Abstract
Atherosclerotic cardiovascular diseases (CVD) are among the leading causes of death in the world. Monocyte‐derived macrophages are key players in the pathophysiology of atherosclerosis. Innate immune memory following exposure of monocytes to atherogenic compounds, such as oxidized low‐density lipoproteins (oxLDL), termed trained immunity, can contribute to atherogenesis. The current study aimed to elucidate intracellular mechanisms of oxLDL‐induced trained immunity. Using untargeted intracellular metabolomics in isolated human primary monocytes, we show that oxLDL‐induced trained immunity results in alterations in the balance of intracellular steroid hormones in monocytes. This was reflected by a decrease in extracellular progesterone concentrations following LPS stimulation. To understand the potential effects of steroid hormones on trained immunity, monocytes were costimulated with oxLDL and the steroid hormones progesterone, hydrocortisone, dexamethasone, β‐estradiol, and dihydrotestosterone. Progesterone showed a unique ability to attenuate the enhanced TNFα and IL‐6 production following oxLDL‐induced trained immunity. Single nucleotide polymorphisms in the nuclear glucocorticoid, progesterone, and mineralocorticoid receptor were shown to correlate with ex vivo oxLDL‐induced trained immunity in 243 healthy volunteers. Pharmacologic inhibition experiments revealed that progesterone exerts the suppression of TNFα in trained immunity via the nuclear glucocorticoid and mineralocorticoid receptors. Our data show that progesterone has a unique ability to suppress oxLDL‐induced trained immunity. We hypothesize that this effect might contribute to the lower incidence of CVD in premenopausal women.
Keywords: cardiovascular disease, oxLDL, progesterone, steroid hormone, trained immunity
Graphical Abstract
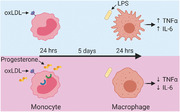
Cytokine production by oxLDL‐induced trained immunity is inhibited by the steroid hormone progesterone.
Abbreviations
- BCG
Bacillus Calmette‐Guérin
- CVD
cardiovascular disease
- DHT
dihydrotestosterone
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- MSEA
metabolite set enrichment analysis
- oxLDL
oxidized LDL
- PR
progesterone receptor
- SNP
single nucleotide polymorphism.
1. INTRODUCTION
Cardiovascular diseases (CVD) are among the deadliest diseases globally. 1 Atherosclerosis is the most important disease mechanism leading to CVD and is characterized by a state of chronic low‐grade inflammation of the arterial wall. Macrophages are the most abundant immune cells in atherosclerotic plaques and contribute to the initiation, progression, and destabilization of these plaques. A series of recent studies showed that the anti‐inflammatory drugs colchicine and canakinumab reduce CVD events, highlighting the important role of inflammation in the pathophysiology of CVD. 2 , 3 , 4
Macrophages preserve a long‐term nonspecific memory of previous encounters with immunologic stimuli, as seen by an augmented response in terms of cytokine production to subsequent immune challenge. This novel phenotype of innate immune adaption termed “trained immunity” is induced in response to a broad range of stimuli, such as β‐glucan, the Bacillus Calmette‐Guérin (BCG) vaccine, as well as endogenous ligands such as oxidized LDL (oxLDL). 5 , 6 , 7 , 8 This new avenue of macrophage activation is being studied for its potential role in chronic low‐grade inflammation, a cornerstone of atherosclerotic disease. OxLDL‐induced trained immunity is mediated, at least in part, by modulation of cellular metabolism and epigenetic remodeling. 7 , 9 This maladaptive innate immune reprogramming has been observed in atherosclerosis‐prone mice fed a Western‐type diet, which persisted even weeks after switching back to a normal diet. 10 Importantly, also in humans with elevated LDL cholesterol concentrations, circulating monocytes have a trained immune phenotype. 11 It is therefore rational to hypothesize that trained immunity contributes to long‐term activation of monocytes in patients with atherosclerosis. Indeed, circulating monocytes of patients with established coronary atherosclerosis show an increased cytokine production capacity, with accompanying epigenetic and metabolic characteristics of trained immunity. 12
Current management of patients with CVD is aimed at risk factor control. However, a considerable number of patients remain inadequately protected under current clinical guidelines, giving rise to a significant residual cardiovascular risk. This urges the identification of novel pharmacologic targets to reduce CVD. With gross metabolic reprogramming of the macrophage serving as an important scaffold for the sustained inflammation seen in atherosclerosis, there is interest in the nature of these metabolic changes for clinical understanding and manipulation. In order to elucidate new immunometabolic processes for the better prediction and management of CVD, we performed an unbiased metabolomics analysis of oxLDL‐trained macrophages. Herein, we found that the intracellular balance of steroid hormones was altered by oxLDL‐induced trained immunity. After investigating the immune modulatory effects of various key steroid hormones, we identified progesterone as an inhibitor of trained immunity, a mechanism that is partly under the control of the nuclear glucocorticoid and mineralocorticoid receptor (GR and MR).
