Abstract
The B. subtilis pyrG gene, which encodes CTP synthetase, is located far from the pyrimidine biosynthetic operon on the chromosome and is independently regulated. The pyrG promoter and 5′ leader were fused to lacZ and integrated into the chromosomes of several B. subtilis strains having mutations in genes of pyrimidine biosynthesis and salvage. These mutations allowed the intracellular pools of cytidine and uridine nucleotides to be manipulated by the composition of the growth medium. These experiments indicated that pyrG expression is repressed by cytidine nucleotides but is largely independent of uridine nucleotides. The start of pyrG transcription was mapped by primer extension to a position 178 nucleotides upstream of the translation initiation codon. A factor-independent termination hairpin lying between the pyrG promoter and its coding region is essential for regulation of pyrG expression. Primer-extended transcripts were equally abundant in repressed and derepressed cells when the primer bound upstream of the terminator, but they were much less abundant in repressed cells when the primer bound downstream of the terminator. Furthermore, deletion of the terminator from pyrG-lacZ fusions integrated into the chromosome yielded elevated levels of expression that was not repressible by cytidine. We suggest that cytidine repression of pyrG expression is mediated by an antitermination mechanism in which antitermination by a putative trans-acting protein is reduced by elevated levels of cytidine nucleotides. Conservation of sequences and secondary structural elements in the pyrG 5′ leaders of several other gram-positive bacteria indicates that their pyrG genes are regulated by a similar mechanism.
The regulation of transcription of the Bacillus subtilis pyr operon, which encodes the enzymes of de novo UMP biosynthesis, has been extensively studied in this laboratory (for a review, see reference 31). Expression of this operon is governed by a transcriptional attenuation mechanism. When uridine nucleotide levels are elevated in the cells, the protein encoded by the first gene in the operon, PyrR, acts to promote termination of transcription at three attenuation sites located in the 5′ end of the operon. PyrR brings about termination by binding to pyr mRNA at specific sites and altering its secondary structure such that formation of a downstream transcription terminator is favored.
By contrast, very little is known about the regulation of expression of pyrG, the gene encoding CTP synthetase, in B. subtilis or other bacteria. The pyrG gene is not part of the pyr operon in B. subtilis. The gene was identified by Trach et al. (32), who called it ctrA, as a gene lying between rpoE, which encodes the δ subunit of RNA polymerase, and spo0F. Expression of B. subtilis pyrG is not coordinated with that of the pyr operon. This conclusion comes from the experiments of Asahi et al. (3), who showed that in wild-type cells both uridine and cytidine repressed the pyr operon enzymes but not CTP synthetase. In a cytidine deaminase-deficient mutant, which cannot convert cytidine to uridine, cytidine was able to repress CTP synthetase but was no longer able to repress genes of the pyr operon. These results suggest that repression of the pyr operon is brought about by uridine nucleotides, in accord with our present understanding of the system (31), but that pyrG is specifically repressed by cytidine nucleotides. Nothing is known about the mechanism of this repression.
The only other study of the regulation of bacterial pyrG of which we are aware is that of West and O'Donovan (36). These authors reported that repression of pyrG by cytidine could be demonstrated only in a Salmonella enterica serovar Typhimurium strain in which the cytidine deaminase gene cdd was inactive and in which a leaky UMP kinase (pyrH) gene brought about abnormally low levels of pyrimidine nucleoside di- and triphosphates. Again, no information about the mechanism of this repression is available.
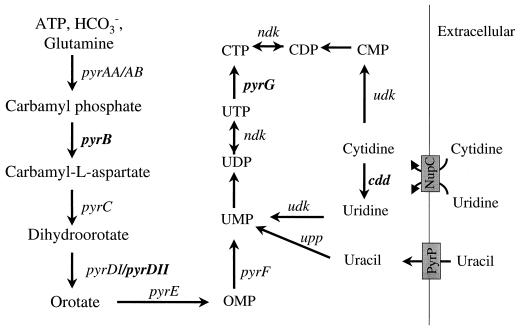
In the present study we identified the site of transcriptional initiation for B. subtilis pyrG. We showed that repression is responsive to cytidine nucleotide levels and is independent of both uridine nucleotides and PyrR-dependent attenuation. To do this, it was necessary to determine pyrG expression in mutant strains in which nucleotide pools could be manipulated by blockage of the de novo biosynthetic pathway (using pyrB and pyrDII mutants) or by inactivation of genes involved in interconversion of uridine and cytidine nucleotides (using cdd and pyrG mutants) (Fig. 1). Primer extension analysis and mutational analysis of the pyrG 5′ leader indicates that repression is brought about by a transcriptional antitermination mechanism that involves an attenuator lying between the pyrG promoter and its coding region.
FIG. 1.
Genes for pyrimidine nucleotide metabolism in B. subtilis. Known pathways for de novo biosynthesis and salvage are shown. Transport proteins implicated in uptake of pyrimidines are indicated by shaded boxes. Genes that were disrupted in strains used in this study to characterize the repression of pyrG are indicated in boldface.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
Bacterial strains used in this study are listed in Table 1. LB medium (22) was used as the rich liquid medium and the solid medium (1.5% agar) for both Escherichia coli and B. subtilis. Buffered minimal medium (19) was used as the chemically defined growth medium. Histidine (50 μg/ml) was used for growth of B. subtilis strain DB104 and its derivatives. To starve B. subtilis strain HC11 for pyrimidines, 100 μg of orotate per ml was used. To grow B. subtilis in the presence of excess pyrimidines, either 50 μg of uracil per ml plus 50 μg of uridine per ml or 200 μg of cytidine per ml was used. For selection of antibiotic resistance, antibiotics were used at the following concentrations: sodium ampicillin, 100 μg/ml; chloramphenicol, 6 μg/ml for B. subtilis and 20 μg/ml for E. coli; spectinomycin, 100 μg/ml; erythromycin 1 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain | Genotype or description | Source (reference) |
