Abstract
Efforts are accelerating to protect and restore ecosystems globally. With trillions of dollars in ecosystem services at stake, no clear framework exists for developing or prioritizing approaches to restore coral reefs even as efforts and investment opportunities to do so grow worldwide. Restoration may buy time for climate change mitigation, but it lacks rigorous guidance to meet objectives of scalability and effectiveness. Lessons from restoration of terrestrial ecosystems can and should be rapidly adopted for coral reef restoration. We propose how the 10 golden rules of effective forest restoration can be translated to accelerate efforts to restore coral reefs based on established principles of resilience, management, and local stewardship. We summarize steps to undertake reef restoration as a management strategy in the context of the diverse ecosystem service values that coral reefs provide. Outlining a clear blueprint is timely as more stakeholders seek to undertake restoration as the UN Decade on Ecosystem Restoration begins.
Keywords: blue carbon, climate change, fragmentation, reef management, spawning, stakeholders, traditional owners, actores, cambio climático, carbono azul, desove, dueños tradicionales, fragmentación, gestión de arrecifes
Abstract
Traducción de las Diez Reglas de Oro de la Reforestación para la Restauración de los Arrecifes de Coral
Resumen
Cada vez son más los esfuerzos para proteger y restaurar los ecosistemas a nivel mundial. Con billones de dólares en servicios ambientales en juego, no existe un marco de trabajo para desarrollar o priorizar estrategias para la restauración de los arrecifes de coral incluso cuando en todo el mundo aumentan los esfuerzos y las oportunidades de inversión. Puede que la restauración gane tiempo para la mitigación del cambio climático, pero carece de las directrices rigurosas para cumplir los objetivos de adaptabilidad y eficacia. Las lecciones que ha brindado la restauración de los ecosistemas terrestres pueden y deben adoptarse rápidamente en la restauración de arrecifes de coral. Proponemos una traducción de las diez reglas doradas de la restauración forestal efectiva para acelerar los esfuerzos para restaurar los arrecifes de coral con base en los principios establecidos de resiliencia, gestión y administración local. Resumimos pasos para emprender la restauración de arrecifes como una estrategia de manejo en el contexto de los valores diversos de los servicios ambientales. Estamos a tiempo de delinear un proyecto conforme más actores buscan restaurar con el inicio de la Década de la ONU para la Restauración de Ecosistemas.
CORALS ARE TO REEFS AS TREES ARE TO FORESTS
Coral reefs and forests are incredibly complex and important ecosystems. More specifically, coral reefs are often compared with rainforests (i.e., corals and trees are primary producers and habitat builders on which entire ecosystem biodiversity rests) (Knowlton, 2001). Forests and reefs persist across vast scales. Estimates of coral population sizes in the Pacific Ocean are similar to tree population sizes in the Amazon (half a trillion) (Dietzel et al., 2021). Both ecosystems are also highly vulnerable to climate extremes, recently evidenced in the mass dieback of mangroves (Avicennia marina) (Duke et al., 2017), mass bleaching‐induced mortality of corals on the Great Barrier Reef (Hughes et al., 2017), and megafires in temperate forests (Nolan et al., 2020). Both ecosystems have large spatial extents and are highly complex (Madin & Madin, 2015).
Climate change mitigation trajectories remain slow and uncertain, and protection‐based strategies alone may therefore no longer be sufficiently rapid to guarantee ecosystem resilience while carbon emissions are curbed (Kleypas et al., 2021). Thus, restoration may buy time for mitigation (Hein et al., 2020), enable local communities to develop more sustainable ecosystem use (Erbaugh et al., 2020), and fast track stabilization of marine biomes (Duarte et al., 2020). Restoration of reefs remains in its infancy relative to reforestation, for which restoration practices have been optimized over decades and energized with funding centered around carbon capture. In the past 2 years, a new body of work on coral reef restoration has emerged (e.g., Boström‐Einarsson et al., 2020) that highlights key priorities to coordinate knowledge sharing between scientists and practitioners (e.g., Hein et al., 2020; Vardi et al., 2021) and provides targeted best practices in, for example, planning and design (Shaver et al., 2020). Although comprehensive, these works have not transparently incorporated lessons learned from terrestrial restoration or provided a clear collective framework needed to develop and prioritize efforts and investment for coral reef restoration. We recommend a framework that not only draws on decades of work in terrestrial systems, but also integrates coral reef restoration within a broader framework of protection and mitigation than has been implemented in the past (Kleypas et al., 2021). Because reef restoration is relatively recent, best practices have only become available in the past few years (Bostrom‐Einarrsson et al., 2020; Hein et al., 2020; Shaver et al., 2020; Vardi et al., 2021), and compared with empirical forestry studies, the application and testing of these methods in the field on coral reefs is only beginning (Howlett et al., 2021; Quigley et al., 2021). Hence, lessons learned from terrestrial‐based restoration can be applied to novel coral reef restoration.
Coral reefs are one of the ecosystems most at risk of disappearing due to climate change (Convention on Biological Diversity, 2020). The United Nations declared a Decade of Ecosystem Restoration, starting in June 2021 (resolution 73/284, UNGA, 2019), to highlight the pressing need to bring many ecosystems back from the brink of collapse through coordinated, scalable efforts that capitalize on the global drive to invest resources into improving ecosystem functioning. Therefore, the critical challenge for coral reef restoration, as the Decade of Ecosystem Restoration begins, is to balance applying the lessons learned (and best practices developed) in other ecosystems while devising solutions specifically tailored for coral reefs.
TRANSLATING REFORESTATION'S 10 GOLDEN RULES TO REEFS
The 10 golden rules were proposed to maximize the recovery of critical ecosystem services that forests provide and the associated livelihoods they support (DiSacco et al., 2021). These rules parallel efforts to implement coral reef restoration at scale, including new frameworks for global reef restoration priorities (e.g., the Coral Restoration Consortium [Vardi et al., 2021]) that support restoration across ecosystems (Aronson et al., 2021). We considered how these rules should apply to the rapid escalation of reef restoration to guide effectiveness and address the challenges of restoring coral reef ecosystems (DiSacco et al., 2021) (Figures 1 & 2).
