Abstract
Objective
Numerous predictive scores have been developed to help determine which patients with epilepsy or seizures of unknown etiology should undergo neural antibody testing. However, their diagnostic advantage compared to only performing testing in patients with “obvious” indications (e.g., broader features of autoimmune encephalitis, characteristic seizure semiologies) requires further study. We aimed to develop a checklist that identifies patients who have “obvious” indications for neural antibody testing and to compare its diagnostic performance to predictive scores.
Methods
We developed the “Obvious” indications for Neural antibody testing in Epilepsy or Seizures (ONES) checklist through literature review. We then retrospectively reviewed patients who underwent neural antibody testing for epilepsy or seizures at our center between March 2019 and January 2021, to determine and compare the sensitivity and specificity of the ONES checklist to the recently proposed Antibody Prevalence in Epilepsy and Encephalopathy (APE2)/Antibodies Contributing to Focal Epilepsy Signs and Symptoms (ACES) reflex score.
Results
One‐hundred seventy patients who underwent neural antibody testing for epilepsy or seizures were identified. Seventy‐four of 170 (43.5%) with a known etiology were excluded from sensitivity/specificity analyses; none had a true‐positive neural antibody. Of the 96 patients with an unknown etiology, 14 (15%) had a true‐positive neural antibody. The proportion of false‐positives was significantly higher among patients with a known etiology (3/3, 100%) compared to an unknown etiology (2/16, 13%; p = .01). There was no significant difference of the APE2/ACES reflex score compared to the ONES checklist with regard to sensitivity (93% for both, p > .99) or specificity (71% vs. 78%, p = .18) for true‐positive neural antibodies.
Significance
Compared to only performing neural antibody testing in patients with epilepsy or seizures of unknown etiology who have “obvious” indications, predictive scores confer no clear diagnostic advantage. Prespecified definitions of what constitutes a true‐positive neural antibody is required in future studies to avoid false‐positives that can confound results.
Keywords: acute symptomatic seizures secondary to autoimmune encephalitis, autoimmune encephalitis, autoimmune epilepsy, autoimmune seizures, autoimmune‐associated epilepsy
Key Points.
We developed the ONES checklist through literature review
There was no difference in sensitivity or specificity of the ONES checklist compared to the recently proposed APE2/ACES reflex score
False‐positive neural antibody results can occur in patients with epilepsy or seizures, the proportion of which is higher in those with known etiology
Studies are needed to clarify which patients, if any, do not have “obvious” indications for testing but would benefit from predictive scores
Defining what constitutes a true‐positive neural antibody is needed in future studies to avoid false‐positives that can confound results
1. INTRODUCTION
Among patients with epilepsy, there has been increasing interest in the detection of neural antibodies that indicate an immune etiology. 1 , 2 The Antibody Prevalence in Epilepsy score, later revised to the Antibody Prevalence in Epilepsy and Encephalopathy (APE2) score, was developed to help determine which patients benefit most from neural antibody testing to diagnose “autoimmune epilepsy.” 3 , 4 , 5 , 6 However, the appropriateness of the term “autoimmune epilepsy” has been questioned, and the need for its conceptual distinction from autoimmune encephalitis has been emphasized. 7 , 8 , 9 Many clinical and neuroimaging items included in predictive scores that are intended to be applied to patients with epilepsy or seizures of unknown etiology are derived from features of autoimmune encephalitis, for which dedicated diagnostic criteria exist. 8 , 10 This is reflective of the finding that whereas some patients with neural antibody‐associated disease may present with seizures in relative isolation, a substantial proportion ultimately develop broader features of autoimmune encephalitis. 8 , 10 , 11 It has recently been suggested that patients who have features that are clearly suspicious for neural antibody positivity, such as those indicative of a broader encephalitis, should be excluded from investigations of neural antibody testing in epilepsy or seizures of unknown etiology. 7 This was attempted in the Antibodies Contributing to Focal Epilepsy Signs and Symptoms (ACES) study, which excluded patients who had features suggesting an immune etiology that were recognized by the referring clinician. 12 Expectedly, the rate of neural antibody positivity in the ACES study was low, at 3.4%, which is in contrast to other studies reporting neural antibody positivity in up to 31.5% of patients with seizures of unknown etiology that did not have this exclusion criterion. 12 , 13 Remarkably, however, among the patients in the ACES study who were neural antibody‐positive, virtually all of them had neuroimaging findings, clinical symptoms, seizure semiologies, or biochemical abnormalities that were characteristic of neural antibody‐associated presentations, but went unrecognized by the referring clinician, which led to study inclusion. 12 This finding highlights the need for an assessment tool that can be used to effectively identify patients with epilepsy or seizures of unknown etiology who have presentations that should raise suspicion for neural antibody positivity, and thus “obviously” merit neural antibody testing. It also lends credence to the hypothesis that after systematic identification of such patients for testing, there may be no additional diagnostic utility of predictive scores.
To evaluate this hypothesis, we developed the “Obvious” indications for Neural antibody testing in Epilepsy or Seizures (ONES) checklist through literature review. It consists of presentations that are individually suspicious for neural antibody positivity, and should therefore prompt consideration of testing. The phrase “epilepsy or seizures” was chosen to highlight the uncertainty surrounding the most appropriate terminology prior to completion of neural antibody testing, the result of which can provide insight into underlying seizure pathophysiology and the likelihood of enduring seizure predisposition. 8 , 9 To evaluate the diagnostic performance of the ONES checklist, we then retrospectively reviewed patients assessed at our center to determine and compare the sensitivity and specificity of the ONES checklist to the APE2/ACES reflex score, which was recently proposed to optimize the performance of predictive scores. 7
2. MATERIALS AND METHODS
2.1. “Obvious” indications for neural antibody testing in patients with epilepsy or seizures: Development of ONES checklist
One of the authors with formal training in autoimmune neurology (A.B.) drafted the ONES checklist, based on review of the literature pertaining to neural antibody‐associated disease and alternative diagnostic considerations. Details regarding the rationale behind each item are provided in Appendix S1 with references. Although paired serum and cerebrospinal fluid (CSF) testing is generally recommended once the decision is made to pursue neural antibody testing, 2 CSF profile (e.g., white blood cell count, protein, oligoclonal bands) was not incorporated in the ONES checklist because of the infrequency of lumbar puncture in epilepsy evaluations. 14 The checklist was brought forth to three of the other authors with formal training in epilepsy (M.N.N, S.M., J.G.B.), and each item was confirmed for inclusion after discussion to achieve consensus. The ONES checklist is shown in Table 1. A guide to its operationalization is provided in Table 2.
TABLE 1.