2. MATERIALS AND METHODS
2.1. Reagents
Water‐soluble progesterone, β‐estradiol, 5α‐dihydrotestosterone (DHT) solution in methanol, hydrocortisone, mifepristone, PF‐02413873, spironolactone, and Escherichia coli LPS (serotype 055:B5) were all obtained from Sigma–Aldrich (St. Louis, MO, USA). LPS was further purified as described previously. 13 Dexamethasone was obtained from Centrafarm (Breda, The Netherlands).
2.2. Preparation of oxidized‐LDL
OxLDL was prepared from LDL as described previously. 14 Briefly, LDL was isolated from serum of healthy subjects via ultracentrifugation. LDL was then oxidized with 20 μM CuSO4 for 16 h in a shaking heat block at 37°C at 600 rpm followed by further dialysis. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). We previously showed that this oxLDL is endotoxin free. 7
2.3. PBMC and monocyte isolation
PBMCs and monocytes were isolated from blood from healthy donors after they provided written informed consent (Sanquin Blood Bank, Nijmegen, The Netherlands) as described previously. 15
2.4. In vitro training of adherent monocytes
Training of adherent monocytes was performed as described previously. 6 Adherent monocytes were incubated with 200 μl RPMI medium supplemented with 10 μg/ml oxLDL in presence or absence of the various test compounds. In experiments with pharmacologic interference with hormone receptors, compounds were added in combination with oxLDL and progesterone. Medium was supplemented with 10% human pooled serum. Cells were left to rest for 5 days with a medium change on day 3. Cells were restimulated with RPMI medium or 10 ng/ml LPS on day 6. After 24 h, supernatants were collected and stored at −20°C until cytokine measurements.
2.5. Cytokine, pregnenolone, and progesterone measurements
Production of TNFα, IL‐6, pregnenolone, and progesterone in supernatants was measured using the IL‐6 and TNF‐α DuoSet ELISA kits (R&D Systems, Minneapolis, MN, USA), Pregnenolone ELISA kit (Abnova, Taipei, Taiwan) and Progesterone Competitive ELISA Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's instructions.
2.6. Metabolomics analysis
Approximately 1 × 107 monocytes were seeded into 10 cm Petri dishes (Greiner, Alphen aan de Rijn, The Netherlands) ± oxLDL (10 μg/mL) in 10 ml medium volumes for 24 h, washed with warm PBS and incubated in normal culture medium at 37°C, 5% CO2. Following 5 days in culture, cells were washed 3 times with 75 mM ammonium carbonate adjusted to pH 7.4 with formic acid, and snap‐frozen in liquid nitrogen. Intracellular metabolites were extracted with 70% ethanol heated to 70°C, supernatants were collected and stored at −80°C. Untargeted analysis of intracellular metabolites was performed by flow injection‐time‐of‐flight mass spectrometry on an Agilent 6550 QTOF Instrument operated in negative mode 4 GHz, high resolution in a mass to charge (m/z) range of 50–1000, as described previously. 16
Data were analyzed using the online platform MetaboAnalyst version 4.0. Metabolite set enrichment analysis (MSEA) was performed on a list of mass spectrometry peaks and fold changes derived from the comparison of oxLDL trained to naïve macrophage metabolites. 17 , 18 Pathway analysis was performed using the manually curated metabolite MFN library original to the mummichog package.
2.7. Genetic analysis
Genotyping was performed on 267 healthy individuals of Western European ancestry from the 300BCG cohort 19 using the commercially available single nucleotide polymorphism (SNP) chip, Infinium Global Screening Array MD v1.0 from Illumina. Adjacent ex vivo training with oxLDL was carried out in PBMCs collected for each individual. The methods for QTL mapping have been described previously. 20
2.8. mRNA extraction and RT‐PCR
Monocytes were cultured as described earlier. mRNA was extracted by TRIzol (Life Technologies, Santa Clara, CA, USA) after 24 h and 6 days of oxLDL training, according to the manufacturer's instructions, and cDNA was synthesized using iScript Reverse Transcriptase (Invitrogen, Waltham, MA, USA). Relative expression was determined using the SYBR Green method (Invitrogen) on an Applied Biosciences StepOne PLUS qPCR machine, and the values are expressed as fold increases in mRNA levels, relative to those in nontrained cells, using 18s as a reference gene. Primers are listed in Table 1.
TABLE 1.
