Abstract
Background and Purpose
Carotid web (CaW) is a cause of recurrent ischemic stroke that remains underdiagnosed using Duplex ultrasound (DUS). Improved methods and description of its ultrasound's features could allow better detection of CaW. Ultrasound microflow imaging (MFI) is a blood flow imaging technique sensitive to slow flow that could increase CaW detection. This study aimed to describe ultrasound features of CaW using B‐mode imaging and MFI.
Methods
In a retrospective monocentric study, patients with CaW on CT angiography who underwent DUS examination of carotid arteries were included. DUS was performed by two nonblinded experienced neurosonologists. The specificity of CaW ultrasound features was evaluated using a group of patients with carotid atherosclerotic plaque (AP).
Results
Twenty‐four patients with CaW were included. Mean age (standard deviation) was 48 years (11). Seventeen (71%) were females. Fifteen (63%) CaWs were symptomatic. MFI was available for 22 patients. B‐mode imaging demonstrated the characteristic CaW appearance in 19/24 (79%) patients as a protruding triangular iso‐hypoechoic lesion on longitudinal view. CaW were detected on axial view in only 9/24 (38%) patients. MFI displayed slow blood flow above CaW during systole and allowed it delineation, appearing as a thin triangular endoluminal defect in 18/22 (82%) cases. Based on MFI and B‐mode, 21/22 (95%) CaWs were visible, including three CaWs only with MFI. These ultrasound features were not found among 24 patients with AP.
Conclusion
We report the ultrasound features from a series of 24 CaW. The use of MFI in addition to B‐mode imaging improved the detection rate of CaW.
Keywords: carotid web, Doppler ultrasound, microflow imaging
INTRODUCTION
Carotid web (CaW), also called atypical fibromuscular dysplasia, corresponds to a focal dysplasia of the carotid artery. 1 , 2 It appears on angiography as a small, membrane‐like, linear filling defect projecting into the lumen of proximal cervical internal carotid artery (ICA), typically arising from the posterior wall. 1 , 2
Efficient screening for CaW after ischemic stroke is of major importance because the risk of stroke recurrence in case of misdiagnosed CaW seems to be high (reaching nearly 20% at 2 years despite the introduction of antithrombotic therapy), 3 whereas case series suggest that a specific treatment based on angioplasty stenting 4 , 5 , 6 or endarterectomy 4 , 6 , 7 might be effective to prevent stroke recurrence.
However, CaW may be underdiagnosed due to its inconspicuous nature and the difficulty of demonstrating it with noninvasive imaging techniques. 1 , 2 , 8
Computed tomography angiography (CTA) is considered the imaging technique of choice for the demonstration of CaW. 1 , 2 , 9 , 10 , 11 It has the advantage of allowing 3‐dimensional reconstructions. However, CTA is irradiating, and potentially allergenic and nephrotoxic.
Duplex ultrasound (DUS) is an inexpensive and harmless imaging technique, particularly suited to the examination of the carotid bulb. It has the advantage to analyze the arterial wall and the hemodynamic impact of endoluminal lesions. Despite these features, previous studies using DUS have shown that a substantial proportion of CaWs are missed or mistaken for atherosclerotic plaque (AP). 2 , 8 , 12 , 13 This limited diagnosis performance could be explained by the subtle nature of the CaW and a lack of awareness among DUS practitioners. 14 However, the ultrasound features of CaW remain poorly described. 2 , 8 , 14 , 15 , 16 , 17
To allow better detection of CaW, improved ultrasound methods and precise description of sonographic features of CaW are, therefore, needed. Based on a clutter removal algorithm that separates slow flow signals from tissue motion artifacts, ultrasound microflow imaging (MFI) is a recent blood flow imaging technique highly sensitive to low velocities blood flow without contrast agents. 18 As slow flow is present in the CaW nidus, 19 MFI has the potential to better delineate the CaW than B‐mode color Doppler imaging.
The objective of the present study was to precisely describe the ultrasound characteristics of CaW using B‐mode imaging and MFI.