|---|---|---|
| Strains | ||
| E. coli DH5α | Φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | Laboratory stock |
| B. subtilis | ||
| DB104 | his nprR2 nprE18 ΔaprA3 | Laboratory stock |
| DB104ΔpyrR | HC11 pyrB+ Specs ΔpyrR | Tumer et al. (33) |
| DB104ΔpyrDII | DB104 ΔpyrDII | Kahler and Switzer (14) |
| HC11 | DB104 pyrB::Specr | Hu and Switzer (11) |
| DB104ΔpyrDIIcdd | DB104 ΔpyrDII cdd::pMX | This study |
| DB104pyrG::cm | DB104 pyrG::Cmr | This study |
| DB104pyrG::erm | DB104 pyrG::Ermr | This study |
| QM201 | DB104 amyE::pMSAF | This study |
| QM202 | DB104 ΔpyrR amyE::pMSAF | This study |
| QM203 | DB104 ΔpyrDII amyE::pMSAF | This study |
| QM204 | DB104 ΔpyrDIIcdd amyE::pMSAF | This study |
| QM205 | HC11 amyE::pMSAF | This study |
| QM206 | DB104 pyrG::erm amyE::pMSAF | This study |
| QM102 | HC11 amyE::pMSAD | This study |
| QM103 | HC11 amyE::pMSAH | This study |
| QM104 | HC11 amyE::pMSAB | This study |
| QM105 | HC11 amyE::pMSCD | This study |
| QM234 | DB104 ΔpyrDIIcdd amyE::pMSAB | This study |
| QM301 | HC11 amyE::pMSANMF | This study |
| Plasmids | ||
| pUC19 | Apr | Yanisch-Perron et al. (38) |
| pDH32 | Apr CmramyE front amyE back lacZ | Grandoni et al. (8) |
| pFL32 | AprrpoE gene in XbaI-HindIII sites of pUC19 | López de Saro et al. (17) |
| pMutin | Apr Emr | Vagner et al. (34) |
| pJH4133 | Cmr, contains pyrG gene | Trach et al. (32) |
| pJH4521 | Apr Cmr, pyrG gene with Cm insertion | Trach et al. (32) |
| pCm::Er | Apr Emr | Steinmetz and Richter (30) |
| pHV502 | pMutin with a 2.4-kb BclI deletion | This study |
| pMX | 186 bp of HindIII-BamHI fragment of cdd gene inserted into HindIII-BamHI-digested pHV502 | This study |
| pUCAN | EcoRI (−96)-SacI (+20) PCR fragment inserted into pUC19 digested with EcoRI and SacI | This study |
| pUCANMF | SacI (+30)-BamHI (+218) PCR fragment inserted into pUCAN digested with SacI and BamHI | This study |
| Integration plasmids | ||
| pMSAF | EcoRI (−96)-BamHI (+218) PCR fragment inserted into pDH32 digested with EcoRI and BamHI | This study |
| pMSAB | EcoRI (−96)-BamHI (+36) PCR fragment inserted into pDH32 digested with EcoRI and BamHI | This study |
| pMSCD | EcoRI (+57)-BamHI (+110) PCR fragment inserted into pDH32 digested with EcoRI and BamHI | This study |
| pMSAD | EcoRI (−96)-BamHI (+110) PCR fragment inserted into pDH32 digested with EcoRI and BamHI | This study |
| pMSAH | EcoRI (−96)-BamHI (+80) PCR fragment inserted into pDH32 digested with EcoRI and BamHI | This study |
| pMSANMF | 0.32-kb EcoRI-BamHI fragment of pUCANMF in pDH32 | This study |
Bacterial transformation, isolation, manipulation, and analysis of DNA and RNA.
Transformation of E. coli was carried out as described by Sambrook et al. (26). Transformation of B. subtilis followed the procedure of Contente and Dubnau (6).
Plasmid DNA from E. coli was isolated as described by Sambrook et al. (26) and purified using the plasmid Miniprep kit from Qiagen. B. subtilis chromosomal DNA was isolated by the protocol of Wilson (37). PCR was performed with VentR or Taq DNA polymerase in a Perkin-Elmer Cetus DNA thermal cycler by the procedure recommended by the manufacturer. DNA sequencing was done at the Genetic Engineering facility in the University of Illinois Biotechnology Center.
Total RNA was extracted from B. subtilis according to the method of Saxild et al. (29). Cells were harvested in exponential phase at a cell density of approximately 90 Klett units except for strain HC11 grown on orotate and DB104 pyrG::erm grown with limiting cytidine, which were harvested at 60 to 65 Klett units. To remove DNA from total RNA samples, the DNA-free kit from Ambion was used as recommended by the supplier.
Strain constructions.
B. subtilis strain DB104 ΔpyrDIIcdd was constructed as follows. Plasmid pMutin was digested with BclI. The 5.9-kb fragment was gel purified and religated, yielding plasmid pHV502, which lacks the lacI gene and part of lacZ. A 186-bp fragment from the cdd gene was amplified from B. subtilis chromosomal DNA by PCR using primers cddHindIII and cddBamHI (Table 2). The PCR product was digested with HindIII and BamHI and ligated to HindIII-BamHI-digested pHV502 to form plasmid pMX. pMX DNA was introduced into strain DB104 ΔpyrDII by transformation, selecting for erythromycin resistance. B. subtilis strain DB104 pyrG::cm was constructed by transforming NdeI-linearized pJH4521 into DB104, selecting for chloramphenicol resistance, and confirming that the transformants were auxotrophic for cytidine. The B. subtilis strain DB104 pyrG::erm was constructed by transforming plasmid pCm::Er into DB104 pyrG::cm.
TABLE 2.
Sequences of deoxyoligonucleotides used in this study
| Primer | Nucleotide sequencea | Coordinate(s)b |
|---|---|---|
| Deletion analysis | ||
| pyrG-A | CCCGAATTCGACTATGATGATGAAGAAGAGG | −96 |
| pyrG-B | TGAAAGGGATCCTAACGTACTATGTTCTC | +36 |
| pyrG-C | CAAGGAATTCAAAGGGAGCTTTCTTATTTTCACCC | +57 |
| pyrG-D | CGGGGATCCTTTTAAATGATTTATAAGAAAAATGAG | +110 |
| pyrG-F | CCGGATCCAGGATACAACTCCCCCGGTTAC | +218 |
| pyrG-H | CCCCGGATCCGGTGAAAATAAGAAAGCTC | +80 |
| Mutant construction | ||
| cddHindIII | GGCCAAGCTTGGAGCCGCTCTTCTTACC | 2610535–2610518 |
| cddBamHI | CGCGGATCCGGCTCCGCACGGAGATACTGG | 2610370–2610350 |
| pyrG-M | GCCGAGCTCATTTACGTTATGCTCCCTTTCAAG | +30 |
| pyrG-N | CGCGAGCTCCTCTTCGTTTTTGAAGAGCC | +20 |
| Primer extension | ||
| Primer C | CGTACTATGTTCTCTCTTCG | +33 |
| Primer B | GTGAGGATACAACTCCCCCGG | +221 |
| Northern blotting | ||
| pyrG-O | CCGGGATCCGACGCCACCTGCATGCCC | +1341 |
| pyrG-P | GGTACTGCAGGAGACATCGAATCACTGCC | +608 |
| rpoE-A | GCGGAATTCGCCGCTTCCTGGCGCTTTCTG | 3811920–3811900 |
| rpoE-B | CCTCTTCTTCATCATCATAGTCTTC | 3811630–3811606 |
| RT-PCR | ||
| pyrG-Q | GACTATGATGATGAAGAAGAGG | −96 |
Underlining indicates the position of restriction sites; boldface indicates nucleotides that differ from the wild-type sequence.
Position of the 5′-proximal nucleotide of the primer, except for primers cddHindIII, cddBamHI, rpoE-A, and rpoE-B, for which the nucleotide numbering of the B. subtilis genome sequence is used.
