Abstract
Microbial necromass is a central component of soil organic matter (SOM), whose management may be essential in mitigating atmospheric CO2 concentrations and climate change. Current consensus regards the magnitude of microbial necromass production to be heavily dependent on the carbon use efficiency of microorganisms, which is strongly influenced by the quality of the organic matter inputs these organisms feed on. However, recent concepts neglect agents relevant in many soils: earthworms. We argue that the activity of earthworms accelerates the formation of microbial necromass stabilized in aggregates and organo‐mineral associations and reduces the relevance of the quality of pre‐existing organic matter in this process. Earthworms achieve this through the creation of transient hotspots (casts) characterized by elevated contents of bioavailable substrate and the efficient build‐up and quick turnover of microbial biomass, thus converting SOM not mineralized in this process into a state more resistant against external disturbances, such as climate change. Promoting the abundance of earthworms may, therefore, be considered a central component of management strategies that aim to accelerate the formation of stabilized microbial necromass in wide locations of the soil commonly not considered hotspots of microbial SOM formation.
Keywords: aggregates, carbon sequestration, casts, concept, hotspot, organo‐mineral associations, substrate quality
We argue that the activity of earthworms accelerates the formation of microbial necromass stabilized in aggregates and organo‐mineral associations and reduces the relevance of the quality of pre‐existing organic matter in this process. Earthworms create transient hotspots (casts) characterized by elevated contents of bioavailable substrate and the efficient build‐up and quick turnover of microbial biomass, thus converting SOM not mineralized in this process into a state more resistant against disturbances. Promoting earthworm abundance may be considered a strategy to accelerate the formation of stabilized microbial necromass in wide locations of the soil commonly not considered hotspots of microbial SOM formation.

1. INTRODUCTION
Management of soil organic carbon (SOC) via modifications to carbon inputs (Amelung et al., 2020; Janzen et al., 2022) has the potential to aid in offsetting carbon dioxide emissions and contribute to the climate targets put forward in the Paris Climate Change Agreement (Paustian et al., 2016). To be effective, it is imperative for such management to follow recent conceptual advances in soil organic matter (SOM) formation and stability. Recent concepts (Cotrufo et al., 2013; Kögel‐Knabner, 2002; Liang et al., 2017) and empirical evidence (Kallenbach et al., 2015; Ludwig et al., 2015; Ma et al., 2018) recognize microbial necromass as an essential pool of SOC (specifically in grassland and arable soil; Angst et al., 2021; Liang et al., 2019). The manipulation of this pool via plant input‐driven modifications to microbial physiology and traits (Kallenbach et al., 2015; Sokol et al., 2022) is thought to be critical in efforts to mitigate climate change. Central to recent concepts and the formation rate of microbial necromass is the efficiency of microbial growth on organic matter compounds (i.e., microbial carbon use efficiency (CUE)—the amount of carbon used for growth related to the carbon heterotrophically respired as CO2), which is directly linked to the quality of these organic compounds (e.g., low C/N and/or lignin/N ratios). The persistence of microbial necromass, in turn, is dependent on stabilization in aggregates and organo‐mineral complexes (Castellano et al., 2015; Cotrufo et al., 2013; Schmidt et al., 2011). While evidence for the validity of recent concepts is steadily increasing (Angst et al., 2021; Ding & Han, 2014; Gillespie et al., 2014; Griepentrog et al., 2014; Liang et al., 2019), they neglect organisms relevant in many soils: earthworms. Earthworms belong to the main soil‐forming agents in multiple soil orders (Kögel‐Knabner & Amelung, 2021), and with a mean biomass of up to 39.2 g/m2 and mean abundances of up to 83 ± 2.0 ind./m2 globally (Figure 1a), they are the most important contributor to invertebrate biomass in many soils (which is ~2% of that of soil microorganisms; Bar‐On et al., 2018; Curry, 2004; Fierer et al., 2009). The high abundance and burrowing activity of mineral soil‐dwelling earthworms (endogeic and anecic species) result in the biophysicochemical alteration of their soil environment (Brown et al., 2000) and the displacement of large amounts of soil, which is estimated to be up to 35 Mg/ha/year in temperate ecosystems (Taylor et al., 2019). Referring to the extent to which earthworms “engineer” their environment (Capowiez, Sammartino, & Michel, 2014; Darwin, 1892; Frouz et al., 2009; Humphreys & Field, 1998; Scheu, 1987; Wilkinson et al., 2009), it is surprising that they have not found their way into recent concepts on SOM dynamics. Here, we argue that earthworms are key to the formation of microbial SOM resistant against external disturbances in wide regions of the mineral soil. Earthworms achieve this by concurrently alleviating constraints on microbial growth in space and time and fostering the formation of aggregates and organo‐mineral associations. Embracing this central role of earthworms in SOM dynamics beyond their bioturbation activity enables a novel view on soil carbon sequestration and provides opportunities for integrated management strategies in the face of climate change.
FIGURE 1.
