Abstract
Living crocodylomorphs have an ossified secondary palate with a posteriorly positioned choana that enables their semi‐aquatic, predatory ecology. In contrast, the earliest branching members of Crocodylomorpha have an open palate with anteriorly positioned choanae. The evolution of an ossified secondary palate and a posteriorly positioned choana features strongly in hypotheses of broad‐scale phylogenetic relationships within Crocodylomorpha. Renewed investigations into palatal morphology among extinct members of the clade show surprising variability in the anatomy of the palate, with at least one and potentially a second independent occurrence of “eusuchian‐type” palate outside of Eusuchia. Understanding the trajectory of crocodylomorph palatal evolution is, therefore, a key to inferring crocodylomorph interrelationships and ecomorphology. To document early‐branching crocodylomorph palatal anatomy, we developed an anatomical comparative dataset using computed tomography scan data and literature, comprising 12 early‐branching crocodylomorph taxa. To understand discrete phenotypic changes in palatal structure, we compiled a phylogenetically broadly sampled character‐taxon matrix from the existing literature, and revised its palatal characters, adding 10 new palatal characters. Our comparative anatomical investigations allow us to propose an adapted hypothesis for the closure of the palate and the posterior migration of the choana. Our phylogenetic findings corroborate previous research showing that non‐crocodyliform crocodylomorphs (“sphenosuchians”) are paraphyletic, with the exclusion of the clade Hallopodidae. Non‐mesoeucrocodylian crocodyliforms (“protosuchians”) are paraphyletic, but form three monophyletic clades: Notochampsoidea, Shartegosuchoidea, and Gobiosuchidae. We find a potential association between secondary palate development and dietary shifts, particularly with regard to hypothesized origins of herbivory.
Keywords: crocodylian, secondary palate, phylogenetics
Living crocodylians are 1 of only 3 groups of vertebrates that evolve an ossified secondary palate. We study the palatal anatomy of fossil crocodylians and its broader implications for the phylogenetics and palaeoecology of the group using micro‐CT data. We find that a secondary palate evolves at least twice and with different configurations of the palatal bones.
1. INTRODUCTION
The ossified secondary palate is an iconic evolutionary innovation that occurs independently in living mammals, turtles, and crocodylians (Gaffney, 1979; Huxley, 1875; Iordansky, 1973; Parrington & Westoll, 1940; Pol et al., 2009; Thomason & Russell, 1986; Turner, 2015; Turner & Pritchard, 2015). Study of the crocodylian secondary palate has a deep history (e.g., Huxley, 1875), and more recent inquiries have documented its evolutionary complexity (e.g., Andrade et al., 2006; Dollman et al., 2018; Pritchard et al., 2013). Despite this work, a gap remains in understanding palatal complexity among the earliest branching members of Crocodylomorpha (the group that includes all extant crocodylians and their closest fossil relatives), partly because of the scarcity of study material and partly because of the challenges of non‐destructively obtaining three‐dimensional (3D) data.
Earlier, pre‐cladistic researchers proposed that the general evolutionary progression of crocodylomorphs could be inferred from their secondary palate structures. These researchers hypothesized three sequential palatal “grades”: the early‐branching “protosuchian,” intermediate “mesosuchian,” and late‐branching “eusuchian” conditions. In that paradigm, a “protosuchian” secondary palate bears an open roof and an anteriorly positioned choana, with the anterior rim of the choana contributed by the maxilla anteriorly and the palatine posteriorly. The intermediate “mesosuchian” palate is characterized by a complete closure of the palate by the maxilla, premaxilla, palatine, and pterygoid, with the choana, positioned farther posteriorly along the palate. The “mesosuchian” palatine forms the anterior margin of the choanal opening and the pterygoid forms the posterior margin. Finally, the “eusuchian” secondary palate features a choana that is positioned posteriorly on the palate and is fully enclosed by the pterygoid (Huxley, 1875; Iordansky, 1973; Langston, 1973).
While many features of the “eusuchian‐type” palate indeed represent synapomorphies of Eusuchia (e.g., Pritchard et al., 2013; Turner & Pritchard, 2015), the homoplastic presence of some derived palatal structures in non‐eusuchian groups (e.g., Shartegosuchidae) suggests a more complex evolutionary history for the palate than the traditional three‐grade system, and invites detailed examination of palatal structures, particularly for early branching taxa (Dollman et al., 2018). For example, the importance of accurately assessing palatal anatomy and homologies of its structures is highlighted by the Susisuchidae, whose phylogenetic position relative to Eusuchia is determined by the interpretation of the choana as either fully bounded by the pterygoid or partially by the palatines (e.g.,de Andrade et al., 2011; Leite & Fortier, 2018; Montefeltro et al., 2019; Salisbury et al., 2006; Turner, 2015; Turner & Pritchard, 2015). Research focusing on more specific palatal structures, such as the choana in Mesoeucrocodylia (Andrade et al., 2006), has shown that understanding specific palatal anatomy has great promise for identifying characters to refine the phylogenetic and systematic analysis of crocodylomorph lineages.
This research aims to expand our understanding of palate evolution in Crocodylomorpha by investigating the palatal anatomy of some of the earliest branching members. First, we review the palatal anatomy of 13 representative crocodylomorphs, including members of most early‐branching clades. Second, we assess the phylogenetic utility of palatal characters and we attempt to provide unambiguous, precise, and accurate descriptions of each character. Finally, we discuss the functional and ecological implications of the diversity in palatal morphologies observed in early crocodylomorphs. Observations made in this study highlight that there is an abundance of systematic and phylogenetic information observable in palatal structures of even these earliest‐branching members.
2. MATERIALS AND METHODS
2.1. Anatomical description
Although we acknowledge that the relationships of earlier‐branching groups are in a state of flux, with major unresolved questions about “sphenosuchian” and “protosuchian” monophyly, we use the terms “sphenosuchian” and “protosuchian” for ease of reading and because they are colloquialisms for those groups. Here, we use “sphenosuchians” to refer to the paraphyletic grade of non‐crocodyliform crocodylomorphs, and “protosuchians” to refer to the paraphyletic grade of non‐mesoeucrocodylian crocodyliforms.
Thirteen early‐branching crocodylomorph specimens are identified for analysis from “sphenosuchian” and the “protosuchian” groups including Notochampsoidea, Shartegosuchoidea, and Gobiosuchidae (Table 1). The palatal features are described based on computed tomography (CT) scan data of crocodylomorph specimens, from previously published descriptions, and personal observation of specimens. The individual palatal bones are analyzed (premaxilla, vomer, maxilla, palatine, pterygoid, ectopterygoid, and jugal) in VG Studio Max 3.0 (Volume Graphics, Heidelberg, Germany) to visualize palatal structures in 3D.
TABLE 1.
List of taxa used for comparisons and palatal reconstructions in this research
| Genus and species | Specimen number | Taxonomic group | Data source | CT scan parameters | Micro CT scanner and scan facility |
|---|---|---|---|---|---|
| Sphenosuchus acutus | SAM‐PK‐K3014 | ‘Sphenosuchia’ |
CT Scan Data |
100 kV 200 μA | General Electric VTomex L240, Stellenbosch MicroCT Scan Facility |
| Dibothrosuchus elaphros | IVPP V 7097 | ‘Sphenosuchia’ |
CT Scan data Wu and Chatterjee (1993) |
Historic scan, scan parameters unavailable | ‐ |
| Litargosuchus leptorhynchus | BP/1/5237 | ‘Sphenosuchia’ |
CT Scan data Clark & Sues (2002) |
150 kV 120 μA | Nikon Metrology XTH 225/320 LC, ESI Micro CT Facility |
| Terrestrisuchus gracilis | NHMUK PV R 7557 | ‘Sphenosuchia’ | Crush (1984) | No CT scan data available | ‐ |
| Orthosuchus stormbergi | SAM‐PK‐K409 |
‘Protosuchia’ Notochampsidae |
CT Scan data |
160 kV 200 μA | General Electric VTomex L240, Stellenbosch MicroCT Scan Facility |
| Protosuchus haughtoni | BP/1/4770 |
‘Protosuchia’ Protosuchidae |
CT Scan data Gow (2000) |
100 kV 109 μA | Nikon Metrology XTH 225/320 LC, ESI Micro CT Facility |
| Unnamed Protosuchid | UCMP 97638 |
‘Protosuchia’ Protosuchidae |
Personal observation, Clark (dissertation, 1986) | No CT scan data available | ‐ |
| Gobiosuchus kielanae | ZPAL MgR‐II—67, 68, 69, 70 |
‘Protosuchia’ Gobiosuchidae |
Osmólska et al. (1997) | No CT scan data available | ‐ |
| Sichuanosuchus shuhanensis | IVPP V10594 |
‘Protosuchia’ Shartegosuchoidea |
Personal observation Wu et al. (1997) |
No CT scan data available | ‐ |
| Nominosuchus mutatinus | IVPP V14392 |
‘Protosuchia’ Shartegosuchidae |
CT Scan data Personal observation |
220 kV 190 μA | AMNH Microscopy and Imaging Facility |
| Shantungosuchus brachycephalus | IVPP V4020 |
‘Protosuchia’ Shartegosuchoidea |
Personal observation Wu et al. (1994) |
No CT scan data available | ‐ |
| Fruitachampsa callisoni | LACM 120455a |
‘Protosuchia’ Shartegosuchidae |
Clark (2011) | No CT scan data available | ‐ |
| Shartegosuchus asperopalatum | IGM 200/50, PIN No. 4174/2 |
‘Protosuchia’ Shartegosuchidae |
CT Scan data Dollman et al. (2018) |
150 kV 160 μA | AMNH Microscopy and Imaging Facility |
Note: Their specimen numbers, taxonomic group, data source, computed tomography (CT) scan parameters, CT scanner, and facility (if applicable) are given.
Institutional Abbreviations: BP: Evolutionary Studies Institute, Johannesburg, South Africa; IGM: Mongolian Institute of Geology, Ulaan Bataar, Mongolia; IVPP: Institute of Vertebrate Paleontology and Palaeoanthropology, Beijing, China; LACM: Los Angeles County Museum of Natural History, California, USA; NHMUK: Natural History Museum, London, UK; PIN: Paleontological Institute of the Russian Academy of Sciences, Moscow, Russia; UCMP: University of California Museum of Paleontology, Berkeley, USA; SAM: Iziko South African Museum, Cape Town, South Africa; ZPAL: Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
The standard Romerian (i.e., non‐veterinary) is employed for direction terminology in our descriptions, using terms like “anterior” and “posterior” to refer to directions hear and tail respectively. The term “palatal view” is used to describe the ventral view of the oral cavity. For descriptive adjectives, where possible the term “length” is reserved for the anteroposterior direction, “width” for the mediolateral direction, and “height” the for dorsoventral direction. When exceptions exist, the modifications are stated where applied.
2.2. Phylogenetic analyses
To examine relationships of the earliest branching members of Crocodylomorpha, we compiled a broadly sampled phylogenetic data matrix with a particular focus on palatal homologies from other previously published data matrices (Clark, 2011; Crush, 1984; Dollman et al., 2018; Gow, 2000; Nash, 1968, 1975; Osmólska et al., 1997; Walker, 1968, 1990; Wu et al., 1994, 1997; Wu & Chatterjee, 1993). We hypothesized 10 original characters that describe putative palatal homologies and we revised five pre‐existing palatal characters (see character discussions in Section 3), to make a final data matrix comprising 42 ingroup taxa, two outgroup taxa (Postosuchus kirkpatricki and Gracilisuchus stipanicicorum), and 523 characters (see Supplementary Information). We investigated the optimizations of characters 9, 11, 48, 89, 97, 306, 492, 502, 503, 504, 505, 506,507, 508, 509, and 510 in order to determine the pattern and polarity of the evolution of palatal features within Crocodylomorpha. The data matrix was compiled and edited in Mesquite version 3.5 (Maddison & Maddison, 2018). Trees were rooted on Gracilisuchus stipanicicorum.
The data matrix was analyzed in TNT version 1.5 to heuristically search for the most parsimonious tree topologies (Goloboff & Catalano, 2016; Goloboff & Nixon, 2008). We used the following protocol: begin with 1,000 Wagner tree builds using a Random Seed of 1, swap on these initial trees using TBR and keep two trees per replication (replacing when more optimal trees were found), and submit the final pool of trees to an additional round of swapping using TBR, and saving up to 10,000 most‐parsimonious topologies.
3. RESULTS
3.1. Description of the palate of “sphenosuchians”
3.1.1. Palatal overview and preservation
The premaxilla does not contribute to any palatal surface in “sphenosuchians.” The vomers are exposed on the palatal surface, and most of the palatal surface is formed by the palatine and maxilla. The choana is positioned anteriorly on the palate with the anterior and posterior margins formed by the maxilla and palatine, respectively. The bones of the palate are not strongly sutured to each other, but rather form overlapping contacts.
The palate of Dibothrosuchus is articulated and mostly complete, but CT scan data show that the bones are fractured within the matrix (Figure 1a). Although fractured, both pairs of vomers, premaxillae, and maxillae are preserved, except for a small missing region of the anterior process of the paired vomers, and the left maxilla at the posteriormost edge of the tooth‐bearing process along the contact with the jugal. The right palatine is complete, but the posterior region of the pterygoid process of the left palatine is not preserved. The right ectopterygoid is missing, except for a small portion of the pterygoid flange process. The left ectopterygoid is more complete and preserves the complete pterygoid flange process and the neck, although the lateral process, which would have contacted the jugal, has not been preserved. The pterygoid is very fractured but mostly completed, except for the quadrate process of the pterygoid which has not been preserved.
FIGURE 1.
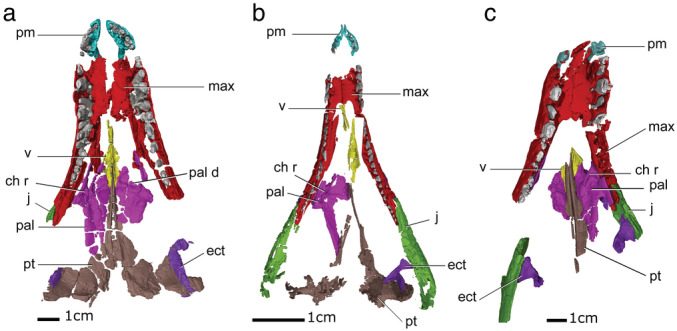
Palatal view of the three‐dimensional (3D) visualizations of the palates of “sphenosuchians” (a) Dibothrosuchus, (b) Litargosuchus, (c) Sphenosuchus. Ch r, choanal ridge; ect, ectopterygoid; j, jugal; max, maxilla; pal, palatine; pal d, palatine depression; pm, premaxilla; pt, pterygoid; v, vomer
The premaxillae of Litargosuchus are both preserved (Figure 1b). Both maxillae are almost completely preserved with the exception of a portion of the anterior surface, and so the incisive foramen septum has not been preserved. The left vomer is complete, but only a small portion of the maxillary process of the right vomer is preserved. The left palatine is not preserved, and the right palatine is missing the vomerine process and a portion of the pterygoid process. The left pterygoid is more complete than the right, which is highly fractured and incomplete. The left pterygoid preserves the vomerine process, the pterygoid flange, and the quadrate process. The right ectopterygoid is missing, but the left ectopterygoid is well preserved and in articulation with the pterygoid flange but is medially removed from the jugal.
