Abstract
Background
In contemporary Cardiac Intensive Care Unit (CICU), bedside intra‐aortic balloon pump (IABP) insertion under echocardiographic guidance may be an attractive option for selected patients with cardiogenic shock (CS). Currently available data on this approach are limited.
Aim
This study aimed to assess the feasibility and safety of bedside IABP insertion, as compared to fluoroscopic‐guided insertion in the Catheterization Laboratory (CathLab), and to describe the clinical features of patients receiving bedside IABP insertion using a standardized technique in real‐world CICU practice.
Methods
We prospectively evaluated all patients admitted the CICU who received transfemoral IABP between June 2020 and October 2021. The overall study cohort was divided according to implant strategy in bedside and CathLab groups. The primary outcome was correct radiographic IABP positioning at the first bedside chest X‐ray obtained after insertion. Secondary outcomes included IABP‐related complications.
Results
Among 115 patients, bedside IABP insertion was performed in 35 (30.4%) cases, mainly presenting with CS‐related to acute decompensated heart failure (ADHF) (68.6 vs 33.8%; p < 0.001), with lower LVEF, higher proportion of right ventricular involvement and higher need of inotropes/vasopressors, compared to those receiving CathLab insertion. Bedside IABP insertion resulted feasible and safe, with similar rates of correct IABP positioning (82.9 vs. 82.5%; p = 0.963) and IABP‐related major vascular complications (5.7 vs. 5.0%; p = 0.874), as compared to CathLab positioning.
Conclusion
This study suggests the feasibility and safety of bedside IABP insertion, which could be of relevant interest in patients with ADHF‐related CS who may not need coronary angiography or other urgent CathLab procedures.
Keywords: bedside, cardiogenic shock, echocardiography, IABP, intra‐aortic balloon pump
1. INTRODUCTION
Cardiogenic shock (CS) is a progressively increasing diagnosis on admission in cardiac intensive care units (CICU) 1 and the use of mechanical circulatory support (MCS) is frequent in these patients. Intra‐aortic balloon pump (IABP) support has been progressively reappraised, especially in the acute decompensated heart failure (ADHF) CS scenario. 2 , 3 Recent data suggest that this device is the most common MCS employed in ADHF‐related CS. 4 IABP features an easy implantation technique and can rapidly be deployed bedside under transthoracic or transesophageal echocardiographic guidance. 2 , 5 , 6 This avoids the need for patient transfer to the catheterization laboratory (CathLab) with the inherent risk of destabilization, and makes it an attractive approach in case of unstable hemodynamics. Furthermore, bedside IABP insertion may be an interesting option in patients who would not otherwise require transfer to the CathLab for invasive coronary angiography (ICA). However, despite decades of IABP utilization, the feasibility and safety of IABP bedside insertion without fluoroscopic guidance has not been systematically evaluated. Currently available data are limited to case reports and small cases series, describing the technique, however without comparison with a reference population. 7 , 8 , 9 Thus, we aimed to assess the feasibility and safety of this approach using a standardized technique, as compared to the fluoroscopic‐guided IABP insertion in the CathLab and to describe the clinical features of patients currently receiving bedside IABP positioning in our single‐center real‐world practice.
2. MATERIALS AND METHODS
2.1. Study design
We prospectively evaluated all CS patients admitted to our Cardiac Intensive Care Unit (CICU) at the IRCCS “San Raffaele Hospital,” Milan, Italy, who received transfemoral IABP. The study period ranged from June 2020 to October 2021. The overall study cohort was divided according to IABP implant strategy in bedside and CathLab groups. Decision on the implant approach was at the discretion of the treating physician. A pragmatic guidance for choosing between bedside or CathLab transfemoral IABP insertion in our Institution is reported in Table 1. All patients received either an Arrow AutoCAT2 (Teleflex) or a CARDIOSAVE (Maquet) IABP device. Balloon size was selected according to manufacturer's instructions.
Table 1.