METHODS
Study design
This was a monocentric, retrospective study, carried out in the Neurosonology Unit of a University Hospital from September 2019 to November 2021.
In a first part, we analyzed the ultrasound characteristics of CaW diagnosed by CTA in a series of patients.
In a second part, we evaluated the specificity of our findings using a control group of patients with carotid AP.
Inclusion criteria
Patients with CaW
All patients presenting a CaW identified on CTA of cervical arteries, and who had undergone a DUS of carotid arteries during the study period were included. Diagnosis of CaW on CTA could be made after ischemic stroke or transient attack (CaW was considered symptomatic if it occurs in the downstream territory of the involved carotid artery and if no alternative causes were identified), or after CTA performed for other reason (asymptomatic CaW). There was no exclusion criterion on age.
Patients with carotid atherosclerotic plaque
Consecutive patients with CTA‐identified nonstenotic AP in the proximal ICA who underwent DUS examination served as controls.
Carotid web definition
CaW was defined on CTA as a smooth, shelf‐like filling defect along the posterior wall of the proximal ICA on oblique sagittal image, and a corresponding septum on axial image. 1 , 10
Duplex ultrasound
Two readers, both neurosonology credentialed and trained stroke neurologists, not blinded to clinical and radiological findings, performed DUS and image interpretation. Images were acquired with an Epiq elite echograph (Philips) using 12‐3 or 18‐4 MHz broadband linear array transducers. The degree of stenosis was determined based on peak‐systolic and end‐diastolic velocities. Common carotid artery, carotid bifurcation, and ICA were systematically examined in the longitudinal and axial planes. The CaW was first identified on B‐mode in the longitudinal plane. The incidence allowing the best view of the CaW was reached by scanning the proximal ICA with anterior, lateral, and postero‐lateral incidences. If the CaW was not visible in B‐mode, the carotid bulb was systematically scanned using MFI. Blood flow velocities were measured in the circulating lumen of the ICA and just above the shelf formed by the CaW. We did not use a contrast agent.
RESULTS
Patients’ characteristics
A total of 24 patients were included.
Mean age (standard deviation) of patients with CaW was 48 years (11). Seventeen (71%) were females. Nine (38%) were current smokers. The prevalence of other atherosclerotic risk factors was low. The CaW was considered symptomatic in 15/24 (63%) cases, including five transient ischemic attacks and 10 ischemic strokes (all classified as undetermined stroke etiology––negative investigation subtype based on Trial of Org 10172 in Acute Stroke Treatment classification criteria). The remaining 9/24 (37%) asymptomatic CaW were identified on CTA performed for headache, stroke in other territory, or other causes (Table 1).
TABLE 1.
Baseline characteristics
| Baseline characteristics | Carotid web, n = 24 | Atherosclerosis plaque, n = 24 |
|---|---|---|
| Mean age (y) ±SD | 48 ± 11 | 66 ± 12 |
| Female sex, n (%) | 17 (71) | 8 (33) |
| Atherosclerotic risk factors: | ||
| Hypertension, n (%) | 2 (8) | 17 (71) |
| Dyslipidemia, n (%) | 2 (8) | 13 (54) |
| Active smoker, n (%) | 9 (38) | 10 (42) |
| Diabetes mellitus, n (%) | 1 (4) | 6 (25) |
| Coronary artery disease, n (%) | 1 (4) | 2 (8) |
| Peripheral artery disease, n (%) | 0 (0) | 2 (8) |
| History of atrial fibrillation, n (%) | 0 (0) | 0 (0) |
| Previous ischemic stroke or transient ischemic attack, n (%) | 4 (17) | 3 (13) |
| Symptomatic carotid web | 15 (64) | na |
| Transient ischemic attack, n (%) | 5 (21) | |
| Ischemic stroke, n (%) | 10 (42) | |
| Asymptomatic carotid web | 9 (36) | na |
| Headache | 3 (12) | |
| Ischemic stroke in another territory | 3 (12) | |
| Other | 3 (12) | |
Abbreviations: n, number of patients; na, nonapplicable; SD, standard deviation; y, years.
