Abstract
Aims
Pulmonary hypertension (PHT) may complicate heart failure with reduced ejection fraction (HFrEF) and is associated with a substantial symptom burden and poor prognosis. Sildenafil, a phosphodiesterase‐5 (PDE‐5) inhibitor, might have beneficial effects on pulmonary haemodynamics, cardiac function and exercise capacity in HFrEF and PHT. The aim of this study was to determine the safety, tolerability, and efficacy of sildenafil in patients with HFrEF and indirect evidence of PHT.
Methods and results
The Sildenafil in Heart Failure (SilHF) trial was an investigator‐led, randomized, multinational trial in which patients with HFrEF and a pulmonary artery systolic pressure (PASP) ≥40 mmHg by echocardiography were randomly assigned in a 2:1 ratio to receive sildenafil (up to 40 mg three times/day) or placebo. The co‐primary endpoints were improvement in patient global assessment by visual analogue scale and in the 6‐min walk test at 24 weeks. The planned sample size was 210 participants but, due to problems with supplying sildenafil/placebo and recruitment, only 69 patients (11 women, median age 68 (interquartile range [IQR] 62–74) years, median left ventricular ejection fraction 29% (IQR 24–35), median PASP 45 (IQR 42–55) mmHg) were included. Compared to placebo, sildenafil did not improve symptoms, quality of life, PASP or walk test distance. Sildenafil was generally well tolerated, but those assigned to sildenafil had numerically more serious adverse events (33% vs. 21%).
Conclusion
Compared to placebo, sildenafil did not improve symptoms, quality of life or exercise capacity in patients with HFrEF and PHT.
Keywords: Heart failure, Pulmonary hypertension, Phosphodiesterase‐5 inhibitors, Quality of life, Exercise capacity, Tolerability
The SilHF trial: sildenafil does not improve symptoms and exercise capacity in patients with heart failure with reduced ejection fraction (HFrEF) and secondary pulmonary hypertension. 6MWT, 6‐min walk test; CI, confidence interval; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; SD, standard deviation.

Introduction
Raised pulmonary artery (PA) pressure is a common finding in patients with heart failure, 1 , 2 , 3 reflecting increases in left atrial pressure, pulmonary vasoconstriction, reductions in pulmonary compliance, increase in endothelin levels and structural changes in the pulmonary arteriole and pulmonary capillary bed. 2 , 4 Endothelial dysfunction leading to impaired nitric oxide (NO) production may make an important contribution to the development of pulmonary hypertension (PHT). 5 Inhibition of the phosphodiesterase‐5 (PDE‐5) enzyme is a potential therapy for patients with heart failure. 6 , 7 , 8 PDE‐5 inhibitors prevent the degradation of cyclic guanosine monophosphate resulting in enhanced NO availability leading to vasodilatation, especially of the pulmonary circulation. 5 , 9 Sildenafil is widely used for the treatment of primary PHT 6 , 10 , 11 but robust evidence of benefit for patients with PHT secondary to left ventricular failure is lacking. Early studies suggested that sildenafil might have favourable effects on pulmonary haemodynamics, particularly in patients with heart failure with reduced ejection fraction (HFrEF). 12 , 13 , 14 , 15 Subsequently, several small, single‐centre trials and a meta‐analysis including 284 patients suggested that sildenafil improves symptoms and exercise capacity in HFrEF. 16 , 17 , 18
Accordingly, we performed a multicentre, placebo‐controlled trial to assess the effects of chronic sildenafil treatment on symptoms and exercise capacity in symptomatic patients with HFrEF and evidence of PHT on echocardiography.
Methods
Study design and protocol
The Sildenafil in Heart Failure (SilHF) trial was a 36‐week, investigator‐initiated, randomized, double‐blind, placebo‐controlled, multinational trial, designed to assess the efficacy, safety, and tolerability of chronic sildenafil treatment in patients with HFrEF. The design and rationale have been published. 8 The trial was funded by local grants from Stavanger University Hospital, and other participating centres, and a ‘Cyber Grant’ from Pfizer. Pfizer also provided sildenafil and matching placebo. The trial was performed in five participating centres in Norway, United Kingdom, Italy and Israel, coordinated from Stavanger University Hospital. An external mediator (J. Kjekshus, University of Oslo) reviewed all serious adverse events (SAE) and fulfilled the role of an unblinded data safety monitoring board. The trial protocol was approved by all members of the Steering Committee (online supplementary Appendix S1 ), and local regulatory approval was obtained by all participating centres. All patients provided written informed consent prior to enrolment in the study.
Patients were randomly assigned to receive sildenafil or placebo in a 2:1 ratio. Active treatment, in the form of sildenafil 20 mg provided by Pfizer, was packaged and blinded at Kragerø Tabelett Produksjon in Norway, with matching placebo. Study medication and placebo was subsequently distributed to all participating sites. The data were entered into an electronic case report form developed by the unit for Applied Clinical Research for the Faculty of Medicine at the Norwegian University of Science and Technology in Trondheim 19 which was also responsible for generating randomization codes.
