Abstract
Purpose
While early treatment of posterior uveal melanoma can save the eye, the effect of early treatment on survival remains unknown. Therefore, we aimed to determine whether the tumour size at diagnosis has changed over time, and if this has affected survival rates of patients with posterior uveal melanoma in Denmark.
Methods
Nationwide retrospective cohort study linking data from registry‐based resources to data from clinical charts and pathology records. Including all Danish patients diagnosed with posterior uveal melanoma from 1943 to 2017. Incidence rates were estimated as annual percentage change (APC) overall and by American Joint Committee on Cancer (AJCC) tumour sizes. The age‐period‐cohort model was applied to estimate the relative risk of calendar period. The cox proportional hazards model, relative survival Kaplan–Meier curves and cumulative incidence curves were applied to estimate the effect of calendar period on survival.
Results
An overall increase in incidence rate of uveal melanoma was found (APC = 0.25%, 0.08–0.42; 95% CI). This was due to increasing incidence rate of AJCC T1 + T2 tumours (APC = 0.97%, 0.57–1.37; 95% CI), whereas no increase in incidence rates of AJCC T3 + T4 tumours was found (APC = −0.01%, −0.26 to 0.25; 95% CI). The disease‐specific survival improved with calendar period for all tumour sizes (HR = 0.988; 0.984–0.993; 95% CI).
Conclusion
Increasing incidence rate and improved survival rate for uveal melanoma was found concordantly with a decrease in tumour size during a 70‐year period.
Introduction
Posterior uveal melanoma accounts for 4% of all malignant melanomas (Chang, Karnell & Menck 1998). It is a rare, but deadly disease in which 45%of the patients die from metastatic dissemination after 15 years (Kujala, Mäkitie & Kivelä 2003). Except from one Swedish study, survival rates have been stable worldwide(Bergman et al. 2003; Singh, Turell & Topham 2011; Isager et al. 2006). While early treatment of uveal melanoma can save the eye, the effect of early treatment on survival remains unknown (Ah‐Fat & Damato 1998). Field et al. questions the effect of an aggressive treatment approach of the primary uveal melanoma, as genomic alterations arise in an early punctuated burst, causing metastatic spread to take place even before the diagnosis the disease (Field et al. 2018). However, genetical analyses of paired primary tumours and metastases from uveal melanoma have shown that the disease continuous to genetically evolve as it progresses (Shain et al. 2019). This suggests that there might be a window where early detection and treatment of uveal melanoma could improve survival.
In this national retrospective cohort study, we aimed to determine whether the incidence of uveal melanoma is increasing in the Danish population, if tumours are detected at an earlier stage and if so, whether survival has improved.
Methods
Study population
Danish patients diagnosed with choroidal and ciliary body melanoma from 1943 to 2017 were included in the study. Several resources were used to identify cases, as illustrated in Flowchart 1. The inclusion of patients has partly been described in a previous study (Bagger et al. 2018). Pathology reports were collected from The Eye Pathology Institute, Copenhagen University Hospital which served as the nationwide ocular pathology service from 1943 to 2015 and from the pathology departments of the treating hospitals. Filed pathology descriptions on choroidal and ciliary body melanomas from 1943 to 2017 were obtained and reviewed. Since the introduction of eye sparing treatment in 1987 using localized radiotherapy (brachytherapy), it has only been performed in two main ocular referral centres in Denmark (Rigshospitalet, Copenhagen University Hospital and Aarhus University Hospital). All clinical records on ciliary body and choroidal melanoma patients who underwent eye sparing treatment during the study period could therefore be accessed and included in the study. The National Danish Cancer Registry and the Registry of Causes of Death was linked to our cohort and used to identify additional cases (Helweg‐Larsen 2011; Gjerstorff 2011).
Unambiguous individual‐level data cross‐linkage is possible in Denmark using the unique 10‐digit personal identification number assigned to every Danish citizen since 1968. Through the personal identification number, date of birth, date of death and migration to and from Denmark is available. Vital status was extracted by the end of the data collection period on the 1st of November 2017 (Schmidt, Pedersen & Sørensen 2014).
Data on tumour characteristics were extracted from pathology reports and clinical records. When tumour size was not available, old pathology slides were obtained and tumours were measured two‐dimensionally on the slide using the Hamamatsu NDP.view v2.6.13 software. Tumour size and tumour stage were assigned in each case in accordance with the American Joint Committee on Cancer (AJCC) staging system, 8th edition (Kivelä T , Simpson ER & Grossniklaus HE 2017).
We complied to the guidelines suggested by the Collaborative Ocular Melanoma Study on cause‐specific mortality coding in uveal melanoma (Collaborative Ocular Melanoma Study Group. 2001). Cause of death was extracted from the Danish Pathology Registry, clinical journals and the Registry of Causes of Death (Helweg‐Larsen 2011). Melanoma‐specific death was registered if patients had either histopathological confirmed liver metastasis, clinical records showing metastatic liver lesions on ultrasonography, magnetic resonance imaging or pet‐computed tomography or if uveal melanoma was registered as the cause of death. If death due to ‘skin cancer’, ‘hepatic cancer’ and ‘non specified cancer’ was registered, data were validated with information from the Cancer Registry. If the diagnosis of secondary primary malignancy could not be validated, it was considered a misclassification and melanoma‐specific death was registered.
The study was conducted in accordance with the tenets of the Declaration of Helsinki. The Regional Research Ethical Committee in Copenhagen waived the need for approval of this retrospective study (Ref: H‐4‐2014‐FSP). Collection and linkage of data was approved by the Danish Data Protection Agency (Ref: 2016‐41‐4897) and the Danish Health Authority (Ref: 3‐3013‐727). The Manuscript was prepared in accordance to the STROBE statements for cohort studies.
