Abstract
Objective
This study was undertaken to evaluate a multicomponent health system intervention designed to reduce escalating disease‐modifying treatment (DMT) expenditures and improve multiple sclerosis (MS) outcomes by increasing use of preferred formulary and highly effective DMTs (HETs).
Methods
We conducted a trend study of treatment utilization and expenditure outcomes prior to (2009–2011) and during (2012–2018) MS Treatment Optimization Program (MSTOP) implementation in Kaiser Permanente Southern California (KPSC) compared to a Kaiser Permanente region of similar size. Annual relapse rates (ARRs) were obtained from KPSC's electronic health records.
Results
Adherence to preferred formulary DMTs increased from 25.4% in 2011 to 72.2% in 2017 following MSTOP implementation in KPSC and 22.1% to 43.8%, respectively, in the comparator. KPSC's annual DMT expenditures in 2018 were less than in 2011 despite an 11.3% increase in DMT‐treated members. The decline in average per patient per year of treatment expenditures from a peak of $43.1 K in 2014 to $26.3 K in 2018 in KPSC was greater than the comparator, which peaked at $52.1 K and declined to $40.0 K in 2018. Over the 7 years following initiation of MSTOP, cumulative MS DMT expenditures were $161.6 million less than the comparator. HET use increased to 62.5% of per patient treatment‐years versus 32.4% in the comparator. This corresponded to a 69% decline in adjusted ARR (95% confidence interval = 64.1–73.2%; p < 0.0001) among DMT‐treated patients in KPSC.
Interpretation
A novel, expert‐led health system intervention reduced MS DMT expenditures despite rising prices while simultaneously reducing MS relapse rates. Our focus on health system progress toward meaningful, measurable targets could serve as a model to improve quality and affordability of MS care in other settings. ANN NEUROL 2022;92:164–172
The predominant approach to multiple sclerosis (MS) treatment in the United States has led to an exponential increase in societal MS treatment expenditures and a 7‐fold increase in patient out‐of‐pocket expenses, without convincing evidence of improved outcomes. 1 The unaffordable prices of MS disease‐modifying treatments (DMTs) also increase inequities by forcing some persons with MS (pwMS) who would benefit from DMTs to go un‐ or undertreated. In addition to pharma's unregulated ability to set and increase drug prices, nonevidence based and cost‐insensitive prescribing practices contribute to these increasing MS DMTs expenditures and their societal consequences.
US health systems have responded to these challenges by creating highly restrictive step therapy formularies based on opaque price negotiations. 2 Highly restrictive step therapy formularies typically require using the lowest cost DMT(s) and provide access to other DMTs only after health care providers complete prior authorization requests documenting treatment failure or contraindications. Whether this approach has successfully reduced DMT spending is unknown, and the impact on MS outcomes is equally unclear. It is possible that these programs could negatively impact outcomes by delaying access to highly effective DMTs (HETs) and creating unnecessary gaps in care when pwMS change health plans.
To address these challenges, we developed and began implementation of a multicomponent health system‐level intervention, the MS Treatment Optimization Program (MSTOP), in 2012. 3 The principal goals of MSTOP are to improve MS outcomes by increasing use of HETs while simultaneously reducing MS DMT expenditures by creating and adhering to an ethical, cost‐sensitive preferred formulary. We provide herein the impact the staggered implementation of MSTOP components had on treatment utilization and outcomes.
Materials and Methods
To determine whether the components of the intervention reduced MS DMT expenditures and improved outcomes, we conducted a trend study that covered the period prior to (2009–2011) and during implementation (2012–2018). During the study period, we examined the association of staggered component interventions with outcomes (average patient treatment‐year MS DMT expenditures and annual MS relapse rates [ARRs]) and treatment utilization process metrics (proportion of DMT‐treated pwMS on [1] preferred formulary DMTs, [2] HETs, or [3] new, low‐value DMTs). To assess whether general practice trends could explain changes in process metrics or DMT expenditures (cumulative and average per patient treatment‐year), we compared Kaiser Permanente Southern California (KPSC) to a Kaiser Permanente (KP) region of similar size with the same drug acquisition costs. MS DMT expenditures were obtained from the KP national pharmacy analytics database, and drug acquisition costs were provided by the KP pharmacy contracting group. For noncontracted products, wholesale acquisition costs were used.
To assess whether unmeasured KPSC practice trends could explain improvements in ARR, we conducted sensitivity analyses comparing a large KPSC medical center (MC) where implementation lagged behind the other 13 KPSC MCs. MS relapses were identified from electronic health records (EHRs).