Symptomatic outpatients aged >18 years with HFrEF (New York Heart Association [NYHA] functional class II–III and left ventricular ejection fraction [LVEF] ≤40%) were screened and enrolled if they had an elevated brain natriuretic peptide (BNP, >100 pg/ml) or N‐terminal proBNP (>400 pg/ml) in the year before enrolment, and PHT at screening, defined as a pulmonary artery systolic pressure (PASP) ≥40 mmHg on echocardiography/Doppler (using tricuspid regurgitation velocity and estimating right atrial pressure using inferior vena cava diameter and collapsibility). 20 All patients were on stable, guideline‐recommended therapy for a minimum of 3 months. The main exclusion criteria were acute coronary syndrome, angiography or hospitalization within last 3 months, angina, severe valvular heart disease, or symptomatic lung disease. Patients were also excluded if the 6‐min walk test (6MWT) distance was >475 m, estimated glomerular filtration was <40 ml/min/1.73 m2, or systemic systolic or diastolic arterial pressures were >160 mmHg and >90 mmHg, respectively. Additional inclusion and exclusion criteria are shown in online supplementary Appendix S1 .
Eligible patients were randomly allocated to either sildenafil or placebo in a ratio of 2:1. Patients were given an initial test dose of 10 mg with a 2‐hour observation period. The dose was then up‐titrated to a target maintenance dose of 40 mg three times a day over the following 2 weeks.
There were visits at screening, inclusion, up‐titration, 8 weeks, 16 weeks, 24 weeks and a final follow‐up telephone call at 36 weeks. Blood tests were performed at screening and at 24 weeks. Side effects and changes to medication were recorded at all visits. Adverse events (AE) and SAE were captured continuously and reviewed by J.K. The 6MWT was performed at both screening and inclusion (baseline) and at 8 and 24 weeks. The 6MWT distance at inclusion was considered baseline. Validated tools such as the Kansas City Cardiomyopathy Questionnaire (KCCQ) and EuroQol‐5D including a visual analogue scale (VAS) were used to assess quality of life 21 at inclusion, week 8 and week 24. Echocardiography was also performed at screening and weeks 8 and 24 (a full echocardiographic protocol is provided in online supplementary Appendix S1 ).
Efficacy and safety measurements
The co‐primary endpoints were patient global assessment as measured by a VAS as part of the EuroQol‐5D and the 6MWT at 24 weeks. Secondary endpoints were safety, tolerability, and changes in symptoms (NYHA functional class), and quality of life (KCCQ and EuroQol‐5D). Changes in renal function and PASP as assessed by echocardiography were also evaluated.
Statistical analysis
The study was designed as a large pilot trial, without a formal sample size calculation. The planned sample size was 210 participants, to be randomized in a 2:1 fashion in order to assess safety in more patients on active treatment. The sample size was mainly derived from the number needed to detect a significant change in the 6MWT. Based on available data at the time, this distance was set at 40 m. 20 However, due to financial, logistical and recruitment problems, only 69 patients were randomized.
At baseline, continuous measures are presented as median and 25th and 75th percentiles. Categorical measures are reported as numbers and percentages. Outcome measures are summarized at each follow‐up point, and as mean change, with standard deviation, from baseline. Outcomes were compared between randomized groups using linear regression, adjusting for baseline measures of the outcome. All analyses were according to the intention to treat principle, i.e. according to randomized group, regardless of treatment compliance. AE and side effects are reported as number of participants experiencing each type of event and are compared between groups using Fisher's exact tests. Missing data were not imputed. No adjustments were made for multiple comparisons, with p‐values <0.05 taken as showing statistical significance. All analyses were performed using R for Windows, v3.6.0.
Results
Recruitment was slower and more challenging than anticipated. Moreover, during the course of the study, supply of placebo and sildenafil was interrupted, which led to a temporary suspension of the trial for 18 months (from September 2015 until February 2017). Of the 69 patients enrolled, 45 were allocated to sildenafil and 24 to placebo (Figure 1 ). Their baseline characteristics are shown in Table 1 . Median age was 68 (Q1–Q3: 62–74) years and 16% were women. Line of identity plots is provided in Figure 2 . More than 50% reported moderate breathlessness on exertion, LVEF was often severely depressed (median 29% [Q1–Q3: 24–35]), median PASP was 45 (Q1–Q3: 42–55) mmHg and median 6MWT distance was 374 (Q1–Q3: 312–427) m. Forty‐six percent of patients were in chronic atrial fibrillation (AF) and 36% of patients had echocardiographic evidence of mitral regurgitation (MR).
Figure 1.
