Abstract
Partial loss-of-function mutations in glycosylation pathways underlie a set of rare diseases called Congenital Disorders of Glycosylation (CDGs). In particular, DPAGT1-CDG is caused by mutations in the gene encoding the first step in N-glycosylation, DPAGT1, and this disorder currently lacks effective therapies. To identify potential therapeutic targets for DPAGT1-CDG, we performed CRISPR knockout screens in Drosophila cells for genes associated with better survival and glycoprotein levels under DPAGT1 inhibition. We identified hundreds of candidate genes that may be of therapeutic benefit. Intriguingly, inhibition of the mannosyltransferase Dpm1, or its downstream glycosylation pathways, could rescue two in vivo models of DPAGT1 inhibition and ER stress, even though impairment of these pathways alone usually causes CDGs. While both in vivo models ostensibly cause cellular stress (through DPAGT1 inhibition or a misfolded protein), we found a novel difference in fructose metabolism that may indicate glycolysis as a modulator of DPAGT1-CDG. Our results provide new therapeutic targets for DPAGT1-CDG, include the unique finding of Dpm1-related pathways rescuing DPAGT1 inhibition, and reveal a novel interaction between fructose metabolism and ER stress.
Author summary
Sugar modifications are important to proper protein folding and function. The biological pathways that control these sugar modifications are called glycosylation pathways. When these pathways are disrupted, such as through genetic mutation, it can cause rare developmental disorders called Congenital Disorders of Glycosylation (CDGs). Most CDGs lack any treatment options, and little is known about interacting genes or pathways that might improve these disorders. Here, we study DPAGT1-CDG which involves mutations in the enzyme-encoding gene, DPAGT1, by using the fruit fly model organism, Drosophila melanogaster. Using inhibitory drugs, we perform two cell-based screens with CRISPR technology to determine which genes are important under DPAGT1 inhibition. We then used a living fly, that models DPAGT1-CDG in the eye, in order to determine which of the top screened genes can improve its impaired eye development. Surprisingly, we found that inhibiting other CDG-causing genes, such as our top hit Dpm1, could improve our DPAGT1-CDG cell and fly models. In addition, loss of certain metabolism genes, and changes in diet, also improved the DPAGT1-CDG model. This study finds multiple new genes and pathways implicated in DPAGT1-CDG and lays the groundwork for new CDG therapies.
Introduction
Glycosylation comprises a variety of sugar-based co- or post-translational modifications that occur primarily in the endoplasmic reticulum (ER) and Golgi apparatus [1]. It includes N-, O-, C-, and S-linked bonds as well as C-terminal-linked GPI anchors. Mutations in glycosylation pathways underlie rare diseases known as Congenital Disorders of Glycosylation (CDGs) [2]. CDGs can be caused by defects of enzymatic machinery or in transport of enzymatic substrates (such as UDP-Gal or sialic acid) [3]. CDGs have limited treatment options. While some treatments correct the cause of the disease, such as dietary sugar supplementation, most only alleviate symptoms [3,4]. Research into the mechanisms of these pathways should help provide better avenues for advancing patient care.
Of the glycosylation pathways, N-linked glycosylation is critical for proper protein folding and function [1,5]. Inhibition of or abnormal N-linked glycosylation causes a build-up of misfolded proteins in the ER lumen, ER stress, and induction of the unfolded protein response (UPR) [5–10]. The UPR is a transcriptional response that attempts to restore homeostasis to the cell, but it can also lead to apoptosis when the stress is not resolved. The initial metabolite required for N-glycosylation is GlcNAc-PP-dolichol, which is synthesized by the dolichyl-phosphate N-acetylglucosamine phosphotransferase 1 (DPAGT1) enzyme [11] (Alg7 in Drosophila, hereafter referred to as DPAGT1). In humans, partial loss-of-function mutations in DPAGT1 can cause DPAGT1-CDG (CDG-Ij) with symptoms including seizures and developmental delay, among others [11,12]. Mutations in DPAGT1 are also associated with congenital myasthenic syndrome due to hypoglycosylated acetylcholine receptors [13,14]. Inhibiting DPAGT1 with the nucleoside Tunicamycin (Tun) reduces GlcNAc-PP-dolichol levels [15,16] and induces ER stress [17]. DPAGT1-CDG patient cells are more sensitive to Tun [12], and DPAGT1 mutant cell lines also show an increased level of chronic ER stress, autophagy, and have signs of senescence [18].
As with most CDGs, there are only symptomatic treatment options and no causative therapeutics available for DPAGT1-CDG [14,19]. Any therapeutic option must be precise, as overexpression of DPAGT1 can also lead to improper N-glycosylation and may underlie some cancer phenotypes [20]. One alternative approach to current therapeutics is to identify modifier genes, where targeting genes that interact with DPAGT1 expression or downstream phenotypes can potentially be used to develop new therapeutic options [21–23]. Moreover, determining if patients have differential baseline expression of these modifier genes may result in better personalized therapeutic solutions [22].
Here we present two genome-wide CRISPR screens for genes that modify phenotypes associated with reduced function of DPAGT1. These screens identify novel genes involved in modifying cellular and physiological outcomes associated with DPAGT1 inhibition and increased ER stress. Of note, we find that knockout of multiple GPI anchor biosynthesis genes improves survival and cell surface glycoprotein levels in Drosophila S2 cells associated with DPAGT1 inhibition and ER stress from tunicamycin. In addition, we find that the mannosyltransferase Dpm1 is one of the strongest modifier genes and inhibition of Dpm1 vastly improves cell survival under the loss of DPAGT1 function and ER stress. We provide evidence that this effect likely occurs through the combined role of Dpm1 in O-mannosylation, N-glycosylation, and GPI anchor biosynthesis pathways. Importantly, the majority of our top candidate genes validate in in vivo models of DPAGT1 inhibition and ER stress. In addition, results from the cell screens suggest that fructose metabolism might affect the loss of DPAGT1 function, and we observed altered fructose metabolism in the in vivo DPAGT1 model, suggesting that dietary fructose supplementation may hold therapeutic promise for these patients. Taken together, we identify numerous new candidate DPAGT1 modifier genes, using Drosophila flies and cells, that are potential therapeutic targets and report the remarkable finding that impairing CDG genes in parallel can result in an improved overall phenotype.
Results
Disruption of glycosylation and glycolytic pathways rescue the effects of DPAGT1 inhibition
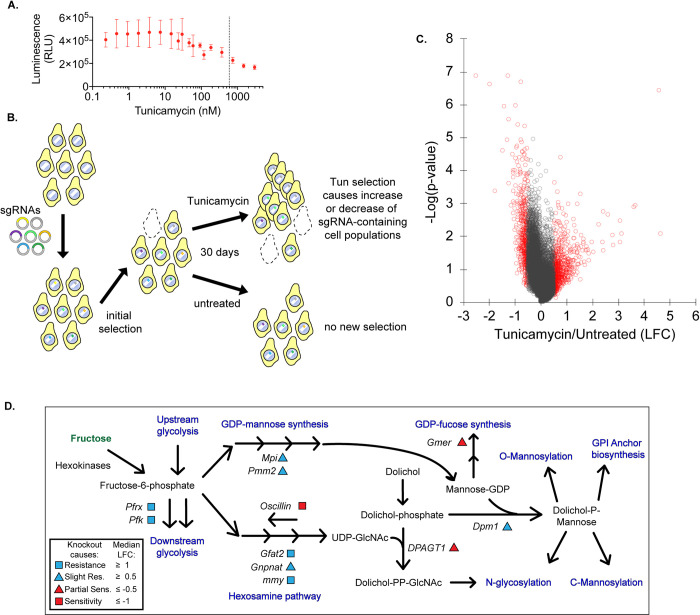
To identify genes impacting the loss of DPAGT1 function and ER stress, we performed a cell-based, genome-wide CRISPR/Cas9 knockout screen (hereafter referred to as "survival screen"). Briefly, we used genome-wide single guide RNA (sgRNA) libraries transfected into a Drosophila S2 cell line to generate a pool of cells in which each cell carried gene knockouts (as described previously [24,25], Fig 1). Each gene tested had at least four different sgRNAs per gene in order to mitigate sgRNA-specific effects. After gene knockout, cells were either untreated or exposed to a sublethal dose of the DPAGT1 inhibitor Tunicamycin (Tun; 500ng/ml [590nM]). This was determined by a titration assay and selection of a dose capable of ~50% reduction in growth rate (Fig 1). After 30 days under DPAGT1 inhibition, cell populations were subjected to deep amplicon sequencing to determine sgRNA abundance. A change in the abundance of an sgRNA in the final population of DPAGT1-inhibited cells vs. untreated cells indicated that knockout of that gene caused resistance or sensitivity. Genes were ranked by the median log-fold change (lfc) in the abundance of each sgRNA targeting that gene (Tables 1 and 2 and S1).
Fig 1. A survival screen reveals hexosamine, glycolysis, and GDP-mannose synthesis in resistance to DPAGT1 loss.
(A) We used a cell titer assay to determine the concentration of Tun capable of reducing cell growth rate by ~50% for use in both follow-up screens. (B) We introduced a whole genome guide RNA library into Drosophila cells stably expressing constitutive Cas9. Pooled cell populations were grown for 30 days either untreated or with Tun. Final cell populations were sequenced for sgRNA abundance to determine candidate genes implicated in Tun resistance or sensitivity. (C) Volcano plot of survival screen. Red dots indicate genes with an absolute lfc value of ≥0.5 or ≤-0.5. (D) A simplified model of one set of highly enriched pathways for gene knockouts causing resistance or sensitivity. Note that only genes whose knockout provided resistance or sensitivity are labeled.
Table 1. Summary of survival screen data.
| Resistance genes | Sensitivity genes | ||||
|---|---|---|---|---|---|
| lfc | Total hits | % total genes | lfc | Total hits | % total genes |
| 2 or higher | 16 | 0.12% | -2 or lower | 1 | 0.01% |
| 1–1.99 | 156 | 1.18% | -1 –-1.99 | 9 | 0.07% |
| 0.5–0.99 | 979 | 7.40% | -0.5 –-0.99 | 241 | 1.82% |
Table 2. Top 20 candidate genes providing resistance and sensitivity.
| Fly Gene | Human ortholog | lfc | Fly Gene | Human ortholog | lfc |
|---|---|---|---|---|---|
| PEK | EIF2AK3 | 4.76 | pnt | ETS1 | -2.41 |
| Gfat2 | GFPT2 | 4.68 | Oscillin | GNPDA2 | -1.87 |
| CG9609 | GTF3A | 3.78 | upSET | KMT2E | -1.66 |
| Hrd3 | SEL1L | 3.71 | CG9590 | FAM114A2 | -1.37 |
| Ide | IDE | 3.25 | GalT1 | B3GALT1 | -1.31 |
| drongo | AGFG1 | 3.19 | Asx | ASXL3 | -1.18 |
| CG30345 | SLC46A1 | 3.17 | CG10984 | ANKRD12 | -1.17 |
| EcR | NR1H3 | 2.94 | Vap-33A | VAPB | -1.12 |
| ham | MECOM | 2.91 | Rab40 | RAB40C | -1.12 |
| srp | GATA1 | 2.72 | Cdk2 | CDK2 | -1.05 |
| sau | GOLPH3 | 2.53 | bel | DDX3X | -0.98 |
| Pten | PTEN | 2.52 | P5cr-2 | PYCR3 | -0.96 |
| Sin3A | SIN3A | 2.52 | Tango14 | NUS1 | -0.95 |
| Cdep | FARP2 | 2.37 | vtd | RAD21 | -0.95 |
| CG10960 | SLC2A8 | 2.16 | CG33110 | ELOVL1 | -0.95 |
| REG | PSME3 | 2.08 | CG32079 | SLC36A2 | -0.95 |
| dl | REL | 1.98 | Tor | MTOR | -0.94 |
| ND-MLRQ | NDUFA4 | 1.97 | Oatp58Da | SLCO1A2 | -0.93 |
| CG2926 | PHRF1 | 1.94 | dre4 | SUPT16H | -0.90 |
| Gpdh1 | GPD1 | 1.90 | sws | PNPLA7 | -0.90 |
Human orthologs are based on the highest DIOPT score [26].
