Abstract
Eosinophilic esophagitis (EoE) is an atopic disease of the esophagus that has shown a significant increase in incidence and prevalence in the last 20 years. The etiology of EoE is unclear, and few studies explore the esophageal microbiota in EoE. The local microbiome has been implicated in the pathogenesis of several allergic and inflammatory diseases, such as asthma and eczema. In this study, we performed a systematic review to evaluate differences in the microbiota profile of patients with EoE compared with controls. MEDLINE, Embase, Cochrane Library, Scopus, and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases were searched to identify studies investigating the microbiota composition in EoE. Three reviewers screened the articles for eligibility and quality. Seven articles underwent full‐text review, and a narrative synthesis was undertaken. The microbiota of the mouth and esophagus are correlated. Patients with active EoE present increased esophageal microbial load and increased abundance in particular species, such as Haemophilus and Aggregatibacter. On the other hand, EoE patients present a decrease in Firmicutes. High microbial load and abundance of Haemophilus are observed in EoE patients, but little evidence exists to demonstrate their influence on inflammation and disease. Understanding microbial signatures in EoE might contribute to the development of novel therapeutic strategies.
Keywords: 16S rRNA, eosinophilic esophagitis, microbiota
Introduction
Eosinophilic esophagitis (EoE) is an increasingly common chronic allergic disease characterized by eosinophilic inflammation in the esophagus and a type 2 immune profile. The pathophysiology of EoE is not entirely understood; however, evidence suggests that an impaired epithelial barrier allows contact between allergens and the esophageal mucosa, leading to the release of alarmins by the epithelium. Alarmins then initiate, through type 2 innate lymphoid cells (ILC2s) and basophils, an immune response that consists of the release of several cytokines, including IL‐4, IL‐5, and IL‐13. 1 Those cytokines induce eosinophilic inflammation and further barrier disruption. In parallel with this process, tissue‐resident antigen‐presenting cells (APC) activate CD4+ T helper type 2 (Th2) cells following contact with the antigen. Through cytokine signaling, Th2 cells recruit and activate eosinophils, mast cells, and plasma cells, which induce the localized production of IgE and IgG4. 2 , 3 The release of IgE then triggers the release of TGF‐β from mast cells, which leads to further inflammation and tissue fibrosis. 4 , 5
EoE causes heartburn, dysphagia, food impaction and, if inflammation is left untreated, can progress to fibrostenosis. 6 In children, EoE can also cause feeding intolerance, nausea, vomiting, and failure to thrive. 7 The pooled incidence is 6.6 per 100 000 cases in children and 7.7 per 100 000 in adults, and the prevalence is 34.4 cases per 100 000 inhabitants. Previous research has demonstrated that sex is a significant risk factor for EoE, where males have three times increased susceptibility in comparison to females. 8 Current treatment options include dietary exclusion of trigger foods, continuous pharmacological treatment with a proton‐pump inhibitor (PPI) or topical corticosteroids, and endoscopic dilations in fibrotic patients. 9
Patients with EoE commonly present with concomitant atopic disorders such as rhinitis, asthma, and eczema. However, these conditions have not been proven to predispose to EoE. 10 The local microbiota is implicated in the pathogenesis of several atopic diseases. 11 , 12 Park et al. published a systematic review in 2020 on the esophageal microbiota in health and disease, including esophageal cancer, Barrett's esophagus, and two EoE articles. 13 The rise in microbiome studies led to a rapid increase in published articles on the microbiome in EoE. Here, we review all available articles to identify and appraise existing information from published peer‐reviewed literature on the local microbiota, specifically in EoE.
Until recently, the esophagus was not considered to have a mucosa‐associated microbiome, although the rise of the small ribosomal subunit (16S rRNA) gene amplicon sequencing has allowed growth‐independent organism characterization, which indicates that this organ contains a resident microbial population that shifts in different health states. 14
We hypothesize that an inflamed esophageal mucosa will impact tissue metabolism, influencing the expression of bacterial virulence factors within the microbiota. It is believed that these virulence factors may contribute to even further inflammation and eosinophil recruitment. To address this hypothesis, we synthetized peer‐reviewed data to investigate the possibility of a distinct microbiome in EoE patients.
Methods
Protocol and registration
We followed the recommended approach described in the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. 15 The protocol for this review has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) under the registration ID CRD42020172862.
Search strategy
MEDLINE, Embase, Cochrane Library, Scopus, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases were searched for relevant studies published within 1993, when EoE was described as a clinical‐pathological disorder distinct from eosinophilic gastroenteritis, 16 and January 2022.
Studies were retrieved and independently screened by three authors. Based on our inclusion and exclusion criteria, relevant studies were selected to be full text screened.
The following search strategy was used for MEDLINE: eosinophilic esophagitis OR eosinophilic oesophagitis OR ee OR eoe OR esophagus OR oesophagus OR esophageal OR oesophageal OR esophagi OR oesophagi; AND microbiota OR microbiome OR microenvironment OR microflora OR flora OR microorganism OR bacteria AND 16S OR RNA OR Ribosomal. This keyword strategy was adapted and reviewed to fit the other databases. Eligible peer‐reviewed articles and gray literature were included in this review. Disagreements were settled by discussion between the authors.
Inclusion and exclusion criteria
Case–control studies that identified the oral and esophageal microbial population by 16S rRNA sequencing of adult and pediatric patients diagnosed with EoE were selected for inclusion in this review. Studies with publication dates prior to 1993 were excluded, as were studies that adopted any microbial identification methods other than rRNA sequencing or that were not published in English. Reviews were also excluded.
Outcome measures
The primary outcome is to identify characteristics in the microbiota of patients with EoE, allowing us to determine if there is a typical bacterial profile in EoE. This could allow a better understanding of how commensal bacteria behave in an EoE environment. Data extracted from each study included title, year of publication, country of study, number of subjects (cases and controls), treatment status, sample type and site, 16S rRNA gene region of analysis, bacterial abundance, bacterial diversity, and final conclusions.
Quality of studies
The quality of the articles was assessed by two reviewers and scored based on the Newcastle‐Ottawa Scale (NOS). 17 The NOS evaluates the quality of group selection, group comparability, and exposure for case–control studies. Each component is awarded zero or one point (marked as a star *), except for the comparability item, which may receive one or one two stars. Studies that sum up 1–3 starts are classified as low quality, 4–6 stars are moderate quality, and 7–9 stars are considered high quality.
