Abstract
Wound healing is a complex process that is critical in restoring the skin's barrier function. This process can be interrupted by numerous diseases resulting in chronic wounds that represent a major medical burden. Such wounds fail to follow the stages of healing and are often complicated by a pro‐inflammatory milieu attributed to increased proteinases, hypoxia, and bacterial accumulation. The comprehensive treatment of chronic wounds is still regarded as a significant unmet medical need due to the complex symptoms caused by the metabolic disorder of the wound microenvironment. As a result, several advanced medical devices, such as wound dressings, wearable wound monitors, negative pressure wound therapy devices, and surgical sutures, have been developed to correct the chronic wound environment and achieve skin tissue regeneration. Most medical devices encompass a wide range of products containing natural (e.g., chitosan, keratin, casein, collagen, hyaluronic acid, alginate, and silk fibroin) and synthetic (e.g., polyvinyl alcohol, polyethylene glycol, poly[lactic‐co‐glycolic acid], polycaprolactone, polylactic acid) polymers, as well as bioactive molecules (e.g., chemical drugs, silver, growth factors, stem cells, and plant compounds). This review addresses these medical devices with a focus on biomaterials and applications, aiming to deliver a critical theoretical reference for further research on chronic wound healing.
Keywords: biomaterial, biomedical device, chronic wound, wound healing

Abbreviations
- 3D
three‐dimensional
- ADSCs
adipose‐derived stem cells
- AgNPs
silver nanoparticles
- bFGF
basic fibroblast growth factor
- BG
bioactive glass
- BMSC
bone marrow‐derived mesenchymal stem cells
- Ca
calcium
- CPP
cell‐penetrating peptide
- ECM
extracellular matrix
- EEP
ethanolic extract of propolis
- EGF
epidermal growth factor
- EPC
endothelial progenitor cells
- ESBL
extended‐spectrum beta‐lactamase
- FDA
Food and Drug Administration
- GFs
Growth factors
- HUVECs
human umbilical vein endothelial cells
- MMP
matrix metalloproteinase
- MRSA
methicillin‐resistant Staphylococcus aureus
- MSCs
mesenchymal stem cells
- NF‐(κ) B
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NPWT
negative pressure wound therapy
- NPWTci
NPWT continuous irrigation
- NPWTi
NPWT instillation
- Nrf2
nuclear factor erythroid 2‐related factor
- NSAID
non‐steroidal anti‐inflammatory drug
- P
phosphorus
- PANi‐EB
polyaniline emeraldine base
- PCL
polycaprolactone
- PDGF
platelet‐derived growth factor
- PDGF‐BB
platelet‐derived growth factor‐BB
- PEG
polyethylene glycol
- PEO
polyethylene oxide
- PL
platelet lysate
- PLA
polylactic acid
- PLGA
poly(lactic‐co‐glycolic acid)
- PLLA
polylactic‐co‐ε‐caprolactone
- PVA
polyvinyl alcohol
- PVP
polyvinylpyrrolidone
- rhEGF
recombinant human epidermal growth factor
- ROS
reactive oxygen species
- Si
silicon
- SSIs
surgical site infections
- TGF‐β
transforming growth factor‐beta
- VEGF
vascular endothelial growth factor
- ZnO
zinc oxide
1. INTRODUCTION
Wounding occurs when the epidermal layer of skin is ruptured and the underlying dermis is thus exposed to the air. Depending on the depth of the skin damage and the area of the skin affected, the tissues exposed to the air range from blood vessels to bone. Thus, wounds are generally classified into three categories. When only the epidermal skin surface is injured, it is regarded as a superficial wound. When the injury involves deeper dermal layers (e.g., blood vessels, sweat glands, and hair follicles), it is known as a partial‐thickness wound. In terms of a full‐thickness wound, this happens when the underlying subcutaneous fat or deeper tissues become ruptured. 1 Burns are common skin injuries that also present significant challenges in restoring functionality and preventing scarring. Burn injuries can be classified as first‐, second‐, and third‐degree, in accordance with superficial, partial‐thickness, full‐thickness wounds. For fourth‐degree burns, the nerve endings are ruptured and there is a loss of feeling in the wound area, and the damage involves underlying tissues, muscles, tendons, ligaments, and even bone. 1
There are four distinct, yet overlapping phases for completing the wound healing process, including hemostasis, inflammation, proliferation, and remodeling (Figure 1). A variety of cell types, enzymes, cytokines, proteins, and hormones participate in the tissue repair processes. 2 Briefly, after an injury is created, in the normal healing process, hemostasis will be triggered to generate blood clots and blood vessels constrict to restrict the blood flow, followed by the secretion of proinflammatory cytokines and growth factors. 3 These growth factors, in turn, stimulate inflammation, facilitated by macrophages, neutrophils, and lymphocytes that are recruited by epithelial cells. Angiogenesis is then induced by growth factors, where reepithelization occurs due to the proliferation of fibroblasts and keratinocytes. The fibroblasts, at a later time point, will further differentiate into myofibroblasts leading to the deposition of extracellular matrix (ECM). 4
FIGURE 1.
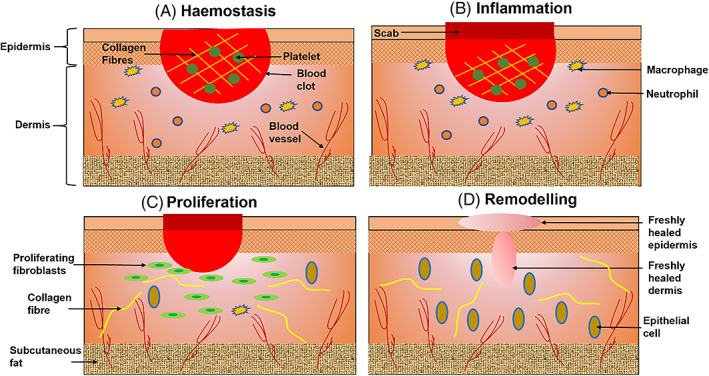
Schematic representation of the cutaneous wound healing. (A) Hemostasis. A wound causes blood clot formation. (B) Inflammation. Recruitment of macrophages and neutrophils. (C) Proliferation. Fibroblast proliferation induces epithelial cell generation. (D) Remodeling. Wound closure
In order to pave the way for promoting wound healing, a comprehensive understanding of the healing process and the potential polymers and bioactive compounds, along with existing medical devices for wound management, are considered important requirements that physicians and technologists must be aware of, to benefit from biomaterial assisted wound healing. The aim of this review is to provide an updated overview of the potential of biomaterials and their applications in wound management and treatment and offer an organized framework for categorizing natural and synthetic polymers, as well as bioactive compounds, that will be useful in biomedical device manufacturing for addressing chronic wound healing. We chose to focus on both biomaterials and their applications because, with increased studies on wound dressings, there is still a lack of reviews updating the latest research findings. Particularly, sutures as the most popular surgical devices, their potential for facilitating wound healing is neglected. In our opinion, we need to be cognizant of wider fields to be able to develop new strategies to tackle the challenges presented by chronic wounds.
2. STUDY METHOD
Firstly, articles that address wound healing with a focus on biomaterials and biomedical devices were searched from the Web of Science, Google Scholar database and related websites for the years 2010 to 2022. Secondly, all search results were exported to a spreadsheet for organized review and the quality evaluation was performed by noting the study type. The relevant articles were identified based on the abstracts of the research. The full text of articles was obtained if they matched the criteria of desire. Search terms used included a combination of the following: chronic wound, wound healing, wound management, skin tissue engineering, biopolymer, phytomedicine, biomaterial application, wound dressing, growth factor, stem cell, silver, curcumin, honey, aloe vera, suture, nanofiber scaffold, hydrogel. During this process, 2940 articles were discovered, 957 articles were selected, and 289 of them were cited in this study.
3. WOUND HEALING IN NORMAL AND CHRONIC WOUNDS
The initial repair of a wound is completed by the formation of a fibrin blood clot to reinforce the wound area and a haemostatic plug is formed to efficiently seal off the underlying tissues from any extended exposure to the air. 2 Serotonin to promote cell proliferation and migration of keratinocytes and fibroblasts, and histamine to accelerate angiogenesis are released by mast cells at the injury site. Furthermore, endothelial cells initiate the recruitment of neutrophils and monocytes for the formation of macrophages at a later stage. 2 Leukocytes are also encouraged to the wound site due to complement activation. Bacteria are phagocytosed by macrophages for eliminating microbes and removing dead cells. 5 Besides bacteria, foreign bodies, and endotoxins can also activate macrophages to release growth factors to contribute to the various cellular reactions. 5 Angiogenesis is a process initiated in the inflammation phase, that involves growing new blood vessels from existing ones. 6 Newly formed epithelial tissue can completely cover the wound site within the first 48 h due to the migration of basal cells from the edge of the wound.
After a few days, macrophages supersede the neutrophils to activate cytokines and produce growth factors. In the following week, transforming growth factor‐beta (TGF‐β) stimulates fibroblast proliferation to infiltrate the wound clot, increasing the number of single collagen fibers. At the initial stage, collagen fibers are soft gel‐like biomaterials that accumulate outside the cells. When the wound becomes mature, collagen fibers are remodeled into a strong fiber placed to cover the whole wound bed. At the same time, a certain number of fibroblasts transform into myofibroblasts for the sake of smooth muscle cell formation. 7 Subsequently, the wound area starts to contract with the help of myofibroblasts and is eventually closed by secondary determination. 7 The cytoplasmic actin filaments are the major components of the myofibroblast, which is the main cause of wound contraction. The wound remodeling phase is a continuous procedure that can progress throughout the lifetime of the patients. 8
Based on the method of wound formation and the duration of the wound healing process, there are typically two types of wounds, including acute and chronic. Amongst healthy patients, an acute wound can be healed completely within 2 to 3 weeks, followed by the remodeling phase with minimal scarring. 9 However, when a wound becomes stuck in one of the phases for a period of 6 to 8 weeks, then the wound is categorized as a chronic wound. 8 In a chronic wound, the site of damaged tissue is greater than the visible area. The tissues beneath and around the wound site are also affected which can further develop into an ulcer. 8
The main causes of acute wounds are ascribed to mechanical injuries (i.e., frictional contact between cutaneous and solid surfaces), which includes penetrating injuries such as surgical wounds caused by incisions. Other causes involve burns, electrical shock and chemical injuries. 10 Chronic wounds, on the other hand, are related to certain diseases, such as diabetes, and do not follow a logical progression. 11 A chronic wound is defined as a wound that fails to heal promptly or a set of healing phases, namely hemostasis, inflammation, proliferation, and remodeling, as in Figure 1. 6
In the early stages of wound repair, the lack of macrophages in the inflammation or proliferation phase results in a reduction of re‐epithelialization and granulation tissue formation or hemorrhage. 12 The M1 subset of macrophages facilitates the formation of pro‐inflammatory mediators and conducts the phagocytic activity. Whereas, the M2 subset of macrophages is associated with the production of the ECM and the formation of anti‐inflammatory mediators. 13 The failure of the transition between M1 and M2 results in chronic wounds (e.g., venous ulcers and diabetic wounds). 14 Chronic wounds frequently remain in the inflammation phase and the duration is dependent upon factors such as bacterial load and the presence of necrotic tissue in the wound site. 11 Additionally, most chronic wounds never heal, even the wounds that are able to heal may take several years. Moreover, there is a high risk for the wound to reappear after healing, unless the foundation issue is solved Ferreira et al., (2006) concluded that an acute or chronic wound that takes a long time to heal should be treated as a complex wound. This type of wound is characterized as having an extensive loss of integument (i.e., skin, hair, and glands), the presence of infection, tissue necrosis, and underlying pathology. 15
For patients with diabetes, the wound healing process halts at the inflammatory phase, which hinders the generation of cytokines, proteins and growth factors that are essential for completing the wound healing process. 16 A chronic wound often results in the formation of an ulcer that can be unhealing, and in the worst case, can lead to amputation. The mechanisms that cause the pathogenesis of chronic wound healing include the continued expression of inflammatory cytokines, nonresponding macrophages and polymorphonuclear leukocytes. 16 Furthermore, the reactive oxygen species become increased in the case of delayed wound healing, which stimulates pro‐apoptotic transcription and stimulates the executioner caspase‐3 to cause apoptosis that irretrievably eventually leads to cell death. 2
Impaired wound healing is a serious medical problem that may lead to increased health care expenses and poor quality of life for patients that may result in repeated re‐hospitalization. 17 Type‐2 diabetics, for example, can cause chronic unhealing wounds and foot ulcers. Chronic wounds are painful and take a long time to heal or fail to heal altogether. They can also cause further complications, including prolonged inflammation, and microbial infections. They may also inhibit angiogenesis and prevent wound closure. 16 In the worst cases, amputation of the affected limb or organ may be required when the available clinical methods have failed to produce the desired results. Thus, studies on wound dressings that have effective healing potential are essential.
4. WOUND INFECTION AND WOUND PAIN
4.1. Wound infection
The presence of micro‐organisms (e.g., bacteria and fungi) can weaken the natural immune system of the host and induce infection in the injured areas. Streptococcus pyogenes, Staphylococcus aureus, Pseudomonas aeruginosa, and some Clostridium species are common bacterial sources that cause delayed wound healing. Other wound infection factors include hypoxia, ischemia and immune deficiencies (e.g., virus and chemotherapy). 18
Additionally, neglected wound care can eventually lead to fatalities from bacteraemia and septicaemia. 10 In healthy individuals, micro‐organisms will multiply on the surface of a wound, but this does not trigger the host immune response or show any clinical symptoms. 19 Unfortunately, when the colonization of the wound invades the living tissues of the host, a series of local and systematic responses are triggered. This can consequently cause purulent discharge and symptomatic cellulitis, which further leads to soft tissue injury. 20 It has been reported that the formation of bacterial biofilms is responsible for the delay of wound healing caused by the high microbial load on the wound site. 19 In terms of burn wounds, roughly 75% of this type of wound are more predisposed to contamination, due to the presence of micro‐organisms already present in the hair follicles, sebaceous glands and the presence of Pseudomonas aeruginosa and Staphylococcus aureus. 21 Chronic wounds are more prone to infection in comparison to acute wounds. This is due to the impaired migration of leukocytes and weakened phagocytosis under the presence of high microbial bioburden. 16
4.2. Wound pain
Pain management is crucial to the recovery of injured patients. Excessive pain can hinder the healing process due to the anxiety‐induced accumulation of stress hormones, leading to prolonged physical and mental burden and extended hospital stays. 22 It is worth noting that the expression of pain from the patient needs to be addressed individually since the pain process largely depends on each patient's perception, not what clinicians believe it should be. There are two types of wound pain, including nociceptive pain and neuropathic pain. Nociceptive pain is a natural physiological response when encountering tissue damage. This type of pain generally lasts within a set period, but when the healing speed slows down, then the inflammatory response might extend. Under this circumstance, primary hyperalgesia (at the wound site) and secondary hyperalgesia (in the surrounding tissue) may experience intensified sensitivity. 23 Neuropathic pain is generated by nerve damage, which can be caused by a primary lesion. The development of neuropathic pain may be related to infection, cancer or metabolic disorders. This can lead to chronic pain over a long period. 24 For the sake of patient comfort and daily task performance, alleviation of pain is the priority for patients with chronic wounds. Efficient wound management can significantly promote a patient's quality of life. 25 Skin transplants, for instance, can cause extreme pain to the patient, due to the impact of the exposure of nerve endings during the skin harvest. Therefore, pain management is also necessary at the secondary wound site. 10
Primary hyperalgesia is a heightened sensitivity caused by a prolonged inflammation or repeated stimulus to the wound and surrounding area, which may be treated by the combination of a non‐steroidal anti‐inflammatory drugs (NSAIDs) combined with a mild opioid in the hospital, to decrease local inflammation and modulate pain at spinal cord level, respectively. 24 Wound dressings provide two basic functions. The first is to cover the wound, preventing infection. The second is to alleviate wound pain, by reducing the bacterial load and hence reducing the inflammatory reaction to the nervous system. Oral administration, on the other hand, can take up to a couple of days to have a significant impact on pain relief. Therefore, topical drug delivery to the wound site is the top choice for pain alleviation. Ibuprofen, in particular has been widely studied due to its excellent local pain reduction effects. 26 Arapoglou et al., (2011) designed a foam dressing loaded with ibuprofen and compared its effect with local best practices on various wound types. 27 They reported that ibuprofen‐loaded foam dressings were associated with significantly higher pain relief based on patient scores. Therefore, they believed that ibuprofen should be applied in chronic and acute wounds for efficient pain reduction, which also suggested that local pain relief by ibuprofen could be a safer alternative to systemic pain treatment. 27
5. POLYMERIC MATERIALS
5.1. Natural polymers
Natural polymers are generally biodegradable or bioabsorbable and biocompatible. Some also manifest antimicrobial or anti‐inflammatory properties such as chitosan and keratin. Hence, natural proteins (e.g., keratin, casein, collagen, and silk) and polysaccharides (e.g., chitosan, alginate, and hyaluronic acid) have received increased interest in wound dressing production, due to their similarity to macromolecules, which are recognized by the human body, achieving ECM biomimicry.