The characteristics of the 24 control patients with carotid plaque are summarized in Table 1. These patients were older (mean age 66 years), included more males, and had more traditional risk factors than patients with CaW.
DUS characteristics of carotid web
In B‐mode, under longitudinal view, the characteristic appearance of CaW, observed in 19/24 (79%) patients, was that of a triangular lesion protruding into the artery lumen, implanted on the wall of the ICA opposite the bifurcation. The lesion was isoechoic or hypoechoic with a hyperechoic border on its inferior edge. No mobile segment was visible. Its size ranged from a small outgrowth of the intima to a large lesion narrowing the arterial lumen (Figures 1 and 2). Two anechoic lesions were not visible in B‐mode and were demonstrated by MFI on longitudinal view. Two other hypoechoic, poorly limited lesions suggested an intraluminal thrombus. In the remaining patient, the lesion could suggest an AP (Table 2).
FIGURE 1.
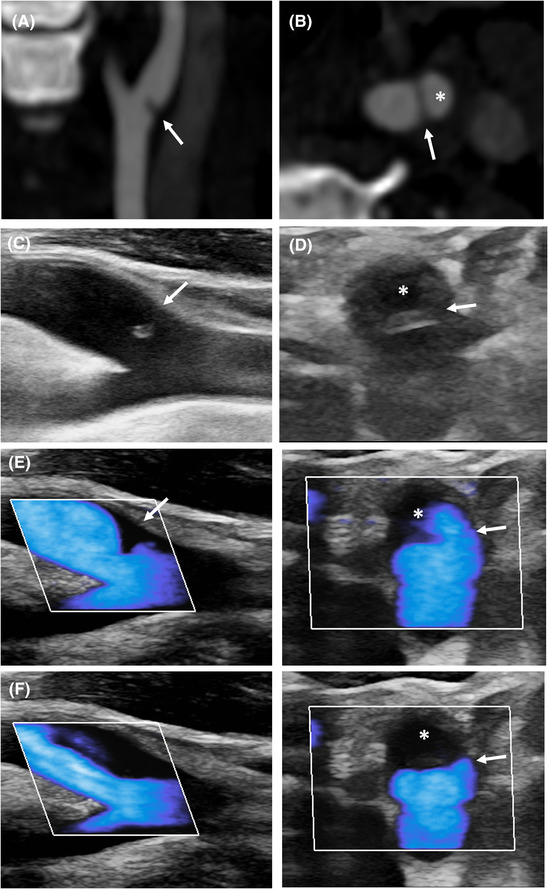
Typical appearance of carotid web. Longitudinal (A) and axial (B) views of carotid web (CaW) on CT‐angiography. The CaW (arrow) delimits a nest (*). (C) Longitudinal view on B‐mode shows an isoechoic lesion protruding into the arterial lumen (arrow). (D) Corresponding axial view on B‐mode reveals a septum (arrow) delimiting a nidus (*). (E) Microflow imaging delineates the CaW during systole (E) with downstream low flow velocities during diastole (F)
FIGURE 2.
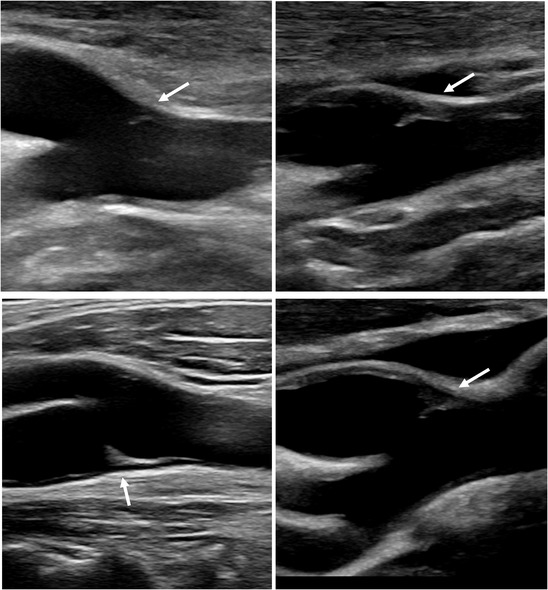
Longitudinal view of carotid bifurcation showing carotid web (CaW) in four patients. The CaW appears as an iso‐ or hypoechoic lesion with a thin hyperechoic inferior edge (arrows)
TABLE 2.