Statistical methods
Information on patient characteristics, demographics and treatment place (centralized/regional) were included for all patients. Descriptive statistics were given by categorized calendar periods (1943–1960, 1960–1980, 1980–2000, 2001–2017) with counts and proportions for categorical variables and mean and standard deviations for continuous variables. Bivariate tests by calendar periods were conducted by chi‐square tests for counts and ANOVA for continuous variables, where appropriate. We obtained person time at risk from Statistics Denmark for all combinations of age, sex and calendar year, which allowed for estimation of incidence rates. This was achieved by applying the Age‐Period‐Cohort framework (Carstensen 2007). In the Age‐Period‐Cohort model, we assumed that the counts of new incident cases followed a Poisson distribution to obtain the individual effects of age, period and cohort. To obtain incidence rates, we included the logarithm of person years at risk as an offset in the regression. This allowed for estimation of the incidence rate of uveal melanoma by attained age, as well as relative risks of calendar period (year of diagnosis in 5‐year intervals), and birth cohort (year of birth). Age and calendar period were included as restricted cubic splines and sex as a categorical variable. For the period, we constrained one estimate to zero (1980) which then provided a baseline for the period effect. The baseline reference for the birth cohort was set to 1940. From the Age‐Period‐Cohort model, the annual percentage change of the age standardized incidence was estimated by the drift, which refers to the net effect of age, period and birth cohort. The drift is the ‘overall slope’ where the incidence either increases or decreases linearly over time.
All estimates were provided with 95% confidence intervals. We investigated whether incidence over time was related to patient characteristics and demographics by testing interaction terms with calendar time and applying likelihood ratio tests. Here, a 5% significance level was applied.
Relative survival among patients diagnosed with uveal melanoma in the following calendar periods (1943–1960, 1961–1980, 1981–2000, 2001–2017) was estimated by the Kaplan–Meier approach with the event of death (all cause). Time since diagnosis was used as time scale and patients who had either emigrated or survived through follow‐up (11/1 2017) were censored. The survival curves were stratified by AJCC tumour size. To account for changes in survival of the background population over time, we calculated the expected survival in the Danish population conditioned on identical distribution of age, sex and birth year. Thus, the excess mortality in the uveal melanoma compared with the mortality in the background population could be interpreted as the disease specific mortality.
To study the effect of patient characteristics and demographics on survival, we conducted a within‐cohort analysis including only the patients with uveal melanoma. Due to limited availability of survival data and death diagnoses before 1968, survival analysis was performed from 1968 to 2017 as illustrated in Fig. 1. We applied the Cox proportional hazards model and included the previously mentioned patient characteristics and demographics. Age and calendar period were included as restricted cubic splines and sex, tumour size, stage and treatment place were included as categorical variables. Effect estimates were reported as hazard ratios with 95% confidence intervals. The proportional hazards assumption was tested based on weighted residuals (Gramsch & Therneau 1994). All analysis was conducted using the statistical software R (R Core Team 2013) and packages survival (Terry, Therneau & Grambsch 2000), rms (Harrel 2015), and ggplot2 (Wickham 2016). The cox proportional hazards model does not take the issue of competing risks into account. Therefore, we conducted a sensitivity analysis using cumulative incidence rates of disease specific survival stratified by year of diagnosis (1968–1970, 1971–1980, 1981–1990, 1991–2000, 2001–2010, 2011–2017).
Fig. 1.
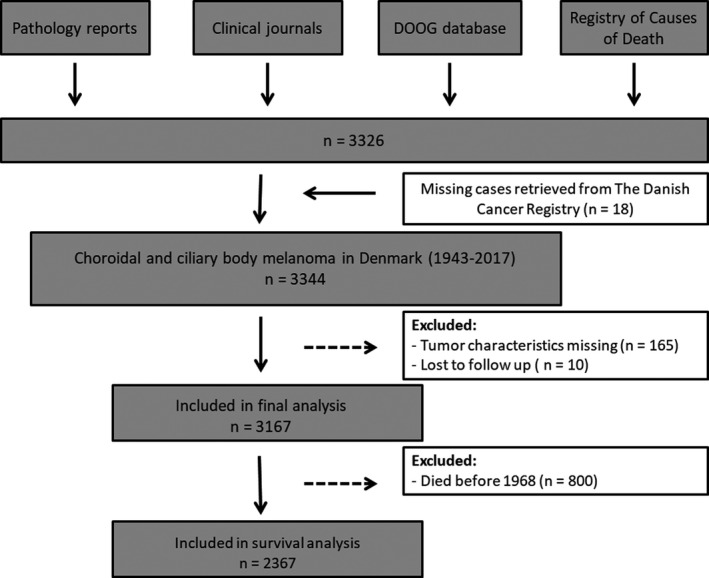
Flowchart illustrating the inclusion of patients to the study. DOOG = Danish Ophthalmologic Oncology Group.
Results
A total number of 3344 patients were diagnosed with choroidal‐ and ciliary body melanoma from 1943 to 2017 in Denmark. Baseline characteristics of the study population by four calendar periods are presented in Table 1. Patient age at diagnosis was significantly older in the later calendar periods (p < 0.001) while sex was almost equally distributed (p = 0.594). An increasing fraction of small‐ (T1 and T2) and low stage (I and II) tumours was found in the later calendar periods (p < 0.001). Similarly, a decreasing fraction of large tumours (T3 and T4) and advanced stage tumours (III and IV) was found in the later calendar periods (p < 0.001). Information on tumour size was missing in 165 patients, accounting for 4.9% of cases. The proportion of patients treated in a specialized ocular oncology unit was significantly increased in the most recent calendar period of 2000–2017 compared with the earlier calendar periods (p < 0.001). At the end of follow up by the 1st of November 2017, 2482 patients had died from any cause (74.2%). Median follow‐up was 5.3 years (Range: 0.01–70 years, IQR: 2.4–12.6). From 1968 to 2017, 10 patients (0.4%) emigrated and were lost to follow up. A total of 1739 patients (52%) were followed for at least five years and 1074 patients (32%) were followed for more than 10 years.
