Abstract
Background
Parallel with the demographic ageing crisis, is a disabling overactive bladder (OAB) crisis (urgency/frequency/nocturia), 30% prevalence in older women, pathogenesis stated as unknown and, according to some learned societies, incurable.
Hypothesis/Aims
To review International Continence Society and Integral System paradigms to test our thesis that OAB per se is not a pathological condition, rather, a prematurely activated uncontrolled micturition; pathogenesis being anatomical damage in a nonlinear feedback control system comprising cortical and peripheral (muscle/ligament) components.
Methods
We examined studies from basic science, anatomy, urodynamics, ultrasonic and video xrays, ligament repairs, from which we created a nonlinear binary model of bladder function. We applied a Chaos Theory feedback equation, Xnext = Xc(1 − X) to test our hypothesis against existing concepts and hypotheses for OAB pathogenesis.
Results
The bladder has ONLY two modes, EITHER closed OR open (micturition). Closure is reflexly controlled cortically and peripherally: muscles contracting against ligaments stretch the vagina to suppress afferent signals to micturate from urothelial stretch receptors. “OAB” can be caused by anatomical damage anywhere in the model, by childbirth or age‐weakened ligaments, which can be repaired to cure all three OAB symptoms. Urodynamic “DO” graphs are interpreted anatomically and by the feedback equation.
Conclusion
OAB is in crisis. Our thesis of OAB as an uncontrolled micturition from anatomical defects in the bladder control system provides fresh directions for further development of new treatments, nonsurgical and surgical, to help break the crisis and bring hope and cure to 600 million women sufferers.
Keywords: bladder control, feedback binary model, integral theory, micturition, OAB, urethral closure, urinary retention
Abbreviations
- DO
urodynamic detrusor overactivity
- DU
urodynamic detrusor underactivity
- IT
integral theory
- LMA
conjoint longitudinal muscle of the anus
- LP
levator plate (LP)
- OAB
overactive bladder
- PCM
pubococcygeus muscle
- PFS
posterior fornix syndrome
- PUL
pubourethral ligament
- SUI
stress urinary incontinence
- UAB
underactive bladder
- UI
urge incontinence
- USL
uterosacral ligament
- “N”
stretch receptors
1. INTRODUCTION
The condition of overactive bladder (“OAB”) as defined by the International Continence Society (ICS) 1 is in crisis: an ageing population, prevalence 30% at 65 years, pathogenesis unknown, no known cure; anticholinergics, the first line of treatment may cause Alzheimer's Disease 2 The first ICS report in 1976 3 defined bladder dysfunctions as stress, urge, reflex, overflow incontinence and urodynamic detrusor instability, (now “DO”), then considered a clinical condition. DO findings in 65% of asymptomatic women on ambulatory urodynamic testing in 1993, 4 seriously questioned DO's legitimacy as a clinical condition. In 2002, the definition for DI was changed to DO, urgency to OAB. 1 The 15 cm rise for DO definition was removed and DO became a wavy pattern on the cystometric graph (see Figure 4).
Figure 4.

“DO”—a struggle for supremacy between opening and closure reflexes. Actual urodynamic graph B = bladder chart; U = urethral chart; CP = closure pressure chart. Because the system is binary, the closure reflex “C” (red unbroken lines) and opening (micturition) reflex “O” (black broken lines) struggle for supremacy. This is a urodynamic graph of a patient with OAB symptoms, with a full bladder, undergoing a handwashing test. The sequence of events is identical to that seen in normal micturition [1] a feeling of urgency, [2] a fall in urethral pressure “X” (graph “U”), [3] a rise in bladder pressure “Y” (graph “B”) [4] urine loss, black arrow, (graph ‘CP’). U = urethral pressure graph; B = bladder pressure graph; CP = closure pressure graph (U minus B). C = closure reflex (red unbroken lines). O = opening (micturition) reflex, (black broken lines) with its components being: Ou = urethral relaxation. Od = detrusor contraction. Om = opening out of the outflow tract by backward/downward striated muscle forces, X‐ray Figure 3. Cm = closure reflex regains control by contraction of pelvic closure muscles, X‐ray Figure 2. The thin blue and red arrows in “B” reflect the struggle between the closure reflex (peak) and the opening reflex (trough)
By 2018, “OAB” pathogenesis was still considered unknown and incurable. 5 Ignoring the large body of evidence indicating otherwise, 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 a series of phenotypes for future research was proposed. 5
The invention of the new term “OAB” in 2002 was not without controversy. Tikinnen, in an important editorial “Does the imprecise Definition of Overactive Bladder Serve Commercial Rather than patient Interests?” 44 presciently quoted John Stuart Mill, “The tendency has always been strong to believe that whatever received a name must be an entity or being, having an independent existence of its own. Urge, frequency, nocturia, still meaningful definitions, 3 are now rarely used.
Zinner 45 trenchantly supported Tikkinen's views, “Creating simplistic definitions and labels leads to “no think.” In so doing we make it easy to tell someone what they have. “You have OAB.” Aha, now I understand. What do we do?” Many experts confronted with a clinical urge incontinence referral, perform urodynamics, which only confirms what they know, does not help the decision for management, but exposes the patients to a 15–20% possibility of urinary tract infection.
Urodynamic “DO,” the “gold standard” for diagnosis of OAB, 1 meets all Sackett's criteria for a failed test, 46 as demonstrated by a well‐documented urodynamic study; 73 women with “OAB” symptoms (urge, frequency, nocturia) treated by a different paradigm, 47 reported 86% cure of urge incontinence following pubourethral and uterosacral slings 23 ; 36/73 had “incurable” DO, whose cure confirmed Kuhn's criteria for a “new paradigm.” 48
1.1. The integral theory (IT) paradigm
The IT paradigm, 47 states that pathogenesis of bladder/bowel dysfunctions is mainly a consequence of collagen deficiency within the organ's suspensory ligaments. One part of the theory, “translational surgery,” using a tape to create a collagenous neoligament (“TVT”) for cure of stress urinary incontinence (SUI) 47 was a radical departure from the surgical principles of the Burch colposuspension. We created a model, Figure 1, based on anatomical, urodynamic, nonlinear studies (Figures 1, 2, 3, 4, 5, 6), plus two “translational” discoveries, urge incontinence was urodynamically identical to a normal micturition, 7 and a consequence 8 of a defective nonlinear, binary feedback control system. 8 We applied the model to challenge our hypothesis that OAB is mainly a premature uncontrolled micturition caused by ligament defects, and so, potentially curable surgically. 47
Figure 1.

