Abstract
Background
Risk factors for local atypical fibroxanthoma (AFX) recurrence and progression to pleomorphic dermal sarcoma (PDS) have not previously been identified.
Objective
To identify risk factors and provide follow‐up suggestions for local AFX recurrence and progression to PDS.
Methods and Materials
A literature search was performed in the PubMed, EMBASE, and Cochrane databases. The PRISMA and MOOSE guidelines were followed. The risks of local AFX recurrence and progression to PDS were presented as Kaplan–Meier plots and risk factors were presented as hazard ratios (HRs) calculated with univariate and multivariate Cox regression.
Results
Five hundred and ninety‐eight patients with AFX from 14 studies were included. Age >74 years and male sex significantly increased the risk of local recurrence (HR: 7.31 [95% confidence interval [CI]: 1.78–30.0], p < 0.01 and HR: 2.89 [95% CI: 1.04–8.01], p < 0.05, respectively). There was no difference when comparing wide local excision and Mohs' micrographic surgery (p = 0.89). The risks of local AFX recurrence and progression to PDS after 2 years were <1%.
Conclusion
A more intensive follow‐up regimen could be considered in patients >74 years old and males due to the higher risk of local AFX recurrence.
Keywords: atypical fibroxanthoma, Mohs, oncology, skin cancer
1. BACKGROUND
Atypical fibroxanthoma (AFX) is a rare cutaneous mesenchymal tumor that can progress to the invasive pleomorphic dermal sarcoma (PDS). Diagnosing AFX is challenging, as AFX shares clinical and histopathological features with other neoplasms, such as spindle‐cell squamous cell carcinoma, desmoplastic malignant melanoma, and basal cell carcinoma. 1 Furthermore, AFX and PDS are difficult to distinguish from one another but features such as invasion of the subcutaneous tissue, vascular or perineural invasion, the presence of necrosis, and aggressive clinical behavior such as local invasion or metastasis suggest PDS. In cases of metastasis it is difficult to determine whether the primary AFX has progressed to PDS or whether the initial tumor should be classified as a primary PDS.
The risk of recurrence after surgical removal of AFX has previously been estimated to be 4.6%–11.3%, and the risk of metastases after initial diagnosis of AFX is 0.5%–3.2%. 2 , 3 , 4 Due to the rarity of the tumor, previous studies have not had sufficient power to assess which patients with primary AFX that should be followed more closely for early detection of local AFX recurrence and progression to PDS.
Meta‐analyses based on individualized participant data have gained popularity over the last decade and are now considered the gold standard. 5 This is due mainly to the increased flexibility, as the analysis is less dependent on the original published data format, which makes data comparable across studies. In this study, we used meta‐analytical methods to estimate the overall risks of local AFX recurrence and progression to PDS and to provide individualized follow‐up suggestions for early detection of local AFX recurrence and progression to PDS.
2. PATIENTS AND METHODS
The meta‐analysis was conducted in accordance with the PRISMA 6 and MOOSE 7 guidelines and the Cochrane handbook of systematic reviews when applicable. 8 The screening of eligible studies was conducted by two independent authors (FLAA and KA), and all discrepancies were discussed with a third author (MØ). All outcomes and statistical analyses were chosen a priori.
2.1. Search strategy and inclusion criteria
The literature search was performed in the PubMed, EMBASE, and Cochrane databases with the search string: “atypical fibroxanthoma” OR “atypical cutaneous fibroxanthoma”OR “malignant histiocytoma” OR “pleomorphic malignant fibrous histiocytoma” OR “pseudosarcomatous reticulohistiocytoma” OR “superficial malignant fibrous histiocytoma” OR “superficial undifferentiated pleomorphic sarcoma.”
Studies were included if they reported on local recurrence or metastasis in cohorts of at least five patients initially diagnosed with AFX validated by histopathology. The current understanding in the literature is that AFX does not metastasize. 9 , 10 As detailed descriptions of the depth of the primary AFX was not available in the included studies, we chose to consider any case of subsequent local invasion or metastasis after initial diagnosis of AFX as a progression to PDS. We only included primary tumors classified as AFX and therefore we excluded patients who presented with primary PDS. Case reports, commentaries, letters, discussions, reviews, and citations in languages other than English, French, German, or Scandinavian were excluded.
