Abstract
Background
The prevalence and risk factors for residual neuromuscular blockade in children remain poorly characterized. We hypothesize that specific patient and anesthetic risk factors may be associated with the administration of additional reversal in children following initial reversal of rocuronium with neostigmine.
Methods
Our electronic health record was queried for patients <18 years of age who received rocuronium and reversal with neostigmine from 2017 through 2020. Patients receiving other nondepolarizing neuromuscular blocking drugs were excluded. The outcome of interest was defined as the administration of additional neostigmine or sugammadex following primary reversal with neostigmine. Time between the last dose of rocuronium and initial dose of neostigmine, and the cumulative dose of rocuronium were dichotomized. These were combined with other covariates including age, weight, sex, racial group, procedure type, ASA physical status, >1 rocuronium dose administered during the procedure, initial neostigmine dose <0.05 mg kg−1, use of train‐of‐four monitoring, duration of anesthesia, inpatient or outpatient, emergency case, neuromuscular disease, and extremes of weight, to assess possible associations with the primary outcome.
Results
During the study period, 101/6373 (1.58%) patients received rocuronium and additional reversal. Dichotomization of time between last dose of rocuronium and neostigmine yielded <28 min since the last dose of rocuronium and cumulative dose of rocuronium >0.45 mg kg−1 hr−1. These were associated with the administration of additional reversal with an OR 1.52 (95% CI, 1.08–2.35) and OR 1.71 (95% CI, 1.10–2.67), respectively. Other risk factors included an initial neostigmine dose <0.05 mg kg−1, OR 4.98 (95% CI, 2.84–6.49), and African American race, OR 1.78 (95% CI, 1.07–2.87).
Conclusion
Risk factors associated with the administration of additional reversal included time <28 min from the last dose of rocuronium to initial dose of neostigmine, cumulative dose of rocuronium >0.45 mg kg−1 hr−1, initial neostigmine dose <0.05 mg kg−1, and African American race.
Keywords: delayed emergence from anesthesia, African American, rocuronium, neostigmine, neuromuscular blockade
What is already known about this topic
Residual neuromuscular blockade defined as a train of four ratio <0.9 following the administration of neostigmine can occur in children receiving rocuronium. Additional reversal may be required to correct this.
What new information this study adds
A cumulative dose of 0.45 mg kg−1 hr−1 of rocuronium, time between the administration of rocuronium and neostigmine of <28 min, African American race, and a neostigmine dose <0.05 mg kg−1 are associated with an increased risk of residual neuromuscular blockade following primary reversal of rocuronium with neostigmine and therefore, the need for administration of additional reversal.
1. INTRODUCTION
The prevalence and risk factors for residual neuromuscular blockade following the administration of an initial dose of neostigmine in children who have received rocuronium remain largely unexplored. In adults, it is clear that inadequate reversal following administration of neostigmine remains relatively common and contributes to postoperative pulmonary complications. 1 , 2 , 3 In children, residual neuromuscular blockade and its consequences remain less well characterized.
Older studies in pediatric patients with longer acting neuromuscular blocking drugs have reported that residual neuromuscular blockade is rare. One study from 1991 found no episodes of train‐of‐four (TOF) ratios <70% on arrival to the recovery room after anesthesia. 4 However, more recent studies using accelerometry have suggested that residual neuromuscular blockade in children may be more common than previously thought. In one prospective study, the investigators found that 27% of pediatric patients had TOF ratios <90% in the recovery room. 5 In another prospective study, 37.5% of pediatric patients who received neostigmine for reversal had TOF ratios <90% prior to tracheal extubation. 6 Interestingly, simply having a TOF ratio <90% in children did not appear to be as clinically significant, as few, if any, of the patients in both of these studies experienced clinically significant sequelae beyond having a TOF ratio <90%.
Therefore, while a lack of twitch response to TOF stimulation likely requires sugammadex to reverse neuromuscular blockade by rocuronium, the optimal choice of reversal agent in other situations where a single twitch or other partial TOF responses are present may be less clear. 7 Further, the risks and benefits of selecting one reversal agent over another and risk factors for inadequate reversal have not been well studied in children. 8 , 9 , 10 Notably, cost may also be a factor when choosing between neostigmine and sugammadex for reversal of rocuronium. 11
The primary aim of this study was to evaluate the prevalence of residual neuromuscular blockade following at least one dose of rocuronium and reversal with neostigmine, and the risk factors associated with the need for additional administration of neostigmine or sugammadex. Further, we hypothesize that specific risk factors for administration of additional reversal may include cumulative rocuronium dose, time between last relaxant and reversal, and an initial reversal dose <0.05 mg kg−1 of neostigmine. Last, we will assess whether patients who received additional administration of neuromuscular blockade reversal suffered any postoperative pulmonary complications.