Binary model summarizes the anatomical basis for OAB control and the chaos equation Xnext = Xc(1 − X). Schematic 3D sagittal view System in normal closed mode. Cortical control of OAB: Afferent impulses ‘X’ from stretch receptors ‘N’ are reflexly suppressed cortically (white arrows). When required, the cortex activates the micturition reflex. Peripheral control of OAB is by a musculo‐elastic mechanism which responds to cortical efferents (small arrows) to stretch vagina in opposite directions to support “N” and decrease afferent impulses “X”. The three directional muscles (large arrows), forward, pubococcygeus muscle “PCM”, backward, levator plate “LP”, and downward, conjoint longitudinal muscle of the anus “LMA” contract against the supporting ligaments, PUL (pubourethral) and USL (uterosacral), to stretch vagina tightly, much like the membrane of a drum. The stretched vagina supports the urine column, preventing activation of the stretch receptors “N”, decreasing afferent impulses to the cortex. Micturition: Central control (white arrows) relaxes, as does PCM (broken circle); this allows the posterior muscles LP and LMA to unrestrictedly open out the posterior wall of urethra (white broken lines) just before bladder evacuation by global detrusor muscle contraction. CX = cervix; CL = cardinal ligament; ATFP = arcus tendineus fascia pelvis. Dysfunction: Weakness in the muscles PCM, LP, LMA and/or the ligaments they contract against, PUL, USL, will affect the ability of the peripheral control mechanism to mechanically close urethra (incontinence), open it (obstructed micturition) or control micturition by bilateral stretching of vagina by the three opposite muscle forces to support “N” (urge incontinence). OAB, overactive bladder
Figure 2.
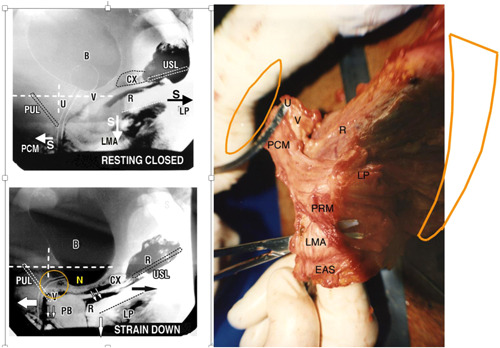
Anatomy and mechanics of the urethral closure reflex. Note: stretching of vagina in opposite directions by the oppositely‐acting muscle vectors both at rest and effort supports stretch receptors “N” from below. Right figure: Anatomical specimen from a female cadaver, cut away from its bony insertions. Bladder and vagina have been excised at the level of bladder neck. U = urethra; V = vagina. PCM = pubococcygeus muscle sweeping behind rectum (R) to merge with the contralateral side to form part of levator plate (LP). PRM = puborectalis muscle; PUL = insertion of pubourethral ligament into PCM; EAS = external anal sphincter. Upper figure: Closure reflex Resting closed ‘S’ denotes slow twitch muscle contraction of the three directional striated muscle forces, forwards PCM, backwards LP, downwards LMA (arrows) which contract against pubourethral ligament (PUL) anteriorly and uterosacral ligament (USL) posteriorly. CX = cervix; U = urethra; LP = levator plate muscle. PCM = pubococcygeus; LMA = conjoint longitudinal muscle of the anus; V = vagina; R = rectum; PB = perineal body. Lower figure: Augmented closure reflex on effort. Straining down Fast twitch directional vector forces stretch the vagina in opposite directions, forwards, backwards, downwards against PUL and USL, to close the distal and proximal urethra. Adequate elasticity is required in the bladder neck area of vagina “Zone of Critical Elasticity' (circle) so as to allow the opposite muscle forces which stretch the vaginal membrane to operate independently of each other
Figure 3.

Mechanics of the urethral opening reflex (Micturition). The forward vector (“S” resting closed) relaxes. The backward/downward vectors stretch the vagina backwards and downwards against USL to open out the posterior urethral wall. Adequate elasticity is required in the vagina to facilitate this action. The enlarged outlet exponentially decreases the resistance to evacuation by detrusor contraction inversely by the 4th power (Poiseuille's Law). Descent of bladder base as a “tug” is exactly as reported by Turner‐Warwick for the unstable bladder in his pioneering video X‐ray urodynamic studies. 8 USL, uterosacral ligament
Figure 5.

3D view of bladder showing zero resultant force 0‐0 at bladder neck. Weak pubourethral ligaments (PUL) weaken forward muscle forces, so the stronger backward muscles pull zero force 0‐0 backwards (blue broken lines). Weak uterosacral ligaments (USL) weaken the backward vector forces, so the stronger forward muscles pull zero force 0‐0 forwards (red broken lines)
Figure 6.

Modes of the bladder iterated by the Chaos Theory equation. Graph of an iterated Chaos Theory feedback equation XNEXT = cX(1 − X) applied to urodynamic experimental findings. 38 Vertical axis = afferent impulses Xnext; horizontal axis = Time. ‘d’(broken lines) = iteration with one variable (central inhibition) and ‘c’(unbroken lines) an inhibitory constant comprising, the sum of two variables, central inhibition plus peripheral suppression as in Figure 1. X = fraction of possible nerve impulses in the micturition circuit. “c” is the inverse of cortical/peripheral inhibition. The whole spectrum of bladder conditions can be graphically expressed by iteration of the feedback equation varying the constant “c”. If vaginal tension is excessive, e.g., Fowler's Syndrome, excessive elevation by Burch colposuspension, peripheral inhibition of stretch receptors “N”, Figure 1, is high, “c” is low. A nominal “c” value of 0.1 is assigned to the iteration and the system goes into retention. Stable closure (normal), micturition quiescent (“c” = 0.2); higher up slope c is low compliance, micturition activated but controlled (“c” = between 2 and 3); at the bifurcation, micturition overcomes closure (“c” > 3) the system swings between open and closed (OAB, DO) as in Figure 2.