2.2. Data extraction and quality assessment
Baseline characteristics were extracted for each included study (e.g., author, year of publication, country, number of patients, and follow‐up). Few of the included studies provided information regarding the specific follow‐up regimen such as the interval or if imaging was employed. The extracted outcomes of interest were the presence of local recurrence or invasion/metastasis, time to event, age at presentation, sex, tumor size, the surgical excision margin, postoperative defect size, type of surgery (wide local excision [WLE] or Mohs' micrographic surgery [MMS]), previous skin cancer and immunosuppression. If authors described a patient with deep‐tissue invasion or metastasis, it was considered a progression to PDS. Excision margins were only extracted in patients undergoing WLE, as the MMS technique is performed in multiple stages until histological margins are clear. 11 The areas of the postoperative defects were extracted for all patients and adjusted for tumor size as a defect/tumor size ratio to compare the amount of tissue removed in WLE and MMS. The quality of the included studies was assessed with the methodological index for non‐randomized studies (MINORS) with a maximal score of 16. 12
2.3. Outcomes
The outcomes of the meta‐analyses were the overall risk of local AFX recurrence, AFX progression to PDS, and risk factors for local AFX recurrence and progression to PDS.
2.4. Statistical analysis
The meta‐analysis of individual participant data was performed as a one‐stage analysis. The overall risks of local AFX recurrence and progression to PDS were estimated using survival analysis with Kaplan–Meier plots. Hazard ratios (HRs) for risk factors (age at presentation, sex, tumor size, excision margin, type of surgery, previous skin cancer, and immunosuppression) were estimated with both univariate and multivariate Cox regression models with robust covariance matrix estimation to adjust for clustering of studies. Significant and borderline significant results (p < 0.1) were included in the multivariate analysis. Age was divided into two groups with a cutoff at 74 years calculated with the maximally selected rank statistic. For patients undergoing WLE, the excision margin was divided into three clinically relevant cutoff points: <5, 5–10, and >10 mm. The assumptions of proportional hazards and linearity were tested and were not violated in any of the models. 13 , 14 A sensitivity analysis of the multivariate Cox regression was performed by leaving out studies before 2002 due to an increased risk of misclassification as the diagnostic criteria of AFX was revised at this time point. 15 An assessment of risk factors for AFX progressing into PDS was not attempted because of too few events. All results are presented as HRs with 95% confidence intervals (CI). The median follow‐up time was estimated with the reverse Kaplan–Meier method. Heterogeneity of the included studies was calculated with tau, I 2‐statistics, and the chi‐squared test of heterogeneity as a two‐stage analysis using the incidence rates (events per total person‐years) of the included studies. A two‐sided p < 0.05 was considered statistically significant. All statistical analyses and plots were performed in R, version 4.0.3 with the packages rms, survival, maxstat, metafor, and ggplot2.
3. RESULTS
3.1. Search results and quality of the included studies
The search resulted in 1740 eligible studies and 8 studies were included directly. The corresponding authors of 20 studies were contacted, and we received additional data from six authors (461 patients) resulting in a total inclusion of 598 patients from 14 studies. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 A flow chart of the screening process is shown in Figure 1. All the included studies were retrospective and observational studies of moderate quality. The mean MINORS score of the included studies was 10.5 (95% CI: 9.34–11.66). The overall heterogeneity of the included studies was significant with an I 2 of 77.3%, p < 0.01. Study characteristics and a forest plot of the included studies are shown in Table 1 and Figure 2.
Figure 1.
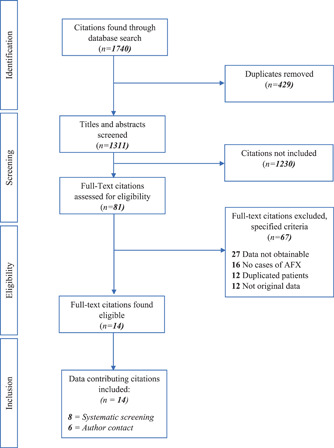
Flow chart of the screening process.