Forward and reverse primer sequences (5′–3′) for genes used for quantitative real‐time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| 18S | GATGGGCGGCGGAAAATAG | GCGTGGATTCTGCATAATGGT |
| PR (PGR) | GGAAGGGCAGCACAACTA | AAGGAATTGTATTAAGAAGTAA |
| GR | ATAGCTCTGTTCCAGACTCAACT | TCCTGAAACCTGGTATTGCCT |
| MR (NR3C2) | CAGCAGTGAAATGGGCAAAG | TCGTACATGCAGGGTAGAGT |
2.9. Statistics
Cytokine and hormone measurements were performed on cells from 6 donors. Data are presented as means ± sem. Trained immunity was expressed as the fold change of cytokine production capacity of oxLDL‐trained cells relative to the untrained cells, as previously reported. 8 , 21 In addition, in Figure S1, we provide the absolute cytokine values. Statistical testing was performed by using the Wilcoxon matched‐pairs signed rank test using GraphPad Prism 6. p Values below 0.05 were considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. Enrichment of steroid hormone metabolites in oxLDL‐trained macrophages
Metabolic reprogramming is a foundational aspect of trained immunity induction. Previous work on monocytes trained with the fungal cell wall component β‐glucan and the live attenuated vaccine BCG revealed marked alterations to the metabolism of the macrophages, with noticeable changes in glycolysis, oxidative phosphorylation, and glutamine utilization. 22 In addition, we recently reported that also oxLDL‐induced trained immunity is dependent on glycolysis 9 and OXPHOS. 23 In order to identify other metabolic pathways important for oxLDL‐trained immunity, we performed untargeted intracellular metabolomics on oxLDL‐trained macrophages. MSEA pathway analysis revealed “C21‐steroid hormone biosynthesis and metabolism” as the most altered pathway (Figure 1(A), adjusted p = 0.22, normalized enrichment score [NES] = −2.03). A NES of −2.03 suggests a lowered presence of intracellular steroid hormones in the trained cells. Additionally, “Androgen and estrogen biosynthesis and metabolism” appeared as the third most altered pathway (p adj = 0.22) with a NES of −1.69 (table with metabolites shown in Table S1 and S2, respectively).
FIGURE 1.
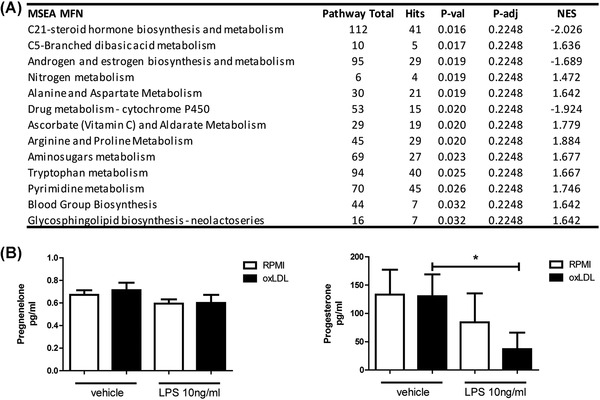
oxLDL‐induced trained immunity alters the abundance of intracellular and extracellular steroid hormones. Adherent monocytes were trained with oxLDL (10 μg/ml) or RPMI for 24 h, washed with PBS and rested in normal culture medium for 5 days. (A) Untargeted metabolomics analysis was performed on intracellular metabolites. Represented are the results of unannotated gene set enrichment (GSEA) pathway analysis of the identified raw m/z score and their respective p values (Table shows total pathway hits, hits from uploaded metabolites data, raw p values, gamma‐adjusted p value, and normalized enrichment score (NES); n = 3). (B) Following 5 days of resting in normal culture media, trained monocytes were stimulated with LPS (10 ng/ml) or RPMI for 24 h. Levels of pregnenolone and progesterone were determined via ELISA. (Wilcoxon matched pairs signed rank test, n = 6)
In an effort to validate the finding that steroid hormones are altered in oxLDL‐trained macrophages, we identified individual hormones of interest from these pathways. Pregnenolone was modestly decreased in the oxLDL‐trained macrophages (data not shown). Pregnenolone is a metabolite that is a precursor for the synthesis of all steroid hormones. Downstream of pregnenolone is progesterone that lays at the crossroads of many steroid hormone metabolic processes, while having well‐characterized effects on biologic processes including inflammation. 24 Progesterone was decreased in our dataset. We validated these findings using commercial ELISA kits, by measuring the concentrations of pregnenolone and progesterone in the supernatants of oxLDL‐trained macrophages (Figure 1(B)). This was done with and without the stimulation of LPS. Interestingly, extracellular levels of progesterone were lowered following LPS restimulation in the oxLDL‐trained macrophages, reminiscent of the MSEA where progesterone was lower in oxLDL‐trained macrophages. The concentration of pregnenolone was not altered by oxLDL training nor by LPS restimulation.
The mechanism by which these cells deplete progesterone from the medium is as yet unknown, though it is interesting to speculate that the hormones are depleted in order to facilitate an increase in proinflammatory cytokine production upon immune challenge. There is some evidence that tissue resident macrophages produce some of the necessary enzymes needed to metabolize hormones, 25 potentially offering clues to how LPS stimulation results in a decrease in hormone levels.