ONES checklist a
| 1a) Perform panel‐based neural antibody testing including anti‐MOG if any of the following are present: | |||
|---|---|---|---|
| Are any of the following present? | Yes | No | Antibody/antibodies of most relevance |
| Brain magnetic resonance imaging b | |||
| Cortical T2‐FLAIR hyperintense lesion(s) with or without involvement of the underlying white matter, in temporal relation to seizure onset | Anti‐MOG, NMDAR, GABAAR, mGluR5 | ||
| Large (>1–2 cm) T2‐FLAIR hyperintense lesion(s) involving the white matter suggestive of non‐MS demyelination, in temporal relation to seizure onset | Anti‐MOG, may overlap with anti‐NMDAR | ||
| Clinical | |||
| Optic neuropathy or myelopathy of unknown etiology, in temporal relation to seizure onset | Anti‐MOG, may overlap with anti‐NMDAR | ||
| 1b) Perform panel‐based neural antibody testing including anti‐GlyR if the following is present: | |||
|---|---|---|---|
| Are any of the following present? | Yes | No | Antibody/antibodies of most relevance |
| Clinical | |||
| Prominent stiffness, spasms, rigidity, and/or hyperekplexia of unknown etiology, in temporal relation to seizure onset | Anti‐GlyR, amphiphysin, DPPX, GAD65 | ||
| 2) If all of the above features are absent, perform panel‐based neural antibody testing excluding anti‐MOG and anti‐GlyR if any of the following are present: | |||
|---|---|---|---|
| Are any of the following present? | Yes | No | Antibody/antibodies of most relevance |
| Brain magnetic resonance imaging b | |||
| T2‐FLAIR hyperintensity restricted to the medial temporal lobe(s) without atrophy, in temporal relation to seizure onset | Various | ||
| Linear radial perivascular enhancement, in temporal relation to seizure onset | Anti‐GFAP, may overlap with anti‐NMDAR | ||
| Biochemical | |||
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, with serum sodium < 130 mEq/L of unknown etiology c , d | Anti‐LGI1 | ||
| Clinical/semiological/electroencephalographical | |||
| Distinguishable central or peripheral nervous system dysfunction of unknown etiology, in temporal relation to seizure onset e | Various | ||
| Musicogenic seizures | Anti‐GAD65 | ||
| Faciobrachial dystonic seizures f | Anti‐LGI1 | ||
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, with pilomotor seizures d | Anti‐LGI1 | ||
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, with paroxysmal dizziness spells d | Anti‐LGI1 | ||
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, beginning after 50 years of age d | Anti‐LGI1, CASPR2 | ||
| Refractory temporal lobe or presumed temporal lobe seizures, with anti‐GAD65‐associated systemic autoimmunity d , g | Anti‐GAD65 | ||
| Historical | |||
| New (within 1 year) seizures, beginning within 2 years of tumor diagnosis h | Various | ||
| New (within 1 year) seizures, beginning within 1 year of last immune checkpoint inhibitor treatment | Various | ||
| New (within 1 year) seizures, beginning or worsening within 3 months of last antiviral treatment for herpes simplex virus encephalitis i | Anti‐NMDAR | ||
Abbreviations: CASPR2, contactin‐associated protein‐like 2; DPPX, dipeptidyl‐peptidase‐like protein 6; FBDS, faciobrachial dystonic seizures; FLAIR, fluid‐attenuated inversion recovery; GABAAR, γ‐aminobutyric acid type A receptor; GAD65, glutamic acid decarboxylase 65; GFAP, glial fibrillary acidic protein; GlyR, glycine receptor; HSV, herpes simplex virus; LGI1, leucine‐rich glioma‐inactivated 1; mGluR5, metabotropic glutamate receptor 5; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NMDAR, N‐methyl‐D‐aspartate receptor; ONES, “Obvious” indications for Neural antibody testing in Epilepsy or Seizures.
The ONES checklist should only be used in patients with epilepsy or seizures of unknown etiology. When pursuing neural antibody testing, serum and cerebrospinal fluid testing is generally recommended to maximize sensitivity and specificity.
All listed neuroimaging abnormalities presume the imaging appearance is not more suggestive of an alternative etiology (e.g., tumor, infection, toxic/metabolic, hypoxic/ischemic, seizure‐related change).
Serum sodium < 130 mEq/L of unknown etiology requires exclusion of competing etiologies (e.g., hypovolemia, medication effect including antiseizure medications and diuretics, heart failure, liver or renal disease, spurious laboratory result).
Temporal lobe seizures with involvement of adjacent regions (e.g., temporoperisylvian) are included.
Examples include cognitive impairment, behavioral changes, psychiatric symptoms, aphasia/speech disturbances, sleep disturbances, movement disorders, brainstem/cerebellar dysfunction, dysautonomia, radiculopathy/neuropathy, neuropathic pain, and peripheral nerve hyperexcitability. For optic neuropathy, myelopathy, or features of stiff‐person spectrum disorders/progressive encephalomyelitis with rigidity and myoclonus, see preceding items.
Includes FBDS with or without basal ganglia T1/T2 hyperintensity, the finding of which on neuroimaging should prompt careful case review for FBDS in the appropriate clinical context.
Examples of anti‐GAD65‐associated systemic autoimmunity include type 1 diabetes mellitus, autoimmune thyroid disease, pernicious anemia, vitiligo, celiac disease, and Addison disease.
Excluding keratinocyte carcinoma, and primary or secondary brain tumor. [Correction added on 17 May 2022, after first online publication: In the preceding sentence, the phrase “nonmelanoma skin cancer” has been replaced with the term “keratinocyte carcinoma”.]
Documentation of negative cerebrospinal fluid polymerase chain reaction for HSV is required. The phrase “beginning or worsening” of seizures is intended to acknowledge that patients may also have seizures related to initial HSV encephalitis, which must be distinguished from new or worsening seizures potentially attributable to post‐HSV autoimmune encephalitis.
TABLE 2.
