Abstract
This review addresses nine areas of knowledge revealed by micromanipulations performed with Paramecium. Microinjection has shown that sexual maturation and senescence of Paramecium caudatum is a programmed process conducted by a specific gene and its product protein. In Paramecium tetraurelia, autogamy was revealed to depend on the number of DNA syntheses rather than the number of cell divisions in clonal aging. The cytoplasmic complementarity test established that microinjection of wild‐type cytoplasm can correct genetic defects of mutants. The concept of complementarity together with protein chemistry revealed compounds that control membrane excitability. In non‐Mendelian inheritance, noncoding small RNAs made from the parental micronucleus regulate the rearrangement of the progeny's macronuclear DNA. The macronucleus has the potential to be used as a factory for genetic engineering. The development and differentiation of progeny's nuclei in mating pairs are controlled by the parental macronucleus. The chemical reaction processes associated with exocytosis have been revealed by microinjection of various enzymes and antibodies. Using the fusion gene of histone H2B and yellow‐fluorescence protein, it was revealed that the fusion gene‐mRNA is transferred between cells during mating. Experiments with endosymbiotic bacteria and the host shed light on the conditions needed to establish sustainable symbiotic relationships.
Keywords: behavioral mutants, complementarity test, conjugation, endosymbiosis, exocytosis, life history, membrane excitation, nucleus differentiation, scnRNA
This review is based on the question: What would we not see if we do not use micromanipulation? Through this, we demonstrate that micromanipulation delivers insights into many areas of biology.
Micromanipulation techniques are divided into microinjection and microsurgery. Microinjection is a method in which a substance is directly injected into living cells. Microsurgery is a method of removing parts of cells such as nuclei or cytoplasm. Both are performed while observing cells under a microscope. The operations are performed using a thin glass needle. Substances injected into Paramecium cells range from various ionic solutions to symbiotic organisms. The transfer of materials into ciliated cells occurs naturally during the process of conjugation and feeding behavior. One of the triggers for the use of microinjection is the phenomenon in which behavioral mutants recover during mating with wild‐type cells (Hiwatashi et al., 1980).
The earliest attempts to manipulate cells under a microscope were summarized by Korzh and Strahle (2002). Marshall Barber developed several sophisticated instruments and used them to isolate and culture bacterial cells (Barber, 1904). The history of micromanipulation experiments in multicellular organisms is described by Xu (2019).
Micromanipulation experiments performed using Paramecium began with the work of Knowles (1974) and Koizumi (1974). An advantage of microinjection is that the action of injected substances can be directly captured as a signal from living cells. By changing the injected volume, the effect of injection can be evaluated quantitatively. It is also possible to compare the effects of injections on individuals of different species.
The categories included in this review are (1) sexual phase transition in the life history, (2) clonal aging, (3) complementarity test by cytoplasmic microinjection, (4) chemical compounds analysis of membrane excitation, (5) non‐Mendelian inheritance, (6) macronucleus as a genetic engineering factory, (7) functional analysis of micronucleus and macronucleus, (8) exocytosis, and (9) endosymbiosis.
METHODS OF MICROINJECTION
Koizumi (1974) developed a way of controlling the quantity of injected material. A calibration curve is prepared in advance by connecting the syringe barrel and needle with a vinyl tube of several tens of centimeters and filling it with liquid paraffin to prevent air from entering. A drop of surfactant and a drop of liquid paraffin are placed about 1 cm apart on a cover glass. The cover glass is inverted and placed in a moist chamber. A small amount of surfactant is drawn into the injection needle, then, paraffin is drawn into the needle and the distance of paraffin is measured with an eyepiece micrometer. The liquid paraffin in the needle is then gently extruded into the surfactant to create a spherical droplet, the diameter of which is measured and volume calculated. The procedure is repeated with different volumes to create a calibration curve. This approach can be used to inject up to about 40 pl in P. caudatum, or about 10% of the cell body (Haga et al., 1982). In the case of liquids, about 20 cells can be injected per hour. The efficiency of injecting cytoplasm is about half that of a liquid sample. The protocol for transforming the vegetative macronucleus of P. tetraurelia by DNA microinjection was given by Beisson et al. (2010).
SEXUAL PHASE TRANSITION IN THE LIFE HISTORY OF PARAMECIUM
The life history of Paramecium caudatum
The life history of P. caudatum is taken to begin with sexual reproduction (Hiwatashi, 1968). Unlike sexuality in most multicellular animals, the formation of reproductive (germ) nuclei and the production of fertilized nuclei take place in a single cell (Figure 1). During conjugation, mating cells form two germ nuclei by meiosis of the micronucleus, and one of them is exchanged with the mating partner (Figure 2). The germ nucleus received from the mating partner fuses with the recipient germ nucleus to form a fusion nucleus (synkaryon) and this generates a new genome. Then, the conjugating cells separate. Each exconjugant cell produces one micronucleus and four macronuclear anlagen from the synkaryon. The exconjugants undergo two cell divisions to become four cells each having one micronucleus and one macronucleus. These are karyonides, and mark the start of a new generation in life history. The first period of the life history is called the immaturity period because cells are not able to mate. In P. caudatum, after about 50 cell divisions, each cell in a clone begins to express mating ability and the life history enters its mature phase. When a mature cell encounters a cell of complementary mating type, they may mate and the transition to the next generation takes place. The mating‐type conversion phase begins after about 150 cell divisions when some cells of Even mating‐type change to O‐type and begin mating with E‐type cells from the same clone. This phenomenon is called selfing conjugation. After about 800 cell divisions, the cell's ability to divide declines, and the cells enter the senile phase. A clone dies when all the cells that make up the clone cannot divide.
FIGURE 1.
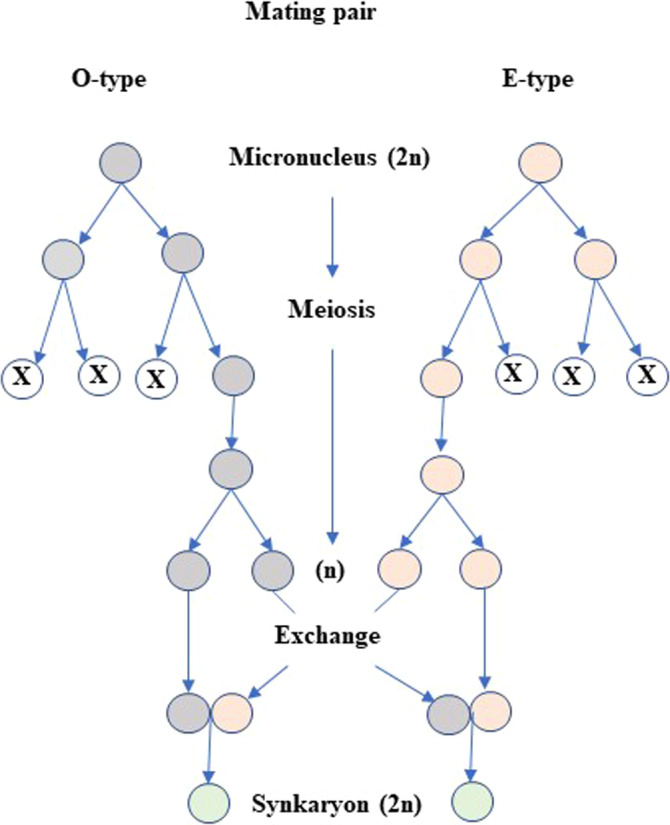
Synkaryon formation during conjugation of Paramecium caudatum. The diagram shows the changes in the micronucleus when cells of complementary mating types (O‐type and E‐type) mate. Micronuclei undergo meiosis in both cells, eventually resulting in two haploid (n) nuclei in each cell. One of them is exchanged with the partner cell, and the nuclei fuse to form a diploid (2n) synkaryon. A circle with an X inside indicates a degenerating nucleus
FIGURE 2.