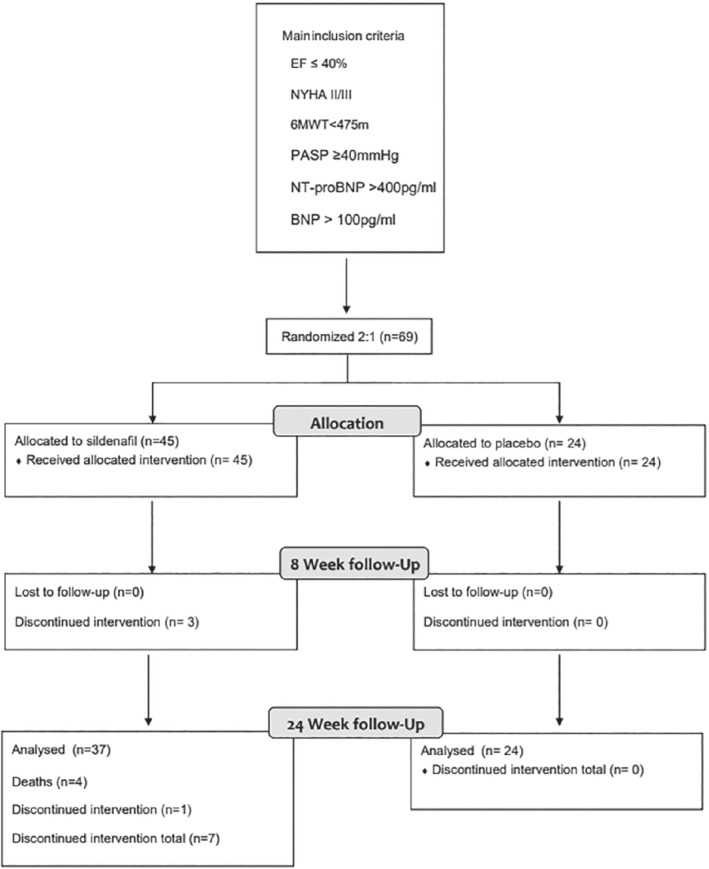
Consort diagram. 6MWT, 6‐min walk test; BNP, brain natriuretic peptide; EF, ejection fraction; NT‐proBP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
Table 1.
Baseline characteristics
| All (n = 69) | Placebo (n = 24) | Sildenafil (n = 45) | |
|---|---|---|---|
| Demographics and medical history | |||
| Age (years) | 68 (62–74) | 69 (63–74) | 67 (62–74) |
| Women | 11 (15.9) | 2 (8.3) | 9 (20.0) |
| CAD | 47 (68.1) | 17 (70.8) | 30 (66.7) |
| Stroke | 4 (5.8) | 0 (0.0) | 4 (8.9) |
| PCI/CABG | 35 (51.5) | 14 (58.3) | 21 (47.7) |
| Smoking | 7 (10.1) | 2 (8.3) | 5 (11.1) |
| Hypertension | 38 (55.1) | 14 (58.3) | 24 (53.3) |
| Dyslipidaemia | 49 (71.0) | 20 (83.3) | 29 (64.4) |
| Diabetes | 24 (34.8) | 10 (41.7) | 14 (31.1) |
| Atrial fibrillation | 32 (46.4) | 9 (37.5) | 23 (51.1) |
| Weight (kg) | 81.0 (70.1–89.5) | 80.0 (73.8–86.7) | 81.0 (70.0–89.6) |
| BMI (kg/m2) | 27.1 (24.0–29.6) | 27.0 (25.8–28.4) | 27.2 (23.8–29.9) |
| SBP (mmHg) | 115 (105–128) | 121 (107–131) | 113 (101–127) |
| DBP (mmHg) | 70 (61–76) | 70 (64–79) | 66 (61–75) |
| Heart rate (bpm) | 71 (65–76) | 70 (67–76) | 73 (64–76) |
| NYHA class II | 32 (46) | 12 (50) | 18 (44) |
| NYHA class III | 37 (54) | 12 (50) | 25 (56) |
| LVEF (%) | 29 (24–35) | 29 (25–35) | 29 (23–35) |
| PASP (mmHg) | 45 (42–55) | 44 (40–62) | 45 (42–54) |
| 6MWT distance (m) | 374 (312–427) | 406 (351–450) | 353 (311–400) |
| EQ‐5D health utility score | 0.796 (0.672–0.883) | 0.805 (0.700–0.883) | 0.779 (0.620–0.850) |
| EQ‐5D VAS | 62.5 (45.8–71.2) | 62.5 (50.0–73.8) | 62.5 (45.0–70.0) |
| KCCQ clinical summary score | 71.9 (58.3–83.3) | 74.5 (64.7–85.4) | 71.3 (52.6–82.3) |
| KCCQ overall summary score | 60.8 (48.2–73.1) | 66.2 (56.5–74.2) | 58.3 (45.3–72.4) |
| Blood results | |||
| Haemoglobin (g/dl) | 13.2 (12.0–14.4) | 13.6 (12.4–15.1) | 12.9 (11.9–14.3) |
| Creatinine (µmol/L) | 103 (88–119) | 89 (79–107) | 112 (95–126) |
| eGFR (ml/min/1.73 m2) | 58 (49–74) | 62 (56–85) | 53 (48–67) |
| NT‐proBNP (pg/ml) | 2109 (1781–2514) | 1668 (1112–2578) | |
| BNP (pg/ml) | 308 (165–762) | 384 (256–806) | |
| Treatment | |||
| Loop diuretic | 60 (87) | 19 (79) | 41 (91.1) |
| Thiazide diuretic | 4 (5.8) | 3 (12.5) | 1 (2.2) |
| Aldosterone antagonist | 54 (79.4) | 15 (65.2) | 39 (86.7) |
| ACEi or ARB | 64 (92.8) | 24 (100.0) | 40 (88.9) |
| Beta‐blocker | 66 (95.7) | 23 (95.8) | 43 (95.6) |
Values are mean (interquartile range), or n (%).
6MWT, six minute walk test; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; CAD, coronary artery disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EQ‐5D, EuroQol‐5 dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; VAS, visual analogue scale.
Figure 2.