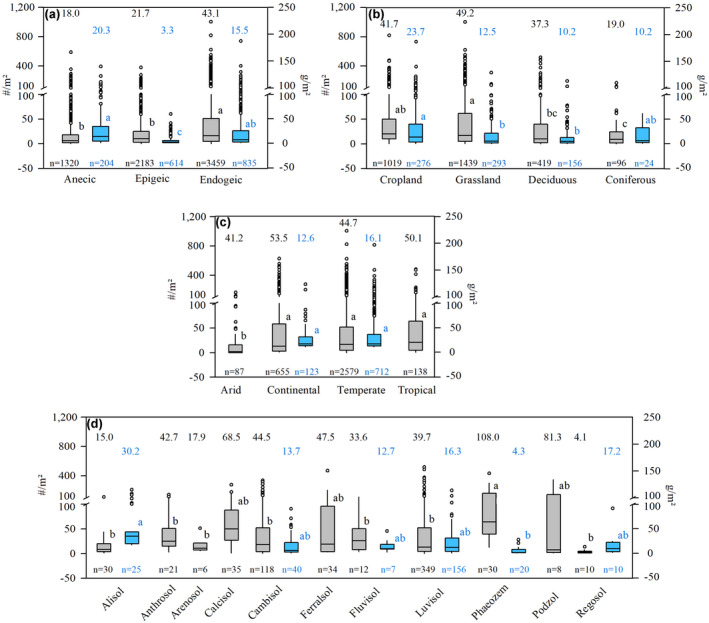
Global earthworm abundance (ind./m2; in grey) and biomass (g/m2; in blue) of three ecological groups [(a); anecic, epigeic, and endogeic earthworms] and abundance and biomass of mineral soil‐dwelling endogeics based on land use [(b); cropland, grassland, and deciduous and coniferous forests], climate [(c); Köppen‐Geiger classification main groups: arid, continental, temperate, and tropical], and reference soil group (d) (IUSS Working Group WRB, 2014). The data were compiled based on the dataset on global earthworm diversity and abundance published by Phillips et al. (2021), including 78 studies and up to ~6900 observations. Lower‐case letters indicate significant differences between abundance or biomass values (p < .05, analysis of variance with Tukey's HSD post‐hoc test); dots indicate outliers, that is, values exceeding 1.5× the interquartile range; means are indicated above each box plot. Note that soil type data, except for Cambisols and Luvisols, are relatively scarce. Panels (b)–(d) for epigeic and anecic species are provided in Figures S1 and S2
2. EARTHWORMS CATALYZE THE FORMATION AND STABILIZATION OF MICROBIAL NECROMASS—CONCEPT AND IMPLICATIONS
During their burrowing activity, earthworms ingest and mix organic matter with mineral soil, add easily decomposable compounds consisting of amino acids, sugars, and glycoproteins (Zhang et al., 2016), and egest this mixture as casts (Scheu, 1991). These casts may account for a major proportion of the whole soil profile, with soil ingestion rates by earthworms of up to 2000 and 500 Mg dry soil/ha/year in tropical and temperate ecosystems, respectively (Lavelle, 1988). Moreover, casts contain >50% higher amounts of water‐soluble compounds and >84% higher contents of available nitrogen and phosphorus as compared to non‐ingested soil (Brown et al., 2000; Van Groenigen et al., 2018). Significant increases in microbial biomass and activity in earthworm casts are well documented (Groffman et al., 2015; Hoeffner et al., 2018; Jouquet et al., 2013). Similarly well documented is the temporary nature of this effect, i.e., microbial biomass and activity in casts approach those in control soil several days after reworking (Brown et al., 2000).
We argue that via the creation of such transient microbial hotspots in casts, earthworms considerably accelerate the formation of microbial necromass (Angst, Mueller, Prater, et al., 2019; Mora et al., 2003; Vidal et al., 2019) by alleviating two factors commonly constraining microbial growth: first, addition of bioavailable compounds and provisioning of nutrients alleviate potential substrate limitations and increase microbial CUE (Barthod et al., 2021; Bohlen et al., 2002), and second, the intimate mixture of mineral soil and organic matter in casts (Scullion & Malik, 2000) co‐locates microorganisms and substrates previously separated (Sokol et al., 2019). This results in the efficient built‐up of microbial biomass and the concurrent and accelerated formation of microbial necromass due to a rapid microbial turnover in casts (Thu Hoang et al., 2020).
This process has important implications for SOM dynamics: first, due to the transient nature of microbial hotspots, microbial activity and, thus, SOM mineralization in casts are increased in the short term, which might result in the loss of bulk SOC (Lubbers et al., 2017). By contrast, the generated microbial necromass becomes stabilized on longer time scales via tight binding to mineral surfaces (Buckeridge, La Rosa, et al., 2020) and occlusion within cast aggregates (Bossuyt et al., 2005), processes that are favored by earthworms (Al‐Maliki & Scullion, 2013; Guhra et al., 2020) and which render the remaining SOC more resistant against disturbances. Second, the abundance of bioavailable substrates in casts enables microbes to grow more efficiently on “recalcitrant” compounds via cometabolism (Marschner et al., 2008), such that microbial necromass formation would be partially independent of the quality of the pre‐existing SOM. This is in contrast to the assumptions of recent concepts that plant input quality is a major driver of the formation rate of microbial necromass (Cotrufo et al., 2013). These concepts may not be unconditionally applicable in soils inhabited by earthworms.
Based on the considerations above, we propose an alternative concept embracing the central role of earthworms in how rapid and how much microbial necromass is generated in mineral soils (Figure 2). This concept aligns recent ideas on the formation of stabilized SOM with the activity of earthworms and provides a novel perspective on the mechanisms underlying microbial necromass formation and stabilization in mineral soil. We specifically emphasize the relevance of this concept in extensive soil regions remote from “classical” hotspots of microbial SOM formation, such as the rhizosphere, detritusphere, or preferential flow paths of dissolved organic matter (Bundt et al., 2001; Kuzyakov & Blagodatskaya, 2015), due to the earthworm's wide sphere of influence (from 0 to more than 100 cm depth, depending on the species). Management strategies that aim to increase soil microbial necromass and SOC sequestration may thus not only want to employ measures aimed at “directly” influencing microbial physiology (Kallenbach et al., 2015), but take a broader approach to also maintain or establish earthworm populations in mineral soils. This could be achieved by implementing various actions at the plot (to landscape) scale that additionally entail benefits for soil health and help adapt to and mitigate climate change (Lehmann et al., 2020; Pörtner et al., 2021). For example, increasing crop diversity, reducing tillage for certain soils, such as those affected by heat or erosion, and applying organic amendments in regions with low crop yields are expected to foster carbon sequestration, have positive effects on plant production, water quality, and human health, and reportedly increase earthworm biomass and abundance (Amelung et al., 2020; Lehmann et al., 2020; Wittwer et al., 2021). Diversification of plant species and planting of legumes in grassland soils, specifically in low‐productivity pastures and savannahs (Tilman et al., 2006), are expected to increase soil carbon sequestration and earthworm biomass and abundance (Eisenhauer et al., 2009; O'Mara, 2012; Singh et al., 2020; Wittwer et al., 2021). Similarly, an increase in tree functional diversity (De Wandeler et al., 2016) and establishment of species with well palatable tissues (e.g., broadleaf vs. coniferous trees; Curry, 2004) and positive influence on the soil's base saturation (such as trees with high tissue Ca concentrations; Angst, Mueller, Eissenstat, et al., 2019; Reich et al., 2005) increase earthworm biomass and abundance in forest soils and can be considered in re‐ or afforestation efforts (Mayer et al., 2020).