Ultrasound features of carotid web
| Incidence | B‐mode | n = 24 |
|---|---|---|
| Longitudinal | Iso/hypoechoic triangular lesion projecting into the arterial lumen, n (%) | 19 (79) |
| Axial | Thin transversal septum delimitating a nest, n (%) | 9 (38) |
| MFI‐mode | n = 22 | |
|---|---|---|
| Longitudinal | Thin triangular endoluminal defect with a downstream low flow area, n (%) | 18 (82) |
| Axial | Transversal linear defect and low flow in the nidus, n (%) | 12 (54) |
Abbreviation: n, number of patients.
The optimal longitudinal incidence to demonstrate CaW was perpendicular to the septum. It corresponded to the slice plane, including the common carotid, internal carotid, and external carotid arteries (Figures 1 and 2). When the septum had a posterior orientation, the operators had to use an anterior incidence to demonstrate CaW.
On axial view, the CaW appeared as a thin, iso or hypoechoic, transversal septum separating the arterial lumen from the nest in 9/24 (38%) patients (Figure 3). The CaW appeared as a thickening of the arterial wall in five cases. It could not be demonstrated in nine patients.
FIGURE 3.
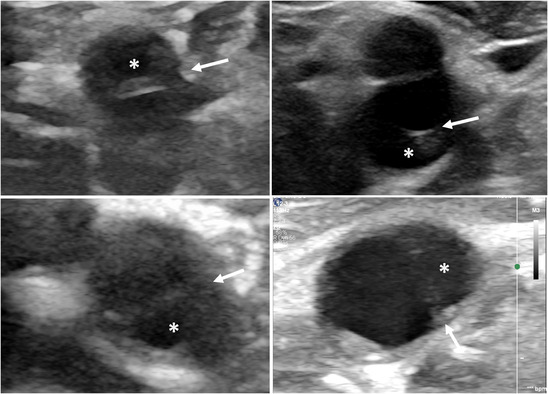
Axial view of internal carotid artery showing a carotid web (CaW) in four patients. The CaW appears as a thin transverse septum (arrows) separating the nest (*) from the circulating lumen
All CaW were detected with the use of a 12‐3 MHz broadband linear array transducers. A 18‐4 MHz transducer permitted to increase imaging contrast and better delineate hypoechoic CaWs. However, it did not detect anechoic CaW that were not detected with a 12‐3 MHz transducer.
MFI was available in 22 patients. On longitudinal view, an area of low velocities and turbulence was demonstrated just above the CaW during systole (Figure 1). This area of low velocities appeared as a filling defect during diastole. MFI allowed the delineation of the CaW during systole as a thin triangular endoluminal filling defect. A CaW that was not visible on B‐mode was demonstrated by MFI in two patients (Figure 3). However, the CaW was not visible on MFI in 4/22 (18%) patients. MFI showed no neovascularization of the CaW.
On axial view, MFI demonstrated the CaW during systole in 12/22 (54%) patients (Figure 1), appearing as a low flow velocity in the nidus and a transversal linear defect. The CaW could be demonstrated only on axial MFI in one patient.
B‐mode imaging and MFI were both available in 22 patients. The CaW was demonstrated in 21/22 (95%) patients, including three CaW that were visualized only in MFI (Figure 4), resulting in a sensitivity of 95%.
FIGURE 4.