Results
A total of 956 articles were identified in the initial literature search. Thirty duplicates were identified and excluded. After the exclusion of duplicates, 926 studies were screened. Thirty‐six relevant studies were selected for full‐text screening, and a final number of seven full texts and three abstracts were included in this review. The majority of studies were excluded because the subjects presented with conditions other than EoE. These results are summarized in Figure 1.
Figure 1.
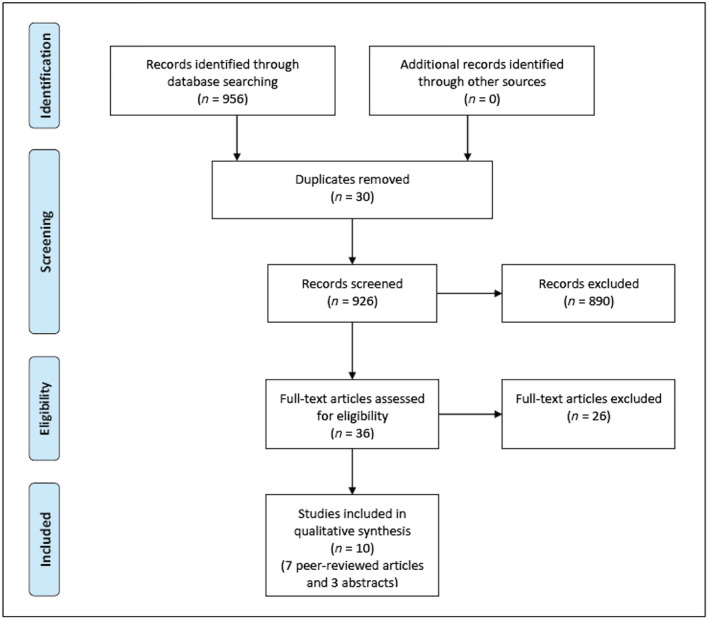
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram of selected studies.
The studies included in this review analyzed esophageal biopsies or brushings, saliva, and oral swabs from adult and children with and without EoE. A total of 471 patients were included, however, due to the diversity in sample type and the employment of different primer regions for bacterial sequencing, the results could not be pooled for a single analysis, instead, we assembled comparable measures among studies. This information is outlined in Table 1, while a summary of the bacteria reported in the studies can be found in Table 2.
Table 1.
General characteristics of the included studies
| Study | Primer region | Sample | Patient number/PPI status | Findings | Quality of the study |
|---|---|---|---|---|---|
| Benitez et al. 18 | V1V2 | Pediatrics oral swab and esophageal biopsy | 68 participants (33 EoE cases, 35 non‐EoE controls),66 were on PPIs |
Non‐EoE controls: ↑ Streptococcus and Atopobium Active EoE: ↑ Neisseria and Corynebacterium genus In addition to food trigger to diet: ↑ Granulicatella and Campylobacter genus |
8* |
| Hiremath et al. 19 | V4 | Pediatrics salivary samples | 45 participants (15 active EoE, 11 inactive EoE, 19 non‐EoE controls), 33 were on PPIs |
Active EoE: ↑ Haemophilus PPI use: ↑ Streptococcus, Corynebacterium, and Rothia Active EoE: ↓ Leptotrichiaceae, Actinomyces, Lactobacillus, and Streptococcus Non‐EoE controls: ↑ Neisseriaceae |
9* |
| Harris et al. 20 | V1V2 | Pediatrics and adults, EoE and GERD using esophageal string test | 70 participants (11 active EoE, 26 inactive EoE, 33 controls), some control subjects and half of the EoE subjects were on PPIs |
↑ Bacterial load in active EoE, non‐active EoE, and GERD EoE: ↑ Haemophilus, Pasteurella, Fusobacterium, and Aggregatibacter EoE: ↓ Actinomyces, Veillonella, and Rothia |
8* |
| Arias et al. 21 | V4 | Adult esophageal biopsy |
20 participants (10 controls, 10 EoE before and after remission) PPI non‐responsive EoE patients |
Active EoE: ↑ bacterial load Microbial load normalized after food elimination diet |
9* |
| Laserna‐Mendieta et al. 22 | V4 | Adult middle esophageal biopsy in active EoE and after remission through different treatments |
40 participants (30 active EoE, 10 PPI responsive, 10 swallowed topical corticosteroids,10 food‐elimination diet, 10 non‐EoE controls) |
Control: ↑ Proteobacteria ↓ Bacteroidetes Active EoE: ↓ Filifactor, ↓ Parvimonas, ↓ Porphyromonas Treated with swallowed topical corticosteroid: ↓ Firmicutes, ↑ Proteobacteria, Bacteroidetes and Fusobacteria Treated with PPIs: ↓ Bacteroidetes and Fusobacteria |
9* |
| Benitez et al. 23 | V1V2 | Pediatric esophageal biopsy | 33 active EoE, 36 inactive EoE, 10 controls (all patient were on PPIs) |
Active EoE: ↑ Haemophilus EoE: ↓ Alloprevotella STC in EoE: ↓ Haemophilus ↑ Candida |
8* |
| Johnson et al. 24 | V3V4 | PPI non‐responsive adults, mid‐esophageal biopsy | 24 PPI non‐responsive EoE patients (all on PPI), 25 non‐EoE controls (16 on PPI) | No significant differences found between EoE cases and controlsPPI use: ↑ Burkholderia, Eikenella, and Kingella | 8* |
| Parashette et al. 25 | Not specified | Pediatric biopsy | 22 healthy, 5 PPI‐responsive EoE, 9 PPI‐non responsive EoE |
PPI non‐responsive EoE: ↑ Gemella, Hallela PPI responsive EoE: ↑ Actinomyces, Catonella, Porphyromonas |
abstract |
| Smith et al. 26 | V4 | Pediatric cytology brush samples | 18 controls, 7 reflux esophagitis, 6 active EoE, 7 treated EoE, 5 untreated IBD, 5 treated IBD |
Esophagus: EoE: ↑ Bacteroidetes Colon: Non‐active EoE: ↑ Clostridia Controls: ↑ Bacteroidia |
abstract |
| Ghisa et al. 27 | Not specified | Not specified | 8 active, 8 inactive |
Active EoE: ↑ Actinobacillus, Alloprevotella, Spirochaetes Non‐active EoE: ↑ Firmicutes |
abstract |
Table 2.
