Summary
Background
Thiopurines remain recommended as maintenance therapy in patients with inflammatory bowel disease (IBD). Despite their widespread use, long‐term effectiveness data are sparse and safety is an increasingly debated topic which thwarts proper delineation in the current IBD treatment algorithm.
Aims
To document effectiveness and safety of thiopurine monotherapy in patients with IBD, using the population‐based IBD South‐Limburg (IBDSL) cohort
Methods
All patients starting thiopurine monotherapy as maintenance between 1991 and 2014 were included. Therapy was defined as effective if there was no escalation to biologicals, no course of corticosteroids, no surgery and no hospitalisation for active disease during treatment. Long‐term effectiveness was assessed by adjusting for differences in follow‐up using Kaplan–Meier analyses. Mid‐ to long‐term safety regarding cancer incidence and clinically relevant liver disease was documented.
Results
In total, 1016 patients (643 Crohn's disease [CD]; 373 ulcerative colitis [UC]) received thiopurine monotherapy at a median of 15.2 (Q1‐Q3 4.2–48.5) months after diagnosis. During follow‐up, effectiveness rates at 1, 5 and 10 years were 64%, 45%, 32%, respectively, in CD and and 66%, 41%, 36%, respectively in UC. No statistically significant differences in effectiveness were observed after stratification for era of initiation (pre‐biological vs biological, CD: p = 0.56; UC: p = 0.43). Sixteen non‐melanoma skin cancers (incidence rate [IR] 3.33/1000 PY), five lymphomas (IR 1.04/1000 PY) and one urinary tract cancer (IR 0.21/1000 PY) were recorded. Two cases of portal hypertension were identified.
Conclusion
In real‐world practice, thiopurine monotherapy remains effective, safe and durable for patients with CD or UC, including in the era of biologics.

1. INTRODUCTION
Thiopurines have held a place in the treatment of Crohn's disease (CD) as well as ulcerative colitis (UC) patients for over 40 years. Thiopurines have proven to be valuable in maintaining (medically induced) remission, corticosteroid‐sparing regimens, reducing post‐operative recurrence, delaying disease progression, and, in combination with anti‐TNF, in preventing anti‐drug antibody formation with or without increasing through levels. 1 , 2 , 3 , 4 , 5 , 6 , 7 Aside from their effectiveness, thiopurines are an attractive treatment option due to their widespread availability, oral administration route, well‐outlined knowledge of potential adverse events, lack of immunogenicity and accessibility due to lower costs compared to biologicals or biosimilars.
Despite the growing armamentarium of biologicals, thiopurines are still recommended as monotherapy in first or second‐line maintenance treatment in patients with moderate‐to‐severe CD and steroid‐dependent UC in current and recent international Inflammatory bowel disease (IBD) treatment guidelines. 8 , 9 , 10 , 11 Although extensively and widely used, effectiveness is an increasingly debated topic, particularly since the introduction of biologicals and the overall quality of evidence for their efficacy remains low. In trials with strongly‐selected CD patients, such as in the RAPID and AZTEC trials, no statistical difference in efficacy was observed when comparing early use of azathioprine to either placebo or conventional thiopurine use respectively. In two older randomised trials conducted in UC patients, no benefit for thiopurines was found. 12 , 13 , 14 , 15 In addition, in the early years of infliximab therapy, combination therapy resulted in higher rates of clinical and endoscopic remission in comparison to monotherapy of thiopurines or infliximab. 5 , 6 In observational studies in real‐world settings it has been suggested that thiopurine monotherapy benefits IBD patients. However, these studies were limited by relatively small numbers or short follow‐up of patients. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Furthermore, in a recent large observational study from the UK, a lower, overall effectiveness rate of thiopurine monotherapy in CD patients as compared to UC patients was reported and the authors suggested a potential re‐evaluation of thiopurine use in CD. 24
Another frequently debated concern over thiopurines is its safety profile, including an increased risk of (viral) infections (e.g. Epstein–Barr virus [EBV]; human papillomavirus [HPV]), potential liver manifestations (e.g. hepatotoxicity and nodular regenerative hyperplasia [NRH]) as well as the long‐term risk of non‐melanoma skin cancers (NMSC), lymphomas, urinary tract cancer and cervical cancer. 25 , 26 , 27 , 28 These safety issues, in combination with the sparsity of long‐term data on thiopurine effectiveness, opened discussions on the benefit–risk balance of thiopurines in comparison to biologicals. However, biologicals and small molecules have also been associated with an increased risk of opportunistic and serious infections, as well as certain cancers, and long‐term population data on safety are still limited. 29 , 30
The aforementioned observations concerning the benefit–risk balance have also fuelled questioning on the position of thiopurines in the IBD treatment algorithm. 26 , 31 Interestingly, particularly in the perspective of consensus guidelines, such as the ECCO's, a recent international survey among more than 400 IBD physicians revealed that the majority of physicians still resort to thiopurines in a substantial proportion of patients and believe that thiopurines will still be an important part of the IBD treatment in the future. Moreover, 70% of respondents (strongly) agreed that thiopurines are effective as monotherapy in both CD and UC. 32
In consideration of the ongoing discussion and limited real‐world long‐term evidence, this study aimed to assess the long‐term effectiveness and safety of thiopurine monotherapy in both CD and UC patients in a population‐based study.
2. MATERIALS AND METHODS
2.1. Setting and patients
All included patients in this multi‐centre retrospective cohort study originated from the population‐based IBD South‐Limburg (IBDSL) cohort. The IBDSL cohort is a longitudinal, population‐based, prospective inception cohort of adult IBD patients, which has been described previously. 33 In brief, this inception cohort was initiated in 1991 and contains all newly‐diagnosed patients with IBD from the age of 18 years and older living in the South Limburg region (the Netherlands). As a result of a multi‐faceted identification strategy and joint cooperation of all regional hospitals (Maastricht University Medical Center, and Zuyderland Medical Centre Heerlen and Sittard), the cohort comprises at least 93% of all patients with IBD in the South Limburg region. In the present study, 2825 IBD patients (1663 UC, 1162 CD) were available for analysis. 33 All patients were prospectively followed from the date of diagnosis to the end of current data collection (2014), date of migration out of area or death. The IBDSL study design has been approved by the Ethics Committee of Maastricht University Medical Center (NL31636.068.10), follows the declaration of Helsinki, and is registered in ClinicalTrial.gov (NCT02130349). 34
Medical records of each individual patient were thoroughly reviewed with regard to demographic data, IBD phenotype (according to the Montreal Classification), hospitalisations (e.g. dates, indications and duration of stay), surgeries (e.g. dates, type and indications) and medication use (e.g. start and stop dates, type of medication, dose and stop reason). All data were extracted by trained physicians, researchers and nurses, using standardised registration forms. In addition, all clinical data, including data on medication, were updated biennially through chart reviews by trained researchers to maintain high level of completeness. 35 Further details on data collection in the IBDSL cohort have been described previously. 33 Data on malignancies were retrieved from the nationwide Dutch Pathology Database (PALGA). Of note, biochemical, endoscopic and histological data were not systematically recorded.
2.2. Design and outcomes
For the purpose of the present study, patients from the IBDSL cohort were eligible for inclusion if thiopurine (Azathioprine [AZA], 6‐mercaptopurine [6‐MP] or 6‐thioguanine [6‐TG]) monotherapy was initiated as maintenance during follow‐up (allowing therapy for post‐operative prophylaxis and perianal disease in CD). UC patients with a history of colectomy prior to therapy initiation were excluded from the analyses as well as patients starting combination therapy or using other immunosuppressive medication (e.g. biologicals, methotrexate) prior to thiopurine initiation to avoid interference from other IBD medical therapies on the study outcome measures.
The primary outcome was therapy effectiveness. Thiopurine monotherapy was defined to be effective if (1) no escalation to biological treatment, (2) no course of systemic corticosteroids (either oral or intravenously; excluding budesonide, beclomethasone and locally acting corticosteroids such as enemas and suppositories), (3) no surgery for active IBD (including (partial) bowel resection as a result active luminal disease, complications such as stricturing and penetrating disease; excluding later perianal procedures (e.g. flap procedures, ligation and stem cell therapy)), and (4) no hospitalisation for active disease (excluding, e.g. drug administrations and elective admissions for endoscopies) occurred whilst on thiopurine treatment. For each patient, data on all outcomes included in the definition were available. Patients who discontinued treatment within 12 weeks after initiation were not considered treatment failures in long‐term effectiveness analyses as it is known that monotherapy may take 12 weeks or more to reach its intended clinical effect. 36 , 37 Dates of thiopurine initiation, discontinuation and reason for discontinuation were retrieved from the medical and pharmacy records. As for the duration of thiopurine treatment, a temporary stop/discontinuation (<3 months) or switch to another thiopurine (e.g. from AZA to 6‐MP or 6‐TG) was allowed and not considered as treatment failure.