1151 gene knockouts (8.7% of genes tested) increased resistance to DPAGT1 inhibition at an lfc value of ≥0.5, while 251 gene knockouts (1.9% of genes tested) increased sensitivity to DPAGT1 inhibition at an lfc value of ≤-0.5 (Table 1). Top resistance genes include the major ER stress sensor PEK (human: PERK; lfc = 4.76), hexosamine enzymes Gfat2 (GFPT2; lfc = 4.68) and mmy (UAP1; lfc = 1.57), the insulin-degrading enzyme Ide (IDE; lfc = 3.25), and the glycolytic kinase Pfk (PFKM; lfc = 1.47) and the kinase/phosphatase Pfrx (PFKFB1; lfc = 1.74) (Tables 2 and S1). Top sensitivity genes include the proto-oncogene pnt (ETS1; lfc = -2.41), the hexosamine enzyme Oscillin (GNPDA2; lfc = -1.87), and the transporter-related genes Rab6 (RAB6A; lfc = -0.85) and Rab40 (RAB40C; lfc = -1.12) (Tables 2 and S1).
Many top candidate genes are Drosophila orthologs of known CDG genes. To explore this further, we queried all known Drosophila CDG orthologs and a CDG genetic panel [27,28] (145 genes, S2 Table). We considered genes for which knockout increased sensitivity (6 genes) separately from genes for which knockout increased resistance (23 genes). We did not perform gene enrichment analysis for the ‘increased sensitivity’ genes due to lack of statistical power. Remarkably, there was a significant enrichment of CDG genes among genes for which knockout increased resistance to DPAGT1 inhibition (23 observed vs. 12.6 expected, p<0.01, S2 Table). This suggests that perturbation of certain CDG-related pathways in parallel can result in an overall improvement to cellular health.
We performed Gene Ontology (GO) analysis on all candidate genes with an absolute lfc ≥ 0.5 compared to untreated cells (Tables 3 and S3). We chose to use both resistance and sensitivity genes in the same analysis, as many pathways include genes that are either positive or negative regulators. The top enriched categories included "nucleotide-sugar biosynthetic process" (GO:0009226) and "pyruvate metabolic process" (GO:0006090). These include genes involved in the hexosamine pathway and glycolysis, respectively, and relate to DPAGT1, as both pathways are critical in synthesizing upstream metabolites for several glycosylation pathways [29]. To further explore these connections, we also examined an adjacent pathway (GO term "GDP-mannose metabolic process", GO:0019673). This pathway involves the creation of GDP-mannose from fructose-6-phosphate, which includes the most common CDG gene Pmm2 (PMM2; lfc = 0.50), and is also important in glycosylation pathways [1]. Here, we found multiple genes at an lfc value cutoff of ±0.5 (4/7 Drosophila orthologs, Fig 1C). Overall, there is strong enrichment of genes in glycolytic, hexosamine, and related pathways, suggesting the hypothesis that suppression of these pathways rescues the effects of DPAGT1 inhibition.
Table 3. Gene ontology analysis of top candidate genes from the survival screen.
| Gene Ontology | Fold Enriched | FDR |
|---|---|---|
| nucleotide-sugar biosynthetic process (GO:0009226) | 6.57 | 3.85E-02 |
| nucleotide-sugar metabolic process (GO:0009225) | 6.34 | 5.26E-03 |
| regulation of feeding behavior (GO:0060259) | 4.38 | 4.40E-02 |
| female germ-line stem cell population maintenance (GO:0036099) | 3.94 | 1.11E-02 |
| larval midgut histolysis (GO:0035069) | 3.52 | 4.55E-02 |
| cuticle pattern formation (GO:0035017) | 3.39 | 3.74E-02 |
| midgut development (GO:0007494) | 3.38 | 2.58E-02 |
| pyruvate metabolic process (GO:0006090) | 3.37 | 1.78E-02 |
| cell surface receptor signaling pathway involved in cell-cell signaling (GO:1905114) | 3.29 | 4.42E-02 |
| synaptic assembly at neuromuscular junction (GO:0051124) | 3.29 | 4.40E-02 |
Candidate genes with an lfc of ≥0.5 or ≤-0.5 were analyzed together.
Loss of GPI anchor biosynthesis rescues DPAGT1 inhibition-induced reduction in cell surface glycoprotein levels
Tun inhibition of DPAGT1 results in reduction of specific glycoproteins from the cell surface such as AchR [30,31] and CASR [32] as well as total cell surface glycosylation [33]. Some candidate modifier genes might rescue the cellular survival phenotype by rescuing cell surface glycoprotein levels. To identify this subset of candidates, we performed a parallel genome-wide screen (Fig 2). We used an identical pool of CRISPR knockout Drosophila cells as described above. Again, cells were either untreated or exposed to Tun and fluorescently-labeled with Concanavalin A (ConA), which binds cell surface glycoproteins [34,35]. Cells were then sorted based on fluorescence, with a higher fluorescent signal indicating more cell surface glycoproteins and vice versa. This assay allowed us to determine if a gene knockout can rescue DPAGT1 inhibition-induced loss of cell surface glycoprotein levels. We note that it is possible that some of these knockouts might be capable of restoring cell surface glycoproteins under non-tunicamycin stress conditions.
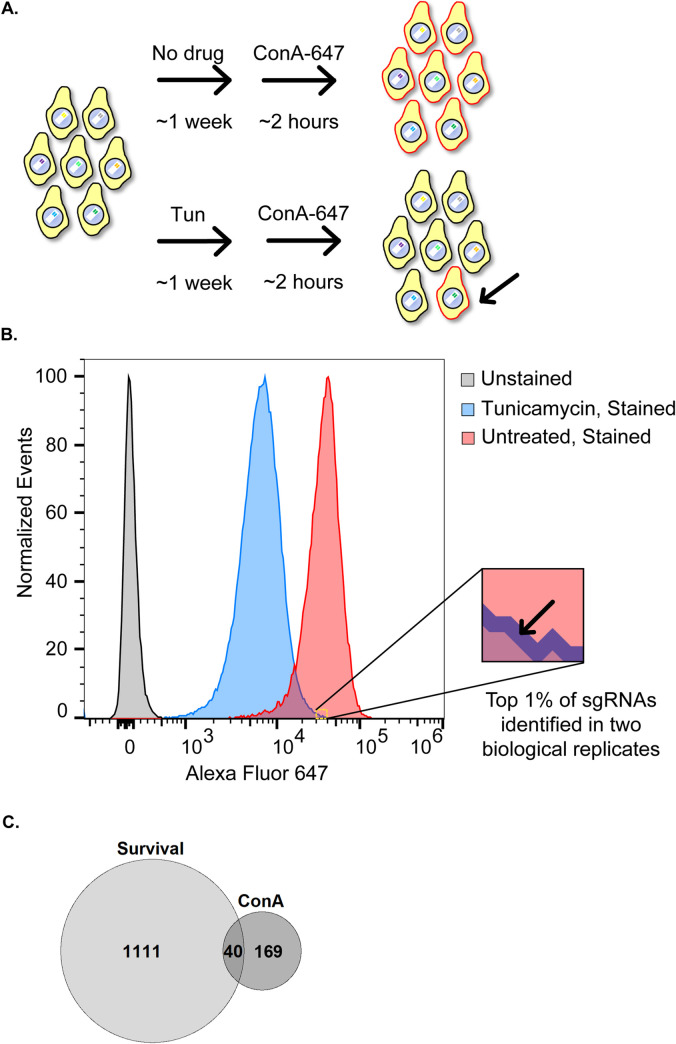
Fig 2. A Concanavalin A screen for gene knockouts capable of restoring cell surface glycoproteins under DPAGT1 inhibition.
(A) CRISPR knockout cells were either untreated or treated with Tun, and then stained with fluorescently-labeled ConA to mark cell-surface glycoproteins. (B) Flow cytometry output of the ConA experiment. Tun treatment caused a ~5-fold decrease in ConA staining intensity on average. The "blown-up" section denotes the start of the gate and encompasses Tun-treated cells capable of consistently restoring cell surface glycoproteins to untreated cell levels (the top ~1% of cells). (C) Venn diagram comparison of the survival and ConA screens. There were 40 genes that restored cell surface glycoproteins and provided resistance to DPAGT1 inhibition.
Under DPAGT1 inhibition, knockout of multiple genes was able to rescue cell surface glycoproteins to the levels observed in untreated cells (S4 Table). We considered genes to be the strongest candidates if they had at least one sgRNA in the highest ~1% of fluorescence of all sgRNAs in two biological replicates (Fig 2 and Tables 4 and S4). This filtering resulted in identification of 209 genes (~1.5% of all genes tested) whose knockout provided strong and consistent rescue of cell surface glycoprotein levels under DPAGT1 inhibition.
Table 4. Top 20 candidate genes from the ConA screen.
| Fly Gene | Human ortholog | # Guides in top ~1% |
|---|---|---|
| Twin | CNOT6L | 10 |
| CG5276 | CANT1 | 7 |
| PIG-S | PIGS | 6 |
| PIG-A | PIGA | 5 |
| e(y)3 | PHF10 | 5 |
| HGTX | NKX6-1 | 5 |
| Slif | SLC7A1 | 5 |
| Ssh | SSH2 | 5 |
| Afti | AFTPH | 4 |
| Bx | LMO1 | 4 |
| CG17140 | VDAC1 | 4 |
| Mvk | MVK | 4 |
| dgt1 | KANSL2 | 4 |
| Kay | FOS | 4 |
| Mbt | PAK4 | 4 |
| Nrg | NRCAM | 4 |
| par-6 | PARD6G | 4 |
| PEK | EIF2AK3 | 4 |
| Skp2 | SKP2 | 4 |
| Uzip | None | 4 |
Human orthologs are based on the highest DIOPT score [26].
GO analysis of the 209 genes that restore cell surface glycoprotein levels indicated that "GPI anchor biosynthetic process" (GO:0006506), "glycolipid metabolic process" (GO:0006664), and "inositol metabolic process" (GO:0006020) were the top enriched categories (Tables 5 and S5). Glycosylphosphatidylinositol (GPI) anchor biosynthesis is a form of glycosylation in which a GPI glycolipid is post-translationally attached to proteins in the ER. The first step in GPI anchor biosynthesis involves the attachment of GlcNAc to phosphatidylinositol, and the final GPI-associated proteins are typically transported to membrane-bound lipid rafts [36,37]. Phosphatidylinositol glycan ("PIG") genes encode a diverse class of enzymes that build the GPI sugar chain [36,37]. Five different PIG genes were among the 209 hits, and all had at least 3 sgRNAs combined from both replicates, placing them in the top ~22% of candidate genes. One of these is PIG-A (PIGA), which was also a hit in the survival screen (lfc = 0.81). The other four were PIG-S (PIGS), PIG-B (PIGB), PIG-H (PIGH), and PIG-O (PIGO). Notably, these five PIG genes are spread throughout the entire GPI anchor biosynthesis process: PIGH and PIGA are both components of the initial GPI-GlcNAc transferase complex that attaches GlcNAc to phosphatidylinositol. PIGB performs an intermediate mannosylation step. PIGO attaches an ethanolamine after PIGB. PIGS is part of the final GPI-transamidase complex which attaches the GPI anchor to a protein. Given their diverse functions, spanning two separate enzyme complexes, it is likely that GPI anchor biosynthesis as a whole can influence the phenotype. Remarkably, the data suggest that impairment of GPI anchor biosynthesis, a process that is typically known to create cell surface glycoproteins, can rescue cell surface glycoprotein levels under DPAGT1 inhibition.