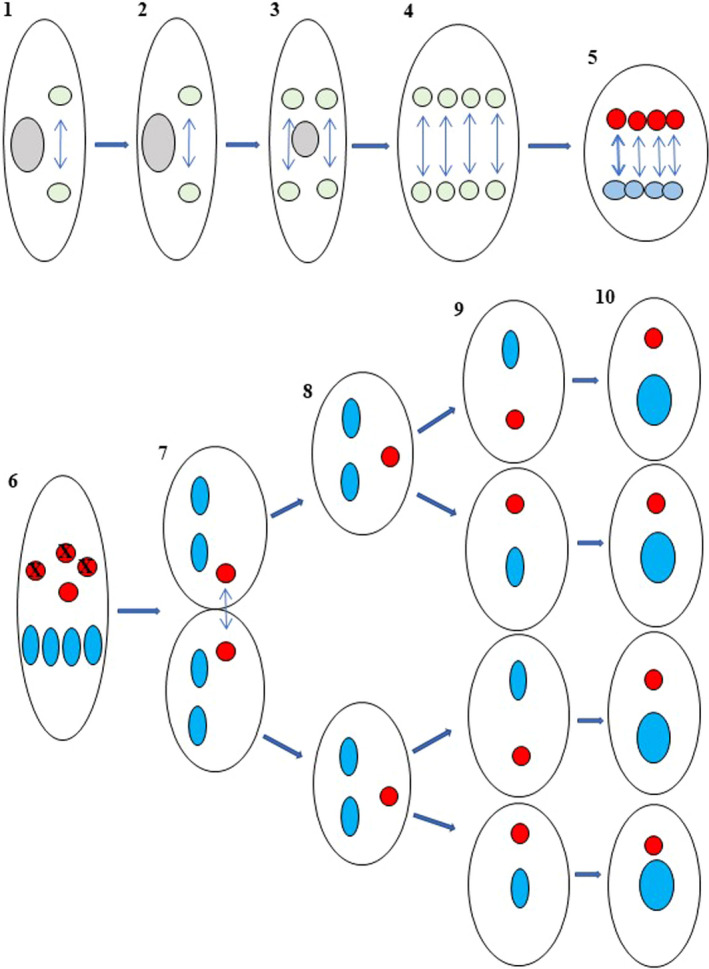
Nuclear differentiation and karyonide formation in Paramecium caudatum. Stages 1 to 4: A synkaryon (light green) undergoes three fissions to form eight nuclei. Stage 5: the most anterior nuclei (red) become micronuclei, and the posterior nuclei (light blue) become the macronuclear anlagen which are destined to become macronuclei. Stage 6: Three micronuclei degenerate and disappear, and the remaining one conveys the genome to the next generation. The macronuclear anlagen (blue) initiates DNA synthesis. Stage 7: Cell division occurs, the micronucleus undergoes mitosis and one product passes to each daughter cell. The macronuclear anlagen do not divide, and two pass to daughter cells (Stage 8). Stage 9: The two cells divide to produce cells each with one micronucleus and one macronuclear anlage. The macronuclear anlage continues DNA synthesis, and at stage 10, four karyonides are completed. This process forms eight karyonides from one mating pair. A circle with an X indicates a degenerating nucleus. Double‐headed arrows indicate nuclear replication
Immaturin: a protein inducing sexual rejuvenation
Microinjection experiments were performed to elucidate the cytoplasmic factors that drive the transition of sexual status in the life history of P. caudatum (Miwa et al., 1975). In one experiment, about 20 pl of the cytoplasm of mature cells were injected into immature cells, but immature cells did not become mature (data not shown). Mature cells were then injected with immature cytoplasm, and the cells lost their mating ability that is, they became immature. The immature state continued for about 10 cell divisions and then returned to maturity (Miwa et al., 1975).
This rejuvenating effect was also verified by microinjection between different closely related species of the genus Paramecium (Miwa, 1979a, 1979b). In addition, the cytoplasmic injection of early mature mutant strains suggested that there are genes that affect the duration of the immaturity period (Hiwatashi & Myohara, 1976; Miwa, 1984). A cytoplasmic factor, immaturin, that produced the rejuvenating effect was isolated and purified from immature cells (Haga & Hiwatashi, 1981). It is a soluble protein with a molecular weight of about 10‐kD as estimated by gel chromatography and sodium dodecyl sulfate–polyacrylamide gel electrophoresis. This purification process involved microinjection bioassays of more than 40 fractions prepared by column chromatography. The discovery of immaturin was a milestone in the study of the phase transition of sexual ability in P. caudatum.
Haga and Karino (1986) explored whether the rejuvenating effect of immaturin depends on the clonal age of the mature cells to be injected. The effect of purified immaturin samples was compared using young mature cells, mature cells that have divided 100 more times than young mature cells, and senile cells with a history of about 700 divisions. No statistically significant difference was observed in the sexual rejuvenation effect between the two groups of mature cells; but, in senile cells, immaturin restored mating ability to the level of mature cells (Haga & Karino, 1986). No rejuvenating effect was observed in other aging traits such as the division rate, the survival rate of the progeny after conjugation, or the incidence of morphologically abnormal cells when compared with control groups. It was concluded that the sexual ability of aged Paramecium cells is restored by cytoplasmic factors from young cells (Haga & Karino, 1986).
Cell structures that record the number of cell divisions in clonal aging
The macronucleus is a source of genetic information necessary for daily life such as cell division and expression of sexual function. The micronucleus has a diploid genomic composition and undergoes meiosis at the time of conjugation to produce the haploid germ nuclei to be transmitted to the progeny. It was demonstrated that the expression of mating ability is associated with the number of cell divisions after conjugation (Miwa, 1973). But it was not yet proven if the state of the macronucleus is associated with the expression of mating ability. A dominant/recessive relationship between an immature macronucleus and a mature macronucleus in the expression of sexual ability was examined by macronuclear transplantation. Using the fusion phenomenon of the host nucleus and the transplanted nucleus that occurs after macronucleus transplantation, it was investigated whether cells with a fusion nucleus of an immature macronucleus and a mature macronucleus become immature or mature (Haga, 1993). Three genetic markers that can be identified in living cells were used to identify immaturity and maturity in the fusion macronucleus. They were: recessive behavioral mutants cnrA (caudatum nonreversal) or cnrB, trichocyst nondischarge (TND) mutants (recessive), tnd1 or tnd2, and mating E‐type (dominant) or O‐type (recessive). The three sets of genes were predistributed to the two macronuclei so that the recombinants could be identified after the macronuclear fusion. For example, for behavioral mutant genes, behavior becomes wild type only if the fusion macronucleus preserves the genes of both macronuclei. This is because the fusion nucleus contains the wild‐type genes for each of the two recessive mutant genes. Similarly, the inability of trichocyst discharge converts to wild type only if both macronuclear genes are conserved. In the case of the mating type, if the macronucleus of E‐type remains, the mating type becomes E‐type, and if it does not remain, it becomes O‐type.
The two macronuclei contribute to the same extent in the formation of fused nuclei (Haga, 1993). The three marker genes are randomly combined and distributed to daughter cells during several cell divisions after the formation of the fusion nucleus. Furthermore, the new gene combination is maintained after the fusion nucleus is stabilized. These results indicate that the dominant/recessive relationship is maintained in the fusion macronucleus. It was evident that the dominant/recessive relationship between the immature macronucleus and the mature macronucleus can be tested by macronuclear fusion experiments.
The dominant/recessive relationship between the immature and mature macronuclei was investigated using immature cells that divided about 20 times after conjugation and mature cells that had divided about 100 times. Both immature and mature cells were used as recipients to assess the effects of cytoplasm. After transplanting the macronucleus, inducing fusion, and dividing about 15 times, the cell was immature regardless of the maturity of the recipients. The immature state lasted for about 30 divisions after fusion occurred and then transformed into the mature state. In the general relationship between dominant and recessive, proteins such as enzymes are often synthesized from genes with dominant traits. From the results, we hypothesized that a protein in the macronucleus of the immature stage is synthesized and suppresses mating ability.
Search for cell structures that record the number of cell divisions in clonal aging
When immature micronuclei which had divided only a dozen times after conjugation, were transplanted into mating cells, they undergo meiosis and differentiate into germ nuclei (Harumoto & Hiwatashi, 1982). In this regard, the potential of the micronucleus as a germ nucleus is equivalent in the immature stage and the mature stage, as both enter meiosis during the conjugation process.
To summarize macronuclear transfer and fusion experiments (Haga, 1993), the macronucleus keeps a record of each selfreplication. The process affects the expression of the immaturin gene. The number of replications increases, the expression of the immaturin gene decreases. When the action of immaturin is reduced, the expression of the mating‐substance gene(s) begins and leads to the sexual maturation of the cells. The ability of the micronucleus to act as a germ nucleus is unchanged regardless of the number of selfreplications. The conclusion is as follows. The macronucleus determines the ability to express sexual activity by counting postconjugation divisions and thereby keeping track of the age of the clone.
Mating type change: intraclonal mating pair formation
Changes to mating type in the life cycle of P. caudatum are related to clonal aging. Approximately 80–120 fissions after conjugation, some E mating‐type cells change to O‐type, allowing intraclonal mating reactions and selfing pair formation. Microinjection of genomic DNA isolated from wild‐type strains induced a mating‐type change in the nonselfer natural mutant. This indicated that the genetic defect in the hypothetical dominant modifier gene, Su (Mt) (Hiwatashi & Myohara, 1976), was restored by wild‐type genomic DNA (Haga & Sato, 1996).
Autogamy and clonal aging
In P. tetraurelia, after completion of conjugation, cells that have undergone a certain number of cell divisions can undergo autogamy, that is the fusion of two reproductive nuclei made in one individual cell. This can start the life history of the next generation. Autogamy is usually induced by starvation (Sonneborn, 1950). An irreversible progression to autogamy requires a 1‐h starvation process (Sonneborn, 1950). Macronucleus transplantation experiments have revealed that autogamy is under the control of the macronucleus (Mikami & Koizumi, 1983b). Furthermore, the number of cell divisions required before the capacity for autogamy develops can be shortened by repeatedly extracting about 3/4 of the macronucleus with a micropipette (Mikami & Koizumi, 1983b). This result supports the hypothesis that the clonal age is determined by the rounds of DNA synthesis rather than by the number of cell divisions and that clonal cells can die.
The macronucleoplasm of autogamy‐competent (mature) cells was transplanted into the macronuclei of cells that were too immature to be able to enter autogamy (Koizumi & Kobayashi, 1989). The immature cells that received transplants underwent autogamy after 3–5 cell divisions. On the other hand, the reverse combination of transplantation experiments had no effect on the initiation of autogamy. This suggests that the macronucleus of mature cells that perform autogamy encodes factors that induce autogamy.
Microinjection of cytoplasm between young and old paramecia
Cytoplasmic microinjection experiments were performed to investigate cytoplasmic factors involved in P. tetraurelia clonal aging (Aufderheide, 1984). When about 5000 µm3 of the cytoplasm of young cells (the volume of which is about 100 pl) was injected into old cells, there was no statistically significant difference in the average number of divisions before cell death occurred. Neither the cytoplasmic injection of old cells into young cells nor the cytoplasmic microinjection of the same cells performed for 3 consecutive days, showed a significant difference.
