAC. 44 Therefore, given subjects with LSBE have a higher risk of progression to EAC, estimated at almost 1% per year, 45 and the results of this meta‐analysis suggest that those with GERD have a six times higher odds of LSBE, it may be worth considering screening individuals with GERD to rule out LSBE.
In conclusion, this systematic review and meta‐analysis has confirmed that GER symptoms are associated with an increased odds of both endoscopically suspected and histologically confirmed BE, with a further increase when weekly symptoms are present, in line with the minimum symptom frequency and severity recommended to make a diagnosis of GERD. A strong association was found between weekly GER symptoms and LSBE, whereas no association was found between weekly GER symptoms and SSBE. This suggests that screening individuals with weekly GER symptoms, which are likely to represent GERD, to exclude LSBE is a worthwhile strategy.
Supporting information
Figure S1: Odds ratio for short segment Barrett's esophagus in subjects with gastro‐esophageal reflux symptoms of any frequency compared with those without.
Figure S2: Odds ratio for long segment Barrett's esophagus in subjects with gastro‐esophageal reflux symptoms of any frequency compared with those without.
Table S1. Quality assessment of included studies using the JBI Critical Appraisal Tool.11
Eusebi, L. H. , Telese, A. , Cirota, G. G. , Haidry, R. , Zagari, R. M. , Bazzoli, F. , and Ford, A. C. (2022) Effect of gastro‐esophageal reflux symptoms on the risk of Barrett's esophagus: A systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology, 37: 1507–1516. 10.1111/jgh.15902.
Declaration of conflict of interest: The authors have no conflict of interest to declare.
Author contribution: LHE, AT, GGC, and ACF made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; LHE, AT, RH, and ACF drafted the work or revised it critically for important intellectual content; all authors approved the version to be published; LHE and ACF agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support: The work reported in this publication was funded by the Italian Ministry of Health, RC‐2022‐2773478.
Data availability statement
The data supporting the findings of this study are available within the article and its supporting information.
References
- 1. Spechler SJ, Souza RF. Barrett's esophagus. N. Engl. J. Med. 2014; 371: 836–845. [DOI] [PubMed] [Google Scholar]
- 2. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet (London, England) 2017; 390: 2383–2396. [DOI] [PubMed] [Google Scholar]
- 3. Fitzgerald RC, di Pietro M, Ragunath K et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014; 63: 7–42. [DOI] [PubMed] [Google Scholar]
- 4. Luigiano C, Iabichino G, Eusebi LH et al. Outcomes of Radiofrequency Ablation for Dysplastic Barrett's Esophagus: A Comprehensive Review. Gastroenterol. Res. Pract. 2016; 2016: 4249510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani‐Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro‐oesophageal reflux symptoms: a meta‐analysis. Gut 2018; 67: 430–440. [DOI] [PubMed] [Google Scholar]
- 6. Eusebi LH, Telese A, Cirota GG et al. Systematic review with meta‐analysis: risk factors for Barrett's oesophagus in individuals with gastro‐oesophageal reflux symptoms. Aliment. Pharmacol. Ther. 2021; 53: 968–976. [DOI] [PubMed] [Google Scholar]
- 7. Eusebi LH, Cirota GG, Zagari RM, Ford AC. Global prevalence of Barrett's oesophagus and oesophageal cancer in individuals with gastro‐oesophageal reflux: a systematic review and meta‐analysis. Gut 2021; 70: 456–463. [DOI] [PubMed] [Google Scholar]
- 8. Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett's esophagus in asymptomatic individuals. Gastroenterology 2002; 123: 461–467. [DOI] [PubMed] [Google Scholar]
- 9. Zagari RM, Eusebi LH, Rabitti S et al. Prevalence of upper gastrointestinal endoscopic findings in the community: A systematic review of studies in unselected samples of subjects. J. Gastroenterol. Hepatol. 2016; 31: 1527–1538. [DOI] [PubMed] [Google Scholar]
- 10. Taylor JB, Rubenstein JH. Meta‐analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett's esophagus. Am. J. Gastroenterol. 2010; 105: 1729: 30–37 quiz 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. JBI Prevalence Critical Appraisal Tool for analytical cross sectional studies. https://jbi.global/sites/default/files/2021‐03/Checklist_for_Analytical_Cross_Sectional_Studies.docx
- 12. Stroup DF, Berlin JA, Morton SC et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 13. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, the Global Consensus Group . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am. J. Gastroenterol. 2006; 101: 1900–1920 quiz 43. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed) 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control. Clin. Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JA, Sutton AJ, Ioannidis JP et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical research ed) 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 18. Mantynen T, Farkkila M, Kunnamo I, Mecklin JP, Juhola M, Voutilainen M. The impact of upper GI endoscopy referral volume on the diagnosis of gastroesophageal reflux disease and its complications: a 1‐year cross‐sectional study in a referral area with 260,000 inhabitants. Am. J. Gastroenterol. 2002; 97: 2524–2529. [DOI] [PubMed] [Google Scholar]
- 19. Rex DK, Cummings OW, Shaw M et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003; 125: 1670–1677. [DOI] [PubMed] [Google Scholar]
- 20. Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett's esophagus: the long and short of it all. Dig. Dis. Sci. 2004; 49: 237–242. [DOI] [PubMed] [Google Scholar]
- 21. Toruner M, Soykan I, Ensari A, Kuzu I, Yurdaydin C, Ozden A. Barrett's esophagus: prevalence and its relationship with dyspeptic symptoms. J. Gastroenterol. Hepatol. 2004; 19: 535–540. [DOI] [PubMed] [Google Scholar]