Construction and integration of pyrG-lacZ fusion integrant strains.
The integration plasmids listed in Table 1 were constructed by PCR amplification of fragments containing the desired region of pyrG using plasmid pJH4133 as the template. The PCR fragments were digested with EcoRI and BamHI and ligated into EcoRI- and BamHI-digested pDH32. The primers used in making each construct are listed in Table 2 as pyrG-A through pyrG-H. For each of the integration plasmids, the last two letters of the name (Table 1) indicate the primers from this series used to construct it. However, pMSANMF was constructed through several steps. First, −96 to +20 of the pyrG sequence was amplified by PCR with primers pyrG-A and pyrG-N. This PCR fragment was digested with EcoRI and SacI and ligated to EcoRI-SacI-digested pUC19 to form pUCAN. Then, +30 to +218 of the pyrG sequence was amplified by PCR with primers pyrG-M and pyrG-F. After digestion with SacI-BamHI, this PCR product was ligated to SacI-BamHI digested pUCAN to form pUCANMF. Because of the overhang and restriction site introduced, nucleotides +21 to +29 of the pyrG 5′ leader sequence were substituted as shown in Fig. 6. A 0.32-kb EcoRI-BamHI fragment of pUCANMF was subcloned into pDH32 to form pMSANMF.
FIG. 6.
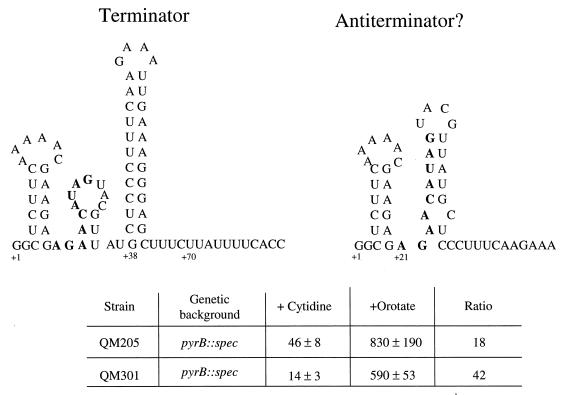
Test of the role of a possible antiterminator hairpin in the pyrG 5′ leader in regulation of pyrG expression. (Top) Two possible ways of folding the pyrG 5′ leader RNA as predicted by MULFOLD. On the left is the more stable predicted structure, which includes a factor-independent transcription terminator. On the right is a less stable alternative structure in which a putative antiterminator hairpin could form. The nucleotides in boldface are those present in the native sequence (QM205) which were replaced by the sequence 5′-GAGCUCAUU-3′ in the mutant strain QM301. Sequencing of the mutant DNA confirmed the correct structure, except that the nucleotide at +5, which is a C in QM205, had also been replaced by a U in QM301. (Bottom) Results of β-galactosidase assays determining the levels of expression of the pyrG-lacZ fusions in QM205 and QM301 under repressing (+cytidine) and derepressing (+orotate) conditions. Cells were grown in the minimal medium described by Lu et al. (19).
pyrG-lacZ fusion integrant strains were constructed as previously described (33). The integration plasmids were linearized with ScaI and PstI and transformed into B. subtilis. Transformants were selected for chloramphenicol resistance, and disruption of amyE was verified on 1% starch–LB plates.
Primer extension, Northern hybridization, and RT-PCR.
Primer extension was performed as described by Saxild et al. (29). Reverse transcription (RT) reactions with avian myeloblastosis virus reverse transcriptase (Promega) were incubated at 42°C for 1 h with 10 μg of total RNA and either primer C or primer B (Table 2). The DNA sequencing ladder used for analysis of primer extension was generated by the dideoxynucleotide method of Sanger et al. (27) using the T7 Sequenase v2.0 DNA sequencing kit (Amersham Life Science) and plasmid pJH4133 as the template.
Northern blot analysis was performed with a modification of the method of Nygaard et al. (24). RNA was separated on a 1.5% agarose gel and transferred to a BrightStar-Plus positively charged nylon membrane (Ambion). After transfer, RNA was cross-linked to the membrane by a UV cross-linker. ULTRAhybe hybridization buffer (Ambion) was used for hybridization. The DNA probes for Northern blot analysis were generated by standard PCR except that unlabeled dCTP was used at 1 μM and [α-32P]dCTP was added at 0.16 μM and a total radioactivity of 50 μCi. Plasmid pJH4133 was used as the template, and pyrG-O and pyrG-P were used as primers for labeling the pyrG probe. EcoRI-HindIII digested pFL32 was used as the template, and rpoE-A and rpoE-B were used as primers for labeling the rpoE probe.
RT-PCR was performed using Superscript II RNase H− reverse transcriptase (Gibco BRL) for RT as recommended by the supplier. Total RNA (0.2 μg) treated with DNase I and 5 ng of primer per μl were used. Two of the 20 μl of RT reaction mixture was used for PCR. Taq polymerase (Gibco BRL) was used for PCR. Plasmid pJH4133 was used as the template for the positive PCR control. Reverse transcriptase was omitted from the normal reaction to provide a negative control. The primers used for RT-PCR are listed in Table 2.
Enzymatic assays.
β-Galactosidase activity was determined by a protocol (19) modified from Miller's method (22). The data in Table 3 are the averages of at least six determinations with the indicated standard deviations. Cells were harvested for assay in the exponential phase of growth at a density of about 90 Klett units, except for slowly growing derivatives of strain HC11 grown on orotate or QM206 grown with limiting cytidine, which were harvested at a density of 35 to 65 Klett units.
TABLE 3.
Regulation of B. subtilis pyrG-lacZ fusions by pyrimidines
| Strain | Genetic background | Addition(s) to minimal mediuma | Doubling time (min) | β-Galactosidase activity (Miller units) |
|---|---|---|---|---|
| QM201 | Wild type | None | 36 | 68 ± 3 |
| Urd, Ura | 38 | 65 ± 7 | ||
| Cyd | 36 | 50 ± 2 | ||
| QM202 | ΔpyrR | None | 47 | 50 ± 1 |
| Urd, Ura | 48 | 50 ± 2 | ||
| Cyd | 45 | 40 ± 4 | ||
| QM203 | ΔpyrDII | None | 58 | 110 ± 15 |
| Urd, Ura | 37 | 55 ± 3 | ||
| Cyd | 38 | 40 ± 4 | ||
| QM204 | ΔpyrDIIcdd | None | 66 | 130 ± 8 |
| Urd, Ura | 38 | 70 ± 8 | ||
| Cyd | 58 | 17 ± 3 | ||
| QM205 | pyrB::spec | Orotate | 85 | 830 ± 190 |
| Urd, Ura | 39 | 55 ± 8 | ||
| Cyd | 38 | 45 ± 8 | ||
| QM206 | pyrG::erm | Cyd | 48 | 440 ± 8 |
| Cyd, 10 μg/ml | 88 | 700 ± 75 | ||
| Cyd, 10 μg/ml; Urd | 180 | 1,900 ± 60 | ||
| Cyd, 10 μg/ml; Ura | 101 | 1,300 ± 120 |
Unless specified, cytidine (Cyd) was used at 200 μg/ml, uridine (Urd) was used at 50 μg/ml, and uracil (Ura) was used at 50 μg/ml.