Table 1.
Baseline characteristics.
| Variable | Level | 1943–1960 | 1961–1980 | 1981–2000 | 2001–2017 | p‐value |
|---|---|---|---|---|---|---|
| n (patients) | 537 | 810 | 916 | 1081 | ||
| Age (mean, SD) | 58 (14) | 61 (14) | 63 (14) | 64 (14) | <0.001 | |
| Gender (%) | Female | 245 (46) | 387 (48) | 436 (48) | 532 (49) | 0.594 |
| Male | 292 (54) | 423 (52) | 480 (52) | 549 (51) | ||
| AJCC stage (%) | I | 88 (16) | 110 (14) | 102 (11) | 265 (25) | <0.001 |
| II | 258 (48) | 382 (47) | 484 (53) | 559 (52) | ||
| III | 135 (25) | 240 (29) | 312 (34) | 217 (20) | ||
| IV | 5 (1) | 6 (1) | 9 (1) | 13 (1) | ||
| Unknown | 51 (10) | 72 (9) | 9 (1) | 27 (3) | ||
| AJCC T (%) | T1 | 106 (20) | 130 (16) | 113 (12) | 299 (28) | <0.001 |
| T2 | 164 (31) | 213 (26) | 295 (32) | 373 (35) | ||
| T3 | 138 (26) | 258 (32) | 307 (34) | 262 (24) | ||
| T4 | 76 (14) | 133 (16) | 192 (21) | 120 (11) | ||
| Unknown | 53 (10) | 76 (9) | 9 (1) | 27 (3) | ||
| Height(median [IQR]) | T1 | 3.8[2.5, 5.0] | 3.0 [2.0, 5.0] | 3.0 [2.5, 4.0] | 2.6 [2.0, 3.0] | <0.001 |
| T2 | 6.0 [4.0, 7.0] | 6.0 [4.0, 7.0] | 5.0 [4.0, 7.0] | 4.8 [3.8, 6.0] | <0.001 | |
| T3 | 9.0 [8.0, 11.0] | 10.0 [8.0, 11.0] | 10.0 [8.0, 11.0] | 8.0 [7.0, 10.0] | <0.001 | |
| T4 | 10.0 [6.0, 19.4] | 12.0 [8.0, 15.0] | 12.0 [8.6, 14.0] | 10.0 [8.0, 12.7] | 0.138 | |
| Treatment place (%) | Central | 134 (25) | 340 (42) | 475 (52) | 1024 (95) | <0.001 |
| Other | 403 (75) | 470 (58) | 441 (48) | 57 (5) | ||
| Death from any cause (%) | Yes | 515 (96) | 757 (94) | 780 (85) | 430 (40) | <0.001 |
| No | 22 (4) | 53 (7) | 136 (15) | 651 (60) |
Categorical variables are presented as counts and percentages. Continuous variables are presented as means and standard deviations. Pearson’s chi‐squared test and ANOVA test were used to compare categorical and continuous data between calendar periods, respectively.
AJCC = American Joint Committee on Cancer, SD = standard deviation, T = AJCC tumour size.
Trends in incidence rates
Crude incidence rates with annual percentage change in Danish patients with ciliary body and choroidal melanoma from 1943 to 2017 are depicted in Fig. 2. During the study period, there were 361.04 million person years at risk. An increasing trend in crude incidence rates was found with an annual percentage change of 0.3% (0.12–0.48; 95% CI), and a maximum crude incidence rate of 13 cases per million years at risk from 2014 to 2017.
Fig. 2.
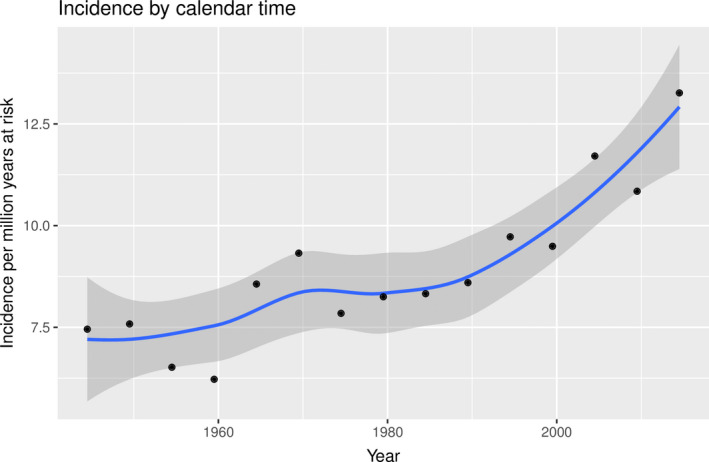
Crude incidence rates and annual percentage change in incidence rates in Danish patients with choroidal and ciliary body melanoma from 1943 through 2017. Black dots denote crude incidence rates by 5‐year periods. Blue line denotes annual percentage change. 95% confidence intervals are presented by the grey area.
Crude incidence rates with annual percentage change are presented for all AJCC stages and – sizes in Fig. 3. Increasing crude incidence rates in AJCC T1 + T2 tumours were observed with an average annual increase of 0.70% (0.45–0.98; 95% CI), whereas no significant trend in incidence rates in T3 + T4 tumours was found with an annual change of −0.12% (−0.37 to 0.14; 95% CI) (Fig. 3B). Trends also applied for AJCC tumour stages with a significant annual increase of 0.56% (0.34–0.79; 95% CI) in tumours classified as stages I and II and no significant trend in tumours classified as stage III and IV with an annual change of −0.31% (−0.61–0.01; 95% CI) (Fig. 3A). In T1 tumours, an annual increase of 0.88% (0.45–1.3; 95% CI) was found (Fig. 3D) and in AJCC stage I tumours an annual increase of 1% (0.58–1.54; 95% CI) was found (Fig. 3C). Likewise, an annual increase of 0.60% (0.28–0.94; 95% CI) was found in T2 tumours (Fig. 3D) and 0.41% (0.16–0.67; 95% CI) was found in stage II tumours (Fig. 3C).