3.2. Progesterone but not hydrocortisone, dexamethasone, β‐estradiol, or DHT modulate oxLDL‐trained immunity in macrophages
Steroid hormones are well characterized as having immune regulatory effects, 24 , 26 therefore we next aimed to investigate the potential impact of adding various steroid hormones on the training response in monocytes stimulated with oxLDL. Progesterone, hydrocortisone, β‐estradiol, and DHT were selected for their central positions in “C21‐Steroid hormone biosynthesis and metabolism” and “Androgen and estrogen biosynthesis and metabolism,” respectively, as well as their well‐described biologic functions. The corticosteroid dexamethasone was also included. Here, adherent monocytes were exposed to RPMI medium or oxLDL combined with progesterone, β‐estradiol, DHT, hydrocortisone, or dexamethasone for 24 h. Following a 5‐day wash‐out period, cytokine production was determined in response to 24 h LPS restimulation (Figures 2(A)–2(E) and Figure S1 for absolute cytokine values).
FIGURE 2.
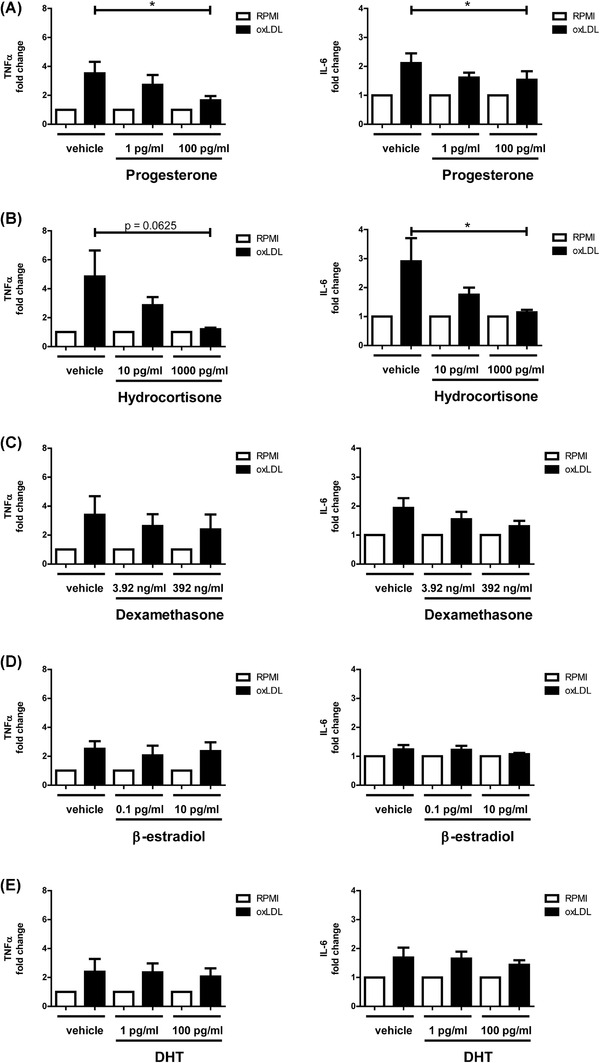
Progesterone inhibits oxLDL‐induced trained immunity. Monocytes were trained with oxLDL (10 μg/ml) or RPMI for 24 h ± (A) progesterone, (B) cortisol, (C) dexamethasone, (D) β‐estradiol, or (E) DHT. Monocytes were then washed with PBS, rested for 5 days in culture media, and subsequently stimulated with LPS (10 ng/ml) for 24 h. Levels of TNFα and IL‐6 were measured in supernatants via ELISA. (Wilcoxon matched pairs signed rank test, n = 6, *p < 0.05)
Progesterone at a concentration of 100 pg/ml significantly lowered TNFα production following oxLDL training, compared with the RPMI control condition (Figure 2(A)). Hydrocortisone also decreased TNFα production in oxLDL‐trained macrophages, although not significantly (p = 0.0625; Figure 2(B)). Progesterone and hydrocortisone significantly reduced IL‐6 production at concentrations of 100 and 1000 pg/ml, respectively. Although dexamethasone reduced cytokine production capacity in general in both trained and untrained cells, it did not specifically interfere with the trained immune response (i.e., did not affect the fold change of cytokine production in the trained cells). No meaningful reduction in either TNFα or IL‐6 production was observed for β‐estradiol or DHT (Figures 2(C)–2(E)). For β‐estradiol and DHT, previous data from de Bree et al. 27 have demonstrated the inability of these sex hormones to interfere with trained immunity induced by BCG.
It is well known that premenopausal women are less likely than men to suffer from CVD as a result of atherosclerosis. 28 This risk increases for postmenopausal women and may even exceed that of men. 29 Estrogen, the primary female sex hormone, is commonly considered to be responsible for the lowered risk of atherosclerosis in premenopausal women, due to its cardioprotective effects. 30 Following menopause, in addition to a reduction in the circulating estrogen concentration, there is a parallel decrease in the production of progesterone by the ovaries. However, research on the effect of progesterone levels on the risk for developing CVD is scarce and most studies on sex differences in CVD focus on the effects of estrogen on atherosclerosis, rather than those of progesterone. This lack of knowledge is present despite clear immune‐modulatory effects of progesterone interfering with signaling induced through pattern‐recognition receptors. 31 , 32 , 33 We now show that progesterone is able to ameliorate oxLDL‐induced trained immunity, which is thought to contribute to the incessant atherosclerotic vascular wall inflammation in CVD. We therefore hypothesize that this effect of progesterone might contribute to the lower incidence of CVD in premenopausal women, and the increase in CVD risk following menopause.