Characteristics of bacteria found in the esophagus
| Phylum | Genus | Characteristics | Results | Pathogenicity status | Previously associated with |
|---|---|---|---|---|---|
| Proteobacteria | Neisseria |
Gram‐negative, aerobic. They extract and import iron from the human host through iron‐binding proteins hemoglobin, lactoferrin, and transferrin 28 |
Contradicting results showed this genus to be enriched in EoE samples 18 and enriched in non‐EoE controls. 19 However, the non‐EoE controls in Hiremath et al's 19 study presented symptoms of esophageal dysfunction. | Pathogen or opportunistic commensal 29 | Abundant in the oral cavity. |
| Proteobacteria | Aggregatibacter | Formerly Haemophilus. Gram‐negative, fastidious, non‐hemolytic, capnophilic 33 | Enriched in EoE samples 20 | Pathogen or opportunistic commensal. | |
| Proteobacteria | Pasteurella | Gram‐negative, anaerobic | Enriched in EoE samples 20 | Pathogen or opportunistic commensal capable of causing infections in humans, domestic and wild animals | |
| Proteobacteria | Actinobacillus | Gram‐negative, aerobic, microaerophilic or facultatively anaerobic, fermenting carbohydrates with the production of acid 38 | Enriched in EoE 39 | Pathogen or opportunistic commensal |
|
| Proteobacteria | Campylobacter | Gram‐negative, microaerophilic, presents flagella (adhesion and invasion) and produces toxins. 40 | Enriched in addition to food trigger to diet 18 | Pathogen or opportunistic commensal |
|
| Proteobacteria | Haemophilus | Gram‐negative, often encapsulated 42 |
Enriched in active EoE, 19 Enriched in EoE samples 20 |
Pathogen or opportunistic commensal | |
| Firmicutes | Streptococcus | Gram‐positive, non‐motile, catalase‐negative, facultative anaerobic. They are classified based on their hemolytic capacity (α‐, β‐, γ‐hemolysis). 42 |
Enriched in non‐EoE control samples, 18 Enriched with PPI use, 19 Decreased in active EoE 19 |
Commensal, beneficial and pathogenic species 46 | |
| Firmicutes | Granulicatella | Gram‐positive, facultative anaerobic 49 | Enriched in addition to food trigger to diet 18 | Normal flora or opportunistic commensal |
|
| Firmicutes | Lactobacillus | Gram‐positive, aero tolerant anaerobes or microaerophilic, non‐spore‐forming | Decreased in active EoE 19 | Commensal, beneficial, commonly used as probiotics 50 | |
| Firmicutes | Veillonella | Gram‐negative, lactate fermenting, anaerobic. 51 | Decreased in EoE samples 20 | Normal flora but have also been associated with oral diseases. |
|
| Firmicutes | Filifactor | Gram‐positive, anaerobic 52 | Decreased in active EoE 22 | Pathogen or opportunistic commensal |
|
| Firmicutes | Parvinomas | Anaerobic, Gram‐positive 53 | Decreased in active EoE 22 | Commensal, found in the oral cavity, respiratory system, gastrointestinal tract and vagina |
|
| Bacteroidetes | Porphyronomas | Anaerobic, Gram‐negative 54 | Decreased in active EoE 22 | Keystone pathogen |
|
| Actinobacteria | Atopobium | Anaerobic, catalase‐negative, Gram‐positive | Enriched in non‐EoE control samples 18 | Normal oral flora, vaginal commensal | |
| Actinobacteria | Actinomyces | Facultative anaerobic, Gram‐positive |
Decreased in active EoE, 19 Decreased in EoE samples 20 |
Commensal inhabitants of the oral cavity, pharynx, gut, genitourinary tract, and skin. Facultative pathogenic |
|
| Actinobacteria | Corynebacterium | Catalase‐positive, aerobic, Gram‐positive bacilli |
Enriched in EoE samples, 18 Enriched with PPI use 19 |
Commensal, opportunistic. |
|
| Actinobacteria | Rothia | Aerobic or facultative anaerobic, non‐motile non‐spore‐forming Gram‐positive cocco‐bacilli |
Decreased with PPI use, 19 |
Normal flora of the human oropharynx and upper respiratory tract, opportunistic. |
|
| Fusobacteria | Leptotrichiaceae | Obligate anaerobic or capnophilic. 61 | Decreased in active EoE 19 | Present in the oral cavities, gastrointestinal or urogenital tracts of humans and animals, containing both members of the resident microbiota and pathogens. | The Leptotrichiaceae family is rarely isolated and are found in mucous membranes, but when introduced into new tissue, they gain pathogenic potential. 61 |
| Fusobacteria | Fusobacterium | Obligate anaerobic Gram‐negative | Enriched in EoE samples 20 | Symbiont, opportunist, commensal of the oral cavity. |
|
| Spirochaete | Not specified | Unique architecture embracing both Gram‐positive and Gram‐negative features. Treponemes and Borrelia lack lipopolysaccharide. 63 | Enriched in active EoE 27 | Parasitic and commensal species |
|
| Proteobacteria | Burkholderia | Gram‐negative, previous part of Pseudomonas genus 64 | Enriched with PPI use 24 | Oportunistic pathogen, obligate pathogen and commensal species. Some possess anti‐fungal properties |
|
| Proteobacteria | Eikenella | Facultative anaerobic, Gram‐negative. 65 | Enriched with PPI use 24 | Commensal of the mouth, intestine and genital tract |
|
| Proteobacteria | Kingella | Gram‐negative, facultative anaerobic 66 | Enriched with PPI use 24 | Commensal organism in the oropharynx |
|
Oral and esophageal microbiota are comparable in eosinophilic esophagitis
Two studies analyzed the oral microbiota in EoE cohorts using 16S rRNA sequencing. Benitez et al. 18 compared the oral and esophageal diversity of 68 (33 cases and 35 controls) pediatric patients and showed a correlation between the two sites across patients (Mantel correlation = 0.16, P = 0.008; Procrustes R2: 0.15, P = 0.009), despite the substantial differences found between oral and esophageal microbiotas. The main differences were that some members of the phylum Firmicutes, including Clostridium, Eubacterium, Megasphaera, Mogibacterium, and Moryella were detected almost exclusively in esophageal samples regardless of disease status. The Atopobium genus of Actinobacteria phylum was predominantly seen in the esophageal biopsy but present in a few oral samples. Neisseria and Corynebacterium were enriched in EoE samples, while Streptococcus and Atopobium genera were consistently enriched in non‐EoE control samples.