Table 5. Gene ontology analysis of candidate genes from the ConA screen.
| Gene Ontology | Fold Enrichment | FDR |
|---|---|---|
| GPI anchor metabolic process (GO:0006505) | 10.82 | 1.46E-02 |
| liposaccharide metabolic process (GO:1903509) | 8.65 | 1.34E-02 |
| glycolipid metabolic process (GO:0006664) | 8.65 | 1.41E-02 |
| glycolipid biosynthetic process (GO:0009247) | 8.52 | 3.74E-02 |
| membrane lipid metabolic process (GO:0006643) | 7.06 | 2.03E-03 |
| membrane lipid biosynthetic process (GO:0046467) | 6.9 | 7.82E-03 |
| phosphatidylinositol metabolic process (GO:0046488) | 6.9 | 8.53E-03 |
| lipoprotein metabolic process (GO:0042157) | 6.31 | 4.67E-02 |
| glycerophospholipid metabolic process (GO:0006650) | 6.07 | 4.54E-03 |
| glycerolipid metabolic process (GO:0046486) | 5.9 | 1.85E-03 |
We hypothesized that some genes rescue the cell survival phenotype (Fig 1) by rescuing cell surface glycoprotein levels. To identify these genes, we compared the 209 genes that rescue cell surface glycoprotein levels to the 1151 genes that increase resistance to DPAGT1 inhibition from the survival screen. Strikingly, 40 genes (~19.1% of ConA hits; S6 Table)—including top resistance hits (lfc > 1.5) PEK, Ide, and Pfrx, as well as PIG-A—overlapped, which is higher than expected by chance (2.2 fold higher, p<0.0001). Thus, a substantial percentage of gene knockouts that restore cell surface glycoproteins also increase resistance. Nevertheless, as the majority of genes that cause increased resistance did not have this effect (1111 genes, 96.5%), the consistent rescue of cell surface glycoprotein levels is not required to increase resistance to DPAGT1 inhibition.
Inhibiting the mannosyltransferase Dpm1 rescues DPAGT1 inhibition and ER stress in vivo
To test candidate genes in vivo, we created a DPAGT1 disease model in Drosophila that expresses DPAGT1 RNAi in the eye—causing a small, rough eye phenotype (hereafter referred to as "DPAGT1 model"; Fig 3A). Initially, we tried using the common GMR-GAL4 eye driver in driving DPAGT1 RNAi [38]. However, GMR driving DPAGT1 RNAi surprisingly slightly increased overall eye size (+3.9%, S7 Table) and did not cause obvious eye defects. We believe this difference is due to eya expression being both stronger and earlier during development (ModEncode transcriptional data [39,40]). Using the DPAGT1 model, we can determine the impact of knocking down candidate genes with RNAi by measuring changes in the eye phenotype both quantitatively by size and qualitatively by observing visible phenotypes such as necrosis. We hypothesized that RNAi knockdown of candidate genes from the previous cell screens, that improved phenotypes under DPAGT1 inhibition, would improve the eye phenotype in the DPAGT1 model. We primarily focused on top significantly-enriched GO categories (FDR<0.05) from the survival and ConA screen (Tables 3 and 5). For validation, we tested multiple RNAi lines against the glycolytic, nucleotide sugar synthesis, GPI anchor pathways, the adjacent GDP-mannose-related pathway, as well as two top resistance hits relating to the ER (S7 Table). We used multiple RNAi lines against each gene when available and used RNAi against multiple genes in the same pathway throughout (see S7 Table for complete RNAi line information).
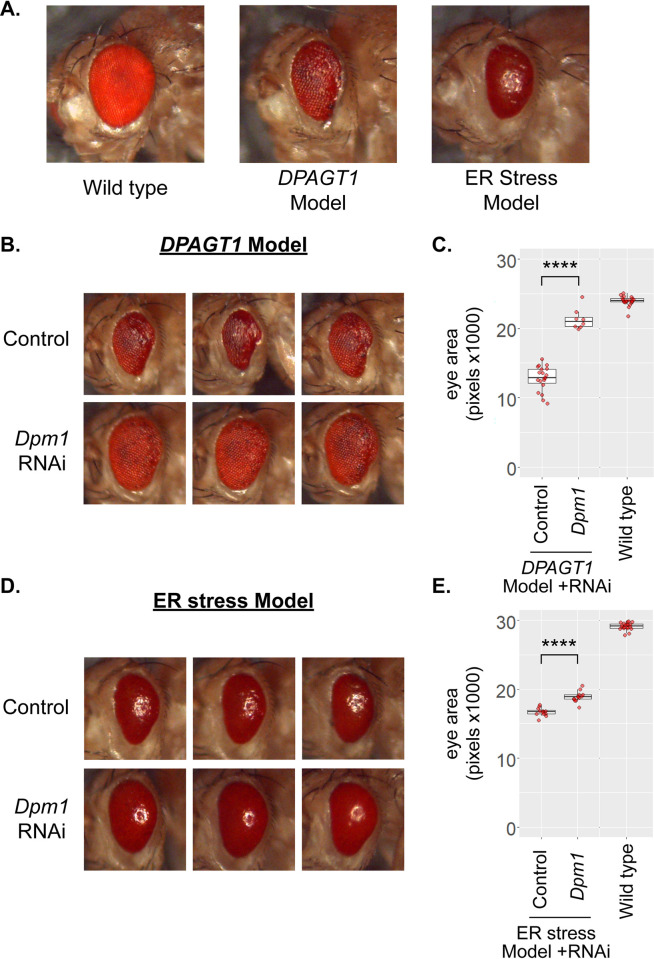
Fig 3. Knockdown of the mannosyltransferase DPM1 rescues the DPAGT1 and ER stress models.
(A) We generated a DPAGT1 model that drives RNAi against DPAGT1 in the fly eye (middle). This causes a small, rough eye phenotype. In addition to the DPAGT1 model, we also used an ER stress fly model that overexpresses the misfolded protein Rh1G69D in the eye (right). (B) RNAi against the mannosyltransferase Dpm1 strongly rescues the DPAGT1 model. The three images are representative from the same experimental cross and RNAi line (BDSC 51396) or control RNAi background (attP2, BDSC 36303). (C) Quantification of Dpm1 rescue of the DPAGT1 model. For reference, a representative DPAGT1 genetically-matched background strain crossed to the control RNAi line is included to indicate the wild type eye size (Wild type).**** p<0.0001 (Student’s t test). (D) Dpm1 RNAi rescues the ER stress model. Eye images are representative from the same experimental cross and RNAi line (BDSC 50713) or control RNAi background (attP40, BDSC 36304). (E) Quantification of Dpm1 rescue of the ER stress model. For reference, a representative ER stress genetically-matched background strain crossed to the control RNAi line is included to indicate the wild type eye size (Wild type). **** p<0.0001 (Student’s t test).
Many of these candidate genes had in vivo effects that were consistent with the outcome from the cell screens, but others had no effect or even the opposite effect (S7 Table). However, some of the strongest candidate genes from the cell screen had a consistent effect in the DPAGT1 model (S7 Table). For example, two of the top resistance genes from the survival screen increased eye size: PEK (PERK) (+14.7% and +27.4%; lfc = 4.76, ConA screen hit) and Hrd3 (SEL1L) (+33.8% and +12%, lfc = 3.7).
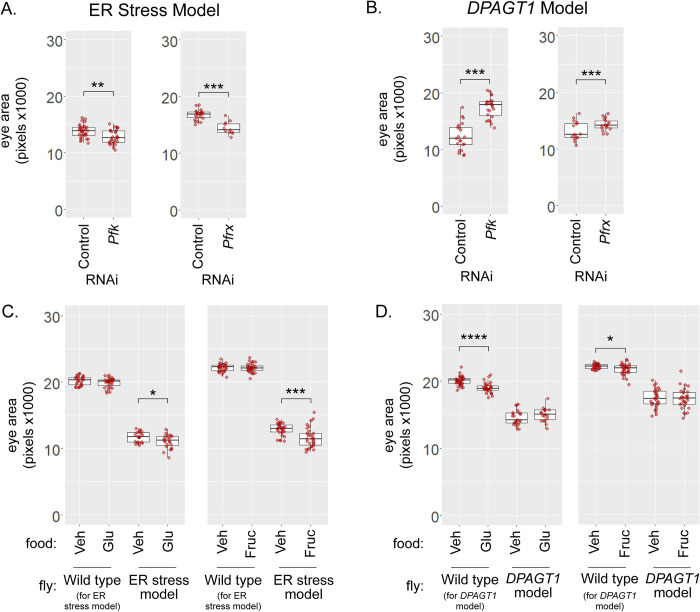
We also tested Pfk (lfc = 1.47) and Pfrx (lfc = 1.74), as they have the highest lfc values in the glycolytic pathway from the survival screen. Pfrx was also a hit in the ConA screen (S4 Table). Matching the strong effect that we observed in the survival screen, RNAi against either of these two enzymes improved the DPAGT1 model (Pfk: +32.2%, Pfrx: +11.3%, averaged across two biological replicates). On the other hand, in the nucleotide sugar synthesis pathway, RNAi against transaminase-encoding Gfat2, despite causing resistance (lfc = 4.68), did not significantly affect the DPAGT1 model (S7 Table). Moreover, RNAi against the deaminase-encoding Oscillin, which caused sensitivity (lfc = -1.87), improved the DPAGT1 model (+28.8%). These differences from the cell screen may be caused by the differing biology of simple replicating cells compared to an in vivo eye model which has to pass through multiple stages of development and differentiation.
We tested RNAi against genes encoding four enzymes in GDP-mannose synthesis, Pmm2, Mpi, CG3792, and Dpm1. One RNAi line against Pmm2 rescued the DPAGT1 model eye size (+22.2%, S7 Table). Mpi RNAi worsened eye size (-9.7%, S7 Table), though we note that Mpi catalyzes a reversible reaction in GDP-mannose synthesis. RNAi against CG3792 (MPDU1), important for bioavailability of downstream dolichol phosphate mannose (Dol-P-Man) [41,42], did not affect DPAGT1 model eye size (S7 Table and S3 and S4 Figs). However, two RNAi lines against the mannosyltransferase Dpm1 (DPM1; lfc = 0.68) showed the strongest rescue of the DPAGT1 model phenotypes (+62.1%; +82.5%) (Fig 3B and 3C and S7 Table and S1 and S2 Figs). Dpm1 encodes dolichol-phosphate mannosyltransferase 1, which, in complex with the membrane-anchored proteins DPM2 and DPM3, synthesizes Dol-P-Man, an essential substrate in N-glycosylation, O- and C-mannosylation, and GPI anchor biosynthesis [43–46] (Fig 1C). Of the two anchor proteins, no RNAi is currently available for Dpm3 in flies. RNAi against Dpm2 had inconsistent but slightly negative effects in the DPAGT1 model (-7.5%, S7 Table and S3 and S4 Figs). The results on MPDU1 and Dpm2 match the cell screen data where neither of these genes were hits, and it suggests that specific loss of the catalytic domain of the DPM1 complex (Dpm1) is most critical for improving DPAGT1 deficiency. In sum, while loss of DPM1 is normally detrimental to cells and patients [12,44,47], knockdown of Dpm1 improved the DPAGT1 model.
To determine if the Dpm1 rescue was specific to DPAGT1 or ER stress in general, we also tested each RNAi line on the well-established Drosophila Rh1G69D ER stress model [48–51] (hereafter referred to as "ER stress model") (Fig 3A and S7 Table). The ER stress model overexpresses a misfolded protein in the eye in order to induce ER stress, apoptosis, and a small, rough eye phenotype. Similar to the DPAGT1 model, Dpm1 RNAi resulted in the strongest rescue of overall eye size in the ER stress model (+18%, Fig 3D and 3E and S7 Table, and S1 and S2 Figs), indicating that Dpm1 inhibition is capable of rescuing the effects of DPAGT1 inhibition and ER stress in general. We also note that Dpm1 RNAi did not significantly impact eye size in either control driver line alone, except for one line in the ER stress control where it slightly reduced eye size (-3%, S7 Table). Despite the fact that Dpm1 is an essential enzyme, inhibiting Dpm1 provides significant benefits to DPAGT1 inhibition and ER stress, and thus, it may be a key factor in rescuing the effects of DPAGT1 deficiency.