FIGURE 1.
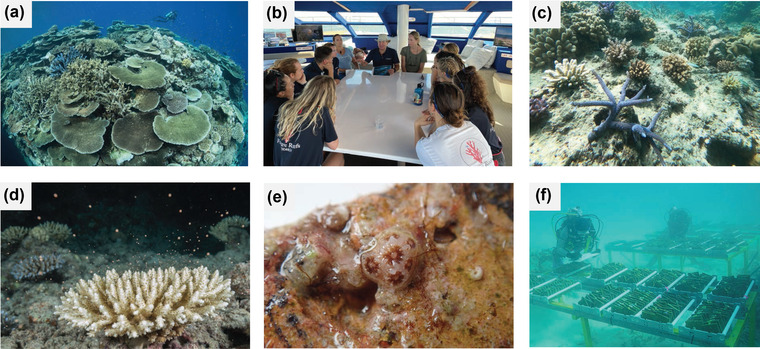
(a) Reef with high coral cover on the Great Barrier Reef (GBR), Australia (photo by J. Freund), (b) tourism partners and researchers working together as part of the Coral Nurture project (photo by D. Suggett), (c) a diversity of coral species planted on a degraded reef (photo by D. Suggett), (d) coral spawning on the GBR (photo by J. Freund), (e) coral juvenile produced from resilient stock on a settlement tile (photo by M. Marzonie, and (f) coral juveniles deployed in the field as part of experiments testing resilient stock on the GBR (photo by K. Green)
FIGURE 2.
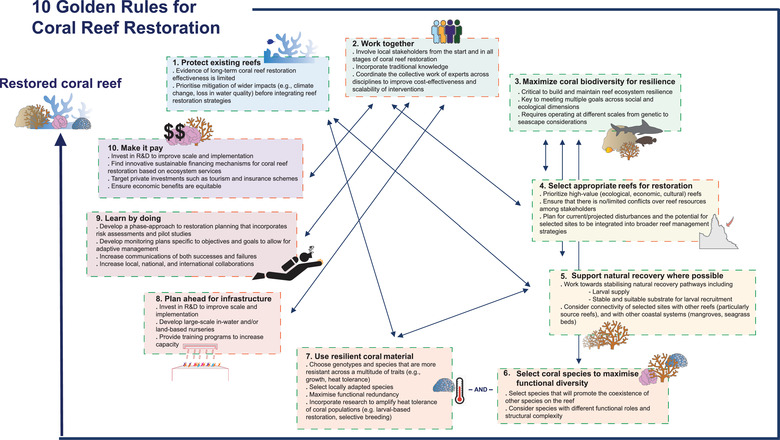
Ten golden rules, each a functional goal, for coral reef restoration. The order of the rules matches the order in which tasks should be considered during project planning and implementation as an iterative prioritization process, although some are interdependent and should be considered in parallel. The color of each box outline corresponds to functional goals (green outlines, ecological‐based rules; orange outlines, socioeconomic rules). Rules are adapted from the rules for reforestation in DiSacco et al. (2021)
Protect existing coral reefs (rule 1)
Coral cover has declined by 30–50% since 1980 (NAS, 2019). Protecting existing coral reefs with the highest ecological, cultural, and economic values should be a priority (Figures 1a & 2). This follows the principle that once degradation reaches a certain point, it is hard to reclaim ecosystem services (Baskett et al., 2014). Important baseline data from long‐term monitoring programs will be critical to help identify these important areas for protection based on habitat or species criteria (rules 5–7). Therefore, we stress that restoration should not be the first but the last type of management action applied (Gann et al., 2019).
Large‐scale efforts aimed at protecting existing habitat highlights the pressing need for carbon sequestration as a primary focus of conservation; restoration is a secondary focus that happens concurrent with action to rapidly curb emissions. Justification for protecting existing habitat is 2‐fold. First, reef degradation is estimated to continue under business‐as‐usual (IPCC RCP 8.5) and modest‐reduction scenarios (IPCC RCP 2.6) (Bindoff et al., 2019). Second, implementing mitigation along with restoration is a priority to safeguard all ecosystems and should be used as an integral part of maintaining and rebuilding habitats (Duarte et al., 2020). Protection of existing habitats also helps ensure restoration efforts will be effective at scale. Although there are encouraging short‐term increases in structural complexity and coral cover after restoration (Hein et al., 2020) and longer‐term predictions of positive restoration outcomes (Condie et al., 2021), overall success (Boström‐Einarsson et al., 2020) and effects on ecological processes are uncertain. No matter how compelling the evidence for the potential positive impact of restoration initiatives, there is no substitute for protection of natural ecosystems.
Work with Traditional Owners and stakeholders (rule 2)
Connecting individuals ensures restoration teams will have the necessary skills and knowledge and an equitable allocation of conservation benefits (e.g., Barnes et al., 2020). Incorporating communities into every stage is essential, where Traditional Owners and stakeholders (Figure 1b) participate in informed approvals, planning, implementation, and monitoring. Local communities working collectively also build operational scale through networks. This collaborative model provides opportunities to minimize conflicts across resources; maximize precious and unique local knowledge; educate and build capacity and stewardship; channel innovations beyond Western thinking (i.e., values are not imposed to restore particular services over others [Gibbs et al., 2021]); and implement common methods for more robust data collection. Many of these factors are analogous to those determining the success of the management efforts in marine protected areas (MPAs) (Giakoumi et al., 2018).
Connecting communities on the front line with experts in specific fields further builds confidence and credibility, especially where successes are documented. Tourism operators and researchers working in partnership scaled propagation efforts in the northern Great Barrier Reef across local reefs (case study in Hein et al., 2020) to validate method and cost‐effectiveness (Suggett et al., 2019, 2020). Similarly, community‐based coral reef restoration in Hawaii showed that the involvement of key stakeholders can result in direct economic benefits to the community and improve reef stewardship by providing a pathway for collective community action (Kittinger et al., 2016).
