Abstract
Aim
Climate change is expected to impact mountain biodiversity by shifting species ranges and the biomes they shape. The extent and regional variation in these impacts are still poorly understood, particularly in the highly biodiverse Andes. Regional syntheses of climate change impacts on vegetation are pivotal to identify and guide research priorities. Here we review current data, knowledge and uncertainties in past, present and future climate change impacts on vegetation in the Andes.
Location: Andes.
Taxon: Plants.
Methods
We (i) conducted a literature review on Andean vegetation responses to past and contemporary climatic change, (ii) analysed future climate projections for different elevations and slope orientations at 19 Andean locations using an ensemble of model outputs from the Coupled Model Intercomparison Project 5, and (iii) calculated changes in the suitable climate envelope area of Andean biomes and compared these results to studies that used species distribution models.
Results
Future climatic changes (2040–2070) are projected to be stronger at high‐elevation areas in the tropical Andes (up to 4°C under RCP 8.5), while in the temperate Andes temperature increases are projected to be up to 2°C. Under this worst‐case scenario, temperate deciduous forests and the grasslands/steppes from the Central and Southern Andes are predicted to show the greatest losses of suitable climatic space (30% and 17%–23%, respectively). The high vulnerability of these biomes contrasts with the low attention from researchers modelling Andean species distributions. Critical knowledge gaps include a lack of an Andean wide plant checklist, insufficient density of weather stations at high‐elevation areas, a lack of high‐resolution climatologies that accommodates the Andes' complex topography and climatic processes, insufficient data to model demographic and ecological processes, and low use of palaeo data for distribution modelling.
Main conclusions
Climate change is likely to profoundly affect the extent and composition of Andean biomes. Temperate Andean biomes in particular are susceptible to substantial area contractions. There are, however, considerable challenges and uncertainties in modelling species and biome responses and a pressing need for a region‐wide approach to address knowledge gaps and improve understanding and monitoring of climate change impacts in these globally important biomes.
Keywords: Andes, climate change, plant biodiversity, plant dynamics, species distribution modelling
1. INTRODUCTION
The Andes (Figure 1) are among the most biodiverse regions on the planet (Myers et al., 2000). Spanning over 9000 km in length (following the mountain ridge) (Graham, 2009) and reaching well over 6000 m in elevation, they are the longest and second highest terrestrial mountain range on Earth after the Himalayas. The Andes are home to an estimated 40,000 plant species and thousands of vertebrate species with exceptionally high levels of species endemism (Kreft & Jetz, 2007; Pennington et al., 2010). In addition, their natural forests, shrublands and grasslands provide critical ecosystem services such as soil protection, carbon storage and—notably—water for millions of people (e.g. Buytaert et al., 2011; Diazgranados et al., 2021; Masiokas et al., 2019; Peña et al., 2018). Understanding the links between climate and natural vegetation, and predicting the impact of future climate change, is thus important for both conservation and human well‐being in the Andes and adjacent lowlands. Given the long latitudinal and steep elevational range, the Andes have a high variability in climate (e.g. Espinoza et al., 2020; Pabón Caicedo et al., 2020) (Figure 1b,c), making them an ideal natural laboratory for studying climate change impacts on plant biodiversity along these gradients.
FIGURE 1.
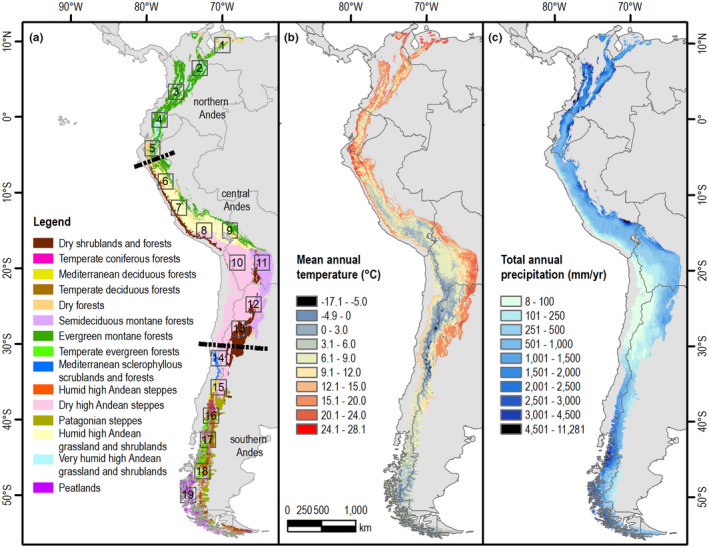
The Andes. (a) Andean biomes based on three vegetation maps (Luebert & Pliscoff, 2018; Oyarzabal et al., 2018; Tovar et al., 2013), major three regions (Northern, Central and Southern Andes) and 19 locations (2° × 2° bounding boxes, indicated by the numbers along the Andes) where climate change projections were analysed for this review, (b) mean annual temperature and (c) total annual precipitation obtained from CHELSA for the period 1979–2013 (Karger et al., 2017). Maps in geographical coordinate system
In the Andes, there are significant knowledge gaps and uncertainties surrounding the effects of climate change on the distribution of species and biomes. Recent papers have contributed to a greater understanding of the topic (e.g. Blundo et al., 2012; Carilla et al., 2018; Duque et al., 2015; Feeley et al., 2011; Srur et al., 2016), but broad‐scale overview studies of the Andes are lacking. Here, we review available data and knowledge on the likely impacts of climate change on plant and biome distributions in the region. Specifically, we combine (i) a literature review on past (long term, medium term and short term) climate change impacts on Andean vegetation, with (ii) an analysis of the outputs of global climate models to characterise future projections for the region and associated uncertainties, and (iii) modelling the potential impacts on the distribution of Andean biomes. Finally, we take a critical look at the limitations of current distribution modelling practice and propose a research agenda to fill knowledge gaps to enable targeted conservation action for plants and biomes in the Andes.
2. MATERIALS AND METHODS
2.1. Literature review: Past climate and vegetation changes
We conducted a literature review of studies on long‐term (thousands of years) and medium‐ to short‐term (last millennium and decades) past climate changes and vegetation responses. Knowledge on past vegetation dynamics over long time‐scales is based on palaeoecological research and specifically fossil pollen records. Around 1650 of such records are known to exist across Latin America based on over 1700 studies (See inventory of fossil pollen records by Flantua et al., 2015 and www.latinamericanpollendb.com) which we complemented with additional studies published between 2015 and 2016. We selected only records located within the Andes (n = 742) using the shapefile of the limits of the Andean biomes (see Section 2.4). In our results, we summarised the main vegetation responses to long‐term climate change based on the spatial coverage of these records.
Medium‐ and short‐term changes were analysed and summarised through a systematic literature review. We conducted a search for peer‐reviewed articles on Scopus on 1st of April 2021 using the following keywords ‘Andes’ or ‘Patagonia’, ‘climate change’ or ‘drought’ or ‘deglaciation’, ‘tree’ or ‘vegetation’ or ‘plant’, and ‘chronosequence’ or ‘plots’ or ‘resurvey’ or ‘tree‐ring’ or ‘dendrochronological’. This allowed us to source publications covering the entire Andes, the main climate changes and the main methods used to analyse vegetation changes over this time‐scale. We obtained 131 studies that were checked to keep only those that recorded changes in climate for a given period and/or a vegetation response to past climate change. We also kept those related to fire events linked to climate conditions. Finally, 58 studies were used (Table S1) to characterise medium‐ and short‐term changes.
2.2. Literature review: Plant distribution modelling
Two main approaches are currently used to model plant species distributions, namely correlative and dynamic models. Correlative models, commonly known as species distribution models (SDMs) or ecological niche models, relate species presence at a certain location to environmental conditions using an algorithm (Guisan & Thuiller, 2005). In contrast, Dynamic Vegetation Models (DVMs) are based on a mechanistic approach, and are able to reflect demographic and ecological processes shaped by physiological constraints and species competition, among others (Guisan & Thuiller, 2005; Snell et al., 2014).
We performed a literature review of plant distribution modelling in the Andes, using either approach. For correlative models, we conducted a search in Scopus (29th of January 2020) using the keywords ‘climate change’ or ‘warming’ and ‘species distribution’ and retrieved 145 publications for 2010–2019. Separate queries were conducted for each Andean country and one additional query used the keywords ‘Andes’ or ‘Andean’ and ‘plant’. After examining all papers, we kept studies that used SDMs to model present, past and/or future distributions of Andean plant species with a final selection of 32 studies (Table S2), while a much lower number of studies used Dynamic Vegetation Models.
2.3. Future climate change projections
To assess projected future climatic change in the Andes, we used an ensemble of model outputs from the Coupled Model Intercomparison Project 5 (CMIP5, https://www.wcrp‐climate.org/wgcm‐cmip/wgcm‐cmip5; Table S3). For this, we selected 19 locations across the Andes, each encompassing a 2° × 2° bounding box covering an area of ~40,000–50,000 km2 each (Table S4; Figure 1a). As the mountain range blocks atmospheric circulation, projected shifts in climate vary not only at different elevations, but also between western and eastern slopes (Arias et al., 2021). Therefore, we used a novel approach to quantify and analyse projected changes in climate along slopes, differentiating areas based on their topography. Annual mean precipitation and near‐surface air temperature data from the CMIP5 models were grouped within each location according to elevation range (discretised by 500 m intervals) and aspect (western slope, peak and eastern slope). In this way, data from different models are only combined if they belong to a given aspect and to the same elevation range (i.e. at each elevation‐aspect combination we got a different ensemble with a different number of contributing models). Finally, since the grid cells of the models only partially coincide with the 2° x 2° bounding box, a weighted average was performed taking into account the percentage of the bounding box covered by each grid cell. In this procedure, the data from the different models are not interpolated to a common grid projection and resolution (see Methods S1 for details). This method was presented by Fita et al. (2019) and was previously used by Pabón‐Caicedo et al. (2020).
Changes in climate were calculated as the ensemble mean difference between projected future (2040–2070, most extreme scenario RCP 8.5) and near‐present conditions (1960–1990, ‘historical scenario’). Scenario RCP 8.5 assumes a drastic increase in the use of coal and was designed to simulate an extreme non‐mitigation situation with increasing population and energy demand. While policy endeavours render this scenario increasingly unlikely, it can be used to understand what could happen in the worst case and which systems/biomes would be potential beneficiaries and where one would expect to see drastic losses. Albeit drastic, RCP 8.5 is not implausible (Schwalm et al., 2020), and it is useful to understand its implication. The robustness of the changes between current and worst‐case future conditions was assessed using the signal‐to‐noise ratio (SNR) (Kendon et al., 2008), which is a measure of models agreement, usually called ‘spread’ (see also details in Methods S2).
2.4. Future projections of vegetation responses
To assess potential climate change impacts on Andean biomes, we first created a unified high‐resolution biome map covering the entire Andean region by combining existing vegetation maps (Luebert & Pliscoff, 2018; Oyarzabal et al., 2018; Tovar et al., 2013). To standardise these maps with different levels of class resolution, we merged several classes to delineate standardised main functional types throughout the Andes. In total, we delineated 15 biomes/vegetation types (Table S5) based on dominant plant functional types (e.g. evergreen/deciduous, tree/shrubs) and dominant climate (e.g. dry, humid). Before assessing potential future changes in the climate envelops of these biomes, we first characterised their current climate envelope using annual mean temperature and total annual precipitation following the widely accepted Whittaker's classification (Whittaker, 1975). For this, we extracted climate data from CHELSA at a resolution of 10 arc minutes (≈18.5 km; Karger et al., 2017) to obtain a higher resolution than the one provided by CMIP5. Then, we applied a simplified delta method (Hay et al., 2000) where the differences in climate obtained from CMIP5 model outputs are added to the observed values from CHELSA for each biome. For each CMIP5 model (Table S3), we estimated the ‘delta‐change’ for the mean annual temperature and total annual precipitation data between the future and present at their original resolution. We used for future climate the 2040–2070 period from the RCP8.5 scenario and the 1960–1990 period as the near‐present climate (‘historical scenario’). Then, the deltas obtained from CMIP5 models were applied to the current climate of each biome (characterised from the CHELSA data) at grid point basis, to estimate their projected changes for the whole Andean region. Results were expressed as relative changes in the extent covered by the present‐day climatic envelope of each biome and assessed using the SNR (Kendon et al., 2008).
3. PAST VEGETATION CHANGE IN THE ANDES
An understanding of how past climatic changes shaped Andean vegetation can inform how biomes might respond to projected future climate. Here, we summarise general findings from palaeoecological studies that cover the last glacial–interglacial cycle (last c. 120,000 years), based on fossil pollen records, and mid‐ and short‐term studies, based on dendrochronology and monitoring (Figure 2).
FIGURE 2.
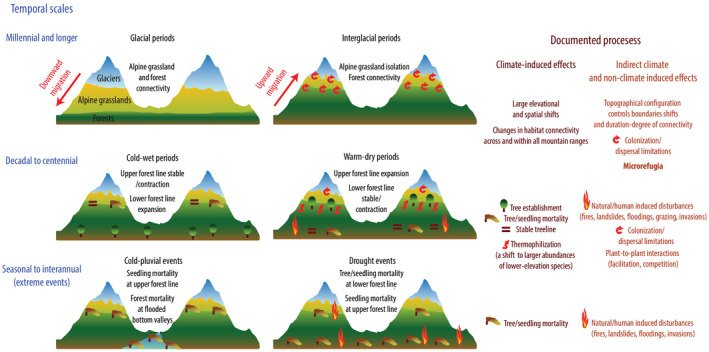
Representation of vegetation responses to past changes in climate at different temporal scales identified in palaeoecological records and plot data from the Andes
3.1. Long‐term changes
Millions of years of geological processes have led to the rise of the Andes, a mountain range that has determined the geography, geomorphology and climate of the whole of South America (Boschman, 2021; Ehlers & Poulsen, 2009; Insel et al., 2010) and the Southern Hemisphere (Falco et al., 2019). Moisture fluxes are concentrated, deviated or blocked along the longitudinal and latitudinal shape of this stretched mountain system (Garreaud, 2009). The spatial and temporal patterns of the Andean climate are affected by ocean–atmosphere interactions, monsoon systems, the seasonal migrations of the Intertropical Convergence Zone (ITCZ) over the Atlantic and Pacific oceans, the southern westerly wind belt (SWW) and storm track at mid and high latitudes (Arias et al., 2021; Garreaud et al., 2009), jointly causing climate variability on interannual to interdecadal scales (e.g. Flantua et al., 2016).