Impairment of O-mannosylation, N-glycosylation, and GPI anchor biosynthesis improves the loss of DPAGT1 function and ER stress outcomes
One hypothesis for how Dpm1 inhibition might rescue DPAGT1 inhibition and ER stress is through the reduction of its product, Dol-P-Man, and the subsequent impairment of Dol-P-Man-dependent glycosylation pathways [46,52]. This hypothesis fits with other screen data. For example, loss of one downstream pathway of Dpm1, GPI anchor biosynthesis, improves cell survival (genes: PIG-A, lfc = 0.81; PIG-T, lfc = 0.57; PIG-K, lfc = 0.50; PGAP1, lfc = 0.61) and rescues cell surface glycoproteins (genes: PIG-A; PIG-H; PIG-O; PIG-B; PIG-S) (Figs 1 and 2 and S1 and S4 Tables). To test this hypothesis, we crossed strains expressing RNAi against the mannosyltransferase enzymes in O- and C-mannosylation, N-glycosylation, and GPI anchor biosynthesis to the DPAGT1 model (S3 and S4 Figs).
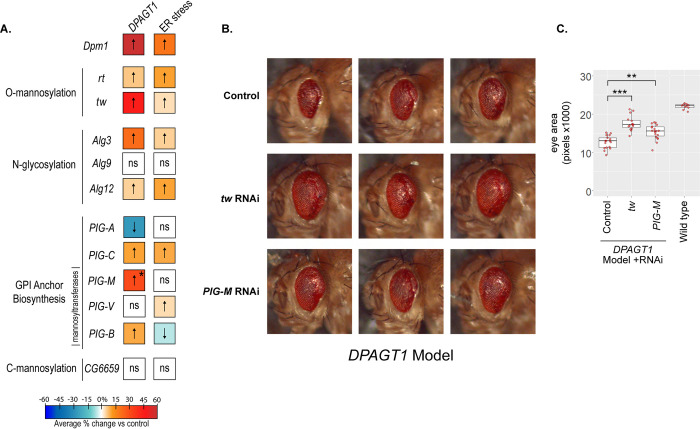
O-mannosylation begins with the addition of mannose to Ser/Thr residues, followed by a lengthening of the chain with additional sugar molecules [53,54]. One important O-mannosylated protein is alpha-dystroglycan, and impairment of O-mannosylation is associated with muscular dystrophy in humans [54]. The first step in O-mannosylation, which attaches the initial mannose from Dol-P-Man, requires an enzyme complex consisting of two O-mannosyltransferases, POMT1 and POMT2. RNAi against rotated abdomen (rt, POMT1) or twisted (tw, POMT2) rescued eye size in both the DPAGT1 (rt: +6.2%; tw: +45.9%) and ER stress (rt: +10.2%; tw: +3.8%) models (Fig 4A–4C and S7 Table and S3 and S4 Figs). These data suggest that loss of O-mannosylation can rescue the loss of DPAGT1 function and ER stress.
Fig 4. Knockdown of glycosylation pathways rescues the DPAGT1 and ER stress models.
(A) A summary of RNAi data performed on downstream DPM1 glycosylation pathways. The majority of mannosyltransferases tested in O-mannosylation, N-glycosylation, and GPI anchor biosynthesis pathways increased eye size in the DPAGT1 model. Colors denote % change of eye size vs their control; warmer colors (or up-arrows) have a stronger effect, and vice versa. Colors are derived from the averages of at least 2 biological replicates with at least one significant replicate and no opposite results (see S7 Table for all RNAi data). * = One PIG-M RNAi line was very strong (BDSC 51890), while a second line was neutral/negative in response (see S7 Table). (B) Representative images of the DPAGT1 model crossed with RNAi against the two strongest hits, tw and PIG-M. Shown are three representative images from the same RNAi line and experimental cross (tw: BDSC 55735, PIG-M: BDSC 51890) or control RNAi background (attP40: BDSC 36304). (C) Quantification of tw and PIG-M rescue shown in B. For reference, the total quantification of that experiment, as well as a representative wild type DPAGT1 genetically-matched background strain crossed to the control RNAi line, is included. ** p<0.01, *** p<0.001 (Student’s t test).
In N-glycosylation, mannosylation steps utilizing Dol-P-Man occur in the ER lumen via the mannosyltransferases ALG3, ALG9, and ALG12 [55]. RNAi against Alg3 (ALG3) and Alg12 (ALG12) improved eye size in both the DPAGT1 (Alg3: +21.4%; Alg12: +4.5%) and ER stress (Alg3: +4.8%; Alg12: +9.9%) models. However, RNAi against Alg9 (ALG9) did not affect either model (Fig 4A and S7 Table and S3 and S4 Figs). Overall, loss of the N-glycosylation mannosyltransferase steps can rescue the loss of DPAGT1 function and ER stress.
In the GPI anchor biosynthesis pathway, most RNAi lines tested improved the DPAGT1 model. These include a subunit of the GPI-GlcNAc transferase complex, PIG-C (PIGC, +22.6%), and the mannosyltransferases PIG-B (PIGB, +11.3%) and PIG-M (PIGM, +31.9%). A second PIG-M RNAi line had a small negative effect (-5.7%). RNAi lines against the mannosyltransferase PIG-V (PIGV) did not have a significant effect. Surprisingly, RNAi against PIG-A was detrimental to the DPAGT1 model (-5.7% and -27.7%, respectively). Unlike the previous two pathways tested, the ER stress model was not as consistent with the DPAGT1 model, though it also had an overall beneficial effect (Fig 4A–4C and S7 Table and S3 and S4 Figs). This may reflect a difference in how GPI anchor biosynthesis affects each model.
C-mannosylation is a less common type of glycosylation that involves the addition of mannose to tryptophan found in thrombospondin type 1 repeats [56]. The four enzymes in humans responsible for C-mannosylation are DPY19L1-4 (DPY19L1 and DPY19L3 being the most functionally important [56]). In Drosophila, CG6659 is the single ortholog of all four human paralogs (DIOPT scores for DPY19L1-4: 10/16, 9/16, 4/16 and 4/16). RNAi against CG6659 did not affect eye size in either model; however, here we note an increased chance of a false negative as only one RNAi line and one gene from this pathway were examined (Fig 4A and S7 Table and S3 and S4 Figs). Thus, it is not immediately clear that impairing C-mannosylation imparts any benefit under the loss of DPAGT1 function or ER stress.
Taken together, disruption of any of the three pathways downstream of Dpm1 and its product Dol-P-Man—O-mannosylation, N-glycosylation, and GPI anchor biosynthesis—can rescue the effects of DPAGT1 inhibition and ER stress. Notably, RNAi against any single gene in these three pathways never reached the same magnitude of rescue provided by Dpm1 RNAi (+62.1%)—with the closest being tw (+45.9%) and PIG-M (+31.9%) RNAi (Fig 4B and 4C). This suggests that the strong rescue of the DPAGT1 model with Dpm1 RNAi may be from the synergy of the combined impairment of the three downstream glycosylation pathways. In addition, for every gene tested, RNAi downregulation in the DPAGT1 model had stronger, more consistent rescue of negative phenotypes than the ER stress model, which suggests an underlying difference between the two models.
Fructose metabolism is differentially altered depending on the source of ER stress
Functions related to glycolysis and metabolism were enriched among our candidate genes (S5 Fig and Tables 3 and S3). In the survival screen (Fig 1), knockout of glycolytic genes generally caused resistance to DPAGT1 inhibition, including the aforementioned enzymes Pfk (lfc = 1.47) and Pfrx (lfc = 1.74), as well as the dehydrogenase Gapdh1 (GAPDH; lfc = 1.14), phosphoglycerate mutase Pglym87 (PGAM2; lfc = 1.21), and pyruvate kinase CG12229 (PKM; lfc = 0.73), among others (S1 Table and S5 Fig). Loss of Pfrx also improved cell surface glycoproteins in the ConA screen (Fig 2). Thus, impairing glycolysis appears to improve survival under the loss of DPAGT1 function.
Surprisingly, knockdown of Pfk and Pfrx worsened the ER stress model (Fig 5A and S7 Table). In agreement with the screen data, knockdown of either of these two enzymes improved the DPAGT1 model (Fig 5B and S7 Table). Pfk and Pfrx both act on the early glycolytic metabolite fructose-6-phosphate to regulate glycolysis (Fig 1C). While Pfrx can have phosphatase activity, one potential hypothesis is that loss of Pfk and Pfrx function might generally increase the pool of fructose-6-phosphate in the cell. Given the shared substrate and differential effects in the two models, one possible reason could be key differences in how fructose-6-phosphate levels and altered metabolism affects the loss of DPAGT1 function and ER stress.
Fig 5. Glucose and fructose metabolism differences between the DPAGT1 and ER stress models.
(A-B) In the ER stress model, knockdown of PFKM/Pfk (BDSC 34336) and PFKFB1/Pfrx (BDSC 57222) causes a worsening of eye size compared to control RNAi (attP2, BDSC 36303; and attP40, BDSC 36304). In contrast, in the DPAGT1 model, knockdown of these genes causes an overall improvement of eye size. (C) 100mM dietary glucose or fructose significantly reduced eye size in the ER stress model. Wild type refers to the genetically-matched background strain. (D) Compared to its background control, feeding of 100mM glucose or fructose was net-positive in the DPAGT1 model. Wild type refers to the genetically-matched background strain. For A-D, 1 replicate is shown of 2 biological replicates (S8 Table), * p< 0.05, ** p<0.01, *** p<0.001 (Student’s t test).
Typically, glucose is metabolized into fructose-6-phosphate using two enzymes, hexokinase and phosphoglucose isomerase [57]. In addition, while in most cases fructose is converted into fructose-1-phosphate by fructokinase, it can also be synthesized directly into fructose-6-phosphate using hexokinase [58–61]. Given that glucose or fructose can be metabolized into fructose-6-phosphate, we tested whether these sugars could differentially affect the DPAGT1 and ER stress models through dietary supplementation. In agreement with the RNAi data, feeding the ER stress model flies dietary glucose or fructose led to a significant decrease in overall eye size (-8.9% and -9.7%, averaged from two biological replicates) (Fig 5C and S8 Table). In contrast, its genetically-matched control was not affected by glucose or fructose supplementation. In the DPAGT1 model, dietary glucose or fructose had no significant reproducible effects on eye size. In contrast, its genetically-matched control had negative effects on eye size from glucose and fructose (-3.0% and -2.4%) (Fig 5D and S8 Table). While the ER stress model showed a net negative effect, compared to its genetically matched control, from sugar supplementation, the DPAGT1 model had an overall net positive effect. Together, the RNAi and sugar feeding data reveal a difference in glycolytic metabolism between the two models.
Discussion
Here, we report hundreds of new candidate genes that modify the effects of DPAGT1 inhibition identified using a cell-based CRISPR screen. Testing these candidate genes in vivo validated many of the strongest hits and demonstrated that knockdown of GPI anchor biosynthesis and downstream DPM1/Dpm1 glycosylation pathways can rescue the effects of DPAGT1 deficiency and ER stress. Even though single gene mutations in these pathways are associated with specific CDGs, knockdown of many of these genes paradoxically improved negative phenotypes associated with DPAGT1 inhibition and ER stress both in vitro and in vivo.