Dietary changes did not influence the composition of the esophageal microbiota (dietary intervention Adonis P‐values: weighted UniFrac P = 0.220; unweighted UniFrac P = 0.450), the addition of food triggers led to an enrichment in Granulicatella and Campylobacter genera in the esophagus of EoE patients (Granulicatella.denovo347: P < 0.0363; Granulicatella.denovo3064: P < 0.0358; Granulicatella: P < 0.0362; Campylobacter: P < 0.0081. Raw Kruskal–Wallis P‐values from significant features detected using LEfSe).
Alpha diversity was represented by sample richness, evenness, and Shannon indexes. Wilcoxon rank‐sum test concluded no significant differences were detected between non‐EoE controls, active EoE, and inactive EoE subjects. 18 The findings of this study suggest that an active, eosinophil‐rich, inflamed tissue is associated with a distinct shift in the relative abundance of crucial esophageal microbes (Neisseria and Corynebacterium), but not in overall community structure. 18
Hiremath et al. 19 analyzed the salivary microbiota of 45 (26 cases and 19 controls) children aged 6 to 18 years old. This study showed that beta diversity was comparable among the three groups: non‐EoE controls, active EoE, and inactive EoE (Bray‐Curtis index; P = 0.93). At the genus level, children with active EoE had a lower relative abundance of Leptotrichiaceae family members (base mean = 12.9726, log2 fold change = −3.3750, q value = 0.04) (base mean = 99.8522, log2 fold change = −1.4859, q value = 0.05), genus Lactobacillus (base mean = 8.2011, log2 fold change = −2.8941, q value = 0.05), and genus Streptococcus (base mean = 2543.5310, log2 fold change = −2.2904, q value = 0.06) compared with non‐EoE controls, while non‐EoE controls had a higher relative abundance for the Neisseriaceae family compared with active EoE (base mean = 75.0051, log2 fold change = 3.5347, q value = 0.006).
Additionally, a significantly higher relative abundance of Haemophilus (base mean = 1858.625, log2 fold change = −3.111, q value = 0.008) was observed when children with active EoE were compared children with inactive EoE. Relative abundance of Haemophilus had a significant correlation with esophageal mucosal abnormalities (base mean = 1942, log2 fold change = 1.4332, q value = 5.370e‐10) and histopathologic severity as assessed by the EoE histology scoring system (base mean = 2014.595, log2 fold change = 5.8667, q value < 0.001).
PPI use was associated with a higher abundance of Streptococcus (base mean = 2687.9599, log2 fold change = 3.1740, q value = 4.28e‐05), Corynebacterium (base mean = 77.8230, log2 fold change = 3.0577, q value = 0.001), and Rothia (base mean = 38.4750, log2 fold change = 1.2574, q value = 0.01). Although the PPI use was not significantly associated with a difference in microbial richness, or alpha or beta diversity, the richness and alpha diversity tended to be lower in children who were using PPIs (all P > 0.20).
Microbial load: A comparison between eosinophilic esophagitis and gastroesophageal reflux disease (GERD)
Two papers were identified in this review as investigating the esophageal microbial load in EoE. 20 , 21 The first by Harris et al. 20 analyzed the microbiota of 70 subjects, including pediatric and adult patients with active EoE, treated EoE, Gastroesophageal reflux disease (GERD) and normal controls using esophageal brushings captured with the “esophageal string test”. EoE patients presented increased abundnance of Haemophilus, Pasteurella, Fusobacterium, and Aggregatibacter and reduced abundance of Actinomyces, Veillonella, and Rothia. Haemophilus was identified as significantly increased in untreated EoE compared with normal control subjects (P = 0.047). All groups were positive for Haemophilus, but the relative abundance was significantly higher in untreated EoE subjects than control subjects or GERD.
In addition, the average bacterial load detected in all subjects with EoE was greater than that determined from normal subjects (P < 0.01). Epithelial eosinophilia did not influence the load of bacteria. The average bacterial load found in GERD subjects was also significantly increased relative to that of the esophagus in control subjects (P < 0.0001), suggesting that the load of bacteria is associated with an inflamed esophagus and not only in EoE patients. However, the bacterial load was increased in subjects with EoE independent of the diagnosis, treatment, or disease activity (P < 0.01), indicating that high microbial load may also be associated with post‐inflammation status or underlying disease processes.
Secondly, Arias et al. 21 analyzed the microbial load of 10 PPI non‐responsive EoE patients, and 10 non‐EoE controls. Biopsies from EoE subjects were taken before and after a 6‐week 6‐food elimination diet (FED) that induced histological and clinical remission. The average bacterial load detected in esophageal samples of subjects with active EoE was 2.85‐fold higher compared with non‐EoE control samples (P < 0.002). Microbial load subsequently normalized (1.16‐fold increase) following six food elimination diet‐induced disease remission (P < 0.005).
Analysis of bacterial abundance for predicted metabolomics content
The most recent published article on the EoE microbiota 22 uses 16S rRNA sequencing and comprehensive bioinformatics techniques to thoroughly interrogate the data. While most other studies reported here focus on the microbiota taxonomy, Laserna‐Mendieta and co‐authors predicted (PICRUSt2) the enzymatic functions and metabolic pathways of the EoE microbiota. Regarding microbiota composition, Firmicutes, Proteobacteria, and Bacteroidetes were the predominant phylum across all groups, respectively. This study showed that Streptococcaceae was the most abundant family in all conditions, and Streptococcus was the most represented genus, conflicting with Benitez et al.'s 18 article where Streptococcus was increased in non‐EoE only.
Even though no substantial differences were seen between groups, control patients presented higher Proteobacteria and lower Bacterioidetes abundance compared with EoE patients. Patients treated with swallowed topical steroids showed a lower abundance of Firmicutes and a higher proportion of Proteobacteria, Bacteroidetes, and Fusobacteria. Patients treated with PPIs presented lower Bacteroidetes and Fusobacteria abundance compared with active EoE patients.
Laserna‐Mendieta et al. 22 also showed that Filifactor, Parvinomas, and Porphyromonas were less abundant in active EoE than controls. Those three genera have not been identified in any of the previous studies. This study also showed that Parvimonas displayed a partial recovery after therapy (adjusted P‐value = 0.679, unadjusted P = 0.036), while Filifactor and Porphyromonas showed a slightly lower abundance after treatment. Porphyromonas was the most abundant among these genera, being detected in 92% of the individuals.