Clinical and logistic factors to guide between CathLab and bedside approach
| Factors favoring CathLab IABP insertion | Factors favoring bedside IABP insertion |
|---|---|
| ACS etiology/need of ICA/need of EMB | ADHF etiology |
| Inadequate aorta visualization on TTE/TEE | Complex CathLab transfer: ongoing CRRT, ongoing VA‐ECMO, invasive hemodynamic monitoring, NIMV‐dependency |
| Common femoral artery diameter <5 mm at bedside ultrasound | |
| Known severe aortic/iliac/femoral disease | Mechanical ventilation allowing for preferred TEE guidance |
| Previous aortic/iliac/femoral vascular surgery/stenting | Unstable patient requiring emergent implant (≤30 min) |
Abbreviations: ACS, acute coronary syndrome; ADHF, acute decompensated heart failure; IABP, intra‐aortic balloon pump; ICA, invasive coronary angiography; CRRT, continuous renal replacement therapy; EMB, endomyocardial biopsy; NIMV, noninvasive mask ventilation; TTE, transthoracic echocardiography; TEE, transesophageal echocardiography; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Bedside IABP insertion was performed in the CICU using a standardized approach (Figure 1), with the patient in the supine position, using a sterile technique, under local or general anesthesia, according to the clinical presentation. After ultrasound assessment of the femoral arteries with a high‐frequency (6–15 MHz) linear transducer, vascular access was obtained using ultrasound‐guided common femoral artery puncture, as previously described. 6 Patients in whom femoral access was deemed complex (due to known vascular disease of abdominal aorta, iliac and femoral arteries or severe atherosclerosis of the femoral arteries identified at bedside ultrasound evaluation) were considered unsuitable for bedside IABP insertion, and received CathLab IABP insertion (Table 1). An 8‐Fr sheath was inserted in all cases. Advancement of the guidewire and IABP positioning were performed under transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE) guidance in spontaneously breathing or mechanically ventilated patients, respectively. Standard TTE views included an off‐axis apical 2‐chamber view of the descending aorta and a suprasternal view of the aortic arch (Figure 2). Standard TEE views included a mid‐esophageal 0° short‐axis view and a 90° long‐axis view of the descending aorta, and an upper‐esophageal 0–10° view of the aortic arch (Figure 2). IABP position was considered appropriate at TTE/TEE if the tip of the balloon catheter was located just distal to the origin of the left subclavian artery. 10 All patients received direct bedside chest X‐ray after either bedside or CathLab IABP insertion to confirm correct IABP positioning.
Figure 1.
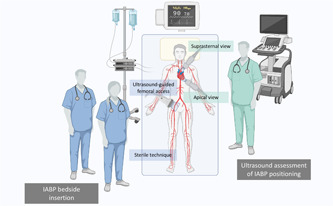
Intra‐aorticballoon pump bedside insertion operative setting
Figure 2.
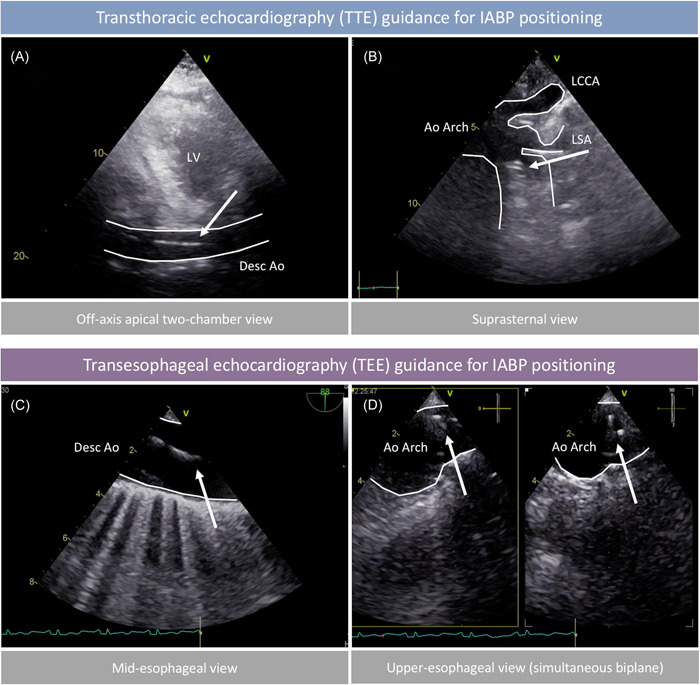
Transthoracic (TTE) and transesophageal (TEE) views for intra‐aorticballoon pump (IABP) insertion guidance. (A) Off‐axis apical 2‐chamber view demonstrating IABP guidewire (arrow) in the descending aorta. (B) Suprasternal view of the aortic arch confirming appropriate position of the IABP tip (arrows) below the left subclavian artery. (C) Mid‐esophageal long‐axis view (approximately 90°) demonstrating IABP guidewire (arrow) in the descending aorta. (D) Mid‐esophageal orthogonal views (approximately 0° and 90°) of the aortic arch confirming appropriate position of the IABP tip (arrows). Ao, aorta; LCCA, left common carotid artery; LSA, left subclavian artery; LV, left ventricle
The primary outcome was correct radiographic IABP positioning at chest X‐ray, defined as the placement of the tip of the balloon catheter at the level of the aortic knob 10 at the first bedside chest X‐ray obtained after IABP insertion (Figure 3). Assessment of chest X‐ray was performed by two authors (Giuseppe Barone and Alessandro Beneduce) blinded to the study groups.