Intervention
The design and implementation of MSTOP is described in detail elsewhere. 3 Briefly, MSTOP goals and strategies, in order of priority are: (1) increase use of HETs to improve outcomes, to be accomplished by implementation of a risk‐stratified treatment algorithm 4 ; (2) reduce DMT expenditures without reducing MS quality of care, to be accomplished by implementation of an ethical, cost‐sensitive preferred formulary 3 ; and (3) minimize uptake of new, low‐value DMTs through proactive counterlaunch campaigns. 3 Low value was defined as a DMT having a negotiated contracting price that exceeded the $150,000 cost per quality‐of‐life‐year gained threshold. 2 , 5 The proactive counterlaunch campaigns are low‐budget efforts and conducted by existing staff pharmacists and MS specialists. We employ academic detailing (informal discussions with individual or groups of providers often over meals), education sessions (2–3 per product), targeted messaging circulated through emails, and quarterly audit and feedback on uptake or lack thereof of low‐value products. We directly address unsubstantiated claims of a drug's superior efficacy or safety that are being disseminated to our providers through pharma's high‐budget, broadscale product launch campaigns. Information is gathered from review of full US Food and Drug Administration (FDA) documents, conference proceedings, Internet searches, and advertising materials in addition to the published literature. 3
Highly effective treatments are those that have demonstrated evidence of superiority to an active comparator in at least 1 head‐to‐head randomized controlled trial (RCT) and/or evidence of potency, defined as a large magnitude of effect in an RCT conducted in a population with highly active relapsing MS or a positive RCT conducted in pwMS who relapsed on modestly effective DMTs (meDMTs). The preferred formulary prioritizes the safest DMTs within the HET or meDMT groups, taking into consideration all available data, and the lowest cost within group DMT when efficacy and safety profiles are similar. 4 Preferred HETs were natalizumab, rituximab, and fingolimod, and preferred meDMTs were the lowest priced interferon‐beta and/or glatiramer acetate products when the preferred formulary was launched in 2013. Fingolimod was later removed as its inferior safety profile emerged. Each year, there was 1 preferred interferon‐beta and 1 preferred glatiramer acetate product on the preferred formulary. However, in the years when the contract negotiated prices between the lowest priced interferon‐beta and glatiramer acetate product were large, only one of these was designated a preferred agent for pwMS first starting a meDMT.
We did not forcibly switch patients with relapsing MS who were stable on a high‐priced, low‐value interferon‐beta product to the lowest priced option. When KP had negotiated a 3 or more‐year contract with a significantly lower price on an interferon‐beta product, neurologists were encouraged to discuss switching to the lowest priced option with the patient with a low threshold for switching back should the patient be unable to tolerate the less expensive version. This strategy, when supported by pharmacists in KP Colorado, showed no evidence of return of disease activity, tolerability was generally high, with few pwMS switching back to the nonpreferred interferon‐beta, and the cost savings were significant. 6 As is standard practice in KP, patients on brand name glatiramer acetate were switched to generic when it became available unless their prescribing neurologist specifically requested a brand name.
Targeted metrics agreed upon by neurology and practice leaders and health plan pharmacists were: (1) ≥60% of DMT‐treated pwMS should be on an HET, as natural history studies show that >60% of relapsing‐onset pwMS will develop long‐term disability 7 ; and (2) ≥90% of prescribed DMTs should be preferred formulary DMTs. 4
Setting
KPSC is a large prepaid health care organization that provides comprehensive health care to >4.7 million members. KPSC uses a comprehensive EHR system that includes all inpatient and outpatient encounters, laboratory and imaging tests, diagnoses, medications, and demographic characteristics. Permanente medical groups have a strong physician‐leadership culture and routinely partner with Kaiser Foundation Hospitals and Health Plan, including its pharmacy team. MS care is provided at 14 MCs by >200 neurologists.
The KP comparator region is similar in size (4.5 million members), structure, demographics, and culture to KPSC and did not fully endorse MSTOP until 2018. Prior to this, it hired 1 full‐time MS clinician (2012) and clinical pharmacists to try to increase use of MSTOP's preferred DMTs (2016–2018). Care is provided to ~5,000 pwMS annually in KPSC and the comparator. Drug acquisition costs are the same for both regions. Automatic substitution of brand name drugs with lower priced generics (once available) is routine practice across KP regions.
Analysis
To account for changes in contracted prices during a calendar year, the average annual price per DMT was used. For expenditure and cost analyses, DMT use was defined by patient treatment‐year, calculated by dividing the total annual expenditures for each DMT by the year‐specific average cost of that DMT. The average cost per patient per year of treatment was calculated by dividing the annual sum of DMT expenditures by the annual sum of patient treatment‐years across DMTs. PwMS who are untreated throughout the calendar year are by definition not included in these expenditure calculations.
To determine whether increased HET use corresponded to decreased ARR (calculated by dividing annual relapse counts by annual person‐years), we measured change over time compared to zero percent change using linear spline Poisson regression, crude and adjusted for age and sex. Nonlinear segmentation joint points were chosen based on visual inspection. Generalized additive models were used to test for linearity of trend.