FIGURE 2.
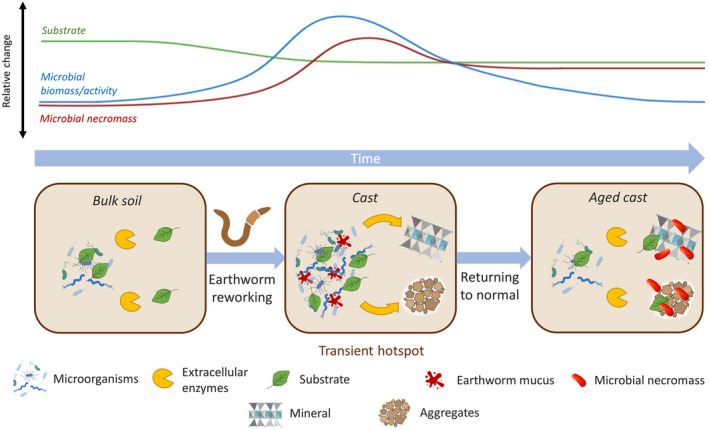
Embracing the central role of earthworms in microbial necromass formation and stabilization. In the initial state, the bulk soil is characterized by a slow formation rate of microbial necromass due to a lack of easily decomposable compounds, a low CUE, and the partial separation of microorganisms and their substrates. Earthworm reworking of this soil co‐locates microorganisms and substrates and provides nutrients and bioavailable compounds in casts. This induces a transient microbial hotspot in which microbial activity and CUE are strongly increased. As a consequence, microbial substrates are partly consumed and microbial biomass quickly and efficiently built‐up, whose necromass is subsequently stabilized in (earthworm‐generated) cast aggregates and by interaction with minerals. With increasing time after casting, microbial biomass, activity, and necromass formation gradually decrease to the initial level, while most of the microbial necromass generated in the transient hotspot is now part of the (stabilized) SOM pool (aged cast). Subsequent reworking of the same soil likely has a smaller effect due to partial re‐synthesis of microbial necromass (Buckeridge, Mason, et al., 2020). The relevance of this concept likely follows a seasonal trend based on the earthworm's life cycle (dormant during cold/hot and dry periods; Gates, 1961) and would be highest for (epi‐)endogeic species, given their abundance, biomass, and specifically, their life strategy (foraging in mineral soil horizons) and temporary burrows, that is, frequent infilling necessitates the continuous reconstruction of burrows (Capowiez, Bottinelli, & Jouquet, 2014; Potvin & Lilleskov, 2017) and higher volumes of affected soil. Factors that influence the stabilization of SOM (Castellano et al., 2015; Li et al., 2022) and the abundance, biomass, and activity of earthworms, such as land use (Figure 1b; Spurgeon et al., 2013), management (e.g., till vs. no‐till; Pelosi et al., 2014; Pérès et al., 2010), ecosystem development (Frouz et al., 2008; Zou & Gonzalez, 1997), climate (Figure 1c; Phillips et al., 2019; Singh et al., 2019), soil group (Figure 1d; Clause et al., 2014), or interaction with other soil fauna (Lubbers et al., 2019), may further modify the relevance of earthworms to the generation and stabilization of microbial necromass
While the central role of earthworms in the formation of microbial SOM is supported by recent experimental evidence (increases in microbial‐derived amino sugars between 37% and 145% have been reported; Angst, Mueller, Prater, et al., 2019; Mora et al., 2003; Nguyen Tu et al., 2020; Vidal et al., 2019), the relevance of our concept to bulk SOC stability and stocks in various environments, for example, different soil groups, land uses, or covers (see also Figure 1), and in the context of a changing climate remains unclear (Phillips et al., 2019; Singh et al., 2019). The potential of earthworms to increase N2O emissions (Drake & Horn, 2007; Lubbers et al., 2013) and the fact that invasive species may initially reduce overall soil carbon stocks (forest floor + mineral soil; Bohlen et al., 2004, but see also Ferlian et al., 2020) have to further be reconciled with the favorable effect of earthworm activity on microbial necromass formation reported here. To solve these unanswered questions, we encourage soil fauna‐related studies to break new ground by combining biomarkers, isotopes, physical fractionations, and a spatially resolved sampling design in integrated field studies, with variations in land use/cover and climate change‐related variables (such as temperature and precipitation) as central elements. Monitoring of carbon and nitrogen fluxes within this context will be of specific importance to disentangle the quantitative role of earthworms with respect to the soil's greenhouse‐gas balance and the whole SOC budget. Finally, we emphasize the necessity to also untwine the role of other, widespread saprophagous invertebrates such as mites, collembolans, and nematodes (van den Hoogen et al., 2019) in the formation of microbial necromass and SOM, which will open up new opportunities for the management of soils as a carbon sink.
AUTHOR CONTRIBUTIONS
Gerrit Angst conceived of the concept and drafted the first version of the manuscript. Ingrid Kögel‐Knabner and Nico Eisenhauer provided specific input on the presented concept and, together with Jan Frouz, Jan Willem van Groenigen, and Stefan Scheu, helped to improve all subsequent versions of the manuscript.
CONFLICTS OF INTEREST
All authors declare no competing interests.
Supporting information
Figure S1
Figure S2
ACKNOWLEDGMENTS
This research was funded by the Deutsche Forschungsgemeinschaft (DFG—German Research Foundation)—grant no. AN 1706/2‐1 to G.A., the Czech Science Foundation (GAČR)—grant nos. 19‐00533Y and 21‐18623S to G.A., the European Research Council (European Union's Horizon 2020 research and innovation program)—grant no. 677232 to N.E., the German Centre for Integrative Biodiversity Research Halle‐Jena‐Leipzig, funded by Deutsche Forschungsgemeinschaft (DFG—German Research Foundation)—FZT 118, 202548816, the Jena Experiment funded by Deutsche Forschungsgemeinschaft (DFG—German Research Foundation)—FOR 5000, and the BonaRes Centre for Soil Research (project BonaRes Module B, grant 031B0511C) “Soil as a Sustainable Resource for the Bioeconomy—BonaRes,” funded by the German Federal Ministry of Education and Research (BMBF). We thank the editor and reviewer for valuable comments on the manuscript.