Table 1.
Baseline characteristics of the included studies.
| Author | Year | Country | Patients, no | Mean age, years | Mean follow‐up, months | Number of recurrences | Number of metastases | Minors score |
|---|---|---|---|---|---|---|---|---|
| Ang | 2009 | USA | 86 | 71.8 | 82.8 | 2 | 0 | 11 |
| Beer | 2010 | Australia | 167 | 75.1 | 56.9 | 2 | 0 | 11 |
| Davidson | 2012 | Canada | 71 | 76.4 | 68.5 | 7 | 3 | 12 |
| Flohil | 2017 | Netherlands | 22 | 71.8 | 24.0 | 2 | 0 | 13 |
| Hudson | 1972 | USA | 15 | 52.3 | 18.5 | 0 | 0 | 7 |
| Iglesias‐Pena | 2020 | Spain | 62 | 81.0 | 47.7 | 4 | 0 | 14 |
| Kempson | 1964 | USA | 19 | 66.3 | 90.5 | 2 | 0 | 13 |
| Koch | 2015 | Germany | 18 | 75.4 | 53.8 | 4 | 1 | 12 |
| Limmer | 1997 | USA | 6 | 71.5 | 32.5 | 0 | 0 | 8 |
| Seavolt | 2006 | USA | 11 | 75.1 | 29.5 | 0 | 0 | 8 |
| Sloane | 2015 | UK | 48 | 79.9 | 26.8 | 9 | 0 | 10 |
| Thum | 2013 | UK | 8 | 75.6 | 52.8 | 0 | 0 | 11 |
| Wollina | 2015 | Germany | 50 | 78.7 | 13.0 | 2 | 4 | 9 |
| Wylie | 2010 | UK | 15 | 80.4 | 30.5 | 1 | 0 | 8 |
Figure 2.
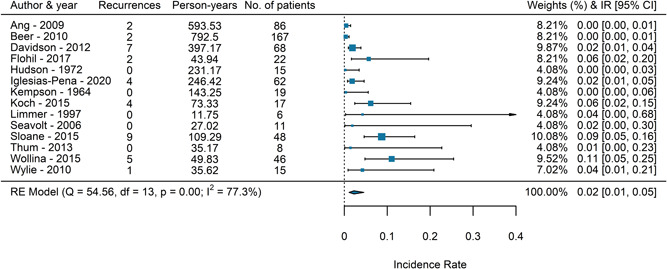
A forest plot of the incidence rates of local AFX recurrence of the included studies. AFX, atypical fibroxanthoma.
3.2. Baseline characteristics
The median age at diagnosis was 77 years (interquartile range [IQR]: 69–83), and the majority were men (78%). The median tumor diameter was 12.8 mm (IQR: 9–20), and tumors were almost exclusively in the head and neck region (94%). In 443 cases (75%) the tumors were excised by WLE in with a median excision margin of 3.0 mm (IQR: 1.0–5.0) and in 147 (25%) the tumors were excised with MMS. Baseline characteristics of the included patients are shown in Table 2.
Table 2.
Baseline characteristics of the included patients.