5.1.1. Chitosan
Chitosan is one of the most widely used ligands. It has been reported to have a positive charge and can improve the absorption of metal anion drugs. 28 Owning to its cationic charge, chitosan has been applied as a coating layer on negatively charged particle surfaces via electrostatic adsorption. 29 Chitosan has also shown its potential for the modulation of drug release behavior. 30 The nanoparticles that are coated by positively charged chitosan attract the negatively charged cell membrane, which enhances the permeation of poorly water‐soluble drugs. 31
The functional versatility of chitosan makes it a popular natural cationic polymer in the production of hydrogels, membranes, and electrospun nanofibers for wound healing. 17 , 32 , 33 Furthermore, chitosan dressings have been selected to treat injured tissues due to their antibacterial properties. 34 Chitosan/alginate hydrogels were produced and loaded with different concentrations of hesperidin. 35 These hydrogel products were evaluated in the full‐thickness dermal wound in a rodent model. The results indicated that the chitosan/alginate hydrogels had a better wound closure rate than the control group of gauze‐treated wounds. 35 In another study, a nanofiber mat that contained chitosan, PVA and zinc oxide (ZnO) demonstrated high antibacterial and antioxidant activities, suggesting its potential for the faster healing of diabetic wounds. 17
5.1.2. Keratin
Keratin is one of the most abundant protein sources found in human or animal parts, such as hair, wool, nails, and feathers. Keratins can be divided into two categories, including epithelial keratins and trichocyte keratins. Epithelial keratins are soft keratins, which contain low sulfur (~1%) and cysteine (<3%). They are the main component in the stratum corneum of the skin and play an important role in stabilizing the cells in epithelia. Trichocyte keratins, as hard keratins, have a higher sulfur (~5%) and cysteine (4%–17%) percentage. They act as structural scaffolds in the areas connecting to the epidermis. 36 Keratin materials have been widely applied in tissue regeneration ascribed to their biocompatibility, biodegradability and structural support. These properties make it possible to produce various wound dressings, such as films, hydrogels and scaffolds, to facilitate the chronic wound healing process. 37
Keratin and HA‐based PCL/PEO fibers can be produced via emulsion and coaxial electrospinning techniques. The results showed that the incorporation of natural polymers promote cell viability and has the potential for wound dressing applications. 38 Keratin (5% w/v) and chitosan (2% w/v)‐based PVA nanofibrous scaffolds have been reported to exhibit desirable tensile strength, swelling ratio, and porosity. These properties were enhanced with the addition of keratin and chitosan. 39 The same research group also utilized the keratin/chitosan (50:50 w/w) blend to produce surgical sutures with anti‐inflammatory properties. The scratch assay showed improved cell proliferation and migration in the presence of the sutures, which indicated the potential of the sutures to be used for facilitating wound healing. 40
5.1.3. Casein
Casein is derived from cow milk makes up 80% of the protein in milk. It has received increasing attention due to its biocompatible, biodegradable, self‐assembling properties. More than half of casein (~55%) contains polar amino acid groups, which benefits hydrogen bond formation to produce films. 41 However, this property also makes it impossible to electrospun casein alone. Therefore, synthetic polymers (e.g., PEG, PVA) have been added to facilitate the casein‐based electrospinning process. 42 , 43 Moreover, cross‐linking agents (e.g., glutaraldehyde and silane) are required during the casein‐based product process, due to the low water stability and mechanical strength of casein caused by its high hydrophilicity. 41
Casein/PVA electrospun nanofibrous mats were successfully fabricated to promote haemostatic activity via thrombin generation. The addition of casein enhanced epithelial proliferation and restitution. 42 Casein/cellulose/chitosan scaffolds have been developed to facilitate wound healing. The presence of casein exposed nucleation sites to bind blood clotting ions, which is essential for hemostasis. 44 A study on casein/alginate‐based injectable hydrogel production was reported. The hydrogels consist of casein, alginate, iron nanoparticles, and bacterial cellulose. The swelling studies revealed a 4000% water uptake of the hydrogels, which indicated its wound dressing potential. Additionally, the porous structure of the hydrogel improved cell viability, suggesting the use of this hydrogel product in wound management. 45
5.1.4. Collagen
Collagen is a biodegradable natural tissue matrix, which provides tensile strength for the skin. It is one of the most important components involved in wound healing and tissue regeneration, which makes collagen a popular choice in the terms of selecting biocompatible and nontoxic materials. 46 Moreover, collagen can facilitate the hemostasis process and has antibacterial properties. 46 Collagen has also been reported to have the ability to enhance dermal and epidermal wound repairs by stimulating granulation tissue formation and activating angiogenesis and the deposition of collagen fibers. 10
As the main component of the ECM and the most abundant protein in the human body, collagen is widely used to produce wound healing biomedical devices. A collagen/chitosan gel was developed and incorporated with a cell‐penetrating peptide (CPP). 47 The authors reported that this collagen/chitosan/CPP gel could retard S. aureus proliferation and showed good wound healing capacity. This result was further confirmed by in vivo study in the mouse where the treatment of collagen/chitosan/CPP gel was compared with a collagen/chitosan gel and a chitosan gel. Amongst all the treatments, collagen/chitosan gel contained CPP demonstrated the highest healing rate and fastest healing speed. 47 In another study, type I collagen was electrospun with PLA and loaded with a drug compound (either triclosan or levofloxacin). 48 This product demonstrated good antimicrobial activities for both E. coli and S. aureus. In addition, an initial burst release was observed due to the presence of the drug at the surface. Furthermore, a controlled drug dissolution profile was achieved after the burst release, which was attributed to the addition of collagen to the fibers that helped shift drug release behavior. 48 Zhou et al. (2017) successfully electrospun bioactive glass and fish collagen to produce nanofiber mats. 49 The mats possessed improved tensile strength and had a certain level of antibacterial activity. Furthermore, the collagen/bioglass mats facilitated the secretion of type I collagen and vascular endothelial growth factor in human dermal fibroblasts, which indicated its potential for effectively inducing skin regeneration. 49
5.1.5. Hyaluronic acid
Hyaluronic acid is a glycosaminoglycan that is one of the components of connective tissues. It is the main integrant of ECM in the skin, which also plays an important role in the tissue regeneration process. 50 Hyaluronic acid maintains structures to support the biological function of organs and it has a high capacity to retain water on the wound surface, which can prevent the injured sites from dryness and promote healing speed. 51 Hyaluronic acid can be easily incorporated into foams or cream to achieve topical drug delivery. Some commercial products contain Hyaluronic acid, including the commercial products Hyalofill® and Hyalomatrix® exhibited positive outcomes in wound repair and pain management. 52
One of the disadvantages of applying hyaluronic acid solutions in the electrospinning process is their very high viscosity even at low concentrations. Despite these limitations, El‐Aassar et al. (2020) fabricated Hyaluronic acid/polygalacturonic acid nanofibrous mats embedded with silver nanoparticles, 53 an in vivo study demonstrated that maximum wound epithelization and collagen deposition was achieved after 14 days, indicating its efficient use for the quick healing of wound infections. 53 In another study, Eskandarinia et al. (2020) designed polyurethane/hyaluronic acid nanofibrous mats enriched with three different concentrations of ethanolic extract of propolis (EEP). 54 The results showed that polyurethane/hyaluronic acid nanofibrous mats with 1% EEP exhibited higher antibacterial activity and higher biocompatibility, as well as an accelerated wound healing process in the animal model in comparison with mats that contained 2% EEP, which promoted their use for further biomedical applications. 54 Hyaluronic acid‐based hydrogels are another focused research topic. Dopamine‐functionalized hyaluronic acid hydrogels incorporated with arginine derivatives were developed with improved antioxidant activity. 55 Enhanced tissue re‐epithelialization and accelerated wound closure were observed in a murine model, suggesting its potential to be used for wound skin repair and regeneration in human patients. 55 The design of photo‐responsive supramolecular hyaluronic acid hydrogels was reported by Zhao et al. in 2020. 56 This hydrogel product displayed excellent biocompatibility and cell viability. Furthermore, a full‐thickness skin defect model revealed superior wound healing efficiency with controlled epidermal growth factor (EGF) release properties, which indicated that it could be a promising wound dressing for clinical treatment. 56
5.1.6. Alginate
Sodium alginate is a natural polysaccharide extracted from brown algae, which has many biological properties that favor wound management. 57 Sodium alginate is widely used in biomedical applications due to its biocompatibility, bio‐absorbability, and ability to form gels. Its high‐water absorption property removes secretions from the injured sites and hence, controls bacterial growth. It is generally utilized in a hydrogel form for facilitating wound healing and tissue engineering. 57 The common way to prepare hydrogel from alginate solution is to add an ionic crosslinking agent. An “egg‐box” shape forms between guluronic acid and adjacent polymer chains due to the interactions between the molecules. 10 The molecular weight ratio of combined mannuronic and guluronic, and the cross‐link extensions have a significant impact on the matrix formation. The amount of α‐l‐guluronic acid that residues in a hydrogel determine the characteristics of the gels (i.e., rigid gels contain high guluronic acid, whereas elastic gels contain low guluronic acid). 58
The high acceptance of alginate in biomedical applications is related to its positive clinical research results. For instance, a group of patients with full‐thickness pressure ulcers were randomized with alginate wound dressing or dextranomer paste treatment for the wounds. It was reported that patients who were treated with alginate wound dressings had relatively better outcomes. 10 Sodium alginate solutions are commonly mixed with PVA to produce hydrogels or nanofibers. An alginate/PVA hydrogel was designed and encapsulated with synthesized green tea polyphenols, 59 which were characterized and applied in a diabetic rat model for regulating the immune response and enhancing wound closure. The results demonstrated that this hydrogel product promoted chronic wound healing by regulating the PI3K/AKT signaling pathway. 59 In another study, a sodium alginate/PVA hydrogel incorporated with PCL microspheres was introduced by Bahadoran et al. (2020) for facilitating wound healing. 60 In vitro results indicated that the increased concentration of alginate was associated with higher porosity and elasticity, but also related to decreased strength and elongation at break. Moreover, the in vivo burn wound rat model showed potential for achieving cell‐induced tissue regeneration and burn wound healing. 60 An alginate/PVA‐based nanofibrous membrane produced via the electrospinning method and incorporated with honey. 61 The results showed that the increased honey content led to improved antioxidant properties, and the alginate/PVA‐based nanofibers did not reveal any cytotoxicity. This study exhibited the biomedical potential of honey loaded alginate/PVA membrane. 61 However, an in vivo animal study needs to be conducted to confirm its clinical safety.
5.1.7. Silk fibroin
Silk fibroin derived from various insects has good biocompatibility, biodegradability, and mechanical properties. 62 Silk fibroin has been widely studied as a biomedical material for regeneration in a wide range of tissues, such as skin, vascular, cartilage, bone, tendon, and ligament. 63 , 64 , 65 , 66 , 67 One type of silk fibroin extracted from silkworm cocoons contains amino acid residues. It has been used in a variety of forms (e.g., hydrogels, films, fibers, and sponges) to enhance collagen synthesis and re‐epithelialisation, which in turn promotes wound healing.
A biopolymer dressing capable of temperature sensing and wound healing was fabricated through electrospinning silk fibroin and nanodiamond. 72 Nanodiamonds are nanoparticles, which are non‐toxic and have a high specific surface area and tunable surface chemistry. 68 According to the authors, the presence of the nanodiamond improved the thermal stability of the silk fibroin‐based membranes. High biocompatibility and rapid wound closure rates were observed in the animal study. Interestingly, this silk fibroin/nanodiamond dressing selectively exhibited antibacterial properties toward gram‐negative bacteria but showed no effect on gram‐positive bacteria. 69 Therefore, its usefulness as a wound dressing is debatable as most gram‐positive bacteria are pathogenic. In another study, a multilayer membrane (silk fibroin/chitosan/alginate) with controlled drug release properties was reported by Pacheco et al. in 2020. 70 Silk fibroin provided excellent mechanical properties for this wound dressing product. It is worth noting that in this study, the authors reported that the incorporation of the drug into the membrane did not weaken its mechanical strength indicating the high performance of the dressings. 70
5.2. Synthetic polymers
Numerous synthetic polymers are used in wound dressings. Amongst the synthetic polymers, aliphatic polyesters, in particular, are well studied, including polyvinyl alcohol (PVA), polyethylene glycol (PEG), polylactic acid (PLA), poly(lactic‐co‐glycolic acid) (PLGA), and polycaprolactone (PCL). 40 , 71 , 72 , 73 , 74 The main properties that these synthetic polymers have in common are high biocompatibility and hydrophilicity, which provides a moist environment and inhibits the accumulation of excess exudates at the wound site, and these polymers generate nontoxic products during the degradation process. 10 In addition, the degradation rate of synthetic polymers can be modulated by adjusting the polymeric composition and the molecular weight. Another common property is that synthetic polymeric materials are adhesive, which renders them with stable mechanical strength and allows them to reside in the tissue for an extended time. 75 The most important reason for them to be chosen in wound care management is not only due to their ability to maintain a moist environment, control the release of various bioactive molecules, resemble skin tissue structures, but importantly these polyesters have approval from the Food and Drug Administration (FDA). 75
5.2.1. Polyvinyl alcohol
PVA exhibits excellent biocompatibility, especially PVA at pharmaceutical grade. PVA is a macromolecular organic substance that has no side effects on the human body, it has been used in various biomedical applications, including artificial joints, 76 contact lenses, 77 cardiovascular devices, 78 and wound dressing. 61 Because of the linear structure of PVA, it can be crosslinked by strategies such as irradiation and chemical agents. Venkataprasanna et al. (2020) applied the chemical glutaraldehyde to crosslink the PVA‐based solutions to increase their mechanical strength and stability. 73
In recent publications, the combination of PVA, chitosan and starch has been a popular topic in the research of wound healing. In one of the studies, PVA/chitosan/starch was electrospun to produce nanofibrous mats, 79 and it was reported that these mats had high cell viability and antibacterial properties. Even though this investigation lacked the in vivo studies necessary to confirm its biological safety, the in vitro scratch assay proved its ability to accelerate wound healing. 79 PVA/chitosan nanofibers that contained the silk protein sericin were fabricated by the electrospinning technique. 80 The low sericin content of the PVA/chitosan nanofibers exhibited higher cell proliferation than PVA/chitosan nanofiber alone. Moreover, the animal study further confirmed their wound repair capability. 80 In another study, PVA/starch was used to make hydrogel membranes. 81 Three different essential oils (clove, oregano, or tea tree oil) were incorporated into the hydrogel membranes. According to the authors, PVA/Starch hydrogels produced with 0.1 ml clove oil in 7% (w/v) starch solution exhibited better antibacterial efficacy compared to the other essential oils, and it was suggested for use as a wound dressing for burn wounds. 81
5.2.2. Polyethylene glycol
PEG is a water‐soluble amphiphilic polymer. It has been approved for use in human intravenous, oral and dermal applications by the FDA, due to its nontoxicity. 82 PEG incorporated with PLA modified the unwanted drawbacks of PLA (e.g., brittleness and low hydrophilicity), to produce PEG‐ and PLA‐based alternating block and random block polyurethane dressings for wound healing. 83 An in vivo rat skin model showed that the dressings demonstrated anti‐inflammatory properties and had a better healing effect than gauze alone. 83 PEG/chitosan hydrogels loaded with silver nanoparticles were reported to be able to accelerate wound healing in diabetic patients. 74 The antimicrobial and antioxidant properties, as well as the wound healing capacity, were shown in both in vitro and the in vivo diabetic rabbit model, 74 which indicated that it can be a promising material for chronic diabetic wound healing.
5.2.3. Polycaprolactone
PCL has good biocompatibility and biodegradability, as well as excellent mechanical strength. It has been widely used in tissue engineering applications. 84 PCL can be electrospun at low voltages to produce scaffolds with good mechanical resistance. 85 PCL/chitosan/curcumin nanofibers fabricated via the electrospinning method were then electro‐sprayed with curcumin‐loaded chitosan nanoparticles. 86 The existence of PCL improved the spinnability of the chitosan and the strength of the electrospun nanofibers. PCL/chitosan/curcumin nanofibers showed antibacterial, antioxidant, and cell proliferation efficiencies, which were confirmed by an in vivo study, indicating that these nanofibers enhanced the wound healing process. 86 Ehterami et al. (2018) electrospun PCL/collagen nanofibers that were then coated with insulin delivering chitosan nanoparticles and tested on a rat model. 87 Compared with sterile gauze, PCL/collagen‐based dressings provided nearly full wound closure within 14 days, which suggested its potential to be applied in clinical practice for wound repair.
5.2.4. Polylactic acid
PLA is an aliphatic polyester directly obtained by the condensation polymerization of lactic acid monomers or the ring‐opening polymerization of the cyclic lactide dimers. 88 PLA is biocompatible, biodegradable and has high versatility. It has been used in tissue engineering, suture production and other biomedical applications. 89 The limitations of PLA, such as its slow degradation rate and low mechanical strength, can be regulated by blending it with other polymers or additives to improve its biological and mechanical properties. 90
In one of the studies, PLA was blended with PCL and loaded with herbal extracts, 91 using the double‐nozzle electrospinning method to produce wound dressings. The optimum scaffold had an average fiber diameter of 638 ± 69 nm and was effective against gram‐positive and gram‐negative bacteria. 91 However, the authors only conducted a proliferation MTT assay to show that although the higher herbal extract led to higher cell viability, there was no in vitro scratch assay or in vivo animal study was done to investigate the effects of the PLA/PCL/herbal extract nanofibers on the wound healing process. Therefore, a further study that can prove its wound healing capacity needs to be included.
In another study, nanofibers of PLA and Poly(γ‐glutamic acid) (γ‐PGA) were fabricated with a core‐shell structure via the coaxial electrospinning method. 92 The authors optimized the electrospinning parameters and demonstrated the biocompatibility and non‐cytotoxicity of the nanofibers. Furthermore, the proliferation of dermal fibroblasts and keratinocytes improved, and re‐epithelialization occurred after 14 days of wound creation in the rat model, which indicated the potential of the core‐shell structured nanofiber for clinical wound repair.
5.2.5. Poly(lactic‐co‐glycolic acid)
PLGA is a biocompatible and biodegradable copolymer polymerized from lactic acid and glycolic acid that has been approved for versatile clinical applications. The ratio of the two monomers determines the mechanical, physicochemical properties, and the degradation time of PLGA. 93 Porporato et al. (2012) utilized PLGA to promote the activity of exogenous lactate to accelerate angiogenesis for wound healing. The results showed that after 10 days of injury, the healing area achieved with the addition of PLGA was 60% greater when compared with the control. 94 Another study of PLGA/gelatine at ratios of 9:1, 7:3, or 5:5 (v/v) was electrospun to fabricate scaffolds. 95 The physical, chemical and biological properties of the scaffolds were investigated by the authors. They reported that the 7:3 (v/v) PLGA/gelatine ratio was the most suitable candidate for chronic wound treatment due to high cell proliferation and the lack of an inflammatory response. 95 However, from our point of view, their research was not robust enough to conclude the suitability of the PLGA/gelatine nanofiber scaffolds in the application of chronic wound healing. Further studies such as scratch assay and animal studies need to be conducted as well.
A novel study was conducted by Gao and his colleagues in 2020. 96 They designed a bilayer PLGA/PVA dressing, which contained silver nanoparticles in the PLGA electrospun film, and the stem cells were seeded into the gelatine modified side of PVA hydrogel. Interestingly, the addition of silver loaded PLGA films improved both the mechanical strength and moisture content of PLGA/PVA dressings. Moreover, the seeded stem cells were able to secrete bioactive growth factors through the dressing to facilitate cell growth and wound healing. 96 This wound dressing has a great potential for skin tissue engineering.
6. BIOACTIVE MOLECULES
The application of bioactive molecules in wound dressing manufacturing brings benefits in accelerating wound healing rate and inhibiting wound infections.
6.1. Chemical drugs
Drugs like NSAIDs are often prescribed to treat the pain that is generated from wounds like mechanical injuries or surgical wounds. Nevertheless, to limit systemic exposure to the drugs, they cannot be given throughout the entire healing process. Therefore, a better way to introduce anti‐inflammatory drugs into a wound healing process is to produce anti‐inflammatory wound dressings or sutures, which enable target drug release to achieve localized pain relief. The common strategies include electrospinning, suture coating and hydrogel production (the details of which are discussed in section 6).
Antibiotic drugs for topical delivery to the wound sites have received increased attention mostly due to the small volumes of drugs required by the local wound treatment compared to systemic administration. Topical application provides high and sustained concentrations of antibiotics to the wound site, which requires a low quantity of drugs compared with systemic therapy. 97 Combining antibiotics with other methods such as adequate nutrition, proper dressing, and elimination of dead tissues can reduce the exogenous microbial infection. 98 However, the unnecessary exposure of the resident microflora to excessive antibiotics can lead to the formation of antibiotic‐resistant strains. 99 Moreover, the antibiotic effect on pre‐existing normal bacterial flora is difficult to control. 100 Apart from the development of resistance, the administration of topical antibiotics may delay hypersensitivity reactions and cause superinfections. 97 Therefore, topical delivery systems need to be designed that can control antibiotic release, this can be achieved by applying polymers to engineer stimuli‐responsive systems, able to trigger antibiotic release under specific changes in physiological conditions (e.g., pH and temperature). 99
Even though the incorporation of chemical drugs into biomedical devices can assist wound repair to some degree, this method is still limited by the inefficient regulation of the initial burst drug release, particularly in relation to the wound healing process. Moreover, antibiotic resistance has become more common within pathogens, and this together with the decreased rate of new antibiotic production has resulted in the search for alternative strategies. Non‐antibiotic components such as silver and plant products have received increased attention for controlling antimicrobial activities in the wound healing process. 101 In addition, combining growth factors and stem cells into biomedical devices, on the other hand, could provide a more bioactive strategy to facilitate wound healing. 102 , 103
6.2. Silver
Silver and its nanoparticles (AgNPs) have been reported as ideal candidates for inhibiting pathogens due to their broad‐spectrum antimicrobial characteristics. The main mechanism behind its antimicrobial properties is due to the binding of thiol groups, which deactivates the enzyme proteins in the cell membrane of bacteria. These proteins are responsible for the transportation of ions and the production of membrane energy. 104 Silver has also been reported to involve catalytic oxidation reactions. Silver catalyzes the oxygen in the cell and the hydrogen from thiol groups forms disulphide bonds, which changes the protein structure of the cell, leading to cell dysfunction. 104
In the past, despite the antimicrobial properties of silver, its use was limited due to the belief that the toxic ions might affect patient health, even though silver is not known to cause cancer or have neurological or reproductive effects. AgNPs with a higher area‐to‐volume ratio have been developed to produce wound dressings, which have higher efficacy of bacterial inhibition and are significantly less toxic to humans. 104 They have been proven to have the ability to inhibit infections on the wound site caused by both gram‐positive and gram‐negative bacteria. Silver has also been found to be toxic against nonbacterial targets such as fungi. Nanofiber dressing loaded with 1% silver is used to treat fungi‐contaminated burn wounds. 105 However, the results were not statistically significant, authors presumed that it was due to the low concentration of silver loaded in the dressing compared with commercial products in the market.
A research study aimed at developing silver‐containing activated carbon fibers was compared with commercial silver dressings to determine their dressing efficacies. 106 The authors found that the silver‐containing activated carbon fibers showed antibacterial properties and the concentration of silver had little effect on cell viability. They concluded that their products could promote wound healing in the early stage, as compared with commercial products. Another study incorporated silver oxide with chitosan and PVP to produce an antibacterial film. 107 The researchers compared this product with other reported chitosan‐based dressings aimed toward wound healing applications, they found that the silver oxide‐loaded chitosan/PVP dressings had good antibacterial activities and healing capacity. Moreover, the transparency of the films enabled wound examination without removing the dressings, which could eliminate patient discomfort.
6.3. Growth factors
Growth factors (GFs) are essential for ECM formation and remodeling in the wound healing process, and they also play a pivotal role in promoting granulation tissue formation and regulating inflammatory responses. 6 GFs are considered ideal candidates for chronic wound treatment. They are secreted by the ECM and have the ability to transfer signals between cells and regulate proliferation, migration, and differentiation of the cells. The lack of interaction amongst GFs, ECM and cells generally lead to chronic wounds. 108 The deficiency of GFs, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), platelet‐derived growth factor (PDGF), EGF, and TGF‐β, can result in chronic pressure ulcers. 108 Additionally, the generation of PDGF was observed to be very low in chronic dermal ulcers compared with acute wounds. 6 This suggests that the deficiency of growth factors during the wound healing process is responsible for chronic wounds and that the incorporation of growth factors into wound dressings might slow or prevent the progression from acute to chronic wounds.
However, in nature, GFs have a short in vivo half‐life, they degrade rapidly under normal physiological conditions. 109 When applied to the wound surface, GFs have difficulties in absorbing through the skin around the wound lesions, and they may also be extruded before reaching the wound area, which limits GFs use in chronic wound applications. 6 Therefore, to extend the effectiveness of GFs, a sustained delivery system, such as the incorporation of GFs into scaffolds, is desired. 109 GFs that are embedded inside scaffolds are protected from wound proteases during the early phase of implantation, but also delayed initial release of the GFs may be achieved. 110 Additionally, microencapsulation and nanoencapsulation techniques have been used to protect the GFs during manufacturing to achieve long‐term exposure for the treatment of chronic wounds. La and Yang et al., (2015) incorporated platelet‐derived growth factor‐BB (PDGF‐BB) into a PLGA‐based wound healing dressing. The results showed that GFs had a prolonged release from the nanosphere form compared to the free form, indicating that the nanoparticles might be ideal to treat chronic wounds. 111
The local application of GFs is important to achieve a therapeutic effect on the wound site. However, continuous injection of GFs is difficult to apply in a clinical setting. Therefore, a topical delivery system, such as gel, cream or ointment, renders direct administration of GFs to the injured site could fulfill the requirements of clinical management. Nevertheless, to date, there is only one gel product (REGRANEX® Gel, becaplermin 0.01%) that has been approved by the FDA for patients with diabetic foot ulcers. 10 Even though the evidence has shown the benefit of becaplermin to chronic wounds is beneficial, the high cost and frequent dressing change requirement limits its use.