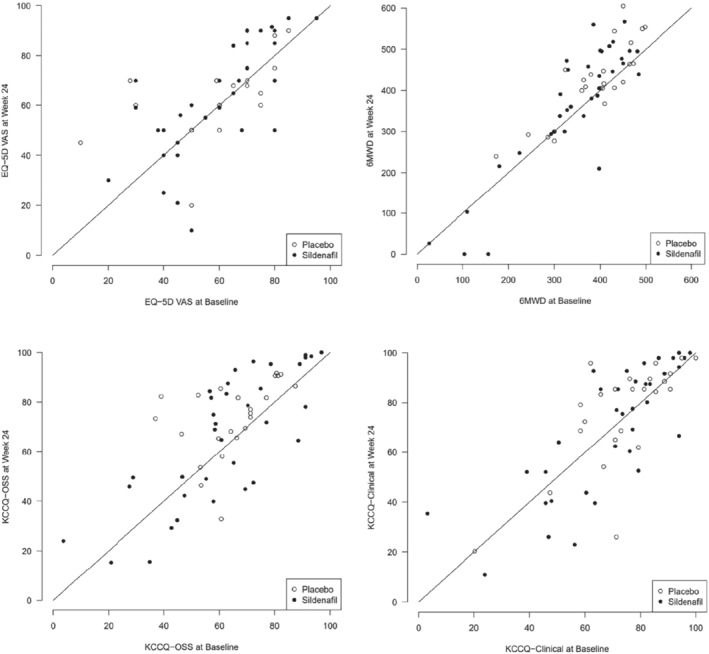
Line of identity plots on different outcome measures at baseline and week 24. 6MWD, 6‐min walk distance; EQ‐5D‐VAS, EuroQol Questionnaire‐5 dimensions visual analogue scale; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire overall summary score.
Outcome measures
There was no significant difference in 6MWT distance at either 8 or 24 weeks for patients assigned to sildenafil as compared to placebo (p = 0.37 and p = 0.88, respectively). At 24 weeks, the mean change in 6MWT distance was +33 (standard deviation [SD] 50) m in those assigned to placebo compared to +24 (SD 75) m for those assigned to sildenafil (Table 2 ).
Table 2.
Outcome measures – 6‐min walk test, EuroQol‐5D and Kansas City Cardiomyopathy Questionnaire
| n | Placebo | Sildenafil | Difference Estimate (95% CI), p‐value | |
|---|---|---|---|---|
| 6MWT distance (m) | ||||
| Participants with data at baseline and 8 weeks | ||||
| Baseline | 64 | 385.8 (83.5) | 336.8 (111.3) | |
| Week 8 | 64 | 396.3 (95.5) | 353.3 (134.4) | |
| Change | 64 | 10.5 (38.9) | 16.5 (50.6) | 11.2 (−13.4, 35.8), p = 0.3656 |
| Participants with data at baseline and 24 weeks | ||||
| Baseline | 58 | 386.8 (81.8) | 341.4 (113.6) | |
| Week 24 | 58 | 420.0 (94.4) | 365.4 (154.3) | |
| Change | 58 | 33.2 (50.2) | 24.0 (75.4) | −2.8 (−38.5, 32.9), p = 0.8765 |
| EQ‐5D visual analogue scale | ||||
| Participants with data at baseline and 8 weeks | ||||
| Baseline | 59 | 60.3 (18.9) | 60.3 (17.4) | |
| Week 8 | 59 | 63.4 (14.0) | 62.9 (18.9) | |
| Change | 59 | 3.1 (15.5) | 2.6 (10.4) | −0.48 (−6.70, 5.73), p = 0.8769 |
| Participants with data at baseline and 24 weeks | ||||
| Baseline | 52 | 59.9 (19.2) | 59.5 (18.9) | |
| Week 24 | 52 | 64.2 (16.6) | 62.4 (22.8) | |
| Change | 52 | 4.3 (17.0) | 2.9 (16.4) | −1.57 (−10.64, 7.50), p = 0.7295 |
| EQ‐5D health utility score | ||||
| Participants with data at baseline and 8 weeks | ||||
| Baseline | 57 | 0.761 (0.219) | 0.705 (0.276) | |
| Week 8 | 57 | 0.826 (0.123) | 0.739 (0.246) | |
| Change | 57 | 0.066 (0.186) | 0.035 (0.198) | −0.056 (−0.144, 0.032), p = 0.2082 |
| Participants with data at baseline and 24 weeks | ||||
| Baseline | 46 | 0.755 (0.236) | 0.777 (0.199) | |
| Week 24 | 46 | 0.740 (0.330) | 0.812 (0.227) | |
| Change | 46 | −0.015 (0.341) | 0.035 (0.179) | 0.058 (−0.089, 0.205), p = 0.4312 |
| KCCQ clinical summary score | ||||
| Participants with data at baseline and 8 weeks | ||||
| Baseline | 66 | 73.2 (17.3) | 68.4 (21.5) | |
| Week 8 | 66 | 77.9 (18.7) | 69.9 (24.7) | |
| Change | 66 | 4.7 (11.4) | 1.5 (15.7) | −3.7 (−11.1, 3.6), p = 0.3138 |
| Participants with data at baseline and 24 weeks | ||||
| Baseline | 60 | 73.2 (17.3) | 70.2 (21.5) | |
| Week 24 | 60 | 75.7 (21.4) | 70.7 (25.5) | |
| Change | 60 | 2.5 (15.1) | 0.5 (15.4) | −2.2 (−10.4, 5.9), p = 0.5879 |
Values are mean (standard deviation).