Angst, G. , Frouz, J. , van Groenigen, J. W. , Scheu, S. , Kögel‐Knabner, I. , & Eisenhauer, N. (2022). Earthworms as catalysts in the formation and stabilization of soil microbial necromass. Global Change Biology, 28, 4775–4782. 10.1111/gcb.16208
Ingrid Kögel‐Knabner and Nico Eisenhauer jointly supervised this work.
DATA AVAILABILITY STATEMENT
The data used to compile Figure 1 and the supplementary figures can be freely accessed at https://doi.org/10.25829/idiv.1880‐17‐3189.
REFERENCES
- Al‐Maliki, S. , & Scullion, J. (2013). Interactions between earthworms and residues of differing quality affecting aggregate stability and microbial dynamics. Applied Soil Ecology, 64, 56–62. 10.1016/j.apsoil.2012.10.008 [DOI] [Google Scholar]
- Amelung, W. , Bossio, D. , de Vries, W. , Kögel‐Knabner, I. , Lehmann, J. , Amundson, R. , Bol, R. , Collins, C. , Lal, R. , Leifeld, J. , Minasny, B. , Pan, G. , Paustian, K. , Rumpel, C. , Sanderman, J. , van Groenigen, J. W. , Mooney, S. , van Wesemael, B. , Wander, M. , & Chabbi, A. (2020). Towards a global‐scale soil climate mitigation strategy. Nature Communications, 11(1), 1–10. 10.1038/s41467-020-18887-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst, G. , Mueller, C. W. , Prater, I. , Angst, Š. , Peterse, F. , & Nierop, K. G. J. (2019). Earthworms act as biochemical reactors to convert labile plant compounds into stabilized soil microbial necromass. Communications Biology, 2(441), 1–7. 10.1038/s42003-019-0684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst, G. , Mueller, K. E. , Eissenstat, D. M. , Trumbore, S. , Freeman, K. H. , Hobbie, S. E. , Chorover, J. , Oleksyn, J. , Reich, P. B. , & Mueller, C. W. (2019). Soil organic carbon stability in forests: Distinct effects of tree species identity and traits. Global Change Biology, 14548, 1529–1546. 10.1111/gcb.14548 [DOI] [PubMed] [Google Scholar]
- Angst, G. , Mueller, K. E. , Nierop, K. G. J. , & Simpson, M. J. (2021). Plant‐ or microbial‐derived? A review on the molecular composition of stabilized SOM. Soil Biology and Biochemistry, 156, 108189. [Google Scholar]
- Bar‐On, Y. M. , Phillips, R. , & Milo, R. (2018). The biomass distribution on earth. Proceedings of the National Academy of Sciences, 115(25), 6506–6511. 10.1073/pnas.1711842115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthod, J. , Dignac, M. F. , & Rumpel, C. (2021). Effect of decomposition products produced in the presence or absence of epigeic earthworms and minerals on soil carbon stabilization. Soil Biology and Biochemistry, 160, 108308. 10.1016/j.soilbio.2021.108308 [DOI] [Google Scholar]
- Bohlen, P. J. , Edwards, C. A. , Zhang, Q. , Parmelee, R. W. , & Allen, M. (2002). Indirect effects of earthworms on microbial assimilation of labile carbon. Applied Soil Ecology, 20(3), 255–261. 10.1016/S0929-1393(02)00027-6 [DOI] [Google Scholar]
- Bohlen, P. J. , Scheu, S. , Hale, C. M. , McLean, M. A. , Migge, S. , Groffman, P. M. , & Parkinson, D. (2004). Non‐native invasive earthworms as agents of change in northern temperate forests. Frontiers in Ecology and the Environment, 2(8), 427–435. 10.1890/1540-9295(2004)002[0427:NIEAAO]2.0.CO;2 [DOI] [Google Scholar]
- Bossuyt, H. , Six, J. , & Hendrix, P. F. (2005). Protection of soil carbon by microaggregates within earthworm casts. Soil Biology and Biochemistry, 37(2), 251–258. 10.1016/j.soilbio.2004.07.035 [DOI] [Google Scholar]
- Brown, G. G. , Barois, I. , & Lavelle, P. (2000). Regulation of soil organic matter dynamics and microbial activityin the drilosphere and the role of interactionswith other edaphic functional domains (Paper presented at the 16th world congress of soil science, 20–26 August 1998, Montpellier, France). European Journal of Soil Biology, 36(3), 177–198. 10.1016/S1164-5563(00)01062-1 [DOI] [Google Scholar]
- Buckeridge, K. M. , La Rosa, A. F. , Mason, K. E. , Whitaker, J. , McNamara, N. P. , Grant, H. K. , & Ostle, N. J. (2020). Sticky dead microbes: Rapid abiotic retention of microbial necromass in soil. Soil Biology and Biochemistry, 149, 107929. 10.1016/j.soilbio.2020.107929 [DOI] [Google Scholar]
- Buckeridge, K. M. , Mason, K. E. , McNamara, N. P. , Ostle, N. , Puissant, J. , Goodall, T. , Griffiths, R. I. , Stott, A. W. , & Whitaker, J. (2020). Environmental and microbial controls on microbial necromass recycling, an important precursor for soil carbon stabilization. Communications Earth & Environment, 1(1), 36. 10.1038/s43247-020-00031-4 [DOI] [Google Scholar]
- Bundt, M. , Widmer, F. , Pesaro, M. , Zeyer, J. , & Blaser, P. (2001). Preferential flow paths: Biological “hot spots” in soils. Soil Biology and Biochemistry, 33(6), 729–738. 10.1016/S0038-0717(00)00218-2 [DOI] [Google Scholar]
- Capowiez, Y. , Bottinelli, N. , & Jouquet, P. (2014). Quantitative estimates of burrow construction and destruction, by anecic and endogeic earthworms in repacked soil cores. Applied Soil Ecology, 74, 46–50. 10.1016/j.apsoil.2013.09.009 [DOI] [Google Scholar]
- Capowiez, Y. , Sammartino, S. , & Michel, E. (2014). Burrow systems of endogeic earthworms: Effects of earthworm abundance and consequences for soil water infiltration. Pedobiologia, 57(4), 303–309. 10.1016/j.pedobi.2014.04.001 [DOI] [Google Scholar]