Biopolymeric wound dressings have been successfully produced with the incorporation of GFs into commonly utilized biomaterials including gelatine, 112 , 113 collagen, 114 , 115 and chitosan. 116 , 117 Electrospinning is a popular technique to produce nanofibers with controlled‐release GFs. GF‐loaded electrospun fibers can be achieved by incorporating GFs into the polymeric nanofibers 118 or conjugating them onto the surface. 119 The difference in the GF loading strategies determines a variation in GF release profiles. There was a novel approach proposed by Kulkarni et al. in 2014. 120 They utilized a layer‐by‐layer assembly method to encapsulate EGF on the surface of poly(acrylic acid)‐modified polyurethane nanofibers. This method successfully prolonged EGF release, which was regulated by adjusting the number of layers deposited onto the surface.
Wound healing involves many complex mechanisms, which sometimes require more than one GF delivery to provide sufficient support. Studies have shown that a combination of different GFs can improve delayed wound repair in diabetes compared with a single GF treatment. 121 Moreover, the spatio‐temporal gradients can also have a considerable impact on treatment efficacy. Lai et al. (2014) either embedded bFGF, EGF, VEGF, and PDGF into collagen/hyaluronic acid‐based electrospun nanofibers or encapsulated them into gelatine nanoparticles. 122 Degradation lasted for a month simulating the temporal release of the normal wound healing process in the human body environment. EGF and bFGF were released in the initial stage mimicking the early phase of angiogenesis of wound repair. The sequent controlled release of VEGF and PDGF imitated the new skin reconstruction phase which was confirmed by an in vivo diabetic rat study.
Platelets are natural sources of multi‐GFs and proteins, which takes part in tissue regeneration. Additionally, platelet derivatives have been reported to facilitate wound healing and accelerate tissue repair. 123 Platelet lysate (PL) is obtained from blood platelets after freezing/thawing cycles. It has been reported that PL can activate various cell types during the wound healing process. 123 In comparison with platelet‐rich plasma or platelet‐rich fibrin, PL has the advantage of limiting the variability of individuals. It has been used to produce sponge‐like dressings, 124 scaffolds, 125 bioactive gels, 126 , 127 and eye drops. 128 , 129 Recently, a core‐shell structure particle‐based dressing was designed with a calcium alginate shell and embedded in an alginate matrix. 130 PL and an antibiotic were loaded in the external matrix as active components for the treatment of chronic skin ulcers. Based on the in vitro and ex vivo studies, the dressings were found to have excellent mechanical properties to handle stress and were able to absorb a high amount of wound extrudate. 130
6.4. Stem cells
Due to complex pre‐existing conditions such as diabetes and ischemia, chronic wounds often appear alongside complications of prolonged inflammation and impaired angiogenesis. 131 The conventional methods for facilitating wound healing such as autograft, xenograft, and allograft have inherent drawbacks of secondary surgeries, the risk of pathogen transfer and limited availability. Cell therapy has been introduced to overcome these difficulties. 132 Stem cells (e.g., mesenchymal stem cells, endothelial progenitor cells [EPC], and epithelial stem cells) have been utilized in wound treatment and their potential discovered for enhancing angiogenesis and tissue regeneration. 133 , 134 , 135 Even though the existing problems such as the high rate of cell death might decrease the treatment efficiency, cells therapy is still a promising strategy, since it integrates environmental signals and transforms them into bio‐factors in the wound sites. 136
For instance, EPCs are bone marrow mononuclear progenitor cells that secret growth factors and cytokines in the wound site to facilitate vascularization and increase angiogenesis. However, previous research on in situ or intravenous injection failed to achieve the target delivery. 132 Hence, Wang and co‐workers developed a bioactive glass (BG) nanoparticle‐based scaffold able to render high biocompatibility and prolong the viability of EPC for promoting wound healing. BGs have received considerable attention on both hard and soft tissue repair due to their unique biocompatibility. 137 Studies have reported that BG nanoparticles can release silicon (Si), Calcium (Ca), and phosphorus (P) ions to provide increased cell response. 138 , 139 It can also improve angiogenesis of human umbilical vein endothelial cells (HUVECs) by regulating growth factors such as VEGF and bFGF, resulting in an improvement in wound healing. 138
Evidence has shown the paracrine effect of stem cells on modulating the level of cytokines and GFs around the wound site. 132 Different from other differentiated cells, stem cells are involved in both acute and chronic wound healing processes, regulating the healing response and synthesizing multiple GFs until the skin is reconstructed. The topical delivery of GFs can be achieved by loading stem cells into scaffolds, which provide robust mechanical strength to sustain stem cell actions and enhance cell proliferation. Various stem cells and techniques can be used for wound healing applications. Amongst them, mesenchymal stem cells (MSCs) especially adipose‐derived stem cells (ADSCs) are probably the most studied stem cells in cutaneous wound healing in the past 5 years. 140 , 141 , 142 , 143 For further reading, Mazini and co‐workers have provided a detailed review of ADSCs in wound healing management. 144
Bone marrow‐derived mesenchymal stem cells (BMSC) are an old fashioned method for facilitating chronic wound healing that has been well studied. 145 , 146 , 147 Guo et al. (2018) recently reported on enhanced diabetic wound healing using ADSC with similar results to BMSC, 148 which was confirmed by conducting an in vivo study on full‐thickness wounds in diabetic mice. Moreover, cell migration and viability of ADSC and BMSC within the biomimetic‐collagen scaffolds were similar. 148 The main challenge of utilizing MSCs in wound management was to maintain their viability and adherence to the wound bed. In situ‐forming injectable hydrogel dressings have been developed to secure a large number of cells at the wound infection site. In addition, the relatively easier “living cell” loading method makes hydrogels a popular choice for implanting cells into complex tissue shapes. 149
6.5. Natural compounds
The development of alternative wound healing products has been a popular topic in recent years. Natural sources, in particular have received increased attention. Natural compounds, such as honey, aloe vera, curcumin and essential oils, have been applied in wound management. 150 , 151 Nevertheless, there is still a lack of standardization methods for the evaluation of the composition of natural products, which makes it difficult to estimate the efficacy of these compounds in wound management.
6.5.1. Curcumin
Curcumin is a natural polyphenolic molecule extracted from the Curcuma longa rhizome widely used in both normal and chronic wound repair. It is a wound‐healing agent and has anti‐inflammatory, anti‐infection, and anti‐oxidant effects. 152 Curcumin can stimulate granulation tissue proliferation and facilitate the transformation of TGF‐β1and proteins into ECM. 153 It acts as an antioxidant to scavenge free radicals since free radicals are considered the major cause of inflammation by activating the downregulation of the PI3K/AKT/NFκB pathway. 154 Studies have shown that the topical application of curcumin on wounds enhanced fibroblast proliferation and vascular density, which in turn promoted epithelial regeneration. 152
One of the major factors that cause chronic wounds involves oxidative stress. Antioxidant therapy focuses on encapsulating antioxidant agents into a wound dressing to eradicate reactive oxygen species (ROS), to promote the healing rate of chronic wounds. 155 Curcumin has been reported to have the potential to decrease ROS and increase healing speed, 152 which can be attributed to the phenolic hydroxyl groups and the diketone structure of curcumin (Figure 2). Its antioxidant efficacy is ascribed to the nuclear factor erythroid 2‐related factor (Nrf2) pathway, which activates the cytoprotective signaling constituents. 154
FIGURE 2.
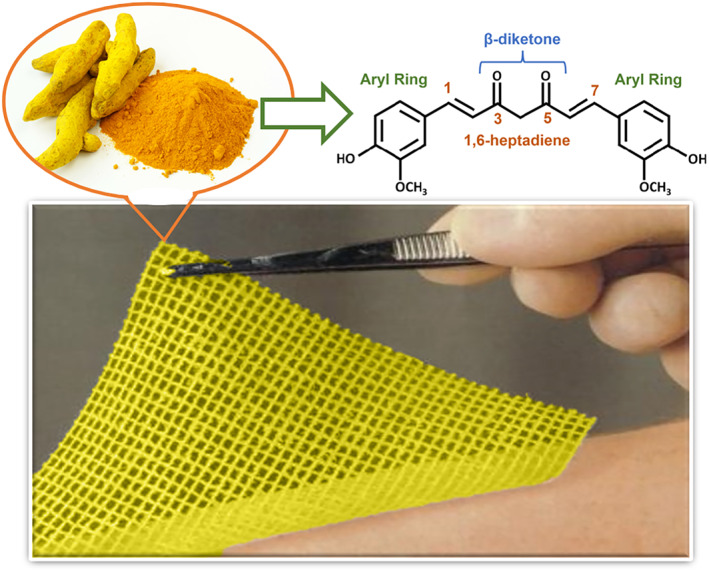
Functional group of curcumin structure in wound management
Additionally, curcumin has also been reported to hinder transcription factor nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐(κ) B), which modulates the genes involved in the inflammatory response. 152 Kinases, including AKT, PI3K, and IKK, regulate the activity of NF‐(κ) B, and curcumin has an impact on these pathways. 152 Mohanty et al. (2012) designed an oleic acid‐based polymeric bandage loaded with curcumin that was placed on the dorsum of injured rats. The addition of curcumin had a downregulation effect on the expression of kinases in the PI3K/AKT/ NF‐(κ)B pathway. 156
Nguyen et al. (2013) designed curcumin‐loaded PLA nanofibers to function as wound‐healing patches. The result showed that the addition of curcumin had a significant impact on the tensile stress of the nanofibers (that increased up to 3.5 MPa), which indicated its suitability as a wound dressing. In the in vivo study, the dorsal wounds on the rats achieved 87% and 99% of wound closure rates on days 7 and 15, respectively. 157
Ranjbar‐Mohammadi et al. (2016) developed a curcumin‐loaded PCL/gum tragacanth nanofiber. The nanofibers were reported to be 85.14% and 99% antibacterial against extended‐spectrum beta‐lactamase (ESBL) and methicillin‐resistant Staphylococcus aureus (MRSA), respectively, with a curcumin content of 3 wt% revealing its potential for treating wounds infected with antibiotic‐resistant organisms. 158
A curcumin‐loaded mat produced by Zahiri et al. (2020) was electrospun with PCL‐gelatine. Sustained release of curcumin was observed from the electrospun mats, and the in vivo study revealed the significant wound closure achievement of curcumin‐loaded nanofiber mats on day 14 (82%), in comparison to the plain nanofibrous mats (73.4%). 159
6.5.2. Honey
Honey is derived from nectar by industrious honeybees and recently has been valued for its biomedical properties in treating diabetic ulcers, burns, and various skin wounds. 160 Instead of being treated as an “alternative medicine” as previous considerations, honey has become one of the main focuses in the terms of wound healing, since the advent of antibiotic resistance to the majority of modern medications. 161 Amongst the different honey types, Medihoney and Manuka honey were studied and showed similar healing properties. 162
Honey is known for its antimicrobial, anti‐inflammatory and antioxidant properties that accelerate wound healing rates. Many studies have shown its use in prohibiting challenging wound infections with its excellent antimicrobial activities. 163 Honey, in nature, contains major (macroelements) and minor (trace elements) ingredients that can perform biomedical activities in various wound healing settings. 163 However, the concentrations of these ingredients vary between different plant species where bees feed and are also influenced by environmental pollution. 164 The main ingredients are sugar (glucose and fructose, 65%) and water (18%) as well as minimal protein and lipid contents. 164 The minerals and heavy metals that honey contains have a significant influence on determining honey qualities. For instance, pale honey only holds 0.04% mineral content, however, it can reach 0.20% in darker honey products. 164 Another important component group of honey are polyphenols (e.g., catechin, quercetin, and taxifolin), which are known for their antioxidant properties. 162
Honey has antimicrobial efficiency against more than 60 bacterial species, including aerobic/anaerobic bacteria, gram‐negative/gram‐positive bacteria, and some fungi. 165 Honey plays an important role in inhibiting biofilm formation, which can be ascribed to its osmotic effect. The osmotic action is the result of the high sugar content in honey, depleting water from the bacterial cells, leading to dehydration and cell death. 166 Unexpectedly, researchers found that diluting honey in the water further increased its antimicrobial properties, they identified that hydrogen peroxide was the key antimicrobial agent in most honey. 167 Moreover, freshly collected honey was reported to have a high level of lysozyme (usually 5–10 mg/ml, occasionally 35–100 mg/ml), which is much higher than extractions from older honey samples. 168
Manuka honey is well known for its use in manufacturing wound dressings. It is produced in New Zealand and Australia from the nectar of the Mānuka tree, and it contains methylglyoxal which is a bioactive molecule that can kill bacteria and reduce the infection rate in biomaterials. 169 Notably, Manuka honey does not contain the hydrogen peroxide found in most other kinds of honey, but rather the methylglyoxal provides the antibacterial effect. Medihoney® dressing was designed for wound healing and used Manuka honey as an active agent. It was the first honey‐based dressing approved by FDA for clinical use. It was accepted for its excellent wound healing for light to moderate wounds such as diabetic foot ulcers, partial/full‐thickness pressure ulcers, and first/second partial‐thickness burns. 160 Moreover, literature has reported that Manuka honey can reduce inflammation and promote fibroblast migration, which provides the potential to enhance the wound healing rate. 162 Additionally, a study by Watanabe et al. (2014) showed that Manuka honey retarded the duplication of influenza virus and improved the effects of antiviral drugs, indicating a potential for the incorporation of honey with an antiviral drug in the future. 170
Surgihoney® is another commercial wound dressing product based on various honey sources. This highly standardized honey has a precise antimicrobial activity level and can steadily deliver oxygen‐free radicals. 171 Its antimicrobial activities own to hydrogen peroxide, which helps the dressing to fight against bacteria at a very low concentration. 162
Even though honey is a natural product and it has high purity, patients who are allergic to honey or bee venom still need to be aware of using honey dressings. Moreover, patients with diabetes need their blood sugar levels to be monitored to avoid putting them at risk of hyperglycaemia. 10
6.5.3. Aloe vera
Aloe vera is a tropical herb that grows in a relatively hot and dry environment. 172 Aloe vera gel extracted from the mucilaginous part of the leaf has been applied in various wound care for centuries. Aloe vera has antioxidant, antibacterial and anti‐inflammatory properties and it stimulates FGF to promote collagen production and secretion. 173 In addition, Aloe vera contains vitamins A, B, C, E, amino acids, enzymes, polysaccharides, and anthraquinones. 173 The glycoprotein fraction isolated from aloe vera was reported to be a bioactive component for wound healing. 174 Aloe vera gel was utilized on sutured incisions in the Wistar rat to study its topical efficacy. 175 It was reported that aloe vera gel had notable wound‐healing effects, which were confirmed by the observation of increased mean numbers of fibroblast proliferation and the mean thickness of the regenerating epithelium after 4 days of surgery. 175
A research group developed nanofibrous scaffolds with a combination of PCL/ aloe vera, PCL/silk fibroin, PCL/aloe vera/silk fibroin, and PCL/aloe vera/silk fibroin/curcumin. 176 These scaffolds successfully mimicked the natural environment of ECM. The total content of aloe vera was 16.6% (w/w), and the scaffolds that contained aloe vera had the best results in terms of porosity and mechanical properties, which indicated their suitability to be used as wound dressings. Aloe vera (25% w/w) was electrospun with silk fibroin and polylactic‐co‐ε‐caprolactone (PLLA) to produce dermal substitutes. 177 The final wound dressing product demonstrated both high wound healing activity and elastic strength, which was a combination of the unique properties of aloe vera and silk fibroin. Another study also used both aloe vera and silk fibroin to produce nanofibers, 178 and the aloe vera content was 10–20% w/w. In that study, vitamin E loaded starch nanoparticles were fabricated and then incorporated into aloe vera/silk fibroin/PVA nanofibers. The addition of aloe vera and vitamin E was ascribed to the largely enhanced cell viability. 178
Garcia‐Orue et al. (2017) developed a PLGA nanofibrous membrane that contained recombinant human epidermal growth factor (rhEGF) and aloe vera extract. 179 The authors reported that this was the first study that used a high proportion of aloe vera (aloe vera/PLGA/rhEGF at a ratio of 1:1:0.4). The addition of aloe vera promoted the proliferation of fibroblasts, which in turn facilitated wound healing. 179 Abdel‐Mohsen et al. (2020) proposed a novel wound dressing that consisted of collagen, chitosan‐glucan complex hollow fibers and aloe vera. This wound dressing was fabricated using a freeze‐drying technique and it demonstrated excellent blood clotting efficiency and wound healing characteristics, which suggests its application in soft tissue engineering. 180
6.5.4. Other phytomedicines
Other plant products, such as green tea, essential oils and grapevine are also used as bioactive molecules for facilitating wound healing. 181 , 182 , 183 Green tea contains high amounts of polyphenols, accounting for nearly 30% of the dry weight of the green tea leaves, which are potently antibacterial, anti‐carcinogenic, and anti‐inflammatory components. 182 There are also catechins within the green tea extract, which inhibit the growth of gram‐positive and gram‐negative bacteria causing damage to the cell wall. 182 All those features suggest that green tea may enhance the wound healing process. An antibacterial microneedle consisting of green tea extract and hyaluronic acid was designed to efficiently deliver green tea extract for facilitating wound healing. 184 In this system, researchers were able to regulate the degradation rate of green tea by modulating hyaluronic acid ratios. Additionally, this delivery system had a high loading capacity, as a 70% green tea load achieved sustained release within 72 h. 184
Essential oils are the largest group of secondary metabolites of plants. 185 They have been used for a variety of medical applications for centuries. 186 Essential oils can be extracted by steam distillation or cold pressing. Their use in cosmetic products and natural medicine products has been increasing. 187 Moreover, due to the anti‐inflammatory, antioxidant, and antibacterial characteristics of essentials oils, they play an important role in dermatological treatment for chronic wounds. 188 García‐Salinas et al. (2020) developed various PCL‐based electrospun patches and loaded them with essential oils thymol and tyrosol to reduce inflammation during wound healing. 181 They showed that essential oil‐loaded PCL patches reduced the size of inflamed cells in immunofluorescence assays, which indicated the alleviation of the inflammatory response, and their potential use for wound healing.
Tea tree oil is one of the most commonly studied essential oils. It is steam‐distilled from Melaleuca alternifolia, which is an Australian native plant. The major component of tea tree oil, which is Terpinen‐4‐ol, has excellent antimicrobial and anti‐inflammatory properties, however, the existence of 1,8‐cineole is a known potential allergic trigger in tea tree oil products. 181 Zhang et al. (2017) developed PLA‐based electrospun nanofibers and encapsulated them with tea tree and manuka essential oils. 189 They observed that tea tree oil worked as a plasticizer for PLA during the manufacturing procedure, which improved the mechanical properties of the PLA‐based electrospun nanofibers.
7. DEVICES FOR WOUND MANAGEMENT
7.1. Wound dressings
Advanced wound dressings are designed to maintain the wound microenvironment, facilitating re‐epithelialization and preventing further skin damage. It is crucial for wound dressings to be porous and breathable, which allows the gaseous exchange to protect the tissues from maceration and promote autolytic debridement. Moreover, the dressings must be able to provide thermal insulation, against infections, and balance the moisture of the wound environment. Furthermore, advanced wound dressings should facilitate wound healing in a way that promotes the natural healing process, including the enhancement of angiogenesis in an ischemic wound, and the acceleration of fibroblasts and keratinocytes migration rate. 190
Wound dressings can be classified as biological dressings, conventional dressings, biosynthetic dressings, and antimicrobial dressings. Amongst them, biological dressings (e.g., cadaver allograft skin, xenograft, and human amnion) and conventional dressings (e.g., Vaseline gauze and silicone sheets) are only used temporarily and cannot be utilized as a permanent skin replacement. 10 Biosynthetic dressings can mimic the natural skin function and replace the epidermis or dermis. For instance, Biobrane® is a biosynthetic skin dressing used on superficial and partial‐thickness wounds, and it has been proven to significantly reduce the healing time of superficial and mid dermal partial‐thickness burns within 12 h. 191 Antimicrobial dressings may contain silver, cadexomer iodine, or honey to prevent wound infection and inflammation. Especially the incorporation of silver compounds in burn wound treatment was a successful case, which effectively reduced the chances of burn‐induced sepsis and possible death. 22
7.1.1. Hydrogels
Hydrogels are swellable dressings made from biocompatible and biodegradable materials such as polyvinylpyrrolidone (PVP) and methacrylate. 192 , 193 Hydrogels contain 80%–90% water molecules and provide a moist environment for the wound site through the release of water molecules. Hydrogels are 3D networks formed by hydrophilic water‐soluble polymers that have a wide range of physicochemical properties. 194 The hydrophilic chains within the hydrogel structure grant it the ability to absorb a large volume of water without changing its gelatinous nature. 193 Hydrogels are nontoxic and nonadherent, and their jelly‐like texture makes hydrogel dressings a common use in wounds with unusual shapes and edges. 195 Recent studies on hydrogel fabrication for wound healing have been summarized in Table 1.
TABLE 1.