To better understand how progesterone modulates trained immunity, we performed a series of additional studies making use of genetic variation in progesterone‐related genes, and pharmacologic inhibitors of steroid hormone receptors.
3.3. SNPs in the hormone receptors and enzymes involved in steroidogenesis correlate with ex vivo oxLDL‐induced trained immunity in PBMCs
In order to gain more insight into the importance of steroid hormones in modulating oxLDL‐trained immunity, we investigated how SNPs (in a window of ±250 kb) in genes encoding for receptors and enzymes in the steroid metabolism pathway correlate with the ex vivo training capability of adherent PBMCs from 243 healthy volunteers, which were included in the 300BCG cohort of the Human Functional Genomic Study (www.humanfunctionalgenomics.org). In all these individuals, isolated PBMCs were exposed to oxLDL ex vivo according to the trained immunity protocol, and the fold change in TNFα and IL‐6 production after restimulation with LPS for 24 h on day 6 was measured and used as a measure for trained immunity. By using this approach in the 300BCG and other cohorts, we have previously identified important roles for enzymes involved in glycolysis and oxidative phosphorylation in oxLDL‐induced trained immunity.
We investigated genes encoding for the steroid hormone receptors as well as genes relevant for steroid metabolism (Figure 3(A)). We observed many SNPs strongly associated with an augmented cytokine response (p < 0.05). The most strongly correlated SNPs were found in the estrogen receptor ERS1 (rs2296254, p = 0.0004) for TNFα and the GR (rs246608, p = 0.0013) for IL‐6. There was also a relatively strong association between a SNP in GR with TNFα responses (rs246432, p = 0.0225), as well as SNPs for the nuclear progesterone receptor (PR) (PGR) for both TNFα and IL‐6 production capacity (rs660541, p = 0.0013; and rs1943758, p = 0.0036, respectively). SNPs around the MR (NR3C2) show nominal significance for TNFα (rs1529935, p = 0.0254) and IL‐6 (rs10032250, p = 0.0108). SNPs in the membrane PR PAQR8 further showed strong correlations with TNFα and IL‐6 production (rs12210492, p = 0.0032 and rs1266823, p = 0.0073, respectively).
FIGURE 3.
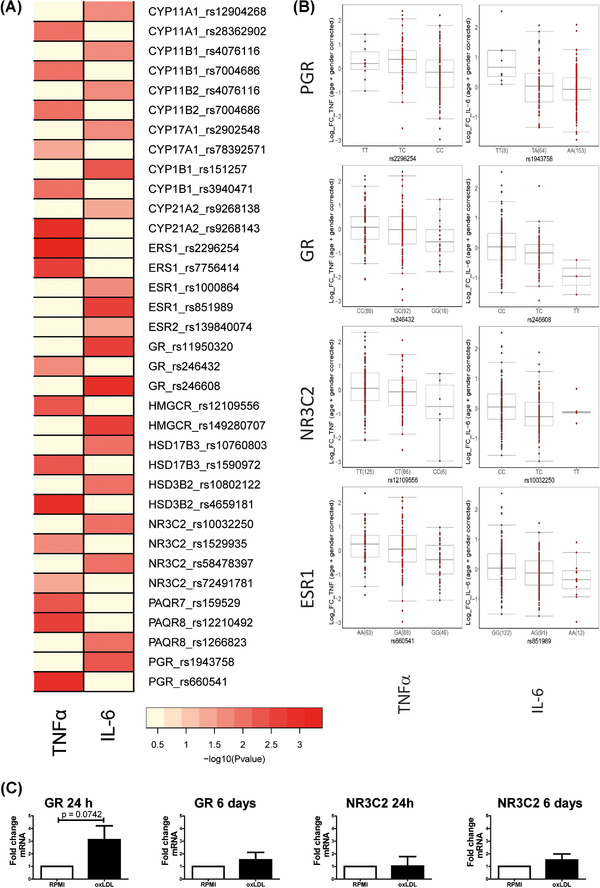
SNPs around the steroidogenesis genes correlate with cytokine production after ex vivo training with oxLDL. (A) Heatmap highlighting SNPs in the steroidogenesis pathways that correlate with levels of TNFα and IL‐6 following ex vivo training with oxLDL (10 μg/ml) and restimulation with LPS (10 ng/ml). The color legend for the heatmap indicates the range of p values from QTL mapping. (B) Boxplots showing the genotype‐stratified cytokine levels for the most strongly correlated SNPS in the PGR, GR, NR3C2, and ESR loci for TNFα and IL‐6, respectively (n = 215 healthy volunteers for TNFα, n = 228 healthy volunteers for IL‐6)
To explore the expression of the GR, MR, and PR, we measured the expression levels with RT‐PCR. We could demonstrate expression of the GR, and MR, but not of the PR. This is in accordance with a lack of expression data of the PR in human whole blood and monocytes/macrophages on the online GTEX platform (www.gtexportal.org) as well as the The Human Protein Atlas database (www.proteinatlas.org). Expression of the GR showed a trend toward being elevated following 24 h of oxLDL stimulation (Figure 3(C)); however, GR transcription was unaltered on day 6. Similarly, the MR (NR3C2) expression was unaltered by oxLDL treatment at both time points.