- Castellano, M. J. , Mueller, K. E. , Olk, D. C. , Sawyer, J. E. , & Six, J. (2015). Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Global Change Biology, 21(9), 3200–3209. 10.1111/gcb.12982 [DOI] [PubMed] [Google Scholar]
- Clause, J. , Barot, S. , Richard, B. , Decaëns, T. , & Forey, E. (2014). The interactions between soil type and earthworm species determine the properties of earthworm casts. Applied Soil Ecology, 83, 149–158. 10.1016/j.apsoil.2013.12.006 [DOI] [Google Scholar]
- Cotrufo, M. F. , Wallenstein, M. D. , Boot, C. M. , Denef, K. , & Paul, E. (2013). The microbial efficiency‐matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Global Change Biology, 19(4), 988–995. 10.1111/gcb.12113 [DOI] [PubMed] [Google Scholar]
- Curry, J. P. (2004). Factors affecting the abundance of earthworms in soils. In Edwards C. A. (Ed.), Earthworm ecology (2nd ed., pp. 91–108). CRC Press. [Google Scholar]
- Darwin, C. (1892). The formation of vegetable mould, through the action of worms: With observations on their habits. John Murray. [Google Scholar]
- De Wandeler, H. , Sousa‐Silva, R. , Ampoorter, E. , Bruelheide, H. , Carnol, M. , Dawud, S. M. , Dănilă, G. , Finer, L. , Hättenschwiler, S. , Hermy, M. , Jaroszewicz, B. , Joly, F. X. , Müller, S. , Pollastrini, M. , Ratcliffe, S. , Raulund‐Rasmussen, K. , Selvi, F. , Valladares, F. , Van Meerbeek, K. , … Muys, B. (2016). Drivers of earthworm incidence and abundance across European forests. Soil Biology and Biochemistry, 99, 167–178. 10.1016/j.soilbio.2016.05.003 [DOI] [Google Scholar]
- Ding, X. , & Han, X. (2014). Effects of long‐term fertilization on contents and distribution of microbial residues within aggregate structures of a clay soil. Biology and Fertility of Soils, 50(3), 549–554. 10.1007/s00374-013-0867-6 [DOI] [Google Scholar]
- Drake, H. L. , & Horn, M. A. (2007). As the worm turns: The earthworm gut as a transient habitat for soil microbial biomes. Annual Review of Microbiology, 61(1), 169–189. 10.1146/annurev.micro.61.080706.093139 [DOI] [PubMed] [Google Scholar]
- Eisenhauer, N. , Milcu, A. , Sabais, A. C. W. , Bessler, H. , Weigelt, A. , Engels, C. , & Scheu, S. (2009). Plant community impacts on the structure of earthworm communities depend on season and change with time. Soil Biology and Biochemistry, 41(12), 2430–2443. 10.1016/j.soilbio.2009.09.001 [DOI] [Google Scholar]
- Ferlian, O. , Thakur, M. P. , Castañeda González, A. , San Emeterio, L. M. , Marr, S. , da Silva Rocha, B. , & Eisenhauer, N. (2020). Soil chemistry turned upside down: A meta‐analysis of invasive earthworm effects on soil chemical properties. Ecology, 101(3), 1–12. 10.1002/ecy.2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer, N. , Strickland, M. S. , Liptzin, D. , Bradford, M. A. , & Cleveland, C. C. (2009). Global patterns in belowground communities. Ecology Letters, 12(11), 1238–1249. 10.1111/j.1461-0248.2009.01360.x [DOI] [PubMed] [Google Scholar]
- Frouz, J. , Pižl, V. , Cienciala, E. , & Kalčík, J. (2009). Carbon storage in post‐mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry, 94(2), 111–121. 10.1007/s10533-009-9313-0 [DOI] [Google Scholar]
- Frouz, J. , Prach, K. , Pižl, V. , Háněl, L. , Starý, J. , Tajovský, K. , Materna, J. , Balík, V. , Kalčík, J. , & Řehounková, K. (2008). Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. European Journal of Soil Biology, 44(1), 109–121. 10.1016/j.ejsobi.2007.09.002 [DOI] [Google Scholar]
- Gates, G. E. (1961). Ecology of some earthworms with special reference to seasonal activity. The American Midland Naturalist, 66(1), 61–86. [Google Scholar]
- Gillespie, A. W. , Diochon, A. , Ma, B. L. , Morrison, M. J. , Kellman, L. , Walley, F. L. , Regier, T. Z. , Chevrier, D. , Dynes, J. J. , & Gregorich, E. G. (2014). Nitrogen input quality changes the biochemical composition of soil organic matter stabilized in the fine fraction: A long‐term study. Biogeochemistry, 117(2–3), 337–350. 10.1007/s10533-013-9871-z [DOI] [Google Scholar]
- Griepentrog, M. , Bodé, S. , Boeckx, P. , Hagedorn, F. , Heim, A. , & Schmidt, M. W. I. (2014). Nitrogen deposition promotes the production of new fungal residues but retards the decomposition of old residues in forest soil fractions. Global Change Biology, 20(1), 327–340. 10.1111/gcb.12374 [DOI] [PubMed] [Google Scholar]
- Groffman, P. M. , Fahey, T. J. , Fisk, M. C. , Yavitt, J. B. , Sherman, R. E. , Bohlen, P. J. , & Maerz, J. C. (2015). Earthworms increase soil microbial biomass carrying capacity and nitrogen retention in northern hardwood forests. Soil Biology and Biochemistry, 87, 51–58. 10.1016/j.soilbio.2015.03.025 [DOI] [Google Scholar]
- Guhra, T. , Stolze, K. , Schweizer, S. , & Totsche, K. U. (2020). Earthworm mucus contributes to the formation of organo‐mineral associations in soil. Soil Biology and Biochemistry, 145, 107785. 10.1016/j.soilbio.2020.107785 [DOI] [Google Scholar]
- Hoeffner, K. , Monard, C. , Santonja, M. , & Cluzeau, D. (2018). Feeding behaviour of epi‐anecic earthworm species and their impacts on soil microbial communities. Soil Biology and Biochemistry, 125(June), 1–9. 10.1016/j.soilbio.2018.06.017 [DOI] [Google Scholar]
- Humphreys, G. S. , & Field, R. (1998). Mixing, mounding and other aspects of bioturbation: Implications for pedogenesis . 16th World Congress of Soil Science, International Society of Soil Science, Montpellier.