One hypothesis for this outcome is that impairing glycosylation pathways might slow down the overall synthesis of glycoproteins, thereby allowing cells to better withstand DPAGT1 inhibition. Similar mechanisms are utilized in the response to ER stress, where the UPR will reduce protein synthesis in response to ER stress, giving the cell time to recover [5,62]. Another mechanism by which impairing glycosylation may rescue the loss of DPAGT1 function is by freeing up cellular machinery that helps fold or degrade misfolded proteins. One such pathway is the calnexin/calreticulin (CNX/CRT) cycle, a process that ensures the proper folding of N-glycosylated proteins in the ER [63]. Recently, the CNX/CRT cycle was found to help mature GPI-associated proteins as well [64]. In one scenario, it is possible that impairing the GPI anchor biosynthesis pathway helps free up machinery in the CNX/CRT cycle, allowing it to fix or clear more misfolded N-glycosylated proteins under loss of DPAGT1 function. ER-associated degradation (ERAD) involves the recognition, translocation, and ubiquitination/degradation of misfolded proteins in the ER through the use of different enzyme complexes [65]. One component of the ERAD Hrd1 complex is the adaptor subunit, Hrd3 (human: SEL1L) [66]. Hrd3 was a strong hit in the cell survival screen (Table 2), and it also strongly rescued the DPAGT1 model (S7 Table). Deficiencies of HRD1 or SEL1L can lead to accumulation of proteins in the ER [67]. As such, one potential hypothesis is that loss of Hrd3 impairs the Hrd1 complex, causing more retention of misfolded proteins in the ER and allowing them longer to properly fold (potentially by the aforementioned CNX/CRT cycle). Finally, as Hrd3 RNAi only improved the DPAGT1 model, but had either no effect or worsened the ER stress model, this suggests another difference between the mechanism of stress between each model and how that stress may be ameliorated. Overall, this might provide a novel approach to DPAGT1-CDG or other glycosylation disorders, where a therapy might be designed to slow down global glycosylation and/or globally activating proteostasis machinery, to allow impacted cells more time to repair or clear misfolded proteins.
Because independent perturbations of three downstream Dpm1-dependent glycosylation pathways alone were capable of partially rescuing the DPAGT1 model, each one could provide a good therapeutic target for DPAGT1-CDG. Although targeting DPM1 itself might be a useful approach, targeting a downstream gene may be better in practice to reduce the effects of elimination of all glycosylation pathways. For example, knockdown of POMT2/tw almost reached the same level of rescue as Dpm1 alone (+45.9% vs. +62.1%), while targeting POMT2 would perturb only O-mannosylation. O-mannosylation affects cadherins, plexins, and alpha-dystroglycan, among other proteins [68], and is associated with multiple CDGs, including POMT2-CDG [69]. Thus, like many other candidate genes identified in our study, care would need to be taken in inhibiting this gene in any potential therapies. Nevertheless, given the multiple target genes throughout these pathways, this approach could allow for better "titration" of genetic or pharmacologic downregulation, which could hopefully provide an effective therapeutic approach. One other hypothesis is that inhibiting Dpm1 might increase the pool of dolichol-phosphate available for DPAGT1 to utilize (including under inhibition). While this would not explain why inhibiting glycosylation pathways downstream of Dpm1 also improves DPAGT1 inhibition, it could be partially responsible for why loss of Dpm1 alone causes stronger rescue than loss of any of its downstream pathways. Alternatively, another hypothesis is that DPAGT1 inhibition may increase flux of dolichol-phosphate through Dpm1 to cause excessive glycosylation, which is then turned off under Dpm1 inhibition in order to cause its rescue effect. However, given that tunicamycin strongly reduced binding of ConA, which binds to mannose/glucose moieties in glycoproteins [70,71], it is unlikely that there is excessive glycosylation under DPAGT1 inhibition.
RNAi against Mpi (MPI), another gene in the GDP-mannose pathway, worsened the DPAGT1 and ER stress models (S7 Table). However, MPI interconverts mannose-6-phosphate and fructose-6-phosphate [72], which means its inhibition could increase or decrease GDP-mannose synthesis. Given that we also see differential effects of fructose in our two models, metabolomic analysis of these pathways may be required to understand the exact mechanism of Mpi loss under DPAGT1 impairment. Of note, Mpi was a resistance hit in the cell survival screen, which suggests there could be differences in metabolite utilization between the in vitro and in vivo models. Surprisingly, loss of neither Dpm2, encoding a protein important for anchoring DPM1, nor CG3792, encoding a protein important for utilizing Dol-P-Man, could improve the DPAGT1 model. Neither gene was a hit in the cell screens, and this suggests it may be more important to directly impair the synthesis of Dol-P-Man—as loss of two other enzymes in the GDP-mannose pathway, Pmm2 and Dpm1, both improve the DPAGT1 model (S7 Table). However, one hypothesis is that there may be another pathway in the fly capable of increasing availability of Dol-P-Man, or that Dpm1 may have some other function, beyond its enzymatic activity, that is important in regulating its downstream glycosylation pathways.
RNAi against many of the GPI anchor biosynthesis genes improved one or both of the in vivo models. However, despite being a hit in both cell screens, in vivo knockdown of PIGA/PIG-A caused a reduction in eye size in the DPAGT1 model and no change in the ER stress model. We hypothesize that PIGA/PIG-A may be too critical for development in vivo, such that developmental effects mask potential protection in either model. In support of this hypothesis, PIGA/PIG-A knockdown alone results in more severe eye phenotypes compared to other PIG genes (S3 and S4 Figs). For example, PIG-B, another PIG gene hit from the ConA screen, was capable of improving the DPAGT1 model, but had a milder phenotype when knocked down alone. There may be a limit on how strongly these downstream pathways can be inhibited before effects change from beneficial to deleterious.
The strongest candidate gene from the cell screen with improved survival under DPAGT1 inhibition was PEK (PERK), which also rescued cell surface glycoproteins in the ConA screen. PEK knockdown also improved the in vivo DPAGT1 model. PEK encodes a protein kinase essential to the cellular ER stress response. Other genes encoding ER stress sensor proteins, Xbp1 and ATF6 [7], did not reach our LFC threshold (lfcs = -0.48 and 0.33, respectively). PEK activates the transcription factor Crc (ATF4), which can regulate both pro-survival or apoptotic-related genes [7]. One hypothesis for why knockout of PEK leads to increased survival is that ER-stress-induced apoptotic-related gene expression might be lower in these cells, which may allow them time to grow and repair despite being stressed. While Drosophila lacks the ATF4 downstream pro-apoptotic target CHOP, Drosophila Crc can activate apoptosis by downregulation of the anti-apoptotic E3 ubiquitin ligase XIAP/Diap1 [73]. Similar to PERK, knockout of crc does provide resistance (lfc = 0.75). Interestingly, Crc is also a co-activator of the hormone receptor FXR/EcR [74], and signaling of the hormone ecdysone through this receptor is critical for proper morphogenesis in the fly [75,76]. Knockout of FXR/EcR also provides resistance (lfc = 2.9). Thus, part of the resistance from PEK and crc knockout might result from its connection to ecdysone signaling.
The majority of genes for which knockout increased resistance to Tun did not overlap with hits from the cell surface glycoprotein ConA screen (S6 Table). This suggests that rescue of surface glycoproteins is not required for cell survival. However, many ConA gene hits did overlap, such as PIG-A, Pfrx, and PEK, which may hint at the mechanisms through which these gene pathways lead to survival. It is possible that loss of a specific protein leads to both Tun resistance and cell surface glycoprotein rescue in these overlapping genes. For example, perturbing GPI anchor biosynthesis genes can improve cell survival, cell surface glycoproteins, and in vivo models of DPAGT1 inhibition, which could indicate that loss of a specific GPI-anchored protein improves these phenotypes. As a different example, loss of PEK also improves these metrics under DPAGT1 inhibition, which suggests the importance of ER stress and/or apoptosis to these phenotypes given the role of PEK noted above [7]. As all gene knockouts occur independently of each other in the CRISPR screens, it is also possible that both of these mechanisms are true in any individual knockout cell.
Not every gene from the cell screens validated in vivo. We believe this is primarily driven by differences in the biology of single cells vs. whole, developing organisms, but it is also possible some opposite results are driven by false discovery. In CRISPR knockout screens, false discovery can be due to off-targeting, poor guide RNA efficiency, or incomplete knockout [77–79]. We employed 4–8 guide RNAs per gene, while utilizing the median LFC value for downstream analysis—both of which should improve the overall false hit rate [78]. However, it is still possible that false positives occur even when multiple guide RNAs are used [77]. For example, the gene Diap1 was a strong hit in our screen, yet loss of Diap1 alone is known to kill S2 cells [80], so this is likely a false positive. We had 8 final guides for Diap1, and 6 of them were in enriched at LFC = 0.5 in the Tun-treated population. As each guide is unique, it is unlikely this is being driven by off-targets (though it may partially explain this result). One possibility is that, in rare circumstances, some cells have only single allelic knockout of Diap1, and that this heterozygosity may improve Tun survival.
There are several other candidate genes that might be informative for understanding DPAGT1 deficiency. For example, another enriched GO pathway from the ConA screen was "inositol metabolic process" (GO:0006020) (Table 3). Inositol has several functions, including growth, immunity, and GTPase signaling [81]. One of these functions may play an important role in DPAGT1 inhibition, unrelated to glycosylation. Other GO enriched categories of note include nucleotide synthesis (Table 3). Activation of the UPR transcription factor ATF4 can promote de novo purine synthesis through the tetrahydrofolate cycle [82,83]. Perhaps modulation of purine synthesis might provide some benefit under DPAGT1 inhibition.
We also found that sugar supplementation had a different effect on the DPAGT1 and ER stress models, and this might be explained by the disease mechanism of each model. The DPAGT1 model ostensibly works by reducing the amount of functional DPAGT1 enzyme (similar to Tun treatment), and DPAGT1 enzymatic activity utilizes UDP-GlcNAc, which is a product of fructose-6-phosphate metabolism through the hexosamine pathway (Fig 1C). Inhibition of DPAGT1 enzymatic activity may result in a complex pattern of feedback that affects both fructose-6-phosphate levels and metabolism. While impairment of this enzyme in cells is associated with ER stress [12,18], it is possible the rough eye phenotype is induced by impaired trafficking or the loss of a specific glycosylated protein. In contrast, the ER stress model overexpresses a single misfolded protein, causing accumulation in the ER lumen and subsequent ER stress [49]. The ER stress model does not directly impair the fructose metabolism pathway, and its misfolded protein landscape is likely dominated by a single misfolded protein. Given these differences, we hypothesize that altering fructose-6-phosphate metabolism may have a differential effect on DPAGT1 because of its metabolic connection to the hexosamine pathway. Metabolomic analysis should be performed to further test this hypothesis—especially considering that the related metabolite mannose-6-phosphate is associated with ER stress [84]; while metabolomic analysis of the fly eye disc is technically challenging due to a low number of relevant cells, this could be further tested in DPAGT1 mutant cell lines [18].
In this study, we used cell culture and in vivo approaches to identify hundreds of new modifier genes affecting DPAGT1 inhibition. Strikingly, we found that Dpm1 and its downstream glycosylation pathways are major enriched sites of these modifier genes. Our screen highlights that genes individually associated with one CDG may also be a source of modifiers of another CDG. If found to be more generally true, this paradoxical relationship between CDG genes could be exploited for therapeutic benefit. Targeting individual parts of these pathways under careful titration with drug or gene therapy may be the answer to better treatments for DPAGT1-CDG. In addition, we found differences in fructose metabolism that may explain differences between models of disease and ER stress and could be key to developing future dietary treatments for this disorder. Taken together, we believe these findings serve as a staging ground for further elucidation of modifier genes of DPAGT1 inhibition and how they affect its underlying metabolism.
Materials and methods
Cell culture
Drosophila melanogaster S2R+ cells are from the Drosophila RNAi Screening Center (Harvard Medical School). A subline expressing SpCas9 and containing an attP integration site, S2R+/NPT005/MT-Cas9 (PT5/Cas9), was described previously [25,85] and is available at the Drosophila Genomics Resource Center (Cell line # 268). Cells were maintained in Schneider’s media (Thermo 21720) supplemented with 1X Penn/Strep (Thermo 15070063) and 10% FBS (Thermo 16140071).
Cell toxicity assay for tunicamycin sensitivity
Tunicamycin (Cayman Chemical # 11445) dissolved in DMSO was serially diluted 2-fold into growth media in 96-well plates. Then, cells were added such that each well contained 10,000 cells in 100 μL. Plates were incubated at 25°C for 4 days and then relative cell titers were determined using Cell Titer Glo (Promega) and a luminescence plate reader (SpectraMax) according to the manufacturer’s instructions.