The anterior edges of the premaxillae of Sphenosuchus are not preserved (Figure 1c). Both maxillae are well preserved, but the rostrum is fractured so that the sagittal midline bends to the right anteriorly. Only the pterygoid processes of both vomers have been preserved, the left vomer preserving a larger surface of the contact than the right. The left palatine is complete, but the right palatine only has a small portion of the pterygoid process preserved. Little of the pterygoid has been preserved, with only the vomerine process remaining. The pterygoid flange is preserved as an impression in the matrix. The pterygoid flange processes of both ectopterygoids are missing, however, the lateral processes are preserved in articulation with the medial surface of the jugals.
The original description of Terrestrisuchus is based on a number of fragmentary individuals that are assembled in a block of the matrix (NHMUK PV R 7557) (Crush, 1984). There are examples of most cranial bones, however, among the material, there was no premaxilla or vomer preserved. Seven maxillae were preserved among the material and so this bone is almost completely known.
3.1.2. Incisive foramen
“Sphenosuchians” have paired incisive foramina that are oval in palatal view, and which vary interspecifically in their length and mediolateral separation from each other (Figure 1). The anterior margin of the incisive foramen is bordered by the premaxilla and the posterior margin by the maxilla. The anterior margin of the incisive foramen of Terrestrisuchus is not preserved, but the posterior margin is visible and extends to the level of the first maxillary tooth. Sphenosuchus has the longest incisive foramen opening, extending almost until the level of the third maxillary tooth. The incisive foramina of Dibothrosuchus are medially separated from each other by a broad incisive foramen septum. The openings in Dibothrosuchus are also short, they only extend between the fifth premaxillary tooth and the first maxillary tooth. The extent of the incisive foramen of Litargosuchus is not known because the region of the maxilla that would demarcate its posterior limit is not preserved.
Crush (1984; Terrestrisuchus) and Walker (1972, 1990; Sphenosuchus) interpreted the premaxilla of “sphenosuchians” as bearing a short, posteriorly extending process that contacts a corresponding anterior process of the maxilla, together forming the incisive foramen septum. However, reconstructed CT scans of Sphenosuchus, Dibothrosuchus and Litargosuchus show that the palatal surface of the premaxilla lacks a posterior process, and we reinterpret it here as the incisive foramen septum being wholly composed by the maxilla (the incisive foramen process of the maxilla).
3.1.3. Choana
The choanae of sphenosuchians are paired, anteroposteriorly long, sub‐oval openings that are positioned anterior to the suborbital fenestrae. The bony contributions to their anterior margins differ between “sphenosuchian” taxa: in Terrestrisuchus and Litargosuchus it is formed entirely by the maxilla; whereas in Dibothrosuchus and Sphenosuchus, the maxilla and palatine both bound the anterior margin. The posterior margin of the choana is mediolaterally narrower than the anterior edge and is bordered by a prominent ridge formed by the palatine in all “sphenosuchian” taxa we investigated.
3.1.4. Suborbital fenestra
The suborbital fenestra is a large opening positioned on the posterolateral edge of the palate. The palatine bounds the anterior margin of the suborbital fenestra, while the palatine and pterygoid bound the medial, the maxilla and jugal the lateral, and the ectopterygoid and pterygoid bound the posterior margins, respectively. The size and shape of the suborbital fenestra also vary across “sphenosuchian” taxa: whereas the fenestra of Litargosuchus is large and mostly oval in outline, the suborbital fenestrae of Dibothrosuchus and Sphenosuchus are smaller and the anterior edge is mediolaterally narrower than the posterior margin. Terrestrisuchus appears to have a proportionally larger suborbital fenestra than both Dibothrosuchus and Sphenosuchus, but this cannot be confirmed without direct observation of the specimen.
3.1.5. Maxilla
The palatal portion of the maxilla has three main processes: an anteriorly projecting medial process that forms the incisive foramen septum (described in Section 3.1.2); the laterally positioned tooth‐bearing portion; and the medially to posteromedially projecting palatal shelf. In all sphenosuchians where the maxilla is known, the tooth‐bearing process of the maxilla forms a loosely overlapping contact with the premaxilla. There is no evidence of a maxillary notch that separates the dentigerous portions of the maxilla and premaxilla, in contrast to the prominent maxillary notch present in most described protosuchians here (Figures 3 and 4). The posterior end of the tooth‐bearing process contacts the jugal, with the jugal overlapping the maxilla laterally. The maxillary tooth row continues along the tooth‐bearing process until the termination of the maxilla at the contact with the jugal across all sphenosuchian taxa. The palatal shelf of the maxilla in sphenosuchians is not as fully developed as later‐branching crocodylomorph members, but it does form an ossified surface that extends from the incisive foramen until the choana and forms the anterior margin of the choana. The posterior edge of the palatal shelf of the maxilla has a short medial projection that underlies the vomer in all “sphenosuchian” taxa.
FIGURE 3.
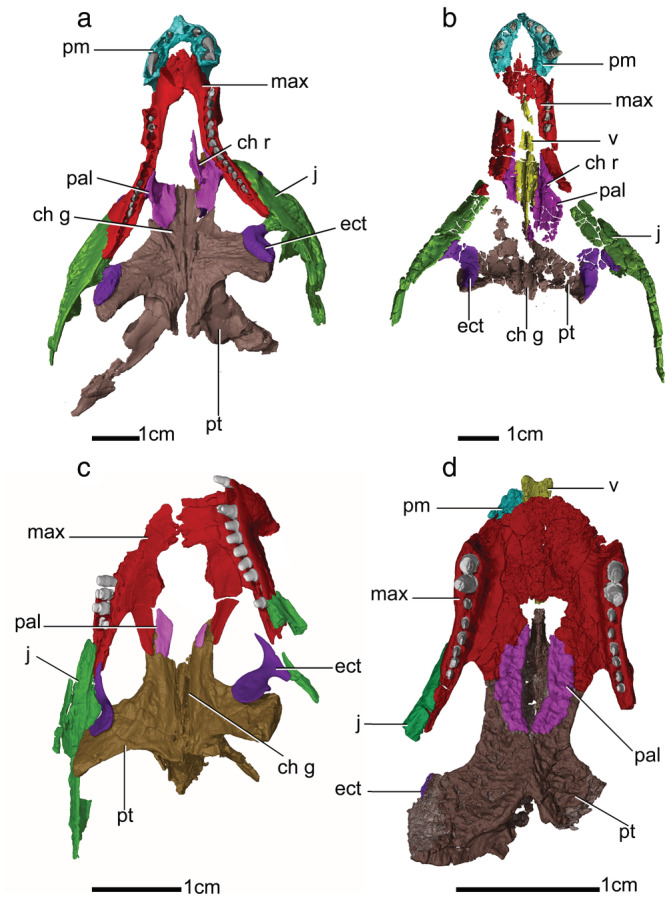
Palatal view of the three‐dimensional (3D) visualizations of the secondary palates of “protosuchians” (a) Protosuchus, (b) Orthosuchus, (c) Nominosuchus, and (d) Shartegosuchus. Ch g, choanal groove; ch r, choanal ridge; ect, ectopterygoid, j, jugal, max, maxilla, pal, palatine, pm, premaxilla, pt, pterygoid, v, vomer
FIGURE 4.
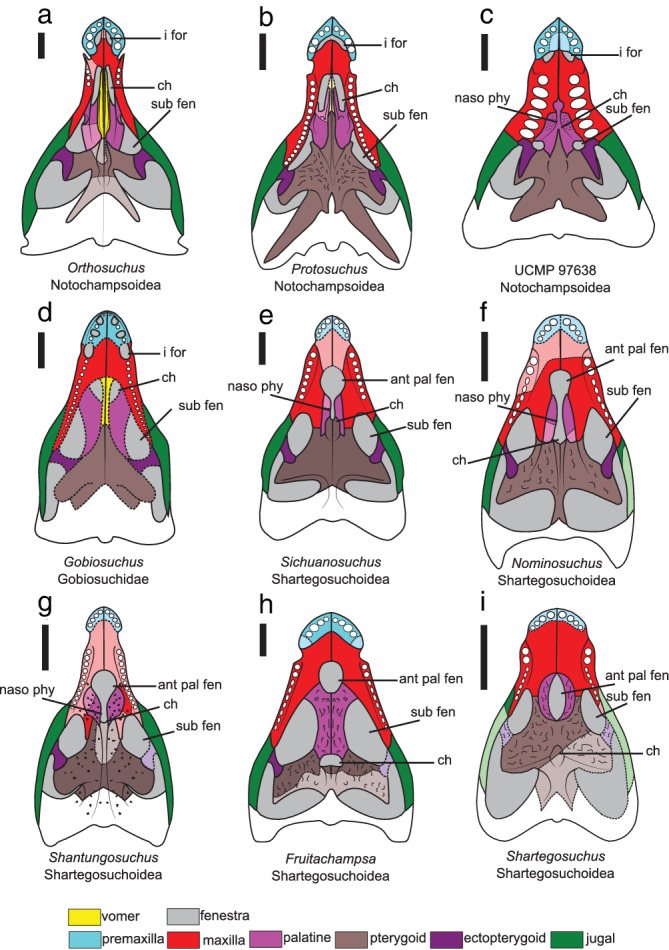
Interpreted anatomical reconstruction of the palatal view of the secondary palate “protosuchians” (a) Orthosuchus, (b) Protosuchus, (c) UCMP 97638, (d) Gobiosuchus, (e) Sichuanosuchus, (f) Nominosuchus, (g) Shantungosuchus, (h) Fruitachampsa, and (i) Shartegosuchus. Missing or incomplete bones are in a lighter shade with a dashed line. Scale bar is 1 cm. Ant pal fen, anterior palatal fenestra; ch, choana; i for, incisive foramen; naso phy, ventrally open nasopharyngeal duct; sub fen, suborbital fenestra
3.1.6. Vomer
The vomer has an anterior maxillary process and a posterior palatopterygoid process, and the overall morphology described below is consistent across all of the “sphenosuchian” taxa. The maxillary process is mediolaterally narrower than the palatopterygoid process, and both are joined by a mediolaterally narrow body of the vomer. The contact between the vomer and maxilla is best preserved in Litargosuchus, and even here the vomer is disarticulated from the maxilla. The vomer appears to overlap the posteriormost edge of the maxillary palatal process for a short distance before terminating. The palatal surface of the body of the vomer is concave, forming a hemicylindrical depression which becomes mediolaterally wider along the palatopterygoid process. The pterygoid, vomer, and palatine overlap each other along the region of the palatopterygoid process of the vomer so that the pterygoid is exposed on the palatal surface, which is then overlapped by the vomer, which is in turn overlapped by the palatine. The vomer is exposed on the palatal surface, a feature which is shared with some “protosuchians” (Orthosuchus, Protosuchus, and Gobiosuchus) but not with shartegosuchoids and most later branching crocodylomorphs.
3.1.7. Palatine
The palatine has two main processes across the “sphenosuchian” taxa: a lateral maxillary process and a mediolaterally broad process that forms the palatal shelves of the palatine. The relative size between the two main processes of the palatine differs between “sphenosuchian” taxa, with Terristrisuchus and Litargosuchus having a much smaller maxillary process relative to the palatal shelf, and with Dibothrosuchus and Sphenosuchus having processes that are closer in size relative to each other.
The maxillary process of the palatine abuts the medial surface of the maxillary body along the toothrow. The anteroposterior length of the maxillary process of Terrestrisuchus is approximately one‐half the anteroposterior length of the palatal shelf of the palatine. The anteroposterior length of the maxillary process of Litargosuchus is proportionately smaller, being approximately one‐third the anteroposterior length of the palatal shelf. Original reconstructions of the palatines of Dibothrosuchus (Wu & Chatterjee, 1993) and Sphenosuchus (Walker, 1990) illustrate the anterior edge of the maxillary process extending farther anterior than the palatal shelf, however, evidence from the CT scans suggests an alternative interpretation that the anterior edge of both the maxillary and palatal shelf end at a point level to each other on the palate.
The palatal shelf of the palatine has a short anterior ramus that contacts the vomer and a much longer posterior ramus that contacts the pterygoid. The ventral surface of the palatal shelf is shallowly concave in each taxon. In Litargosuchus and Terrestrisuchus, the palatal shelf is lenticular in the palatal view. A distinct ridge (the choanal ridge) is present along the anterior edge of the palatal shelf, bounding the posterior edge of the choana across all “sphenosuchian” taxa. The choanal ridge is most prominent in Sphenosuchus, forming a distinct lip on the palatal surface of the palatine (Figure 2c). Uniquely in Dibothrosuchus, a depression on the palatal surface on both the palatine and vomer forms a large triangular outline when the bones are paired (Figure 2a).
FIGURE 2.