Fig. 3.
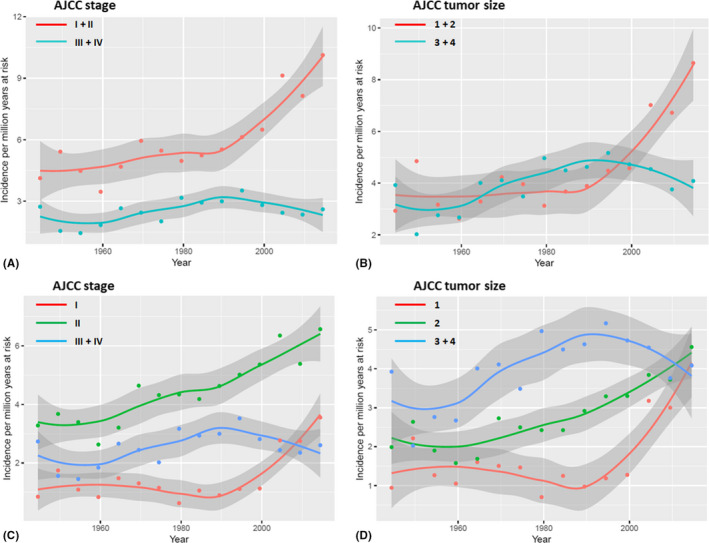
Crude incidence rates and annual percentage change in Danish patients with uveal melanoma from 1943 through 2017 subdivided into AJCC tumour stages and – sizes. Dots denote crude rates by 5 year intervals and lines denote annual percentage change. Grey areas represent 95% confidence intervals. (A) Red line denotes incidence rates in low stage tumours (AJCC stage I and II) and turquoise line denotes advanced stage tumours (AJCC stage III and IV). (B) Red line denotes incidence rates in small tumours (AJCC T1 and T2) and turquoise line denotes large tumours (AJCC T 3 and T4). (C) Blue line denotes incidence rates in advanced stage tumours (AJCC stage III and IV). Low stage tumours are subdivided into AJCC stage I (red line) and AJCC stage II (green line). (D) Blue line denotes incidence rates in large tumours (AJCC T3 and T4). Small tumours are subdivided into AJCC T1 (red line) and AJCC T2 (green line). AJCC = American Joint Committee on Cancer, T = AJCC tumour size.
Age‐period‐cohort models revealed a significant effect of calendar period on small (T1 + T2) ‐ and low stage (I + II) tumours and overall incidence rates when adjusted for age and birth cohort. No significant effect of calendar period was found in large (T3 + T4) and advanced stage (III + IV) tumours. APC models for small and large AJCC tumour sizes is shown in Fig. 4. Estimates of drift with 95% confidence intervals are presented in Table 2. Tumour height decreased with increasing calendar periods in T1, T2 and T3 tumours (p < 0.001), but not in T4 tumours (p = 0.8) (Table 1).
Fig. 4.
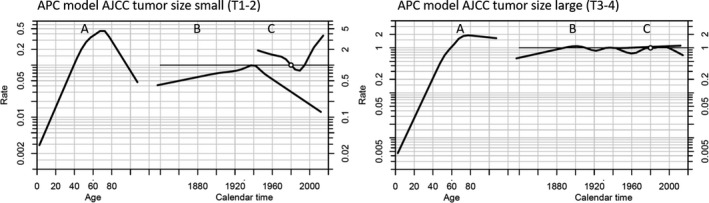
Age Period Cohort (APC) models for small (T1‐2) and large (T3‐4) AJCC tumour sizes. Reference cohort is 1940 and reference for the calendar period is 1980. The model shows the individual effects of age, period and cohort by three lines A, B and C, respectively on the incidence of posterior uveal melanoma. A, The incidence according to age increased until the age of approximately 70‐years in both models. B, There was no significant effect of birth cohort on the incidence in both models. C, The incidence according to calendar period increased significantly after 1990 for small sized tumours but remained stable for large sized tumours throughout the period. The estimations of drift (the slope) are shown in Table 2. AJCC = American Joint Committee on Cancer.
Table 2.
Estimates of drift in calendar period by AJCC tumour stages and sizes presented with 95% confidence intervals
| Estimate of drift | (95% CI) | |
|---|---|---|
| All tumours | 1.0025* | (1.000757; 1.004161) |
| AJCC T1 and T2 | 1.0097* | (1.005662; 1.013681) |
| AJCC T3 and T4 | 0.9999 | (0.9974666; 1.002532) |
| AJCC stage I and II | 1.0112* | (1.006866; 1.015521) |
| AJCC stage III and IV | 0.9980 | (0.9947825; 1.001158) |
Age‐period‐cohort models were used to calculate estimates of drift and APC models according to AJCC tumour size are sown in Fig. 4.
AJCC = American Joint Committee on Cancer, CI = confidence interval, T = AJCC tumour size.
Statistical significance.
Trends in disease specific mortality
In cox proportional hazards regression, a statistically significant relationship between calendar period and disease specific mortality was found (HR = 0.988, (0.984–0.993; 95% CI), p < 0.001) from 1968 to 2017. Other statistically significant prognostic factors were age, sex and tumour size, as presented in Table 3. Logarithmic relative hazard plots are presented in the appendix (Fig. S1) and confirm that the assumption of proportional hazards was met. An improvement of disease specific mortality with calendar period was found in both patients with small (T1 + T2) and large (T3 + T4) tumours, although the diminished hazards were more pronounced in patients with small tumours, as depicted in Fig. 5. Kaplan–Meier plots on relative survival categorized by calendar periods are depicted in Fig. 6. The Kaplan–meier plots are based on overall survival and thus represents death from any cause among the patients with uveal melaoma and the background population respectively. The difference between the blue line (background population) and the coloured lines (AJCC tumour size T1‐4) represents the excess mortality (relative survival) among the study population and isused as an estimate of the cause specific mortality. The overall survival among patients with uveal melanoma improved relative to the expected survival in the background population with calendar period. In patients with T1 tumours, the survival was comparable to the background population in the later calendar periods (1980–2000 and 2000–2017).