Figure 3.
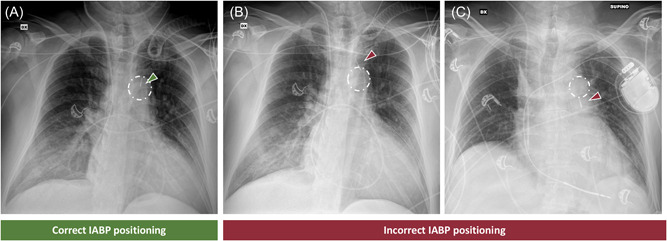
Intra‐aortic balloon pump (IABP) position assessment on chest X‐ray. (A) correct IABP positioning with the tip (green arrowhead) inside the aortic knob (white circle); (B) incorrect IABP positioning with the tip (red arrowhead) above the aortic knob (white circle); (C) incorrect IABP positioning with the tip (red arrowhead) below the aortic knob (white circle)
Secondary outcomes were in‐hospital mortality, IABP‐related vascular complications, CICU stay, need for MCS escalation or renal replacement therapy (RRT), sepsis, and stroke/transient ischemic attack (TIA). Major vascular complication was defined as any thoracic aortic dissection, access‐related vascular injury leading to either death, unplanned percutaneous/surgical intervention, need for ≥4 blood unit transfusions, amputation for distal embolization, or irreversible end‐organ damage. Minor vascular complication was defined as any vascular complication not meeting criteria for major vascular complication. Assessment of secondary endpoints was performed by an author (Alessandro Beneduce) blinded to the study groups.
2.2. Statistical analyses
Categorical variables are reported as proportions, while continuous variables are reported as means and standard deviation (SD). Normality was checked with the Shapiro–Wilk test. Continuous variables from independent groups were compared by the Student's t‐test and the categorical variables with the Fisher's Exact test. A p < 0.05 was considered statistically significant. All analyses were performed with RStudio (Version 1.3.1093, RStudio, PBC).
3. RESULTS
3.1. Study population
A total of 115 patients were included in this study (Figure 4). Overall, median age was 69.4 ± 12.8 years and 27 (23.5%) patients were females. Etiology of CS was acute coronary syndrome (ACS) in 64 patients (55.7%) and ADHF in 51 patients (44.3%). Serum lactate on admission was 4.2 ± 3.6 mmol/l. In 3 (2.6%) patients not otherwise requiring transfer to the CathLab, IABP implant was a‐priori scheduled in the CathLab due to severe peripheral artery diseases leading to anticipated difficult femoral access. A total of 35 (30.4%) patients received bedside IABP insertion. In 15 (42.9%) cases TEE was used to guide the procedure. No patient with attempted bedside IABP positioning required conversion to CathLab procedure for poor or inadequate echocardiographic window. One patient (1/35; 2.9%), without known history of peripheral vascular disease, required CathLab transfer for impossibility to advance the IABP shaft due to extremely tortuous ilio‐femoral axis, after uneventful femoral puncture. IABP was eventually placed with the aid of a 8‐Fr flexible sheath. Age, sex, and serum lactate on admission were balanced in the bedside and CathLab groups. The bedside cohort featured a higher proportion of ADHF etiology (68.6 vs. 33.8%; p < 0.001), more frequent pre‐existing HF (65.7 vs. 37.5%; p = 0.005) and lower systolic blood pressure (102.3 ± 22.1 vs. 119.7 ± 25.6 mmHg; p = 0.002) on admission. On echocardiography, these patients presented with worse left ventricular ejection fraction (LVEF; 21.4 ± 8.4 vs. 27.5 ± 9.6%; p = 0.002), higher proportion of right ventricular failure (60.0 vs. 22.5%; p < 0.001), severe tricuspid regurgitation (25.7 vs. 7.5%; p = 0.008), and severe mitral regurgitation (40.0 vs. 15.0%; p = 0.003). Laboratory tests demonstrated lower hemoglobin in the bedside group (11.9 ± 2.0 vs. 13.2 ± 2.3 g/dl; p = 0.007). Bedside IABP cohort more frequently required inotropic support (100.0 vs. 82.5%; p = 0.008). The use of invasive mechanical ventilation was high and similar between groups (65.7 vs. 61.2%; p = 0.649).