| No recurrence (N = 554) | Local AFX recurrence (N = 36) | Progression to PDS (N = 8) | Total (N = 598) | |
|---|---|---|---|---|
| Age, years | ||||
| Median | 76.0 | 81.0 | 80.0 | 76.7 |
| IQR | 68.9, 83.0 | 77.5, 85.5 | 78.0, 83.0 | 69.0, 83.1 |
| Sex | ||||
| Female | 112 (95.7%) | 3 (2.6%) | 2 (1.7%) | 117 (22.0%) |
| Male | 387 (90.6%) | 27 (6.3%) | 2 (0.5%) | 416 (78.0%) |
| Location | ||||
| Head and neck | 462 (90.1%) | 32 (6.2%) | 8 (1.6%) | 502 (93.8%) |
| Upper limbs | 15 (100.0%) | 0 (0.0%) | 0 (0.0%) | 15 (2.8%) |
| Truncus | 5 (100.0%) | 0 (0.0%) | 0 (0.0%) | 5 (0.9%) |
| Lower limbs | 13 (100.0%) | 0 (0.0%) | 0 (0.0%) | 13 (2.4%) |
| Previous skin cancer | ||||
| No | 132 (91.7%) | 9 (6.2%) | 3 (2.1%) | 144 (52.6%) |
| Yes | 116 (89.2%) | 9 (6.9%) | 5 (3.8%) | 130 (47.4%) |
| Surgery | ||||
| MMS | 136 (92.5%) | 7 (4.8%) | 4 (2.7%) | 147 (24.9%) |
| WLE | 410 (90.3%) | 29 (6.4%) | 4 (0.9%) | 443 (75.1%) |
| Size, mm | ||||
| Median | 13.0 | 12.8 | 4.9 | 12.8 |
| IQR | 9.0, 19.9 | 10.0, 20.0 | 4.9, 4.9 | 9.0, 20.0 |
| Margin, mm | ||||
| Median | 3.0 | 3.0 | 0.0 | 3.0 |
| IQR | 1.0, 5.0 | 1.5, 5.0 | 0.0, 0.0 | 1.0, 5.0 |
| <5 mm | 96 (86.5%) | 13 (11.7%) | 1 (0.9%) | 111 (64.9%) |
| 5–10 mm | 34 (87.2%) | 5 (12.8%) | 0 (0.0%) | 39 (22.8%) |
| >10 mm | 20 (95.2%) | 1 (4.8%) | 0 (0.0%) | 21 (12.3%) |
Note: Percentages in the three subgroups (no recurrence, local AFX recurrence, and progression to PDS). are compared to the total.
Abbreviations: AFX, atypical fibroxanthoma; IQR, interquartile range; MMS, Mohs' micrographic surgery; PDS, pleomorphic dermal sarcoma; WLE, wide local excision.
3.3. Overall risk of recurrence and progression to PDS
A total of 36 recurrences were reported, resulting in a 1‐year risk of 3.2% (95% CI: 1.4–5.1) and a 5‐year risk of 7.4% (95% CI: 1.7–13). The median time to recurrence was 13 months (95% CI: 11–16). The risk of recurrence after 2 years was 0.7% (95% CI: 0–1.3). A total of eight patients developed metastases and were considered to have progressed to PDS, resulting in a 1‐year risk of 0.54% (95% CI: 0–1.30) and a 5‐year risk of 1.72% (95% CI: 0–3.96). The median time to progression to PDS was 24 months (95% CI: 11.8–24). The risk of progression to PDS after 2 years was 0.26% (95% CI: 0–0.73). The median follow‐up was 48 months (IQR: 18.2–84.0). Kaplan–Meier plots are shown in Figures 3A,B.
Figure 3.
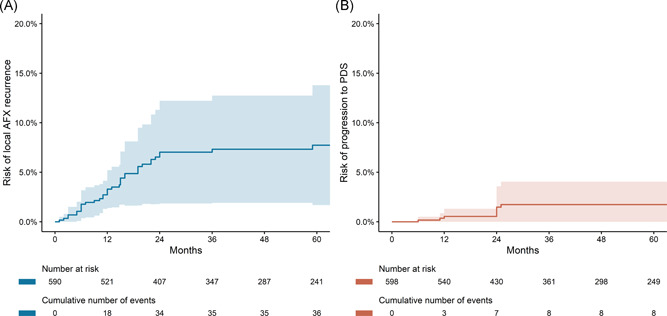
Kaplan–Meier plots showing risk of local AFX recurrence (A) and risk of progression to PDS (B). AFX, atypical fibroxanthoma; PDS, pleomorphic dermal sarcoma.