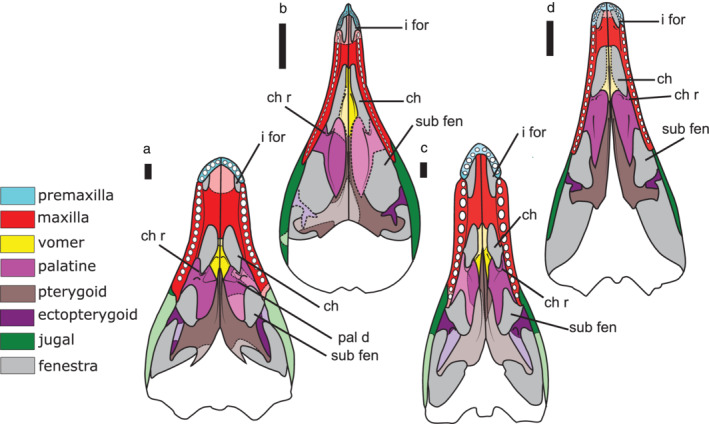
Interpreted anatomical reconstruction of the palatal view of the secondary palates of ‘sphenosuchians’ (a) Dibothrosuchus, (b) Litargosuchus, (c) Sphenosuchus, and (d) Terrestrisuchus. Missing or incomplete bones are in a lighter shade with a dashed line. Scale bar is 1 cm. Ch, choana; ch r, choanal ridge; i for, incisive foramen; pal d, palatine depression; sub fen, suborbital fenestra
3.1.8. Pterygoid
The pterygoids are unfused and have two main processes pertinent to the palate, an anterior vomerine process, and the pterygoid flange. The vomerine process of the pterygoid narrows mediolaterally as it extends anteriorly along the palate, terminating in a point in all “sphenosuchian” taxa. The vomerine process of the pterygoid is overlapped dorsolaterally by the vomer and then the palatine. There is a distinct ridge on the vomerine process along the inter‐pterygoid contact extending anteroposteriorly along the palatal midline. The ridges diverge laterally as they extend posteriorly toward the pterygoid flange and grade into the ventral surface in all taxa. In Dibothrosuchus, Sphenosuchus, and Litargosuchus, the paired pterygoids are reconstructed here from CT scan data as contacting almost along their entire length on the midline of the palate. However, in Terrestrisuchus, Crush (1984) reconstructs the pterygoids as separated by the basisphenoid rostrum.
The pterygoid flanges of “sphenosuchians” are anteroposteriorly broad and rectangular. They are positioned posteriorly on the palate, far posterior to the maxillary tooth row. This posterior separation from the maxillary tooth‐row is most prominent in Litargosuchus and Terrestrisuchus. The pterygoid flanges of all “sphenosuchian” taxa are inclined relative to the transverse plane of the palate so that the palatal surface of the pterygoid flange is facing anterolaterally. The posterolateral‐most tip of the pterygoid flange of Dibothrosuchus has a distinct posteriorly projecting lip. The pterygoid flange lip is also reconstructed from the impression of the pterygoid flange of Sphenosuchus, but it is not present in Litargosuchus or Terrestrisuchus.
3.1.9. Ectopterygoid
The ectopterygoid has a lateral process and a pterygoid process with a neck joining them. The lateral process of the ectopterygoid contacts the medial surface of the jugal in “sphenosuchians” (Figure 2), in some later‐branching taxa the lateral process contacts both the jugal and maxilla. The pterygoid process contacts the lateral edge of the pterygoid flange. The pterygoid process of the ectopterygoid of Dibothrosuchus is mediolaterally narrow and extends along almost the entire length of the lateral edge of the pterygoid flange (Figure 2a). The pterygoid processes of Litargosuchus and Terrestrisuchus are forked, with a medial projection that extends a short distance toward the midline of the palate, and a lateral projection that extends a short distance along the lateral edge of the pterygoid flange (Figure 2b,d). The neck joining the jugal and pterygoid processes of the ectopterygoid are slender. The medial surface of the neck of the ectopterygoid of Sphenosuchus has a deep fossa that faces posteromedially (Figure 1c). Pissarrachampsa (a baurusuchid) also has a fossa on the ectopterygoid, but this is positioned farther anteriorly, along the ectopterygoid/jugal contact (Montefeltro et al., 2011). The fossa is in a similar position to the fossa with a foramen described for the antorbital sinus (or potentially a separate ectopterygoid sinus, Witmer (1997)) that is observed in some theropods (Gold et al., 2013) and phytosaurs (Lautenschlager & Butler, 2016).
3.1.10. Summary of “sphenosuchian” palatal anatomy
Below, we summarize the similarities in the composition of the “sphenosuchian” palate and highlight the major points of distinction. The incisive foramen is prominent and separated by a septum formed wholly by the maxilla in all “sphenosuchian” taxa. The incisive foramina of Dibothrosuchus are the smallest and are most broadly separated relative to the other “sphenosuchians.” In all “sphenosuchian” taxa, the choana is positioned anteriorly on the palate and bounded by the maxilla, vomer, and palatine. The suborbital fenestra is large and bounded by the maxilla, palatine, pterygoid, ectopterygoid, and jugal. The maxilla presents a palatal shelf that meets the contralateral element along the midline of the palate, forming an ossified surface between the incisive foramen and choana, and there is no caniniform notch to separate the maxillary from the premaxillary toothrow. The maxillary tooth row is extensive, extending the entire length of the tooth‐bearing process of the maxilla until the contact with the jugal in all taxa. The vomer is exposed on the palatal surface, the plesiomorphic condition for Archosauria. The paired palatines have large medial palatal shelves that do not contact along the midline of the palate. The palatal shelves of Terrestrisuchus and Litargosuchus are particularly broad and lenticular in ventral view. The palatine in all taxa has a distinct ridge bounding the choana posteriorly, described here as the choanal ridge, which is most prominent in Sphenosuchus. Uniquely, Dibothrosuchus has a large, triangular depression that extends between the paired palatines and vomers. The pterygoid has a long vomerine process that extends anteriorly along the midline of the palate in all taxa. The paired pterygoids generally contact along the midline of the palate for most of their length, except in Terrestrisuchus where they are described as separated. The pterygoid flange is broad, with Dibothrosuchus and Sphenosuchus having a prominent lip at the posterolater‐most edge of the flange. The ectopterygoid has a lateral process that contacts the jugal, and a medial process that contacts the pterygoid flange in all taxa. The pterygoid flange process of the ectopterygoid either extends almost along the entire length of the lateral edge of the pterygoid flange (Dibothrosuchus and Sphenosuchus) or has a forked process (Litargosuchus and Terrestrisuchus). The lateral process of the ectopterygoid of Sphenosuchus has a deep fossa that is possibly related to the antorbital sinus system or another distinct ectopterygoid sinus (Witmer, 1997).
3.2. “Protosuchians”
3.2.1. Preservation
The palate of Protosuchus (BP/1/4770) is mostly complete, but missing the vomers entirely and the anterior region of the palatal shelf of the right palatine (Figure 3a). The palate of the Orthosuchus holotype (SAM‐PK‐K409) is highly fractured, but the fragments retain their original positions and it is almost complete except for a portion of the posterior surface of the right palatine, a portion of the medial surface of the pterygoid, and a section of the tooth‐bearing process of the maxilla (Figure 3b). The palate of UCMP 97638 (an unnamed protosuchid) is well preserved with only a small portion of the right anteromedial edge of the premaxilla missing. Osmólska et al. (1997)'s reconstruction of the palate of Gobiosuchus is based on two complete skulls. One of these skulls (ZPAL MgR‐II/67) has a complete palatal surface exposed that is only missing the left premaxilla. The palate of Sichuanosuchus (IVPP V10594) is complete and mostly observable, except for the premaxillary and anterior surface of the maxillary palatal shelf because they are covered by the occluded dentary symphyseal region. The palate of Nominosuchus (IVPP 14392) is slightly disarticulated and is missing both premaxillae, the vomers, a portion of the anterior surface of the maxillary palatal shelf, and the anterior surface of the palatines (Figure 3c). The palate of Shantungosuchus brachycephalus (IVPP V10097) is fractured, with portions of the maxillary palatal shelf and the vomerine process of the pterygoid not preserved. Additionally, the anterior region of the maxillary palatal shelf, tooth‐bearing process, and the premaxilla is obscured by the occluded mandible. The reconstruction of the palate of Fruitachampsa is based on Clark (2011)'s description of several specimens recovered from the Morrison Formation, Colorado, USA. The palate of Fruitachampsa is complete and exposed anterior to the choana, but the region around the pterygoid flanges and posterior to the choana is not preserved. The reconstruction of the palate of Shartegosuchus (IGM 200/50) is based on a partial snout that is well preserved, but missing the premaxilla, the posteriormost section of the tooth‐bearing process of the left maxilla, both ectopterygoids except for a small portion of the left pterygoid flange process, and the right pterygoid flange (Figure 3d).
3.2.2. Overview of skull and skull openings
The incisive foramen is observable in Orthosuchus, Protosuchus, and UCMP 97638 (Figure 4a–c). It is absent in Gobiosuchus. Within the shartegosuchoids, the incisive foramen is not observable either because the region is occluded by the mandibular symphysis (Sichuanosuchus and Shantungosuchus) or because the premaxillary region is poorly preserved (Nominosuchus, Fruitachampsa, and Shartegosuchus). In Orthosuchus, Protosuchus, and UCMP 97638, the incisive foramen is small and sub‐oval, with its long axis parallel to the palatal midline. The anterior margin is formed by the premaxilla and the posterior margin is formed by the maxilla. The incisive foramen septum is formed by the maxilla, as in “sphenosuchians,” which extends a short process anteriorly along the midline of the palate to contact the posterior edge of the palatal surface of the premaxilla.
3.2.3. Anterior palatal fenestra
The anterior palatal fenestra is present in the shartegosuchoids Sichuanosuchus, Nominosuchus, Shantungosuchus, Fruitachampsa, and Shartegosuchus, and in the protosuchid UCMP 97638, but it is not present in any other “protosuchian” (Figure 4). In all taxa investigated here (except potentially Shartegosuchus—see maxilla 3.2.7 and palatine 3.2.9 description), the anterior palatal fenestra is enclosed by the maxilla anteriorly and the palatine posteriorly. In shartegosuchoids, the anterior palatal fenestra is large and sub‐oval in outline. In UCMP 97638, the anterior palatal fenestra is an autapomorphically small, circular, nearly indiscernible feature. But given its position, relation to the choana, and the relative size of the suborbital fenestra, it is likely that this small circular feature is homologous to the anterior palatal fenestra (Figure 4c). The fenestra is linked to the choana through a ventrally open nasopharyngeal duct in UCMP 97638, Sichuanosuchus, Nominosuchus, and Shantungosuchus (Figure 4c,e,f). In both Fruitachampsa and Shartegosuchus, the anterior palatal fenestra is isolated from the choana by palatal shelves, but these vary in being formed by either the palatine (Fruitachampsa) or the pterygoid (Shartegosuchus) (Figure 4h,i).
3.2.4. Choana
The shape, position, and size of the choana (or choanae in taxa with paired openings) vary across “protosuchians.” In the following sections, we highlight if there are one or two openings in each taxon, but describe the anatomy of only one to simplify singular versus plural terminology.
In Orthosuchus, Protosuchus, and Gobiosuchus, the choanae are paired and the choanal septum is formed by the paired vomers. In these taxa, the choana is positioned anteriorly on the palate and is bounded anteriorly by the maxilla and the palatine posteriorly. The choanal shapes of Orthosuchus and Protosuchus are ovate in ventral view, with the long axis orientated along the sagittal axis of the palate. In comparison, the choana of Gobiosuchus is almost equal in anteroposterior and mediolateral length. UCMP 97638 has only a single choanal opening that is positioned posteriorly relative to the level of the suborbital fenestra. Additionally, the choana of UCMP 97638 opens posteriorly through a deep midline depression that extends from the maxilla and partitions the palatines.
The single choana of most shartegosuchoids is mediolaterally positioned on the midline of the palate between the suborbital fenestrae, but varies in its anteroposterior position, being either approximately at the level of their anterior margin, (Sichuanosuchus and Shantungosuchus), the level of their midpoint (Nominosuchus), or the level of their posteriormost edge (Fruitachampsa). Only the choana of Shartegosuchus is positioned posterior to the suborbital fenestra. Sichuanosuchus, Nominosuchus, and Shantungosuchus share a choana that is linked to the anterior palatal fenestra through a ventrally open nasopharyngeal duct. Personal observation of Shantungosuchus brachycephalus (IVPP V4020) shows that there is a prominent ridge that extends anteroposteriorly along the midline of the ventrally open nasopharyngeal duct from the choana until at least the level of the palatines. There is a fragment of bone positioned inside the anterior palatal fenestra of IVPP V4020, but it is unclear whether this is a fragment of the palatine or the anterior end of a choanal septum (potentially formed by the vomer). If it is the latter, it suggests that the choanal septum extends as a distinct ridge from the maxilla until the pterygoid, to a level posterior to the pterygoid flange. The anterior margins of the choanae of Sichuanosuchus, Nominosuchus, and Shantungosuchus are not clearly demarcated because of the ventrally open nasopharyngeal duct, but given the size of the depression on the pterygoid, the anterior margin of the choana is likely to level with the posteriormost edge of the palatine. The choana of Fruitachampsa is mediolaterally wider than it is anteroposteriorly long. Its anterior margin is clearly formed by the palatines and its posterior margin by the pterygoid. The choana of Shartegosuchus is oval, with its major axis orientated anteroposteriorly. The margins of the choana of Shartegosuchus are formed solely by the pterygoid.
3.2.5. Suborbital fenestra
The suborbital fenestrae of notochampsoids are smaller in the area relative to gobiosuchids and shartegosuchoids, especially those of UCMP 97638, which appear to be apomorphically reduced in comparison to all other taxa. The suborbital fenestrae of Orthosuchus and Fruitachampsa are bordered by the palatine anteriorly and anteromedially, the maxilla anterolaterally, the pterygoid posteromedially and posteriorly, and the jugal and ectopterygoid posterolaterally (similar to the condition in “sphenosuchians”). In UCMP 97638, Protosuchus, Gobiosuchus, and Nominosuchus, the ectopterygoid is positioned farther anteriorly and it contacts the medial surface of the maxilla, which excludes the jugal from the margins of the suborbital fenestra. A unique feature of most shartegosuchoids, except Fruitachampsa, is that the palatines are excluded from the margin of the suborbital fenestra by a contact between the maxilla anteriorly and pterygoid posteriorly.
3.2.6. Premaxilla
The premaxilla of most early‐diverging crocodyliforms contributes a smaller portion to the secondary palate than in the later‐branching members of Mesoeucrocodylia. The condition is not well known in Nominosuchus, Sichuanosuchus, and Shartegosuchus because the palatal surface of the premaxilla is either not preserved, or is concealed by the dentary symphyseal region (and CT scan data are not available). The palatal surfaces of the premaxillae in Orthosuchus, Protosuchus, Shantungosuchus, and Fruitachampsa are separated along the midline of the palate posterior to the tooth‐bearing region. Only the paired premaxillae of UCMP 97638 and Gobiosuchus possess palatal shelves that meet along the midline of the palate and form a portion of a secondary palate. Gobiosuchus is unique among the early branching crocodyliforms in that the premaxilla has two smaller foramina followed by a much larger foramen that receives an enlarged dentary caniniform (Figure 4d). These openings are positioned in line with the tooth row on the palatal surface, with the first smaller foramen positioned posterior to the first premaxillary tooth, the second smaller foramen posterior to the second premaxillary tooth, and the largest foramen posterior to the third premaxillary tooth and positioned between the premaxilla—maxilla contact.