Unpublished DNA sequence data.
Preliminary sequence data for portions of the pyrG genes from several bacteria listed in Fig. 7 were obtained from The Institute for Genomic Research (TIGR) at http://www.tigr.org.
FIG. 7.
Conserved sequences in the 5′ untranslated leader regions of the pyrG genes from 10 gram-positive bacteria. For each sequence the first conserved segment is located 7 nt downstream from a reasonable −10 sequence for a promoter and is likely to be at or near the 5′ end of the pyrG mRNA. The second and third conserved sequences are complementary and are predicted to form part of the stem of a rho-independent transcription terminator that serves as the pyrG attenuator, which is followed by several U residues (polyU). Sequences and their sources: Bsu, B. subtilis (16); Bha, Bacillus halodurans, GenBank accession no. AP001520; Ban, B. anthracis, TIGR; Bst, B. stearothermophilus, TIGR; Efa, E. faecalis, TIGR; Spy, Streptococcus pyogenes, GenBank accession no. AE006614; Spn, S. pneumoniae, TIGR; Sau, Staphylococcus aureus, GenBank accession no. AP003364; Llac, Lactococcus lactis subsp. lactis, GenBank accession no. AE006284; Lcre, L. lactis subsp. cremoris, GenBank accession no. AJ010153.
RESULTS
Characterization of pyrG transcripts.
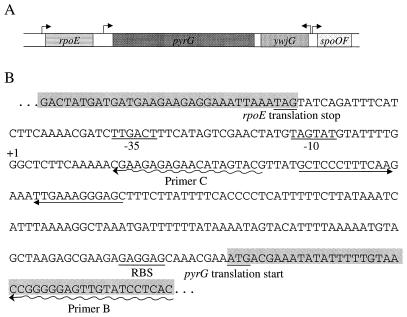
A map of the B. subtilis genome in the region surrounding pyrG (16, 32) is shown in Fig. 2A. The pyrG gene lies immediately downstream of the rpoE gene and is transcribed in the same direction. The next gene downstream of pyrG is ywjG, a gene of unknown function transcribed in the opposite direction. Examination of the DNA sequence of the intercistronic region between rpoE and pyrG (Fig. 2B) revealed a reasonable ςA-dependent promoter sequence upstream of a possible factor-independent transcription terminator sequence, which presumably functions as the terminator for the rpoE gene. The site of initiation of pyrG transcription was determined by primer extension analysis using two single-stranded deoxyoligonucleotide primers. Primer B was complementary to mRNA from the pyrG coding region and downstream from the terminator sequence, whereas primer C was complementary only to an mRNA sequence upstream of the terminator (Fig. 2B).
FIG. 2.
(A) Structure of the rpoE-pyrG region of the B. subtilis chromosome. Shaded bars indicate open reading frames. The genes shown are described in the text. Arrows indicate the location and direction of transcription of putative promoters for the genes. (B) Nucleotide sequence of the rpoE-pyrG intercistronic region. Shaded bars denote the 3′ end of the rpoE coding region and the 5′ end of the pyrG coding region. Translation stop and start codons are underlined, as is the ribosome-binding site (RBS) for pyrG. −35 and −10 denote the ςA recognition sequence for the pyrG promoter. Straight arrows denote complementary sequences that form a factor-independent terminator hairpin, and wavy arrows show sequences complementary to primers B and C used for primer extension experiments in this work (see Fig. 3).
Template RNA for primer extension reactions using both primer B and primer C was extracted from HC11 (pyrB::spec) cells grown in the presence of 200 μg of cytidine per ml, which represses pyrG expression, and from HC11 cells grown in the presence of 100 μg of orotate per ml, a poor pyrimidine source that causes severe pyrimidine starvation (19). For primer extension reactions using primer B, RNA was also extracted from DB104 pyrG::erm cells grown in the presence of 10 μg of cytidine per ml plus 50 μg of uridine per ml. As shown below for experiments with strain QM206 in Table 3, this is a second method to derepress pyrG without resorting to starvation of a pyrimidine auxotroph.
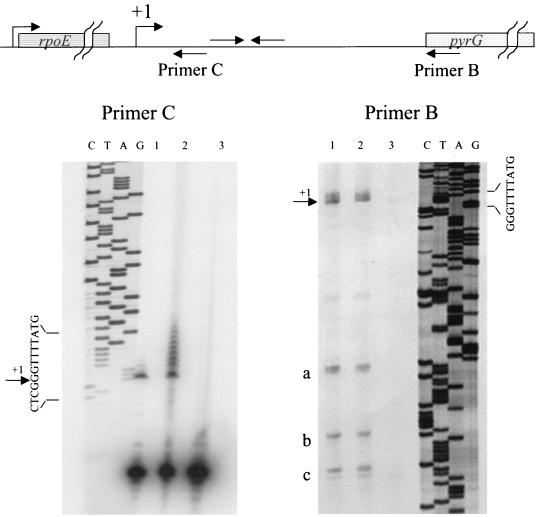
In all cases where a primer-extended product was detectable, the lengths of the extended reverse transcripts from both primers mapped the major site of transcription initiation to the G residue indicated as +1 in Fig. 2B (Fig. 3). For primer extension reactions using primer B (located downstream of the terminator), both methods used to derepress pyrG expression led to equal amounts of primer-extended product (Fig. 3, right, lanes 1 and 2), whereas RNA extracted from cells grown under conditions that repress pyrG gave only traces of primer-extended product (Fig. 3, right, lane 3). This result suggests that repression by cytidine acts by increasing transcription termination at the terminator that lies just downstream from the pyrG promoter. For primer extension reactions using primer C (located upstream of the terminator), RNA from cells grown under both repressing and derepressing conditions yielded approximately equal amounts of primer-extended products (Fig. 3, left, lanes 1 and 2), as expected if repression is mediated by the terminator downstream from the primer site.
FIG. 3.
Identification of the start site of pyrG transcription by primer extension. (Left) Lane 1, 10 μg of total RNA from strain HC11 grown with 200 μg of cytidine per ml was used; lane 2, 10 μg for total RNA from strain HC11 grown with 100 μg of orotate per ml was used; lane 3, no primer extension (primer C only was subjected to electrophoresis). The left side of the panel shows the results of dideoxy sequencing of the rpoE-pyrG region with primer C to allow identification of the end of the primer-extended product. (Right) Lane 1, 10 μg of total RNA from strain DB104 pyrG::erm grown on minimal medium with 10 μg of cytidine and 50 μg of uridine per ml was used; lane 2, 10 μg of total RNA from strain HC11 grown with 100 μg of orotate per ml was used; lane 3, 10 μg of total RNA from strain HC11 grown with 200 μg of cytidine per ml was used. Prematurely terminated primer extension products are indicated by a, b, and c; product a probably results from blockage of reverse transcriptase at the terminator hairpin, and products b and c occur in A- and U-rich regions of the reverse transcriptase template. The right side of the panel shows the results of dideoxy sequencing of the rpoE-pyrG region with primer B.