- IUSS Working Group WRB . (2014). World Reference Base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps . World Soil Resources Reports No. 106 (Vol. 43, Issue 02). FAO. 10.1017/S0014479706394902 [DOI]
- Janzen, H. H. , van Groenigen, K. J. , Powlson, D. S. , Schwinghamer, T. , & van Groenigen, J. W. (2022). Photosynthetic limits on carbon sequestration in croplands. Geoderma, 416(March), 115810. 10.1016/j.geoderma.2022.115810 [DOI] [Google Scholar]
- Jouquet, P. , Maron, P. A. , Nowak, V. , & Tran Duc, T. (2013). Utilization of microbial abundance and diversity as indicators of the origin of soil aggregates produced by earthworms. Soil Biology and Biochemistry, 57, 950–952. 10.1016/j.soilbio.2012.08.026 [DOI] [Google Scholar]
- Kallenbach, C. M. , Grandy, A. S. , Frey, S. D. , & Diefendorf, A. F. (2015). Microbial physiology and necromass regulate agricultural soil carbon accumulation. Soil Biology and Biochemistry, 91, 279–290. 10.1016/j.soilbio.2015.09.005 [DOI] [Google Scholar]
- Kögel‐Knabner, I. (2002). The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry, 34(2), 139–162. 10.1016/S0038-0717(01)00158-4 [DOI] [Google Scholar]
- Kögel‐Knabner, I. , & Amelung, W. (2021). Soil organic matter in major pedogenic soil groups. Geoderma, 384(October 2020), 114785. 10.1016/j.geoderma.2020.114785 [DOI] [Google Scholar]
- Kuzyakov, Y. , & Blagodatskaya, E. (2015). Microbial hotspots and hot moments in soil: Concept & review. Soil Biology and Biochemistry, 83, 184–199. 10.1016/j.soilbio.2015.01.025 [DOI] [Google Scholar]
- Lavelle, P. (1988). Earthworm activities and the soil system. Biology and Fertility of Soils, 6(3), 237–251. 10.1007/BF00260820 [DOI] [Google Scholar]
- Lehmann, J. , Bossio, D. A. , Kögel‐Knabner, I. , & Rillig, M. C. (2020). The concept and future prospects of soil health. Nature Reviews Earth and Environment, 1(10), 544–553. 10.1038/s43017-020-0080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Meador, T. , Sauheitl, L. , Guggenberger, G. , & Angst, G. (2022). Substrate quality effects on stabilized soil carbon reverse with depth. Geoderma, 406(October 2021), 115511. 10.1016/j.geoderma.2021.115511 [DOI] [Google Scholar]
- Liang, C. , Amelung, W. , Lehmann, J. , & Kästner, M. (2019). Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biology, 25(11), 3578–3590. 10.1111/gcb.14781 [DOI] [PubMed] [Google Scholar]
- Liang, C. , Schimel, J. P. , & Jastrow, J. D. (2017). The importance of anabolism in microbial control over soil carbon storage. Nature Microbiology, 2(8), 1–6. 10.1038/nmicrobiol.2017.105 [DOI] [PubMed] [Google Scholar]
- Lubbers, I. M. , Berg, M. P. , De Deyn, G. B. , van der Putten, W. H. , & van Groenigen, J. W. (2019). Soil fauna diversity increases CO2 but suppresses N2O emissions from soil. Global Change Biology, 26(3), 1886–1898. 10.1111/gcb.14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers, I. M. , Pulleman, M. M. , & Van Groenigen, J. W. (2017). Can earthworms simultaneously enhance decomposition and stabilization of plant residue carbon? Soil Biology and Biochemistry, 105, 12–24. 10.1016/j.soilbio.2016.11.008 [DOI] [Google Scholar]
- Lubbers, I. M. , Van Groenigen, K. J. , Fonte, S. J. , Six, J. , Brussaard, L. , & Van Groenigen, J. W. (2013). Greenhouse‐gas emissions from soils increased by earthworms. Nature Climate Change, 3(3), 187–194. 10.1038/nclimate1692 [DOI] [Google Scholar]
- Ludwig, M. , Achtenhagen, J. , Miltner, A. , Eckhardt, K. U. , Leinweber, P. , Emmerling, C. , & Thiele‐Bruhn, S. (2015). Microbial contribution to SOM quantity and quality in density fractions of temperate arable soils. Soil Biology and Biochemistry, 81, 311–322. 10.1016/j.soilbio.2014.12.002 [DOI] [Google Scholar]
- Ma, T. , Zhu, S. , Wang, Z. , Chen, D. , Dai, G. , Feng, B. , Su, X. , Hu, H. , Li, K. , Han, W. , Liang, C. , Bai, Y. , & Feng, X. (2018). Divergent accumulation of microbial necromass and plant lignin components in grassland soils. Nature Communications, 9, 3480. 10.1038/s41467-018-05891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner, B. , Brodowski, S. , Dreves, A. , Gleixner, G. , Gude, A. , Grootes, P. M. , Hamer, U. , Heim, A. , Jandl, G. , Ji, R. , Kaiser, K. , Kalbitz, K. , Kramer, C. , Leinweber, P. , Rethemeyer, J. , Schaeffer, A. , Schmidt, M. W. I. , Schwark, L. , & Wiesenberg, G. L. B. (2008). How relevant is recalcitrance for the stabilization of organic matter in soils? Journal of Plant Nutrition and Soil Science, 171(1), 91–110. 10.1002/jpln.200700049 [DOI] [Google Scholar]