- 22. Kim JY, Kim YS, Jung MK et al. Prevalence of Barrett's esophagus in Korea. J. Gastroenterol. Hepatol. 2005; 20: 633–636. [DOI] [PubMed] [Google Scholar]
- 23. Ronkainen J, Aro P, Storskrubb T et al. Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology 2005; 129: 1825–1831. [DOI] [PubMed] [Google Scholar]
- 24. Amano Y, Kushiyama Y, Yuki T et al. Prevalence of and risk factors for Barrett's esophagus with intestinal predominant mucin phenotype. Scand. J. Gastroenterol. 2006; 41: 873–879. [DOI] [PubMed] [Google Scholar]
- 25. Ward EM, Wolfsen HC, Achem SR et al. Barrett's esophagus is common in older men and women undergoing screening colonoscopy regardless of reflux symptoms. Am. J. Gastroenterol. 2006; 101: 12–17. [DOI] [PubMed] [Google Scholar]
- 26. Johansson J, Hakansson HO, Mellblom L et al. Risk factors for Barrett's oesophagus: a population‐based approach. Scand. J. Gastroenterol. 2007; 42: 148–156. [DOI] [PubMed] [Google Scholar]
- 27. Tseng PH, Lee YC, Chiu HM et al. Prevalence and clinical characteristics of Barrett's esophagus in a Chinese general population. J. Clin. Gastroenterol. 2008; 42: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 28. Zagari RM, Fuccio L, Wallander MA et al. Gastro‐oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general population: the Loiano‐Monghidoro study. Gut 2008; 57: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 29. Odemis B, Cicek B, Zengin NI et al. Barrett's esophagus and endoscopically assessed esophagogastric junction integrity in 1000 consecutive Turkish patients undergoing endoscopy: a prospective study. Dis. Esophagus: Off. J. Int. Soc. Dis. Esophagus 2009; 22: 649–655. [DOI] [PubMed] [Google Scholar]
- 30. Peng S, Cui Y, Xiao YL et al. Prevalence of erosive esophagitis and Barrett's esophagus in the adult Chinese population. Endoscopy 2009; 41: 1011–1017. [DOI] [PubMed] [Google Scholar]
- 31. Kuo CJ, Lin CH, Liu NJ, Wu RC, Tang JH, Cheng CL. Frequency and risk factors for Barrett's esophagus in Taiwanese patients: a prospective study in a tertiary referral center. Dig. Dis. Sci. 2010; 55: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 32. Xiong LS, Cui Y, Wang JP et al. Prevalence and risk factors of Barrett's esophagus in patients undergoing endoscopy for upper gastrointestinal symptoms. J. Dig. Dis. 2010; 11: 83–87. [DOI] [PubMed] [Google Scholar]
- 33. Zou D, He J, Ma X et al. Epidemiology of symptom‐defined gastroesophageal reflux disease and reflux esophagitis: the systematic investigation of gastrointestinal diseases in China (SILC). Scand. J. Gastroenterol. 2011; 46: 133–141. [DOI] [PubMed] [Google Scholar]
- 34. Ege B, Dinc T, Yildiz BD, Balci Z, Bozkaya H. Utility of endoscopy for diagnosis of barrett in a non‐Western society: endoscopic and histopathologic correlation. Int. Surg. 2015; 100: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sami SS, Dunagan KT, Johnson ML et al. A randomized comparative effectiveness trial of novel endoscopic techniques and approaches for Barrett's esophagus screening in the community. Am. J. Gastroenterol. 2015; 110: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quach DT, Pham QTT, Tran TLT et al. Prevalence, clinical characteristics, and risk factors of Barrett esophagus in Vietnamese patients with upper gastrointestinal symptoms. Medicine (Baltimore) 2020; 99: e21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zagari RM, Eusebi LH, Galloro G, Rabitti S, Neri M, Pasquale L, Bazzoli F. Attending Training Courses on Barrett's Esophagus Improves Adherence to Guidelines: A Survey from the Italian Society of Digestive Endoscopy. Dig. Dis. Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma P, Dent J, Armstrong D et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology 2006; 131: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 39. Peters FP, Curvers WL, Rosmolen WD et al. Surveillance history of endoscopically treated patients with early Barrett's neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis. Esophagus: Off. J. Int. Soc. Dis. Esophagus 2008; 21: 475–479. [DOI] [PubMed] [Google Scholar]
- 40. Thrift AP, Kramer JR, Qureshi Z, Richardson PA, El‐Serag HB. Age at onset of GERD symptoms predicts risk of Barrett's esophagus. Am. J. Gastroenterol. 2013; 108: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spechler SJ. Barrett's esophagus: is the goblet half empty? Clin. Gastroenterol. Hepatol. 2012; 10: 1237–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhat S, Coleman HG, Yousef F et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population‐based study. J. Natl. Cancer Inst. 2011; 103: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hvid‐Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch‐Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N. Engl. J. Med. 2011; 365: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 44. Hamade N, Vennelaganti S, Parasa S et al. Lower Annual Rate of Progression of Short‐Segment vs Long‐Segment Barrett's Esophagus to Esophageal Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2019; 17: 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hage M, Siersema PD, van Dekken H, Steyerberg EW, Dees J, Kuipers EJ. Oesophageal cancer incidence and mortality in patients with long‐segment Barrett's oesophagus after a mean follow‐up of 12.7 years. Scand. J. Gastroenterol. 2004; 39: 1175–1179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Odds ratio for short segment Barrett's esophagus in subjects with gastro‐esophageal reflux symptoms of any frequency compared with those without.
Figure S2: Odds ratio for long segment Barrett's esophagus in subjects with gastro‐esophageal reflux symptoms of any frequency compared with those without.
Table S1. Quality assessment of included studies using the JBI Critical Appraisal Tool.11
Data Availability Statement
The data supporting the findings of this study are available within the article and its supporting information.


