Abstract
Background
An effective regenerative protocol is key to reestablish and maintain the hard and soft tissue dimensions over time. The choice of the graft material and its properties also could have an impact on the results. To prevent alveolar ridge dimensional changes, since numerous graft materials have been suggested and in the past years, a growing interest in teeth material has been observed as a valuable alternative to synthetic biomaterials.
Aim
The aim of the study was to explore the histomorphometric outcomes of tooth derivative materials as used as bone substitute material in socket preservation procedure.
Methods
After alveolar socket preservation (ASP) procedures using autologous demineralized tooth as graft material prepared by means of an innovative device, was evaluated. A total of 101 histological samples, from 96 subjects, were analyzed by evaluating the total amount of bone (BV), residual tooth material (residual graft, TT), and vital bone (VB). The section from each sample was then split in nine subsections, resulting in 909 subsections, to allow statistical comparison between the different areas.
Results
It was not noticed a statistically significant difference between maxillary and mandibular sites, being the amount of VB in upper jaw sites 37.9 ± 21.9% and 38.0 ± 22.0% in lower jaw sites and the amount of TT was 7.7 ± 12.2% in maxilla and 7.0 ± 11.1% in mandibles. None of the other considered parameters, including defect type and section position, were statistically correlated to the results of the histomorphometric analysis.
Conclusions
ASP procedure using demineralized autologous tooth‐derived biomaterial may be a predictable procedure to produce new vital bone potentially capable to support dental implant rehabilitation.
Keywords: alveolar ridge reconstruction, autogenous, biomaterials, bone, bone augmentation, bone grafting, bone substitutes, histological analysis, implantology, prospective
Summary Box.
Alveolar socket preservation often requires the positioning of one bone substitute material to fill the socket, in order to create, after the healing phase, the conditions for placing one dental implant.
Autogenous teeth material, derived from processing the dentin of one extracted tooth, could be consider as a source of bone grafting material due to its characteristics, that are very similar to bone tissue.
On the basis of the histomorphometric outcomes found in the study, the use of autogenous teeth material grafting resulted in:
low quantities of residual graft material (TT%) over time
no signs of foreign body reactions and presented patterns of osteoconductivity and osteoindections
relatively high amount of vital new bone VB%, that we can hypothesize resulted by the high resorption/substitution rate of teeth material.
1. INTRODUCTION
Tooth extraction always implies the alveolar bone resorption, due to remodeling process of the tooth socket. In general terms, as reported in published literature, the amount of bone resorption following tooth extraction is 1.76 ± 2.03 mm vertically and 3.87 mm horizontally, on average. 1 Moreover, the scientific literature proved that the process of alveolar socket remodeling reaches the highest rate during the first year after extraction, and it also leads to a significant and clinically detectable decrease of soft tissue trophism, that is directly related to bone reduction. 2 One systematic review of the literature published in 2011 affirmed that the width reduction during the healing phase could be of 2.6 and 4.6 mm and between 0.4 and 3.9 mm in height without the positioning of any bone substitute material. 3 Moreover, the amount of bone volume loss during the healing phase of extraction sockets in non‐molar sites is strictly dependent on the vestibular bone width and integrity, being lower when the bundle bone is thick. 4
The loss of alveolar bone volume in the tooth extraction area could be a very relevant issue, particularly in cases when there is the need of placing dental implants to substitute the missing tooth. Several techniques were proposed in order to maintain the bone volume and reduce bone resorption during the healing period, thus reducing the physiological bone reduction. 5 Moreover, the literature explored in depth the efficacy of placing in the extraction socket one biomaterial (autogenous, allogenic, or xenogenic) in terms of clinical and histomorphometric outcomes. 6
In general terms, ideal bone substitute should be safe, cost‐effective, and absolutely biocompatible. It should prevent foreign body reaction or adverse inflammatory reaction. It must be sterile and should induce or not limit bone formation process during the healing period. It should completely or almost completely re‐absorbable by the organism, leaving the space for newly formed bone. 7
For such purposes, different types of graft material were used. Didactically, they could be divided in deproteinized animal matrices, mostly with bovine or porcine derivation, industrial matrices (hydroxyapatites and glasses) and, new on the market, sugar derived matrices. 8
While all materials presented good results in terms of bone regeneration and volume maintenance, a recent literature systematic review with network meta‐analysis, suggested animal matrices as the best material for post‐extraction site development. 9
However, different studies highlighted as this group of graft materials may produce a delayed bone healing as it was hypothesized that the bone substitute material, although providing a scaffold for bone regeneration, is not substituted easily by newly formed bone. 8