The secondary outcome was drug safety. Safety was assessed in a non‐specific umbrella concept, distinguishing short‐term drug tolerability and mid‐ to long‐term drug safety. The latter was assessed in terms of threatening complications, particularly of oncogenesis (lymphomas, non‐melanoma skin cancers [NMSCs], urinary tract cancers and cervical cancers) and portal hypertension due to liver disease. In order to be considered for mid‐ to long‐term safety analyses, a patient had to have at least 12 months of cumulative thiopurine exposure. Exposure duration was based on previous studies which demonstrated that the risk of both NMSCs and lymphomas becomes apparent after 1 year of exposure. 38 , 39 Incidence rates of NMSCs, lymphomas, urinary tract cancers and cervical cancers were calculated. In addition, patient records were screened to identify events of nodular regenerative hyperplasia (NRH) and clinically relevant manifestations of portal hypertension as potential NRH (excluding primary sclerosing cholangitis) using an automated search query on the corresponding ICD‐10 codes (e.g. Portal hypertension [K76.6]; NRH [K76.89]; Toxic liver disease [K71.9]; and Oesophageal varices with and without bleeding [I85.0, I85.9]) in the electronic medical patient files from both hospitals.
2.3. Statistical analyses
Clinical characteristics are presented as means with standard deviations (SD) or as medians with quartiles (Quartile 1 [Q1]–Quartile 3 [Q3]) for numerical variables, depending on normality of the underlying distribution; and as frequencies with corresponding percentages for categorical variables. Descriptive data, if applicable, were compared using the independent samples t‐test (normal distribution) or Mann–Whitney U test (nonparametric distribution) for numerical variables, and the chi‐square test for categorical variables.
The long‐term effectiveness of thiopurine monotherapy was estimated in time‐to‐event [‘time‐to‐failure’] analyses and illustrated in survival curves using Kaplan Meier survival statistics to adjust for differences in follow‐up between patients. Subsequently, stratified analyses (IBD subtype [CD vs UC], and era of treatment initiation [CD: pre‐biological 1991–1998 vs biological ≥1999; UC: pre‐biological 1991–2005 vs biological ≥2006]) were performed and strata were compared for statistical significance using log‐rank testing. Cox proportional hazards models were built, adjusting for established clinical characteristics, to determine the association of several predictors in time‐to‐event analyses and reported as hazard ratio (HR) with corresponding 95% confidence intervals (95% CI). Model covariates included age at diagnosis, gender, IBD subtype (CD vs UC), time between diagnosis and treatment initiation, era of treatment initiation, smoking status (only in the CD model, not available for UC) and disease phenotype according to the Montreal classification. Background knowledge strategy was used as model building strategy, incorporating risk factors described in literature along with potential confounders. 40 , 41 The proportional hazards assumption was checked for all included variables.
With regard to safety analyses, incidence rates of NMSCs, lymphomas, urinary tract cancers and cervical cancers were calculated as the ratio between events and patient‐years of thiopurine exposure. Rates were expressed per 1000 patients‐years and 95% CIs were calculated using the Mid‐P exact test. Data on liver manifestations were reported descriptively.
All statistical analyses were conducted using SPSS (Version 26.0, SPSS Inc.) and survival curves were produced using R statistics (version 4.0.3) using the ggplot2 package. Two‐sided p‐values of ≤0.05 were considered statistically significant.
3. RESULTS
3.1. Study population
Out of the 2825 patients in the IBDSL cohort, 1078 (688 CD; 390 UC) were treated with thiopurines at some point during follow‐up. Of these, a total of 1016 patients (643 CD [63.3%]; 373 UC [36.7%]) initiated thiopurine monotherapy and were included. In the CD cohort, 595 of 643 (92.5%) initiated thiopurine monotherapy as maintenance after/concurrent with steroid induction, 21 of 643 (3.3%) as post‐operative prophylaxis, and 27 of 643 (4.2%) for perianal disease. In the UC cohort, all patients initiated thiopurine monotherapy as maintenance. Flowcharts outlining both study populations are shown in Figures 1 and 2.
FIGURE 1.
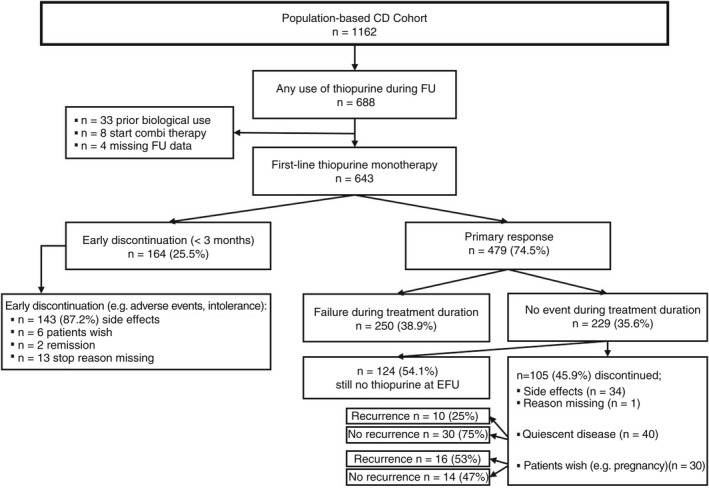
Flowchart outlining study population and main findings of thiopurine monotherapy in CD patients.
FIGURE 2.
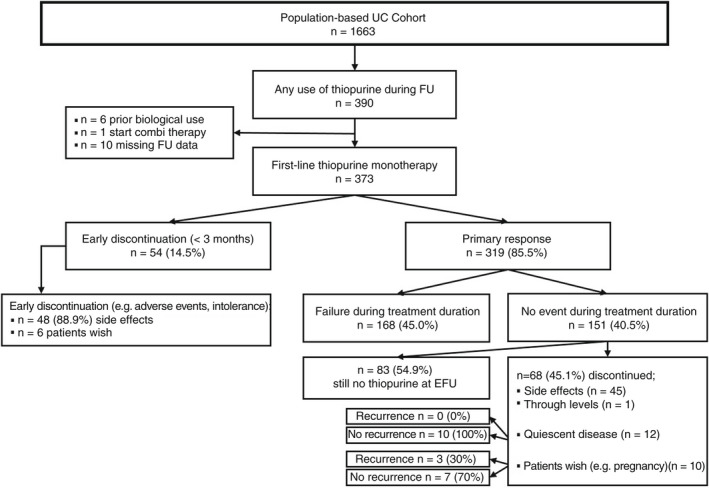
Flowchart outlining study population and main findings of thiopurine monotherapy in UC patients.
Median time of follow‐up was 8.5 years (Q1–Q3 5.0–13.2) and 9.8 years (Q1–Q3 5.8–14.6) in CD and UC patients, respectively (total follow‐up of 9860 patient‐years [PY]/total thiopurine exposure 3731 PY). CD patients were younger at the time of thiopurine monotherapy initiation (median 35.0 years [Q1–Q3 25.0–46.4] vs 44.9 years [Q1–Q3 33.8–57.4]) and started therapy earlier in their disease course (median 9.7 months [Q1–Q3 3.2–38.8] vs 28.2 months [Q1–Q3 8.4–63.5]) as compared to UC patients. Baseline characteristics of the study population are summarised in Table 1 and baseline characteristics of the CD cohort stratified for indication of thiopurine monotherapy are shown in Table S1.
TABLE 1.