2. METHODS
After institutional IRB approval (IRB00068834), our electronic health records were queried for patients <18 years of age who underwent general endotracheal anesthesia from January 1, 2017, through December 31, 2020. This cohort of patients was further refined to patients who received rocuronium and neostigmine for reversal. Patients who received succinylcholine for initial intubation, who subsequently received rocuronium during the procedure were also included. Patients who received exclusively sugammadex for reversal, patients who received no reversal, and patients who remained intubated following their procedure were excluded. We also excluded any case in which the initial reversal and second dose of reversal were given to facilitate neuromonitoring or other circumstances requiring full reversal prior to the end of the procedure. Other exclusion criteria included age ≥18 years and administration of another nondepolarizing neuromuscular blockade agent in addition to rocuronium. The Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) checklist was used in the preparation of this manuscript. 12
2.1. Primary outcome and controls
The primary outcome of interest was the administration of additional reversal as a potential surrogate for clinically significant residual neuromuscular blockade, either with sugammadex following initial reversal with neostigmine or a second dose of neostigmine. The control group consisted of patients administered rocuronium followed by a single dose of reversal with neostigmine followed by successful extubation.
2.2. Covariates
Demographic data including age, weight, sex, race, procedure type, and ASA physical status were collected from the electronic health record for cases and controls. Race was self‐reported by the patient's parents. Surgical procedures were binned into the following 4 classes: otolaryngology (ENT), general surgery, orthopedic surgery, and other. In addition, we collected the initial dose of neostigmine. This was dichotomized to <0.05 mg kg−1 or ≥0.05 mg kg−1. 13 Other covariates included the total cumulative dose of rocuronium in mg kg−1 hr−1, as well as the interval between the last dose of rocuronium and dose of neostigmine, more than one rocuronium dose administered during the procedure, duration of anesthesia, high end‐tidal agent concentration defined as end‐tidal sevoflurane >0.4%, or end‐tidal isoflurane >0.3%, or end‐tidal desflurane >1.5%. High end‐tidal agent concentration was selected as a covariate to attempt to potentially identify situations in which additional reversal was administered in the setting of elevated potent inhalational agent and delayed emergence separate and apart from situations in which there was presumably clinically significant residual neuromuscular blockade. Agent concentration was extracted at the administration of a second dose of reversal in the primary outcome group as this would have been the point where the clinician would have made the determination in this group about whether the patient had residual neuromuscular blockade or was simply slow to emerge. Agent concentration was extracted 1 min prior to extubation in the control group as this would have been the level prior to extubation. This time point was selected because in some cases the inhalational agent concentration was missing at the point of extubation as these values are recorded in our electronic medical record as the average over that minute. In several patients who received a second dose of reversal following extubation, the end‐tidal agent concentration was extracted 1 min prior to extubation as this value is not likely to be captured accurately with a native airway. In several other cases where a second dose of reversal was given prior to the beginning of emergence during surgical closure, the end‐tidal agent concentration was recorded at the time of extubation as elevated concentrations at the point of administration of additional reversal would be necessary to maintain akinesis during closure and not reflective of those that would be found at emergence. Additional covariates included inpatient versus outpatient status, emergency case status, time of day, neuromuscular disease, extremes of weight, and the use of intraprocedural TOF monitoring. The presence of intraprocedural qualitative TOF monitoring was defined as the documentation of at least one qualitative measure of TOF 15 min prior to emergence. In addition, the TOF count value, when present, was also examined in the initial univariate analysis. Duration of anesthesia was binned into the following 3 categories: <60 min, 60–120 min, and >120 min. Neuromuscular disease was defined as the presence of at least one of the following: multiple sclerosis, cerebral palsy, Parkinson disease, muscular dystrophy, myotonic disorder, and mitochondropathy. Time of day was dichotomized to off hours and business hours where off hours was defined as the administration of reversal from 6 pm to 7 am during the week and/or Saturday and Sunday. Extremes of weight were defined as having a weight <5th percentile or >95th percentile according to the Center for Disease Control's standards for age and sex.
2.3. Complications
All records in which the patient received additional reversal were manually reviewed for documentation of any complications including oxygen desaturation, defined as oxygen saturation <90% for ≥1 min, reintubation, and increased level of post‐procedure care beyond what had initially been anticipated, or a new oxygen requirement in the post anesthesia care unit (PACU). Delayed emergence was defined as >20 min between the provider defined start of emergence and extubation.
2.4. Data validation
All cases identified by electronic review as having the outcome of interest were manually reviewed by study staff following data extraction to ensure the accuracy of covariates and the presence of the primary outcome measure.