The use of tooth material as autogenous bone substitute was proposed more than 50 years ago, considering the characteristics of the dentin and enamel and in search of absolute biocompatibility. 10 Indeed, dentin is made of 65% of inorganic component made of 3Ca3(PO4)2Ca(OH)2 hydroxyapatite with crystals about 10 times larger than bone and 300 times smaller than enamel, similar in shape to those that constitute the radicular cement. 11 The organic component (35%) was made by collagen (mainly type I) and other non‐collagenic proteins. In general, dentin presents many similarities to bone tissue, thus justifying the possibility of using it as bone substitute material. 12 Moreover, the higher density of tooth materials, as compared to the bone, could allow to achieve a slow resorption rate which is favorable in the bone healing process. 13 Several techniques and devices were proposed and described for using the tooth material as bone substitute. 14 , 15 , 16
One procedure, developed in Italy, can predictably fragmentate, decontaminate, and demineralize the tooth material to be grafted when needed in the same subject. 17
The clinical performances of the material derived from such procedure were inquired in several studies of the same research group, performed over time, also in a multi‐centric manner. 18 , 19
The aim of the present study was to better evaluate the histomorphometric outcomes of the application of tooth‐derived autogenous bone substitute material in a large cohort of patients treated with alveolar socket preservation (ASP), with particular focus on the amount of VB% that resulted over time.
2. MATERIALS AND METHODS
2.1. Patient selection
Subject were recruited from patients requiring tooth extraction in the upper and lower maxillae between April 2019 and January 2021, in 11 private dental clinics. The study was performed following the standard protocol for socket preservation and implant placement without adopting any experimental procedure. All data were anonymized. The present study was carried out following the principles embodied in the Helsinki Declaration, in its latter form. 20
On March 21st 2019, the University of Chieti Ethics Committee authorized the clinical study protocol on a human model registered under the number: 638—21/3/19.
2.2. Inclusion and exclusion criteria
The histomorphometric analysis was performed on samples selected according to the following inclusion criteria:
subjects who underwent surgical intervention for tooth removal and ASP by using only tooth‐derived bone substitute (Tooth Transformer®—Tooth Transformer srl).
subjects who underwent implant placement in the same site of socket preservation.
subjects with partially missing of buccal or palatal bone walls.
subjects who did not present any systemic diseases and conditions that could cause an impairment of the bone metabolism.
Cases referring to pregnant or lactating women were excluded.
2.3. Surgical protocol
All the surgeries were performed by trained expert clinicians, with more than 10 years of clinical training in the field. Briefly, the preparation of the tooth‐derived substitute material followed the procedure here described. First, the extracted tooth was accurately cleaned from residual calculus and thoroughly polished by using a diamond drill (ref. 6855—Dentsply Maillefer) with abundant saline solution irrigation. The procedure required the complete remotion of any root filling material from the selected tooth. Afterwards, the tooth was cut in small pieces and the fragments were placed in the mill (Tooth Transformer®—Tooth Transformer srl) previously prepared, following the procedure described in previously published studies. 17
In general terms, the entire tooth was used. In particular, in most cases (endodontically treated teeth and teeth that served as prosthetic support) the enamel was missing because removed during the preparation procedure. Moreover, most of the cement was removed during the cleansing procedure. So, the vast majority of the tooth‐derived material is made of dentin.
After 25 min, the material was ready to be placed in the recipient area, prepared by performing cortical perforations with a 1.5–2 mm spiral drill, to enhance the bone healing process. 21
Once grafted, the defect was covered with a resorbable membrane (Osseo guard—Zimmer Biomet).
Dental implants were placed in the grafted area after a healing period that ranged between 3 and 12 months. For the preparation of implant site, a 3 mm trephine bur (Meisinger) was used under copious irrigation with saline solution associated or not with other specific drills, following the standard protocol. Implants were positioned with different torque values. The healing period was not shorter than 3 months.
2.4. Histological technique
The specimens were decalcified and the paraffin‐embedded and cut. Samples were fixed in 10% neutral buffered formalin (37% formaldehyde solution 10 ml, NaCl 0.8 g, Potassium phosphate monobasic 0.4 g, potassium phosphate dibasic 0.65 g, distilled water 90 ml) for 7 days. Decalcification was carried out with disodium EDTA pH 7 until total decalcification, the endpoint was determined physically. Specimens were then dehydrated in ethanol of rising concentration from 70% to 100%, cleared with xylene, and embedded in paraffin; all the chemical use were manufactured from Carlo Erba reagents. Paraffin slides were obtained with a Lecia RM2245 rotatory microtome and placed on superfrost microscope glass slides e mounted with Biomout HM bio‐optica. The histological images obtained from the transmitted light microscope (Olympus) were digitized through a digital camera and analyzed by means of an image analysis software IAS 2000 (QEA).