SNPs in genes relating to steroid metabolic processes correlated less strongly with cytokine production after oxLDL‐induced trained immunity. However, there was a correlation between a SNP in hydroxy‐delta‐5‐steroid dehydrogenase, 3 beta‐ and steroid delta‐isomerase 2 (HSD3B2) and TNFα (rs4659181, p = 0.0011). This gene encodes an enzyme that is responsible for the conversion of pregnenolone into progesterone. Another SNP in the HMG‐CoA reductase (HMGCR) enzyme that is responsible for the synthesis of cholesterol, which serves as the backbone of all steroid hormones, was correlated with IL‐6 production (rs149280707, p = 0.0029). HMGCR is an important rate limiting enzymes in the mevalonate pathway, which has previously been shown by our group to play an important role in trained immunity. 34
These findings underscore our in vitro findings that the steroid hormones are relevant for oxLDL‐induced trained immunity in human monocytes.
3.4. Nuclear hormone receptors are responsible for the immune modulatory effects of progesterone on oxLDL‐induced trained immunity
Pharmacologic interference of progesterone binding to its cognate receptors provides important evidence on the pathways through which progesterone leads to an inhibition of oxLDL‐induced trained immunity. Although SNPs around ESR1 correlated strongly with TNFα and IL‐6 production following oxLDL‐induced trained immunity, we have demonstrated that β‐estradiol does not modulate oxLDL‐induced trained immunity. To date, there are no reports of progesterone exerting any effects through the ESR1. Additionally, there are no commercially available inhibitors for the membrane PRs, and downstream targets of these G protein‐coupled receptors are ubiquitous to other cellular processes, which have already been implicated in having other, nonrelated roles in trained immunity. 8 , 35 With the unclear role that ESR1 and membrane PRs may play in macrophage responses toward progesterone, we decided to focus on the contributions of the nuclear receptors to the progesterone‐mediated suppression of oxLDL training, which we have shown to be expressed by these cells (Figure 3(C)).
To this end, the nuclear PR and GR antagonist mifepristone was added in conjunction with oxLDL and progesterone costimulation. Mifepristone restored oxLDL training in the presence of progesterone for TNFα, while having no restorative effect on IL‐6 production (Figure 4(A)). To further differentiate between the PR and GR, the selective PR antagonist PF‐02413873 was used. This PGR antagonist could not prevent the inhibiting effect of progesterone on oxLDL‐induced trained immunity (Figure 4(B)). Accordingly, we could not detect any PGR mRNA expression in oxLDL‐treated (24 h) and ‐trained (6 days) macrophages. These data suggest that the effects of mifepristone are largely conferred via the GR. Progesterone is also known to bind to the MR, which has recently been demonstrated to play a role in induction of trained immunity in monocytes stimulated with aldosterone. 36 Pharmacologic inhibition of the MR with spironolactone resulted in a small restoration of TNFα cytokine production upon the addition of progesterone, so that progesterone in the presence of spironolactone did not significantly lower TNFα production in oxLDL‐trained cells (Figure 4(C)). This finding suggests that, in addition to the GR, the MR is also involved in the inhibiting effect of progesterone on trained immunity.
FIGURE 4.