3.4. Risk factors for local AFX recurrence
The risk of local recurrence significantly increased with age at presentation above 74 years in both the univariate analysis and multivariate analysis (HR: 7.31 [95% CI: 1.78–30.0], p < 0.01). Males also had a significantly higher risk of local recurrence (HR: 2.89 [95% CI: 1.04–8.01], p < 0.05). In the case of WLE, there was no significant effect of an excision margin of 5–10 mm (p = 0.23) or >10 mm (p = 0.21). There was no significant difference between WLE and MMS (p = 0.39). The defect/tumor size ratios were 1.96 (IQR: 1.44–3.36) for WLE and 1.68 (IQR: 1.23–2.80) for MMS (p = 0.07). In a subgroup analysis, there was no significant difference between MMS and the three WLE subgroups (excision margin <5, 5–10, or >10 mm). Tumor size or previous skin cancer was not associated with the risk of recurrence. Age above 74 years remained significant in the sensitivity analysis, p < 0.05, whereas male sex did not, p = 0.05. The results from the Cox regression are shown in Table 3.
Table 3.
Results of the univariate, multivariate Cox regression and sensitivity analysis.
| Univariate analysis | Multivariate analysis | Sensitivity analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N, studies | HR (95% CI) | p value | N, studies | HR (95% CI) | p value | N, studies | HR (95% CI) | p value |
| Margin (mm) | 169, 5 | 529, 12 | 489, 9 | ||||||
| <5 | ‐ | ‐ | |||||||
| 5–10 | 1.27 (0.86–1.88) | 0.23 | |||||||
| >10 | 0.35 (0.07–1.83) | 0.21 | |||||||
| Surgery | 582, 14 | ||||||||
| WLE | ‐ | ‐ | |||||||
| MMS | 0.89 (0.16–4.99) | 0.89 | |||||||
| Age (years) | 575, 13 | ||||||||
| <74 | ‐ | ‐ | ‐ | ‐ | |||||
| >74 | 5.88 (1.85–18.7) | <0.01* | 7.31 (1.78–30.0) | <0.01* | 6.46 (1.56–26.8) | <0.05* | |||
| Sex | 529, 12 | ||||||||
| Female | ‐ | ‐ | ‐ | ‐ | |||||
| Male | 2.66 (0.87–8.19) | 0.09 | 2.89 (1.04–8.01) | <0.05* | 2.70 (0.99–7.33) | 0.05 | |||
| Size (mm) | 456, 11 | ||||||||
| <10 | ‐ | ‐ | |||||||
| 10–20 | 1.42 (0.56–3.55) | 0.46 | |||||||
| >20 | 1.81 (0.51–6.49) | 0.36 | |||||||
| Previous skin cancer | 266, 8 | ||||||||
| No | ‐ | ‐ | |||||||
| Yes | 1.16 (0.67–2.00) | 0.60 | |||||||
Note: Asterisk indicates statistical significance. Immunosuppression could not be estimated due to the small sample size.
Abbreviations: CI, confidence interval; HR, hazard ratio; MMS, Mohs' micrographic surgery; WLE, wide local excision.
3.5. Progression to PDS
A total of eight patients initially diagnosed with AFX subsequently developed metastasis and were therefore considered to have progressed to PDS. The median age at diagnosis was 80 years (IQR: 78–83) with a 1:1 male to female ratio. The primary AFX was excised with WLE in 50% of the cases. The excision margin was only reported in one patient. Five of the eight patients developed local AFX recurrence before metastasis. This results in an overall risk of subsequently progressing to PDS after local AFX recurrence of 13% (95% CI: 5.2–30). The regional metastases were localized to the skin and subcutis (n = 3), regional lymph nodes (n = 1), and parotid gland (n = 2), and distant metastases were localized to the lungs (n = 2), suggesting both lymphatic and hematogenous spread.