For cells grown under derepressing conditions, a ladder of somewhat longer primer-extended products (1 to 10 nucleotides [nt] longer than the primary extended product) was clearly observed with primer C (Fig. 3, left, lane 2) and possibly also with primer B (Fig. 3, right, lanes 1 and 2). This observation raised the possibility that some pyrG-containing transcripts might originate from the upstream rpoE promoter, i.e., that under derepressing conditions some rpoE-pyrG bicistronic transcripts are formed. Alternatively, the longer primer-extended products seen with primer C could have resulted from extension of the extreme 3′ end of rpoE transcripts if they end at the terminator in the pyrG 5′ leader. In that case, the laddering of products would suggest that the rpoE transcripts are partially degraded.
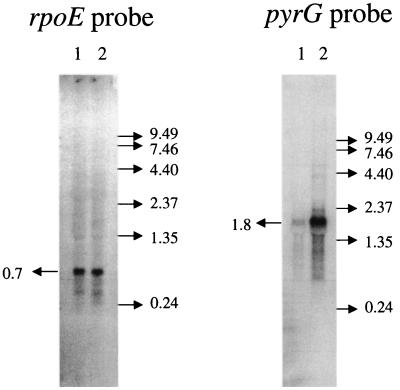
The length of pyrG and rpoE transcripts was probed further by Northern hybridization analysis. Bulk RNA was isolated from HC11 cells grown with cytidine and with orotate, which are repressing and derepressing conditions for pyrG. Probes designed to hybridize specifically to transcripts from the pyrG and rpoE coding regions (Fig. 2A) detected transcripts that were 1.8 and 0.7 knt in length, respectively (Fig. 4). These are the sizes expected for separate pyrG and rpoE mRNAs. The abundance of the rpoE transcripts was not affected by pyrimidine starvation, but the pyrG transcripts were much more abundant under these conditions (Fig. 4). This observation indicates that most or all of the pyrG transcripts whose abundance is regulated by cytidine originate from the promoter we have mapped to the rpoE-pyrG intercistronic region. In no case was a transcript of 2.5 knt, corresponding to a full-length rpoE-pyrG bicistronic transcript, detected. However, the pyrG transcripts show evidence of considerable degradation, and the presence of a small amount of hybridizing material larger than 1.8 knt may indicate that an rpoE-pyrG transcript, if one was formed, could have been degraded under the conditions used.
FIG. 4.
Northern hybridization analysis of rpoE and pyrG transcripts. Total RNA (10 μg) from strain HC11 grown on minimal medium with 200 μg of cytidine per ml (lane 1) or 100 μg of orotate per ml (lane 2) was subjected to electrophoresis, electroblotted onto nylon membranes, and hybridized with 32P-labeled deoxyoligonucleotide probes. The positions of RNA size standards (in kilonucleotides) are on the right of each panel.
The question of rpoE-pyrG cotranscription was examined further by RT-PCR analysis. RNA from derepressed cells (strain HC11 growth with orotate) was used as a source of pyrG mRNA template for RT-PCR in which primer B or primer C (Fig. 2B) and a primer complementary to the 3′ end of the rpoE coding region (pyrG-Q) (Table 2) were used for amplification. RT-PCR products of the expected sizes were obtained with primer C but not with primer B (data not shown). This result indicates that rpoE transcripts terminate at or very close to the terminator located in the pyrG 5′ leader, but no evidence was found for transcripts that include both rpoE and pyrG coding sequences.
Regulation of pyrG-lacZ expression by cytidine nucleotides.
We constructed a series of strains in which the entire rpoE-pyrG intercistronic region was fused to a lacZ reporter gene and the fusion was integrated in a single copy into the chromosomes at the amyE locus of several B. subtilis strains. These fusions allowed us to study regulation of B. subtilis pyrG expression by supplementation of the growth medium with pyrimidine nucleotides or cultivation of the bacteria under conditions of pyrimidine starvation. The lacZ reporter gene permitted pyrG expression to be readily determined from β-galactosidase assays, whereas we were unable to assay CTP synthetase activity reliably in crude extracts of B. subtilis cells. When the pyrG-lacZ fusion was integrated into a wild-type (strain DB104) background and the cells were grown on minimal medium with or without pyrimidines, a small but statistically reliable repressive effect of cytidine was observed (Table 3). Cells grown with cytidine expressed 25 to 30% lower levels of β-galactosidase than cells grown without supplementation. Uracil and uridine did not cause significant repression.
When these experiments were repeated with the pyrG-lacZ fusion integrated into a strain with an in-frame deletion of the pyrR gene (33), expression levels were reduced by about 30% compared to expression in wild-type cells and exhibited only very small repressive effects of cytidine. Since deletion of the pyrR gene has been shown to lead to very high, constitutive overexpression of the genes of the pyr operon (33), the low expression of the pyrG-lacZ fusion in the ΔpyrR strain indicates that pyrR is not involved in regulation of pyrG expression. The reduced level of pyrG expression in the ΔpyrR strain probably results from very high levels of intracellular pyrimidine nucleotides (including CTP) in this strain (21, 33).
Regulation of pyrG expression could be demonstrated more convincingly by integrating the pyrG-lacZ fusion into strains that could be starved for pyrimidines. A derivative of DB104 with an in-frame deletion of the pyrDII gene grows slowly in the absence of pyrimidines and exhibits derepressed pyr genes because of reduced intracellular pyrimidine nucleotide levels (14). Expression of pyrG-lacZ was elevated 1.7-fold in a ΔpyrDII background (strain QM203) relative to the wild-type strain QM201 (Table 3). Addition of pyrimidines to this strain brought about repression of pyrG, twofold repression by uracil plus uridine, and almost threefold repression by cytidine. Since the repressive effects of cytidine might be masked by deamination of this nucleoside by cytidine deaminase (cdd), pyrG-lacZ expression was also determined in a ΔpyrDIIcdd background (strain QM204). Repression by uracil plus uridine was not altered by the cdd mutation, but cytidine became a more effective repressor (seven- to eightfold repression). These observations suggest that a cytidine nucleotide is the most effective metabolite for repression of pyrG and that uridine nucleotides may act only via conversion to cytidine nucleotides. The largest derepression of pyrG-lacZ expression observed in our studies was obtained by growth of a pyrimidine auxotroph, strain QM205 (pyrB::spec) (11), on orotate, which is taken up slowly by the cells and results in slow growth and highly derepressed pyr gene expression (19). As seen in Table 3, pyrG-lacZ was expressed at very high levels in the pyrB::spec background when the cells were starved for pyrimidines by growth on orotate and was repressed 20-fold when the cells were grown with cytidine. Growth of strain QM205 with excess uracil plus uridine brought about 12-fold repression.