2.5. Statistical analysis
An initial query at the time of project development indicated there might be between 100 and 120 cases available. Using 10 events per predictor, we estimated that we could develop a model with 10–12 covariates. Given that this was an exploratory analysis attempting to evaluate more than 20 covariates, we decided a priori to implement a least absolute shrinkage and selection operator (LASSO) multivariable regression model. 14
A data analysis plan was developed prior to accessing the data. Patients who received additional reversal were compared with the control group. All categorical covariates were evaluated initially using the Chi‐squared test to determine their association with the primary outcome at a univariate level. Where the cell counts were <5, Fisher's exact test was used. Continuous covariates were initially evaluated for normalcy using Shapiro–Wilk Normality test and Kolmogorov–Smirnov goodness‐of‐fit test. Normally distributed data are presented as means with standard deviation, and data that were not normally‐distributed are presented as medians with interquartile ranges. Normally distributed continuous covariates were compared at the univariate level using an independent, two sample, two‐tailed t‐test, while the Mann–Whitney U test was used for comparison of nonparametric data. Significance for the univariate analysis was established at p < 0.05. Prior to execution of the multivariable regression model, the covariates of cumulative dose of rocuronium and the time interval between the last dose of rocuronium and dose of neostigmine were dichotomized to maximize sensitivity and specificity using Youden's index. Selective inference (SI) techniques designed for the LASSO model were used to determine parameter inference on the model coefficients. 15 , 16 , 17 Following this analysis, all covariates except TOF count values, because they were not present in all patients, were entered into a LASSO regression model. Odds ratios were calculated with p < 0.05 considered significant. The lambda values used for analysis ranged from 0.00001 to 0.1. The lambda value for the final LASSO multivariable regression model was 0.0026. In addition, we internally validated the regression model using 10‐fold cross‐validation to test that the model performed well across randomly selected validation samples from the dataset.
We also performed several post‐hoc sensitivity analyses. The first of these was to exclude those patients from both groups in which the TOF count value was not documented and repeated the LASSO analysis adding in the TOF count values closest in time to the initial dose of neostigmine to the LASSO regression model. In addition, we performed a repeat analysis in which the time interval between relaxant and reversal and cumulative dose of rocuronium were evaluated as continuous variables along with other covariates using another LASSO regression model.
We performed several additional post‐hoc analyses to evaluate potential relationships between race and clinician behavior. These additional analyses included a comparison of the time from last dose of relaxant to reversal, cumulative dose of rocuronium, and the initial dose of neostigmine between African American and all other races combined in the group with residual neuromuscular blockade. We also compared the TOF count value closest to initial reversal with neostigmine. In addition, we compared the cumulative dose of rocuronium per hour using ideal body weight in the group requiring additional reversal. Finally, we performed an analysis of attending clinicians to determine if one or more attendings were significantly overrepresented in the group with the primary outcome as compared with the control group leading to selection bias.
Statistical analyses were performed in R v3.6.1 (R Foundation for Statistical Computing) using RStudio environment v1.1.456 (RStudio).
3. RESULTS
3.1. The patients
A query of our electronic health record revealed 6373 patients who received neostigmine for neuromuscular blockade reversal following the administration of rocuronium. Of these, 101/6373 (1.58%) patients received additional reversal, with 36 receiving sugammadex and 65 receiving a second dose of neostigmine. The control cohort consisted of 6272 patients. The prevalence of delayed emergence in the group that received additional reversal, defined by the study as an emergence time >20 min, was 13/101 (13%). Not surprisingly, the prevalence of delayed emergence in the control group was lower 390/6272 (6.22%): mean difference 6.78% (95% CI: [6.55%–6.75%], p = 0.0001). The prevalence of patients who received additional reversal 101/6373 (1.58%) was thus lower than the prevalence of those with delayed emergence in the whole cohort 403/6373 (6.32%): mean difference 4.78% (95% CI: [4.69%–4.81%], p = 0.0001).
Maximizing sensitivity and specificity using Youden's index to dichotomize time from the last dose of rocuronium to the initial dose of neostigmine and cumulative dose of rocuronium, yielded values of 28 min, and a cumulative dose of 0.45 mg kg−1 hr−1 of rocuronium. The median dose of sugammadex rescue was 2.1 mg kg−1 (range 0.2–5.5 mg kg−1). Finally, 11/101 (11%) received the additional dose of reversal after extubation. Unfortunately, documentation supporting the clinical reasoning for administering additional reversal was present in only one case. In that case, the clinician stated in the electronic health record that the patient had 4/4 twitches prior to extubation but had significant obstruction following extubation and therefore, additional sugammadex was administered with immediate relief of symptoms.
No one attending anesthesiologist was significantly overrepresented in either the control or the group with the primary outcome (Figure 1).
FIGURE 1.
Case distribution of top 10 providers by volume with all other providers grouped together as “Others.” Control group versus Additional Reversal Group. The numbers in each pie section represent the total number of cases performed by each provider within both the control group and those that received additional reversal
3.2. Univariate analysis
In the univariate analysis, age, increasing weight, extremes of weight, emergency case status, African American race, interval between the last dose of rocuronium and reversal administration <28 min, total cumulative dose of rocuronium >0.45 mg kg−1 hr−1, and TOF count value =0 were significantly associated with the administration of additional reversal. These results are summarized in Table 1. A histogram incorporating African American race and time between last dose of rocuronium and neostigmine is shown in Figure 2. As time from the last dose of rocuronium increased, the proportion of African American patients increased in the group of patients who received additional reversal.
TABLE 1.