3.2.7. Maxilla
The palatal surface of the maxilla has two major processes, a lateral tooth‐bearing process and a medial process that forms the palatal shelves of the maxilla. Shartegosuchoids (excluding Fruitachampsa) have an additional maxillary process that projects posteromedially to contact the pterygoid.
The tooth‐bearing process among some “protosuchians” contacts the jugal (Orthosuchus, Sichuanosuchus, Shantungosuchus, Figure 4a,e,g). In other “protosuchians” the tooth‐bearing process of the maxilla contacts the jugal and ectopterygoid. The maxillary tooth row is separated from the premaxillary tooth row by a maxillary notch (in all taxa except Gobiosuchus) or by a caniniform foramen (Gobiosuchus). Unlike “sphenosuchians,” the number, shape, and size of the maxillary teeth vary among taxa. Orthosuchus has only three maxillary teeth whereas Protosuchus, UCMP 97638, and Gobiosuchus have a tooth row that extends posteriorly from the maxillary notch until the termination of the tooth‐bearing process at the maxillary–jugal contact. The remaining “protosuchian” taxa have a tooth row that extends almost along the entire length of the tooth‐bearing process of the maxilla, terminating just before the maxillary–jugal contact.
The medial contact between the palatal shelves of the maxilla of the notochampsoids Protosuchus and Orthosuchus and the shartegosuchoid Fruitachampsa terminates posteriorly at a level anterior to the first maxillary tooth. The contact between palatal shelves of UCMP 97638 terminates at a level with the second maxillary tooth and in Shantungosuchus it ends at a level between the fifth and sixth maxillary tooth. It is unclear where the medial contact between the maxillary palatal shelves of Shantungosuchus, Nominosuchus, and Shartegosuchus ends because in Shantungosuchus, that region is covered by the mandibular symphysis, in Nominosuchus the anterior portion of the maxillary palatal surface has not preserved, and in Shartegosuchus there is a breakage along the posteromedial portion of the maxillary palatal shelves. However, in Shantungosuchus brachycephalus (IVPP V4020), the medial contact between the palatal shelves of the maxilla extends until between the fifth and sixth maxillary tooth, in Nominosuchus the contact extends until between the fourth and fifth preserved maxillary tooth, and in Shartegosuchus the contact is preserved until between the second and third maxillary tooth.
The pterygoid process of the maxilla is either a distinct process from the palatal shelf, being separated by the anterior palatal fenestra (Sichuanosuchus, Nominosuchus, Shantungosuchus, Figure 4e,g), or confluent with the shelf (Shartegosuchus, Figure 4i). Only Shantungosuchus hangjinensis (Figure 4g) has notable sculpturing on the palatal surface of the maxilla, which is depicted by Wu et al. (1994) as taking the form of large circular pits that lack interconnecting ridges and grooves. The closely related species S. brachycephalus (IVPP V4020) is devoid of any palatal sculpturing.
3.2.8. Vomer
Only the vomer of Orthosuchus, Shartegosuchus, and Gobiosuchus (Osmólska et al., 1997) are observable. The vomers of Orthosuchus are paired. They are anteroposteriorly long and mediolaterally narrow and exposed on the palatal surface. The vomer is u‐shaped in transverse cross‐section, as in “sphenosuchians,” forming a hemicylinder that extends between maxilla, palatine, and pterygoid. The CT scan data of Orthosuchus shows that the contact between the vomer, palatine, and pterygoid resembles the “sphenosuchian” condition, with the pterygoid overlapped by the vomer, which is in turn overlapped by the palatine.
The vomer of Orthosuchus extends much farther posteriorly along the pterygoid than in other “protosuchians,” terminating at a level between the midpoint of the suborbital fenestra. The vomer of Gobiosuchus resembles Orthosuchus in that it is exposed on the palatal surface and forms the choanal septum. The vomer of Shartegosuchus is only partially preserved on the posteromedial surface of the maxillary palatal shelves. Given the anterior extension of the pterygoid over the anterior palatal fenestra, it is likely that the posterior edge of the vomer would have contacted the pterygoid (as in other “protosuchians”), but would not have been exposed on the ventral surface of the palate.
3.2.9. Palatine
In Protosuchus and Orthosuchus, the palatine has a lateral maxillary process and a palatal shelf that contacts the vomer anteromedially and the pterygoid posteromedially (as described in section 3.1.7 “sphenosuchians”). The maxillary process forms a strong, buttressing contact with the medial surface of the tooth‐bearing portion of the maxilla in the Orthosuchus, Protosuchus, UCMP 97638, and Gobiosuchus. In shartegosuchoids, Sichuanosuchus, Nominosuchus, and Shantungosuchus, the palatines loosely underlap the maxillary palatal shelves.
The surface of the palatal shelf of Protosuchus that borders the choana laterally and medially has a distinct choanal ridge (Figure 3a). Whereas in Orthosuchus, the choanal ridge on the palatine borders the choana laterally (Figure 3b), but both clearly show the posterior passage of the air passage. The surface of the palatal shelf posterior to the choana of Orthosuchus is strongly dorsally concave (Figure 3b).
The palatine of UCMP 97638 has a small palatal shelf that does not connect along the midline of the palate. It appears that in UCMP 97638, the palatine curves dorsomedially above the nasopharyngeal duct to form a roof over the choanal region (Figure 4c), a feature not shared with any other “protosuchian” or “sphenosuchian,” to the best of our knowledge.
The palatine palatal shelves of Gobiosuchus do not contact along the midline of the palate and are separated by the pterygoid and vomer (Figure 4d). The palatal shelf of Gobiosuchus is obliquely inclined toward the contact with the pterygoids, so that posterior to choana, along the margins of the suborbital fenestra, the palatal surface is vaulted dorsally.
The palatine of most shartegosuchoids, excluding Fruitachampsa, is very small in size in comparison to other bones of the palate. The palatines of Sichuanosuchus, Nominosuchus, and Shantungosuchus loosely underlap the maxilla and the pterygoid. In Shartegosuchus, the contact between the palatine and maxilla/pterygoid is less distinguishable in cross‐section because the bones are firmly sutured together. The palatines of Sichuanosuchus, Nominosuchus, and Shantungosuchus contribute to the posteromedial margin of the anterior palatal fenestra, the lateral margin of the ventrally open nasopharyngeal duct, and the anterior margin of the choana. The palatine of Fruitachampsa forms the posteromedial margin of the anterior palatal fenestra and the anterior margin of the choana (a ventrally open nasopharyngeal duct is not present). In only Shartegosuchus, the palatine forms the lateral margins of the anterior palatal fenestra.
The palatines of most shartegosuchoids do not contact along the midline of the palate. They are either separated by a ventrally open nasopharyngeal duct (i.e., Sichuanosuchus, Nominosuchus, Shantungosuchus), or they are separated by the anterior palatal fenestra (i.e., Shartegosuchus). However, in Fruitachampsa the palatal shelves are mediolaterally broad and meet along the midline of the palate, contacting the maxilla anteriorly and the pterygoid posteriorly.
The palatal surface of the palatines of UCMP 97638, Shantungosuchus hangjinensis, Fruitachampsa, and Shartegosuchus are sculptured, while Shantungosuchus brachycephalus lacks palatal sculpturing. In UCMP 97638 and Shantungosuchus, the sculpturing consists of small circular, isolated pits that are evenly distributed over the palatal surface. The sculpturing on the palatine of Fruitachampsa and Shartegosuchus consists of ridges and deep crevasses.
3.2.10. Pterygoid
The midline suture between the paired pterygoids is not easily distinguishable in cross‐section, and together with the paired pterygoids present as a single, fused structure. The pterygoid has an anterior vomerine process, a lateral pterygoid flange, and a posterolateral quadrate process (which does not contribute to the secondary palate). The vomerine process of the pterygoid is only visible in Orthosuchus and Shartegosuchus. In Orthosuchus, the vomerine process of the pterygoid extends along the dorsal surface of the vomer until the choanal opening. In Shartegosuchus, the vomerine process of the pterygoid extends over the anterior palatal fenestra toward the maxilla and vomer (Dollman et al., 2018). In shartegosuchoids, the vomerine process of the pterygoid contacts the maxillary palatal shelf.
The pterygoid flange is at an oblique angle to the body of the pterygoid so that the palatal surface faces anteroventrally. The position of the pterygoid flange relative to the maxillary tooth row differs among the taxa. The pterygoid flanges of Protosuchus, UCMP 97638, and Gobiosuchus are at a level immediately posterior to the distal‐most tooth of the maxillary row. The pterygoid flanges of Orthosuchus, Nominosuchus, Sichuanosuchus, Shantungosuchus, Fruitachampsa, and Shartegosuchus are positioned posterior to the distal‐most tooth of the maxillary row.
Sculpturing is present on the pterygoid palatal surface of Protosuchus, Nominosuchus, Shantungosuchus hangjinensis (but not Shantungosuchus brachycephalus), Fruitachampsa, and Shartegosuchus. The sculpturing on the pterygoid of Protosuchus, Nominosuchus, Fruitachampsa, and Shartegosuchus consists of ridges and crevasses, which are most prominent and extensive in Fruitachampsa and Shartegosuchus. The sculpturing on the palatal surface of the pterygoid of Shantungosuchus is reconstructed by Wu et al. (1994) as small, individual, evenly distributed pits.
Across “protosuchians” there is a choanal groove that extends posteriorly from the choana along the midline of the paired pterygoids (Figure 3). In Gobiosuchus, Osmólska et al. (1997) describes a “longitudinal trough” on the vomerine process that ends anterior to the level of the suborbital fenestra. In the other “protosuchians,” a distinct ridge is present along the midline of the trough, extending along the length of the palatal surface of the pterygoid (this feature is not described or illustrated in Gobiosuchus).
3.2.11. Ectopterygoid
The ectopterygoid has a lateral process and a medial pterygoid flange process that are connected by a narrow neck. The pterygoid flange process contacts the anterolateral edge of the pterygoid flange. The pterygoid process in notochampsoids extends almost along the entire lateral edge of the pterygoid flange (Figures 3a,b and 4a–c), resembling Sphenosuchus and Dibothrosuchus. In the other “protosuchians,” the pterygoid process only contacts the anterolateral‐most edge of the pterygoid flange, with a short, and ovate suture (Figures 3b and 4d–i).
The lateral process of the ectopterygoid extends anterolaterally to contact either the jugal only or the maxilla and jugal (as in eusuchians). The ectopterygoid of Orthosuchus, Sichuanosuchus, and Shantungosuchus contacts the jugal. The ectopterygoid of Protosuchus, UCMP 97638, Gobiosuchus, Nominosuchus, and Fruitachampsa contact both the maxilla and jugal. This process is not preserved in Shartegosuchus.
3.2.12. Summary of “protosuchian” palatal anatomy
The palatal construction between “protosuchian” taxa differs substantially, and below we highlight the most salient observations. Some “protosuchians” have an additional opening of the palate, the anterior palatal fenestra, that is either linked to the choana through a ventrally open nasopharyngeal duct (UCMP 97638, Sichuanosuchus, Nominosuchus, Shantungosuchus) or is separated from the choana (Fruitachampsa and Shartegosuchus). The choanae are positioned anterior to the suborbital fenestra (Orthosuchus, Protosuchus, Gobiosuchus), or between the medial point of the suborbital fenestra (Sichuanosuchus, Nominosuchus, Shantungosuchus, UCMP 97638), or closer to the posterior end of the palate and posterior to or at a level with the posterior end of the suborbital fenestra (Fruitachampsa and Shartegosuchus). The suborbital fenestrae also differ among taxa, for example, the fenestrae of UCMP 97638 are very small. The suborbital fenestrae are bounded by different palatal bones across the “protosuchian” taxa. In Orthosuchus, the suborbital fenestra is bounded by the maxilla, palatine, pterygoid, ectopterygoid, and jugal. In Protosuchus, UCMP 97638 and Fruitachampsa, the jugal does not participate in its borders. The palatine is excluded from the borders of the suborbital fenestra in Sichuanosuchus, Shantungosuchus, Nominosuchus, and Shartegosuchus. The palate of Gobiosuchus is reconstructed with the pterygoid excluded from the boundary of the suborbital fenestra.
Only the premaxillae of UCMP 97638 and Gobiosuchus have palatal shelves that contribute to the secondary palate. “Protosuchians” have maxillary palatal shelves that meet along the midline of the palate. Some “protosuchians” have a pterygoid process of the maxillary palate which projects toward the pterygoid posterior to the anterior palatal fenestra. Only the vomers of Orthosuchus, Protosuchus, and Gobiosuchus among the “protosuchians” are exposed on the palatal surface. The palatines of Orthosuchus, Protosuchus, and Gobiosuchus have palatal shelves but they do not meet along the midline of the palate because they are separated by the vomer and pterygoid. The palatine palatal shelves of UCMP 97638, Sichuanosuchus, Nominosuchus, Shantungosuchus, and Shartegosuchus are small and do not meet along the midline of the palate because they are separated by a ventrally open nasopharyngeal duct and the anterior palatal fenestra. Fruitachampsa has large palatine palatal shelves that meet along the midline of the palate.
The position of the pterygoid flange relative to the maxillary tooth row differs among the taxa. Some taxa have a pterygoid flange that is positioned further anteriorly and closer in proximity to the maxillary toothrow (Protosuchus, UCMP 97638, Gobiosuchus), and in other taxa, the pterygoid flange is positioned further posteriorly away from the maxillary toothrow (Orthosuchus, Nominosuchus, Sichuanosuchus, Shantungosuchus, Fruitachampsa, and Shartegosuchus). In some notochampsoids, the pterygoid flange process of the ectopterygoid extends almost along the entire lateral edge of the pterygoid flange (Orthosuchus, Protosuchus, UCMP 97638), whereas in the other “protosuchian” taxa it contacts only the anterolateral‐most edge. The lateral process of the ectopterygoid contacts either only the jugal (Orthosuchus, Sichuanosuchus, Shantungosuchus), or the maxilla and jugal (Protosuchus, UCMP 97638, Gobiosuchus, Nominosuchus, and Fruitachampsa). Palatal sculpturing is observed in some of the “protosuchians,” including Protosuchus, Nominosuchus, Shantungosuchus, Fruitachampsa, and Shartegosuchus.
3.3. Character description
Here, we reinvestigate and modify the existing characters that describe phenotypic changes in palatal structures, and we add eight new and review nine palatal characters derived from our study of “sphenosuchian” and “protosuchian” anatomy. All these characters are detailed below and presented with detailed character descriptions.