Maximize coral biodiversity for resilience (rule 3)
Goals for restoration include socioeconomic (e.g., increasing fisheries productivity), ecological (i.e., restoring critical ecosystem functions for specific fishes species or recruitment space for corals), and climatological (i.e., mitigate climate change effects) (Hein et al., 2020) (Figures 1c & 2). However, achieving one or multiple goals in parallel requires building resilience through functional redundancy and uniqueness (i.e., maximizing biodiversity [Rockström et al., 2009]). This allows for the increased probability that species will resist current and projected disturbances, enabling faster recovery and buffering against phase shifts (Baskett et al., 2014).
Maximizing biodiversity can operate at the landscape, reef, phenotype, species, and genetic scales and be incorporated into diverse restoration designs. In the reef landscape, sites are connected to important sources of larvae or key nursery habitats in the seascape, such as mangroves (rule 4). Efforts at individual reefs account for ecological processes, including organisms beyond corals (fish, invertebrates, and microbes). Considerations of phenotypic diversity are essential, and include choosing species from a range of functional groups and growth forms to maximize structural complexity and habitats for diversity and refugia. Although coral species boundaries are complex (e.g., Cowman et al., 2020), maintaining high biodiversity can be paired with maintaining functional or phenotypic diversity, resulting in greater protection of important ecosystem attributes (Ladouceur et al., 2021) (rule 6). Maintaining high genetic diversity is critical to sustain processes of sexual reproduction and natural cycles of coral recruitment, maximize the chances of building resistance, and prevent extinctions (e.g., Baums et al., 2019).
Select appropriate reefs for restoration (rule 4)
Not all areas can or should be restored. Restoration represents only one strategy with potentially limited spatial and temporal efficacy. Although MPAs do not sufficiently slow reef declines due to climate change (e.g., Hughes et al., 2017), they are critical to relieve multiple local pressures (Donavan et al., 2021). Just as the selection of MPAs should follow a targeted approach to maximize resilience, restoration sites should also be selected using quantitative criteria. Prioritization of locations of high value amenable to restoration is needed, for example, reefs with the highest survival likelihoods under climate change (Beyer et al., 2018) or reefs that are highly connected and intact (Hock et al., 2017) (rule 3) or “bright spot” reefs for human needs (Cinner et al., 2016). Targeting high‐value reefs for stakeholder benefit builds in historical and socioeconomic factors (e.g., restoration at resorts in the Maldives [Hein et al., 2019]).
Location selection should meet long‐term goals (increasing fisheries, tourism, or reestablishing function and structure) and affects the potential efficiency and efficacy of the restoration effort. Care should be taken to assure that there is no conflict over reef resources (rule 2), that the sites are not likely to be heavily affected by current and potential future disturbances, and that efforts are integrated within broader reef‐management strategies. Ultimately, site selection should be made with resilience and climate in mind (Shaver et al., 2020) and provide opportunities to scale up efforts (mixed methods and connecting small‐scale projects to increase positive ecological footprint). Selection should be an iterative process that involves stakeholders’ assessments of relevance to restoration goals, potential to improve site condition, and likelihood of coral survival (Shaver et al., 2020).
Support natural recovery where possible (rule 5)
Supporting natural recovery should involve the removal of barriers of recovery processes and prioritization of approaches that leverage natural resilience. Natural regeneration should not be disregarded as a pathway to reef resilience. This includes conventional management to remove acute and chronic pressures on reefs, thereby reinforcing natural resilience. For example, targeted local management can reduce the negative impacts of climate‐driven heat waves that induce mass coral bleaching (Donovan et al., 2021). In cases where active approaches are needed, restoration should be nature based to harness existing natural capital (rule 3) (Figure 1d) and minimize risks associated with artificial selection.
Practitioners should prioritize identification and amplification of natural regeneration pathways. For example, initial causes of degradation need to be identified and assessed as to whether they can be mitigated (Edwards & Clark, 1998) (rule 1). Other questions include what may slow natural recovery rates and can they be prioritized within the restoration framework? One critical example is where reef recovery (regeneration) fundamentally rests on larval supply and settlement but there is insufficient stock generation and supply (connectivity [Hock et al., 2017, Quigley et al., 2019]) or substrate availability and stability (Hughes et al., 2018). Self‐recruitment rates are generally low in corals and therefore may not regenerate high‐diversity reefs. Hence, the lack of recruitment and limited survival of recruits and subsequent juveniles can be 2 barriers to natural recovery. Both barriers can be addressed through targeted restoration methods. Without natural regeneration, recovery rests on local asexual propagation and the ability of local corals to reach sexual maturity, likely limiting the capacity for adaptation through sexual recombination.
Select coral species to maximize functional diversity (rule 6)
Species like trees and corals engineer the environment to allow the existence of other species. To maximize biodiversity, restoration should include species that promote species coexistence. Tabular plate corals preferentially promote other species via enhancement of recruitment and therefore recovery (Ortiz et al., 2021). Selection should also maximize species’ functional roles (rule 3) (McWilliams et al., 2018; Bellwood et al., 2019; Madin et al., 2021), including local species, and incorporate taxonomically cryptic species (Cowman et al., 2020).
Guidelines for coral species selection are emerging (Madin et al., 2021; Shaver et al., 2020), and decision‐making should be informed by knowledge of local species’ ecologies. Years of reforestation provide evidence of the power of a clear framework for selecting species (DiSacco et al., 2021). For example, 20–30 pioneer and climax species of trees are recommended that typically have high survival, can grow and reproduce rapidly, and attract seed‐dispersing wildlife or that can “recapture” the site after disturbance (DiSacco et al., 2021). An “objective‐based prioritization” (Ladouceur et al., 2021) or “goal‐based” approach (Shaver et al., 2020), in which the outcome of an intended restoration activity is used as a guideline for action, has been developed for tree seed mixes, methods that logically carry over to coral restoration prioritization. For example, defining objectives for different restoration goals informs selection; a trait‐based approach helps define what species should be chosen given specific coral traits (Madin et al., 2021).