Baseline characteristics in the 1016 IBD patients who started on thiopurine monotherapy
| CD (n = 643/6092 PY) | UC (n = 373/3768 PY) | |
|---|---|---|
| Age at diagnosis in years, median [Q1‐Q3] | 32.5 [22.9–43.5] | 40.2 [29.8–53.6] |
| Era of diagnosis, n (%) | ||
| 1991–1998 | 146 (22.7) | 116 (31.1) |
| 1999–2005 | 239 (37.2) | 159 (42.6) |
| 2006–2011 | 258 (40.1) | 98 (26.3) |
| Time to thiopurine in months, median [Q1‐Q3] | 9.7 [3.2–38.8] | 28.2 [8.4–63.5] |
| Follow‐up in years, median [Q1‐Q3] | 8.5 [5.0–13.2] | 9.8 [5.8–14.6] |
| Thiopurine dose in mg/day a , median [Q1‐Q3] | ||
| Azathioprine | 125 [100–150] | 150 [100–150] |
| 6‐MP | 50 [50–62.5] | 50 [50–75] |
| 6‐TG | 20 [20–20] | 20 [20–20] |
| Gender, n (%) | ||
| Male | 232 (36.1) | 205 (55.0) |
| Female | 411 (63.9) | 168 (45.0) |
| Smoking at diagnosis b , n (%) | ||
| Yes | 307 (52.1) | |
| No | 282 (47.9) | |
| Disease location at diagnosis c , n (%) | ||
| UC | ||
| E1: proctitis | 78 (20.9) | |
| E2: left‐sided | 193 (51.7) | |
| E3: extensive | 102 (27.3) | |
| CD | ||
| L1: ileal | 265 (41.2) | |
| L2: colonic | 191 (29.7) | |
| L3: ileocolonic | 178 (27.7) | |
| L4: isolated upper GI disease | 9 (1.4) | |
| P: Perianal involvement | 55 (8.6) | |
| Disease behaviour at diagnosis c , n (%) | ||
| B1: non‐stricturing, non‐penetrating | 482 (75.0) | |
| B2: stricturing | 114 (17.7) | |
| B3: penetrating | 47 (7.3) | |
Abbreviations: CD, Crohn's disease; PY, patient years; n, number of patients; Q1‐Q3, quartile 1 and quartile 3; UC, ulcerative colitis.
Median dose of thiopurine monotherapy per mg/day, stratified for the type of thiopurine. Q1 and Q3 overlap with median in some instances due to the range of prescribed doses.
No data available on smoking status in UC patients, missing smoking data in 54 CD patients.
Phenotype at diagnosis according to Montreal Classification.
3.2. Thiopurine monotherapy effectiveness
Of the 1016 patients treated with thiopurine monotherapy, treatment was effective for the total duration of the treatment without the need for escalation to biologicals, corticosteroids, surgery and hospitalisation due to active disease in 229 of 643 (35.6%) CD patients and 151 of 373 (40.5%) UC patients. Moreover, 54% of these CD and UC patients were still on thiopurine monotherapy at the time of data lock (Figures 1 and 2).
As for the duration of treatment effectiveness, median duration of thiopurine monotherapy survival on time‐to‐event analyses was 3.2 years (95% CI 2.1–4.3) in CD and 3.3 years (95% CI 2.0–4.6) in UC patients, corresponding to estimated effectiveness rates (i.e. the proportion of patients still on monotherapy without therapy failure) of 64%, 45%, 32% and 66%, 41%, 36% at 1, 5 and 10 years after treatment initiation in CD and UC patients, respectively (Table 2, Figure 3A,B). Effectiveness rates over time did not differ between CD and UC patients (p = 0.78, Table 2, Figure 4A,B). In addition, over time, after stratification for era of treatment initiation (pre‐biological vs biological era), no statistically significant differences in estimated effectiveness rates were observed between eras in either group (CD: p = 0.56; UC: p = 0.43). Furthermore, a sensitivity analysis was performed that only included CD patients that initiated thiopurine monotherapy for maintenance of remission (595/643 CD patients, 92.5%). In this main subgroup, median duration of thiopurine monotherapy survival was 3.1 years (95% CI 2.3–4.0), corresponding to effectiveness rates of 64%, 44% and 30% at 1, 5 and 10 years after treatment initiation, respectively, consistent with the outcomes of the total CD cohort (n = 643) (Tables S1 and S2). No sensitivity analysis was performed in the UC cohort as all patients started thiopurine monotherapy for maintenance of remission.
TABLE 2.
Estimated thiopurine effectiveness rates on time‐to‐event analyses
| CD | UC | |
|---|---|---|
| Median survival, years (95% CI) | 3.2 (2.1–4.3) | 3.3 (2.0–4.6) |
| % of patients still on thiopurine monotherapy without event a (years) | ||
| 1 | 64.4 | 65.8 |
| 5 | 45.0 | 40.8 |
| 10 | 31.9 | 35.6 |
| Log‐rank testing CD vs UC b : p = 0.78 | ||
Abbreviations: CI, confidence interval; p, p value.
Estimated percentage of patients still on monotherapy 1, 5 and 10 years after initiation without need for escalation to biologicals, corticosteroids, surgery and hospitalisation due to active disease.
Comparison of estimated effectiveness over time between CD and UC patients.
FIGURE 3.
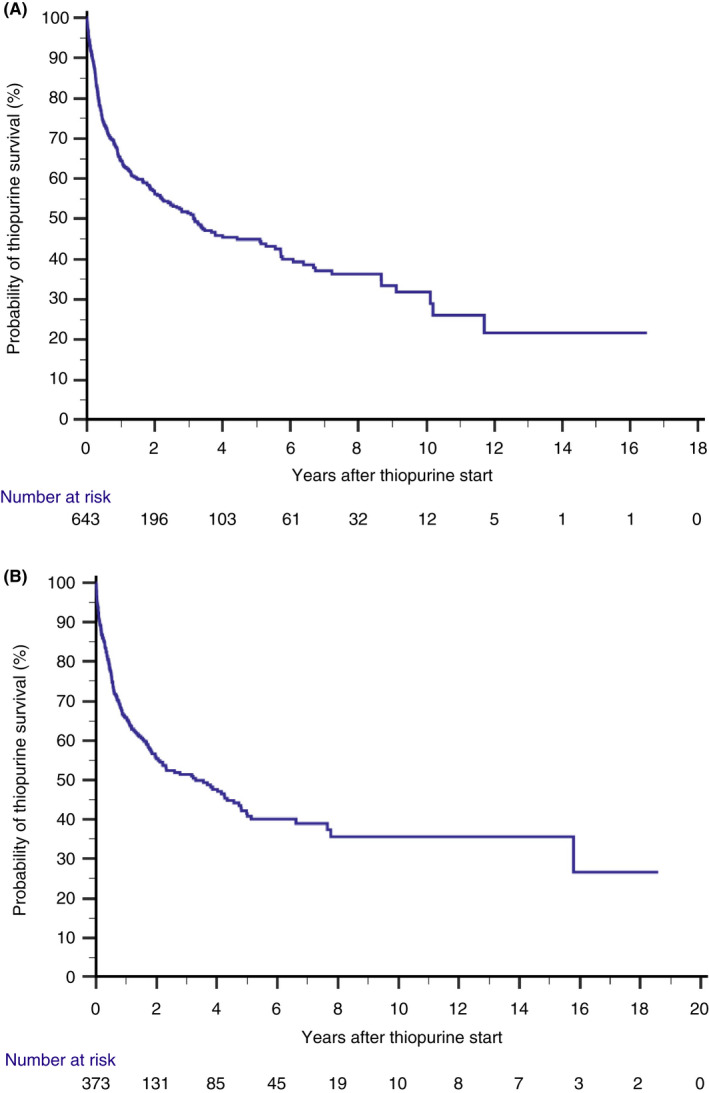
(A) Kaplan–Meier plot showing the probability of thiopurine monotherapy effectiveness in all Crohn's disease patients initiated on therapy (1991–2014). (B) Kaplan–Meier plot showing the probability of thiopurine monotherapy effectiveness in all ulcerative colitis patients initiated on therapy (1991–2014).
FIGURE 4.