Hydrogels in wound management
| Materials | Crosslinking engine | Bioassay | Wound healing assay | Properties/results | References |
|---|---|---|---|---|---|
| GelMA | Chemical | — | In vitro | The cells were able to grow in each layer, which rendered epidermis‐like thin cell layers for keratin/chitosan nanofibers, and dermis‐like cell‐laden for the GelMA hydrogel. | [200] |
| Sodium alginate/ gelatine/ AgNPs | Physical | Pseudomonas aeruginosa/ Staphylococcus aureus | In vitro/in vivo | AgNPs loaded sodium alginate/gelatine hydrogels accelerate skin tissue formation and stimulate early collagen scar generation in 14 days. | [201] |
| Chitosan/ PEG/ AgNPs | Chemical | Pseudomonas aeruginosa/ Escherichia coli/ Staphylococcus aureus/ Bacillus subtilis/ Bacillus pumilus | In vivo | AgNPs incorporated chitosan/PEG hydrogels exhibited sustained AgNPs release and achieved fast healing in the diabetic rabbit study. | [74] |
| PEG/PAH/gold nanoparticles/poloxamer® 407 | — | Staphylococcus aureus/Pseudomonas aeruginosa | In vivo | AuNPs with different shapes and surface modifications were loaded into PEG or PAH blended poloxamer® 407 hydrogels. AuNPs demonstrated slow release over 48 h. Hydrogels promoted skin re‐epithelization and achieved almost complete wound healing in 14 days. | [202] |
| BAPEG/DCS/VEGF | Hybrid | Staphylococcus aureus/Escherichia coli | In vitro/in vivo | BAPEG/DCS hybrid hydrogel repaired acute tissue immediately, due to adhesion and hemostasis of the hydrogel. Chronic wound healing could be achieved by VEGF‐loaded hydrogel. | [197] |
| QCS‐C/PLEL | Physical | Staphylococcus aureus/Escherichia coli | In vivo | An injectable adhesive thermo‐sensitive hydrogel was fabricated. In vivo study displayed superior ability to heal complicated skin defects compared with the suture and fibrin glue within 7 days. | [203] |
| Chitosan/PVA/AgNPs | Physical | Pseudomonas aeruginosa/Staphylococcus aureus | In vivo | AgNPs loaded chitosan/PVA hydrogel achieved 98 ± 4 to 99 ± 1% wound closure on day 12. | [204] |
| Gelatine/quercetin/Carbopol® | — | — | In vivo | Quercetin loaded liposomal hydrogel showed a wound reduction of 98.93% on day 12. Whereas, simple liposome‐hydrogel achieved 52.26% wound closure on day 4. | [205] |
| Chitosan/PLGA/PDGF/β‐glycerophosphate | — | — | In vivo | PDGF/PLGA hydrogel demonstrated better performance than PDGF alone in wound closure and granulation tissue formation. Moreover, autophagy levels were decreased in the PDGF/PLGA hydrogel group in comparison with control groups at day 8. | [206] |
| Quaternized chitosan (QCS)/polydopamine‐coated reduction graphene oxide (rGO‐PDA)/ poly(N‐isopropylacrylamide) (PNIPAm) | Chemical | Staphylococcus aureus/Escherichia coli | In vitro/in vivo | Self‐healing QCS/rGO3‐PDA/PNIPAm hydrogel with thermo‐responsive properties was successfully fabricated. The increased content of rGO‐PDA led to reduced drug release within 10 days, due to the decrease of swelling ratios. However, more drug (about 40%) were released in initial stage (8.5 h) with high rGO‐PDA loading. | [207] |
| Ag/Ag@AgCl/ ZnO | Hybrid | Staphylococcus aureus/Escherichia coli | In vivo | Ag/ Ag@AgCl/ ZnO hybrid hydrogel killed 95.95% E. coli and 98.49% S. aureus within 20 min. A sustained release of silver and zinc ions was observed in 21 days. Moreover, 90% Zn2+ was released in acidic conditions after 3 days, whereas, only 10% was released after 21 days in neutral conditions. | [208] |
| PH/PVA | Physical | — | In vitro | PH/PVA hydrogel demonstrated higher swelling and cell proliferation rate compared to the PVA hydrogel after 1 and 5 days. | [209] |
| BG/OSA | Chemical | — | In vivo | BG/OSA hydrogel completed the healing process on day 21 in a rat model. The presence of BG imparted tissue adhesiveness to the OSA hydrogel, due to alkaline ion release. | [210] |
| PDA/PUE/PEG‐DA | Hybrid | — | In vivo | PEG‐DA/PDA/PUE hydrogel absorbed 2000 times more solution than its own weight and completed wound healing in a rat model in 20 days. | [211] |
Abbreviations: AgNPs, silver nanoparticles; AuNPs, gold nanoparticles; BAPEG, benzaldehyde‐terminated PEG; BG, bioglass; DCS, dodecyl‐modified chitosan; GelMA, gelatine methacrylate; OSA, oxidized sodium alginate; PAH, polyallylamine hydrochloride; PDA, polydopamine; PDGF, platelet‐derived growth factor receptor; PEG, polyethylene glycol; PEG‐DA, polyethylene glycol diacrylate; PH, phlorotannins; PLEL, poly(d,l‐lactide)‐poly(ethylene glycol)‐poly(d,l‐lactide); PNIPAm, poly(N‐isopropylacrylamide; PUE, puerarin; PVA, Polyvinyl alcohol; QCS, quaternized chitosan; QCS‐C, catechol modified quaternized chitosan; rGO‐PDA, polydopamine‐coated reduction graphene oxide; VEGF, vascular endothelial growth factor.
Hydrogel wound dressings have been widely studied for their wound healing potential in wounds with minimal exudates (Figure 3). Hydrogels are not able to absorb exudates as hydrocolloids do. Thus, they are not suitable for wound treatment that involves high exudate production. 196 One of the main drawbacks of hydrogels is their low mechanical properties, which are correlated with the plasticising effect of the water contained in the polymer network. Therefore, the same structure feature that renders hydrogels with permeability and other unique properties (e.g., improved transmission of moist vapor and oxygen), however, at the same time, causes poor tear strength and a tendency to deformation. 196
FIGURE 3.

Hydrogel wound dressing production and application
Chen et al. (2018) proposed a benzaldehyde‐terminated PEG and dodecyl‐modified chitosan hybrid hydrogel system, loading, and controlling the release of VEGF. 197 This hybrid hydrogel was able to heal acute wounds immediately and it also facilitated chronic wound healing on a full‐thickness skin model by enhancing angiogenesis, collagen deposition, macrophage polarization, and granulation tissue formation. 197
Wang et al. (2019) developed an injectable polypeptide‐based hydrogel and loaded it with adipose‐derived mesenchymal stem cells exosomes to promote chronic wound healing and skin tissue regeneration. 198 This hydrogel product significantly improved the proliferation and migration of HUVEC cells. An in vivo diabetic animal study proved that this hydrogel product accelerated granulation tissue formation and collagen remodeling, which eventually promoted chronic wounds to re‐epithelialize and achieve full skin regeneration. 198
Annabi et al. (2017) reported on a sprayable hydrogel with broad‐spectrum antimicrobial activity to treat chronic wounds. 199 This hydrogel was engineered with gelatine methacryloyl and methacryloyl‐substituted recombinant human tropoelastin by visible‐light‐induced crosslinking. The results showed that this hydrogel product was comparatively superior to commercial tissue adhesive products and demonstrated minimal inflammatory response in a murine animal model, 199 which indicated its potential as an alternative suture for promoting chronic wound healing and preventing subsequent wound infections.
7.1.2. Nanofiber scaffolds
A scaffold is a structure that provides support and holds tissues together. They can be naturally generated by the injured body, but artificially engineered scaffolds can further promote the healing process. 212 To achieve the optimal activity, engineered scaffolds have to mimic the biological properties of the natural ECM, to maintain basic mechanical support and modulate cellular activity. 212 Various cells and growth factors may be incorporated into three‐dimensional (3D) scaffolds to enhance tissue regeneration. In cutaneous wound healing, scaffolds stimulate proliferation, migration, remodeling, and scar formation processes. 196 Numerous synthetic and natural polymers with biocompatible and biodegradable properties have been applied to develop scaffolds for skin tissue regeneration. Recent studies on nanofiber fabrication for wound healing have been summarized in Table 2.
TABLE 2.
Nanofibrous scaffolds in wound management
| Methods | Materials | Bioactive molecules | Flow rate (ml/h) | Voltage (kV) | Tip‐collector distance (cm) | Fiber diameter | Properties/results | Reference |
|---|---|---|---|---|---|---|---|---|
| Uniaxial electrospinning | PVA/keratin/chitosan | — | 0.4 | 16 | 10 | 120–151 nm | Nanofibrous scaffolds comprised of 5% w/v keratin and 2% w/v chitosan were successfully produced without deteriorating the nanofiber morphology. | [39] |
| PVA/ GT/ MoS2 | TCH | 0.7 | 16 | 12 | 50–100 nm |
TCH loaded PVA/GT/MoS2/TCH nanofibers demonstrated excellent tensile strength and a slow release due to the presence of MoS2. Nanofibers exhibited non‐toxic effects on fibroblast cells and good antimicrobial activity against bacteria. |
[220] | |
| Silk fibroin | Fenugreek | 0.5 | 25 | 10 | 309 ± 83 nm | Fenugreek incorporated silk fibroin nanofiber was fabricated. Fenugreek release was 21.5 ± 0.9% in 24 h. | [221] | |
| PCL | PCE | — | 18 | 15 | 212 ± 40–450 ± 100 nm | PCL/PCE hybrid nanofibrous matrix exhibited high antibacterial properties and similar tensile elastomeric modulus to human skin tissue. The water contact angle of pure PCL nanofibers was 130 ± 3°, and PCL‐30%PCE nanofibers were 41 ± 1°. | [222] | |
| PCL/ Col I | — | 1 | 14 | — | Random (556.3 ± 36 nm), aligned (583.3 ± 55 nm), crossed (573.3 ± 91 nm) | PCL/Col I nanofibrous scaffolds were fabricated to resemble the organization of collagen fibrils in native skin. Diabetic rat models revealed the ability of scaffolds to enhance wound healing. | [223] | |
| PCL/collagen | Bioactive glass nanoparticles | 10 μl/min | 15 | 1.4 | 300–500 nm | ECM‐biomimetic nanofibrous scaffolds were fabricated from the composition of PCL/collagen/bioactive glass for enhancing wound healing in diabetes. The wound recovery achieved 90% in 14 days. | [224] | |
| Gelatine | Curcumin | 1.5 | 15 | 10 | — | Curcumin/gelatine‐blended nanofibrous mats were fabricated to enhance the bioavailability of curcumin for wound repair. Decreased wound area (2%) was seen at 15 days in a rat model. | [225] | |
| Cellulose acetate/gelatine | Hydroxyapatite | 0.8 | 18 | 13 | 316 ± 115 nm |
Cellulose acetate/gelatine/hydroxyapatite nanocomposite mats were fabricated as wound dressings. The wound closure rate reached 66% in 7 days and 93% in 14 days. |
[226] | |
| PHB/ gelatine/ collagen | OSA | 1.5 | 1.5 | 12 | 80 ± 10 nm | PHB/ gelatine/ OSA nanofibers were coated with collagen for wound healing application. The degradation rate of the scaffolds was 71.8% in 12 h. | [227] | |
| PCL | — | 0.1 | 21 | 6 | 250–3000 nm | An electrospun nanofibrous mat with a human skin pattern was successfully fabricated. In vitro cell culture had a proliferation of 7 days. | [228] | |
| CA | — | 3 | 25 | 10 | 1.0 μm | Electrospun cellulose nanofiber mats demo high adsorption of multiple microorganisms. Uptake capacity of nanofiber mats collected 420 times more E. coli than control. | [229] | |
| PCL | — | 1 | 18 | 15 | — | Electrospun PCL membranes fabricated as a skin substitute material. 5% PCL membrane had a modulus of 2.46 ± 0.26 MPa. Whereas, 15% PCL membrane was 3.84 ± 0.25 MPa. | [230] | |
| Coaxial electrospinning | PCL (8 wt%)/gelatine (4 wt%) | Plant extracts/minocycline antibiotics | 1.2 | 13 | 12 | 302 ± 44 nm | PCL/gelatine nanofibrous mats with core‐shell structure containing antibiotics and natural extracts were fabricated with excellent antibacterial activities and enhanced proliferation of fibroblasts and keratinocytes. Higher keratinization was observed in the mat samples loaded with antibiotics and natural extracts. | [231] |
| RSF | Curcumin/doxorubicin hydrochloride | 0.9 | 30 | 15 | 1224 nm | Dual drug loaded RSF nanofibers were fabricated. Curcumin and doxorubicin hydrochloride achieved a sustained release of 30% in 10 h. | [232] | |
| PLGA/ GT | TCH | 1 and 0.2 | 15 | 15 | 180–460 nm | TCH‐loaded PLGA/GT nanofibers with a core‐shell structure exhibited excellent tensile strength in both dry and wet conditions, and a drug release of 20% in 2 h. | [233] | |
| PCL/ Col I | DMOG | 5 and 10 μl/min | 15 | 15 | 200–500 nm | PCL/Col nanofibrous mats were fabricated and loaded with DMOG. Drug release for simply blended nanofibers was 53.3 ± 2.7% in 12 h. Whereas, core‐shell nanofibers achieved 17 ± 2.1% drug release in 12 h and 36.1 ± 4.2% in 24 h. | [234] | |
| PLLA/ mesoporous silica nanoparticles | DMOG | 0.025 ml/min | 10 | 10 | — | PLLA/ mesoporous silica nanofibers loaded with DMOG were fabricated. The wound healing ratio was 97% in 15 days, and the cumulative release achieved 0.04 mg/mL in 12 days. | [235] | |
| PCL | Ampicillin | 0.20–0.60 | 12–24 | 11 | 464 ± 214 nm | Ampicillin‐loaded AL‐BSA membranes were produced. The drug release rate reached 94.8% within 72 h. | [236] | |
| PCL/zein | MNA | 0.7 to 1.4 | 23–25 | 18 | 0.6 ± 0.04 μm | MNA‐loaded PCL/zein coaxial nanofiber membranes were fabricated. The drug release rate was 12.2% in 2 h. | [237] | |
| SA/ RCSPs | Calcium ions | — | 20 | 15 | 87.58 nm | SA/RCSPs/Calcium ions composite was fabricated based on the gelation reaction of calcium ions with alginate. The wound healing rate in the rat model was 46.6% on the 5th day and wound closure in 15 days. | [238] | |
| Gelatine/ OC | — | 2 | 20–22 | 15–18 | 150–400 nm | Cellular nanofibers had a water contact angle of 80° and a swelling rate of 380%. | [239] | |
| PCL/gelatine | — | 0.4–1.2 | 8–15 | — | 666 ± 164 nm | PCL/gelatine composite scaffolds were created and the wound closure rate in the animal model achieved 38% in 21 days. | [240] | |
| PCL/ BC | — | 1–4 | 28–29.4 | 13 | 384.24 nm | PCL/BC composite nanofibers were able to regulate scaffold hydrophilicity, which significantly increased cell proliferation. 20%PCL/5%BC nanofibers demonstrated 100% cell viability in 72 h, same as control. | [241] | |
| Triaxial electrospinning | PCL/cellulose acetate/ PVP | Nisin | 0.6, 1.2, and 0.2 | 12–14 | 20 | 0.8 μm |
Triaxial electrospun nisin‐containing membranes exhibited excellent antimicrobial properties for more than 5 days under damp conditions. Whereas, single blended nisin membranes immobilized nisin did not show antimicrobial activities. |
[242] |
| PCL/ gelatine | Doxycycline hydrophilic | 2–3 and 1–1.5 | 17–18 | 17–19 | 30.0 ± 17.0 μm | PCL/ gelatine fibers were electrospun into triaxial configuration. Doxycycline hydrophilic achieved controlled release, and the presence of GT allowed viable cells to attach to the fibers. | [243] |
Abbreviations: BC, bacterial cellulose; CA, cellulose acetate; Col I, type I collagen; DMOG, dimethyloxalylglycine; GT, gum tragacanth; MNA, metronidazole; MoS2, molybdenum disulphide; OC, oleoyl chitosan; OSA, ostholamide; PCE, poly(citrate)‐ε‐polylysine; PCL, polycaprolactone; PHB, polyhydroxybutyrate; PLGA, poly(lactic‐co‐glycolic acid); PLLA, Poly‐L‐lactic acid; PVA, polyvinyl alcohol; PVP, Polyvinylpyrrolidone; RCSPs, Rana chensinensis skin peptides; RSF, regenerated silk fibroin; SA, sodium alginate; TCH, tetracycline.
Skin injury often is a complicated problem in a clinical setting. Conventional methods including autograft and allograft cannot achieve an ideal result. Electrospun nanofibers as an alternative for skin tissue regeneration have received increased attention in the last decade. 213 This fabrication process is shown in Figure 4. Electrospun nanofibers have porous hydrophilic surfaces. Its unique features assist in oxygen diffusion, maintain a moist environment and absorb exudates from the injured site. 214 Electrospun fibers share a similar structure and physiochemical properties to natural ECM that obtains sufficient mechanical structure support from nanofiber scaffolds while rendering a dynamic 3D internal network environment for the cells to settle. 215 Cell signal transmission between the nucleus and the ECM enables the attachment, proliferation, differentiation, and migration of the cells, as well as regulating apoptosis, growth‐factor release, and intracellular signal activation. 216
FIGURE 4.
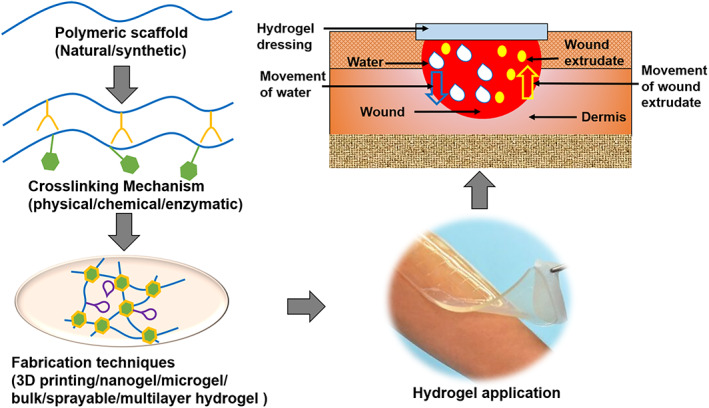
The nanofiber fabrication process for wound dressing manufacturing
Many synthetic and natural polymers have been trialed for producing biodegradable electrospun nanofibers. For example, collagen scaffolds are the most biomimetic substitutes for natural skin, as it promotes cell proliferation and enables cells to penetrate the ECM. 217 Compared with composite polymeric scaffolds, scaffolds produced from a single polymer cause a “fishnet effect” (i.e., high fiber density), which reduces cellular penetration. 218 In the comparison of natural and synthetic polymers, natural polymeric scaffolds are known to enhance cell adherence and growth, whereas synthetic polymers can render robust mechanical structures for the scaffolds, hence the reason for combining the two polymers. 214
Nanofibrous mats electrospun by PLGA/silk fibroin and loaded with zinc oxide nanoparticles demonstrated mild anti‐oxidant activity and showed activity against both gram‐positive and gram‐negative bacteria. Furthermore, an in vivo study revealed its potential for treating chronic wounds. 71 Hajikhani et al. (2021) proposed a coaxial PLA based electrospun nanofiber to treat infectious wounds. 72 They prepared a core solution with PLA/PEO and loaded it with cefazolin, PVP and collagen as the shell solution. This scaffold achieved tunable drug release and had sufficient antimicrobial properties. Moreover, a further in vivo study supported its ability to accelerate wound healing rates. 72 In another study, different molecular weights and content of PEG were added into PLA and electrospun into nanofibers, curcumin as the model drug was encapsulated. 219 The results showed that the lower molecular weight and higher content of PEG intensified drug release. Additionally, curcumin‐loaded scaffolds were found suitable as wound dressings. 219
7.2. Wearable wound monitors
Wearable wound monitors provide real‐time information related to the specific wound healing state, which can prevent time lag and chronic wound formation. It is important for clinicians to make appropriate treatment decisions based on feedback from the monitors. Sensors are the important components of wearable monitors. They trace the biological or chemical analytes and convert them into signals, such as optical and electrical signals, to deliver useful information to the clinician. 244 Many wearable sensors/systems have been developed by incorporating conventional wound dressings with sensors based on optical (e.g., fluorescence, colorimetry) and/or electrical (e.g., potentiometry, amperometry) mechanisms, to detect and convert these biomarkers for achieving real‐time monitoring of wound status. 245 The wearable wound monitor technology related to temperature and pH detection has been growing rapidly and consequently has improved wound diagnosis and treatment.
Temperature fluctuation is associated with wound infection and inflammation, as abnormal temperature changes can affect a series of chemical and enzymatic reactions during the wound healing process. A sudden temperature increase in the acute wound area is a signal of infection. It is caused by local vascular expansion, which promotes oxygen and nutrients to be transported to the injured site. It was reported that 2.2°C fluctuations in wound temperature may indicate approaching wound deterioration. 246
Normal and healthy skin pH is between 4 and 6. The slight acidity can prevent bacterial proliferation. Whereas, in the injured wound site, the pH is maintained at 7.4, which creates opportunities for bacteria to grow proliferate and may also trigger further wound infection. The pH of chronic wounds is between 7 and 9, which is more alkaline. It indicates that chronic wounds are more susceptible to bacterial colonization. 247 Therefore, temperature and pH are important parameters for assessing wound status, and their sensors have great potential to be used as efficient wound diagnosing methods.