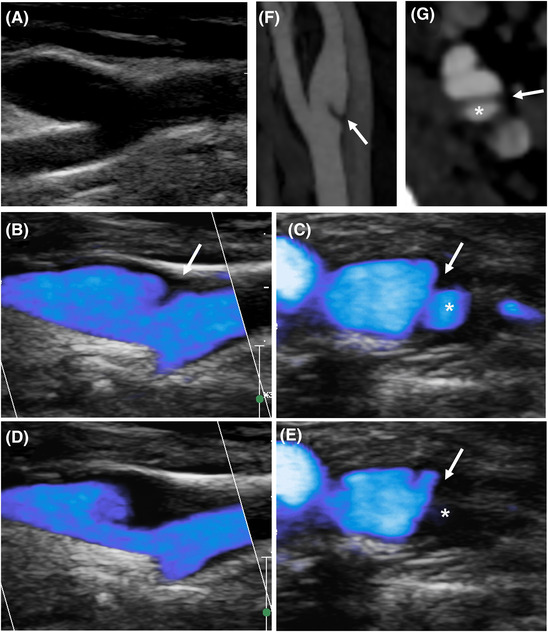
Anechoic carotid web (CaW) revealed by microflow imaging (MFI) mode. The CaW cannot be demonstrated on B‐mode imaging (A) and is revealed by MFI. Longitudinal and axial view during systole (B and C) and during diastole (D and E). CaW appearance on CT‐angiography in the same patient: longitudinal (F) and axial (G) view. Arrow: carotid web; *: nest
Color‐coded Doppler showed an area of turbulence and low flow velocities downstream of the CaW in 17/22 (77%) patients. No significant changes in flow velocities were detected in the circulating arterial lumen.
Comparison with atherosclerotic plaque
None of the 24 patients with AP showed ultrasound features suggestive of CaW in B‐mode and MFI‐mode. The specificity of the ultrasound features of CaW was thus 100%. APs were more often heterogeneous, with hypo and hyperechoic areas. Small calcifications were present in 10/24 (40%). A hyperechoic edge could be seen in 3/24 (13%). Localization was rarely limited to the posterior wall of the proximal ICA. Circumferential atherosclerotic infiltration lesion was seen in 12/24 (50%).
Three plaques protruded into the arterial lumen. These protruding plaques were thicker and more echoic than CaW, and without nidus on longitudinal or axial view.
MFI allowed a good delineation of the anechoic plaques. On axial incidence, anechoic plaques appeared as convex filling (whereas caw was rectilinear) defect without nidus (Figure 5).
FIGURE 5.
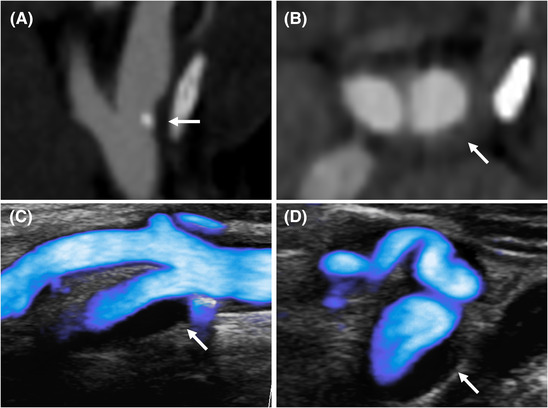
Atherosclerotic plaque on microflow imaging (MFI). Atherosclerotic plaque on CTA (A and B) and MFI (C and D). The MFI‐mode during systole delineates a mostly anechoic plaque with a small calcification and without area of low flow on longitudinal view (C). The axial incidence shows a convex anechoic thickening of the artrial wall without nidus (D)
DISCUSSION
We report the sonographic features of CaW from a series of 24 patients and compared to 24 APs.
The typical appearance of CaW on B‐mode imaging in the longitudinal view is a motionless, triangular lesion appended to the wall of the ICA opposite the carotid bifurcation. The lesion is hypo‐ or isoechoic and can present a hyperechoic border along its inferior edge. Its size is variable, from an inconspicuous to a large lesion. The optimal longitudinal slice plane for detecting CaW was, in most cases, the slice plane, including the common carotid, internal carotid, and external carotid arteries. MFI, which is sensitive to slow flow in the CaW nidus, allowed good delineation of CaW and could reveal anechoic lesions that were not demonstrated on B‐mode imaging. Using B‐mode imaging plus MFI‐mode, we were able to demonstrate CaW in 95% of cases.