The foregoing experiments demonstrated repression of pyrG expression by pyrimidines in the growth medium and suggested that cytidine nucleotides were more effective than uridine nucleotides as corepressors, but they did not allow the cytidine and uridine nucleotide pools to be manipulated separately. This was accomplished by studying pyrG-lacZ expression when the fusion was integrated into a strain in which the normal chromosomal pyrG gene was disrupted by insertion of an erythromycin resistance gene. This strain, QM206, was a cytidine auxotroph; uracil, uridine, and cytosine did not support growth. Interestingly, this strain had a normal cdd gene, which demonstrates that in B. subtilis—unlike enteric bacteria (23)—cytidine deaminase is not sufficiently active to prevent growth of a pyrG strain on cytidine. Strain QM206 grew more slowly with 10 than with 200 μg of cytidine per ml; cytidine limitation resulted in derepression of an integrated pyrG-lacZ fusion (Table 3). Addition of 50 μg of uridine per ml did not increase the slow growth brought about by 10 μg of cytidine per ml; in fact, it caused the cells to grow more slowly and resulted in further derepression of pyrG. This effect can be explained by the fact that cytidine and uridine are known to share a common uptake protein in B. subtilis, NupC (15, 28). Thus, addition of uridine results in competitive inhibition of cytidine uptake, decreased pools of intracellular cytidine nucleotides, and further derepression of pyrG. Uracil, which is believed to be taken up by the PyrP carrier in B. subtilis (33), caused modest inhibition of growth and pyrG derepression. These experiments indicate that pyrG expression is regulated largely, if not entirely, by intracellular cytidine nucleotide pools, because the highest expression was obtained in pyrG::erm cells that were the most severely starved for cytidine but would be predicted to have high uridine nucleotide pools.
Mutational analysis of the pyrG leader indicates an antitermination mechanism for repression but does not identify an antiterminator in the pyrG RNA.
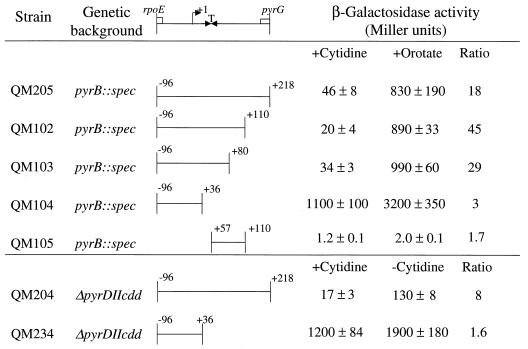
Additional insight into the elements present in the rpoE-pyrG intercistronic region that are important for pyrG expression and its repression by cytidine was obtained by characterizing the expression of a series of deletions of the QM205 pyrG-lacZ fusion that was studied for Table 3. The parent fusion and the deletions shown in Fig. 5 were integrated into strain HC11 (pyrB::spec) and grown under repressing and derepressing conditions, i.e., in minimal medium with either 200 μg of cytidine or 100 μg of orotate per ml, respectively. Normal expression and regulation were obtained with all fusion-integrant strains that contained sequences from the 3′ end of rpoE to +80 in the pyrG leader transcript. However, further deletion of pyrG leader sequences from +37 to +80, which specify a putative factor-independent transcription terminator, led to highly derepressed pyrG expression, which was not reduced by cytidine in the medium (Fig. 5, strain QM104). The threefold further increase in expression in cells of this strain that were starved by growth on orotate may be a nonspecific effect of this extreme starvation, as has been previously observed for the pyr operon (19). This conclusion is supported by the observation that an alternative mode of pyrG derepression, growth of a ΔpyrDIIcdd strain without pyrimidines, yielded only 1.6-fold derepression in the QM234 strain containing the same deletion of the terminator but 8-fold derepression in strain QM204 (Fig. 5). When a downstream segment from the rpoE-pyrG intercistronic region, +57 to +110, was fused to lacZ and integrated into strain HC11, no detectable β-galactosidase was expressed. These findings support the conclusions from the primer extension experiments that the only active pyrG promoter lies upstream of transcription terminator that is predicted to lie between nt +38 and +70 of the pyrG transcript leader (Fig. 2B). Furthermore, the repression of pyrG expression by cytidine requires the presence of this terminator. This leads us to suggest that termination at this site is efficient when cytidine nucleotides are abundant in the cell and that cytidine starvation suppresses termination.
FIG. 5.
Deletion analysis of pyrG-lacZ fusion integrants. The portions of the rpoE-pyrG intercistronic region shown were fused to lacZ in pDH32 and integrated into the B. subtilis chromosome at amyE in the indicated strains. The cells were grown under repressing (+cytidine) or derepressing (+orotate or −cytidine) conditions and harvested during exponential growth. The β-galactosidase activities of the cells were assayed to determine the degree of pyrG expression.
What mechanism could account for regulation of termination in the pyrG 5′ leader by cytidine nucleotides? Clearly, some mechanism for preventing transcription termination must act when the cytidine nucleotide pool is low. We searched the sequence of the 5′ leader with the MULFOLD program (12, 13, 39) for a possible RNA antiterminator hairpin and identified only a single, relatively weak alternative RNA structure that would prevent the more stable terminator hairpin from forming (Fig. 6). However, a mutagenesis experiment in which nucleotides 21 through 29 were replaced with nucleotides that cannot base-pair to form the putative antiterminator hairpin in a pyrG-lacZ fusion integrant gave regulation of β-galactosidase expression by cytidine that was similar to that of the native pyrG-lacZ fusion integrant (Fig. 6).
DISCUSSION
Prior to the present study, little was known about the regulation of pyrG expression in B. subtilis. Our results demonstrate that pyrG transcription is regulated by cytidine nucleotides, although the repressive effects of cytidine are very small unless the cells are starved for pyrimidines and are enhanced when the gene encoding cytidine deaminase is inactivated. This suggests that the intracellular level of the regulatory metabolite formed by de novo biosynthesis is high enough to almost fully repress pyrG. Experiments with a strain in which conversion of UTP to CTP was blocked by a pyrG disruption allowed us to starve the cells for cytidine nucleotides while providing abundant uridine nucleotides. Under these conditions pyrG was fully derepressed, which indicated that the cytidine nucleotides are the repressing metabolites for this gene. While we have not measured nucleotide pools directly in our experiments, it seems most probable to us that the actual regulatory metabolite is CTP, since this nucleotide is the product of the enzyme encoded by pyrG and CTP is the cytidine nucleotide used directly in biosynthetic pathways.