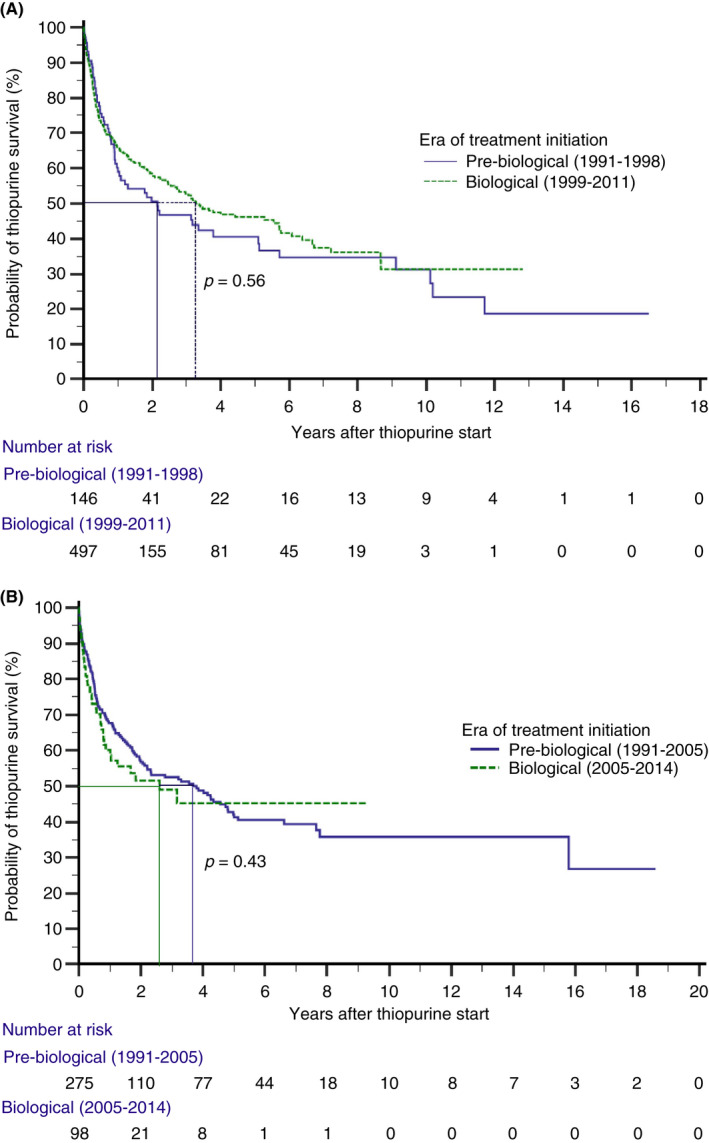
(A) Kaplan–Meier plot showing the probability of thiopurine monotherapy effectiveness in Crohn's disease patients stratified for the era of treatment initiation (pre‐biological vs biological era). (B) Kaplan–Meier plot showing the probability of thiopurine monotherapy effectiveness in ulcerative colitis patients stratified for the era of treatment initiation (pre‐biological vs biological era).
Regarding therapy failure, 250 CD and 168 UC patients failed therapy after initial response, respectively. In CD, 82/250 (33.2%) patients were escalated to biologicals, 9/250 (3.6%) had surgery, 78/250 (31.2%) were hospitalised and 80/250 (32.0%) received systemic steroids. Of CD patients that were initially hospitalised or received steroids, 43 and 45 progressed to biologicals and surgery within the year, respectively. In UC, 28/168 (16.7%) patients were escalated to biologicals, 1/168 (0.6%) had surgery, 38/168 (22.6%) were hospitalised and 101/168 (60.1%) received systemic steroids. Of UC patients that were initially hospitalised or received steroids, 30 and 14 progressed to biologicals and surgery within the year, respectively. In the Cox regression analyses, a stricturing phenotype (HR 1.42, 95% CI 1.01–1.99, p = 0.044), upper GI involvement (HR 1.53, 95% CI 1.02–2.28, p = 0.040) at diagnosis, and younger age at treatment initiation (HR 0.99, 95% CI 0.98–1.00, p = 0.048) were identified as risk factors for thiopurine monotherapy failure in CD patients. In UC, a longer duration between diagnosis and start of therapy (HR 0.94, 95% CI 0.90–0.99, p = 0.015) was associated with a lower risk of therapy failure (Tables 3 and 4).
TABLE 3.
Cox regression analysis of factors affecting time to thiopurine monotherapy failure in CD patients
| Adjusted HR [95% CI] | p | |
|---|---|---|
| Age at thiopurine initiation | 0.99 [0.98–1.00] | 0.048* |
| Era of treatment initiation | ||
| 1991–1998 | REF | |
| 1999–2011 | 0.80 [0.57–1.14] | 0.22 |
| Time between diagnosis and start thiopurine in years | 0.96 [0.91–1.01] | 0.79 |
| Smoking at diagnosis | 1.29 [0.99–1.67] | 0.056 |
| Disease location at diagnosis a | ||
| L1: ileal | REF | |
| L2: ileocolonic | 0.92 [0.65–1.30] | 0.63 |
| L3: colonic | 1.26 [0.91–1.75] | 0.17 |
| Disease behaviour at diagnosis a | ||
| B1: inflammatory | REF | |
| B2: stricturing | 1.42 [1.01–1.99] | 0.044* |
| B3: penetrating | 1.26 [0.76–2.11] | 0.37 |
| Upper GI involvement at diagnosis | 1.53 [1.02–2.28] | 0.040* |
| Perianal disease at diagnosis | 1.34 [0.90–2.00] | 0.15 |
| First thiopurine type | ||
| AZA | REF | |
| 6‐MP/6‐TG | 1.01 [0.73–1.39] | 0.95 |
| Gender, female | 1.18 [0.88–1.59] | 0.28 |
Abbreviations: 6‐MP, 6‐mercaptopurine; 6‐TG, 6‐thioguanine; AZA, azathioprine; CD, Crohn's disease; CI, confidence interval; HR, Hazard ratio; p, p value; REF, reference category.
Disease location and behaviour based on Montreal Classification.
Significant p < 0.05.
TABLE 4.
Cox regression analysis of factors affecting time to thiopurine monotherapy failure in UC patients
| Adjusted HR [95% CI] | p | |
|---|---|---|
| Age at thiopurine initiation | 1.00 [0.99–1.01] | 0.37 |
| Era of treatment initiation | ||
| 1991–2005 | REF | |
| 2005–2011 | 0.96 [0.64–1.43] | 0.82 |
| Time between diagnosis and start thiopurine in years | 0.94 [0.90–0.99] | 0.015* |
| Disease location at diagnosis a | ||
| E1: proctitis | REF | |
| E2: left‐sided | 1.09 [0.74–1.61] | 0.65 |
| E3: extensive | 0.88 [0.56–1.38] | 0.58 |
| First thiopurine type | ||
| AZA | REF | |
| 6‐MP/6‐TG | 1.10 [0.56–1.38] | 0.55 |
| Gender, female | 1.01 [0.74–1.37] | 0.96 |
Abbreviations: 6‐MP, 6‐mercaptopurine; 6‐TG, 6‐thioguanine; AZA, azathioprine; CI, confidence interval; HR, hazard ratio; p, p value; REF, reference category; UC, ulcerative colitis.
Disease location based on Montreal Classification.
Significant p < 0.05.
In total, 52 patients (76.9% CD, 53.8% female, 20.0% L3) discontinued thiopurine monotherapy because of sustained quiescent disease as judged by physician global assessment (median duration thiopurine 3.26 years, Q1‐Q3 1.33–6.57). Of these, 10 patients (19.2%) experienced disease recurrence after a median time of 1.84 years (Q1‐Q3 0.91–3.49; median follow‐up after thiopurine discontinuation: 3.1 years). In contrast, treatment discontinuation due to patients' wish (n = 40; e.g. pregnancy, worry about potential side effects; 75.0% CD, 67.5% female, 43.3% L3; median duration thiopurine 1.46 years Q1‐Q3 0.47–3.67) resulted in disease recurrence in 19/40 (47.5%, p = 0.004 on comparison) patients after a median of 1.61 years (Q1‐Q3 0.63–2.48; median follow‐up after thiopurine discontinuation: 3.3 years), depicted in Figures 1 and 2. Baseline characteristics of these groups are presented in Table S3.
3.3. Safety
3.3.1. Tolerability
Of the 1016 patients in which thiopurine monotherapy was initiated, 710 (69.9% [CD 70.6%, UC 68.6%]) were treated with AZA, 302 (29.7% [CD 29.2%, UC 30.6%]) with 6‐MP and 4 (0.4% [CD 0.2%, UC 0.8%]) with 6‐TG as their first thiopurine. In the first 3 months, 218 (21.5%) patients discontinued treatment (CD 164 [25.5%], UC 54 [14.5%]), 191 (87.6%) because they were unable to tolerate thiopurines due to side effects (Figures 1 and 2). In an effort to identify clinical characteristics associated with early discontinuation, on multivariable logistic regression a CD diagnosis (odds ratio [OR] 1.96, 95% CI 1.37–2.82, p < 0.001) and a diagnosis after 2005 (OR 2.23, 95% CI 1.38–3.59, p = 0.001) were both associated with treatment discontinuation in the first 3 months (Table S4).