1.2. Hypothesis
Three oppositely‐acting involuntary pelvic muscles contract reflexly against competent pubourethral (PUL) and uterosacral (USL) ligaments, to tension vagina to support urothelial stretch receptors “N” from below, Figure 1. Weak PUL or USL weaken these forces: “N” are cells with “neuron‐like” properties which respond to mechanical and chemical stimuli. 17 Excess afferent impulses to the brain are interpreted as “urge.” Sensitization and cross‐talk among urothelial cells and disturbed afferent signaling were later described in multiple studies. 49 , 50 , 51 , 52
2. METHODS
We aimed to validate the models' predictions for function and dysfunction by anatomical, video X‐ray, ultrasound, urodynamic, surgical and nonsurgical studies.
3. RESULTS
Key to the IT and every discovery based on the IT, are the three oppositely‐acting reflex striated muscle forces which act autonomically, Figure 1.
Closure reflex Figure 2 shows cadaveric anatomy of the three reflex muscles hypothesized to drive the three directional forces in the X‐ray: m. pubococcygeus (PCM), levator plate (LP), conjoint longitudinal muscle of the anus (LMA). The X‐ray is from a video myogram. It shows three oppositely‐acting muscle forces contracting against PUL and USL to close urethra distally and at bladder neck 6 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 47 (Video 1, ultrasound: https://www.youtube.com/watch?v=3vJx2OvUYe0).
Opening reflex, micturition X‐ray, myogram Figure 3. Under the influence of the micturition reflex, forward vector “PCM” relaxes; LP/LMA contract backwards against USL. (arrows) to stretch open the posterior urethral wall. External opening exponentially reduces resistance to urine flow, 6 , 9 , 10 , 11 , 13 , 15 , 16 as per Video 2 (micturition:https://www.youtube.com/watch?v=eiF4G1mk6EA%26feature=youtu.be). 13 , 15 , 16
Stretching vagina bilaterally to support bladder base stretch receptors. It is clear from Video 1 and comparing top and bottom xrays, Figure 2, that the opposite muscle forces have stretched the vagina bilaterally to tension it. This would support the urothelial stretch receptors “N” from below, as hypothesized, Figure 1.
3.1. Urodynamic studies prove DO is the same as an uncontrolled micturition
The first experiment, 7 demonstrated that the sequence of events in urodynamic DO was identical with Tanagho's observations in normal micturition 18 : sensation of urgency, fall in proximal urethral pressure, rise in detrusor pressure, urine loss. These are well demonstrated in Figure 4.
In the second experiment, 8 handwashing provoked sudden urine loss in 13/16 low compliance group with an unstable pattern. This was attributed to interruption of cortical suppression allowing the micturition reflex to dominate. In a 3rd experiment, digital support of bladder base caused sensory urgency to disappear in 18/20 women and in six, detrusor contraction also. These apparently disparate results were reconciled by a neurologically driven feedback control system consistent with a nonlinear Chaos Theory feedback equation XNEXT = cX(1 − X). 8 The equation was able to explain each bladder mode UAB, low compliance, normal closed state, OAB. 8
An important “translational” experiment by Drake 19 described autonomous “micromotions" which coalesce on demand to contract the detrusor. 19 This filled a gap in the description of the original feedback circuit, 8 that cortical control could not only directly suppress afferent impulses “X”, Figure 1, but also, facilitate AND suppress efferent impulses at the level of detrusor.
The Binary Model for bladder function and dysfunction 8 , 47 Figure 1, has two reflexes, EITHER closed OR open (micturition), mutually exclusive, cortically controlled, with 3 components, cortical, peripheral (striated pelvic muscle/ligaments), smooth muscle. It works like an electric switch controlling two different circuits, one at a time, either closed or open. Though control is autonomic, the cortex exerts voluntary control over both closure and evacuation reflexes. 35 , 36
The closure reflex is dominant and has three cortically co‐ordinated components.
1. Central reflex inhibition (white arrows, Figure 1) blocks afferent signals from the urothelial stretch receptors “N” reaching the cortex to activate the micturition reflex. 8
2. Peripheral control of micturition is by reflex action of three oppositely acting muscle forces (Figure 1) (Video 1: https://www.youtube.com/watch?v=3vJx2OvUYe0).
The directional muscles PCM, LP, LMA, stretch vagina in opposite directions against competent pubourethral (PUL) and uterosacral (USL) ligaments, Figure 2, much like a trampoline; the now tensioned vagina counteracts the hydrostatic pressure of urine to reduce afferent signals from “N” to cortex, Figure 1. 47
3. Suppression/activation of detrusor smooth muscle by efferent signals.
Efferent suppression signals are sent to detrusor by the cortex, Figure 1. Drake 19 viewed the detrusor as groups of smooth muscle controlled by a peripheral myovesical plexus, consisting of intramural ganglia and interstitial cells. The individual smooth muscles have characteristics which predispose to tonic contraction and urine loss. These include cell to cell propagation of the electrical signal, unstable all‐or‐none action potentials, low‐resistance pathways between cells and modification by excitatory or inhibitory nerves. 37 All exhibit “micromotions”, inherent spontaneous contractions occurring even in vitro. The cells are “en garde,” ready to expand under influence of cholinergic drugs or reduced by adrenergic drugs. 19 Bladder smooth muscles contract tonically when activated by the micturition reflex: see Video 2 (micturition: https://www.youtube.com/watch?v=eiF4G1mk6EA%26feature=youtu.be).