Demographics and univariate analysis of risk factors for administration of additional reversal following reversal of rocuronium with neostigmine
| Demographics | Control (n = 6272) | RNMB (n = 101) | p‐Value |
|---|---|---|---|
| Median [IQR] | Median [IQR] | ||
| Age (year) | 9.69 [4.01, 14.38] | 14.00 [8.00, 16.00] | 1.12*10−4 a |
| Weight (kg) | 32.66 [15.70, 58.06] | 58.97 [21.32, 77.40] | 1.05*10−4 a |
| Demographics | N (%) | N (%) | p |
|---|---|---|---|
| ASA ≥3 | 1570 (25.0) | 34 (33.7) | 0.062 |
| Weight, <5th percentile or >95th percentile | 1942 (31.0) | 41 (40.6) | 0.049 |
| Neuromuscular disease | 12 (0.2) | 1 (1.0) | 0.513 |
| Male | 3386 (54.0) | 48 (47.5) | 0.233 |
| Inpatient | 1968 (31.4) | 34 (33.7) | 0.702 |
| Emergency | 941 (15.0) | 26 (25.7) | 0.004 |
| Demographics (Ethnicity) | |||
| Hispanic | 858 (13.7) | 16 (15.8) | 0.631 |
| Demographics (Race) | 0.045 | ||
| African American | 990 (15.8) | 25 (24.8) | ‐ |
| Caucasian | 3609 (57.5) | 54 (53.5) | ‐ |
| Other | 1673 (26.7) | 22 (21.8) | ‐ |
| Surgical case type | 0.409 | ||
| ENT | 454 (7.2) | 9 (8.9) | ‐ |
| General | 2727 (43.5) | 45 (44.6) | ‐ |
| Orthopedic | 1474 (23.5) | 17 (16.8) | ‐ |
| Other | 1617 (25.8) | 30 (29.7) | ‐ |
| Case length | 0.677 | ||
| Case length < 60 min | 952 (15.2) | 16 (15.8) | ‐ |
| Case length 60–120 min | 2568 (40.9) | 37 (36.6) | ‐ |
| Case length > 120 min | 2752 (43.9) | 48 (47.5) | ‐ |
| Other variables | |||
| Off Hours Case | 995 (15.9) | 14 (13.9) | 0.682 |
| Elevated End‐Tidal Agent Concentration | 1181 (18.8) | 10 (9.9) | 0.031 |
| Neuromuscular blockade variables | |||
| Initial neostigmine dose administered <28 min after last rocuronium dose | 1930 (30.8) | 46 (45.6) | 0.002 |
| Rocuronium re‐dosed | 2497 (39.8) | 56 (55.4) | 0.002 |
| Rocuronium dose >0.45 mg kg−1 hr−1 | 2639 (42.1) | 54 (53.5) | 0.028 |
| Initial neostigmine dose <0.05 mg kg−1 | 477 (7.6) | 21 (20.8) | 7.05*10−4 |
| TOF monitored intraoperatively | 3924 (62.6) | 85 (84.2) | 6.40*10−4 |
| TOF count = 0 | 513 (8.2) | 45 (44.6) | 7.54*10−4 |
| TOF count = 1 | 251 (4.0) | 8 (7.9) | 0.085 |
| TOF count = 2 | 243 (3.9) | 8 (7.9) | 0.069 |
| TOF count = 3 | 134 (2.1) | 2 (2.0) | 0.998 |
| TOF count = 4 | 2783 (44.4) | 22 (21.8) | 9.62*10−4 |
Note: p < 0.05 considered significant.
Abbreviations: ASA, American society of anesthesiologist physical status; ENT, otolaryngologic or airway procedure; LASSO, least absolute shrinkage and selection operator; RNMB, residual neuromuscular blockade; TOF, train‐of‐four.
Data were not normally distributed.
FIGURE 2.
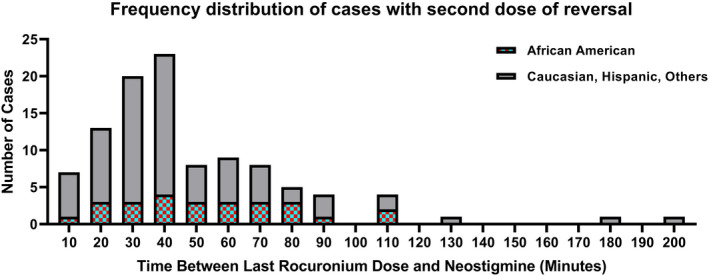
Histogram of cases requiring more than one dose of reversal broken down by African American race and all other patients as a function of time from the last dose of rocuronium
3.3. Multivariable analyses
The LASSO multivariable regression model demonstrated significant associations with an interval of <28 min from the last dose of rocuronium to the primary dose of neostigmine as well as an association with a cumulative dose of rocuronium >0.45 mg kg−1 hr−1. Other factors associated with the administration of additional reversal were an additional dose of rocuronium during the procedure, a neostigmine dose <0.05 mg kg−1 and African American race. Covariates not associated with the administration of additional reversal included extremes of weight, case length, case type, and off hours case. These results are summarized in Table 2. Sensitivity analyses in which time between relaxant and initial reversal and cumulative dose were treated as continuous variables were associated with a 22% reduction in odds (p < 0.044) for additional reversal every 20 min. These results are summarized in Table S1. In another sensitivity analysis incorporating number of twitches in proximity to initial reversal with neostigmine, a TOF count value of 0, 1, and 2 was associated with the need for additional reversal. These results are summarized in Table S2.