Genome-wide CRISPR–Cas9 screening for resistance to tunicamycin
The Drosophila CRISPR genome-wide knockout library was previously described and is available from Addgene (134582–4) [25,85]. In brief, PT5/Cas9 cells were transfected with an equal-parts mixture of pLib6.4 containing an sgRNA library as well as pBS130 (26290, Addgene) using Effetene (301427, Qiagen) following the manufacturer’s instructions. The library was delivered in three parts, sublibrary group 1, 7,956 gRNAs for 995 genes; sublibrary group 2, 17,827 gRNAs for 2979 genes; and sublibrary group 3, 59,406 gRNAs for 9954 genes. After 4 days, the transfected cell library was selected with 5 μg/mL puromycin (540411, Calbiochem) for 12 additional days, subculturing every 4 days. After the stable sgRNA-expressing cell library was established, 1000 cells per sgRNA were subcultured in each passage to maintain the expected diversity of the sgRNA library. The cells were exposed to 590 nM tunicamycin (500 ng/ml, Cayman Chemical # 11445) for 30 days, passaging the same number of cells (~8×107) to four new 15 cm dishes every 4 days. Following the final passage, aliquots of the cells were collected, and their genomic DNA was extracted using a Zymo Quick-gDNA Miniprep kit (D3025, Zymo Research). DNA fragments containing the sgRNA sequences were amplified by PCR using at least 1000 genomes per sgRNA as template for each sample. The in-line barcoding strategy and sequences were as previously described [85]. Next generation sequencing (Illumina NextSeq) was performed at the Biopolymers Facility at Harvard Medical School. Following barcode demultiplexing, a readcount table was prepared from the raw sequencing files using MAGeCK version 0.5.9.4 [86], count subprogram, using standard parameters with 22 bp 5’ trimming and median-normalizing each sublibrary to 10,000 reads. The median log2 fold-change reported throughout was calculated from this data directly by measuring log2 of treated readcount divided by untreated readcount and then determining the median value among all sgRNAs targeting the same gene. For the volcano plot, the log2 fold-change and robust rank aggregation (RRA) p-value measures were determined using MAGeCK version 0.5.9.4 software, test subprogram, using standard parameters.
FACS-based selection for altered concanavalin A staining intensity
After generating puromycin-resistant pools of cells expressing a genome-wide library CRISPR sgRNA library (88,627 sgRNAs targeting 13,685 Drosophila genes), the cells were again subjected to treatment with tunicamycin (590 nM) in normal growth medium for 1 week. Next, 1×108 cells were placed into suspension in 10 mL and labeled live with 10 μg/mL Alexa Fluor 647 Conjugated Concanavalin A (Thermo # C21421) for at least 2 hours by diluting the stock 1:500 into full growth medium at room temperature with occasional inversion. 1 mL aliquots of labeled cells were transferred individually to 5 mL 40 μm filter-cap vials to mechanically declump the cells. Cells were then subjected to fluorescence-activated cell sorting (FACS) using a Sony MA900 FACS machine guided by gates established by tunicamycin-untreated cells stained in parallel and unstained cells. Approximately 1% of cells that exhibited the greatest intensity of staining in the AlexaFluor 647-A filter (corresponding to the average intensity of tunicamycin-untreated cells) were sorted into full growth media. Gating was reestablished after each 1 mL to account for increased uptake of the dye over the course of the experiment (~6 hours). Finally, collected cells were transferred to a 6-well dish well and gently spun down at 100 x g for 10 min and their genomic DNA was extracted using the Zymo Quick-gDNA Miniprep kit, then subjected to PCR and Illumina sequencing as described. Amplicon sequencing was carried out at the MGH DNA core. A readcount table was prepared from raw sequencing files using MAGeCK version 0.5.9.4, count subprogram, using standard parameters with 22 bp 5’ trimming. Readcounts were sorted to reveal genes in the top 1000 of all sgRNAs (minimum of 4 or more sgRNAs).
Fly stocks and maintenance:
All flies in this study were maintained at room temperature and fed a standard fly diet based on the Bloomington Drosophila Stock Center Standard Cornmeal Medium with malt and without soy flour (unless otherwise noted). Stocks obtained from the Bloomington Drosophila Stock Center and the Vienna Drosophila Resource Center [87] were used in this study (listed in S7 Table). The w-;; eya composite-GAL4 line was a gift from Justin Kumar (Indiana University Bloomington) and was characterized previously [88]. The "ER stress model" contains GMR-GAL4 and UAS-Rh1G69D on the second chromosome and has been previously described [49–51].
The DPAGT1 model was generated as follows. The eya composite-GAL4 and UAS-Alg7 RNAi line (BDSC #53264) were crossed to create an eya composite-GAL4/UAS-Alg7 RNAi (III) line. This line was then crossed to a balancer +/TM3, Dfd-YFP, Sb (III) and progeny were examined for crossover events. Progeny with small, rough eyes and Sb were collected, maintained for stability of the phenotype, and referred to as the DPAGT1 model (w-, y-, v-, sc-, sev-; +/+; (eya composite-GAL4, w+, P[sc+, y+, v+, Alg7 RNAi])/TM3, Dfd-YFP, Sb).
Eye imaging and quantification:
Adult female flies (2–7 days old) were collected under CO2 anesthesia, then placed on ice and transferred to -80°C for later imaging. Eyes were imaged at 3x magnification (Leica EC3 Camera). Eye area was determined as previously described [50]. All measurements were done blinded to the RNAi line used, and one replicate from each model was measured by a second observer. Qualitative images of all control flies are also included as a reference (S2 and S4 Figs). Note that when two RNAi lines were assayed together, the same control was compared to each. When possible, we used two different RNAi lines to limit the possibility of reagent-specific effects. Complete information on lines used can be found in S7 Table and representative images can be found in S1–S4 Figs. In circumstances where only one RNAi line was available, when possible, multiple genes from the same pathway were tested in order to draw conclusions on the pathway as a whole in order to further limit any reagent-specific effects. For Dpm1, the BDSC 50713 RNAi line was lethal in the DPAGT1 model and its control (S7 Table), and studies with the Dpm1 BDSC 51396 RNAi line were performed at 18°C in the w-;; eya composite-GAL4 control line to increase viability. All Dpm1 RNAi lines were viable when crossed with the ER stress model.
Sugar supplementation
To make the glucose- and fructose-supplemented media, our standard media was melted in a microwave and maintained at 95°C on a stir plate. We used this melted media as-is for the control and added glucose or fructose to a final concentration of 100mM to a separate set of vials and stirred until it was fully dissolved to a total volume of ~8.5ml/vial. The sugar adds approximately 0.6 calories/tube (that are ~6 calories per 8.5ml [89], when accounting for our added malt), indicating near-isocaloric levels (within ~10%) between each tube. Flies were mated in these vials and removed after egg laying, allowing progeny to feed on sugar-supplemented media from hatching. Timing and quantitative imaging of these progeny was performed as described above.
Statistics
Genetic overlap representation factors and probability statistics were calculated via "Nematode bioinformatics" (http://nemates.org). For the CDG gene overlap analysis, we used the DIOPT ortholog finder (https://www.flyrnai.org/cgi-bin/DRSC_orthologs.pl) [26] and analyzed any hits with a DIOPT score of 5 or greater.
Gene Ontology (GO) analysis was performed with the PANTHER Overrepresentation test using the "GO biological process complete" data set, compared to all Drosophila genes, and using the default parameters (Fisher’s Exact, False Discovery Rate) [90,91]. We also indicate in S7 Table if individual gene orthologs are associated with other relevant GO terms in H. sapiens (via http://www.pantherdb.org/).
Eye size comparisons were analyzed using the Student’s t test. When comparing groups of RNAi lines to a single control, Bonferroni multiple testing correction was used. Venn diagram and graphs of eye size comparisons were made using the statistical software R [92].
Supporting information
Representative images from two replicates of RNAi line crosses to both the DPAGT1 and ER stress models are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set. Quantification of these eye crosses are included in S7 Table.
(TIF)
Representative images from two replicates of RNAi line crosses to both the eya composite-GAL4 and GMR-GAL4 control lines are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set.
(TIF)
Representative images from two replicates of RNAi line crosses to both the DPAGT1 and ER stress models are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set. Quantification of these eye crosses is included in S7 Table.
(TIF)
Representative images from two replicates of RNAi line crosses to both the eya composite-GAL4 and GMR-GAL4 control lines are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set.
(TIF)
Fly gene names are listed, as these genes are derived from the S2 CRISPR knockout guide RNA library. All resistance and sensitivity metrics are based on lfc values from the survival screen.
(PDF)
The first tab includes the raw sgRNA enrichment scores (tunicamycin-vs-untreated) 4–8, as well as the median values of those sgRNAs used in subsequent analyses. The second tab includes the adjusted lfc values and p-values used to create the volcano plot in Fig 1.
(XLSX)
The first tab includes the full list of human CDGs derived from The Essentials of Glycobiology and Invitae panel, the derived fly orthologs via their DIOPT score, a finalized list removing duplicate orthologs alongside their lfc value from the survival screen, and finally the overrepresentation test analysis performed. The second tab includes human CDGs for which no fly ortholog was found at a DIOPT score of <5.
(XLSX)
This includes the output of the PANTHER gene list analysis from the combined input of resistance and sensitivity candidate genes at an lfc of ≥0.5 or ≤-0.5 from the survival screen in Fig 1 (with re-labeled columns to improve readability). Categories are sorted by fold enrichment.
(XLSX)
The first tab includes only genes that had at least one sgRNA in the highest ~1% of fluorescence of all sgRNAs in two biological replicates. This includes information on specific guides, and the final list of genes found (FBgn number and Fly gene name). The second tab is the raw data from the ConA experiment, including information on specific guide RNAs and their abundance in either replicate.
(XLSX)
This includes the output of the PANTHER gene list analysis from the input of candidate genes that improved ConA phenotypes in Fig 2 (with re-labeled columns to improve readability). Categories are sorted by fold enrichment.
(XLSX)
This includes the list of resistance candidate genes from the survival screen (S1 Table), all hits from the ConA screen (S4 Table), and the overlapping genes between them (FBgn number and Fly gene name).
(XLSX)
Quantitative results of all RNAi cross replicates to both the DPAGT1 and ER stress model are listed here. This table includes information on the Drosophila gene (name and FBgn number), closest human ortholog, the reasoning on why the gene was tested (GO category, validation, relation to Dpm1, etc.), if the gene was a hit in one or both screens, the specific RNAi stock used in each cross, as well as the related pathway function. % change in eye size, confidence interval (derived from the standard deviation of change and listed N), as well as the Student’s t test p-value are indicated for each replicate.
(XLSX)
This lists the data on two replicates of the sugar feeding experiments on the eya composite-GAL4 and GMR-GAL4 control lines and DPAGT1 and ER stress models. % change in eye size, confidence interval (derived from the standard deviation of change and listed N), as well as the Student’s t test p-value are indicated for each replicate.