Figure 4.
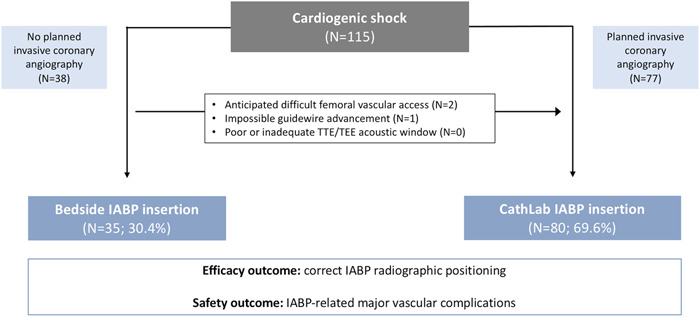
Study flow‐chart
Baseline clinical characteristics are summarized in Table 2.
Table 2.
Study cohort characteristics
| Overall (N = 115) | Bedside (N = 35) | Cath Lab (N = 80) | p‐value | |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age (years) | 69.4 (12.8) | 69.1 (10.5) | 69.5 (13.7) | 0.881 |
| Female sex | 27 (23.5%) | 10 (28.6%) | 17 (21.2%) | 0.394 |
| History of HF | 53 (46.1%) | 23 (65.7%) | 30 (37.5%) | 0.005* |
| Hypertension | 71 (61.7%) | 22 (62.9%) | 49 (61.2%) | 0.870 |
| Diabetes mellitus | 41 (35.7%) | 13 (37.1%) | 28 (35.0%) | 0.825 |
| History of CKD (eGFR < 60 ml/min/1.73 m2) | 44 (38.8%) | 20 (57.1%) | 24 (30.0%) | 0.006* |
| eGFR (ml/min/1.73 m2) | 51.6 (30.8%) | 39.3 (29.6) | 57.0 (29.9) | 0.005* |
| Peripheral artery disease | 27 (23.5%) | 7 (20.0%) | 20 (25.0%) | 0.561 |
| Previous stroke/TIA | 12 (10.4%) | 3 (8.6%) | 9 (11.2%) | 0.665 |
| CAD | 71 (61.7%) | 20 (57.1%) | 51 (63.8%) | 0.502 |
| Previous MI | 28 (24.3%) | 12 (34.3%) | 16 (20.0%) | 0.100 |
| Previous PCI | 34 (29.6%) | 12 (34.3%) | 22 (27.5%) | 0.463 |
| Previous CABG | 6 (5.2%) | 2 (5.7%) | 4 (5.0%) | 0.874 |
| Clinical presentation | ||||
| CS Etiology | <0.001* | |||
| ACS | 64 (55.7%) | 11 (31.4%) | 53 (66.2%) | |
| ADHF | 51 (44.3%) | 24 (68.6%) | 27 (33.8%) | |
| SCAI CS stage | 0.031* | |||
| B | 56 (48.7%) | 10 (28.6%) | 46 (57.5%) | |
| C | 52 (45.2%) | 22 (62.9%) | 30 (37.5%) | |
| D | 7 (6.1%) | 3 (8.6%) | 4 (5.0%) | |
| Acute pulmonary edema | 32 (27.8%) | 12 (34.3%) | 20 (25.0%) | 0.307 |
| Systolic arterial pressure (mmHg) | 114.3 (25.9) | 102.3 (22.1) | 119.7 (25.6) | 0.002* |
| Diastolic arterial pressure (mmHg) | 61.5 (15.6) | 59.1 (12.7) | 62.5 (16.7) | 0.329 |
| Heart rate (bpm) | 90.1 (22.8) | 95.5 (23.0) | 87.7 (22.5) | 0.114 |
| SpO2 (%) | 97.6 (3.9) | 96.8 (4.5) | 97.9 (3.5) | 0.187 |
| Echocardiographic characteristics | ||||
| LVEF (%) | 25.6 (9.6) | 21.4 (8.4) | 27.5 (9.6) | 0.002* |
| Severe MR | 26 (22.6%) | 14 (40.0%) | 12 (15.0%) | 0.003* |
| Significant RV failure | 39 (33.9%) | 21 (60.0%) | 18 (22.5%) | <0.001* |
| Estimated sPAP (mmHg) | 44.6 (26.2) | 49.1 (19.0) | 42.5 (28.8) | 0.260 |
| Severe TR | 15 (13.0%) | 9 (25.7%) | 6 (7.5%) | 0.008* |
| Estimated CVP (mmHg) | 10.2 (5.8) | 12.0 (6.3%) | 9.3 (5.4) | 0.032* |
| Laboratory findings | ||||
| Serum lactate (mmol/L) | 4.2 (3.6) | 5.1 (3.1) | 3.8 (3.8) | 0.119 |
| Creatinine (mg/dl) | 2.0 (1.6) | 2.4 (1.7) | 1.8 (1.6) | 0.089 |
| INR | 1.5 (0.8) | 1.8 (1.0) | 1.4 (0.6) | 0.056 |