Use resilient coral material (rule 7)
Even if global action is mobilized and carbon emissions end immediately, warming will continue (Donner, 2009). Restoration aimed at preparing reefs for warmer futures must be considered (Kleypas et al., 2021). Selecting biological material with naturally high heat tolerance would enhance overall resilience through improved survival during stress. Restoring reefs accordingly should include multiple strategies, including maximizing genetic diversity and function (rule 3) and amplifying natural stress tolerances. Methods could include selective breeding of different coral populations (Figure 1e) (Quigley et al., 2021) and different species (Chan et al., 2019) or using larval capture methods that increase genotypic diversity (NAS, 2019). Identification of resilient stock to harness natural adaptation is essential (see rule 5) and should focus on genotypes and species resilient across multiple traits (e.g., growth and heat tolerance) to increase the probability of long‐term success under climate change.
Corals are complex, and restoration methods incorporating multiple adaptive mechanisms targeting their different biological aspects (e.g., host and symbiont [Voolstra et al., 2021]) offer multiplicative benefits. For example, combining heat‐tolerant symbionts and heat‐tolerant coral genotypes results in 26 times more heat tolerance relative to without symbionts (Quigley et al., 2020). The genetic diversity of the host can also be maximized through selection of an optimized number of genotypes for breeding designs and restoration deployments. Maximizing genotype use limits the creation of genetic bottlenecks and thus genetic drift, further declines in population size, and loss of adaptive capacity. The use of 30–50 individuals per population is recommended for trees (DiSacco et al., 2021) and corals (Baums, 2008). Finally, the danger of disease should also be considered (Baums et al., 2019).
Plan ahead for infrastructure, capacity, and coral biomass supply (rule 8)
Ensuring access to sufficient coral material for restoration where coral populations are greatly reduced requires planning of equitable resource sharing across traditional owners and stakeholders (rule 2). In the short term, corals or coral fragments may serve as stock (Suggett et al., 2019), but stock needs to be retained and generated at rates that exceed natural derivation. In‐water nurseries could produce colony numbers from asexual fragments. When well planned, nurseries provide a high return on investment (Stewart‐Sinclair et al., 2021).
Ensuring that restoration methods are scalable within resource constraints is important. Although scaling up is technically and financially challenging for some coral restoration methods, investment in sexual propagation is key to improving scale and overcoming genetic bottlenecks from purely asexual propagation (see rule 7), but it is largely limited to practices that coincide with annual spawning. Land‐based production facilities provide environments where corals can be reared under controlled conditions, employ sexual propagation for out‐of‐season spawning (O'Neil et al., 2021), cryopreserve gametes for future use (e.g., Daly et al., 2018; analogous to seed banks), or fuse microfragments to fast‐track growth to sexual production (Forsman et al., 2015). All are critical to complement in‐water propagation, but there are drawbacks, including operational costs and significant environmental footprints, that undermine implementation at scale. Perhaps the most challenging aspect of planning ahead is how much coral material will persist to be propagated. Managers may have to consider whether and how to transfer material across biogeochemical (and geopolitical) boundaries. This will inevitably require new approaches and consideration of time in the planning process.
Learn by doing coral restoration (rule 9)
Although general guidelines for ecosystem restoration (e.g. Gann et al., 2019) and for coral reef restoration exist (e.g., Hein et al., 2020; Shaver et al., 2020; Goergen et al., 2020), the outcomes of any restoration efforts are likely to be context and site specific. Many of these restoration actions have been undertaken for decades with little formal documentation. As a starting point, it is logical to adopt methods that have been deemed effective elsewhere during the establishment of new programs (e.g., Suggett et al., 2019), but planning that integrates local and traditional owner knowledge and concerns is critical (rule 2) to account for decades (if not centuries) of knowledge. Such an approach is considered relatively low risk and ensures early feasibility by conducting local controlled trials. Other efforts are required for strict validation, often under methods designed for increased risk but larger scale. Large initiatives, such as the Reef Restoration and Adaptation Program (RRAP) in Australia and the National Academies of Science, Engineering, and Medicine's National Committee on Interventions to Increase the Resilience of Coral Reef in the United States, have implemented a so‐called fast‐fails approach and many smaller‐scale tests of feasibility before large‐scale implementation with a staged approach (NAS, 2019). For example, carefully incorporating risk assessments including small field trials (Figure 1f) (Quigley et al., 2021) is necessary to quantify and therefore plan to mitigate risks associated with the translocation of genetic material, non‐native species, and pests.
A phased, iterative approach (initial pilot studies to broader, long‐term objectives) (Shaver et al., 2020) will minimize the risks of failing while helping gain public trust and social license to operate (Gibbs et al., 2021). The first phase of adaptive management requires carefully designing a monitoring plan specific to goals and objectives with clear timelines and thresholds (Goergen et al., 2020; Shaver et al., 2020), planning in advance for capacity and funding necessary to change during restoration if necessary. Hence, learning by doing improves the cost‐efficiency and scalability of efforts, but requires pathways to communicate successes and failures because transparency builds public trust and knowledge transfer (e.g., Vardi et al., 2021).
Make coral restoration pay (rule 10)
Novel restoration financing is needed for coral reefs. For forests, there are numerous financing schemes that emphasize tree planting as an organic carbon capture intervention to mitigate current and future climate emissions (Bastin et al., 2019). However, the danger may be that restoration may be viewed as a “burn now, pay later” strategy that deprioritizes nations away from carbon mitigation (Dyke et al., 2021).