(XLSX)
Acknowledgments
We thank Emily Coelho and Shayna Scott for technical assistance with fly management.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by NIGMS R35 GM124780 to CYC and P41 GM132087 to NP, and by a grant from the Primary Children’s Hospital Center for Personalized Medicine to CYC. HMD was supported by an NIGMS NRSA award (F32 GM136057). JSZ was supported by the Undergraduate Research Opportunities Program (UROP) through the University of Utah Office of Undergraduate Research. NP is an investigator of the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019. Jun 1;15(6):346–66. doi: 10.1038/s41581-019-0129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Péanne R, de Lonlay P, Foulquier F, Kornak U, Lefeber DJ, Morava E, et al. Congenital disorders of glycosylation (CDG): Quo vadis? Eur J Med Genet. 2018. Nov 1;61(11):643–63. doi: 10.1016/j.ejmg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 3.Brasil S, Pascoal C, Francisco R, Marques-da-Silva D, Andreotti G, Videira PA, et al. CDG therapies: From bench to bedside. Int J Mol Sci. 2018. May 1;19(5). doi: 10.3390/ijms19051304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipiński P, Tylki-Szymańska A. Congenital Disorders of Glycosylation: What Clinicians Need to Know? Front Pediatr. 2021. Sep 3;9. doi: 10.3389/fped.2021.715151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherepanova N, Shrimal S, Gilmore R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol. 2016. Aug 1;41:57–65. doi: 10.1016/j.ceb.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Zhao Y, Zhou K, Freeze HH, Zhang YW, Xu H. Insufficient ER-stress response causes selective mouse cerebellar granule cell degeneration resembling that seen in congenital disorders of glycosylation. Mol Brain. 2013. Dec 4;6(1):52. doi: 10.1186/1756-6606-6-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134 [DOI] [PubMed] [Google Scholar]
- 8.Shang J, Körner C, Freeze H, Lehrman MA. Extension of lipid-linked oligosaccharides is a high-priority aspect of the unfolded protein response: Endoplasmic reticulum stress in Type I congenital disorder of glycosylation fibroblasts. Glycobiology. 2002;12(5):307–17. doi: 10.1093/glycob/12.5.307 [DOI] [PubMed] [Google Scholar]
- 9.Rita Lecca M, Wagner U, Patrignani A, Berger EG, Hennet T. Genome-wide analysis of the unfolded protein response in fibroblasts from congenital disorders of glycosylation type-I patients. FASEB J. 2005. Feb 15;19(2):1–21. doi: 10.1096/fj.04-2397fje [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016. Jan 20;529(7586):326–35. doi: 10.1038/nature17041 [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Rush JS, Karaoglu D, Krasnewich D, Lubinsky MS, Waechter CJ, et al. Deficiency of UDP-GlcNac:dolichol phosphate N-acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type Ij. Hum Mutat. 2003;22(2):144–50. doi: 10.1002/humu.10239 [DOI] [PubMed] [Google Scholar]
- 12.Yuste-Checa P, Vega AI, Martín-Higueras C, Medrano C, Gámez A, Desviat LR, et al. DPAGT1-CDG: Functional analysis of disease-causing pathogenic mutations and role of endoplasmic reticulum stress. Lewin AS, editor. PLoS One. 2017. Jun 29;12(6):e0179456. doi: 10.1371/journal.pone.0179456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selcen D, Shen XM, Brengman J, Li Y, Stans AA, Wieben E, et al. DPAGT1 myasthenia and myopathy: Genetic, phenotypic, and expression studies. Neurology. 2014. May 20;82(20):1822. doi: 10.1212/WNL.0000000000000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibáñez-Micó S, Domingo Jiménez R, Pérez-Cerdá C, Ghandour-Fabre D. Congenital myasthenia and congenital disorders of glycosylation caused by mutations in the DPAGT1 gene. Neurologia. 2019. Mar 1;34(2):139–41. [DOI] [PubMed] [Google Scholar]
- 15.Dong YY, Wang H, Pike ACW, Cochrane SA, Hamedzadeh S, Wyszyński FJ, et al. Structures of DPAGT1 Explain Glycosylation Disease Mechanisms and Advance TB Antibiotic Design. Cell. 2018. Nov 1;175(4):1045–1058.e16. doi: 10.1016/j.cell.2018.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heifetz A, Keenan RW, Elbein AD. Mechanism of Action of Tunicamycin on the UDP-GlcNAc:Dolichyl-Phosphate GlcNAc-1 -Phosphate Transferase. Biochemistry. 1979;18(11):2186–92. doi: 10.1021/bi00578a008 [DOI] [PubMed] [Google Scholar]
- 17.Oslowski CM, Urano F. Measuring ER Stress and the Unfolded Protein Response Using Mammalian Tissue Culture System. In: Methods in enzymology. 2011. p. 71–92. doi: 10.1016/B978-0-12-385114-7.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebedeva I V., Wagner M V., Sahdeo S, Lu Y-F, Anyanwu-Ofili A, Harms MB, et al. Precision genetic cellular models identify therapies protective against ER stress. Cell Death Dis 2021 128. 2021. Aug 5;12(8):1–11. doi: 10.1038/s41419-021-04045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basiri K, Belaya K, Liu WW, Maxwell S, Sedghi M, Beeson D. Clinical features in a large Iranian family with a limb-girdle congenital myasthenic syndrome due to a mutation in DPAGT1. Neuromuscul Disord. 2013. Jun;23(6):469–72. doi: 10.1016/j.nmd.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nita-Lazar M, Noonan V, Rebustini I, Walker J, Menko AS, Kukuruzinska MA. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009. Jul 15;69(14):5673. doi: 10.1158/0008-5472.CAN-08-4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riordan JD, Nadeau JH. From Peas to Disease: Modifier Genes, Network Resilience, and the Genetics of Health. Am J Hum Genet. 2017. Aug 3;101(2):177–91. doi: 10.1016/j.ajhg.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahit KMTH, Tarailo-Graovac M. Genetic Modifiers and Rare Mendelian Disease. Genes (Basel). 2020. Feb 25;11(3). doi: 10.3390/genes11030239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow CY. Bringing genetic background into focus. Nat Rev Genet. 2016. Feb 14;17(2):63–4. doi: 10.1038/nrg.2015.9 [DOI] [PubMed] [Google Scholar]
- 24.Okamoto N, Viswanatha R, Bittar R, Li Z, Haga-Yamanaka S, Perrimon N, et al. A Membrane Transporter Is Required for Steroid Hormone Uptake in Drosophila. Dev Cell. 2018. Nov 5;47(3):294–305.e7. doi: 10.1016/j.devcel.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanatha R, Li Z, Hu Y, Perrimon N. Pooled genome-wide CRISPR screening for basal and context-specific fitness gene essentiality in Drosophila cells. Elife. 2018. Jul 27;7. doi: 10.7554/eLife.36333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Flockhart I, Vinayagam A, Bergwitz C, Berger B, Perrimon N, et al. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinforma 2011 121. 2011. Aug 31;12(1):1–16. doi: 10.1186/1471-2105-12-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al. Essentials of Glycobiology. Cold Spring Harb. 2017;823. [Google Scholar]
- 28.Test | Invitae Congenital Disorders of Glycosylation Panel. (Available from https//www.invitae.com/en/providers/test-catalog/test-06155).
- 29.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol 2019 171. 2019. Jul 4;17(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prives J, Bar-Sagi D. Effect of tunicamycin, an inhibitor of protein glycosylation, on the biological properties of acetylcholine receptor in cultured muscle cells—PubMed. J Biol Chem. 1983;258(3):1775–80. [PubMed] [Google Scholar]
- 31.Olden K, Law J, Hunter VA, Romain R, Parent JB. Inhibition of fusion of embryonic muscle cells in culture by tunicamycin is prevented by leupeptin. J Cell Biol. 1981;88(1):199–204. doi: 10.1083/jcb.88.1.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan G, Goldsmith PK, Collins R, Dunn CK, Krapcho KJ, Rogers K V., et al. N-linked glycosylation of the human Ca2+ receptor is essential for its expression at the cell surface. Endocrinology. 1997;138(5):1916–22. doi: 10.1210/endo.138.5.5131 [DOI] [PubMed] [Google Scholar]
- 33.Peiris D, Spector AF, Lomax-Browne H, Azimi T, Ramesh B, Loizidou M, et al. Cellular glycosylation affects Herceptin binding and sensitivity of breast cancer cells to doxorubicin and growth factors. Sci Rep. 2017. Feb 22;7. doi: 10.1038/srep43006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olden K, Pratt RM, Yamada KM. Role of carbohydrates in protein secretion and turnover: Effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978;13(3):461–73. doi: 10.1016/0092-8674(78)90320-3 [DOI] [PubMed] [Google Scholar]
- 35.Ryczko MC, Pawling J, Chen R, Abdel Rahman AM, Yau K, Copeland JK, et al. Metabolic Reprogramming by Hexosamine Biosynthetic and Golgi N-Glycan Branching Pathways. Sci Rep. 2016. Mar 14;6. doi: 10.1038/srep23043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinoshita T. Biosynthesis and biology of mammalian GPI-anchored proteins. Open Biol. 2020. Mar 1;10(3). doi: 10.1098/rsob.190290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: A complex membrane-anchoring structure for proteins. Biochemistry. 2008. Jul 8;47(27):6991–7000. doi: 10.1021/bi8006324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li WZ, Li SL, Zheng HY, Zhang SP, Xue L. A broad expression profile of the GMR-GAL4 driver in Drosophila melanogaster. Genet Mol Res. 2012;11(3):1997–2002. doi: 10.4238/2012.August.6.4 [DOI] [PubMed] [Google Scholar]
- 39.Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, Yang L, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011. Mar 24;471(7339):473–9. doi: 10.1038/nature09715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelbart WM, Emmert DB. FlyBase Reference Report: FlyBase High Throughput Expression Pattern Data. FlyBase. 2013;(Available from: https://flybase.org/reports/FBrf0221009.html). [Google Scholar]
- 41.Kranz C, Denecke J, Lehrman MA, Ray S, Kienz P, Kreissel G, et al. A mutation in the human MPDU1 gene causes congenital disorder of glycosylation type If (CDG-If). J Clin Invest. 2001. Dec 1;108(11):1613–9. doi: 10.1172/JCI13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastaki F, Bizzari S, Hamici S, Nair P, Mohamed M, Saif F, et al. Single-center experience of N-linked Congenital Disorders of Glycosylation with a Summary of Molecularly Characterized Cases in Arabs. Ann Hum Genet. 2018. Jan 1;82(1):35–47. doi: 10.1111/ahg.12220 [DOI] [PubMed] [Google Scholar]
- 43.Schenk B, Fernandez F, Waechter CJ. The ins(ide) and outs(ide) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 2001;11(5). [DOI] [PubMed] [Google Scholar]
- 44.Yang AC, Ng BG, Moore SA, Rush J, Waechter CJ, Raymond KM, et al. Congenital disorder of glycosylation due to DPM1 mutations presenting with dystroglycanopathy-type congenital muscular dystrophy. Mol Genet Metab. 2013. Nov;110(3):345–51. doi: 10.1016/j.ymgme.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda Y, Tanaka S, Hino J, Kangawa K, Kinoshita T. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. EMBO J. 2000. Jun 6;19(11):2475. doi: 10.1093/emboj/19.11.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefeber DJ, Schönberger J, Morava E, Guillard M, Huyben KM, Verrijp K, et al. Deficiency of Dol-P-Man Synthase Subunit DPM3 Bridges the Congenital Disorders of Glycosylation with the Dystroglycanopathies. Am J Hum Genet. 2009. Jul 10;85(1):76. doi: 10.1016/j.ajhg.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imbach T, Schenk B, Schollen E, Burda P, Stutz A, Grünewald S, et al. Deficiency of dolichol-phosphate-mannose synthase-1 causes congenital disorder of glycosylation type Ie. J Clin Invest. 2000;105(2):233–9. doi: 10.1172/JCI8691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palu RAS, Dalton HM, Chow CY. Decoupling of apoptosis from activation of the ER stress response by the drosophila metallopeptidase superdeath. Genetics. 2020. Apr 1;214(4):913–25. doi: 10.1534/genetics.119.303004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryoo HD, Domingos PM, Kang M-J, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 2007. Jan 10;26(1):242–52. doi: 10.1038/sj.emboj.7601477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chow CY, Kelsey KJP, Wolfner MF, Clark AG. Candidate genetic modifiers of retinitis pigmentosa identified by exploiting natural variation in Drosophila. Hum Mol Genet. 2016. Feb 15;25(4):651–9. doi: 10.1093/hmg/ddv502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palu RAS, Chow CY. Baldspot/ELOVL6 is a conserved modifier of disease and the ER stress response. Lin JH, editor. PLOS Genet. 2018. Aug 6;14(8). doi: 10.1371/journal.pgen.1007557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labeau A, Simon-Loriere E, Hafirassou M-L, Bonnet-Madin L, Tessier S, Zamborlini A, et al. A Genome-Wide CRISPR-Cas9 Screen Identifies the Dolichol-Phosphate Mannose Synthase Complex as a Host Dependency Factor for Dengue Virus Infection. J Virol. 2020. Mar 17;94(7). doi: 10.1128/JVI.01751-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loibl M, Strahl S. Protein O-mannosylation: What we have learned from baker’s yeast. Biochim Biophys Acta—Mol Cell Res. 2013. Nov 1;1833(11):2438–46. doi: 10.1016/j.bbamcr.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 54.Dobson CM, Hempel SJ, Stalnaker SH, Stuart R, Wells L. O-Mannosylation and human disease. Cell Mol Life Sci. 2013. Aug;70(16):2849–57. doi: 10.1007/s00018-012-1193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walski T, De Schutter K, Van Damme EJM, Smagghe G. Diversity and functions of protein glycosylation in insects. Insect Biochem Mol Biol. 2017. Apr 1;83:21–34. doi: 10.1016/j.ibmb.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 56.Shcherbakova A, Tiemann B, Buettner FFR, Bakker H. Distinct C-mannosylation of netrin receptor thrombospondin type 1 repeats by mammalian DPY19L1 and DPY19L3. Proc Natl Acad Sci. 2017. Mar 7;114(10):2574–9. doi: 10.1073/pnas.1613165114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ros S, Schulze A. Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism. Cancer Metab. 2013. Dec;1(1). doi: 10.1186/2049-3002-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Legeza B, Marcolongo P, Gamberucci A, Varga V, Bánhegyi G, Benedetti A, et al. Fructose, Glucocorticoids and Adipose Tissue: Implications for the Metabolic Syndrome. Nutrients. 2017. May 1;9(5). doi: 10.3390/nu9050426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maser D, Johnson L, Lee CY. Multiple forms of Drosophila hexokinase. Purification, biochemical and immunological characterization. J Biol Chem. 1980;255:4673–9. [PubMed] [Google Scholar]