| Plt (*103/ml) | 220.3 (79.3) | 205.5 (90.0) | 227.2 (73.4) | 0.181 |
| Hb (g/dl) | 12.8 (2.3) | 11.9 (2.0) | 13.2 (2.3) | 0.007* |
| Management | ||||
| Inotropes use | 191 (87.8%) | 25 (100%) | 66 (82.5%) | 0.008* |
| Vasopressors use | 66/104 (63.5%) | 24/32 (75.0%) | 42/72 (58.3%) | 0.103 |
| Invasive mechanical ventilation | 72 (62.6%) | 23 (65.7%) | 49 (61.2%) | 0.649 |
Abbreviations: ACS, acute coronary syndrome; ADHF, acute decompensated heart failure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; CS, cardiogenic shock; CVP, central venous pressure; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; PCI, percutaneous coronary intervention; RV, right ventricle; SCAI, Society for Cardiovascular Angiography and Interventions; sPAP, systolic pulmonary artery pressure; SpO2, peripheral oxygen saturation; TR, tricuspid regurgitation; Categorial variables are expressed as count and proportions, continuous variable as means (standard deviations). *Significant at the 0.05 level.
3.2. Clinical outcomes
The occurrence of the primary endpoint of correct IABP positioning at the chest X‐ray was observed in 82.6% of patients overall and was similar between the two groups (82.9 vs. 82.5%; p = 0.963). Mean distance of IABP tip from aortic knob in patients with wrong IABP positioning was 18.2 ± 12.3 mm. All patients with incorrect IABP positioning had the device successfully repositioned bedside in the CICU, with immediate chest X‐ray confirmation. In‐hospital death did not significantly differ according to implant strategy (28.6 vs. 17.5%; p = 0.179). Duration of IABP support was similar between the bedside and CathLab groups (7.2 ± 6.3 vs. 5.4 ± 7.5 days; p = 0.230). No differences in need for MCS escalation to Impella or VA‐ECMO, need for RRT, sepsis, stroke or transient ischemic attack (TIA) were observed between groups. On the opposite, bedside IABP group experienced longer CICU stay (18.0 vs. 11.1 days; p = 0.017) and a higher rate of left ventricular assist device (LVAD) implantation (17.1 vs. 0.0%; p < 0.001) during index hospitalization. IABP‐related major vascular complications (5.7 vs. 5.0%; p = 0.874), IABP‐related pseudoaneurysm (5.7 vs. 5.0%; p = 0.874), IABP‐related arterio‐venous fistula (0.0 vs. 2.5%; p = 0.345), IABP‐related critical limb ischemia (8.6 vs. 5.0%; p = 0.461), IABP‐related access‐site bleeding/hematoma (2.9 vs. 2.5%; p = 0.912), IABP‐related arterial dissection (2.9 vs. 1.2%; p = 0.544) were similar between groups. In‐hospital outcomes are summarized in Table 3.