With regards to the effect of switching thiopurine type on treatment tolerability, a total of 145 patients were switched from thiopurine during the course of treatment, in 119 due to intolerance (80/119 [67.2%] from AZA to 6‐MP; 39/119 [32.7%] from either AZA or 6‐MP to 6‐TG). Of the patients being switched as a result of intolerance, 79/119 (66.4%) were able to tolerate a second thiopurine (44/79 [55.7%] 6‐MP; 35/79 [44.3%] 6‐TG). Of note, among the 191 intolerant patients in the first 3 months of treatment, only 33 were switched.
3.3.2. Cancer (NMSCs, lymphoma) and liver manifestations (compatible with NRH)
A total of 653 patients met the criteria for at least 12 months of cumulative thiopurine monotherapy exposure, accounting for 4799 PYs of follow‐up. During this period, 16 NMSC events (12 basal cell carcinoma, 4 squamous cell carcinoma; median age at event, 63.0 years; 62.5% male; median thiopurine duration, 8.0 years), 5 lymphoma events (median age at event, 56.0 years; 80% male; median thiopurine duration, 5.4 years), and one urinary tract cancer event (59‐year‐old male, 4.9 years after start of thiopurine) were recorded corresponding to incidence rates of 3.33 (95% CI 1.97–5.30) per 1000 PY, 1.04 (95% CI 0.38–2.31) per 1000 PY and 0.21 (95% CI 0.01–1.03) per 1000 PY, respectively (Table 5). Details on lymphoma events are presented in Table S5. No cases of cervical cancer were identified.
TABLE 5.
Incidence rate of non‐melanoma skin cancers (NMSC) and lymphoma in patients with at least 12 months of cumulative thiopurine exposure
| N | Time at risk (patient years, PY) | N events | Incidence rate per 1000 PY (95% CI) | |
|---|---|---|---|---|
| Outcome | ||||
| NMSC a | 653 | 4799 | 16 | 3.33 (1.97–5.30) |
| Lymphoma b | 653 | 4799 | 5 | 1.04 (0.38–2.31) |
| Urinary tract cancer | 653 | 4799 | 1 | 0.21 (0.01–1.03) |
Note: No cases of cervical cancer were identified.
Abbreviations: CI, confidence interval; N, number of patients; PY, patient year; N events, number of events; NMSC, non‐melanoma skin cancer.
Twelve cases of basal cell carcinoma (BCC), and 4 cases of squamous cell carcinoma.
Details on lymphoma events are presented in Table S3.
As for mid‐ to long‐term liver manifestations, one event of NRH was identified in a 55‐year‐old male patient after 3.1 years of AZA use, and one event of portal hypertension as potential NRH of the liver in a 54‐year‐old male patient after 16.9 years of AZA use.
4. DISCUSSION
In this real‐world cohort of 1016 IBD patients with 9860PYs of follow‐up, we observed a median duration of maintenance thiopurine monotherapy survival of 3.2 years in CD and 3.3 years in UC, corresponding to effectiveness rates of over 40% (45% CD, 41% UC) and 30% (32% CD, 36% UC) at 5 and 10 years after treatment initiation, respectively. Interestingly, since the introduction of biologicals, no differences in these effectiveness rates were observed. Moreover, of patients in whom therapy was deemed effective, a substantial proportion (54.5%) were still using thiopurine monotherapy at the time of data lock (median of 9 years).
In two meta‐analyses of randomised controlled trials, it has been concluded that sustained remission rates of 73% in CD and 57% in UC for thiopurine maintenance versus placebo may be expected. 1 , 2 In a number of observational cohort studies, it has been reported that therapeutic benefit may be observed in 40%–75% of patients using thiopurine monotherapy in CD or UC with various definitions for effectiveness (e.g. [steroid‐free] clinical remission, drug persistence and prevention of disease progression). 16 , 17 , 18 , 19 , 20 , 21 , 22 , 42 , 43 However, most of these studies were limited as a result of modest sample sizes, duration of follow‐up or both. Effectiveness defined as clinical remission, regardless of corticosteroid use, escalation to biological or surgery, was reported in a Dutch cohort study of 363 patients with observed effectiveness rates of 63%, 51% and 42% at 1, 2 and 5 years, respectively. 18 In another, Italian study, it was observed that steroid‐free clinical remission rates were 87% and 53% at 1 and 5 years in a cohort of 192 UC patients. 21 More recently, a Belgian tertiary IBD referral centre observed 34% thiopurine monotherapy effectiveness at 1 year in a cohort of 780 CD patients. 44
In the largest and most recent study to date, thiopurine monotherapy effectiveness was investigated in a cohort of 11,928 IBD patients with 68,132 PY of thiopurine exposure in the UK. 24 The primary outcome was overall effectiveness for the total duration of treatment based on clinical judgement and was also evaluated in terms of time from initiation of thiopurine monotherapy to treatment escalation with biologicals or surgery on time‐to‐event analysis as secondary outcome. The authors observed lower overall effectiveness rates when comparing CD with UC patients (34.2% vs 52.7%). An important question, however, is if treatment recommendations should be based on comparisons in effectiveness between CD and UC, given the differences in natural disease course and indication for thiopurines in both diseases.
Overall, thiopurine monotherapy effectiveness rates in the aforementioned studies range from 34%–70% at 1 year to 40%–50% at 5 years in CD patients, and from 60%–87% at 1 year to 38%–64% at 5 years in UC patients. The observed effectiveness rates in our population‐based cohort are in line with the rates presented in most of these previous studies, including similar effectiveness rates over time in our CD cohort (1 year: 64%; 5 years: 45%; 10 years: 32%) compared to the UK‐CD cohort (1 year: 69%; 5 years: 45%; 10 years: 29%). In addition, and similar to others, we did not detect statistically significant differences in effectiveness rates over time between UC and CD patients. 18 The differences in reported effectiveness among these studies may be explained by several factors. First, the use of various definitions for thiopurine effectiveness. Using a fairly strict definition for therapy failure, we observed relatively high effectiveness rates compared to studies using only clinical remission or drug persistence as a proxy for effectiveness. This may raise the question of whether the population in our study had sufficient disease activity to require treatment in the first place, which may have led to overtreatment. However, relapse rates after thiopurine discontinuation due to quiescent disease resulted in relapse in only 19% of patients in our cohort, whereas discontinuation due to patient's wish (e.g. pregnancy, side effects) resulted in relapse in 47.5% of patients, indicating a correct indication for treatment.
Another explanation for the variability in effectiveness rates may be related to differences in study populations between studies. The low effectiveness rates observed in the tertiary referral centre from Belgium at 1 year in CD patients, may be the result of the overrepresentation of patients with a more refractory disease course, and may in turn lead to an early use of biological treatment in these patients (i.e. defined as failures of thiopurines). The differences in phenotypical characteristics are further supported by the risk factors for therapy failure identified in our cohort, including a stricturing phenotype, upper GI involvement and younger age at treatment initiation in CD, as well as a shorter time between diagnosis and treatment initiation in UC. These risk factors have all been described previously and presumably reflect the fact that thiopurines are less effective in patients with risk factors for disease progression and severe disease. 18 , 24 , 44 In contrast, the high effectiveness rates observed in the UC cohort from the UK, as the authors stated, may be the result of potential selection bias with underrepresentation of more severe UC cases and possibly more escalation to biologicals in recent years. Altogether, these observations underscore that patients with a more favourable risk profile, potentially or even likely, benefit from thiopurines, whereas patients with an unfavourable profile might benefit from the early introduction of biologicals.
In recent years, thiopurines have been increasingly questioned for their potentially inferior effectiveness compared to biologicals. Since biologicals have now also become the main drivers of direct healthcare costs in IBD, many low‐income countries cannot afford this type of treatment, inducing worrisome disparities for patients. 45 , 46 A recent Danish study investigated trends in biological use in a population‐based cohort of over 6000 biological‐naïve IBD patients. Strikingly, persistence rates of first‐line biologicals were low with only 44% and 17% of UC, and 60% and 34% of CD patients still on treatment after 1 and 3 years. 47 Similar secondary loss of response (LOR) rates have been described in other real‐world observational cohorts and even though biological users are generally characterised by higher disease severity, these LOR rates are all considerably higher than the rates observed in our and other thiopurine cohorts. 48 , 49 , 50 , 51 While discontinuation of biologicals may also reflect remission, relapse rates following biological cessation for (clinical) remission are high, ranging between 30 and 40% at 1 year and up to 50% after 2 years. 52 , 53 These observations once again seem to indicate a role for thiopurines in selected patient groups and highlight the need for adequate stratification.