TABLE 2.
Odds ratios for increased likelihood of administration of additional reversal derived from LASSO multivariable regression
| Variable | OR | 95% CI | p‐Value |
|---|---|---|---|
| Demographics | |||
| ASA Classification ≥3 | 1.40 | 0.88–2.18 | 0.146 |
| Weight, <5th percentile or >95th percentile | 1.40 | 0.91–2.13 | 0.124 |
| Male | 0.71 | 0.47–1.06 | 0.091 |
| Case type | |||
| General a | ‐ | ‐ | ‐ |
| ENT | 1.04 | 0.46–2.13 | 0.927 |
| Orthopedics | 0.68 | 0.37–1.21 | 0.207 |
| Other | 1.12 | 0.68–1.81 | 0.657 |
| Race/Ethnicity | |||
| Hispanic | 1.72 | 0.83–3.47 | 0.140 |
| African American | 1.78 | 1.07–2.87 | 0.021 |
| Caucasian a | ‐ | ‐ | ‐ |
| Other | 0.75 | 0.38–1.41 | 0.380 |
| Case length | |||
| Case length >120 min | 0.63 | 0.34–1.22 | 0.157 |
| Case length 60–120 min | 0.95 | 0.53–1.77 | 0.859 |
| Other variables | |||
| Off Hours Case | 0.78 | 0.42–1.35 | 0.399 |
| Elevated End‐Tidal Agent Concentration | 0.43 | 0.21–0.8 | 0.014 |
| Neuromuscular blockade variables | |||
| Initial neostigmine dose administered <28 min after last rocuronium dose | 1.52 | 1.08–2.35 | 0.039 |
| Rocuronium re‐dosed | 1.66 | 1.06–2.63 | 0.028 |
| Rocuronium dose >0.45 mg kg−1 hr−1 | 1.71 | 1.1–2.67 | 0.018 |
| TOF monitored intraoperatively | 3.04 | 1.8–5.47 | 3.336*10−4 |
| Initial neostigmine dose <0.05 mg kg−1 | 4.98 | 2.84–6.49 | 2.84*10−5 |
Note: p < 0.05 considered significant. ENT = otolaryngologic or airway procedure;
Abbreviations: ASA, American society of anesthesiologist physical status; LASSO, least absolute shrinkage and selection operator; TOF, train‐of‐four.
Reference category.
3.4. Race and additional reversal
A comparison of the average time from last dose of rocuronium to initial neostigmine reversal in patients with residual neuromuscular blockade was 55.8 ± 26.6 min versus 44.6 ± 34.7 min (p = 0.230) in children of African American race versus the other race groups combined. In addition, the mean cumulative dose of rocuronium was 0.46 ± 0.14 mg kg−1 hr−1 versus 0.48 ± 0.24 mg kg−1 hr−1 (p = 0.713) in children of African American race versus the other race groups combined. When adjusted for ideal body weight, the difference again was not statistically significant. The median TOF count value in the African American group requiring additional reversal was 0 versus 1 in the Non‐African American group requiring reversal. This, however, did not achieve statistical significance (p = 0.378). An overview of TOF count value data in both the control group and those receiving additional reversal broken down by African American and Non‐African American is presented in Figure 3. Finally, the average initial dose of neostigmine in all other children receiving additional reversal was 0.05 ± 0.02 mg kg−1 versus 0.05 ± 0.03 mg kg−1 (p = 0.957) in children of African American race. These results are summarized in Table 3.
FIGURE 3.
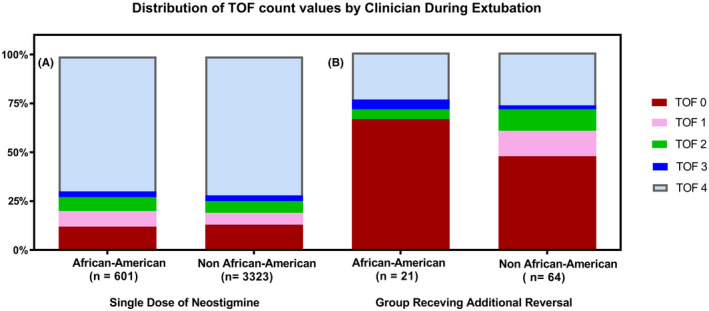
Distribution of train‐of‐four count values for African American and Non‐African American in the control group (A) and those who received additional reversal (B). The median train‐of‐four count value for African American patients was 0/4 compared with 1/4 in other patients in the residual neuromuscular blockade group (p = 0.378). TOF, train‐of‐four count value
TABLE 3.