COMPLEMENTARITY TEST: TESTING RECESSIVE MUTANTS BY MICROINJECTION OF CYTOPLASM
Cytoplasmic and genetic complementation in pawns and CNRs
Paramecium has an electrically excitable cell membrane that can be evaluated by the swimming behavior of the cell because changes in potential across the cell membrane and the ciliary movement are coupled. Studies using P. tetraurelia to elucidate the mechanism of cell membrane excitement use mutants that show abnormal swimming behavior. The experimental protocol that reveals a causal relationship by interfering with physiological actions using mutations is called genetic dissection. In the 1970s, Kung artificially induced temperature‐sensitive behavioral mutants in P. tetraurelia (Chang & Kung, 1973a, 1973b) to classify the functions of various ion channels.
In this area, we discuss experimental systems in which microinjection played an important role in connecting genetics, electrophysiology, and cell behavior. The functional background is as follows. Action potentials in the cell membrane of Paramecium are generated by the instantaneous influx of calcium ions through voltage‐gated Ca2+‐channels (Eckert et al., 1976). The excitability of cell membranes is determined by the action of calcium ion channels causing the reversal of ciliary beat (Dunlap, 1977). Many behavioral mutants were classified by crossbreeding experiments and divided into complementary groups. Among them, the most important were mutants called pawns. Pawn cells are recessive mutants in which the calcium channels do not function. Pawns cannot swim backward in a test solution (40 mM KCl in Dryl's solution) because the reversal of ciliary movements does not occur. The cilia of pawn cells had a reversal reaction when the concentration of calcium ions was increased (Chang & Kung, 1973a), confirming that the motor functions of the cilia were normal. Electrophysiological analyses revealed that the function of the membrane voltage‐gated calcium channel had been lost (Oertel et al., 1977). In this way, genetic dissection helps clarify the sequence and causal relationships of each event by suppressing the action of a specific gene product in complex systems. Taking the example of pawn, the steps in the system include (1) reception of stimulus, (2) generation of membrane receptor potential over the nonciliary (somatic) cell membrane, (3) depolarization of the ciliary membrane potential, (4) activation of the calcium channel, (5) influx of calcium ions from the external fluid into the cell, (6) generation of action potentials, (7) transient increase in intracellular calcium ion concentration, (8) induction of ciliary reversal movement, and (9) backward swimming of cells (Kung & Saimi, 1982). The mutated genes affect the functional expression of the calcium channel in the ciliary membrane.
Complementarity tests by crossbreeding revealed that pawns belong to one of three complementarity groups (Kung, 1971). A complementarity group indicates whether the mutation occurs at the same or at a different locus. In the latter half of the 1970s, Takahashi introduced many recessive CNR (caudatum nonreversal) mutants in P. caudatum, which, like pawn, cannot swim backward (Takahashi, 1979). Four types, cnrA, cnrB, cnrC, and cnrD are now recognized (Takahashi et al., 1985). Hiwatashi discovered that when a Paramecium belonging to cnrC forms a mating pair with the wild type, the ciliary reversal reaction recovers after a few hours and swims in the same way as the wild type (Hiwatashi et al., 1980). It was speculated that some of the wild‐type cytoplasm migrated to cnrC cells during conjugation and compensated for the genetic deficiency of this mutant. This led to experiments in which wild‐type cytoplasm was introduced into cnrC by microinjection (Hiwatashi et al., 1980). The backward swimming ability of the injected cnrC recovered to wild‐type levels after about 8 h. The cytoplasmic factor that restores the genetic deficiency of cnrC is called the cnrC‐curing factor. The gene for the cnrC‐curing factor has been found to belong to the Pccentrin1p gene family, a member of the Ca2+‐binding EF‐hand protein superfamily (Gonda et al., 2004).
In crossbreeding complementarity tests, two mutant genes are placed in the progeny to see if the progeny's function is restored to wild type. If the progeny traits were restored to wild type, the conclusion is that the two mutations originated from different loci. The outcome of complementarity tests may be caused not by a gene but by the gene product. We can test if the gene product is sufficient for the complementarity test using cytoplasmic microinjection.
In one series of experiments (Haga et al., 1982), cytoplasmic microinjection was performed by injecting cytoplasm of wild‐type cells into behavioral mutants in P. tetraurelia. The swimming behavior recovered to the wild type 7–8 h after injection in all complementary groups of pawns. Recovery of the function of membrane voltage‐gated calcium channel was confirmed by electrophysiology (Haga et al., 1982). All three pawn traits, A to C, were cured by injection of wild‐type cytoplasm. Combinations of cells belonging to the same genetic complementarity group were used as control experiments, and all possible combinations were tested. In all cases of complementation, pawns converted to wild type (Haga et al., 1982). On the other hand, all remained pawns in the control experimental group. Furthermore, when the complementarity test by microinjection was performed using the pawns that had not been genetically analyzed and compared with the results of the genetic analysis, all the complementarity groups agreed in terms of both behavior and electrophysiology. This confirms that complementarity tests can be performed by cytoplasmic microinjection. In the genetic experiments, complementarity tests took 3–7 days, whereas in the microinjection studies the result was found in 8 h (Haga et al., 1982).
Complementarity tests were conducted between the artificially induced behavioral CNR and pawn mutants (Haga et al., 1983). Since P. caudatum and P. tetraurelia are of different species, they cannot be crossed, but complementarity can be examined by cytoplasmic microinjection. Cytoplasmic microinjection was performed between the four complementarity groups of CNRs and three complementarity groups of pawns. Swimming behavior and voltage‐gated calcium channel functions were restored to wild type in all combinations. In detail, we found that all these mutations originated at different loci and that in P. caudatum and P. tetraurelia the functional expression of the voltage‐gated calcium channel is supported by at least 7 genes.
Stoichiometric analysis of cnrC curing factors
Microinjection analyses were performed to explore if the action of cnrC curing factor stoichiometrically regulates the activity of voltage‐gated calcium channels (Haga et al., 1984a). If the curing factor binds to the calcium channel at a constant rate, the activity of the calcium channel should increase in proportion to the injected amount of the curing factors. The concentration of the curing factor was changed stepwise and injected, and after a certain period, the activity of the calcium channel was quantitatively measured by the influx of calcium ions. The activity of the calcium channel was shown to be proportional to the injected amount of the curing factor. This confirms that the cnrC curing factor stoichiometrically controls the activity of the calcium channel (Haga et al., 1984a).
The site of action of the curing factors was explored by a deciliation method (Haga et al., 1984a). First, cnrC cells were injected with a curing factor. In the swimming behavior test after 1 h, the function of the calcium channel had hardly recovered. When the cytoplasm of this cell was injected into another cnrC cell, the injected cell recovered to wild type after about 8 h. This indicated that curing factors are present in the cytoplasm of the first recipient. The calcium channels of Paramecium are localized to the ciliary membrane on the cell surface. Cilia can be removed from cells in which the calcium channel function has not yet recovered after injection. Next, about 8 h after the injection of the cnrC curing factor, the cytoplasm of the cell in which the function of the calcium channel was completely restored was injected into another cnrC cell. In this experiment, cnrC cells did not recover to wild type. It suggests that the curing factors do not persist in free form in the cytoplasm of cells with fully restored calcium channel function. To confirm this, the cnrC cells were again injected with a curing factor. After about 8 h, when the function of the calcium channel was completely restored, the deciliation treatment was performed. Examination of the function of the calcium channel in the regenerated cilia after 4–5 h revealed that it had returned to mutant levels. Calcium channel function maintained wild‐type levels in cells of the control group without deciliation. It is suggested that the injected curing factor was removed from the cells by deciliation.
Biochemical characterization of the curing factors of Pawns
In pawns, the curing factors are proteins contained in intracellular membranes (Haga et al., 1984b). They exhibit different properties in thermal stability, optimal pH, and divalent ion sensitivity. The specific activity of the pwC curing factor was purified 180 times or more by the standard intracellular membrane fractionation method. The pwB curing factor was found to be amplified in vivo by a sequential transfer of cytoplasm between pwB cells.
The method of microinjection and sorting a genomic library revealed the genetic information of the DNA fragment that complements pwB mutation. The translated product of the DNA fragment has two putative transmembrane domains. Mutation sites in some strains have single base substitutions, whereas the strain de‐662 (previously pwD) has a 44‐bp insertion that matches an internal elimination sequence in the wild‐type germline DNA except for a single C‐to‐T transition. The location of the pwB protein visualized by GFP‐fusion found no obvious signal in the cilia (Haynes et al., 2000). After deciliation, a bright signal was visualized along the membrane of the nucleus and near the cortex below the plasma membrane. No direct binding to cilia is compatible with genetic analyses (below).
Voltage‐gated calcium channels in Paramecium tetraurelia
The P. tetraurelia ciliary membrane proteome includes three subtypes of voltage‐gated calcium channels (Lodh et al., 2016). RNA interference experiments show that these calcium channels are responsible for the ciliary reversal beating. The ciliary membrane proteome of the Pawn (pw) mutants does not contain any of the three calcium channels. The introduction of the wild‐type PW gene into the macronucleus of mutants rescued the mutant phenotypes. These results support the correlation of the calcium channels with the backward swimming through ciliary reversal. The pwB protein directly interacts with the calcium channels suggesting a function in trafficking the channel protein. The pwA mutant lacks the ability to transport calcium channels to cilia, nor does it appear to interact directly with channel proteins in biochemical analyses (Lodh et al., 2016), in agreement with our own results (see above).