Unlike terrestrial forests—or indeed marine habitats, such as mangroves, seagrasses, and kelp forests—coral reefs are not effective carbon sinks (Smith & Gattuso, 2009) and therefore do not fall under the growing (green or blue) carbon market economies. However, coral reefs carry immense economic value associated with diverse critical ecosystem services. For example, in the United States, the value of reefs for flood risk reduction alone is estimated at over US$1.8 billion/year (Reguero et al., 2021), and the GBR in Australia is valued at US$56 billion in economic, social, and asset value (O'Mahony et al., 2017). Such high‐value ecosystem services need to be incorporated into funding schemes for coral reef restoration to generate long‐term private investment. As such, efforts toward and investment in rebuilding reefs to offset the rate of reef loss (as emissions reduction strategies remain slow and unpredictable) should safeguard this value to the economy provided by reefs. Rebuilding and retaining productive and biodiverse reef systems—the blue economy—fundamentally overrules the value of reefs to the carbon market economy.
Financial sustainability is also critical. A key to securing this with multiple, diverse, long‐term sustainable funding models is to increase the evidence of the worth of coral reef restoration. This can be achieved through improving cost‐effectiveness, scalability, and long‐term monitoring to identify components of coral reef restoration effectiveness (Hein et al., 2020) in which restoration already outweighs the costs (Stewart‐Sinclair et al., 2021). Ensuring investments meet returns may include focusing on high‐value reefs (Howlett et al., 2021), targeting private investments through tourism or reef insurance policies (TNC, 2020), or promoting biological diversity on infrastructure, such as artificial reefs (Airoldi et al., 2021). Comprehensive socioeconomic monitoring is essential to ensure equity and sharing of economic benefits with local community members and stakeholders (Wells et al., 2021) by incorporating economists in planning and design for sustainable, profitable, equitable coral reef restoration.
CONCLUSION
Reef ecosystem management is at a historical turning point in which active interventions such as restoration are being increasingly adopted to assist existing protection strategies to slow reef declines under climate change. The loss of reefs would be devastating for the millions of people globally that rely on them for food (Bellwood et al., 2004) and for the coastal protection they afford (Ferrario et al., 2014). Conception, implementation, and evolution of coral reef restoration efforts are already supported by a number of practical guides produced by experts through first‐hand experience (e.g., Goergen et al., 2020; Shaver et al., 2020), but these can be further bolstered with the lessons learned through decades of terrestrial restoration. By applying the 10 golden rules for reforestation to coral reef restoration, we suggest a framework that should be at the core of restoration efforts and that captures the ever‐increasing evidence of drivers of reef resilience.
Here, we sought to harness the knowledge gained from decades of restoration of forest systems with the hopes of avoiding the same growing pains for the relatively younger field of coral reef restoration. This type of cross‐disciplinary perspective is sorely needed. Although these rules should help create a more effective framework for restoration, important differences exist between forests and reefs that will require innovation and novel approaches. One includes financing aligned with different ecosystem service values (e.g., carbon capture by forests but not reefs). The second is the relative infancy of data generation for reefs compared with terrestrial systems, including the genomic data vital for rules 3 and 7. Importantly, forests and coral reefs differ fundamentally in their connectivity and dispersal patterns, which will greatly affect the replenishment and recovery of restored systems, thereby necessitating novel thinking around rule 5. Further, some of the primary threats facing reefs and forests are also weighted fundamentally differently—notably the severity and extent of loss from direct (i.e., ecosystem clearance through deforestation vs. coral extraction) versus indirect (climate change) factors will also differ. The mitigation of direct factors is arguably more feasible, which suggests the importance of methods needed to restore at scale [rules 2, 8, 9, and 10].) Finally, the Bonn Challenge aims to restore 350 million ha of forest, 10 times the size of the GBR, itself the size of Italy. This is an ambitious but necessary goal. The coral reef community, be it scientists, tourist operators, traditional owners, or governments, needs an equally ambitious goal globally to secure the persistence of healthy coral reefs in the future.
ACKNOWLEDGMENTS
We thank the many peers, collaborators, and colleagues for the conversations that contributed to the perspectives generated in this article. We also thank DiSacco et al. (2021) for their timely review, which we used as an outline for this current work.
Quigley, K. M. , Hein, M. , & Suggett, D. J. (2022). Translating the 10 golden rules of reforestation for coral reef restoration. Conservation Biology, 36, e13890. 10.1111/cobi.13890
Article impact statement: There are 10 rules for protection, management, and repair to guide effective and equitable restoration of coral reefs.