To assess whether the observed decrease in total annual expenditures over the study period could be due to increase in proportion of untreated MS patients, an MS patient was defined as a member with at least 1 International Classification of Diseases (ICD) code for MS during that calendar year.
Identifying Relapse Episodes
An MS relapse was defined as receiving 3 to 5 days of intravenous methylprednisolone or an inpatient admission with MS as the primary discharge diagnosis. Clinically significant MS relapses in KPSC are typically treated at outpatient infusion centers. To allow for weekend and holiday interruptions, infusions occurring within 8 days of each other were counted as 1 episode. Inpatient hospitalizations occurring within 24 hours of each other were also counted as 1 relapse to account for interhospital transfers. Outpatient oral prednisone prescriptions were not included, because chart reviews throughout the study period showed these to be given primarily to patients for other indications (eg, allergies) or pseudorelapses.
To account for the delayed onset of action of MS DMTs, relapses were defined as those occurring 6 months or more after first DMT infusion/dispensed prescription during the study period. Relapses occurring in the ever‐treated population were retained during periods of noncompliance to reflect the real‐world problems of drug cessation relapses.
For the purposes of these analyses, KPSC members treated with any MS DMT during the study period and with at least 6 consecutive months of membership after starting a DMT were included. To eliminate transient DMT users where no effect on ARR would be expected, DMT use was defined based on the onset of action as follows: at least 4 consecutive infusions of natalizumab, 1 infusion of rituximab, 2 dispensed prescriptions of fingolimod, and at least 3 consecutive dispensed prescriptions for each of the following: interferon‐betas, glatiramer acetates, dimethyl fumarate, and teriflunomide. Members treated with MS‐specific DMTs (interferon‐betas, glatiramer acetates, dimethyl fumarate, teriflunomide) according to these criteria were considered to have MS. Because rituximab and natalizumab are not MS‐specific DMTs, charts of those with at least 1 MS or MS‐like ICD‐9 diagnostic code were abstracted to confirm MS diagnosis.
Person‐years contributions began at start of the first DMT during the study period and ended at membership termination, death, or DMT discontinuation as follows: 24 months since last rituximab or other B‐cell–depleting drug, 12 months after last natalizumab infusion, or the last dispensed prescription for all other DMTs. Patients who switched from an meDMT to an HET midyear were counted as being on an HET during that year to incentivize this desired change.
All statistical tests were 2‐sided, with p < 0.05 considered statistically significant. Analyses were conducted using SAS statistical software, version 9.4 (Cary, North Carolina).
Results
Adherence to the Expert‐Led Preferred Formulary and MS DMT Expenditures
Increased uptake of preferred DMTs (Fig 1) was accompanied by substantial reductions in spending on MS DMTs (Fig 2) in both KPSC and the comparator region. Use of preferred DMTs and total and per patient‐year DMT expenditures were similar in these regions until 2013, when MSTOP was endorsed by practice leaders and implemented across KPSC but not the comparator. In 2013, DMT expenditures began to diverge, with expenditures continuing to increase in the comparator region while flattening in KPSC. Following the comparator's assignment of clinical pharmacists to implement MSTOP's preferred formulary (2016–2018) and endorsement of MSTOP (2018), use of preferred DMTs increased (see Fig 1), total annual DMT expenditures stabilized, and the average cost per patient per year of treatment declined (see Fig 2).
FIGURE 1.
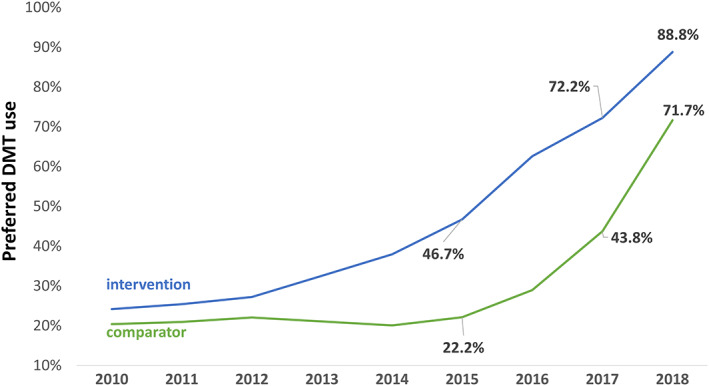
Use of preferred disease‐modifying treatments (DMTs). This graph depicts the annual proportion of patients treated with preferred DMTs as defined by the 2013 expert‐led preferred formulary (lowest priced interferon‐beta and/or glatiramer acetate product, natalizumab and rituximab) in the MS Treatment Optimization Program (MSTOP) intervention region (Kaiser Permanente Southern California [KPSC], blue line) and the Kaiser Permanente (KP) comparator (green line). Adherence to the preferred formulary within KPSC improved following MSTOP endorsement and spread in 2013. In the comparator, adherence to the preferred formulary improved from 2016 to 2018 with the assignment of clinical pharmacy support and endorsement of MSTOP in 2018. The timeline begins in 2010 because this is when the first DMT was designated as the preferred KP agent (by health plan pharmacy based on price).