3.2. The opening reflex (micturition)
As bladder fills, urothelial stretch receptors “N”, Figure 1, send afferent signals to the pons. 17 The impulses constitute the afferent limb of a feedback loop. At this point, the opening (micturition) reflex is quiescent, kept under control by the closure reflex, centrally and peripherally. At a certain fullness, afferents escape from inhibition and reach the cortex. This sensation, sensory urgency, is the 1st part of the normal micturition. 7 , 8 , 18 If convenient, the full micturition reflex is activated. Cortical suppression (white arrows, Figure 1) ceases; the cortex accelerates the efferent loop of the reflex which causes the forward vector muscle PCM to relax (red circle, Figure 1); urethral pressure falls. 7 , 18 Fall in urethral pressure is the 2nd part of the micturition reflex. 18 Efferent nerve suppression of micromotions in the detrusor is replaced by activation; the micromotions expand and coalesce 19 LP and LMA, Figures 1 and 3, contract and pull open the posterior wall of urethra 9 , 10 , 11 , 13 , 47 immediately before detrusor contraction. These mechanical events precede detrusor contraction and urine expulsion, Figure 3. These are the 3rd and 4th parts of a normal micturition cycle, 18 as demonstrated urodynamically, Figure 4. Competent posterior ligaments (cardinal/uterosacral) are required so that the posterior muscles, LP/LMA, which contract against them, Figure 3, can exert their full opening force on the posterior wall of the urethra. Once full urine flow is established, posterior muscles relax; urine being incompressible, urethra remains open until micturition is completed. 16
3.3. Pathogenesis according to the binary model
Anatomical damage or dysfunction in any component of the binary model, Figure 3, stretch receptors “N” (infection, cancer), ligament weakness, muscle damage, afferent/efferent nerve injury, brain (bladder cancer, trauma, degeneration, enlarged prostate pressing on “N”) may interfere with the binary control system making it unstable. Degree of imbalance depends on site and extent. Because collagen maybe damaged at birth or by age, weak ligaments are the achilles heel in the system, and therefore, the major cause of bladder dysfunction. 47
3.4. Sensation of urge according to the binary model
Whatever the underlying cause, urge sensations reflect excess or uninhibited afferent impulses “X” from bladder stretch receptors “N”, Figure 1, e.g., from an “overfull” normal continent woman, urodynamic DO, inflammation or cancer irritating “N”, inability of the directional muscles to stretch vagina to support “N" because ligaments are loose, multiple sclerosis in efferent nerves or damage to cortical inhibitory centers.
3.5. The binary model explains urodynamic DO as an uncontrolled micturition
The binary control model interprets “detrusor overactivity” (DO) as a struggle for dominance between the closure and opening (micturition) reflexes, Figure 4. 8
The sequence of events in the urodynamic “DO” tracing, Figure 4, fit Tanagho's description for normal micturition, 18 confirming the basis of this work, that “OAB” is actually an activated micturition reflex which cannot be controlled by the central and peripheral mechanisms, Figure 1.
Because control is binary, the bladder can only be in one mode at any one time, open or closed. Inability of the closure reflex to maintain constant closure allows the opening reflex to dominate, even if only briefly, (urodynamic graph, Figure 4). The wavy pressure curves, Figure 4, graphically represent the struggle for control between the two states, closure “C” (red lines—pressure rise) and opening “O”. This is defined as “DO”. 4 The wave form is a consequence of the time interval to change from one mode to the other. The sudden steep fall in pressure labeled “Om” (opening muscles) is attributed to the active opening of the posterior urethral wall by the LP/LMA muscles, Figure 4. CP falls to 0 and 1.0 gm of urine is (temporarily) lost, until the closure reflex “Cm” (closure muscles), regains control.
Urodynamically The wavy form on the urodynamic graph at “3”, Figure 4, is the definition for DO and is a consequence of the time delay in any binary system, when it switches from one mode “open” to the other “closed” mode and vice versa. The thin blue and red arrows in “B”, Figure 4, reflect the struggle between closure reflex (peak) and opening reflex (trough).
3.6. Why urodynamics only diagnoses DO in 50% of cases
With reference to Figure 4, the micturition reflex “O” is activated ‘1” (sensory urgency.) At each stage, the closure reflex “C”, intervenes to reverse the micturition reflex. Even at the final micturition stage “4”, only 1 gm of urine is lost, before the urine leakage was reversed by the closure reflex. In 1993, 7 of 115 women with a history of urge incontinence subjected to a urodynamically monitored hand‐washing test, 93 had sensory urgency, 79 had fall in proximal urethral pressure, 49 had detrusor contraction, (DO) and 45 had urine loss. Of 93 with cystometric sensory urgency, 49 had DO, i.e., just above 50%. 7 As in Figure 4, the closure reflex “C” was able to inhibit the micturition reflex sufficiently to prevent progression to Stage 3 of the reflex (detrusor contraction) and Stage 4 (urine loss).
3.7. Unbalanced forces in binary control cause urge, SUI, emptying dysfunctions
With reference to Figure 5, The 3 reflex forward and backward muscles pull against each other and cancel out at zero point “0”, which is at bladder neck. 47 A weak PUL weakens the forward vector forces which contract against it; the now dominant backward vector forces unbalance the system, so zero point moves backwards (blue broken lines) to open out (funnel) the urethra, so urine is lost on effort (SUI); the weakened forward vector's contribution to vaginal stretching could not support “N”, causing urge as well as SUI (“mixed incontinence”). 8 Weak USLs also weaken backward vector contribution to supporting “N, to cause urge. Nor can weakened posterior forces open urethra; detrusor contracts against an unopened urethra: “obstructed micturition”. Weak USLs unbalance the system; relatively stronger forward forces move zero point forwards (red broken lines); vagina is stretched more tightly, supports “N” more tightly, which now requires higher urine volumes to activate micturition, ultimately leading to retention.
3.8. Challenging validity of the binary model by a Chaos feedback equation
As the binary feedback model, Figure 1, is the very basis for challenging existing OAB concepts, it, too, was challenged for validity by the nonlinear Chaos Theory feedback equation Xnext = Xc(1 − X), Figure 6, where “c” inversely represents reflex cortical plus peripheral suppression of afferent urothelial impulses “X” from urothelial stretch receptors “N”. X = fraction of possible nerve impulses emanating from “N” in the micturition circuit of the model. Numeral “1” = maximum possible number of impulses in the circuit 8 ; “c” represents the closure reflex's inhibition of micturition, central and peripheral, in the Chaos equation.
How “c” and “X” were calculated.