Table 3.
Effect estimates of prognostic factors on disease specific mortality reported as hazard ratios with 95% confidence intervals.
| Hazard ratio | (95% CI) | |
|---|---|---|
| Age | 1.025* | (1.020; 1.030) |
| Diagnosis year | 0.988* | (0.984; 0.993) |
| Male sex | 1.119 | (0.989; 1.266) |
| AJCC T2 | 2.100* | (1.650; 2.671) |
| AJCC T3 | 3.520* | (2.782; 4.454) |
| AJCC T4 | 5.384* | (4.187; 6.923) |
Hazard ratios were calculated by cox proportional hazards regression analysis. References were female sex and AJCC T1 tumours.
AJCC = American Joint Committee on Cancer, CI = confidence interval, HR = hazard ratio, T = AJCC tumour size.
Statistical significance.
Fig. 5.
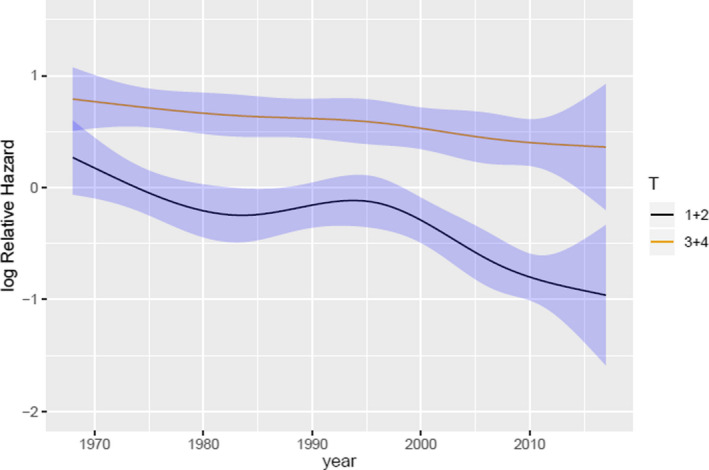
Logarithmic relative hazard in small and large tumours from 1968 through 2017 in relation to calendar period. Black line depicts small tumours (AJCC T1 and T2) and yellow line depicts large tumours (AJCC T3 and T4). 95% confidence intervals are presented by blue areas. The figure graphically presents the log relative hazard (RH) of small and large tumours, respectively, relative to calendar year when all other covariates are fixed (age = 60 years, sex = male). In 1980 (reference year) the RH of small tumours was 1 (logRH = 0) and in 2007 the RH had decreased to 0.6 (logRH = (−0.5)). Thus, the model demonstrates a 40% decreased risk of disease specific mortality in posterior uveal melanomas diagnosed in 2007 compared to 1980. T = AJCC tumour size.
Fig. 6.
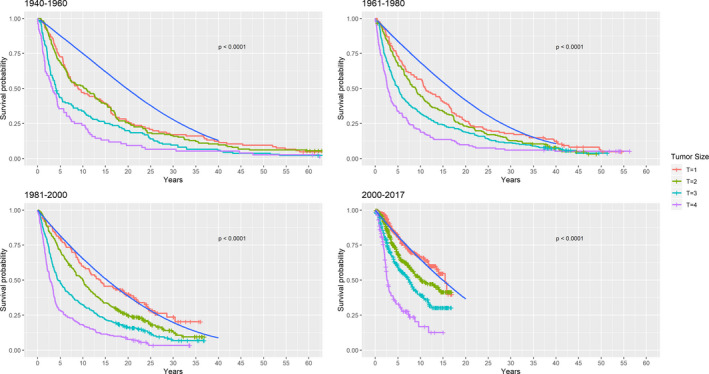
Relative survival categorized by calendar periods. The coloured lines represent all‐cause mortality of the study population according to AJCC tumour sizes. The blue solid line depicts survival probability in the Danish background population conditioned on identical distribution of age, sex and birth year. The distance between the coloured lines and the blue line represents excess mortality which can be interpreted as the disease specific mortality. T = AJCC tumour size.
Discussion
This national retrospective cohort study on patients with ciliary body and choroidal melanoma during a 70‐year period, showed increasing incidence of small tumours (AJCC T1‐2) while the incidence of large tumours (AJCC T3‐4) remained stable. Simultaneously, decreasing disease‐specific mortality and improved relative survival rates from 1943 to 2017 was found.