FIGURE 2.
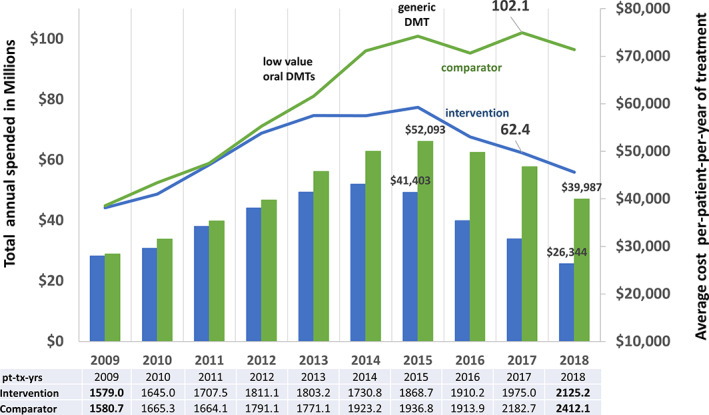
Annual multiple sclerosis (MS) disease‐modifying treatment (DMT) expenditures. This figure depicts the total annual expenditures in millions (left vertical axis) in the MS Treatment Optimization Program (MSTOP) intervention region (Kaiser Permanente Southern California [KPSC]; blue line) compared to a Kaiser Permanente (KP) region of similar size (comparator, green line); and the average annual cost per patient per year of treatment (US dollars, right vertical axis) in KPSC (blue bars) and comparator (green bars). Drug prices are the same across the KP regions. This figure shows that implementation of MSTOP starting in 2012 corresponds with a leveling off and then significant reduction in total and average per patient per year treatment expenditures on MS DMTs. In contrast, the total and average per patient per year treatment expenditures in the comparator continued to rise. Introduction of 2 low‐value oral modestly effective DMTs in September 2012 (teriflunomide) and March 2013 (dimethyl fumarate) corresponded with an increase in the comparator's but not the intervention's expenditures. Introduction of a generic DMT (glatiramer acetate) in mid‐2015 corresponds with reduced expenditures at the intervention site and an unsustained reduction in the comparator region. The total annual patient treatment‐years (pt‐tx‐yrs) increased during the study period in KPSC and the comparator region (shown below the figure).
The marked increase in use of preferred DMTs corresponded with a decrease in per‐patient DMT expenditures in KPSC starting in 2016, and by 2018 in the comparator. In 2017, the total and per patient per year of treatment expenditures in the comparator region were $39.7 million US dollars and $15,181 greater than KPSC, respectively. In the same year, the use of preferred DMTs by the comparator was 28.4% lower than KPSC.
The annual number of patient treatment‐years increased in both regions throughout the study period (see Fig 2), although the proportion of untreated pwMS was similar during the study period in KPSC (59.1% and 60.7% in 2010 and 2018, respectively) and the comparator (58.7% and 58.2% in 2010 and 2018, respectively).
Uptake and Expenditures on New, Low‐Value DMTs
The impact of the first proactive, counterlaunch campaign is shown in Figure 3. KPSC had significantly less uptake of these low‐value meDMTs (4.1–5.0% of DMT‐treated patients receiving teriflunomide or dimethyl fumarate) than the comparator (14.1–17.4%). This resulted in KPSC spending $81.9 and $99.8 million less than the comparator from FDA approval of these DMTs through 2018 and 2019, respectively. This difference in prescribing behavior contributed to a rise in total and per‐patient annual expenditures by the comparator but not KPSC (see Fig 2). The use of these low‐value, nonpreferred DMTs by the comparator negated the expected reduction in DMT expenditures following FDA approval of the first generic preferred meDMT (see Fig 2).
FIGURE 3.
