Abstract
Paternal age and past mating effort by males are often confounded, which can affect our understanding of a father's age effects. To our knowledge, only a few studies have standardized mating history when testing for effects of paternal age, and none has simultaneously disentangled how paternal age and mating history might jointly influence offspring traits. Here, we experimentally manipulated male mating history to tease apart its effects from those of paternal age on female fertility and offspring traits in the eastern mosquitofish (Gambusia holbrooki). Male age did not affect female fertility. However, males with greater past mating effort produced significantly larger broods. Paternal age and mating history interacted to affect sons' body size: sons sired by old‐virgin males were larger than those sired by old‐mated males, but this was not the case for younger fathers. Intriguingly, however, sons sired by old‐virgin males tended to produce fewer sperms than those sired by old‐mated males, indicating a potential trade‐off in beneficial paternal effects. Finally, neither paternal age nor mating history affected daughter's fitness. Our results highlight that variation in offspring traits attributed to paternal age effect could partly arise due to a father's mating history, and not simply to his chronological age.
Looking for predictable similarities between parents and their offspring is an age‐old concept. The usual source of similarity is shared genes, but similarities or, indeed, predictable differences between parents and offspring can also be due to nongenetic effects of parents on their descendants, formally known as “parental effects” (Wolf et al. 1999; Badyaev and Uller 2009). Maternal effects have been studied in a broader range of research fields, while studies on paternal effects have only become popular comparatively recently (Rutkowska et al. 2020). This is partly because the proximate mechanism underpinning maternal effects is usually obvious: namely, greater provisioning of eggs or transfer of nutrients to offspring after birth (e.g., providing more maternal care; Mousseau and Fox 1998). This is less often the case for paternal effects given that males usually do little more than transfer sperm when mating, and then have no subsequent interactions with their offspring (Bonduriansky and Day 2018). Even so, there is evidence that paternal effect can arise due to variation among fathers in, for instance, paternal age, social interactions, body condition, and the environment they have experienced (e.g., diet, temperature, stress; Curley et al. 2011; Rando 2012; Bonduriansky and Day 2013; Crean and Bonduriansky 2014). Paternal effects have been documented for a range of offspring traits, and these can be attributed to either direct effects of the paternal environment (i.e., paternal care or levels of parental investment) or to indirect mechanisms (e.g., changes in ejaculate traits that affect early offspring development, or epigenetic changes to the paternal genome that offspring then inherit which affect subsequent gene expression; Crean and Bonduriansky 2014; Evans et al. 2019). Theory suggests that variation in offspring traits due to paternal effects can have major consequences for evolution by sexual selection (e.g., Wolf et al. 1999; Bonduriansky and Day 2013), and the general rate of evolution (Badyaev and Uller 2009; Roper et al. 2021). It is, therefore, important to identify paternal effects, specify their origins, and distinguish them from other sources of variation among offspring (Crean and Bonduriansky 2014; Rutkowska et al. 2020).
Male age is a paternal characteristic that is known to be related to offspring traits that affect their fitness (Priest et al. 2002; Bonduriansky et al. 2008; Noguera et al. 2018; Lee et al. 2019; Cholewa et al. 2021; Noguera 2021; also reviewed in Monaghan et al. 2020). General life‐history theory predicts that, older individuals tend to invest more than younger individuals into their current reproduction, as the potential opportunity cost for future reproduction decreases over time (Williams 1966). This can result in higher quality offspring due to improved paternal care or other forms of provisioning of offspring by older males (Brooks and Kemp 2001). In contrast, classical evolutionary theories of ageing explain senescence as a consequence of the strength of natural selection declining with age (Medawar 1952; Hamilton 1966; Stearns and Partridge 2001). General theory of senescence suggests that, all else being equal, offspring fitness should decline with parental age because older parents are less capable of provisioning offspring and/or are less effective at repairing DNA damage or epigenetic markers that cause offspring to inherit genomes that function less effectively. Recent theoretical studies, mainly focusing on maternal age, have attempt to reconcile these opposing effects of parental age on offspring, and have led to renewed interest in understanding the effect of parental age on offspring fitness (Gillespie et al. 2013; Moorad and Nussey 2016; Barks and Laird 2020; Hernández et al. 2020).
Both general interest in, and evolutionary theories about, ageing have given rise to many empirical studies asking how paternal age is linked to offspring performance. In humans, studies have generally confirmed that older fathers sire offspring with lower fitness (Kong et al. 2012; also reviewed in Sartorius and Nieschlag 2009; Sharma et al. 2015; du Fossé et al. 2020; Fang et al. 2020). Studies on other animals have, however, given rise to more equivocal results. Many studies have reported that older fathers produce lower quality offspring (e.g., bulb mite: Prokop et al. 2007; mice: García‐Palomares et al. 2009; cabbage beetles: Liu et al. 2011; nerid fly: Wylde et al. 2019; bustards: Vuarin et al. 2019, 2021), partly due to higher offspring mortality rates (e.g., ungulates: Ruiz‐López et al. 2010; zebra finch: Noguera et al. 2018; mice: Xie et al. 2018; field crickets: Noguera 2021). On the other hand, some studies have shown that offspring fathered by older males have better early life and adult performance (e.g., fruitflies: Krishna et al. 2012; Lee et al. 2019; European blackbirds: Cholewa et al. 2021), including a higher rate of early life survival (e.g., butterfly: Ducatez et al. 2012; zebra fish: Johnson et al. 2018; superb fairy‐wren: Cooper et al. 2020) and longevity (e.g., antler flies: Angell et al. 2022). Other studies simply report no detectable effect of paternal age on offspring traits (e.g., ants: Heinze et al. 2018; fruitflies: Lee et al. 2019; monoandrous moth: Lai et al. 2020; common gulls: Sepp et al. 2021; burying beetle: Cope et al. 2021). These equivocal findings about how a father's age affects offspring fitness raise concerns that there are confounding variables that mask any consistent, direct effect of male age on offspring fitness. This gives rise to a question: how does male age itself affect offspring fitness if we control for obvious sources of variation?
One factor that might play an important role in determining the correlation between a father's age and his offspring performance is his past reproductive effort (review: Johnson and Gemmell 2012; Monaghan et al. 2020). After all, in nature, older males are likely to have made more mating effort in the past than have younger males, which might result in a decline in paternal investment due to the well‐known costs of reproduction. Most studies testing for a trade‐off between past mating effort and subsequent paternal investment have shown a decline in direct paternal provisioning of offspring (review: Stiver and Alonzo 2009). In species without paternal care, however, a father's ejaculate can also transmit environmental information to offspring via nongenetic or epigenetic mechanisms (Bonduriansky and Day 2018; Evans et al. 2019). In mice, for example, sperm from aging males and body tissue from their offspring both contain some promoters that are methylated in the same way, and these promoters are involved in the regulation of evolutionarily conserved longevity pathways (Xie et al. 2018). Moreover, repeated mating leads to more frequent replenishment of sperm (i.e., higher rates of meiosis), which could create more frequent DNA damage and the accumulation of deleterious germline mutations that are inherited by offspring as a by‐product of greater paternal mating effect (reviewed in: Pizzari et al. 2008; Ruiz‐López et al. 2010; Simon and Lewis 2011; Velando et al. 2011; Monaghan and Metcalfe 2019). It is, therefore, necessary to tease apart how male age and mating effort affect offspring quality to determine the extent, if any, to which changes in offspring fitness that are attributed to male age are, in fact, due to a male's past mating effort.
To explicitly test for paternal effects attributable to male age and/or their mating history, it is important to consider other sources of variation that might affect paternal investment. For instance, maternal age in Gambusia holbrooki can affect offspring size (Vega‐Trejo et al. 2018). Therefore, experimental elimination of any assortative mating based on age can better control for the influence of confounding maternal effect. This can be done by minimizing variation in female age and mating status and randomly assigning females to males (Aich et al. 2020a). Female preference in the form of cryptic female choice can also lead to maternal provisioning that affects fitness‐related traits in offspring (i.e., differential allocation; Benowitz et al. 2013; Alonzo et al. 2016). Using artificial insemination in a laboratory environment can eliminate differential maternal allocation that is based on female assessment of mates (Evans et al. 2003; Alonzo et al. 2016). Finally, stochastic variation in male resources, such as food availability, can affect paternal investment (e.g., ejaculate content), which might, in turn, affects a male's effect on female fecundity and offspring fitness (Macartney et al. 2018). The experimental minimization of such variation allows for more powerful tests of the effects of male age and past mating effort on their mate's breeding performance and offspring traits.