4. DISCUSSION
4.1. Key results
To the best of our knowledge, this is the first attempt to identify risk factors and provide follow‐up suggestions for early detection of local AFX recurrence and progression to PDS. The 5‐year risk of local recurrence was 7.4% (95% CI: 1.7–12.9), and the 5‐year risk of progression to PDS was 1.72% (95% CI: 0–3.96). The risks of local AFX recurrence and progression to PDS after 2 years were 0.7% (95% CI: 0–1.3) and 0.26% (95% CI: 0–0.73). Age at presentation above 74 years and male sex significantly and independently increased the risk of local recurrence (HR: 7.31 [95% CI: 1.78–30.0], p < 0.01 and HR: 2.89 [95% CI: 1.04–8.01], p < 0.05, respectively). There was no association between increasing excision margin (5–10 mm, p = 0.23 and >10 mm, p = 0.21) and the risk of local recurrence. There was no difference between WLE and MMS with regard to local recurrence (p = 0.89). None of the other investigated risk factors were significantly associated with the risk of local recurrence.
4.2. Follow‐up of patients with AFX
A follow‐up period of at least 2 years could be considered, as both the risk of local AFX recurrence and progression to PDS after this time point is <1%. Patients older than 74 years at presentation and male patients should be followed more closely, as these groups have a significantly increased risk of local recurrence. Metastases were found both locoregionally in the skin, regional lymph nodes, and parotid gland and in the lungs suggesting both lymphatic and hematogenous spreading of the tumor. As 13% of the patients with local AFX recurrence subsequently progress to PDS, a routine radiological examination of all patients with local AFX recurrence could be considered. There was no difference between WLE and MMS. The popularity of MMS has been due primarily to the greater degree of skin preservation. In our cohort, the median defect/tumor size ratios were 1.96 for WLE and 1.68 for MMS (p = 0.07), suggesting more tissue preservation after MMS. We found no difference in the risk of recurrence between MMS and WLE, and therefore MMS may be reasonable to use when the tumor is localized in anatomical regions with close proximity to important structures such as the ears, nose, lips, or near the eyes. 11
4.3. Progression to PDS
The differentiation between AFX and PDS is challenging since the tumors resemble each other both histopathologically and immunohistochemically. PDS is distinguished from AFX by features such as histological invasion of the subcutaneous tissue, vascular or perineural invasion, the presence of necrosis or local invasion/metastases. 31 , 32 The current consensus is that AFX does not metastasize 9 , 10 ; therefore, we chose to consider the cases of metastasis as a progression of the primary AFX diagnosis to a PDS. Whether this means that the patients in our cohort who initially were diagnosed with AFX should have been classified as a primary PDS or that the patients had a nonradically excised primary AFX that subsequently progressed to a PDS is therefore debatable. We did not include patients diagnosed with primary PDS, as the purpose of the study was to evaluate the risk of progression in a cohort of patients solely with primary AFX.
4.4. Limitations
The results of our meta‐analysis have some limitations. First, AFX is an exclusion diagnosis with an inherent risk of misclassification. Some of the tumors presented in our meta‐analysis could potentially represent tumors other than AFX, and other excluded tumors could have been AFXs misclassified as other tumors. However, it is most likely that non‐AFX tumors would have been diagnosed either clinically, histopathologically, or immunohistochemically, and with 598 patients included, the risk of misclassifications in both directions is assumed to be balanced. Second, the excision margins were often described as the most proximal distance to the border of the tumor. A more detailed description of both lateral and deep margins, the underlying tissue (e.g., fascia and periosteum) and the number of cases with positive margins on final pathology, and the subsequent number of re‐excisions due to infiltrated margins was not reported sufficiently to be included in the analysis. AFX has previously been described as difficult to excise radically due to its protuberant growth pattern. Some of the tumors in our data set could therefore have been non‐radically excised, which could explain the nonsignificant effect of a wide excision margin. Third, there is a risk that metastases/progression to PDS is not being reported, as some authors consider this tumor a different entity than AFX. This could underestimate the risk of progression to PDS. However, metastasis after excision of a tumor is considered a serious event and is therefore assumed to be reported if this occurred. Fourth, the analysis was limited by missing data despite the large sample size. The missing data was handled by analyzing only complete cases. In the multivariate analysis the proportion of missing data was approximately 10% and the estimates were considered. However, the proportions of missing data in the estimates involving excision margin and previous skin cancer were severe (>50%) and should be interpreted with caution. Fifth, the included studies showed substantial heterogeneity, and the exact diagnostic criteria of AFX where not specifically stated in the included studies. The results of the meta‐analysis should be interpreted with caution and further studies with homogenous AFX populations diagnosed with strict diagnostic criteria are needed to verify the results of this meta‐analysis.