Guide to operationalization of the ONES checklist
| 1. The ONES checklist should only be used in patients with epilepsy or seizures of unknown etiology, after appropriate evaluation (e.g., clinical history, physical examination, brain magnetic resonance imaging, electroencephalography) to exclude more likely alternative diagnoses. |
| 2. The ONES checklist restricts anti‐MOG and anti‐GlyR testing to patients with typical disease phenotypes. The tiered approach to the checklist should be followed, with progression from one tier to the next only if the answer is “No” to all preceding items. In the rare patient with seizures and features of both anti‐MOG and anti‐GlyR, panel‐based testing that includes both of these antibodies should be performed, hence their listing as (1a) and (1b) on the ONES checklist. |
| 3. Nervous system dysfunction or neuroimaging findings that are ictal or postictal phenomena should not be the reason for answering “Yes” to relevant items on the ONES checklist. Close clinical and/or neuroimaging follow‐up can aid in making these distinctions and is encouraged. |
| 4. The phrase “in temporal relation to seizure onset” used throughout the ONES checklist emphasizes the importance of evaluating clinical symptoms and neuroimaging findings as they relate to seizure onset, because a temporal relationship supports a shared etiology. |
| 5. The term “distinguishable” and the phrase “of unknown etiology” used throughout the ONES checklist are intended to emphasize the importance of distinguishing dysfunction possibly attributable to neural antibody associated‐disease not only from alternative diagnoses, but also from neuropsychiatric symptoms that are common among patients with epilepsy. Inquiry into the impact of such symptoms on activities of daily living, collection of ancillary clinical history from friends or relatives, and formal cognitive assessment/neuropsychometric testing can help make these determinations and are encouraged. |
| 6. The term “refractory” refers to failure of two or more antiseizure medications (either as monotherapies or in combination). In patients with high seizure frequency, timely identification using the ONES checklist relies on expedient determination of seizure refractoriness. |
| 7. Medial temporal lobe T2‐FLAIR hyperintensity with atrophy suggestive of MTS is not included in the ONES checklist, because of the frequency of MTS in nonimmune temporal lobe epilepsy. In patients with MTS, however, it is critical to review any previously available neuroimaging to look for T2‐FLAIR hyperintensity restricted to the medial temporal lobe(s) without atrophy that is suggestive of autoimmune limbic encephalitis in temporal relation to seizure onset, which is included in the ONES checklist. |
| 8. Where “temporal lobe” seizure localization is specified, review of clinical information (e.g., seizure semiology) and ancillary test data (e.g., electroencephalography) is critical to identify supportive evidence for this localization. Temporal lobe seizures with involvement of adjacent regions (e.g., temporoperisylvian) are included. Thorough review is particularly important for patients with recurrent generalized tonic–clonic seizures, in whom temporal lobe seizure origin may not be immediately apparent. |
| 9. The term “presumed temporal lobe seizures” is intended to identify rare patients with recurrent seizures for whom there are no clinical or ancillary test data that definitively aid in seizure localization. These are patients who could, however, reasonably be presumed to have temporal lobe seizures in the absence of evidence to suggest otherwise. For this reason, patients with non‐temporal lobe symptoms/semiologies, or electroencephalographic findings suggesting exclusively extratemporal/independent extratemporal multifocal spike foci should not be considered to have “presumed temporal lobe seizures.” |
| 10. In patients with seizures and one or more historical features, clinicians are likely to pursue neural antibody testing even prior to definitive determination of seizure localization or refractoriness, so no qualifiers regarding these aspects are included. However, the use of the word “seizures” (plural) should be kept in mind, to avoid incorrect application of these items to single provoked seizures that may occur in this setting. Patients with a single seizure and historical feature(s) may still be considered for neural antibody testing, but often have other items on the checklist that raise suspicion for neural antibody positivity (e.g., other nervous system dysfunction, neuroimaging abnormalities). Thorough review to exclude more likely alternative diagnoses is particularly important in these medically complex patients. |
Abbreviations: FLAIR, fluid‐attenuated inversion recovery; GlyR, glycine receptor; MOG, myelin oligodendrocyte glycoprotein; MTS, mesial temporal sclerosis; ONES, “Obvious” indications for Neural antibody testing in Epilepsy or Seizures (ONES).
Special consideration was given to testing for antibodies against myelin oligodendrocyte glycoprotein (MOG) and glycine receptor (GlyR). Although some have recommended expanded neural antibody testing that includes testing for anti‐MOG and anti‐GlyR routinely in patients with epilepsy or seizures, 13 there are potential issues with this approach. The positive predictive value of anti‐MOG has been shown to decrease substantially when testing patients with atypical phenotypes for MOG‐associated disease. 15 Meanwhile, anti‐GlyR has specificity for stiff‐person spectrum disorders (SPSD)/progressive encephalomyelitis with rigidity and myoclonus (PERM), but low levels have been found in diverse syndromes, and false‐positivity has been reported in up to 4% of healthy controls. 16 , 17 Anti‐GlyR has also been detected in a variety of epileptic presentations, sometimes without features of SPSD/PERM and with variable response to immunotherapy, raising further questions regarding its clinical significance in this context. 18 , 19 For these reasons, the ONES checklist restricts testing for anti‐MOG and anti‐GlyR to patients with characteristic features of these antibodies, which is intended to avoid false‐positives associated with more indiscriminate testing.
2.2. Evaluating diagnostic performance of ONES checklist compared to APE2/ACES reflex score
Two authors (A.B. and Y.‐C.C.) independently reviewed the electronic medical records (EMRs) of all patients at London Health Sciences Centre who had serum and/or CSF neural antibody testing ordered as part of neurological evaluation for epilepsy or seizures in the outpatient clinic, elective admission (epilepsy monitoring unit [EMU]) setting, inpatient ward, or intensive care unit (ICU) between March 2019 and January 2021, inclusive. Patients with a more likely nonimmune etiology (e.g., idiopathic generalized/genetic epilepsy, toxic/metabolic derangement, malformation of cortical development), or with a more likely immune etiology not associated with neural antibody positivity (e.g., Rasmussen encephalitis, multiple sclerosis), were classified as having a known etiology and excluded from sensitivity/specificity analyses as shown in Figure 1. The number of true‐positive neural antibody results and the proportion of false‐positive results (described further below) in patients with a known etiology were compared to those with an unknown etiology to assess for any significant difference. Clinical data required to complete the APE2 score, ACES score, and ONES checklist were independently extracted from the EMR of each patient with epilepsy or seizures of unknown etiology by A.B and Y.‐C.C., with discussion to achieve consensus in discrepant cases. A “Yes” to one or more items was classified as positive for the ONES checklist, and an APE2 score of ≥4 or an APE2 score of ≤3 followed by a ACES score ≥2 was classified as positive for the APE2/ACES reflex score. 7 Each patient's neural antibody status classification, ONES checklist classification, and APE2/ACES reflex score classification as positive or negative (Figure 1) allowed for sensitivity and specificity calculations.
FIGURE 1.