Interestingly, the majority of previous studies have reported unchanged survival rates in patients with uveal melanoma through the last three decades (Virgili et al. 2008; Singh, Turell & Topham 2011). The finding of improved survival rates in the present study is in accordance with Bergman et al. (2003), who reported improved survival rates from 1960 to 1998 in Sweden. The authors speculated if improved survival could be explained by early recognition of small melanomas due to increased awareness with diabetic retinopathy screening and expanding cataract surgery causing earlier treatment of tumours. However, no data on tumour characteristics were available in the Swedish study. Our study found a significant decrease in tumour height in AJCC T1‐T3 tumours over time supporting treatment of smaller tumours. The effect of early detection on survival has been previously emphasized by Shields et al and Hussain et al (Shields et al. 2004; Hussain et al. 2020), and the relationship between low AJCC stage and a favourable prognosis has been thoroughly validated (Kujala et al. 2003). Furthermore, it has been shown that small posterior uveal melanomas are less likely to harbour genetic alterations, than larger ones (Bagger et al. 2014; Shields et al. 2018) It could be questioned whether increased awareness of the disease has caused the detection of more nevi to be misdiagnosed as small melanomas, thereby biasing the survival rate. The challenge of differentiating melanocytic nevi from small uveal melanomas have been previously emphasized (Shields et al. 2004) and with treatment shifting towards eye‐sparing techniques, the problem remains relevant (Singh, Turell & Topham 2011). Although it is possible that tumours below 2 mm in height are in fact nevi being misdiagnosed as uveal melanomas, the simultaneous increase in incidence of T2 tumours is highly unlikely to have been caused by misdiagnosed nevi. Furthermore, according to current clinical guidelines, melanocytic lesions with a height below 2 mm were only included in the study if tumour growth was documented (Shields et al. 1995). Biopsies have also been performed regularly in Danish patients with uveal melanoma since 1985 and histopathological diagnostic criteria remains unchanged (Bagger et al. 2018). In addition, the metastatic potential of small melanomas is relevant and 3% of melanomas, with a height below 3 mm and documented tumour growth, metastasized during a 4 year period (Shields et al. 1995). The AJCC staging system (8th edition) relies mainly on tumour size, but there are currently no strict guidelines regarding which measurement modalities should be used in order to obtain accurate and reproducible data. In the present study, tumour measurements were available from ULB scan when the patients underwent brachytheraphy and from histology measurements after formalin fixation when the patients were enucleated. It is well known that tumour tissue can undergo shrinkage in the process of formalin fixation and paraffin embedding, but the reported rates of tissue shrinkage varies greatly and ranges from 4% up to 20% (Park et al. 2017; Kawai & Morikawa 2018). One study on lung cancer reported not only tissue shrinkage but tissue enlargement was observed in 4.8% after formalin fixation (Park et al. 2017). Thus, reporting tumour size from different measurements poses a potential bias where histology measurements could be underestimated, but as there is no consensus of an exact shrinkage factor it was not possible to modify the data to compensate for potential tissue shrinkage. Interestingly, Park et al. only reported a shift in AJCC tumour stage in 3.7% despite an observed tissue shrinkage in 48.8%. Park et al. (2017) reported on lung cancer but there is no reason to think this observation should be different for uveal melanoma. Our data relies solely on histology measurements until 1985 where brachytherapy was introduced, thus an adjustment for a shrinkage factor would increase tumour size in the early decades and thereby increase the significance of our results making the conclusion less reliable.
The metastatic potential is primarily caused by specific genetic alterations (Ewens et al. 2013; Dogrusöz et al. 2017), and it would therefore have been ideal to include data on tumour genetics in the analyses. It was, however, beyond the scope of this study.
The only significant changes in the management of patients with ciliary body and choroidal melanoma in Denmark have been the introduction of brachytherapy to university hospitals in 1987 and the gradually centralization of workup and treatment to highly specialized departments. The COMS large randomized clinical trial failed to show difference in survival between eye conserving brachytherapy and enucleation for medium sized uveal melanomas (The Collaborative Ocular Melanoma Study Group 2001). It is therefore less likely that the improved survival, observed in this study, can be explained by introduction of brachytherapy.
The majority of epidemiologic studies in posterior uveal melanoma have reported stable incidence rates (Teikari & Raivo 1985; Isager et al. 2005; Singh, Turell & Topham 2011; Jensen 1982). After adjusting for population ageing in Denmark, a small annual increase of 0.24% in incidence rates during the observation period was found. Only Aronow & Topham (2018) have evaluated incidence rates after the millennium, and they likewise found an annual percentage increase of 0.5% among the white population in America from 1973 to 2017. Environmental factors which could explain this increase are not known for uveal melanomas. Ultraviolet radiation might increase the risk of iris melanoma, but this study only included choroidal and ciliary body melanomas, which arise behind the UV‐light absorbing lens (Singh et al. 2004). In addition, genetic analysis of choroidal and ciliary melanoma reveals the lack of UV damage signatures (Karlsson et al. 2020).
This nationwide study present compelling arguments of earlier detection of uveal melanoma and improved survival. This introduces the risk of lead time bias which is survival time added to a patient’s survival because of earlier diagnosis irrespective of a potential postponed time of death. Lead time bias is a recurrent and well‐known issue in retrospective cohort studies but is especially relevant if screening of a particular cancer is introduced (Andersson, Rutherford & Humphreys 2017). It is, however, unlikely that the marked improved survival relative to the background population, found in this study, could be explained solely by lead time bias as the survival improved in all AJCC tumour categories (T1‐T3) except T4. The Cox regression model allowed for the adjustment of relevant covariates and directly quantified the isolated effect size (hazard ratio) of year of diagnosis on the risk of disease specific death. Thus, in the present study, the risk of metastatic death significantly decreased 1% for each calendar year of diagnosis (Table 3). The cox regression model does not differentiate between competing events which are all treated as censored observations. This can cause an overestimation of disease specific mortality especially among patients with a high proportion of death due to other causes (Kujala, Mäkitie & Kivelä 2003). Other available risk regression models like the Fine and Gray account for competing events by maintaining subjects in the ‘at risk group’ even though they experience a competing event (e.g. died from other causes). Due to this fact, the regression coefficient from this model cannot be directly interpreted as a hazard ratio. These models are relevant in prognostic studies as they provide estimated probabilities of an event given the characteristics of the individual patient. However, for etiological studies, evaluating a causal relationship of risk factors and outcome, the cox regression model is more appropriate as it directly quantifies the hazard ratios among the individuals who are at risk of developing the event (Noordzij et al. 2013). To mitigate the issue of competing risks, especially as the mean age increased in the later patient cohorts, we used relative survival rates as a measurement of melanoma‐specific mortality in different time periods (Fig. 6). This avoids the issue of competing risks and the relative risk analysis is independent of cause of death and thereby diminish the questionable validity of death certificates.