The intensity and mean latitude of the ITCZ, atmospheric convective systems and the trade winds were influenced by Quaternary (last 2.6 million years) climate changes, causing changes in precipitation regimes over South America and especially along the eastern tropical Andes (Novello et al., 2017, 2019). In the Southern Andes, the changes in the intensity and latitudinal position of the SWW caused strong variations in rainfall during the last glacial–interglacial cycle (Lamy et al., 2010; Rojas et al., 2009). Paleotemperatures have been recorded on glacier snowline reconstructions and fossil pollen records during the Quaternary, evidencing a remarkable variability of geo‐climatic scenarios. For example, during the last glacial–interglacial cycle, several periods of relatively warmer climates (interstadials) were interrupted by periods of cooler climates (stadials) with sharp temperature declines during glacial advances in the Northern (Groot et al., 2013) and Southern (Kaiser et al., 2005; Villagrán et al., 2019) Andes. Temperature ranges over a full glacial–interglacial cycle could have reached 5–10°C in the high Northern and Central Andes above 2500 m (e.g. Flantua et al., 2019; Groot et al., 2011; Hooghiemstra & Flantua, 2019; Klein et al., 1995; Mark et al., 2005; Valencia et al., 2010), while at mid‐latitudes of the Southern Andes, a decrease in mean summer temperatures of 6–8°C below modern values has been estimated (e.g. Heusser et al., 1981). Temperature estimates of periods warmer than present, such as the early to mid‐Holocene climatic optimum (c. 10,000–6000 years ago depending on the region) and the last interglacial are scarce, but along the coast of the Central and Southern Andes, for instance, temperature is estimated to have been 3–4°C higher than today (Heusser et al., 1981; see more in Mayle et al., 2004).
Fossil pollen records have contributed to understanding biome responses to long‐term paleoclimate fluctuations at long and short time‐scales. While there is a relatively high density of fossil pollen records for the Northern Andes and the tip of the Patagonian Andes (Figure S1), only few records cover the last glacial–interglacial cycle as most fossil pollen records reach only the Holocene (last 11,700 years) (Flantua et al., 2015). In the Southern Andes, only few continental records span the last glacial maximum (LGM) and beyond due to the massive extent of glaciers in this part of the Andes (Palacios et al., 2020). The scarce long fossil pollen records from the Northern and Central Andes have, however, provided evidence of the sensitivity of Andean biomes to Quaternary climate fluctuations, responding with elevational shifts of biomes and taxa over long time‐scales (e.g. Hooghiemstra & Flantua, 2019). These elevational shifts caused substantial changes in habitat fragmentation for the high‐elevation grass biome (páramos) of the Northern Andes, likely contributing to the build‐up of its exceptional biodiversity over the course of the Quaternary (Flantua et al., 2019). Andean forests in the Northern Andes, on the other hand, show little change in species composition over long time‐scales, that is, between glacial–interglacial cycles (Felde et al., 2016), likely the effect of a continuous high habitat connectivity unaffected by the Quaternary climate fluctuations (Flantua & Hooghiemstra, 2018).
In the Southern Andes, during the stadials, glacial vegetation north of 42° S in the western Andes was dominated by Nothofagus and conifer forests together with Magellanic moorlands (Abarzúa et al., 2014; Villagrán, 2001; Villagrán et al., 2019). In contrast, Patagonia (southern to 42°S) was dominated by a cold and dry steppe vegetation during the glacial period, up to 51,000 years BP (Recasens et al., 2012). During the warmer periods, the Nothofagus and coniferous forests expanded further south and to lower elevations in the western cordillera (Heusser et al., 1981; Villagrán et al., 2019). Today, these forests exist only as relicts at mountain summits in north Patagonia or in sub‐Antarctic forest formations.
Over shorter time‐scales (last 30,000 years), high‐resolution palaeoecological records provide abundant evidence of gradual replacement and temporal disappearance of taxa in response to shifts in climate conditions (e.g. thermophilisation; Bogotá et al., 2016; González‐Carranza et al., 2012; Groot et al., 2013). Also these records provide evidence of non‐analogue biome composition as the result of altitudinal shifts and mixes of high‐ and low‐land taxa in response to climate change (e.g. in eastern forest of the Central Andes, Mayle et al., 2004, 2009) and the presence of microrefugia (e.g. Valencia et al., 2010). At the level of biomes, the spatial distribution differed substantially between the present and that of the LGM at the scale of South America, with altitudinal shifts between forest and grassland biomes in most Andean regions with changes in species composition (Marchant et al., 2009; Mayle et al., 2009; Villagrán, 2001). Under warmer periods than present, there were elevational shifts of biomes to higher elevations. For instance, montane cloud forest expanded upwards in the Northern Andes (Niemann & Behling, 2008). Also, after the glacier retreatment, a mixture of Nothofagus forest and shrubland/steppe developed on the east side of the Southern Andes, while temperate rainforests developed on the west (Abarzúa et al., 2004; Whitlock et al., 2006). As temperature increased during the warmer and drier early‐Holocene, fire became an important component of these modern forest biomes in the Southern Andes (e.g. Kitzberger & Veblen, 2003; Whitlock et al., 2006), but also in the high‐elevation grasslands of the Northern and Central Andes (e.g. Villota et al., 2012; Weng et al., 2006).
At millennial time‐scales, Andean biomes have reached their modern diversity and distribution over the late Holocene, according to the increase in climate variability, but also the human impact along the Andes (Armesto et al., 2010; Flantua et al., 2016; Niemann & Behling, 2010).
3.2. Mid‐ to short‐term changes
Few high‐resolution regional studies currently exist on vegetation responses to climate changes observed in the last 1000 years (Table S1). Given the heterogeneity in climate and vegetation across the Andes, we briefly discuss reported changes for the Northern, Central and Southern Andes separately.
While positive trends in surface temperatures have been reported across the Andes in the last 100 years, observed trends of precipitation have been both negative and positive (Pabón Caicedo et al., 2020). In Ecuador, one of the oldest climatic datasets shows increasing temperatures since the mid‐1800 (Morueta‐Holme et al., 2015). As a result, an upward shift of 215–266 m in the upper limit of the alpine vegetation has been observed (Moret et al., 2019) in comparison to Humboldt's observations in 1802 (von Humboldt and Bonpland, 1807). Forest vegetation has also shown changes due to increasing temperatures. Thermophilisation, a shift in composition towards greater relative abundances of species from lower and warmer elevations, is reported to be widespread in Northern Andean forests, affecting both adult and juvenile tree communities (Duque et al., 2015; Fadrique et al., 2018). Observed changes seem related to higher than normal tree mortality among cold‐adapted species, which also lead to a decrease in species richness of adult trees (Duque et al., 2015). Such changes in tree mortality due to climate variations have also been observed in fossil pollen records (see e.g. González‐Carranza et al., 2012) and can result in temporal or permanent change in biome composition. The local persistence of species (and hence species richness) is likely to depend on the presence or absence of microrefugia where taxa may reside until climate favours their expansion again, also influenced by the heterogeneity of the Andean ‘mountain fingerprint’ (Flantua & Hooghiemstra, 2018) that may facilitate or inhibit migration along the Andes.
In the Central Andes, positive temperature trends have been reported for example in Peru (Lavado Casimiro et al., 2013; Schauwecker et al., 2014) and Bolivia (Seiler et al., 2014) over the last 100 years. Warming has led to both thermophilisation and primary succession in recently deglaciated areas. Thermophilisation of Peruvian Andean forests at the genus level has been registered over periods of time as short as 4 years (Feeley et al., 2011). Similar results were reported from a network of plots installed across tropical Andean montane forests over the last 15 years (Fadrique et al., 2018). Yet, the rates of thermophilisation are heterogeneous, with areas at intermediate elevations having more species that seem to be less sensitive to temperature increases, possibly due to certain plant traits (e.g. wider thermal tolerances), but also to other factors such as local site conditions (e.g. topgraphy and soil characteristics). Deglaciation has also rapidly increased in the Central Andes since the late 1970s, leading to the formation of new species assemblages (Zimmer et al., 2018). However, succession in response to warming has been slow due to the over‐representation of wind‐dispersed species (initial colonisers) and the low numbers and maturity of nurse plants, whose facilitation role is crucial in alpine ecosystems (Zimmer et al., 2018). Precipitation patterns have been more variable with both negative and positive trends in the last decades. Based on Polylepis tree‐ring reconstructions, an unprecedented decrease in precipitation over the past 700 years has been recorded over the arid Altiplano since mid‐20th century (Morales et al., 2012). A similar trend is found in the humid eastern Peruvian Andes (11° S) based on a Cedrela‐J uglans reconstruction that dates to 1817 (Humanes‐Fuente et al., 2020). On the contrary, the northern Argentinian Andes (eastern slope) has experienced sustained increasing precipitation and river streamflow in the last decades, which is unprecedented for the last three centuries (Ferrero et al., 2015; Villalba et al., 1998).
In the Southern Andes, tree‐ring‐based climate reconstructions show a consistent increase in temperature and a marked precipitation decrease in the last century. In the southernmost part of the Andes (47–52° S), tree‐ring chronologies from Nothofagus pumilio indicate that over the past 400 years the highest temperatures were found after the beginning of the 20th century (Villalba et al., 2003). Tree rings also reveal unprecedented high summer (December–February) temperatures during the last decades over the last 1000 years in northern Patagonia (37–44°S) (Pabón Caicedo et al., 2020; Villalba, 1990), together with a distinct decrease in precipitation over the last 50 years (Villalba et al., 2012). As a result of the increasing frequency of drought events, tree mortality in the Nothofagus forests has risen and tree growth declined since the mid‐1970s, particularly at the lower elevations in the eastern slope of the Andes (Rodríguez‐Catón et al., 2016; Srur et al., 2018; Suarez et al., 2004). In the Mediterranean region of the Chilean Andes (34°S), Nothofagus macrocarpa forests show an unprecedent decrease in growth since 1980 compared to the last two centuries (Venegas‐González et al., 2019) as a result of the same climatic trend. Massive mortality events in mesic‐wet forests of Austrocedrus chilensis coincided with hot and dry summers in 1912–1913, 1942–1943, 1956 and 1962 (Villalba & Veblen, 1997). Although a substantial Austrocedrus establishment occurred during the cool and wet conditions in the region between 1963 and 1979, no new episodes of tree establishment at the lower forest line have been observed since the 1980s, when warmer and drier climatic conditions have prevailed across the Southern Andes. In contrast, the recent warmer temperatures have allowed the establishment of new Nothofagus pumilio trees above the upper forest line and on deglaciated terrains across Patagonia (Garibotti et al., 2011; Srur et al., 2018). All the recorded impacts of climate change on tree demographic rates highlight the need to identify the climatic thresholds that regulate establishment and mortality along Andean forests.
4. FUTURE CLIMATE CHANGE PROJECTIONS
Our results show that while projected future temperatures consistently increase across the Andes, projected changes in precipitation are regionally variable (Figure 3), consistent with Arias et al. (2021). In some regions, total annual precipitation is projected to change by as much as 150 mm. Most regions may experience both increasing and decreasing precipitation depending on slope orientation (Figure 3a). A consistent direction in changes in precipitation across all combinations of elevation and aspect was projected for only a handful of locations, namely in the tropical Andes (e.g. Cali‐Huila, Loja and Quito located between 4.5°N and 3°S and Cotahuasi at 14°S), where precipitation was projected to ubiquitously increase, and in the Southern Andes (e.g. Villarica‐Lanin at 38.5°S and O'Higgins at 49°S) where precipitation was projected to ubiquitously decrease. A few more general patterns emerged: while the Northern‐Central Andes from Cali‐Huila to Titicaca‐Madidi (4.35°N–15°S) may predominantly experience increasing precipitation, areas located in the far north (Mérida; 10.5°N), far south (>39°S) and some areas in the centre (Uyuni to Salta‐Jujuy; 18°S–24°S) may predominantly get drier. However, for most combinations of elevation and aspect, precipitation changes could not be computed with confidence due to the large spread in individual model outputs (Figure 3a).
FIGURE 3.
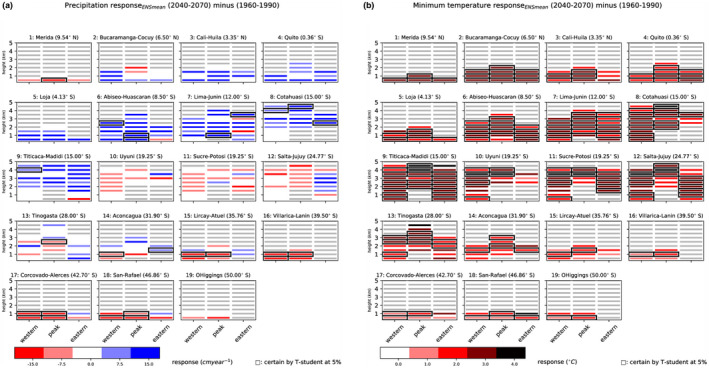
Future projections of precipitation and minimum annual temperature in 19 locations along the Andes indicated in Figure 1a for 2040–2070 RCP8.5. (a) total annual precipitation and (b) annual mean near‐surface air temperature for each location split by 500 m elevation intervals (y‐axis) and aspect (W = western, pk = peak, E = eastern in the x‐axis). The changes are calculated using an ensemble of CMIP5 GCMs, as differences between the future (2040–2070, RCP8.5 scenario) and near‐present conditions (1960–1990). Black edge lines highlight confident changes (SNR at 95%), while grey cells are combinations of elevation and aspect without data. Latitude values represent the geographical coordinates of the top left corner of the bounding box defining each location (see Table S4)
Both the minimum (Figure 3b) and maximum temperature (Figure S2) were projected to increase across all combinations of elevation and aspect, mostly with high confidence (i.e. with a high signal to noise ratio). The warming trend was slightly stronger for minimum than for maximum temperature. This may be due to the different way in which certain factors, such as cloudiness and increasing water vapour in the atmosphere, alter the surface energy balance during the day (affecting maximum temperature) and during the night (affecting minimum temperature) (IPCC AR4, 2007). Overall, temperature increases are expected to be larger in the Northern and Central Andes than in the Southern Andes. Several factors could be related to this pattern, but the complexity of the South American climate system prevents definite conclusions. One example of this complexity are the different main modes of variability of the climate system affecting the Andes, such as the North Atlantic Oscillation (NAO), El Niño‐Southern Oscillation (ENSO) and Southern Annular Mode (SAM), which affect temperature in the north, north/central and south of the continent, respectively (Flantua et al., 2016; see e.g. Box 1, Figure 1 IPCC AR5, 2013). With respect to topography, warming is generally projected to be stronger at higher than at lower elevations, with some aspect‐elevation combinations at mid‐to‐high elevations facing projected temperatures increases of up to 4°C for minimum temperature. This was the case for Cotahuasi, Titicaca‐Madidi, Uyuni, Sucre‐Potosí and Salta‐Jujuy, all located between 14°S and 24°S. There was no consistent difference in patterns of change between slope directions. The relationship between elevation and climate change is also complex, and processes related to albedo‐snow, cloud, water vapour and aerosol feedbacks contribute to an elevation‐dependent climate response (Mountain Research Initiative EDW Working Group, 2015), with generally greater warming trends at high elevations (Rangwala & Miller, 2012). However, there is not yet full agreement on this topic across the Andes (Pabón‐Caicedo et al., 2020).