Demographics of children who received additional neuromuscular blockade reversal with comparison of African American children to Non‐African American children
| Demographics | African American (n = 25) | Non‐African American (n = 76) | p‐Value |
|---|---|---|---|
| Median [IQR] | Median[IQR] | ||
| Age (year) | 13.0 [1.00, 16.10] | 14.3 [9.43, 16.00] | 0.420 |
| Weight (kg) | 56.52 [12.02, 67.59] | 60.10 [35.02, 77.70] | 0.238 |
| Median train‐of‐four count value prior to initial reversal | 0 [0,3.5] (n = 21) |
1 [0,4] (n = 64) |
0.378 |
| N (%) | N (%) | ||
|---|---|---|---|
| Male | 12 (48.0) | 36 (47.4) | 0.890 |
| Inpatient | 7 (28.0) | 27 (35.5) | 0.627 |
| Emergency | 6 (24.0) | 20 (26.3) | 0.880 |
| Weight, <5th percentile or > 95th percentile | 8 (32.0) | 33 (43.4) | 0.439 |
| Elevated end‐tidal agent concentration | 3 (12.0) | 7 (9.2) | 0.985 |
| Off hours | 3 (12.0) | 11 (14.5) | 0.982 |
| Surgical case type | 0.367 a | ||
| ENT | 4 (16.0) | 5 (6.6) | ‐ |
| General | 11 (44.0) | 34 (44.7) | ‐ |
| Orthopedics | 5 (20.0) | 12 (15.8) | ‐ |
| Other | 5 (20.0) | 25 (32.9) | ‐ |
| Mean (SD) | Mean (SD) | ||
|---|---|---|---|
| Case length (minutes) | 135.56 (71.74) | 139.42 (81.59) | 0.833 |
| Initial neostigmine dose (mg kg−1) | 0.05 (0.02) | 0.05 (0.03) | 0.957 |
| Time from last rocuronium dose to initial neostigmine dose (minutes) | 55.80 (26.55) | 44.64 (34.67) | 0.230 |
| Cumulative rocuronium dose (mg kg−1 hr−1) | 0.46 (0.14) | 0.48 (0.24) | 0.713 |
| Cumulative rocuronium dose using ideal body weight | 0.73 (0.66) | 0.65 (0.44) | 0.541 |
Note: p < 0.05 considered significant. ENT = otolaryngologic or airway procedure.
p‐values based on 2 × 3 or 2 × 4 Chi‐square analysis.
3.5. Complications in patients receiving additional reversal
A manual review of all 101 patients with residual neuromuscular blockade revealed one episode of oxygen desaturation of <90% for >1 min. This episode occurred during emergence from anesthesia at the time of additional reversal administration. There were eight episodes of new oxygen requirements after surgery in the PACU. There was one episode of reintubation, which occurred 10 h after discharge from PACU.
4. DISCUSSION
The primary finding of this retrospective case–control study is that although the administration of additional reversal and clinically significant residual neuromuscular blockade is uncommon, it does occur. The prevalence of the primary outcome in this study is sufficient enough that the clinician should be aware of specific risk factors associated with the need for additional reversal when using neostigmine. In addition, these results suggest that should the clinician decide to proceed with neostigmine in situations at higher risk for inadequate reversal, the clinician should likely use at least 0.05 mg kg−1. These considerations may be especially important in situations where the patient may be at higher risk of perioperative respiratory adverse events as a result of underlying patient factors including comorbidities, the accumulation of metabolic debt, or location and type of procedure.
Intuitively, there are several factors that would appear to have the greatest impact on this decision, including a shorter interval between the last dose of relaxant and the cumulative dose of rocuronium. We found it surprising that 28 min was the interval of time between relaxant and reversal with neostigmine that placed the patient potentially at increased risk of administration of additional reversal. Initially, we presumed that it might be significantly shorter. Interestingly, the cumulative dose of rocuronium associated with additional administration of neuromuscular blockade reversal was fairly low. Generally, most clinicians do not think in terms of a cumulative dose of relaxant, as they will often titrate to effect using TOF monitoring to guide administration. Looking at these findings, even a relatively low intubation dose of 0.5 mg kg−1 in a case lasting 45 min where the relaxant is not re‐dosed could put the patient at risk for residual neuromuscular blockade following reversal with neostigmine. These data are consistent with the study by Klucka et al. 5 in which shorter time intervals between the administration of relaxant and reversal agent were found to be a risk factor for residual neuromuscular blockade. Specifically, there was a 19% risk reduction for residual neuromuscular blockade with every additional 10 min from the administration of a neuromuscular blockade agent. It should be clarified, that in this study, the primary outcome was a TOF ratio of <0.9 in the operating room and PACU rather than an additional dose of reversal. Importantly, the discrepancy in the prevalence of the primary outcomes between their study and our study (27% vs. 1.58%) and the lack of significant pulmonary complications associated with a TOF <0.9 in their study suggest that current definitions of clinically significant residual neuromuscular blockade in children may need to be reexamined.