Character 9: Anterior palatal fenestra: (0) absent; (1) present. Modified from Wu et al. (1997) character 128.
The anterior palatal fenestra is the opening between the oral and nasal cavities that is positioned anteriorly on the palate with respect to the choana, and is either linked to the choana through a ventrally open nasopharyngeal duct or is separated from the choana by a bony bridge formed by either the palatine or pterygoid (Figure 4). The anterior palatal fenestra is a feature that is commonly present within shartegosuchoids and also known within the protosuchid UCMP 97638. It is absent in Junggarsuchus, suggesting that it first appeared in Crocodyliformes. An anterior palatal fenestra has also been observed in other later branching crocodylomorphs, such as Notosuchus (MPCA‐Pv‐237).
Character 11: Medial palatal contact between the palatines in the region medial to the suborbital fenestrae: (0) forms a smooth, not reinforced palatal suture; (1) forms a rugose, reinforced palatal suture. Modified from Montefeltro et al. (2011) character 42.
The medial sutures between the palatines of all crocodylomorph taxa with secondary palates are interdigitated, but in general, the suture is level with the surface of the palate. In Shartegosuchus and in nearly all baurusuchids (Darlim et al., 2021) where this region is preserved (excluding Cynodontosuchus rothi and Gondwanasuchus scabrosus), the suture is strongly interdigitated and thickened so that it forms a raised ridge that extends along the entire length of the contact (Figure 3d). This character is amended here from its original description to clarify the palatal suture is reinforced.
Character 48: Palatal shelves of the premaxilla: (0) do not meet posteriorly to the incisive foramen; (1) meet posterior to the incisive foramen, forming a continuous surface with the palatal shelves of the maxilla. Modified from Clark (1994) character 7.
In “sphenosuchians” and most “protosuchians” (excluding UCMP 97638 and Gobiosuchus), the premaxillae lack palatal shelves and only contact along their symphysis. The premaxilla of mesoeucrocodylians has palatal shelves that meet along the midline of the palate and contact the maxillary palatal shelves posteriorly and together form a broad ossified secondary palate. (Figure 4). This character description was edited from the previous usage (in, for example, Clark, 1994; Pol et al., 2013) “palatal parts of the premaxilla” to “the palatal shelves of the premaxilla” to maintain consistency in palatal anatomical nomenclature throughout the text. Also, the description of state 1 was edited to clarify the contact between the palatal shelves of the premaxilla and maxilla. The presence of premaxillary palatal shelves is synapomorphic for mesoeucrocodylians but lost independently in Baurusuchus.
Character 78: Maxillary palatal shelves: (0) contact on the midline of the palate for a short distance and do not form a broad ossified secondary palate surface; (1) meet on the midline of the palate to form an anteroposteriorly extensive ossified secondary palate. Modified from Pol et al. (2013) character 10 and Clark (1994) character 10.
The maxillae in “sphenosuchians” and some “protosuchians” only meet along the midline of the palate for a short distance, generally extending posteriorly to the level of the first few maxillary teeth. In these taxa, the contact between the palatal shelves of the maxillae is interrupted by an anteriorly positioned choana or an anterior palatal fenestra. In later‐branching crocodyliformes, the contact between the palatal shelves of the maxilla is more extensive and continues from the premaxilla/maxilla contact until the maxilla/palatine contact. In this phylogenetic analysis, an ossified secondary palate is independently known in only a few “protosuchians,” including UCMP 97638, Gobiosuchus, and Shartegosuchus. An ossified secondary palate is then synapomorphic for a Mesoeucrocodylia that includes Hsisosuchus (if Hsisosuchus is considered to be included in Mesoeucrocodylia).
Character 89: Rugose surface on palatal shelves of maxilla posterior to the level of the posteriormost maxillary tooth: (0) absent; (1) present. Modified from Pol and Powell (2011) character 291.
In most crocodylomorphs, the palatal shelves of the maxilla lack any sculpturing. In Shartegosuchus, Fruitachampsa, and Baurusuchus, the palatal shelves of the maxilla are thickened and bear sculpturing (Figures 3 and 4). This feature is a synapomorphy of the clade Fruitachampsa + Shartegosuchus and appears independently in Baurusuchus.
Character 97 (new, ordered): Anterior edge (s) of the choana(e) is (are): (0) situated between the suborbital fenestrae (or anterior to it); (1) situated near the posterior edge of the suborbital fenestra; (2) posterior to the level of the posterior margin of the suborbital fenestrae (reaching in some cases the edge of the pterygoid flange).
Relating the position of the choana to the suborbital fenestra is a solution to clarifying the position of the choana along the palate when palatal construction is so variable. Although the size of the suborbital fenestra can vary greatly across taxa (e.g., UCMP 97638 compared to Litargosuchus), it does not greatly influence the ability to identify the choana as more anterior or more posterior between taxa (Figures 2 and 4). The plesiomorphic condition for crocodylomorphs is an anteriorly positioned choana, well anterior to the position of the suborbital fenestrae. In Zosuchus, notosuchians, thalattosuchians, and goniopholids, the choana is more posteriorly positioned, near the posterior edge of the suborbital fenestra. Fruitachampsa and Shartegosuchus, together with Baurusuchus and the crown clade (Gavialis, Alligator, and Crocodylus) have a choana positioned posterior to the suborbital fenestra. The choana positioned near the posterior edge of the suborbital fenestra optimizes as convergently acquired in Zosuchus and mesoeucrocodylians. A choana positioned posterior to the level of the posterior margin of the suborbital fenestra optimizes as convergently acquired in later branching shartegosuchoids, Baurusuchus, and the crown clade. The character‐state transitions of the choana from an anterior to a posterior position are ordered because the choana must pass through a position closer to the anterior edge of the suborbital fenestra before it is positioned more posteriorly.
Character 306 (ordered): Palatines: (0) do not meet medially on palate ventral to the narial passage; (1) meet medially along the sagittal midline of the palate; (2) meet ventral to the narial passage, forming palatal shelves that form part of the secondary palate. Modified from Pol et al. (2013) character 37 and Clark (1994) character 37.
This character is edited here to capture the transition of the palatine from lacking conjoined palatal shelves to possessing broad palatal shelves that contact along the midline of the palate. The plesiomorphic condition for this character in Crocodylomorpha is palatines that do not meet along the midline of the palate and do not contribute to an ossified secondary palate. The intermediate condition is the palatines that do meet along the midline of the palate but do not form broad palatal shelves, as in Hsisosuchus (Gao, 2001). A palatine that forms broad palatal shelves that meet along the midline and contribute to an ossified secondary palate is a synapomorphy of mesoeucrocodylians. The optimization of this character on the MPTs shows clearly that palatine palatal shelves evolve independently in Zosuchus, Fruitachampsa, and mesoeucrocodylians.
Character 492: Anterior palatal fenestra (0) linked to choana through a ventrally open nasopharyngeal duct; (1) or anterior palatal fenestra and choana separated. Modified from Dollman et al. (2018) character 262.
An anterior palatal fenestra is only known in UCMP 97638, Sichuanosuchus, Nominosuchus, Shantungosuchus, Shartegosuchus, Fruitachampsa, and Notosuchus among taxa sampled for this analysis. Of these taxa, only Shartegosuchus, Fruitachampsa, and Notosuchus have an anterior palatal fenestra that is fully separated from the choana (Figure 4). In other taxa, the choana is either positioned anteriorly on the palate in the position of the anterior palatal fenestra (the plesiomorphic condition of crocodylomorphs), or the maxilla and palatine have palatal shelves that meet along the midline of the palate forming an ossified secondary palate that separates the oral from the nasal cavity. This character was separated into two characters to independently homologize the position of the choana and whether the anterior palatal fenestra and choana are conjoined.
Character 502 (new, ordered): The anterior margin of the choanal opening is formed by the: (0) maxilla; (1) palatine; (2) pterygoid.
This character captures variation in the construction of the margins of the choana, which is potentially independent from the position of the choana on the palate (e.g., character 97, Figure 4). The plesiomorphic condition is the maxilla forming the anterior margin of the choana. Among some “protosuchians,” the palatines form the anterior margin of the choana (UCMP 97638 and Fruitachampsa). The palatines forming the anterior margin of the choana are a synapomorphy of mesoeucrocodylians. Among “sphenosuchians,” only Shartegosuchus has a pterygoid that forms the anterior margin of the choana. Outside of Shartegosuchus, this state is a synapomorphy of Eusuchia. The choana must pass through the intermediate state of the palatine forming the anterior margin of the opening before it can transition into the derived condition of the pterygoid forming the anterior edge, so we consider this character to be ordered. The palatine forming the anterior margin of the choana optimizes as evolving independently within protosuchids, shartegosuchoids, and mesoeucrocodylians.
Character 503 (new): The posterior margin of the choanal opening is formed by the: (0) maxilla; (1) pterygoid.
Plesiomorphically, the maxilla forms the posterior margin of the choana and is potentially independent of the position of the choana (Figures 2 and 4). UCMP 97638, shartegosuchoids, and mesoeucrocodylians have a pterygoid that forms the posterior margin of the choana. State 1 is reconstructed as evolving once in UCMP 97638 and then again at the node that includes shartegosuchoids and Mesoeucrocodylia.
Character 504 (new): Choanal groove: (0) absent; (1) present.
A choanal groove is a depression in the bone immediately anterior or posterior to the choanal opening. The choanal groove is a depression that is associated with the soft tissues of the nasal passage as it passes between the nasal cavity and throat (Figure 4). Prior analysis has not clearly documented the presence of this feature within the crocodylomorph tree, and so we included it in this analysis to investigate how this feature varies with other palatal structures, that is, the position of the choana. The presence of a choanal groove is homoplastically distributed throughout the tree. The presence of a choanal groove is traced as originating at the node that includes Junggarsuchus and all later‐branching crocodylomorphs, but this optimization is uncertain given the lack of data of the choanal region throughout this part of the tree.
Character 505 (new): Choanal groove present and positioned: (0) anterior to the choana; (1) posterior to the choana.
This character is developed to test the relationship between the position of the choanal groove and the choana (Figure 4).
Character 506 (new): choanal septum: (0) present; (1) absent.
The choanal septum is a slender sheet of bone that partitions the paired nasal passages as they pass through the nasal cavity into the throat via the choana. The choanal septum is reconstructed as synapomorphic for Crocodylomorpha but is independently lost within UCMP 97638, shartegosuchoids, and Gavialis.
Character 507 (ordered): Sculpturing on the palatine is: (0) absent; (1) small pitting on the palatines; (2) large pitting and ridges on the palatines. Modified from Clark (2011) and Dollman et al. (2018).
The term “sculpturing” describes structures such as ridges, pitting, and grooves that forms on the surface of the bone, in this case referring to the palatal surface of the palatal bones (Figures 3 and 4). Clark (2011) originally created a character to code the presence or absence of sculpturing generally on the palate, this was later refined by Dollman et al. (2018) to refer to the palatal bones that are sculptured (the pterygoid and palatines). In this revision of the character, it is broken down into two separate characters to assess their independent occurrences. This character is ordered because it is assumed that in order to progress from an unsculptured condition to a heavily sculptured condition, the taxa must first transition through an intermediate state of small pitting with shallow or no grooves. Only UCMP 97638, Shantungosuchus, Shartegosuchus, and Fruitachampsa are known to have sculpturing on the palatines. UCMP 97638 and Shantungosuchus have small pitting on the palatines whereas Shartegosuchus and Fruitachampsa have large pitting and ridges on the palatines. On our MPTs, lack of sculpturing optimizes as the plesiomorphic condition. Sculpturing on the palatine is reconstructed as evolving independently in UCMP 97638 and again in the later‐branching shartegosuchoid clade (Shantungosuchus + Fruitachampsa + Shartegosuchus).
Character 508 (ordered): Sculpturing on the pterygoid is: (0) absent; (1) small pitting on the pterygoid; (2) large pitting and ridges on the pterygoid. Modified from Clark (2011) and Dollman et al. (2018).
This is the second character (see character 507) testing the presence of sculpturing on the palate in Crocodylomorpha (Figure 4). Similar to character 507, it is assumed that the taxa must transition through a state of small pitting with shallow or no grooves before developing large pitting and ridges on the pterygoid. Only Protosuchus haughtoni, Shantungosuchus, Shartegosuchus, and Fruitachampsa are known to have sculpturing on the pterygoid, and all of them have large pits and ridges. The plesiomorphic condition is optimized as no sculpturing present on the pterygoid. Sculpturing on the pterygoid is reconstructed as evolving independently within Protosuchus and again in later branching shartegosuchoids (Shantungosuchus + Fruitachampsa + Shartegosuchus). UCMP 97638 has sculpturing of the palatines, but no sculpturing on the pterygoid. Nominosuchus and Protosuchus have sculptured on the pterygoid, but none on the palatine. Shantungosuchus, Fruitachampsa, and Shartegosuchus have sculptured on both the pterygoid and palatine. These observations indicate that there is no discernible pattern between which bones are sculptured.
Character 509 (new, ordered): Anterior margin of the suborbital fenestra is formed by the: (0) palatine; (1) palatine and maxilla; (2) maxilla.
In this analysis, the plesiomorphic condition is optimized as the palatine forming the anterior margin of the suborbital fenestra (Figure 2). This character is ordered because the taxa must pass through a state where both the maxilla and palatine contribute to the anterior margin before the palatal shelves of the maxilla have extended enough posteromedially, and the palatal shelves of the palatine have reduced enough medially so that the maxilla contributes solely to the anterior margin of the suborbital fenestra (Figure 4). Only shartegosuchoids have an anterior margin of the suborbital fenestra that is formed solely by the maxilla, excluding Fruitachampsa. The anterior margin of the suborbital fenestra of Fruitachampsa and mesoeucrocodylians is formed by both the palatine and maxilla.
Character 510 (new, ordered): The lateral margin of the suborbital fenestra is formed by the: (0) jugal and maxilla; (1) ectopterygoid and maxilla.
In this analysis, the plesiomorphic condition is optimized as the jugal and maxilla form the lateral margin of the suborbital fenestra (Figure 2). In Gobiosuchus, shartegosuchoids (excluding Sichuanosuchus) and in mesoeucrocodylians, the lateral margin of the suborbital fenestra is formed by both the ectopterygoid and maxilla (Figure 4).