A pH‐sensitive electrospun film was developed by incorporating curcumin into the PCL matrix. 248 Curcumin is a natural polyphenol with yellow color and it exhibits keto‐enol tautomerism. The keto form and enol form are stable in an acidic and alkaline environment respectively. Therefore, curcumin can show obvious chemical structure change with pH change. The results showed that the curcumin‐loaded fibrous mat changed color from yellow to red‐brown in a pH range of 6 to 9. 248 This wound dressing has the advantage that the color change can be easily detected without requiring medical training, however, it cannot quantify the wound pH value precisely. In another study, omniphobic paper‐based smart bandages were designed to monitor the pH of chronic wounds and the formation of pressure ulcers. 249 Ag/AgCl electrodes were printed on the omniphobic paper and a layer of Ag/polyaniline emeraldine base (PANi‐EB) mixture was used to separate the electrodes. Because PANi‐EB is sensitive to pH changes in nature, the measurement of the wound extrudate could be conducted with a low voltage of 100 mV. The results indicated that this device can be applied to qualify the pH range of 5.5 to 8.5 in the wound site. 249
A wearable flexible intelligent bandage was reported to be able to detect not only temperature, pH, but also can electronically release drugs to eliminate bacterial proliferation. 250 It was designed using a carbon‐based working electrode and counter electrode, and the reference electrode was Ag/AgCl. The temperatures were measured at a range of 25–45°C with a wireless patch‐embedded thermostat. The results showed that the temperature sensor had a precise accuracy, suggesting the use of the temperature sensor in the wound dressing to monitor wound healing status in real‐time. 250 A flexible electrochemical sensing bandage was developed to monitor temperature, pH, uric acid, as well as Na+, K+, Ca2+ at the wound site. Electrode substrates of Au, Ag, and Pt were selected, using magnetron sputtering technology to prepare the sensor. The results indicated that the sensor had a sensitivity of 0.16 Ω/°C in the temperature between 25 and 45°C and a sensitivity of 47.33 mV/pH in the pH between 4 and 10. 251 In another recent study, a three‐layer smart dressing was designed. The layers included a biomimetic nanofiber membrane, an electronic system, and a crosslinked hydrogel. In vivo rabbit study showed that the temperature at the local wound site was slightly high in the first 3 to 4 days, which may be attributed to the wound inflammation. During the remodeling phase, the wound temperature returned to a normal and stable plateau. 252
7.3. Negative pressure wound therapy
Negative pressure wound therapy (NPWT), also known as vacuum‐assisted closure, has received much attention in wound care for both open wounds and closed incisions. 253 NPWT is a powerful design for wounds that cannot undergo surgery. After facilitating wound cleanliness and helping to increase vascularity, NPWT can speed up the secondary intention healing. The wound healing mechanism of NPWT includes macro‐deformation, micro‐deformation, modulation of the wound environment, and fluid removal. 254 Macro‐deformation is a process that involves wound size‐reduction, achieved from the centripetal forces of the dressing and increased pressure on wound bed tissues Micro‐deformation indicates that the wound tissue interacts with dressings on a microscopic level. A porous foam dressing promotes cellular differentiation and makes inflammatory control possible. Lastly, NPWT regulates the wound environment by withdrawing fluid and maintaining moisture to reduce bacterial load.
An NPWT device is composed of a vacuum source, drainage tubing, and dressing (Figure 5). The dressing can seal the wound securely with the help of adhesive tape. In general, these devices range from home units for simple wound care, to complex machines for more severe acute wounds. They are also applied to complicated surgical wounds and are reported to be able to reduce the volume of the wound, which simplified the repair process. 255 In comparison with the conventional dressings that are usually changed 2 or 3 times each day, NPWT devices only require to be changed every 2 to 3 days, which can reduce the chances of cross‐contamination and save costs. Moreover, they can modulate the moisture of the wound environment, withdraw exudates, regulate blood flow, and apply pressure to facilitate wound closure. 255 These devices are capable of offering crucial factors that may lack in chronic wounds, and they were also reported to be associated with infection reduction in the wound sites. 254
FIGURE 5.
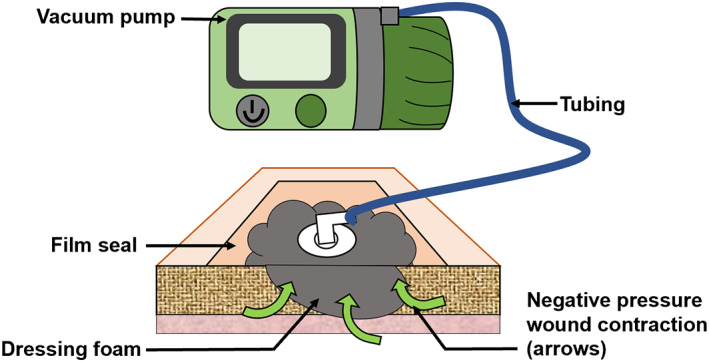
Illustration of negative pressure wound therapy
In a recent clinical study, NPWT was utilized along with other surgical treatments of debridement, maggot therapy, and silver foam dressing to treat patients with diabetic foot ulcers. After 1 month of hospitalization and five sessions of NPWT home treatment, the patient's conditions improved significantly. 256 It was suggested that the aforementioned treatment combination should be used to prevent the spread of the wound and control chronic infection.
One type of NPWT device was developed with the instillation of different solutions, where a substance was added drop by drop from saline to antibiotics, that would irrigate the wound sites. This relatively novel variation applied the technology of negative pressure and automated instillation of solutions with different volumes to saturate the dressing. Each process takes 10 to 20 min, repeats every 1.5 to 2 h, and the whole treatment may last 2 to 10 days. 253 NPWT instillation (NPWTi) allows the solution of desire to be instilled cyclically into the wound covered by NPWT dressing. Whereas, NPWT continuous irrigation (NPWTci) is not a cyclical process. It functions with continuous irrigation of solution into the wound bed while applying negative pressure suction at the same time. 22 It was reported that NPWTi may accelerate the rate of tissue granulation compared with the treatment results of NPWT. 257 Moreover, when antimicrobial solutions were utilized, the NPWTi dressing exhibited reduced bacterial load compared to normal standard dressings saturated with the equal antimicrobial solution, 22 which suggested the antimicrobial efficiency of NPWTi in wound therapy.
7.4. Surgical sutures
A surgical suture is another important wound healing device that can be manufactured in a way that can accelerate wound healing processes. Sutures are biomedical devices made of natural or synthetic polymers used for providing mechanical strength and holding tissues together in a surgical procedure. Even though suturing has a very long history applied in wound management, along with staples, tape and adhesive, sutures are still the most widely used medical devices in terms of surgery. 258 Recent studies on suture fabrication for wound healing have been summarized in Table 3.
TABLE 3.
Surgical sutures in wound management
| Polymers | Absorbability | Bioactive molecules | Process technique | Duration of release/degradation | Type of analysis done | Properties/results | References |
|---|---|---|---|---|---|---|---|
| PCL/collagen | Absorbable | bFGF | Electrospinning | Over 21 days (bFGF release) | Morphology analysis, mechanical testing, growth factor release and in vivo study. | bFGF loading rate reached 33.3%, and PCL/collagen nanofibers were highly aligned, which offered a high surface area to improve mechanical strength. | [102] |
| PDO | Non‐absorbable | Fibronectin | Dip‐coating | 24 h (fibronectin release) | Physicochemical testing, fibronectin release, in vitro cell culture and in vivo study. | Fibronectin‐coated suture reduced tissue drag, improved wound healing, and limited scar formation through enhanced re‐epithelialization. | [261] |
| DAC | Absorbable | — | Wet spinning | 42 days (suture degradation) | Physicochemical testing, mechanical testing and in vivo study. | DAC suture had tensile strength for conventional suturing and significantly reduced wound healing time on a full‐thickness wound model. | [273] |
| PLGA | — | mRNA | Dip‐coating | — | Physicochemical testing, mechanical testing, real‐time Quantitative PCR (qPCR,) in vitro cell culture and hemocompatibility testing. | mRNA‐PLGA coated suture did not affect cell viability and stimulated keratinocyte growth factor and functional fluorescent protein expression, which accelerated the wound healing process. | [274] |
| Nylon (braided) | Non‐absorbable | Rifampicin/ trans‐resveratrol | Coating | 5 weeks (drug release) | Morphology analysis, mechanical testing, in vitro drug release, antimicrobial, and anti‐inflammatory testing. | Drug coated sutures achieved drug release within 5 weeks and demonstrated excellent antimicrobial and anti‐inflammatory properties. | [262] |
| PCL (twisted) | Absorbable | Gentamicin/Ag | Electrospinning | Over 5 weeks (drug/silver release) | Morphology analysis, mechanical testing, in vitro drug/silver release, in vitro cell culture and antimicrobial testing | Drug/Ag‐coated PCL sutures had sustained release over 5 weeks after an initial burst and exhibited no influence on skin cell migration. Sutures inhibited bacterial growth rather than silver or drug alone loaded sutures. | [275] |
| AASF | Non‐absorbable | AMOX | Soaking | 336 h (AMOX release) | In vitro drug release, antibacterial test, haemolysis assay, in vivo animal model | O2 plasma‐treated AASF sutures had an increase in drug loading to 16.7% and showed bacterial inhibition to S aureus and E coli. Sustained AMOX release was observed within 336 hours after a 24 h burst release. The addition of O2 plasma treatment and AMOX did not affect the hemocompatibility of AASF suture but reduced wound healing time. | [276] |
| PP | Absorbable | Ag particles (3–5 wt%) | Grafting | — | Physicochemical testing, Morphology analysis, mechanical testing, antimicrobial test, cytocompatibility test | Radiation‐grafted and Ag‐loaded PP sutures demonstrated antimicrobial activity without adverse effects on cell viability. | [277] |
| Fibrin | Absorbable | hMSCs |
Solution coextrusion |
1 week (hMSCs delivery) | hMSC attachment quantification, In vivo animal model. | Fibrin biological sutures were developed to deliver cells to infarcted tissue. 100,000 cells per suture seeded for 12–24 h. The authors were not confident in using hMSCs for regenerating contractile cardiomyocytes. |
[278] |
| PP, PET, PDO, glycolic acid. | Non‐absorbable/ absorbable | Oxygen plasma | Coating | 60–90 days (suture degradation) | Morphology analysis, antimicrobial testing, tissue drag testing, mechanical testing, in vitro degradation, in vivo animal model. | Nanostructures were generated on the surface of commercial sutures via oxygen plasma treatment, which inhibited biofilm formation. | [279] |
| PCL | Absorbable | Tadalafil | Electrospinning | 15 days (tadalafil release) | Morphology analysis, mechanical testing, in vitro drug release, in vivo animal model | Tadalafil loaded PCL suture demonstrated a sustained release, with no toxic effect on the systemic circulation. The number of blood vessels, fibroblast and epithelization significantly improved, which in turn enhanced the wound healing process. | [280] |
| Polyglactin 910 | Absorbable | PDGF‐BB | Dip‐coating | 48 h (PDGF‐BB delivery) | In vitro PDGF‐BB release, in vivo animal model, in vivo uniaxial tensile biomechanical analysis |
PDGF‐BB coated commercial Vicryl sutures enhanced the remodeling phase in a rat model at 4 weeks following surgery. |
[281] |
| Polyglactin 910 | Absorbable | BMSC | Coating | — | In vivo animal model | BMSC coated commercial Vicryl sutures with stem cells attached to the surface, became unattached and migrated into the wound site to support soft tissue regeneration. | [282] |
| Polyglactin 910/PLGA/PEG | Absorbable | Diclofenac sodium salt | Dip‐coating | 7 days (diclofenac release) | Physicochemical testing, Morphology analysis, in vitro drug release, in vitro cell culture, In vivo evaluation of anti‐inflammatory effects. | Diclofenac loaded PLGA nanoparticles with PEG and coated them onto commercial Vicryl suture surface. Diclofenac was released sustainably and significantly reduced the inflammatory reactions of tissues around the suture in the rat. | [283] |
| PLLA (core)/PLGA (shell) | Absorbable | Aceclofenac (15%)/ insulin (4%) | Electrospinning |
10 days (aceclofenac release), 7 days (insulin release). 4 weeks (sheath degradation), 1 year (core degradation). |
Morphological analysis, Mechanical testing, in vitro degradation study, in vitro drug release, in vitro cell culture, in vivo animal model. | Aceclofenac loaded PLLA/PLGA core‐shell structural sutures were released within 10 days and reduced inflammation reactions in the animal model. Insulin loaded PLLA/PLGA core‐shell structural sutures were released within 7 days and accelerated the wound healing process. | [284] |
| Polyglactin 910 | Absorbable | Silver | Soaking | 21 days (silver release & suture degradation) | Physicochemical testing, Morphology analysis, in vitro degradation, in vitro silver release, antimicrobial test, in vitro cell culture, MTT assay, live/dead assay, scratch assay. | Silver coated polyglactin 910 sutures exhibited antibacterial activities and promoted cell migration and proliferation, which indicates its potential for facilitating wound healing. | [285] |
| Fibrin | Absorbable | hMSCs | Solution coextrusion |
1–2 weeks (suture degradation) |
In vitro live/dead cell viability assay, in vivo animal model, in vivo global mechanical function, in vivo cell delivery. | hMSCs incorporated fibrin biological sutures reduced fibrosis and enhanced mechanical properties. | [286] |
| PCL (core)/PEG–PLA (sheath) | Absorbable | Curcumin | Electrospinning | 160 h (curcumin release) | Morphological analysis, mechanical testing, in vitro curcumin release. | Core‐sheath structured sutures had more drug release when the fibers had finer cores. The drug release time frame was controlled by adjusting the parameters of the electrospun process. | [287] |
Abbreviations: AASF, Antheraea assama silk fibroin; Ag, silver; AMOX, amoxicillin trihydrate; BMSC, bone‐marrow‐derived mesenchymal stem cells; DAC, diacetyl chitin; hMSCs, human mesenchymal stem cells; mRNA, messenger ribonucleic acid; PCL, polycaprolactone; PDGF‐BB, platelet‐derived growth factor‐BB; PDO, polydioxanone; PEG, Polyethylene glycol; PET, poly(ethylene terephthalate); PLGA, poly(lactide‐co‐glycolide acid); PLLA, poly‐L‐lactic acid; PP, polypropylene.
According to the origin of the suture materials, sutures can be categorized as natural or synthetic sutures. Based on the degradability of sutures in the human body, sutures are again classified as absorbable and nonabsorbable sutures. The structures that sutures are composed of providing different mechanical properties, the different types of sutures include monofilament and multifilament (braided or twisted). A complex literature review based on suture types and materials, as well as their manufacturing techniques, has been published by Deng et al. 259
Despite the variety of suture materials and forms available, the main task of a suture is to provide adequate tensile strength to support wound healing until the tissue regains its strength. To fulfill this purpose, sutures are implanted in direct contact with the wound, which makes a suture an ideal device for local delivery of bioactive molecules to further promote wound healing. 259 It is particularly useful as surgical site infections (SSIs) are still a common phenomenon, which can cause prolonged pain in patients or lead to death in severe cases. 260 Sutures can also be a contamination source, which is particularly common in braided sutures, due to the increased surface to volume ratio. 259
Three dynamic phases that a wound healing process goes through (i.e., inflammation, proliferation, and remodeling) require the participation of fibroblasts. 261 At the late stage of inflammation and proliferation, fibrin clotting is inhibited by fibroblasts via the release of matrix metalloproteinase (MMP), followed by ECM compound formation. 11 After the ECM is fully restored, the newly formed tissue regains its strength and function. Most research on the delivery of bioactive molecules via surgical sutures has been focused on coating techniques. 262 , 263 In previous studies, suture surfaces bound with MMP inhibitor and coated with ECM compounds (e.g., collagen and laminin), resulted in the successful restoration of ECM organization. 264
However, one of the major drawbacks of the coating strategy is that the bioactive molecules are almost fully exposed to the surrounding tissues, which causes a rapid bolus release in the initial stage. A controlled release can be achieved by using a variety of carriers; however, the release profiles have been reported to still be within a relatively short period. 265 For instance, a study utilized fatty acid as the carrier to achieve the sustained release of antiseptic via braided sutures. The total release time only reached 100 h. 265 Another disadvantage of the coating method that is worth mentioning is that the number of bioactive molecules that can be successfully loaded on the suture surface is limited. Additionally, there is an added risk for the coating to peel off during handling due to weak binding. 103
For the sake of reducing or even eliminating bacterial attachment and colonization on the suture surface, while avoiding potential coating drawbacks, researchers have incorporated antimicrobial molecules (i.e., antibiotics, silver nanoparticles, antiseptic, and anti‐inflammatory drugs) to impart antimicrobial properties to the sutures (Figure 6), especially for braided or twisted sutures. Deng et al. (2021) recently developed a PEG/PCL/chitosan‐keratin based suture loaded with diclofenac potassium. 40 The suture products demonstrated high cellular viability and achieved a better wound healing rate compared with the sutures that did not contain the drug.
FIGURE 6.
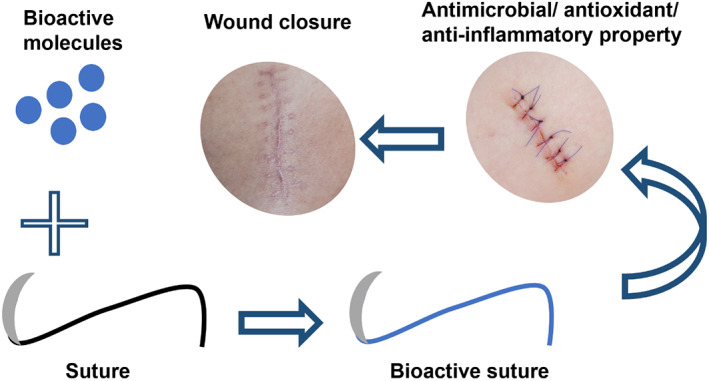
Bioactive molecule incorporated suture for wound management
In terms of reducing tissue drag in suture applications, fibronectin or synovial fluid has the potential to provide lubricating properties. 266 Fibronectin is an abundant ECM compound found in anionic proteoglycan‐rich extracellular regions. 267 In the later stage of the wound healing process, globular cellular fibronectins released by fibroblasts form a 3D fibrillar structure to modulate the composition of the ECM. It also regulates the deposition of ECM components (e.g., collagen types I and III, fibrinogen, and laminin). 268 Fibronectin has been reported to be successfully incorporated into the suture surfaces by applying polystyrene sulfonate and UV/ozone irradiation. This method renders negative charges on the suture surface to generate electrostatic interactions. 269 Moreover, this strategy can be widely applied in any type of suture with any structure or absorbability.
Clinical data indicated that suture materials left within the wound are the main source of wound infection. 270 Multifilament sutures even further facilitate bacterial attachment and allow the bacteria to penetrate the gaps between the threads, which further ascribes to the hindered immunological response of the host. Therefore, nonabsorbable monofilaments are preferred and should be removed within the biologically accepted time. Unless the bacteriostatic substance is coated or embedded on/into the absorbable sutures (e.g., Vicryl Plus is a commercial absorbable suture coated with triclosan). 270
Sutures have also been used to deliver GFs, stem cells, enzymes and other bioactive molecules. Casado et al. (2014) coated different absorbable/nonabsorbable sutures with mesenchymal stem cells. 271 The stem cell coated sutures enhanced collagen deposition and facilitated injured tissue regeneration. Centeno‐Cerdas et al. (2018) seeded photosynthetic genetically modified microalgae into commercially available sutures achieving controlled release of growth factors (VEGF, PDGF‐BB, or SDF‐1α) at the wound site. 272 This method has the potential to impart bioactive properties to the commercially available sutures, to further enhance their wound healing results.
It is well known that suturing is particularly necessary when mechanical stability is required for the wound. However, the friction that occurs between the suture and the tissue during surgery can cause secondary damage to soft tissues and lead to inflammation and infection at the surgical site, which can prolong the wound healing process. 259 To overcome this, lubricating a braided silk suture with chitosan not only reduced tissue drag, but the sliding speed of the sutures also improved. 259 Thus, in the terms of suture surface modification, the reduction of the dynamic coefficient of friction is one of the most important factors to consider, to minimize scar formation and enhance wound healing. 261
8. CONCLUSIONS AND FUTURE PERSPECTIVES
The main aim of this review was to highlight the various natural and synthetic polymers, bioactive molecules and drugs, as well as biomedical devices in wound healing management. Many polymers can be modified to mimic the skin in the form of scaffolds, which renders suitable conditions and structures to different wound types. Moreover, the incorporation of growth factors, stem cells, silver, active agents, and drugs can facilitate wound healing and further enhance wound treatment. Many biomedical devices have been developed to fulfill this purpose, such as nanofibrous scaffolds, hydrogels, wearable wound monitors, negative pressure wound therapy devices, and surgical sutures.
Wound healing is a complex ordered physiological process, which involves cell growth, re‐epithelialization, deposition of collagen fibers, and tissue regeneration. Therefore, selecting suitable polymers, bioactive compounds and wound dressings that can enhance and facilitate the wound healing process is crucial. Considering the different wound exudate production rates and the various wound surface shapes, there is no single wound dressing that can be effectively applied to all wound types. Thus, the future challenge is to develop a smart wound dressing that has antimicrobial, anti‐inflammatory, and antioxidant properties, most importantly, can positively affect almost all wound types. On the other hand, the combination of polymers and bioactive molecules has a significant effect on accelerating wound repair. The use of natural products for wound healing has been widely explored, but still only relatively few have been commercialized or used in clinical practice. Therefore, it is important to conduct more preclinical research on discovering the potential of natural bioactive molecules in skin tissue regeneration. Future studies need to be performed to discover new natural bioactive products and their facilitation in the wound healing process and their ability to act as alternatives to modern‐day antibiotics.
ACKNOWLEDGEMENT
Open access publishing facilitated by University of Otago, as part of the Wiley ‐ University of Otago agreement via the Council of Australian University Librarians.
Deng X, Gould M, Ali MA. A review of current advancements for wound healing: Biomaterial applications and medical devices. J Biomed Mater Res. 2022;110(11):2542‐2573. doi: 10.1002/jbm.b.35086
[Correction added on 20 July 2022, after first online publication: Funding statement has been added.]