Table 3.
In‐hospital management and outcomes
| Overall (N = 115) | Bedside (N = 35) | Cath Lab (N = 80) | p‐value | |
|---|---|---|---|---|
| Correct IABP radiographic position | 95 (82.6%) | 29 (82.9%) | 66 (82.5%) | 0.963 |
| IABP support duration (days) | 6.0 (7.1) | 7.2 (6.3) | 5.4 (7.5) | 0.230 |
| CICU stay (days) | 13.0 (12.6) | 18.0 (11.8) | 11.1 (12.4) | 0.017* |
| In‐hospital death | 24 (20.9%) | 10 (28.6%) | 14 (17.5%) | 0.179 |
| Renal replacement therapy | 19 (16.5%) | 8 (22.9%) | 11 (13.8%) | 0.226 |
| Escalation to Impella 2.5/CP | 11 (9.6%) | 5 (14.3%) | 6 (7.5%) | 0.255 |
| Escalation to VA‐ECMO | 5 (4.3%) | 3 (8.6%) | 2 (2.5%) | 0.142 |
| IABP‐related major vascular complication | 6 (5.2%) | 2 (5.7%) | 4 (5.0%) | 0.874 |
| IABP‐related minor vascular complication | 12 (10.4%) | 4 (11.4%) | 8 (10.0%) | 0.818 |
| IABP‐related pseudoaneurysm | 6 (5.2%) | 2 (5.7%) | 4 (5.0%) | 0.874 |
| IABP‐related arterio‐venous fistula | 2 (1.7%) | 0 (0.0%) | 2 (2.5%) | 0.345 |
| IABP‐related critical limb ischemia | 7 (6.1%) | 3 (8.6%) | 4 (5.0%) | 0.461 |
| IABP‐related access‐site bleeding/hematoma | 3 (2.6%) | 1 (2.9%) | 2 (2.5%) | 0.912 |
| IABP‐related arterial dissection | 2 (1.7%) | 1 (2.9%) | 1 (1.2%) | 0.544 |
| IABP‐related stroke/TIA | 1 (0.9%) | 1 (2.9%) | 0 (0.0%) | 0.129 |
| Non‐IABP related major bleeding | 17 (14.8%) | 8 (22.9%) | 9 (11.2%) | 0.107 |
| Sepsis | 33/88 (39.8%) | 13/28 (46.4%) | 22/60 (36.7%) | 0.383 |
| LVAD implantation | 6 (5.2%) | 6 (17.1%) | 0 (0.0%) | <0.001* |
Abbreviations: IABP, intra‐aortic balloon pump; ICU, intensive care unit; LVAD, left ventricular assist device; TIA, transient ischemic attack; VA‐ECMO, venoarterial extracorporeal membrane oxygenation; Categorial variables are expressed as count and proportions, continuous variable as means (standard deviations). *Significant at the 0.05 level.
4. DISCUSSION
The main findings of this real‐world, observational, single‐center study may be summarized as follows:
‐ Bedside IABP insertion was feasible and safe, with similar rates of correct IABP positioning and IABP‐related complications as compared to CathLab positioning;
‐ Bedside insertion was performed in 30.4% of all patients undergoing MCS with transfemoral IABP, and was chiefly reserved for ADHF‐related CS patients, representing a complex and sicker population, as highlighted by lower LVEF, higher proportion of right ventricular involvement and higher need of inotropes/vasopressors.