Relative survival is a useful method to evaluate the impact of changes in cancer management on the survival of cancer patients and allows for a direct comparison to the expected survival in a similar cohort matched on age, sex and birthyear. Thereby, it also accounts for the issue of varying survival rates and compositions in the background population over time. As a sensitivity analyses, we computed cumulative incidence functions with death from other causes as competing risk (Fig. 7), which confirmed the results. Thus, improved survival since 1980 was demonstrated in this study by tree independent and thoroughly validated statistical methods.
Fig. 7.
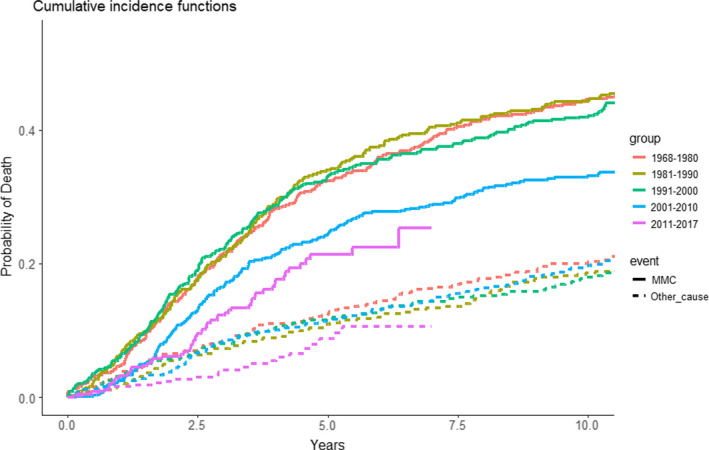
Cumulative incidence functions of disease specific mortality with death from other causes as a competing event, Fine and Grey model. Curves are shown as disease specific survival by year of diagnosis in the following periods: 1968–1980, 1981–1990, 1991–2000, 2001–2010 and 2011–2017. The solid lines represents death due to MMC and the dotted lines represents death due to other causes.
Estimation of incidence was obtained by performing a national collection of all cases of uveal melanoma within a 70‐year period. While Denmark offers excellent registries for epidemiological studies, the registries have limitations. Therefore, data were collected using several registries and clinical databases to ensure optimal detection of cases, in addition cases were internally validated between the different registries to ensure the validity of the data (Fig. 1). The composition of a population differs over time, thus traditionally standardized cancer incidence has been reported using an international standard population. However, in addition to age and sex, the incidence of cancer can differ between populations due to lifestyle, environmental, genetic or other unpredictable factors. Thus, the evaluation of changes in cancer incidence should optimally be assessed separately in different populations (Chia et al. 2005). The age‐period‐cohort analysis can be used to separate the different effects of chronological age, calender period (year of diagnosis), and birth cohort (year of birth).
In conclusion, this study showed improved disease specific survival in patients diagnosed with both small‐ and medium sized posterior uveal melanomas in Denmark from 1968 to 2017. This finding can be related to a simultaneous decrease in tumour height, indicating that tumours are detected at an earlier stage.
Supporting information
Fig S1 Logarithmic relative hazard on prognostic factors on relative survival: age, sex, AJCC tumour size and year of diagnosis.
We thank Hajer Ahmad Al‐Abaiji for helping with the data collection. This work was supported by grants from Fight for Sight Denmark; The Synoptic Foundation; and the Danish Cancer Society. The funding sources had no influence in the design and conduct of the study.
References
- Ah‐Fat F & Damato B (1998): Delays in the diagnosis of uveal melanoma and effect on treatment. Eye 12: 781–782. [DOI] [PubMed] [Google Scholar]
- Andersson TML, Rutherford MJ & Humphreys K (2017): Assessment of lead‐time bias in estimates of relative survival for breast cancer. Cancer Epidemiol 46: 50–56. [DOI] [PubMed] [Google Scholar]
- Aronow ME & Topham AKSA (2018): Uveal melanoma: 5‐year update on incidence, treatment, and survival (SEER 1973–2013). Ocul Oncol Pathol 4: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger M, Andersen MT, Andersen KK, Heegaard S, Andersen MK & Kiilgaard JF (2014): The prognostic effect of American Joint Committee on Cancer staging and genetic status in patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci 56: 438–444. [DOI] [PubMed] [Google Scholar]
- Bagger M, Smidt‐Nielsen I, Andersen MK, Jensen PK, Heegaard S, Andersen KK, Friis S & Kiilgaard JF (2018): Long‐term metastatic risk after biopsy of posterior uveal melanoma. Ophthalmology 1–8. [DOI] [PubMed] [Google Scholar]
- Bergman L, Seregard S, Nilsson B, Lundell G, Ringborg U & Ragnarsson‐Olding B (2003): Uveal melanoma survival in Sweden from 1960 to 1998. Invest Opthalmol Vis Sci 44: 3282. [DOI] [PubMed] [Google Scholar]
- Carstensen B (2007): Age‐period‐cohort models for the Lewis diagram. Stat Med 26: 3018–3045. [DOI] [PubMed] [Google Scholar]
- Chang A, Karnell L & Menck H (1998): The National Cancer Data Base Report on Cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeopns Commision on Cancer and the American Cancer Society. Cancer 8: 1664–1678. [DOI] [PubMed] [Google Scholar]
- Chia K‐S, Reilly M, Tan C‐S, Lee J, Pawitan Y, Adami H‐O, Hall P & Mow B (2005): Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: A comparative population‐based study in Singapore and Sweden. Int J Cancer 113: 302–306. [DOI] [PubMed] [Google Scholar]
- Collaborative Ocular Melanoma Study Group . (2001): Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol (Chicago, Ill 1960) 119: 670–676. [DOI] [PubMed] [Google Scholar]
- Dogrusöz M, Bagger M, van Duinen SG et al. (2017): The prognostic value of AJCC staging in uveal melanoma is enhanced by adding chromosome 3 and 8q status. Invest Ophthalmol Vis Sci 58: 833–842. [DOI] [PubMed] [Google Scholar]