The tolerability of thiopurines is a relevant topic, as approximately one fifth of patients (21.5%) in the current study discontinued thiopurines within 3 months after treatment, primarily due to adverse events such as GI intolerance (27.7%), hyperlipasemia (29.3%), liver tests abnormalities (11.5%) and cytopenia (6.3%). Comparable early discontinuation rates, ranging between 20 and 30%, have been reported in a number of other studies. 4 , 18 , 54 , 55 , 56 Of the patients who were rechallenged with another thiopurine after discontinuation, nearly two‐thirds (66.4%) were able to tolerate a second thiopurine, consistent with reported rates in previous studies. 24 , 56 , 57 The high rate of tolerability seen with switching to 6‐TG in our cohort (35/39 patients), underlines the potential of optimisation strategies, as documented before. 57 Aside from switching, other optimisation strategies, including co‐administration with allopurinol, have also proven valuable in overcoming therapy toxicity. 58 , 59
Concerns regarding safety contribute to a significant extent to the discussion around the position of thiopurine treatment. The incidence rate of lymphoma in our cohort was 1.04 (95% CI 0.38–2.31) per 1000 PY, which is in line with incidence rates in the CESAME (0.90 per 1000 PY) and other cohorts. 26 , 60 , 61 As for NMSCs, studies have consistently reported increased incidence rates in patients receiving thiopurines. 39 , 62 The CESAME cohort observed an incidence rate of 2.59 per 1000PY for NSMCs in patients aged 50–65 years on thiopurine monotherapy, which is slightly lower than the NMSCs incidence rate in our cohort (3.33 [95%CI 1.97–5.30] per 1000 PY) and most likely explained by the limited number of events in both cohorts. 63 Although an increased risk for these specific malignancies has been established with thiopurine use, their absolute risk remains low and is even further reduced with adequate stratification (e.g. EBV serology testing) and precautionary measures (e.g. dermatological screening and sunscreen use). 25 , 64 Besides that, in the largest cohort to date from France, thiopurine monotherapy was not associated with a higher risk of lymphoma compared to anti‐TNF monotherapy in 189,000 IBD patients, whereas the relative risk with combination therapy was substantially increased. 65 With regard to mid‐ to long‐term liver manifestations, one confirmed case of NRH and one potential case of NRH were identified. Although this is lower than the reported incidence of NRH in literature, it is important to stress that these data should be interpreted with caution, given the observational nature of our study. However, its absolute risk seems limited. 66 , 67 , 68
Another important point to consider when discussing safety is to recognise the timespan required to adequately address long‐term safety. The safety profile of thiopurines is well‐documented and no new safety signals are expected with thiopurines due to the accrued PY of exposure. In contrast, biologicals and small molecules, such as JAK inhibitors, were registered more recently and their long‐term safety profiles are still likely to change over time. Thiopurines, for instance, have been used for over decades and it took 20 years to detect the lymphoma signal, whereas the FDA has issued a black box warning for JAK inhibitors only 3 years after market approval. 69
Strengths of the current study are its population‐based design, the use of strict definitions for thiopurine effectiveness, and the inclusion of a large number of patients with almost 10,000 PY of follow‐up. These strengths enabled the assessment of long‐term thiopurine effectiveness and comparison of outcomes between the pre‐biological and biological eras. Nonetheless, our study certainly has limitations. First, due to the retrospective nature of the study, we had to use a definition for effectiveness which did not include biochemical (CRP, calprotectin) and endoscopic data. However, by using relatively strict clinical and composite assessments of disease remission, including escalation to biologicals, corticosteroids, surgery, as well as hospitalisations for active disease, we reduced the risk of missing instances of disease activity. Second, as with almost all observational studies, data on thiopurine methyltransferase (TPMT) testing and therapeutic drug monitoring (TDM) were applied according to clinical judgement rather than as per protocol and were therefore not available. Third, a potential (beneficial) effect of smoking in UC patients on therapy effectiveness could not be evaluated as it was not consistently recorded. Last, data on concomitant 5‐aminosalicylic acid (5‐ASA) use, although probably of limited effect, were incompletely available. However, studies have also reported that concomitant 5‐ASA use in patients using thiopurines did not improve disease outcomes. 70 , 71
As a result of the increasingly growing armamentarium of biologicals and small molecules, attention for the benefits of thiopurines has diminished whilst potential detrimental effects have been highlighted with little nuance. A thiopurine‐free IBD treatment has been advocated by some, while current guidelines, recent international surveys and others underline their usefulness. 72 Moreover, alternative therapies to thiopurines, comprising corticosteroids, biologicals and the new small molecules (to come) have limitations such as relevant LOR rates over time and accompany specific safety concerns. Considering the balance between the benefits of thiopurines, including effectiveness and affordability and the risks recognised over decades of use in IBD patients, there remains an essential place for thiopurine monotherapy in the modern therapeutic era for selected patients with mild‐to‐moderate, (steroid dependent) IBD. In particular, when personalising thiopurine monotherapy with TDM, TPMT and after identification of patients with an established IBD risk profile indicative of thiopurine therapy failure who would benefit from biologicals.
In conclusion, long‐term real‐world data from this population‐based study demonstrated that maintenance thiopurine monotherapy was an effective, safe and durable treatment option, with over 40% and 30% of IBD patients, irrespective of treatment era, continuing therapy without the need for biologicals, corticosteroids, surgery or hospitalisation due to active disease at 5, and 10 years after treatment initiation.
AUTHOR CONTRIBUTIONS
Ashkan Rezazadeh Ardabili: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); software (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Steven Jeuring: Conceptualization (equal); data curation (equal); formal analysis (supporting); investigation (equal); methodology (supporting); resources (supporting); supervision (lead); visualization (equal); writing – review and editing (lead). Zlatan Mujagic: Data curation (supporting); investigation (supporting); supervision (supporting); visualization (supporting); writing – review and editing (equal). Liekele E. Oostenbrug: Data curation (supporting); investigation (supporting); writing – review and editing (equal). Mariëlle J Romberg‐Camps: Data curation (equal); investigation (supporting); writing – review and editing (equal). Daisy Jonkers: Conceptualization (supporting); funding acquisition (lead); supervision (supporting); writing – review and editing (equal). Ad A van Bodegraven: Conceptualization (equal); data curation (equal); investigation (equal); methodology (equal); supervision (equal); visualization (equal); writing – review and editing (equal). Marie Pierik: Conceptualization (lead); funding acquisition (lead); investigation (equal); methodology (supporting); resources (equal); supervision (lead); writing – review and editing (lead). All authors approved the final version of the manuscript.
FUNDING INFORMATION
This work was in part supported by funding from the European Union Seventh Framework Programme (FP7/2012–2017, Grant number: 305564) as the IBDSL cohort involved in the Sysmed‐IBD consortium.
AUTHORSHIP
Guarantor of this article: Marieke Pierik.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGEMENT
Declaration of personal interests: MP reports grants and non‐financial support from Falk Pharma, grants from European commission, grants from ZonMw (Dutch national research fund), grants and non‐financial support from Takeda, grants and non‐financial support from Johnson and Johnson, grants and non‐financial support from Abbvie, non‐financial support from Ferring, non‐financial support from Immunodiagnostics, non‐financial support from MSD, all outside the submitted work. DJ reports grants from Top Knowledge Institute (Well on Wheat), grants from Horizon 2020 DISCOvERIE, grants from NWO‐CCC Partnership programme (Carbokinetics), all outside the submitted work. ZM reports grants form MLDS, Niels Stensen Fellowship and Galapagos. AvB has served as speaker, adviser and/or principal investigator for AbbVie, Arandal, Arena, Celgene, Ferring, Galapagos, Janssen, MSD, Pfizer, Roche, Takeda, TEVA and received research grants from Pfizer, TEVA, Eurostars funding, ZonMw, all outside the submitted work. The other authors have nothing to disclose relevant to this publication.
Rezazadeh Ardabili A, Jeuring S, Mujagic Z, Oostenbrug L, Romberg‐Camps M, Jonkers D, et al. Classic drugs in the time of new drugs: Real‐world, long‐term outcomes of thiopurine monotherapy in 1016 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;56:1030–1043. 10.1111/apt.17128
Conference presentation: Part of this work was presented at the ECCO’21 Congress (Digital Oral Presentation 54).
The Handling Editor for this article was Dr Cynthia Seow, and it was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
A data management plan for IBDSL is made in collaboration with DataHub MUMC+. DataHub works according to the FAIR principles. Metadata of IBDSL is available for interested researchers and data is available after approval of research proposals by the IBDSL committee (contact: m.pierik@mumc.nl).