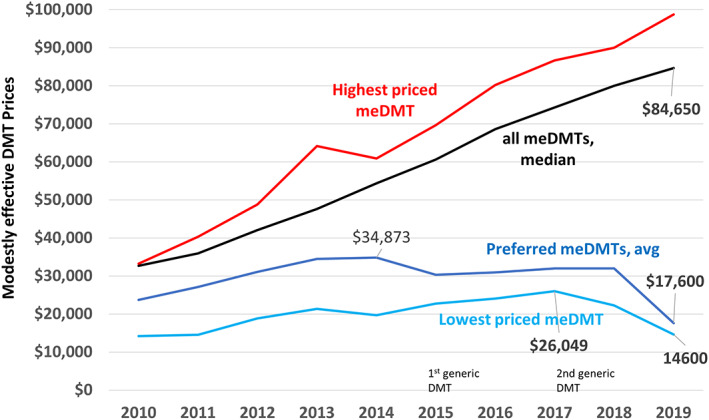
Prices of modestly effective disease‐modifying treatments (DMTs). This graph shows that average contract‐negotiated prices of the MS Treatment Optimization Program (MSTOP) designated preferred modestly effective DMTs (meDMTs; dark blue line; lowest priced interferon‐beta + lowest priced glatiramer acetate product) improved following MSTOP initiation despite continual and rapidly increasing prices of most meDMTs. The highest priced meDMT per year is shown by the red line and the median annual Kaiser Permanente–negotiated price of all meDMTs by the black line. Introduction of a generic DMT (glatiramer acetate) in mid‐2015 did not result in a decreased price of either the lowest priced meDMT (light blue line) or the average price of the preferred meDMTs. However, both of these prices came down following the introduction of a second generic glatiramer acetate product in October of 2017.
Reduced Contract Negotiated Prices of Preferred meDMTs
Despite marked increases in the prices of most meDMTs, the average annual negotiated cost of the preferred meDMTs (lowest priced interferon‐beta and lowest priced daily glatiramer acetate product) remained relatively stable between 2015 and 2018 ($30,342–$31,983) and dropped to $17,600 in 2019 (Fig 4). Introduction of the first generic glatiramer acetate in 2015 was not associated with a drop in price of the lowest priced meDMT; rather, the price increased from $19,707 to $26,049 between 2014 and 2017 (see Fig 4). Following introduction of the second generic glatiramer acetate product in October 2017, the lowest priced preferred meDMT declined to $14,600 annually.
FIGURE 4.
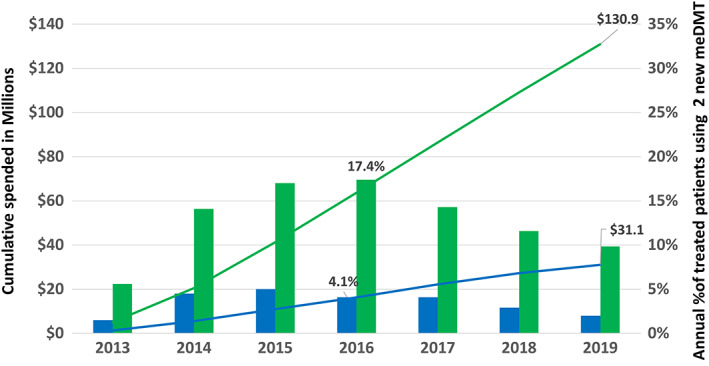
Expenditures and uptake of new, low‐value, modestly effective disease‐modifying treatments (meDMTs). Depicted are the differences in the cumulative expenditures (in millions, US dollars, left vertical axis) on 2 low‐value oral DMTs (lines) and annual percentage of DMT‐treated patient‐years on either of these DMTs (bars, right vertical axis) in the MS Treatment Optimization Program–intervention region (Kaiser Permanente Southern California [KPSC]; blue) and the comparator region (green). This figure shows that the active counterlaunch campaign conducted in KPSC but not the comparator region significantly reduced uptake of these new, low‐value products (teriflunomide and dimethyl fumarate), leading to $99.8 million US dollars less in spending in KPSC than the comparator through 2019. Teriflunomide was US Food and Drug Administration approved in September 2012 and dimethyl fumarate in March 2013. The drug costs are the same for KPSC and the comparator region.
Increased Utilization of HETs and MS Relapses
HET use increased from 11.6% to 43.2% of patient treatment‐years between 2012 and 2016 and reached 62.5% by the end of 2018 in KPSC. In contrast, HET use rose very slowly in the comparator region (from 12.2% to 16.8%, 2012–2016) until its endorsement of the treatment algorithm in 2018. By the end of 2018, the comparator's HET use increased to 39.8% of patient treatment‐years. Approximately 80% of patients on HETs in both the comparator and intervention groups were treated with rituximab at the end of the study period in 2018.
The key milestones of MSTOP development and implementation, annual incidence of MS relapses and annual proportion of pwMS on an HET in KPSC, are depicted in Figure 5. In the year prior to leadership's decision to hire an MS clinician‐researcher (2009), only 4.9% of DMT‐treated MS patients received an HET and the ARR was 182.2 (95% confidence interval [CI] = 165.0–199.4) per 1,000 patient‐years (see Fig 5). The annual MS relapse rate was stable between 2009 and 2011, and HET use rose to 11.3%. The age‐ and sex‐adjusted ARR declined by 17.6% (95% CI = 11.2–23.6%, p < 0.0001) between 2012 and 2014, and HET use rose to 19.5%. HET use accelerated in 2015, and ARR declined 37.0% (95% CI = 30.5–42.8%, adjusted) between 2015 and 2018. In 2018, 59.5% of DMT‐treated MS patients were receiving an HET, and the ARR was 67.3/1,000 patient‐years (95% CI = 58.8–75.9) for a total decline of 69.0% (adjusted, 95% CI = 64.1–73.2%, p < 0.0001) between 2011 and 2018. The average age increased, the proportion of females decreased slightly, and the number of DMT‐treated pwMS increased.