- 60.Varma V, Boros LG, Nolen GT, Chang CW, Wabitsch M, Beger RD, et al. Metabolic fate of fructose in human adipocytes: a targeted 13C tracer fate association study. Metabolomics. 2015. Jun 1;11(3):529. doi: 10.1007/s11306-014-0716-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller CO, Yang X, Lu K, Cao J, Herath K, Rosahl TW, et al. Ketohexokinase knockout mice, a model for essential fructosuria, exhibit altered fructose metabolism and are protected from diet-induced metabolic defects. Am J Physiol Endocrinol Metab. 2018. Sep 1;315(3):E386–93. doi: 10.1152/ajpendo.00027.2018 [DOI] [PubMed] [Google Scholar]
- 62.Lin JH, Walter P, Yen TSSB. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol Mech Dis. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q, Groenendyk J, Michalak M. Glycoprotein Quality Control and Endoplasmic Reticulum Stress. Molecules. 2015. Aug 1;20(8):13689. doi: 10.3390/molecules200813689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo XY, Liu YS, Gao XD, Kinoshita T, Fujita M. Calnexin mediates the maturation of GPI-anchors through ER retention. J Biol Chem. 2020. Nov 27;295(48):16393–410. doi: 10.1074/jbc.RA120.015577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruggiano A, Foresti O, Carvalho P. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol. 2014;204(6):869–79. doi: 10.1083/jcb.201312042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun S, Shi G, Han X, Francisco AB, Ji Y, Mendonça N, et al. Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proc Natl Acad Sci U S A. 2014. Feb 4;111(5):E582–91. doi: 10.1073/pnas.1318114111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhattacharya A, Qi L. ER-associated degradation in health and disease—from substrate to organism. J Cell Sci. 2019;132(23). doi: 10.1242/jcs.232850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, et al. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci. 2013. Dec 24;110(52):21018–23. doi: 10.1073/pnas.1313446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sparks SE. Congenital protein hypoglycosylation diseases. Appl Clin Genet. 2012. Jul 4;5:43. doi: 10.2147/TACG.S18673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maupin KA, Liden D, Haab BB. The fine specificity of mannose-binding and galactose-binding lectins revealed using outlier motif analysis of glycan array data. Glycobiology. 2012. Jan;22(1):160. doi: 10.1093/glycob/cwr128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cavada BS, Pinto-Junior VR, Osterne VJS, Nascimento KS. ConA-Like Lectins: High Similarity Proteins as Models to Study Structure/Biological Activities Relationships. Int J Mol Sci. 2019. Jan 1;20(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lieu EL, Kelekar N, Bhalla P, Kim J. Fructose and Mannose in Inborn Errors of Metabolism and Cancer. Metabolites. 2021. Aug 1;11(8). doi: 10.3390/metabo11080479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demay Y, Perochon J, Szuplewski S, Mignotte B, Gaumer S. The PERK pathway independently triggers apoptosis and a Rac1/Slpr/JNK/Dilp8 signaling favoring tissue homeostasis in a chronic ER stress Drosophila model. Cell Death Dis 2014 510. 2014. Oct 9;5(10):e1452–e1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gauthier SA, VanHaaften E, Cherbas L, Cherbas P, Hewes RS. Cryptocephal, the Drosophila melanogaster ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2. PLoS Genet. 2012. Aug;8(8). doi: 10.1371/journal.pgen.1002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas HE, Stunnenberg HG, Stewart AF. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature. 1993;362(6419):471–5. doi: 10.1038/362471a0 [DOI] [PubMed] [Google Scholar]
- 76.Yao TP, Segraves WA, Oro AE, McKeown M, Evans RM. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell. 1992. Oct 2;71(1):63–72. doi: 10.1016/0092-8674(92)90266-f [DOI] [PubMed] [Google Scholar]
- 77.Perez AR, Sala L, Perez RK, Vidigal JA. CSC software corrects off-target mediated gRNA depletion in CRISPR-Cas9 essentiality screens. Nat Commun. 2021. Dec 1;12(1). doi: 10.1038/s41467-021-26722-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bodapati S, Daley TP, Lin X, Zou J, Qi LS. A benchmark of algorithms for the analysis of pooled CRISPR screens. Genome Biol. 2020. Mar 9;21(1). doi: 10.1186/s13059-020-01972-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meyers RM, Bryan JG, McFarland JM, Weir BA, Sizemore AE, Xu H, et al. Computational correction of copy-number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat Genet. 2017. Dec 1;49(12):1779. doi: 10.1038/ng.3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, et al. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science. 2004. Feb 6;303(5659):832–5. doi: 10.1126/science.1091266 [DOI] [PubMed] [Google Scholar]
- 81.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017. Aug 10;170(4):605. doi: 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 Induces Purine Synthesis Through Control of the Mitochondrial Tetrahydrofolate Cycle. Science. 2016. Feb 12;351(6274):728. doi: 10.1126/science.aad0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reich S, Nguyen CDL, Has C, Steltgens S, Soni H, Coman C, et al. A multi-omics analysis reveals the unfolded protein response regulon and stress-induced resistance to folate-based antimetabolites. Nat Commun 2020 111. 2020. Jun 10;11(1):1–15. doi: 10.1038/s41467-020-16747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao N, Shang J, Huynh D, Manthati VL, Arias C, Harding HP, et al. Mannose-6-phosphate regulates destruction of lipid-linked oligosaccharides. Mol Biol Cell. 2011. Sep 1;22(17):2994–3009. doi: 10.1091/mbc.E11-04-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viswanatha R, Brathwaite R, Hu Y, Li Z, Rodiger J, Merckaert P, et al. Pooled CRISPR Screens in Drosophila Cells. Curr Protoc Mol Biol. 2019. Dec 6;129(1). doi: 10.1002/cpmb.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014;15(12):554. doi: 10.1186/s13059-014-0554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007. Jul 12;448(7150):151–6. doi: 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- 88.Weasner BM, Weasner BP, Neuman SD, Bashirullah A, Kumar JP. Retinal Expression of the Drosophila eyes absent Gene Is Controlled by Several Cooperatively Acting Cis-regulatory Elements. PLOS Genet. 2016. Dec 1;12(12):e1006462. doi: 10.1371/journal.pgen.1006462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lesperance DNA, Broderick NA. Meta-analysis of Diets Used in Drosophila Microbiome Research and Introduction of the Drosophila Dietary Composition Calculator (DDCC). G3 Genes|Genomes|Genetics. 2020. Jul 1;10(7):2207. doi: 10.1534/g3.120.401235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mi H, Muruganujan A, Huang X, Ebert D, Mills C, Guo X, et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc 2019 143. 2019. Feb 25;14(3):703–21. doi: 10.1038/s41596-019-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mi H, Ebert D, Muruganujan A, Mills C, Albou L-P, Mushayamaha T, et al. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021. Jan 8;49(D1):D394–403. doi: 10.1093/nar/gkaa1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.R Core Team. R: A language and environment for statistical computing. 2013;(Available from: http://www.r-project.org/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative images from two replicates of RNAi line crosses to both the DPAGT1 and ER stress models are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set. Quantification of these eye crosses are included in S7 Table.
(TIF)
Representative images from two replicates of RNAi line crosses to both the eya composite-GAL4 and GMR-GAL4 control lines are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set.
(TIF)
Representative images from two replicates of RNAi line crosses to both the DPAGT1 and ER stress models are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set. Quantification of these eye crosses is included in S7 Table.
(TIF)
Representative images from two replicates of RNAi line crosses to both the eya composite-GAL4 and GMR-GAL4 control lines are shown here. This also includes representative images from the RNAi line-specific controls for each cross. RNAi lines used, including specific stock numbers, are included to the left of each image set.
(TIF)
Fly gene names are listed, as these genes are derived from the S2 CRISPR knockout guide RNA library. All resistance and sensitivity metrics are based on lfc values from the survival screen.
(PDF)
The first tab includes the raw sgRNA enrichment scores (tunicamycin-vs-untreated) 4–8, as well as the median values of those sgRNAs used in subsequent analyses. The second tab includes the adjusted lfc values and p-values used to create the volcano plot in Fig 1.
(XLSX)
The first tab includes the full list of human CDGs derived from The Essentials of Glycobiology and Invitae panel, the derived fly orthologs via their DIOPT score, a finalized list removing duplicate orthologs alongside their lfc value from the survival screen, and finally the overrepresentation test analysis performed. The second tab includes human CDGs for which no fly ortholog was found at a DIOPT score of <5.
(XLSX)
This includes the output of the PANTHER gene list analysis from the combined input of resistance and sensitivity candidate genes at an lfc of ≥0.5 or ≤-0.5 from the survival screen in Fig 1 (with re-labeled columns to improve readability). Categories are sorted by fold enrichment.
(XLSX)
The first tab includes only genes that had at least one sgRNA in the highest ~1% of fluorescence of all sgRNAs in two biological replicates. This includes information on specific guides, and the final list of genes found (FBgn number and Fly gene name). The second tab is the raw data from the ConA experiment, including information on specific guide RNAs and their abundance in either replicate.
(XLSX)
This includes the output of the PANTHER gene list analysis from the input of candidate genes that improved ConA phenotypes in Fig 2 (with re-labeled columns to improve readability). Categories are sorted by fold enrichment.
(XLSX)
This includes the list of resistance candidate genes from the survival screen (S1 Table), all hits from the ConA screen (S4 Table), and the overlapping genes between them (FBgn number and Fly gene name).
(XLSX)
Quantitative results of all RNAi cross replicates to both the DPAGT1 and ER stress model are listed here. This table includes information on the Drosophila gene (name and FBgn number), closest human ortholog, the reasoning on why the gene was tested (GO category, validation, relation to Dpm1, etc.), if the gene was a hit in one or both screens, the specific RNAi stock used in each cross, as well as the related pathway function. % change in eye size, confidence interval (derived from the standard deviation of change and listed N), as well as the Student’s t test p-value are indicated for each replicate.
(XLSX)
This lists the data on two replicates of the sugar feeding experiments on the eya composite-GAL4 and GMR-GAL4 control lines and DPAGT1 and ER stress models. % change in eye size, confidence interval (derived from the standard deviation of change and listed N), as well as the Student’s t test p-value are indicated for each replicate.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.