This single‐center experience provides insights on current bedside IABP insertion rates in modern CICU and suggests the feasibility and safety of this approach in the setting of CS related to ACS or ADHF. In our all‐comers population of IABP recipients, we found a 30.4% rate of bedside insertion. Bedside insertion was left at the discretion of the treating physician, according to our clinical practice (Table 1). Interestingly, despite decades of IABP utilization, no study specifically compared this approach to the fluoroscopic‐guided insertion. We compared the bedside IABP cohort with a reference population of patients who underwent IABP insertion in the CathLab during the same study period, and we found a similar rate of correct IABP positioning at the first chest X‐ray obtained after the procedure (82.9 vs. 82.5%, for bedside and CathLab approach, respectively). The relatively low correct positioning achieved was due to the strict radiological definition, chosen to provide a quantitative comparison for both groups and implying correct advancement of IABP shaft in the aorta. Wrong IABP position/height was always immediately corrected bedside, with chest X‐ray confirmation of final position. In addition, in patients without correct IABP position, the device tip was still relatively close to the aortic knob at a mean distance of 18 mm. IABP insertion was successfully achieved in all patients with attempted bedside implant, with the exception of one patient with extremely tortuous ilio‐femoral axis, resulting in a bedside implant feasibility rate of 97.1%. Finally, TTE or TEE allowed IABP insertion guidance in all patients with attempted bedside approach and no case required CathLab transfer for inadequate acoustic window. Importantly, IABP‐related complications were low and similar in both cohorts, with IABP‐related major vascular complications occurring in 5.7% and 5.0%. In general, the rates of vascular complications were similar between groups, and comparable to those reported in previous studies. 11 , 12 , 13
The relatively low profile of IABP insertion sheath, coupled with its easy implant technique make it an attractive device for rapid bedside deployment. 2 , 5 The clinical outlook of this report is strengthened by the recent reappraisal of the value of IABP in specific settings of CS, 14 and by the increasing use of IABP in the setting of ADHF with hypoperfusion. 2
Indeed, patients who received bedside IABP at our Institution more often presented with ADHF‐related CS and had history of pre‐existing HF. This population was sicker, as highlighted by the worse arterial systolic pressure at presentation and the higher rate of inotrope use. On echocardiography, they presented with findings of biventricular dysfunction and mitral and tricuspid severe regurgitation. The ADHF cohort has been identified as an ideal candidate for counterpulsation and feasibility of bedside implant may enhance IABP adoption in this setting, avoiding the need of patient transfer to the CathLab and the related costs. Indeed, ICA and CathLab transfer would otherwise be seldom required in AHF patients (~20% all admissions), and this proportion may be even smaller in those featuring a “cold” hypoperfused phenotype (15%–17%). 15
The major limitation of this study is the small sample size. However, it represents the first description of bedside IABP implant to inform on the feasibility and safety of this technique. The ultrasound‐guided femoral artery puncture may have contributed to the observed findings and these results may not be generalizable to “blinded” femoral vessel puncture. In rare but possible situations the echocardiographic window may impede correct visualization of the guidewire and CathLab implant may be the only possible option. In addition, expertise in arterial puncture and Seldinger technique is necessary to perform this procedure and may require a learning curve for untrained personnel. Finally, a mobile C‐arm may offer the unique possibility to attempt bedside insertion while maintaining a direct real‐time fluoroscopic view during guidewires manipulation and IABP shaft advancement: if available, this method combined with echocardiography may be the preferred approach for bedside IABP implant.