6MWT, 6‐min walk test; CI, confidence interval; EQ‐5D, EuroQol‐5 dimensions; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Changes in VAS score (EuroQol‐5D) were similar in those assigned placebo or sildenafil at both 8 and 24 weeks. At 24 weeks, the mean change in VAS was +4.3 (SD 17.0) in those assigned to placebo compared to +2.9 (SD 16.4) for those assigned to sildenafil. The changes in VAS were not significant.
Quality of life, as assessed by the mean differences in KCCQ overall summary score and EuroQol‐5D, did not change significantly during 24 weeks of follow‐up in either group. No significant differences were observed between patients assigned to sildenafil compared to placebo. This also applied to the KCCQ clinical summary score.
Renal function tended to decline in both groups during the course of the study, but no between group differences were observed. In patients randomized to sildenafil, mean systolic blood pressure did not change between baseline and 24 weeks. Mean systolic blood pressure for the sildenafil group was 115 (SD 17) mmHg and 115 (SD 22) mmHg at inclusion and week 24, respectively, and 121 (SD 17) mmHg and 120 (SD 18) mmHg in the control group. No significant differences in echocardiographic variables were observed (Table 3 ).
Table 3.
Main echocardiography measures and creatinine at baseline and 24 weeks
| Placebo | Sildenafil | |
|---|---|---|
| PASP inclusion (mmHg) | 52 (16) | 49 (9) |
| PASP week 24 (mmHg) | 45 (15) | 41 (13) |
| EF inclusion (%) | 29 (8) | 29 (8) |
| EF Week 24 (%) | 31 (8) | 30 (12) |
| E/E′ inclusion | 18 (11) | 21 (10) |
| E/E′ Week 24 | 15(7) | 20 (11) |
| Creatinine baseline (µmol/L) | 93 (30) | 110 (23) |
| Creatinine Week 24 (µmol/L) | 100 (20) | 118 (28) |
Values are mean (standard deviation).
EF, ejection fraction; PASP, pulmonary artery systolic pressure.
Adverse events
The proportions of patients with SAE were similar in each group, 5 (21%) with placebo and 15 (33%) with sildenafil. AE were also similar in each group (22% with sildenafil vs. 17% with placebo) (Table 4 ). There were no deaths in the placebo group, but four (8.9%) in those assigned to sildenafil including two unwitnessed out‐of‐hospital sudden deaths, for which the cause was not known to the principal investigator, one death from worsening heart failure and one death with lung cancer and pneumonia. There was a significant difference in temporary withdrawals from treatment, with 10 occurring in the sildenafil group and none in the placebo group (p = 0.01).
Table 4.
Safety outcomes
| Placebo | Sildenafil | p‐value a | |
|---|---|---|---|
| Patients with ≥1 SAE | 5 (21%) | 15 (33%) | 0.4 |
| SAE deemed related to intervention | 1 (4%) | 0 (0%) | 0.3 |
| Total number of SAE | 6 (25%) | 17 (38%) | |
| Patients with ≥1 AE | 4 (17%) | 10 (22%) | 0.8 |
| Hospitalizations | 5 (21%) | 13 (29%) | 0.6 |
| Deaths | 0 (0%) | 4 (9%) | 0.3 |
| Side effects | 1 (4%) | 10 (22%) | 0.08 |
| Temporary withdrawn due to any event | 0 (0%) | 10 (22%) | 0.01 |
AE, adverse event; SAE, serious adverse event.
Fisher's exact test.
Discussion
We did not detect any beneficial clinical or haemodynamic effects from chronic treatment with sildenafil in patients with symptomatic HFrEF and non‐invasive evidence of PHT.
The acute effects of sildenafil on the pulmonary circulation have been well documented, 21 and several small trials suggest that short‐term PDE‐5 inhibitor treatment may improve exercise capacity, symptoms and quality of life. 17 , 22 Chronic treatment with sildenafil was also reported to improve pulmonary haemodynamics, suggesting that the adverse vascular remodelling associated with PHT might be attenuated and even reversed. 7
Lewis and co‐workers 16 assessed sildenafil (25–75 mg three times a day for 12 weeks) in 34 patients with HFrEF and PHT, and found that sildenafil improved exercise capacity (measured by peak oxygen uptake [VO2] and 6MWT) and quality of life, but did not affect pulmonary artery pressures measured by right heart catheterization. Guazzi and colleagues 1 assessed the long‐term effects of sildenafil on brachial artery flow‐mediated dilatation (FMD) and exercise capacity in 46 patients with HFrEF and PHT. In addition to reductions in PASP and improvements in FMD, both breathlessness and exercise capacity (peak VO2) improved with sildenafil over 6 months. Behling and colleagues 22 also reported that sildenafil improved exercise capacity and reduced echocardiographic PASP in a randomized, placebo‐controlled trial of 19 patients with HFrEF.