The median section of each sample was split in nine subsections in order to evaluate if differences existed between different portion of the samples (lateral ones versus central ones or upper vs bottom).
With histomorphometric analysis we have distinguished:
BV% which represented the percentage of mineralized tissue with exclusion of medullary tissues.
TT% which represented the percentage of the volume occupied by the remaining graft, namely dentin.
VB% which represented the percentage of vital bone, excluding medullary tissues.
The amount of BV% was the sum of TT% and VB%.
Each subsection was measured using ImageJ program. In total, 909 subsections were measured.
2.5. Statistical methods
Descriptive statistics is provided through mean, median and standard deviation for continuous variables. For non‐continuous categorical variables, frequencies were calculated. The normality of variables distribution was evaluated by Shapiro–Wilk test.
Differences between sex, location (maxilla vs mandible), type of bone defect, and healing time were calculated using ANOVA and Student's t‐test, for normally distributed variables BV%, TT%, VB%).
Linear regression analysis was adopted in order to evaluate the influence of baseline parameters (sex, healing time, location, defect type) on histomorphometric parameters, namely BV%, TT%, VB%. The position of the examined subsection was considered as a further parameter and its influence on histologic outcomes was calculated.
The level of significance was p < 0.05.
3. RESULTS
A total of 101 biopsies belonging to different sites from 96 subjects (50 females and 46 males), aged 56.3 ± 14.7 years, were retrieved and analyzed. The distribution of sites is presented in Figure 1.
FIGURE 1.
A total of 101 biopsies belonging to different sites from 96 subjects. The figure shows the distribution of sites. There is a strong prevalence of the first molar sites
The mean healing time was 5.2 ± 1.9 months. The summary of the results of histomorphometric analysis are presented in Table 1. No statistically significant difference was registered between maxillary and mandibular sites, being the amount of VB% in upper jaw sites 37.9 ± 21.9% and 38.0 ± 22.0% in lower jaw sites and the amount of TT% 7.7 ± 12.2% in maxilla and 7.0 ± 11.1% in mandible.
TABLE 1.
A total of 101 histological samples, from 96 subjects, were analyzed.
| Without bone substitute (N = 101) | |
|---|---|
| % Vital bone | 38.0 ± 21.9% |
| % TT | 7.5 ± 11.9% |
Note: This table indicates the mean cumulative results of histomorphometric analysis.
None of the other considered parameters (including defect type and section position) were statistically correlated to the results of histomorphometric analysis.
The amount of VB% and TT% were evaluated to be significantly correlated by the statistical point of view (Pearson correlation −0.448, p < 0.001).
Figure 2 shows the percentage of bone in relation to healing time, while Table 2 provides the results of all sections analyzed. No differences in histomorphometric outcomes were observed for more coronal sections or lateral ones, as compared to the central ones, as reported in Table 2.
FIGURE 2.

The figure represents the percentage of bone in relation to healing time. A total of 101 biopsies were compared at the collecting time.
TABLE 2.
Each sample of the 101 histological samples was split into nine subsections.
| Section 1 | Section 2 | Section 3 |
|---|---|---|
| %Vital Bone: 25.6 ± 22.9 | %Vital Bone: 31.4 ± 21.7 | %Vital Bone: 37.0 ± 23.7 |
| %TT: 14.2 ± 14.5 | %TT: 12.6 ± 16.2 | %TT: 10.3 ± 12.6 |
| Section 1, 2, 3 | ||
| %Vital Bone: 32.4 ± 19.6 | ||
| %TT: 13.1 ± 12.9 | ||
| Section 4 | Section 5 | Section 6 |
|---|---|---|
| %Vital Bone: 39.3 ± 22.9 | %Vital Bone: 42.5 ± 21.4 | %Vital Bone: 42.3 ± 19.8 |
| %TT: 8.2 ± 12.3 | %TT: 7.0 ± 10.3 | %TT: 5.3 ± 9.0 |
| Section 4, 5, 6 | ||
|---|---|---|
| %Vital Bone: 42.1 ± 19.4 | ||
| %TT: 7.0 ± 9.5 |
| Section 7 | Section 8 | Section 9 |
|---|---|---|
| %Vital Bone: 43.8 ± 20.7 | %Vital Bone: 41.1 ± 20.7 | %Vital Bone: 32.4 ± 17.6 |
| %TT: 3.9 ± 8.2 | %TT: 2.6 ± 6.4 | %TT: 2.0 ± 4.8 |
| Section 7, 8, 9 | ||
| %Vital Bone: 39.2 ± 16.4 | ||
| %TT: 2.5 ± 5.1 | ||
Note: Therefore, 909 subsections were histomorphometrically analyzed and the table shows the results of all sections. It is interesting to note the amount of mean vital bone in each subsection.