Based on the microbiota composition and how its products may influence the host metabolism and affect the health state, Laserna and colleagues performed function predictions. It was identified that oxidation/reduction of sulfur groups via a ferricytochrome acceptor was notably different between treatments. The increase of this predicted oxidoreductase enzyme (EC 1.8.2) was observed in the post‐swallowed topical corticosteroids (STC) group relative to EoE baseline (P = 0.082), post‐PPI (P = 0.060), and post‐FED groups (P = 0.048). PICRUSt2 assigned these functions to Proteobacteria and Bacteroidetes, particularly to ASVs in the genus Pseudomonas and several unidentified ASVs in the Burkholderiaceae family. Metabolism of amino acids arginine and ornithine (pathways ARGSYN and GLUTORN) were higher in active EoE than in controls. Controls indicated higher degradation of 4‐aminobutanoate (pathway 5022) than active EoE and inactive post‐PPI and FED. Peptidoglycan synthesis and β‐lactam resistance (pathway 6470) were higher in post‐FED samples than after STC treatment.
Effect of swallowed topical corticosteroid on bacteria and fungal communities
Benitez et al. 23 published a second paper on the microbiome of EoE, being the first to report the fungal microbiome in EoE. Streptococcus, Prevotella, and Alloprevotella were predominant in all groups. Despite the bacterial community composition not being significantly different between groups, Alloprevotella was decreased in both active (q = 0.02) and inactive EoE (q = 0.001), and the abundance of Haemophilus was increased in active EoE subjects, compared with non‐EoE controls (q = 0.02).
This study also investigated the effect of STC on microbial communities. No significant differences associations with STC were found in regards to bacteria. However, the relative abundance of Actinobacillus was lower in the presence of STC, compared with steroid‐naïve patients. The relative abundance of Haemophilus was lower in active STC non‐responders compared with active STC‐naïve subjects (P = 0.004), suggesting that STC reduces the Haemophilus signature in active EoE. In regard to fungus, Candida, Cladosporiaceae, and Malassezia were predominant fungal taxa across all groups. While Agaricomycetes, Candida, Cladosporiaceae, and Peniophora were most present in control samples. Candida was increased in controls compared with steroid‐naïve EoE patients (P = 0.002). Candida was significantly increased in STC‐treated in comparison to untreated subjects (P = 0.007), as expected based on previous observations of a higher Candida infection rate during STC therapy.
Microbial response to proton‐pump inhibitors
A study lead by Johnson et al. 24 analyzed 24 PPI non‐responsive EoE cases and 25 non‐EoE controls and found no significant differences in the esophageal microbiome between cases and controls or within EoE cases based on clinical features.
However, the use of PPIs was significantly associated with five taxa, including SR1 at the phylum level and Burkholderia, Eikenella, and Kingella at the genus level in cases and controls. All cases and nine controls were on PPIs at the time of endoscopy, which prevented further exploration of additional clinical features and PPI use.
Gray literature
Parashete et al. 25 analyzed esophageal biopsies from 22 normal controls, 5 PPI‐responsive esophageal eosinophilia, and 9 PPI‐non responsive EoE subjects. There was a high presence of Gemella (P < 0.01) and Hallela (P < 0.01) in the EoE group and Neisseria in the control group.
Another published abstract 26 analyzed the esophageal microbiotas of 18 controls, six active EoE patients, and seven treated EoE patients and showed that untreated EoE patients had a higher average proportion of Bacteroidetes than controls (24.8% vs 10.4%, P = 0.13). Ghisa et al. 27 showed, for the first time in EoE patients, the presence of Spirochaetes in the mouth, esophagus, and stomach, in addition to higher abundance of this phylum in active EoE. We speculate the Spirocheates to be Treponema, as it is commonly found in dental plaque. However, further studies are needed to confirm.
The gray literature studies presented interesting results supporting findings from peer‐reviewed papers such as the increase of Bacteroidetes in active EoE 22 , 26 and report of specific genus for the first time as significantly contrasting on EoE studies, such as Spirochaetes. 27
Discussion
The microbiota has been linked with the initiation and/or perpetuation of inflammation in mucosal surfaces. Thus, it is compelling that we investigate the role of the microbiota in esophageal mucosa inflammation. However, the microbiota in EoE has not been widely studied. Here, we gather all EoE microbiota sequencing studies published thus far. Our main findings showed that patients with active EoE have increased microbial load, as well as increased abundance of Haemophilus and decrease of specific members of the Firmicutes phylum.
Arias et al. 21 showed that bacterial load and specific Toll‐like receptors (TLR) are overexpressed in the esophagus of EoE patients compared with controls and that those changes were normalized after 6‐FED and mucosal healing. TLRs are a type of microbial pattern recognition present on epithelial and lamina propria cells, and are capable of differentiating pathogens and commensal microorganisms. 21 This suggests that increased exposure of the microbiota and microbial products to the impaired esophageal mucosal barrier may increase the release of alarmins by the epithelial cells in the esophagus, resulting in the advancement of esophageal inflammation.
Our systematic review highlights that the oral and esophageal microbiotas are correlated 18 ; it is likely that the oral microbiota shapes the esophageal microbiota by the swallowing of microorganisms and associated products. Also, microbial load is consistently shown to be increased in the esophagus of active EoE patients. Masterson et al. 67 exhibited that Hypoxia‐inducible factor (HIF)‐1α is decreased in biopsies of active EoE patients compared with controls. The decrease of HIF‐1α leads to the decrease of β‐Defensins, antimicrobial peptides secreted by the epithelium. 68 We speculate that the decrease in β‐Defensins, caused by the diminished HIF‐1α leads to the increase microbial load in the esophageal mucosa of EoE patients.
However, in inactive EoE, the microbial load results are contradictory between studies. Harris et al. 20 showed that microbial load is increased independently of disease status and treatment, while Arias et al. 21 showed that microbial load normalized after remission was achieved. These conflicting results could have occurred due to different methodologies used for bacterial quantitative estimation or sample heterogeneity, with both studies only having a limited sample size. Harris et al. 20 used quantitative PCR with pan‐bacterial primers targeting the small subunit ribosomal RNA (SSU‐rRNA) to evaluate the mucosa of adults and children with EoE (different treatments approaches), GERD and normal mucosa controls. While Arias et al. 21 used primers for the V4 region of the 16S rRNA to access the mucosa of adults before and after remission through FED and normal mucosa controls. Interestingly, EoE patients from both studies were non‐responsive to PPI treatment; however, half of EoE subjects in the Arias et al. paper were on PPIs at the time of endoscopy, while none of EoE subjects in the Harris et al. study were on PPIs at the time of microbiota characterization. Further studies on the microbial load and PPI status are required to clarify the connection between bacterial load, disease status and treatment in EoE.