5. CONCLUSION
The 5‐year risks of local AFX recurrence and progression to PDS were relatively low (7.4% and 1.7%, respectively). A closer follow‐up of patients >74 years old and males could be considered due to an increased risk of local AFX recurrence. MMS could be a reasonable option in cosmetically sensitive areas due to the higher degree of tissue preservation.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SYNOPSIS
The purpose of the meta‐analysis is to investigate risk factors associated with local atypical fibroxanthoma recurrence and progression to pleomorphic dermal sarcoma. Age >74 years and male sex were significantly associated with a higher risk of AFX recurrence and a closer follow‐up of this patient group could be considered.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank the following authors for contributing data and clarifying data regarding their studies: Dr. John Davidson, Dr. Daniel Demsey, Dr. Nicolas Iglesias‐Pena, Dr. Trevor Beer, Dr. Renate Van Den Bos, and Dr. James Sloane.
Ørholt M, Aaberg FL, Abebe K, et al. Risk factors for local atypical fibroxanthoma recurrence and progression to pleomorphic dermal sarcoma: a meta‐analysis of individualized participant data. J Surg Oncol. 2022;126:555‐562. 10.1002/jso.26898
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Ziemer M. Atypical fibroxanthoma. J der Dtsch Dermatologischen Gesellschaft. 2012;10(8):537‐548. [DOI] [PubMed] [Google Scholar]
- 2. Tolkachjov SN, Kelley BF, Alahdab F, Erwin PJ, Brewer JD. Atypical fibroxanthoma: systematic review and meta‐analysis of treatment with Mohs micrographic surgery or excision. J Am Acad Dermatol. 2018;79(5):929‐934. [DOI] [PubMed] [Google Scholar]
- 3. Phan K, Onggo J. Time to recurrence after surgical excision of atypical fibroxanthoma‐updated systematic review and meta‐analysis. Australas J Dermatol. 2019;60(3):e220‐e222. [DOI] [PubMed] [Google Scholar]
- 4. Polcz MM, Sebaratnam DF, Fernández‐Peñas P. Atypical fibroxanthoma management: recurrence, metastasis and disease‐specific death. Australas J Dermatol. 2018;59(1):10‐25. [DOI] [PubMed] [Google Scholar]
- 5. Riley RD, Lambert PC, Abo‐Zaid G. Meta‐analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:340‐c221. [DOI] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:2535. https://www.bmj.com/content/bmj/339/bmj.b2535.full.pdf [PMC free article] [PubMed] [Google Scholar]
- 7. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008‐2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 8. Higgins J, Sally G. Cochrane handbook for systematic reviews of interventions version 5.1.0; 2011.