The most abundant pyrG transcripts found in B. subtilis cells originate from a promoter which we identified in the rpoE-pyrG intercistronic region, and these became more abundant in cells that were starved for pyrimidines. Bicistronic rpoE-pyrG transcripts were not detected, however. Most rpoE transcripts were the size of monocistronic transcripts, and their abundance was not altered by starvation for pyrimidines. This is consistent with the report of López de Saro et al. (18) that the rpoE promoter is expressed at substantial levels under all conditions examined.
The most important new finding in our work is that B. subtilis pyrG expression is regulated by a factor-independent transcription terminator located in the pyrG 5′ leader downstream from the pyrG promoter. Termination at this site is regulated by cytidine nucleotides over an 8- to 10-fold range if the effects on pyrG-lacZ fusion integrants can be taken to reflect levels of pyrG mRNA. Evidence for the role of this terminator was of two kinds. First, primer-extended transcripts originating from a primer upstream of the terminator (primer C in Fig. 3) were equally abundant in cells grown under repressing and derepressing conditions, whereas primer-extended transcripts originating from a primer downstream of the terminator (primer B in Fig. 3) were much less abundant in cells grown with excess cytidine. More direct evidence was provided by deletion analysis of the pyrG leader region in pyrG-lacZ transcriptional fusions (Fig. 5).
The presence of a regulated transcription terminator upstream of the coding region of the gene being regulated is the hallmark of an attenuation system. In two of the best-studied cases of biosynthetic operons regulated by attenuation in B. subtilis, the trp operon (2) and the pyr operon (31), a more stable antiterminator hairpin forms in the leader RNA before the terminator hairpin can form. This condition prevents termination and allows expression of the downstream genes. In the presence of an end product of the operon, a regulatory protein—trp RNA-binding attenuation protein plus tryptophan in the case of the trp operon and PyrR plus UMP or UTP in the case of the pyr operon—binds to a specific site in the 5′ stem of the antiterminator hairpin and destabilizes it, allowing formation of the terminator.
In other antitermination systems, the terminator hairpin is the most stable secondary structure in the leader mRNA, and antitermination is brought about by the binding of a regulatory protein to sequences upstream of the terminator, which prevents terminator formation. In some cases, such as the E. coli bgl operon (1) and the B. subtilis sacB operon (20, 35), the regulatory protein, BglG or SacY, respectively, binds to an RNA antiterminator hairpin that overlaps the sequence of the terminator hairpin. Antitermination in the lambdoid phages is mechanistically similar but involves direct interactions with RNA polymerase and the Nus proteins (10). In other cases, such as the nasF operon of Klebsiella (4), there is no identifiable antiterminator hairpin that overlaps the terminator. Instead, the antiterminator protein NasR appears to bind to a hairpin that lies upstream of the terminator hairpin and is separated from it by a 9-nt linker (4). The pyrG leader RNA resembles the latter group of antitermination systems because the terminator structure is predicted to be the most stable secondary structure. We suggest that a hypothetical regulatory protein binds upstream of this terminator when the level of a cytidine nucleotide, most likely CTP, is low and that the binding of CTP at high concentrations causes the protein to dissociate from the pyrG mRNA, allowing transcriptional termination. The RNA sequences of the pyrG leader upstream of its terminator have no homology to any of the known antitermination systems. We searched the sequence of the pyrG 5′ leader for antiterminator hairpin structures that overlap with the terminator and identified only one candidate (Fig. 6). However, mutation of the sequence of this region so as to prevent the antiterminator from forming did not reduce the ability of pyrG to be repressed by cytidine.
A comparison of the pyrG 5′ leader sequences from 10 different gram-positive bacteria suggests that the mechanism for pyrG regulation we have identified is found in these other bacteria as well (Fig. 7). All of the leader RNA sequences are capable of forming transcription terminator structures. Furthermore, three sequence segments are conserved in the RNA of the pyrG leaders. The first of these, GGGC(U/A)C, is consistently located at the very 5′ end of the pyrG transcript. In some cases this sequence may form part of the stem of a hairpin structure, as suggested for the B. subtilis pyrG leader (Fig. 6), but this did not appear to always be the case. Two additional conserved segments were observed in each of the leaders, typically GCUCCC and GGGAGC; these were consistently base-paired to form the base of the stem of a terminator hairpin, as shown for B. subtilis pyrG in Fig. 6. Only in the case of Lactococcus lactis subsp. cremoris is the terminator so truncated that its formation is questionable. The presence of these conserved sequence elements in the gram-positive pyrG leaders provides a hint that they are important for regulation of expression, either by serving as recognition elements for a regulatory protein or by participation in a higher-order RNA folding structure that we have not yet identified.
An unusual feature of our model is that the pyrG attenuator also functions as the transcription terminator for the upstream rpoE gene, so that under conditions of cytidine starvation some rpoE-pyrG bicistronic transcripts should be formed. Other instances of transcription terminators that appear to also function as attenuators for downstream genes have been described for the B. subtilis gltX-cysES (7) and acsA-tyrS (9) operons, but in the former case there is not a second promoter immediately upstream of the terminator. From our observations it appears that the majority of pyrG transcripts originate from the pyrG promoter, not the rpoE promoter, and, furthermore, that any bicistronic transcripts that may be formed are too unstable in vivo to be detected. Evidence for the cleavage of the B. subtilis gltX-cysES (25) and acsA-tyrS (5) transcripts at sites near the intercistronic terminators has been presented. Such cleavage may be a general feature of such transcripts.
Our hypothesis for the regulation of pyrG by an antitermination mechanism obviously needs further experimental testing. Such work is currently under way in our laboratory.
ACKNOWLEDGMENTS
We gratefully acknowledge Jim Hoch and Marta Perego for providing the pyrG-containing plasmids pJH4133 and pJH4521and John Helmann for providing the rpoE-containing plasmid pFL32. Jan Martinussen is gratefully acknowledged for calling our attention to conserved sequence features in the pyrG leaders from various bacteria and for sharing his unpublished observations with us. We also thank Xianmin Zeng for valuable suggestions concerning experimental design and John Cronan for helpful comments on the manuscript.