X was given a mean value of 0.5, being a sufficient number for input to the micturition centre from the urothelial stretch receptors “N”, Figure 1. “c” is inversely proportional to the quantum of inhibition. The clinical conditions were fitted as estimates into known “c” values from the Chaos Equation: Fowler's Syndrome (retention) with a very low “c” 0.1, normal (normally suppressed) 0.2, low compliance (activated but controlled) 2.0, urge incontinence (uncontrolled unstable) 3.6, example below.
Note: The calculation figures are given only as an example. At 3 decimal places, they are inherently inaccurate, as Lorenz's original discovery was based on inclusion of the 5th decimal number. We have only calculated on three.
| |
| 1st iteration: Xnext = 0.5 × 3.6 (1 − 0.5) = 0.9. | |
| 2nd iteration: Xnext = 0.9 × 3.6 (1 − 0.9) = 0.324. | |
| 3rd iteration: Xnext = 0.324 × 3.6 (1 − 0.324) = 0.788. | |
| 4th iteration: Xnext = 0.788 × 3.6 (1 − 0.788) = 0.601 | |
| 5th iteration: Xnext = 0.601 × 3.6 (1 − 0.399) = 0.864. | |
| 6th iteration: Xnext = 0.864 × 3.6 (1 − 0.136) = 0.423. | |
Normal function With reference to Figure 6, along the curve ‘c’, the micturition reflex has been activated from the urothelial stretch receptors “N”. If the peripheral and central control mechanisms of the model are all working properly, the system quickly goes into normal closed retention mode, and the micturition reflex is shut down.
Low compliance” and “DO” were consistent with a partially activated but controlled micturition reflex. 8 However, at the uppermost point on the upward trajectory “c”, Figure 6, the afferents “X” from the stretch receptors “N” exceed the combined ability of all cortical and peripheral inhibitors of the micturition reflex “c” in the model; the system swings temporarily into “Open Mode (bifurcation on line “c” Figure 6)”; the bladder has now entered the unstable “CHAOTIC ZONE”; it begins to swing between the “closed” and “open” attractors, splitting into larger fractions (4, 8, 16, etc.).
Urodynamically The swings between OPENunstable and CLOSEDunstable in Figure 6, are reflected urodynamically by a wavy form on urodynamic graphs, which is actually the definition for DO, “urodynamic detrusor overactivity”. The wavy form is a consequence of the time delay in any binary system, when it switches from one mode “open” to the other “closed” mode and vice versa, and reflects the struggle for dominance between closure reflex (peak) and opening reflex (trough).
4. OTHER CHALLENGES FOR THE MODEL TO EXPLAIN
4.1. Contradictory DO findings in asymptomatic women
DO, said to be the “gold standard” for OAB [3] was positive in 65% of asymptomatic continent women who had ambulatory urodynamics [4]. Prima facie this finding invalidated DO as a gold standard. Viewed via Figure 6, it was an activated but controlled micturition reflex varying somewhere along “stable closed” on the graph, Figure 6.
4.2. Period related OAB
Hormonal changes perimenstrually depolymerize collagen to relax the cervix, so as to allow egress of menstrual blood. USL attachment to cervix is also concomitantly loosened which also loosens USL's. Loose USLs restrict the capacity of vector forces to stretch vagina support ‘N’, Figures 1 and 5; the afferents XNEXT increase and the system may be pushed from “stable closed” into the Chaotic Zone at period time.
4.3. A thought experiment—Can a continent person fulfil threshold definition for OAB?
It is well known that a normal person pushed to breaking point by holding on too long has “an uncontrollable desire to pass urine,” and must run to the toilet. This immediately satisfies the definition for OAB. 1 A continent person wetting with turning on a tap or putting a key in the front door fulfils the definition for “urge incontinence”; by giving a signal to the micturition centre to remove cortical inhibition. Using filling cystometry, Van Meel and Wyndaele 53 found volumes, at which different sensations of bladder filling (first sensation of filling, first desire to void, strong desire to void) were very significantly higher in controls than in OAB patients. These findings are consistent with the Theory's contention that “OAB” is essentially the same as a normal, albeit, less controlled, micturition activated at a lower volume because of inability of the peripheral muscle/ligament mechanism to sufficiently dampen the flow of afferent impulses.
Challenging the model directly with “simulated operations” which mechanically support ligaments thought to be weak, Figure 7, For USL. Gently inserting the lower blade of a bivalve speculum into vaginal apex “speculum test,” 38 supports USL and diminishes urge and chronic pelvic pain 38 about 70–80% of the time. Decrease in pain and urge, even nocturia can be achieved by inserting a large menstrual tampon, or a roll gauze 41 into the posterior fornix to support the USLs. For PULs, a weak PUL will also weaken vaginal stretching to support “N”, Figure 5. A hemostat on one side of midurethra mechanically supports PUL to control not only SUI but also urge symptoms if their origin is a weak PUL (Video 3, mechanical control of SUI: https://youtu.be/0UZuJtajCQU) (VIdeo 4, mechanical control activated micturition reflex: https://youtu.be/1VO7kY7T7Ec).
Figure 7.

Diminution of urge by “simulated operations” “Simulated” operations work by mechanically supporting loose or damaged ligaments. The speculum is very gently inserted into the apex of the vagina. This stretches the vagina and supports the uterosacral ligaments (USL). The mechanical support restores firmness to the USL insertion point, so the vagina can now be stretched to support “N” and diminish the afferents to the brain. Left figure Gentle digital support of bladder base supports the hydrostatic pressure of the urine and prevents it activating the stretch receptors “N” at bladder base. The number of afferents to the cortex are therefore diminished and the feeling of urge subsides.Right figure Inserting the lower half of a bivalve speculum supports USLs. It relieves urge by restoring the strength of the opposite pelvic muscles; it relieves chronic pelvic pain by supporting the USLs which mechanically support the Frankenhauser and sacral plexuses
4.4. Challenging the model to explain OAB improvement by exercises
Table 1 Skilling found squatting‐based exercises in the female 20 strengthen the 3 reflex muscles and PUL and USL which they contract against, Figure 1. These exercises improve urge, nocturia and SUI symptoms by 50% in premenopausal women, 20 Table 2. Modified for young children, they cured day/night enuresis (OAB/nocturia equivalents) in 86% of children. 39 This was attributed to strengthening directional muscles, Figure 1 and collagenopoiteic effect of the exercises on PUL/USL. In the male, Burgio 54 found pelvic floor muscle training (PFMT) is effective for incontinence, as well as urgency, frequency, and nocturia.