- 9. Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma: adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol. 2012;36(9):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 10. Soleymani T, Hollmig ST. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18(8):1‐11. [DOI] [PubMed] [Google Scholar]
- 11. Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261‐1271. [DOI] [PubMed] [Google Scholar]
- 12. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. [DOI] [PubMed] [Google Scholar]
- 13. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515‐526. [Google Scholar]
- 14. Therneau TM, Grambsch PM, Fleming TR. Martingale‐based residuals for survival models. Biometrika. 1990;77(1):147‐160. [Google Scholar]
- 15. Fletcher CDM, Unni K, Mertens F, World Health Organization. Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press; 2002. [Google Scholar]
- 16. Ang GC, Roenigk RK, Otley CC, Kim Phillips P, Weaver AL. More than 2 decades of treating atypical fibroxanthoma at Mayo Clinic: what have we learned from 91 patients? Dermatol Surg. 2009;35(5):765‐772. [DOI] [PubMed] [Google Scholar]
- 17. Beer TW, Drury P, Heenan PJ. Atypical fibroxanthoma: a histological and immunohistochemical review of 171 cases. Am J Dermatopathol. 2010;32(6):533‐540. [DOI] [PubMed] [Google Scholar]
- 18. Davidson JS, Demsey D. Atypical fibroxanthoma: clinicopathologic determinants for recurrence and implications for surgical management. J Surg Oncol. 2012;105(6):559‐562. [DOI] [PubMed] [Google Scholar]
- 19. Flohil SC, van Lee CB, Beisenherz J, et al. Mohs micrographic surgery of rare cutaneous tumours. J Eur Acad Dermatology Venereol. 2017;31(8):1285‐1288. [DOI] [PubMed] [Google Scholar]
- 20. Hudson AW, Winkelmann RK. Atypical fibroxanthoma of the skin: a reappraisal of 19 cases in which the original diagnosis was spindle‐cell squamous carcinoma. Cancer. 1972;29(2):413‐422. [DOI] [PubMed] [Google Scholar]
- 21. Iglesias‐Pena N, López‐Solache L, Martínez‐Campayo N, et al. Incidence rate and clinicopathological features of 62 atypical fibroxanthomas in a North‐Western Spanish population. Australas J Dermatol. 2020;61(1):e22‐e27. [DOI] [PubMed] [Google Scholar]
- 22. Kempson RL, McGavran MH. Atypical fibroxanthomas of the skin. Cancer. 1964;17(11):1463‐1471. [DOI] [PubMed] [Google Scholar]
- 23. Koch M, Freundl AJ, Agaimy A, et al. Atypical fibroxanthoma‐histological diagnosis, immunohistochemical markers and concepts of therapy. Anticancer Res. 2015;35(11):5717‐5735. [PubMed] [Google Scholar]
- 24. Limmer BL, Clark DP. Cutaneous micrographic surgery for atypical fibroxanthoma. Dermatol Surg. 1997;23(7):553‐557. [DOI] [PubMed] [Google Scholar]
- 25. Seavolt M, McCall M. Atypical fibroxanthoma: review of the literature and summary of 13 patients treated with Mohs micrographic surgery. Dermatol Surg. 2006;32(3):435‐441. [DOI] [PubMed] [Google Scholar]
- 26. Sloane J, Patel D, Walsh S. Atypical fibroxanthoma of the head and neck: a UK clinicopathologic study of 110 patients. Int J Oral Maxillofac Surg. 2015;44:e146. [Google Scholar]
- 27. Thum C, Husain EA, Mulholland K, Hornick JL, Brenn T. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17(6):502‐507. [DOI] [PubMed] [Google Scholar]
- 28. Wang W‐L, Torres‐Cabala C, Curry JL, et al. Metastatic atypical fibroxanthoma: a series of 11 cases including with minimal and no subcutaneous involvement. Am J Dermatopathol. 2015;37(6):455‐461. [DOI] [PubMed] [Google Scholar]
- 29. Wollina U, Schönlebe J, Ziemer M, et al. Atypical fibroxanthoma: a series of 56 tumors and an unexplained uneven distribution of cases in southeast Germany. Head Neck. 2015;37(6):829‐834. [DOI] [PubMed] [Google Scholar]
- 30. Wylie J, Hampton N, Telfer MR, Clarke AMT. Atypical fibroxanthoma: case series of 16 patients. Br J Oral Maxillofac Surg. 2010;48(6):466‐468. [DOI] [PubMed] [Google Scholar]
- 31. Toll A, Gimeno J, Baró T, Hernández‐Muñoz MI, Pujol RM. Study of epithelial to mesenchymal transition in atypical fibroxanthoma and undifferentiated pleomorphic sarcoma to discern an epithelial origin. Am J Dermatopathol. 2016;38(4):270‐277. [DOI] [PubMed] [Google Scholar]
- 32. Hanlon A, Stasko T, Christiansen D, Cyrus N, Galan A. LN2, CD10, and Ezrin do not distinguish between atypical fibroxanthoma and undifferentiated pleomorphic sarcoma or predict clinical outcome. Dermatol Surg. 2017;43(3):431‐436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Data are available upon reasonable request.