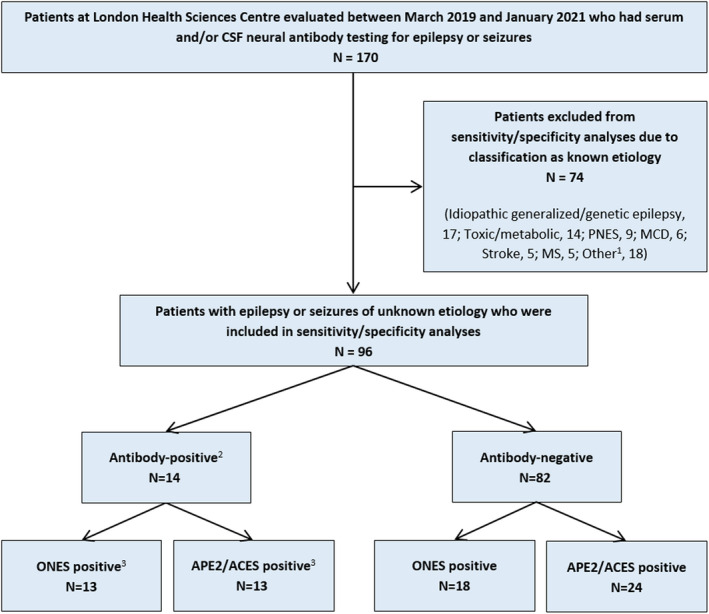
Identification of patients for inclusion in specificity/sensitivity analyses and their classifications. 1Other known etiologies included Rasmussen encephalitis (n = 3), vasculitis (n = 2), neurodegenerative (n = 2), infectious (n = 2), developmental/epileptic encephalopathy (n = 2), posterior reversible encephalopathy syndrome (n = 2), posttraumatic (n = 1), cavernous malformation (n = 1), glioma (n = 1), delayed radiation‐induced leukoencephalopathy (1), and chronic‐appearing frontal lesion not otherwise specified (n = 1). 2Only patients with true‐positive neural antibody results (see text) were classified as neural antibody‐positive for sensitivity/specificity analyses. True‐positive neural antibody results consisted of anti‐leucine‐rich glioma‐inactivated 1 (n = 5), anti‐glutamic acid decarboxylase 65 (GAD65; n = 3), anti‐myelin oligodendrocyte glycoprotein (n = 2), anti‐contactin‐associated protein‐like 2 (CASPR2; n = 2), anti‐N‐methyl‐D‐aspartate receptor (n = 1), and unclassified neural‐specific antibody (n = 1). False‐positive neural antibody results (classified as neural antibody‐negative for sensitivity/specificity analyses) consisted of isolated weak serum positivity for anti‐CASPR2 (n = 2). 3One anti‐GAD65 patient who was negative by both the “Obvious” indications for Neural antibody testing in Epilepsy or Seizures (ONES) checklist and the Antibody Prevalence in Epilepsy and Encephalopathy (APE2)/Antibodies Contributing to Focal Epilepsy Signs and Symptoms (ACES) reflex score is described in the text. CSF, cerebrospinal fluid; MCD, malformation of cortical development; MS, multiple sclerosis; PNES, psychogenic nonepileptic seizures
2.3. Neural antibody test methodologies employed
Patients underwent comprehensive panel‐based testing that included composite mouse brain/nonbrain tissue indirect immunofluorescence (TIIF) to screen for neural‐specific antibodies against intracellular and extracellular antigens as previously described, 20 fixed cell‐based assays (CBAs) for anti‐N‐methyl‐D‐aspartate receptor (NMDAR), leucine‐rich glioma‐inactivated 1 (LGI1), contactin‐associated protein‐like 2 (CASPR2), dipeptidyl‐peptidase‐like protein 6, γ‐aminobutyric acid type B receptor (GABABR), and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor (EUROIMMUN), and testing for high levels of antibodies against glutamic acid decarboxylase 65 (GAD65). Between March 2019 and September 2020, high levels of anti‐GAD65 were determined by fixed CBA (EUROIMMUN), whereas from October 2020 onward this was replaced by enzyme‐linked immunosorbent assay (ELISA) using a serum cutoff of >10 000 IU/ml and a CSF cutoff of >100 IU/ml (KRONUS). 21 , 22 Only high levels of anti‐GAD65 were considered positive. Patients with TIIF staining indicative of anti‐Hu, Yo, Ri, Ma2, amphiphysin, collapsin response mediator protein 5/CV2, or SOX1 were considered positive only if confirmed by immunoblot (EUROIMMUN). Patients with TIIF staining indicative of antibodies against GABAAR or metabotropic glutamate receptor type 5 were planned to be reflexed to confirmatory fixed CBA (EUROIMMUN), whereas patients with TIIF staining indicative of anti‐glial fibrillary acidic protein were planned to be sent out for confirmatory CBA (Mayo Clinic). 23 , 24 , 25 , 26 Any patient with neural‐specific staining on TIIF that was not indicative of any of the aforementioned antibodies was considered to have an unclassified neural‐specific antibody. Panel‐based testing therefore incorporated use of TIIF, CBAs, assays to determine high levels of anti‐GAD65, and demonstration of paraneoplastic antibody positivity by two assays, in keeping with recommended best practices. 7 , 22 , 27 , 28 All fixed CBAs were performed at a dilution of 1:10 for serum and undiluted for CSF. Isolated serum weak positivity for anti‐NMDAR or anti‐CASPR2 by CBA at 1:10 dilution was considered a false‐positive and thus classified as a negative neural antibody result for the purposes of sensitivity/specificity analyses, given previous findings by us and other groups that suggest false‐positivity/clinical irrelevance when detecting only low serum levels of these antibodies by CBA. 29 , 30 , 31 All other neural antibody results determined as outlined above were considered true‐positive results. Separate from panel‐based neural antibody testing, testing for anti‐MOG was performed by fixed CBA (EUROIMMUN), and anti‐GlyR was planned to be sent out for CBA (Mayo Clinic) 17 in patients with characteristic features of these antibodies.
2.4. Statistical methodologies employed
Sensitivities, specificities, and their 95% confidence intervals of the APE2 score, ACES score, APE2/ACES reflex score, and ONES checklist for true‐positive neural antibodies were determined. The McNemar test was used to compare significance of differences in sensitivity and specificity between the APE2/ACES reflex score and ONES checklist. 32 Categorical variables were compared using Fisher exact test. Probability values of <.05 were considered statistically significant. Analyses were performed using SAS Studio.
3. RESULTS
3.1. True‐positive neural antibody results were not observed in patients with epilepsy or seizures of known etiology
One‐hundred seventy patients underwent neural antibody testing for epilepsy or seizures. Seventy‐four of 170 (43.5%) who were classified as having a known etiology were excluded from sensitivity/specificity analyses (Figure 1), none of whom had a true‐positive neural antibody result.
3.2. Patients with epilepsy or seizures of unknown etiology who underwent neural antibody testing were predominantly adults evaluated in outpatient/elective admission (EMU) setting
Of the remaining 96 patients with epilepsy or seizures of unknown etiology, 77 (80%) were adults (age ≥ 18 years) at time of seizure onset and 44 (46%) were female. The median age of seizure onset was 28 years (range = 1–76 years). Seventy‐one of 96 (74%) were evaluated in the outpatient/elective admission (EMU) setting, whereas 25 of 96 (26%) were evaluated in the inpatient ward/ICU setting. The median time from seizure onset to neural antibody testing was 2 years (range = 0–41 years), and 36 of 96 (38%) were tested within 1 year of seizure onset. Forty of 96 (42%) underwent serum and CSF testing, 54 of 96 (56%) underwent serum testing only, and two of 96 (2%) underwent CSF testing only.