BOX 1. Climate data and climate models: Details on the climate datasets, the common approaches employed for their generation, and details about their reliability and uncertainties. Glossary of acronyms and specialised terms are available on‐line at https://www.ipcc‐data.org/guidelines/pages/glossary/glossary_b.html .
Observational data
Different types of climate data are used to produce climate information. The way in which each data type is obtained determines their characteristics and thus the correct interpretation and use. In‐situ observations (e.g. weather station data) represent a measured variable at one spatial point. Other data type is remotely sensed data, which are provided on a spatial–temporal grid. These usually have global coverage, where each grid cell represents an area of, for example, 5–100 km2 depending on the variable and instrument. In‐situ and remotely sensed data can also be assimilated into Global Climate Models (GCMs) to produce global gridded and physically consistent datasets (called ‘reanalysis’). Reanalyses are considered ‘quasi‐observational’ data and play an increasingly important role in applied studies, such as the identification of relationships between current climate and natural processes such as glacier fluctuations, river discharges, vegetation dynamics and ecosystem services (Li et al., 2020). They have also been used as surrogates of in‐situ observed climate in data‐sparse regions (Doblas‐Reyes et al., 2021), notably in mountain areas.
Global and Regional Climate models
GCMs are coupled mechanistic models, which simulate past, present and future climate and contribute to a better understanding of climate variability and change. These models simulate the key components of the climate—for example, the atmosphere, oceans, ice and land masses as well as the interactions between them. The results of a GCM are provided on a global spatiotemporal grid (at spatial resolutions well above 0.5° = ~55 km). Due to their distinct construction and resolution, different GCMs simulate unalike future regional responses to anthropogenic global warming. The uncertainties are exacerbated in mountain regions (Flato et al., 2013), where the spatial resolution of climate models is a limitation to adequately represent the height of peaks, valleys, and slopes, and the complex mountain atmosphere. Dynamic downscaling techniques such as Regional Climate Models (RCMs) overcome some of the GCMs uncertainties along the Andes (Falco et al., 2019; Urrutia & Vuille, 2009). Nevertheless, the current spatial resolution of both the GCMs and RCMs makes it challenging to use their products to represent ecological processes that occur at a much finer scale than ~55 km. An alternative or complementary to dynamic downscaling is the application of statistical downscaling, a technique that consists of using different statistical methods to generate regional projections. The performance of models and methods for producing information about regional climate change, particularly for mountainous regions, has recently been assessed by the Intergovernmental Panel on Climate Change (Doblas‐Reyes et al., 2021).
Very high‐resolution climate data used in ecological sciences
The very high‐resolution global climate datasets (~ 1 km) WorldClim (Hijmans et al., 2005), WorldClim2 (Fick & Hijmans, 2017) and CHELSA (Karger et al., 2017) are primarily based on interpolated weather station data, and these datasets are widely used for ecological research. Although they have not been rigorously tested along the Andes, in other regions of the world questions arose about their capacity to represent the climate in topographically complex regions (Bedia et al., 2013), and in areas where there is a low density of weather stations—both of which is the case in the Andes. More recent climatologies attempted to improve data accuracy by integrating additional information. For instance, while the WorldClim baseline climatology is based on weather station data only, CHELSA is based on quasi‐mechanistic statistical downscaling of an atmospheric reanalysis, in which satellite information was additionally included to correct biases, and the more recent WorldClim2 also includes satellite information. Karger et al. (2017) claim that CHELSA performs better than WorldClim for the prediction of the orographic precipitation patterns. A dense, high‐quality network of weather station data would be beneficial for resolving continued uncertainty over data quality and which dataset is best to use.
Future climate projections in these climate datasets are obtained from models used in the Coupled Model Intercomparison Project (https://www.wcrp‐climate.org/wgcm‐cmip). Climate change is computed as the difference between the GCMs output for the baseline climatology and for the targeted years (future period) in each grid point of the climate model (typical resolution ~200 km). These changes are interpolated to the high (~1 km) resolution grid and are added to the baseline climatology in high resolution (Fick & Hijmans, 2017). The assumption made is that the change in climate is stable over space (high spatial autocorrelation), a premise not achieved in regions with strong gradients as the Andean mountains as well as in other mountain ranges of the world (Maraun et al., 2017).
Sound data on current climate and projected future changes are vital for accurate projections of species distributions. Two of the most commonly used datasets in biodiversity studies are ‘WorldClim’ (Fick & Hijmans, 2017; Hijmans et al., 2005) and ‘CHELSA’ (Karger et al., 2017). Both are available at a high resolution (30 arc seconds≈1 km). While WorldClim is primarily based on interpolated weather station data, augmented and/or corrected using other data sources, CHELSA is based on statistical downscaling of the ERA‐Interim reanalysis (Box 1). In the Andes, these data are limited by the low number of weather stations, meaning that the region's climatic complexity with its strong gradients and associated climate processes is likely to be poorly represented (Box 1). This limitation particularly affects precipitation. According to Karger et al. (2017), CHELSA performs better than WorldClim at representing orographic rainfall patterns in topographically complex areas; however, a known bias is that orographic precipitation may be overestimated on flat terrain, and as for WorldClim, uncertainty values are not provided. Consequently, freely available high‐resolution climate data for the Andes, though widely used, need to be interpreted with care as these often might not be suitable for the used purposes of modelling in topographic complex regions. Our analyses were based on outputs from Global Climate Models (GCMs). Both GCMs and Regional Climate Models (RCMs) provide ‘physically robust’ climate data, but, among other things, are limited by their coarse resolution (~140 km for GCMs and ~20–50 km for RCMs; Box 1).
5. FUTURE VEGETATION RESPONSE PROJECTIONS
5.1. Biome level
For each Andean biome, we first characterised their climatic niche (Figure 4a). From the 15 identified biomes, the broadest climate niche is found for the Evergreen montane forest and the narrowest for the Humid high Andean steppes in Patagonia. Dry shrublands and forest, Semideciduous montane forest, Dry forests and Evergreen montane forests all have mean temperatures above 12°C but differ in precipitation. Dry high Andean steppes and Humid high Andean grasslands and shrublands (locally known as dry Puna and humid Puna respectively) occupy mean temperatures between 0 and 12°C and receive total annual precipitation below 800 mm. Temperate deciduous and Temperate evergreen forests occur at temperatures between 4 and 10°C with an annual precipitation between 400–2200 and 800–2800 mm, respectively. Scattered peatlands exist along the Northern and Central Andes, but the Peatlands in southern Patagonia are a well identified vegetation unit spanning 400 km (Figure 1) with temperatures between 4 and 8°C and high levels of precipitation which are mostly above 1400 mm (Figure 4a).
FIGURE 4.
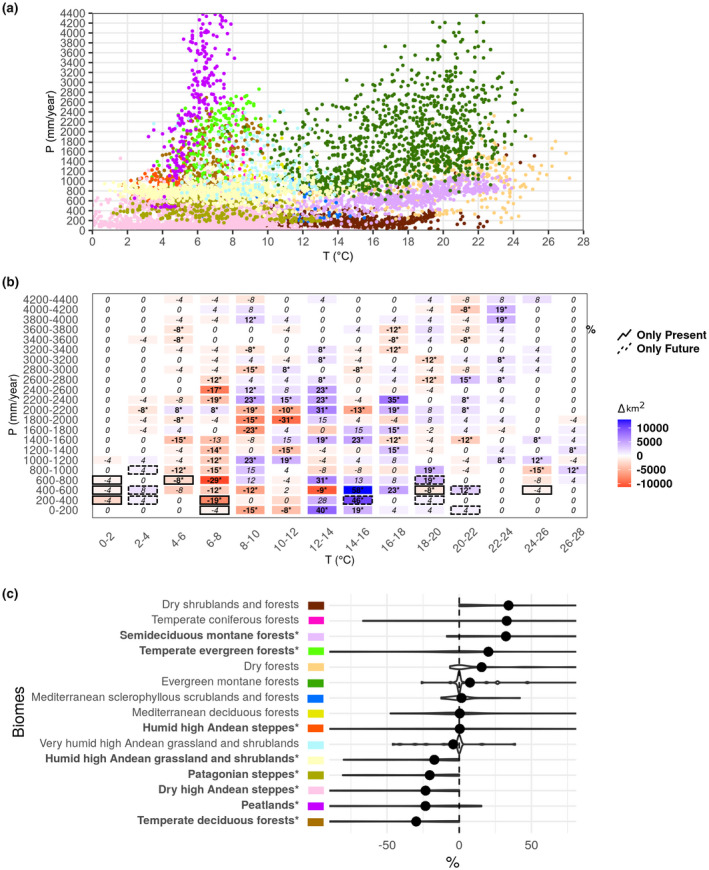
Projected changes in the climatic envelope area of Andean biomes. (a) Climatological classification of Andean biomes using annual mean temperature (°C) and total annual precipitation (mm) from CHELSA at 10‐min resolution (Karger et al. 2017) using bins measuring 2° temperature and 200 mm rainfall. Each point is a pixel of a given biome as indicated by the colour code displayed in the bottom panel. Axis y was truncated to 4400 mm (b) Projected change in the extent covered by specific combinations of annual mean temperature and total annual precipitation (bins measuring 2° and 200 mm rainfall). Changes are calculated as the difference between the future (2040–2070; RCP8.5 scenario) and near‐present conditions (1960–1990), using an ensemble of CMIP5 GCMs. Changes are expressed in absolute values (km2, coloured boxes) and relative values (% of change respect to 1960–1990, numbers inside boxes). Stars indicate levels of confidence (SNR at the 0.01 level). Full and dashed edge lines highlight combinations of temperature and precipitation found only in the historical and future scenarios, respectively. (c) Violin plots showing the projected relative change in the climatic envelope area for each biome using the climate classification shown in (a) in combination with the expected changes in (b). Each violin shows the distribution of the multi‐GCMs projected changes in the area covered by the present‐day climatic envelope of a biome (expressed in % of change relative to 1960–1990) where the dot is the ensemble mean. Stars indicate highly confident changes (SNR at the 0.01 level)
The CMIP5 climate experiments project that areas with present‐day temperatures ranging from 6 to 8°C and with total annual precipitation lower than 1600 mm will decrease in extent (Figure 4b). In contrast, regions with present‐day temperatures of 14–18°C and total annual precipitation lower than 800 mm are projected to increase in extent. Some combinations of temperature and precipitation, for example 20–22°C and precipitation 400–600 mm, are not recorded at present but are projected to occur in the future (Figure 4b) while areas in the colder and drier extreme (<−2°C and below 800 mm) are projected to disappear. Given the coarse resolution of CMIP models, these values may slightly vary and should be used with caution.
Overlaying the projected climate changes with the current climatic niches highlighted differential impacts on the different biomes. The climatic envelope area of the Humid high Andean steppes might remain unchanged (though with high range of estimates), while for five biomes it will significantly likely decrease (Figure 4c), namely Temperate deciduous forest (−30%), Peatlands (−23%), Dry high Andean steppes (−23%), Patagonian steppes (−20.6%) and Humid high Andean grassland and shrublands (−17%). This decrease is in agreement with results from a previous study for the Dry high Andean steppes (Xeric puna) and the Humid high Andean grassland and shrublands (Humid puna; Tovar et al., 2013) but the magnitude of change in our results is higher. Another study by Ramirez‐Villegas et al. (2014) projected a decrease in species richness for the Humid Puna as a result of decrease in the available suitable climate area. For Temperate deciduous forest, Peatlands and Patagonian steppes, our study is the first to provide estimates of changed in climate envelope area under future climate conditions.
Our results also show that the climatic envelope area of Semideciduous montane forest (Selva Tucumano‐Boliviana) and Temperate evergreen forest (Bosque Valdiviano) will likely increase by 30% and 21%, respectively (Figure 4c). Previous studies have shown contrasting projections for the Semideciduous montane forest biome. On one hand, it is projected to have a substantial decrease in extent for the Argentinian side (Pacheco et al., 2010), in suitable area for key species (e.g. Alnus acuminata, Betulaceae; Wicaksono et al., 2017) and in plant species richness (Ramirez‐Villegas et al., 2014). On the other hand, stable areas under future climate change have been projected for the Bolivian side (Tovar et al., 2013). We did not find any previous studies projecting plant distributions for the Temperate evergreen forest.
Lastly, for seven biomes namely Dry shrublands and forests, Temperate coniferous forests, Dry forests, Evergreen montane forests, Mediterranean sclerophyllous scrublands and forests, Mediterranean deciduous forests and Very humid Andean grassland and shrublands, the uncertainty between climate model outputs is too large to reach a conclusion.
Previous studies using distribution models to analyse responses to warming in the Andes have mostly focused on the Evergreen montane forests (Table S2; Figure 5a). Evergreen montane forests are already experiencing an upward migration, with mid‐elevation species suffering area loss (Feeley et al., 2011; Feeley & Silman, 2010). In addition, these forests have been projected to have a decrease in area and species richness (Ramirez‐Villegas et al., 2014; Tovar et al., 2013) and their tree species have been projected to have 15% increased risk of extinction (Tejedor Garavito et al., 2015). However, in our study, projections of change show a large spread between climate models for this biome. Differences in the projections could be attributed to the different modelling approaches (see next section).
FIGURE 5.
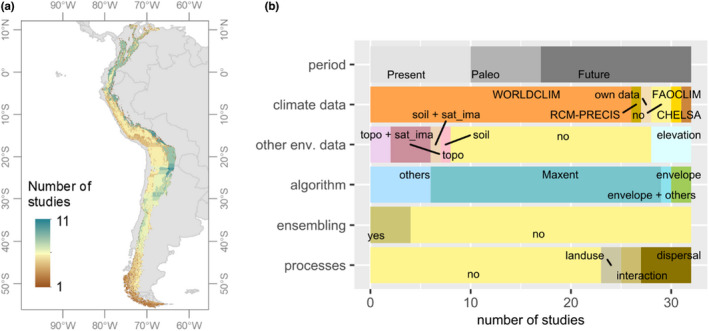
Summary of studies that have used species distribution models (SDMs) in Andean regions. (a) Geographical distribution of the 32 studies carried out in the Andes that used SDMs published between 2010 and 2019. (b) Model details used by these studies considering the studied period, the climatic data used, whether other environmental variables in addition to climatic data were used, the algorithm, whether model ensemble was applied or not, and whether other biological processes beyond climate were used. Topo = topographical, sat_ima = variables derived from satellite images. Details of the different studies are found in Table S2
Our results provide the first regional assessment of projected climate change impacts for the whole Andes (2040–2070). They are based on a worst‐case scenario in which radiative forcing (= net change in energy flux in the atmosphere) reaches 8.5 Watts per m2 by 2100, leading to a mean temperature increase over continental areas of around 5°C relative to pre‐industrial times (Collins et al., 2013). If pledges made at COP26 are held, then a current best‐case scenario would be a warming of 1.8°C, which would significantly reduce threats to thermally sensitive systems such as high Andean vegetation and temperature deciduous forests. However, although the magnitude of changes will vary according to the level of warming, the analyses here serve to highlight the biomes most sensitive to climatic change in the Andes. Future research should consider a combined modelling approach (rather than modelling individual biomes) and include a range of plausible scenarios, as well as an uncertainty analysis. In some cases, the large inter‐GCMs spread noted here was due to a few models performing as outliers. Future studies should identify the best subset of GCMs to be used for the Andes.