Other ion channel mutations
The P. tetraurelia mutant, pantophobiacA, has a defect that results in an in vivo loss of calcium‐dependent potassium efflux channel activity (Schaefer et al., 1987). There is a one amino acid sequence difference between the two Paramecium calmodulins under consideration: phenylalanine in the mutant protein at residue 101 instead of a serine (Schaefer et al., 1987). This change is at a calcium‐ligand residue in the third of the four calcium‐binding loops. Comparatively subtle changes in the structure of calmodulin can result in quantitative alterations of in vivo activity. This finding provides insights into the in vivo roles of calmodulin and the regulation of ion channels. Functional alterations of calmodulin are not necessarily lethal (Schaefer et al., 1987).
Genetic and microinjection data show that the calmodulin mutant cam 101 in P. tetraurelia is an intragenic suppressor of cam 1. The cam101 calmodulin may provide the basis for further genetic analysis of structure–function relationships in the calmodulin molecule (Hinrichsen et al., 1991).
Exchange of genetic information between conjugating cells
In the P. tetraurelia B system of mating‐type determination, cytoplasmic factors determine the mating type of cells (Sonneborn, 1975). The mating type of cytoplasm is determined and maintained by the action of the macronucleus. This cytoplasmic state determines the mating type of macronucleus formed after autogamy. However, the mating type of the micronucleus is determined before the formation of the macronucleus, and the new macronucleus is thought to maintain the mating type of the micronucleus during the conjugation process. Microinjection was used to transplant a micronucleus of known mating type into experimentally denucleated cells, and autogamy was induced in the recipient. A macronucleus was produced from the transplanted micronucleus. The mating type of the recipient cell represents its original mating type rather than the mating type of the transplanted micronucleus. This indicates that the mating type of micronuclei in the asexual cycle has not been determined. O‐type macronuclei were transplanted into E‐type recipients from which the macronucleus had been removed. The mating type of the recipient cell quickly changed from E to O, even though autogamy had not yet occurred. The mating type of the cells in which the autogamy occurred and where the new macronucleus was formed remained O. The transplanted macronucleus determines the cytoplasm to be an O‐type state, and a newly formed macronucleus after autogamy is O‐type. It is unlikely that the micronucleus was determined to be O or E during the asexual cycle (Mikami & Koizumi, 1982b).
The macronuclear content of autogamy mature cells was transplanted into the autogamy immature macronuclei (Kosciuszko & Koizumi, 1983). The transplanted cells underwent autogamy after 3–5 cell divisions. On the other hand, the reverse combination of transplantation experiments had no effect on the initiation of autogamy. It was suggested that the macronuclei of mature cells contain factors that induce autogamy (Kosciuszko & Koizumi, 1983).
NON‐MENDELIAN CYTOPLASMIC INHERITANCE
Surface antigen proteins
Expression of surface immobilization antigens (I‐antigens, meaning that when an antibody against this substance is administered to living Paramecium cells, the ciliary movement of paramecia is stopped) occurs if appropriate genes are present. A plasmid (16‐kilobase) incorporating the I‐antigen A gene of wild‐type P. tetraurelia was injected into the macronucleus of a mutant lacking this gene (Godiska et al., 1987). I‐antigen A was expressed in about 40% of the injected cells. In many clones derived from the recipients, this trait remained stable until autogamy occurred. 45,000–135,000 copies of the injected plasmid were retained in the macronucleus of the transformants. It was not integrated into the macronucleus genome. The linear plasmids were of various lengths, thought to have been caused by breakage at many different points. The results indicate that the injected plasmid maintains the genetic information required for gene expression and the information replicates autonomously (Godiska et al., 1987).
There are mutants in which the A antigen gene in the micronucleus is lost in the process of making the macronucleus. Microinjection of the macronucleoplasm or cytoplasm of wild‐type cells into the cytoplasm of this mutant converts it to wild type. The transformant maintained the transformed trait during vegetative growth, and the transformed trait was inherited by the progeny after autogamy (Koizumi & Kobayashi, 1989). These experiments show the cytoplasmic inheritance of the I‐antigen.
MACRONUCLEUS AS A MOLECULAR FACTORY
Efficient antisense RNAi
3′‐Hydroxyhexylphosphate was applied to introduce antisense oligodeoxyribnucleotides (ODNs) which were complementary to synthesized calmodulin mRNA of P. tetraurelia. The microinjection of the calmodulin antisense ODNs efficiently reduced the Ca2+/calmodulin‐dependent Na+ current. The 3′‐modified‐antisense ODNs are useful as tools for reducing the expression of specific gene products and provide a powerful model system with which to study and develop antisense ODN technology (Hinrichsen et al., 1992).
Posttranslational regulation
In P. tetraurelia, a gene family encodes K+‐channel pore‐forming subunits. Microinjection of the DNA isolated from a mutant belonging to this gene family induces clonal transformants to exhibit hyperexcitable swimming behavior. The two different Ca2+‐activated K+‐specific currents are reduced in the transformants. Because mRNA is clearly produced from the injected transgenes, the reduction of K+‐currents by the expression of the K+‐channel transgenes is likely to be the consequence of a posttranslational event (Ling et al., 2001).
Forward genetics approach
The membrane of P. tetraurelia possesses a large Mg2+‐selective current and exhibits a corresponding backward swimming behavior. Eccentric mutants are missing both characters. The DNA fragment that complements the eccentric mutations was isolated from a wild‐type genomic library. The Mg2+‐currents and behavior are restored fully in transformants (Haynes et al., 2002). The conceptually translated protein has some similarities to K+‐dependent Na+/Ca2+ exchangers (Haynes et al., 2002). This study revealed that mutations in the exchanger gene produce channels with altered ion specificity. This article proposes a "forward genetics approach" by understanding mutant phenotypes in vivo without prejudice (Haynes et al., 2002). Once the mutant gene is identified and a known gene with high homology to that gene is searched from the sequence information, a method for knowing the functional characteristics of the mutant trait will be opened. This provides a path to the origin of genes that function as wild type in the organism.
Telomere production reaction
The telomeres of P. tetraurelia are made up of variable repeats, whereas in P. caudatum telomeric repeats are largely invariant. To investigate variable repeat synthesis in P. tetraurelia, mutated telomerase RNA genes were expressed in vivo (McCormick‐Graham et al., 1997). P. caudatum telomerase RNA can participate in telomere synthesis when expressed in P. tetraurelia macronucleus despite a 24% difference in RNA sequences. P. tetraurelia telomerase fidelity is dramatically affected by template substitutions and misincorporation at a single templating position is likely to account for the majority of P. tetraurelia telomeric DNA variability. Fidelity is not solely a function of the RNA moiety, as the P. caudatum telomerase RNA does not impart high fidelity to the chimeric enzyme. Differences in telomere sequence diversity between P. tetraurelia and P. caudatum may be due to enzymatic differences in telomeres (McCormick‐Graham et al., 1997).
The mechanism of choice of telomere addition sites was investigated by microinjection of a cloned telomeric gene into Paramecium species. Telomere addition regions in the process of macronuclear differentiation are not strictly determined by the micronuclear sequences but are partially controlled by the old macronucleus. Microinjection of a high copy number of a plasmid containing the G surface antigen gene into the macronucleus of wild‐type cells specifically modifies the processing of the G gene‐bearing micronuclear chromosome at the following autogamy. The telomeric repeats are added upstream of the gene, rather than at the wild‐type position, resulting in the deletion of the gene from the new macronucleus. This macronuclear mutation is unstable following autogamy. However, after several successive autogamy cycles, cell lines can be obtained in which the telomeres reproducibly form in the same region. These macronuclear mutations show cytoplasmic inheritance (Meyer, 1992).
DNA fragments harboring the his3 gene of Saccharomyces cerevisiae were injected into the P. primaurelia macronucleus. The injected molecules were mostly inserted at their ends at multiple points in the genome. However, insertion sites were not totally at random. Roughly 30% of the molecules were integrated near telomeric repeats. The telomeric repeats occupy an internal or interstitial position (Katinka & Bourgain, 1992).
The fate of DNA molecules injected in the Paramecium macronucleus was analyzed using P. tetraurelia DNA containing the A‐antigen gene. The linear DNA with telomere sequences at both ends was protected from degradation when injected into the macronucleus. Furthermore, preexisting telomeric repeats prevent end‐to‐end fusions (Bourgain & Katinka, 1991).
Macronucleoplasm transplantation
Macronucleoplasm refers to the contents of the macronucleus. It is a viscous material containing chromosomes and nucleoli. Transplantation experiments were performed between cells with three types of genetic markers, and the transformation pattern was clarified in P. caudatum (Harumoto & Hiwatashi, 1992). There were two types of transformation, a stable transmission to daughter cells and an unstable one. It was suggested that the transformation may be influenced by the stage of the cell cycle of the donor and recipient during the transplantation of macronucleoplasm. Induction of stable transformation was observed in the transformation between closely related species of the same genus. Cross‐species macronucleoplasm transplantation experiments can evaluate the maintenance of gene products and the commonality of gene expression (Harumoto & Hiwatashi, 1992).
Plasmid or DNA fragments injection into the macronucleus
A plasmid incorporating the serotype A surface antigen gene was injected into the macronucleus of the mutant lacking the A gene to create a transformant expressing the A antigen. The injected plasmid was maintained during replication cycles, changed to linear form, with Paramecium‐type telomere sequences added to both ends without the need for a specific recognition sequence (Gilley et al., 1988).
Kanabrocki and colleagues reported a method for increasing the efficiency of transformation by DNA microinjection in P. tetraurelia (Kanabrocki et al., 1991). Mutants with a one‐amino acid substitution in the calmodulin molecule have a Ca2+/CaM‐dependent potassium current. A DNA fragment containing the wild‐type CAM gene was injected into the macronucleus of this behavioral mutant called cam2. Cloned cells generated after injection recovered to wild type. In vitro addition of Tetrahymena telomeres to both ends of this DNA, fragment increased the frequency and quality of transformation induction. Injection of 5 × 104 copies of DNA fragments induced stable transformations. Many clones lost the traits after autogamy.