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated
REFERENCES
- 1. Jahromi MAM, Zangabad PS, Basri SMM, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajendran NK, Kumar SSD, Houreld NN, Abrahamse H. A review on nanoparticle based treatment for wound healing. J Drug Delivery Sci Technol. 2018;44:421‐430. doi: 10.1016/j.jddst.2018.01.009 [DOI] [Google Scholar]
- 3. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4(3):119‐136. doi: 10.1089/wound.2013.0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linnerz T, Hall CJ. The diverse roles of phagocytes during bacterial and fungal infections and sterile inflammation: lessons from zebrafish. Front Immunol. 2020;11:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JW, Hwang SR, Yoon IS. Advanced growth factor delivery Systems in Wound Management and Skin Regeneration. Molecules. 2017;22(8):1259‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5(3):119‐136. doi: 10.1089/wound.2014.0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano‐drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnol. 2019;17(1):82. doi: 10.1186/s12951-019-0514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560‐582. doi: 10.1089/wound.2015.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci. 2015;104(11):3653‐3680. [DOI] [PubMed] [Google Scholar]
- 11. Naskar A, Kim KS. Recent advances in nanomaterial‐based wound‐healing therapeutics. Pharmaceutics. 2020;12(6):499‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sindrilaru A, Scharffetter‐Kochanek K. Disclosure of the culprits: macrophages‐versatile regulators of wound healing. Adv Wound Care. 2013;2(7):357‐368. doi: 10.1089/wound.2012.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro‐wound healing phenotypes. Front Physiol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mirza RE, Fang MM, Novak ML, et al. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J Pathol. 2015;236(4):433‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira MC, Tuma P Jr, Carvalho VF, Kamamoto F. Complex wounds. Clinics (Sao Paulo). 2006;61(6):571‐578. [DOI] [PubMed] [Google Scholar]
- 16. Holl J, Kowalewski C, Zimek Z, et al. Chronic diabetic wounds and their treatment with skin substitutes. Cell. 2021;10(3):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed R, Tariq M, Ali I, et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol. 2018;120:385‐393. doi: 10.1016/j.ijbiomac.2018.08.057 [DOI] [PubMed] [Google Scholar]
- 18. Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi‐professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8(1):23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ladhani HA, Yowler CJ, Claridge JA. Burn wound colonization, infection, and sepsis. Surg Infect (Larchmt). 2021;22(1):44‐48. [DOI] [PubMed] [Google Scholar]
- 20. Scalise A, Bianchi A, Tartaglione C, et al. Microenvironment and microbiology of skin wounds: the role of bacterial biofilms and related factors. Seminars in Vascular Surgery. Vol 28. Elsevier; 2015:151‐159. [DOI] [PubMed] [Google Scholar]
- 21. Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19(1):243. doi: 10.1186/s13054-015-0961-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3‐17. [DOI] [PubMed] [Google Scholar]
- 23. Gardner SE, Abbott LI, Fiala CA, Rakel BA. Factors associated with high pain intensity during wound care procedures: a model. Wound Repair Regen. 2017;25(4):558‐563. doi: 10.1111/wrr.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pham TL, Bazan HEP. Docosanoid signaling modulates corneal nerve regeneration: effect on tear secretion, wound healing, and neuropathic pain. J Lipid Res. 2021;62:100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13(Suppl 2):5‐15. doi: 10.1111/iwj.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fogh K, Andersen MB, Bischoff‐Mikkelsen M, et al. Clinically relevant pain relief with an ibuprofen‐releasing foam dressing: results from a randomized, controlled, double‐blind clinical trial in exuding, painful venous leg ulcers. Wound Repair Regen. 2012;20(6):815‐821. doi: 10.1111/j.1524-475X.2012.00844.x [DOI] [PubMed] [Google Scholar]
- 27. Arapoglou V, Katsenis K, Syrigos K, et al. Analgesic efficacy of an ibuprofenreleasing foam dressing compared with local best practice for painful exuding wounds. J Wound Care. 2011;20(7):319‐325. [DOI] [PubMed] [Google Scholar]
- 28. Makita‐Chingombe F, Kutscher HL, DiTursi SL, Morse GD, Maponga CC. Poly(lactic‐co‐glycolic) acid‐chitosan dual loaded nanoparticles for antiretroviral Nanoformulations. J Drug Delivery. 2016;2016:3810175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen J, Lee K, Choi S, Qu W, Wang Y, Burgess DJ. A reproducible accelerated in vitro release testing method for PLGA microspheres. Int J Pharm. 2016;498(1–2):274‐282. doi: 10.1016/j.ijpharm.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajabi M, McConnell M, Cabral J, Ali MA. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr Polym. 2021;260:117768. [DOI] [PubMed] [Google Scholar]
- 31. Badran MM, Alomrani AH, Harisa GI, Ashour AE, Kumar A, Yassin AE. Novel docetaxel chitosan‐coated PLGA/PCL nanoparticles with magnified cytotoxicity and bioavailability. Biomed Pharmacother. 2018;106:1461‐1468. doi: 10.1016/j.biopha.2018.07.102 [DOI] [PubMed] [Google Scholar]
- 32. Liu H, Wang CY, Li C, et al. A functional chitosan‐based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018;8(14):7533‐7549. doi: 10.1039/c7ra13510f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abbas M, Hussain T, Arshad M, et al. Wound healing potential of curcumin cross‐linked chitosan/polyvinyl alcohol. Int J Biol Macromol. 2019;140:871‐876. doi: 10.1016/j.ijbiomac.2019.08.153 [DOI] [PubMed] [Google Scholar]
- 34. Moeini A, Pedram P, Makvandi P, Malinconico M, Gomez d'Ayala G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan‐based wound dressings: a review. Carbohydr Polym. 2020;233:115839. [DOI] [PubMed] [Google Scholar]
- 35. Bagher Z, Ehterami A, Safdel MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Delivery Sci Technol. 2020;55:101379. [Google Scholar]
- 36. Rajabi M, Ali A, McConnell M, Cabral J. Keratinous materials: structures and functions in biomedical applications. Mater Sci Eng C. 2020;110:110612. [DOI] [PubMed] [Google Scholar]
- 37. Konop M, Rybka M, Drapała A. Keratin biomaterials in skin wound healing, an old player in modern medicine: a mini review. Pharmaceutics. 2021;13(12):2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Su S, Bedir T, Kalkandelen C, et al. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur Polym J. 2021;142:110158. [Google Scholar]
- 39. Islam MT, Laing RM, Wilson CA, McConnell M, Ali MA. Fabrication and characterization of 3‐dimensional electrospun poly (vinyl alcohol)/keratin/chitosan nanofibrous scaffold. Carbohydr Polym. 2022;275:118682. [DOI] [PubMed] [Google Scholar]
- 40. Deng X, Gould M, Ali MA. Fabrication and characterisation of melt‐extruded chitosan/keratin/PCL/PEG drug‐eluting sutures designed for wound healing. Mater Sci Eng C Mater Biol Appl. 2021;120:111696. [DOI] [PubMed] [Google Scholar]
- 41. Akhmetova A, Heinz A. Electrospinning proteins for wound healing purposes: opportunities and challenges. Pharmaceutics. 2020;13(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Biranje S, Madiwale P, Adivarekar R. Porous electrospun casein/PVA nanofibrous mat for its potential application as wound dressing material. J Porous Mater. 2019;26(1):29‐40. [Google Scholar]
- 43. Selvaraj S, Thangam R, Fathima NN. Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int J Biol Macromol. 2018;120:1674‐1681. [DOI] [PubMed] [Google Scholar]
- 44. Biranje SS, Sun J, Cheng L, et al. Development of cellulose nanofibril/casein‐based 3D composite hemostasis scaffold for potential wound‐healing application. ACS Appl Mater Interfaces. 2022;14:3792‐3808. [DOI] [PubMed] [Google Scholar]
- 45. Patwa R, Zandraa O, Capáková Z, Saha N, Sáha P. Effect of iron‐oxide nanoparticles impregnated bacterial cellulose on overall properties of alginate/casein hydrogels: potential injectable biomaterial for wound healing applications. Polymers. 2020;12(11):2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gaspar‐Pintiliescu A, Stanciuc A‐M, Craciunescu O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: a review. Int J Biol Macromol. 2019;138:854‐865. [DOI] [PubMed] [Google Scholar]
- 47. Li M, Han M, Sun Y, Hua Y, Chen G, Zhang L. Oligoarginine mediated collagen/chitosan gel composite for cutaneous wound healing. Int J Biol Macromol. 2019;122:1120‐1127. doi: 10.1016/j.ijbiomac.2018.09.061 [DOI] [PubMed] [Google Scholar]
- 48. Barrientos IJH, Paladino E, Szabo P, et al. Electrospun collagen‐based nanofibres: a sustainable material for improved antibiotic utilisation in tissue engineering applications. Int J Pharm. 2017;531(1):67‐79. doi: 10.1016/j.ijpharm.2017.08.071 [DOI] [PubMed] [Google Scholar]
- 49. Zhou T, Sui B, Mo X, Sun J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int J Nanomedicine. 2017;12:3495‐3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vigani B, Rossi S, Sandri G, Bonferoni MC, Caramella CM, Ferrari F. Hyaluronic acid and chitosan‐based nanosystems: a new dressing generation for wound care. Expert Opin Drug Deliv. 2019;16(7):715‐740. [DOI] [PubMed] [Google Scholar]
- 51. Catanzano O, D'Esposito V, Acierno S, et al. Alginate‐hyaluronan composite hydrogels accelerate wound healing process. Carbohydr Polym. 2015;131:407‐414. doi: 10.1016/j.carbpol.2015.05.081 [DOI] [PubMed] [Google Scholar]
- 52. Aduba DC, Yang H. Polysaccharide fabrication platforms and biocompatibility assessment as candidate wound dressing materials. Bioengineering. 2017;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. El‐Aassar MR, Ibrahim OM, Fouda MMG, El‐Beheri NG, Agwa MM. Wound healing of nanofiber comprising Polygalacturonic/hyaluronic acid embedded silver nanoparticles: in‐vitro and in‐vivo studies. Carbohydr Polym. 2020;238:116175. [DOI] [PubMed] [Google Scholar]
- 54. Eskandarinia A, Kefayat A, Gharakhloo M, et al. A propolis enriched polyurethane‐hyaluronic acid nanofibrous wound dressing with remarkable antibacterial and wound healing activities. Int J Biol Macromol. 2020;149:467‐476. doi: 10.1016/j.ijbiomac.2020.01.255 [DOI] [PubMed] [Google Scholar]
- 55. Zhang SH, Hou JY, Yuan QJ, et al. Arginine derivatives assist dopamine‐hyaluronic acid hybrid hydrogels to have enhanced antioxidant activity for wound healing. Chem Eng J. 2020;392:123775. [Google Scholar]
- 56. Zhao W, Li Y, Zhang X, et al. Photo‐responsive supramolecular hyaluronic acid hydrogels for accelerated wound healing. J Control Release. 2020;323:24‐35. doi: 10.1016/j.jconrel.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 57. De Silva RT, Mantilaka M, Goh KL, Ratnayake SP, Amaratunga GAJ, De Silva KMN. Magnesium oxide nanoparticles reinforced electrospun alginate‐based Nanofibrous scaffolds with improved physical properties. Int J Biomater. 2017;2017:1391298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goh CH, Heng PWS, Chan LW. Cross‐linker and non‐gelling Na+ effects on multi‐functional alginate dressings. Carbohydr Polym. 2012;87(2):1796‐1802. doi: 10.1016/j.carbpol.2011.09.097 [DOI] [Google Scholar]
- 59. Chen G, He L, Zhang P, et al. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater Sci Eng C Mater Biol Appl. 2020;110:110686. [DOI] [PubMed] [Google Scholar]
- 60. Bahadoran M, Shamloo A, Nokoorani YD. Development of a polyvinyl alcohol/sodium alginate hydrogel‐based scaffold incorporating bFGF‐encapsulated microspheres for accelerated wound healing. Sci Rep. 2020;10(1):7342. doi: 10.1038/s41598-020-64480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang YD, Lan XZ, Liang CF, et al. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr Polym. 2019;219:113‐120. doi: 10.1016/j.carbpol.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 62. Sogawa H, Ifuku N, Numata K. 3,4‐Dihydroxyphenylalanine (DOPA)‐containing silk fibroin: its enzymatic synthesis and adhesion properties. ACS Biomater Sci Eng. 2019;5(11):5644‐5651. doi: 10.1021/acsbiomaterials.8b01309 [DOI] [PubMed] [Google Scholar]
- 63. Farokhi M, Mottaghitalab F, Samani S, et al. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol Adv. 2018;36(1):68‐91. doi: 10.1016/j.biotechadv.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 64. Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A, Reis RL, Kaplan DL, Kundu SC. Silk fibroin for skin injury repair: where do things stand? Adv Drug Deliv Rev. 2020;153:28‐53. [DOI] [PubMed] [Google Scholar]
- 65. Hong H, Seo YB, Kim DY, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. [DOI] [PubMed] [Google Scholar]
- 66. Liu Q, Ying G, Jiang N, et al. Three‐dimensional silk fibroin microsphere‐nanofiber scaffolds for vascular tissue engineering. Med Novel Technol Devices. 2021;9:100051. [Google Scholar]
- 67. Tellado SF, Chiera S, Bonani W, et al. Heparin functionalization increases retention of TGF‐β2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament‐to‐bone tissue engineering. Acta Biomater. 2018;72:150‐166. [DOI] [PubMed] [Google Scholar]
- 68. Bondon N, Raehm L, Charnay C, Boukherroub R, Durand JO. Nanodiamonds for bioapplications, recent developments. J Mater Chem B. 2020;8(48):10878‐10896. [DOI] [PubMed] [Google Scholar]
- 69. Khalid A, Bai D, Abraham AN, et al. Electrospun Nanodiamond‐silk fibroin membranes: a multifunctional platform for biosensing and wound‐healing applications. ACS Appl Mater Interfaces. 2020;12(43):48408‐48419. [DOI] [PubMed] [Google Scholar]
- 70. Pacheco MS, Kano GE, Paulo LD, Lopes PS, De Moraes MA. Silk fibroin/chitosan/alginate multilayer membranes as a system for controlled drug release in wound healing. Int J Biol Macromol. 2020;152:803‐811. doi: 10.1016/j.ijbiomac.2020.02.140 [DOI] [PubMed] [Google Scholar]
- 71. Khan AUR, Huang K, Zhao JZ, et al. Exploration of the antibacterial and wound healing potential of a PLGA/silk fibroin based electrospun membrane loaded with zinc oxide nanoparticles. J Mater Chem B. 2021;9(5):1452‐1465. doi: 10.1039/d0tb02822c [DOI] [PubMed] [Google Scholar]
- 72. Hajikhani M, Emam‐Djomeh Z, Askari G. Fabrication and characterization of mucoadhesive bioplastic patch via coaxial polylactic acid (PLA) based electrospun nanofibers with antimicrobial and wound healing application. Int J Biol Macromol. 2021;172:143‐153. doi: 10.1016/j.ijbiomac.2021.01.051 [DOI] [PubMed] [Google Scholar]
- 73. Venkataprasanna KS, Prakash J, Vignesh S, et al. Fabrication of chitosan/PVA/GO/CuO patch for potential wound healing application. Int J Biol Macromol. 2020;143:744‐762. doi: 10.1016/j.ijbiomac.2019.10.029 [DOI] [PubMed] [Google Scholar]
- 74. Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan‐PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23‐36. doi: 10.1016/j.ijpharm.2019.01.019 [DOI] [PubMed] [Google Scholar]
- 75. Janouskova O. Synthetic polymer scaffolds for soft tissue engineering. Physiol Res. 2018;67(Suppl 2):S335‐S348. doi: 10.33549/physiolres.933983 [DOI] [PubMed] [Google Scholar]
- 76. Cui LL, Chen JY, Yan CQ, Xiong DS. Articular cartilage inspired the construction of LTi‐DA‐PVA composite structure with excellent surface wettability and low friction performance. Tribol Lett. 2021;69(2):1‐10. doi: 10.1007/s11249-021-01416-y [DOI] [Google Scholar]
- 77. Wu C, Or PW, Chong JIT. Controllable release of pirfenidone by polyvinyl alcohol film embedded soft contact lenses in vitro and in vivo. Drug Deliv. 2021;28(1):634‐641. doi: 10.1080/10717544.2021.1895911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pereira BC, Isreb A, Forbes RT, et al. 'Temporary Plasticiser': a novel solution to fabricate 3D printed patient‐centred cardiovascular 'Polypill' architectures. Eur J Pharm Biopharm. 2019;135:94‐103. doi: 10.1016/j.ejpb.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 79. Adeli H, Khorasani MT, Parvazinia M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int J Biol Macromol. 2019;122:238‐254. doi: 10.1016/j.ijbiomac.2018.10.115 [DOI] [PubMed] [Google Scholar]
- 80. Bakhsheshi‐Rad HR, Ismail AF, Aziz M, et al. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: in vitro and in vivo assessment. Int J Biol Macromol. 2020;149:513‐521. doi: 10.1016/j.ijbiomac.2020.01.139 [DOI] [PubMed] [Google Scholar]
- 81. Altaf F, Niazi MBK, Jahan Z, et al. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J Polym Environ. 2021;29(1):156‐174. [Google Scholar]
- 82. Kutikov AB, Song J. Biodegradable PEG‐based amphiphilic block copolymers for tissue engineering applications. ACS Biomater Sci Eng. 2015;1(7):463‐480. doi: 10.1021/acsbiomaterials.5b00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li L, Liu X, Niu Y, et al. Synthesis and wound healing of alternating block polyurethanes based on poly(lactic acid) (PLA) and poly(ethylene glycol) (PEG). J Biomed Mater Res B Appl Biomater. 2017;105(5):1200‐1209. doi: 10.1002/jbm.b.33670 [DOI] [PubMed] [Google Scholar]
- 84. Mochane MJ, Motsoeneng TS, Sadiku ER, Mokhena TC, Sefadi JS. Morphology and properties of electrospun PCL and its composites for medical applications: a mini review. Appl Sci. 2019;9(11):2205. [Google Scholar]
- 85. Croisier F, Atanasova G, Poumay Y, Jerome C. Polysaccharide‐coated PCL nanofibers for wound dressing applications. Adv Healthc Mater. 2014;3(12):2032‐2039. doi: 10.1002/adhm.201400380 [DOI] [PubMed] [Google Scholar]
- 86. Fahimirad S, Abtahi H, Satei P, Ghaznavi‐Rad E, Moslehi M, Ganji A. Wound healing performance of PCL/chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr Polym. 2021;259:117640. [DOI] [PubMed] [Google Scholar]
- 87. Ehterami A, Salehi M, Farzamfar S, et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin‐chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol. 2018;117:601‐609. [DOI] [PubMed] [Google Scholar]
- 88. Nofar M, Salehiyan R, Ray SS. Rheology of poly (lactic acid)‐based systems. Polym Rev. 2019;59(3):465‐509. [Google Scholar]
- 89. Scaffaro R, Lopresti F, Marino A, Nostro A. Antimicrobial additives for poly (lactic acid) materials and their applications: current state and perspectives. Appl Microbiol Biotechnol. 2018;102(18):7739‐7756. [DOI] [PubMed] [Google Scholar]
- 90. Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical applications. J Appl Microbiol. 2019;127(6):1612‐1626. doi: 10.1111/jam.14290 [DOI] [PubMed] [Google Scholar]
- 91. Sharifi M, Bahrami SH, Nejad NH, Milan PB. Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing. J Appl Polym Sci. 2020;137(46):49528. [Google Scholar]
- 92. Fang Y, Zhu X, Wang N, et al. Biodegradable core‐shell electrospun nanofibers based on PLA and γ‐PGA for wound healing. Eur Polym J. 2019;116:30‐37. [Google Scholar]
- 93. Valderrama MAM, Van Putten RJ, Gruter GJM. The potential of oxalic—and glycolic acid based polyesters (review). Towards CO2 as a feedstock (carbon capture and utilization ‐ CCU). Eur Polym J. 2019;119:445‐468. [Google Scholar]
- 94. Porporato PE, Payen VL, De Saedeleer CJ, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis. 2012;15(4):581‐592. doi: 10.1007/s10456-012-9282-0 [DOI] [PubMed] [Google Scholar]
- 95. Vazquez N, Sanchez‐Arevalo F, Maciel‐Cerda A, et al. Influence of the PLGA/gelatin ratio on the physical, chemical and biological properties of electrospun scaffolds for wound dressings. Biomed Mater. 2019;14(4):045006. [DOI] [PubMed] [Google Scholar]
- 96. Gao T, Tian C, Ma Z, Chu Z, Wang Z, Zhang P. Stem cell seeded and silver nanoparticles loaded bilayer PLGA/PVA dressings for wound healing. Macromol Biosci. 2020;20(10):e2000141. [DOI] [PubMed] [Google Scholar]
- 97. Kaiser P, Wächter J, Windbergs M. Therapy of infected wounds: overcoming clinical challenges by advanced drug delivery systems. Drug Deliv Transl Res. 2021;11(4):1545‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gámez‐Herrera E, García‐Salinas S, Salido S, et al. Drug‐eluting wound dressings having sustained release of antimicrobial compounds. Eur J Pharm Biopharm. 2020;152:327‐339. [DOI] [PubMed] [Google Scholar]
- 99. Saghazadeh S, Rinoldi C, Schot M, et al. Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev. 2018;127:138‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ray P, Singh S, Gupta S. Topical antimicrobial therapy: current status and challenges. Indian J Med Microbiol. 2019;37(3):299‐308. [DOI] [PubMed] [Google Scholar]
- 101. Llorens E, Calderon S, del Valle LJ, Puiggali J. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater Sci Eng C Mater Biol Appl. 2015;50:74‐84. doi: 10.1016/j.msec.2015.01.100 [DOI] [PubMed] [Google Scholar]