5.2. Species level: State of the art of distribution modelling of Andean plants
5.2.1. Species Distribution Models (SDMs)
Most studies using SDMs have been conducted on species from the Evergreen montane forest biome and the Semideciduous montane forests, whereas species in the biomes of the Southern Andes are the least studied (Table S2; Figure 5a). Most of these studies have used only climatic data, without considering topographic and soil data (Figure 5b). For climate, the WorldClim dataset (Hijmans et al., 2005) was most widely used, while few studies have used alternative datasets such as Regional Climate Models used in the Mediterranean region of Chile (Bambach et al., 2013). The more recently published climate dataset, CHELSA (Karger et al., 2017), has yet to be used more widely in studies modelling Andean species. Important to note here is that a recent study shows little agreement between different climate datasets for the Andes (Morales‐Barbero & Vega‐Álvarez, 2019), that is, the choice of climate data will impact the model outputs.
Although there is a variety of algorithms with different levels of complexity for SDMs, only a limited number of algorithms are being applied (Table S2; Figure 5b). One of the simplest approaches is the ‘Envelope model’, where the species' niche is defined by the lower and upper bounds of environmental values at the locations where the species has been recorded (Guisan & Zimmermann, 2000). An example is the use of the species elevational range to model the present‐day distribution and potential changes under climate change scenarios of Andean trees (Feeley & Silman, 2010). While envelope models may be most intuitive, the most popular algorithm is MAXENT, a machine‐learning algorithm which iteratively matches the environment at the locations of projected occurrence to the environment at the actual occurrences while ensuring the solution has maximum entropy (i.e. probability distribution is closest to uniform) (Elith et al., 2011). Ensemble models, where two or more algorithms are combined (Hao et al., 2019), have been rarely used in Andean studies. Although the best practices of how to perform ensemble models have yet to be refined (Hao et al., 2019), they are useful to account for model uncertainty, especially in complex regions such as the Andes.
Biotic interactions (e.g. competition and mutualism) are rarely incorporated into SDMs in general, and studies focusing on the Andes are no exception. The few studies we identified for the Andes modelled interacting species separately and then assessed the spatial overlap of their distributions. For example, a study of the future projected distribution of 11 Argentinian cactus species and their pollinators found little mismatch between them under future warming (Gorostiague et al., 2018). Another study focusing on palaeodistributions of Calceolaria species in combination with their pollinators predicted floral traits divergence in Patagonia (Sosa‐Pivatto et al., 2017). Another key ecological process, dispersal, has been incorporated into modelling studies by only considering two extreme scenarios: full (unlimited) or null (restricted) dispersal (e.g. Bambach et al., 2013; Gorostiague et al., 2018; Ramirez‐Villegas et al., 2014). In an unlimited dispersal scenario, projections of future distributions use all suitable new areas, whereas in a null‐dispersal scenario, future distributions are only projected in areas where the species currently exists.
Among the main limitations of these models is the effect of niche truncation (when species occurrence records only represent a fraction of the climatic conditions the species could tolerate) on future projections (Peterson et al., 2018). Although improved sampling could help solve this issue, future conditions in the Andes might be non‐analogous to present‐day conditions (see Section 4) and thus palaeoecological data could be another valuable source of information (see Section 3). Lastly, our search did not identify any SDM studies for invasive species despite their potential negative impacts on biodiversity (but see Martin‐Gallego et al., 2020 on invasive species in temperate forests).
5.2.2. Dynamic Vegetation Models (DVMs)
Dynamic vegetation models have emerged as an alternative to SDMs to simulate plant species ranges (Gutiérrez et al., 2016; Snell et al., 2014). DVMs include demographic processes and biotic interactions, which influence plant range dynamics, to project future vegetation composition and structure under climate change (Bugmann, 2001, 2014). For example, they explicitly include competition using parameters such as light interception of tree crowns and demographic rates such as plant growth, recruitment and mortality rates, and the influence of climate on these processes (Snell et al., 2014). Recent DVM development has also included dispersal to simulate species distributions (Snell, 2014). DVMs have successfully predicted range shifts driven by demographic processes in tree species (Bykova et al., 2012; Snell, 2014; Vanderwel et al., 2013). Despite the potential for DVMs to simulate plant ranges, one of their main limitations is the large number of parameters needed, which requires expert knowledge or empirical information on the ecology of species. DVM applications are often conducted in data‐rich regions (e.g. North America or Europe), and/or run for a limited set of well‐known or dominant species or plant functional types (Köhler & Huth, 1998; Rüger et al., 2020).
In the Andes, DVMs have been used to predict current vegetation composition and the structure of forest stands. For example, the first DVM modelled the dynamics of tree species in a low‐elevation tropical forest in the eastern Andes of Venezuela (Ramirez‐Angulo et al., 1997). Kammesheidt et al. (2001) studied the effect of different management strategies on forest composition using DVM, also in Venezuela. A similar study in south‐central Chile demonstrated how unsustainable logging impacted the composition of an old‐growth temperate rainforest (Rüger et al., 2007). In Bolivia, a DVM was used to simulate the ecotone between evergreen and deciduous forests (Seiler et al., 2014), and in Ecuador to study landslide impacts on forest structure in a tropical montane forest (Dislich et al., 2009). In south‐central Chile, DVMs have been applied to predict forest composition in several species‐rich forest stands near the Andes (Gutiérrez & Huth, 2012). The same DVM (FORMIND) was then applied to predict the influence of increased drought conditions driven by climate change on forest structure by 2100 (Gutiérrez et al., 2014).
The DVM applications discussed above have been conducted at local scales (e.g. forest stands <100 ha). To the best of our knowledge, there is no application of DVMs to study the dynamics of species distributions at a regional level for the Andes. However, there are examples for the use of DVMs at large spatial scales in South America to model changes in the forest carbon budgets of the Amazon (Brinck et al., 2017). Notwithstanding the promise of these techniques, challenges remain for the dynamic modelling of species ranges in the Andes, notably the paucity of data on dispersal and recruitment rates of individual plant species (Singer et al., 2016; a detailed discussion can be found in Snell et al., 2014).
6. RESEARCH PRIORITIES
To better understand Andean vegetation responses to climate change, several data and methodological gaps need to be filled. Below we summarise our view on the main research priorities. This is to enable the development of a coordinated research agenda to fill critical knowledge gaps (Table 1).
TABLE 1.
Uncertainties and gaps in our understanding of past, present and future plant species distributions in the Andes and priorities for research
| Topic | Subtopic | Uncertainties and gaps | Priorities |
|---|---|---|---|
| Observations | Species data |
|
|
| |||
|
|
||
| |||
| Climate data |
|
|
|
| |||
| |||
|
|
||
| Models | Climate models |
|
|
| |||
|
|
||
| |||
| Plant distribution models |
|
|
|
| |||
|
|
||
| |||
|
|
||
|
|
||
|
|
6.1. Filling biological and climate data gaps
6.1.1. Plant species data
A plant list for the whole of the Andes should be one of the first priorities. Although a recent list identifies around 28,700 species, this likely underestimates the real number of species as it is based solely on georeferenced records (Pérez‐Escobar et al., 2022). This list should contain information on estimated ranges and georeferenced locations of native and non‐native species, and should allow to estimate the number of species per biome. There is also a need to collect species occurrence data more widely, especially in remote areas and those with high endemism, including non‐native species that are spreading into the most remote ecosystems. In addition, many regional herbaria have yet to complete specimens' digitisation and currently key distributional records are only accessible through direct communication with them. Digitisation will allow participation in global (e.g. GBIF) or regional (e.g. SIB‐Colombia) initiatives of data storage and sharing.
6.1.2. Invasive species
In the Andes, modelling non‐native plants, including those that are considered invasive species should be a priority given that many of them are spreading in the region, putting biodiversity and ecosystem services at high risk (Alexander et al., 2016; Pauchard et al., 2009). Examples include the invasion of non‐native conifers such as many Pinus species, alien shrubs like Rosa rubiginosa and herbs (Fuentes‐Lillo & Pauchard, 2019). Modelling the distribution of invasive species that are not at equilibrium is challenging, and recent studies have used co‐occurring native community members to improve predictions (Briscoe Runquist et al., 2021). Information about non‐native species' niches, associated species, demographic rates, dispersal capacity, residence time and on‐the‐ground microclimatic conditions is required for improved risk assessments of the spread and potential impact of such species.
6.1.3. Climate data
A larger number of weather stations across the entire elevational gradient, aiming to collect information at high frequency, is needed to adequately capture climatic processes in the Andes and to increase the resolution along climatic gradients. Weather stations above the upper forest line in the Central Andes are scarce, limiting understanding of the climate in these vulnerable regions. At the same time, we should also aim to monitor microclimatic variations at local scales, such as initiated by the SoilTemp network using soil temperature sensors (Lembrechts et al., 2020).
As these data are collected, efforts should be made to share them widely. Either by developing regional platforms based on country efforts (e.g. BIOMODELOS, Colombia Velásquez‐Tibatá et al., 2019) or by contributing to existing global datasets (e.g. GBIF, BIEN, NOAA). Only a concerted effort between different research groups committed to generating and transferring knowledge will allow filling data gaps and mobilising data.
6.2. Advancing climate modelling
The advances in climate modelling are steps along a continuous revision and improvement process. Although state‐of‐the‐art GCMs simulate the first‐order statistics of large‐scale climatology appropriately, computational resources constrain GCMs to a simplified description of many physical processes, such as air flowing over the Andes range. Small‐scale details could be tracked using RCMs with increasing resolution/complexity as a higher spatial resolution is the priority for improved predictions of plant–climate interaction under future climate scenarios.
The global climate scientific community agrees that the largest uncertainties in climate models are associated with the representation of both cloud (sub‐grid) processes and land‐surface processes (including land cover and its management; Flato et al., 2013). Modelling the climate of the Andes is challenging because of the complexity of the processes and feedbacks to be simulated and because of the computational cost associated with the increased spatial resolution to appropriately represent its complex orography. Therefore, complementing the global simulations performed with GCMs with dynamic and statistical downscaling techniques should be the next step (see Box 1). However, this requires higher density and quality of observational data.
6.3. Improving plant distribution models
Detailed standards for distribution modelling have been recently published (e.g. Araújo et al., 2019). In the Andes, besides improving climate data and their resolution, other aspects deserve further attention in future studies such as incorporating key ecological, biological and palaeoecological knowledge into the models and their interpretations.
6.3.1. Dispersal
One way to incorporate dispersal in SDMs, beyond using the extreme scenarios of null and full dispersal, is to use dispersal distances (e.g. migclim R package, Engler et al., 2012). This can be estimated using plant functional traits related to dispersal (Tamme et al., 2014). A similar approach can be followed for DVMs, coupling mechanistic seed dispersal models into plant regeneration modules (Snell, 2014). A less computationally and data‐intensive approach would be to group species by dispersal types and other functional traits and to model these entities instead (Tamme et al., 2014). However, only a handful of studies have collected dispersal traits in the Andes (e.g. Tovar et al., 2020), and further quantification of dispersal distances in the field is required.
6.3.2. Demographic processes
There is a need to improve our understanding of how plant demographic processes, such as individual establishment and mortality, are being impacted by climate change. Long‐term vegetation monitoring using permanent plots is of particularly relevance to study demographic rates and to assess the influence of climate on them (Rüger et al., 2018). Current monitoring networks (e.g. RBA (https://redbosques.condesan.org/), MIREN (https://www.mountaininvasions.org/), GLORIA (https://redgloria.condesan.org/)) can help address this.
6.3.3. Land use and fire regimes
Disturbances such as fire have been, and are, ubiquitous in the Andes. Representing disturbance dynamics such as fire regimes, logging, road construction and urbanisation, and other natural disturbances such as insect and pathogen outbreaks and landslides is thus necessary for a prediction of range dynamics. Incorporating disturbance and land‐use changes into plant distribution models adds important contextual information and is needed for both SDMs and DVMs.
6.3.4. Spatial representation of DVMs
In the Andean temperate zone, where DVMs can be run at species level, future research can prioritise on how to expand the spatial scope without losing detail at local scales using an optimised species parameterisation procedure (Gutiérrez et al., 2016). Additional research should particularly focus on an improved definition of species niches, and factors affecting demographic processes, which shape the geographical distribution of species.
6.3.5. Intraspecific adaptations, niches and traits
The distribution of species and biomes in the Andes has allowed the identification of species/biomes at risk and those showing higher resilience (e.g. Ramirez‐Villegas et al., 2014; Tovar et al., 2013). However, intraspecific variability in response to climate has been shown elsewhere, suggesting adaptive differences and response at the population level should be also analysed (Razgour et al., 2019). Given the large elevation gradient in the Andes, individual populations may show different levels of resilience to climate change through differentiated traits (such as thermal niches), and thus distributions should be projected separately for distinct populations. However, trait values are mostly obtained from only a few individuals, at species level or are averaged to obtain community‐trait‐weighted means, and thus trait data collection should consider intraspecific variation.
6.3.6. Use of palaeoecological knowledge in SDMs
More cross‐disciplinary ground‐work should be done on integrating palaeoecological knowledge in predictive models—one of the spear points of the emerging discipline of Conservation Paleobiology (e.g. Dietl et al., 2015). This starts with considering palaeoecological knowledge during the stage of hypothesis development and validating outputs from species distributions models for past climate conditions with palaeoecological records (see more suggestions in supplementary material in Hooghiemstra & Flantua, 2019). In addition, fossil pollen data have shown that taxa can occupy different realised niches in the past, for example in the Andes, during the LGM, palaeo‐atmospheric pCO2 was different from present‐day values (Boom et al., 2002). Therefore, a series of recent papers warn against fitting only present‐day niches to reconstruct or predict species distributions for the past and future (e.g. Nogués‐Bravo, 2009; Veloz et al., 2012). SDMs can therefore be substantially improved if knowledge on past distributions is used for calibration and validation of the models.