3.4. Phylogenetic results
The analysis found six most parsimonious tree (MPTs), length = 1,263, consistency index (CI) = 0.446, and retention index (RI) = 0.654 (strict consensus is shown in Figure 5). “Sphenosuchia” is paraphyletic with some of its taxa more closely related to Crocodyliformes than others. As found by Leardi et al. (2017), “Sphenosuchia” includes one monophyletic group, Hallopodidae, which consists of Macelognathus and Almadasuchus and is the sister group to Crocodyliformes. A synapomorphy of Hallopodidae is the axis of the femoral head and the axis of the condyles that join the fibular and medial condyles at the distal end of the femur are parallel to each other (character 442, state 1). Other features that support Hallopodidae in this analysis include the quadrate, otoccipital, and squamosal enclose the cranioquadrate passage near the lateral edge of the skull (character 175, state 1), which hallopodids share with thalattosuchians. Hallopodids also share a prominent medially directed femoral head (character 441, state 2), which is also observed in other “sphenosuchians” Terrestrisuchus and Hesperosuchus. Additionally, hallopodids share a lesser trochanter that is observed as a long ridge on the anterolateral surface of the proximal end of the femur (character 442, state 0); and the presence of basioccipital recesses that are observed as a deep depression on the ventral surface of the basioccipital (character 514, state 1). Both are observed more broadly throughout the crocodylomorph lineage, the former characteristic is present also in Kayentasuchus and the notosuchians Baurusuchus and Notosuchus. The latter is present broadly among earlier branching “sphenosuchians,” including Litargosuchus, Terrestrisuchus, Hesperosuchus, and Sphenosuchus.
FIGURE 5.
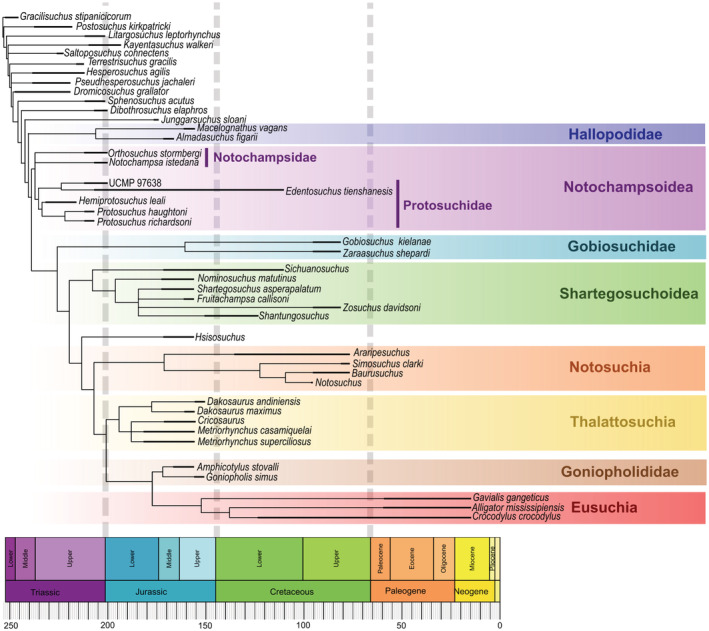
Time calibrated strict consensus of the six MPTs (length = 1,263, CI = 0.446, and RI = 0.654)
“Protosuchia” includes monophyletic groups Notochampsoidea, Gobiosuchidae, and Shartegosuchoidea. The interrelationships found among these groups are generally consistent with other prior analyses (Clark, 2011; Dollman et al., 2018, 2021; Osmólska et al., 1997) but are summarized below. Within Notochampsoidea in four of the six MPTs, Notochampsidae is a sister group to Protosuchidae. In the remaining two MPTs, the relationship of Notochampsa and Orthosuchus with Protosuchidae is unresolved. The monophyly of Notochampsoidea is supported by the following synapomorphies: the postorbital process of the jugal is positioned anteriorly to the postorbital bar of the postorbital (character 116, state 0), and the transverse process of the posterior dorsal vertebrae are dorsoventrally high (character 466, state 1). The former characteristic is not observed in notochampsoid Edentosuchus, and the latter characteristic is only observed in notochampsoids Orthosuchus and Protosuchus.
Several features support Gobiosuchidae, many of which are synapomorphies of the group. Gobiosuchidae includes Gobiosuchus and Zaraasuchus, and is the sister‐group to Shartegosuchoidea (Figure 5). These include three curved ridges on the dorsal surface of the posterolateral region of the squamosal (character 269, state 1); a lack of a supratemporal fenestra (character 270, state 1); palpebral that are sutured to each other and the frontal (character 364, state 1); the dorsal surface of the osteoderms are ornamented with anterolaterally and anteromedially directed ridges (character 374, state 1); the cervical region has lateral and ventral osteoderms which are sutured to the dorsal elements (character 375, state 1); and the presence of appendicular osteoderms (character 375, state 1).
Shartegosuchoidea is monophyletic in our analysis and includes Sichuanosuchus, Shantungosuchus, Nominosuchus, Shartegosuchus, Fruitachampsa, and Zosuchus. Shartegosuchoidea is the sister group to Hsisosuchus and Mesoeucrocodylia (Figure 5). Features that support Shartegosuchoidea in this analysis include a choana that opens through a choanal groove (character 93, state 1), but this feature is also known in Goniopholis, Edentosuchus, and UCMP97638. Additionally, the palatines of shartegosuchoids are excluded from the margins of the suborbital fenestrae (character 309, state 1), but this is not observed in Fruitachampsa and Zosuchus; and the absence of a choanal septum (character 506, state 0), but this condition is also observed in UCMP 97638 and Gavialis.
The relationship of Zosuchus with other shartegosuchoids remains largely unresolved. In a separate analysis, a posteriori exclusion of Zosuchus shows that its uncertain position obscures resolvable relationships within Shartegosuchoidea. This analysis found two MPTs, length = 1,234, CI = 0.456, and RI = 0.668 (see Supplementary Information for MPTs). With Zosuchus excluded a posteriori, Shartegosuchoidea is supported additionally by the palatine forming the anterior margin of the choana (character 502, state 1), which is not observed in Shartegosuchus and is a feature broadly present among mesoeucrocodylians. The result from this analysis shows that Shantungosuchus is included in Shartegosuchidae, as it is more closely related to Shartegosuchus than it is to Sichuanosuchus. This is due to the presence of sculpturing on the palate of Shantungosuchus hangjinensis (character 507, state 2 and character 508, state 2). This association with Shartegosuchidae should invite new investigations of this genus, given the presence of palatal sculpturing in Shantungosuchus hangjinensis and the apparent lack thereof in closely related species Shantungosuchus brachycephalus.
4. DISCUSSION
4.1. Palatal construction and evolution of an ossified secondary palate
Prior research hypothesized a sequence of palate ossification evolution that can now be tested using broad anatomical observations from the early‐branching crocodylomorphs. These observations document the difficulties in summarizing palate evolution into only three categories (i.e., “protosuchian”‐, mesosuchian‐, and eusuchian‐type secondary palates) because of the appearance of complex and often independent histories of palatal structures, such as an anterior palatal fenestra and a ventrally open nasopharyngeal duct.
There is a relationship between the position of the choana on the palate and the palatal bone that forms its anterior margin (maxilla, palatine, or pterygoid). “Sphenosuchians” express the plesiomorphic palatal construct, with the choana positioned anteriorly and the maxilla bounding the anterior margin of it. However, among “protosuchians” only Protosuchus, Orthosuchus, and Gobiosuchus have the construct of the choana historically ascribed to early crocodylomorphs (Figure 6). Most of the remaining ‘protosuchians’ have a choana which is bounded anteriorly by the palatine and the choana positioned further posteriorly. Fruitachampsa is unique among shartegosuchoids because it maintains broad palatine palatal shelves, which are reduced in other shartegosuchoids. Perhaps these fundamental differences in shartegosuchoid palatal anatomy can be attributed to the geographic distance between Fruitachampsa (Morrison Formation, Colorado, USA) and the remaining shartegosuchoids (recovered from Mongolia, Siberia, and China).
FIGURE 6.
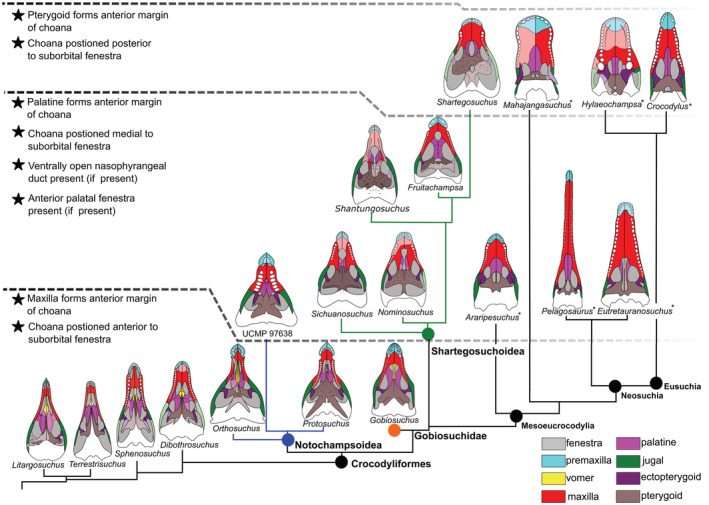
Major palatal configurations in palate evolution of crocodylomorphs represented by 19 skulls in palatal view. Palatal configurations were reconstructed based on CT scan data, or published data of known crocodylomorphs. Asterisks indicate crocodylomorph taxa not sampled in this analysis. Areas of the palate not preserved or not observable are illustrated with outlines used dashed lines and transparent coloring. Notable changes in anatomical palatal structures are bullet‐pointed, and the evolutionary stages are separated by black dashed lines. Palatal bones are colored, ectopterygoid, purple; jugal, green; maxilla, red; palatine, pink; premaxilla, light blue; pterygoid, brown
Among ‘protosuchians’, Shartegosuchus uniquely has a choana bounded exclusively by the pterygoid and positioned posterior to the level of the suborbital fenestra, the choana of other ‘protosuchians’ are bounded either by the maxilla and palatine or by the palatine and pterygoid (Dollman et al., 2018). In all crocodylomorphs with the choana positioned posterior to the level of the suborbital fenestra, the choana is bounded by the pterygoid only, which is perhaps the most posterior position achievable before impeding deglutition (i.e., Shartegosuchus and eusuchians).
In the eusuchian lineage, the palatines form palatal shelves that contribute largely to the secondary palate, and they have an internal cavity that encloses the nasal passage. The palatines observed in the shartegosuchoid clade (excluding Fruitachampsa) are small plates that neither contribute greatly to the secondary palate nor enclose the nasal passage. Rather, this function in shartegosuchoids is performed by the pterygoid which forms a large portion of the secondary palate and encloses the nasal passage. The overall contribution of palatal bones to the palate does not greatly change among crocodylomorph taxa as the choana moves posteriorly, with the exclusion of the palatine.
Shartegosuchoids also share another unusual palatal structure, an anterior palatal fenestra, and ventrally open nasopharyngeal duct. The anterior palatal fenestra and ventrally open nasopharyngeal duct are formed by a separation in the palatine and maxillary palatal shelves along the midline of the palate. An anterior palatal fenestra (otherwise also called “maxilla‐palatine fenestra”) is independently developed in the mesoeucrocodylian Notosuchus (Barrios et al., 2018) but not again (within sampled taxa for this analysis) in the lineage leading up to the crown clade. Baurusuchus also presents an additional palatal fenestra, but these are paired openings that are medially separated by the pterygoid and maxilla (de Souza et al., 2005, 2011). An anterior palatal fenestra that is linked to the choana through a ventrally open nasopharyngeal duct is reconstructed as the ancestral state (if the anterior palatal fenestra is present within a group), with an anterior palatal fenestra that is separated from the choana evolving independently in later‐branching shartegosuchoids and Notosuchus only.
The anterior palatal fenestra has no obvious modern analog. The presence of both incisive foramina and anterior palatal fenestrae in a few taxa, for example, Notosuchus (Barrios et al. (2018) preclude their potential homology (via conjunction sensu de Pinna, 1991). The incisive foramen (or “subnarial foramen” as it is sometimes referred in crocodylians) is for the transmittance of blood vessels and the nasopalatine nerves (Porter et al., 2016; Sedlmayr, 2002; Thakur et al., 2013). The anterior palatal fenestra and nasopharyngeal duct would likely have been floored by soft tissues, which implies an intermediate soft tissue stage in palate evolution. Extant crocodylians do not have a soft tissue palate component during or after palatogenesis (Ferguson, 1981; Witmer, 1995, 1997). Squamates, which may serve as a functional analog for a soft tissue palate, have two flaps of tissue that are supported by ectochoanal cartilages that overlap and separate the nasal from the oral cavity (Rieppel et al., 2008; Savage, 1963).
Ferguson's (1981) work on pre‐hatchling Alligator mississipiensis shows that in ovo ossification of the secondary palate occurs early and quickly (day 17–24 of an approximately 80‐day incubation period). They observe an anterior‐to‐posterior direction of palatal closure and state that “the alligator nasopharyngeal duct arises anteriorly by the fusion of the maxillary palatal shelves with a septum‐like downgrowth from the base of the skull, so forming a partitioned duct” (Ferguson, 1981, p. 431). The presence of a ventrally open nasopharyngeal duct partitioning the palatines is described, but there is no mention of an anterior palatal fenestra during palatogenesis. Therefore, while some of the features of a developing embryo resemble intermediate phylogenetic stages, other features of those stages are lacking (i.e., the anterior palatal fenestra). The resemblance therefore could simply be a structural consequence of midline closure during embryonic growth rather than being recapitulatory (Müller, 1967).
By synthesizing these observations, it is clear that the palate ossification encompasses more than just a posterior shift of the choana and medially expanding maxillary and palatine palatal shelves. Any future hypothesis on palate evolution must therefore incorporate palatal structures such as the anterior palatal fenestra and a ventrally open nasopharyngeal duct.
In most non‐mesoeucrocodylians, the choana is positioned anteriorly on the palate with the maxilla forming its anterior margin and the palatine forming its posterior margin. In shartegosuchoids, the palatines reduce in size and the existing palatal shelves of the maxillae expand posteriorly and contact the pterygoid. In mesoeucrocodylians leading up to Eusuchia, the palatines develop broad palatal shelves and the existing palatal shelves of the maxillae expand posteriorly. In both lineages, the choana moves posteriorly along the palate, and the choana is linked to the anterior palatal fenestra through a ventrally open nasopharyngeal duct in some intermediate taxa. In shartegosuchoids, if a ventrally open nasopharyngeal duct is not present, either the paired palatines (Fruitachampsa) or pterygoid (Shartegosuchus) separate the choana from the anterior palatal fenestra. In mesoeucrocodylians, the maxillary and palatine palatal shelves meet along the midline along the entire length of the palate and the choana is positioned posteriorly on the palate with either the palatine or pterygoid forming the anterior margin. It is interesting to observe that both lineages, shartegosuchoids and mesoeucrocodylians, share a condition at some point where there is both an anterior palatal fenestra and a choana that are linked through a ventrally open nasopharyngeal duct. It is worth considering that there is a conserved intermediate palatal condition wherein the palate is more open as the choana moves posteriorly.