Figures 3 and 4 show some examples of histologic analysis.
FIGURE 3.

Example of histologic analysis. The sample was subdivided in nine subsections following the marginal dimension of the image. The dentin granules (indicated with X) are homogeneously present in all sections of the image. Magnification 50×: stain hematoxylin and eosin
FIGURE 4.

Example of a single subsection histologic analysis. X refers to the dentin granules. The bone is pink colored. The yellow lines surrounding the surfaces are useful to evaluate the relative percentage of individual histology components. Magnification 100×: stain hematoxylin and eosin
In Figures 5 and 6, it can be observed, with high magnification histologic images, the tooth granules totally buried in the bone and in intimate contact with it. In some cases (Figure 7), it is possible to found osteoclasts during the reabsorption phase with no differences between tooth and bone.
FIGURE 5.

Newly formed tissue is closely linked to the tooth bone graft substitute (indicated with X). It is possible to see how osteoblasts are able to deposit new tissue around the entire perimeter of the granule, regardless the presence of irregularities. Magnification 200×: stain hematoxylin and eosin
FIGURE 6.

The new bone matrix deeply penetrates into the non‐resorbed dentin granules. In the upper part of the image, there is newly formed bone. In the underlying part there is dentin, recognizable by the dentinal tubules firmly attached to the bone. As you can see, the junction between the two tissues does not present any kind of discontinuity. Magnification 1000×: stain hematoxylin and eosin (reproduced with permission from Minetti E. Bone regeneration in implantology: tooth as a graft. ©Edra, Milano 2021)
FIGURE 7.

Characteristic signs of resorption on dentin granule profile (indicated with X) due to osteoclasts (indicated with O). The granule is completely surrounded by the new bone with total continuity between the two different types of tissue (graft and bone) called cement line 200× magnification: stain hematoxylin and eosin (reproduced with permission from Minetti E. Bone regeneration in implantology: tooth as a graft. ©Edra, Milano 2021).
One hundred and one histological samples were analyzed splitting in nine subsections for each core.
The purpose of these subsections is to allow statistical comparison analysis between the different sampling area. It is interesting to note that each subsection has mean VB% ranging from 25.6% (section 1) to 43.8% (section 7), showing a high homogeneity in all 909 sections measured. The TT% highest quantity was 14.2% (section 1) and the minimum quantity was 2.0% (section 9) (Table 2). Analyzing all the subsections using the dispersion graphic, it is possible to note that the values are homogenous in their dispersion and concentrated in a zone where the residual graft is lower than 20% (Figure 8).
FIGURE 8.
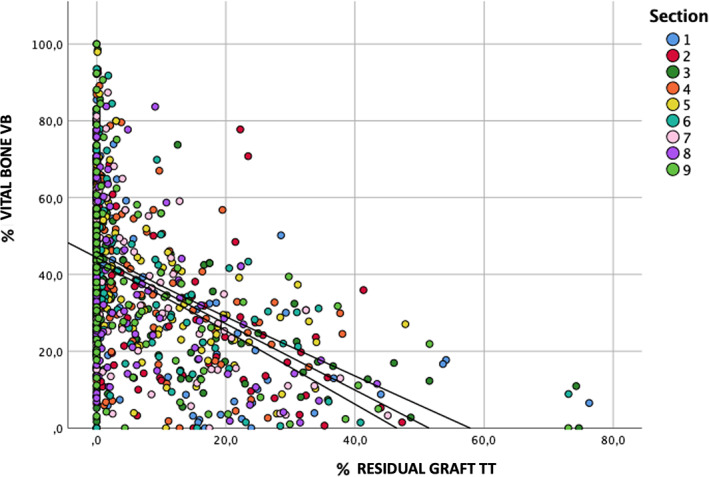
Dispersion graph showing the distribution of residual %TT dentin graft values related to %Bone. The colors represent the different subsections of the samples. It is noticeable that the higher the proportion of residual TT the lower the proportion of new bone. As it is observable, the vast majority of the results are located in the area that represents a percentage of residual TT lower than 20%. We found no differences among different sections.