The detection rate of CaW was much lower in the axial view, as previously reported. 17 The septum that separates the nidus from the artery lumen may be difficult to detect in small lesions. With a cross‐sectional plane through the base of the lesion, the CaW can be mistaken for an AP or thrombus. In addition, artifacts are more frequent in the axial view, especially in the case of retropharyngeal ICA, which limits the ability to visualize a hypoechoic septum.
The high rate of CaW detected in our study suggests that DUS could be a reliable tool for CaW detection. However, some previous studies have shown that the agreement between DUS and CT angiography was only moderate. 2 , 13 , 20 This discrepancy may be partly explained by the use of MFI, which improved the sensitivity of DUS in our study. In addition, operators were aware of the presence of CaW and the examination protocol was adapted to CaW screening. Indeed, the proximal ICA was carefully scanned from an antero‐posterior to a postero‐lateral incidence, which resulted in an extended duration of examination. It is, therefore, possible that some CaW would have been missed if we had used a standard examination protocol. Further blinded studies are needed to properly assess the accuracy of the methods we have used.
We report a high specificity of DUS to detect CaW. However, this result must be interpreted with caution, as DUS were not performed blinded to CTA results. In addition, only 3/24 carotid plaques used as controls protruded into the artery lumen. These protruding plaques were more difficult to differentiate from CaW than nonprotruding plaques. The protruding plaques were more echoic than CaW, nontriangular, and without nidus.
MFI also permitted to demonstrate typical anechoic atherosclerotic infiltration around the protruding lesion.
Hyperechoic edging on the lower side of the CaW could be explained by the interface between blood and intima. 21 , 22 Although this feature was inconstant, it was useful to reveal hypoechoic CaWs.
We found no CaW microvasculature on MFI, which is consistent with previously reported data. 8 However, in contrast to some previous studies, 8 , 18 we also did not detect microvasculature in patients with AP. Therefore, this feature could not be used to differentiate CaW and AP in our study. This could be explained by the fact that we selected only nonstenotic AP.
In agreement with previous reports, CaW was not responsible for hemodynamic stenosis in any patient. 10 , 23
The use of a 18‐4 MHz transducer did not increase CaW detection, but it was useful to better delineate hypoechoic lesion.
Our study has several limitations. Because DUS were not performed blinded to CTA findings, the sensitivity and specificity of the CaW ultrasound features that we have described may be overestimated. Moreover, there was no histopathologic examination of CaW. However, our findings provide a refined description of the ultrasound characteristics of CaW and the optimal examination protocol to detect this lesion. Thus, our findings could be used in future validation studies. The use of a contrast agent could have potentially resulted in a higher detection rate of CaW. 8 , 15 However, contrast agents are expensive and time‐consuming to use.
In conclusion, we report the ultrasound features of CaW. The use of MFI in addition to B‐mode imaging improved the detection rate of CaW. Further study is needed to prospectively assess the sensitivity and specificity of DUS based on these findings.
ACKNOWLEDGMENTS AND DISCLOSURE
None.
Fontaine L, Guidolin B, Viguier A, Gollion C, Barbieux M, Larrue V. Ultrasound characteristics of carotid web. J Neuroimaging. 2022;32:894–901. 10.1111/jon.13022
Funding informationNone.