Members of the Firmicutes phylum, such as Streptococcus, Lactobacillus, Veillonela, and Parvimonas 18 , 19 , 20 , 22 are shown to be decreased in the esophagus of EoE subjects. A previous study by Holvoet et al. 69 showed that supplementation with Lactococcus lactis NCC 2287 decrease esophageal and bronchoalveolar eosinophilia in a murine model of EoE. While the link that determines the cause and effect of the microbial composition in EoE is not clear, pre‐clinical studies could be performed using those organisms as potential probiotics to replenish the microbiota with genera that are reduced in EoE patients.
Three studies 19 , 20 , 23 demonstrated that the abundance of Haemophilus was significantly higher in EoE subjects. Laserna‐Mendieta et al. 22 only detected Haemophilus in one sample, however, they have shown an enrichment in Actinobacillus and Aggregatibacter, which are closely related to Haemophilus, and suggested that microbial analysis could be interpreted differently by the studies. The use of 16S amplicon sequencing provides lower taxonomic resolution and may be responsible for this variation in reported results. To overcome this, future studies should aim to characterize the microbiota populations using shotgun metagenomic sequencing (none primer‐based approach, data provided for the total DNA of a given sample), which is capable of providing a non‐biased species‐level resolution of the community and would be necessary to validate the relationship between Haemophilus and EoE.
The capability of Haemophilus to enter epithelial cells 70 suggests that these organisms may be able to take advantage of the impaired barrier in EoE, which in turn contributes to chronic inflammation. Haemophilus is associated with a range of other Th2‐mediated conditions, including recurrent pediatric asthma, chronic obstructive pulmonary disease, and rhinosinusitis, 45 , 71 , 72 strengthening the argument that this genus could be associated with the propagation of inflammation in EoE.
To our knowledge, this is the first systematic review of emerging associations between the local bacterial population and EoE. The main limitations of this review were the low number of total studies, exclusion of articles published in languages other than English, the different methodologies applied to analyze the results, for example the primer region of choice varied between studies and software of author's choice, and diverse approach in reporting the data. Given this heterogeneity, we could not perform a meta‐analysis, which was our initial goal. Another limitation is the method used to sequence the microbiota, 16S rRNA sequencing, has its associated drawbacks and limitations. Firstly, the choice of sequencing primers targeting the 16S rRNA variable regions each comes with its own bias and microbiota profiles will differ based on this. Secondly, the taxonomic resolution achieved with 16S rRNA sequencing is less than that achieved with shotgun metagenomic sequencing (MGS).
In most cases 16S rRNA sequencing reads cannot be assigned to the species level, this is mostly due to the short reads generated with this technique and subsequent mapping to microbial databases limits full taxonomic assignment. Finally, 16S rRNA sequencing tends to overlook the mycobiome and virome, which can be captured with the MGS approach. From our review, studies on the local microbiota in EoE are limited. Further studies analyzing bacterial strain and metabolomics are essential to help us characterize the effect of the microbiota in EoE and possibly help identify new targets for EoE and other esophageal diseases. To improve the literature, shotgun metagenomic studies of the esophageal microbiota will be crucial to linking microbial composition to functional contributions to inflammation, and different EoE endotypes previously described by Rothenberg and colleagues. 73
In conclusion, this systematic review suggests that the microbiota of EoE is similar in composition to the mouth. Patients with active EoE have increased microbial load and increased abundance of Haemophilus. These findings suggest Haemophilus may represent an opportunistic pathogen in EoE that is linked with esophageal inflammation.
Acknowledgment
Open access publishing facilitated by The University of Newcastle, as part of the Wiley ‐ The University of Newcastle agreement via the Council of Australian University Librarians.
Angerami Almeida, K. , de Queiroz Andrade, E. , Burns, G. , Hoedt, E. C. , Mattes, J. , Keely, S. , and Collison, A. (2022) The microbiota in eosinophilic esophagitis: A systematic review. Journal of Gastroenterology and Hepatology, 37: 1673–1684. 10.1111/jgh.15921.
Declaration of conflict of interest: The authors report no conflict of interest.
Author contribution: KAA meditated the project, reviewed papers and drafted the paper. EQA and GB contributed to the design of the study and review of papers identified at each stage. EH, JM, SK and AC provided manuscript concept, revised the drafts and critically edited the manuscript. All authors participated in the drafting and agreed with this final manuscript for submission.
Financial support: This work was supported by the Hunter Children's Research Foundation under Grant 2020/0649 and the Priority Research Centre GrowUpWell at the University of Newcastle.
Contributor Information
Kaylani Angerami Almeida, Email: kaylani.almeida@uon.edu.au.
Adam Collison, Email: adam.collison@newcastle.edu.au.