Interestingly, Pritchard et al. (2013) observe that goniopholids appear to evolve an open secondary palate with a ventrally open nasopharyngeal duct from an ossified secondary palate, that is, Calsoyasuchus and Eutretauransosuchus (Pritchard et al., 2013; Tykoski et al., 2002). Pritchard et al. (2013) optimized characters within Goniopholididae and noted the order of morphological transformations leading to an open palate. A number of these features are observed also in lineages evolving an ossified secondary palate. Characters included in their assessment are (1) the posteriormost processes of the maxilla meet or fail to meet on the midline; (2) palatine palatal shelves meet or fail to meet on the midline; (3) ventral surface of the choanal septum is smooth or has bilateral vomerine septal laminae. It is worth noting that the posterior processes of the maxilla do not meet in Sunosuchus because of paired openings that are bounded by the maxilla and palatine (Wu et al., 1996), which resembles the anterior palatal fenestra described in other taxa.
4.2. The implications of a shift to herbivory on secondary palate evolution
One of the prevailing hypotheses for the evolution of a secondary palate in mammals is that it is a functional adaptation for increased chewing ability, particularly of tough plant parts (King, 1996). The palatal data collected here, paired with information from recent research hypothesizing that crocodylomorph teeth repeatedly adapted for herbivory (Melstrom & Irmis, 2019) warrants evaluation of herbivory as a causative model for palate ossification in Crocodylomorpha.
Within Crocodylomorpha, Melstrom and Irmis (2019) hypothesize that herbivory evolves within notochampsoids, specifically Edentosuchus and UCMP 97638. They propose either a single origin for herbivory as ancestral for Notosuchia, or alternatively at least three independent occurrences of herbivory within that group (Simosuchus, Pakasuchus, and Chimaerasuchus). Finally, the earliest diverging eusuchians, the clade Hylaeochampsidae, are considered herbivores in their analysis. Other potential herbivorous early crocodylomorphs that were not in their study include Phyllodontosuchus and Macelognathus (Göhlich et al., 2005; Harris et al., 2000).
Among early‐branching crocodylomorphs, the development of an ossified secondary palate appears to coincide with the three main dietary shifts that Melstrom and Irmis (2019) describe in protosuchidae (UCMP 97638), Notosuchia, and Hylaeochampsidae. Interestingly, shartegosuchoids show the development of the palatal region, from a palatine secondary palate in the early‐branching members of this group, to a pterygoid secondary palate in Shartegosuchus. Shartegosuchoids were not sampled in Melstrom and Irmis (2019)'s analysis and so further speculation on their ecology cannot be completed, but they do express unique dental cusp morphology that indicates at least a shift in diet away from hypercarnivory (Clark, 2011).
The evolution of herbivory is a complex process and requires a number of additional adoptions, including but not limited to improved thermoregulation and digestive processes capable of breaking down plant compounds such as cellulose (O'Grady et al., 2005). The evolution of a secondary palate could be beneficial for a herbivorous diet because it provides structural strength for herbivores that process hard substrates (King, 1996; Sharp, 2015). However, some successful herbivorous clades lack an ossified secondary palate, such as squamates and some dinosaurian clades.
In squamates, the development of the palatal bones is related to chemosensory abilities, tongue morphology, and their feeding mechanics (Rieppel, 1990). Herbivorous squamates, such as liolaemids, iguanids, and agamids (Espinoza et al., 2004; O'Grady et al., 2005), do not have a complete secondary palate (Rieppel et al., 2008). Herrel et al. (2007) discuss that herbivorous squamates shorten their rostrum and shift the orientation of jaw adductor musculature to improve mechanical performance by reducing the out‐lever to closing in‐lever length ratio without needing to substantially increase jaw adductor musculature mass. Furthermore, analysis of feeding kinematics of herbivorous squamates shows that they do not employ any substantial degree of oral processing and rather focus on hindgut digestion (Herrel & Vree, 1999; O'Grady et al., 2005).
There are several independent origins of herbivory in dinosaurs, including in Ornithischia, Sauropodomorpha, and multiple times in Theropoda (Butler et al., 2009; Zanno et al., 2009; Zanno & Makovicky, 2011 etc.). Among these lineages, an ossified secondary palate is only sometimes present. In theropod clades that evolve herbivory, they do not evolve dental occlusion or oral processing nor do they evolve a complete secondary palate, for example, Kobayashi et al. (1999); Zanno et al. (2009); Zanno and Makovicky (2011); Novas et al. (2015); and Lautenschlager (2017). In ornithischians, which are ancestrally herbivorous, only some derived members (hadrosaurs) evolve dental occlusion, oral processing, and a vaulted ossified secondary palate (Reichel, 2010; Rybczynski et al., 2008). Therefore, secondary palates appear to be ubiquitous among archosaurian lineages that develop herbivory with oral processing, in turn suggesting that some early‐branching crocodylomorphs with secondary palates are masticators.
Another possible palatal adaptation for oral processing is a thickening of the palate which might be achieved by sculpturing of the palate, as observed in many “protosuchians”. Rugosity or thickening and sculpturing of the palatal bones could assist in structural reinforcement of the palate. For example, an investigation of the phenotypic diversity in palate ontogenetic ossification development in rabbits showed that thicker palates developed in individuals that were exposed to a diet of a harder substrate than those exposed to a softer diet. This result indicates that differences in palatal thicknesses are related to changes in masticatory stress loads (Menegaz et al., 2009), and can possibly be extended to adaptations of thickness over evolutionary timescales. The sculpturing observed among sampled crocodylomorph taxa does vary in topology, some taxa have ridges and grooves (e.g., Protosuchus and Shartegosuchus), and others have small circular pits (e.g., UCMP 97638 and Shantungosuchus) which could have served as small openings for transmission of blood vessels to the soft secondary palate (Ősi, 2014). No vascular passages were observed in Protosuchus or Shartegosuchus, but that does not preclude that functional possibility in other taxa with different sculpturing morphology.
The observation that oral processing and herbivory are closely correlated with ossification of the secondary palate in a range of vertebrates, as well as the crocodylomorphs described herein, suggests a common causative hypothesis for palatal closure. That is, ossified secondary palates are structural modifications that occur in amniote taxa that evolve the oral processing of plant matter. This study presents a novel hypothesis for secondary palate evolution in addition to already known hypotheses which consider the development of an ossified secondary palate as a supportive structure for platyrostral and longistrine rostral shapes (Busbey, 1995; McHenry et al., 2006; Pierce et al., 2008; Rayfield & Milner, 2008). It is possible that the evolution of an ossified secondary palate within Crocodylomorpha has multiple causative models and for remarkably different evolutionary reasons, such as herbivory, hypercarnivory, and rostral shape.
5. CONCLUSION
There is marked variation in the palatal structures of early branching members of Crocodylomorpha. This variation is a fruitful area for hypothesizing systematic characters. In particular, the most significant areas are the position of the choana and the palatal bones that contribute to the border of the opening, the contribution of the palatine bones to the ossified secondary palate, and other additional palatal structures such as thickening or sculpturing of the palate.
In this research, an amendment to the evolutionary developmental hypothesis of an ossified secondary palate within Crocodylomorpha is presented. Future hypotheses of palatal evolution must include the palatal structures of an anterior palatal fenestra and ventrally open nasopharyngeal duct. These are structures that have been observed in independent lineages within Crocodylomorpha that present transitional developmental changes in the composition of the secondary palate, either through further ossification or deossification of the palate (i.e., “protosuchians,” notosuchians, and goniopholids).
Selective hypotheses for the evolution of an ossified secondary palate in Crocodylomorpha have previously centered on the need for torsional stress management in longistrine or platyrostral snout shapes. Here, we present a novel hypothesis that the causative model for palate ossification throughout Crocodylomorpha may stem from very different ecological factors, such as oral processing requirements for herbivory or, indeed, changes in rostral morphology. This research presents the conceptual foundation for testing many of the above‐mentioned questions and concludes that even in the earliest branching members, crocodylomorphs presented innovative evolutionary solutions to ecological pressures.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
Kimi Chapelle and Serjoscha Evers are thanked for their discussions on this topic. The Evolutionary Studies Institute (University of Witwatersrand, South Africa), American Museum of Natural History (New York), the Institute of Vertebrate Paleontology and Paleoanthropology (Beijing, China), and the Iziko South African Museum (Cape Town, South Africa) are thanked for allowing access to research specimens. This research was funded by grants from the NRF African Origins Platform (98800 and 118974 to J.N.C.) and Competitive Programme for Rated Researchers (98906 to J.N.C.), and the NSF grant EAR 1636753. The support of the DST‐NRF Centre of Excellence in Palaeosciences (CoE‐Pal) University of the Witwatersrand, Johannesburg (Grant 86073) toward this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the CoE.
Dollman, K. N. , & Choiniere, J. N. (2022). Palate evolution in early‐branching crocodylomorphs: Implications for homology, systematics, and ecomorphology. The Anatomical Record, 305(10), 2766–2790. 10.1002/ar.24993
Grant sponser(s): NRF AOP grants (98800 and 118794), DSI‐NRF Centre of Excellence in Palaeosciences, University of the Witwatersrand, Johannesburg (Grant 86073).
Funding information DSI‐NRF Centre of Excellence in Palaeosciences, Grant/Award Number: 86073; NRF‐AOP, Grant/Award Numbers: 118794, 98800
REFERENCES
- Andrade, M. , Bertini, R. , & Pinheiro, A. (2006). Observations on the palate and choanae structures in mesoeucrocodylia (Archosauria, Crocodylomorpha): Phylogenetic implications. Revista Brasileria de Paleontologia, 9, 323–332. [Google Scholar]
- Barrios, F. , Bona, P. , Paulina‐Carabajal, A. , & Gasparini, Z. (2018). Re‐description of the cranio‐mandibular anatomy of Notosuchus terrestris (Crocodyliformes, mesoeucrocodylia) from the upper cretaceous of Patagonia. Cretaceous Research, 83, 3–39. [Google Scholar]
- Busbey, A. B. (1995). The structural consequences of skull flattening in crocodilians. In Thomason J. J. (Ed.), Functional morphology in vertebrate paleontology (pp. 173–192). Cambridge Press. [Google Scholar]
- Butler, R. , Barrett, P. , Kenrick, P. , & Penn, M. (2009). Diversity patterns amongst herbivorous dinosaurs and plants during the cretaceous: Implications for hypotheses of dinosaur/angiosperm co‐evolution. Journal of Evolutionary Biology, 22, 446–459. [DOI] [PubMed] [Google Scholar]
- Clark, J. M. (1994). Patterns of evolution in Mesozoic Crocodyliformes. In Fraser N. C. & Sues H. (Eds.), In the shadow of dinosaurs (pp. 84–97). Cambridge University Press. [Google Scholar]
- Clark, J. M. (2011). A new shartegosuchid crocodyliform from the upper Jurassic Morrison formation of Western Colorado. Zoological Journal of the Linnean Society, 163, S152–S172. [Google Scholar]
- Clark, J. M. , & Sues, H. D. (2002). Two new basal crocodylomorph archosaurs from the Lower Jurassic and the monophyly of the Sphenosuchia. Zoological Journal of the Linnean Society, 136(1), 77–95. [Google Scholar]
- Crush, P. J. (1984). A late upper Triassic sphenosuchid crocodilian from Wales. Paléo, 27, 27. [Google Scholar]
- Darlim, G. , Montefeltro, F. , & Langer, M. (2021). 3D skull modelling and description of a new baurusuchid (Crocodyliformes, mesoeucrocodylia) from the late cretaceous (Bauru Basin) of Brazil. Journal of Anatomy, 239, 622–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade, M. , Edmonds, R. , Benton, M. , & Schouten, R. (2011). A new Berriasian species of Goniopholis (mesoeucrocodylia, Neosuchia) from England, and a review of the genus. Zoological Journal of the Linnean Society, 163, S66–S108. [Google Scholar]
- De Pinna, M. C. (1991). Concepts and tests of homology in the cladistic paradigm. Cladistics, 7(4), 367–394. [Google Scholar]
- de Souza, C. I. , Campos, A. , & Nobre, P. (2005). Baurusuchus salgadoensis, a new crocodylomorpha from the Bauru Basin (cretaceous), Brazil. Gondwana Research, 8, 11–30. [Google Scholar]
- de Souza, C. I. , Teixeira, V. P. A. , Ferraz, M. L. F. , Ribeiro, L. C. B. , Martinelli, A. G. , Neto, F. M. , Sertich, J. J. , Cunha, G. C. , Cunha, I. C. , & Ferraz, P. F. (2011). Campinasuchus dinizi gen. et sp. nov., a new late cretaceous baurusuchid (Crocodyliformes) from the Bauru Basin, Brazil. Zootaxa, 2871, 19–42. [Google Scholar]
- Dollman, K. N. , Clark, J. M. , Norell, M. A. , Xu, X. , & Choiniere, J. N. (2018). Convergent evolution of a Eusuchian‐type secondary palate within Shartegosuchidae. American Museum Novitates, 3901, 1–23. [Google Scholar]