Table 1.
Testing the model with the Chaos Theory Equation Xnext = Xc(1 − X)
| Mathematical iterations of clinical bladder phases |
|---|
| Normal inhibition |
| 1st iteration: Xnext = 0.5 × 0.2 (1 − 0.5) = 0.05 |
| 2nd iteration: Xnext = 0.05 × 0.2 (1 − 0.05) = 0.019 |
| 3rd iteration: Xnext = 0.019 × 0.2 (1 − 0.019) = 0.0037 |
| Partial inhibition (low compliance group) |
| 1st iteration: Xnext = 0.5 × 2 (1 − 0.5) = 0.5 |
| 2nd iteration: Xnext = 0.5 × 2 (1 − 0.5) = 0.5 |
| 3rd iteration: Xnext = 0.5 × 2 (1 − 0.5) = 0.5 |
| Inadequate inhibition (bladder instability group “OAB”, “DO”) |
| 1st iteration: Xnext = 0.5 × 3.6 (1 − 0.5) = 0.9. |
| 2nd iteration: Xnext = 0.9 × 3.6 (1 − 0.9) = 0.324. |
| 3rd iteration: Xnext = 0.324 × 3.6 (1 − 0.324) = 0.788. |
| 4th iteration: Xnext = 0.788 × 3.6 (1 − 0.788) = 0.601 |
| 5th iteration: Xnext = 0.601 × 3.6 (1 − 0.399) = 0.864. |
| 6th iteration: Xnext = 0.864 × 3.6 (1 − 0.136) = 0.423. |
Table 2.
Results from Skilling's squatting bases exercises in premenopausal women
| Fate of individual symptoms (n = 78) | ||
|---|---|---|
| condition | n | >50% improvement |
| stress incontinence | 69 | 57 (82%) |
| urge incontinence | 44 | 33 (68%) |
| frequency only | 12 | 10 (83%) |
| nocturia | 32 | 29 (90%) |
| pelvic pain | 17 | 13 (76%) |
| residual urine 202 ml | 23 | 71 ml |
4.5. Challenging the model directly to explain OAB cure with ligament repair operations
Tapes which create new collagen to repair PUL or USL in women with OAB/UAB, test the model directly. PUL and USL are the anchoring points of the 3 directional muscles, Figures 1 and 5, It is known that a tape at midurethra (midurethral sling) cures urgency as well as SUI (mixed incontinence) 50% of the time. 21 Grouped USL‐related symptoms, urge, frequency, nocturia, abnormal emptying, chronic pelvic pain (known as the “Posterior Fornix Syndrome”) have been cured or improved by USL repair, either native ligament 41 or slings. 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33
Challenging the model to explain OAB “phenotypes” was done in the context where the authors 5 stated the pathogenesis of “OAB” was unknown and there was no cure. 5 Instead, a series of phenotypes for future research was proposed. 5 Each is introduced and addressed in turn.
Underactive bladder “UAB” 40 (“urinary retention”) Model: weak USLs weaken downward opening vector (LMA Figures 1 and 5); detrusor empties against an unopened urethra.
Overactive bladder (OAB) Model: weak USLs weaken LP/LMA vectors which cannot stretch vagina to support “N”, Figures 1 and 5; “N” fires afferent impulses at lower bladder volume to activate micturition, sensed as an “urge” (OAB).
Myogenic dysfunction 5 caused by nerve damage was hypothesized by Brading and Turner to cause urgency but cannot explain OAB cure by USL repair. 5
Urotheliogenic hypothesis 5 Model: anything pressuring “N”, Figure 1, be it cancer, inflammation, will increase afferents to the cortex and be perceived as urgency. Botox may prevent coalescence of micromotions 19 or desensitize “N”. Sacral nerve stimulation (SNS) may inhibit afferents from “N” to the cortex.
Urethrogenic hypothesis 5 Model: lax vagina or ligaments may allow urine into the proximal urethra to activate the micturition reflex.
Supraspinal lesions, stroke, tumor, dementia 5 Model: interference with cortical suppression of an activated micturition reflex.
Damage to the nerve circuits 5 Model: depending on site, either OAB (efferent nerve damage) and/or retention, UAB (afferent nerve damage).
Obesity causation of OAB 5 Model: extra weight of abdominal contents on bladder and vagina, requires extra force from the directional muscles to stretch vagina to support “N”, Figure 1. Inability to do so may cause closure, OAB and emptying dysfunctions, Figure 1.
4.6. Comparison with computer model micturition switching circuit of the Groat et al.
De Groat et al. 17 proposed a computer model switching circuits, pontine micturition center (ascending limbs) and periaqueductal gray (PAG) descending limbs. The de Groat model is consistent with the cortical part of our binary model, Figure 1. There is no discussion about the role of the reflex muscles in their model, 17 which is core to explaining preceding hypotheses for OAB, urodynamic tests for DO and observed surgical cures.
5. CONCLUSIONS
The whole concept of OAB is in crisis. The population is ageing. An estimated 20% of the 3.5 billion women on the planet (30% for aged) have OAB. Elite bodies, 1 , 3 , 5 leaders in incontinence, have little to offer, except definitions, and even these are misleading. Anticholinergics, the first line of OAB treatment are relatively ineffective and known to cause Alzheimer's Disease. 2 OAB is stated “incurable with pathogenesis unknown” 5 ignoring multiple studies since 1997 which prove otherwise 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 ; up to 85% cure of OAB symptoms are achieved with daycare ligament repair surgery. How is it possible for expert bodies 1 , 5 to be unaware of the available cures outlined in this work? Is Tikinnen's editorial, 44 “Does the imprecise Definition of Overactive Bladder Serve Commercial Rather than patient Interests?” still relevant?