Significant secondary findings in our study included that an initial neostigmine dose of <0.05 mg kg−1 was also associated with a higher risk of administration of additional neuromuscular blockade reversal. Notably, the manufacturer labeling identifies the usual dose of neostigmine for neuromuscular blockade reversal as 0.03–0.07 mg kg−1. 7 Interestingly, older research had shown that infants and children require less neostigmine than adults to antagonize d‐tubocurarine or rocuronium‐induced neuromuscular blockade. 13 , 18 In a 1991 study, no episodes of TOF ratios <70% were identified in pediatric patients in the recovery room after anesthesia; however, this study only included patients who received 0.06 mg kg−1 of neostigmine before extubation. 4 In a 2015 study, only one episode of TOF ratio <70% was identified out of 32 pediatric patients who received 0.08 mg kg−1 of neostigmine before extubation. 6 Therefore, in situations where the clinician has chosen to use neostigmine for reversal, a dose of at least 0.05 mg kg−1 should be considered.
In addition, African American race was independently associated with a higher risk of administration of additional neuromuscular blockade reversal. This was an unexpected finding that may be multifactorial. In most cases, racial differences in medicine and disparities in care are related to cultural biases and clinician behavior; however, multiple analyses including timing of administration of neostigmine, cumulative dose of relaxant, and similar doses of initial reversal with neostigmine, did not yield an adequate behavioral related explanation. It is possible that these analyses may have had insufficient numbers to demonstrate any differences in behavior that were present. Pharmacogenetic differences may play a role in the higher risk of administration of additional reversal agent among African American children. While the potency and duration of action of rocuronium have been noted to vary by ethnic background, a prolonged effect of rocuronium in African Americans has not been previously reported in either adults or children. 19 However, steroid molecules have a longer half‐life and reduced clearance in African Americans, and this same mechanism could occur with rocuronium. 20 , 21 Single‐nucleotide polymorphisms in the organic anion transporting polypeptide (OATP) family have also been identified as a potential source of variability in rocuronium dose requirements, as rocuronium is a known substrate of OATP1A2 and allelic frequency appears to vary by race. 22 , 23 , 24
Interestingly, TOF monitoring was also found to be associated with the need for additional reversal. We felt, however, this is likely related to a selection bias because clinicians who are concerned that, either they may have given too much relaxant or have not had adequate time for the relaxant to wear off, may be more likely to apply a qualitative TOF monitor to attempt to assess depth of neuromuscular blockade and further reassure themselves that the patient is in a good position to be reversed with neostigmine. Further, we know that the use of qualitative TOF monitoring by pediatric anesthesia clinicians is haphazard at best and this is reflected in the lower utilization in the control group. 25
4.1. Limitations
Limitations of our study included the smaller sample size and single center nature of the cohort. In addition, the administration of additional neostigmine or sugammadex after initial administration of neostigmine may have been a clinical action that was taken in response to delayed emergence, which may or may not have represented actual residual neuromuscular blockade. Importantly though, the incidence of administering additional reversal was low (1.58%) when compared with the incidence of delayed emergence (6.22%) in the control group as defined by the study suggesting that there were other clinical indicators including potentially low tidal volumes or a lack of movement despite low end‐tidal agent concentration suggestive of residual neuromuscular blockade necessitating additional reversal. Conversely, we potentially misclassified some patients with residual neuromuscular blockade who did not receive additional reversal. Given published rates of residual neuromuscular blockade in children, defined strictly as a TOF <0.9, it is possible and perhaps even likely that this occurred. 5 , 6 Interestingly, all patients in the control group were successfully extubated suggesting that the traditional definition of residual neuromuscular blockade may be too strict and less clinically applicable in children. Finally, these findings cannot be generalized to patients who have received other nondepolarizing neuromuscular blocking drugs or a combination of rocuronium and other nondepolarizing neuromuscular blocking drugs.
5. CONCLUSION
In summary, clinically significant residual neuromuscular blockade occurs in children and is increased in situations where the interval between the last dose of rocuronium and neostigmine administration is <28 min and the cumulative dose of rocuronium exceeds 0.45 mg kg−1 hr−1. In addition, lower doses of neostigmine reversal (<0.05 mg kg−1) and African American race also appear to be associated with an increased risk of administration of additional neuromuscular blockade reversal. Future studies will be needed to corroborate these findings and likely further refine definitions of clinically significant residual neuromuscular blockade in children.
CONFLICT OF INTEREST
The authors declare no competing interests.
Supporting information
Tables S1 and S2
Vishneski SR, Saha AK, Fram MR, et al. Risk factors for administration of additional reversal following neuromuscular blockade with rocuronium in children: A retrospective case–control study. Pediatr Anaesth. 2022;32:916‐925. doi: 10.1111/pan.14463
Section Editor: Francis Veyckemans
Funding information
Funding was provided solely from department resources.
DATA AVAILABILITY STATEMENT
The research data will not be shared due to institutional restrictions.