6.4. Understanding species distributions across evolutionary time‐scales
6.4.1. Changes in diversification rates on the Andes
Global climatic oscillations during the Pleistocene appeared to have influenced the speciation of endemic Andean plant groups in the Pleistocene onwards (Flantua et al., 2019), such as the genus Espeletia (Compositeae; Pouchon et al., 2018). A key research topic in the Andes is how diversification rates in lineages have changed across different evolutionary time‐scales in response to abiotic variables like climatic oscillations in the Andes. Modelling speciation and extinction rates as a function of time and paleo‐climatic variables provide a unique opportunity to understand how past climatic dynamics have affected the distribution of Andean plant lineages and the assemblies of their floras into discrete ecosystems (Condamine et al., 2013). More importantly, by integrating species occurrence data with speciation and extinction rates, it is also feasible to identify geographical areas that have the potential to serve as species pumps (i.e. areas with high speciation rates) or sinks (i.e. areas with high extinction rates; Forest et al., 2007; Pérez‐Escobar et al., 2017). The projection of speciation and extinction rates on geographical areas coupled with species distribution modelling supported by long fossil pollen records could enable the assessment of the survival of areas of importance for conservation because of their ‘evolutionary potential’ in the face of projected climatic conditions.
7. CONCLUSIONS
In this review, we have presented an analysis of projected climate change across the Andes and have summarised current existing information on climate change impacts on Andean vegetation.
First, we reviewed findings from Andean palaeoecological, dendrochronological and plot monitoring studies. We found that biome responses to climate change are and have been highly heterogeneous across the Andes. Main responses were, among others, (i) changes in elevational distributions of grassy/shrubs biomes, (ii) altered species composition due to upward shifts of warm‐adapted species or internal forest dynamics, (iii) primary succession in recently deglaciated areas, and (iv) changes in tree demographic patterns (recruitment and mortality) negatively affecting population viability.
Second, by exploring the projected climate changes for different regions across the Andes, we found that increasing temperatures are projected to be higher in the tropical Andes and at higher elevations (up to 4°C for 2040–2070, CMIP5 RCP8.5). Precipitation patterns are projected to be highly variable with clear differences between eastern and western slopes, yet, with large uncertainties in specific regions given the complex topography of the Andes. Climate change is likely to impact the distribution and extent of the Andean biomes. Projections for a worst‐case scenario (RCP 8.5) would result in a reduction of 17%–23% in the climate envelope area of the grassland/steppe biomes from the Central and Southern Andes, and 30% in that of Temperate deciduous forest, while the climatic envelope area for Temperate evergreen forest and Semideciduous montane forest may increase by 21% and 30%, respectively. Although current policy endeavours render this high‐forcing scenario as unlikely, it is useful to understand which biomes would be the winners, and which systems would be the losers and hence may deserve further attention, particularly given that there is still uncertainty around the sensitivity of the climate system. Most of the SDM studies we reviewed have been conducted in Montane evergreen forest while many vulnerable biomes such as the dry steppes of the Southern Andes remain understudied. Simultaneously, critical gaps in biological and climate data need to be covered. Lastly, mechanistic models, such as DVMs, have yet to be widely used in the Andes but offer a great potential especially for forested biomes.
Third, we identified four main research priorities: (i) Fill data gaps by working towards a comprehensive list of plant species for the Andean region, increasing species occurrence data, installing more weather stations above upper forest line and sharing data widely, (ii) advance climate modelling by representing key features in climate models (e.g. sharp rainfall gradients) and generating high‐resolution climate data with models that better represent the complex Andean topography, (iii) improve plant distribution models by including key ecological/biological processes, data on plant traits and available palaeoecological data and knowledge, but also by accounting for disturbance regimes and land‐use changes, and (iv) increase understanding of the locations and conditions which promote species diversification to support the integration of evolutionary refugia into conservation planning. Only a concerted effort between botanists, ecologists and climatologists working in the region will help achieve the proposed interdisciplinary research agenda.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
BIOSKETCH
The authors are an interdisciplinary group including botanists, ecologists, geneticists, geographers and climatologists focusing on understanding plant dynamics and climate change in the Andes. This paper is one of the outputs of a Newton Fund Workshop organised in 2018 in Mendoza, Argentina. AA, AGG, AP, CT, DA, LF, OPE, PG and PF conceived the idea and outlined the paper, climate change analyses were designed by AAS, AFC, CGM, LF, PF, PZ, RCR and run by LF and PZ, the biome map was collated by AB, CT and JC. The writing was led by CT, AFC, AAG and SGAF. All authors wrote and/or contributed to the different sections of the manuscript based on their expertise.
Supporting information
DataS1
ACKNOWLEDGEMENTS
We would like to thank Lucia Hudson for helping in collating information from the SDM studies. This work was the result of a Newton Fund Workshop organised in 2018 in Mendoza, Argentina, funded by a Researcher Links grant, ID 2017‐RLWK9‐359514245 from the UK Department of Business, Energy and Industrial Strategy (BEIS) and CONICET/MINCyT and delivered by the British Council and the Argentinean National Council of Research (CONICET). Most co‐authors, except for A.A. and S.G.A.F. attended the workshop. A.S, A.F.C., R.R., P.Z, C.G.M., L.F. and P.F. were partially supported by ANPCyT (PICT‐2018‐02511, PICT‐2017‐3020), CONICET (PIP‐112‐2015‐0100402CO, 112‐2020‐0102141CO) and UBACYT (20020170100620BA) projects, and by the French National Programme LEFE/INSU (AO 2020‐12962). O.A.P.E. was supported by the Lady Sainsbury Orchid Fellowship and the Swiss Orchid Foundation. A.G.G. was supported by the FONDECYT Chile grant 1200468 and REDI‐CONICYT 170321. D.A. was supported by FONDECYT Chile 3200675 grant. A.P. was funded by FONDECYT Chile 1180205. A.P. and A.G.G. were funded by ANID/BASAL FB21000. M.E.F. was partially supported by FONDECYT Peru ‐ Banco Mundial 039‐2019‐FONDECYT‐BM‐INV. S.G.A.F. was supported by the European Research Council under the EU Horizon 2020 Research 479 and Innovation Programme (grant 741413 HOPE) Humans on Planet Earth—Long‐term impacts on biosphere dynamics. No permits were needed to carry out this research.
Tovar, C. , Carril, A. F. , Gutiérrez, A. G. , Ahrends, A. , Fita, L. , Zaninelli, P. , Flombaum, P. , Abarzúa, A. M. , Alarcón, D. , Aschero, V. , Báez, S. , Barros, A. , Carilla, J. , Ferrero, M. E. , Flantua, S. G. , Gonzáles, P. , Menéndez, C. G. , Pérez‐Escobar, O. A. , Pauchard, A. … Hollingsworth, P. M. (2022). Understanding climate change impacts on biome and plant distributions in the Andes: Challenges and opportunities. Journal of Biogeography, 49, 1420–1442. 10.1111/jbi.14389
Handling Editor: Carina Hoorn
DATA AVAILABILITY STATEMENT
Data used in the climate diagnostics is publicly available from any of the open nodes of the 'Earth System Grid Federation' (ESGF) for example the node https://esgf.llnl.gov. Diagnostics used in the manuscript making use of this numerical climate data are described in detail in the methods. The results of the searches in the SCOPUS database are provided in the Supporting Information and the details of keywords and timeframe used for this are described in the methods.
REFERENCES
- Abarzúa, A. M. , Pinchicura, A. G. , Jarpa, L. , Martel‐Cea, A. , Sterken, M. , Vega, R. , & Pino, Q. M. (2014). Environmental responses to climatic and cultural changes. In Dillehay T. D. (Ed.), The teleoscopic polity (Vol. 38, pp. 123–141). Springer International Publishing. 10.1007/978-3-319-03128-6_6 [DOI] [Google Scholar]
- Abarzúa, A. M. , Villagrán, C. , & Moreno, P. I. (2004). Deglacial and postglacial climate history in east‐central Isla Grande De Chiloé, Southern Chile (43°S). Quaternary Research, 62(1), 49–59. 10.1016/j.yqres.2004.04.005 [DOI] [Google Scholar]
- Alexander, J. M. , Lembrechts, J. J. , Cavieres, L. A. , Daehler, C. , Haider, S. , Kueffer, C. , Liu, G. , McDougall, K. , Milbau, A. , Pauchard, A. , Rew, L. J. , & Seipel, T. (2016). Plant invasions into mountains and alpine ecosystems: Current status and future challenges. Alpine Botany, 126(2), 89–103. 10.1007/s00035-016-0172-8 [DOI] [Google Scholar]
- Araújo, M. B. , Anderson, R. P. , Márcia Barbosa, A. , Beale, C. M. , Dormann, C. F. , Early, R. , Garcia, R. A. , Guisan, A. , Maiorano, L. , Naimi, B. , O'Hara, R. B. , Zimmermann, N. E. , & Rahbek, C. (2019). Standards for distribution models in biodiversity assessments. Science Advances, 5(1), eaat4858. 10.1126/sciadv.aat4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias, P. A. , Garreaud, R. , Poveda, G. , Espinoza, J. C. , Molina‐Carpio, J. , Masiokas, M. , Viale, M. , Scaff, L. , & van Oevelen, P. J. (2021). Hydroclimate of the Andes part II: Hydroclimate variability and sub‐continental patterns. Frontiers in Earth Science, 8, 505467. 10.3389/feart.2020.505467 [DOI] [Google Scholar]
- Armesto, J. J. , Manuschevich, D. , Mora, A. , Smith‐Ramirez, C. , Rozzi, R. , Abarzúa, A. M. , & Marquet, P. A. (2010). From the Holocene to the Anthropocene: A historical framework for land cover change in southwestern South America in the past 15,000 years. Land Use Policy, 27(2), 148–160. 10.1016/j.landusepol.2009.07.006 [DOI] [Google Scholar]
- Bambach, N. , Meza, F. J. , Gilabert, H. , & Miranda, M. (2013). Impacts of climate change on the distribution of species and communities in the Chilean Mediterranean ecosystem. Regional Environmental Change, 13(6), 1245–1257. 10.1007/s10113-013-0425-7 [DOI] [Google Scholar]
- Bedia, J. , Herrera, S. , & Gutiérrez, J. M. (2013). Dangers of using global bioclimatic datasets for ecological niche modeling. Limitations for future climate projections. Global and Planetary Change, 107, 1–12. 10.1016/j.gloplacha.2013.04.005 [DOI] [Google Scholar]
- Blundo, C. , Malizia, L. R. , Blake, J. G. , & Brown, A. D. (2012). Tree species distribution in Andean forests: Influence of regional and local factors. Journal of Tropical Ecology, 28(1), 83–95. 10.1017/S0266467411000617 [DOI] [Google Scholar]
- Bogotá, A. R. G. , Hooghiemstra, H. , & Berrio, J. C. (2016). North Andean environmental and climatic change at orbital to submillennial time‐scales: Vegetation, water‐levels and sedimentary regimes from Lake Fúquene between 284 and 130 ka. Review of Palaeobotany and Palynology, 226, 91–107. 10.1016/j.revpalbo.2015.09.007 [DOI] [Google Scholar]
- Boom, A. , Marchant, R. , Hooghiemstra, H. , & Sinninghe Damsté, J. S. (2002). CO2‐ and temperature‐controlled altitudinal shifts of C4‐ and C3‐dominated grasslands allow reconstruction of palaeoatmospheric pCO2 . Palaeogeography, Palaeoclimatology, Palaeoecology, 177(1–2), 151–168. 10.1016/S0031-0182(01)00357-1(01)00357‐1 [DOI] [Google Scholar]
- Boschman, L. M. (2021). Andean mountain building since the Late Cretaceous: A paleoelevation reconstruction. Earth‐Science Reviews, 220, 103640. 10.1016/j.earscirev.2021.103640 [DOI] [Google Scholar]
- Brinck, K. , Fischer, R. , Groeneveld, J. , Lehmann, S. , Dantas De Paula, M. , Pütz, S. , Sexton, J. O. , Song, D. , & Huth, A. (2017). High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nature Communications, 8(1), 14855. 10.1038/ncomms14855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe Runquist, R. D. , Lake, T. A. , & Moeller, D. A. (2021). Improving predictions of range expansion for invasive species using joint species distribution models and surrogate co‐occurring species. Journal of Biogeography, 48(7), 1693–1705. 10.1111/jbi.14105 [DOI] [Google Scholar]
- Bugmann, H. (2001). A review of forest gap models. Climatic Change, 51(3/4), 259–305. 10.1023/A:1012525626267 [DOI] [Google Scholar]
- Bugmann, H. (2014). Forests in a greenhouse atmosphere: Predicting the unpredictable? In Coomes D. A., Burslem D. F. R. P., & Simonson W. D. (Eds.), Forests and global change (pp. 359–380). Cambridge University Press. 10.1017/CBO9781107323506.017 [DOI] [Google Scholar]
- Buytaert, W. , Cuesta‐Camacho, F. , & Tobón, C. (2011). Potential impacts of climate change on the environmental services of humid tropical alpine regions. Global Ecology and Biogeography, 20(1), 19–33. [Google Scholar]
- Bykova, O. , Chuine, I. , Morin, X. , & Higgins, S. I. (2012). Temperature dependence of the reproduction niche and its relevance for plant species distributions. Journal of Biogeography, 39(12), 2191–2200. 10.1111/j.1365-2699.2012.02764.x [DOI] [Google Scholar]