Here, we disentangle the effects of paternal age and mating effort on both female reproductive output and a series of offspring fitness components in the eastern mosquitofish (G. holbrooki) by experimentally manipulating the mating history of young and old males. In the laboratory, we bred males in groups that were 12 weeks apart in age to generate “old” and “young” males. These two age classes of males were then provided with one of two different mating treatments. One group of males had full access to females with whom they could mate freely. They became our old and young mated males with high mating effort. The other group of males only had visual contact with females without being able to mate. These became our old and young naive males with lower mating effort. We collected equal numbers of sperm bundles from each male and then artificially inseminated two virgin females per male. We minimized variation in female age to quantify any effects that are attributable to paternal age or mating history (Vega‐Trejo et al. 2018).
We tested for the independent effects of sire age and past mating effort (and their interaction) on their mate's breeding success (i.e., any effect on female fecundity). More importantly, we then tested for any paternal effects on: early offspring survival, growth, and immunocompetence; sons’ attractiveness, mating behavior and ejaculate traits; and daughters’ fecundity.
Methods
ORIGIN AND MAINTENANCE OF THE FISH
To examine the effects of paternal age and mating history on their mate's breeding success and the fitness of any offspring sired in G. holbrooki, we bred “young” and “old” males in the laboratory. We then assigned males in each age group to one of two mating treatments. The full details of the methods used to produce old and young males with different mating histories are described in Aich et al. (2020a). In brief, we produced “old” males from the offspring of a population of 400 breeding adult males and females. We then raised them in individual tanks and recorded their date of birth and date of maturity. We then used a new set of 400 breeding adult males and females 12 weeks later to rear a second set of offspring to produce “young” males. Mosquitofish females have an average postmaturation lifespan of 12–15 months, but far less for males (see Aich et al. 2021a for details). Autumn‐born females in this species often breed alongside their spring‐born offspring the following year, but this seasonal overlap is absent for males (Pyke 2005; Kahn et al. 2013). In our field population, it is unlikely that adult male mosquitofish live longer than 6 months. The time to maturity itself varies from 25 to 120 days in the laboratory (Vega‐Trejo et al. 2016). We, therefore, selected an age difference whereby “old” males had been adults for 12–13 weeks longer than “young” males.
After creating these two sets of adult males, we then included an experimental mating treatment. We manipulated whether or not each male could mate with a female. “Naïve” males were allowed visual contact with a female, but with a mesh barrier separating them from the female to prevent any mating. Therefore, naive males had a low past mating effort. In contrast, “mated” males were housed with a female that they could mate: these males had higher past mating effort. Equal numbers of “young” and “old” males were assigned to each mating treatment for 2 weeks, to create four treatments (“old/mated”; “young/mated”; “old/Naïve”; and “young/Naïve”; n = 63 × 4 treatments = 252 males). Male exposure to females for a prolonged period, even in the absence of mating, might be costly for males, because sexually primed males produce more sperm (e.g., guppies: Cattelan et al. 2016) or the perception of female can affect survival and physiological cost (e.g., Drosophila: Harvanek et al. 2017). However, this pattern is absent in mosquitofish, where the availability of females does not affect male sperm production rate (Chung et al. 2019). Each test male was housed in a 7 L tank (17 × 28 × 15 cm) with a female. Each male was provided with a new female after 7 days to maintain his sexual interest, and avoid any “Coolidge effect” (Vega‐Trejo et al. 2014). The females that we used in this part of the experiment were collected from the wild 3 months earlier, and held in 90 L female‐only aquaria until used.
After 2 weeks in their assigned mating treatment, all males were isolated for 5 days to replenish their sperm reserves (O'Dea et al. 2014) before we collected sperm to artificially inseminate virgin females. We collected sperms when the old and young males were approximately 15 and 3 weeks postmaturity, respectively. This age difference is biologically relevant as a previous study have found a significant decline in sperm traits (i.e., sperm replenishment rate and sperm velocity) from weeks 3 to 14 (Vega‐Trejo et al. 2019), which also fits with the natural lifespan of mosquitofish males in the field (Kahn et al. 2013). The females that were used in this part of the experiment were virgin, laboratory‐born offspring of wild‐caught mothers.
SPERM COLLECTION AND ARTIFICIAL INSEMINATION
We used artificial insemination (AI) to quantify the ability of males to achieve successful fertilization of a virgin female. We then tested for differences among the four types of males in their paternal effects on offspring traits related to fitness.
The males were stripped of sperm before being put in the mating treatment to reduce variation in sperm age (i.e., all sperm were a maximum of 19 days old). The process of stripping males to obtain sperm followed the methods of Vega‐Trejo et al. (2016). Each male was anaesthetized and placed under a dissecting microscope on a glass slide coated with 1% polyvinyl alcohol solution. The gonopodium was swung forward and gentle pressure was applied to the abdomen to eject all the available sperm. The fish was then removed from the slide and 100 μL of 0.9% NaCl solution pipetted onto the ejaculate to keep it hydrated. From each ejaculate, we collected two separate samples of 10 sperm bundles and then artificially inseminated two virgin females per male. To artificially inseminate a virgin female, she was anaesthetized by putting her in an ice‐slurry, then she was placed ventral side up in a polystyrene cradle. Using a micropipette, we injected 10 bundles of sperm into her gonopore, following the protocol of Marsh et al. (2017). Previous study on these four types of experimental males found no difference in sperm velocity (Aich et al. 2021b).
EFFECT OF MALE AGE AND MATING HISTORY ON FEMALE FERTILITY
After insemination, females were transferred to individual 1 L aquaria containing a mesh barrier to provide a refuge for newborn fry. After 18 days (the gestation period for G. holbrooki is usually 22–25 days: Pyke 2005), tanks were inspected twice daily for the presence of offspring. For each female, we recorded whether or not she gave birth, her gestation period (days to give birth after AI), and the brood size (number of offspring).
EFFECT OF MALE AGE AND MATING HISTORY ON OFFSPRING TRAITS
The size of offspring at birth was recorded by photographing them from above in a petri dish placed over 1 mm graph paper. We then transferred the offspring to individual 1 L tank, where they grew until mature. Average time to maturity for offspring was 87.3 ± 30.4 days (sons: 90.2 ± 25 days, n = 184; daughters: 83.8 ± 35.6 days, n = 152). We rephotographed offspring 21 days later to measure their growth rate. We also recorded offspring early‐ and late‐life survival (before 21 days and from 21 days to adulthood). Offspring were inspected three times a week for sexual maturation. For sons, this was defined by the presence of a fully formed gonopodium (including distal spines); and for daughters by the presence of a visible gravid spot on the ventral flank. We recorded the time to maturity and body size 5 weeks after maturity for all surviving offspring. Upon maturity, we also measured sex‐specific offspring traits related to fitness.
MEASUREMENT OF SON'S FITNESS
Attractiveness and mating behavior
We measured the attractiveness of sons 4 weeks after they had matured. We ran a two‐choice trial where a virgin female could choose between the focal male (son) and a nonfocal male of average size. At the start of each trial, a female was placed in a clear plastic cylinder in the center of a three‐compartment choice tank (49 × 20 × 22cm). A single male was placed in each end compartments, and these were separated from the middle compartment by a fixed mesh screen and a removable opaque screen (to minimize male–male and male–female interactions before the trial). After a 5 min acclimation period, the opaque screens were removed and the female was released from the cylinder. We recorded her movements for 10 min (Booksmythe et al. 2013). Male attractiveness was then quantified as the time she spent <6 cm from each male compartment (equivalent to approximately two male body lengths). At the end of the trial, the male compartments were covered with opaque screens, and the males were switched between compartments, the female was returned to the central cylinder and the fishes were left for 5 minutes. Then the screens were again removed from the male compartments and the focal female was released and her movements recorded for another 10 min. This process allowed us to quantify the repeatability of her choice, and account for any side bias or female lateralization (Vinogradov et al. 2021).
Following the choice trials, the focal male was placed in a 3 L tank with a virgin female to observe his mating behavior. After 10 min to acclimate, the focal male was observed for 10 min and his behavior was recorded, namely: time spent within two body lengths of the female and the number of copulation attempts.
Investment in ejaculates
After the behavior trial, the sons were given 1 week to replenish their sperm before we measured their sperm velocity and sperm count. Each son was stripped following the method described above for sires. For sperm velocity assays, two samples of three individual sperm bundles were extracted using a 3 μL pipette and transferred to a microtube containing 2 μL of extender medium (207 mM NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 0.49 mM MgCl2, 0.41 mM MgSO4, 10 mM Tris, pH 7.5). For sperm count measures, the remainder of the ejaculate was transferred to a 1.5 mL Eppendorf tube containing 100−900 μL of extender medium using a 100 μL pipette, with the quantity of extender medium used depending on the amount of ejaculate released by the male.