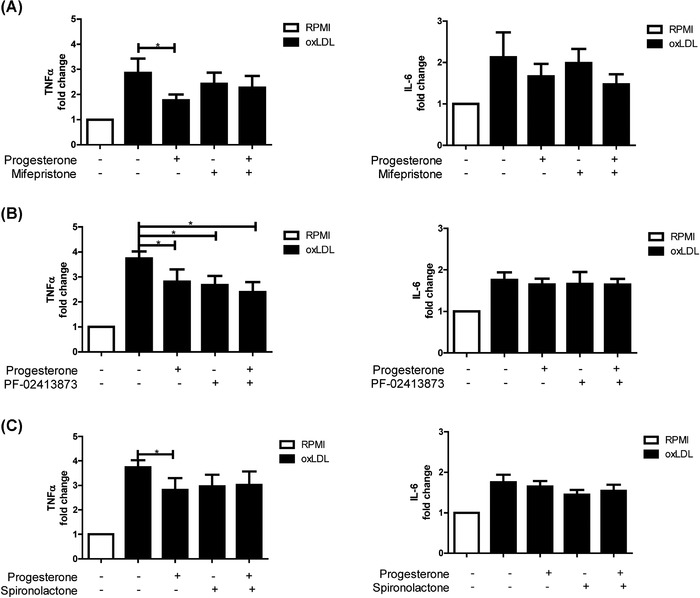
Nuclear progesterone, glucocorticoid, and mineralocorticoid receptors are important for the inhibition of oxLDL training by the steroid hormone progesterone. Monocytes were trained with oxLDL (10 μg/ml) or RPMI for 24 h ± progesterone in the presence of either (A) the combined PR and GR antagonist mifepristone (1 μM), (B) the selective PR antagonist PF‐02413873 (150 nM), or (C) the MR antagonist spironolactone (1 μM). Monocytes were then washed with PBS, rested for 5 days in culture media, and subsequently stimulated with LPS (10 ng/ml) for 24 h. Levels of TNFα and IL‐6 were measured in supernatants via ELISA. (Wilcoxon matched pairs signed rank test, n = 6, *p < 0.05)
Progesterone confers at least part of its inhibitory functions via the nuclear GR and MR, related pathways that are well studied in interfering with immune responses. The GR, once bound to progesterone, translocates into the nucleus where it interferes with the binding of NF‐κB and AP‐1 to their cognate gene targets. 37 Additionally, the GR acts as a transcription factor in its own right, driving the expression of many immune modulatory genes. Wang et al. 38 have demonstrated that addition of the synthetic glucocorticoid triamcinolone acetonide in a set‐up reminiscent of trained immunity, with a 24 h treatment followed by a 5 day wash out period, resulted in broad changes of the chromatin architecture which lead to the increased transcription of a number of anti‐inflammatory genes.
In summary, oxLDL induces a long‐term proinflammatory response in macrophages in vitro, 7 and this long‐term inflammation is believed to contribute to the pathogenesis of atherosclerosis and CVD. 12 The present study shows that progesterone specifically interferes with the enhanced cytokine production capacity in oxLDL‐trained macrophages. These effects are shown to be under the influence of the GR and the MR. Given the accumulating evidence that trained immunity contributes to the pathophysiology of atherosclerosis, we hypothesize that this interfering effect of progesterone might contribute to the lower CVD risk in premenopausal women, who have higher progesterone concentrations, but this needs to be further substantiated in future studies.
DISCLOSURE
The authors declare no conflicts of interest.
AUTHORSHIP
L.G. and N.P.R. conceived and designed the experiments. L.G., D.E.V., C.D.C.C.H, V.M., L.C.B. S.J.C.F.M.M., V.A.C.M.K, V.P.M., S.T.K., and J.H.P. performed the experiments. L.G. and D.E.V. analyzed the results. L.G. and D.E.V. wrote the manuscript. L.A.B.J., M.G.N., and N.P.R. critically read the manuscript.
Supporting information
Supplementary information
Supplementary information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
N.P.R. received funding from the European Union Horizon 2020 research and innovation program REPROGRAM under grant agreement No 667837, an IN‐CONTROL CVON grant (CVON2018‐27) of the Netherlands Heart Foundation, and a JTC2018 grant (“MEMORY”) from the European Research Area Network on Cardiovascular Disease (ERA‐CVD). Graphical abstract was created with BioRender.com.
Groh LA, Verel DE, van der Heijden CDCC, et al. Immune modulatory effects of progesterone on oxLDL‐induced trained immunity in monocytes. J Leukoc Biol. 2022;112:279–288. 10.1002/JLB.3AB1220-846R
Contributor Information
Laszlo A. Groh, Email: Laszlo.A.Groh@Radboudumc.nl.
Niels P. Riksen, Email: Niels.Riksen@Radboudumc.nl.
REFERENCES
- 1. Organization, W.H. , Noncommunicable diseases country profiles 2018. 2018.