References
- 1. Davis BP. Pathophysiology of Eosinophilic Esophagitis. Clin Rev Allergy Immunol 2018; 55: 19–42. [DOI] [PubMed] [Google Scholar]
- 2. Muir AB, Wang JX, Nakagawa H. Epithelial‐stromal crosstalk and fibrosis in eosinophilic esophagitis. J. Gastroenterol. 2019; 54: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clayton F, Fang JC, Gleich GJ et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014; 147: 602–609. [DOI] [PubMed] [Google Scholar]
- 4. Hogan SP, Mishra A, Brandt EB et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat. Immunol. 2001; 2: 353–360. [DOI] [PubMed] [Google Scholar]
- 5. Mishra A, Rothenberg ME. Intratracheal IL‐13 induces eosinophilic esophagitis by an IL‐5, eotaxin‐1, and STAT6‐dependent mechanism. Gastroenterology 2003; 125: 1419–1427. [DOI] [PubMed] [Google Scholar]
- 6. Furuta GT, Liacouras CA, Collins MH et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133: 1342–1363. [DOI] [PubMed] [Google Scholar]
- 7. O'Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, Rothenberg ME. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology 2018; 154: 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navarro P, Arias Á, Arias‐González L, Laserna‐Mendieta EJ, Ruiz‐Ponce M, Lucendo AJ. Systematic review with meta‐analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population‐based studies. Aliment. Pharmacol. Ther. 2019; 49: 1116–1125. [DOI] [PubMed] [Google Scholar]
- 9. Yaxley JP, Chakravarty B. Eosinophilic oesophagitis‐‐a guide for primary care. Aust. Fam. Physician 2015; 44: 723–727. [PubMed] [Google Scholar]
- 10. Lucendo AJ, Molina‐Infante J, Arias Á et al. Guidelines on eosinophilic esophagitis: evidence‐based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol. J. 2017; 5: 335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sokolowska M, Frei R, Lunjani N, Akdis CA, O'Mahony L. Microbiome and asthma. Asthma Res. Pract. 2018; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paller AS, Kong HH, Seed P et al. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019; 143: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park CH, Lee SK. Exploring Esophageal Microbiomes in Esophageal Diseases: A Systematic Review. J. Neurogastroenterol. Motil. 2020; 26: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corning B, Copland AP, Frye JW. The Esophageal Microbiome in Health and Disease. Curr. Gastroenterol. Rep. 2018; 20: 39. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig. Dis. Sci. 1993; 38: 109–116. [DOI] [PubMed] [Google Scholar]
- 17. Wells A, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses, 2000. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 18. Benitez AJ, Hoffmann C, Muir AB, Dods KK, Spergel JM, Bushman FD, Wang ML. Inflammation‐associated microbiota in pediatric eosinophilic esophagitis. Microbiome 2015; 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hiremath G, Shilts MH, Boone HH et al. The Salivary Microbiome Is Altered in Children With Eosinophilic Esophagitis and Correlates With Disease Activity. Clin. Transl. Gastroenterol. 2019; 10: e00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris JK, Fang R, Wagner BD et al. Esophageal microbiome in eosinophilic esophagitis. PLoS ONE 2015; 10: e0128346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arias A, Vicario M, Bernardo D et al. Toll‐like receptors‐mediated pathways activate inflammatory responses in the esophageal mucosa of adult eosinophilic esophagitis. Clin. Transl. Gastroenterol. 2018; 9: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laserna‐Mendieta EJ, FitzGerald J, Arias‐Gonzalez L, Ollala JM, Bernardo D, Claesson MJ, Lucendo AJ. Esophageal microbiome in active eosinophilic esophagitis and changes induced by different therapies. Sci. Rep. 2021; 11: 7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benitez AJ, Tanes C, Mattei L et al. Effect of topical swallowed steroids on the bacterial and fungal esophageal microbiota in eosinophilic esophagitis. Allergy 2021; 76: 1549–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson J, Dellon E, McCoy AN, Sun S, Jensen ET, Fodor AA, Keku TO. Lack of association of the esophageal microbiome in adults with eosinophilic esophagitis compared with non‐EoE controls. J. Gastrointestin. Liver Dis. 2021; 30: 17–24. [DOI] [PubMed] [Google Scholar]
- 25. Parashette KR, Sarsani V, Toh E, Hon EC, Janga SC, Nelson D, Gupta SK. Mo1202 Esophageal Microbiome in Healthy Children and Eosinophilic Esophagitis: A Prospective Study. Gastroenterology 2015; 148: S‐637–S‐638. [Google Scholar]
- 26. Smith E, CaJacob N, Ptacek T, Kumar R, Morrow C, Dimmitt R. Su1105 Eosinophilic Esophagitis: Analyzing the Esophageal and Colonic Microbiome. Gastroenterology 2015; 148: S‐409. [Google Scholar]
- 27. Ghisa M, Facchin S, Caldart F et al. Characterization of Salivary, Gastric and Esophageal Microbiota in Patients with Eosinophilic Esophagitis. Gastroenterology 2020; 158: S‐837. [Google Scholar]
- 28. Noinaj N, Buchanan SK, Cornelissen CN. The transferrin‐iron import system from pathogenic Neisseria species. Mol. Microbiol. 2012; 86: 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu G, Tang CM, Exley RM. Non‐pathogenic Neisseria: members of an abundant, multi‐habitat, diverse genus. Microbiology 2015; 161: 1297–1312. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Y, Lin P, Li Q et al. Analysis of the microbiota of sputum samples from patients with lower respiratory tract infections. Acta Biochim. Biophys. Sin. 2010; 42: 754–761. [DOI] [PubMed] [Google Scholar]
- 31. Zeigler CC, Persson GR, Wondimu B, Marcus C, Sobko T, Modéer T. Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity 2012; 20: 157–164. [DOI] [PubMed] [Google Scholar]
- 32. Kageyama S, Takeshita T, Takeuchi K et al. Characteristics of the Salivary Microbiota in Patients With Various Digestive Tract Cancers. Front. Microbiol. 2019; 10: 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai C‐C, Ho YP, Chou YS, Ho KY, Wu YM, Lin YC. Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human periodontitis – A historic review with emphasis on JP2. Kaohsiung J. Med. Sci. 2018; 34: 186–193. [DOI] [PubMed] [Google Scholar]
- 34. Yang M, Sun B, Li J et al. Alteration of the intestinal flora may participate in the development of Graves' disease: a study conducted among the Han population in southwest China. Endocr. Connect. 2019; 8: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan X, Alekseyenko AV, Wu J et al. Human oral microbiome and prospective risk for pancreatic cancer: a population‐based nested case‐control study. Gut 2018; 67: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng Z, Wang X, Zhou R, Chen H, Wilson BA, Wu B. Pasteurella multocida: Genotypes and Genomics. Microbiol. Mol. Biol. Rev. 2019; 83: e00014‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katechakis N, Maraki S, Dramitinou I, Marolachaki E, Koutla C, Ioannidou E. An unusual case of Pasteurella multocida bacteremic meningitis. J. Infect. Public Health 2019; 12: 95–96. [DOI] [PubMed] [Google Scholar]