FIGURE 5.
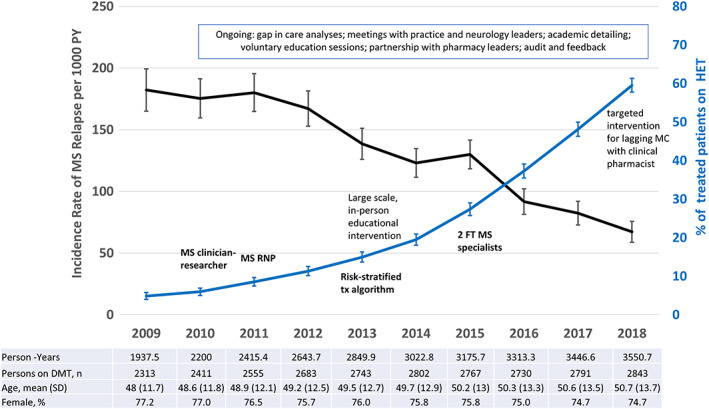
Multiple sclerosis (MS) program implementation, highly effective disease‐modifying treatments (DMTs), and MS relapses. Depicted are the key milestones of the multicomponent MS intervention (in text), the annual proportion of MS patients treated with highly effective DMTs (HETs) among persons treated with any DMT with 95% confidence intervals (CIs; blue line, right vertical axis) and the annual MS relapse rate (ARR) per 1,000 person‐years with 95% CI (black line, left vertical access) among MS DMT‐treated patients. The figure shows that the increase in use of HETs corresponds to a decrease in the ARR and that both accelerated in 2015 corresponding with the hiring of 2 additional MS specialists. A targeted intervention for a large medical center (MC) that lagged in adopting the MS Treatment Optimization Program began in September of 2017 and included series of local education sessions for neurologists and training of a clinical pharmacist supervised by an MS specialist remotely. MS ARR was stable prior to implementation (2009–2011), and declined by 69.0% (95% CI = 64.1–73.2%, p < 0.0001, adjusted for age and sex) between 2011 and 2018. FT = full time; SD = standard deviation; RNP = registered nurse practitioner; tx = treatment.
To assess whether the decline in ARR was due to background practice trends in KPSC rather than increasing HET use, we compared the large MC that lagged in HET use (27.8% in 2016) to the other 13 MCs (39.2% in 2016). Although a targeted intervention in 2017–2018 led to increased HET use at the large MC (59.5% by the end of 2018), this lag in implementation resulted in a 49.0% decline in ARR between 2011 and 2018, (95% CI = 35.4–59.8%) compared to a 75.5% decline at the other MCs with more rapid uptake (95% CI = 70.5–79.7%, age‐ and sex‐adjusted).
Discussion
Our novel physician‐led approach simultaneously reduced MS DMT expenditures and the frequency of MS relapses. We know of no other health system intervention that has reduced MS DMT expenditures or improved outcomes. Annual DMT expenditures in 2018 were less than those in 2011, even with an 11.3% increase in DMT‐treated pwMS. In contrast, Medicare's MS DMT costs rose on average 12.8% annually during the same timeframe. 1 In 2016, Medicare spent an estimated $4.4 billion primarily on meDMTs that are unlikely to have improved outcomes, as the majority of Medicare recipients are 65 years of age or older. At these ages, several observational studies have found no benefit of treatment with these DMTs, 8 , 9 , 10 and RCT data to suggest otherwise do not exist. 11 In contrast, we focused on increasing use of HETs, treatments that by definition are more effective than meDMTs.
Unique aspects of our approach that we believe underpin its success are that clinical experts led the development and implementation of the risk‐stratified, cost‐sensitive formulary rather than administrators; that the formulary adheres to ethical principles of step therapy design 12 ; that we provided quarterly audit and feedback on easily measured process metrics that are closely tied to the desired outcomes; that we expanded access to MS providers to implement MSTOP; and that proactive counterlaunch campaigns were created to curtail escalating expenditures.
Setting step therapy formularies that prioritize or require the use of the lowest cost DMT(s) before providing access to others is how US health systems approach MS care. This was true for KP as well, prior to MSTOP. How well this approach works in curbing expenditures and its impact on outcomes have not previously been reported. Our findings show that this approach was not successful, with total and per patient per year of treatment expenditures continuing to rise and no impact on relapse rates. In our system, adherence to this cost‐only preferred formulary was low.