The whole ICS construct was broken forever by a recent study of 2933 pelvic organ prolapse surgeries 55 which reported 75% cure of “bothersome” urinary urge incontinence, further emphasized by an editorial which described this as “an emperor with no clothes” moment. 56
We present our thesis of OAB as an uncontrolled micturition as a starting solution to the OAB crisis. It is a fresh new direction which brings hope and the potential for cure, to OAB sufferers.
AUTHOR CONTRIBUTIONS
Substantial contributions to conception and design: all authors. Drafting and revising the article critically for important intellectual content: all authors. Final approval of the version to be published: all authors.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Open access publishing facilitated by The University of Western Australia, as part of the Wiley‐The University of Western Australia agreement via the Council of Australian University Librarians.
Petros P, Quaghebeur J, Wyndaele J‐J. Defining urge as an uncontrolled micturition explains pathogenesis, informs cure and helps solve the burgeoning OAB crisis. Neurourol Urodyn. 2022;41:1281‐1292. 10.1002/nau.24990
REFERENCES
- 1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub‐committee of the International Continence Society. Neurourol Urodyn. 2002;21:167‐178. [DOI] [PubMed] [Google Scholar]
- 2. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurology. 2016;73:721‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bates P. First report on the standardization of terminology of lower urinary tract function. urinary incontinence: procedures related to the evaluation of urine storage‐cystometry, urethral closure pressure profile, units of measurement. Br J Urol. 1976;48:39‐42.944601 [Google Scholar]
- 4. van Waalwijk van Doorn , E.S.C. 209 (Rijksuniversiteit Limburg, Maastricht, 1993).
- 5. Peyronnet B, Mironska E, Chapple C, et al. A comprehensive review of overactive bladder pathophysiology: on the way to tailored treatment. Eur Urol. 2019;75:988‐1000. [DOI] [PubMed] [Google Scholar]
- 6. Petros PE, Ulmsten UI. An integral theory and its method for the diagnosis and management of female urinary incontinence. Acta Obstet Gynecol Scand Suppl. 1990;153:1‐93. [PubMed] [Google Scholar]
- 7. Petros PE, Ulmsten U. Bladder instability in women: a premature activation of the micturition reflex. Neurourol Urodyn. 1993;12:235‐239. [DOI] [PubMed] [Google Scholar]
- 8. Petros PE. Detrusor instability and low compliance may represent different levels of disturbance in peripheral feedback control of the micturition reflex. Neurourol Urodyn. 1999;18:81‐91. [DOI] [PubMed] [Google Scholar]
- 9. Petros PE, Ulmsten U. Role of the pelvic floor in bladder neck opening and closure II: vagina. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:69‐73. [DOI] [PubMed] [Google Scholar]
- 10. Petros PE, Ulmsten U. Role of the pelvic floor in bladder neck opening and closure I: muscle forces. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:74‐80. [DOI] [PubMed] [Google Scholar]
- 11. Petros PE, Ulmsten U. Urethral and bladder neck closure mechanisms. Am J Obstet Gynecol. 1995;173:346‐348. [DOI] [PubMed] [Google Scholar]
- 12. Petros PP, Von Konsky B. Anchoring the midurethra restores bladder‐neck anatomy and continence. Lancet. 1999;354:997‐998. [DOI] [PubMed] [Google Scholar]
- 13. Bush MB, Messner‐Pellenc L, Petros P, Moron C, Millard R. A mechanical model for the opening of the human female urethra. Biomed Eng. 2005;00:210‐213. [Google Scholar]
- 14. Petros PE, Ulmsten U. Urethral pressure increase on effort originates from within the urethra, and continence from musculovaginal closure. Neurourol Urodyn. 1995;14:337‐346. [DOI] [PubMed] [Google Scholar]
- 15. Bush MB, Petros PE, Barrett‐Lennard BR. On the flow through the human female urethra. J Biomech. 1997;30:967‐969. [DOI] [PubMed] [Google Scholar]
- 16. Petros PEP, Bush MB. A mathematical model for micturition gives new insights into pressure measurement and function. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:103‐107. [DOI] [PubMed] [Google Scholar]
- 17. de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urol. 2004;64:7‐11. [DOI] [PubMed] [Google Scholar]
- 18. Tanagho EA. The anatomy and physiology of micturition. Clin Obstet Gynaecol. 1978;5:3‐26. [PubMed] [Google Scholar]
- 19. Drake MJ, Kanai A, Bijos DA, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int. 2017;119:22‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skilling PM, Petros P. Synergistic non‐surgical management of pelvic floor dysfunction: second report. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:106‐110. [DOI] [PubMed] [Google Scholar]
- 21. Rezapour M, Ulmsten U. Tension‐Free vaginal tape (TVT) in women with mixed urinary incontinence–a long‐term follow‐up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S15‐S18. [DOI] [PubMed] [Google Scholar]
- 22. Petros PE, Ulmsten U. The posterior fornix syndrome: a multiple symptom complex of pelvic pain and abnormal urinary symptoms deriving from laxity in the posterior fornix. Scand J Urol Nephrol. 1993;27:89‐93.8493476 [Google Scholar]
- 23. Petros PE. New ambulatory surgical methods using an anatomical classification of urinary dysfunction improve stress, urge and abnormal emptying. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:270‐277. [DOI] [PubMed] [Google Scholar]
- 24. Wagenlehner F, Muller‐Funogea I‐A, Perletti G, et al. Vaginal apical prolapse repair using two different sling techniques improves chronic pelvic pain, urgency and nocturia: a multicentre study of 1420 patients. Pelviperineol. 2016;35:99‐104. [Google Scholar]
- 25. Liedl B, Goeschen K, Yassouridis A, et al. Cure of underactive and overactive bladder symptoms in women by 1,671 apical sling operations gives fresh insights into pathogenesis and need for definition change. Urol Int. 2019;103:228‐234. [DOI] [PubMed] [Google Scholar]
- 26. Goeschen K, Gold DM. Surgical cure of chronic pelvic pain, associated bladder & bowel symptoms by posterior sling in 198 patients validates the Pescatori Iceberg principle of pelvic symptom co‐occurrence. Pelviperineol. 2017;36:84‐88. [Google Scholar]