Microinjection of a high copy number of plasmids containing only the coding region of a gene into the Paramecium macronucleus led to a marked reduction in the expression of the corresponding endogenous genes (Ruiz et al., 1998) The silencing effect is stably maintained throughout vegetative growth. The effects were observed for a single‐copy gene (ND7; ND for nontrichocyst discharge) and members of multigene families such as centrin genes and trichocysts matrix protein genes.
Wild‐type genomic DNA of P. caudatum was injected into the macronucleus of the recessive mutant (Endoh et al., 1995). The injected DNA contains three genetic markers affecting exocytosis, behavior, and mating type. The recipient has triple recessive homozygosity for the corresponding genes. The DNA microinjection transformed trichocyst‐exocytosis into a wild type, but other traits did not change. The transformation occurred more efficiently when the recipient was in the S phase than when it was in the G1 phase.
Expression of a vector harboring different genes in codon‐usage
A vector incorporating a transformation marker, the bacterial antibiotic resistance gene aminoglycoside 3′‐phosphotransferase‐II (APH‐3′‐II) expressed in Paramecium was prepared for microinjection (Haynes et al., 1995). Injection of approximately 10–20 pl of the linear plasmid into the macronucleus induces transformation at 100%. It was found that the antibiotic resistance gene of this vector has a base sequence derived from prokaryotes but is expressed in Paramecium with different codon usage.
Microinjection of a linearized vector containing a GFP (green fluorescent protein) open reading frame into the macronucleus of P. caudatum induced the expression of GFP mRNA, but protein expression was not detected. After the extensive optimization of GFP codons, the protein expression was increased, resulting in the GFP‐derived fluorescence in the recipients (Takenaka et al., 2002).
Paramecium promoter analysis
The regions of DNA necessary for immobilization antigen A expression in P. tetraurelia have been defined and the approximate position of the A‐antigen promoter has been located (Martin et al., 1994). This has enabled a precise mutational analysis of these regions and the first detailed characterization of a Paramecium promoter (Martin et al., 1994).
Two types of nickel‐inducible genes, in P. caudatum, were isolated by subtractive hybridization. This technology allows for polymerase chain reaction (PCR)‐based amplification of only the cDNA fragments that differ between control and experimental transcriptome. The presence of different mechanisms was suggested in the induction of these two genes. The nickel‐responsive element is involved in the putative PcGST1 (P. caudatum glutathione S‐transferase gene) promoter. The nickel‐inducible system may be applicable to the efficient expression of proteins that require temporal control (Takenaka et al., 2014).
MICRONUCLEUS‐MACRONUCLEUS DIFFERENTIATION
Micronucleus as a germ nucleus
The micronuclei in the premeiotic S phase were transplanted into vegetative cells of P. caudatum (Fujishima & Hiwatashi, 1978). The transplanted micronuclei stopped progressing to meiosis, and when the recipient underwent cell division, they also performed mitosis. The commitment to the transition of the micronucleus to meiosis occurs after the completion of premeiotic DNA synthesis (Fujishima & Hiwatashi, 1978).
Micronuclei were transplanted into the prophase cells of P. caudatum (Fujishima & Hiwatashi, 1981). The G1‐phase micronucleus did not transition to meiosis. Micronuclei that were not committed to meiosis during premeiotic DNA synthesis transitioned to meiosis. It was suggested that the cytoplasm in the prophase has a factor that shifts the micronucleus in the premeiotic DNA synthesis phase to meiosis.
Synkaryon (zygotic nucleus)
The question of whether the synkaryon can function as a reproductive nucleus was investigated in a nuclear transfer experiment in P. caudatum (Harumoto & Hiwatashi, 1982). A synkaryon was transplanted into an amicronucleate cell in the asexual cycle. The transplanted synkaryon underwent both mitosis and meiosis. The nuclear exchange process proceeded in the same way as with the normal germ nucleus. It was concluded that no special differentiation process is required for the synkaryon to function as a germ nucleus. The reproductive nucleus lineage continued for generations (Harumoto & Hiwatashi, 1982).
Changes to the micronucleus in mating pairs proceed synchronously. Macronucleus removal experiments were performed to identify the nuclei that control the synchronous changes in the micronuclei in P. caudatum (Mikami, 1992). When the macronucleus was removed from both cells of the mating pair, the micronuclear changes stopped after 1–1.5 h. It was found that most of the changes in the micronucleus proceed under the control of the macronucleus. Furthermore, it was suggested that the macronucleus gene product was carried to the mating partner. There are factors that promote or suppress the fission of the micronucleus. An unidentified gene product of the macronucleus is involved in the synchronization of micronucleus changes (Mikami, 1992).
Nuclear differentiation
The next generation of macronucleus and micronucleus are created during the conjugation process of P. caudatum. Four macronuclear anlagen and one micronucleus are produced (Figure 2). To investigate the problem of how this nuclear differentiation is carried out, a micronucleus was removed (Mikami, 1980). The nucleus located at the anterior end of the cell was found to differentiate into the micronucleus and the nucleus located at the posterior end of the cell differentiates into the macronucleus. This experiment led to the important discovery that location information in the cytoplasm determines what becomes macronuclei and micronuclei. The anteroposterior differentiation first occurs in the cytoplasm at a specific time during the conjugation process. Subsequently, eight postzygotic nuclei differentiate into micronuclei or macronuclei under the influence of the surrounding cytoplasm (Mikami, 1980).
Nuclei located at the posterior end of conjugating cells cannot become micronuclei if the four nuclei at the anterior end are removed (Mikami, 1982a). There is no distinction between the four micronucleus candidates at the anterior end. Even if three are randomly removed, the remaining one undergoes fission and becomes a micronucleus. If the presumptive micronucleus is transplanted into asexually reproducing cells, it functions as a micronucleus. There is usually only one micronucleus. When micronuclei are transplanted to create a multimicronucleus state, the extra nuclei are eventually eliminated and the state establishes one micronucleus.
The micronucleus usually forms a macronucleus through meiosis, mitosis, and fusion during sexual reproduction. The micronuclei of vegetative cells were transplanted into the early exconjugant cells. As a result, the micronucleus differentiated directly into the macronucleus (Mikami & Ng, 1983a). The developmental process of the micronucleus is flexible and changes the developmental process under the influence of cytoplasmic conjugation‐specific factors.
A new generation of macronucleus develops in exconjugant cells. The development of a macronucleus causes an increase in the amount of DNA. Heterokaryons were prepared for the purpose of investigating the timing of gene expression in the early macronucleus (Mikami, 1987). A micronucleus of P. caudatum was removed from cells with 5 types of recessive mutations. After the cells had divided several times, a micronucleus carrying wild‐type genes was transplanted into the cells. The heterokaryons and recessive mutants were used to induce conjugation and the timing of conversion of the five traits of the exconjugant from mutant to wild type was measured. Approximately 18 h after the determination of macronucleus differentiation, the earliest wild‐type trait appeared. Other traits showed conversion to wild type after about 24 and 40 h. Mikami also observed that some special gene (see below) was expressed when the amount of DNA in anlagen began to increase.
The A gene in the micronucleus of mutants that cannot express the serotype A protein on the cell surface is intact in the micronuclear genome of P. tetraurelia. A large deletion has occurred in this gene during the process of macronuclear development. The permanent rescue was achieved in a completely wild type by microinjection of the internal A gene fragment (4.5 kb) (Jessop‐Murray et al., 1991). This method does not require control at the transcription level, intact mRNA, or serotype protein. There is no evidence to suggest a small molecule stable RNA or RNA encoding a cytoplasmic factor involved in this effect (Jessop‐Murray et al., 1991).
The mutant strain d48 of P. tetraurelia maintains the gene for antigen A in the micronuclei, but not in the macronucleus. Transplantation of wild‐type macronucleoplasm into the mutant induced reversion to wild‐type after autogamy (Harumoto, 1986). Transplantation experiments including macro‐, micronuclei, or cytoplasm suggested that the micronucleus of d48 has a normal antigen A gene and hereditary determinants responsible for the d48 trait are in the macronucleus. The revertant of d48 possessed the antigen A gene in the macronucleus.
Generational epigenetics: Paramecium tetraurelia mating‐type inheritance
Paramecium conjugations occur between O‐type and E‐type cells. In P. tetraurelia, the nucleus of the progeny receives the same genome in both cells involved in the conjugation, but the mating type of the progeny is expressed in the same way as the mating type of each parent. The mechanism has long been the subject of research. Removal of transposable elements and their single‐copy remnants occurs during the development and differentiation of the somatic cell nucleus from the germ nucleus. It is key for the inheritance of the mating type. ScnRNA (small scan RNA) produced during meiosis of the germinal nucleus scans the parental macronucleus genome to identify missing base sequences. Then, it acts on the macronucleus derived from the zygotic nucleus, which will be the macronucleus of next‐generation, and corrects the missing defect. This process has been identified in the mating‐type determination and its inheritance of P. tetraurelia (Singh et al., 2014).
Internal elimination sequences in the micronucleus of Paramecium tetraurelia
The germline genome of ciliates is extensively rearranged during the development of a new somatic macronucleus from the germline micronucleus. Single‐copy internal eliminated sequences (IESs) are precisely excised from coding sequences and intergenic regions. ScanRNAs (scnRNAs) produced during micronuclear meiosis by a developmentally regulated RNAi pathway have been proposed to mediate this transnuclear cross‐talk (Lepère et al., 2008). Microinjection experiments provided direct evidence that 25‐nucleotide (nt) scnRNAs promote IES excision. Noncoding RNAs are produced from the somatic parental genome, both during vegetative growth and during sexual events. A scnRNA/macronuclear RNA scanning model is supported (Lepère et al., 2008).