- Mayer, M. , Prescott, C. E. , Abaker, W. E. A. , Augusto, L. , Cécillon, L. , Ferreira, G. W. D. , James, J. , Jandl, R. , Katzensteiner, K. , Laclau, J. P. , Laganière, J. , Nouvellon, Y. , Paré, D. , Stanturf, J. A. , Vanguelova, E. I. , & Vesterdal, L. (2020). Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. Forest Ecology and Management, 466(January), 118127. 10.1016/j.foreco.2020.118127 [DOI] [Google Scholar]
- Mora, P. , Seugé, C. , Chotte, J. L. , & Rouland, C. (2003). Physico‐chemical typology of the biogenic structures of termites and earthworms: A comparative analysis. Biology and Fertility of Soils, 37(4), 245–249. 10.1007/s00374-003-0592-7 [DOI] [Google Scholar]
- Nguyen Tu, T. T. , Vidal, A. , Quenea, K. , Mendez‐Millan, M. , & Derenne, S. (2020). Influence of earthworms on apolar lipid features in soils after 1 year of incubation. Biogeochemistry, 147, 243–258. 10.1007/s10533-020-00639-w [DOI] [Google Scholar]
- O'Mara, F. P. (2012). The role of grasslands in food security and climate change. Annals of Botany, 110(6), 1263–1270. 10.1093/aob/mcs209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paustian, K. , Lehmann, J. , Ogle, S. , Reay, D. , Robertson, G. P. , & Smith, P. (2016). Climate‐smart soils. Nature, 532(7597), 49–57. 10.1038/nature17174 [DOI] [PubMed] [Google Scholar]
- Pelosi, C. , Pey, B. , Hedde, M. , Caro, G. , Capowiez, Y. , Guernion, M. , Peigné, J. , Piron, D. , Bertrand, M. , & Cluzeau, D. (2014). Reducing tillage in cultivated fields increases earthworm functional diversity. Applied Soil Ecology, 83, 79–87. 10.1016/j.apsoil.2013.10.005 [DOI] [Google Scholar]
- Pérès, G. , Bellido, A. , Curmi, P. , Marmonier, P. , & Cluzeau, D. (2010). Relationships between earthworm communities and burrow numbers under different land use systems. Pedobiologia, 54(1), 37–44. 10.1016/j.pedobi.2010.08.006 [DOI] [Google Scholar]
- Phillips, H. R. P. , Bach, E. M. , Bartz, M. L. C. , Bennett, J. M. , Beugnon, R. , Briones, M. J. I. , Brown, G. G. , Ferlian, O. , Gongalsky, K. B. , Guerra, C. A. , König‐Ries, B. , Krebs, J. J. , Orgiazzi, A. , Ramirez, K. S. , Russell, D. J. , Schwarz, B. , Wall, D. H. , Brose, U. , Decaëns, T. , … Eisenhauer, N. (2021). Global data on earthworm abundance, biomass, diversity and corresponding environmental properties. Scientific Data, 8(1), 136. 10.1038/s41597-021-00912-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, H. R. P. , Guerra, C. A. , Bartz, M. L. C. , Briones, M. J. I. , Brown, G. , Crowther, T. W. , Ferlian, O. , Gongalsky, K. B. , van den Hoogen, J. , Krebs, J. , Orgiazzi, A. , Routh, D. , Schwarz, B. , Bach, E. M. , Bennett, J. , Brose, U. , Decaëns, T. , König‐Ries, B. , Loreau, M. , … Eisenhauer, N. (2019). Global distribution of earthworm diversity. Science, 366(6464), 480–485. 10.1126/science.aax4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner, H.‐O. , Scholes, R. J. , Agard, J. , Archer, E. , Arneth, A. , Bai, X. , Barnes, D. , Burrows, M. , Chan, L. , Cheung, W. L. (William), Diamond, S. , Donatti, C. , Duarte, C. , Eisenhauer, N. , Foden, W. , Gasalla, M. A. , Handa, C. , Hickler, T. , Hoegh‐Guldberg, O ., … Ngo, H . (2021). Scientific outcome of the IPBES‐IPCC co‐sponsored workshop on biodiversity and climate change. IPBES Secretariat, Bonn, Germany. 10.5281/ZENODO.5101125 [DOI]
- Potvin, L. R. , & Lilleskov, E. A. (2017). Introduced earthworm species exhibited unique patterns of seasonal activity and vertical distribution, and Lumbricus terrestris burrows remained usable for at least 7 years in hardwood and pine stands. Biology and Fertility of Soils, 53(2), 187–198. 10.1007/s00374-016-1173-x [DOI] [Google Scholar]
- Reich, P. B. , Oleksyn, J. , Modrzynski, J. , Mrozinski, P. , Hobbie, S. E. , Eissenstat, D. M. , Chorover, J. , Chadwick, O. A. , Hale, C. M. , & Tjoelker, M. G. (2005). Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecology Letters, 8(8), 811–818. 10.1111/j.1461-0248.2005.00779.x [DOI] [Google Scholar]
- Scheu, S. (1987). The role of substrate feeding earthworms (Lumbricidae) for bioturbation in a beechwood soil. Oecologia, 72(2), 192–196. 10.1007/BF00379266 [DOI] [PubMed] [Google Scholar]
- Scheu, S. (1991). Mucus excretion and carbon turnover of endogeic earthworms. Biology and Fertility of Soils, 12(3), 217–220. 10.1007/BF00337206 [DOI] [Google Scholar]
- Schmidt, M. W. I. , Torn, M. S. , Abiven, S. , Dittmar, T. , Guggenberger, G. , Janssens, I. A. , Kleber, M. , Kögel‐Knabner, I. , Lehmann, J. , Manning, D. A. C. , Nannipieri, P. , Rasse, D. P. , Weiner, S. , & Trumbore, S. E. (2011). Persistence of soil organic matter as an ecosystem property. Nature, 478(7367), 49–56. 10.1038/nature10386 [DOI] [PubMed] [Google Scholar]
- Scullion, J. , & Malik, A. (2000). Earthworm activity affecting organic matter, aggregation and microbial activity in soils restored after opencast mining for coal. Soil Biology and Biochemistry, 32(1), 119–126. 10.1016/S0038-0717(99)00142-X [DOI] [Google Scholar]