REFERENCES
- 1. Kheterpal S, Vaughn MT, Dubovoy TZ, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020;132:1371‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grosse‐Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non‐depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012;345:e6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bulka CM, Terekhov MA, Martin BJ, Dmochowski RR, Hayes RM, Ehrenfeld JM. Nondepolarizing neuromuscular blocking agents, reversal, and risk of postoperative pneumonia. Anesthesiology. 2016;125:647‐655. [DOI] [PubMed] [Google Scholar]
- 4. Baxter MR, Bevan JC, Samuel J, Donati F, Bevan DR. Postoperative neuromuscular function in pediatric day‐care patients. Anesth Analg. 1991;72:504‐508. [DOI] [PubMed] [Google Scholar]
- 5. Klucka J, Kosinova M, Krikava I, Stoudek R, Toukalkova M, Stourac P. Residual neuromuscular block in paediatric anaesthesia. Br J Anaesth. 2019;122:e1‐e2. [DOI] [PubMed] [Google Scholar]
- 6. Ledowski T, O'Dea B, Meyerkort L, Hegarty M, von Ungern‐Sternberg BS. Postoperative residual neuromuscular paralysis at an Australian tertiary children's hospital. Anesthesiol Res Pract. 2015;2015:410248. doi: 10.1155/2015/410248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloxiverz Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204078s000lbl.pdf. Accessed October 14, 2021.
- 8. Gaver RS, Brenn BR, Gartley A, Donahue BS. Retrospective analysis of the safety and efficacy of sugammadex versus neostigmine for the reversal of neuromuscular blockade in children. Anesth Analg. 2019;129:1124‐1129. [DOI] [PubMed] [Google Scholar]
- 9. Liu G, Wang R, Yan Y, Fan L, Xue J, Wang T. The efficacy and safety of sugammadex for reversing postoperative residual neuromuscular blockade in pediatric patients: a systematic review. Sci Rep. 2017;7:5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsui M, Konishi J, Suzuki T, Sekijima C, Miyazawa N, Yamamoto S. Reversibility of rocuronium‐induced deep neuromuscular block with sugammadex in infants and children‐A randomized study. Biol Pharm Bull. 2019;42:1637‐1640. [DOI] [PubMed] [Google Scholar]
- 11. Hurford WE, Welge JA, Eckman MH. Sugammadex versus neostigmine for routine reversal of rocuronium block in adult patients: a cost analysis. J Clin Anesth. 2020;67:110027. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453‐1457. [DOI] [PubMed] [Google Scholar]
- 13. Abdulatif M, Mowafi H, Al‐Ghamdi A, El‐Sanabary M. Dose–response relationships for neostigmine antagonism of rocuronium‐induced neuromuscular block in children and adults. Br J Anaesth. 1996;77:710‐715. [DOI] [PubMed] [Google Scholar]
- 14. Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Statist Soc B. 2011;73:273‐282. [Google Scholar]
- 15. Lee JD, Sun DL, Sun Y, Taylor JE. Exact post‐selection inference, with application to the lasso. Ann Stat. 2016;44:907‐927. [Google Scholar]
- 16. Taylor J, Tibshirani R. Post‐selection inference for‐penalized likelihood models. Can J Stat. 2018;46:41‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor J, Tibshirani RJ. Statistical Learning and Selective Inference. Proc Natl Acad Sci U S A. 2015;112(25):7629‐7634. doi: 10.1073/pnas.1507583112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher DM, Cronnelly R, Miller RD, Sharma M. The neuromuscular pharmacology of neostigmine in infants and children. Anesthesiology. 1983;59:220‐225. [DOI] [PubMed] [Google Scholar]
- 19. Dahaba AA, Perelman SI, Moskowitz DM, et al. Geographic regional differences in rocuronium bromide dose–response relation and time course of action: an overlooked factor in determining recommended dosage. Anesthesiology. 2006;104:950‐953. [DOI] [PubMed] [Google Scholar]
- 20. Magee MH, Blum RA, Lates CD, Jusko WJ. Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol. 2001;41:1180‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tornatore KM, Reed KA, Venuto RC. Racial differences in the pharmacokinetics of methylprednisolone in black and white renal transplant recipients. Pharmacotherapy. 1993;13:481‐486. [PubMed] [Google Scholar]
- 22. Ahlstrom S, Bergman P, Jokela R, et al. First genome‐wide association study on rocuronium dose requirements shows association with SLCO1A2. Br J Anaesth. 2021;126:949‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP‐C: identification of multiple allelic variants associated with altered transport activity among European‐ and African‐Americans. J Biol Chem. 2001;276(38):35669‐35675. [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Zhou MT, Chen CY, et al. Increased renal clearance of rocuronium compensates for chronic loss of bile excretion, via upregulation of Oatp2. Sci Rep. 2017;7:40438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meretoja OA. Neuromuscular block and current treatment strategies for its reversal in children. Pediatric Anesthesia. 2010;20(7):591‐604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2
Data Availability Statement
The research data will not be shared due to institutional restrictions.