This research was supported by Public Health Service grant GM47112 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Amster-Choder O, Wright A. Transcriptional regulation of the bgl operon of Escherichia coli involves phosphotransferase system-mediated phosphorylation of a transcriptional antiterminator. J Cell Biochem. 1993;51:83–90. doi: 10.1002/jcb.240510115. [DOI] [PubMed] [Google Scholar]
- 2.Antson A A, Dodson E J, Dodson G, Greaves R B, Chen X-P, Gollnick P. Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA. Nature. 1999;401:235–242. doi: 10.1038/45730. [DOI] [PubMed] [Google Scholar]
- 3.Asahi S, Doi M, Tsunemi Y, Akiyama S. Regulation of pyrimidine nucleotide biosynthesis in cytidine deaminase-negative mutants of Bacillus subtilis. Agric Biol Chem. 1989;53:97–102. [Google Scholar]
- 4.Chai W, Stewart V. RNA sequence requirements for NasR-mediated, nitrate-responsive transcription antitermination of the Klebsiella oxytoca M5aI nasF operon leader. J Mol Biol. 1999;292:203–216. doi: 10.1006/jmbi.1999.3084. [DOI] [PubMed] [Google Scholar]
- 5.Condon C, Putzer H, Grunberg-Manago M. Processing of the leader mRNA plays a major role in the induction of thrS expression following threonine starvation in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:6992–6997. doi: 10.1073/pnas.93.14.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contente S, Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979;167:251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon Y, Breton R, Putzer H, Pelchat M, Grunberg-Manago M, Lapointe J. Clustering and co-transcription of the Bacillus subtilis genes encoding the aminoacyl-tRNA synthetases specific for glutamate and for cysteine and the first enzyme for cysteine biosynthesis. J Biol Chem. 1994;269:7473–7482. [PubMed] [Google Scholar]
- 8.Grandoni J A, Fulmer S B, Brizzio V, Zahler S A, Calvo J M. Regions of the Bacillus subtilis ilv-leu operon involved in regulation by leucine. J Bacteriol. 1993;175:7581–7593. doi: 10.1128/jb.175.23.7581-7593.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy F J, Waters D A, Takova T Y, Henkin T M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol. 1993;10:259–271. doi: 10.1111/j.1365-2958.1993.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 10.Henkin T M. Control of transcriptional termination in prokaryotes. Annu Rev Genet. 1996;30:35–57. doi: 10.1146/annurev.genet.30.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Hu P, Switzer R L. Evidence for substrate stabilization in regulation of the degradation of Bacillus subtilis aspartate transcarbamylase in vivo. Arch Biochem Biophys. 1995;316:260–262. doi: 10.1006/abbi.1995.1036. [DOI] [PubMed] [Google Scholar]
- 12.Jaeger J A, Turner D H, Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci USA. 1989;86:7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaeger J A, Turner D H, Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1989;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- 14.Kahler A E, Switzer R L. Identification of a novel gene of pyrimidine nucleotide biosynthesis, pyrDII, that is required for dihydrooroate dehydrogenase activity in Bacillus subtilis. J Bacteriol. 1996;178:5013–5016. doi: 10.1128/jb.178.16.5013-5016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloudová A, Fucik V. Transport of nucleoside in Bacillus subtilis: characteristics of cytidine uptake. Nucleic Acids Res. 1974;1:629–637. doi: 10.1093/nar/1.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 17.López de Saro F J, Woody A M, Helmann J D. Structural analysis of the Bacillus subtilis factor δ: a protein polyanion which displaces RNA from RNA polymerase. J Mol Biol. 1995;252:189–202. doi: 10.1006/jmbi.1995.0487. [DOI] [PubMed] [Google Scholar]
- 18.López de Saro F J, Yoshikawa N, Helmann J D. Expression, abundance, and RNA polymerase binding properties of the δ factor of Bacillus subtilis. J Biol Chem. 1999;274:15953–15958. doi: 10.1074/jbc.274.22.15953. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Turner R J, Switzer R L. Roles of the three transcriptional attenuators of the Bacillus subtilis pyrimidine biosynthetic operon in the regulation of its expression. J Bacteriol. 1995;177:1315–1325. doi: 10.1128/jb.177.5.1315-1325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manival X, Yang Y, Strub M P, Kochoyan M, Steinmetz M, Aymerich S. From genetic to structural characterization of a new class of RNA-binding domain within the SacY/BglG family of antiterminator proteins. EMBO J. 1997;16:5019–5029. doi: 10.1093/emboj/16.16.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinussen J, Glaser P, Andersen P S, Saxild H H. Two genes encoding uracil phosphoribosyltransferase are present in Bacillus subtilis. J Bacteriol. 1995;177:271–274. doi: 10.1128/jb.177.1.271-274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Neuhard J, Nygaard P. Purines and pyrimidines. In: Neidhardt F C, editor. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1987. pp. 445–473. [Google Scholar]
- 24.Nygaard P, Duckert P, Saxild H H. Role of adenine deaminase in purine salvage and nitrogen metabolism and characterization of the ade gene in Bacillus subtilis. J Bacteriol. 1996;178:846–853. doi: 10.1128/jb.178.3.846-853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelchat M, Lapointe J. In vivo and in vitro processing of the Bacillus subtilis transcript coding for glutamyl-tRNA synthetase, serine acetyltransferase, and cysteinyl-tRNA synthetase. RNA. 1999;5:281–289. doi: 10.1017/s1355838299980858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxild H H, Andersen L N, Hammer K. dra-nupC-pdp operon of Bacillus subtilis: nucleotide sequence, induction by deoxyribonucleosides, and transcriptional regulation by the deoR-encoded DeoR repressor protein. J Bacteriol. 1996;178:424–434. doi: 10.1128/jb.178.2.424-434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxild H H, Jacobsen J H, Nygaard P. Functional analysis of the Bacillus subtilis purT gene encoding formate-dependent glycinamide ribonucleotide transformylase. Microbiology. 1995;141:2211–2218. doi: 10.1099/13500872-141-9-2211. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz M, Richter R. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 31.Switzer R L, Turner R J, Lu Y. Regulation of the Bacillus subtilis pyrimidine biosynthetic operon by transcriptional attenuation: control of gene expression by an mRNA-binding protein. Prog Nucleic Acids Res Mol Biol. 1999;62:329–367. doi: 10.1016/s0079-6603(08)60512-7. [DOI] [PubMed] [Google Scholar]
- 32.Trach K, W. C J, Piggot P L D, Hoch J A. Complete sequence and transcriptional analysis of the spo0F region of the Bacillus subtilis chromosome. J Bacteriol. 1988;170:4194–4208. doi: 10.1128/jb.170.9.4194-4208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner R J, Lu Y, Switzer R L. Regulation of the Bacillus subtilis pyrimidine biosynthetic (pyr) gene cluster by an autogenous transcriptional attenuation mechanism. J Bacteriol. 1994;176:3708–3722. doi: 10.1128/jb.176.12.3708-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vagner V, Dervyn E, Ehrlich S D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 35.Van Tilbeurgh H, Manival X, Aymerich S, Lhoste J-M, Dumas C, Kochoyan M. Crystal structure of a new RNA-binding domain from the antiterminator protein SacY of Bacillus subtilis. EMBO J. 1997;16:5030–5036. doi: 10.1093/emboj/16.16.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West T P, O'Donovan G A. Repression of cytidine triphosphate synthetase in Salmonella typhimurium by pyrimidines during uridine nucleotide depletion. J Gen Microbiol. 1982;128:895–899. doi: 10.1099/00221287-128-4-895. [DOI] [PubMed] [Google Scholar]
- 37.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology, supplement 9. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1990. pp. 2.4.1–2.4.2. [Google Scholar]
- 38.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 39.Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981;9:133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]