To estimate sperm velocity, we analyzed the two samples per ejaculate per male. For each sample, we collected 3 μL of diluted sperm (see above) and place it in the center of a cell of a 12‐cell multitest slide (MP Biomedicals, Aurora, OH, USA). The sample was then activated using activator medium (3 μL solution of 150 mM KCl and 2 mg/mL bovine serum albumin) and covered with a coverslip. We analyzed sperm velocity within 30 s of activation. We recorded two standard measures of sperm velocity: (1) average path velocity (VAP), which estimates the average velocity of sperm cells over a smoothed cell path and (2) curvilinear velocity (VCL), the actual velocity along the trajectory using a CEROS Sperm Tracker. We only analyzed VCL, which is the more biologically relevant measure (Boschetto et al. 2011). VCL and VAP were highly correlated (r = 0.99, N = 177, p <0.00001). Sperm velocities for each son were calculated as the average of the two samples, weighting by the number of sperm tracked per sample (Aich et al. 2020b).
To measure total sperm count, we vortexed the contents of the 1.5 mL Eppendorf tube for 1 min to break up sperm bundles and distribute the sperm evenly throughout the sample. We then pipetted 3 μL of solution onto a 20‐micron capillary slide (Leja) and used the CEROS Sperm Tracker to count the number of sperm in five separate fields of view under the microscope (100× magnification). We used the average of these five subsamples for further analyses. We estimated the total size of a male's sperm reserves by multiplying the count by the appropriate dilution factor. We also corrected for the six bundles (2 × 3 bundles) that were removed from each sample to estimate sperm velocity.
MEASUREMENT OF DAUGHTERS’ FITNESS
Five weeks after maturity, daughters were euthanized in MS222 to estimate their reproductive investment. Individuals were photographed laterally alongside a scale bar, and then dissected, their eggs transferred to a glass slide, counted and photographed under a dissecting microscope with a reference scale following Aich et al. (2020b). From these images, we measured eggs using the ImageJ tool and calculated their average diameter.
MEASUREMENT OF OFFSPRING IMMUNE RESPONSE
We estimated the immunocompetence of offspring of both sexes using a phytohaemagglutinin injection assay (PHA test) following Iglesias‐Carrasco et al. (2018). The PHA test measures T‐cell‐dependent immunocompetence in vivo, and has been used in many vertebrates including G. holbrooki (Iglesias‐Carrasco et al. 2019). After the measurement of sperm traits, sons were anesthetized in iced water and we measured the thickness of their body between the dorsal and caudal fin with a pressure‐sensitive spessimeter (accuracy: 0.01 mm; we use an average of five measurements per fish). Immediately afterwards we injected 0.01 mg of PHA dissolved in 0.01 mL PBS which causes swelling of the skin due to the immune response that disappears after around 72 h (Iglesias‐Carrasco et al. 2018). The sons were then placed back in their individual aquaria. After 24 h, we again anaesthetized the males and measured their body thickness at the same point to calculate the difference between pre‐ and postinjection measures (i.e., the degree of inflammation). The immunity response of the daughters was measured using the same method 5 weeks after their maturity, on the day before they were dissected to count eggs. All individuals behaved normally and fed after recovering from the anesthesia.
All experimental procedures were performed and trials were scored by UA who was blinded to paternal age and mating treatment. At the conclusion of the experiment, all focal individuals were euthanized in MS222 as Australian legislation prevents the release of pest species into the wild.
STATISTICAL ANALYSES
All analyses were performed in RStudio version 3.6.0. (R Core Team 2019) using either lme4 (Bates et al. 2015) or glmmTMB (Brooks et al. 2017) package. We quantified the effect of paternal age and past mating effort on female fertility traits, and offspring fitness using linear mixed effect models, and generalized linear mixed effect models. In all models analyzing offspring traits for both sexes, we included paternal age, mating history, and offspring sex as fixed factors. Initial models included all two‐way interactions. For tractability of interpretation, we excluded three‐way interactions. We included male (paternal) ID as a random factor for all the female traits we measured as we inseminated two females per male. For all the offspring traits, we included both paternal and maternal ID as random factors to account for the measurement of several offspring per brood. We excluded nonsignificant interactions from final models to test for main effects. If any interaction was significant, we retained it in our final model and conducted post‐hoc pairwise comparison tests using the emmeans package (Lenth R. and Lenth M. R. 2018). In all relevant cases we tested for, and dealt with overdispersion and zero inflation. For all count and continuous data, we choose the error distribution that best fit the data. All model terms were tested for significance using the Anova function in the car package specifying Type III Wald chi‐square tests.
Female fertility traits
We assessed the effect of paternal age and past mating effort on female fertility traits, namely, (a) whether the female gave birth, and if she did, (b) the gestation period, and (c) the brood size. We included male age, past mating effort, and their two‐way interaction as fixed effects, mean‐centered female body size was a covariate, and male (paternal) ID was a random factor. We assumed a binomial error distribution for the success or failure of the female giving birth. Data for both female gestation period and first brood size were right skewed and zero‐inflated. We, therefore, used negative‐binomial models with zero inflation to analyses female gestation period and first brood size.
Offspring fitness
To test for the effects of paternal age and past mating effort on offspring life‐history traits, we ran mixed effect models for: (a) early survival, (b) survival to adulthood, (c) early growth rate, (d) growth rate to maturity (log‐transformed), (e) size at birth, (f) size at 21 days, and (g) time to maturity. To test for offspring early survival, we included paternal age, past mating effort and their interaction as fixed factors. In all other cases, we also included offspring sex and all two‐way interactions as fixed factors. As stated above, we excluded nonsignificant interactions from the final model to test for main effects. We also included mean‐centered maternal body size as a fixed covariate, and paternal and maternal ID as random factors. We ran models with a binomial error distribution for the success or failure of early and late survival; Gaussian error distribution for growth rate and offspring size; and a negative‐binomial model for time to maturity.
We tested the effect of paternal age and past mating effort on sons’ traits, namely, (a) size at 5 weeks after maturity, (b) gonopodium size, (c) sperm velocity, (d) sperm count, (e) immune response, (f) time spent near the female, (g) number of copulation attempts, and (h) attractiveness in the mate choice trials (proportion of time spent with the focal male). We included paternal age, past mating effort and their two‐way interaction as fixed effects, and paternal and maternal ID as random factors. Mothers’ mean‐centered body size was added as covariate for all the traits. For son's gonopodium size, we always included their body size as a covariate as these traits are highly correlated (r = 0.8). We then ran exploratory analysis for sons’ traits adding mean‐centered sons’ body size as a covariate. This was done to check if variation in son's traits was solely mediated by their body size and if paternal age and mating history effect persisted when sons’ body size is accounted for. We used a Poisson error distribution for son's sperm count, negative‐binomial error distributions for the time spent near a female and the number of copulation attempts; a beta‐binomial distribution for sons’ attractiveness; and Gaussian error distributions for the other traits. We included sons’ ID as a random factor for sons’ attractiveness to account for it being measured twice.
Finally, we tested the effect of paternal age and past mating effort on daughters’ traits, namely, (a) size at maturity, (b) immune response, (c) egg size, and (d) number of eggs. Similar to the analyses of sons’ traits, we included paternal age, past mating effort, and their two‐way interaction as fixed effects, and paternal and maternal ID as random factors. Mothers’ mean‐centered body size was included as a covariate for all the traits. As above, we then ran exploratory analysis for daughters’ traits adding mean‐centered daughters’ body size as an additional covariate. Daughters’ body size, immune response, and egg size were analyzed in models using a Gaussian error structure. We used Poisson error distribution and accounted for zero inflation to analyses the number of eggs produced by daughters.
We conducted 22 separate tests for female and offspring traits. We accounted for multiple testing for each main factor (paternal age and mating effort) and their interaction, for three datasets (females, sons, and daughters) using the Benjamini‐Hochberg False Discovery Rate method with a false discovery rate of 10% (Benjamini and Hochberg 1995). Two initially statistically significant results when we used a conservative alpha value of 0.01 remained significant based on the false discovery rate analysis. Two other results where the initial p value was 0.01 < p < 0.05 was not significant based on the false discovery rate analysis. We, therefore, describe 0.01 < p < 0.05 as moderate‐to‐weak evidence using the reporting approach of Muff et al. (2021), and we advise readers to interpret this result with caution.
RESULTS
FEMALE FERTILITY TRAITS
Following AI, we found no evidence of an interaction between paternal age and mating history affecting their partner's fertility, gestation period, or brood size (all p > 0.05). There was no evidence that male age nor a male's past mating history had an effect on their partner's fertility (χ1 2 = 0.516, p = 0.472 and χ1 2 = 0.532, p = 0.466, respectively) or gestation period (χ1 2 = 0.019, p = 0.892 and χ1 2 = 2.082, p = 0.149, respectively). A female's body size also had no effect on her fertility or gestation period (both p > 0.05). Although paternal age had no effect on brood size (χ1 2 = 0.336, p = 0.562), there was strong evidence that females inseminated by previously mated males produced larger broods (χ1 2 = 7.284, p = 0.007; Fig. 1). Larger females also tended to produce a larger brood (χ1 2 = 3.998, p = 0.046). The model details and parameter estimates are in Supporting information Tables S1 and S2.
Figure 1.
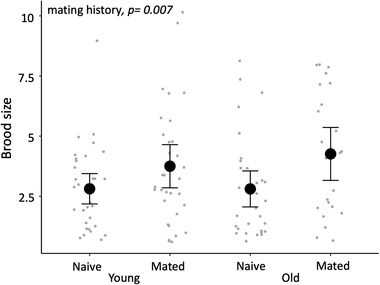
The effect of paternal age and mating history on female brood size. Error bars represent the 95% confidence interval of the mean (n = 118 broods of 91 fathers).
OFFSPRING EARLY‐LIFE TRAITS
There was no evidence of interactions between paternal age and mating history affecting any of the seven measured offspring early‐life traits, namely early survival and survival to adulthood, early growth rate, growth rate to maturity, size at birth, size at 21 days, and time to maturity (all p > 0.05, Supporting information Table S1). Likewise, there was no evidence of the two‐way interaction between offspring sex and either paternal age or past mating history affecting these offspring traits (all p > 0.05, Supporting information Tables S3, S4). There were also no main effects of paternal age or past mating history on any of the offspring early‐life traits (all p > 0.05, Supporting information Table S2). Similarly, maternal body size had no significant effect on offspring early‐life traits (Supporting information Table S2).
In contrast, our data provided evidence that sons and daughters differed for three of the seven offspring early‐life traits (Supporting information Table S2). Daughters had both a higher early‐growth rate and growth rate to maturity (χ1 2 = 8.987, p = 0.003 and χ1 2 = 124.890, p ≤ 0.0001, respectively), and matured sooner than sons (χ1 2 = 13.117, p = 0.0002). Daughters also tended to be larger at 21 days (χ1 2 = 6.113, p = 0.013). Sons and daughters did not, however, differ in their survivability or size at birth (all p > 0.05, Supporting information Table S2).
OFFSPRING ADULT TRAITS: SONS
We found strong evidence that paternal age and mating history had an interactive effect on a son's adult body size (Supporting information Table S5). Sons of old, naive males were larger than those of young, naive males, while the pattern was reversed for the sons of old and young mated males (χ1 2 = 6.787, p = 0.009; Fig. 2). However, post‐hoc pairwise comparison did not reveal any difference among the four paternal treatment groups (all p > 0.05, Supporting information Table S6). Similarly, we found some weak evidence that paternal age and past mating history had an interactive effect on a son's sperm count (Fig. 3, Supporting information Table S5): sons of old, mated males produced more sperm than those of old, naive males, while the pattern was reversed for sons sired by young mated versus naive males (χ1 2 = 3.912, p = 0.048). Again, post‐hoc pairwise comparison did not reveal any difference among the four paternal treatment groups (all p > 0.05 Supporting information Table S7). There was no evidence of an interaction between paternal age and mating history affecting a son's gonopodium length, sperm velocity, immune response, mating behavior, or attractiveness (all p > 0.05, Supporting information Table S5). There was also no evidence that either paternal age or male mating history had any effect on five of the six traits measured in sons (all p> 0.05, Supporting information Table S8). The exception was that sons of older males made slightly more copulation attempts (χ1 2 = 4.460, p = 0.033; Fig. 4; Supporting information Table S8); but the result does not hold if we account for multiple testing. Paternal mating history had no detectable effect on son's copulation attempts (χ1 2 = 0.59, p = 0.442). Maternal body size had a moderate negative effect on son's gonopodium size (χ1 2 = 5.464, p = 0.019), but none of the other traits were related to maternal body size (all p > 0.05, Supporting information Table S8).
Figure 2.
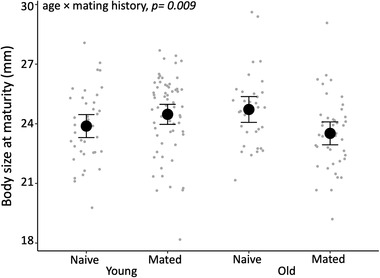
The interactive effect of paternal age and mating history on sons’ body size postmaturity. Error bars represent the 95% confidence interval of the mean (n = 178 sons from 92 females sired by 80 males).
Figure 3.
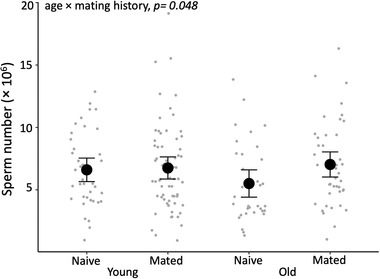
The interactive effect of paternal age and mating history on sons’ sperm production. Error bars represent the 95% confidence interval of the mean (n = 178 sons from 92 females sired by 80 males).
Figure 4.
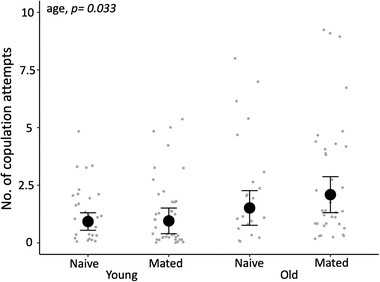
The effects of paternal age and mating history on a son's number of copulation attempts. Error bars represent the 95% confidence interval of the mean (n = 178 sons from 92 females sired by 80 males).
OFFSPRING ADULT TRAITS: DAUGHTERS
There was no evidence of an interaction between paternal age and mating history affecting a daughter's adult body size, egg number, egg size, or immune response (all p > 0.05, Supporting information Table S9). Paternal age and past mating history also had no independent effects on these four traits (all p > 0.05, see Supporting information Table S10). Similarly, maternal body size had no detectable effect on daughter's traits (all p > 0.05, Supporting information Table S10).
Exploratory analysis with son's and daughter's body size added as covariates did not alter the effects of paternal age and mating history described in the previous models (Supporting information Table S11‐S14). However, son's and daughter's body size were strongly related to some traits (Supporting information Table S12, S14). Larger sons had higher sperm velocity (χ1 2 = 262.921, p < 0.0001), a greater sperm count (χ1 2 = 1072300, p < 0.0001), and a marginally stronger immune response (χ1 2 = 4.032, p = 0.045), while there was no effect of a son's body size on his mating behavior or attractiveness (all p > 0.05, Supporting information Table S12). Larger daughters produced larger eggs (χ1 2 = 26.390, p ≤ 0.0001), but a daughter's body size did not affect her fecundity or immune response (χ1 2 = 0.150, p = 0.699, and χ1 2 = 0.016, p = 0.898 respectively; Supporting information Table S14).
Discussion
Most theories about the evolution of aging do not consider the possibility that the fitness of offspring might depend on parental age. Recently, however, there has been renewed theoretical interest in how offspring quality varies with parental age, especially that of mothers (Gillespie et al. 2013; Moorad and Nussey 2016; Barks and Laird 2020; review in Roper et al. 2021). Experimental studies testing for paternal age effect on offspring survival and fitness have shown a general decline associated with older fathers, but this pattern is not universal (Monaghan et al. 2020; Angell et al. 2022). Variation in results among correlational studies could potentially be driven by maternal effects and by other factors confounded with paternal age that are often overlooked (Johnson and Gemmell 2012; Vega‐Trejo et al. 2018; Cholewa et al. 2021; Aich et al. 2020b). Here, we experimentally separated the effects of paternal age and past mating history on fertility and key offspring life‐history traits in G. holbrooki using AI of sperm from four different types of males. We could, therefore, determine whether paternal age, past mating effort, or their interaction affect offspring fitness when variation due to maternal effect (i.e., maternal age, mating status, and differential allocation) and paternal ejaculate size was experimentally controlled. We found no decline in realized female fecundity due to greater male age. However, females produced larger brood when inseminated with ejaculates of males with greater past mating effort. We also found that neither paternal age nor past mating effort influenced offspring survival and other early‐life traits. There were, however, interactions between paternal age and mating history that affected some traits of sons. Sons of old, naive males were larger than those of old, previously mated male, while the opposite was true for the sons of young males. There was also weak evidence that an interaction between paternal age and mating history affected son's sperm count. There were no comparable interactions affecting daughter's traits. Finally, like their fathers, sons of older males tended to make more copulation attempts than those of younger males. Overall, we found no consistent evidence that older males produce less successful offspring. This study, in conjunction with others on G. holbrooki (e.g., Aich et al. 2020b) does, however, provide some evidence for paternal effects.
We found no effect of male age on female fertility (i.e., breeding success, gestation period, and brood size). This result contrast with other studies that generally report lower female fertility when paired with older males (e.g., zebra fish: Johnson et al. 2018; houbara bustards: Preston et al. 2015; Vuarin et al. 2019; broad‐horned flour beetle: Okada et al. 2020; spider mites: Morita et al. 2021; but see: Queensland fruit fly: Tasnin et al. 2021). However, our result is not totally unexpected as we used AIs to control for sperm number, which might mediate male age effects on fecundity in other studies. Also, a previous study on mosquitofish has shown that, on average, old and young males with either higher or lower past mating effort produce sperm with similar swimming speeds (Aich et al. 2021b). Our results further agree with studies in other taxa that experimentally controlled for male mating history, and then found no detectable effect of male age on female fertility (e.g., Mexican Fruit Fly: Pérez‐Staples et al. 2010; monandrous moth: Lai et al. 2020). Unexpectedly, in contrast to our earlier study (Aich et al. 2020b), we found that female G. holbrooki had larger brood when inseminated by males with greater past mating effort. One distinction between our current study and the earlier one is that mated males in our current study were in the mating treatment for 2 weeks rather than for their entire life. These males, therefore, incurred lower costs of repeatedly mating and replenishing sperm (Chung et al. 2021). It is possible that the assigned period of mating was insufficient to reveal negative effects associated with repeated mating for some traits. Although, we minimized variation in post‐meiotic sperm age, and sperm velocity did not differ with mating treatment (Aich et al. 2021b), differences in other ejaculate traits, such as sperm viability and seminal fluid content, might explain the larger brood size of females mated to more experienced males (Borziak et al. 2016; Cardozo et al. 2020; Ramm 2020; Sepil et al. 2020; Cattelan and Gasparini 2021).
Paternal age, mating history, and their interaction had no effect on the survival, early‐life traits, or immunity of offspring in G. holbrooki. These results are in contrast to other studies that have reported an effect of paternal age on offspring early‐life traits in a wide range of taxa (reviewed in Monaghan et al. 2020; also see Cooper et al. 2020; Depeux et al. 2020; Vuarin et al. 2021; Travers et al. 2021). However, our results align with an earlier study on G. holbrooki which found no paternal effect, but reported a maternal age effect on offspring early‐life traits (Vega‐Trejo et al. 2018). This highlights the importance of standardizing maternal age, when explicitly testing for paternal age effect. In contrast, we found no detectable effect of paternal mating history on offspring life‐history traits which is inconsistent with a previous experimental study on G, holbrooki that reported greater past male mating effort extends daughters’ time to maturity (Aich et al. 2020b). This earlier study manipulated lifetime male mating effort, which suggests that higher levels of past reproductive investment are more likely to have detrimental effects on offspring life‐history traits. If so, this again raises the concern that paternal effects attributed to male age per se based on correlational data could really be due to older males having mated more often than younger males.
We found some sex‐specific paternal effect on offspring late‐life reproductive traits. First, paternal age and past mating history significantly interacted to determine sons’ adult body size. Sons of older males with low past mating effort were larger than those of older fathers with high past mating effort; while the pattern tended to be reversed for sons of younger fathers. Body size in mosquitofish is under sexual selection (see Kim et al. 2021 for a review), although individual studies report mixed results (e.g., Bisazza and Marin 1991; Bisazza et al. 2001; Kahn et al. 2010; Kahn et al. 2012; Booksmythe et al. 2013; Head et al. 2015; Aich et al. 2020a). Our findings, therefore, support our suggestion that paternal age and mating history might have independent and/or interacting effects on offspring fitness. Second, there was very weak evidence (p = 0.048) that paternal age and past mating history interacted to affect sons’ sperm counts. There was a trend for sons of older males with higher past mating effort to produce more sperm than those of older fathers with low past mating effort. This suggests that there is a less‐clear pattern of trade‐off in the effect of paternal age between son's size and sperm count. Third, irrespective of paternal mating history, the sons of older males, to some extent, made more copulation attempts than sons of younger males (p = 0.033). This implies there is evidence of epigenetic heritability of this behavioral trait as we have elsewhere shown that older fathers make more mating attempts (Aich et al. 2021a). Overall, our results provide evidence that paternal effects might alter the strength of sexual selection on precopulatory and postcopulatory reproductive traits, establishing an intergenerational link between ageing and sexual selection (Monaghan et al. 2020; Vuarin et al. 2021). Finally, we found no effects of paternal age or mating history on daughters’ reproductive traits. In an earlier study of G. holbrooki, we also found that while there was a maternal age effect, paternal age had no effect on daughters’ traits (Vega‐Trejo et al. 2018). In combination with previous studies on G. holbrooki (Aich et al. 2020b; Vega‐Trejo et al. 2018), our current findings suggest that sex‐specific paternal effect can differ depending on the offspring developmental stages, offspring sex, and interactions between the paternal traits of age and mating effort. This complexity is important to bear in mind when considering the contrasting evidence for negative effects of paternal age from studies of other taxa. Crucially, most of these studies are correlational and fail to control for male mating effort or other confounding sources of paternal variation.
Empirical studies based on natural correlations often find a general decline in offspring quality with greater paternal age (reviewed in: Johnson and Gemmell 2012; Gaillard and Lemaître 2017; Monaghan et al. 2020; also see: Noguera 2021; Vuarin et al. 2021). In our experimental study, we found no robust evidence of paternal senescence in the ability to sire high‐quality offspring once we disentangled paternal mating history from age. This difference in results could arise for many reasons. Here, we note two possibilities as to why there was no negative effect of paternal age. They are specific to our experimental design, rather than more obvious differences between correlational and experimental studies related to causality. First, in our study, males were reproductively isolated from females until they were placed in a mating treatment for 2 weeks. It is possible that 2 weeks of mating was too short to reveal interactive negative effects of past reproductive investment which are then only manifest in older males (see Poizat et al. 1999; Creighton et al. 2009; Harvanek et al. 2017). This explanation is further supported by our recent experimental studies of the same mosquitofish population where older males achieved significantly higher paternity success than young males (Aich et al. 2021a, 2021b). These results are intriguing, because they support our hypothesis that old male mosquitofish do not show any decline in reproduction success or offspring quality once past mating effort is experimentally controlled. Second, mosquitofish males only provide ejaculates and there is no paternal provisioning, suggesting that maternal effect will have a much stronger influence on offspring quality (Curley et al. 2011; Crean and Bonduriansky 2014) Our decision to use AI reduced any variation in offspring quality that could arise due to assortative mating and/or cryptic female choice based on male age (Alonzo et al. 2016; Firman et al. 2017). We recommend that future studies account for confounding variations in maternal (or paternal) traits associated with paternal age that could lead to an overestimation of any age‐dependent decline in offspring traits.
Conclusions
In sum, we have provided experimental evidence that when parental age and past mating history are experimentally disentangled, there is no obvious decline in offspring fitness when sired by older males. However, there was weak evidence that older fathers with low and higher past mating effort had a counter‐balancing effect on two fitness‐related traits of their sons, namely their adult body size and sperm production. Overall, the main and interactive effects of paternal age and mating history suggests there are paternal effect on offspring phenotypic, reproductive, and behavioral traits in G. holbrooki mediated by age‐related changes in ejaculates, but they are not consistently biased toward greater fitness for the offspring of younger sires. Our study adds to the growing evidence for complex paternal effects, even in species lacking parental care. It highlights that there is a clear need for comparative analyses to identify what factors moderate paternal age effects and determine whether they elevate or lower offspring fitness.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
RESEARCH FUNDING
Australian Research Council Discovery Project. Grant Numbers: DP160100285, DP190100279.
AUTHOR CONTRIBUTIONS
UA and MDJ conceived the project. UA designed the study and collected the data. UA and SC curated the data. UA analyzed the data and drafted the manuscript. MDJ and UA contributed to subsequent revisions of the manuscript. All authors approved the final version of the manuscript.
Associate Editor: S. Foitzik
Handling Editor: A. McAdam
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank the staff of ANU Animals Services, Rebecca J Fox, Lauren Harrison, Meng‐Han Chung, and Ivan Vinogradov for help with fish maintenance.
DATA AVAILABILITY STATEMENT
Data available in OSF (https://osf.io/v25xg/) and also in Dryad Digital Repository: https://doi.org/10.5061/dryad.63xsj3v4j
LITERATURE CITED
- Aich, U. , Bonnet, T. , Fox, R.J. & Jennions, M.D. (2020a) An experimental test to separate the effects of male age and mating history on female mate choice. Behavioral ecology : official journal of the International Society for Behavioral Ecology, 31, 1353–1360. [Google Scholar]
- Aich, U. , Jennions, M.D. & Fox, R.J. (2020b) An experimental test of the role of male mating history on paternal effects in the livebearer fish Gambusia holbrooki . Biol. Lett, 16, 20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aich, U. , Bonnet, T. , Head, M.L. & Jennions, M.D. (2021a) Disentangling the effects of male age and mating history: contrasting effects of mating history on pre‐copulatory mating behaviour and paternity success. Evolution; Internation Journal of Organic Evolution, 75, 2867–2880. [DOI] [PubMed] [Google Scholar]
- Aich, U. , Head, M.L. , Fox, R.J. & Jennions, M.D. (2021b) Male age alone predicts paternity success under sperm competition when effects of age and past mating effort are experimentally separated. Proc. R. Soc. B, 288, 20210979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo, S.H. , Stiver, K.A. & Marsh‐Rollo, S.E. (2016) Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun, 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell, C.S. , Janacek, R. & Rundle, H.D. (2022) Maternal and paternal age effects on male antler flies: a field experiment. Am. Nat, 199, 436–442. [DOI] [PubMed] [Google Scholar]
- Badyaev, A.V. & Uller, T. (2009) Parental effects in ecology and evolution: mechanisms, processes and implications. Philos. Trans. R. Soc. B, 364, 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barks, P.M. & Laird, R.A. (2020) Parental age effects and the evolution of senescence. Am. Nat, 195, 886–898. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Machler, M. , Bolker, B. & Walker, S. (2015) Fitting linear mixed‐effects models using lme4. J. Stat. Softw, 67, 1–48. [Google Scholar]
- Benjamini, Y. & Hochberg, Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B, 57, 289–300. [Google Scholar]
- Benowitz, K.M. , Head, M.L. , Williams, C.A. , Moore, A.J. & Royle, N.J. (2013) Male age mediates reproductive investment and response to paternity assurance. Proc. R. Soc. B, 280, 20131124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza, A. & Marin, G. (1991) Male size and female mate choice in the eastern mosquitofish (Gambusia holbrooki: Poeciliidae). Copeia, 1991: 730–735. [Google Scholar]
- Bisazza, A. , Vaccari, G. & Pilastro, A. (2001) Female mate choice in a mating system dominated by male sexual coercion. Behav. Ecol, 12, 59–64. [Google Scholar]
- Bonduriansky, R. , Maklakov, A. , Zajitschek, F. & Brooks, R. (2008) Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol, 22, 443–453. [Google Scholar]
- Bonduriansky, R. & Day, T. (2013) Nongenetic inheritance and the evolution of costly female preference. J. Evol. Biol, 26, 76–87. [DOI] [PubMed] [Google Scholar]
- Bonduriansky, R. & Day, T. (2018) Extended heredity. Princeton, NJ: Princeton University Press. [Google Scholar]
- Booksmythe, I. , Backwell, P.R.Y. & Jennions, M.D. (2013) Competitor size, male mating success and mate choice in eastern mosquitofish, Gambusia holbrooki . Anim. Behav, 85, 371–375. [Google Scholar]
- Borziak, K. , Álvarez‐Fernández, A. , Karr, T.L. , Pizzari, T. & Dorus, S. (2016) The seminal fluid proteome of the polyandrous Red junglefowl offers insights into the molecular basis of fertility, reproductive ageing and domestication. Science Reports, 6, 35864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschetto, C. , Gasparini, C. & Pilastro, A. (2011) Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol, 65, 813–821. [Google Scholar]
- Brooks, R. & Kemp, D.J. (2001) Can older males deliver the good genes? Trends Ecol. Evol, 16, 308–313. [DOI] [PubMed] [Google Scholar]
- Brooks, M.E. , Kristensen, K. , van Benthem, K.J. , Magnusson, A. , Berg, C.W. , Nielsen, A. , Skaug, H.J. , Machler, M. & Bolker, B.M. (2017) glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R J, 9, 378–400. [Google Scholar]
- Cardozo, G. , Devigili, A. , Antonelli, P. & Pilastro, A. (2020) Female sperm storage mediates post‐copulatory costs and benefits of ejaculate anticipatory plasticity in the guppy. J. Evol. Biol, 33, 1294–1305. [DOI] [PubMed] [Google Scholar]
- Cattelan, S. & Gasparini, C. (2021) Male sperm storage impairs sperm quality in the zebrafish. Science Reports, 11, 16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattelan, S. , Evans, J.P. , Pilastro, A. & Gasparini, C. (2016) The effect of sperm production and mate availability on patterns of alternative mating tactics in the guppy. Anim. Behav, 112, 105–110. [Google Scholar]
- Cholewa, M. , Jankowiak, Ł. , Szenejko, M. , Dybus, A. , Śmietana, P. & Wysocki, D. (2021) The effects of parental age difference on the offspring sex and fitness of European blackbirds. PeerJ, 9, e10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, M. , H, J. , Jennions, M.D. & Fox, R.J. (2019) Novel ablation technique shows no sperm priming response by male eastern mosquitofish to cues of female availability. Behavioral Ecology and Sociobiology, 73, 167. [Google Scholar]
- Chung, M. , H, J. , Fox, R.J. & Jennions, M.D. (2021) Quantifying the costs of pre‐ and post‐copulatory traits for males: evidence that costs of ejaculation are minor relative to mating effort. Evol. Lett, 5, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, E.B. , Bonnet, T. , Osmond, H. , Cockburn, A. & Kruuk, L.E. (2020) Do the ages of parents or helpers affect offspring fitness in a cooperatively breeding bird?. J. Evol. Biol, 33, 1735–1748. [DOI] [PubMed] [Google Scholar]
- Cope, H. , Ivimey‐Cook, E.R. , &, Moorad, J. (2021) Biparental age effects in the burying beetle Nicrophorus vespilloides . bioRxiv.,. 10.1101/2021.09.08.459445 [DOI] [Google Scholar]
- Crean, A.J. & Bonduriansky, R. (2014) What is a paternal effect?. Trends Ecol. Evol, 29, 554–559. [DOI] [PubMed] [Google Scholar]
- Creighton, J.C. , Heflin, N.D. & Belk, M.C. (2009) Cost of reproduction, resource quality, and terminal investment in a burying beetle. Am. Nat, 174, 673‐684. [DOI] [PubMed] [Google Scholar]
- Curley, J.P. , Mashoodh, R. & Champagne, F.A. (2011) Epigenetics and the origins of paternal effects. Horm. Behav, 59, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depeux, C. , Lemaître, J.F. , Moreau, J. , Dechaume‐Moncharmont, F.X. , Laverre, T. , Pauhlac, H. , Gaillard, J.M. & Beltran‐Bech, S. (2020) Reproductive senescence and parental effects in an indeterminate grower. J. Evol. Biol, 33, 1256–1264. [DOI] [PubMed] [Google Scholar]
- du Fossé, N.A. , Van der Hoorn, M.L.P. , van Lith, J.M. , le Cessie, S. & Lashley, E.E. (2020) Advanced paternal age is associated with an increased risk of spontaneous miscarriage: a systematic review and meta‐analysis. Human Reproduction Update, 26, 650–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez, S. , Baguette, M. , Stevens, V.M. , Legrand, D. & Fréville, H. (2012) Complex interactions between paternal and maternal effects: parental experience and age at reproduction affect fecundity and offspring performance in a butterfly. Evolution; Internation Journal of Organic Evolution, 66, 3558–3569. [DOI] [PubMed] [Google Scholar]
- Evans, J.P. , Wilson, A.J. , Pilastro, A. & Garcia‐Gonzalez, F. (2019) Ejaculate‐mediated paternal effects: evidence, mechanisms and evolutionary implications. Reproduction (Cambridge, England), 157, R109‐R126. [DOI] [PubMed] [Google Scholar]
- Evans, J.P. , Zane, L. , Francescato, S. & Pilastro, A. (2003) Directional postcopulatory sexual selection revealed by artificial insemination. Nature, 421, 360‐363. [DOI] [PubMed] [Google Scholar]