Competition in DNA synthesis between new and old macronuclei
When Paramecium enters the conjugation process, the macronuclear fragments of the parent's generation and the macronuclear anlagen for the progeny's generation may coexist in the cell for some time. In cells from which the macronuclear anlagen were removed with a micropipette, DNA synthesis of the parental macronucleus fragment, as measured using tritium‐labeled thymidine, was no longer inhibited (Mikami, 1979b). There is a competition for DNA synthesis between the two macronuclei, and the macronuclear anlagen takes up more precursors favoring the next generation of macronucleus formation. Competition for the limited amount of DNA precursors plays an important role in the onset of the selective suppression of DNA synthesis.
Micronucleus in a stomatogenesis
The function of micronuclei was investigated using Paramecium when the micronuclei of which were removed by a micropipette. An abnormality occurred in the morphogenesis of the oral apparatus of the exconjugant, resulting in a shallower shape (Mikami, 1979a). It was suggested that the micronucleus plays a primary role in normal oral region formation.
The function of micronuclei in vegetative growth was investigated by micronucleus removal and reimplantation experiments in P. caudatum. In nonmicronucleated cells, the cell cycle time was prolonged, food vacuole formation was reduced, and the buccal cavity became shorter (Fujishima & Watanabe, 1981). Upon re‐transplantation of the micronucleus, these anomalous properties returned to normal type. Thus, micronuclei play an important role in vegetative growth. Similar roles were confirmed in Paramecium bursaria and Paramecium jenningsi (Fujishima & Watanabe, 1981).
The zygotic nucleus or postzygotic nuclei were removed from mating paramecia with a micropipette. During subsequent cell division, many abnormal morphologies occurred in the oral region (Ng & Fujishima, 1989).
EXOCYTOSIS
Chemical reactions involved in exocytosis in Paramecium
Microinjection of antibodies against calcineurin was performed to investigate the possible involvement of protein phosphorylation in the exocytosis of trichocysts (Momayezi et al., 1987). Anti‐CaN AB microinjection inhibited exocytosis. Alternatively, Ca2+–CaM–CaN complex and alkaline phosphatase injection‐induced exocytosis. Similar effects were observed in an in vitro system using isolated cortices. The membrane fusion that occurs during exocytosis requires an appropriate molecular assembly that parallels dephosphorylation of 65‐kDPP. This step is crucial for the induction of Paramecium exocytosis. It is suggested that Ca2+–CaM‐stimulated CaN‐like phosphoprotein phosphatase is probably involved in vivo.
Intramembrane particles at the trichocyst docking site
Cooperative action of two TND genes is required when trichocysts perform stable docking to the surface of P. caudatum. Two recessive mutants, tnd1 and tnd2, were examined for rescue both by microinjection and by conjugation (Watanabe & Haga, 1996). Either way, tnd2 was rescued but tnd1 was not. Freeze‐fracture electron microscopy of the plasma membrane showed characteristic intramembrane particles at the trichocyst docking site in the cortex. As the tnd2 mutant could be rescued by partial cell fusion during conjugation with tnd1 cells, it was concluded that the cooperative action of the two TND genes is necessary for stable docking of the trichocysts to the cortical sites.
Secretory organelle (trichocyst) docking
In the trichocyst docking process, trichocysts are carried along microtubules just below the cell surface. Microtubules, which are transiently formed, start at the ciliary basal body near the docking site (Glas‐Albrecht et al., 1991). The transport of trichocyst proceeds from the plus end to the minus end of microtubules, with the tip of the trichocyst facing the cell surface. In contrast, in gland cells (bovine adrenal medulla), organelles move in the opposite direction, from the minus end of the microtubule at the centrosome to the plus end. When an artificial trichocyst redocking system is used, trichocyst docking occurs synchronously. To investigate the transport specificity of trichocysts, chromaffin granules were isolated from the bovine adrenal medulla and injected into P. tetraurelia cells (Glas‐Albrecht et al., 1991). Chromaffin granules were not transported along the microtubules of the injected paramecia, perhaps because of the inverse polarity of the microtubules.
A model system for exocytosis in vitro in conjunction with microinjection
An isolated cortex that consists of the surface membrane with trichocysts still attached has been used to investigate the effects of chemical compounds on trichocyst exocytosis (Lumpert et al., 1990). ATP and its nonhydrolyzable analogs inhibited exocytosis, whereas GTP and its analogs slightly induced exocytosis. In microinjected intact cells (in vivo), ATP suppressed exocytosis, but GTP promoted it. In in vivo experiments, GTP hydrolysis occurred immediately after exocytosis. It will be important to determine the exact site of action of GTP during signal transduction in the exocytosis process (Lumpert et al., 1990). At that time, monomeric GTP‐binding proteins with GTPase function had been described in the literature as relevant for exocytosis performance.
CONJUGATING CELLS
Histone mRNA transport between mating pairs
To investigate the transport of macromolecules between mating Paramecium cells, an expression vector was constructed in which the histone H2B gene was fused with a yellow fluorescent protein (PcVenus) gene (Takenaka et al., 2007). Transformants produced by microinjecting this vector proliferated normally, expressed mating ability, and conjugated with the cells of the complementary mating type (Takenaka et al., 2007). Significant fluorescent signals derived from histone H2B‐PcVenus were detected in the macronucleus and micronucleus of transformants. During the conjugation process, PcVenus signals were observed in the macronucleus, micronucleus, and macronucleus anlagen of the transformant, as well as in the nontransformed mating cells. This suggests that during meiosis and subsequent nuclear differentiation and development, conjugating cells share histone H2B produced from the parental gene in all types of nuclei such as macronucleus, micronucleus, and macronuclear anlagen. Single‐cell reverse transcription‐PCR (reverse transcription‐polymerase chain reaction) reveals the presence of H2B‐PcVenus mRNA in the nontransformed cell, suggesting the transport of mRNA from transformants to nontransformed cells (Takenaka et al., 2007).
Microinjection of Ca2+/EGTA buffer into mating reactive cells
The earliest nuclear event in the process of conjugation of P. caudatum was investigated by microinjection of Ca2+/EGTA buffer (Xianyu & Haga, 1993). The early micronuclear migration (EMM) was induced by microinjection of about 40 pl of the 1.5 × 10−5 M free Ca2+ buffer. The inductions were observed in mating reactive cells but not in nonreactive cells, suggesting that the increase in intracellular free calcium concentration initiates EMM in the mating reactive cells. The sensitivity to calcium increase may be coupled with the expression of mating reactivity (Xianyu & Haga, 1993).
ENDOSYMBIOSIS
Gram‐negative endosymbiotic bacteria Holospora obtuse and Holospora undulata infect the macronucleus and micronucleus of P. caudatum, respectively. They have two different types of cellular forms, infectious and reproductive. The microinjection of the infectious form of these bacteria from the nucleus of host cells to the macronucleus of aposymbiotic P. caudatum showed that the infectious form has no ability to differentiate into the reproductive form in either case (Skovorodkin et al.., 2001). Symbiosis was established only with bacteria through the host digestive vacuole. Also, the bacteria that settled in the micronucleus did not settle in the macronucleus, and vice versa.
CONCLUSIONS
Micromanipulation techniques help to understand the roles of "parts" of a cell and their relationship with the "whole‐cell." These methods applied to Paramecium have provided significant insights in three broad areas. First, the process of sexual maturation and aging in Paramecium's life history is a programmed process based on the interaction of genes with macronuclear functions. Micromanipulation methods have allowed an exploration of aging in terms of genes, cytoplasm, or macronucleus as independent functional units. Secondly, cytoplasmic complementarity tests introduced in the study of cell membrane ion channels with recessive behavioral mutants revealed the presence of multiple unexpected ion channels. Cross‐species complementarity tests revealed that the genes that guarantee the function of the voltage‐gated Ca2+‐channels in more than one species. Thirdly, applying the micromanipulation method to non‐Mendelian inheritance has revealed that the problems specific to Paramecium are closely related to the general problems relating to the function of RNA molecules. It is shown that as progeny nuclei are produced from parental reproductive nuclei, small nontranscribed RNAs mediate interactions between the micronucleus and the macronucleus genomic DNA. The actions of the small nontranscribed RNA may be common to the general mechanism of RNA interference.
Beyond this, many other discoveries resulted when various substances were injected into the macronucleus. There is potential to develop the macronucleus as a new type of manufacturing factory for nanomachines combined with modern RNA/DNA technology to address issues such as telomere synthesis reactions, expression of vectors containing genes with different codon‐usage, efficient antisense RNA interference, internal elimination sequence system, forward genetic approach, posttranslational regulation, plasmid or DNA fragments engineering, histone mRNA transport, micronuclear migration in conjugation, and macro and micronuclear endosymbionts.
Microinjection studies suggest that sexual maturation relates to the number of DNA replications or the number of chromosome replications rather than the number of cell divisions. Microinjection has also provided insights into the molecular mechanisms of exocytosis.
ACKNOWLEDGMENTS
I thank Prof. David Patterson for his generous help in editing this manuscript. I also received a lot of appropriate advice from Prof. Helmut Plattner to complete this review. I am deeply grateful.