LITERATURE CITED
- Airoldi, L. , Beck, M. W. , Firth, L. B. , Bugnot, A. B. , Steinberg, P. D. , & Dafforn, K. A. (2021). Emerging solutions to return nature to the urban ocean. Annual Review of Marine Science, 13, 445–477. [DOI] [PubMed] [Google Scholar]
- Aronson, J. , Goodwin, N. , Orlando, L. , Eisenberg, C. , & Cross, A. T. (2021). A world of possibilities: Six restoration strategies to support the United Nations Decade on Ecosystem Restoration. Restoration Ecology, 28(4), 730–736. [Google Scholar]
- Barnes, M. L. , Wang, P. , Cinner, J. E. , Graham, N. A. J. , Guerrero, A. M. , Jasny, L. , Lau, J. , Sutcliffe, S. R. , & Zamborain‐Mason, J. (2020). Social determinants of adaptive and transformative responses to climate change. Nature Climate Change, 10, 823–828. [Google Scholar]
- Baskett, M. L. , Fabina, N. S. , & Gross, K. (2014). Response diversity can increase ecological resilience to disturbance in coral reefs. The American Naturalist, 184(2), E16–E31. [DOI] [PubMed] [Google Scholar]
- Bastin, J. F. , Finegold, Y. , Garcia, C. , Mollicone, D. , Rezende, M. , Routh, D. , Zohner, C. M. , & Crowther, T. W. (2019). The global tree restoration potential. Science, 365(6448), 76–79. [DOI] [PubMed] [Google Scholar]
- Baums, I. B. (2008). A restoration genetics guide for coral reef conservation. Molecular Ecology, 17(12), 2796–811, [DOI] [PubMed] [Google Scholar]
- Baums, I. B. , Baker, A. C. , Davies, S. W. , Grottoli, A. G. , Kenkel, C. D. , Kitchen, S. A. , Kuffner, I. B. , LaJeunesse, T. C. , Matz, M. V. , Miller, M. W. , Parkinson, J. E. , & Shantz, A. A. (2019). Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecological Applications, 29(8), e01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellwood, D. R. , Hughes, T. P. , Folke, C. , & Nyström, M. , (2004). Confronting the coral reef crisis. Nature, 429(6994), 827–833. [DOI] [PubMed] [Google Scholar]
- Bellwood, D. R. , Streit, R. P. , Brandl, S. J. , & Tebbett, S. B. (2018). The meaning of the term ‘function’ in ecology: A coral reef perspective. Functional Ecology, 33(6), 948–961 [Google Scholar]
- Beyer, H. L. , Kennedy, E. V. , Beger, M. , Chen, C. A. , Cinner, J. E. , Darling, E. S. , Gates, R. D. , Heron, S. F. , Knowlton, N. , Obura, D. O. , Palumbi, S. R. , Possingham, H. P. , Puotinen, M. , Runting, R. K. , Skirving, W. J. , Spalding, M. , Wilson, K. A. , Wood, S. , Veron, J. E. , & Hoegh‐Guldberg, O. (2018). Risk‐sensitive planning for conserving coral reefs under rapid climate change. Conservation Letters, 11(6), e12587. [Google Scholar]
- Bindoff, N. L. , Cheung, W. W. L. , & Kairo, J. G. (2019). Changing ocean, marine ecosystems, and dependent communities. In Pörtner D. C. R., Masson‐Delmotte H.‐O., Zhai V., Tignor P., Poloczanska M., & Mintenbeck E. (Eds.), IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (pp. 447–587). IPCC. [Google Scholar]
- Boström‐Einarsson, L. , Babcock, R. C. , Bayraktarov, E. , Ceccarelli, D. , Cook, N. , Ferse, S. C. A. , Hancock, B. , Harrison, P. , Hein, M. , Shaver, E. , Smith, A. , Suggett, D. , Stewart‐Sinclair, P. J. , Vardi, T. , & McLeod, I. M. (2020). Coral restoration ‐ A systematic review of current methods, successes, failures and future directions. PLoS ONE, 15, e0226631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, W. Y. , Hoffmann, A. , & van Oppen, M. J. H. (2019). Hybridization as a conservation management tool. Conservation Letters, 12(5), e12652. [Google Scholar]
- Cinner, J. , Huchery, C. , MacNeil, M. , Graham, N. A. J. , McClanahan, T. R. , & Maina, J. (2016). Bright spots among the world's coral reefs. Nature, 535, 416–419. [DOI] [PubMed] [Google Scholar]
- Condie, S. A. , Anthony, K. R. , Babcock, R. C. , Baird, M. E. , Beeden, R. , Fletcher, C. S. , Gorton, R. , Harrison, D. , Hobday, A. J. , Plagányi, É. E. , & Westcott, D. A. (2021). Large‐scale interventions may delay decline of the Great Barrier Reef. Royal Society Open Science, 8(4), 201296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman, P. F. , Quattrini, A. M. , Bridge, T. C. L. , Watkins‐Colwell, G. J. , Fadli, N. , Grinblat, M. , Roberts, T. E. , McFadden, C. S. , Miller, D. J. , & Baird, A. H. (2020). An enhanced target‐enrichment bait set for Hexacorallia provides phylogenomic resolution of the staghorn corals (Acroporidae) and close relatives. Journal of Molecular and Phylogenetic Evolution, 153:106944. [DOI] [PubMed] [Google Scholar]
- Daly, J. , Zuchowicz, N. , Nunez Lendo, I. , Khosla, K. , Lager, C. , Henley, M. , …, & Hagerdon, M. (2018). Successful cryopreservation of coral larvae using vitrification and laser warming. Scientific Reports, 8, 15714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel, A. , Bode, M. , Connolly, S. R. , & Hughes, T. P. (2021). The population sizes and global extinction risk of reef‐building coral species at biogeographic scales. Nature Ecology & Evolution, [DOI] [PubMed] [Google Scholar]
- DiSacco, A. , Hardwick, K. A. , Blakesley, D. , Brancalion, P. H. S. , Breman, E. , Rebola, L. C. ,…, & Antonelli, A. (2021). Ten golden rules for reforestation to optimise carbon sequestration, biodiversity recovery and livelihood benefits. Global Change Biology, 27(7), 1328–1348. [DOI] [PubMed] [Google Scholar]
- Donavan, M. K. , Burkepile, D. E. , Kratochwill, C. , Shlessinger, T. , Sully, S. , Oliver, T. A. , …, & van Woesik, R. (2021). Local conditions magnify coral loss after marine heatwaves. Science, 372(6545), 977–980. [DOI] [PubMed] [Google Scholar]