- 38. Phillips JE. Actinobacillus. In: Carter GR, Cole JR, eds. Diagnostic Procedure in Veterinary Bacteriology and Mycology, Fifth edn. San Diego: Academic Press, 1990; 143–149. [Google Scholar]
- 39. Dellon ES, McCoy N, Barnes C, Arrington A, Covington J, McGee SJ, Keku TO. Sa1156 ‐ The Esophageal Microbiome Differes in Adults with Eosinophilic Esophagitis Compared with Non‐Eoe Controls. Gastroenterology 2018; 154: S‐261. [Google Scholar]
- 40. Bolton DJ. Campylobacter virulence and survival factors. Food Microbiol. 2015; 48: 99–108. [DOI] [PubMed] [Google Scholar]
- 41. Costa D, Iraola G. Pathogenomics of Emerging Campylobacter Species. Clin. Microbiol. Rev. 2019; 32: e00072–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kayser FH, Thieme E. MedOne, Medical microbiology. Stuttgart, New York, NY, Georg Thieme Verlag, 2005. [Google Scholar]
- 43. Musher DM. In: Baron S, ed. Haemophilus Species, Medical Microbiology. Galveston: University of Texas Medical Branch, 1996. [PubMed] [Google Scholar]
- 44. Segal LN, Clemente JC, Tsay JCJ et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat. Microbiol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lal D, Keim P, Delisle J et al. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int. Forum Allergy Rhinol. 2017; 7: 561–569. [DOI] [PubMed] [Google Scholar]
- 46. Abranches J, Zeng L, Kajfasz JK et al. Biology of Oral Streptococci. Microbiol. Spectr. 2018; 6: 6‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vitetta L, Llewellyn H, Oldfield D. Gut Dysbiosis and the Intestinal Microbiome: Streptococcus thermophilus a Key Probiotic for Reducing Uremia. Microorganisms 2019; 7: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR. The Gastric and Intestinal Microbiome: Role of Proton Pump Inhibitors. Curr. Gastroenterol. Rep. 2017; 19: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cargill JS, Scott KS, Gascoyne‐Binzi D, Sandoe JAT. Granulicatella infection: diagnosis and management. J. Med. Microbiol. 2012; 61: 755–761. [DOI] [PubMed] [Google Scholar]
- 50. Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP. The Lactobacillus casei Group: History and Health Related Applications. Front. Microbiol. 2018; 9: 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Knapp S, Brodal C, Peterson J, Qi F, Kreth J, Merritt J. Natural Competence Is Common among Clinical Isolates of Veillonella parvula and Is Useful for Genetic Manipulation of This Key Member of the Oral Microbiome. Front. Cell. Infect. Microbiol. 2017; 7: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aja E, Mangar M, Fletcher HM, Mishra A. Filifactor alocis: Recent Insights and Advances. J. Dent. Res. 2021; 100: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watanabe T, Hara Y, Yoshimi Y, Fujita Y, Yokoe M, Noguchi Y. Clinical characteristics of bloodstream infection by Parvimonas micra: retrospective case series and literature review. BMC Infect. Dis. 2020; 20: 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olsen I, Lambris JD, Hajishengallis G. Porphyromonas gingivalis disturbs host–commensal homeostasis by changing complement function. J. Oral Microbiol. 2017; 9: 1340085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Q, Rao Y, Guo X et al. Oral Microbiome in Patients with Oesophageal Squamous Cell Carcinoma. Sci. Rep. 2019; 9: 19055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perera M, al‐hebshi NN, Perera I et al. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018; 97: 725–732. [DOI] [PubMed] [Google Scholar]
- 57. Li J, Li Y, Zhou Y, Wang C, Wu B, Wan J. Actinomyces and Alimentary Tract Diseases: A Review of Its Biological Functions and Pathology. Biomed. Res. Int. 2018; 2018: 3820215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takahashi K, Nishida A, Fujimoto T et al. Reduced Abundance of Butyrate‐Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease. Digestion 2016; 93: 59–65. [DOI] [PubMed] [Google Scholar]
- 59. McMullen AR, Anderson N, Wallace MA, Shupe A, Burnham CAD. When Good Bugs Go Bad: Epidemiology and Antimicrobial Resistance Profiles of Corynebacterium striatum, an Emerging Multidrug‐Resistant, Opportunistic Pathogen. Antimicrob. Agents Chemother. 2017; 61: e01111‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramanan P, Barreto JN, Osmon DR, Tosh PK. Rothia Bacteremia: a 10‐Year Experience at Mayo Clinic, Rochester, Minnesota. J. Clin. Microbiol. 2014; 52: 3184–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eisenberg T, Fawzy A, Nicklas W, Semmler T, Ewers C. Phylogenetic and comparative genomics of the family Leptotrichiaceae and introduction of a novel fingerprinting MLVA for Streptobacillus moniliformis. BMC Genomics 2016; 17: 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brennan CA, Garrett WS. Fusobacterium nucleatum — symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019; 17: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haake DA. Spirochetes. In: Schaechter M, ed. Encyclopedia of Microbiology, Third edn. Oxford: Academic Press, 2009; 278–292. [Google Scholar]
- 64. Woods DE, Sokol PA. The genus Burkholderia. In: Dworkin M et al., eds. The Prokaryotes, Vol. 5, Proteobacteria: Alpha and Beta Subclasses. New York, NY: Springer New York, 2006; 848–860. [Google Scholar]
- 65. Oztoprak N, Bayar U, Celebi G, Basaran M, Cömert F. Eikenella corrodens, cause of a vulvar abscess in a diabetic adult. Infect. Dis. Obstet. Gynecol. 2009; 2009: 63565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yagupsky P. Kingella kingae: carriage, transmission, and disease. Clin. Microbiol. Rev. 2015; 28: 54–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Masterson JC, Biette KA, Hammer JA et al. Epithelial HIF‐1α/claudin‐1 axis regulates barrier dysfunction in eosinophilic esophagitis. J. Clin. Invest. 2019; 129: 3224–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kelly CJ, Glover LE, Campbell EL et al. Fundamental role for HIF‐1α in constitutive expression of human β defensin‐1. Mucosal Immunol. 2013; 6: 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Holvoet S, Doucet‐Ladevèze R, Perrot M, Barretto C, Nutten S, Blanchard C. Beneficial effect of Lactococcus lactis NCC 2287 in a murine model of eosinophilic esophagitis. Allergy 2016; 71: 1753–1761. [DOI] [PubMed] [Google Scholar]
- 70. Clementi CF, Murphy TF. Non‐typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front. Cell. Infect. Microbiol. 2011; 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bisgaard H, Hermansen MN, Buchvald F et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007; 357: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 72. Chalermwatanachai T, Vilchez‐Vargas R, Holtappels G et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci. Rep. 2018; 8: 7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shoda T, Wen T, Aceves SS et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: a cross‐sectional study. Lancet Gastroenterol. Hepatol. 2018; 3: 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]