3.3. True‐positive neural antibody results were observed in 15% of patients with epilepsy or seizures of unknown etiology
Fourteen of 96 patients with epilepsy or seizures of unknown etiology (15%) had a true‐positive neural antibody result (anti‐LGI1, n = 5; anti‐GAD65, n = 3; anti‐MOG, n = 2; anti‐CASPR2, n = 2; anti‐NMDAR, n = 1; unclassified neural‐specific antibody, n = 1). The patient with an unclassified neural‐specific antibody met diagnostic criteria for definite autoimmune limbic encephalitis. 10 The number of true‐positive neural antibody results was significantly higher among patients classified as having an unknown etiology for epilepsy or seizures (14/96, 15%) compared to a known etiology (0/74, 0%; p = .0003).
3.4. Proportion of false‐positive neural antibody results was significantly higher in patients with epilepsy or seizures of known etiology
Three patients with epilepsy or seizures of known etiology were considered false‐positives based on isolated weak serum staining for anti‐NMDAR or anti‐CASPR2 by CBA (malformation of cortical development, n = 1; neurodegenerative, n = 1; delayed radiation‐induced leukoencephalopathy, n = 1). Meanwhile, two patients with epilepsy or seizures of unknown etiology were considered false‐positives based on isolated weak serum staining for anti‐CASPR2 by CBA (medically controlled temporal lobe epilepsy without other clinical features of autoimmune encephalitis, n = 2). The proportion of positive results that were considered false‐positives was significantly higher among patients classified as having a known etiology for epilepsy or seizures (3/3, 100%) compared to an unknown etiology (2/16, 13%; p = .01).
3.5. No significant difference was found when comparing sensitivity and specificity of APE2/ACES reflex score to ONES checklist
There was no statistically significant difference found when comparing the sensitivity of the APE2/ACES reflex score to the ONES checklist for true‐positive neural antibodies (93%, 95% confidence interval [CI] = 79%–100% for both, p > .99). One patient with new onset temporal lobe seizures, who was unable to undergo brain magnetic resonance imaging due to deep brain stimulator implantation for anorexia nervosa, had high levels of anti‐GAD65 and was missed by both the APE2/ACES reflex score and ONES checklist. Her APE2 score was 3 (1 point for new onset seizure activity, 2 points for elevated CSF protein) and her ACES score was 1 (1 point for autoimmune diseases, vitiligo). This patient had ongoing seizures despite a therapeutic dose of lacosamide and monthly intravenous immunoglobulin, but deferred further antiseizure medication or immunotherapy. If the patient had trialed and failed another antiseizure medication (thus classifying her as refractory), she would have been captured by both the APE2/ACES reflex score (APE2 score = 5) and the ONES checklist (refractory temporal lobe seizures with anti‐GAD65‐associated systemic autoimmunity). There was also no statistically significant difference found when comparing the specificity of the APE2/ACES reflex score (71%, 95% CI = 61%–81%) to the ONES checklist (78%, 95% CI = 69%–87%) for true‐positive neural antibodies (p = .18). Sensitivities and specificities are provided in Table 3. For each item of the ONES checklist, the proportion of patients with a positive checklist item who had true‐positive neural antibodies is shown in Table 4.
TABLE 3.
Sensitivities and specificities of predictive scores and the ONES checklist for neural antibody positivity
| APE2 score | ACES score | APE2/ACES reflex score | ONES checklist | p a | |
|---|---|---|---|---|---|
| Sensitivity, % (95% CI) | 86 (67–100) | 64 (39–89) |

|
>.99 | |
| Specificity, % (95% CI) | 72 (62–82) | 90 (84–97) |

|
.18 | |
Abbreviations: ACES, Antibodies Contributing to Focal Epilepsy Signs and Symptoms; APE2, Antibody Prevalence in Epilepsy and Encephalopathy; CI, confidence interval; ONES, “Obvious” indications for Neural antibody testing in Epilepsy or Seizures.
Probability values are for comparisons of sensitivity and specificity of APE2/ACES reflex score to ONES checklist.
TABLE 4.
Proportion of patients with a positive antibody for each ONES checklist item
| ONES checklist item | Proportion of patients with item who had a positive antibody a |
|---|---|
| Cortical T2‐FLAIR hyperintense lesion(s) with or without involvement of the underlying white matter, in temporal relation to seizure onset | 3/7 (43%) b |
| Large (>1–2 cm) T2‐FLAIR hyperintense lesion(s) involving the white matter suggestive of non‐MS demyelination, in temporal relation to seizure onset | 0/0 (‐) |
| Optic neuropathy or myelopathy of unknown etiology, in temporal relation to seizure onset | 0/0 (‐) |
| Prominent stiffness, spasms, rigidity, and/or hyperekplexia of unknown etiology, in temporal relation to seizure onset | 0/0 (‐) |
| T2‐FLAIR hyperintensity restricted to the medial temporal lobe(s) without atrophy, in temporal relation to seizure onset | 4/8 (50%) b |
| Linear radial perivascular enhancement, in temporal relation to seizure onset | 0/0 (‐) |
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, with serum sodium < 130 mEq/L of unknown etiology | 0/0 (‐) |
| Distinguishable central or peripheral nervous system dysfunction of unknown etiology, in temporal relation to seizure onset | 11/21 (52%) c |
| Musicogenic seizures | 0/1 (0%) d |
| Faciobrachial dystonic seizures | 2/2 (100%) |
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, with pilomotor seizures | 0/0 (‐) |
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, with paroxysmal dizziness spells | 0/0 (‐) |
| New (within 1 year), refractory, temporal lobe or presumed temporal lobe seizures, beginning after 50 years of age | 1/1 (100%) |
| Refractory temporal lobe or presumed temporal lobe seizures, with anti‐GAD65‐associated systemic autoimmunity | 2/3 (67%) |
| New (within 1 year) seizures, beginning within 2 years of tumor diagnosis | 0/0 (‐) |
| New (within 1 year) seizures, beginning within 1 year of last immune checkpoint inhibitor treatment | 0/0 (‐) |
| New (within 1 year) seizures, beginning or worsening within 3 months of last antiviral treatment for herpes simplex virus encephalitis | 0/0 (‐) |
Abbreviations: FLAIR, fluid‐attenuated inversion recovery; GAD65, glutamic acid decarboxylase 65; MS, multiple sclerosis; ONES, “Obvious” indications for Neural antibody testing in Epilepsy or Seizures.
Eleven patients were positive for more than one item on the ONES checklist. Only true‐positive neural antibodies are considered positive antibodies for the purposes of this table (see text for more information).
One patient with cortical T2‐FLAIR hyperintensity and two patients with medial temporal lobe T2‐FLAIR hyperintensity without atrophy had neuroimaging abnormalities that were possibly seizure‐related changes, but lack of close (e.g., within 4 weeks) neuroimaging follow‐up to assess for resolution resulted in findings being considered of unknown onset. None of these three patients had a true‐positive neural antibody result.