REFERENCES
- 1. Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2015;10:CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;5:CD000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peyrin‐Biroulet L, Deltenre P, Ardizzone S, D'haens G, Hanauer SB, Herfarth H, et al. Azathioprine and 6‐mercaptopurine for the prevention of postoperative recurrence in Crohn's disease: a meta‐analysis. Off J Am College Gastroenterol. 2009;104:2089–96. [DOI] [PubMed] [Google Scholar]
- 4. Mowat C, Arnott I, Cahill A, Smith M, Ahmad T, Subramanian S, et al. Mercaptopurine versus placebo to prevent recurrence of Crohn's disease after surgical resection (TOPPIC): a multicentre, double‐blind, randomised controlled trial. Lancet Gastroenterol Hepatol. 2016;1:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362(15):1383–95. [DOI] [PubMed] [Google Scholar]
- 6. Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400.e3. [DOI] [PubMed] [Google Scholar]
- 7. Magro F, Rodrigues‐Pinto E, Coelho R, Andrade P, Santos‐Antunes J, Lopes S, et al. Is it possible to change phenotype progression in Crohn's disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol. 2014;109(7):1026–36. [DOI] [PubMed] [Google Scholar]
- 8. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. Crohn's obotE, Organisation C. ECCO Guidelines on Therapeutics in Crohn's disease: medical treatment. J Crohn's Colitis. 2019;14(1):4–22. [DOI] [PubMed] [Google Scholar]
- 9. Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn's Colitis. 2021. 16(1):2–17. [DOI] [PubMed] [Google Scholar]
- 10. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn's disease in adults. Off J Am College Gastroenterol. 2018;113(4):481–517. [DOI] [PubMed] [Google Scholar]
- 11. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Off J Am College Gastroenterol. 2019;114(3):384–413. [DOI] [PubMed] [Google Scholar]
- 12. Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4(5945):627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: one‐year, placebo‐controlled, randomized trial. Indian J Gastroenterol. 2000;19(1):14–6. [PubMed] [Google Scholar]
- 14. Cosnes J, Bourrier A, Laharie D, Nahon S, Bouhnik Y, Carbonnel F, Allez M, Dupas JL, Reimund JM, Savoye G, Jouet P, Moreau J, Mary JY, Colombel JF. Early administration of azathioprine vs conventional management of Crohn's disease: a randomized controlled trial. Gastroenterology 2013;145(4):758‐65.e2; quiz e14‐5, 765.e2. [DOI] [PubMed] [Google Scholar]
- 15. Panés J, López‐Sanromán A, Bermejo F, García‐Sánchez V, Esteve M, Torres Y, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn's disease. Gastroenterology. 2013;145(4):766–74.e1. [DOI] [PubMed] [Google Scholar]
- 16. Barber GE, Hendler S, Choe M, Keyashian K, Lechner S, Limketkai BN, et al. Thiopurine monotherapy is effective in maintenance of mild‐moderate inflammatory bowel disease. Dig Dis Sci. 2021;67:1287–94. [DOI] [PubMed] [Google Scholar]
- 17. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50(4):485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8‐year intercept cohorts. Inflamm Bowel Dis. 2010;16(9):1541–9. [DOI] [PubMed] [Google Scholar]
- 19. Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, et al. Long‐term effectiveness of azathioprine in IBD beyond 4 years: a European multicenter study in 1176 patients. Dig Dis Sci. 2006;51(9):1516–24. [DOI] [PubMed] [Google Scholar]
- 20. Glazier KD, Palance AL, Griffel LH, Das KM. The ten‐year single‐center experience with 6‐mercaptopurine in the treatment of inflammatory bowel disease. J Clin Gastroenterol. 2005;39(1):21–6. [PubMed] [Google Scholar]
- 21. Pugliese D, Aratari A, Festa S, Ferraro PM, Monterubbianesi R, Guidi L, et al. Sustained clinical efficacy and mucosal healing of thiopurine maintenance treatment in ulcerative colitis: a real‐life study. Gastroenterol Res Pract. 2018;2018:4195968–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suárez Ferrer C, González‐Lama Y, González‐Partida I, Calvo Moya M, Vera Mendoza I, Matallana Royo V, et al. Usefulness of thiopurine monotherapy for Crohn's disease in the era of biologics: a long‐term single‐center experience. Dig Dis Sci. 2019;64(3):875–9. [DOI] [PubMed] [Google Scholar]
- 23. Saibeni S, Virgilio T, D'Incà R, Spina L, Bortoli A, Paccagnella M, et al. The use of thiopurines for the treatment of inflammatory bowel diseases in clinical practice. Dig Liver Dis. 2008;40(10):814–20. [DOI] [PubMed] [Google Scholar]
- 24. Stournaras E, Qian W, Pappas A, Hong YY, Shawky R, Investigators UIB, et al. Thiopurine monotherapy is effective in ulcerative colitis but significantly less so in Crohn's disease: long‐term outcomes for 11 928 patients in the UK inflammatory bowel disease bioresource. Gut. 2021;70(4):677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanauer SB, Sandborn WJ, Lichtenstein GR. Evolving considerations for thiopurine therapy for inflammatory bowel diseases‐a clinical practice update: commentary. Gastroenterology. 2019;156(1):36–42. [DOI] [PubMed] [Google Scholar]
- 26. de Boer NKH, Peyrin‐Biroulet L, Jharap B, Sanderson JD, Meijer B, Atreya I, et al. Thiopurines in inflammatory bowel disease: new findings and perspectives. J Crohns Colitis. 2017;12(5):610–20. [DOI] [PubMed] [Google Scholar]
- 27. Bourrier A, Carrat F, Colombel JF, Bouvier AM, Abitbol V, Marteau P, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016;43(2):252–61. [DOI] [PubMed] [Google Scholar]
- 28. Hazenberg H, de Boer NKH, Mulder CJJ, Mom SH, van Bodegraven AA, Tack MP, et al. Neoplasia and precursor lesions of the female genital tract in IBD: epidemiology, role of immunosuppressants, and clinical implications. Inflamm Bowel Dis. 2018;24(3):510–31. [DOI] [PubMed] [Google Scholar]
- 29. Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15:879–913. [DOI] [PubMed] [Google Scholar]
- 30. Greuter T, Vavricka S, König AO, Beaugerie L, Scharl M. Malignancies in inflammatory bowel disease. Digestion. 2020;101(suppl 1)(1):136–45. [DOI] [PubMed] [Google Scholar]
- 31. Gargallo‐Puyuelo CJ, Laredo V, Gomollón F. Thiopurines in inflammatory bowel disease. How to optimize thiopurines in the biologic era? Front Med. 2021;8:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sousa P, Ministro P, Armuzzi A, Dignass A, Høivik ML, Barreiro‐de Acosta M, et al. Thiopurines: use them or lose them? International survey on current and future use of thiopurines in inflammatory bowel disease. Dig Liver Dis. 2021;53:1571–9. [DOI] [PubMed] [Google Scholar]
- 33. van den Heuvel TR, Jonkers DM, Jeuring SF, Romberg‐Camps MJ, Oostenbrug LE, Zeegers MP, et al. Cohort profile: the inflammatory bowel disease south limburg cohort (IBDSL). Int J Epidemiol. 2017;46(2):e7. [DOI] [PubMed] [Google Scholar]
- 34. World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 35. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Canadian Journal of Gastroenterology = Journal canadien de gastroenterologie. 2005;19 Suppl A:5a–36a. [DOI] [PubMed] [Google Scholar]
- 36. Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn's disease with 6‐mercaptopurine. A long‐term, randomized, double‐blind study. N Engl J Med. 1980;302(18):981–7. [DOI] [PubMed] [Google Scholar]
- 37. Gisbert JP, Niño P, Cara C, Rodrigo L. Comparative effectiveness of azathioprine in Crohn's disease and ulcerative colitis: prospective, long‐term, follow‐up study of 394 patients. Aliment Pharmacol Ther. 2008;28(2):228–38. [DOI] [PubMed] [Google Scholar]
- 38. Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, Loftus EV, Jr. , Peyrin‐Biroulet L, Blonski WC, Van Domselaar M, Chaparro M, Sandilya S, Bewtra M, Beigel F, Biancone L, Lichtenstein GR. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6‐mercaptopurine: a meta‐analysis. Clin Gastroenterol Hepatol. 2015;13(5):847‐58.e4; quiz e48‐50. [DOI] [PubMed] [Google Scholar]