The distribution of the newly formed VB% in relation to the healing time is presented in Figure 9. The amount of new formed VB% changes over time and is greater between 6 and 7 months and decrease after 10 months (Figure 2).
FIGURE 9.

Dispersion graph showing the distribution of residual %TT values related to %Bone. The colors represent different healing times. It is noticeable that the higher the proportion of residual TT the lower the proportion of new bone. As it is observable, the vast majority of the results are located in the area that represents a percentage of residual TT lower than 20%.
Subjectively, the operators referred no different in their feelings and sensation while preparing the sites as compared to native bone, but we have not collected quantitative measures about it.
4. DISCUSSION
The present study is the first prospective histomorphometric evaluation to assess the effectiveness of the tooth as a graft in ASP procedures.
The processes of healing of the post‐extraction sites after bone grafting procedures has been widely studied by a large number of clinical trials analyzing different graft materials. 22 , 23 , 24 , 25
Recent systematic reviews and meta‐analysis demonstrated overall intriguing performances of xenogenic materials. Regarding the residual graft material, the lowest rates are displayed by procedures with allografts (12.4%–21.11%), while those using xenografts and alloplasts showed higher results at 7 months (37.14 and 37.23%). 26 From a clinical standpoint, in fact, it was clarified that, if sealed using a collagen membrane, this family of graft may reduce the bony crest tridimensional shrinkage. However, from a histological point of view, those materials, due to their production process, may result in an incomplete resorption also in the long run.
Medicine is increasingly evolving toward personalized minimally invasive therapies that reduce the impact on patients. 27 , 28 , 29 Thus, the maintenance and reuse of tissues or the creation of “biomimetic” materials should be considered as important issues aiming at reducing the impact on the subjectivity of the patients of surgical procedures. Considering this aspect, there has been a growing interest in teeth and tooth derivatives as valuable alternatives to synthetic biomaterials for bone‐grafting procedures, such as ASP. 30 , 31 Furthermore, the idea of recycling compromised teeth that need to be extracted, instead of discarding them, thereby avoiding the use of expensive heterologous or synthetic bone substitutes, is expected to be well‐accepted by patients.
This graft derives from the patient extracted tooth and is produced by processing the tooth itself with a recently introduced device, able to shred and fully decontaminate dental materials and transform them into a grafting material usable for the treatment of different types of bone defects in oral surgery procedures. 32 Following the procedure, that requires one to 5 min for cleaning and cutting the tooth (depending on tooth conditions) and 25 min for processing, there is the availability of 0.5–3 grams of material, depending on the tooth.
The strong points in favor of using such material are as following: it is totally autogenous; it does not require an additional surgical site for harvesting bone graft; the dentin structure and composition is very similar to that of bone 33 ; it contains BMP‐2, that is made available by the demineralization procedure, giving to the material osteoinductive properties (in addition to the osteoconductive features, provided by the porous 3‐dimensional matrix). 13 , 34 , 35 In addition to these points, there is the possibility to store the extracted teeth for a long time before the surgery. 36 , 37
In the present study, whereas each histology was carried out in cavities completely filled with dental granules, the fact that there is no difference between the subsections can be interpreted as a predictive factor of a more uniform bone consistency at the time of the implant placement, even if the study was not designed specifically to demonstrate this aspect. Moreover, the newly formed tissue is closely connected with the tooth‐derived material and the distribution appears uniform. As it can be seen from the detail in Figure 5, there is a total continuity between the two different types of tissue, the graft and the bone. 38
The bone regeneration scaffold is supposed to provide structural support to the newly forming bone, share mechanical properties similar to the host bone, be biocompatible, and biodegrade at a rate synergistic with bone remodeling. Considering this issue, controlling pore size and number is essential in creating a scaffold that meets the needs of the specific repair site 39 ; indeed, bone is naturally porous, with pore sizes ranging from 1 to 100 μm. 40 , 41 , 42
Thanks to the dentin tubules enlarged by demineralization, as shown by Tanoue, 43 the new bone matrix is able to penetrate deeply into the non‐resorbed dentin granules as shown in Figure 6 and this is also due to the fact that dentin is recognized by the body as its own bone tissue. 44 , 45
The granules, when not completely resorbed, show characteristic signs of resorption on their profile as due to the work of the osteoclasts (Figure 7). 46
Furthermore, they act as the basis for the deposition of a new bone matrix, which is generated by the osteoblasts present around the not resorbed granules.