- Fang, Y. , Wang, Y. , Peng, M. , Xu, J. , Fan, Z. , Liu, C. , Zhao, K. & Zhang, H. (2020) Effect of paternal age on offspring birth defects: a systematic review and meta‐analysis. Aging, 12, 25373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firman, R.C. , Gasparini, C. , Manier, M.K. & Pizzari, T. (2017) Postmating female control: 20 years of cryptic female choice. Trends in Ecology & Evolution, 32, 368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, J.M. & Lemaître, J.F. (2017) The Williams' legacy: a critical reappraisal of his nine predictions about the evolution of senescence. Evolution; Internation Journal of Organic Evolution, 71, 2768–2785. [DOI] [PubMed] [Google Scholar]
- García‐Palomares, S. , Navarro, S. , Pertusa, J.F. , Hermenegildo, C. , García‐Pérez, M.A. , Rausell, F. , Cano, A. & Tarín, J.J. (2009) Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring. Biology of Reproduction, 80, 343–349. [DOI] [PubMed] [Google Scholar]
- Gillespie, D.O.S. , Trotter, M.V. , Krishna‐Kumar, S. & Tuljapurkar, S.D. (2013) Birth‐order differences can drive natural selection on aging. Evolution; Internation Journal of Organic Evolution, 68, 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, W.D. (1966) The moulding of senescence by natural selection. J. Theor. Biol, 12, 12–45. [DOI] [PubMed] [Google Scholar]
- Harvanek, Z.M. , Lyu, Y. , Gendron, C.M. , Johnson, J.C. , Kondo, S. , Promislow, D.E. & Pletcher, S.D. (2017) Perceptive costs of reproduction drive ageing and physiology in male Drosophila . Nat. Ecol. Evol, 1, 0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head, M.L. , Vega‐Trejo, R. , Jacomb, F. & Jennions, M.D. (2015) Predictors of male insemination success in the mosquitofish (Gambusia holbrooki). Ecol. Evol, 5, 4999–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze, J. , Hanoeffner, M. , Delabie, J.H. & Schrempf, A. (2018) Methuselah's daughters: paternal age has little effect on offspring number and quality in Cardiocondyla ants. Ecol. Evol, 8, 12066–12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández, C.M. , van Daalen, S.F. , Caswell, H. , Neubert, M.G. & Gribble, K.E. (2020) A demographic and evolutionary analysis of maternal effect senescence. PNAS, 117, 16431‐16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias‐Carrasco, M. , Head, M.L. & Cabido, C. (2018) Effect of an immune challenge on the anti‐predator response of the green Iberian frog (Pelophylax perezi): the influence of urban habitats. Biol. J. Linn. Soc, 124, 447–455. [Google Scholar]
- Iglesias‐Carrasco, M. , Fox, R.J. , Vincent, A. , Head, M.L. & Jennions, M.D. (2019) No evidence that male sexual experience increases mating success in a coercive mating system. Animal Behaviour, 150, 201–208. [Google Scholar]
- Johnson, S.L. & Gemmell, N.J. (2012) Are old males still good males and can females tell the difference? Bioessays, 34, 609–619. [DOI] [PubMed] [Google Scholar]
- Johnson, S.L. , Zellhuber‐McMillan, S. , Gillum, J. , Dunleavy, J. , Evans, J.P. , Nakagawa, S. & Gemmell, N.J. (2018) Evidence that fertility trades off with early offspring fitness as males age. Proc. R. Soc. B, 285, 20172174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, A.T. , Mautz, B. & Jennions, M.D. (2010) Females prefer to associate with males with longer intromittent organs in mosquitofish. Biol. Lett, 6, 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, A.T. , Livingston, J.D. & Jennions, M.D. (2012) Do females preferentially associate with males given a better start in life? Biol. Lett, 8, 362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, A. , Kokko, H. & Jennions, M.D. (2013) Adaptive sex allocation in anticipation of changes in offspring mating opportunities. Nat. Commun, 4, 1603. [DOI] [PubMed] [Google Scholar]
- Kim, B. , Moran, N.P. , Reinhold, K. & Sánchez‐Tójar, A. (2021) Male size and reproductive performance in three species of livebearing fishes (Gambusia spp.): a systematic review and meta‐analysis. J. Anim. Ecol, 90, 2431‐2445. [DOI] [PubMed] [Google Scholar]
- Kong, A. , Frigge, M.L. , Masson, G. , Besenbacher, S. , Sulem, P. , Magnusson, G. , Gudjonsson, S.A. , Sigurdsson, A. , Jonasdottir, A. , Jonasdottir, A. , et al (2012) Rate of de novo mutations and the importance of father's age to disease risk. Nature, 488, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, M.S. , Santhosh, H.T. & Hegde, S.N. (2012) Offspring of older males are superior in Drosophila bipectinata . Zool. Stud, 51, 72–84. [Google Scholar]
- Lai, M. , Zhang, S.Y. , Zhang, Y.F. Liu, X.P. (2020) Male age affects female mating preference but not fitness in the monandrous moth Dendrolimus punctatus Walker (Lepidoptera: Lasiocampidae). Physiol. Entomol, 45, 22–29. [Google Scholar]
- Lee, J.H. , Seo, W. , Lee, S.H. , Lee, H.Y. & Min, K.J. (2019) Strain‐specific effects of parental age on offspring in Drosophila melanogaster . Entomol. Res, 49, 187–202. [Google Scholar]
- Lenth, R. & Lenth, M.R. (2018) Package ‘lsmeans’. Am. Stat, 34, 216–221. [Google Scholar]
- Liu, X.P. , Xu, J. , He, H.M. , Kuang, X.J. & Xue, F.S. (2011) Male age affects female mate preference and reproductive performance in the cabbage beetle, Colaphellus bowringi . J. Insect. Behav, 24, 83–93. [Google Scholar]
- Macartney, E.L. , Crean, A.J. & Bonduriansky, R. (2018) Epigenetic paternal effects as costly, condition‐dependent traits. Heredity, 121, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, J.N. , Vega‐Trejo, R. , Jennions, M.D. & Head, M.L. (2017) Why does inbreeding reduce male paternity? Effects on sexually selected traits. evolving, 71, 2728–2737. [DOI] [PubMed] [Google Scholar]
- Medawar, P.B. (1952) An unsolved problem of biology. London: H. K. Lewis. [Google Scholar]
- Monaghan, P. & Metcalfe, N.B. (2019) The deteriorating soma and the indispensable germline: gamete senescence and offspring fitness. Proc. R. Soc. B, 286, 20192187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan, P. , Maklakov, A.A. & Metcalfe, N.B. (2020) Intergenerational transfer of ageing: parental age and offspring lifespan. Trends Ecol. Evol, 35, 927–937. [DOI] [PubMed] [Google Scholar]
- Moorad, J.A. & Nussey, D.H. (2016) Evolution of maternal effect senescence. PNAS, 113, 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, A. , Ullah, M.S. , Sugawara, R. & Gotoh, T. (2021) Effects of male and female age on mating success in Tetranychus urticae Koch (Acari: Tetranychidae). System. Appl Acarol., 26, 1280–1292. [Google Scholar]
- Mousseau, T.A. & Fox, C.W. (1998) The adaptive significance of maternal effects. Trends Ecol. Evol, 13, 403–407. [DOI] [PubMed] [Google Scholar]
- Muff, S. , Nilsen, E.B. , O'Hara, R.B. & Nater, C.R. (2021) Rewriting results sections in the language of evidence. Trends Ecol. Evol, 37, 203–210. [DOI] [PubMed] [Google Scholar]
- Noguera, J.C. , Metcalfe, N.B. & Monaghan, P. (2018) Experimental demonstration that offspring fathered by old males have shorter telomeres and reduced lifespans. Proc. R. Soc. B, 285, 20180268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera, J.C. (2021) Heterogenous effects of father and mother age on offspring development. Behav. Ecol, 32, 349–358. [Google Scholar]
- O'Dea, R. , Jennions, M.D. & Head, M.L. (2014) Male size and condition affects sperm number and production rates in mosquitofish Gambusia holbrooki . J. Evol. Biol, 27, 2739–2744. [DOI] [PubMed] [Google Scholar]
- Okada, K. , Katsuki, M. , Kiyose, K. & Okada, Y. (2020) Older males are more competitive in male fights and more aggressive toward females in the broad‐horned flour beetle Gnatocerus cornutus . Behav. Ecol. Sociobiol, 74, 1–10. [Google Scholar]
- Pérez‐Staples, D. , Martínez‐Hernández, M.G. & Aluja, M. (2010) Male age and experience increases mating success but not female fitness in the mexican fruit fly. ethologist, 116, 778–786. [Google Scholar]
- Pizzari, T. , Dean, R. , Pacey, A. , Moore, H. & Bonsall, M.B. (2008) The evolutionary ecology of pre‐and post‐meiotic sperm senescence. Trends Ecol. Evol, 23, 131–140. [DOI] [PubMed] [Google Scholar]
- Poizat, G. , Rosecchi, E. & Crivelli, A.J. (1999) Empirical evidence of a trade–off between reproductive effort and expectation of future reproduction in female three‐spined sticklebacks. Proc. R. Soc. B, 266, 1543–1548. [Google Scholar]
- Preston, B. , Saint Jalme, M. , Hingrat, Y. , Lacroix, F. & Sorci, G. (2015) The sperm of aging male bustards retards their offspring's development. Nature Communication, 6, 6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest, N.K. , Mackowiak, B. & Promislow, D.E. (2002) The role of parental age effects on the evolution of aging. Evolution; Internation Journal of Organic Evolution, 56, 927–935. [DOI] [PubMed] [Google Scholar]
- Prokop, Z.M. , Stuglik, M. , Żabińska, I. & Radwan, J. (2007) Male age, mating probability, and progeny fitness in the bulb mite. Behav. Ecol, 18, 597–601. [Google Scholar]
- Pyke, G.H. (2005) A Review of the Biology of Gambusia affinis and G. holbrooki . Rev. Fish Biol. Fisheries, 15, 339–365. [Google Scholar]
- R Core Team (2019) R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing.