To motivate changes in prescribing behavior in real time and create an intervention that can be spread to other settings, we carefully chose process metrics that are closely tied to improved affordability and quality and can be easily measured in real‐world settings. Strictly defined relapses and sustained disability progression (measured by the Expanded Disability Status Scale) as used in MS RCTs are impractical in real‐world settings. Capturing these outcomes requires regular, lengthy in‐person follow‐ups, and even when assessed at irregularly spaced in‐person visits, they are not entered as discrete data elements. Although these outcomes can be obtained via manual chart abstractions in KP, this is costly and time‐consuming and not possible in many US health care systems. Using claims for mobility aides, although easy to obtain, is uninformative over annual intervals, as disability in MS typically accumulates slowly over decades.
Highly effective treatments, as we define them, have RCT evidence demonstrating superior evidence to meDMT, either by direct comparison or by potency. 4 Thus, it is not surprising that increasing use of HETs would correspond to declining relapse rates. Although we did not measure disability progression, since the initial inception of our risk‐stratified algorithm in 2012, there is growing real‐world evidence that increasing utilization of HETs reduces the risk of intermediate 13 and long‐term disability. 14
Similarly, improvements in the adherence to the ethical, cost‐sensitive preferred formulary metric is expected to reduce expenditures by design. However, simply creating this preferred formulary was not enough to improve adherence or reduce expenditures in the comparator region. Not until staff was assigned and trained to implement MSTOP did expenditures decline.
The proactive counterlaunch campaigns conducted as part of MSTOP were very effective in limiting the uptake of new, low‐value DMTs, whereas the launch of the first generic DMT had less impact than anticipated. Conversion from brand name to the first generic preferred meDMT decreased expenditures in KPSC but not the comparator. Negotiated prices of the preferred meDMTs did not improve. The MS market space, like many other conditions, is crowded with branded products that drive up prices of all DMTs as they come to market. Like other US pharmaceutical market spaces, the price of the branded DMT increases significantly in the year prior to introduction of its generic competitor, which then launches at high price, albeit lower than the current brand price. Coinciding with the first generic DMT was the launch of 2 heavily advertised first in class, low‐value DMTs that negated potential cost savings from brand name to generic conversions in the comparator region.
By successfully adhering to the preferred formulary, we were able to create competition in the heavily populated meDMT market space, particularly after the second generic launched. This enabled the health plan to negotiate lower prices for both preferred classes of meDMTs. Unlike Medicare's 12.8% mean annual increased costs, KP's average cost of preferred meDMTs decreased by 49.9% over 5 years.
The biggest limitation of this study is whether MSTOP can be adapted to non‐KP settings. This will depend upon insurers' and clinicians' willingness to work together. Systems with similarly close relationships between providers and the insurance carrier like the Department of Veterans Affairs and Department of Defense may be the most feasible places to pilot an adapted version of MSTOP. Public county health systems that provide care for the most vulnerable pwMS and have extremely tight budgets should also strongly consider adapting MSTOP to their cost structure.
Another less formidable barrier to MSTOP spread is increasing use of the lowest cost B‐cell–depleting treatment, including rituximab or its biosimilars. Citing lack of FDA approval, some neurologists may be reluctant to prescribe rituximab because they believe that B‐cell–depleting agents that are specifically FDA‐approved for MS (ocrelizumab and subcutaneous ofatumumab) are somehow more effective or safer than off‐label use of rituximab. Although neither of these products were available during the course of this study, we have overcome this barrier by providing clinicians with health systems science education 3 and providing them with ongoing review of the safety of rituximab use in MS. The FDA approval process does not assess whether products are safer or more effective than others unless head‐to‐head RCTs are conducted. To date, there is no evidence that the FDA‐approved products are safer or more effective than rituximab or its biosimilars.
In addition, many payers set up barriers to coverage for rituximab or its biosimilars for the treatment of MS. 2 This, combined with the lack of pharma‐sponsored copay assistance for rituximab, can result in unaffordable patient out‐of‐pocket expenses, leading some well‐intentioned neurologists to prescribe the high‐priced branded products with copay assistance programs. This type of unintended consequence hurts both consumers and the health plan, as it drives up costs for all health plan members and underscores the importance of insurers' working directly with clinicians to adapt MSTOP to their cost structure.
Another potential limitation is that the decline in ARR could be explained by unmeasured practice trends rather than the increased use of HETs. If this were the case, we should have seen similar ARR decline across KPSC MCs regardless of HET use. Defining MS relapses by methylprednisolone infusions and hospitalizations likely underestimates the true ARR by missing mild, untreated relapses, but this does not explain the decline over time or differences between MCs. A limitation of the HET use metric is that it does distinguish between patients with relapsing versus nonrelapsing forms of MS. However, this would not explain the decrease in relapse rates among DMT‐treated pwMS over time or between the large MC that lagged in HET use, as these MS subtypes were treated with meDMT prior to MSTOP implementation.