- 27. Liedl B, Inoue H, Sekiguchi Y, et al. Is overactive bladder in the female surgically curable by ligament repair? Cent European J Urol. 2017;70:53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inoue H, Kohata Y, Fukuda T, et al. Repair of damaged ligaments with tissue fixation system minisling is sufficient to cure major prolapse in all three compartments: 5‐year data. J Obstet Gynaecol Res. 2017;43:1570‐1577. [DOI] [PubMed] [Google Scholar]
- 29. Inoue H, Yutaka K, Yuki S, et al. The TFS minisling restores major pelvic organ prolapse and symptoms in aged Japanese women by repairing damaged suspensory ligaments 12‐48 month data. Pelviperineol. 2015;34:79‐83. [Google Scholar]
- 30. Abendstein B, Brugger C, Furtschegger A, Rieger M, Rieger P. Study No. 12: role of the uterosacral ligaments in the causation of rectal intussusception, abnormal bowel emptying, and fecal incontinence. A prospective study. Pelviperineol. 2008;27:118‐121. [Google Scholar]
- 31. Petros PEP, Richardson PA. TFS posterior sling improves overactive bladder, pelvic pain and abnormal emptying, even with minor prolapse. A prospective urodynamic study. Pelviperineol. 2010;29:52‐55. [Google Scholar]
- 32. Himmler M, Rakhimbayeva A, Sutherland SE, Roovers JP, Yassouridis A, Liedl B. The impact of sacrospinous ligament fixation on pre‐existing nocturia and co‐existing pelvic floor dysfunction symptoms. Int Urogynecol J. 2021;32:919‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson P. Surgical cure of nocturia using 4 different methods based on strengthening the structural supports of the vaginal apex—a short review. Pelviperineol. 2015;34:92‐93. [Google Scholar]
- 34. Liedl B, Goeschen K, Sutherland SE, Roovers JP, Yassouridis A. Can surgical reconstruction of vaginal and ligamentous laxity cure overactive bladder symptoms in women with pelvic organ prolapse? BJU Int. 2019;123:493‐510. [DOI] [PubMed] [Google Scholar]
- 35. Barrington FJF. The relation of the hind‐brain to micturition. Brain. 1921;44:23‐53. [Google Scholar]
- 36. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Creed KE. Functional diversity of smooth muscle. Br Med Bull. 1979;35:243‐247. [DOI] [PubMed] [Google Scholar]
- 38. Chen YJ, Shie MY, Hung CJ, et al. Mechanical support of the posterior fornix relieved urgency and suburethral tenderness. Pelviperineol. 2013;32:55‐56. [Google Scholar]
- 39. Garcia‐Fernandez A, Petros PE. A four month squatting‐based pelvic exercise regime cures day/night enuresis and bowel dysfunction in children aged 7‐11 years. Cent European J Urol. 2020;73:307‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petros P, Abendstein B, Swash M. Retention of urine in women is alleviated by uterosacral ligament repair: implications for Fowler's syndrome. Cent European J Urol. 2018;71:436‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shkarupa D, Zaytseva A, Kubin N, Kovalev G, Shapovalova E. Native tissue repair of cardinal/uterosacral ligaments cures overactive bladder and prolapse, but only in pre‐ menopausal women. Cent European J Urol. 2021;74:372‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piñango‐Luna S, Level‐Córdova L, Petros PE, Yassouridis A. A low cost artisan tension‐free tape technique cures pelvic organ prolapse and stress urinary incontinence—proof of concept. Cent European J Urol. 2020;73:490‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abendstein B, Petros P. Role of the uterosacral ligaments in the causation of urinary and bowel dysfunction. Neurourol Urodyn. 2011;30:630. [DOI] [PubMed] [Google Scholar]
- 44. Tikkinen KA, Auvinen A. Does the imprecise definition of overactive bladder serve commercial rather than patient interests? Eur Urol. 2012;61:746‐748. [DOI] [PubMed] [Google Scholar]
- 45. Zinner NR. OAB. Are we barking up the wrong tree? A lesson from my dog. Neurourol Urodyn. 2011;30:1410‐1411. [DOI] [PubMed] [Google Scholar]
- 46. Sackett, D , et al. The interpretation of diagnostic tests. Clinical epidemiology. Little Brown and Co; 1985:59‐138. [Google Scholar]
- 47. Petros PE, Ulmsten U. An integral theory of female urinary incontinence. Acta Obstet Gynecol Scand. 1990;00(Supp 153):1‐79. [DOI] [PubMed] [Google Scholar]
- 48. Kuhn T The structure of scientific revolutions University of Chicago Press, Chicago, (1962).
- 49. Merrill L, Gonzalez EJ, Girard BM, Vizzard MA. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol. 2016;13:193‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Birder LA, Kullmann FA. Role of neurogenic inflammation in local communication in the visceral mucosa. Semin Immunopathol. 2018;40:261‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Birder LA, Andersson KE, Kanai AJ, Hanna‐Mitchell AT, Fry CH. Urothelial mucosal signaling and the overactive bladder‐ICI‐RS 2013. Neurourol Urodyn. 2014;33:597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Persyn S, De Wachter S, Wyndaele M, Birder L, Wyndaele JJ. Mechanisms of pelvic organ cross‐talk: impact of urethral ligation on the inhibitory rectovesical reflex. J Urol. 2014;192:1574‐1579. [DOI] [PubMed] [Google Scholar]
- 53. Van Meel TD, Wyndaele JJ. Reproducibility of urodynamic filling sensation at weekly interval in healthy volunteers and in women with detrusor overactivity. Neurourol Urodyn. 2011;30:1586‐1590. [DOI] [PubMed] [Google Scholar]
- 54. Burgio KL. Update on behavioral and physical therapies for incontinence and overactive bladder: the role of pelvic floor muscle training. Curr Urol Rep. 2013;14:457‐464. [DOI] [PubMed] [Google Scholar]
- 55. Karjalainen PK, Tolppanen AM, Mattsson NK, et al. Pelvic organ prolapse surgery and overactive bladder symptoms‐a population‐based cohort (FINPOP) . Int Urogynecol J. 2022;33:95‐105. 10.1007/s00192-021-04920- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petros PE. Editorial, The Emperor has no clothes. International Urogynecology Journal, 202in press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