- Ramm, S.A. (2020) Seminal fluid and accessory male investment in sperm competition. Phil. Trans. R. Soc. B, 375, 20200068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando, O.J. (2012) Daddy issues: paternal effects on phenotype. Cell, 151, 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper, M. , Capdevila, P. & Salguero‐Gómez, R. (2021) Senescence: why and where selection gradients might not decline with age. Proc. R. Soc. B, 288, 20210851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐López, M.J. , Espeso, G. , Evenson, D.P. , Roldan, E.R. & Gomendio, M. (2010) Paternal levels of DNA damage in spermatozoa and maternal parity influence offspring mortality in an endangered ungulate. Proc. R. Soc. B, 277, 2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska, J. , Lagisz, M. , Bonduriansky, R. & Nakagawa, S. (2020) Mapping the past, present and future research landscape of paternal effects. Bmc Biology, 18, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius, G.A. & Nieschlag, E. (2009) Paternal age and reproduction. Human Reproduction Update, 16, 65–79. [DOI] [PubMed] [Google Scholar]
- Sepil, I. , Hopkins, B.R. , Dean, R. , Bath, E. , Friedman, S. , Swanson, B. , Ostridge, H.J. , Harper, L. , Buehner, N.A. , Wolfner, M.F. , et al (2020) Male reproductive aging arises via multifaceted mating‐dependent sperm and seminal proteome declines, but is postponable in Drosophila . Proc. Natl. Acad. Sci, 117, 17094–17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp, T. , Meitern, R. , Heidinger, B. , Noreikiene, K. , Rattiste, K. , Hõrak, P. , Saks, L. , Kittilson, J. , Urvik, J. & Giraudeau, M. (2021) Parental age does not influence offspring telomeres during early life in common gulls (Larus canus). Mol. Ecol, 1‐11. 10.1111/mec.15905 [DOI] [PubMed] [Google Scholar]
- Sharma, R. , Agarwal, A. , Rohra, V.K. , Assidi, M. , Abu‐Elmagd, M. & Turki, R.F. (2015) Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol, 13, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, L. & Lewis, S.E. (2011) Sperm DNA damage or progressive motility: which one is the better predictor of fertilization in vitro?. Syst. Biol. Reprod. Med, 57, 133–138. [DOI] [PubMed] [Google Scholar]
- Stearns, S.C. & Partridge, L. (2001) The genetics of aging in Drosophila . in Masoro E. J. and Austad S. (Eds). The handbook of aging, 5th edition. , London: Academic Press, Pp. 345–360. [Google Scholar]
- Stiver, K.A. & Alonzo, S.H. (2009) Parental and mating effort: is there necessarily a trade‐off? Ethology, 115, 1101–1126. [Google Scholar]
- Tasnin, M.S. , Kay, B.J. , Peek, T. , Merkel, K. & Clarke, A.R. (2021) Age‐related changes in the reproductive potential of the Queensland fruit fly. J. Insect Physiol, 131, 104245. [DOI] [PubMed] [Google Scholar]
- Travers, L.M. , Carlsson, H. , Lind, M.I. & Maklakov, A.A. (2021) Beneficial cumulative effects of old parental age on offspring fitness. bioRxiv.,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐Trejo, R. , Fox, R.J. , Iglesias‐Carrasco, M. , Head, M.L. & Jennions, M.D. (2019) The effects of male age, sperm age and mating history on ejaculate senescence. Funct. Ecol, 33, 1267–1279. [Google Scholar]
- Vega‐Trejo, R. , Head, M.L. & Jennions, M.D. (2016) Inbreeding depression does not increase after exposure to a stressful environment: a test using compensatory growth. BMC Evol. Biol, 16, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐Trejo, R. , Kruuk, L.E. , Jennions, M.D. , & Head, M.L. (2018) What happens to offspring when parents are inbred, old or had a poor start in life?: interactions between multiple causes of parental effects. J. Evol. Biol, 31, 1138–1151. [DOI] [PubMed] [Google Scholar]
- Vega‐Trejo, R. , O'Dea, R.E. , Jennions, M.D. & Head, M.L. (2014) The effects of familiarity and mating experience on mate choice in mosquitofish, Gambusia holbrooki . Behav. Ecol, 25, 1205–1211. [Google Scholar]
- Velando, A. , Noguera, J.C. , Drummond, H. & Torres, R. (2011) Senescent males carry premutagenic lesions in sperm. J. Evol. Biol, 24, 693–697. [DOI] [PubMed] [Google Scholar]
- Vinogradov, I.M. , Jennions, M.D. , Neeman, T. & Fox, R.J. (2021) Repeatability of lateralisation in mosquitofish Gambusia holbrooki despite evidence for turn alternation in detour tests. Anim. Cogn, 24, 765–775. [DOI] [PubMed] [Google Scholar]
- Vuarin, P. , Bouchard, A. , Lesobre, L. , Levêque, G. , Chalah, T. , Saint Jalme, M. , Frédéric, L. , Hingrat, Y. & Sorci, G. (2019) Post‐copulatory sexual selection allows females to alleviate the fitness costs incurred when mating with senescing males. Proc. R. Soc. B, 286, 20191675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuarin, P. , Lesobre, L. , Levêque, G. , Saint Jalme, M. , Lacroix, F. , Hingrat, Y. & Sorci, G. (2021) Paternal age negatively affects sperm production of the progeny. Ecol. Lett, 24, 719–727. [DOI] [PubMed] [Google Scholar]
- Wolf, J.B. , Brodie, E.D. & Moore, A.J. (1999) The role of maternal and paternal effects in the evolution of parental quality by sexual selection. J. Evol. Biol, 12, 1157–1167. [Google Scholar]
- Williams, G.C. (1966) Natural selection, the cost of reproduction and a refinement of Lack's priniciple. Am. Nat, 100, 687–690. [Google Scholar]
- Wylde, Z. , Spagopoulou, F. , Hooper, A.K. , Maklakov, A.A. & Bonduriansky, R. (2019) Parental breeding age effects on descendants’ longevity interact over 2 generations in matrilines and patrilines. PLoS. Biol, 17, e3000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, K. , Ryan, D.P. , Pearson, B.L. , Henzel, K.S. , Neff, F. , Vidal, R.O. , Hennion, M. , Lehmann, I. , Schleif, M. , Schröder, S. , et al (2018) Epigenetic alterations in longevity regulators, reduced life span, and exacerbated aging‐related pathology in old father offspring mice. Proc. Natl. Acad. Sci, 115, E2348‐E2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Data available in OSF (https://osf.io/v25xg/) and also in Dryad Digital Repository: https://doi.org/10.5061/dryad.63xsj3v4j