The strengths of this study are the importance of the question, the comprehensive focus on overcoming barriers to appropriate DMT use, and the assessment of both expenditures and outcomes. HETs continue to be underutilized even at US academic MS centers, 15 and MS DMT costs continue to rise. Our experience suggests that physician‐led rational prescribing programs could lead to substantial improvements in quality and affordability in the care of pwMS in other settings.
Author Contributions
A.L.‐G. and M.H.K. contributed to the conception and design of the study; S.C.C., B.H.L., and J.B.S. contributed to the collection and interpretation of data included in the article; A.L.‐G. and J.B.S. contributed to drafting the text and preparing the figures.
The KPSC MS Specialist Group at the time this work was done consisted of Brandon E. Beaber, MD, Downey Medical Center, Department of Neurology, Southern California Permanente Medical Group; Sonu M. Brara, MD, Panorama City Medical Center, Department of Neurology, Southern California Permanente Medical Group; and Allen Scott Nielsen, MD, Fontana Medical Center, Department of Neurology, Southern California Permanente Medical Group.
Potential Conflicts of Interest
Nothing to report.
ACKNOWLEDGMENTS
This study was funded by Southern California Permanente Medical Group.
We thank Dr M. K. Gould for helpful feedback throughout the development and implementation of MSTOP, Drs B. Mittman and S. A. Klocke for helpful input during the development of MSTOP, and E. G. Gonzales for help with manuscript preparation.
References
- 1. San‐Juan‐Rodriguez A, Good CB, Heyman RA, et al. Trends in prices, market share, and spending on self‐administered disease‐modifying therapies for multiple sclerosis in Medicare Part D. JAMA Neurol 2019;76:1386–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Institute for Clinical and Economic Review . Disease‐modifying therapies for relapsing‐remitting and primary progressive multiple sclerosis: effectiveness and value. Final evidence report. March 6, 2017. Available at: http://icerorg.wpengine.com/wp-content/uploads/2020/10/CTAF_MS_Final_Report_030617.pdf. Accessed September 1, 2020.
- 3. Langer‐Gould A, Cheng SC, Li BH, Kanter MH. The multiple sclerosis treatment optimization program. Ann Clin Transl Neurol 2021;8:2146–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langer‐Gould A, Klocke S, Beaber B, et al. Improving quality, affordability, and equity of multiple sclerosis care. Ann Clin Transl Neurol 2021;8:980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Institute for Clinical and Economic Review . Siponimod for the treatment of secondary progressive multiple sclerosis: effectiveness and value. Final evidence report. June 20, 2019. Available at: https://34eyj51jerf417itp82ufdoe-wpengine.netdna-ssl.com/wp-content/uploads/2020/10/ICER_MS_Final_Evidence_Report_062019.pdf. Accessed September 1, 2020.
- 6. Hahn N, Palmer KE, Klocke S, Delate T. Therapeutic interferon interchange in relapsing multiple sclerosis lowers health care and pharmacy expenditures with comparable safety. Perm J 2018;30:18‐046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pittock SJ, McClelland RL, Mayr WT, et al. Clinical implications of benign multiple sclerosis: a 20‐year population‐based follow‐up study. Ann Neurol 2004;56:303–306. [DOI] [PubMed] [Google Scholar]
- 8. Hua LH, Fan TH, Conway D, et al. Discontinuation of disease‐modifying therapy in patients with multiple sclerosis over age 60. Mult Scler 2019;25:699–708. [DOI] [PubMed] [Google Scholar]
- 9. Yano H, Gonzalez C, Healy BC, et al. Discontinuation of disease‐modifying therapy for patients with relapsing‐remitting multiple sclerosis: effect on clinical and MRI outcomes. Mult Scler Relat Disord 2019;35:119–127. [DOI] [PubMed] [Google Scholar]
- 10. McFaul D, Hakopian N, Smith J, Langer‐Gould A. Stopping disease modifying therapy in patients over age 50 with presumed benign multiple sclerosis. Neurology 2020;94:2057. [Google Scholar]
- 11. Butler M, Forte ML, Schwehr N, et al. AHRQ comparative effectiveness reviews. Decisional dilemmas in discontinuing prolonged disease‐modifying treatment for multiple sclerosis. Rockville, MD: Agency for Healthcare Research and Quality, 2015. [PubMed] [Google Scholar]
- 12. Nayak RK, Pearson SD. The ethics of 'fail first': guidelines and practical scenarios for step therapy coverage policies. Health Aff (Millwood) 2014;33:1779–1785. [DOI] [PubMed] [Google Scholar]
- 13. Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing‐remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol 2021;78:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease‐modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019;76:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGinley M, Thompson N, Weber M, et al. Trends in the use of highly effective disease modifying treatments in multiple sclerosis over 12 years across 10 sites. 2019;92:S26.003. [Google Scholar]