Pulmonary hypertension secondary to left heart disease (PH‐LHD) usually reflects increases in left atrial pressure, with backwards hydrostatic pressure into the pulmonary circulation, called isolated post‐capillary pulmonary hypertension (ipcPH). However, this can progress to pulmonary vasoconstriction and changes in the pulmonary arterioles and microvasculature. Arteriolar constriction is followed by hypertrophy of arteriolar smooth muscle and vascular remodelling leads to increases in pulmonary vascular resistance and pressure, which is called combined pre‐ and post‐capillary pulmonary hypertension (cpcPH). Both forms are differentiated using by right heart catheterization. 2 , 3
As in the study performed by Lewis, 16 but in contrast to others, 1 , 22 we found no significant reduction in PASP with chronic administration of sildenafil. It is possible that this reflects differences in patient selection. Our trial included a high proportion of patients with permanent AF who are likely to have chronically elevated left atrial pressures. Patients with AF have been excluded from several other trials of sildenafil in patients with HFrEF (Table 5 ). 1 , 16 , 17 , 22 , 23 , 24 , 25 , 26 , 27 , 28 This difference may partially explain why our patients had, on average, a higher PASP than those investigated by Lewis or Guazzi. Pulmonary arteriolar constriction may have progressed to a component of fixed pulmonary arteriolar remodelling in our patients suggesting that we had missed the window of opportunity to reverse PHT. Another possible cause for discrepancies between studies may be the effect of MR. In our study, 38% of patients in the sildenafil group (31% in placebo) had echocardiographic evidence of MR. Pulmonary vasodilators such as sildenafil, might increase MR, thus increasing the backward pulsatile flow into the pulmonary circulation. 4 Previous studies have shown that PHT often persists after surgical correction of mitral valve disease and that acute improvements in haemodynamics with sildenafil for such patients may not be sustained over time. 23
Table 5.
Studies assessing phosphodiesterase‐5 inhibitors in heart failure
| SilHF | Guazzi 1 | Guazzi 17 | Lewis 16 | Amin 9 | Behling 22 | Bermejo 23 | Guazzi 24 | RELAX 25 | Belyavskiy 26 | Hoendermis 27 | Andersen 28 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial design | ||||||||||||
| Population | HFrEF | HFrEF | HFrEF | HFrEF | HFrEF | HFrEF | Valve disease | HFpEF | HFpEF | HFpEF | HFpEF | Post‐MI |
| Length of treatment | 24 weeks | 24 weeks | 52 weeks | 12 weeks | 12 weeks | 4 weeks | 6 months | 52 weeks | 24 weeks | 6 months | 12 weeks | 9 weeks |
| Target dose | 40 mg tds | 50 mg bd | 50 mg tds | 75 mg tds | 50 mg tds | 50 mg tds | 40 mg tds | 50 mg tds | 60 mg tds | 50 mg tds | 60 mg tds | 40 mg tds |
| Comparator | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | Placebo | UC | Placebo | Placebo |
| Key demographics | ||||||||||||
| Patients, n | 69 | 46 | 45 | 34 | 106 | 19 | 200 | 44 | 216 | 50 | 52 | 70 |
| Age (years) | 68 | 63 | 61 | 58 | 51 | 48 | 72 | 72 | 69 | 71 | 74 | 62 |
| Men (%) | 84 | 100 | 100 | 85 | 74 | 68 | 23 | 80 | 52 | 48 | 29 | 87 |
| AF (%) | 46 | 0 | 16 | NR | NR | 0 | 77 | 0 | 51 | 30 | 62 | Excluded |
| LVEF (%) | 29 | 32 | 30 | 20 | NR | 28 |
<55%: 52 ≥55%:133 |
60 | 60 | 61 | 58 | 56 |
| PASP (mmHg) | 45 | 33 | 38 | 31 a | NR | 59 | 62 | 53 | 41 | 57 | 52 | 27 |
| 6MWD (m) | 374 | NR | NR | 366 | 340 | NR | ∼350 | NR | 308 | ∼400 | NR | ∼560 |
| Key results (sildenafil vs placebo) | ||||||||||||
| Main findings |
↔VAS score ↔ 6MWD ↔QoL |
↑ PeakVO2 ↓ PASP a |
↑ PeakVO2 ↑ QoL ↑LV diastolic function echo |
↑ PeakVO2 ↔mPAP |
↔Tolerability ↔6MWD |
↑ PeakVO2 ↓ PASP |
↓ Clinically ↔6MWD ↔ PASP |
↓ PASP a ↑ RV function ↑ QoL |
↔PeakVO2 ↔6MWD ↔Clinical status |
↑ 6MWD ↑ NYHA ↑ Exercise |
↔mPAP a ↔PCWP ↔PeakVO2 |
↔PCWP ↔ mPAP |
| AE/SAE | ↔ SAEs | ↔ | ↔ | ↑ AE (headache) | ↔ | ↔ | ↑ HFH | NR | ↔ | NR | ↔ |
↑ AE (dyspepsia/headache) |
|
Deaths, n (%) |
||||||||||||
| Sildenafil | 4 (9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (3) | NR | 3 (3) | 0 (0) | 1 (4) | 0 (0) |
| Placebo |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
2 (4) |
1 (9) |
2 (2) |
NR |
0 (0) |
0 (0) |
1 (4) |
0 (0) |
6MWD, 6‐min walk distance; AE, adverse event; bd, twice a day; FMD, brachial artery flow‐mediated dilatation; HFH, heart failure hospitalization; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; mPAP, mean pulmonary artery pressure measured by right heart catheterization; NR, not recorded; NYHA, New York Heart Association; PASP, systolic pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; QoL, quality of life; RV, right ventricular; SAE, serious adverse event; SVR, systemic vascular resistance; tds, three times a day; UC, usual care; VAS, visual analogue scale; VO2, oxygen uptake.
Mean pulmonary artery pressure measured by echocardiography.