- Singh, J. , Cameron, E. , Reitz, T. , Schädler, M. , & Eisenhauer, N. (2020). Grassland management effects on earthworm communities under ambient and future climatic conditions. European Journal of Soil Science, 72(1), 343–355. 10.1111/ejss.12942 [DOI] [Google Scholar]
- Singh, J. , Schädler, M. , Demetrio, W. , Brown, G. G. , & Eisenhauer, N. (2019). Climate change effects on earthworms ‐ a review. Soil Organisms, 91(3), 114–138. 10.25674/so91iss3pp114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol, N. W. , Sanderman, J. , & Bradford, M. A. (2019). Pathways of mineral‐associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Global Change Biology, 25(1), 12–24. 10.1111/gcb.14482 [DOI] [PubMed] [Google Scholar]
- Sokol, N. W. , Slessarev, E. , Marschmann, G. L. , Nicolas, A. , Blazewicz, S. J. , Brodie, E. L. , Firestone, M. K. , Foley, M. M. , Hestrin, R. , Hungate, B. A. , Koch, B. J. , Stone, B. W. , Sullivan, M. B. , Zablocki, O. , Trubl, G. , McFarlane, K. , Stuart, R. , Nuccio, E. , Weber, P. , … Pett‐Ridge, J. (2022). Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nature Reviews Microbiology. 10.1038/s41579-022-00695-z [DOI] [PubMed] [Google Scholar]
- Spurgeon, D. J. , Keith, A. M. , Schmidt, O. , Lammertsma, D. R. , & Faber, J. H. (2013). Land‐use and land‐management change: Relationships with earthworm and fungi communities and soil structural properties. BMC Ecology, 13(1), 46. 10.1186/1472-6785-13-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. R. , Lenoir, L. , Vegerfors, B. , & Persson, T. (2019). Ant and earthworm bioturbation in cold‐temperate ecosystems. Ecosystems, 22(5), 981–994. 10.1007/s10021-018-0317-2 [DOI] [Google Scholar]
- Thu Hoang, D. T. , Maranguit, D. , Kuzyakov, Y. , & Razavi, B. S. (2020). Accelerated microbial activity, turnover and efficiency in the drilosphere is depth dependent. Soil Biology and Biochemistry, 147, 107852. 10.1016/j.soilbio.2020.107852 [DOI] [Google Scholar]
- Tilman, D. , Hill, J. , & Lehman, C. (2006). Carbon‐negative biofuels from low‐input high‐diversity grassland biomass. Science, 314(5805), 1598–1600. 10.1126/science.1133306 [DOI] [PubMed] [Google Scholar]
- van den Hoogen, J. , Geisen, S. , Routh, D. , Ferris, H. , Traunspurger, W. , Wardle, D. A. , de Goede, R. G. M. , Adams, B. J. , Ahmad, W. , Andriuzzi, W. S. , Bardgett, R. D. , Bonkowski, M. , Campos‐Herrera, R. , Cares, J. E. , Caruso, T. , de Brito Caixeta, L. , Chen, X. , Costa, S. R. , Creamer, R. , … Crowther, T. W. (2019). Soil nematode abundance and functional group composition at a global scale. Nature, 572(7768), 194–198. 10.1038/s41586-019-1418-6 [DOI] [PubMed] [Google Scholar]
- Van Groenigen, J. W. , Van Groenigen, K. J. , Koopmans, G. F. , Stokkermans, L. , Vos, H. M. J. , & Lubbers, I. M. (2018). How fertile are earthworm casts? A meta‐analysis. Geoderma, 338(October 2018), 525–535. 10.1016/j.geoderma.2018.11.001 [DOI] [Google Scholar]
- Vidal, A. , Watteau, F. , Remusat, L. , Mueller, C. W. , Nguyen Tu, T. T. , Buegger, F. , Derenne, S. , & Quenea, K. (2019). Earthworm cast formation and development: A shift from plant litter to mineral associated organic matter. Frontiers in Environmental Science, 7(April), 1–15. 10.3389/fenvs.2019.00055 [DOI] [Google Scholar]
- Wilkinson, M. T. , Richards, P. J. , & Humphreys, G. S. (2009). Breaking ground: Pedological, geological, and ecological implications of soil bioturbation. Earth‐Science Reviews, 97(1–4), 257–272. 10.1016/j.earscirev.2009.09.005 [DOI] [Google Scholar]
- Wittwer, R. A. , Bender, S. F. , Hartman, K. , Hydbom, S. , Lima, R. A. A. , Loaiza, V. , Nemecek, T. , Oehl, F. , Olsson, P. A. , Petchey, O. , Prechsl, U. E. , Schlaeppi, K. , Scholten, T. , Seitz, S. , Six, J. , & Van Der Heijden, M. G. A. (2021). Organic and conservation agriculture promote ecosystem multifunctionality. Science Advances, 7(34), 1–13. 10.1126/sciadv.abg6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Chen, Y. , Ma, Y. , Guo, L. , Sun, J. , & Tong, J. (2016). Earthworm epidermal mucus: Rheological behavior reveals drag‐reducing characteristics in soil. Soil and Tillage Research, 158, 57–66. 10.1016/j.still.2015.12.001 [DOI] [Google Scholar]
- Zou, X. , & Gonzalez, G. (1997). Changes in earthworm density and community structure during secondary succession in abandoned tropical pastures. Soil Biology and Biochemistry, 29(3–4), 627–629. 10.1016/S0038-0717(96)00188-5 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Data Availability Statement
The data used to compile Figure 1 and the supplementary figures can be freely accessed at https://doi.org/10.25829/idiv.1880‐17‐3189.