- Donner, S. D. (2009). Coping with commitment: Projected thermal stress on coral reefs under different future scenarios. PLoS ONE, 4(6), e5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, C. M. , Agusti, S. , Barbier, E. , Britten, G. L. , Castilla, J. C. , Gattuso, J. P. , …, & Worm, B. (2020). Rebuilding marine life. Nature, 580, 39–51. [DOI] [PubMed] [Google Scholar]
- Duke, N. C. , Kovacs, J. M. , Anthony, B. , Griffiths, D. , Preece, C. L. , D, H. , D. J. E., B., van Oosterzee, P. D. E. , Jock Mackenzie J. A., Morning, H. S. , & Burrows, A. D. (2017). Large‐scale dieback of mangroves in Australia's Gulf of Carpentaria: A severe ecosystem response, coincidental with an unusually extreme weather event. Marine and Freshwater Research, 68(10), 1816–1829. [Google Scholar]
- Dyke, J. , Watson, R. , & Knorr, W. (2021). Climate scientists: Concept of net zero is a dangerous trap. The Conversation. [Google Scholar]
- Edwards, A. J. , & Clark, S. (1998). Coral transplantation: A useful management tool or misguided meddling? Marine Pollution Bulletin, 37, 474–487. [Google Scholar]
- Erbaugh, J. T. , Pradhan, N. , Adams, J. , Oldekop, J. A. , Agrawal, A. , Brockington, R. , Pritchard, R. , & Chhatre, A. (2020). Global forest restoration and the importance of prioritizing local communities. Nature Ecology & Evolution, 4, 1472–1476. [DOI] [PubMed] [Google Scholar]
- Ferrario, F. , Beck, M. W. , Storlazzi, C. D. , Micheli, F. , Shepard, C. C. , & Airoldi, L. (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nature Communications, 5, 3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman, Z. H. , Page, C. A. , Tooned, R. J. , & Vaughan, D. (2015). Growing coral larger and faster: Micro‐colony‐fusion as a strategy for accelerating coral cover. PeerJ, 3, e1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gann, G. D. , McDonald, T. , Walder, B. , Aronson, J. , Nelson, C. R. , Johnson, J. , Hallett, J. G. , Eisenberg, C. , Guariguata, M. R. , Liu, J. , Hua, F. , Echeverría, C. , Gonzales, E. , Shaw, N. , Decleer, K. , & Dixon, K. W. (2019). International principles and standards for the practice of ecological restoration. Society for Ecological Restoration. [Google Scholar]
- Giakoumi, S. , McGowan, J. , Mills, M. , Beger, M. , Bustamante, R. H. , Charles, A. , …, & Possingham, H. P. (2018). Revisiting “success” and “failure” of marine protected areas: A conservation scientist perspective. Frontiers in Marine Science, 5, 223. [Google Scholar]
- Gibbs, M. T. , Gibbs, B. L. , Newlands, M. , & Ivey, J. (2021). Scaling up the global reef restoration activity: Avoiding ecological imperialism and ongoing colonialism. PLoS ONE, 16(5), e0250870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goergen, E. , Schopmeyer, S. , Moulding, A. L. , Moura, A. , Kramer, P. , & Viehman, T. S. (2020). Coral reef restoration monitoring guide: Methods to evaluate restoration success from local to ecosystem scales. NOAA Technical Memorandum NOS NCCOS 279.
- Hein, M. Y. , Birtles, A. , Willis, B. L. , Gardiner, N. , Beeden, R. , & Marshall, N. A. (2019). Coral restoration: Socio‐ecological perspectives of benefits and limitations. Biological Conservation, 229, 14–25. [Google Scholar]
- Hein, M. Y. , McLeod, I. M. , Shaver, E. C. , Vardi, T. , Pioch, S. , Boström‐Einarsson, L. , Ahmed, M. , & Grimsditch, G. (2020). Coral Reef Restoration as a strategy to improve ecosystem services –A guide to coral restoration methods. United Nations Environment Program. [Google Scholar]
- Hock, K. , Wolff, N. H. , Ortiz, J. C. , Condie, S. A. , Anthony, K. R. N. , Blackwell, P. G. , & Mumby, P. J. (2017). Connectivity and systemic resilience of the Great Barrier Reef. PLoS Biology, 15(11), e2003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, L. , Camp, E. F. , Edmondson, J. , Henderson, N. , & Suggett, D. J. (2021). Coral growth, survivorship and return‐on‐effort within nurseries at high‐value sites on the Great Barrier Reef. PLoS ONE, 16(1), e0244961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. , Barnes, M. , Bellwood, D. , Cinner, J. E. , Cumming, G. S. , Jackson, J. B. C. , …, & Scheffer, M. (2017). Coral reefs in the Anthropocene. Nature, 546, 82–90 (2017). [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. , Kerry, J. T. , Baird, A. H. , Connoly, S. R. , Dietzel, A. , Eakin, C. M. , …, & Torda, G. (2018). Global warming transforms coral reef assemblages. Nature, 556, 492–496. [DOI] [PubMed] [Google Scholar]
- Kittinger, J. N. , Bambico, T. M. , Minton, D. , Miller, A. , Meija, M. , Kalei, N. , Wong, B. , & Glazier, E. W. (2016). Restoring ecosystems, restoring community: Socioeconomic and cultural dimensions of a community‐based coral reef restoration project. Regional Environmental Change, 16, 301–313. [Google Scholar]
- Kleypas, J. , Allemand, D. , Anthony, K. , Baker, A. C. , Beck, M. W. , Zeitlin Hale, L. , …, & Gattuso, J. P. (2021). Designing a blueprint for coral reef survival. Biological Conservation, 257, 109107, [Google Scholar]
- Knowlton, N. (2001). The future of coral reefs. Proceedings of the National Academy of Sciences of the United States of America, 98(10), 5416–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur, E. , McGowan, J. , Huber, P. , Possingham, H. , Scridel, D. , van Klink, R. , …, & Jimenez‐Alfaro, B. (2021). An objective‐based prioritization approach to improve trophic complexity through ecological restoration. bioRxiv. 10.1101/2021.03.09.434521 [DOI] [Google Scholar]
- Madin, J. S. , & Madin, E. (2015). The full extent of the global coral reef crisis. Conservation Biology, 29, (6), 1724–1726. [DOI] [PubMed] [Google Scholar]