Trials assessing patients with heart failure with preserved ejection fraction (HFpEF) and PHT have met with little success. 29 One small trial by Guazzi and colleagues 24 showed improvements in exercise capacity and in pulmonary haemodynamics as measured by echocardiography. A larger trial performed by Redfield and colleagues, 25 which included patients with HFpEF with and without PHT, found no benefit on symptoms, exercise capacity or pulmonary haemodynamics. A study conducted by Hoendermis and co‐workers, assessing sildenafil in patients with HFpEF and mainly post‐capillary PHT, found no improvement in symptoms, exercise capacity or invasive haemodynamic measurements at 12 weeks. 27
Vericiguat, a novel oral soluble guanylate cyclase stimulator, also increases NO production by a different pathway from sildenafil. The VICTORIA trial, 30 a large multicentre trial assessing vericiguat in patients with advanced HFrEF, demonstrated a modest reduction in the primary composite endpoint of hospitalizations for worsening heart failure or cardiovascular mortality. The positive results of this trial may ensure continued interest in the NO pathway as a potential target for treatment in HFrEF with PHT.
Sildenafil was generally well tolerated, with rates of AE and SAE similar to placebo. There was a higher proportion of permanent discontinuations in the sildenafil group, but this was not statistically significant. This was mainly due to non‐cardiovascular causes, including prosthetic joint infection, two patients with urinary retention, subdural haematoma following a fall, oesophageal cancer, obstructive jaundice and the four deaths. None of these events were deemed likely related to the intervention by the attending clinician. All four deaths occurred in patients assigned to sildenafil. Temporary discontinuations of study drug was significantly higher, and all occurred in patients who were in the sildenafil group. Only one in three patients was randomized to placebo and therefore the numerical difference in deaths and temporary discontinuations may have arisen by chance due to the small sample size, especially of the placebo group. Temporary withdrawals were generally short, usually only lasting a few days due to minor side effects, most often headaches. This would also be in keeping with previous studies (Table 5 ). Several other studies where PDE‐5 inhibition has been given for other indications, such as erectile dysfunction, have also demonstrated safety and tolerability in patients with heart failure. 31 , 32 , 33
Limitations
There are several important limitations in our study. We randomized only 69 patients, which was substantially less than planned and underpowered the study. However, in contrast to trials evaluating clinical events, in which most patients do not contribute to the endpoints, all patients in SilHF contributed to the outcomes such as symptoms, exercise capacity and quality of life. Interventions that offer substantial clinical benefits on such outcomes do not need large trials to show important effects; indeed, large trials may detect effects that are statistically significant but clinically irrelevant for the majority of the patients. Trials of modest size also provide valuable information to estimate the likely magnitude and variability in treatment effect sizes, which can be used to determine how large a trial would be required to provide a definitive answer. Failure to detect a beneficial trend for any outcome in our trial makes it unlikely that a much larger trial would detect a clinically important effect. Additionally, as shown in Table 5 , our trial is one of the largest trial assessing PDE‐5 inhibition in HFrEF, and to our knowledge the first multicentre international trial. This was an investigator‐initiated research effort and the administration and logistics were not funded by the pharmaceutical industry. There were several major logistical delays, and obstacles in setting up research centres in the participating countries. Lastly, there were substantial delays in receiving further sildenafil and placebo when the first batch expired due to slow recruitment.
We did not require right heart catheterization to enrol patients, but used echo‐Doppler to detect and quantify PHT non‐invasively. Although the correlation between invasive and non‐invasive measurements is generally good, 34 we did not perform right heart catheterization and were unable to assess the reversibility of PHT, or differentiate between ipcPH and cpcPH. Patients with HFrEF and cpcPH might be a subgroup of patients who could obtain more benefit from chronic PDE‐5 inhibition.
Conclusion
Chronic treatment with sildenafil did not improve symptoms, quality of life or exercise capacity for patients with HFrEF and moderate PHT assessed by echocardiography. Based on this relatively small dataset, routine administration of sildenafil to this population is not recommended.
Supporting information
Appendix S1. Supplementary Information.