Included cognitive impairment (13 patients), behavioral change/cognitive impairment (two patients), aphasia (two patients), behavioral change/cognitive impairment/dysautonomia (one patient), behavioral change (one patient), psychosis (one patient), and visual field deficit (one patient).
One patient had musicogenic seizures as well as seizures triggered by tactile stimuli, suggesting a broader reflex epilepsy.
4. DISCUSSION
We found no clear diagnostic advantage of the APE2/ACES reflex score when compared to only performing neural antibody testing in patients who had “obvious” indications for testing as identified by the ONES checklist, with no significant difference in sensitivity or specificity for true‐positive neural antibodies. Benefits of the ONES checklist are its relative ease of use compared to sequential predictive scores and its highlighting of presentations suspicious for neural antibody positivity that may go unrecognized by clinicians. 12 Our study suggests that there is no additional diagnostic utility of currently available predictive scores after the systematic identification of patients with “obvious” indications for neural antibody testing. This finding should be incorporated into the design of future studies that aim to investigate neural antibody prevalence, predictive scores for neural antibody testing, and novel immunologic biomarkers in patients with epilepsy or seizures. 7
It should be emphasized that neural antibody positivity is not the diagnostic gold standard for all neuroinflammatory diseases that can cause epilepsy or seizures, some of which may have immune mechanisms independent from autoantibody production that underpin their pathogenesis. 33 Therefore, patient exclusion by the ONES checklist does not exclude the possibility of an immune etiology for epilepsy or seizures. As an example, concern for an immune etiology may be raised in patients who present with epilepsia partialis continua (EPC), given the association of EPC with neuroinflammatory diseases such as Rasmussen encephalitis and, rarely, multiple sclerosis. 34 , 35 However, outside of patients who have cortical T2‐fluid‐attenuated inversion recovery hyperintensity, which is included in the ONES checklist primarily because of its association with anti‐MOG, the presentation of EPC in isolation has not been reproducibly associated with neural antibody positivity and is thus not included in the ONES checklist. 2 , 35 , 36 , 37 This example highlights the primary intent of the ONES checklist, which is to identify patients with epilepsy or seizures of unknown etiology who have neural antibody‐associated disease specifically, and not all immune causes more generally; it for this reason that patients with non‐neural antibody‐associated forms of neuroinflammatory disease (e.g., Rasmussen encephalitis, multiple sclerosis) were classified as having epilepsy or seizures of known etiology in this study and excluded from sensitivity/specificity analyses. Because a negative neural antibody result in isolation cannot definitively exclude an immune etiology for epilepsy or seizures, consideration of this diagnostic possibility should persist in patients in whom there is a high index of suspicion clinically; in such patients, the judicious use of immunotherapy trials may have both therapeutic and diagnostic utility, bearing in mind when interpreting the outcomes of such trials that some nonimmune epilepsies may respond to immunotherapy as well. 38
One could argue that restricting the development of the ONES checklist to described presentations of established neural antibodies limits its ability to identify novel disease phenotypes of as yet undiscovered neural antibodies. Although this is a valid theoretical concern, comprehensive tissue‐based neural antibody testing in studies of patients with epilepsy or seizures of unknown etiology have not robustly demonstrated novel neural antibodies of clear clinical relevance in this patient population. 12 , 13 This is in keeping with our study, in which only one patient had an unclassified neural antibody by TIIF; this patient met criteria for definite autoimmune limbic encephalitis and was captured by the ONES checklist. Meanwhile, an opposing and often underappreciated concern is that broadly performing neural antibody testing in hopes of identifying novel disease phenotypes can dramatically increase the proportion of false‐positive results; this is due to the lowering of positive predictive value that occurs when performing testing in low‐probability scenarios with assays in widespread clinical use that have high but imperfect specificity. 15 , 17 , 30 , 39 This issue is exemplified in our study by the significantly higher proportion of false‐positive results in patients who were classified as having epilepsy or seizures of known etiology, and who thus had an intuitively lower probability of neural antibody‐associated disease. The possibility of false‐positives takes on particular importance when attempting to interpret previous studies of neural antibody testing in patients with epilepsy or seizures, which have reported patients with isolated serum positivity for certain neural antibodies (e.g., anti‐NMDAR, CASPR2, GlyR) and atypical disease phenotypes. 19 , 40 , 41 The risk of false‐positives is increasingly being recognized when neural antibody testing is performed in low‐probability scenarios, 15 , 17 , 28 , 29 , 30 , 39 , 42 , 43 , 44 , 45 highlighting the importance of appropriate patient selection. To this end, tools like the ONES checklist can serve not only to enhance clinician recognition of neural antibody‐associated presentations, but also to trigger scrutiny of possible false‐positive results in patients with atypical disease phenotypes who may have indiscriminately undergone neural antibody testing. Improved neural antibody reporting practices by the testing laboratory can also help to avoid misinterpretation of clinically irrelevant or false‐positive results in patients with epilepsy or seizures; as an example, the inclusion of interpretative comments when reporting isolated low serum levels of certain antibodies, such as serum anti‐GAD65 detected by ELISA at values < 10 000 IU/ml or serum anti‐NMDAR as well as anti‐CASPR2 detected by CBA with only weak positivity at 1:10 dilution, can serve to educate clinicians that these results typically lack relevance in patients with neurological symptoms. 21 , 29 , 30 , 46
4.1. Limitations
This was a retrospective study, and lack of systematic data collection at the time of patient assessment could have impacted the performance of a checklist or predictive score. However, the high sensitivity of both the ONES checklist and APE2/ACES reflex score is reassuring in this regard. Because of the retrospective nature of data collection, blinding to the neural antibody result during EMR review was not possible, given that neural antibody status was highlighted across clinical notes. However, independent review by two clinicians was performed to minimize bias. The ONES checklist was found to have high sensitivity in our patient cohort that consisted primarily of patients in the outpatient/elective admission (EMU) setting, but it does place emphasis on seizure refractoriness. Its sensitivity could therefore be lower when applied to patients who first present with seizures to hospital. It is, however, well suited for an epilepsy clinic, where patients are more likely to have failed antiseizure medications, prompting referral. Anti‐MOG and anti‐GlyR were not tested routinely in patients who did not have typical disease features, so some patients who would have been positive for these antibodies could theoretically have been missed. However, our tiered approach minimizes the possibility of false‐positives that can occur when testing for these antibodies in low‐probability scenarios. There is an element of subjectivity to some items included in the ONES checklist that aim to distinguish relevant findings from those due to other disease etiologies, epilepsy comorbidities, and ictal or postictal phenomena. In particular, distinguishing the neuropsychiatric symptoms secondary to neural antibody‐associated disease from those that are common among patients with epilepsy can be exceptionally challenging. 47 Although formal cognitive assessment can be useful in this regard, validated instruments to aid in this differentiation are lacking. This represents a knowledge gap that could be addressed through future study, as improved ability to make this distinction clinically would be advantageous to the diagnostic evaluation of patients with epilepsy and directly result in increased specificity of the ONES checklist.