The main limitation of the present study was represented by the fact that the regenerated volumes and their evolution over time were not considered. Moreover, All the biopsies were made in the regenerated bone independently to the residual volume. However, a tridimensional analysis may be helpful for a complete comparison to other graft materials. The quantity of new bone and residual graft are very similar to autologous bone tissue. 47
Further studies must be promoted with many surgeries, and a long observation time could be needed to evaluate and understand the real impact of the demineralized tooth graft materials in bone regeneration of oral and maxillofacial procedures. 48
The data collected were included in a dispersion graphic where each subsection was measured for values of newly vital bone quantity and graft residual.
Therefore, it is possible to note a high spot thickening in the zone from 5 to 0% of residual graft, while the central oblique line indicates the trend mean and the two lateral lines the confidence interval trend. We should observe that as the TT% decreases, the amount of VB% increases.
It can also be seen that the density is higher at the top of the curve where the confidence interval is very narrow and suggest the variability is greater when the graft material residue is greater.
On the opposite, when the graft residue is smaller, the variability is lower and therefore indicates the greater accuracy of the measurement.
The bone density after a regenerative procedure is conditioned by healing time, patient age and gender, jaw (maxilla, mandible), and location in mouth (posterior, anterior). 49
At the same time, the new regenerated tissue, with tooth as a graft, meets a sort of homologation with the receiving tissue since the stimulation resulting from the reabsorption of the granules is exhausted.
This process could be called proximity homologation and is determined by the normal modality of bone metabolism. The tooth seems to behave like autologous bone where the resorptive pattern may tend to follow the natural anatomy of the original ridge. 50
5. CONCLUSIONS
Within the limitations of the present study, natural teeth could be considered as a source of bone substitute material.
Further studies must be promoted with a large number and a long observation time, comparing with a gold standard, could be needed to evaluate and understand the real impact of the demineralized tooth graft materials in bone regeneration of oral and maxillofacial procedures.
AUTHOR CONTRIBUTIONS
Elio Minetti: concept, design, acquisition data. Stefano Corbella: drafting, data analysis interpretation. Silvio Taschieri: critical revision of article, acquisition data. Luigi Canullo: critical revision of article, approval final version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge: Dr. Marcello Contessi, Dr. Fabrizio Carini, Dr. Ugo Gambardella, Dr. Edoardo Giacometti, Dr. Johannes H. Schmitz, Dr. Mauro Libertucci for their assistance in surgeries and Dr. Paolo Savadori for his histological analysis. Open Access Funding provided by Universita degli Studi di Milano within the CRUI‐CARE Agreement.
Minetti E, Corbella S, Taschieri S, Canullo L. Tooth as graft material: Histologic study. Clin Implant Dent Relat Res. 2022;24(4):488‐496. doi: 10.1111/cid.13097
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Van Der Weijden F, Dell'Acqua F, Slot DE. Alveolar bone dimensional changes of post‐extraction sockets in humans: a systematic review. J Clin Periodontol. 2009;36:1048‐1058. [DOI] [PubMed] [Google Scholar]
- 2. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single‐tooth extraction: a clinical and radiographic 12‐month prospective study. Int J Periodontics Restorative Dent. 2003;23:313‐323. [PubMed] [Google Scholar]
- 3. Ten Heggeler JM, Slot DE, Van der Weijden GA. Effect of socket preservation therapies following tooth extraction in non‐molar regions in humans: a systematic review. Clin Oral Implants Res. 2011;22(8):779‐788. [DOI] [PubMed] [Google Scholar]
- 4. Chappuis V, Engel O, Reyes M, Shahim K, Nolte L‐P, Buser D. Ridge alterations post‐extraction in esthetic zone:a 3D analysis with CBCT. J Dent Res. 2013;92(12 Supl):195S‐201S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Del Fabbro M, Bucchi C, Lolato A, Corbella S, Testori T, Taschieri S. Healing of postextraction sockets preserved with autologous platelet concentrates. A systematic review and meta‐analysis. J Oral Maxillofac Surg. 2017;75(8):1601‐1615. doi: 10.1016/j.joms.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 6. Canellas JVDS, Ritto FG, Figueredo CMDS, et al. Histomorphometric evaluation of different grafting materials used for alveolar ridge preservation: a systematic review and network meta‐analysis. Int J Oral Maxillofac Surg. 2020;49(6):797‐810. doi: 10.1016/j.ijom.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 7. Fernandez de Grado G, Keller L, Idoux‐Gillet Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018;4(9):2041731418776819. doi: 10.1177/2041731418776819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanz M, Dahlin C, Apatzidou D, et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J Clin Periodontol. 2019;46(Suppl 21):82‐91. [DOI] [PubMed] [Google Scholar]
- 9. Canullo L, Pesce P, Antonacci D, et al. Soft tissue dimensional changes after alveolar ridge preservation using different sealing materials: a systematic review and network meta‐analysis. Clin Oral Investig. 2022;26(1):13‐39. doi: 10.1007/s00784-021-04192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999‐1008. [DOI] [PubMed] [Google Scholar]
- 11. Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed). 2011;3:711‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boskey AL. Mineralization of bone and teeth. Elements. 2007;3:387‐393. [Google Scholar]
- 13. Nampo T, Watahiki J, Enomoto A, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010. Sep;81(9):1264‐1272. doi: 10.1902/jop.2010.100016 [DOI] [PubMed] [Google Scholar]
- 14. Kim KW. Bone induction by demineralized dentin matrix in nude mouse muscles. Maxillofac Plast Reconstr Surg. 2014;36(2):50‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu D, Zhou L, Lin J, Chen J, Huang W, Chen Y. Immediate implant placement in anterior teeth with grafting material of autogenous tooth bone vs xenogenic bone. BMC Oral Health. 2019;19:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Binderman I, Hallel G, Nardy C, Yaffe A, Sapoznikov L. A novel procedure to process extracted teeth for immediate grafting of autogenous teeth. J Interdiscipl Med Dent Sci. 2014;2:6. [Google Scholar]
- 17. Minetti E, Casasco A, Casasco M, Corbella S., Giacometti, E , HKL Ho, Palermo A, Savadori P, Taschieri S. Bone regeneration in implantology: tooth as a graft. 2021 EDRA ed. ISBN: 978‐88‐214‐5353‐
- 18. Minetti E, Giacometti E, Gambardella U, et al. Alveolar socket preservation with different autologous graft materials: preliminary results of a multicenter pilot study in human. Materials. 2020;13:1153. doi: 10.3390/ma13051153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minetti E, Celko M, Contessi M, et al. Implants survival rate in regenerated sites with innovative graft biomaterials: 1 year follow‐up. Materials. 2021;14:5292. doi: 10.3390/ma14185292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21. Danesh‐Sani SA, Tarnow D, Yip JK, Mojaver R. The influence of cortical bone perforation on guided bone regeneration in humans. Int J Oral Maxillofac Surg. 2017;46(2):261‐266. doi: 10.1016/j.ijom.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 22. Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda). 2016;31(3):233‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacBeth N, Trullenque‐Eriksson A, Donos N, Mardas N. Hard and soft tissue changes following alveolar ridge preservation: a systematic review. Clin Oral Implants Res. 2017;28(8):982‐1004. [DOI] [PubMed] [Google Scholar]
- 24. Atieh MA, Alsabeeha NH, Payne GT, Duncan W, Faggion CM, Esposito M. Interventions for replacing missing teeth: alveolar ridge preservation techniques for dental implant site development. Cochrane Database Syst Rev. 2015;2015(5):CD010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corbella S, Taschieri S, Francetti L, Weinstein R, del Fabbro M. Histomorphometric results after postextraction socket healing with different biomaterials: a systematic review of the literature and meta‐analysis. Int J Oral Maxillofac Implants. 2017;32(5):1001‐1017. [DOI] [PubMed] [Google Scholar]
- 26. De Risi V, Clementini M, Vittorini G, Mannocci A, De Sanctis M. Alveolar ridge preservation techniques: a systematic review and meta‐analysis of histological and histomorphometrical data. J Implants Res. 2015;26(1):50‐68. doi: 10.1111/clr.12288 [DOI] [PubMed] [Google Scholar]
- 27. Nibali L, Koidou V, Salamone S, et al. Minimally invasive non‐surgical vs. surgical approach for periodontal intrabony defects: a randomised controlled trial. Trails. 2019;20(1):461. doi: 10.1186/s13063-019-3544-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ower P. Minimally‐invasive non‐surgical periodontal therapy. Dent Update. 2013;40(4):289‐290. doi: 10.12968/denu.2013.40.4.289 [DOI] [PubMed] [Google Scholar]
- 29. Gual‐Vaques P, Polis‐Yanes C, Estrugo‐Devesa A, Ayuso‐Montero R, Mari‐Roig A, Lopez Lopez J. Autogenous teeth used for bone grafting: a systematic review. Med Oral Patol Oral Cir Bucal. 2018;23(1):e112‐e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park M, Mah YJ, Kim DH, Kim ES, Park EJ. Demineralized deciduous tooth as a source of bone graft material: its biological and physicochemical characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(3):307‐314. [DOI] [PubMed] [Google Scholar]
- 31. Bono N, Parsini P, Candiani G. BMP‐2 and type I collagen preservation in human deciduous teeth after demineralization. J Appl Biomater Funct Mater. 2019;17(2):2280800018784230. [DOI] [PubMed] [Google Scholar]
- 32. Yeomans JD, Urist MR. Bone induction by decalcified dentin implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999‐1008. [DOI] [PubMed] [Google Scholar]
- 33. Kumar GS. Orban's Oral Histology & Embryology. 14th ed. Elsevier; 2015. [Google Scholar]
- 34. Bang G, Urist MR. Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg. 1967;94:781‐789. [DOI] [PubMed] [Google Scholar]
- 35. Bessho K, Tanaka N, Matsumoto J, Tagawa T, Murata M. Human dentin‐matrix‐derived bone morphogenetic protein. J Dent Res. 1991;70:171‐175. [DOI] [PubMed] [Google Scholar]
- 36. Schmidt‐Schultz TH, Schultz M. Intact growth factors are conserved in the extracellular matrix of ancient human bone and teeth: a storehouse for the study of human evolution in health and disease. Biol Chem. 2005;386:767‐776. [DOI] [PubMed] [Google Scholar]
- 37. Włodarski KH, Szczęsny G, Kuzaka B, Włodarski PK. Long‐term preservation of bone morphogenetic activity in stored demineralized murine incisors. Pol Orthop Traumatol. 2013;4(78):97‐100. [PubMed] [Google Scholar]
- 38. Rauwolf M, Turyanskaya A, Roschger A, et al. Synchrotron radiation micro X‐ray fluorescence spectroscopy of thin structures in bone samples: comparison of confocal and color X‐ray camera setups. J Synchrotron Rad. 2017;24:307‐311. doi: 10.1107/S1600577516017057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474‐5491. doi: 10.1016/j.biomaterials.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 40. Klawitter JJ, Bagwell JG, Weinstein AM, Sauer BW. An evaluation of bone growth into porous high density polyethylene. J Biomed Mater Res. 1976;10(2):311‐323. [DOI] [PubMed] [Google Scholar]
- 41. Hing KA, Best SM, Tanner KE, Bonfield W, Revell PA. Mediation of bone ingrowth in porous hydroxyapatite bone graft substitutes. J Biomed Mater Res A. 2004;68A:187‐200. [DOI] [PubMed] [Google Scholar]
- 42. Simske SJ, Ayers RA, Bateman TA. Porous materials for bone engineering. Mater Sci Forum. 1997;250:151‐182. [Google Scholar]
- 43. Tanoue R, Ohta K, Miyazono Y, et al. Three‐dimensional ultrastructural analysis of the interface between an implanted demineralised dentin matrix and the surrounding newly formed bone. Sci Rep. 2018;8:2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adamo S, De Felici M, Dolfi A, et al. Istologia di Monesi. 7a edizione ed. Padova; 2018. [Google Scholar]
- 45. Young B, O'Dowd G, Woodford P. Wheater – Istologia e anatomia microscopica. 6a edizione ed. Milano; 2014. [Google Scholar]
- 46. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504‐1508. doi: 10.1126/science.289.5484.1504 [DOI] [PubMed] [Google Scholar]
- 47. Papageorgiou SN, Papageorgiou PN, Deschner J, Götz W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: systematic review and network meta‐analysis of parallel and cluster randomized controlled trials. J Dent. 2016;48:1‐8. doi: 10.1016/j.jdent.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 48. Elakkiya S, Ramesh AS, Prabhu K. Systematic analysis on the efficacy of bone enhancement methods used for success in dental implants. J Indian Prosthodont Soc. 2017;17:219‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deeb JG, Reichert A, Carrico CK, Laskin DM, Deeb GR. Effect of biologic materials on the outcomes of horizontal alveolar ridge augmentation: a retrospective study. Clin Exp Dent Res. 2021;7:147‐155. doi: 10.1002/cre2.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naenni N, Lim HC, Papageorgiou SN, Hämmerle CHF. Efficacy of lateral bone augmentation prior to implant placement: a systematic review and meta‐analysis. J Clin Periodontol. 2019;46:287‐306. doi: 10.1111/jcpe.13052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