- Carilla, J. , Halloy, S. , Cuello, S. , Grau, A. , Malizia, A. , & Cuesta, F. (2018). Vegetation trends over eleven years on mountain summits in NW Argentina. Ecology and Evolution, 8(23), 11554–11567. 10.1002/ece3.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, M. , Knutti, R. , Arblaster, J. , Dufresne, J.‐L. , Fichefet, T. , Friedlingstein, P. , Gao, X. , Gutowski, W. J. , Johns, T. , Krinner, G. , Shongwe, M. , Tebaldi, C. , Weaver, A. J. , & Wehner, M. (2013). Long‐term climate change: Projections, commitments and irreversibility. In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate Change 2013: The physical ccience basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change (pp. 1029–1136). Cambridge University Press. [Google Scholar]
- Condamine, F. L. , Rolland, J. , & Morlon, H. (2013). Macroevolutionary perspectives to environmental change. Ecology Letters, 16, 72–85. 10.1111/ele.12062 [DOI] [PubMed] [Google Scholar]
- Diazgranados, M. , Tovar, C. , Etherington, T. R. , Rodríguez‐Zorro, P. A. , Castellanos‐Castro, C. , Galvis Rueda, M. , & Flantua, S. G. A. (2021). Ecosystem services show variable responses to future climate conditions in the Colombian páramos. PeerJ, 9, e11370. 10.7717/peerj.11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietl, G. P. , Kidwell, S. M. , Brenner, M. , Burney, D. A. , Flessa, K. W. , Jackson, S. T. , & Koch, P. L. (2015). Conservation paleobiology: Leveraging knowledge of the past to Inform conservation and restoration. Annual Review of Earth and Planetary Sciences, 43(1), 79–103. 10.1146/annurev-earth-040610-133349 [DOI] [Google Scholar]
- Dislich, C. , Günter, S. , Homeier, J. , Schröder, B. , & Huth, A. (2009). Simulating forest dynamics of a tropical montane forest in South Ecuador. Erdkunde, 63(4), 347–364. 10.3112/erdkunde.2009.04.05 [DOI] [Google Scholar]
- Doblas‐Reyes, F. J. , Sörensson, A. A. , Almazroui, M. , Dosio, A. , Gutowski, W. J. , Haarsma, R. , Hamdi, R. , Hewitson, B. , Kwon, W.‐T. , Lamptey, B. L. , Maraun, D. , Stephenson, T. S. , Takayabu, I. , Terray, L. , Turner, A. , & Zuo, Z. (2021). Linking global to regional climate change. In Masson‐Delmotte V., Zhai P., Pirani A., Connors S. L., Péan C., Berger S., Caud N., Chen Y., Goldfarb L., Gomis M. I., Huang M., Leitzell K., Lonnoy E., Matthews J. B. R., Maycock T., Waterfield T., Yelekci O., Yu R., & Zhou B. (Eds.), Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- Duque, A. , Stevenson, P. R. , & Feeley, K. J. (2015). Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proceedings of the National Academy of Sciences of the United States of America, 112(34), 10744–10749. 10.1073/pnas.1506570112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers, T. A. , & Poulsen, C. J. (2009). Influence of Andean uplift on climate and paleoaltimetry estimates. Earth and Planetary Science Letters, 281(3–4), 238–248. 10.1016/j.epsl.2009.02.026 [DOI] [Google Scholar]
- Elith, J. , Phillips, S. J. , Hastie, T. , Dudík, M. , Chee, Y. E. , & Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists: Statistical explanation of MaxEnt. Diversity and Distributions, 17(1), 43–57. 10.1111/j.1472-4642.2010.00725.x [DOI] [Google Scholar]
- Engler, R. , Hordijk, W. , & Guisan, A. (2012). The MIGCLIM R package—Seamless integration of dispersal constraints into projections of species distribution models. Ecography, 35(10), 872–878. 10.1111/j.1600-0587.2012.07608.x [DOI] [Google Scholar]
- Espinoza, J. C. , Garreaud, R. , Poveda, G. , Arias, P. A. , Molina‐Carpio, J. , Masiokas, M. , Viale, M. , & Scaff, L. (2020). Hydroclimate of the Andes part I: Main climatic features. Frontiers in Earth Science, 8, 64. 10.3389/feart.2020.00064 [DOI] [Google Scholar]
- Fadrique, B. , Báez, S. , Duque, Á. , Malizia, A. , Blundo, C. , Carilla, J. , Osinaga‐Acosta, O. , Malizia, L. , Silman, M. , Farfán‐Ríos, W. , Malhi, Y. , Young, K. R. , Cuesta, C. F. , Homeier, J. , Peralvo, M. , Pinto, E. , Jadan, O. , Aguirre, N. , Aguirre, Z. , & Feeley, K. J. (2018). Widespread but heterogeneous responses of Andean forests to climate change. Nature, 564(7735), 207–212. 10.1038/s41586-018-0715-9 [DOI] [PubMed] [Google Scholar]
- Falco, M. , Li, L. Z. X. , Menéndez, C. G. , & Carril, A. F. (2019). The influence of South American regional climate on the simulation of the Southern Hemisphere extratropical circulation. Climate Dynamics, 53(9–10), 6469–6488. 10.1007/s00382-019-04940-9 [DOI] [Google Scholar]
- Feeley, K. J. , & Silman, M. R. (2010). Modelling the responses of Andean and Amazonian plant species to climate change: The effects of georeferencing errors and the importance of data filtering. Journal of Biogeography, 37, 733–740. [Google Scholar]
- Feeley, K. J. , Silman, M. R. , Bush, M. B. , Farfan, W. , Garcia Cabrera, K. , Malhi, Y. , Meir, P. , Salinas Revilla, N. , Raurau Quisiyupanqui, M. N. , & Saatchi, S. (2011). Upslope migration of Andean trees. Journal of Biogeography, 38, 783–791. [Google Scholar]
- Felde, V. A. , Hooghiemstra, H. , Torres‐Torres, V. , & Birks, H. J. B. (2016). Detecting patterns of change in a long pollen‐stratigraphical sequence from Funza, Colombia – A comparison of new and traditional numerical approaches. Review of Palaeobotany and Palynology, 234, 94–109. 10.1016/j.revpalbo.2016.08.003 [DOI] [Google Scholar]
- Ferrero, M. E. , Villalba, R. , De Membiela, M. , Ferri Hidalgo, L. , & Luckman, B. H. (2015). Tree‐ring based reconstruction of Río Bermejo streamflow in subtropical South America. Journal of Hydrology, 525, 572–584. 10.1016/j.jhydrol.2015.04.004 [DOI] [Google Scholar]
- Fick, S. E. , & Hijmans, R. J. (2017). WorldClim 2: New 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315. 10.1002/joc.5086 [DOI] [Google Scholar]
- Fita, L. , Carril, A. F. , Flombaum, P. , Menéndez, C. G. , Ruscica, R. , Sörensson, A. , & Zaninelli, P. (2019). Results of a new methodology to analyze the climate change uncertainty on a high step mountain range. In Application at the Andes. International conference on regional climate. WCRP‐CORDEX, Beijing, China. https://icrc‐cordex2019.cordex.org/wp‐content/uploads/sites/2/2019/11/09_CARRIL_Andrea_SessionC2.pdf [Google Scholar]
- Flantua, S. G. A. , & Hooghiemstra, H. (2018). Historical connectivity and mountain biodiversity. In Hoorn C., Perrigo A., & Antonelli A. (Eds.), Mountains, climate and biodiversity (pp. 171–185). John Wiley & Sons. [Google Scholar]
- Flantua, S. G. A. , Hooghiemstra, H. , Grimm, E. C. , Behling, H. , Bush, M. B. , González‐Arango, C. , Gosling, W. D. , Ledru, M.‐P. , Lozano‐García, S. , Maldonado, A. , Prieto, A. R. , Rull, V. , & Van Boxel, J. H. (2015). Updated site compilation of the Latin American Pollen Database. Review of Palaeobotany and Palynology, 223, 104–115. 10.1016/j.revpalbo.2015.09.008 [DOI] [Google Scholar]
- Flantua, S. G. A. , Hooghiemstra, H. , Vuille, M. , Behling, H. , Carson, J. F. , Gosling, W. D. , Hoyos, I. , Ledru, M. P. , Montoya, E. , Mayle, F. , Maldonado, A. , Rull, V. , Tonello, M. S. , Whitney, B. S. , & González‐Arango, C. (2016). Climate variability and human impact in South America during the last 2000 years: Synthesis and perspectives from pollen records. Climate of the Past, 12(2), 483–523. 10.5194/cp-12-483-2016 [DOI] [Google Scholar]
- Flantua, S. G. A. , O'Dea, A. , Onstein, R. E. , Giraldo, C. , & Hooghiemstra, H. (2019). The flickering connectivity system of the north Andean páramos. Journal of Biogeography, 13607. 10.1111/jbi.13607 [DOI] [Google Scholar]
- Flato, G. , Marotzke, J. , Abiodun, B. , Braconnot, P. , Chou, S. C. , Collins, W. , Cox, P. , Driouech, F. , Emori, S. , Erying, V. , Forest, C. , Gleckler, P. , Guilyardi, E. , Jakob, C. , Kattsov, V. , Reason, C. , & Rummukainen, M. (2013). Evaluation of climate models. In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- Forest, F. , Grenyer, R. , Rouget, M. , Davies, T. J. , Cowling, R. M. , Faith, D. P. , Balmford, A. , Manning, J. C. , Procheş, Ş. , van der Bank, M. , Reeves, G. , Hedderson, T. A. J. , & Savolainen, V. (2007). Preserving the evolutionary potential of floras in biodiversity hotspots. Nature, 445(7129), 757–760. 10.1038/nature05587 [DOI] [PubMed] [Google Scholar]
- Fuentes‐Lillo, E. , & Pauchard, A. (2019). Invasiones en montañas: ¿Cuánto hemos avanzado en los últimos 10 años y cuáles son los desafíos para los ecosistemas de los Andes? Gayana. Botánica, 76(2), 141–155. 10.4067/S0717-66432019000200141 [DOI] [Google Scholar]
- Garibotti, I. A. , Pissolito, C. I. , & Villalba, R. (2011). Spatiotemporal pattern of primary succession in relation to meso‐topographic gradients on recently deglaciated terrains in the Patagonian Andes. Artic and Alpine Research, 43(4), 555–567. [Google Scholar]
- Garreaud, R. D. (2009). The Andes climate and weather. Advances in Geosciences, 22, 3–11. 10.5194/adgeo-22-3-2009 [DOI] [Google Scholar]
- Garreaud, R. D. , Vuille, M. , Compagnucci, R. , & Marengo, J. (2009). Present‐day South American climate. Palaeogeography, Palaeoclimatology, Palaeoecology, 281(3–4), 180–195. 10.1016/j.palaeo.2007.10.032 [DOI] [Google Scholar]
- González‐Carranza, Z. , Hooghiemstra, H. , & Vélez, M. I. (2012). Major altitudinal shifts in Andean vegetation on the Amazonian flank show temporary loss of biota in the Holocene. The Holocene, 22(11), 1227–1241. 10.1177/0959683612451183 [DOI] [Google Scholar]
- Gorostiague, P. , Sajama, J. , & Ortega‐Baes, P. (2018). Will climate change cause spatial mismatch between plants and their pollinators? A test using Andean cactus species. Biological Conservation, 226, 247–255. 10.1016/j.biocon.2018.07.003 [DOI] [Google Scholar]
- Graham, A. (2009). The Andes: A geological overview from a biological perspective. Annals of the Missouri Botanical Garden, 96(3), 371–385. 10.3417/2007146 [DOI] [Google Scholar]
- Groot, M. H. M. , Bogotá, R. G. , Lourens, L. J. , Hooghiemstra, H. , Vriend, M. , Berrio, J. C. , Tuenter, E. , Van der Plicht, J. , Van Geel, B. , Ziegler, M. , Weber, S. L. , Betancourt, A. , Contreras, L. , Gaviria, S. , Giraldo, C. , González, N. , Jansen, J. H. F. , Konert, M. , Ortega, D. , … Westerhoff, W. (2011). Ultra‐high resolution pollen record from the northern Andes reveals rapid shifts in montane climates within the last two glacial cycles. Climate of the Past, 7(1), 299–316. 10.5194/cp-7-299-2011 [DOI] [Google Scholar]
- Groot, M. H. M. , Hooghiemstra, H. , Berrio, J. C. , & Giraldo, C. (2013). North Andean environmental and climatic change at orbital to submillennial time‐scales: Vegetation, water levels and sedimentary regimes from Lake Fúquene 130–27 ka. Review of Palaeobotany and Palynology, 197, 186–204. 10.1016/j.revpalbo.2013.04.005 [DOI] [Google Scholar]
- Guisan, A. , & Thuiller, W. (2005). Predicting species distribution: Offering more than simple habitat models. Ecology Letters, 8, 993–1009. [DOI] [PubMed] [Google Scholar]
- Guisan, A. , & Zimmermann, N. (2000). Predictive habitat distribution models in ecology. Ecological Modelling, 135, 147–186. [Google Scholar]
- Gutiérrez, A. G. , Armesto, J. J. , Díaz, M. F. , & Huth, A. (2014). Increased drought impacts on temperate rainforests from southern South America: Results of a process‐based, dynamic forest model. PLoS ONE, 9(7), e103226. 10.1371/journal.pone.0103226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez, A. G. , & Huth, A. (2012). Successional stages of primary temperate rainforests of Chiloé Island, Chile. Perspectives in Plant Ecology, Evolution and Systematics, 14(4), 243–256. 10.1016/j.ppees.2012.01.004 [DOI] [Google Scholar]
- Gutiérrez, A. G. , Snell, R. S. , & Bugmann, H. (2016). Using a dynamic forest model to predict tree species distributions: Predicting tree species distribution. Global Ecology and Biogeography, 25(3), 347–358. 10.1111/geb.12421 [DOI] [Google Scholar]
- Hao, T. , Elith, J. , Guillera‐Arroita, G. , & Lahoz‐Monfort, J. J. (2019). A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Diversity and Distributions, 25(5), 839–852. 10.1111/ddi.12892 [DOI] [Google Scholar]
- Hay, L. E. , Wilby, R. L. , & Leavesley, G. H. (2000). A comparison of delta change and downscaled GCM scenarios for three mountainous bains in the United States. Journal of the American Water Resources Association, 36(2), 387–397. 10.1111/j.1752-1688.2000.tb04276.x [DOI] [Google Scholar]
- Heusser, C. J. , Streeter, S. S. , & Stuiver, M. (1981). Temperature and precipitation record in southern Chile extended to ∼43,000 yr ago. Nature, 294(5836), 65–67. 10.1038/294065a0 [DOI] [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- Hooghiemstra, H. , & Flantua, S. G. A. (2019). Colombia in the Quaternary: An overview of environmental and climatic change. Chapter 2. In Gomez‐Tapias J. (Ed.), The geology of Colombia Book: Vol. Volumen 4 Quaternary (pp. 33–57). Servicio Geológico Colombiano. [Google Scholar]
- Humanes‐Fuente, V. , Ferrero, M. E. , Muñoz, A. A. , González‐Reyes, Á. , Requena‐Rojas, E. J. , Barichivich, J. , Inga, J. G. , & Layme‐Huaman, E. T. (2020). Two centuries of hydroclimatic variability reconstructed from tree‐ring records over the Amazonian Andes of Peru. Journal of Geophysical Research: Atmospheres, 125(18), e2020JD0325665. 10.1029/2020JD032565 [DOI] [Google Scholar]