- Madin, J. S. , McWilliam, M. , Quigley, K. , Bay, L. K. , Bellwood, D. , Doropoulos, C. , Fernandes, L. , Harrison, P. , Hoey, A. S. , Mumby, P. J. , Richards, Z. T. et al., (2021). Selecting species for restoration in foundational assemblages. bioRxiv. 10.1101/2021.11.03.467181 [DOI] [Google Scholar]
- McWilliam, M. , Hoogenboom, M. O. , Baird, A. H. , Kuo, C. Y. , Madin, J. S. , & Hughes, T. P. (2018). Biogeographical disparity in the functional diversity and redundancy of corals. Proceedings of the National Academy of Sciences, 115(12), 3084–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine . (2019). A research review of interventions to increase the persistence and resilience of coral reefs. The National Academies Press. [Google Scholar]
- Nolan, R. H. , Boer, M. M. , Collins, L. , Resco de Dios, V. , Clarke, H. , Jenkins, M. , …, & Bradstock, R. A. (2020). Causes and consequences of eastern Australia's 2019–2020 season of mega‐fires. Global Change Biology, 26 (3), 1039–1041. [DOI] [PubMed] [Google Scholar]
- O'Neil, K. L. , Serafin, R. M. , Patterson, J. T. , & Craggs, J. R. K. (2021). Repeated ex situ spawning in two highly disease susceptible corals in the family Meandrinidae. Frontiers in Marine Science, 8, 463. [Google Scholar]
- O'Mahony, J. , Simes, R. , Redhill, D. , Heaton, K. , Atkinson, C. , Hayward, E. , & Nguyen, M. (2017). At what price? The economic, social and icon value of the Great Barrier Reef . Deloitte Access Economics. https://elibrary.gbrmpa.gov.au/jspui/bitstream/11017/3205/1/deloitte‐au‐economics‐great‐barrier‐reef‐230617.pdf. [Google Scholar]
- Ortiz, J. C. , Pears, R. J. , Beeden, R. , Dryden, J. , Nicholas, J. , Wolff, H. , Gomez Cabrera, M. , & Mumby, P. J. (2021). Important ecosystem function, low redundancy and high vulnerability: The trifecta argument for protecting the Great Barrier Reef's tabular Acropora . Conservation Letters, 14(50), e12817. 10.1111/conl.12817 [DOI] [Google Scholar]
- Quigley, K. M. , Bay, L. K. , & van Oppen, M. J. (2019). The active spread of adaptive variation for reef resilience. Ecology and evolution, 9(19), 11122–11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley, K. M. , Bay, L. K. , & van Oppen, M. J. H. (2020). Genome‐wide SNP analysis reveals an increase in adaptive genetic variation through selective breeding of coral. Molecular Ecology, 29, (12), 2176–2188. [DOI] [PubMed] [Google Scholar]
- Quigley, K. M. , Marzonie, M. , Ramsby, B. , Abrego, D. , Milton, G. , van Oppen, M. J. H. , & Bay, L. K. (2021). Variability in fitness trade‐offs amongst coral juveniles with mixed genetic backgrounds held in the wild. Frontiers in Marine Science, 8, 636177. 10.3389/fmars.2021.636177 [DOI] [Google Scholar]
- Reguero, B. G. , Storlazzi, C. D. , Gibbs, A. E. , Shope, J. B. , Cole, A. D. , Cumming, K. A. , & Beck, M. W. (2021). The value of US coral reefs for flood risk reduction. Nature Sustainability, 4, 688–698. [Google Scholar]
- Rockström, J. , Steffen, W. , Noone, K. , Persson, A. , Chappin III, F. S. , Lambin, E. F. , …, & Foley, J. A. (2009). A safe operating space for humanity. Nature, 461, 472–475. [DOI] [PubMed] [Google Scholar]
- Shaver, E. C. , Courtney, C. A. , West, J. M. , Maynard, J. , Hein, M. , Wagner, C. , …, & Koss, J. (2020). A manager's guide to coral reef restoration planning and design. Technical Memorandum CRCP. Coral Reef Conservation Program, National Oceanic and Atmospheric Administration. [Google Scholar]
- Smith, S. V. , & Gattuso, J. P. (2009). Coral reefs. In: Laffoley, D. & Grimsditch, G. (eds). The management of natural coastal carbon sinks (pp. 39–45). IUCN. [Google Scholar]
- Stewart‐Sinclair, P. J. , Klein, C. J. , Bateman, I. J. , & Lovelock, C. E. (2021). Spatial cost–benefit analysis of blue restoration and factors driving net benefits globally. Conservation Biology, 35(6), 1850–1860. [DOI] [PubMed] [Google Scholar]
- Suggett, D. J. , Camp, E. F. , Edmondson, J. , Boström‐Einarsson, L. , Ramler, V. , Lohr, K. , & Patterson, J. T. (2019). Optimizing return on‐effort for coral nursery and outplanting practices to aid restoration of the Great Barrier Reef. Restoration Ecology, 27(3), 683–93. [Google Scholar]
- Suggett, D. J. , Edmondson, J. , Howlett, L. , & Camp, E. F. (2020). Coralclip®: A low‐cost solution for rapid and targeted out‐planting of coral at scale. Restoration Ecology, 28(2), 289–296. [Google Scholar]
- The Nature Conservancy (TNC) (2020). Coral reef insurance policy triggered by hurricane Delta . https://www.nature.org/en‐us/newsroom/coral‐reef‐insurance‐policy‐triggered/.
- United Nations General Assembly (UN) . (2019). Resolution 73/284. United Nations Decade on Ecosystem Restoration (2021–2030) . https://documents‐dds‐ny.un.org/doc/UNDOC/GEN/N19/060/16/PDF/N1906016.pdf?OpenElement.
- Vardi, T. , Hoot, W. C. , Levy, J. , Shaver, E. C. , Winters, R. S. , Banaszack, A. T. , …, & Montoya‐Maya, P. H. (2021). Six priorities to advance the science and practice of coral reef restoration worldwide. Restoration Ecology, 29(8), e13498. [Google Scholar]
- Voolstra, C. R. , Suggett, D. J. , Peixoto, R. S. , Parkinson, J. E. , Quigley, K. M. , Silveira, C. B. , Sweet, M. et al. (2021). Extending the natural adaptive capacity of coral holobionts. Nature Reviews Earth & Environment, 2, 747–762. [Google Scholar]
- Wells, H. B. M. , Kirobi, E. H. , Chen, C. L. , Winowiecki, L. A. , Vagen, T. , Ahmad, M. N. , Stringer, L. C. , & Dougill, A. J. (2021). Equity in ecosystem restoration. Restoration Ecology, 29(5), e13385. [Google Scholar]