- Dollman, K. N. , Clark, J. M. , Viglietti, P. A. , Browning, C. , & Choiniere, J. N. (2021). Revised anatomy, taxonomy and biostratigraphy of Notochampsa istedana broom, 1904, a lower Jurassic crocodyliform from the Clarens formation (Stormberg group), and its implications for early crocodyliform phylogeny. Journal of Systematic Palaeontology, 19(9), 651–675. [Google Scholar]
- Espinoza, R. E. , Wiens, J. J. , & Tracy, C. R. (2004). Recurrent evolution of herbivory in small, cold‐climate lizards: Breaking the ecophysiological rules of reptilian herbivory. Proceedings of the National Academy of Sciences, 101, 16819–16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, M. W. J. (1981). The structure and development of the palate in Alligator mississippiensis . Archives of Oral Biology, 26, 427–443. [DOI] [PubMed] [Google Scholar]
- Gaffney, E. S. (1979). Comparative cranial morphology of recent and fossil turtles. Bulletin of the AMNH, 164, article 2. [Google Scholar]
- Gao, Y. H. (2001). A new species of Hsisosuchus from Dashanpu, Zigong, Sichuan. Vertebrata PalAsiatica, 39, 177–184. [Google Scholar]
- Göhlich, U. B. , Chiappe, L. M. , Clark, J. M. , & Sues, H.‐D. (2005). The systematic position of the late Jurassic alleged dinosaur Macelognathus (Crocodylomorpha: Sphenosuchia). Canadian Journal of Earth Sciences, 42, 307–321. [Google Scholar]
- Gold, M. E. L. , Brusatte, S. L. , & Norell, M. A. (2013). The cranial pneumatic sinuses of the tyrannosaurid Alioramus (Dinosauria: Theropoda) and the evolution of cranial pneumaticity in theropod dinosaurs. American Museum Novitates, 2013, 1–46. [Google Scholar]
- Goloboff, P. A. , & Catalano, S. A. (2016). TNT version 1.5 including a full implementation of phylogenetic morphometrics. Cladistics, 32, 221–238. [DOI] [PubMed] [Google Scholar]
- Goloboff, P. A. , & Nixon, K. C. (2008). TNT, a free program for phylogenetic analysis. Cladistics, 24, 774–786. [Google Scholar]
- Gow, C. E. (2000). The skull of Protosuchus haughtoni, an early Jurassic crocodyliform from southern Africa. Journal of Vertebrate Paleontology, 20, 49–56. [Google Scholar]
- Harris, J. D. , Lucas, S. G. , Estep, J. W. , & Li, J. (2000). A new and unusual sphenosuchian (Archosauria: Crocodylomorpha) from the lower Jurassic Lufeng formation, People's Republic of China. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 215, 47–68. [Google Scholar]
- Herrel, A. , Schaerlaeken, V. , Meyers, J. J. , Metzger, K. A. , & Ross, C. F. (2007). The evolution of cranial design and performance in squamates: Consequences of skull‐bone reduction on feeding behavior. Integrative and Comparative Biology, 47, 107–117. [DOI] [PubMed] [Google Scholar]
- Herrel, A. , & Vree, F. (1999). Kinematics of intraoral transport and swallowing in the herbivorous lizard Uromastix acanthinurus . Journal of Experimental Biology, 202, 1127–1137. [DOI] [PubMed] [Google Scholar]
- Huxley, T. H. (1875). On Stagnolepis robertsoni, and on the evolution of the Crocodilia. Quarterly Journal of the Geological Society of London, 41, 423–438. [Google Scholar]
- Iordansky, N. N. (1973). The skull of crocodilia. In Gans C. & Parsons T. S. (Eds.), Biology of Reptilia (pp. 201–262). Academic Press. [Google Scholar]
- King, G. M. (1996). Reptiles and herbivory. Springer Science & Business Media. [Google Scholar]
- Kobayashi, Y. , Lu, J.‐C. , Dong, Z.‐M. , Barsbold, R. , Azuma, Y. , & Tomida, Y. (1999). Herbivorous diet in an ornithomimid dinosaur. Nature, 402, 480–481.10591205 [Google Scholar]
- Langston, W. (1973). The crocodilian skull in historical perspective. In Gans C. & Parsons T. S. (Eds.), Biology of Reptilia. Academic Press. [Google Scholar]
- Lautenschlager, S. (2017). Functional niche partitioning in Therizinosauria provides new insights into the evolution of theropod herbivory. Palaeontology, 60, 375–387. [Google Scholar]
- Lautenschlager, S. , & Butler, R. J. (2016). Neural and endocranial anatomy of Triassic phytosaurian reptiles and convergence with fossil and modern crocodylians. PeerJ, 4, e2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leardi, J. M. , Pol, D. , & Clark, J. M. (2017). Detailed anatomy of the braincase of Macelognathus vagans marsh, 1884 (Archosauria, Crocodylomorpha) using high resolution tomography and new insights on basal crocodylomorph phylogeny. PeerJ, 5, e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite, K. J. , & Fortier, D. C. (2018). The palate and choanae structure of the Susisuchus anatoceps (Crocodyliformes, Eusuchia): Phylogenetic implications. PeerJ, 6, e5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison, W. P. , & Maddison, D. R. (2018). Mesquite: A modular system for evolutionary analysia, version. Mesquite, 3, 40. [Google Scholar]
- McHenry, C. R. , Clausen, P. D. , Daniel, W. J. T. , Meers, M. B. , & Pendharkar, A. (2006). Biomechanics of the rostrum in crocodilians: A comparative analysis using finite‐element modeling. The Anatomical Record Part A, 288A, 827–849. [DOI] [PubMed] [Google Scholar]
- Melstrom, K. M. , & Irmis, R. B. (2019). Repeated evolution of herbivorous crocodyliforms during the age of dinosaurs. Current Biology, 29, 2389–2395.e3. [DOI] [PubMed] [Google Scholar]
- Menegaz, R. A. , Sublett, S. V. , Figueroa, S. D. , Hoffman, T. J. , & Ravosa, M. J. (2009). Phenotypic plasticity and function of the hard palate in growing rabbits. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 292, 277–284. [DOI] [PubMed] [Google Scholar]
- Montefeltro, F. C. , Bronzati, M. , Langer, M. C. , & Anelli, L. E. (2019). A new specimen of Susisuchus anatoceps (Crocodyliformes, Neosuchia) with a non‐eusuchian‐type palate. Journal of Vertebrate Paleontology, 39, e1716240. [Google Scholar]
- Montefeltro, F. C. , Larsson, H. C. , & Langer, M. C. (2011). A new baurusuchid (Crocodyliformes, mesoeucrocodylia) from the late cretaceous of Brazil and the phylogeny of Baurusuchidae. PLoS One, 6, e21916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, F. (1967). On the embryonal development of the head in Crocodylus cataphractus CUV. Revue Suisse de Zoologie: Annales de la Societe Zoologique suisse et du Museum d'histoire Naturelle de Geneve, 74, 189–294. [PubMed] [Google Scholar]
- Nash, D. (1968). A crocodile from the upper Triassic of Lesotho. Journal of Zoology, 156, 163–179. [Google Scholar]
- Nash, D. (1975). The morphology and relationships of a crocodilian, Orthosuchus stormbergi, from the upper Triassic of Lesotho. Annals of the South African Museum, 67, 227–239. [Google Scholar]
- Novas, F. E. , Salgado, L. , Suárez, M. , Agnolín, F. L. , Ezcurra, M. D. , Chimento, N. R. , De La Cruz, R. , Isasi, M. P. , Vargas, A. O. , & Rubilar‐Rogers, D. (2015). An enigmatic plant‐eating theropod from the late Jurassic period of Chile. Nature, 522, 331–334. [DOI] [PubMed] [Google Scholar]
- O'Grady, S. P. , Morando, M. , Avila, L. , & Dearing, M. D. (2005). Correlating diet and digestive tract specialization: Examples from the lizard family Liolaemidae. Zoology, 108, 201–210. [DOI] [PubMed] [Google Scholar]
- Ősi, A. (2014). The evolution of jaw mechanism and dental function in heterodont crocodyliforms. Historical Biology, 26, 279–414. [Google Scholar]
- Osmólska, H. , Hua, S. , & Buffetaut, E. (1997). Gobiosuchus kielanae [Protosuchia] from the late cretaceous of Mongolia: Anatomy and relationships. Acta Palaeontologica Polonica, 42, 257–289. [Google Scholar]
- Parrington, F. R. , & Westoll, T. S. (1940). On the evolution of the mammalian palate. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 230, 305–355. [Google Scholar]
- Pierce, S. E. , Angielczyk, K. D. , & Rayfield, E. J. (2008). Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: A combined geometric morphometric and finite element modeling approach. Journal of Morphology, 269, 840–864. [DOI] [PubMed] [Google Scholar]
- Pol, D. , & Powell, J. E. (2011). A new sebecid mesoeucrocodylian from the Rio Loro formation (Palaeocene) of North‐Western Argentina. Zoological Journal of the Linnean Society, 163, S7–S36. [Google Scholar]
- Pol, D. , Rauhut, O. W. M. , Lecuona, A. , Leardi, J. M. , Xu, X. , & Clark, J. M. (2013). A new fossil from the Jurassic of Patagonia reveals early basicranial evolution and the origins of Crocodyliformes. Biological Reviews, 88, 862–872. [DOI] [PubMed] [Google Scholar]
- Pol, D. , Turner, A. H. , & Norell, M. A. (2009). Morphology of the late cretaceous crocodylomorph Shamosuchus djadochtaensis and a discussion of neosuchian phylogeny as related to the origin of Eusuchia. Bulletin of the American Museum of Natural History, 2009, 1–103. [Google Scholar]
- Porter, W. R. , Sedlmayr, J. C. , & Witmer, L. M. (2016). Vascular patterns in the heads of crocodilians: Blood vessels and sites of thermal exchange. Journal of Anatomy, 229, 800–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard, A. C. , Turner, A. H. , Allen, E. R. , & Norell, M. A. (2013). Osteology of a north American Goniopholidid (Eutretauranosuchus delfsi) and palate evolution in Neosuchia. Am Mus Nov, 3783, 1–56. [Google Scholar]
- Rayfield, E. J. , & Milner, A. C. (2008). Establishing a framework for archosaur cranial mechanics. Paleobiology, 34, 494–515. [Google Scholar]
- Reichel, M. (2010). A model for the bite mechanics in the herbivorous dinosaur Stegosaurus (Ornithischia, Stegosauridae). Swiss Journal of Geosciences, 103, 235–240. [Google Scholar]
- Rieppel, O. (1990). Ontogeny a way forward for systematics a way backward for phylogeny. Biological Journal of the Linnean Society, 39, 177–191. [Google Scholar]
- Rieppel, O. , Gauthier, J. , & Maisano, J. (2008). Comparative morphology of the dermal palate in squamate reptiles, with comments on phylogenetic implications. Zoological Journal of the Linnean Society, 152, 131–152. [Google Scholar]
- Rybczynski, N. , Tirabasso, A. , Bloskie, P. , Cuthbertson, R. , & Holliday, C. (2008). A three‐dimensional animation model of Edmontosaurus (Hadrosauridae) for testing chewing hypotheses. Palaeontologia Electronica, 11, 9A. [Google Scholar]
- Salisbury, S. W. , Molnar, R. E. , Frey, E. , & Willis, P. M. (2006). The origin of modern crocodyliforms: New evidence from the cretaceous of Australia. Proceedings of the Royal Society B: Biological Sciences, 273, 2439–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JM. 1963. Studies on the lizard family Xantusiidae IV. The genera. Los Angeles County Museum. [Google Scholar]
- Sedlmayr, J. C. (2002). Anatomy, evolution, and functional significance of cephalic vasculature in Archosauria. Ohio University. [Google Scholar]
- Sharp, A. C. (2015). Comparative finite element analysis of the cranial performance of four herbivorous marsupials. Journal of Morphology, 276, 1230–1243. [DOI] [PubMed] [Google Scholar]
- Thakur, A. R. , Burde, K. , Guttal, K. , & Naikmasur, V. G. (2013). Anatomy and morphology of the nasopalatine canal using cone‐beam computed tomography. Imaging Science in Dentistry, 43, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason, J. , & Russell, A. (1986). Mechanical factors in the evolution of the mammalian secondary palate: A theoretical analysis. Journal of Morphology, 189, 199–213. [DOI] [PubMed] [Google Scholar]
- Turner, A. H. (2015). A review of Shamosuchus and Paralligator (Crocodyliformes, Neosuchia) from the cretaceous of Asia. PLoS One, 10, e0118116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, A. H. , & Pritchard, A. C. (2015). The monophyly of Susisuchidae (Crocodyliformes) and its phylogenetic placement in Neosuchia. PeerJ, 3, e759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykoski, R. S. , Rowe, T. B. , Ketcham, R. A. , & Colbert, M. W. (2002). Calsoyasuchus valliceps, a new crocodyliform from the early Jurassic Kayenta formation of Arizona. Journal of Vertebrate Paleontology, 22, 593–611. [Google Scholar]
- Walker, A. (1968). Protosuchus, Proterochampsa, and the origin of phytosaurs and crocodiles. Geological Magazine, 105, 1–14. [Google Scholar]
- Walker, A. D. (1972). New light on the origin of birds and crocodiles. Nature, 237(5353), 257–263. [Google Scholar]
- Walker, A. (1990). A revision of Sphenosuchus acutus Haughton, a crocodylomorph reptile from the Elliot formation (late Triassic or early Jurassic) of South Africa. Philosophical Transactions of the Royal Society of London B, 330, 1–20. [Google Scholar]
- Witmer, L. M. (1995). Homology of facial structures in extant archosaurs (birds and crocodilians), with special reference to paranasal pneumaticity and nasal conchae. Journal of Morphology, 225, 269–327. [DOI] [PubMed] [Google Scholar]
- Witmer, L. M. (1997). The evolution of the antorbital cavity of archosaurs: A study in soft‐tissue reconstruction in the fossil record with an analysis of the function of pneumaticity. Journal of Vertebrate Paleontology, 17, 1–76. [Google Scholar]
- Wu, X. , Brinkman, D. B. , & Lu, J. (1994). A new species of Shantungosuchus from the lower cretaceous of Inner Mongolia (China), with comments of S, chuhsienensis young, 1961 and the phylogenetic position of the genus. Journal of Vertebrate Palaeontology, 14, 210–229. [Google Scholar]
- Wu, X. , & Chatterjee, S. (1993). Dibothrosuchus elaphros, a crocodylomorph from the lower Jurassic of China and the phylogeny of the Sphenosuchia. Journal of Vertebrate Paleontology, 13, 58–89. [Google Scholar]
- Wu, X. , Sues, H. , & Dong, Z. (1997). Sichuanosuchus shuhanensis, a new? Early cretaceous protosuchian (Archosauria: Crocodyliformes) from Sichuan (China), and the monophyly of Protosuchia. Journal of Vertebrate Paleontology, 17, 89–103. [Google Scholar]
- Wu, X.‐C. , Brinkman, D. B. , & Russell, A. P. (1996). Sunosuchus junggarensis sp. nov.(Archosauria: Crocodyliformes) from the upper Jurassic of Xinjiang, People's Republic of China. Canadian Journal of Earth Sciences, 33, 606–630. [Google Scholar]
- Zanno, L. E. , Gillette, D. D. , Albright, L. B. , & Titus, A. L. (2009). A new north American therizinosaurid and the role of herbivory in ‘predatory’ dinosaur evolution. Proceedings of the Royal Society B: Biological Sciences, 276, 3505–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanno, L. E. , & Makovicky, P. J. (2011). Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proceedings of the National Academy of Sciences, 108, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