REFERENCES
- 1. Choi PMC, Singh D, Trivedi A, et al. Carotid webs and recurrent ichemic strokes in the era of CT angiography. Am J Neuroradiol 2015;36:2134‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joux J, Chausson N, Jeannin S, et al. Carotid‐bulb atypical fibromuscular dysplasia in young Afro‐Caribbean patients with stroke. Stroke 2014;45:3711‐3. [DOI] [PubMed] [Google Scholar]
- 3. Guglielmi V, Compagne KCJ, Hossein A, et al. Assessment of recurrent stroke risk in patients with a carotid web. JAMA Neurol 2021;78:826‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang AJ, Dhruv P, Choi P, et al. A systematic literature review of patients with carotid web and acute ischemic stroke. Stroke 2018;49:2872‐6. [DOI] [PubMed] [Google Scholar]
- 5. Haussen DC, Grossberg JA, Koch S, et al. Multicenter experience with stenting for symptomatic carotid web. Intervent Neurol 2018;7:413‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SJ, Nogueira RG, Haussen DC. Current understanding and gaps in research of carotid webs in ischemic strokes: a review. JAMA Neurol 2019;76:355. [DOI] [PubMed] [Google Scholar]
- 7. Multon S, Denier C, Charbonneau P et al. Carotid webs management in symptomatic patients. J Vasc Surg 2021;73:1290‐7. [DOI] [PubMed] [Google Scholar]
- 8. Chaari D, Baud J‐M, Deschamps L, et al. Carotid diaphragm: atypical fibromuscular dysplasia or atheromatous lesions? Rev Neurol 2017;173:230‐3. [DOI] [PubMed] [Google Scholar]
- 9. Sajedi PI, Gonzalez JN, Cronin CA, et al. Carotid bulb webs as a cause of “cryptogenic” ischemic stroke. Am J Neuroradiol 2017;38:1399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coutinho JM, Derkatch S, Potvin A, et al. Carotid artery web and ischemic stroke. Neurology 2017;88:65‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SJ, Allen JW, Bouslama M, et al. Carotid webs in cryptogenic ischemic strokes: a matched case‐control study. J Stroke Cerebrovasc Dis 2019;28:104402. [DOI] [PubMed] [Google Scholar]
- 12. Zhu C, Li Z, Ju Y, et al. Detection of carotid webs by CT angiography, high‐resolution MRI, and ultrasound. J Neuroimaging 2020;31:71‐5 [DOI] [PubMed] [Google Scholar]
- 13. Madaelil TP, Grossberg JA, Nogueira RG, et al. Multimodality imaging in carotid web. Front Neurol 2019;10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu W, Crockett A, Low G, Patel V. Internal carotid artery web: Doppler ultrasound with CT angiography correlation. Radiol Case 2015;9:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo X, Li Z. Ultrasonic risk stratification of carotid web. Echocardiography 2019;36:2103‐7. [DOI] [PubMed] [Google Scholar]
- 16. Kliewer MA, Carroll BA. Ultrasound case of the day: internal carotid artery web (atypical fibromuscular dysplasia). Radiographics 1991;11:504‐5. [DOI] [PubMed] [Google Scholar]
- 17. Ben Z, Wang J, Zhan J, Chen S. Ultrasonic characteristics of carotid webs. Neuroradiology 2022;64:95‐8. [DOI] [PubMed] [Google Scholar]
- 18. Zhang H, Du J, Wang H, et al. Comparison of diagnostic values of ultrasound micro‐flow imaging and contrast‐enhanced ultrasound for neovascularization in carotid plaques. Exp Ther Med 2017;14:680‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Compagne KCJ, Dilba K, Postema EJ, et al. Flow patterns in carotid webs: a patient‐based computational fluid dynamics study. Am J Neuroradiol 2019;40:703‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haussen DA, Madaelil T, Barreira C, et al. Ultrasonography in carotid webs. Presented at the 10th Society of Vascular and Interventional Neurology: November 8–11, 2017; Boston. [Google Scholar]
- 21. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima‐Media Thickness Task Force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93‐1. [DOI] [PubMed] [Google Scholar]
- 22. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986;74:1399‐406. [DOI] [PubMed] [Google Scholar]
- 23. Haussen DC, Grossberg JA, Bouslama M, et al. Carotid web (intimal fibromuscular dysplasia) has high stroke recurrence risk and is amenable to stenting. Stroke 2017;48:3134‐7. [DOI] [PubMed] [Google Scholar]