5. CONCLUSIONS
Compared to only performing neural antibody testing in patients with epilepsy or seizures of unknown etiology who had “’obvious” indications for testing as identified by the ONES checklist, we found that predictive scores conferred no clear diagnostic advantage. The diagnostic utility of predictive scores should be examined critically in future prospective studies to systematically determine which patients, if any, do not have “obvious” indications for neural antibody testing but would still benefit from their use. Prespecified definitions of what constitutes a true‐positive neural antibody, particularly if testing patients with atypical disease phenotypes or if using neural antibody tests with imperfect specificity, is required in such studies to avoid false‐positives that can confound results.
CONFLICT OF INTEREST
S.M. reports that he is on the advisory boards and speaker bureaus for UCB Canada, Eisai, and Sunovion Pharmaceuticals Canada. He is in the editorial board of Epilepsy & Behavior Reports and Frontiers in Neurology. J.G.B. reports that he holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. A.B. reports that he holds the London Health Sciences Centre and London Health Sciences Foundation Chair in Neural Antibody Testing for Neuro‐Inflammatory Diseases. Neither of the other authors has any conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Yiu‐Chia Chang: Conceptualization, investigation, writing–review & editing. Maryam N. Nouri: Conceptualization, writing–review & editing. Seyed Mirsattari: Conceptualization, writing–review & editing. Jorge G. Burneo: Conceptualization, writing–review & editing. Adrian Budhram: Conceptualization, methodology, investigation, formal analysis, writing–original draft, supervision.
Supporting information
App S1
ACKNOWLEDGMENT
None.
Chang Y‐C, Nouri MN, Mirsattari S, Burneo JG, Budhram A. “Obvious” indications for Neural antibody testing in Epilepsy or Seizures: The ONES checklist. Epilepsia. 2022;63:1658–1670. 10.1111/epi.17238
The authors have not published, posted, or submitted any related manuscripts from the same study.
Funding information
No funding was received for this research.
REFERENCES
- 1. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Budhram A, Dubey D, Sechi E, Flanagan EP, Yang L, Bhayana V, et al. Neural antibody testing in patients with suspected autoimmune encephalitis. Clin Chem. 2020;66(12):1496–509. 10.1093/clinchem/hvaa254 [DOI] [PubMed] [Google Scholar]
- 3. Dubey D, Singh J, Britton JW, Pittock SJ, Flanagan EP, Lennon VA, et al. Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia. 2017;58(7):1181–9. 10.1111/epi.13797 [DOI] [PubMed] [Google Scholar]
- 4. Dubey D, Kothapalli N, McKeon A, Flanagan EP, Lennon VA, Klein CJ, et al. Predictors of neural‐specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62–72. 10.1016/j.jneuroim.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 5. Dubey D, Alqallaf A, Hays R, Freeman M, Chen K, Ding K, et al. Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol. 2017;74(4):397–402. 10.1001/jamaneurol.2016.5429 [DOI] [PubMed] [Google Scholar]
- 6. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367–9. 10.1111/epi.14649 [DOI] [PubMed] [Google Scholar]
- 7. Steriade C, Gillinder L, Rickett K, Hartel G, Higdon L, Britton J, et al. Discerning the role of autoimmunity and autoantibodies in epilepsy: a review. JAMA Neurol. 2021;78(11):1383. 10.1001/jamaneurol.2021.3113 [DOI] [PubMed] [Google Scholar]
- 8. Geis C, Planagumà J, Carreño M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. 2019;129(3):926–40. 10.1172/jci125178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune‐associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341–51. 10.1111/epi.16571 [DOI] [PubMed] [Google Scholar]
- 10. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. 10.1016/s1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson J, Bi M, Murchison AG, Makuch M, Bien CG, Chu K, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141(2):348–56. 10.1093/brain/awx323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Bruijn M, Bastiaansen AEM, Mojzisova H, Sonderen A, Thijs RD, Majoie MJM, et al. Antibodies contributing to focal epilepsy signs and symptoms score. Ann Neurol. 2021;89(4):698–710. 10.1002/ana.26013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bozzetti S, Rossini F, Ferrari S, Delogu R, Cantalupo G, Marchioretto F, et al. Epileptic seizures of suspected autoimmune origin: a multicentre retrospective study. J Neurol Neurosurg Psychiatry. 2020;91(11):1145–53. 10.1136/jnnp-2020-323841 [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Tymchuk S, Barry J, Muppidi S, Le S. Antibody prevalence in epilepsy before surgery (APES) in drug‐resistant focal epilepsy. Epilepsia. 2021;62(3):720–8. 10.1111/epi.16820 [DOI] [PubMed] [Google Scholar]
- 15. Sechi E, Buciuc M, Pittock SJ, Chen JJ, Fryer JP, Jenkins SM, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol. 2021;78(6):741–6. 10.1001/jamaneurol.2021.0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carvajal‐González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137(Pt 8):2178–92. 10.1093/brain/awu142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hinson SR, Lopez‐Chiriboga AS, Bower JH, Matsumoto JY, Hassan A, Basal E, et al. Glycine receptor modulating antibody predicting treatable stiff‐person spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2018;5(2):e438. 10.1212/nxi.0000000000000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekizoglu E, Baykan B, Sezgin M, Erdag E, Gundogdu‐Unverengil G, Vanlı‐Yavuz EN, et al. Follow‐up of patients with epilepsy harboring antiglycine receptor antibodies. Epilepsy Behav. 2019;92:103–7. 10.1016/j.yebeh.2018.09.034 [DOI] [PubMed] [Google Scholar]
- 19. Brenner T, Sills GJ, Hart Y, Howell S, Waters P, Brodie MJ, et al. Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia. 2013;54(6):1028–35. 10.1111/epi.12127 [DOI] [PubMed] [Google Scholar]
- 20. Jitprapaikulsan J, Klein CJ, Pittock SJ, Gadoth A, McKeon A, Mills JR, et al. Phenotypic presentations of paraneoplastic neuropathies associated with MAP1B‐IgG. J Neurol Neurosurg Psychiatry. 2020;91(3):328–30. 10.1136/jnnp-2019-322175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muñoz‐Lopetegi A, de Bruijn MAAM, Boukhrissi S, Bastiaansen AEM, Nagtzaam MMP, Hulsenboom ESP, et al. Neurologic syndromes related to anti‐GAD65: clinical and serologic response to treatment. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e696. 10.1212/nxi.0000000000000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graus F, Saiz A, Dalmau J. GAD antibodies in neurological disorders—insights and challenges. Nat Rev Neurol. 2020;16(7):353–65. 10.1038/s41582-020-0359-x [DOI] [PubMed] [Google Scholar]