- 2. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119‐1131. [DOI] [PubMed] [Google Scholar]
- 3. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838‐1847. [DOI] [PubMed] [Google Scholar]
- 4. Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low‐dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497‐2505. [DOI] [PubMed] [Google Scholar]
- 5. Arts RJW, Carvalho A, La Rocca C, et al. Immunometabolic pathways in BCG‐induced trained immunity. Cell Rep. 2016;17:2562‐2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bekkering S, Blok BA, Joosten LA, Riksen NP, van Crevel R, Netea MG. In vitro experimental model of trained innate immunity in human primary monocytes. Clin Vaccine Immunol. 2016;23:926‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, Riksen NP. Oxidized low‐density lipoprotein induces long‐term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol. 2014;34:1731‐8. [DOI] [PubMed] [Google Scholar]
- 8. Cheng SC, Quintin J, Cramer RA, et al. mTOR‐ and HIF‐1alpha‐mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keating ST, Groh L, Thiem K, et al. Rewiring of glucose metabolism defines trained immunity induced by oxidized low‐density lipoprotein. J Mol Med (Berl). 2020;8:819‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christ A, Günther P, Lauterbach MAR, et al. Western diet triggers NLRP3‐dependent innate immune reprogramming. Cell. 2018;172:162‐175.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekkering S, Stiekema LCA, Bernelot Moens S, et al. Treatment with statins does not revert trained immunity in patients with familial hypercholesterolemia. Cell Metab. 2019;30:1‐2. [DOI] [PubMed] [Google Scholar]
- 12. Bekkering S, van den Munckhof I, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. 2016;254:228‐236. [DOI] [PubMed] [Google Scholar]
- 13. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll‐like receptor 2. J Immunol. 2000;165:618‐22. [DOI] [PubMed] [Google Scholar]
- 14. van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro‐inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel‐like factor 2. Atherosclerosis. 2011;214:345‐9. [DOI] [PubMed] [Google Scholar]
- 15. Repnik U, Knezevic M, Jeras M. Simple and cost‐effective isolation of monocytes from buffy coats. J Immunol Methods. 2003;278:283‐92. [DOI] [PubMed] [Google Scholar]
- 16. Fuhrer T, Heer D, Begemann B, Zamboni N. High‐throughput, accurate mass metabolome profiling of cellular extracts by flow injection‐time‐of‐flight mass spectrometry. Anal Chem. 2011;83:7074‐80. [DOI] [PubMed] [Google Scholar]
- 17. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia J, Wishart DS. MSEA: a web‐based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010;38:W71‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koeken VA, de Bree LCJ, Mourits VP, et al. BCG vaccination in humans inhibits systemic inflammation in a sex‐dependent manner. J Clin Invest. 2020;130:5591‐5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keating ST, Groh L, van der Heijden C, et al. The Set7 lysine methyltransferase regulates plasticity in oxidative phosphorylation necessary for trained immunity induced by β‐glucan. Cell Rep. 2020;31:107548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Heijden C, Groh L, Keating ST, et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ Res. 2020;127:269‐283. [DOI] [PubMed] [Google Scholar]
- 22. Groh L, Keating ST, Joosten LAB, Netea MG, Riksen NP. Monocyte and macrophage immunometabolism in atherosclerosis. Semin Immunopathol. 2018;40:203‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Groh LA, Ferreira AV, Helder L, et al. oxLDL‐induced trained immunity is dependent on mitochondrial metabolic reprogramming. Immunometabolism. 2021;3:e210025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. dos Santos RL, da Silva FB, Ribeiro RF Jr, Stefanon I. Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig. 2014;18:89‐103. [DOI] [PubMed] [Google Scholar]
- 25. Chadwick JA, Swager SA, Lowe J, et al. Myeloid cells are capable of synthesizing aldosterone to exacerbate damage in muscular dystrophy. Hum Mol Genet. 2016;25:5167‐5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59:1‐14. [DOI] [PubMed] [Google Scholar]
- 27. de Bree LCJ, Janssen R, Aaby P, et al. The impact of sex hormones on BCG‐induced trained immunity. J Leukoc Biol. 2018;104:573‐578. [DOI] [PubMed] [Google Scholar]
- 28. Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21:1024‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathur P, Ostadal B, Romeo F, Mehta JL. Gender‐related differences in atherosclerosis. Cardiovasc Drugs Ther. 2015;29:319‐27. [DOI] [PubMed] [Google Scholar]
- 30. Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89:12E‐17E. discussion 17E‐18E. [DOI] [PubMed] [Google Scholar]
- 31. Jones LA, Anthony J‐P, Henriquez FL, et al. Toll‐like receptor‐4‐mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125:59‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lei K, Chen L, Georgiou EX, et al. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL‐1beta‐induced COX‐2 expression in human term myometrial cells. PLoS One. 2012;7:e50167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Su L, Sun Y, Ma F, Lü P, Huang H, Zhou J. Progesterone inhibits Toll‐like receptor 4‐mediated innate immune response in macrophages by suppressing NF‐kappaB activation and enhancing SOCS1 expression. Immunol Lett. 2009;125:151‐5. [DOI] [PubMed] [Google Scholar]
- 34. Bekkering S, Arts RJW, Novakovic B, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;172:135‐146.e9. [DOI] [PubMed] [Google Scholar]
- 35. Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte‐to‐macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Heijden C, Keating ST, Groh L, Joosten LAB, Netea MG, Riksen NP. Aldosterone induces trained immunity: the role of fatty acid synthesis. Cardiovasc Res. 2020;116:317‐328. [DOI] [PubMed] [Google Scholar]
- 37. Escoter‐Torres L, Caratti G, Mechtidou A, Tuckermann J, Uhlenhaut NH, Vettorazzi S. Fighting the fire: mechanisms of inflammatory gene regulation by the glucocorticoid receptor. Front Immunol. 2019;10:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang C, Nanni L, Novakovic B, et al. Extensive epigenomic integration of the glucocorticoid response in primary human monocytes and in vitro derived macrophages. Sci Rep. 2019;9:2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Supplementary information
Supplementary information