- von Humboldt, A. , & Bonpland, A. (1807). Essai sur la géographie des plantes, accompagné d'un tableau physique des régions équinoxiales. Chez Levrault, Schoell et compagnie, libraires. 10.5962/bhl.title.9309 [DOI] [Google Scholar]
- Insel, N. , Poulsen, C. J. , & Ehlers, T. A. (2010). Influence of the Andes Mountains on South American moisture transport, convection, and precipitation. Climate Dynamics, 35(7–8), 1477–1492. 10.1007/s00382-009-0637-1 [DOI] [Google Scholar]
- IPCC AR4 (2007). In Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., & Miller H. L. (Eds.), Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- IPCC AR5 (2013). In Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- Kaiser, J. , Lamy, F. , & Hebbeln, D. (2005). A 70‐kyr sea surface temperature record off southern Chile (Ocean Drilling Program Site 1233). Paleoceanography, 20(4), PA4009. 10.1029/2005PA001146 [DOI] [Google Scholar]
- Kammesheidt, L. , Kohler, P. , & Huth, A. (2001). Sustainable timber harvesting in Venezuela: A modelling approach. Journal of Applied Ecology, 38(4), 756–770. 10.1046/j.1365-2664.2001.00629.x [DOI] [Google Scholar]
- Karger, D. N. , Conrad, O. , Bohner, J. , Kawohl, T. , Kreft, H. , Soria‐Auza, R. W. , Zimmermann, N. , Linder, H. P. , & Kessler, M. (2017). Climatologies at high resolution for the earth's land surface areas. Scientific Data, 4(1), 170122. 10.1038/sdata.2017.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendon, E. J. , Rowell, D. P. , Jones, R. G. , & Buonomo, E. (2008). Robustness of future changes in local precipitation extremes. Journal of Climate, 21(17), 4280–4297. 10.1175/2008JCLI2082.1 [DOI] [Google Scholar]
- Kitzberger, T. , & Veblen, T. T. (2003). Influences of climate on fire in northern Patagonia, Argentina. In Veblen T. T., Baker W. L., Montenegro G., & Swetnam T. W. (Eds.), Fire and climatic change in temperate ecosystems of the western Americas (Vol. 160, pp. 296–321). Springer‐Verlag. 10.1007/0-387-21710-X_10 [DOI] [Google Scholar]
- Klein, A. G. , Isacks, B. L. , & Bloom, A. (1995). Modern and Last Glacial Maximum snowline in Peru and Bolivia: Implications for regional climatic change. Bulletin de l'Institut Français d'études Andines, 24(3), 607–617. [Google Scholar]
- Köhler, P. , & Huth, A. (1998). The effects of tree species grouping in tropical rainforest modelling: Simulations with the individual‐based model Formind. Ecological Modelling, 109(3), 301–321. 10.1016/S0304-3800(98)00066-0(98)00066‐0 [DOI] [Google Scholar]
- Kreft, H. , & Jetz, W. (2007). Global patterns and determinants of vascular plant diversity. Proceedings of the National Academy of Sciences of the United States of America, 104(14), 5925–5930. 10.1073/pnas.0608361104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy, F. , Kilian, R. , Arz, H. W. , Francois, J.‐P. , Kaiser, J. , Prange, M. , & Steinke, T. (2010). Holocene changes in the position and intensity of the southern westerly wind belt. Nature Geoscience, 3(10), 695–699. 10.1038/ngeo959 [DOI] [Google Scholar]
- Lavado Casimiro, W. S. , Labat, D. , Ronchail, J. , Espinoza, J. C. , & Guyot, J. L. (2013). Trends in rainfall and temperature in the Peruvian Amazon‐Andes basin over the last 40 years (1965‐2007). Hydrological Processes, 27(20), 2944–2957. 10.1002/hyp.9418 [DOI] [Google Scholar]
- Lembrechts, J. J. , Aalto, J. , Ashcroft, M. B. , De Frenne, P. , Kopecký, M. , & Lenoir, J. (2020). SoilTemp: A global database of near‐surface temperature. Global Change Biology, 26(11), 6616–6629. 10.1111/gcb.15123 [DOI] [PubMed] [Google Scholar]
- Li, M. , Wu, P. , & Ma, Z. (2020). Comprehensive evaluation of soil moisture and soil temperature from third‐generation atmospheric and land reanalysis datasets. International Journal of Climatology, 6549. 10.1002/joc.6549 [DOI] [Google Scholar]
- Luebert, F. , & Pliscoff, P. A. (2018). Sinopsis bioclimática y vegetacional de Chile (2nd ed.). Editorial Universitaria. [Google Scholar]
- Maraun, D. , Shepherd, T. G. , Widmann, M. , Zappa, G. , Walton, D. , Gutiérrez, J. M. , Hagemann, S. , Richter, I. , Soares, P. M. M. , Hall, A. , & Mearns, L. O. (2017). Towards process‐informed bias correction of climate change simulations. Nature Climate Change, 7(11), 764–773. 10.1038/nclimate3418 [DOI] [Google Scholar]
- Marchant, R. , Cleef, A. M. , Harrison, S. P. , Hooghiemstra, H. , Markgraf, V. , van Boxel, J. H. , Ager, T. , Almeida, L. , Anderson, R. , Baied, C. , Behling, H. , Berrio, J. C. , Burbridge, R. E. , Bjorck, S. , Byrne, R. , Bush, M. B. , Duivenvoorden, J. F. , Flenley, J. R. , De Oliveira, P. , … Wille, M. (2009). Pollen‐based biome reconstructions for Latin America at 0, 6000 and 18 000 radiocarbon years ago. Climate of the Past, 5, 725–767. [Google Scholar]
- Mark, B. G. , Harrison, S. P. , Spessa, A. , New, M. , Evans, D. J. A. , & Helmens, K. F. (2005). Tropical snowline changes at the last glacial maximum: A global assessment. Quaternary International, 138–139, 168–201. 10.1016/j.quaint.2005.02.012 [DOI] [Google Scholar]
- Martin‐Gallego, P. , Aplin, P. , Marston, C. , Altamirano, A. , & Pauchard, A. (2020). Detecting and modelling alien tree presence using Sentinel‐2 satellite imagery in Chile's temperate forests. Forest Ecology and Management, 474, 118353. 10.1016/j.foreco.2020.118353 [DOI] [Google Scholar]
- Masiokas, M. H. , Cara, L. , Villalba, R. , Pitte, P. , Luckman, B. H. , Toum, E. , Christie, D. A. , Le Quesne, C. , & Mauget, S. (2019). Streamflow variations across the Andes (18°–55°S) during the instrumental era. Scientific Reports, 9(1), 17879. 10.1038/s41598-019-53981-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle, F. E. , Beerling, D. J. , Gosling, W. D. , & Bush, M. B. (2004). Responses of Amazonian ecosystems to climatic and atmospheric carbon dioxide changes since the last glacial maximum. Philosophical Transactions of the Royal Society B: Biological Sciences, 359, 499–514. 10.1098/rstb.2003.1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle, F. E. , Burn, M. J. , Power, M. , & Urrego, D. H. (2009). Vegetation and fire at the last glacial maximum in tropical south America. In Vimeux F., Sylvestre F., & Khodri M. (Eds.), Past climate variability in south America and surrounding regions (Vol. 14, pp. 89–112). Springer. [Google Scholar]
- Morales, M. S. , Christie, D. A. , Villalba, R. , Argollo, J. , Pacajes, J. , Silva, J. S. , Alvarez, C. A. , Llancabure, J. C. , & Soliz Gamboa, C. C. (2012). Precipitation changes in the South American Altiplano since 1300 AD reconstructed by tree‐rings. Climate of the Past, 8(2), 653–666. 10.5194/cp-8-653-2012 [DOI] [Google Scholar]
- Morales‐Barbero, J. , & Vega‐Álvarez, J. (2019). Input matters matter: Bioclimatic consistency to map more reliable species distribution models. Methods in Ecology and Evolution, 10(2), 212–224. 10.1111/2041-210X.13124 [DOI] [Google Scholar]
- Moret, P. , Muriel, P. , Jaramillo, R. , & Dangles, O. (2019). Humboldt's Tableau Physique revisited. Proceedings of the National Academy of Sciences of the United States of America, 116(26), 12889–12894. 10.1073/pnas.1904585116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morueta‐Holme, N. , Engemann, K. , Sandoval‐Acuña, P. , Jonas, J. D. , Segnitz, R. M. , & Svenning, J.‐C. (2015). Strong upslope shifts in Chimborazo's vegetation over two centuries since Humboldt. Proceedings of the National Academy of Sciences of the United States of America, 112(41), 12741–12745. 10.1073/pnas.1509938112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain Research Initiative EDW Working Group . (2015). Elevation‐dependent warming in mountain regions of the world. Nature Climate Change, 5(5), 424–430. 10.1038/nclimate2563 [DOI] [Google Scholar]
- Myers, N. , Mittermeier, R. , Mittermeier, C. , Fonseca, G. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Niemann, H. , & Behling, H. (2008). Late Quaternary vegetation, climate and fire dynamics inferred from the El Tiro record in the southeastern Ecuadorian Andes. Journal of Quaternary Science, 23(3), 203–212. 10.1002/jqs.1134 [DOI] [Google Scholar]
- Niemann, H. , & Behling, H. (2010). Late Holocene environmental change and human impact inferred from three soil monoliths and the Laguna Zurita multi‐proxi record in the southeastern Ecuadorian Andes. Vegetation History and Archaeobotany, 19(1), 1–15. 10.1007/s00334-009-0226-6 [DOI] [Google Scholar]
- Nogués‐Bravo, D. (2009). Predicting the past distribution of species climatic niches. Global Ecology and Biogeography, 18(5), 521–531. 10.1111/j.1466-8238.2009.00476.x [DOI] [Google Scholar]
- Novello, V. F. , Cruz, F. W. , McGlue, M. M. , Wong, C. I. , Ward, B. M. , Vuille, M. , Santos, R. A. , Jaqueto, P. , Pessenda, L. C. R. , Atorre, T. , Ribeiro, L. M. A. L. , Karmann, I. , Barreto, E. S. , Cheng, H. , Edwards, R. L. , Paula, M. S. , & Scholz, D. (2019). Vegetation and environmental changes in tropical South America from the last glacial to the Holocene documented by multiple cave sediment proxies. Earth and Planetary Science Letters, 524, 115717. 10.1016/j.epsl.2019.115717 [DOI] [Google Scholar]
- Novello, V. F. , Cruz, F. W. , Vuille, M. , Stríkis, N. M. , Edwards, R. L. , Cheng, H. , Emerick, S. , de Paula, M. S. , Li, X. , Barreto, E. d. S. , Karmann, I. , & Santos, R. V. (2017). A high‐resolution history of the South American Monsoon from Last Glacial Maximum to the Holocene. Scientific Reports, 7(1), 44267. 10.1038/srep44267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarzabal, M. , Clavijo, J. , Oakley, L. , Biganzoli, F. , Tognetti, P. , Barberis, I. , Maturo, H. M. , Aragón, R. , Campanello, P. I. , Prado, D. , Oesterheld, M. , & León, R. J. C. (2018). Unidades de vegetación de la Argentina. Ecología Austral, 28(1), 40–63. 10.25260/EA.18.28.1.0.399 [DOI] [Google Scholar]
- Pabón‐Caicedo, J. D. , Arias, D. , Carril, A. F. , Espinoza, J. C. , Goubanova, K. , Fita, L. , Lavado, W. , Masiokas, M. , Solman, S. , & Villalba, R. (2020). Observed and projected hydroclimate changes in the Andes. Frontiers in Environmental Sciences, 8, 61. 10.3389/feart.2020.00061 [DOI] [Google Scholar]
- Pacheco, S. , Malizia, L. R. , & Cayuela, L. (2010). Effects of climate change on subtropical forests of South America. Tropical Conservation Science, 3(4), 423–437. 10.1177/194008291000300407 [DOI] [Google Scholar]
- Palacios, D. , Stokes, C. R. , Phillips, F. M. , Clague, J. J. , Alcalá‐Reygosa, J. , Andrés, N. , Angel, I. , Blard, P.‐H. , Briner, J. P. , Hall, B. L. , Dahms, D. , Hein, A. S. , Jomelli, V. , Mark, B. G. , Martini, M. A. , Moreno, P. , Riedel, J. , Sagredo, E. , Stansell, N. D. , … Ward, D. J. (2020). The deglaciation of the Americas during the Last Glacial Termination. Earth‐Science Reviews, 203, 103113. 10.1016/j.earscirev.2020.103113 [DOI] [Google Scholar]
- Pauchard, A. , Kueffer, C. , Dietz, H. , Daehler, C. C. , Alexander, J. , Edwards, P. J. , Arévalo, J. R. , Cavieres, L. A. , Guisan, A. , Haider, S. , Jakobs, G. , McDougall, K. , Millar, C. I. , Naylor, B. J. , Parks, C. G. , Rew, L. J. , & Seipel, T. (2009). Ain't no mountain high enough: Plant invasions reaching new elevations. Frontiers in Ecology and the Environment, 7(9), 479–486. 10.1890/080072 [DOI] [Google Scholar]
- Peña, M. A. , Feeley, K. J. , & Duque, A. (2018). Effects of endogenous and exogenous processes on aboveground biomass stocks and dynamics in Andean forests. Plant Ecology, 219(12), 1481–1492. 10.1007/s11258-018-0895-2 [DOI] [Google Scholar]
- Pennington, R. T. , Lavin, M. , Särkinen, T. , Lewis, G. P. , Klitgaard, B. B. , & Hughes, C. E. (2010). Contrasting plant diversification histories within the Andean biodiversity hotspot. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13783–13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Escobar, O. A. , Chomicki, G. , Condamine, F. L. , Karremans, A. P. , Bogarín, D. , Matzke, N. J. , Silvestro, D. , & Antonelli, A. (2017). Recent origin and rapid speciation of Neotropical orchids in the world's richest plant biodiversity hotspot. New Phytologist, 215(2), 891–905. 10.1111/nph.14629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Escobar, O. A. , Zizka, A. , Bermúdez, M. A. , Meseguer, A. S. , Condamine, F. L. , Hoorn, C. , Hooghiemstra, H. , Pu, Y. , Bogarín, D. , Boschman, L. M. , Pennington, R. T. , Antonelli, A. , & Chomicki, G. (2022). The Andes through time: Evolution and distribution of Andean floras. Trends in Plant Science, 27(4), 364–378. 10.1016/j.tplants.2021.09.010 [DOI] [PubMed] [Google Scholar]
- Peterson, A. T. , Cobos, M. E. , & Jiménez‐García, D. (2018). Major challenges for correlational ecological niche model projections to future climate conditions: Climate change, ecological niche models, and uncertainty. Annals of the New York Academy of Sciences, 1429(1), 66–77. 10.1111/nyas.13873 [DOI] [PubMed] [Google Scholar]
- Pouchon, C. , Fernández, A. , Nassar, J. M. , Boyer, F. , Aubert, S. , Lavergne, S. , & Mavárez, J. (2018). Phylogenomic analysis of the explosive adaptive radiation of the Espeletia complex (Asteraceae) in the Tropical Andes. Systematic Biology, 67(6), 1041–1060. 10.1093/sysbio/syy022 [DOI] [PubMed] [Google Scholar]
- Ramirez‐Angulo, H. , Torres‐Lezama, A. , & Acevedo, M. F. (1997). Simulación de la dinámica de grupos especies vegetales en un bosque de los llanos occidentales venezolanos. Ecotropicos, 10, 9–20. [Google Scholar]