- 102. Hu J, Song Y, Zhang C, et al. Highly aligned electrospun collagen/Polycaprolactone surgical sutures with sustained release of growth factors for wound regeneration. ACS Appl Bio Mater. 2020;3(2):965‐976. doi: 10.1021/acsabm.9b01000 [DOI] [PubMed] [Google Scholar]
- 103. Li J, Linderman SW, Zhu C, Liu H, Thomopoulos S, Xia Y. Surgical sutures with porous sheaths for the sustained release of growth factors. Adv Mater. 2016;28(23):4620‐4624. doi: 10.1002/adma.201506242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials. 2019;12(16):2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ciloglu NS, Mert AI, Dogan Z, et al. Efficacy of silver‐loaded nanofiber dressings in Candida albicans‐contaminated full‐skin thickness rat burn wounds. J Burn Care Res. 2014;35(5):e317‐e320. doi: 10.1097/BCR.0b013e3182aa7143 [DOI] [PubMed] [Google Scholar]
- 106. Lin YH, Hsu WS, Chung WY, Ko TH, Lin JH. Evaluation of various silver‐containing dressing on infected excision wound healing study. J Mater Sci‐Mater Med. 2014;25(5):1375‐1386. doi: 10.1007/s10856-014-5152-1 [DOI] [PubMed] [Google Scholar]
- 107. Archana D, Singh BK, Dutta J, Dutta PK. Chitosan‐PVP‐nano silver oxide wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol. 2015;73:49‐57. doi: 10.1016/j.ijbiomac.2014.10.055 [DOI] [PubMed] [Google Scholar]
- 108. Catanzano O, Quaglia F, Boateng JS. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin Drug Deliv. 2021;18(6):737‐759. [DOI] [PubMed] [Google Scholar]
- 109. Bruggeman KF, Williams RJ, Nisbet DR. Dynamic and responsive growth factor delivery from electrospun and hydrogel tissue engineering materials. Adv Healthc Mater. 2018;7(1):1700836. [DOI] [PubMed] [Google Scholar]
- 110. Losi P, Briganti E, Errico C, et al. Fibrin‐based scaffold incorporating VEGF‐ and bFGF‐loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9(8):7814‐7821. doi: 10.1016/j.actbio.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 111. La WG, Yang HS. Heparin‐conjugated poly(lactic‐co‐glycolic acid) nanospheres enhance large‐wound healing by delivering growth factors in platelet‐rich plasma. Artif Organs. 2015;39(4):388‐394. doi: 10.1111/aor.12389 [DOI] [PubMed] [Google Scholar]
- 112. Joshi A, Xu Z, Ikegami Y, et al. Exploiting synergistic effect of externally loaded bFGF and endogenous growth factors for accelerated wound healing using heparin functionalized PCL/gelatin co‐spun nanofibrous patches. Chem Eng J. 2021;404:126518. [Google Scholar]
- 113. Augustine R, Hasan A, Dalvi YB, et al. Growth factor loaded in situ photocrosslinkable poly (3‐hydroxybutyrate‐co‐3‐hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater Sci Eng C. 2021;118:111519. [DOI] [PubMed] [Google Scholar]
- 114. Kim J, Lee K‐M, Han SH, et al. Development of stabilized dual growth factor‐loaded hyaluronate collagen dressing matrix. J Tissue Eng. 2021;12:2041731421999750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sarrigiannidis SO, Rey JM, Dobre O, González‐García C, Dalby MJ, Salmeron‐Sanchez M. A tough act to follow: collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater Today Bio. 2021;10:100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Feng P, Luo Y, Ke C, et al. Chitosan‐based functional materials for skin wound repair: mechanisms and applications. Front Bioeng Biotechnol. 2021;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee Y‐H, Hong Y‐L, Wu T‐L. Novel silver and nanoparticle‐encapsulated growth factor co‐loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C. 2021;118:111385. [DOI] [PubMed] [Google Scholar]
- 118. Asiri A, Saidin S, Sani MH, Al‐Ashwal RH. Epidermal and fibroblast growth factors incorporated polyvinyl alcohol electrospun nanofibers as biological dressing scaffold. Sci Rep. 2021;11(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mbese Z, Alven S, Aderibigbe BA. Collagen‐based nanofibers for skin regeneration and wound dressing applications. Polymers. 2021;13(24):4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kulkarni A, Diehl‐Jones W, Ghanbar S, Liu S. Layer‐by‐layer assembly of epidermal growth factors on polyurethane films for wound closure. J Biomater Appl. 2014;29(2):278‐290. doi: 10.1177/0885328214523058 [DOI] [PubMed] [Google Scholar]
- 121. Nurkesh A, Jaguparov A, Jimi S, Saparov A. Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front Cell Dev Biol. 2020;8:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lai HJ, Kuan CH, Wu HC, et al. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014;10(10):4156‐4166. doi: 10.1016/j.actbio.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 123. Shariati A, Moradabadi A, Azimi T, Ghaznavi‐Rad E. Wound healing properties and antimicrobial activity of platelet‐derived biomaterials. Sci Rep. 2020;10(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mori M, Rossi S, Ferrari F, et al. Sponge‐like dressings based on the Association of Chitosan and Sericin for the treatment of chronic skin ulcers. II. Loading of the Hemoderivative platelet lysate. J Pharm Sci. 2016;105(3):1188‐1195. doi: 10.1016/j.xphs.2015.11.043 [DOI] [PubMed] [Google Scholar]
- 125. Sandri G, Bonferoni MC, Rossi S, et al. Platelet lysate embedded scaffolds for skin regeneration. Expert Opin Drug Deliv. 2015;12(4):525‐545. doi: 10.1517/17425247.2015.961421 [DOI] [PubMed] [Google Scholar]
- 126. Mendes BB, Gomez‐Florit M, Araujo AC, et al. Intrinsically bioactive Cryogels based on platelet lysate nanocomposites for hemostasis applications. Biomacromolecules. 2020;21(9):3678‐3692. doi: 10.1021/acs.biomac.0c00787 [DOI] [PubMed] [Google Scholar]
- 127. Fortunato TM, Beltrami C, Emanueli C, De Bank PA, Pula G. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: tissue engineering implications. Sci Rep. 2016;6(1):25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang J, Crimmins D, Faed JM, Flanagan P, McGhee CNJ, Patel DV. Characteristics of platelet lysate compared to autologous and allogeneic serum eye drops. Transl Vis Sci Technol. 2020;9(4):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pezzotta S, Del Fante C, Scudeller L, et al. Long‐term safety and efficacy of autologous platelet lysate drops for treatment of ocular GvHD. Bone Marrow Transplant. 2017;52(1):101‐106. doi: 10.1038/bmt.2016.221 [DOI] [PubMed] [Google Scholar]
- 130. Rossi S, Mori M, Vigani B, et al. A novel dressing for the combined delivery of platelet lysate and vancomycin hydrochloride to chronic skin ulcers: hyaluronic acid particles in alginate matrices. Eur J Pharm Sci. 2018;118:87‐95. doi: 10.1016/j.ejps.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 131. Wang CG, Wang QQ, Gao WD, et al. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full‐thickness wound healing. Acta Biomater. 2018;69:156‐169. doi: 10.1016/j.actbio.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 132. Duscher D, Barrera J, Wong VW, et al. Stem cells in wound healing: the future of regenerative medicine? A Mini‐Review. Gerontology. 2016;62(2):216‐225. doi: 10.1159/000381877 [DOI] [PubMed] [Google Scholar]
- 133. Zhang JY, Chen CY, Hu B, et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 2016;12(12):1472‐1487. doi: 10.7150/ijbs.15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Li Y, Zhang J, Yue J, Gou X, Wu X. Epidermal stem cells in skin wound healing. Adv Wound Care. 2017;6(9):297‐307. doi: 10.1089/wound.2017.0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Golchin A, Farahany TZ, Khojasteh A, Soleimanifar F, Ardeshirylajimi A. The clinical trials of mesenchymal stem cell therapy in skin diseases: An update and concise review. Curr Stem Cell Res Ther. 2019;14(1):22‐33. doi: 10.2174/1574888X13666180913123424 [DOI] [PubMed] [Google Scholar]
- 136. Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017;64:176‐186. doi: 10.1016/j.actbio.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9(1):4457‐4486. doi: 10.1016/j.actbio.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 138. Miguez‐Pacheco V, Hench LL, Boccaccini AR. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1‐15. doi: 10.1016/j.actbio.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 139. Xue Y, Du Y, Yan J, et al. Monodisperse photoluminescent and highly biocompatible bioactive glass nanoparticles for controlled drug delivery and cell imaging. J Mater Chem B. 2015;3(18):3831‐3839. doi: 10.1039/c5tb00204d [DOI] [PubMed] [Google Scholar]
- 140. Gadelkarim M, Abushouk AI, Ghanem E, Hamaad AM, Saad AM, Abdel‐Daim MM. Adipose‐derived stem cells: effectiveness and advances in delivery in diabetic wound healing. Biomed Pharmacother. 2018;107:625‐633. doi: 10.1016/j.biopha.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 141. Li X, Xie X, Lian W, et al. Exosomes from adipose‐derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1‐14. doi: 10.1038/s12276-018-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Casado‐Diaz A, Quesada‐Gomez JM, Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol. 2020;8:146‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Motegi SI, Ishikawa O. Mesenchymal stem cells: the roles and functions in cutaneous wound healing and tumor growth. J Dermatol Sci. 2017;86(2):83‐89. doi: 10.1016/j.jdermsci.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 144. Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and limits of adipose‐derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020;21(4):1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lei Z, Singh G, Min Z, et al. Bone marrow‐derived mesenchymal stem cells laden novel thermo‐sensitive hydrogel for the management of severe skin wound healing. Mater Sci Eng C Mater Biol Appl. 2018;90:159‐167. doi: 10.1016/j.msec.2018.04.045 [DOI] [PubMed] [Google Scholar]
- 146. Shou K, Niu Y, Zheng X, et al. Enhancement of bone‐marrow‐derived mesenchymal stem cell Angiogenic capacity by NPWT for a combinatorial therapy to promote wound healing with large defect. Biomed Res Int. 2017;2017:7920265. doi: 10.1155/2017/7920265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yao M, Zhang J, Gao F, et al. New BMSC‐laden gelatin hydrogel formed in situ by dual‐enzymatic cross‐linking accelerates dermal wound healing. ACS Omega. 2019;4(5):8334‐8340. doi: 10.1021/acsomega.9b00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Guo JM, Hu HD, Gorecka J, et al. Adipose‐derived mesenchymal stem cells accelerate diabetic wound healing in a similar fashion as bone marrow‐derived cells. Am J Physiol‐Cell Physiol. 2018;315(6):C885‐C896. doi: 10.1152/ajpcell.00120.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mantha S, Pillai S, Khayambashi P, et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ibrahim NI, Wong SK, Mohamed IN, et al. Wound healing properties of selected natural products. Int J Environ Res Public Health. 2018;15(11):2360‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid Based Complement Alternat Med. 2019;2019:2684108. doi: 10.1155/2019/2684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int J Mol Sci. 2019;20(5):1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rujirachotiwat A, Suttamanatwong S. Curcumin upregulates transforming growth factor‐β1, its receptors, and vascular endothelial growth factor expressions in an in vitro human gingival fibroblast wound healing model. BMC Oral Health. 2021;21(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Alven S, Nqoro X, Aderibigbe BA. Polymer‐based materials loaded with curcumin for wound healing applications. Polymers. 2020;12(10):2286‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm des. 2013;19(11):2101‐2113. [PubMed] [Google Scholar]
- 156. Mohanty C, Das M, Sahoo SK. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol Pharm. 2012;9(10):2801‐2811. doi: 10.1021/mp300075u [DOI] [PubMed] [Google Scholar]
- 157. Nguyen TTT, Ghosh C, Hwang SG, Tran LD, Park JS. Characteristics of curcumin‐loaded poly (lactic acid) nanofibers for wound healing. J Mater Sci. 2013;48(20):7125‐7133. doi: 10.1007/s10853-013-7527-y [DOI] [Google Scholar]
- 158. Ranjbar‐Mohammadi M, Rabbani S, Bahrami SH, Joghataei M, Moayer F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε‐caprolactone) electrospun nanofibers. Mater Sci Eng C. 2016;69:1183‐1191. [DOI] [PubMed] [Google Scholar]
- 159. Zahiri M, Khanmohammadi M, Goodarzi A, et al. Encapsulation of curcumin loaded chitosan nanoparticle within poly (ε‐caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int J Biol Macromol. 2020;153:1241‐1250. [DOI] [PubMed] [Google Scholar]
- 160. Saikaly SK, Khachemoune A. Honey and wound healing: An update. Am J Clin Dermatol. 2017;18(2):237‐251. doi: 10.1007/s40257-016-0247-8 [DOI] [PubMed] [Google Scholar]
- 161. Carter DA, Blair SE, Cokcetin NN, et al. Therapeutic Manuka honey: no longer so alternative. Front Microbiol. 2016;7:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Minden‐Birkenmaier BA, Bowlin GL. Honey‐based templates in wound healing and tissue engineering. Bioengineering. 2018;5(2):46‐47. doi: 10.3390/bioengineering5020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hixon KR, Klein RC, Eberlin CT, et al. A critical review and perspective of honey in tissue engineering and clinical wound healing. Adv Wound Care. 2019;8(8):403‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Solayman M, Islam MA, Paul S, et al. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: a comprehensive review. Compr Rev Food Sci Food Saf. 2016;15(1):219‐233. doi: 10.1111/1541-4337.12182 [DOI] [PubMed] [Google Scholar]
- 165. Nezhad‐Mokhtari P, Javanbakht S, Asadi N, et al. Recent advances in honey‐based hydrogels for wound healing applications: towards natural therapeutics. J Drug Delivery Sci Technol. 2021;66:102789. [Google Scholar]
- 166. Proaño A, Coello D, Villacrés‐Granda I, et al. The osmotic action of sugar combined with hydrogen peroxide and bee‐derived antibacterial peptide Defensin‐1 is crucial for the antibiofilm activity of eucalyptus honey. LWT. 2021;136:110379. [Google Scholar]
- 167. Bucekova M, Buriova M, Pekarik L, Majtan V, Majtan J. Phytochemicals‐mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci Rep. 2018;8(1):1‐9. doi: 10.1038/s41598-018-27449-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Martinotti S, Ranzato E. Honey, wound repair and regenerative medicine. J Funct Biomater. 2018;9(2):34‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Frydman GH, Olaleye D, Annamalai D, et al. Manuka honey microneedles for enhanced wound healing and the prevention and/or treatment of methicillin‐resistant Staphylococcus aureus (MRSA) surgical site infection. Sci Rep. 2020;10(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N. Anti‐influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch Med Res. 2014;45(5):359‐365. doi: 10.1016/j.arcmed.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 171. Cooke J, Dryden M, Patton T, Brennan J, Barrett J. The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Res Notes. 2015;8(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Garcia‐Orue I, Gainza G, Garcia‐Garcia P, et al. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int J Pharm. 2019;556:320‐329. doi: 10.1016/j.ijpharm.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 173. Hashemi SA, Madani SA, Abediankenari S. The review on properties of aloe Vera in healing of cutaneous wounds. Biomed Res Int. 2015;2015:714216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Liang J, Cui L, Li J, Guan S, Zhang K, Li J. Aloe vera: a medicinal plant used in skin wound healing. Tissue Eng Part B Rev. 2021;27(5):455‐474. [DOI] [PubMed] [Google Scholar]
- 175. Tarameshloo M, Norouzian M, Zarein‐Dolab S, Dadpay M, Mohsenifar J, Gazor R. Aloe vera gel and thyroid hormone cream may improve wound healing in Wistar rats. Anat Cell Biol. 2012;45(3):170‐177. doi: 10.5115/acb.2012.45.3.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Karuppuswamy P, Venugopal JR, Navaneethan B, Laiva AL, Sridhar S, Ramakrishna S. Functionalized hybrid nanofibers to mimic native ECM for tissue engineering applications. Appl Surf Sci. 2014;322:162‐168. doi: 10.1016/j.apsusc.2014.10.074 [DOI] [Google Scholar]
- 177. Suganya S, Venugopal J, Ramakrishna S, Lakshmi BS, Dev VR. Naturally derived biofunctional nanofibrous scaffold for skin tissue regeneration. Int J Biol Macromol. 2014;68:135‐143. doi: 10.1016/j.ijbiomac.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 178. Kheradvar SA, Nourmohammadi J, Tabesh H, Bagheri B. Starch nanoparticle as a vitamin E‐TPGS carrier loaded in silk fibroin‐poly(vinyl alcohol)‐Aloe vera nanofibrous dressing. Colloids Surf B‐Biointerfaces. 2018;166:9‐16. doi: 10.1016/j.colsurfb.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 179. Garcia‐Orue I, Gainza G, Gutierrez FB, et al. Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm. 2017;523(2):556‐566. doi: 10.1016/j.ijpharm.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 180. Abdel‐Mohsen AM, Frankova J, Abdel‐Rahman RM, et al. Chitosan‐glucan complex hollow fibers reinforced collagen wound dressing embedded with aloe vera. II. Multifunctional properties to promote cutaneous wound healing. Int J Pharm. 2020;582:119349. [DOI] [PubMed] [Google Scholar]
- 181. García‐Salinas S, Evangelopoulos M, Gámez‐Herrera E, et al. Electrospun anti‐inflammatory patch loaded with essential oils for wound healing. Int J Pharm. 2020;577:119067. [DOI] [PubMed] [Google Scholar]
- 182. Zhao X, Pei D, Yang Y, et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18):2009442. [Google Scholar]
- 183. Tedesco S, Fevereiro P, Kragler F, Pina A. Plant grafting and graft incompatibility: a review from the grapevine perspective. Sci Hortic. 2022;299:111019. [Google Scholar]
- 184. Park SY, Lee HU, Lee YC, et al. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater Sci Eng C Mater Biol Appl. 2014;42:757‐762. doi: 10.1016/j.msec.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 185. Pérez‐Recalde M, Arias IER, Hermida ÉB. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine. 2018;38:57‐65. [DOI] [PubMed] [Google Scholar]
- 186. Ty‐Kisera M. Acupressure with Essential Oils: A Self‐Care Guide to Enhance your Health and Lift your Spirit—Includes 24 Common Conditions. North Atlantic Books; 2019. [Google Scholar]
- 187. Manion CR, Widder RM. Essentials of essential oils. Am J Health Syst Pharm. 2017;74(9):e153‐e162. doi: 10.2146/ajhp151043 [DOI] [PubMed] [Google Scholar]
- 188. Semeniuc CA, Pop CR, Rotar AM. Antibacterial activity and interactions of plant essential oil combinations against gram‐positive and gram‐negative bacteria. J Food Drug Anal. 2017;25(2):403‐408. doi: 10.1016/j.jfda.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Zhang WW, Huang C, Kusmartseva O, Thomas NL, Mele E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React Funct Polym. 2017;117:106‐111. doi: 10.1016/j.reactfunctpolym.2017.06.013 [DOI] [Google Scholar]
- 190. Das S, Baker AB. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol. 2016;4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Harish V, Li Z, Maitz PK. The optimal timing of outpatient biobrane™ application for superficial and mid dermal partial thickness burns: evidence for the '12‐hour rule'. Burns. 2019;45(4):936‐941. [DOI] [PubMed] [Google Scholar]
- 192. Kalantari K, Mostafavi E, Saleh B, Soltantabar P, Webster TJ. Chitosan/PVA hydrogels incorporated with green synthesized cerium oxide nanoparticles for wound healing applications. Eur Polym J. 2020;134:109853. [Google Scholar]
- 193. Liang Y, Chen B, Li M, He J, Yin Z, Guo B. Injectable antimicrobial conductive hydrogels for wound disinfection and infectious wound healing. Biomacromolecules. 2020;21(5):1841‐1852. [DOI] [PubMed] [Google Scholar]
- 194. Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14(7):737‐744. doi: 10.1038/nmat4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Dhivya S, Padma VV, Santhini E. Wound dressings—a review. Biomedicine. 2015;5(4):24‐28. doi: 10.7603/s40681-015-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shah SA, Sohail M, Khan S, et al. Biopolymer‐based biomaterials for accelerated diabetic wound healing: a critical review. Int J Biol Macromol. 2019;139:975‐993. doi: 10.1016/j.ijbiomac.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 197. Chen GP, Yu YR, Wu XW, Wang GF, Ren JN, Zhao YJ. Bioinspired multifunctional hybrid hydrogel promotes wound healing. Adv Funct Mater. 2018;28(33):1801386. [Google Scholar]
