Abstract
Nodding syndrome (NS) is a poorly understood form of childhood‐onset epilepsy that is characterized by the pathognomonic ictal phenomenon of repetitive vertical head drops. To evaluate the underlying ictal neurophysiology, ictal EEG features were evaluated in nine participants with confirmed NS from South Sudan, Tanzania, and Uganda and ictal presence of high frequency gamma oscillations on scalp EEG were assessed. Ictal EEG during the head nodding episode predominantly showed generalized slow waves or sharp‐and‐slow wave complexes followed by electrodecrement. Augmentation of gamma activity (30–70 Hz) was seen during the head nodding episode in all the participants. We confirm that head nodding episodes in persons with NS from the three geographically distinct regions in sub‐Saharan Africa share the common features of slow waves with electrodecrement and superimposed gamma activity. ANN NEUROL 2022;92:75–80
Introduction
Nodding syndrome (NS) is a childhood onset neurological disorder of unknown etiology characterized by pathognomonic repetitive episodes of vertical head drops, progressive cognitive decline and often generalized tonic–clonic seizures. 1 NS was initially reported among the Wapagoro tribe in Tanzania in the 1960s and, 2 subsequently, a higher occurrence was found amid certain communities in South Sudan and northern Uganda.3, 4 At present, the diagnosis of NS is made based on epidemiological and clinical characteristics as per the 2012 WHO Kampala consensus. 5 It remains unknown if the cases of NS found in Tanzania, South Sudan and Uganda represent indeed the same or similar neurological disorder, since the course of the disease is different between these countries with a more benign course in Tanzania. 6
Prior studies have found consistent interictal electrographic abnormalities of generalized spike‐and‐wave discharges and background slowing in persons with NS, although the ictal phenomena during head nodding spells remain poorly understood with inconsistent findings: electrodecremental response with and without preceding slow wave or brief fast activity. 7 , 8 Importantly, ictal recordings during head nodding episodes have not yet been reported in NS subjects from Tanzania. 7
In this study, we quantitatively analyzed the neurophysiological changes on scalp EEG during the ictal event of head nodding and compared the ictal phenomena across children with NS from geographically separate endemic groups. We hypothesize that the ictal event of head nodding is associated with gamma activity on scalp EEG, as reported as a biomarker in other forms of epilepsy. 9
Methods
Subjects
A convenient sample of subjects from sites in South Sudan, Tanzania, and Uganda underwent scalp EEG and met the following inclusion criteria: (1) Confirmed cases of NS per the 2012 WHO Kampala consensus (probable case per the 2012 WHO Kampala consensus + head nodding observed/ recorded on EEG by physician/healthcare worker)5; and (2) the presence of characteristic head nodding during the EEG recording.
Ethics
Consent from the caregivers, in addition to, assent from the minor subjects were obtained. IRB approvals were obtained: (1) Uganda: Lacor Hospital Institutional Research and Ethics Committee, Uganda National Council for Science and Technology, Uganda, and University of California, Los Angeles IRB, United States; (2) Tanzania: Ethics committees Muhimbili University of Health and Allied Sciences, University of Heidelberg and the University of Tübingen, Germany; (3) South Sudan: Ethics Committee of Scientific Institute Eugenio Medea.
EEG Recording and Qualitative Analysis
The scalp video‐EEGs were recorded with a digital sampling frequency of 256 Hz using the following systems: (1) Brain Quick – EEG Line (Micromed) in South Sudan; (2) Twin Grass Telefactor in Tanzania; (3) Lifelines Neuro Trackit MK3 ambulatory system in Uganda. The international 10–20 system was used for scalp EEG placement. Participants underwent activation maneuvers as follows: hyperventilation (7 out of 9), photic stimulations (4 out of 9), and food (4 out of 9). Recordings were independently evaluated by three experienced epileptologists (DE, JE, BDB), who were blinded to the child's medical history and clinical information. The interpretations were adjudicated through consensus.
Time‐Frequency Analysis of Ictal‐Head Nodding EEG Data
The time‐frequency analysis for each subject was performed using BESA EEG software (BESA GmbH, Gräfelfing, Germany. A common average referential montage was used for the analysis as referential montages allow better localization. Each head nodding was identified visually in the time‐locked video and the largest negative peak of slow wave seen during the head nodding episode was set as a trigger point for the time‐frequency analyses. 10 The trigger point was set at 0 ms and changes in delta and gamma frequency was analyzed from −1,000 to +1,000 ms. Gamma was evaluated in 10 ms bins between 10–90 Hz in 5 Hz bins and delta changes were assessed in 100 ms bins between 1–4 Hz in 0.5 Hz bins. The baseline interval was defined between −1,000 and −800 ms. Gamma and delta band was defined as 30 Hz to 70 Hz and 1–4 Hz, respectively. We investigated the maximum percent change in amplitude or the augmentation of amplitude of the gamma frequency band in each time bin compared to the baseline amplitude for each electrode location (averaged across all head nodding events). Time and regions of maximal augmentation of amplitude were also statistically evaluated using BESA, which uses a bootstrapping approach and corrects for multiple testing. 11 , 12 Onset latency of the gamma augmentation was also determined. To limit any contamination due to muscle artifacts, we limited the analysis to parasagittal and midline channels (F3, F4, C3, C4, P3, P4, O1, O2, Fz, Cz, Pz). Gamma augmentation was considered statistically significant only if amplitude modulations involved at least 20 Hz in width and lasted for 10 oscillations, thus limiting the probability of a type‐1 error. 13
Results
Patient Characteristics and Qualitative EEG Analysis
Nine participants from three NS‐endemic regions of South Sudan (4 cases), Tanzania (3 cases), and Uganda (2 cases) had a mean age of 13.2 years (range = 7–18 years, Std.dev = 3.5 years). Average age of onset of head nodding was 7.3 years (range = 5–12 years, Std.dev = 2.4 years). Detail demographic information, interictal and ictal findings are described in Table S1.
During the cluster of the head nodding episodes, the ictal background activity was significantly different from the interictal EEG background. In all but 1 subject, the generalized 2–3‐Hz spike and wave discharges that were seen interictally did not appear during the head nodding cluster. Ictal EEG was dominated by periodic slow wave or sharp‐and‐slow wave complexes followed by electrodecrement, which accompanied head drops (Video S1). In 1 subject from Tanzania, the generalized spike‐and‐wave discharges persisted between the head drops (Figure 1).
FIGURE 1.
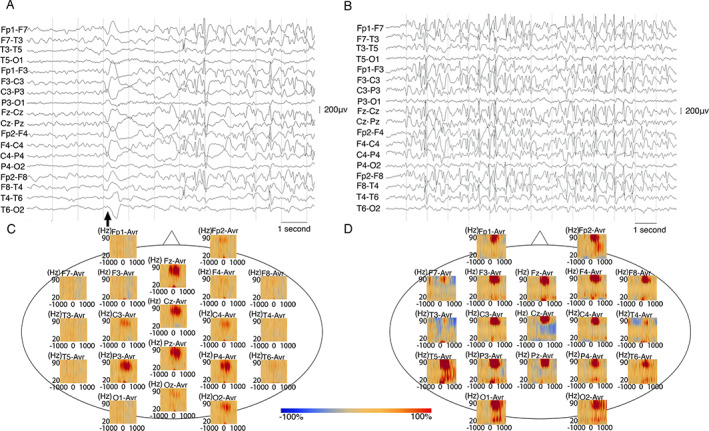
(A and B) A 7 year‐old male with NS (participant s3) onset at age 6 years. (A) Ictal EEG shows generalized slow wave followed by electrodecrement. EEG background between each head nod is marked by generalized spike and wave discharges. (B) Interictal EEG shows 2‐ 3 Hz generalized spike and wave discharges. Both EEGs are shown in bipolar montage. Low frequency filter: 0.053 Hz, high frequency filter: 70 Hz Black arrows indicate head drops. C and D: Time frequency analysis of ictal head nod showed augmentation of gamma activity on scalp EEG of children with NS from South Sudan and Tanzania. (C) In an 18 year‐old male with NS (participant s8) from South Sudan, time‐frequency plot derived from 70 head nodding episodes showed maximal augmentation of gamma (30 Hz‐70 Hz) at the centro‐parietal electrodes during the head drops. (D) In a 12 year‐old male (participant s4) from Tanzania, time‐frequency plot derived from 17 head nodding episodes showing augmentation of gamma‐activity. Blue color indicates attenuation of amplitude and red color indicates augmentation of amplitude in the corresponding time‐frequency bin relative to the baseline.
Time‐Frequency Analysis of the Ictal Events
The maxima of gamma and delta amplitude changes and their location on scalp EEG is noted in Table for each subject. 663 of 908 (73%, range = 59–100%) head nodding events from nine subjects were evaluated for changes in gamma (30–70 Hz) and slow wave (delta) component using time‐frequency analysis. Augmentation of gamma activity was noted during the head nodding episodes in all subjects from all three different regions (Figs 1 and 2). Maximal augmentation of gamma activity was most commonly noted in the parietal region (n = 5, 56%), followed by occipital (n = 3, 33%) and central (n = 1, 11%) regions. The location of the maximal amplitude change in delta was discordant to location of the maximal gamma augmentation in 89% of participants. The onset latency of the gamma activity occurred at an average of 216 ms before the trigger point (range −610 to 30 ms), which was visually set at the largest negative peak of the slow wave seen during the head nodding episode. The peak latency of gamma augmentation occurred at 42 ms (range −220 to 270 ms). The peak latency was temporally related and followed the gamma augmentation on average 240 ms after the trigger point. The augmentation of gamma oscillation did not reach pre‐defined statistical significance in 4 of the 9 and for these individuals onset latency of the gamma activity was not calculated (Table 1).
FIGURE 2.
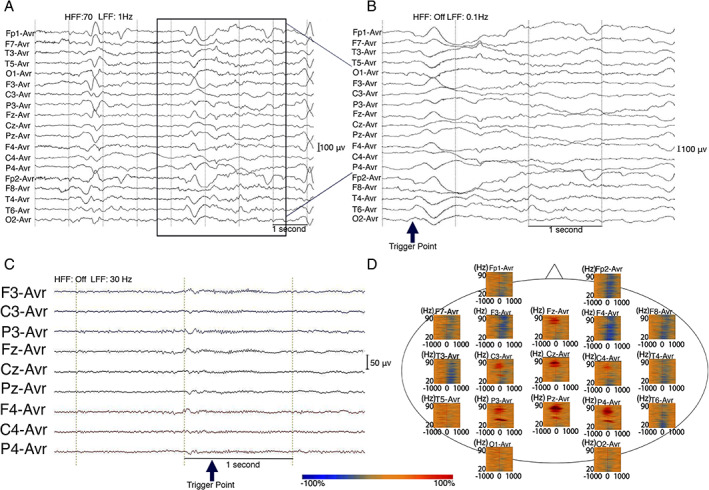
Time frequency analysis of ictal head nod showed augmentation of gamma activity on scalp EEG of a 17 year‐old male (participant s1) with NS from Uganda. EEG sampling frequency 256 Hz. (A) Tracing with average EEG montage. Low frequency filter (LFF): 1 Hz, High frequency filter (HFF): 70 Hz; (B) Temporally expanded trace of EEG shown in panel A LFF: 0.1 Hz, HFF: Off. The Trigger point marked by blue arrow was visually set at the deflection point of the largest negative peak of slow wave during head nodding episode. (C) EEG filtered with LFF 30 Hz and HFF off. (D) Time‐frequency plot derived from 100 head nodding episodes. There is maximal augmentation of gamma (30 Hz‐70 Hz) at the centro‐parietal electrodes during the head drops. Blue color indicates attenuation of amplitude and red color indicates augmentation of amplitude in the corresponding time‐frequency bin relative to the baseline.
TABLE 1.
Time‐Frequency Analysis of Ictal Head Nodding
| ID | Peak Gamma Channel | Gamma Maximum Amplitude Change (%) | Onset Latency of Gamma (ms) | Peak Latency Gamma (ms) | Peak Delta Channel | Delta Maxima‐Amplitude Change (%) | Peak Latency of Delta (ms) |
|---|---|---|---|---|---|---|---|
| s1 | Pz | 47.9 | −310 | −220 | Pz | 126 | 0 |
| s2 | P3 | 74.5 | −610 | −210 | F4 | 400 | 0 |
| s3 | Pz | 169 | a | a | C3 | 176 | a |
| s4 | O1 | 98.7 | a | a | C3 | 253 | a |
| s5 | O1 | 177 | 10 | 270 | P4 | 567 | 1,000 |
| s6 | O2 | 161 | a | a | O1 | 110 | a |
| s7 | C4 | 189 | a | a | P3 | 435 | a |
| s8 | P4 | 63.7 | −140 | 240 | Fz | 150 | 100 |
| s9 | P3 | 45 | −30 | 130 | P4 | 525 | 100 |
For subjects s3, s4, s6, s7, gamma augmentation did not reach statistical significance and onset latency could not be calculated.
Discussion
Here, we report the first ictal EEG of head nodding from Tanzania and compare the electrophysiological changes in children with confirmed NS from the three hyperendemic regions in sub‐Saharan Africa. Our findings confirm that the head nodding episodes in NS from the three non‐contiguous regions share common features of slow waves with electrodecrement and superimposed gamma activity. Similar to our findings, prior reports from South Sudan also reported slow waves followed by electrodecrement responses during ictal head nods.8 This study confirms that NS represents a new form of childhood onset epilepsy that shares ictal EEG feature with epileptic spasms.
The combination of ictal EEG with electrodecrement response and the occurrence of head nodding episodes in clusters strongly resembles characteristics of late‐onset epileptic spasms. The median age at onset of late‐onset spasms is around 3 years and usually occurs between the ages of 12 months and 12 years. 14 Similarly, the age at onset of NS ranged between 5–12 years among our participants. The ictal clustering of head drops in NS resembling epileptic spasms likely represents a novel disease that shares electro‐clinical features within the spectrum of developmental and epileptic encephalopathies and common mechanism of ictogenesis. We found that there is a statistically significant increase in gamma frequency that could be recorded on scalp EEG during the ictal period of head drops, a phenomenon that has been reported in other developmental and epileptic encephalopathies. 9 , 11 The presence of diffuse ictal cortical high frequency rhythms suggest recruitment of a widely distributed epileptic network, involving multifocal cortical areas and deep brain structures including thalamus and brainstem as reported in infantile spasms and Lennox–Gastaut syndrome. 15 , 16
In this study, we found that maximal augmentation of gamma activity mostly occurred in the parietal region (Figs 1 and 2). The ictal augmentation of gamma preceded the trigger point, which was the best visual estimation of the clinical head drop. Moreover, the gamma augmentation was temporally related to the change in the delta wave and always preceded it. This temporal relationship between fast and slow wave components has been also noted in epileptic spasms. 17 The location of the delta and gamma maxima was discordant among most participants. In epileptic spasms with intracranial recordings, ictal slow wave have been noted to propagate beyond the ictal onset zone consistently suggesting a widespread epileptogenic network and having a limited localizing value. 17 The time‐frequency analysis involves averaging of all the head nodding episodes for each participant. Thus, the location, onset, and the percentage augmentation of ictal gamma activity during the head nodding episodes compared to the baseline is an average across the entire cluster rather than during each individual head drop. In 4 cases, predefined statistical significance for the gamma augmentation was not reached likely due to the small numbers of head nodding events. Gamma oscillations on scalp EEG have been described to delineate the ictal onset zone within the cortical region and could serve as objective neurophysiological biomarker to identify vulnerable cortical regions that are involved in epileptogenesis in NS. 18 However, in this study, particularly in the absence of intracranial recording and concurrent imaging, we are unable to assess if the seizure onset zone in NS is focal or generalized.
Our study has few limitations. Children with NS often have multiple other clinical seizure types, including absence, and generalized tonic–clonic seizures, which were not recorded in our study. 1 , 4 , 7 Due to a lack of long‐term EEG monitoring and prospective follow‐up of the children with NS through the course of the disease, we are unable to correlate the EEG patterns with the natural history and severity of the disease. NS is postulated to be an immune‐mediated epilepsy caused by the parasite Onchocerca volvulus, and our study did not evaluate the presence of any inflammatory marker to investigate its role in driving the ictal physiology. Our study was also limited by lack of concurrent electromyography, which limits interpretation of the peripheral changes in muscle leading to the semiology of head nodding. EKG lead did not show any increased myographic activity as might be seen with epileptic spasms.
We are first to confirm that the head nodding episodes in NS from the three non‐contiguous regions share common features of slow waves with electrodecrement and superimposed gamma activity. The results of this study could be further used to expand on the current WHO‐based case definition of confirmed NS, which currently does not include any neurophysiological information. 5 , 6
Potential Conflict of Interest
The authors do not have any conflict of interest.
Author Contributions
R.M., A.S.W., A.D., P.B., G.D.P., T.W., W.M., E.S., J.K., D.E., D.K.L., J.E. contributed to the conception and design of the study; A.D., P.B., G.D.P.,T.W., J.K., R.M., D.K.L., D.E. contributed to the acquisition of data and analysis was performed by D.E., J.E., B.D.B., H.N., R.M. Drafting the text and preparing the figures were performed by R.M., A.S.W., H.N., D.K.L., A.D., D.E., J.E., B.D.B., P.B., G.D.P., T.W., W.M., E.S., H.L., J.S., J.K.