References
- 1. Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long‐term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–44. [DOI] [PubMed] [Google Scholar]
- 2. Guazzi M. Pulmonary hypertension and heart failure: a dangerous liaison. Heart Fail Clin. 2018;14:297–309. [DOI] [PubMed] [Google Scholar]
- 3. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al.; ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2015;37:67–119. [DOI] [PubMed] [Google Scholar]
- 4. Al‐Omary MS, Sugito S, Boyle AJ, Sverdlov AL, Collins NJ. Pulmonary hypertension due to left heart disease: diagnosis, pathophysiology, and therapy. Hypertension. 2020;75:1397–408. [DOI] [PubMed] [Google Scholar]
- 5. Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–90. [DOI] [PubMed] [Google Scholar]
- 6. Prasad S, Wilkinson J, Gatzoulis MA. Sildenafil in primary pulmonary hypertension. N Engl J Med. 2000;343:1342. [DOI] [PubMed] [Google Scholar]
- 7. Guazzi M, Labate V. Group 2 PH: medical therapy. Prog Cardiovasc Dis. 2016;59:71–7. [DOI] [PubMed] [Google Scholar]
- 8. Cooper TJ, Guazzi M, Al‐Mohammad A, Amir O, Bengal T, Cleland JG, et al. Sildenafil in Heart failure (SilHF). An investigator‐initiated multinational randomized controlled clinical trial: rationale and design. Eur J Heart Fail. 2013;15:119–22. [DOI] [PubMed] [Google Scholar]
- 9. Amin A, Mahmoudi E, Navid H, Chitsazan M. Is chronic sildenafil therapy safe and clinically beneficial in patients with systolic heart failure? Congest Heart Fail. 2013;19:99–103. [DOI] [PubMed] [Google Scholar]
- 10. Pepke‐Zaba J, Gilbert C, Collings L, Brown MC. Sildenafil improves health‐related quality of life in patients with pulmonary arterial hypertension. Chest. 2008;133:183–9. [DOI] [PubMed] [Google Scholar]
- 11. Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al.; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group . Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. [DOI] [PubMed] [Google Scholar]
- 12. Katz SD, Balidemaj K, Homma S, Wu H, Wang J, Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow‐mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36:845–51. [DOI] [PubMed] [Google Scholar]
- 13. Hirata K, Adji A, Vlachopoulos C, O'Rourke MF. Effect of sildenafil on cardiac performance in patients with heart failure. Am J Cardiol. 2005;96:1436–40. [DOI] [PubMed] [Google Scholar]
- 14. Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase‐5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–48. [DOI] [PubMed] [Google Scholar]
- 15. Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta‐adrenergic‐stimulated cardiac contractility in humans. Circulation. 2005;112:2642–9. [DOI] [PubMed] [Google Scholar]
- 16. Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–62. [DOI] [PubMed] [Google Scholar]
- 17. Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1 year, prospective, randomized, placebo‐controlled study. Circ Heart Fail. 2011;4:8–17. [DOI] [PubMed] [Google Scholar]
- 18. Barnes H, Brown Z, Burns A, Williams T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst Rev. 2019;1:CD012621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WebCRF—Unit for Applied Clinical Research, Faculty of Medicine, Norwegian University of Science and Technology, Trondheim. https://webcrf.medisin.ntnu.no
- 20. Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Triangulating clinically meaningful change in the six‐minute walk test in individuals with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2012;23:5–15. [PMC free article] [PubMed] [Google Scholar]
- 21. Damy T, Hobkirk J, Walters M, Ciobanu A, Rigby AS, Kallvikbacka‐Bennett A, et al. Development of a human model for the study of effects of hypoxia, exercise, and sildenafil on cardiac and vascular function in chronic heart failure. J Cardiovasc Pharmacol. 2015;66:229–38. [DOI] [PubMed] [Google Scholar]
- 22. Behling A, Rohde LE, Colombo FC, Goldraich LA, Stein R, Clausell N. Effects of 5′‐phosphodiesterase four‐week long inhibition with sildenafil in patients with chronic heart failure: a double‐blind, placebo‐controlled clinical trial. J Card Fail. 2008;14:189–97. [DOI] [PubMed] [Google Scholar]
- 23. Bermejo J, Yotti R, García‐Orta R, Sánchez‐Fernández PL, Castaño M, Segovia‐Cubero J, et al.; Sildenafil for Improving Outcomes after VAlvular Correction (SIOVAC) Investigators . Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. Eur Heart J. 2018;39:1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction. Circulation. 2011;124:164–74. [DOI] [PubMed] [Google Scholar]
- 25. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al.; RELAX Trial . Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belyavskiy E, Ovchinnikov A, Potekhina A, Ageev F, Edelmann F. Phosphodiesterase 5 inhibitor sildenafil in patients with heart failure with preserved ejection fraction and combined pre‐ and postcapillary pulmonary hypertension: a randomized open‐label pilot study. BMC Cardiovasc Disord. 2020;20:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoendermis ES, Liu LCY, Hummel YM, van der Meer P, de Boer RA, Berger RMF, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565–73. [DOI] [PubMed] [Google Scholar]
- 28. Andersen MJ, Ersbøll M, Axelsson A, Gustafsson F, Hassager C, Køber L, et al. Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction: the Sidenafil and Diastolic Dysfunction After Myocardial Infarction (SIDAMI) trial. Circulation. 2013;127:1200–8. [DOI] [PubMed] [Google Scholar]
- 29. Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(Suppl):D100–8. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al.; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–93. [DOI] [PubMed] [Google Scholar]
- 31. Webster LJ, Michelakis ED, Davis T, Archer SL. Use of sildenafil for safe improvement of erectile function and quality of life in men with New York Heart Association classes II and III congestive heart failure: a prospective, placebo‐controlled, double‐blind crossover trial. Arch Intern Med. 2004;164:514–20. [DOI] [PubMed] [Google Scholar]
- 32. Katz SD, Parker JD, Glasser DB, Bank AJ, Sherman N, Wang H, et al. Efficacy and safety of sildenafil citrate in men with erectile dysfunction and chronic heart failure. Am J Cardiol. 2005;95:36–42. [DOI] [PubMed] [Google Scholar]
- 33. Bocchi EA, Guimarães G, Mocelin A, Bacal F, Bellotti G, Ramires JF. Sildenafil effects on exercise, neurohormonal activation, and erectile dysfunction in congestive heart failure. Circulation. 2002;106:1097–103. [DOI] [PubMed] [Google Scholar]
- 34. D'Alto M, Romeo E, Argiento P, D'Andrea A, Vanderpool R, Correra A, et al. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol. 2013;168:4058–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Information.