5. CONCLUSION
In this real‐world study, optimal IABP positioning at chest X‐ray after implant was similar between patients who received bedside and CathLab IABP insertion, as were rates of IABP‐related vascular complications. Bedside insertion was performed in 30.4% of all patients undergoing MCS with IABP for CS in a modern CICU. Patients requiring bedside IABP insertion more often presented with ADHF‐related CS, worse biventricular function and higher need of inotropes. This study suggests the feasibility and safety of bedside IABP insertion, which could be of relevant interest in patients with ADHF‐related CS who may not need coronary angiography or other urgent CathLab procedures.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Baldetti L, Beneduce A, Boccellino A, et al. Bedside intra‐aortic balloon pump insertion in cardiac intensive care unit: A single‐center experience. Catheter Cardiovasc Interv. 2022;99:1976‐1983. 10.1002/ccd.30197
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jentzer JC, Ahmed AM, Vallabhajosyula S, et al. Shock in the cardiac intensive care unit: changes in epidemiology and prognosis over time. Am Heart J. 2021;232:94‐104. 10.1016/j.ahj.2020.10.054 [DOI] [PubMed] [Google Scholar]
- 2. Baldetti L, Pagnesi M, Gramegna M, et al. Intra‐aortic balloon pumping in acute decompensated heart failure with hypoperfusion: from pathophysiology to clinical practice. Circ Hear Fail. 2021;14:14. 10.1161/CIRCHEARTFAILURE.121.008527 [DOI] [PubMed] [Google Scholar]
- 3. den Uil CA, Van Mieghem NM, B Bastos M, et al. Primary intra‐aortic balloon support versus inotropes for decompensated heart failure and low output: a randomised trial. EuroIntervention. 2019;15:586‐593. 10.4244/EIJ-D-19-00254 [DOI] [PubMed] [Google Scholar]
- 4. Hernandez‐Montfort J, Sinha SS, Thayer KL, et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ Hear Fail. 2021;14:e007924. 10.1161/CIRCHEARTFAILURE.120.007924 [DOI] [PubMed] [Google Scholar]
- 5. Kimman JR, Van Mieghem NM, Endeman H, et al. Mechanical support in early cardiogenic shock: what is the role of intra‐aortic balloon counterpulsation? Curr Heart Fail Rep. 2020;17:247‐260. 10.1007/s11897-020-00480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez Lima DR, Duran EJ, Rojas Díaz EL, Pinilla Rojas DI, Mercado Díaz MA, Bustos Martínez YF. Ultrasound‐guided insertion of intra‐aortic balloon counterpulsation in intensive care: description of the technique. Ultrasound J. 2020;12:23. 10.1186/s13089-020-00166-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishioka T, Friedman A, Cercek B, et al. Usefulness of transesophageal echocardiography for positioning the intraaortic balloon pump in the operating room. Am J Cardiol. 1996;77:105‐106. 10.1016/S0002-9149(97)89148-5 [DOI] [PubMed] [Google Scholar]
- 8. Klopman MA, Chen EP, Sniecinski RM. Positioning an intraaortic balloon pump using intraoperative transesophageal echocardiogram guidance. Anesth Analg. 2011;113:40‐43. 10.1213/ANE.0b013e3182140b9a [DOI] [PubMed] [Google Scholar]
- 9. Šustic A, Medved I, Šimic O. Ultrasound‐guided placement of intra‐aortic balloon pump. Eur J Anaesthesiol. 2002;19:149. 10.1017/S0265021502230261 [DOI] [PubMed] [Google Scholar]
- 10. Hyson E, Ravin C, Kelley M, Curtis A. Intraaortic counterpulsation balloon: radiographic considerations. Am J Roentgenol. 1977;128:915‐918. 10.2214/ajr.128.6.915 [DOI] [PubMed] [Google Scholar]
- 11. Thiele H, Zeymer U, Neumann F‐J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287‐1296. 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 12. Poirier Y, Voisine P, Plourde G, et al. Efficacy and safety of preoperative intra‐aortic balloon pump use in patients undergoing cardiac surgery: a systematic review and meta‐analysis. Int J Cardiol. 2016;207:67‐79. 10.1016/j.ijcard.2016.01.045 [DOI] [PubMed] [Google Scholar]
- 13. de Jong MM, Lorusso R, Al Awami F, et al. Vascular complications following intra‐aortic balloon pump implantation: an updated review. Perfusion. 2018;33:96‐104. 10.1177/0267659117727825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baldetti L, Gramegna M, Beneduce A, et al. Strategies of left ventricular unloading during VA‐ECMO support: a network meta‐analysis. Int J Cardiol. 2020;312:16‐21. 10.1016/j.ijcard.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 15. Chioncel O, Mebazaa A, Maggioni AP, et al. Acute heart failure congestion and perfusion status—impact of the clinical classification on in‐hospital and long‐term outcomes; insights from the ESC‐EORP‐HFA heart failure long‐term registry. Eur J Heart Fail. 2019;21:1338‐1352. 10.1002/ejhf.1492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