Supporting information
Table S1 Demographic characteristics with interictal and ictal qualitative EEG findings.
Video S1 Time‐locked video‐EEG recording of head nodding episodes.
Acknowledgments
We thank the participating children and their families. We thank Dr. Jessica Pasqua for her assistance with formatting the figures. R.M. was supported by International Outreach Scholarship from the American Neurological Association. D.E. and R.M. were supported by Seed grant from the American Epilepsy Society. The study in Tanzania was supported by German Centre for Infection Research” (DZIF) and “Prof. Dr. Peter and the Jytte Wolf Foundation for Epilepsy.” J.K. was supported by a doctoral scholarship from the German Society for Epileptology (DGfE). We do not want to conclude this paper without mentioning the sad loss of Prof. Louise Jilek Aall, one of the greatest supporters of the people with nodding syndrome, who sadly passed away on 4 January 2022. Louise will always be remembered as an exceptional and warm human being, a knowledgeable and caring doctor and an inspiring and thoughtful colleague.
References
- 1. Winkler AS, Friedrich K, Konig R, et al. The head nodding syndrome‐clinical classification and possible causes. Epilepsia 2008;49:2008–2015. [DOI] [PubMed] [Google Scholar]
- 2. Aall Jilek LM. Epilepsy in the Wapogoro tribe in Tanganyika. Acta Psychiat Scand 1965;41:57–86. [Google Scholar]
- 3. Spencer PS, Vandemaele K, Richer M, et al. Nodding syndrome in Mundri county, South Sudan: environmental, nutritional and infectious factors. Afr Health Sci 2013;13:183–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sejvar JJ, Kakooza AM, Foltz JL, et al. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol 2013;12:166–174. [DOI] [PubMed] [Google Scholar]
- 5.WHO. International scientific meeting on nodding syndrome. 2012. Available from: https://www.who.int/neglected_diseases/diseases/Nodding_syndrom_Kampala_Report_2012.pdf?ua=1.
- 6. Dowell SF, Sejvar JJ, Riek L, et al. Nodding syndrome. Emerg Infect Dis 2013;19:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winkler AS, Wallner B, Friedrich K, et al. A longitudinal study on nodding syndrome‐a new African epilepsy disorder. Epilepsia 2014;55:86–93. [DOI] [PubMed] [Google Scholar]
- 8. de Polo G, Romaniello R, Otim A, et al. Neurophysiological and clinical findings on nodding syndrome in 21 south Sudanese children and a review of the literature. Seizure 2015;31:64–71. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi K, Oka M, Akiyama T, et al. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia 2004;45:488–496. [DOI] [PubMed] [Google Scholar]
- 10. Nagasawa T, Juhasz C, Rothermel R, et al. Spontaneous and visually driven high‐frequency oscillations in the occipital cortex: intracranial recording in epileptic patients. Hum Brain Mapp 2012;33:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nariai H, Beal J, Galanopoulou AS, et al. Scalp EEG ictal gamma and beta activity during infantile spasms: evidence of focality. Epilepsia 2017;58:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoechstetter K, Bornfleth H, Weckesser D, et al. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr 2004;16:233–238. [DOI] [PubMed] [Google Scholar]
- 13. Nagasawa T, Rothermel R, Juhasz C, et al. Cortical gamma‐oscillations modulated by auditory‐motor tasks‐intracranial recording in patients with epilepsy. Hum Brain Mapp 2010;31:1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auvin S, Lamblin MD, Pandit F, et al. Infantile epileptic encephalopathy with late‐onset spasms: report of 19 patients. Epilepsia 2010;51:1290–1296. [DOI] [PubMed] [Google Scholar]
- 15. Dalic LJ, Warren AEL, Young JC, et al. Cortex leads the thalamic centromedian nucleus in generalized epileptic discharges in Lennox‐Gastaut syndrome. Epilepsia 2020;61:2214–2223. [DOI] [PubMed] [Google Scholar]
- 16. Lado FA, Moshe SL. Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? Int Rev Neurobiol 2002;49:115–140. [DOI] [PubMed] [Google Scholar]
- 17. Nariai H, Matsuzaki N, Juhász C, et al. Ictal high‐frequency oscillations at 80‐200 Hz coupled with delta phase in epileptic spasms. Epilepsia 2011;52:e130–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrade‐Valenca LP, Dubeau F, Mari F, et al. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 2011;77:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographic characteristics with interictal and ictal qualitative EEG findings.
Video S1 Time‐locked video‐EEG recording of head nodding episodes.