- 39. Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non‐melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8(3):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heinze G, Wallisch C, Dunkler D. Variable selection – a review and recommendations for the practicing statistician. Biom J. 2018;60(3):431–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Commun Health. 2020;8(1):e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chebli JM, Gaburri PD, De Souza AF, Pinto AL, Chebli LA, Felga GE, et al. Long‐term results with azathioprine therapy in patients with corticosteroid‐dependent Crohn's disease: open‐label prospective study. J Gastroenterol Hepatol. 2007;22(2):268–74. [DOI] [PubMed] [Google Scholar]
- 43. Bayoumy AB, van Liere E, Simsek M, Warner B, Loganayagam A, Sanderson JD, et al. Efficacy, safety and drug survival of thioguanine as maintenance treatment for inflammatory bowel disease: a retrospective multi‐centre study in the United Kingdom. BMC Gastroenterol. 2020;20(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verstockt B, Boets L, Sabino J, Vermeire S, Ferrante M. Thiopurine monotherapy has a limited place in treatment of patients with mild‐to‐moderate Crohn's disease. Gut. 2021;70(7):1416–8. [DOI] [PubMed] [Google Scholar]
- 45. van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti‐TNFα therapy: results from the COIN study. Gut. 2014;63(1):72–9. [DOI] [PubMed] [Google Scholar]
- 46. Burisch J, Vardi H, Schwartz D, Friger M, Kiudelis G, Kupčinskas J, et al. Health‐care costs of inflammatory bowel disease in a pan‐European, community‐based, inception cohort during 5 years of follow‐up: a population‐based study. Lancet Gastroenterol Hepatol. 2020;5(5):454–64. [DOI] [PubMed] [Google Scholar]
- 47. Zhao M, Sall Jensen M, Knudsen T, Kelsen J, Coskun M, Kjellberg J, et al. Trends in the use of biologicals and their treatment outcomes among patients with inflammatory bowel diseases – a Danish nationwide cohort study. Aliment Pharmacol Ther. 2021;55:541–57. [DOI] [PubMed] [Google Scholar]
- 48. Narula N, Kainz S, Petritsch W, Haas T, Feichtenschlager T, Novacek G, et al. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti‐TNF‐α naïve Crohn's disease. Aliment Pharmacol Ther. 2016;44(2):170–80. [DOI] [PubMed] [Google Scholar]
- 49. Chen C, Hartzema AG, Xiao H, Wei YJ, Chaudhry N, Ewelukwa O, et al. Real‐world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25(8):1417–27. [DOI] [PubMed] [Google Scholar]
- 50. Helwig U, Kostev K, Schmidt C. Comparative analysis of 3‐year persistence with vedolizumab compared with antibodies against tumor necrosis factor‐alpha in patients with inflammatory bowel disease in germany: retrospective analysis of a large prescription database. J Clin Gastroenterol. 2021;55(1):e1–7. [DOI] [PubMed] [Google Scholar]
- 51. Angelison L, Almer S, Eriksson A, Karling P, Fagerberg U, Halfvarson J, et al. Long‐term outcome of infliximab treatment in chronic active ulcerative colitis: a Swedish multicentre study of 250 patients. Aliment Pharmacol Ther. 2017;45(4):519–32. [DOI] [PubMed] [Google Scholar]
- 52. Gisbert JP, Marín AC, Chaparro M. The risk of relapse after anti‐TNF discontinuation in inflammatory bowel disease: systematic review and meta‐analysis. Am J Gastroenterol. 2016;111(5):632–47. [DOI] [PubMed] [Google Scholar]
- 53. Kennedy NA, Warner B, Johnston EL, Flanders L, Hendy P, Ding NS, et al. Relapse after withdrawal from anti‐TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta‐analysis. Aliment Pharmacol Ther. 2016;43(8):910–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vasudevan A, Parthasarathy N, Con D, Nicolaides S, Apostolov R, Chauhan A, et al. Thiopurines vs methotrexate: comparing tolerability and discontinuation rates in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52(7):1174–84. [DOI] [PubMed] [Google Scholar]
- 55. Macaluso FS, Renna S, Maida M, Dimarco M, Sapienza C, Affronti M, et al. Tolerability profile of thiopurines in inflammatory bowel disease: a prospective experience. Scand J Gastroenterol. 2017;52(9):981–7. [DOI] [PubMed] [Google Scholar]
- 56. Meijer B, Mulder CJ, Peters GJ, van Bodegraven AA, de Boer NK. Efficacy of thioguanine treatment in inflammatory bowel disease: a systematic review. World J Gastroenterol. 2016;22(40):9012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simsek M, Deben DS, Horjus CS, Bénard MV, Lissenberg‐Witte BI, Buiter HJC, et al. Sustained effectiveness, safety and therapeutic drug monitoring of tioguanine in a cohort of 274 IBD patients intolerant for conventional therapies. Aliment Pharmacol Ther. 2019;50(1):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoentjen F, Seinen ML, Hanauer SB, de Boer NKH, Rubin DT, Bouma G, et al. Safety and effectiveness of long‐term allopurinol–thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2012;19(2):363–9. [DOI] [PubMed] [Google Scholar]
- 59. Meijer B, Seinen ML, van Egmond R, Bouma G, Mulder CJJ, van Bodegraven AA, et al. Optimizing thiopurine therapy in inflammatory bowel disease among 2 real‐life intercept cohorts: effect of allopurinol comedication? Inflamm Bowel Dis. 2017;23(11):2011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet (London, England). 2009;374(9701):1617–25. [DOI] [PubMed] [Google Scholar]
- 61. Tominaga K, Sugaya T, Tanaka T, Kanazawa M, Iijima M, Irisawa A. Thiopurines: recent topics and their role in the treatment of inflammatory bowel diseases. Front Pharmacol. 2021;11:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Solitano V, D'Amico F, Correale C, Peyrin‐Biroulet L, Danese S. Thiopurines and non‐melanoma skin cancer: partners in crime in inflammatory bowel diseases. Br Med Bull. 2020;136(1):107–17. [DOI] [PubMed] [Google Scholar]
- 63. Peyrin‐Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, Carbonnel F, Colombel JF, Dupas JL, Godeberge P, Hugot JP, Lémann M, Nahon S, Sabaté JM, Tucat G, Beaugerie L. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011;141(5):1621‐28.e1‐5, 1628.e5. [DOI] [PubMed] [Google Scholar]
- 64. Louis E, Irving P, Beaugerie L. Use of azathioprine in IBD: modern aspects of an old drug. Gut. 2014;63(11):1695–9. [DOI] [PubMed] [Google Scholar]
- 65. Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vernier‐Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, et al. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. 2007;56(10):1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Asseldonk DP, Jharap B, Verheij J, den Hartog G, Westerveld DB, Becx MC, et al. The prevalence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with thioguanine is not associated with clinically significant liver disease. Inflamm Bowel Dis. 2016;22(9):2112–20. [DOI] [PubMed] [Google Scholar]
- 68. De Boer NK, Tuynman H, Bloemena E, Westerga J, Van Der Peet DL, Mulder CJ, et al. Histopathology of liver biopsies from a thiopurine‐naïve inflammatory bowel disease cohort: prevalence of nodular regenerative hyperplasia. Scand J Gastroenterol. 2008;43(5):604–8. [DOI] [PubMed] [Google Scholar]
- 69. FDA . FDA requires warnings about increased risk of serious heart‐related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions 2021. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐requires‐warnings‐about‐increased‐risk‐serious‐heart‐related‐events‐cancer‐blood‐clots‐and‐death.
- 70. Campbell S, Ghosh S. Effective maintenance of inflammatory bowel disease remission by azathioprine does not require concurrent 5‐aminosalicylate therapy. Eur J Gastroenterol Hepatol. 2001;13(11):1297–301. [DOI] [PubMed] [Google Scholar]
- 71. Andrews JM, Travis SP, Gibson PR, Gasche C. Systematic review: does concurrent therapy with 5‐ASA and immunomodulators in inflammatory bowel disease improve outcomes? Aliment Pharmacol Ther. 2009;29(5):459–69. [DOI] [PubMed] [Google Scholar]
- 72. de Boer NKH. Thiopurine therapy in inflammatory bowel diseases: making new friends should not mean losing old ones. Gastroenterology. 2019;156(1):11–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Data Availability Statement
A data management plan for IBDSL is made in collaboration with DataHub MUMC+. DataHub works according to the FAIR principles. Metadata of IBDSL is available for interested researchers and data is available after approval of research proposals by the IBDSL committee (contact: m.pierik@mumc.nl).