- 23. Fang B, McKeon A, Hinson SR, Kryzer TJ, Pittock SJ, Aksamit AJ, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297–307. 10.1001/jamaneurol.2016.2549 [DOI] [PubMed] [Google Scholar]
- 24. Spatola M, Sabater L, Planagumà J, Martínez‐Hernandez E, Armangué T, Prüss H, et al. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology. 2018;90(22):e1964–72. 10.1212/wnl.0000000000005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spatola M, Petit‐Pedrol M, Simabukuro MM, Armangue T, Castro FJ, Barcelo Artigues MI, et al. Investigations in GABA(A) receptor antibody‐associated encephalitis. Neurology. 2017;88(11):1012–20. 10.1212/wnl.0000000000003713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Connor K, Waters P, Komorowski L, Zekeridou A, Guo CY, Mgbachi VC, et al. GABA(A) receptor autoimmunity: a multicenter experience. Neurol Neuroimmunol Neuroinflamm. 2019;6(3):e552. 10.1212/nxi.0000000000000552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graus F, Vogrig A, Muñiz‐Castrillo S, Antoine JCG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1014. 10.1212/nxi.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruiz‐García R, Martínez‐Hernández E, Saiz A, Dalmau J, Graus F. The diagnostic value of onconeural antibodies depends on how they are tested. Front Immunol. 2020;11:1482. 10.3389/fimmu.2020.01482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Budhram A, Mirian A, McFadden S, Edmond P, Bhayana V, Yang L. Neural antibody testing for autoimmune encephalitis: a Canadian single‐centre experience. Can J Neurol Sci. 2021;48:859–63. 10.1017/cjn.2021.23 [DOI] [PubMed] [Google Scholar]
- 30. Bien CG, Mirzadjanova Z, Baumgartner C, Onugoren MD, Grunwald T, Holtkamp M, et al. Anti‐contactin‐associated protein‐2 encephalitis: relevance of antibody titres, presentation and outcome. Eur J Neurol. 2017;24(1):175–86. 10.1111/ene.13180 [DOI] [PubMed] [Google Scholar]
- 31. Steiner J, Teegen B, Schiltz K, Bernstein HG, Stoecker W, Bogerts B. Prevalence of N‐methyl‐D‐aspartate receptor autoantibodies in the peripheral blood: healthy control samples revisited. JAMA Psychiatry. 2014;71(7):838–9. 10.1001/jamapsychiatry.2014.469 [DOI] [PubMed] [Google Scholar]
- 32. McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Hinson SR, Kryzer TJ, et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol. 2009;66(9):1134–8. 10.1001/archneurol.2009.178 [DOI] [PubMed] [Google Scholar]
- 33. Campos‐Bedolla P, Feria‐Romero I, Orozco‐Suárez S. Factors not considered in the study of drug‐resistant epilepsy: drug‐resistant epilepsy: assessment of neuroinflammation. Epilepsia Open. 2022. Online ahead of print. 10.1002/epi4.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, et al. Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13(2):195–205. 10.1016/s1474-4422(13)70260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jain J, Son M, Debicki DB, Jog M, Casserly CS, Burneo JG, et al. Epilepsia partialis continua in relapsing‐remitting multiple sclerosis: a possible distinct relapse phenotype. Clin Neurol Neurosurg. 2022;213:107099. 10.1016/j.clineuro.2021.107099 [DOI] [PubMed] [Google Scholar]
- 36. Budhram A, Mirian A, Le C, Hosseini‐Moghaddam SM, Sharma M, Nicolle MW. Unilateral cortical FLAIR‐hyperintense lesions in anti‐MOG‐associated encephalitis with seizures (FLAMES): characterization of a distinct clinico‐radiographic syndrome. J Neurol. 2019;266(10):2481–7. 10.1007/s00415-019-09440-8 [DOI] [PubMed] [Google Scholar]
- 37. Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, et al. MOG antibody‐positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e322. 10.1212/nxi.0000000000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toledano M, Britton JW, McKeon A, Shin C, Lennon VA, Quek AMl, et al. Utility of an immunotherapy trial in evaluating patients with presumed autoimmune epilepsy. Neurology. 2014;82(18):1578–86. 10.1212/wnl.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Budhram A, Yang L, Bhayana V, Mills JR, Dubey D. Clinical sensitivity, specificity, and predictive value of neural antibody testing for autoimmune encephalitis. J Appl Lab Med. 2022;7(1):350–6. 10.1093/jalm/jfab127 [DOI] [PubMed] [Google Scholar]
- 40. Wright S, Geerts AT, Jol‐van der Zijde CM, Jacobson L, Lang B, Waters P, et al. Neuronal antibodies in pediatric epilepsy: clinical features and long‐term outcomes of a historical cohort not treated with immunotherapy. Epilepsia. 2016;57(5):823–31. 10.1111/epi.13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGinty RN, Handel A, Moloney T, Ramesh A, Fower A, Torzillo E, et al. Clinical features which predict neuronal surface autoantibodies in new‐onset focal epilepsy: implications for immunotherapies. J Neurol Neurosurg Psychiatry. 2021;92(3):291–4. 10.1136/jnnp-2020-325011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balint B, Bhatia KP, Dalmau J. "Antibody of unknown significance" (AUS): the issue of interpreting antibody test results. Mov Disord. 2021;36(7):1543–7. 10.1002/mds.28597 [DOI] [PubMed] [Google Scholar]
- 43. Budhram A, Mills JR, Shouman K, Dyck PJB, Hassan A, Zalewski NL. False‐positive anti‐neuronal nuclear antibody type 1 in a patient with RFC1 repeat expansion: preventing "phenotype creep in autoimmune neurology. J Neurol Sci. 2020;416:117018. 10.1016/j.jns.2020.117018 [DOI] [PubMed] [Google Scholar]
- 44. Déchelotte B, Muñiz‐Castrillo S, Joubert B, Vogrig A, Picard G, Rogemond V, et al. Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e701. 10.1212/nxi.0000000000000701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Budhram A, Nicolle MW, Yang L. The positive predictive value of onconeural antibody testing: a retrospective review. Can J Neurol Sci. 2018;45(5):577–9. 10.1017/cjn.2018.74 [DOI] [PubMed] [Google Scholar]
- 46. Garrido Sanabria ER, Zahid A, Britton J, Kraus GJ, López‐Chiriboga AS, Zekeridou A, et al. CASPR2‐IgG‐associated autoimmune seizures. Epilepsia. 2022;63(3):709–22. 10.1111/epi.17164 [DOI] [PubMed] [Google Scholar]
- 47. Steriade C. The search for autoimmune‐associated epilepsy continues—are we getting closer to our target? Epilepsy Curr. 2021;21(4):255–7. 10.1177/15357597211010816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