Haga, N. (2022) Micromanipulation in Paramecium: From non‐mendelian inheritance to the outlook for versatile micromachines. Journal of Eukaryotic Microbiology, 69, e12909. Available from: 10.1111/jeu.12909
REFERENCES
- Aufderheide, K. (1984) Clonal aging in Paramecium tetraurelia. Absence evidence for a cytoplasmic factor. Mechanisms of Ageing and Development, 28, 57–66. [DOI] [PubMed] [Google Scholar]
- Barber, M. (1904) A new method of isolating microorganisms. The Journal of the Kansas Medical Society, 4, 489–494. [Google Scholar]
- Beisson, J. , Bétermier, M. , Bré, M.‐H. , Cohen, J. , Duharcourt, S. , Duret, L. et al. (2010) DNA microinjection into the macronucleus of Paramecium . Cold Spring Harbor Protocols, 2010. Available from: 10.1101/pdb.Prot5364 [DOI] [PubMed] [Google Scholar]
- Bourgain, F. & Katinka, M.D. (1991) Telomeres inhibit end‐to‐end fusion and enhance the maintenance of linear DNA molecules injected into the Paramecium primaurelia macronucleus. Nucleic Acids Research, 19, 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.T. & Kung, C. (1973a) Genetic analysis of heat‐sensitive pawn mutants of Paramecium aurelia . Genetics, 75, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.Y. & Kung, C. (1973b) Temperature‐sensitive pawns: conditional behavioral mutants of Paramecium aurelia . Science, 180, 1197–1199. [DOI] [PubMed] [Google Scholar]
- Dunlap, K. (1977) Localization of calcium channels in Paramecium caudatum . Journal of Physiology, 271, 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, R. , Naitoh, Y. & Machemer, H. (1976) Calcium in the bioelectric and motor functions of Paramecium. In: Duncan, C.J. (Ed.) Calcium in biological systems. New York, NY: Cambridge University Press, pp. 233–255. [Google Scholar]
- Endoh, H. , Mikami, K. , Hori, M. & Takahashi, M. (1995) Injection of total genomic DNA and restoration of wild type phenotype in trichocyst non‐discharge mutant TND of Paramecium caudatum . Japanese Journal of Genetics, 70, 633–642. [Google Scholar]
- Fujishima, M. & Hiwatashi, K. (1978) Transplantation of germ nucleus in Paramecium caudatum. I. Nuclei in the pre‐meiotic S phase can enter mitotic cycle. Experimental Cell Research, 111, 468–471. [DOI] [PubMed] [Google Scholar]
- Fujishima, M. & Hiwatashi, K. (1981) Transplantation of germ nuclei in Paramecium caudatum. II. Induction of meiosis in transplanted interphase nucleus. Experimental Cell Research, 131, 63–71. [DOI] [PubMed] [Google Scholar]
- Fujishima, M. & Watanabe, T. (1981) Transplantation of germ nuclei in Paramecium caudatum. III. Role of germinal micronucleus in vegetative growth. Experimental Cell Research, 132, 47–56. [DOI] [PubMed] [Google Scholar]
- Gilley, D. , Preer, J.R. Jr , Aufderheide, K.J. & Polisky, B. (1988) Autonomous replication and addition of telomere‐like sequences to DNA microinjected into Paramecium tetraurelia macronuclei. Molecular and Cellular Biology, 8, 4765–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas‐Albrecht, R. , Kaesberg, B. , Knoll, G. , Allmann, K. , Pape, R. & Plattner, H. (1991) Synchronized secretory organelle docking in Paramecium. Saltatory movement along microtubules transiently formed from ciliary basal bodies and selective exclusion of microinjected heterologous organelles. Journal of Cell Science, 100, 45–54. [Google Scholar]
- Godiska, R. , Aufderheide, K.J. , Gilley, D. , Hendrie, P. , Fitzwater, T. , Preer, L.B. et al. (1987) Transformation of Paramecium by microinjection of a cloned serotype gene. Proceedings of the National Academy of Sciences of the United States of America, 84, 7590–7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda, K. , Yoshida, A. , Oami, K. & Takahashi, M. (2004) Centrin is essential for the activity of the ciliary reversal‐coupled voltage‐gated Ca2+ channels. Biochemical and Biophysical Research Communications, 323, 891–897. [DOI] [PubMed] [Google Scholar]
- Haga, N. (1993) Elucidation of nucleus‐cytoplasm interaction: change in ability of the nucleus to express sexuality according to clonal age in Paramecium . Journal of Cell Science, 108, 3671–3676. [DOI] [PubMed] [Google Scholar]
- Haga, N. , Forte, M. , Saimi, Y. & Kung, C. (1982) Microinjection of cytoplasm as a test of complementation. Journal of Cell Biology, 82, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga, N. , Forte, M. , Ramanathan, R. , Hennessey, T. , Takahashi, M. & Kung, C. (1984a) Characterization and purification of a soluble protein controlling Ca‐channel activity in Paramecium . Cell, 39, 71–78. [DOI] [PubMed] [Google Scholar]
- Haga, N. , Forte, M. , Saimi, Y. & Kung, C. (1984b) Characterization of cytoplasmic factors which complement Ca2+ channel mutations in Paramecium tetraurelia . Journal of Neurogenetics, 1, 259–274. [DOI] [PubMed] [Google Scholar]
- Haga, N. & Hiwatashi, K. (1981) A protein called immaturin controlling sexual immaturity in Paramecium . Nature, 289, 177–179. [DOI] [PubMed] [Google Scholar]
- Haga, N. & Karino, S. (1986) Microinjection of immaturin rejuvenates sexual activity of old Paramecium . Journal of Cell Science, 86, 263–271. [DOI] [PubMed] [Google Scholar]
- Haga, N. , Saimi, Y. , Takahashi, M. & Kung, C. (1983) Intra‐ and interspecific complementation of membrane‐inexcitable mutants of Paramecium . Journal of Cell Biology, 97, 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga, N. & Sato, J. (1996) Induction of mating‐type change in a non‐selfer mutant of Paramecium by microinjecting wild‐type genomic DNA. European Journal of Protistology, 19, 32–36. [Google Scholar]
- Harumoto, T. (1986) Induced change in a non‐mendelian determinant by transplantation of macronucleoplasm in Paramecium tetraurelia . Molecular and Cellular Biology, 6, 3498–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto, T. & Hiwatashi, K. (1982) Transplantation of synkaryon in Paramecium caudatum . Experimental Cell Research, 137, 476–481. [DOI] [PubMed] [Google Scholar]
- Harumoto, T. & Hiwatashi, K. (1992) Stable and unstable transformation by microinjection of macronucleoplasm in Paramecium . Developmental Genetics, 13, 118–125. [DOI] [PubMed] [Google Scholar]
- Haynes, W.J. , Kung, C. , Saimi, Y. & Preston, R. (2002) An exchanger‐like protein underlies the large Mg2+current in Paramecium . Proceedings of the National Academy of Sciences of the United States of America, 99, 15717–15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, W.J. , Ling, K.‐Y. , Saimi, Y. & Kung, C. (1995) Induction of antibiotic resistance in Paramecium tetraurelia by the bacterial gene APH‐3’‐II. Journal of Eukaryotic Microbiology, 42, 83–91. [DOI] [PubMed] [Google Scholar]
- Haynes, W.J. , Ling, K.Y. , Preston, R.R. , Saimi, Y. & Kung, C. (2000) The cloning and molecular analysis of pawn‐B in Paramecium tetraurelia . Genetics, 155, 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen, R.D. , Fraga, D. & Reed, M.W. (1992) 3’‐Modified antisense oligodeoxyrionucleotides complementary to calmodulin mRNA alter behavioral response in Paramecium . Proceedings of the National Academy of Sciences of the United States of America, 89, 8601–8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen, R.D. , Pollock, M. , Hennessey, T. & Russell, C. (1991) An intragenic suppressor of a calmodulin mutation in Paramecium: genetic and biochemical characterization. Genetics, 129, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatashi, K. (1968) Determination and inheritance of mating type in Paramecium caudatum . Genetics, 58, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatashi, K. , Haga, N. & Takahashi, M. (1980) Restoration of membrane excitability in a behavioral mutant of Paramecium caudatum during conjugation and by microinjection of wild‐type cytoplasm. Journal of Cell Biology, 84, 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatashi, K. & Myohara, K. (1976) A modifier gene involved in the expression of the dominant mating type allele in Paramecium caudatum . Genetical Research, 27, 135–141. [DOI] [PubMed] [Google Scholar]
- Jessop‐Murray, H. , Martin, L.D. , Gilley, D. , Preer, J.R. & Polisky, B. (1991) Permanent rescue of a non‐mendelian mutation of Paramecium by microinjection of specific DNA sequences. Genetics, 129, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabrocki, J.A. , Saimi, Y. , Preston, R.R. , Haynes, W.J. & Kung, C. (1991) Efficient transformation of cam2, a behavioral mutant of Paramecium tetraurelia, with the calmodulin gene. Proceedings of the National Academy of Sciences of the United States of America, 88, 10845–10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka, M.D. & Bourgain, F.M. (1992) Interstitial telomerase are hotspots for illegitimate recombination with DNA molecules injected into the macronucleus of Paramecium primaurelia . EMBO Journal, 11, 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, J.K. (1974) An improved microinjection technique in Paramecium aurelia: transfer of mitochondria conferring erythromycin‐resistance. Experimental Cell Research, 88, 79–87. [DOI] [PubMed] [Google Scholar]