- Ramirez‐Villegas, J. , Cuesta, F. , Devenish, C. , Peralvo, M. , Jarvis, A. , & Arnillas, C. A. (2014). Using species distributions models for designing conservation strategies of Tropical Andean biodiversity under climate change. Journal for Nature Conservation, 22(5), 391–404. 10.1016/j.jnc.2014.03.007 [DOI] [Google Scholar]
- Rangwala, I. , & Miller, J. R. (2012). Climate change in mountains: A review of elevation‐dependent warming and its possible causes. Climatic Change, 114(3–4), 527–547. 10.1007/s10584-012-0419-3 [DOI] [Google Scholar]
- Razgour, O. , Forester, B. , Taggart, J. B. , Bekaert, M. , Juste, J. , Ibáñez, C. , Puechmaille, S. J. , Novella‐Fernandez, R. , Alberdi, A. , & Manel, S. (2019). Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proceedings of the National Academy of Sciences, 116(21), 10418–10423. 10.1073/pnas.1820663116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens, C. , Ariztegui, D. , Gebhardt, C. , Gogorza, C. , Haberzettl, T. , Hahn, A. , Kliem, P. , Lisé‐Pronovost, A. , Lücke, A. , Maidana, N. , Mayr, C. , Ohlendorf, C. , Schäbitz, F. , St‐Onge, G. , Wille, M. , Zolitschka, B. , & Sience Team, P. (2012). New insights into paleoenvironmental changes in Laguna Potrok Aike, southern Patagonia, since the Late Pleistocene: The PASADO multiproxy record. The Holocene, 22(11), 1323–1335. 10.1177/0959683611429833 [DOI] [Google Scholar]
- Rodríguez‐Catón, M. , Villalba, R. , Morales, M. , & Srur, A. (2016). Influence of droughts on Nothofagus pumilio forest decline across northern Patagonia, Argentina. Ecosphere, 7(7), e01390. 10.1002/ecs2.1390 [DOI] [Google Scholar]
- Rojas, M. , Moreno, P. , Kageyama, M. , Crucifix, M. , Hewitt, C. , Abe‐Ouchi, A. , Ohgaito, R. , Brady, E. C. , & Hope, P. (2009). The Southern Westerlies during the last glacial maximum in PMIP2 simulations. Climate Dynamics, 32(4), 525–548. 10.1007/s00382-008-0421-7 [DOI] [Google Scholar]
- Rüger, N. , Comita, L. S. , Condit, R. , Purves, D. , Rosenbaum, B. , Visser, M. D. , Wright, S. J. , & Wirth, C. (2018). Beyond the fast‐slow continuum: Demographic dimensions structuring a tropical tree community. Ecology Letters, 21(7), 1075–1084. 10.1111/ele.12974 [DOI] [PubMed] [Google Scholar]
- Rüger, N. , Condit, R. , Dent, D. H. , DeWalt, S. J. , Hubbell, S. P. , Lichstein, J. W. , Lopez, O. R. , Wirth, C. , & Farrior, C. E. (2020). Demographic trade‐offs predict tropical forest dynamics. Science, 368(6487), 165–168. 10.1126/science.aaz4797 [DOI] [PubMed] [Google Scholar]
- Rüger, N. , Gutiérrez, Á. G. , Kissling, W. D. , Armesto, J. J. , & Huth, A. (2007). Ecological impacts of different harvesting scenarios for temperate evergreen rain forest in southern Chile—A simulation experiment. Forest Ecology and Management, 252(1–3), 52–66. 10.1016/j.foreco.2007.06.020 [DOI] [Google Scholar]
- Schauwecker, S. , Rohrer, M. , Acuña, D. , Cochachin, A. , Dávila, L. , Frey, H. , Giráldez, C. , Gómez, J. , Huggel, C. , Jacques‐Coper, M. , Loarte, E. , Salzmann, N. , & Vuille, M. (2014). Climate trends and glacier retreat in the Cordillera Blanca, Peru, revisited. Global and Planetary Change, 119, 85–97. 10.1016/j.gloplacha.2014.05.005 [DOI] [Google Scholar]
- Schwalm, C. R. , Glendon, S. , & Duffy, P. B. (2020). RCP8.5 tracks cumulative CO2 emissions. Proceedings of the National Academy of Sciences of the United States of America, 117(33), 19656–19657. 10.1073/pnas.2007117117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler, C. , Hutjes, R. W. A. , Kruijt, B. , Quispe, J. , Añez, S. , Arora, V. K. , Melton, J. R. , Hickler, T. , & Kabat, P. (2014). Modeling forest dynamics along climate gradients in Bolivia. Journal of Geophysical Research: Biogeosciences, 119(5), 758–775. 10.1002/2013JG002509 [DOI] [Google Scholar]
- Singer, A. , Johst, K. , Banitz, T. , Fowler, M. S. , Groeneveld, J. , Gutiérrez, A. G. , Hartig, F. , Krug, R. M. , Liess, M. , Matlack, G. , Meyer, K. M. , Pe'er, G. , Radchuk, V. , Voinopol‐Sassu, A.‐J. , & Travis, J. M. J. (2016). Community dynamics under environmental change: How can next generation mechanistic models improve projections of species distributions? Ecological Modelling, 326, 63–74. 10.1016/j.ecolmodel.2015.11.007 [DOI] [Google Scholar]
- Snell, R. S. (2014). Simulating long‐distance seed dispersal in a dynamic vegetation model: Incorporating seed dispersal into a DVM. Global Ecology and Biogeography, 23(1), 89–98. 10.1111/geb.12106 [DOI] [Google Scholar]
- Snell, R. S. , Huth, A. , Nabel, J. E. M. S. , Bocedi, G. , Travis, J. M. J. , Gravel, D. , Bugmann, H. , Gutiérrez, A. G. , Hickler, T. , Higgins, S. I. , Reineking, B. , Scherstjanoi, M. , Zurbriggen, N. , & Lischke, H. (2014). Using dynamic vegetation models to simulate plant range shifts. Ecography, 37(12), 1184–1197. 10.1111/ecog.00580 [DOI] [Google Scholar]
- Sosa‐Pivatto, M. , Cosacov, A. , Baranzelli, M. C. , Iglesias, M. R. , Espíndola, A. , & Sérsic, A. N. (2017). Do 120,000 years of plant–pollinator interactions predict floral phenotype divergence in Calceolaria polyrhiza? A reconstruction using species distribution models. Arthropod‐Plant Interactions, 11(3), 351–361. 10.1007/s11829-016-9490-4 [DOI] [Google Scholar]
- Srur, A. M. , Villalba, R. , Rodríguez‐Catón, M. , Amoroso, M. M. , & Marcotti, E. (2016). Establishment of Nothofagus pumilio at upper treelines across a precipitation gradient in the northern Patagonian Andes. Arctic, Antarctic, and Alpine Research, 48(4), 755–766. 10.1657/AAAR0016-015 [DOI] [Google Scholar]
- Srur, A. M. , Villalba, R. , Rodríguez‐Catón, M. , Amoroso, M. M. , & Marcotti, E. (2018). Climate and Nothofagus pumilio establishment at upper treelines in the Patagonian Andes. Frontiers in Earth Science, 6, 57. 10.3389/feart.2018.00057 [DOI] [Google Scholar]
- Suarez, M. L. , Ghermandi, L. , & Kitzberger, T. (2004). Factors predisposing episodic drought‐induced tree mortality in Nothofagus‐ site, climatic sensitivity and growth trends. Journal of Ecology, 92(6), 954–966. 10.1111/j.1365-2745.2004.00941.x [DOI] [Google Scholar]
- Tamme, R. , Götzenberger, L. , Zobel, M. , Bullock, J. M. , Hooftman, D. A. P. , Kaasik, A. , & Pärtel, M. (2014). Predicting species' maximum dispersal distances from simple plant traits. Ecology, 95(2), 505–513. 10.1890/13-1000.1 [DOI] [PubMed] [Google Scholar]
- Tejedor Garavito, N. , Newton, A. C. , Golicher, D. , & Oldfield, S. (2015). The relative impact of climate change on the extinction risk of tree species in the montane tropical Andes. PLoS One, 10(7), e0131388. 10.1371/journal.pone.0131388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar, C. , Arnillas, C. A. , Cuesta, F. , & Buytaert, W. (2013). Diverging responses of tropical Andean biomes under future climate conditions. PLoS ONE, 8(5), e63634. 10.1371/journal.pone.0063634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar, C. , Melcher, I. , Kusumoto, B. , Cuesta, F. , Cleef, A. , Meneses, R. I. , Halloy, S. , Llambi, L. D. , Beck, S. , Muriel, P. , Jaramillo, R. , Jacome, J. , & Carilla, J. (2020). Plant dispersal strategies of high tropical alpine communities across the Andes. Journal of Ecology, 108(5), 1910–1922. 10.1111/1365-2745.13416 [DOI] [Google Scholar]
- Urrutia, R. , & Vuille, M. (2009). Climate change projections for the tropical Andes using a regional climate model: Temperature and precipitation simulations for the end of the 21st century. Journal of Geophysical Research, 14, D02108. [Google Scholar]
- Valencia, B. G. , Urrego, D. H. , Silman, M. R. , & Bush, M. B. (2010). From ice age to modern: A record of landscape change in an Andean cloud forest. Journal of Biogeography, 37, 1637–1647. 10.1111/j.1365-2699.2010.02318.x [DOI] [Google Scholar]
- Vanderwel, M. C. , Lyutsarev, V. S. , & Purves, D. W. (2013). Climate‐related variation in mortality and recruitment determine regional forest‐type distributions: Forest distributions from demography. Global Ecology and Biogeography, 22(11), 1192–1203. 10.1111/geb.12081 [DOI] [Google Scholar]
- Velásquez‐Tibatá, J. , Olaya‐Rodríguez, M. H. , López‐Lozano, D. , Gutiérrez, C. , González, I. , & Londoño‐Murcia, M. C. (2019). BioModelos: A collaborative online system to map species distributions. PLoS ONE, 14(3), e0214522. 10.1371/journal.pone.0214522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloz, S. D. , Williams, J. W. , Blois, J. L. , He, F. , Otto‐Bliesner, B. , & Liu, Z. (2012). No‐analog climates and shifting realized niches during the late quaternary: Implications for 21st‐century predictions by species distribution models. Global Change Biology, 18(5), 1698–1713. 10.1111/j.1365-2486.2011.02635.x [DOI] [Google Scholar]
- Venegas‐González, A. , Roig, F. A. , Peña‐Rojas, K. , Hadad, M. A. , Aguilera‐Betti, I. , & Muñoz, A. A. (2019). Recent consequences of climate change have affected tree growth in distinct Nothofagus macrocarpa (DC.) FM Vaz & Rodr age classes in central Chile. Forests, 10(8), 653. 10.3390/f10080653 [DOI] [Google Scholar]
- Villagrán, C. (2001). Un modelo de la historia de la vegetación de la Cordillera de La Costa de Chile central‐sur: La hipótesis glacial de Darwin. Revista Chilena de Historia Natural, 74(4), 793–803. 10.4067/S0716-078X2001000400007 [DOI] [Google Scholar]
- Villagrán, C. , Abarzúa, A. M. , & Armesto, J. J. (2019). Nuevas evidencias paleobotánicas y filogeográficas de la historia Cuaternaria de los bosques subtropical‐templados de la Cordillera de la Costa de Chile. In Smith‐Ramírez C. & Squeo F. A. (Eds.), Biodiversidad y Ecología de los bosques costeros de Chile (pp. 3–30). Universidad de los Lagos. [Google Scholar]
- Villalba, R. (1990). Climatic fluctuations in northern Patagonia during the last 1000 years as inferred from tree‐ring records. Quaternary Research, 34(3), 346–360. 10.1016/0033-5894(90)90046-N(90)90046‐N [DOI] [Google Scholar]
- Villalba, R. , Grau, H. R. , Boninsegna, J. A. , Jacoby, G. C. , & Ripalta, A. (1998). Tree‐ring evidence for long‐term precipitation changes in subtropical South America. International Journal of Climatology, 18(13), 1463–1478. [Google Scholar]
- Villalba, R. , Lara, A. , Boninsegna, J. A. , Masiokas, M. , Delgado, S. , Aravena, J. C. , Roig, F. A. , Schmelter, A. , Wolodarsky, A. , & Ripalta, A. (2003). Large‐scale temperature changes across the southern Andes: 20th‐century variations in the context of the past 400 Years. Climatic Change, 59(1/2), 177–232. 10.1023/A:1024452701153 [DOI] [Google Scholar]
- Villalba, R. , Lara, A. , Masiokas, M. H. , Urrutia, R. , Luckman, B. H. , Marshall, G. J. , Mundo, I. A. , Christie, D. A. , Cook, E. R. , Neukom, R. , Allen, K. , Fenwick, P. , Boninsegna, J. A. , Srur, A. M. , Morales, M. S. , Araneo, D. , Palmer, J. G. , Cuq, E. , Aravena, J. C. , … LeQuesne, C. (2012). Unusual southern hemisphere tree growth patterns induced by changes in the Southern Annular Mode. Nature Geoscience, 5(11), 793–798. 10.1038/ngeo1613 [DOI] [Google Scholar]
- Villalba, R. , & Veblen, T. T. (1997). Regional patterns of tree population age structures in northern Patagonia: Climatic and disturbance influences. The Journal of Ecology, 85(2), 113. 10.2307/2960643 [DOI] [Google Scholar]
- Villota, A. , León‐Yánez, S. , & Behling, H. (2012). Vegetation and environmental dynamics in the Páramo of Jimbura region in the southeastern Ecuadorian Andes during the late Quaternary. Journal of South American Earth Sciences, 40, 85–93. 10.1016/j.jsames.2012.09.010 [DOI] [Google Scholar]
- Weng, C. , Bush, M. B. , Curtis, J. H. , Kolata, A. L. , Dillehay, T. D. , & Binford, M. W. (2006). Deglaciation and Holocene climate change in the western Peruvian Andes. Quaternary Research, 66, 87–96. [Google Scholar]
- Whitlock, C. , Bianchi, M. M. , Bartlein, P. J. , Markgraf, V. , Marlon, J. , Walsh, M. , & McCoy, N. (2006). Postglacial vegetation, climate, and fire history along the east side of the Andes (lat 41–42.5°S), Argentina. Quaternary Research, 66(2), 187–201. 10.1016/j.yqres.2006.04.004 [DOI] [Google Scholar]
- Whittaker, R. H. (1975). Communities and ecosystems (2nd ed.). Macmillan USA. [Google Scholar]
- Wicaksono, C. Y. , Aguirre‐Guiterrez, J. , Nouhra, E. , Pastor, N. , Raes, N. , Pacheco, S. , & Geml, J. (2017). Contracting montane cloud forests: A case study of the Andean alder (Alnus acuminata) and associated fungi in the Yungas. Biotropica, 49(2), 141–152. 10.1111/btp.12394 [DOI] [Google Scholar]
- Zimmer, A. , Meneses, R. I. , Rabatel, A. , Soruco, A. , Dangles, O. , & Anthelme, F. (2018). Time lag between glacial retreat and upward migration alters tropical alpine communities. Perspectives in Plant Ecology, Evolution and Systematics, 30, 89–102. 10.1016/j.ppees.2017.05.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DataS1
Data Availability Statement
Data used in the climate diagnostics is publicly available from any of the open nodes of the 'Earth System Grid Federation' (ESGF) for example the node https://esgf.llnl.gov. Diagnostics used in the manuscript making use of this numerical climate data are described in detail in the methods. The results of the searches in the SCOPUS database are provided in the Supporting Information and the details of keywords and timeframe used for this are described in the methods.