- Ewens KG, Kanetsky PA, Richards‐Yutz J, Al‐Dahmash S, De Luca MC, Bianciotto CG, Shields CL & Ganguly A (2013): Genomic profile of 320 uveal melanoma cases: chromosome 8p‐loss and metastatic outcome. Invest Ophthalmol Vis Sci 54: 5721–5729. [DOI] [PubMed] [Google Scholar]
- Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, Kurtenbach S & Harbour JW (2018): Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun 9: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstorff ML (2011): The Danish cancer registry. Scand J Public Health 39: 42–45. [DOI] [PubMed] [Google Scholar]
- Gramsch P & Therneau T (1994): Proportional hazards test and diagnostics based on weighted residuals. Biometrika 81: 515–526. [Google Scholar]
- Harrel F (2015): Regression Modelling Strategies. Springer. [Google Scholar]
- Helweg‐Larsen K (2011): The Danish register of causes of death. Scand J Public Health 39: 26–29. [DOI] [PubMed] [Google Scholar]
- Hussain R, Czanner G, Taktak A, Damato B, Praidou A & Heimann H (2020): Mortality of patients with uveal melanoma detected by diabetic retinopathy screening. Retina 40: 2198–2206. [DOI] [PubMed] [Google Scholar]
- Isager P, Engholm G, Overgaard J & Storm H (2006): Uveal and conjunctival malignant melanoma in denmark 1943–97: observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol 13: 85–96. [DOI] [PubMed] [Google Scholar]
- Isager P, Østerlind A, Engholm G, Heegaard S, Lindegaard J, Overgaard J & Storm HH (2005): Uveal and conjunctival malignant melanoma in Denmark, 1943–97: incidence and validation study. Ophthalmic Epidemiol 12: 223–232. [DOI] [PubMed] [Google Scholar]
- Jensen O (1982): Malignant melanomas of the human uvea: 25 year follow up of cases in Denmark, 1943–1952. Acta Ophthalmol 60: 161–182. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Nilsson LM, Mitra S et al. (2020): Molecular profiling of driver events in metastatic uveal melanoma. Nat Commun 11: 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K & Morikawa T (2018): The effect of formalin fixation on the size of pelvic sidewall lymph nodes. Int J Colorectal Dis 33: 1493–1495. [DOI] [PubMed] [Google Scholar]
- Kivelä T, Simpson ER & Grossniklaus HE et al. (2017): Uveal Melanoma. In: Amin MB, Edge SB & Fl G et al. (ed.) Am Jt Comm Cancer AJCC Cancer Staging Man. 8th Ed. New York: Springer; 805–817. [Google Scholar]
- Kujala E, Damato B, Coupland SE, Desjardins L & Bechrakis NE (2003): Staging of ciliary body and choroidal melanomas based on anatomic extent. Clin J Oncol 31: 2825–2831. [DOI] [PubMed] [Google Scholar]
- Kujala E, Mäkitie T & Kivelä T (2003): Very long‐term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci 44: 4651–4659. [DOI] [PubMed] [Google Scholar]
- Noordzij M, Leffondré K, Van Stralen KJ, Zoccali C, Dekker FW & Jager KJ (2013): When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28: 2670–2677. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee S, Haam S & Lee GD (2017): Effect of formalin fixation and tumour size in small‐sized non‐small‐cell lung cancer: a prospective, single‐centre study. Histopathology 71: 437–445. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: http://www.R‐project.org/. (Accessed on 15 Jan 2021). [Google Scholar]
- Schmidt M, Pedersen L & Sørensen HT (2014): The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 29: 541–549. [DOI] [PubMed] [Google Scholar]
- Shain AH, Bagger MM, Yu R et al. (2019): The genetic evolution of metastatic uveal melanoma. Nat Genet 51: 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CL, Demirci H, Materin MA, Marr BP, Mashayekhi A & Shields JA (2004): Clinical factors in the identification of small choroidal melanoma. Can J Ophthalmol 39: 351–357. [DOI] [PubMed] [Google Scholar]
- Shields CL, Say EAT, Hasanreisoglu M et al. (2018): Cytogenetic abnormalities in uveal melanoma based on tumor features and size in 1059 patients the 2016 W. Richard Green Lecture. Ophthalmology 124: 609–618. [DOI] [PubMed] [Google Scholar]
- Shields CL, Shields JA, Kiratli H, De PP & Cater JR (1995): Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 102: 1351–1361. [PubMed] [Google Scholar]
- Singh AD, Rennie IG, Seregard S, Giblin M & Mckenzie J (2004): Sunlight exposure and pathogenesis of uveal melanoma. Surv Ophthalmol 49: 419–428. [DOI] [PubMed] [Google Scholar]
- Singh AD, Turell ME & Topham AK (2011): Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology 118: 1881–1885. [DOI] [PubMed] [Google Scholar]
- Teikari J & Raivo I (1985): Incidence of choroidal malignant melanoma in Finland in the years 1973–1980. Acta Ophthalmol 63: 661–665. [DOI] [PubMed] [Google Scholar]
- Terry M, Therneau T & Grambsch P (2000): Modeling Survival Data: Extending the Cox Model. New York: Springer. [Google Scholar]
- The Collaborative Ocular Melanoma Study Group (2001): The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. Arch Ophthalmol 119: 969–982. [DOI] [PubMed] [Google Scholar]
- Virgili G, Gatta G, Ciccolallo L et al. (2008): Survival in patients with uveal melanoma in Europe. Arch Ophthalmol (Chicago, Ill 1960) 126: 1413–1418. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016): ggplot2: Elegant Graphics for Data Analysis. New York: Springer‐Verlag. Available at: https://ggplot2.tidyverse.org. (Accessed on 15 Jan 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1 Logarithmic relative hazard on prognostic factors on relative survival: age, sex, AJCC tumour size and year of diagnosis.