- Koizumi, S. (1974) Microinjection and transfer of cytoplasm in Paramecium: experiment on the transfer of kappa particles into cells at different stages. Experimental Cell Research, 88, 74–78. [DOI] [PubMed] [Google Scholar]
- Koizumi, S. & Kobayashi, S. (1989) Microinjection of plasmid DNA encoding the A surface antigen of Paramecium tetraurelia restores the ability to regenerate a wild‐type macronucleus. Molecular and Cellular Biology, 9, 4398–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzh, V. & Strahle, U. (2002) Marshall Barber and the century of microinjection: from cloning of bacteria to cloning of everything. Differentiation, 70, 221–226. [DOI] [PubMed] [Google Scholar]
- Kosciuszko, H. & Koizumi, S. (1983) Induction of autogamy by transfer of macronuclear karyoplasm in Paramecium tetraurelia . Experimental Cell Research, 146, 436–438. [DOI] [PubMed] [Google Scholar]
- Kung, C. (1971) Genic mutations with altered system of excitation in Paramecium aurelia. II. Mutagenesis, screening, and genetic analysis of the mutants. Genetics, 69, 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung, C. & Saimi, Y. (1982) The physiological basis of taxes in Paramecium . Annual Review of Physiology, 44, 519–534. [DOI] [PubMed] [Google Scholar]
- Lepère, G. , Bétermier, M. , Meyer, E. & Duharcourt, S. (2008) Maternal noncoding transripts antagonize the targeting of DNA elimination by scanRNAs in Paramecium tetraurelia . Genes & Development, 22, 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, K.‐Y. , Haynes, W.J. , Oesterle, L. , Kung, C. , Preston, R.R. & Saimi, Y. (2001) K+‐channel transgenes reduce K+ currents in Paramecium, probably by a post‐translational mechanism. Genetics, 159, 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodh, S. , Yano, J. , Valentine, M.S. & Van Houten, J.L. (2016) Voltage‐gated calcium channels of Paramecium cilia. Journal of Experimental Biology, 219, 3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpert, C.J. , Kersken, H. & Plattner, H. (1990) Cell surface complexes (‘cortices’) isolated from Paramecium tetraurelia cells as a model system for analyzing exocytosis in vitro in conjunction with microinjection studies. The Biochemical Journal, 269, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L.D. , Pollack, S. , Preer, J.R. Jr & Polisky, B. (1994) DNA sequence requirements for the regulation of immobilization antigen A expression in Paramecium tetraurelia . Developmental Genetics, 15, 443–451. [DOI] [PubMed] [Google Scholar]
- McCormick‐Graham, M. , Haynes, W.J. & Romero, D.P. (1997) Variable telomeric repeat synthesis in Paramecium tetraurelia is consistent with misincorporation by telomerase. EMBO Journal, 16, 3233–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, E. (1992) Induction of specific macronuclear developmental mutations by microinjection of a cloned telomeric gene in Paramecium primaurelia . Genes & Development, 6, 211–222. [DOI] [PubMed] [Google Scholar]
- Mikami, K. (1979a) Stomatogenesis during sexual and asexual reproduction in an amiconucleate strain of Paramecium caudatum . Journal of Experimental Zoology, 208, 121–128. [DOI] [PubMed] [Google Scholar]
- Mikami, K. (1979b) Internuclear control of DNA synthesis in exconjugant cells of Paramecium caudatum . Chromosoma, 73, 131–142. [DOI] [PubMed] [Google Scholar]
- Mikami, K. (1980) Differentiation of somatic and germinal nuclei correlated with intracellular localization in Paramecium caudatum exconjugants. Developmental Biology, 80, 46–55. [DOI] [PubMed] [Google Scholar]
- Mikami, K. (1982a) Nuclear transplant studies on the reduction in numbers of presumptive germ nuclei in exconjugants of Paramecium caudatum . Journal of Cell Science, 56, 453–460. [DOI] [PubMed] [Google Scholar]
- Mikami, K. (1987) Macronuclear development and gene expression in the exconjugants of Paramecium caudatum . Developmental Biology, 123, 161–168. [Google Scholar]
- Mikami, K. (1992) Behavior of germinal micronuclei under control of the somatic macronucleus during conjugation in Paramecium caudatum . Developmental Biology, 149, 317–326. [DOI] [PubMed] [Google Scholar]
- Mikami, K. & Koizumi, S. (1982b) Nuclear transplant studies of the determination of mating type in germ nuclei of Paramecium tetraurelia . Experimental Cell Research, 137, 397–402. [DOI] [PubMed] [Google Scholar]
- Mikami, K. & Koizumi, S. (1983b) Microsurgical analysis of the clonal age and the cell‐cycle stage required for the onset of autogamy in Paramecium tetraurelia . Developmental Biology, 100, 127–132. [DOI] [PubMed] [Google Scholar]
- Mikami, K. & Ng, S.F. (1983a) Nuclear differentiation in Paramecium tetraurelia. Transplantation of vegetative micronuclei into early exconjugants. Experimental Cell Research, 144, 25–30. [DOI] [PubMed] [Google Scholar]
- Miwa, I. (1973) Difference of culture method and length of immaturity in Paramecium caudatum . Science Reports of the Tohoku University, Series 4 (Biology), 36, 217–222. [Google Scholar]
- Miwa, I. (1979a) Specificity of the immaturity substances in Paramecium . Journal of Cell Science, 36, 253–260. [DOI] [PubMed] [Google Scholar]
- Miwa, I. (1979b) Immaturity substances in Paramecium primaurelia and their specificity. Journal of Cell Science, 38, 193–199. [DOI] [PubMed] [Google Scholar]
- Miwa, I. (1984) Destruction of immaturin activity in early mature mutants of Paramecium caudatum . Journal of Cell Science, 72, 111–120. [DOI] [PubMed] [Google Scholar]
- Miwa, I. , Haga, N. & Hiwatashi, K. (1975) Immaturity substances: material basis for immaturity in Paramecium . Journal of Cell Science, 19, 369–378. [DOI] [PubMed] [Google Scholar]
- Momayezi, M. , Lumpert, C.J. , Kersken, H. , Gras, U. , Plattner, H. , Krinks, M.H. et al. (1987) Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. Journal of Cell Biology, 105, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, S.F. & Fujishima, M. (1989) Control of development of the oral apparatus of Paramecium during sexual reproduction: an embryological perspective. Developmental Biology, 134, 317–326. [DOI] [PubMed] [Google Scholar]
- Oertel, D. , Schein, S.J. & Kung, C. (1977) Separation of membrane currents using a Paramecium mutant. Nature, 268, 120–124. [DOI] [PubMed] [Google Scholar]
- Ruiz, F. , Vayssié, L. , Klotz, C. , Sperling, L. & Madeddu, L. (1998) Homology‐dependent gene silencing in Paramecium . Molecular Biology of the Cell, 9, 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, W.H. , Hinrichsen, R.D. , Burgess‐Cassler, A. , Kung, C. , Blair, I.A. & Watterson, D.M. (1987) A mutant Paramecium with a defective calcium‐dependent potassium conductance has an altered calmodulin: a nonlethal selective alteration in calmodulin regulation. Proceedings of the National Academy of Sciences of the United States of America, 84, 3931–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, D.P. , Saudemont, B. , Guglielmi, G. , Arnaiz, O. , Goût, J.‐F. , Prajer, M. et al. (2014) Genome‐defense small RNAs exapted for epigenetic mating‐type inheritance. Nature, 22, 441–452. [DOI] [PubMed] [Google Scholar]
- Skovorodkin, I.N. , Fokin, S.I. & Fujishima, M. (2001) Fates of the endonuclear symbiotic bacteria Holospora obtusa and Holospora undulata into the macronucleus of Paramecium caudatum . European Journal of Protistology, 37, 137–145. [Google Scholar]
- Sonneborn, T.M. (1950) Methods in the general biology and genetics of Paramecium tetraurelia . Journal of Experimental Zoology, 113, 87–147. [Google Scholar]
- Sonneborn, T.M. (1975) Handbook of genetics 2 (ed King R.C.), p. 469. New York, NY: Plenum Press. [Google Scholar]
- Takahashi, M. (1979) Behavioral mutants in Paramecium caudatum . Genetics, 9, 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M. , Haga, N. , Hennessey, T. , Hinrichsen, R.D. & Hara, R. (1985) A gamma ray‐induced non‐excitable membrane mutant in Paramecium caudatum: a behavioral and genetic analysis. Genetics Research Cambridge, 46, 1–10. [DOI] [PubMed] [Google Scholar]
- Takenaka, Y. , Haga, N. , Harumoto, T. , Matsuura, T. & Mitsui, Y. (2002) Transformation of Paramecium caudatum with a novel expression vector harboring codon‐optimized GFP gene. Gene, 284, 233–240. [DOI] [PubMed] [Google Scholar]
- Takenaka, Y. , Haga, N. , Inoue, I. , Nakano, T. , Ikeda, M. , Katayama, S. et al. (2014) Identification of two nickel ion‐induced genes, NCI16 and PcGST1, in Paramecium caudatum . Eukaryotic Cell, 13, 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, Y. , Yanagi, A. , Masuda, H. , Mitsui, Y. , Mizuno, H. & Haga, N. (2007) Direct observation of histone H2B‐YFP fusion proteins and transport of their mRNA between conjugating Paramecia. Gene, 395, 108–115. [DOI] [PubMed] [Google Scholar]
- Watanabe, T. & Haga, N. (1996) Genetic characterization of the secretory mutants of Paramecium caudatum . Protoplasma, 192, 11–19. [Google Scholar]
- Xianyu, Y. & Haga, N. (1993) Initiation of the earliest nuclear event in fertilization of Paramecium by the microinjection of calcium buffer. Zoological Science, 10, 859–862. [Google Scholar]
- Xu, W. (2019) Microinjection and micromanipulation: a historical perspective. Microinjection: methods and protocols. Methods in Molecular Biology, 1874, 1–16. [DOI] [PubMed] [Google Scholar]