- 198. Wang C, Wang M, Xu T, et al. Engineering bioactive self‐healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65‐76. doi: 10.7150/thno.29766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Annabi N, Rana D, Shirzaei Sani E, et al. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials. 2017;139:229‐243. doi: 10.1016/j.biomaterials.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kim JW, Kim MJ, Ki CS, Kim HJ, Park YH. Fabrication of bi‐layer scaffold of keratin nanofiber and gelatin‐methacrylate hydrogel: implications for skin graft. Int J Biol Macromol. 2017;105(Pt 1):541‐548. doi: 10.1016/j.ijbiomac.2017.07.067 [DOI] [PubMed] [Google Scholar]
- 201. Diniz FR, Maia R, Rannier L, et al. Silver nanoparticles‐composing alginate/Gelatine hydrogel improves wound healing in vivo. Nanomaterials. 2020;10(2):390‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mahmoud NN, Hikmat S, Abu Ghith D, et al. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: effect of nanoparticles' shape and surface modification. Int J Pharm. 2019;565:174‐186. doi: 10.1016/j.ijpharm.2019.04.079 [DOI] [PubMed] [Google Scholar]
- 203. Zheng Z, Bian S, Li Z, et al. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo‐sensitive hydrogel for wound healing. Carbohydr Polym. 2020;249:116826. [DOI] [PubMed] [Google Scholar]
- 204. Nguyen TD, Nguyen TT, Ly KL, et al. In vivo study of the antibacterial chitosan/polyvinyl alcohol loaded with silver nanoparticle hydrogel for wound healing applications. Int J Polym Sci. 2019;2019:7382717. doi: 10.1155/2019/7382717 [DOI] [Google Scholar]
- 205. Jangde R, Srivastava S, Singh MR, Singh D. In vitro and in vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int J Biol Macromol. 2018;115:1211‐1217. doi: 10.1016/j.ijbiomac.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 206. Xu K, An N, Zhang H, et al. Sustained‐release of PDGF from PLGA microsphere embedded thermo‐sensitive hydrogel promoting wound healing by inhibiting autophagy. J Drug Delivery Sci Technol. 2020;55:101405. [Google Scholar]
- 207. Li M, Liang YP, He JH, Zhang HL, Guo BL. Two‐pronged strategy of biomechanically active and biochemically multifunctional hydrogel wound dressing to accelerate wound closure and wound healing. Chem Mater. 2020;32(23):9937‐9953. doi: 10.1021/acs.chemmater.0c02823 [DOI] [Google Scholar]
- 208. Mao CY, Xiang YM, Liu XM, et al. Photo‐inspired antibacterial activity and wound healing acceleration by hydrogel embedded with ag/ag@AgCl/ZnO nanostructures. ACS Nano. 2017;11(9):9010‐9021. doi: 10.1021/acsnano.7b03513 [DOI] [PubMed] [Google Scholar]
- 209. Park HH, Ko SC, Oh GW, et al. Fabrication and characterization of phlorotannins/poly (vinyl alcohol) hydrogel for wound healing application. J Biomater Sci Polym Ed. 2018;29(7–9):972‐983. doi: 10.1080/09205063.2017.1374030 [DOI] [PubMed] [Google Scholar]
- 210. Gao L, Zhou YL, Peng JL, et al. A novel dual‐adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 2019;11(1):1‐11. doi: 10.1038/s41427-019-0168-0 [DOI] [Google Scholar]
- 211. Zhang S, Ou Q, Xin P, Yuan Q, Wang Y, Wu J. Polydopamine/puerarin nanoparticle‐incorporated hybrid hydrogels for enhanced wound healing. Biomater Sci. 2019;7(10):4230‐4236. doi: 10.1039/c9bm00991d [DOI] [PubMed] [Google Scholar]
- 212. Gao C, Zhang L, Wang J, et al. Electrospun nanofibers promote wound healing: theories, techniques, and perspectives. J Mater Chem B. 2021;9(14):3106‐3130. [DOI] [PubMed] [Google Scholar]
- 213. Chen S, Liu B, Carlson MA, Gombart AF, Reilly DA, Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine (Lond). 2017;12(11):1335‐1352. doi: 10.2217/nnm-2017-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Liu M, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Mater Sci Eng C Mater Biol Appl. 2017;76:1413‐1423. doi: 10.1016/j.msec.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 215. Ambekar RS, Kandasubramanian B. Advancements in nanofibers for wound dressing: a review. Eur Polym J. 2019;117:304‐336. doi: 10.1016/j.eurpolymj.2019.05.020 [DOI] [Google Scholar]
- 216. Memic A, Abudula T, Mohammed HS, Joshi Navare K, Colombani T, Bencherif SA. Latest Progress in electrospun nanofibers for wound healing applications. ACS Appl Bio Mater. 2019;2(3):952‐969. doi: 10.1021/acsabm.8b00637 [DOI] [PubMed] [Google Scholar]
- 217. Law JX, Liau LL, Saim A, Yang Y, Idrus R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng Regener Med. 2017;14(6):699‐718. doi: 10.1007/s13770-017-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Mim LM, Sultana N, Hasbullah H, Aziz M. Nanofiber electrospun membrane based on biodegradable polymers for biomedical and tissue engineering application. In: Ismail AF, Hilal N, Jaafar J, Wright CJ, Eds. Nanofiber Membranes for Medical, Environmental, Energy Applications. 1st ed. CRC Press, Boca Raton; 2019;37‐38. [Google Scholar]
- 219. Moradkhannejhad L, Abdouss M, Nikfarjam N, Shahriari MH, Heidary V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J Drug Delivery Sci Technol. 2020;56:101554. [Google Scholar]
- 220. Khaledian S, Kahrizi D, Balaky STJ, Arkan E, Abdoli M, Martinez F. Electrospun nanofiber patch based on gum tragacanth/polyvinyl alcohol/molybdenum disulfide composite for tetracycline delivery and their inhibitory effect on gram plus and gram‐ bacteria. J Mol Liq. 2021;334:115989. [Google Scholar]
- 221. Selvaraj S, Fathima NN. Fenugreek incorporated silk fibroin nanofibers‐a potential antioxidant scaffold for enhanced wound healing. ACS Appl Mater Interfaces. 2017;9(7):5916‐5926. doi: 10.1021/acsami.6b16306 [DOI] [PubMed] [Google Scholar]
- 222. Xi Y, Ge J, Guo Y, Lei B, Ma PX. Biomimetic elastomeric polypeptide‐based Nanofibrous matrix for overcoming multidrug‐resistant bacteria and enhancing full‐thickness wound healing/skin regeneration. ACS Nano. 2018;12(11):10772‐10784. doi: 10.1021/acsnano.8b01152 [DOI] [PubMed] [Google Scholar]
- 223. Sun L, Gao W, Fu X, et al. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater Sci. 2018;6(2):340‐349. doi: 10.1039/c7bm00545h [DOI] [PubMed] [Google Scholar]
- 224. Gao W, Jin W, Li Y, et al. A highly bioactive bone extracellular matrix‐biomimetic nanofibrous system with rapid angiogenesis promotes diabetic wound healing. J Mater Chem B. 2017;5(35):7285‐7296. doi: 10.1039/c7tb01484h [DOI] [PubMed] [Google Scholar]
- 225. Dai XY, Liu J, Zheng HY, et al. Nano‐formulated curcumin accelerates acute wound healing through Dkk‐1‐mediated fibroblast mobilization and MCP‐1‐mediated anti‐inflammation. NPG Asia Mater. 2017;9(3):e368. doi: 10.1038/am.2017.31 [DOI] [Google Scholar]
- 226. Samadian H, Salehi M, Farzamfar S, et al. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif Cells Nanomed Biotechnol. 2018;46(1):964‐974. [DOI] [PubMed] [Google Scholar]
- 227. Kandhasamy S, Perumal S, Madhan B, et al. Synthesis and fabrication of collagen‐coated Ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl Mater Interfaces. 2017;9(10):8556‐8568. doi: 10.1021/acsami.6b16488 [DOI] [PubMed] [Google Scholar]
- 228. Kim JH, Jang J, Jeong YH, Ko TJ, Cho DW. Fabrication of a nanofibrous mat with a human skin pattern. Langmuir. 2015;31(1):424‐431. doi: 10.1021/la503064r [DOI] [PubMed] [Google Scholar]
- 229. Rieger KA, Thyagarajan R, Hoen ME, Yeung HF, Ford DM, Schiffman JD. Transport of microorganisms into cellulose nanofiber mats. RSC Adv. 2016;6(29):24438‐24445. doi: 10.1039/c6ra01394e [DOI] [Google Scholar]
- 230. Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, Thomas S. Electrospun poly(epsilon‐caprolactone)‐based skin substitutes: in vivo evaluation of wound healing and the mechanism of cell proliferation. J Biomed Mater Res B Appl Biomater. 2015;103(7):1445‐1454. doi: 10.1002/jbm.b.33325 [DOI] [PubMed] [Google Scholar]
- 231. Ramalingam R, Dhand C, Mayandi V, et al. Core‐Shell structured antimicrobial nanofiber dressings containing herbal extract and antibiotics combination for the prevention of biofilms and promotion of cutaneous wound healing. ACS Appl Mater Interfaces. 2021;13(21):24356‐24369. doi: 10.1021/acsami.0c20642 [DOI] [PubMed] [Google Scholar]
- 232. Li HJ, Zhu JX, Chen S, Jia L, Ma YL. Fabrication of aqueous‐based dual drug loaded silk fibroin electrospun nanofibers embedded with curcumin‐loaded RSF nanospheres for drugs controlled release. RSC Adv. 2017;7(89):56550‐56558. doi: 10.1039/c7ra12394a [DOI] [Google Scholar]
- 233. Ranjbar‐Mohammadi M, Zamani M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2016;58:521‐531. doi: 10.1016/j.msec.2015.08.066 [DOI] [PubMed] [Google Scholar]
- 234. Gao W, Sun L, Fu X, et al. Enhanced diabetic wound healing by electrospun core‐sheath fibers loaded with dimethyloxalylglycine. J Mater Chem B. 2018;6(2):277‐288. doi: 10.1039/c7tb02342a [DOI] [PubMed] [Google Scholar]
- 235. Ren X, Han Y, Wang J, et al. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018;70:140‐153. doi: 10.1016/j.actbio.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 236. Kabay G, Meydan AE, Kaleli Can G, Demirci C, Mutlu M. Controlled release of a hydrophilic drug from electrospun amyloid‐like protein blend nanofibers. Mater Sci Eng C Mater Biol Appl. 2017;81:271‐279. doi: 10.1016/j.msec.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 237. He M, Jiang H, Wang R, Xie Y, Zhao C. Fabrication of metronidazole loaded poly (ε‐caprolactone)/zein core/shell nanofiber membranes via coaxial electrospinning for guided tissue regeneration. J Colloid Interface Sci. 2017;490:270‐278. [DOI] [PubMed] [Google Scholar]
- 238. Li R, Cheng Z, Wen R, et al. Novel SA@ ca 2+/RCSPs core–shell structure nanofibers by electrospinning for wound dressings. RSC Adv. 2018;8(28):15558‐15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Datta S, Rameshbabu AP, Bankoti K, et al. Oleoyl‐chitosan‐based nanofiber Mats impregnated with amniotic membrane derived stem cells for accelerated full‐thickness excisional wound healing. ACS Biomater Sci Eng. 2017;3(8):1738‐1749. [DOI] [PubMed] [Google Scholar]
- 240. Anjum F, Agabalyan NA, Sparks HD, Rosin NL, Kallos MS, Biernaskie J. Biocomposite nanofiber matrices to support ECM remodeling by human dermal progenitors and enhanced wound closure. Sci Rep. 2017;7(1):10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Aydogdu MO, Altun E, Crabbe‐Mann M, et al. Cellular interactions with bacterial cellulose: Polycaprolactone nanofibrous scaffolds produced by a portable electrohydrodynamic gun for point‐of‐need wound dressing. Int Wound J. 2018;15(5):789‐797. doi: 10.1111/iwj.12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Han D, Sherman S, Filocamo S, Steckl AJ. Long‐term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017;53:242‐249. [DOI] [PubMed] [Google Scholar]
- 243. Khalf A, Madihally SV. Modeling the permeability of multiaxial electrospun poly(epsilon‐caprolactone)‐gelatin hybrid fibers for controlled doxycycline release. Mater Sci Eng C Mater Biol Appl. 2017;76:161‐170. doi: 10.1016/j.msec.2017.03.093 [DOI] [PubMed] [Google Scholar]
- 244. Gianino E, Miller C, Gilmore J. Smart wound dressings for diabetic chronic wounds. Bioengineering. 2018;5(3):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Tang N, Zheng Y, Jiang X, et al. Wearable sensors and systems for wound healing‐related ph and temperature detection. Micromachines. 2021;12(4):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Wijlens AM, Holloway S, Bus SA, Van Netten JJ. An explorative study on the validity of various definitions of a 22 C temperature threshold as warning signal for impending diabetic foot ulceration. Int Wound J. 2017;14(6):1346‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Qin M, Guo H, Dai Z, Yan X, Ning X. Advances in flexible and wearable pH sensors for wound healing monitoring. J Semicond. 2019;40(11):111607. [Google Scholar]
- 248. Pan N, Qin J, Feng P, Li Z, Song B. Color‐changing smart fibrous materials for naked eye real‐time monitoring of wound pH. J Mater Chem B. 2019;7(16):2626‐2633. [DOI] [PubMed] [Google Scholar]
- 249. Pal A, Goswami D, Cuellar HE, Castro B, Kuang S, Martinez RV. Early detection and monitoring of chronic wounds using low‐cost, omniphobic paper‐based smart bandages. Biosens Bioelectron. 2018;117:696‐705. [DOI] [PubMed] [Google Scholar]
- 250. Xu G, Lu Y, Cheng C, et al. Battery‐free and wireless smart wound dressing for wound infection monitoring and electrically controlled on‐demand drug delivery. Adv Funct Mater. 2021;31(26):2100852. [Google Scholar]
- 251. Liu Z, Liu J, Sun T, et al. Integrated multiplex sensing bandage for in situ monitoring of early infected wounds. ACS Sens. 2021;6(8):3112‐3124. [DOI] [PubMed] [Google Scholar]
- 252. Zhang Y, Li T, Zhao C, et al. An integrated smart sensor dressing for real‐time wound microenvironment monitoring and promoting angiogenesis and wound healing. Front Cell Dev Biol. 2021;9:2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Anghel EL, Kim PJ. Negative‐pressure wound therapy: a comprehensive review of the evidence. Plast Reconstr Surg. 2016;138(3S):129S‐137S. [DOI] [PubMed] [Google Scholar]
- 254. Norman G, Goh EL, Dumville JC, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2020;6:CD009261. doi: 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Parizad N, Hajimohammadi K, Goli R. Surgical debridement, maggot therapy, negative pressure wound therapy, and silver foam dressing revive hope for patients with diabetic foot ulcer: a case report. Int J Surg Case Rep. 2021;82:105931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Yang C, Goss SG, Alcantara S, Schultz G, LI JC. Effect of negative pressure wound therapy with instillation on bioburden in chronically infected wounds. Wounds: Compend Clin Res Pract. 2017;29(8):240‐246. [PubMed] [Google Scholar]
- 258. Pillai CK, Sharma CP. Review paper: absorbable polymeric surgical sutures: chemistry, production, properties, biodegradability, and performance. J Biomater Appl. 2010;25(4):291‐366. doi: 10.1177/0885328210384890 [DOI] [PubMed] [Google Scholar]
- 259. Deng X, Qasim M, Ali A. Engineering and polymeric composition of drug‐eluting suture: a review. J Biomed Mater Res A. 2021;109(10):2065‐2081. doi: 10.1002/jbm.a.37194 [DOI] [PubMed] [Google Scholar]
- 260. Wu X, Kubilay NZ, Ren J, et al. Antimicrobial‐coated sutures to decrease surgical site infections: a systematic review and meta‐analysis. Eur J Clin Microbiol Infect Dis. 2017;36(1):19‐32. doi: 10.1007/s10096-016-2765-y [DOI] [PubMed] [Google Scholar]
- 261. Setiawati A, Jang D, Cho D, et al. An accelerated wound‐healing surgical suture engineered with an extracellular matrix. Adv Healthc Mater. 2021;10(6):e2001686. [DOI] [PubMed] [Google Scholar]
- 262. Haley RM, Qian VR, Learn GD, von Recum HA. Use of affinity allows anti‐inflammatory and anti‐microbial dual release that matches suture wound resolution. J Biomed Mater Res A. 2019;107(7):1434‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Baygar T. Characterization of silk sutures coated with propolis and biogenic silver nanoparticles (AgNPs); an eco‐friendly solution with wound healing potential against surgical site infections (SSIs). Turk J Med Sci. 2020;50(1):258‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Camenzind RS, Tondelli TO, Götschi T, Holenstein C, Snedeker JG. Can genipin‐coated sutures deliver a collagen crosslinking agent to improve suture pullout in degenerated tendon? An ex vivo animal study. Clin Orthop Relat Res. 2018;476(5):1104‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Obermeier A, Schneider J, Wehner S, et al. Novel high efficient coatings for anti‐microbial surgical sutures using chlorhexidine in fatty acid slow‐release carrier systems. PLoS One. 2014;9(7):e101426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Andresen Eguiluz RC, Cook SG, Tan M, et al. Synergistic interactions of a synthetic Lubricin‐mimetic with fibronectin for enhanced Wear protection. Front Bioeng Biotechnol. 2017;5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J. 2015;12(3):313‐316. doi: 10.1111/iwj.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re‐epithelialization. Matrix Biol. 2019;75:12‐26. [DOI] [PubMed] [Google Scholar]
- 269. Ahn S, Deravi LF, Park SJ, et al. Self‐organizing large‐scale extracellular‐matrix protein networks. Adv Mater. 2015;27(18):2838‐2845. doi: 10.1002/adma.201405556 [DOI] [PubMed] [Google Scholar]
- 270. Burkhardt R, Lang NP. Influence of suturing on wound healing. Periodontol 2000. 2015;68(1):270‐281. doi: 10.1111/prd.12078 [DOI] [PubMed] [Google Scholar]
- 271. Casado JG, Blazquez R, Jorge I, et al. Mesenchymal stem cell‐coated sutures enhance collagen depositions in sutured tissues. Wound Repair Regen. 2014;22(2):256‐264. doi: 10.1111/wrr.12153 [DOI] [PubMed] [Google Scholar]
- 272. Centeno‐Cerdas C, Jarquin‐Cordero M, Chavez MN, et al. Development of photosynthetic sutures for the local delivery of oxygen and recombinant growth factors in wounds. Acta Biomater. 2018;81:184‐194. doi: 10.1016/j.actbio.2018.09.060 [DOI] [PubMed] [Google Scholar]
- 273. Shao K, Han B, Gao J, et al. Fabrication and feasibility study of an absorbable diacetyl chitin surgical suture for wound healing. J Biomed Mater Res B Appl Biomater. 2016;104(1):116‐125. doi: 10.1002/jbm.b.33307 [DOI] [PubMed] [Google Scholar]
- 274. Link A, Haag H, Michel T, et al. Development of a novel polymer‐based mRNA coating for surgical suture to enhance wound healing. Coatings. 2019;9(6):9060374. [Google Scholar]
- 275. Chen SX, Ge LP, Mueller A, et al. Twisting electrospun nanofiber fine strips into functional sutures for sustained co‐delivery of gentamicin and silver. Nanomed Nanotechnol Biol Med. 2017;13(4):1435‐1445. doi: 10.1016/j.nano.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Choudhury AJ, Gogoi D, Chutia J, et al. Controlled antibiotic‐releasing Antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery. 2016;159(2):539‐547. doi: 10.1016/j.surg.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 277. López‐Saucedo F, Flores‐Rojas GG, López‐Saucedo J, et al. Antimicrobial silver‐loaded polypropylene sutures modified by radiation‐grafting. Eur Polym J. 2018;100:290‐297. [Google Scholar]
- 278. Hansen KJ, Favreau JT, Guyette JP, et al. Functional effects of delivering human mesenchymal stem cell‐seeded biological sutures to an infarcted heart. Biores Open Access. 2016;5(1):249‐260. doi: 10.1089/biores.2016.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Serrano C, Garcia‐Fernandez L, Fernandez‐Blazquez JP, et al. Nanostructured medical sutures with antibacterial properties. Biomaterials. 2015;52:291‐300. doi: 10.1016/j.biomaterials.2015.02.039 [DOI] [PubMed] [Google Scholar]
- 280. Soufdoost RS, Mosaddad SA, Salari Y, et al. Surgical suture assembled with Tadalafil/Polycaprolactone drug‐ delivery for vascular stimulation around wound: validated in a preclinical model. Biointerface Res Appl Chem. 2020;10(5):6317‐6327. [Google Scholar]
- 281. Cummings SH, Grande DA, Hee CK, et al. Effect of recombinant human platelet‐derived growth factor‐BB‐coated sutures on Achilles tendon healing in a rat model: a histological and biomechanical study. J Tissue Eng. 2012;3(1):2041731412453577. doi: 10.1177/2041731412453577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Horvathy DB, Vacz G, Cselenyak A, Weszl M, Kiss L, Lacza Z. Albumin‐coated bioactive suture for cell transplantation. Surg Innov. 2013;20(3):249‐255. doi: 10.1177/1553350612451353 [DOI] [PubMed] [Google Scholar]
- 283. Kim H, Kim BH, Huh BK, et al. Surgical suture releasing macrophage‐targeted drug‐loaded nanoparticles for an enhanced anti‐inflammatory effect. Biomater Sci. 2017;5(8):1670‐1677. doi: 10.1039/c7bm00345e [DOI] [PubMed] [Google Scholar]
- 284. Padmakumar S, Joseph J, Neppalli MH, et al. Electrospun polymeric Core‐sheath yarns as drug eluting surgical sutures. ACS Appl Mater Interfaces. 2016;8(11):6925‐6934. doi: 10.1021/acsami.6b00874 [DOI] [PubMed] [Google Scholar]
- 285. Gallo AL, Paladini F, Romano A, et al. Efficacy of silver coated surgical sutures on bacterial contamination, cellular response and wound healing. Mater Sci Eng C Mater Biol Appl. 2016;69:884‐893. doi: 10.1016/j.msec.2016.07.074 [DOI] [PubMed] [Google Scholar]
- 286. Tao ZW, Favreau JT, Guyette JP, et al. Delivering stem cells to the healthy heart on biological sutures: effects on regional mechanical function. J Tissue Eng Regen Med. 2017;11(1):220‐230. doi: 10.1002/term.1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Sharifisamani E, Mousazadegan F, Bagherzadeh R, Latifi M. PEG‐PLA‐PCL based electrospun yarns with curcumin control release property as suture. Polym Eng Sci. 2020;60(7):1520‐1529. doi: 10.1002/pen.25398 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated


